Abstract
Objectives
The aim of this analysis was to assess the effect of the coronary revascularization strategy during index admission on clinical outcomes among patients undergoing percutaneous coronary intervention (PCI) with multivessel coronary artery disease (MVD).
Background
The value of complete revascularization (CR) over incomplete revascularization (IR) in MVD patients is not fully established.
Methods
Patients with MVD defined as ≥2 major epicardial vessels with ≥50% stenosis were selected from the observational all‐comer e‐Ultimaster registry. Patients were treated with a sirolimus‐eluting thin‐strut coronary stent. Completeness of revascularization was physician assessed at the index procedure or an eventually staged procedure during the index hospitalization. Outcomes measures at 1 year were target lesion failure (TLF) (composite of cardiac death, target vessel‐related myocardial infarction [MI], and clinically driven target lesion revascularization [TLR]), and patient‐oriented composite endpoint (POCE) (all‐cause mortality, MI, or revascularization). The inverse probability of treatment weights (IPTW) methodology was used to perform a matched analysis.
Results
The registry recruited 37,198 patients of whom 15,441 (41.5%) had MVD. CR on hospital discharge was achieved in 7413 (48.0%) patients and IR in 8028 (52.0%) patients. Mean age was 64.6 ± 11.1 versus 65.7 ± 11.0 years (p < 0.01), male gender 77.9% and 77.3% (p = 0.41) and diabetes 31.3% versus 33.4% (p = 0.01) for CR and IR, respectively. Chronic stable angina patients more commonly underwent CR (47.6% vs. 36.8%, p < 0.01). After propensity weighted analysis, 90.5% of CR patients were angina‐free at 1 year compared with 87.5% of IR patients (p < 0.01). TLF (3.3% vs. 4.4%; p < 0.01), POCE (6.8% vs. 10.8%; p < .01), and all‐cause mortality (2.3% vs. 3.1%; p < .01) were all lower in CR patients.
Conclusions
A physician‐directed use of a CR strategy utilizing sirolimus‐eluting thin‐strut stent results in optimized clinical outcomes and less angina in an all‐comer population. Our findings suggest that a CR should be aimed for.
Keywords: all‐comers, drug‐eluting stent, multivessel disease, percutaneous coronary intervention, revascularization strategy
- Abbreviations: ACC/AHA
American College of Cardiology/American Heart Association
- ACS
acute coronary syndrome
- CABG
coronary artery bypass graft
- CAD
coronary artery disease
- CR
complete revascularization
- DES
drug‐eluting stent
- IR
incomplete revascularization
- IPTW
inverse probability of treatment weights
- MI
myocardial infarction
- MVD
multivessel disease
- (N)STEMI
(non) ST elevation myocardial infarction
- PCI
percutaneous coronary intervention
- POCE
patient‐oriented composite endpoint
- PSM
propensity score matching
- RCT
randomized controlled trial
- ST
stent thrombosis
- TLF
target lesion failure
- TLR
target lesion revascularization
- TV
target vessel
- TVF
target vessel failure
- TVR
target vessel revascularization
1. INTRODUCTION
Current guidelines identify completeness of revascularization as a major consideration in patients being considered for either coronary artery bypass graft (CABG) surgery or percutaneous coronary intervention (PCI). 1 In the absence of prospective, randomized studies, this Class IIa recommendation with the level of evidence B is based upon data coming from either CABG or PCI studies or studies comparing both treatment modalities that are reporting on complete revascularization (CR) versus incomplete revascularization (IR). A large meta‐analysis among patients undergoing PCI or CABG demonstrated that CR was associated with reduced mortality in both groups. 2 Similarly, posthoc analysis of the SYNTAX trial showed an association of IR with worse long‐term outcomes after both CABG and PCI. 3 , 4 Another meta‐analysis of 14 observational studies showed that CR was associated with lower all‐cause and cardiovascular mortality. 5 However, a recent smaller randomized study (CORRECT‐II) did not find a difference between the two strategies at 5‐year follow‐up. 6 The question remains as to whether complete revascularization in patients undergoing PCI offers greater benefit than incomplete revascularisation with a contemporary DES. 7
The e‐Ultimaster registry recruited patients worldwide who underwent PCI with the cobalt‐chromium thin‐strut sirolimus‐eluting stent with an abluminal bioresorbable polymer coating (Ultimaster). A subset of patients within this study had multivessel disease, and the revascularization strategy was physician‐directed. We analyzed the data from this large international registry to assess the effect of revascularization strategy during index admission on clinical outcomes among patients undergoing PCI with MVD.
