Abstract
Background and Aims
Porto‐sinusoidal vascular disorder (PSVD) is a rare and commonly overlooked cause of portal hypertension. The interest of CT analysis, including quantification of liver surface nodularity (LSN) for PSVD diagnosis has not been established. This study aimed at assessing the performance of LSN and CT features for a PSVD diagnosis in patients with signs of portal hypertension.
Approach and Results
This retrospective case‐control study included a learning cohort consisting of 50 patients with histologically proven PSVD, according to VALDIG criteria, and 100 control patients with histologically proven cirrhosis, matched on ascites. All patients and controls had at least one sign of portal hypertension and CT available within 1 year of liver biopsy. Principal component analysis of CT features separated patients with PSVD from patients with cirrhosis. Patients with PSVD had lower median LSN than those with cirrhosis (2.4 vs. 3.1, p < 0.001). Multivariate analysis identified LSN < 2.5 and normal‐sized or enlarged segment IV as independently associated with PSVD. Combination of these two features had a specificity of 90% for PSVD and a diagnostic accuracy of 84%. Even better results were obtained in an independent multicenter validation cohort including 53 patients with PSVD and 106 control patients with cirrhosis (specificity 94%, diagnostic accuracy 87%).
Conclusions
This study that included a total of 103 patients with PSVD and 206 patients with cirrhosis demonstrates that LSN < 2.5 combined with normal‐sized or enlarged segment IV strongly suggests PSVD in patients with signs of portal hypertension.

Abbreviations
- AUROC
receiver operating characteristic curve
- CT
contrast‐enhanced CT
- IQR
interquartile range
- LSN
liver surface nodularity
- MELD
Model for End‐Stage Liver Disease
- PSVD
porto‐sinusoidal vascular disorder
INTRODUCTION
Porto‐sinusoidal vascular disorder (PSVD) is a group of rare diseases causing portal hypertension and characterized by the absence of cirrhotic modification of liver parenchyma.[ 1 ] This group of entities was previously largely known as idiopathic noncirrhotic portal hypertension. Liver histological lesions found in patients with PSVD include obliterative portal venopathy, hepatoportal sclerosis, nodular regenerative hyperplasia, and incomplete septal cirrhosis.[ 2 , 3 ] Although the widespread use of noninvasive tests for liver fibrosis might contribute to a better recognition of PSVD, particularly by showing a contrast between portal hypertension and low liver stiffness values,[ 4 , 5 ] PSVD still remains commonly overlooked and misdiagnosed as cirrhosis.[ 6 , 7 , 8 ] Moreover, histologic changes may be subtle at liver histological analysis, and the diagnosis may not be recognized in the absence of strong clinical suspicion.[ 9 ] Differentiating PSVD from cirrhosis has clinical implications, as management and prognosis differ between these two entities.[ 1 ] Identifying additional noninvasive tools raising suspicion of PSVD would therefore be useful in clinical practice. Few studies described liver morphological changes associated with PSVD, including intrahepatic portal vein radicle irregularities, lack of visualization of intrahepatic portal vein branches, non‐occlusive thrombosis of main portal vein, altered liver morphology, and absence of nodular liver surface.[ 10 , 11 , 12 , 13 , 14 ] Recently, a software was developed to quantify liver surface nodularity (LSN) on routine CT images, providing quantitative measurement of irregularities of the liver surface.[ 15 , 16 , 17 ] LSN quantification is associated with a high reproducibility, and increased agreement compared with visual assessment.[ 18 ] LSN quantification is able to differentiate cirrhotic from noncirrhotic livers[ 15 ] and is associated with clinically significant portal hypertension in patients with cirrhosis,[ 17 ] risk of hepatic decompensation in patients with compensated cirrhosis, and increased risk of post‐hepatectomy liver failure in patients with resectable HCC.[ 19 ] Potential interest of LSN for PSVD diagnosis has not been tested. Moreover, clinical features and histological lesions associated with morphological changes at CT have not been investigated. The aims of this study were (1) to assess diagnostic performance of LSN and CT features to diagnose PSVD in patients with signs of portal hypertension, (2) to identify clinical features and histological lesions associated with CT changes in patients with PSVD, and (3) to evaluate the prognostic value of CT changes in patients with PSVD.
PATIENTS AND METHODS
We designed a retrospective case‐control study.
Patients with PSVD in the learning cohort
We included all patients with PSVD and signs of portal hypertension (at least one among ascites, thrombocytopenia, splenomegaly, gastro‐esophageal varices, porto‐systemic collaterals at imaging[ 1 ]) who underwent transjugular liver biopsy between January 2011 and June 2018 at Beaujon Hospital (Clichy, France) and a multiphasic contrast‐enhanced CT within 1 year of the liver biopsy. The protocol was performed in accordance with ethical guidelines of the 1975 Declaration of Helsinki and was approved by the institutional review board (CPP Ile de France IV, Paris, France). None of the patients refused permission for use of their case records for medical research.
Diagnosis of PSVD with portal hypertension was based on the criteria proposed by VALDIG.[ 1 ] Noninclusion criteria were other causes of portal hypertension, as defined by VALDIG, or a history of transjugular intrahepatic portosystemic shunt. All biopsies from patients with PSVD were reviewed by an expert pathologist to rule out cirrhosis and to assess the presence of elementary lesions classified according to prespecified criteria (Table S1). Extrahepatic conditions associated with PSVD were classified into categories detailed in Table S2, according to previous reports.[ 20 , 21 , 22 , 23 ]
Control patients with cirrhosis in the learning cohort
Each patient with PSVD was randomly matched according to severity of ascites and to year of liver catheterization with 2 patients with histologically proven cirrhosis and signs of portal hypertension (same as patients with PSVD), who also underwent hepatic vein catheterization between 2011 and June 2018 at our center. We chose to stratify on ascites, because this feature is the only one associated with survival both in patients with PSVD and in patients with cirrhosis.[ 24 ] Liver samples were either liver biopsy samples obtained during hepatic vein catheterization or surgical specimen (liver resection or transplantation). We included only patients with cirrhosis related to excessive alcohol consumption, chronic B or C virus infection, and metabolic associated fatty liver disease, as it represents most of the patients with cirrhosis seen in clinical practice. Noninclusion criteria were history of transjugular intrahepatic porto‐systemic shunt, HCC outside Milan criteria, infection within the 15 days before the inclusion, and alcohol‐associated hepatitis. As for the learning cohort, patients with cirrhosis were included if they had undergone a CT within 1 year of liver sample collection.
