Abstract
Combined heavy‐ and light‐load ballistic training is often employed in high‐performance sport to improve athletic performance and is accompanied by adaptations in muscle architecture. However, little is known about how training affects muscle‐tendon unit (MTU) kinematics during the execution of a sport‐specific skill (e.g., jumping), which could improve our understanding of how training improves athletic performance. The aim of this study was to investigate vastus lateralis (VL) MTU kinematics during a countermovement jump (CMJ) following combined ballistic training. Eighteen young, healthy males completed a 10‐week program consisting of weightlifting derivatives, plyometrics, and ballistic tasks under a range of loads. Ultrasonography of VL and force plate measurements during a CMJ were taken at baseline, mid‐test, and post‐test. The training program improved CMJ height by 11 ± 13%. During the CMJ, VL's MTU and series elastic element (SEE) length changes and velocities increased from baseline to post‐test, but VL's fascicle length change and velocity did not significantly change. It is speculated that altered lower limb coordination and increased force output of the lower limb muscles during the CMJ allowed more energy to be stored within VL's SEE. This may have contributed to enhanced VL MTU work during the propulsion phase and an improved CMJ performance following combined ballistic training.
Keywords: muscle mechanics, performance, resistance exercise, Ultrasonography, weightlifting
1. INTRODUCTION
The ability to jump high following a short ground contact time is fundamental to success in many sports and requires high work and power outputs from the lower limb muscles. Mechanically, muscle fibers generate maximum power output only at ~1/3 of their maximum shortening velocity. However, in athletic tasks such as jumping, rapid movements are required for optimal performance and thus if muscle fibers were required to contract at higher velocities, this would reduce the amount of power the muscle fibers could produce. 1 To enable muscle fibers to contract at lower shortening velocities during fast in vivo movements, long elastic structures (i.e., series elastic element, SEE) in‐series with the muscle (i.e., contractile element, CE) amplify power output of the muscle‐tendon unit (MTU) by storing and then rapidly releasing energy during a stretch‐shorten cycle, 2 like a catapult. This power amplification is crucial for enhancing jumping performance, even for squat jumps without a stretch‐shortening cycle at the MTU level. 3
The ability of the SEE to amplify power during jumping is facilitated by the decoupling of SEE and CE length changes. A decoupling that involves the SEE undergoing the majority of the length change has been typically observed during jumping, 3 , 4 and can be used to increase both SEE and CE power outputs. This is because SEE power output increases with the velocity of shortening and amount of energy stored, while CE power output is maximized at a slower shortening velocity. 5 In a maximal countermovement jump (CMJ), the SEE stores energy during the countermovement and later, using a catapult‐like mechanism, rapidly recoils when the MTU shortens to amplify MTU power. 3 These SEE kinematics prevent rapid shortening of the CE, which consequently enhances the MTU's power output. 3 , 6
While our understanding of muscle‐tendon interaction during an explosive task (such as jumping) is improving, we still have limited understanding of how resistance training alters MTU kinematics during explosive tasks that are crucial for successful sport performance. A popular type of training adopted by strength and conditioning coaches and athletes to enhance jump performance is ballistic training. Ballistic training emphasizes the use of low‐load exercises that allow velocity to be maximized toward the end of the movement (e.g., medicine ball throws, jump squats). 7 While this training method is commonly adopted in elite athletes, 8 , 9 , 10 to our knowledge there are no studies that have examined changes in MTU kinematics during a sport‐specific task in response to ballistic training. There has been one study that has investigated muscle architecture changes in response to 5 weeks of sprint and jump training and found an increase in resting vastus lateralis (VL) and rectus femoris fascicle lengths, but no increase in jump height. 11 Whether changes in MTU kinematics arose from these muscle architecture adaptations during a sport‐specific task, like jumping, were not explored. However, quantifying MTU interaction during sport‐specific tasks before and after ballistic training could provide important insights into why jumping performance is or is not improved following training involving combined traditional resistance training and ballistic exercises (i.e., multi‐modal training).
We therefore aimed to quantify changes in VL MTU kinematics during a CMJ during (mid‐training) and following a multi‐modality heavy‐ and light‐load resistance training intervention. We hypothesized that the training intervention would increase CMJ jump height due to higher ground reaction forces over a similar movement time. 12 We also hypothesized that ballistic training would increase VL's SEE stretch and shortening velocities, but not muscle fascicle velocities, during a CMJ. This hypothesis was based on the assumption that higher SEE velocities reflect enhanced power outputs, whereas higher muscle fascicle velocities do not necessarily reflect enhanced muscle power outputs.
2. METHODS
2.1. Participants
Eighteen healthy males (body mass: 80.1 ± 11.0 kg; height: 1.79 ± 0.08 m; age: 25.6 ± 4.0 years; 1‐RM squat: 126.2 ± 32.9 kg; resistance training experience: 2.0 ± 1.4 years), who had no pre‐existing injuries or recent lower limb injuries, voluntarily participated in the study after providing written informed consent. All participants could competently perform a back squat and had undertaken a minimum of two resistance training session per week for the previous 6 months. The study protocol was approved by the Bellberry Human Research Ethics Committee (Application ID: HREC2016‐04‐269) and conducted in accordance with the Declaration of Helsinki.
2.2. Experimental design
2.2.1. Training program
All participants completed a 10‐week combined heavy‐ and light‐load maximal ballistic training program of the lower body. The training program was organized into two five‐week blocks that were separated by 1 week. Participants completed three supervised one‐hour sessions per week within each training block. The training program incorporated weightlifting derivatives (i.e., modified weightlifting exercises that require less skill than full weightlifting movements, for example, power clean, hang power clean), 13 lower‐body plyometrics and ballistic movements, which were completed under a range of loads (Table 1). Progression from block 1 to block 2 was based on the principles of periodisation. This included an increasing load for weightlifting derivatives, more complex (e.g., progressing from double to split stance) plyometrics, and a reduced load for jump squats (thus a presumed shift toward loads that produced higher peak positive power outputs). Plyometric and ballistic tasks were performed with the intent of maximizing jump height.
TABLE 1.
Ten‐week combined heavy‐ and light‐load ballistic‐training program
| Weeks 1–5 | Weeks 6–10 | ||||
|---|---|---|---|---|---|
| Exercise | Sets/Reps | Loading a | Exercise | Sets/Reps | Loading |
| Days 1 and 3 | Days 1 and 3 | ||||
| Power clean | 5 × 5 | 70% 1RM | Jump squat | 5 × 5 |
0%1RM (D1) 30%1RM (D3) |
| Jump squat | 5 × 5 | 40% 1RM (D1), 50% 1RM (D3) | Power clean | 5 × 4 | 85% 1RM |
| Depth jump (5‐s between repetition recovery) | b | Unloaded | |||
| Day 2 | Day 2 | ||||
| Hang power clean | 4 × 5 | 55% 1RM (of the PC) | Hang power clean | 5 × 4 | 70% 1RM (of the PC) |
| Snatch grip pull | 4 × 5 | 70% 1RM (of the PC) | Snatch grip pull | 5 × 4 | 85% 1RM (of the PC) |
| Plyometric rebound split squat | 4 × 3 | Unloaded | |||
Abbreviations: 1RM, 1 repetition maximum; D1, day 1; D2, day 2; D3, day 3; PC, power clean.
Loading refers to the amount of weight placed onto the bar for each activity. In all activities, the load of body weight is present.
Depth jump volume progressed in the following fashion (sets/reps): Week 6: 3 × 3; Week 7: 3 × 4; Week 8: 4 × 4: Weeks 9 and 10: 5 × 4.
