Abstract
Background
Basal cell carcinoma (BCC) is the most common cancer in the world and has a rising incidence. Current guidelines for low‐risk BCC including superficial BCC (sBCC) recommend several treatment options including destructive treatment methods, such as cryosurgery with or without prior curettage or curettage and electrodesiccation. Curettage only (i.e. without subsequent cryosurgery or electrodesiccation) is a simple and quick destructive treatment method used for many benign skin lesions but has not been sufficiently evaluated for the treatment of sBCCs.
Objectives
The objective was to compare the effectiveness of curettage vs. cryosurgery for sBCCs in terms of overall clinical clearance rates after 1 year as well as wound healing times.
Methods
A single‐centre non‐inferiority clinical trial was conducted. Non‐facial sBCCs with a diameter of 5–20 mm were randomised to either cryosurgery using one freeze–thaw cycle or curettage. At follow‐up visits, treatment areas were evaluated regarding the presence of residual tumour after 3–6 months and recurrence after 1 year. Further, wound healing times were assessed.
Results
In total, 228 sBCCs in 97 patients were included in the analysis. At 3–6 months, no residual tumours were seen in any of the treated areas. After 1 year, the clinical clearance rates for curettage and cryosurgery were 95.7% and 100%, respectively (P = 0.060). However, the non‐inferiority analysis was inconclusive. Wound healing times were shorter for curettage (4 weeks) compared to cryosurgery (5 weeks; P < 0.0001). Overall, patient satisfaction at 1 year was high.
Conclusions
Both treatment methods showed high clinical clearance rates after 1 year, whilst curettage reduced the wound healing time.
Introduction
Basal cell carcinoma (BCC) is the most common tumour amongst humans and incidence is rising. 1 Reports on incidence trends also confirm a relative increase of superficial BCCs (sBCC) in favour of other subtypes. 2 The majority of sBCCs are located outside cosmetically sensitive areas, which alters the spectrum of tumours needed to treat. The high prevalence and need for diagnosis and treatment entail an increased burden on healthcare. 3 , 4
The treatment options for BCCs are many. Surgery with or without intraoperative margin evaluation is regarded as the cornerstone of treatment. 6 , 7 , 8 For low‐risk non‐facial BCCs, however, destructive treatment methods can be good alternatives. For sBCC, cryosurgery using one freeze–thaw cycle, with or without prior curettage as well as curettage and electrodesiccation is commonly performed. 6
Curettage only, that is, not combined with cryosurgery or electrodesiccation (hereinafter ‘curettage’), is an alternative destructive method that is simple, quick and inexpensive. It is often used for benign skin lesions but has been insufficiently studied as a destructive treatment option for BCC. 9 , 10 , 11 There are no prospective, randomised and controlled trials that compare curettage with cryosurgery for the treatment of BCCs, let alone such studies on sBCCs specifically. In Sweden, the most common protocol for cryosurgery for sBCCs includes a single freeze–thaw cycle without prior curettage. 12
The main objective of this study was to compare curettage with cryosurgery for the treatment of sBCCs regarding the clinical tumour clearance rate after 1 year. The secondary objective was to compare wound healing times for the two treatment methods.
Patients and methods
The study was approved by the Regional Ethical Review Board in Gothenburg (Approval number 743–17) and adhered to the Declaration of Helsinki—ethical principles for medical research involving human subjects. Participants gave written consent prior to inclusion. The trial is registered on http://www.researchweb.org/ (project number 259511).
Study design and setting
This study was a single‐centre, randomised and controlled non‐inferiority trial to compare the effectiveness of curettage vs. cryosurgery for treating sBCC. Participants were recruited from the Department of Dermatology and Venereology at Sahlgrenska University Hospital in Gothenburg, Sweden, between November 2017 and May 2020. Follow‐up visits were performed from December 2017 to March 2021. The study was conducted over four appointments, that is, the treatment visit and follow‐up visits 1–3 (Fig. 1).
Figure 1.