Validation cohort
An independent multicenter validation cohort included 53 and 106 patients with biopsy‐proven PSVD and cirrhosis, respectively. All patients in this validation group met the same inclusion and noninclusion criteria as those of the learning cohort, except that patients with PSVD could be included despite a duration between CT and biopsy exceeding 1 year, provided the histological diagnosis of PSVD with portal hypertension was made before CT. Patients were included in Clichy (France) between June 2018 and 2020 (13 with cirrhosis and 14 with PSVD), Barcelona (Spain) between 2003 and 2021 (21 with cirrhosis and 25 with PSVD), Tours (France) between 2011 and 2021 (25 with cirrhosis and 14 with PSVD), and Geneva (Switzerland) between 2011 and 2017 (47 with cirrhosis). This validation cohort was used to test the ability of the combination of size of segment IV with LSN to diagnose PSVD.
Pooled learning and validation cohorts
For sensitivity analysis, we pooled learning and validation cohorts, matched patients with PSVD 1:1 with patients with cirrhosis (according to age [±5 years], sex, ascites, and Model for End‐Stage Liver Disease [MELD] [±3]), and tested the ability of the combination of size of segment IV with LSN to diagnose PSVD.
CT acquisition protocol and image analysis
CT acquisition protocol is detailed in the Supporting Methods. CT examinations from patients with PSVD and cirrhosis were anonymized, shuffled, and presented randomly to two radiologists (4 years of experience for each radiologist) for independent image review. Readers were thus not aware of clinical information nor of patients’ groups. Readers evaluated images qualitatively using prespecified criteria and classification (Table S3) on a PACS station (Directview; Carestream Health Inc.). Volume assessment of segment IV was performed by the visual impression of the reader. Another abdominal radiologist (5 years of experience) also measured transversal size of segment IV. Given the absence of established definition of segment IV atrophy, a cutoff of 40‐mm transversal size of segment IV was chosen.[ 25 ]
LSN quantification
LSN quantification was performed on portal venous phase CT images using semiautomated CT software (LSN Software, version 0.88; Liver Nodularity LLC) by two abdominal radiologists (5 and 8 years of experience) blinded to clinical data. The radiologist drew a region of interest of 1–2 cm in diameter across the margin of the left liver surface. LSN was only measured on the left hepatic lobe, because LSN on the right and left hepatic lobes have been shown to be highly correlated.[ 18 ] Moreover, by choosing a unique anatomic region, the user is not inclined to choose a portion of liver with the greatest perceived surface nodularity, which may reduce observational bias.[ 26 ] The software automatically detected the liver edge compared with adipose tissue on the selected section, and on upward and downward continuous sections by propagating the painted region of interest. The software automatically generated a smooth polynomial line to mimic the expected normal liver surface. The distance between the detected liver margin and the polynomial line was measured on a pixel‐by‐pixel basis, expressed in tenths of a millimeter. At least eight valid margin measurements were obtained according to previous studies.[ 16 , 27 ] The software calculated the arithmetical mean of the measurements. Less than eight valid measurements were considered to be LSN quantification failure.[ 16 , 27 ]
Statistical analysis
Results are presented as median (interquartile range [IQR]) or absolute number (percentage). Comparisons between continuous variables were performed using Mann‐Whitney U test. Comparisons between categorical variables were performed using the chi‐square or Fisher exact test, when appropriate. Correlations between quantitative variables were performed using a Spearman’s test. For LSN, a cutoff value of 2.5 was chosen based on previously published data showing that this value differentiates the presence and absence of advanced liver fibrosis.[ 26 , 28 , 29 ] The discriminative ability of LSN for the identification of PSVD was assessed by measuring the area under the receiver operating characteristic curve (AUROC) with its 95% CI. Comparison between AUROCs was performed by using the DeLong method.
Interreader agreement for CT features was assessed with kappa coefficients: 0.00–0.20 indicated slight agreement; 0.21–0.40, fair agreement; 0.41–0.60, moderate agreement; 0.61–0.80 substantial agreement; and 0.81–1.00 almost perfect agreement. Interreader variability for LSN measurement was assessed with the intraclass correlation coefficient.
Principal component analysis of CT features was performed. To identify CT features associated with PSVD, we fitted a multivariate binary logistic regression analysis including CT features having a different prevalence between patients with PSVD and with cirrhosis at univariate analysis with alpha‐level <0.05 after Bonferroni’s adjustment for both readers and with data available for all patients. A model was also constructed with the regression coefficients of the variables that were independently associated with PSVD.
The potential ability of LSN and CT features to predict cumulative incidence of liver‐related events and cumulative incidence of liver transplantation or death was tested by fitting Cox proportional HRs in patients with PSVD. Patients dying without liver‐related events were censored at death. Statistical analyses were performed using SPSS 20 (IBM, Chicago, IL, USA). All tests were two‐sided and used a significance level of 0.05.
RESULTS
Patients of the learning cohort
Fifty patients with PSVD were included and compared with 100 control patients with cirrhosis. Their characteristics at liver biopsy are summarized in Table 1 and detailed in Supporting Results and Table S2.[ 30 ]
TABLE 1.
Characteristics of the patients of the learning cohort at liver biopsy
| Characteristics | Cirrhosis (n = 100) | PSVD (n = 50) | p value | ||
|---|---|---|---|---|---|
| n a | Number (%) or median (IQR) | n a | Number (%) or median (IQR) | ||
| Clinical features | |||||
| Age, years | 100 | 61 (54–65) | 50 | 58 (39–65) | 0.067 |
| Male gender | 100 | 78 (78) | 50 | 27 (54) | 0.002 |
| Diabetes mellitus | 98 | 38 (38) | 46 | 7 (14) | 0.004 |
| Ascites | 100 | 50 | |||
| Absent | 80 (80) | 40 (80) | |||
| Moderate or controlled with diuretics | 12 (12) | 6 (12) | 1 | ||
| Tense | 8 (8) | 4 (8) | |||
| History of HE | 100 | 17 (17) | 50 | 1 (2) | 0.008 |
| History of variceal bleeding | 100 | 23 (23) | 50 | 11 (22) | 0.89 |
| Beta blockers use | 99 | 41 (41) | 47 | 20 (43) | 1 |
| Gastroesophageal varices b | 59 | 42 | |||
| Absent | 21 (36) | 14 (33) | |||
| Small | 15 (25) | 6 (15) | 0.224 | ||
| Medium or large or history of band ligation | 23 (39) | 22 (52) | |||
| HCC | 100 | 45 (45) | 50 | 0 (0) | <0.0001 |
| Laboratory data | |||||
| Serum creatinine, µmol/l | 97 | 69 (60–80) | 50 | 73 (61–85) | 0.404 |
| Serum bilirubin, µmol/l | 99 | 23 (12–46) | 50 | 13 (9–18) | <0.0001 |
| Serum albumin, g/l | 96 | 33 (27–39) | 47 | 36 (31–41) | 0.044 |
| INR | 99 | 1.3 (1.1–1.6) | 49 | 1.1 (1.0–1.2) | <0.0001 |
| Platelet count, ×109/l | 99 | 115 (72–154) | 48 | 122 (71–209) | 0.498 |
| MELD | 95 | 11 (9–16) | 49 | 8 (7–10) | <0.001 |
| Child‐Pugh score | 95 | 46 | |||
| Child‐Pugh A | 50 (53) | 33 (72) | |||
| Child‐Pugh B | 27 (28) | 11 (24) | 0.031 | ||
| Child‐Pugh C | 18 (19) | 2 (4) | |||
Results are presented as median (interquartile range) or absolute number (percentage). Patients with cirrhosis and PSVD were matched on the severity for ascites. Bold indicates significant differences.