2.2.2. CMJ testing
CMJ testing was performed in the week before the training program commenced (baseline), during the week between block 1 and block 2 (mid‐test), and in the week following the end of block 2 (post‐test) to assess jump performance metrics and muscle fascicle kinematics using force plate measurements and ultrasound imaging, respectively. Participants commenced each testing session with a general then specific dynamic warmup, which included a variety of squat and lunging movements and submaximal CMJs with progressively increasing effort. A minimum of three unloaded CMJs, with at least 3 s between each attempt, were then performed while participants had their hands on their hips and were instructed to achieve maximal height. Participants were instructed to perform the CMJ with a depth corresponding to an approximate knee flexion angle of ~85° as this represents a common self‐selected depth. 14 Participants were provided with an opportunity to practice jumping with this countermovement depth using feedback provided by a webcam (model C270, recording at 30 Hz, Logitech, Switzerland), where knee flexion angle was measured using 2D motion analysis software (V0.8.15, Kinovea).
2.3. Data acquisition procedures
2.3.1. Performance and kinetics metrics
The CMJs were performed by participants on a force platform (Bertec Corporation) that sampled at 2000 Hz (NI USB‐6259 BNC, National Instruments), and the data were saved using custom software (V.12.0f3, National Instruments) for offline analysis. The data were then imported into Matlab (The Mathworks, Inc.) where custom‐written scripts, modified from the method employed by Wade et al., 15 were used to calculate jump height. The modified method to calculate jump height first involved cropping vertical force between the start of the countermovement (defined as the point where vertical force first dropped below the force of body weight) to the instant of take‐off (defined as the point where vertical force dropped below 40 N) to minimize integration drift errors. The force of body weight was calculated as the average force over 20 frames, in a period prior to the start of the countermovement while the participant was standing still. Net vertical force was calculated from the vertical ground reaction force, by subtracting the force of body weight, measured prior to each testing session, from the vertical ground reaction force. The acceleration of the participant's center of mass was calculated by dividing the net vertical force by the body mass measured prior to each testing session. The acceleration was then integrated to identify the participant's velocity at take‐off. Using projectile motion equations and the participant's velocity at take‐off, jump height was calculated as the vertical displacement of the center of mass between take‐off and the apex of the jump. The vertical ground reaction force trace was integrated to construct velocity‐ and displacement‐time curves. The jump containing the highest peak positive (i.e., upward) velocity was then used in further analysis.
2.3.2. Muscle kinematic measurements
Fascicle lengths of the right leg's vastus lateralis (VL) were measured from images obtained via ultrasonography during the CMJ. A linear, 96‐element, multi‐frequency ultrasound transducer (LV7.5/60/96, Telemed, Lithuania) connected to a PC‐based ultrasound system (Echoblaster 128, UAB, Telemed) was used to capture ultrasound images at a sound frequency of 6 MHz, with a field of view of 60 × 65 mm (width × depth), a focus range of 18–26 mm and a frame rate of 80 Hz using Echowave II software (Telemed). While this single transducer is not able to image complete VL muscle fascicles, this method was shown to be as accurate as a dual‐transducer approach for assessing VL muscle fascicle length changes during a dynamic task. 16 A 5 V signal was sent from the unit to the same AD board as that used to sample force to synchronize the ultrasound and force plate data. The transducer was positioned on the lateral aspect of the thigh at the mid‐point between the greater trochanter and lateral epicondyle of the femur and was oriented perpendicular to the skin and in line with the muscle fascicles. To ensure consistent placement of the transducer across testing sessions, the location of the transducer was marked on an elastic garment along with relevant anatomical landmarks, and multiple images were taken of the instrument's location on the thigh. An elastic adhesive compression bandage (Coban, 3‐M Health Care) was used to secure the transducer to the thigh and prevent movement of the transducer relative to the skin. These procedures have been used before in the investigators' laboratory for a variety of actions, including walking 17 and jumping tasks. 18
Fascicle length and pennation angle were measured directly from the ultrasound images with a semi‐automated procedure in Matlab that uses a Lucas‐Kanade optical flow algorithm with an affine optic flow extension. 19 This algorithm (albeit for the medial gastrocnemius) has been shown to be reliable and in good agreement with manual tracking of fascicle lengths during walking and jogging, 20 while it has also been used to measure changes in lateral gastrocnemius and soleus fascicle lengths during CMJs. 18 This algorithm tracks movement of user‐defined muscle fascicle endpoints throughout the captured sequence of images within a user‐defined region of interest. The fascicle length was measured as the calibrated distance between the fascicle endpoints while the pennation angle was defined as the angle of a line drawn between the two muscle fascicle endpoints relative to the horizontal. To reduce the error associated with initially defining the length and pennation angle of a representative fascicle within the ultrasound image field‐of‐view across testing sessions, the coordinates of the fascicle and region of interest defined in the baseline measurements were saved, and later overlayed on the ultrasound images captured at mid‐test and post‐test while participants assumed the same quiet standing posture. Manual adjustments were made to the fascicle endpoints if they were visibly different to those defined at baseline.
To calculate muscle‐tendon unit (MTU) length, kinematic data of the right leg were captured throughout each countermovement jump using a four‐camera, infrared motion analysis system (OptiTrack, NaturalPoint, Inc.). Single reflective markers were placed on the right greater trochanter, medial and lateral epicondyles of the right knee and the medial and lateral malleoli of the right ankle, and marker clusters (groups of four markers) were placed on the right thigh and shank segments. The motion capture data were sampled at 200 Hz using computer software (Motive, OptiTrack) and then processed offline (Visual 3D, C‐Motion Inc.), which allowed fascicle data to be combined and synchronized with the kinematic and kinetic data. Using a custom‐written script (MatLab, Mathworks), the MTU length of VL, normalized to thigh length (NLMTU), was calculated according to the following regression equation:
| (1) |
where C0, C2, and C3 are correlation coefficients derived from Hawkins & Hull. 21 β represents the knee flexion angle as measured in degrees. To estimate the absolute MTU length of VL, the normalized MTU length was multiplied by the thigh length of each individual as determined from the motion capture data. 21
To estimate the length of the SEE (i.e., the length of all elastic material in series with the muscle, predominantly consisting of the free tendon and aponeurosis; LSEE), the following equation was used 22 :
| (2) |
where LMTU is the absolute MTU length of the individual as calculated from Equation 3 above, LFAS is the fascicle length of VL and θ is the pennation angle of VL.
CMJ kinetics and muscle kinematics were analyzed within each of the following three phases of the CMJ: (1) Unloading phase—from the instant when vertical ground reaction force drops below the force of body weight (i.e., the beginning of the countermovement) to the instant when the ground reaction force is the smallest; (2) Braking phase—from the end of the unloading phase to the instant where the COM velocity is 0 m/s (i.e., the end of the countermovement), and: (3) Propulsion phase—from the end of the braking phase to the instant where maximum COM velocity occurs.
Positive impulse was calculated as the area under the vertical ground reaction force–time curve within each phase. Negative impulse was calculated by multiplying the force due to body weight by the same time intervals as for positive impulse. Subsequently, the net impulse for each phase was calculated by summing the positive and negative impulses together.
2.4. Statistical analysis
Two‐way repeated‐measures mixed‐effects analyses were performed to identify differences in vertical ground reaction force, contact duration, net impulse, knee rotation amplitude and velocity, and VL's MTU, SEE, and fascicle length change amplitudes and velocities between CMJ testing sessions and CMJ phases (time × phase). One‐way repeated measures mixed‐effects analyses were used to identify differences between CMJ testing sessions in CMJ jump height, CMJ depth, body mass, mean vertical ground reaction force over the three CMJ phases, and total CMJ contact duration. The Greenhouse–Geisser (epsilon hat) method was used to correct for violations of sphericity as sphericity was not assumed. 23 When a significant main effect or significant interaction was identified, Tukey post hoc comparisons were performed to determine the specific time point during the training period when differences were surprising under the assumption of no difference. The alpha level was set at 5%, and statistical analysis was performed using commercially‐available software (GraphPad Prism 9.1.2, USA). Data are presented as mean ± standard deviation in the text.