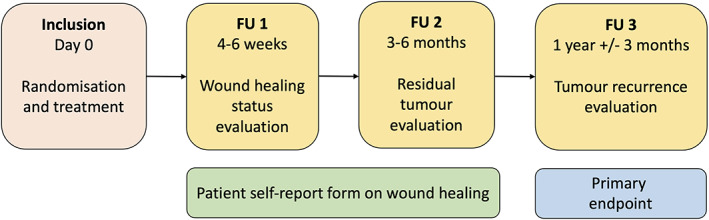
Overview of study visits with performed procedures. Evaluations at follow‐up (FU) visits were performed by a dermatologist except for FU 1, which was performed by a nurse. The tumours were documented with both clinical and dermoscopic photos at inclusion and FU visits, except at FU 1, when only clinical photos were taken. Once a week, the patient did a self‐evaluation of the wound healing until the wound was completely healed.
Participants
The eligibility criteria were as follows: patients ≥18 years with ≥1 previously untreated sBCCs with diameters ranging from 5 to 20 mm and located between the neck and the knees. Only clinically superficially growing non‐aggressive BCCs were included, and all lesions were confirmed with either dermoscopic examination or histopathological analysis, in accordance with our clinical practice. Patients with Gorlin syndrome or with a life expectancy <1 year were ineligible for study inclusion. Immunosuppression was not listed as an exclusion criterium.
Interventions
After patients had signed the informed consent form, each included lesion was randomly assigned to either curettage or cryosurgery. Five dermatologists (four experienced resident physicians at the time of the study initiation and one board‐certified dermatologist) employed at our department performed the treatments according to a pre‐specified protocol.
Curettage
Tumour boundaries were identified using a DermLite DL200 Hybrid or DL4 dermoscope (3Gen, San Juan Capistrano, CA, U.S.A.) and demarcated with a surgical marker. Under local anaesthesia (lidocaine 10 mg/mL and adrenaline 5 μg/mL), curettage was performed using disposable dermal curettes of two different sizes: 7 and 4 mm (Kai Medical, Tokyo, Japan). The sharp side of the instrument was applied in different directions across the surface of the lesion until the marked area was completely eliminated and a clean white dermal surface with evenly distributed bleeding was achieved (Video S1). When necessary, a 50% ferric chloride cutaneous solution (Apotek Produktion & Laboratorier, Stockholm, Sweden) was applied to achieve haemostasis. If the diagnosis was not previously confirmed by histopathology, the scraped‐off material was sent for histopathological verification. If the histopathological diagnosis did not confirm a non‐aggressive BCC subtype, the lesion was excluded.
Cryosurgery
Tumour boundaries were identified and demarcated as described above. A CryoPro Mini liquid nitrogen unit (Cortex Technology, Hadsund, Denmark) with a ‘B’ nozzle was used to freeze the tumours in one session with an open cone spray technique. The lesion was isolated with a neoprene cone (Cortex Technology, Hadsund, Denmark) with a wall thickness of 3 mm and an inner diameter of at least the same diameter as the lesion. The spray gun was held at a perpendicular angle to the skin surface at approximately 1 cm whilst spraying. During the procedure, the nozzle was moved in a circular motion across the demarcated tumour area inside of the cone until a frost halo of approximately 4 mm was achieved in the surrounding clinically healthy skin (Video S2). The freeze time and the time it took for the halo to thaw were recorded. The halo thawing time was only considered acceptable if ≥60 s after the freezing was terminated. If the halo thawing time was <60 s, the freeze–thaw cycle was repeated once more after complete thawing of the lesion.
Following all treatments, treated areas were covered with a simple surgical dressing (no moisture) and patients were told to change dressings as long as the wound was oozing. The patients also received a self‐report form (SRF) to record wound healing status once per week. The patients were informed to report if the wound was oozing, covered with a crust or healed (Fig. 2).
Figure 2.
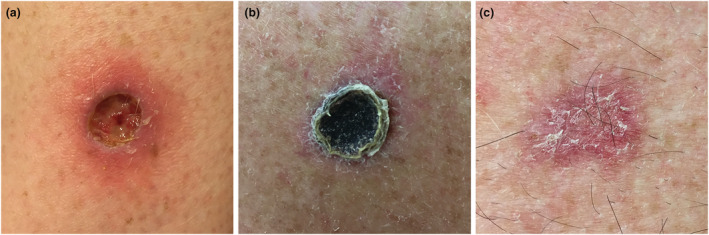
Clinical appearance of wounds that are: (a) oozing, (b) covered with crust or (c) healed.
Objectives
The primary objective was to compare the clinical clearance rates after 1 year and to test if curettage was non‐inferior to cryosurgery, allowing for an 8% difference in clearance rates. The secondary objective was to compare wound healing times for the two treatment groups.