Abbreviations: IQR, interquartile range; INR, international normalized ratio; MELD, Model for End‐Stage Liver Disease.
n represents the number of patients with available data.
Only endoscopies performed within 12 months before or after CT were considered here.
LSN quantification in the learning cohort
Overall, 3 (2%) patients (1 with PSVD and 2 with cirrhosis) had LSN quantification failure (<8 valid measurements) because of an insufficient adipose tissue‐to‐liver interface. LSN was lower in patients with PSVD than in those with cirrhosis (median: 2.4 [IQR 2.2–2.9] vs. 3.1 [2.6–3.5]) (Figure 1). AUROC (95% CI) of LSN for the diagnosis of PSVD was 0.77 (0.68–0.85). Twenty‐nine (59%) patients with PSVD had LSN < 2.5 versus 19 (19%) patients with cirrhosis (p < 0.001). Therefore, LSN < 2.5 identified PSVD with a sensitivity of 59% and a specificity of 81% (Table S4). Overall, this cutoff value correctly classified 108 of 147 (73%) patients. A second reader also found lower LSN in patients with PSVD than in those with cirrhosis (median: 2.4 [2.2–2.9] vs. 2.9 [2.5–3.4], respectively; p < 0.001). Intraclass correlation coefficient for interreader variability was 0.97 (95% CI, 0.96–0.98).
FIGURE 1.
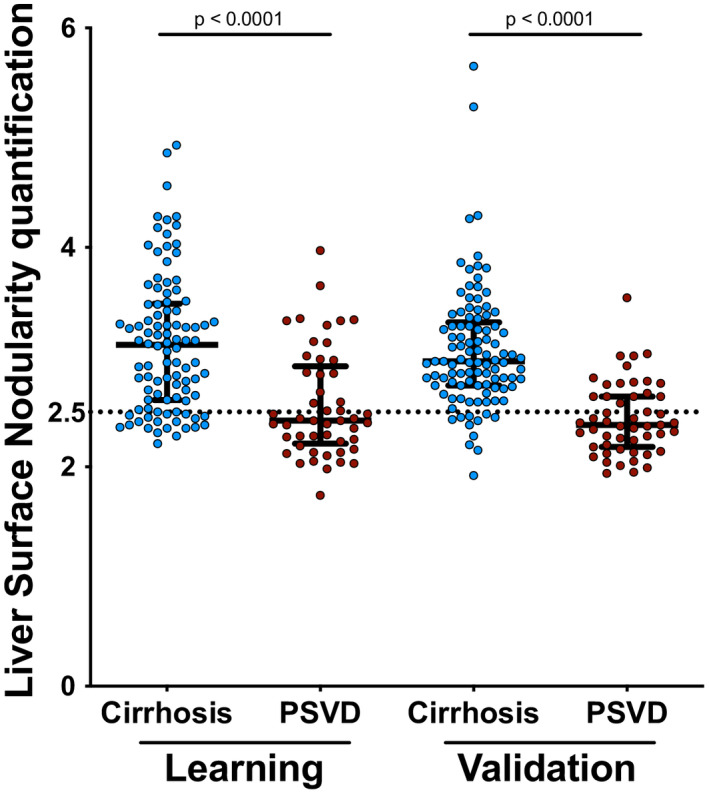
Liver surface nodularity quantification in patients with porto‐sinusoidal vascular disorder (PSVD) (learning cohort n = 49; validation cohort n = 51) and cirrhosis (learning cohort n = 98; validation cohort n = 101). Three and 7 patients in the learning cohort and validation cohorts did not have valid liver surface nodularity quantification, respectively
Morphological CT features associated with PSVD in the learning cohort
For both readers, as given in Table 2, patients with PSVD had twice more commonly intrahepatic and/or extrahepatic portal vein abnormalities (details are presented in Table S5), 6‐fold more commonly splenic and/or mesenteric vein thrombosis, and 6‐fold more commonly venous collaterals between hepatic veins than patients with cirrhosis (Figure S1). Spleen height was significantly higher in patients with PSVD than in patients with cirrhosis (16 cm vs. 14 cm and 17 cm vs. 14cm, p = 0.010 and p = 0.006 for reader 1 and 2, respectively). For both readers, patients with PSVD had 4‐fold less commonly visual nodular liver surface, 3‐fold less commonly simultaneous hypertrophy of caudate lobe with atrophy of segment IV, twice less commonly hepatic artery diameter larger than splenic artery diameter, and twice less commonly arterioportal or arteriovenous shunts, than patients with cirrhosis. When analyzing caudate lobe and segment IV separately, we observed that prevalence of atrophy of segment IV strongly differed between PSVD and cirrhosis, while hypertrophy of caudate lobe did not (Table 2, Figure 2). We thereafter focused on segment IV.
TABLE 2.