2. METHODS
2.1. Study design
The e‐Ultimaster registry (Clinicaltrials.gov NCT02188355) was a prospective, multicenter, observational registry examining the use of the bioresorbable polymer‐coated sirolimus‐eluting Ultimaster stent. Recruitment was worldwide and accepted all‐comer patients aged over 18, irrespective of presentation. Patients were followed up clinically at 3 months and 1 year after their procedure. Antiplatelet therapy was at the discretion of the operator. Predefined groups were selected for subgroup analysis from the overall registry, including patients with multivessel disease defined as the presence of a >50% diameter stenosis on angiography in two or three major epicardial coronary vessels or bypass grafts. The registry was conducted in accordance with the Declaration of Helsinki and country‐specific regulatory requirements. All patients signed a consent form reviewed and approved by the Institutional Review Board/Ethics Committee of each participating center.
Completeness of revascularization in patients with the multivessel coronary disease was physician assessed at the index procedure and eventually staged procedure performed during the index hospitalization. For this analysis, patients were divided based upon the completeness of revascularization either during the index procedure or during the index hospitalization.
2.2. Study device
The Ultimaster stent (Terumo Corporation, Tokyo, Japan) 8 is an 80 μm‐thick cobalt‐chromium device mounted on a semi‐compliant balloon, with an abluminal, gradient coating of bioresorbable polymer (PDLLA‐PCL: poly (d, l) lactic acid‐polycaprolactone) as a delivery system for the immunosuppressant sirolimus. The polymer degrades over 3–4 months. Stent sizes are available from 2.25 to 4 mm diameter, in lengths of 9, 12, 15, 18, 24, 28, 33, and 38 mm.
2.3. Outcomes
The primary outcome measure was target lesion failure (TLF) at 1 year, defined as a composite of cardiac death, target vessel (TV) related myocardial infarction (MI), and clinically driven (CD) target lesion revascularization (TLR). Secondary outcomes at 3 months and 1 year included all‐cause mortality, cardiac mortality, MI, TLR, target vessel revascularization (TVR), freedom from angina, patient‐oriented composite endpoint (POCE; all‐cause mortality, any MI or any coronary revascularization), and definite and probable stent thrombosis (ST), defined according to the Academic Research Consortium (ARC) definitions. An independent clinical event committee reviewed and adjudicated all endpoint‐related adverse events.
2.4. Statistical analysis
Patient demographics, risk factors, procedural, and lesion characteristics are summarized with mean, standard deviation, and percentages for discrete variables. A propensity score analysis was performed to reduce the effect of baseline differences between the two groups. Propensity scores were calculated using a logistic regression model with the subgroup (CR vs. IR) as the outcome and the variables, which needed to be matched, as independent variables. The probability of belonging to one of the two groups was used as the propensity score. Variables to be entered into the model were predefined based on any possible impact on the outcomes and include: age, gender, smoking, diabetes, hypertension, renal failure, family history of heart disease, history of MI, previous PCI, previous CABG, ACS, STEMI, left main, graft treated, type B2 and C lesions (according to the classification of American College of Cardiology/American Heart Association/[ACC/AHA]), bifurcation, moderate to severe calcification, chronic total occlusion, in‐stent restenosis, long lesions (≥25 mm), small vessels (≤2.75 mm), radial access, and post‐dilatation. The inverse probability of treatment weights (IPTW) methodology was used to perform a matched analysis.