Outcomes
Primary outcome
The primary outcome was clinical tumour clearance after 1 year. Early treatment failure was evaluated at follow‐up 2 and tumour recurrence was assessed at follow‐up 3. Treated areas were evaluated clinically and with dermoscopy and, if residual tumour or recurrence was suspected, a tissue sample for histopathology was acquired. At follow‐up 2, lesions with a low suspicion of residual tumour were not biopsied but marked as having ‘uncertain clearance’ with active surveillance until follow‐up 3. At each follow‐up visit, clinical and dermoscopic photographs were taken of each treated area.
Secondary outcome
At follow‐up 1, patient‐reported wound healing times were recorded as well as a research nurse's assessment of the wound healing status. The research nurse assessed the wound healing status using the same categories as in the SRF. If not completely healed, another SRF was handed out to the patient for further weekly evaluations.
Randomisation and blinding allocation and outcome assessment
Block randomisation (block size 4) was applied using R version 3.0.3 (https://www.r‐project.org/). For individuals with multiple included lesions, these were numbered and randomised according to body site using the following rule: front before back, then from top to bottom and, if tied, from right to left. The random allocation sequence was generated by a statistician; lesions were identified by a dermatologist and assigned a number by a research nurse; all employed at our department. Due to the different approaches of the two treatment methods, it was not possible to blind the patients or the physicians during the interventions. At the follow‐up visits, however, patient identities were masked and only lesion numbers were used to fill out the case report forms in order to blind physicians during outcome assessment.
Statistical methods
Sample size
Assuming that an estimated 95% of lesions would show complete response to cryosurgery and that the response to curettage would be the same in the study population, it was estimated that 184 lesions, 92 lesions per group, would be required to demonstrate that curettage was no more than 8% inferior to cryosurgery with a 95% one‐sided confidence interval (CI) and a power of 80%.
Statistical analysis
All data were analysed using R version 3.5.3 (https://www.r‐project.org/). The non‐inferiority hypothesis was tested by calculating a one‐sided 95% CI for the difference in 1‐year clearance rates between the two treatments (Wang's exact method). 13 Fisher’s exact test was used to test for a significant difference in clearance rates and to compare the proportion of healed lesions at follow‐up 1 for each treatment group. Wilcoxon rank‐sum test was used to compare the patient‐reported wound healing times. Further, a multiple linear regression analysis was performed with wound healing time as the dependent variable and treatment, lesion diameter, location, smoking, diabetes and immunosuppression as independent variables. All tests were two sided, except when comparing the CI regarding non‐inferiority, which was one sided. P‐values <0.05 were considered significant.
Results
Recruitment and participant characteristics
Overall, 240 tumours in 102 patients were included and treated. However, 12 tumours in five patients were excluded from the analyses. Four lesions were excluded prior to follow‐up due to histopathology not confirming BCC (n = 1), cryo‐unit lacking nozzle (n = 1), halo thaw time <60 s despite two attempts (n = 1) and biopsy‐proven nodular BCC in advance (n = 1). Another eight tumours were lost to follow‐up: five tumours in one patient due to deteriorating health impeding follow‐up, one tumour in another patient who changed residence to another region and two tumours in two patients who had follow‐up visits postponed due to the COVID‐19 pandemic. Thus, 228 tumours in 97 patients were included in the final analysis (per protocol) with 115 assigned to curettage and 113 to cryosurgery (Fig. 3).
Figure 3.
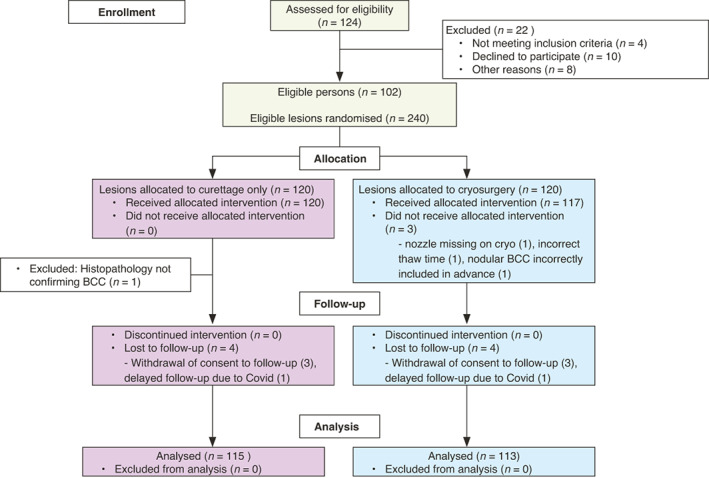
Study flow chart.