Morphological CT features in patients with PSVD and with cirrhosis of the learning cohort
| CT features | Reader 1 | Reader 2 | Kappa (%) | ||||
|---|---|---|---|---|---|---|---|
| Cirrhosis (n = 100) | PSVD (n = 50) | p value | Cirrhosis (n = 100) | PSVD (n = 50) | p value | ||
| Intrahepatic and/or extrahepatic portal vein abnormalities a | 11 (11) | 13 (26) | 0.018 | 8 (8) | 11 (22) | 0.015 | 76 |
| Splenic and/or mesenteric vein thrombosis | 2 (2) | 7 (14) | 0.007 | 3 (3) | 9 (18) | 0.003 | 85 |
| Porto‐systemic shunts | 89 (89) | 43 (86) | 0.594 | 95 (95) | 43 (86) | 0.106 | 48 |
| Venous collateral between hepatic veins | 3 (3) | 7 (14) | 0.031 | 1 (1) | 6 (12) | 0.005 | 56 |
| Not assessable | 6 (6) | 2 (4) | 0 (0) | 1 (2) | |||
| Hepatic veins near the liver capsule | 12 (12) | 11 (22) | 0.114 | 8 (8) | 13 (26) | 0.002 | 46 |
| Not assessable | 5 (5) | 2 (4) | 0 (0) | 1 (2) | |||
| Hepatic artery diameter (mm) | 6 (5–7) | 5 (4–6) | <0.0001 | 5 (5–6) | 5 (4–6) b | 0.006 | |
| Hepatic artery diameter ≥ splenic artery diameter | 76 (76) | 24 (48) | 0.004 | 80 (80) | 22(44) | <0.0001 | 77 |
| Not assessable | 0 (0) | 4 (8) | 0 (0) | 6 (12) | |||
| Heterogeneous liver hyperenhancement on arterial phase | 35 (35) | 8 (16) | 0.076 | 51 (51) | 10 (20) | 0.009 | 57 |
| Not assessable | 1 (1) | 10 (20) | 0 (0) | 12 (24) | |||
| Arterioportal or arteriovenous shunts | 33 (33) | 6 (12) | 0.029 | 42 (42) | 9 (18) | 0.046 | 61 |
| Not assessable | 1 (1) | 10 (20) | 0 (0) | 12 (24) | |||
| Abdominal portosystemic shunts >10 mm | 29 (29) | 22 (44) | 0.068 | 30 (30) | 19 (38) | 0.325 | 88 |
| Atrophy of segment IV | 80 (80) | 14 (28) | <0.0001 | 85 (85) | 12 (24) | <0.0001 | 90 |
| Hypertrophy of caudate lobe | 91 (91) | 41 (82) | 0.11 | 93 (93) | 41 (82) | 0.04 | 93 |
| Hypertrophy of caudate lobe with atrophy of segment IV | 76 (76) | 13 (26) | <0.0001 | 82 (82) | 11 (22) | <0.0001 | 89 |
| Global atrophy | 17 (17) | 2 (4) | 0.024 | 17 (17) | 3 (6) | 0.062 | 50 |
| Nodular liver surface | 74 (74) | 9 (18) | <0.0001 | 77 (77) | 10 (20) | <0.0001 | 86 |
| Focal retraction of liver surface | 44 (44) | 11 (22) | 0.008 | 28 (28) | 9 (18) | 0.18 | 54 |
| Spleen height (cm) c | 14 (12–16) | 16 (12–20) | 0.01 | 14 (14–16) | 17 (11–20) | 0.006 | |
| Fibrosis band | 32 (32) | 8 (16) | 0.127 | 41 (41) | 8 (16) | 0.132 | 66 |
| Not assessable | 1 (1) | 9 (18) | 8 (8) | 22 (44) | |||
Results are presented as median (IQR) or absolute number (percentage). Bold indicates significant differences.
Details are provided in Table S4.
Available in 49 patients
Available in 46 patients with PSVD; 4 patients were splenectomized; available in all patients with cirrhosis.
FIGURE 2.
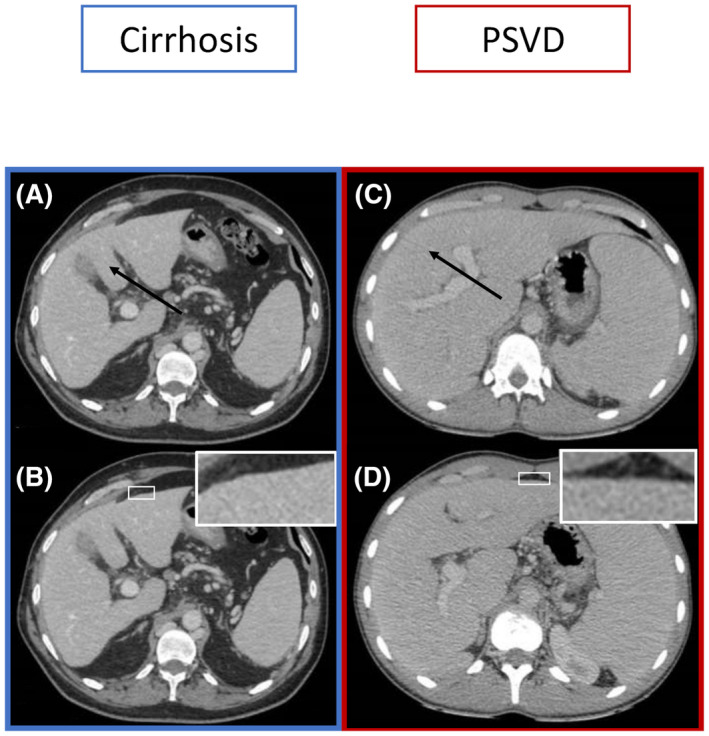
CT images in patients with cirrhosis (A,B) and in patients with PSVD (C,D). (A) Axial contrast‐enhanced CT during the portal venous phase shows an atrophy of segment IV (black arrow). (B) Axial contrast‐enhanced CT during the portal venous phase in the same patient shows a nodular liver surface (white box). (C) Axial contrast‐enhanced CT during the portal venous phase shows a normal‐sized or enlarged segment IV (black arrow). (D) Axial contrast‐enhanced CT during the portal venous phase in the same patient shows a smooth liver surface (white box)
According to only one of the two readers, hepatic veins near the capsule were more common, whereas heterogeneous liver perfusion pattern on hepatic arterial phase, global atrophy, and focal retraction of liver surface were significantly less common in patients with PSVD than in patients with cirrhosis. For both readers, there was no difference between the two groups regarding the presence of porto‐systemic shunts or abdominal porto‐systemic shunts >10 mm, and fibrosis bands.
Combination of LSN and morphological CT features in the learning cohort
Principal component analysis was then applied to LSN and CT features, and separated patients with PSVD from patients with cirrhosis (Figure 3).
FIGURE 3.