Weighted χ 2 tests are used for binary or categorical data, weighted Wilcoxon rank‐sum tests were used for continuous data, and time‐to‐event data were analyzed using weighted Kaplan–Meier analysis according to Xie and Liu. 9 For subgroup analyses, weighted relative risks were calculated using logistic regression. A p‐value less than 0.05 was considered significant. Statistical analyses were performed using SAS software, version 9.4 (SAS Institute, Inc.).
3. RESULTS
3.1. Patient, lesions, and procedural characteristics
In total, 37,198 patients were enrolled between October 2014 and June 2018 (Appendix Figure 1), of which 15,441 (41.5%) presented with multivessel coronary disease. Of this subgroup, 7413 (48.0%) were CR and 8028 (52.0%) were IR at their index procedure or in a staged procedure whilst an inpatient. Patient characteristics are shown in Table 1. Patients in the completely revascularised group were younger (mean age 64.6 ± 11.3 vs. 65.7 ± 11.0 year; p < 0.0001), had a lower prevalence of hypertension (69.8% vs. 73.8%; p < 0.0001), renal impairment (7.5% vs. 10.7%; p < 0.0001) or a previous MI (24.3% vs. 28.2%; p < 0.001). The CR group had a higher proportion of current smokers (23.5% vs. 22.0%; p = 0.04) and higher number of patients with a family history of heart disease (37.0% vs. 31.6%; p < .0001). In the CR group, 16.4% presented with a STEMI, while this was 22.9% in the IR group (p < 0.00001). The clinical presentation was more often an ACS in the IR group (52.3% vs. 63.1%, p < 0.0001).
Figure 1.
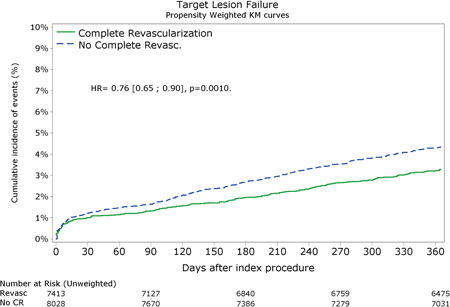
Propensity adjusted Kaplein–Meier curve for the primary outcome of target lesion failure up to 1 year follow‐up [Color figure can be viewed at wileyonlinelibrary.com]
Table 1.
Baseline patient characteristics
| Unadjusted | |||
|---|---|---|---|
|
CR n = 7413 |
IR n = 8028 |
p‐value | |
| Age, years | 64.6 ± 11.3 | 65.7 ± 11.0 | <0.0001 |
| Male gender | 77.9 | 77.3 | 0.41 |
| Current smoker | 23.5 | 22.0 | 0.04 |
| Diabetes mellitus | 31.3 | 33.4 | 0.01 |
| Hypertension | 69.8 | 73.8 | <0.0001 |
| Hypercholesterolemia | 63.2 | 62.3 | 0.27 |
| Renal impairment | 7.5 | 10.7 | <0.0001 |
| Family history of heart disease | 37.0 | 31.6 | <0.0001 |
| Previous MI | 24.3 | 28.2 | <0.001 |
| Previous PCI | 28.1 | 28.9 | 0.25 |
| Chronic coronary syndrome | 47.6 | 36.8 | <0.0001 |
| Acute coronary syndrome | 52.3 | 63.1 | <0.0001 |
| STEMI | 16.4 | 22.9 | <0.0001 |
| NSTEMI | 24.3 | 26.8 | <0.001 |
| Unstable angina pectoris | 11.6 | 13.4 | 0.001 |
Note: Values are mean ± SD or percentages.
Abbreviations: ACS, acute coronary syndrome; CR, complete revascularization; IR, incomplete revascularization; MI, myocardial infarction; PCI, percutaneous coronary intervention; SD, standard deviation; STEMI, ST elevation myocardial infarction.
