Abstract
Objectives
To report the clinical, radiographic, esthetic, and patient‐reported outcomes after placement of a newly developed narrow‐diameter implant (NDI) in patients with congenitally missing lateral incisors (MLIs).
Materials and methods
Patients with MLIs with a mesio‐distal distance between the canine and the central incisor of 5.9–6.3 mm received a dental implant with a diameter of 2.9 mm (Test), while a diameter of 3.3 mm (Control) was used when the distance was 6.4–7.1 mm. After healing, a cement‐retained bi‐layered zirconia crown was fabricated. At the 1‐year follow‐up (T2), implant survival rate, marginal crestal bone level (CBL) changes, biological and technical complications were registered. The esthetic outcome was assessed by using the Copenhagen index score, and the patient‐reported outcomes were recorded using the OHIP‐49 questionnaire.
Results
One hundred patients rehabilitated with 100 dental implants Ø2.9 mm (n = 50) or Ø3.3 mm (n = 50) were included. One Ø3.3 mm implant was lost, and seven patients dropped out of the study, yielding an implant survival rate of 99% (p = 1.000). At T2 a. CBL of −0.19 ± 0.25 mm (Test) and −0.25 ± 0.31 mm (Control) was detected, with no statistically significant difference between the groups (p = .342). Good to excellent esthetic scores (i.e., 1–2) were recorded in most of cases. Technical complications (i.e., loss of retention, abutment fracture, and chipping of veneering ceramic) occurred once in three patients with no statistically significant difference between the groups (p > .05). OHIP scores did not differ significantly at follow‐ups between groups (p = .110).
Conclusion
The use of Ø2.9 mm diameter implants represents as reliable a treatment option as Ø3.3 mm implants, in terms of CBL changes, biological and technical complications. Favorable esthetics and patient‐reported outcomes were recorded for both groups.
Keywords: clinical research, clinical trials, dental implants, patient centered outcomes, prosthodontics
1. INTRODUCTION
Congenitally missing teeth, also known as tooth agenesis, is a frequent developmental dental anomaly, which may cause esthetic and functional challenges if left untreated (Khalaf et al., 2014; Rakhshan & Rakhshan, 2016). The prevalence has been estimated to be almost 7%, and the most frequently missing teeth are the mandibular second premolars (29.9%) followed by the maxillary lateral incisors (MLI) (24.3%) and second premolars (13.7%) (Khalaf et al., 2014; Rakhshan & Rakhshan, 2016). The etiopathogenesis of tooth agenesis has been correlated with specific genetic syndrome (Matalova et al., 2008; Vastardis, 2000), even though non‐syndromic hypodontia is a more frequent finding (Nieminen et al., 1995).
Oral rehabilitation of patients with congenitally missing teeth most often requires an interdisciplinary collaboration and different well‐documented treatment options frequently exist. More specifically, either space closure by canine substitution or prosthetic rehabilitation represent the most frequently applied treatment modalities (Beyer et al., 2007; Kafantaris et al., 2020; Kern et al., 2017; Kiliaridis et al., 2016; Priest, 2019).