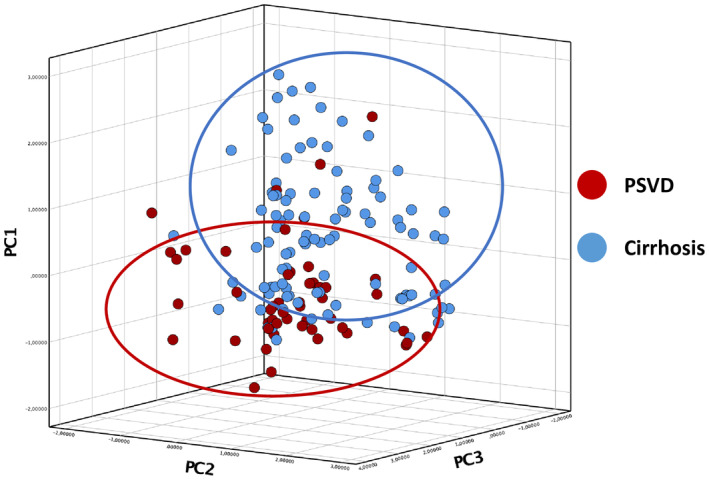
Principal component analysis of CT features, including liver surface nodularity quantification, in patients with PSVD and in patients with cirrhosis. Abbreviation: PC, principal component
We then sought to determine whether LSN together with morphological CT features could help identify PSVD in patients with portal hypertension in clinical practice. We performed a multivariate binary logistic regression analysis including LSN and CT features associated with PSVD at univariate analysis after Bonferroni adjustment (i.e., p < 0.003) and with data available in all patients, namely only normal‐sized or enlarged segment IV. Both LSN < 2.5 and normal‐sized or enlarged segment IV were independently associated with PSVD (Table S6).
For reader 1, 24 of 33 (73%) patients with both LSN < 2.5 and a normal‐sized or enlarged segment IV had PSVD (Figure 4). This combination had 91% specificity (95% CI, 0.81–0.95) for the diagnosis of PSVD (Table S4). Similar results were obtained with Reader 2 (Figure S2). When defining segment IV atrophy as a segment IV < 40 mm, 26 of 34 (76%) of patients with both LSN < 2.5 and a normal‐sized or enlarged segment IV had PSVD. This combination had 92% specificity (95% CI, 0.85–0.96) and an AUROC (95% CI) of 0.72 (0.63–0.82) for the diagnosis of PSVD. This AUROC was not different from that obtained when combining LSN < 2.5 and a visual impression of normal‐sized or enlarged segment IV (p = 0.509 and p = 0.503 for Reader 1 and Reader 2, respectively). Sensitivity analysis showed that combination of LSN < 2.5 with normal‐sized or enlarged segment IV remained associated with PSVD when restricting the analyses to patients without HCC (specificity 90% [95% CI, 0.79–0.97]), or to patients with Child‐Pugh A liver disease (specificity 88% [95% CI, 0.75–0.95]). When restricting the analysis to patients fulfilling idiopathic noncirrhotic portal hypertension criteria, the same results were also obtained (specificity 91% [95% CI, 0.83–0.96]).
FIGURE 4.
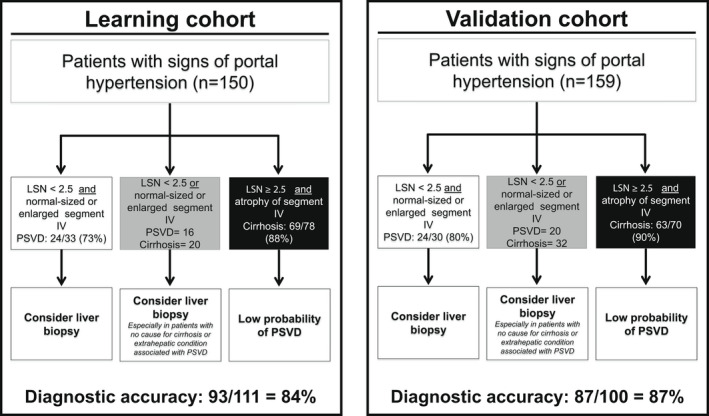
Interest of normal‐sized or enlarged segment IV combined with LSN < 2.5 for the diagnosis of PSVD in patients with signs of portal hypertension. Three and 7 patients in the learning cohort and validation cohort, respectively, did not have valid LSN quantification
LSN ≥ 2.5 combined with atrophy of segment IV had a 70% (95% CI, 0.60–0.80) sensitivity, 82% (95% CI, 0.68–0.91) specificity, a positive and negative predictive value of 88% (95% CI, 0.81–0.93) and 58% (95% CI, 0.50–0.66) respectively, and a positive and negative likelihood ratios of 3.83 (95% CI, 2.10–7.01) and 0.36 (0.26–0.51), respectively, for cirrhosis. AUROC (95% CI) of LSN ≥ 2.5 combined with atrophy of segment IV for the diagnosis of cirrhosis was 0.76 (0.68–0.84). The 9 patients with PSVD with both LSN ≥ 2.5 and an atrophy of segment IV had features suggesting a more severe liver disease than the 40 patients without such combination (Table S7). Among these 9 patients, 3 had a cause for cirrhosis, but without incomplete septal fibrosis at liver biopsy.
Overall, the size of segment IV and LSN had a diagnostic accuracy of 84% (Figure 4).
Of the 49 patients with PSVD, 34 had available liver stiffness measurement using transient elastography. Twenty‐two of them had liver stiffness measurement <10 kPa. Of the 12 patients with liver stiffness measurement ≥10 kPa, 4 had LSN < 2.5 combined with a normal‐sized or enlarged segment IV, and none had LSN ≥ 2.5 combined with atrophy of segment IV.
Clinical, laboratory, and hemodynamic and histological features associated with CT features in patients with PSVD in the learning cohort
We then performed a detailed analysis of clinical and laboratory data associated with CT features. As shown in Figure S3, intrahepatic and or extrahepatic vein abnormalities, splenic and/or mesenteric vein thrombosis, and global atrophy of the liver were associated with ascites with or without low serum albumin and increased serum creatinine. Abdominal portosystemic shunts >10 mm, atrophy of segment IV, and LSN ≥ 2.5 were associated with features of liver dysfunction including high serum bilirubin level with or without increased international normalized ratio. Conditions associated with PSVD (immunological conditions vs. other causes) and HVPG (≥10 mm Hg) were not associated with any CT features (Table S8). The only association between pathological findings with CT features was portal venules stenosis with LSN (p = 0.015). For all other pathological features, there was no association or a strong disagreement between the two readers (Figure S4).
Validation cohort
We then tested the ability of LSN < 2.5 alone or in combination with normal‐sized or enlarged segment IV to diagnose PSVD in an independent cohort including 53 patients with PSVD and 106 patients with cirrhosis, matched on ascites. Characteristics of the patients are presented in Table S9.
Valid LSN quantification was obtained in 51 (96%) patients with PSVD and 101 patients (95%) with cirrhosis. LSN was lower in patients with PSVD than in those with cirrhosis (Figure 1). AUROC (95% CI) of LSN for PSVD was 0.84 (0.77–0.91). Thirty‐three (65%) patients with PSVD had LSN < 2.5, versus 11 (11%) patients with cirrhosis (p < 0.001). Therefore, LSN < 2.5 identified PSVD with a sensitivity of 65% and a specificity of 89% (Table S4). Overall, this cutoff value correctly classified 123 of 152 (81%) patients.