At inclusion, the median age of the patients was 70 years. The majority were male (67%), 4% were smokers, 11% were immunosuppressed and 9% had diabetes (Table 1). In all, 56 patients had one tumour, 12 patients had two, 28 patients had three to seven tumours and one patient had 18 tumours.
Table 1.
Patient characteristics at study inclusion
| Patient characteristics at inclusion | Total (n = 97) |
|---|---|
| Age at the initial treatment, yrs | |
| Median (Range) | 70 (29–88) |
| Gender | |
| Male | 65 (67%) |
| Female | 32 (33%) |
| Tumours per patient, n | |
| Mean (SD) | 2.4 (2.4) |
| Median (Range) | 1 (1–18) |
| Smoking habits | |
| Smoker | 4 (4%) |
| Former smoker | 27 (28%) |
| Never smoked | 59 (61%) |
| Missing data | 7 (7%) |
| Immunosuppression | |
| Yes | 11 (11%) |
| No | 83 (86%) |
| Missing data | 3 (3%) |
| Diabetes | |
| Yes | 9 (9%) |
| No | 86 (86%) |
| Missing data | 5 (5%) |
SD, standard deviation; yrs, years.
The tumour characteristics were comparable between the two study groups (Table 2). Overall, the most frequent tumour location was the trunk (71.5%) and the mean tumour diameter was 10.7 mm. There was no significant difference in tumour location (P = 0.13) or diameter (P = 0.31) between the two study groups. The BCCs were included upon either dermoscopic evaluation (n = 86) or histopathological verification (n = 142). The histopathological verification was based on a punch biopsy prior to the intervention in 60 cases or by examination of tissue obtained after curettage in the remaining 82 cases. In the curettage group, 97.2% (n = 112) of the tumours were histopathologically verified as compared to 26.5% (n = 30) in the cryosurgery group (P < 0.001), in which the majority of the lesions were included based on the dermoscopic assessment only. Amongst the 82 lesions in the curettage group enrolled without a biopsy prior to the intervention, 26 BCCs showed a non‐aggressive histopathological growth pattern other than sBCC, including 13 lesions with an unspecified growth pattern. Only one lesion proved to be another diagnosis than BCC.
Table 2.
Tumour characteristics
| Curettage | Cryosurgery | Total | |
|---|---|---|---|
| Tumours, n (%) | 115 (50.4) | 113 (49.6) | 228 |
| Location, n (%) | |||
| Neck | 1 (0.9) | 5 (4.4) | 6 (2.6) |
| Trunk | 78 (67.8) | 85 (75.2) | 163 (71.5) |
| Upper limbs | 27 (23.5) | 17 (15.0) | 44 (19.3) |
| Thigh | 9 (7.8) | 6 (5.3) | 15 (6.6) |
| Mean diameter in mm, (SD) | 11.1 (3.8) | 10.4 (3.1) | 10.7 (3.5) |
| Range (mm) | 5–20 | 5–19 | 5–20 |
| Histopathologically verified lesions, n (%) | 112 (97.4) | 30 (26.5) | 142 (63.2) |
| Verified prior to intervention | 30 | 30 | 60 |
| Histopathological subtype, n (%) | |||
| Superficial | 86 (76.8) | 30 (100) | 116 (81.7) |
| Nodular | 10 (8.9) | 10 (7.0) | |
| Superficial & nodular | 3 (2.7) | 3 (2.1) | |
| Unspecified non‐aggressive growth pattern | 13 (11.6) | 13 (9.2) | |
mm, millimetres; SD, standard deviation.
For transparency, clinical and dermoscopic pictures of all lesions (n = 86) included without histopathologic confirmation are presented in the (Appendix S1).
The median freeze time in the cryosurgery group was 20 s (range 11–30 s) and the median halo thaw time was 80 s (range 35–184 s). In four cases, the halo thaw time was <60 s and therefore repeated. There was a positive correlation between the maximum diameter and the freeze time necessary to achieve the halo thawing time ≥60 s with longer freeze times required for lesions with a larger maximum diameter (P < 0.001).