3. RESULTS
3.1. CMJ performance
The ten‐week combined ballistic training program significantly improved CMJ jump height (F 1.90,32.32 = 6.69, p = 0.004) from baseline to post‐test (change in jump height: 3.2 ± 4.2 cm [95% CI: 0.7 to 5.7], p = 0.011), but not from baseline to mid‐test (change in jump height: 1.0 ± 3.4 cm [−1.1 to 3.1], p = 0.442) or from mid‐test to post‐test (change in jump height: 2.2 ± 3.9 cm [−0.1 to 4.6], p = 0.066; Figure 1). CMJ depth also changed significantly over the course of the training program (F 1.70,28.81 = 6.17, p = 0.008), where CMJ depth significantly increased from mid‐test to post‐test (change in depth: 4.1 ± 1.1 cm [1.3 to 6.8], p = 0.004), but no significant changes occurred from baseline to mid‐test (change in depth: −1.2 ± 4.5 cm [−3.9 to 1.5], p = 0.520) or from baseline to post‐test (change in depth: 2.9 ± 6.0 cm [−0.8 to 6.5], p = 0.134). Body mass did not significantly change over the training period (F 1.53,26.02 = 0.41, p = 0.612; change in body mass between baseline and post‐test: ≤0.3 kg).
FIGURE 1.
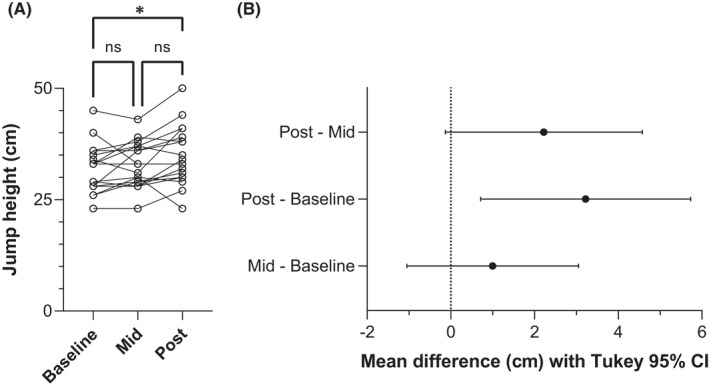
(A) Individual vertical jump heights (open circles, n = 18) and B) mean paired differences (point estimate ±95% confidence interval [CI]) between countermovement jumps performed at baseline, mid‐test (Mid), and post‐test (Post) of the 10‐week combined ballistic training program. Solid lines link the same participant. * and a 95% CI that does not cross zero indicates a significant difference between baseline and post‐test (p = 0.011), whereas “ns” and an overlapping 95% CI with zero indicates no significant difference between baseline and mid‐test (p = 0.442) and mid‐test and post‐test (p = 0.066). Tukey post hoc comparisons controlled the family‐wise error rate at 5% and were computed with individual variances.
3.2. CMJ kinetics
To jump higher, participants significantly reduced their mean vertical ground reaction force (time × phase interaction: F 2.30,39.09 = 15.49, p < 0.001) during the unloading phase (mean mid‐test and post‐test reduction from baseline: 68 ± 103 N [5 to 130], (p = 0.032) and 127 ± 78 N [79 to 174], (p < 0.001), respectively), while significantly increasing their mean vertical ground reaction forces during the braking (mean mid‐test and post‐test increase from baseline: 75 ± 78 N [27 to 122], (p = 0.002) and 95 ± 64 N [56 to 133], (p < 0.001), respectively) and propulsion phases (mean mid‐test and post‐test increase from baseline: 70 ± 88 N [17 to 123], (p = 0.010) and 59 ± 85 N [7 to 110], (p = 0.024), respectively; Figure 2). These changes led to a significant increase in the mean vertical ground reaction force during the combined unloading, braking, and propulsion phases (F 1.90,32.21 = 22.64, p < 0.001) from baseline to mid‐test (change in mean vertical ground reaction force: 62 ± 59 N [26 to 98], p = 0.001) and from baseline to post‐test (change in mean vertical ground reaction force: 82 ± 52 N [50 to 113], p < 0.001).
FIGURE 2.
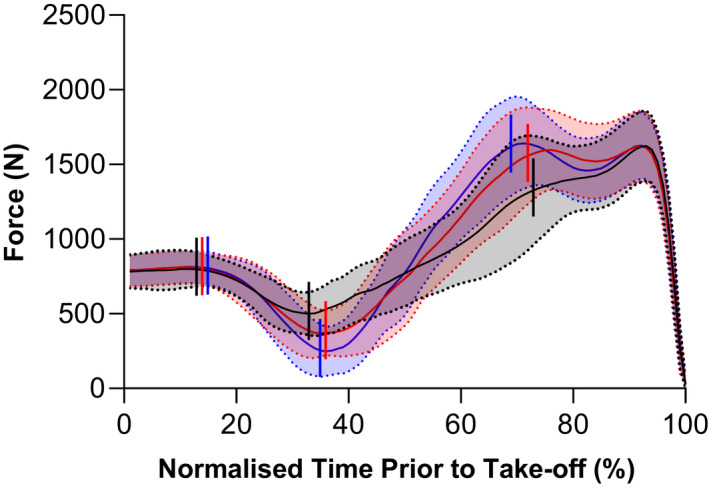
Mean (solid lines) ± standard deviation (shaded areas with dotted lines) vertical ground reaction force during the countermovement jumps (CMJs) performed at baseline (black), mid‐test (red), and post‐test (blue) of the 10‐week ballistic training program. As the duration of the CMJ varied between participants, group traces were constructed by time normalizing the data to 100 points using linear interpolation over a period corresponding to 20 frames before the initiation of the countermovement (0%) to take‐off (100%). Vertical lines (left to right) represent the start of the unloading, braking, and propulsion phases, respectively.
The total CMJ contact duration significantly decreased (F 1.85,31.39 = 12.01, p < 0.001) from baseline to mid‐test (−0.17 ± 0.21 s [−0.30 to −0.04], p = 0.008) and from baseline to post‐test (−0.20 ± 0.19 s [−0.32 to −0.09], p = 0.001) due to a significantly faster braking phase (see kinematics). This resulted in a significantly earlier onset of the propulsion phase (time × phase interaction: F 1.77,30.07 = 12.53, p < 0.001) at post‐test (0.60 ± 0.08 s [0.09 to 0.30], p < 0.001) compared with baseline (0.80 ± 0.19 s). The mean net impulse (time × phase interaction: F 1.47,24.95 = 6.64, p = 0.009), which was negative during the unloading phase, significantly increased (i.e., became more negative) from baseline to mid‐test (change in mean net impulse: −19 ± 16 N s [−28 to −9], p < 0.001) and from baseline to post‐test (change in mean net impulse: −26 ± 15 N·s [−35 to −17], p < 0.001). The mean net impulses, which were positive during the braking and propulsion phases, significantly increased only from baseline to post‐test (change in mean net braking impulse: 25 ± 17 N·s [15 to 35], p < 0.001; change in mean net propulsion impulse: 16 ± 17 N·s [5 to 26], p = 0.003).