Twenty‐four of the 30 (80%) patients with LSN < 2.5 combined with normal‐sized or enlarged segment IV had PSVD. This combination had a specificity of 94% (95% CI, 0.88–0.98) for the diagnosis of PSVD (Table S4). LSN ≥ 2.5 combined with atrophy of segment IV had a 63% sensitivity (95% CI, 0.52–0.72), 86% specificity (95% CI, 0.73–0.94), a positive and negative predictive value of 90% (95% CI, 0.82–0.95) and 54% (95% CI, 0.47–0.60), respectively, and a positive and negative likelihood ratio of 4.54 (95% CI, 2.25–9.19) and 0.44 (0.33–0.57), respectively, for cirrhosis. AUROC (95% CI) of LSN ≥ 2.5 combined with atrophy of segment IV for the diagnosis of cirrhosis was 0.74 (0.66–0.82).
Overall, LSN and size of segment IV had a diagnostic accuracy of 87% (Figure 4).
Pooled learning and validation cohorts
For sensitivity analyses, 54 patients with PSVD were matched on age, sex, ascites, and MELD to 54 patients with cirrhosis. We observed that LSN was lower in patients with PSVD than in those with cirrhosis (median: 2.4 [IQR 2.2–2.7] vs. 2.9 [2.5–3.3], p < 0.001). AUROC (95% CI) of LSN for the diagnosis of PSVD was 0.76 (0.66–0.85). LSN < 2.5 combined with normal‐sized or enlarged segment IV was more common in patients with PSVD (26 of 54, 48%) than in patients with cirrhosis (7 of 54, 13%) (p < 0.001) and had a specificity of 87% (95% CI, 0.75–0.95) for diagnosis of PSVD (Table S4).
Model based on LSN and CT features to identify PSVD
LSN and normal‐sized or enlarged segment IV being independently associated with PSVD, we built the following model: 1.277 × LSN + 1.832 × Segment IV based on Reader’s 1 assessment. Segment IV was quoted 1 when atrophy of segment IV was present, and 0 otherwise. AUROC of this model was 0.82 (0.75–0.90) and 0.83 (0.76–0.90) in the learning and validation cohort respectively for reader 1. Using Delong method, AUROC of this score was better than AUROC of LSN alone for diagnosis of PSVD in the learning cohort (p = 0.03), but not in the validation cohort (p = 0.75).
Outcomes in patients with PSVD
Median duration of follow‐up after PSVD diagnosis was 42 (1–53) months. During that period of time, 12 patients had liver‐related events, including portal hypertension hemorrhage (n = 3), appearance (n = 4) or worsening (n = 4) of ascites, and HE (n = 2). One patient simultaneously developed ascites and HE. Four patients underwent liver transplantation and 13 patients died (including 7 after liver‐related events). All liver transplantations occurred after liver‐related events. LSN, as a continuous variable, tended to be associated with cumulative incidence of liver‐related events (p = 0.075) and was associated with cumulative incidence of liver transplantation or death (HR: 2.6, 95% CI 1.0–6.5, p = 0.049). However, these associations with patient’s outcome were not observed when analyzing LSN as a binary variable, using 2.5 as a threshold (data not shown).
DISCUSSION
Despite the rarity of PSVD, the present retrospective study—by joining the efforts of four reference centers for vascular liver disease—was able to include a large number of patients with PSVD with portal hypertension and CT. This allowed for identifying and validating CT features useful to suspect PSVD in the diagnostic work‐up of patients with portal hypertension, namely, combination of LSN < 2.5 with normal‐sized or enlarged segment IV. We also observed that global imaging pattern of PSVD was different from cirrhosis and that some CT features were associated with PSVD severity.
The first major finding of the present study was that LSN strongly differs between patients with PSVD and those with cirrhosis, LSN < 2.5 suggesting PSVD in patients with signs of portal hypertension. Absence of visual nodular liver surface has been described as associated with PSVD by several independent groups.[ 11 , 13 , 14 ] However, diagnostic accuracy of this finding for PSVD was not tested. LSN was initially developed as a noninvasive test for diagnosis and prognosis of cirrhosis.[ 15 , 18 , 26 ] This score reflects major architectural changes occurring during cirrhosis development, including progressive liver fibrosis and formation of regenerative liver nodules.[ 26 ] Our group extended LSN field of application and showed a high diagnostic performance of LSN for detecting clinically significant portal hypertension.[ 16 , 17 ] LSN has several advantages as compared with other existing tests: the possibility of performing a retrospective analysis at routine CT without dedicated acquisition protocol, rapid measurement (<2 min) without advanced postprocessing, and excellent reproducibility and interobserver agreement in cirrhosis.[ 17 ] We demonstrate here that LSN also has an excellent interobserver agreement in patients with PSVD. In the present study, contrary to most previous studies,[ 10 , 11 , 12 , 13 , 14 ] patients with PSVD and cirrhosis were matched on severity of liver disease reflected by ascites. Indeed, ascites is the only feature associated with survival both in patients with PSVD and in patients with cirrhosis.[ 24 ] As patients with PSVD have a more preserved liver function and are supposed to develop ascites later in the disease course than patients with cirrhosis, we can speculate that a higher portal pressure is required to produce a similar level of ascites in patients with PSVD than in patients with cirrhosis. If this hypothesis were true, it would further reinforce the value of our findings. Indeed, in patients with cirrhosis, LSN correlates with portal pressure.[ 17 ] Therefore, if patients with cirrhosis had a lower portal pressure than patients with PSVD, it would translate into lower LSN score. We actually observed the opposite. Furthermore, sensitivity analyses performed after matching patients with PSVD and cirrhosis on age, sex, and MELD on top of ascites gave similar results, attesting to the robustness of this finding.