Primary outcome
At 3–6 months, no treated areas revealed residual tumour. At 1 year, five tumours in the curettage group and no tumours in the cryosurgery group showed histopathologically verified tumour recurrence resulting in a clearance rate of 95.7% for curettage and 100% for cryosurgery (P = 0.060; Table 3).
Table 3.
Descriptive statistics of the recurrent tumours after 1 year
| Variable | Curettage | 95% CI | Cryosurgery | 95% CI | P‐value |
|---|---|---|---|---|---|
| Tumours, n | 115 | 113 | |||
| Recurrences at 1 year n (%) | 5 (4.3) | 1.4–9.9 | 0 (0) | 0.0–3.2 | 0.06 |
| Mean time (days) to FU 3 (SD) | 372 (60) | 383 (65) | |||
| Location, n | |||||
| Neck | – | – | |||
| Trunk | 3 | – | |||
| Upper limbs | 1 | – | |||
| Thigh | 1 | – | |||
| Diameter at inclusion (mm) | |||||
| Median (range) | 14 (6–20) | – | |||
| Histopathological subtype, n | – | ||||
| sBCC | 4 | – | |||
| Mix (sBCC + SCCis) | 1 | – | |||
CI, confidence interval; FU, follow up visit; sBCC, superficial basal cell carcinoma; SCCis, Squamous cell carcinoma in situ; SD, standard deviation.
The non‐inferiority analysis was inconclusive, that is, a non‐inferiority could not be ensured as the lower margin of the 95% CI for the difference between curettage and cryosurgery was below the non‐inferiority cut‐off limit of 8% (10.5% with the one‐sided 95% CI test and 12.3% two‐sided 95% CI‐test; Fig. 4). Clinical and dermoscopic pictures of the recurrent lesions are presented in the Appendix S2.
Figure 4.
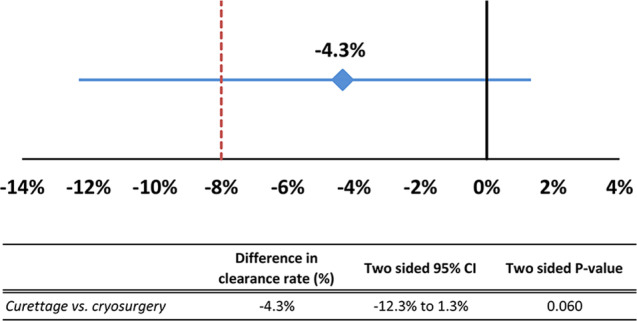
Absolute difference in effectiveness between curettage and cryosurgery 1 year after treatment (−4.3%). The horizontal line represents the 95% CI (−12.3% to 1.3%). The lower boundary of the 95% CI crosses the non‐inferiority limit of 8%. CI, confidence interval.
Secondary outcomes
The median time (interquartile range) to follow‐up 1 was 30 days (28–35), with no significant difference between the two groups (P = 0.95). At this time, more wounds were completely healed in the curettage group (65.5%, n = 74) in comparison with the cryosurgery group (45.5%, n = 50; P < 0.01; Table 4). The median self‐reported wound healing times were shorter with curettage (4 weeks) than with cryosurgery (5 weeks) (P < 0.0001). Further, the median self‐reported time with an oozing wound was 0.8 weeks for curettage vs. 1.6 weeks for cryosurgery (P < 0.0001).
Table 4.
Wound healing status at 4 weeks assessed by a research nurse and self‐reported wound healing times
| Wound Status at FU 1 | Curettage n = 115 | Cryosurgery n = 113 | Total n = 228 | P‐value |
|---|---|---|---|---|
| Wound healing status at FU 1, n (%) | ||||
| Healed | 74 (65.5) | 50 (45.5) | 124 (55.6) | 0.003 |
| Crust | 36 (31.9) | 56 (50.9) | 92 (41.3) | 0.004 |
| Oozing | 3 (2.6) | 4 (3.5) | 7 (3.1) | 0.72 |
| Missing data | 2 | 3 | 5 | |
| Self‐reported wound healing | Curettage | Cryosurgery | Total | P‐value |
|---|---|---|---|---|
| Complete wound healing time, weeks | ||||
| Mean (SD) | 4.3 (1.3) | 5.1 (1.9) | 4.8 | <0.0001 |
| Median (range) | 4 (2–8) | 5 (3–14) | 4 (2–14) | |
| Missing data | 13 | 16 | 29 | |
| Time with oozing wound, weeks | ||||
| Mean (SD) | 0.8 (0.9) | 1.6 (1.0) | 1.2 | <0.0001 |
| Median (range) | 0 (0–3) | 2 (0–5) | 1 (0–5) | |
| Missing data | 6 | 4 | 10 | |
FU, follow‐up visit.