Characteristics of the procedures and lesions are shown in Table 2. The CR group patients had fewer lesions per patient identified (mean 2.4 ± 0.9 vs. 2.9 ± 1.1; p < 0.0001), but with more bifurcation disease (15.5% vs. 13.4%; p = 0.01), left main stem disease (5.8% vs. 4.4%; p < 0.0001), chronic total occlusions (6.1% vs. 5.1%; p = 0.01), and more long lesions requiring ≥25 mm stent length implanted (44.2% vs. 40.6%; p < 0.0001) and more smaller vessels requiring a stent diameter ≤2.75 mm (53.4% vs. 45.3%; p < 0.0001).
Table 2.
Procedure and lesion characteristics (per patient)
| Unadjusted | |||
|---|---|---|---|
|
CR n = 7413 |
IR n = 8028 |
p‐value | |
| Left main | 5.8 | 4.4 | <0.0001 |
| Right coronary artery | 46.6 | 35.5 | <0.0001 |
| Left anterior descending | 60.8 | 46.9 | <0.0001 |
| Left circumflex | 47.4 | 31.3 | <0.0001 |
| Bifurcation | 15.5 | 13.4 | 0.01 |
| In‐stent restenosis | 6.7 | 5.1 | <0.0001 |
| Stent length ≥ 25 mm | 44.2 | 40.6 | <0.0001 |
| Stent diameter ≤ 2.75 mm | 53.4 | 45.3 | <0.0001 |
| Chronic total occlusion | 6.1 | 5.1 | 0.01 |
| Venous or arterial graft | 1.4 | 2.1 | <0.001 |
| Calcified lesion (severe/moderate) | 22.7 | 23.1 | 0.54 |
| Ostial lesion | 10.3 | 9.1 | <0.01 |
| Radial access | 74.8 | 81.2 | <0.0001 |
| N of lesions identified, n | 2.4 ± 0.9 | 2.9 ± 1.1 | <0.0001 |
| N of lesions treated, n | 1.8 ± 0.8 | 1.3 ± 0.6 | <0.0001 |
| N of stents implanted, n | 2.2 ± 1.1 | 1.6 ± 0.9 | <0.0001 |
| Total stent length, mm | 39.4 ± 24.6 | 32.5 ± 20.3 | <0.0001 |
| Pre‐dilatation | 71.0 | 72.5 | 0.05 |
| Post‐dilatation | 52.4 | 50.6 | <0.03 |
Note: Values are mean ± SD or percentages. Type B2 and C lesions according to ACC/AHA lesion classification.
Abbreviations: ACC/AHA, American College of Cardiology/American Heart Association; CR, complete revascularization; IR, incomplete revascularization; SD, standard deviation.
3.2. Clinical outcomes
Dual antiplatelet use (DAPT) pre‐procedure (76.7% vs. 77.2%; p = 0.47) and at index procedure discharge (97.5% vs. 97.5%; p = 0.90) was similar in the two groups. More patient in the CR group were on DAPT at 3 month (92.6 vs. 91.3%; p < 0.001) and at 1 year (68.3% vs. 65.7%, p < 0.001).
After Propensity weighted analysis, the primary outcome (Table 3 and Figure 1) of TLF at 1 year follow‐up was lower after CR (3.3% vs. 4.4%; p = 0.001). All‐cause mortality (2.3% vs. 3.1%; p < 0.01) and cardiac mortality (1.3% vs. 1.9%; p < 0.01) were lower in CR group compared with the IR group. Rates of any MI and TV‐MI in the CR versus IR group were 1.3% versus 1.6% (p = 0.22) and 1.0 versus 1.1% (p = 0.35), respectively. The rates of clinically driven target lesion revascularization were 1.7% versus 2.2%; p < 0.05. The overall incidence of target vessel revascularization (2.6% versus 2.9%; p = 0.25) was similar between the two groups. Non target vessel revascularisation occurred less in CR patients (2.4% versus 5.6%; p < 0.0001). POCE was lower (6.8% vs. 10.8%; p < 0.0001) after CR. Unadjusted clinical outcomes at 3 months and 1 year are shown in Table S1.
Table 3.