The second major finding of this study was that LSN < 2.5 combined with normal‐sized or enlarged segment IV has over 90% specificity for PSVD in patients with signs of portal hypertension. Although previous studies pointed to the absence of simultaneous hypertrophy of the caudate lobe and atrophy of segment IV being associated with PSVD, we showed here that all diagnostic information was carried by segment IV size. This might further simplify applicability of these criteria in routine practice. Assessment of segment IV size performed by the visual impression of the reader was as accurate as a measurement. Although specificity of these features for diagnosis of PSVD was high, a note of caution is needed. Prevalence of cirrhosis being much higher in the general population than that of PSVD, identifying these two CT features does not deter from performing a liver biopsy in patient with signs of portal hypertension. Indeed, the high prevalence of PSVD in our study, related to the case‐control design, might lead to a spectrum bias affecting sensitivity and specificity of the diagnostic criteria we propose.[ 31 ] These imaging features should rather be viewed as tools to be added to the set of features that should raise a suspicion of PSVD. In particular, this association can be complementary to liver stiffness measurement using transient elastography. Indeed, 4 of the 12 patients with PSVD and liver stiffness measurement ≥10 kPa had LSN < 2.5 combined with normal‐sized or enlarged segment IV. Such a contrast between liver stiffness measurement and CT features should question diagnosis of cirrhosis and prompt physicians to perform a liver biopsy. Other CT features rarely observed in patients with cirrhosis could help raise suspicion of PSVD (i.e., extension of portal vein thrombosis to splenic and/or mesenteric veins and venous collaterals between hepatic veins). The latter likely corresponds to the hepatic vein‐to‐vein communications reported at hepatic venography in 50% of patients with PSVD and rarely in patients with cirrhosis.[ 32 ]
The third major finding of the present study was the correlation between CT features and liver‐disease severity. Patients with PSVD, but harboring CT features normally found in cirrhosis patients (i.e., LSN ≥ 2.5 or atrophy of segment IV) had more advanced liver disease as attested by more frequent ascites and higher serum bilirubin and international normalized ratio levels. Moreover, we observed that LSN was associated with cumulative incidence of liver transplantation or death. These results are reminiscent of a previous study by Krishnan et al., who studied CT and MRI features in 18 patients with PSVD. Nodular liver surface was observed in all 4 patients who underwent liver transplantation versus only 1 of the 14 other patients who did not.[ 13 ] In contrast, almost no correlation was found between CT features and liver histological lesions, suggesting that mechanisms driving macroscopic changes in PSVD might differ from those leading to microscopic modifications.
In conclusion, in patients with signs of portal hypertension, LSN < 2.5, combined with normal‐sized or enlarged segment IV on CT raise the suspicion of PSVD and should therefore encourage physicians to perform a liver biopsy. Some CT features classically associated with cirrhosis are associated with liver‐disease severity in patients with PSVD.
CONFLICT OF INTEREST
Dr. Durand consults for Behring and Biotest. Dr. Garcia Pagan consults for Cook Medical, Shionogi, WL Gore & Associates, Vifor Pharma, and Boehringer Ingelheim. He received grants from Mallinckrodt and Noorik.
AUTHOR CONTRIBUTIONS
Study concept and design: Maxime Ronot, Vilgrain Valérie, and Pierre‐Emmanuel Rautou. Data acquisition: Shantha Ram Valainathan, Riccardo Sartoris, Marco Dioguardi Burgio, Silvia Pellegrino, Arianna Nivolli, Marco Dioguardi Burgio, Laure Elkrief, LS, Sylvain Terraz, Nicolas Drilhon, Marie Lazareth, and Julia Herrou. Data analysis and interpretation: Shantha Ram Valainathan and Pierre‐Emmanuel Rautou. Manuscript draft: Shantha Ram Valainathan and Pierre‐Emmanuel Rautou. Critical revision of the article for important intellectual content and final approval: Onorina Bruno, Audrey Payance, Aurélie Plessier, François Durand, Maxime Ronot, Dominique‐Charles Valla, Valérie Paradis, Juan Carlos Garcia‐Pagan, Valérie Vilgrain, and Pierre‐Emmanuel Rautou.
Supporting information
Supplementary Material
Valainathan SR, Sartoris R, Elkrief L, Magaz M, Betancourt F, Pellegrino S, et al. Contrast‐enhanced CT and liver surface nodularity for the diagnosis of porto‐sinusoidal vascular disorder: A case‐control study. Hepatology. 2022;76:418–428. 10.1002/hep.32367
Funding information
Rio Hortega grant from Instituto de Salud Carlos III, Spain
REFERENCES
- 1. De Gottardi A, Rautou P‐E, Schouten J, Rubbia‐Brandt L, Leebeek F, Trebicka J, et al. Porto‐sinusoidal vascular disease: proposal and description of a novel entity. Lancet Gastroenterol Hepatol. 2019;4:399–411. [DOI] [PubMed] [Google Scholar]
- 2. de Franchis R, Faculty BVI. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: Stratifying Risk and Individualizing Care for Portal Hypertension. J Hepatol. 2015;63:743–52. [DOI] [PubMed] [Google Scholar]
- 3. Elkrief L, Rautou P‐E. Idiopathic non‐cirrhotic portal hypertension: the tip of the obliterative portal venopathies iceberg? Liver Int. 2016;36:325–7. [DOI] [PubMed] [Google Scholar]
- 4. Berzigotti A. Non‐invasive evaluation of portal hypertension using ultrasound elastography. J Hepatol. 2017;67:399–411. [DOI] [PubMed] [Google Scholar]
- 5. Elkrief L, Lazareth M, Chevret S, Paradis V, Magaz M, Blaise L, et al. Liver stiffness by transient elastography to detect porto‐sinusoidal vascular liver disease with portal hypertension. Hepatology. 2021;74:364–78. [DOI] [PubMed] [Google Scholar]
- 6. Krasinskas AM, Eghtesad B, Kamath PS, Demetris AJ, Abraham SC. Liver transplantation for severe intrahepatic noncirrhotic portal hypertension. Liver Transpl. 2005;11:627–34. discussion 610–611. [DOI] [PubMed] [Google Scholar]
- 7. Ataide EC, Dos Santos IN, Martins DL, Pereira TS, de Souza Almeida JR, Stucchi RSB, et al. Liver failure and the need for transplantation in 6 patients with hepatoportal sclerosis. Transplant Proc. 2013;45:1907–9. [DOI] [PubMed] [Google Scholar]