3.3. CMJ kinematics
The knee flexion amplitude (time × phase interaction: F 2.28,38.91 = 3.16, p = 0.047) during the unloading phase significantly increased from baseline to post‐test (change in knee flexion amplitude: 7.4 ± 11.4° [0.5 to 14.4], p = 0.034), but not from baseline to mid‐test (change in knee flexion amplitude: 5.4 ± 9.3° [−0.2 to 11.0], p = 0.062). There were no significant changes in knee flexion amplitude during the braking phase from baseline to mid‐test (change in knee flexion amplitude: −1.6 ± 28.8° [−19.0 to 15.9], p = 0.971) or from baseline to post‐test (change in knee flexion amplitude: 2.3 ± 23.8° [−12.1 to 16.7], p = 0.912). During the propulsion phase, the knee extension amplitude significantly increased from baseline to post‐test (change in knee flexion amplitude: 13.0 ± 16.3° [3.1 to 22.9], p = 0.010), but not from baseline to mid‐test (change in knee flexion amplitude: 3.8 ± 21.9° [−9.5 to 17.0], p = 0.751).
In line with the significant increases in knee range of motion during the unloading and propulsion phases from baseline to post‐test only, significant time × phase interactions were identified for both VL's MTU length change amplitude (F 3.19,54.22 = 3.53, p = 0.019) and SEE length change amplitude (F 3.03,51.48 = 3.33, p = 0.026; Figures 3, 4). For the unloading phase, the SEE lengthening amplitude increased significantly between baseline and post‐test (change in SEE lengthening amplitude: 9.1 ± 13.4 mm [0.9 to 17.2], p = 0.028) and between baseline and mid‐test (change in SEE lengthening amplitude: 7.4 ± 11.9 mm [0.2 to 14.6], p = 0.044). However, a significant increase in the MTU lengthening amplitude was not identified (mean mid‐test and post‐test change from baseline: 6.7 ± 13.3 mm [−1.3 to 14.7], (p = 0.112) and 8.6 ± 15.0 mm [−0.4 to 17.7], (p = 0.064), respectively). In the propulsion phase, both MTU and SEE increased their shortening amplitudes between baseline and post‐test (change in MTU shortening amplitude: 9.5 ± 10.3 mm [3.3 to 15.8], p = 0.003; change in SEE shortening amplitude: 10.8 ± 15.2 mm [1.6 to 19.9], p = 0.020). However, VL's muscle fascicles did not lengthen or shorten significantly more during the respective unloading or propulsion phases, or during the braking phase (main effect of time: F 1.88,31.90 = 0.78, p = 0.458; time × phase interaction: F 2.38,40.47 = 1.07, p = 0.362).
FIGURE 3.
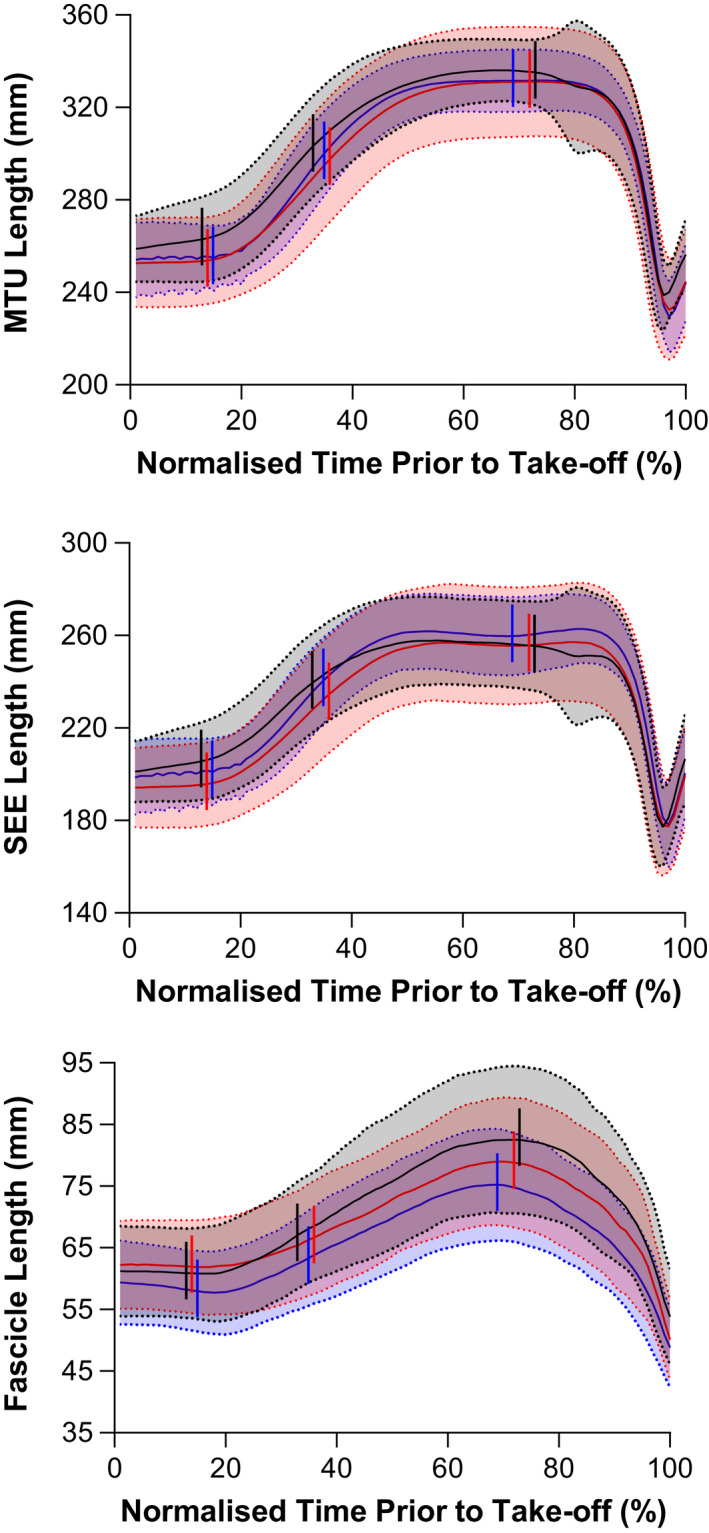
Mean (solid lines) ± standard deviation (shaded areas with dotted lines) vastus lateralis (TOP) muscle‐tendon unit (MTU), (MIDDLE) series elastic element (SEE), and (BOTTOM) vastus lateralis fascicle lengths during the countermovement jumps (CMJs) performed at baseline (black), mid‐test (red), and post‐test (blue) of the 10‐week ballistic training program. As the duration of the CMJ varied between participants, group traces were constructed by time normalizing the data to 100 points using linear interpolation over a period corresponding to 20 frames before the initiation of the countermovement (0%) to take‐off (100%). Vertical lines (left to right) represent the start of the unloading, braking, and propulsion phases, respectively.
FIGURE 4.