Incidence of propensity‐adjusted clinical outcomes and anginal status at 3 months and 1 year
| 3‐month outcomes | 1‐year outcomes | |||||
|---|---|---|---|---|---|---|
|
CR n = 7413 |
IR n = 8028 |
p‐value |
CR n = 7413 |
IR n = 8028 |
p‐value | |
| TLF | 1.4 | 1.7 | 0.11 | 3.3 | 4.4 | 0.001 |
| POCE | 2.3 | 3.3 | 0.001 | 6.8 | 10.8 | <0.0001 |
| All‐cause death | 1.0 | 1.2 | 0.16 | 2.3 | 3.1 | <0.01 |
| Cardiac death | 0.7 | 0.9 | 0.47 | 1.3 | 1.9 | <0.01 |
| All MI | 0.6 | 0.9 | 0.11 | 1.3 | 1.6 | 0.22 |
| Target vessel MI | 0.5 | 0.7 | 0.17 | 1.0 | 1.1 | 0.35 |
| Clinically driven TLR | 0.5 | 0.6 | 0.26 | 1.7 | 2.2 | <0.05 |
| Clinically driven TVR | 0.7 | 0.8 | 0.35 | 2.6 | 2.9 | 0.25 |
| Non‐TVR | 0.7 | 1.3 | 0.001 | 2.4 | 5.6 | <0.0001 |
| Non‐TVR, re‐PCI | 0.7 | 1.1 | 0.02 | 2.3 | 5.2 | <0.0001 |
| Non‐TVR, CABG | 0.01 | 0.2 | <0.001 | 0.2 | 0.5 | <0.0001 |
| Definite/probable ST | 0.6 | 0.7 | 0.38 | 0.7 | 0.9 | 0.22 |
| Angina status | <0.0001 | <0.0001 | ||||
| No angina | 90.9 | 88.4 | 90.5 | 87.5 | ||
| Silent ischemia | 0.4 | 1.0 | 0.6 | 1.1 | ||
| Stable angina | 6.9 | 8.7 | 6.4 | 8.5 | ||
| Unstable angina | 0.6 | 0.5 | 0.8 | 0.8 | ||
Note: Values are percentages.
Abbreviations: CABG, coronary artery bypass graft; MI, myocardial infarction; PCI, percutaneous coronary intervention; POCE, patient‐oriented composite endpoint of all‐cause death, any MI and any coronary revascularization; TL, target lesion; TLF, target lesion failure, a composite of cardiac death, TV‐MI and clinically driven TLR; TLR, target lesion revascularization; TVR, target vessel revascularization; ST, stent thrombosis.
At 3 months and 1 year follow‐up, CR patients reported more freedom from angina (90.9% vs. 88.4% at 3 months and 90.5% vs. 87.5% at 1 year; both p < 0.0001).
3.3. Subgroup analysis
Figure 2 illustrates the propensity‐adjusted relative risk and 95% confidence interval (CI) for TLF at 1 year according to subgroups including diabetes mellitus, left main, long lesions (stent length ≥25 mm), small vessels (stent diameter ≤2.75 mm), bifurcation and acute coronary syndrome (ACS). A significant interaction (p = 0.03) was found for bifurcations with a higher risk for TLF in no bifurcation and no CR (p < 0.01). Furthermore, no CR was associated with a higher risk for most of the subgroups, that is, diabetes, no diabetes, no left main, stent length <25 mm, stent diameter ≤ and >2.75 mm, and no ACS patients.
Figure 2.
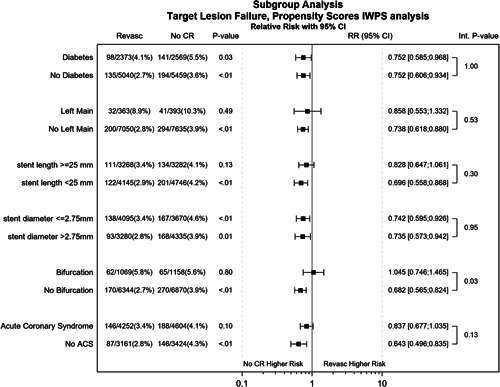
Subgroup analysis showing propensity‐adjusted relative risk with 95% confidence interval (CI) of TLF at 1‐year follow‐up. TLF, target lesion failure
4. DISCUSSION
In this analysis of all‐comer patients with multivessel disease undergoing PCI for a range of indications with a contemporary drug‐eluting stent, complete revascularisation at index hospitalization was associated with better patient outcomes. At 1 year, rates of all‐cause mortality, target lesion failure, and patient‐oriented composite outcome were lower in the CR group. Patients who had CR had less angina at 12 months follow‐up.