- 8. Meijer B, Simsek M, Blokzijl H, Man RA, Coenraad MJ, Dijkstra G, et al. Nodular regenerative hyperplasia rarely leads to liver transplantation: a 20‐year cohort study in all Dutch liver transplant units. United Eur Gastroenterol J. 2017;5:658–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Isabel Fiel M, Thung SN, Hytiroglou P, Emre S, Schiano TD. Liver failure and need for liver transplantation in patients with advanced hepatoportal sclerosis. Am J Surg Pathol. 2007;31:607–14. [DOI] [PubMed] [Google Scholar]
- 10. Dhiman RK, Chawla Y, Vasishta RK, Kakkar N, Dilawari JB, Trehan MS, et al. Non‐cirrhotic portal fibrosis (idiopathic portal hypertension): experience with 151 patients and a review of the literature. J Gastroenterol Hepatol. 2002;17:6–16. [DOI] [PubMed] [Google Scholar]
- 11. Glatard A‐S, Hillaire S, d’Assignies G, Cazals‐Hatem D, Plessier A, Valla DC, et al. Obliterative portal venopathy: findings at CT imaging. Radiology. 2012;263:741–50. [DOI] [PubMed] [Google Scholar]
- 12. Matsutani S, Maruyama H, Akiike T, Kobayashi S, Yoshizumi H, Okugawa H, et al. Study of portal vein thrombosis in patients with idiopathic portal hypertension in Japan. Liver Int. 2005;25:978–83. [DOI] [PubMed] [Google Scholar]
- 13. Krishnan P, Fiel MI, Rosenkrantz AB, Hajdu CH, Schiano TD, Oyfe I, et al. Hepatoportal sclerosis: CT and MRI appearance with histopathologic correlation. AJR Am J Roentgenol. 2012;198:370–6. [DOI] [PubMed] [Google Scholar]
- 14. Kang JH, Kim DH, Kim SY, Kang HJ, Lee JB, Kim KW, et al. Porto‐sinusoidal vascular disease with portal hypertension versus liver cirrhosis: differences in imaging features on CT and hepatobiliary contrast‐enhanced MRI. Abdom Radiol (NY). 2021;46:1891–903. [DOI] [PubMed] [Google Scholar]
- 15. Smith AD, Branch CR, Zand K, Subramony C, Zhang H, Thaggard K, et al. Liver surface nodularity quantification from routine CT images as a biomarker for detection and evaluation of cirrhosis. Radiology. 2016;280:771–81. [DOI] [PubMed] [Google Scholar]
- 16. Souhami A, Sartoris R, Rautou P‐E, Cauchy F, Bouattour M, Durand F, et al. Similar performance of liver stiffness measurement and liver surface nodularity for the detection of portal hypertension in patients with hepatocellular carcinoma. JHEP Rep. 2020;2:100147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sartoris R, Rautou P‐E, Elkrief L, Pollorsi G, Durand F, Valla D, et al. Quantification of liver surface nodularity at CT: utility for detection of portal hypertension. Radiology. 2018;289:698–707. [DOI] [PubMed] [Google Scholar]
- 18. Smith AD, Zand KA, Florez E, Sirous R, Shlapak D, Souza F, et al. Liver surface nodularity score allows prediction of cirrhosis decompensation and death. Radiology. 2017;283:711–22. [DOI] [PubMed] [Google Scholar]
- 19. Hobeika C, Cauchy F, Sartoris R, Beaufrère A, Yoh T, Vilgrain V, et al. Relevance of liver surface nodularity for preoperative risk assessment in patients with resectable hepatocellular carcinoma. Br J Surg. 2020;107:878–88. [DOI] [PubMed] [Google Scholar]
- 20. Cazals‐Hatem D, Hillaire S, Rudler M, Plessier A, Paradis V, Condat B, et al. Obliterative portal venopathy: portal hypertension is not always present at diagnosis. J Hepatol. 2011;54:455–61. [DOI] [PubMed] [Google Scholar]
- 21. Schouten JNL, Nevens F, Hansen B, Laleman W, van den Born M, Komuta M, et al. Idiopathic noncirrhotic portal hypertension is associated with poor survival: results of a long‐term cohort study. Aliment Pharmacol Ther. 2012;35:1424–33. [DOI] [PubMed] [Google Scholar]
- 22. Siramolpiwat S, Seijo S, Miquel R, Berzigotti A, Garcia‐Criado A, Darnell A, et al. Idiopathic portal hypertension: natural history and long‐term outcome. Hepatology. 2014;59:2276–85. [DOI] [PubMed] [Google Scholar]
- 23. Bissonnette J, Garcia‐Pagán JC, Albillos A, Turon F, Ferreira C, Tellez L, et al. Role of the transjugular intrahepatic portosystemic shunt in the management of severe complications of portal hypertension in idiopathic noncirrhotic portal hypertension. Hepatology. 2016;64:224–31. [DOI] [PubMed] [Google Scholar]
- 24. Schouten JNL, Nevens F, Hansen B, Laleman W, van den Born M, Komuta M, et al. Idiopathic noncirrhotic portal hypertension is associated with poor survival: results of a long‐term cohort study. Aliment Pharmacol Ther. 2012;35:1424–33. [DOI] [PubMed] [Google Scholar]
- 25. Lafortune M, Matricardi L, Denys A, Favret M, Déry R, Pomier‐Layrargues G. Segment 4 (the quadrate lobe): a barometer of cirrhotic liver disease at US. Radiology. 1998;206:157–60. [DOI] [PubMed] [Google Scholar]
- 26. Pickhardt PJ, Malecki K, Kloke J, Lubner MG. Accuracy of liver surface nodularity quantification on MDCT as a noninvasive biomarker for staging hepatic fibrosis. AJR Am J Roentgenol. 2016;207:1194–9. [DOI] [PubMed] [Google Scholar]
- 27. Sartoris R, Lazareth M, Nivolli A, Dioguardi Burgio M, Vilgrain V, Ronot M. CT‐based liver surface nodularity for the detection of clinically significant portal hypertension: defining measurement quality criteria. Abdom Radiol (NY). 2020;45:2755–63. [DOI] [PubMed] [Google Scholar]
- 28. Catania R, Furlan A, Smith AD, Behari J, Tublin ME, Borhani AA. Diagnostic value of MRI‐derived liver surface nodularity score for the non‐invasive quantification of hepatic fibrosis in non‐alcoholic fatty liver disease. Eur Radiol. 2021;31:256–63. [DOI] [PubMed] [Google Scholar]
- 29. Lubner MG, Jones D, Said A, Kloke J, Lee S, Pickhardt PJ. Accuracy of liver surface nodularity quantification on MDCT for staging hepatic fibrosis in patients with hepatitis C virus. Abdom Radiol (NY). 2018;43:2980–6. [DOI] [PubMed] [Google Scholar]
- 30. Schouten JNL, Garcia‐Pagan JC, Valla DC, Janssen HLA. Idiopathic noncirrhotic portal hypertension. Hepatology. 2011;54:1071–81. [DOI] [PubMed] [Google Scholar]
- 31. Leeflang MMG, Rutjes AWS, Reitsma JB, Hooft L, Bossuyt PMM. Variation of a test’s sensitivity and specificity with disease prevalence. CMAJ. 2013;185:E537–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Seijo S, Reverter E, Miquel R, Berzigotti A, Abraldes JG, Bosch J, et al. Role of hepatic vein catheterisation and transient elastography in the diagnosis of idiopathic portal hypertension. Dig Liver Dis. 2012;44:855–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