Large lesion diameter was significantly associated with longer wound healing time (P < 0.01), but no association was found between wound healing and location, freeze times, diabetes, immunosuppression or smoking.
Patient satisfaction
Preliminary data on satisfaction with the cosmetic result were available for 92 patients with 220 lesions (data were missing for five patients with four lesions treated with curettage and four with cryosurgery). Eighty‐eight patients were satisfied with the cosmetic result. Two patients were unsatisfied (one with a single lesion treated with cryosurgery and one with a single lesion treated with curettage). Two patients considered the scars to be irrelevant (one with a single lesion treated with cryosurgery and one with three lesions treated with cryosurgery and one with curettage).
Adverse events
Overall, 83 adverse events, including five severe adverse events, were reported from follow‐up 1 to follow‐up 3, but none were related to the study interventions. No secondary wound infections requiring antibiotic treatment were observed.
Discussion
This is the first prospective, randomised and controlled study comparing the effectiveness of curettage and cryosurgery in the treatment of sBCC. Although both treatments resulted in high 1‐year clinical clearance rates, the non‐inferiority analysis was inconclusive, as the lower limit of the 95% confidence interval for the difference was below 8%. Wound healing was more often complete after 4 weeks following curettage. Furthermore, self‐reported wound healing times were shorter with curettage, both in terms of complete wound healing time and time with an oozing wound.
Curettage for BCCs (regardless of their subtype) has not been evaluated in prospective comparative studies before. Although longer follow‐up is warranted, our results are consistent with a retrospective study on curettage for non‐aggressive BCCs by Barlow et al. showing 96% 5‐year clearance rates 9 and a prospective, non‐controlled study on curettage for mainly nodular BCCs by McDaniel et al. demonstrating 91% five‐year clearance rates. 10
The clearance rates for cryosurgery vary considerably between different published studies. In line with our results, 5‐year clearance rates of 95–100% have been reported from early retrospective reports and prospective, non‐controlled studies. 14 , 15 , 16 , 17 , 18 On the other hand, two more recent randomised controlled studies on low‐risk BCCs, comparing cryosurgery to photodynamic therapy, have reported 1‐year clearance rates of 87% and 5‐year clearance rates of 80%, respectively. 19 , 20 There are few studies performed on cryosurgery for sBCC specifically, especially on cryosurgery without prior curettage. Mallon and Dawber only observed one recurrence amongst 31 clinically diagnosed truncal sBCCs treated with cryosurgery in one session without prior curettage with follow‐up ranging from approximately 1 to 7 years. 21 Peikert et al. reported a 99% 5‐year clearance rate for curettage with subsequent cryosurgery in one session in a prospective, non‐randomised study including mainly non‐facial sBCCs. 22 Comparisons are difficult to make in general due to different inclusion criteria in these studies and especially since previous studies lack standardized and well‐described protocols for cryosurgery. Cryosurgery can be performed with different freeze techniques, including different nozzles on the spray unit, different distances between lesion and nozzle, different ways of measuring thaw times and so forth, which can probably result in different outcomes. To avoid this limitation, we have presented the precise technique used in this study along with two Videos S1 and S2.
Curettage resulted in shorter wound healing times (compared to cryosurgery). This result is supported by earlier studies on both treatment methods, although no comparative studies on curettage vs. cryosurgery for BCC have been performed earlier. 10 , 23 Long wound healing times are often highlighted as a disadvantage of destructive treatment methods. This is partly true, though in our experience, patients are primarily bothered by the period in which they have an oozing wound. This period is comparable to the 1‐2 weeks in which a surgical wound, for example, requires special attention. The mean times for oozing wounds in this study, regardless of treatment method, were comparable with the normal duration of having sutures in place following surgery. For non‐invasive therapies such as imiquimod, 5‐fluorouracil and photodynamic therapy, erosions and crusting in the treated areas last several weeks.