Our analysis includes similar real‐world patients to a systematic review and meta‐analysis including over 89,000 patients which reviewed outcomes in CR versus IR. 7 In this large meta‐analysis CR was associated with lower long‐term mortality (risk ratio [RR]: 0.71, 95% CI: 0.65–0.77; p < 0.001), lower rates of MI (RR: 0.78, 95% CI: 0.68–0.90; p < 0.001), and repeat revascularization (RR: 0.75, 95% CI: 0.65–0.83; p < 0.001). A pooled analysis of trials comparing PCI to CABG outcomes has similarly shown that results with CR are favorable to those with IR regardless of the modality of intervention, suggesting that the ability to achieve CR is important in improving outcomes. 10 Analysis of patients in the SYNTAX trial comparing CR vs IR showed higher mortality, revascularization, stent thrombosis, and major adverse cardiac events up to 4 years follow‐up after IR. 4
There are no direct randomized trials of CR versus IR in patients presenting with NSTEMI. An observational multicenter study of 21,857 NSTEMI patients with MVD showed superior long‐term mortality rates after CR despite initial higher mortality ratesrates. 11
Multiple studies have demonstrated benefits of CR versus infarct‐related artery only revascularization in the context of STEMI. 12 , 13 , 14 , 15 A meta‐analysis of CR demonstrated an advantage of this strategy with a reduction in MACE rates. 16 Long‐term outcomes are less clear, however, with most of the reduction in MACE coming from reduced rates of urgent revascularization, and an absence of benefit for mortality or MI. The optimal timing of the revascularization (immediate or staged) to achieve CR is less evident and needs further investigation. In patients with cardiogenic shock, however, a multivessel PCI approach during the index procedure was associated with increased mortality. 17
The definition of completeness or revascularization is not uniform. 18 This necessitates some caution in interpreting the data fully until a universal definition is adopted, especially given the variation in the use of intracoronary imaging and fractional flow reserve assessment in place during routine practice. In e‐Ultimaster, the definition of multivessel coronary disease was operator dependent and could be diagnosed angiographically, or on invasive functional assessment.
Another pitfall in interpreting results from investigations comes from the rapid advance in technology available for coronary intervention in contemporary practice. A meta‐analysis demonstrated improved outcomes in more recent publications regarding CR due to a significant reduction in relative risk of MI compared to older studies. 2 A possible reason includes advancing technology that enables more frequently complete percutaneous revascularization in complex anatomies, such as chronic total occlusions. 19 , 20 The Ultimaster DES platform had efficacy and safety demonstrated in two early trials, CENTURY and CENTURY II 21 , 22 , 23 before the initiation of e‐Ultimaster registry to provide further validation in a worldwide real‐world cohort of patients. Results in CR of patients in this cohort with multivessel disease using a contemporary DES are positive, supporting a role for complete revascularization in patients with multivessel disease. This adds to the growing evidence for the role of CR during PCI treatment of patients with multivessel coronary disease.
5. LIMITATIONS
Propensity matching has its limitations. When groups are different at the outset, matching will only correct for visible rather than all confounding variables. This is a limitation of our study and is in keeping with many other studies where propensity matching is used to try to improve the similarity of two groups. Incomplete revascularization can be considered to be a surrogate marker for sicker or more complex patients and therefore this must be borne in mind when interpreting our analysis.
Second, the multivessel disease was based on angiographic rather than ischemic indices. Many of the lesions that were noted to be >50% might not in fact have been flow‐limiting. Thirdly as this study reflected standard clinical practice across many geographies, there was no uniformity of procedural techniques or antiplatelet regimens. Neither was there a standardized definition of complete revascularisation used in the e‐CRF and it is possible that discrepancies exist in this regard.