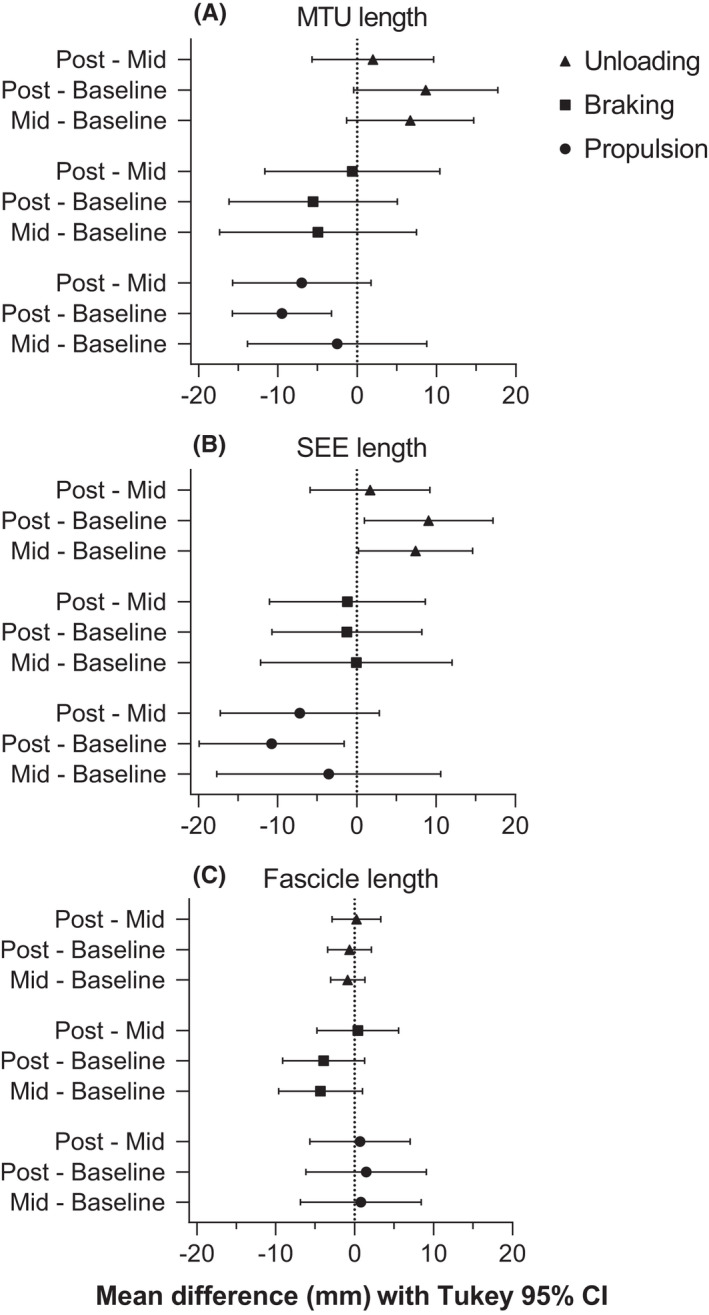
Mean paired differences (point estimate ±95% confidence interval [CI]) in vastus lateralis' (A) muscle‐tendon unit (MTU), (B) series elastic element (SEE), and (C) fascicle length changes between unloading, braking, and propulsion phases during countermovement jumps performed at baseline, mid‐test (Mid), and post‐test (Post) of the 10‐week combined ballistic training program. As the propulsion phase specifically resulted in shortening (i.e., a negative length change), negative differences indicate more shortening in the first listed testing session, whereas negative differences in the unloading and braking phases indicate less lengthening. A 95% CI that does not cross zero indicates a significant difference between testing sessions, and descriptive results are reported in the text. Tukey post hoc comparisons controlled the family‐wise error rate at 5% and were computed with individual variances.
A significant time × phase interaction effect was found for both contact duration (F 3.07,52.21 = 5.64, p = 0.002) and knee flexion velocity (F 3.14,53.40 = 12.56, p < 0.001). Despite a significant increase in knee flexion amplitude during the unloading phase from baseline to post‐test, there was no significant difference in the unloading duration (−0.04 ± 0.11 s [−0.10 to 0.03], p = 0.335), and therefore, knee flexion velocity during this phase significantly increased from baseline to post‐test (change in knee flexion velocity: 45.1 ± 53.8°·s [12.5 to 77.6], p = 0.007). In line with these changes, VL's MTU velocity (time × phase interaction: F 2.59,43.95 = 5.88, p = 0.003) and SEE velocity (time × phase interaction: F 2.74,46.62 = 5.43, p = 0.004) also significantly increased during the unloading phase from baseline to post‐test (change in MTU lengthening velocity: 54.4 ± 69.0 mm·s [12.7 to 96.2], p = 0.010; change in SEE lengthening velocity: 51.9 ± 67.0 mm·s [11.4 to 92.4], p = 0.012; Figure 5).
FIGURE 5.
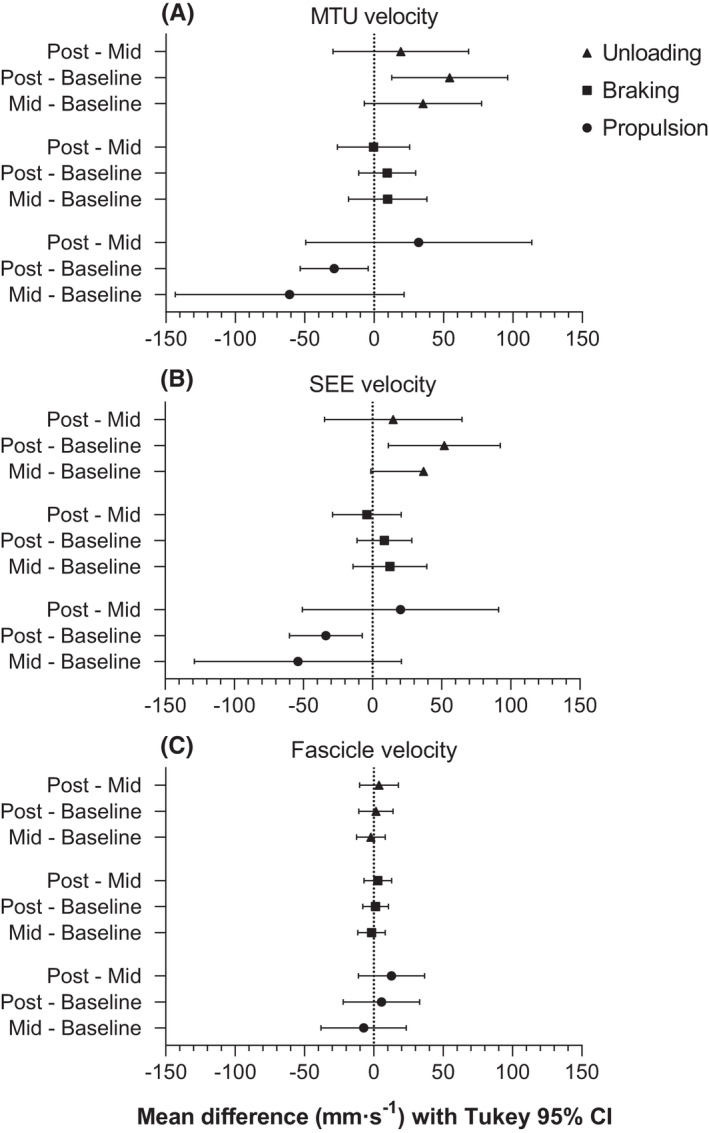
Mean paired differences (point estimate ±95% confidence interval [CI]) in vastus lateralis' (A) muscle‐tendon unit (MTU), (B) series elastic element (SEE), and (C) fascicle velocity changes between unloading, braking, and propulsion phases during countermovement jumps performed at baseline, mid‐test (Mid), and post‐test (Post) of the 10‐week combined ballistic training program. As the propulsion phase specifically resulted in shortening (i.e., a negative velocity), negative differences indicate a higher shortening velocities in the first listed testing session, whereas negative differences in the unloading and braking phases indicate lower lengthening velocities. A 95% CI that does not cross zero indicates a significant difference between testing sessions, and descriptive results are reported in the text. Tukey post hoc comparisons controlled the family‐wise error rate at 5% and were computed with individual variances.
As the braking phase duration significantly decreased from baseline to mid‐test (0.12 ± 0.16 s [0.02 to 0.22], p = 0.015) and from baseline to post‐test (0.16 ± 0.12 s [0.08 to 0.23], p < 0.001), the knee flexion velocities during this phase also increased; albeit the increase was only significant from baseline to post‐test (mean mid‐test and post‐test increase from baseline: 32.9 ± 68.7°s [−8.7 to 74.4] (p = 0.135) and 47.8 ± 43.6°s [21.5 to 74.2] (p = 0.001), respectively). However, these knee flexion velocity changes during the braking phase were not accompanied by significant increases in VL's MTU and SEE lengthening velocities between baseline and mid‐test (change in MTU lengthening velocity: 9.4 ± 46.8 mm·s [−11.1 to 29.9], p = 0.655; change in SEE lengthening velocity: 12.6 ± 44.1 mm·s [−14.1 to 39.3], p = 0.463) or between baseline and post‐test (change in MTU lengthening velocity: 9.4 ± 33.9 mm·s [−11.1 to 29.9], p = 0.483; change in SEE lengthening velocity: 8.5 ± 32.9 mm·s [−11.3 to 28.4], p = 0.525).