Limitations
This was a single‐centre study with only five dermatologists involved. Only clinically superficial BCCs located between the neck and the knees with a 5–20 mm diameter were included. Recurrences were assessed by dermoscopic evaluation and were biopsied only in case of clinical suspicion of recurrence. When evaluating self‐reported wound healing times, 12–14% of participants failed to bring their SRFs back at follow‐up 2 resulting in missing data which could have had a certain impact on the median complete wound healing times. As BCCs are slow‐growing, further follow‐up is needed to provide long‐term data on effectiveness. Therefore, patients in this study will be followed for 5 years. Furthermore, long‐term data on cosmetic outcome and patient satisfaction will be analyzed. The majority of BCCs randomised to cryosurgery were included based on the dermoscopic diagnosis and not histopathologically verified. Nevertheless, a recent meta‐analysis showed that the sensitivity and specificity for making a dermoscopic diagnosis of BCC are 91.2% and 95%, respectively. 24 Further, in this study, only one out of 83 lesions included prior to histopathological verification proved not to be a BCC.
Conclusion
Several international guidelines and review articles on BCC management highlight the lack of randomised controlled trials performed on destructive treatment methods, as well as the lack of well‐described treatment protocols. Further, studies on specific subtypes of BCCs have been requested. This study provides new evidence that simple destructive treatment methods can be used to treat sBCC effectively. Both curettage and cryosurgery in a single freeze–thaw cycle show promising 1‐year results regarding clinical clearance. In addition, curettage provides shorter wound healing times compared to cryosurgery.
Supporting information
Video S1. Video presentation of the precise treatment protocol for curettage of superficial basal cell carcinoma used in the study.
Video S2. Video presentation of the precise treatment protocol for cryosurgery of superficial basal cell carcinoma used in the study.
Appendix S1. Clinical and dermoscopic pictures of all lesions included without histopathologic confirmation.
Appendix S2. Clinical and dermoscopic pictures at inclusion and follow‐up visits of the five recurrent lesions. White arrows indicate areas of BCC recurrence and the black arrow indicates scarring from a previous punch biopsy.
Conflicts of interest
None reported.
Funding sources
The study was financed by grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF agreement (ALFGBG‐728261).
Trial number: Researchweb.org, project number 259511 available at: https://www.researchweb.org/is/vgr/project/259511
Data availability statement
All data is not available in the manuscript and supplementary material, but can be made available from the corresponding author on reasonable request.
References
- 1. Lomas A, Leonardi‐Bee J, Bath‐Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol 2012; 166: 1069–1080. [DOI] [PubMed] [Google Scholar]
- 2. Arits AH, Schlangen MH, Nelemans PJ, Kelleners‐Smeets NW. Trends in the incidence of basal cell carcinoma by histopathological subtype. J Eur Acad Dermatol Venereol 2011; 25: 565–569. [DOI] [PubMed] [Google Scholar]
- 3. Rogers HW, Coldiron BM. Analysis of skin cancer treatment and costs in the United States Medicare population, 1996–2008. Dermatol Surg 2013; 39: 35–42. [DOI] [PubMed] [Google Scholar]
- 4. Gordon LG, Rowell D. Health system costs of skin cancer and cost‐effectiveness of skin cancer prevention and screening: a systematic review. Eur J Cancer Prev 2015; 24: 141–149. [DOI] [PubMed] [Google Scholar]
- 5. Eriksson TTG. Samhällskostnader för hudcancer 2011. Swedish radiation safety authority. Rapportnummer: 2014;49: 25–35. ISSN:2000–0456 Available from www.stralsakerhetsmyndigheten.se [Google Scholar]
- 6. Peris K, Fargnoli MC, Garbe C et al. Diagnosis and treatment of basal cell carcinoma: European consensus‐based interdisciplinary guidelines. Eur J Cancer 2019; 118: 10–34. [DOI] [PubMed] [Google Scholar]
- 7. Telfer NR, Colver GB, Morton CA. Guidelines for the management of basal cell carcinoma. Br J Dermatol 2008; 159: 35–48. [DOI] [PubMed] [Google Scholar]