Patients were included in the CR arm here if CR was achieved during the index procedure or index hospitalization. Patients with IR during the index procedure or index hospitalization may have been fully revascularized at a staged procedure later than their index hospitalization. Although this does not alter the results presented here, we cannot comment on the effect that delayed complete revascularisation may have had on outcomes.
6. CONCLUSION
In this large analysis of patients undergoing PCI with a contemporary DES platform, physician‐directed complete revascularization in patients with the multivessel disease was associated with better clinical outcomes and less angina during follow‐up.
CONFLICT OF INTERESTS
Except for the research grant received in the framework of the e‐Ultimaster study, the authors have no conflict of interests to declare.
Supporting information
Supporting information.
Supporting information.
ACKNOWLEDGMENT
e‐Ultimaster was funded by Terumo Europe.
Williams T, Mittal A, Karageorgiev D, et al. Complete revascularization optimizes patient outcomes in multivessel coronary artery disease: data from the e‐Ultimaster registry. Catheter Cardiovasc Interv. 2022;99:961‐967. 10.1002/ccd.30042
REFERENCES
- 1. Neumann FJ, Sousa‐Uva M. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40(287):87‐165. [DOI] [PubMed] [Google Scholar]
- 2. Zimarino M, Ricci F, Romanello M, et al. Complete myocardial revascularization confers a larger clinical benefit when performed with state‐of‐the‐art techniques in high‐risk patients with multivessel coronary artery disease: a meta‐analysis of randomized and observational studies. Catheter Cardiovasc Interv. 2016;87(1):3‐12. [DOI] [PubMed] [Google Scholar]
- 3. Farooq V, Serruys PW, Bourantas CV, et al. Quantification of incomplete revascularization and its association with five‐year mortality in the synergy between percutaneous coronary intervention with taxus and cardiac surgery (SYNTAX) trial validation of the residual SYNTAX score. Circulation. 2013;128(2):141‐151. [DOI] [PubMed] [Google Scholar]
- 4. Farooq V, Serruys PW, Garcia‐Garcia HM, et al. The negative impact of incomplete angiographic revascularization on clinical outcomes and its association with total occlusions: the SYNTAX (Synergy Between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery) trial. J Am Coll Cardiol. 2013;61(3):282‐294. [DOI] [PubMed] [Google Scholar]
- 5. Ando T, Takagi H, Grines CL. Complete versus incomplete revascularization with drug‐eluting stents for multi‐vessel disease in stable, unstable angina or non‐ST‐segment elevation myocardial infarction: a meta‐analysis. J Interv Cardiol. 2017;30(4):309‐317. [DOI] [PubMed] [Google Scholar]
- 6. Fagel ND, van Nooijen FC, Maarse M, et al. Five‐year results of the complete versus culprit vessel percutaneous coronary intervention in multivessel disease using drug‐eluting stents II (CORRECT II) study: a prospective, randomised controlled trial. Netherlands Heart J. 2019;27:310‐320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garcia S, Sandoval Y, Roukoz H, et al. Outcomes after complete versus incomplete revascularization of patients with multivessel coronary artery disease: a meta‐analysis of 89,883 patients enrolled in randomized clinical trials and observational studies. J Am Coll Cardiol. 2013;62(16):1421‐1431. [DOI] [PubMed] [Google Scholar]
- 8. Chisari A, Pistritto AM, Piccolo R, La Manna A, Danzi GB. The Ultimaster biodegradable‐polymer sirolimus‐eluting stent: an updated review of clinical evidence. Int J Mol Sci. 2016;17(9):e1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xie J, Liu C. Adjusted Kaplan‐Meier estimator and log‐rank test with inverse probability of treatment weighting for survival data. Stat Med. 2005;24(20):3089‐3110. [DOI] [PubMed] [Google Scholar]