Despite a significant increase in knee extension amplitude during the propulsion phase from baseline to post‐test, there was no significant change in the propulsion duration (−0.01 ± 0.04 s [−0.03 to 0.02], p = 0.860), and therefore, knee extension velocity during this phase significantly increased from baseline to post‐test (change in knee extension velocity: 40.1 ± 34.4°·s [19.3 to 60.9], p < 0.001). In line with these changes, MTU and SEE lengthening velocities also significantly increased during the propulsion phase from baseline to post‐test (change in MTU lengthening velocity: 28.7 ± 40.5 mm·s [4.2 to 53.2], p = 0.021; change in SEE lengthening velocity: 33.7 ± 43.6 mm·s [7.3 to 60.1], p = 0.012). VL's fascicle lengthening velocities during the unloading and braking phases, as well as VL's fascicle shortening velocity during the propulsion phase, did not significantly change over the training period (main effect of time: F 1.59,27.02 = 1.25, p = 0.296; time × phase interaction: F 2.35,40.00 = 0.29, p = 0.782).
4. DISCUSSION
This study investigated changes to MTU kinematics of the VL during a CMJ following 10 weeks of multi‐modality, combined heavy‐ and light‐load ballistic training. This training modality is commonly used in sports performance settings, but little is known about the underlying changes in MTU kinematics during a sport‐specific task (e.g., jumping) following training. CMJ height increased by ~3 cm, on average, after 10 weeks of training and this was underpinned by significant increases in vertical ground reaction force during the braking and propulsion phases of the CMJ. Force also significantly decreased during the unloading phase of the CMJ, which was accompanied by increases in knee flexion amplitude and velocity, as well as increases in the amount and rate of SEE lengthening. Subsequently, knee extension amplitude and velocity, as well as the amount and rate of SEE and MTU shortening were increased during the propulsion phase of the CMJ following training. However, muscle fascicle kinematics did not significantly change over the training period. It is speculated that the CMJ kinetic and kinematic changes arose from altered lower limb coordination during the CMJ, which is supported by changes to the CMJ force profile (Figure 2), and increased force capacities of the lower limb muscles following combined ballistic training, which subsequently enhanced positive work output of the lower limb MTUs, including VL.
4.1. CMJ performance
The ten‐week combined ballistic training program improved CMJ performance as indicated by a 11 ± 13% increase in jump height from baseline to post‐test. This is in line with findings from previous studies that demonstrated significant and meaningful improvements in CMJ variables following light‐ballistic activities, 24 heavy‐strength training, 25 weightlifting derivatives, 26 , 27 and mixed‐method training plans. 7 , 28 Although CMJ depth increased from baseline to post‐test, the total contact duration before take‐off was significantly reduced, indicating that participants performed the CMJ faster. A faster CMJ was permitted because participants reduced their vertical ground reaction force during the unloading phase, which resulted in a significantly faster downwards center of mass velocity. A shorter braking phase following training was also observed and, along with the lower vertical ground reaction force entering the braking phase, this required participants to generate significantly higher forces during the braking and propulsion phases to increase their jump height. It is speculated that enhanced lower limb muscle force output following training allowed these higher forces to be produced following training.
Altered CMJ coordination over the training period was evident from the vertical ground reaction force profile changing from unimodal to bimodal‐primary, which has previously been shown in a 12‐week power training study. 12 The type of force profile has been shown to distinguish between athletes of different abilities and/or development stages, 12 , 29 and it has been suggested that a bimodal pattern of force production reflects a disassociation in the timing of knee extension, ankle plantarflexion, and peak agonist muscle activation. 30 It has been speculated that a greater countermovement depth, as observed here, could require knee extension (and thus the propulsion phase) to occur earlier to overcome the greater amount of knee flexion prior to ankle plantarflexion and the onset of plantarflexion muscle activation. 30 Although our results do not provide information about the timing of knee extension relative to plantarflexion, we observed that the propulsion phase started ~0.2 s earlier, which supports the suggestion that knee extension occurs earlier for greater countermovement depths following training. 30 The changes in CMJ coordination potentially arose from enhanced lower limb muscle force capacities following the combined ballistic training and improved intermuscular coordination during the CMJ; however, this would need to be verified in future studies by inverse dynamics and electromyography analyses.
4.2. Unloading phase
During the unloading phase, participants increased their knee flexion range of motion following the ten‐week combined ballistic training program. As a result, the VL MTU and SEE lengthening amplitudes increased by a similar magnitude (MTU: 8.6 ± 15.0 mm; SEE: 9.1 ± 13.4 mm), but the difference in MTU lengthening did not reach significance (MTU: p = 0.064; SEE: p = 0.028). The significant increase in SEE, but not MTU lengthening amplitude, could reflect greater muscle activity and less VL muscle fascicle lengthening during the unloading phase following training, despite no significant decrease (−0.7 ± 4.6 mm) in VL's fascicle lengthening amplitude. This occurs due to the presence of MTU compliance and muscle activation, where the muscle fascicles can shorten to stretch the SEE, without changing MTU length. 31 Taken together, these findings suggest that the increased kinetic energy arising from a faster countermovement following training was not dissipated by VL's muscle fascicles and could be effectively stored within VL's SEE.
4.3. Braking phase
There were no significant changes in VL's MTU, SEE or muscle fascicle lengthening amplitudes or velocities during the braking phase of the CMJ following training, despite participants' knee flexion velocity increasing from baseline to post‐test. It is a little surprising that MTU and SEE lengthening velocities did not increase with the increase in knee flexion velocity; however, this might be due to the viscoelastic properties of the MTU and/or because of the variability of the repeated measurements. In support of the speculation that measurement variability precluded differences from being observed, VL fascicle lengthening amplitudes were reduced from baseline to mid‐test and post‐test by on average 4.1 mm, which could be considered a meaningful difference, but the average standard deviation of this difference was 8.7 mm (Cohen's dz = 0.46 to 0.49). To detect a difference of this magnitude with 90% power and a two‐tailed alpha level of 5%, we would have needed to test at least 46–52 individuals. However, with our sample size of 18 participants, we could only detect significant standardized mean differences of greater than 0.81. Thus, to detect significant effect sizes of smaller magnitudes in the future with reasonable power (at least 90%), we recommend a much larger sample size. Alternatively, a more accurate methodology for measuring fascicle length changes over repeated sessions could be developed. For now though, it appears reasonable to speculate that during the braking phase, because of the higher vertical ground reaction forces produced following training, the VL muscle was activated more, which subsequently reduced the amount of active VL muscle fascicle lengthening a meaningful amount compared with baseline. This speculation is supported by the finding that VL's MTU lengthening velocity increased ~15% from baseline to post‐test, but its muscle fascicle lengthening velocity increased three times less (~5%).
4.4. Propulsion phase
During the propulsion phase, a greater knee extension velocity was observed following the 10‐week training program due to an increased knee extension range of motion without a concomitant increase in phase duration. Subsequently, VL's MTU and SEE shortening amplitudes and velocities increased during the propulsion phase following training. However, no significant changes in VL's fascicle shortening amplitude or velocity were observed. It is speculated that greater VL MTU work, which arose due to a greater VL MTU shortening amplitude and increased VL muscle activation and force production (which is assumed based on the increased vertical ground reaction forces observed following training), contributed to the increased knee extension velocity and increased jump height following training. Additionally, greater elastic energy storage within VL's SEE, which could have increased due to higher muscle forces and increased kinetic energy arising from a faster countermovement, might have also contributed to increased CMJ performance by allowing faster SEE recoil and more favorable velocities for the muscle fascicles to produced force during the propulsion phase. This is supported by the finding that VL's muscle fascicles shortened 8% slower following training while its MTU shortened 10% faster.