It is widely accepted that implant‐supported single unit crowns represent a reliable treatment option for the replacement of missing teeth with favorable outcomes in terms of high implant survival rate and stable peri‐implant marginal bone levels (Jung et al., 2012; Sailer et al., 2012; Wittneben et al., 2014). Nevertheless, in clinical scenarios with limited mesio‐distal space of the edentulous area, implant placement might be challenging due to root proximity of the adjacent teeth (Richardson & Russell, 2001). Patients with congenitally missing teeth often present with reduced tooth dimensions and limited space in the jaws compared to patients with all permanent teeth formed (McKeown et al., 2002). To overcome these problems, and to provide patients with an implant‐supported restoration with a harmonious emergence profile mimicking the contralateral tooth (Roccuzzo et al., 2018), narrow‐diameter implants (NDIs) have been introduced (Zarone et al., 2006). More specifically, following the 2018 ITI Consensus Conference, three categories of NDIs were proposed (i.e., (1): Ø < 3.0 mm [“Mini‐implants”]; (2): Ø3–3.25 mm; (3): Ø 3.3–3.5 mm) (Schiegnitz & Al‐Nawas, 2018). In areas of full load, concerns regarding risk of fracture of the fixtures especially after long‐term of function have been raised (Galindo‐Moreno et al., 2017; Ioannidis et al., 2015; Jung et al., 2018; Ma et al., 2019; Schiegnitz & Al‐Nawas, 2018), even though this risk potentially may be reduced by the introduction of new alloys such as titanium–zirconium alloy (Chiapasco et al., 2012). However, in areas of limited load, implants with a reduced diameter have shown comparable results compared with standard diameter implants. In addition, a potential advantage of using NDIs may be a reduced need for bone augmentation procedures due to the reduced diameter of the required osteotomy (Roccuzzo et al., 2021). So far, however, the generalizability of the reported clinical, radiographic, and esthetic outcomes of NDIs might be questionable due to the limited number of treated patients (Zarone et al., 2006) or without a control group (Lacarbonara et al., 2021).
Therefore, the aim of this study was to test the reliability of a newly developed narrow‐diameter implant to replace congenitally missing lateral incisors (MLIs) in terms of implant survival rate, peri‐implant marginal bone level changes, esthetic outcome, and patient‐reported outcome measures (PROMS) compared with the same type of implant with a diameter of 3.3 mm in a large population. The null hypothesis (H0) was no statistical difference in peri‐implant marginal bone level changes between implants with a diameter of 2.9 and 3.3 mm during a 1‐year follow‐up period, when used to replace MLIs.
2. MATERIALS AND METHODS
Data reporting has been performed according to the STROBE guidelines.
2.1. Study design
This study was designed as a prospective non‐randomized controlled clinical trial with two parallel study groups, a duration period of 5 years, and was conducted at the Department of Oral & Maxillofacial Surgery, Copenhagen University Hospital, Copenhagen, Denmark. Approval to perform the study was provided by the Danish Data Protection Agency (approval number: 2012‐58‐0004). The investigation was conducted according to the revised principles of the Helsinki Declaration. Informed consent was obtained from each patient before beginning of the study.
2.2. Study population
From August 2016 to December 2018, patients with MLIs (i.e., 12 and/or 22) referred to the Department of Oral & Maxillofacial Surgery, Copenhagen University Hospital, Copenhagen, Denmark were consecutively enrolled and included in this study according to the following inclusion criteria:
age ≥ 18 years
patients with systemic health or controlled medical conditions
arrested skeletal growth as documented by two body height measurements at least one year apart not indicating continuous growth (Jensen, 2019)
The following exclusion criteria were applied:
patients with MLI's with the canine situated in the MLI region
2.3. Study group allocation
Patients' allocation to one of the two groups of the study was determined based on the mesio‐distal distance (MD) between the canine and the central incisor measured with a calliper. Patients with a MD of 5.9 to 6.3 mm received a dental implant with a diameter of 2.9 mm [Ø2.9 mm] (Straumann BLT implant, Roxolid®, SLActive®, Straumann AG), while patients presenting with MD of 6.4 to 7.1 mm received a dental implant with a diameter of 3.3 mm [Ø3.3 mm] (Straumann BLT implant, Roxolid®, SLActive®).
2.4. Surgical procedure
All the surgical procedures were performed according to the manufacturers' recommendations under sterile conditions in an outpatient environment by one of the authors (S.S.J.), with more than 20 years of experience in implant dentistry.