- 8. Kim JYS, Kozlow JH, Mittal B, Moyer J, Olencki T, Rodgers P. Guidelines of care for the management of basal cell carcinoma. J Am Acad Dermatol 2018; 78: 540–559. [DOI] [PubMed] [Google Scholar]
- 9. Barlow JO, Zalla MJ, Kyle A, DiCaudo DJ, Lim KK, Yiannias JA. Treatment of basal cell carcinoma with curettage alone. J Am Acad Dermatol 2006; 54: 1039–1045. [DOI] [PubMed] [Google Scholar]
- 10. McDaniel WE. Therapy for basal cell epitheliomas by curettage only. Further Study. Arch Dermatol 1983; 119: 901–903. [PubMed] [Google Scholar]
- 11. Reymann F. 15 years' experience with treatment of basal cell carcinomas of the skin with curettage. Acta Derm Venereol Suppl (Stockh) 1985; 120: 56–59. [PubMed] [Google Scholar]
- 12. Swedish guidelines for basal cell carcinoma and squamous cell carcinoma. (in Swedish). Last updated 2016. Available at: https://www.ssdv.se/images/pdf/SDKOs_Riktlinjer_for_SCC__BCC_2016.pdf
- 13. Wang W. On construction of the smallest one‐sided confidence interval for the difference of two proportions. Annals Stat 2010; 38: 1227–1243. [Google Scholar]
- 14. Zacarian SA. Cryosurgery of cutaneous carcinomas. An 18‐year study of 3022 patients with 4228 carcinomas. J Am Acad Dermatol 1983; 9: 947–956. [DOI] [PubMed] [Google Scholar]
- 15. Kuflik EG. Cryosurgery for skin cancer: 30‐year experience and cure rates. Dermatol Surg 2004; 30: 297–300. [DOI] [PubMed] [Google Scholar]
- 16. Nordin P, Larkö O, Stenquist B. Five‐year results of curettage‐cryosurgery of selected large primary basal cell carcinomas on the nose: an alternative treatment in a geographical area underserved by Mohs' surgery. Br J Dermatol 1997; 136: 180–183. [PubMed] [Google Scholar]
- 17. Lindgren G, Larkö O. Cryosurgery of eyelid basal cell carcinomas including 781 cases treated over 30 years. Acta Ophthalmol 2014; 92: 787–792. [DOI] [PubMed] [Google Scholar]
- 18. Lindemalm‐Lundstam B, Dalenbäck J. Prospective follow‐up after curettage‐cryosurgery for scalp and face skin cancers. Br J Dermatol 2009; 161: 568–576. [DOI] [PubMed] [Google Scholar]
- 19. Wang I, Bendsoe N, Klinteberg CA et al. Photodynamic therapy vs. cryosurgery of basal cell carcinomas: results of a phase III clinical trial. Br J Dermatol 2001; 144: 832–840. [DOI] [PubMed] [Google Scholar]
- 20. Basset‐Seguin N, Ibbotson SH, Emtestam L et al. Topical methyl aminolaevulinate photodynamic therapy versus cryotherapy for superficial basal cell carcinoma: a 5 year randomized trial. Eur J Dermatol 2008; 18: 547–553. [DOI] [PubMed] [Google Scholar]
- 21. Mallon E, Dawber R. Cryosurgery in the treatment of basal cell carcinoma. Assessment of one and two freeze‐thaw cycle schedules. Dermatologic Surg 1996; 22: 854–858. [PubMed] [Google Scholar]
- 22. Peikert JM. Prospective trial of curettage and cryosurgery in the management of non‐facial, superficial, and minimally invasive basal and squamous cell carcinoma. Int J Dermatol 2011; 50: 1135–1138. [DOI] [PubMed] [Google Scholar]
- 23. Ahmed I, Berth‐Jones J, Charles‐Holmes S, O'Callaghan CJ, Ilchyshyn A. Comparison of cryotherapy with curettage in the treatment of Bowen's disease: a prospective study. Br J Dermatol 2000; 143: 759–766. [DOI] [PubMed] [Google Scholar]
- 24. Reiter O, Mimouni I, Gdalevich M et al. The diagnostic accuracy of dermoscopy for basal cell carcinoma: a systematic review and meta‐analysis. J Am Acad Dermatol 2019; 80: 1380–1388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. Video presentation of the precise treatment protocol for curettage of superficial basal cell carcinoma used in the study.
Video S2. Video presentation of the precise treatment protocol for cryosurgery of superficial basal cell carcinoma used in the study.
Appendix S1. Clinical and dermoscopic pictures of all lesions included without histopathologic confirmation.
Appendix S2. Clinical and dermoscopic pictures at inclusion and follow‐up visits of the five recurrent lesions. White arrows indicate areas of BCC recurrence and the black arrow indicates scarring from a previous punch biopsy.
Data Availability Statement
All data is not available in the manuscript and supplementary material, but can be made available from the corresponding author on reasonable request.