4.5. Limitations
In the present study, no direct measures of free tendon length change during the CMJs were made, nor were muscle activation patterns investigated, lower limb joint kinetics computed, or patellar tendon forces estimated. Muscle activation measurements would provide useful information to evaluate how participants shifted from a unimodal to bimodal CMJ force profile over the training period. In the interpretation of the above findings, it has been assumed that a faster countermovement resulted in greater knee extension moments and patellar tendon forces (i.e., greater MTU forces) due to increased kinetic energy during the countermovement and higher VL muscle forces. We believe this is a reasonable assumption and is supported by previous findings showing both increased resting tendon forces and increased negative knee joint work under higher gravitational forces. 15
Naturally, factors other than greater knee joint moments could have made a greater contribution to the CMJ performance improvement observed, such as a more upright position at take‐off (i.e., full extension of all joints), greater work output at another joint (e.g., hip), a joint‐work redistribution (e.g., from the knee to hip), and/or increased biarticular muscle energy transfer from proximal to distal joints. Further research is thus recommended to investigate changes in joint work and power contributions following training, as well as tendinous tissue adaptations. Nevertheless, we are confident that part of the CMJ performance gain was due to increased positive work of VL's MTU as reflected by changes in its kinematics. As this study had no control group, we are unable to conclude whether the increase in CMJ height arose from the combined ballistic training or solely from CMJ repetition and improved coordination across the repeated testing sessions. However, the latter effect seems unlikely based on data showing a similar increase (~3 cm) in CMJ height following 8 weeks of countermovement drop jump training compared with no improvements following bounce drop jump training or no training in young varsity‐level males. 32
In conclusion, a 10‐week heavy‐ and light‐load combined ballistic training program increased vertical CMJ height in a group of young, male adults. Underpinning this improvement were higher vertical ground reaction forces during the braking and propulsion phases of the CMJ, as well as significant increases in VL's MTU and SEE shortening amplitudes and velocities during the propulsion phase. It is speculated that increased VL muscle activation and a faster countermovement allowed more energy to be initially stored within VL's SEE following training and that this contributed to increased VL MTU work during the propulsion phase and a higher jump height. The faster countermovement may have been permitted following training because participants were better able to coordinate the activation of their lower limb muscles due to CMJ and other jump practice as evidenced by changes in their CMJ force profile, and because participants improved the force‐producing capacity of their lower limb muscles due to the combined ballistic training.
5. PERSPECTIVE
The findings from the current study provide novel insights into how muscle and tendon kinematics of one lower limb MTU change during a sport‐specific task in response to heavy‐ and light‐load combined ballistic training, which is a common training modality used to improve athletic performance. Our results support the idea that starting the propulsion phase with a higher ground reaction force results in a higher jump, which has been suggested to be the dominant factor explaining the difference in CMJ and squat jump heights. 33
The increase in jump height observed following the 10‐week combined ballistic training program (3.2 ± 4.2 cm, 11 ± 13%) is consistent with the increases observed following plyometric and weightlifting training programs (~8–9%) of similar duration (~9 weeks), but greater than the improvements observed following traditional resistance training programs (~2%). 34 At least 10 weeks of training and more than 20 sessions that involve high‐intensity jump exercises potentially maximize the probability of improving CMJ performance. 35 As most of our significant changes relative to baseline occurred at post‐test, rather than mid‐Test, our results indicate that a minimum of 10 weeks of combined ballistic training is also required to induce adaptations that enhance CMJ performance. Consequently, the combined ballistic training program used in this study is recommended over a traditional resistance training program to improve CMJ height. To maximize the probability of improving CMJ height, CMJs should also be added or substituted into the training program (Table 1). The combined ballistic training program is also recommended over a weightlifting training program to athletes unfamiliar with the snatch or clean and jerk as the weightlifting derivates incorporated are easier to perform and should subsequently take less time to learn.
CONFLICT OF INTEREST
The authors wish to disclose that there are no professional relationships with companies or manufacturers that could benefit from the results of the present study. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Supporting information
Appendix S1
ACKNOWLEDGEMENTS
The authors thank Dr. Glen Lichtwark and Dr. Logan Wade for their input throughout the study. We also thank the participants for donating their time to this project. Open access publishing facilitated by University of Southern Queensland, as part of the Wiley ‐ University of Southern Queensland agreement via the Council of Australian University Librarians.
Hoffman BW, Raiteri BJ, Connick MJ, et al. Altered countermovement jump force profile and muscle‐tendon unit kinematics following combined ballistic training. Scand J Med Sci Sports. 2022;32:1464‐1476. doi: 10.1111/sms.14211
Ben W. Hoffman and Brent J. Raiteri should be considered as joint first authors.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Hill AV. The heat of shortening and the dynamic constants of muscle. Proc R Soc B Biol Sci. 1938;126(843):136‐195. doi: 10.1098/rspb.1938.0050 [DOI] [Google Scholar]
- 2. Galantis A, Woledge RC. The theoretical limits to the power output of a muscle‐tendon complex with inertial and gravitational loads. Proc R Soc B Biol Sci. 2003;270(1523):1493‐1498. doi: 10.1098/rspb.2003.2403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Farris DJ, Lichtwark GA, Brown NAT, Cresswell AG. The role of human ankle plantar flexor muscle‐tendon interaction and architecture in maximal vertical jumping examined in vivo. J Exp Biol. 2016;219(4):528‐534. doi: 10.1242/jeb.126854 [DOI] [PubMed] [Google Scholar]
- 4. Kurokawa S, Fukunaga T, Fukashiro S. Behavior of fascicles and tendinous structures of human gastrocnemius during vertical jumping. J Appl Physiol. 2001;90(4):1349‐1358. doi: 10.1152/jappl.2001.90.4.1349 [DOI] [PubMed] [Google Scholar]
- 5. Roberts TJ, Azizi E. Flexible mechanisms: the diverse roles of biological springs in vertebrate movement. J Exp Biol. 2011;214(3):353‐361. doi: 10.1242/jeb.038588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aeles J, Lichtwark G, Peeters D, Delecluse C, Jonkers I, Vanwanseele B. Effect of a prehop on the muscle‐tendon interaction during vertical jumps. J Appl Physiol. 2018;124(5):1203‐1211. doi: 10.1152/japplphysiol.00462.2017 [DOI] [PubMed] [Google Scholar]