In cases of fenestration‐ or dehiscence‐type defects, simultaneous contour augmentation was performed using guided bone regeneration (GBR) by means of locally harvested autogenous bone chips applied on the exposed implant threads and subsequently covered with demineralized bovine bone mineral (DBBM) (Bio‐Oss® Granules 0.25–1 mm, Geistlich Pharma AG). Thereafter, the grafted area was covered by a double‐layer collagen membrane (Bio‐Gide®, Geistlich Pharma AG) as described by (Buser et al., 2009). In cases of facial bone wall thickness after implant osteotomy of <1.7 mm, GBR was performed using DBBM alone covered with a double‐layer collagen membrane on the buccal aspect to protect against additional resorption and to support the soft tissue contour. Finally, a conical healing abutment (Straumann SC Healing Abutment narrow and standard connections, 2) was mounted, and the flap was repositioned and sutured to allow a tension‐free healing. Details of the surgical procedures have been previously reported (Roccuzzo et al., 2021).
2.5. Prosthetic procedure
All the prosthetic procedures followed the manufacturer's instructions for the type of reconstruction in question and were performed by one experienced prosthodontist (J.L.) with more than 10 years of experience in implant prosthodontics.
All restorations were fabricated by the same Dental Laboratory (CC Dent) by the same experienced dental technician, using identical materials and technical procedure. After a healing period of 3 months (Type 4‐C placement and loading according to [Gallucci et al., 2018]), impressions were taken at fixture level using a polyether material (Impregum; 3 M Espe) to fabricate provisional screw‐retained, laboratory cemented PolyMethylMethAcrylate (PMMA) single‐unit crowns on temporary abutments (Straumann NC and SC, 1‐3 mm gingival height), which were mounted and adjusted to slight or no occlusion.
After an additional period of 3 months, a second impression of the mucosal tissue was taken and a new mucosa model, on the original master model, was made. The patient was referred to the laboratory and color measurement was performed. A Straumann CARES Titanium abutment was CAD/CAM designed and manufactured and a feldspatic‐ceramic‐veneered‐zirconia crown was fabricated. Final adjustment of color was performed by the laboratory. The crown was returned to the prosthodontist. The provisional crown was removed, and abutment was mounted and torqued according to the manufacturer’s instructions. The screw channel was blocked with sterilized PTFE‐tape and cemented with zinc‐phosphate cement either Hoffmann's Phosphate cement, Color 03 (Hoffmann Dental Manufactur GMBH) or Detray zinc‐phosphate cement (Dentsply Detrey GmbH). Excess cement was carefully removed with a periodontal probe and dental floss (Oral B, Superfloss, Procter & Gamble UK), and complete removal was thereafter checked radiographically. Finally, impression in Alginot (Kerr Corporation) was made, and a 1.5 mm thermoplastic protection night‐guard (Scheu Dental GmbH) was fabricated for the upper jaw. Patients were finally given careful instructions on proper oral hygiene. This appointment was considered the baseline examination (T1).
2.6. Supportive periodontal/peri‐implant program and follow‐up examination
At the completion of the rehabilitation phase (T1), patients were referred to their private dental practioners for individual maintenance programs. Patients were invited for a follow‐up examination 1‐year after crown delivery which was considered the 1‐year examination (T2).
2.7. Outcomes measures
For the record and analysis of the investigated outcomes, three different time points were defined:
T0: time of the implant placement. Pre‐ and intraoperative clinical measurements, radiographic crestal bone level (CBL)
T1: delivery of the final reconstruction (i.e., baseline). Radiographic crestal bone level (CBL), esthetic assessment of the Copenhagen index score.
T2: follow‐up examination (i.e., 1‐year after baseline). Assessment of implant survival rate, radiographic crestal bone level (CBL), esthetic assessment
Oral Health Impact Profile‐49 (OHIP‐49) questionnaires were filled in just before initiation of the prosthetic phase at impression taking for the temporary implant‐supported fixed dental prosthesis (FDP) approximately 3 months after T0 and at T2.
2.8. Implant and reconstruction survival
Implant survival rate was calculated at patient level, which was identical to implant level since only one implant was included per patient, and was defined as the presence of the installed implant in the oral cavity. Reconstruction survival rate was defined as the presence of the implant‐supported single‐unit crown on the implant.