- 10. Ahn JM, Park DW, Lee CW, et al. Comparison of stenting versus bypass surgery according to the completeness of revascularization in severe coronary artery disease: patient‐level pooled analysis of the SYNTAX, PRECOMBAT, and BEST Trials. JACC Cardiovasc Interv. 2017;10(14):1415‐1424. [DOI] [PubMed] [Google Scholar]
- 11. Rathod KS, Koganti S, Jain AK, et al. Complete versus culprit‐only lesion intervention in patients with acute coronary syndromes. J Am Coll Cardiol. 2018;72(17):1989‐1999. [DOI] [PubMed] [Google Scholar]
- 12. Wald DS, Morris JK, Wald NJ, et al. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med. 2013;369(12):1115‐1123. [DOI] [PubMed] [Google Scholar]
- 13. Gershlick AH, Khan JN, Kelly DJ, et al. Randomized trial of complete versus lesion‐only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol. 2015;65(10):963‐972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Engstrøm T, Kelbæk H, Helqvist S, et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST‐segment elevation myocardial infarction and multivessel disease (DANAMI‐3‐PRIMULTI): an open‐label, randomised controlled trial. Lancet. 2015;386(9994):665‐671. [DOI] [PubMed] [Google Scholar]
- 15. Smits PC, Abdel‐Wahab M, Neumann FJ, Boxma‐de Klerk BM, Lunde K, Schotborgh CE, et al. Fractional flow reserve‐guided multivessel angioplasty in myocardial infarction. N Engl J Med. 2017;376(13):1234‐1244. [DOI] [PubMed] [Google Scholar]
- 16. Elgendy IY, Mahmoud AN, Kumbhani DJ, Bhatt DL, Bavry AA. Complete or culprit‐only revascularization for patients with multivessel coronary artery disease undergoing percutaneous coronary intervention: a pairwise and network meta‐analysis of randomized trials. JACC Cardiovasc Interv. 2017;10(4):315‐324. [DOI] [PubMed] [Google Scholar]
- 17. Thiele H, Akin I, Sandri M, et al. PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med. 2017;377(25):2419‐2432. [DOI] [PubMed] [Google Scholar]
- 18. Gossl M, Faxon DP, Bell MR, Holmes DR, Gersh BJ. Complete versus incomplete revascularization with coronary artery bypass graft or percutaneous intervention in stable coronary artery disease. Circ Cardiovasc Interv. 2012;5(4):597‐604. [DOI] [PubMed] [Google Scholar]
- 19. Jones DA, Rathod KS, Pavlidis AN, et al. Outcomes after chronic total occlusion percutaneous coronary interventions: an observational study of 5496 patients from the Pan‐London CTO Cohort. Coron Artery Dis. 2018;29(7):557‐563. [DOI] [PubMed] [Google Scholar]
- 20. Werner GS, Martin‐Yuste V, Hildick‐Smith D, et al. A randomized multicentre trial to compare revascularization with optimal medical therapy for the treatment of chronic total coronary occlusions. Eur Heart J. 2018;39(26):2484‐2493. [DOI] [PubMed] [Google Scholar]
- 21. Barbato E, Salinger‐Martinovic S, Sagic D, et al. A first‐in‐man clinical evaluation of Ultimaster, a new drug‐eluting coronary stent system: CENTURY study. EuroIntervention. 2015;11:541‐548. [DOI] [PubMed] [Google Scholar]
- 22. Saito SV‐CM, Richardt G, Moreno R, et al. A randomized, prospective, intercontinental evaluation of a bioresorbable polymer sirolimus‐eluting coronary stent system: the CENTURY II (Clinical Evaluation of New Terumo Drug‐Eluting Coronary Stent System in the Treatment of Patients with Coronary Artery Disease) trial. Eur Heart J. 2014;35(30):2021‐2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wijns W, Valdes‐Chavarri M, Richardt G, et al. Long‐term clinical outcomes after bioresorbable and permanent polymer drug‐eluting stent implantation: final five‐year results of the CENTURY II randomised clinical trial. EuroIntervention. 2018;14(3):e343‐e351. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.