- 7. James LP, Haff GG, Kelly VG, Connick M, Hoffman B, Beckman EM. The impact of strength level on adaptations to combined weightlifting, plyometric and ballistic training. Scand J Med Sci Sports. 2018;28(5):1494‐1505. doi: 10.1111/sms.13045 [DOI] [PubMed] [Google Scholar]
- 8. Ebben WP, Carroll RM, Simenz CJ. Strength and conditioning practices of national hockey league strength and conditioning coaches. J Strength Cond Res. 2004;18(4):889‐897. doi: 10.1519/14133.1 [DOI] [PubMed] [Google Scholar]
- 9. Ebben WP, Hintz MJ, Simenz CJ. Strength and conditioning practices of major league baseball strength and conditioning coaches. J Strength Cond Res. 2005;19(3):538‐546. doi: 10.1519/R-15464.1 [DOI] [PubMed] [Google Scholar]
- 10. Simenz CJ, Dugan CA, Ebben WP. Strength and conditioning practices of national basketball association strength and conditioning coaches. J Strength Cond Res. 2005;19(3):495‐504. doi: 10.1519/15264.1 [DOI] [PubMed] [Google Scholar]
- 11. Blazevich AJ, Gill ND, Bronks R, Newton RU. Training‐specific muscle architecture adaptation after 5‐wk training in athletes. Med Sci Sports Exerc. 2003;35(12):2013‐2022. doi: 10.1249/01.MSS.0000099092.83611.20 [DOI] [PubMed] [Google Scholar]
- 12. Cormie P, Mcbride JM, Mccaulley GO. Power‐time, force‐time, and velocity‐time curve analysis of the countermovement jump: Impact of training. J Strength Cond Res. 2009;23(1):177‐186. doi: 10.1519/JSC.0b013e3181889324 [DOI] [PubMed] [Google Scholar]
- 13. Suchomel TJ, Comfort P, Stone MH. Weightlifting pulling derivatives: rationale for implementation and application. Sport Med. 2015;45(6):823‐839. doi: 10.1007/s40279-015-0314-y [DOI] [PubMed] [Google Scholar]
- 14. Petushek E, Richter C, Donovan D, Jensen RL. Comparison of 2D video and electrogoniometry measurements of knee flexion angle during a countermovement jump and landing task. Sport Eng. 2012;15:159‐166. doi: 10.1007/s12283-012-0094-7 [DOI] [Google Scholar]
- 15. Wade L, Lichtwark G, Farris DJ. Movement strategies for countermovement jumping are potentially influenced by elastic energy stored and released from tendons. Sci Rep. 2018;8(1):1‐11. doi: 10.1038/s41598-018-20387-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brennan SF, Cresswell AG, Farris DJ, Lichtwark GA. In vivo fascicle length measurements via B‐mode ultrasound imaging with single vs dual transducer arrangements. J Biomech. 2017;64:240‐244. doi: 10.1016/j.jbiomech.2017.09.019 [DOI] [PubMed] [Google Scholar]
- 17. Hoffman BW, Cresswell AG, Carroll TJ, Lichtwark GA. Muscle fascicle strains in human gastrocnemius during backward downhill walking. J Appl Physiol. 2014;116(11):1455‐1462. doi: 10.1152/japplphysiol.01431.2012 [DOI] [PubMed] [Google Scholar]
- 18. Wade L, Lichtwark GA, Farris DJ. The influence of added mass on muscle activation and contractile mechanics during submaximal and maximal countermovement jumping in humans. J Exp Biol. 2019;222(2):jeb194852. doi: 10.1242/jeb.194852 [DOI] [PubMed] [Google Scholar]
- 19. Farris DJ, Lichtwark GA. UltraTrack: software for semi‐automated tracking of muscle fascicles in sequences of B‐mode ultrasound images. Comput Methods Programs Biomed. 2016;128:111‐118. doi: 10.1016/j.cmpb.2016.02.016 [DOI] [PubMed] [Google Scholar]
- 20. Cronin NJ, Carty CP, Barrett RS, Lichtwark G. Automatic tracking of medial gastrocnemius fascicle length during human locomotion. J Appl Physiol. 2011;111(5):1491‐1496. doi: 10.1152/japplphysiol.00530.2011 [DOI] [PubMed] [Google Scholar]
- 21. Hawkins D, Hull ML. A method for determining lower extremity muscle‐tendon lengths during flexion/extension movements. J Biomech. 1990;23(5):487‐494. doi: 10.1016/0021-9290(90)90304-L [DOI] [PubMed] [Google Scholar]
- 22. Fukunaga T, Kubo K, Kawakami Y, Fukashiro S, Kanehisa H, Maganaris CN. In vivo behaviour of human muscle tendon during walking. Proc R Soc B Biol Sci. 2001;268(1464):229‐233. doi: 10.1098/rspb.2000.1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maxwell SE, Delaney HD. Designing Experiments and Analyzing Data: A Model Comparison Perspective. 2nd ed. Lawrence Erlbaum Associates Publishers; 2004. [Google Scholar]
- 24. Cormie P, McGuigan MR, Newton RU. Influence of strength on magnitude and mechanisms of adaptation to power training. Med Sci Sports Exerc. 2010;42(8):1566‐1581. doi: 10.1249/MSS.0b013e3181cf818d [DOI] [PubMed] [Google Scholar]
- 25. Cormie P, McGuigan MR, Newton RU. Adaptations in athletic performance after ballistic power versus strength training. Med Sci Sport Exerc. 2010;42(8):1582‐1598. doi: 10.1249/MSS.0b013e3181d2013a [DOI] [PubMed] [Google Scholar]
- 26. Arabatzi F, Kellis E, De Villarreal E‐S. Vertical jump biomechanics after plyometric, weight lifting, and combined (weight lifting + plyometric) training. J Strength Cond Res. 2010;24(9):2440‐2448. doi: 10.1519/JSC.0b013e3181e274ab [DOI] [PubMed] [Google Scholar]
- 27. Suchomel TJ, McKeever SM, McMahon JJ, Comfort P. The effect of training with weightlifting catching or pulling derivatives on squat jump and countermovement jump force‐time adaptations. J Funct Morphol Kinesiol. 2020;5(2):28. doi: 10.3390/jfmk5020028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. James LP, Comfort P, Suchomel TJ, Kelly VG, Beckman EM, Haff GG. Influence of power clean ability and training age on adaptations to weightlifting‐style training. J Strength Cond Res. 2019;33(11):2936‐2944. doi: 10.1519/JSC.0000000000002534 [DOI] [PubMed] [Google Scholar]
- 29. McMahon JJ, Murphy S, Rej SJE, Comfort P. Countermovement‐jump‐phase characteristics of senior and academy rugby league players. Int J Sports Physiol Perform. 2017;12(6):803‐811. doi: 10.1123/ijspp.2016-0467 [DOI] [PubMed] [Google Scholar]
- 30. Sahrom SB, Wilkie JC, Nosaka K, Blazevich AJ. The use of yank‐time signal as an alternative to identify kinematic events and define phases in human countermovement jumping: Yank‐time signal to analyse jumping. R Soc Open Sci. 2020;7(8):192093. doi: 10.1098/rsos.192093rsos192093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Griffiths R. Shortening of muscle fibres during stretch of the active cat medial gastrocnemius muscle: the role of tendon compliance. J Physiol. 1991;436(1):219‐236. doi: 10.1113/jphysiol.1991.sp018547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Marshall BM, Moran KA. Which drop jump technique is most effective at enhancing countermovement jump ability, “countermovement” drop jump or “bounce” drop jump? J Sports Sci. 2013;31(12):1368‐1374. doi: 10.1080/02640414.2013.789921 [DOI] [PubMed] [Google Scholar]
- 33. Bobbert MF, Gerritsen KGM, Litjens MCA, Van Soest AJ. Why is countermovement jump height greater than squat jump height? Med Sci Sports Exerc. 1996;28(11):1402‐1412. doi: 10.1097/00005768-199611000-00009 [DOI] [PubMed] [Google Scholar]
- 34. Berton R, Lixandrão ME, Pinto e Silva CM, Tricoli V. Effects of weightlifting exercise, traditional resistance and plyometric training on countermovement jump performance: a meta‐analysis. J Sports Sci. 2018;36(18):2038‐2044. doi: 10.1080/02640414.2018.1434746 [DOI] [PubMed] [Google Scholar]
- 35. de Villarreal ES‐S, Kellis E, Kraemer WJ, Izquierdo M. Determining variables of plyometric training for improving vertical jump height performance: a meta‐analysis. J Strength Cond Res. 2009;23(2):495‐506. doi: 10.1519/JSC.0b013e318196b7c6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