2.9. Biological and technical complications
At the 1‐year follow‐up (T2) clinical examination, performed by an experienced dental hygienist under supervision of the senior author (S.S.J.), the clinical examination included the assessment at each implant site of the following biological parameters:
Peri‐implant probing depth in mm at six sites per implant
Plaque Score: the presence or absence at implant site (0/1)
Exudation or suppuration after probing (0/1)
Presence of fistula
Pain
Necrosis of the neighboring teeth
The following technical complications were recorded:
Loosening or fracture of the abutment
Loss of retention of the reconstruction (i.e., decementation of the reconstruction)
Fracture or chipping of the veneering ceramic
2.10. Radiographic assessment
Peri‐apical radiographs were taken after implant placement (T0), after crown delivery (T1) and at the 1‐year follow‐up examinations (T2). Digital non‐standardized and non‐individualized intraoral radiographs were obtained using the paralleling technique. Images were then imported in a dedicated software (Zen pro, Carl Zeiss AG). The known implant lengths (i.e., 10 or 12 mm) were used to calibrate the images (Figure 2). Crestal bone levels (CBL) were assessed on both the mesial and the distal peri‐implant surface as the linear distance between the implant shoulder and the first bone to implant contact. Changes in CBL were calculated by subtracting the T1 value from the T2 value. Therefore, all positive values indicated bone gain, while bone loss was defined by negative values. All radiographic measurements were taken independently and in duplicate by two of the authors (A.R. and J‐C.I.) not involved in any part of the treatment and follow‐up examinations.
FIGURE 2.

Crestal bone levels were determined by measuring linear distance between the implant shoulder and the first bone to implant contact. The distance is calibrated to the known implant length
2.11. Esthetic assessment (Copenhagen index score and supplementary implant‐related esthetic parameters)
The esthetic assessment was performed from frontal and semi‐axial clinical photographs of the restorations, including the adjacent teeth and marginal peri‐implant mucosa taken at T1 and T2 (Roccuzzo et al., 2020). To assess the esthetic outcomes, the Copenhagen index score (CPHI) was used as described by (Dueled et al., 2009) and (Hosseini & Gotfredsen, 2012) with all the evaluated parameters scored from 1 (i.e., optimal) to 4 (i.e., not‐sufficient) (Table 4).
TABLE 4.
Frequency of esthetic scores within the Ø2.9 mm and Ø3.3 mm groups at baseline (T1) and 1‐year follow‐up examination (T2)
| T1 | p‐value | T2 | p‐value | T2‐T1 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Ø2.9 mm | Ø3.3 mm | Ø2.9 mm | Ø3.3 mm | Ø2.9 mm | Ø3.3 mm | ||||
| n = 47 | n = 45 | n = 47 | n = 45 | ||||||
| Symmetry/harmony | 1 | 39.1% | 31.8% | .769 | 39.1% | 33.3% | .698 | 1.000 | 1.000 |
| 2 | 41.3% | 63.6% | 41.3% | 61.9% | |||||
| 3 | 19.6% | 4.5% | 19.6% | 4.8% | |||||
| Crown morphology | 1 | 69.6% | 97.7% | <.001 | 69.6% | 97.6% | <.001 | 1.000 | 1.000 |
| 2 | 30.4% | 2.3% | 30.4% | 2.4% | |||||
| Crown color | 1 | 50.0% | 25.0% | .006 | 47.8% | 26.2% | .023 | 1.000 | 1.000 |
| 2 | 50.0% | 70.5% | 52.2% | 71.4% | |||||
| 3 | 0.0% | 4.5% | 0.0% | 2.4% | |||||
| Soft tissue color | 1 | 47.8% | 43.2% | .978 | 39.1% | 35.7% | .747 | .097 | .125 |
| 2 | 43.5% | 56.8% | 47.8% | 61.9% | |||||
| 3 | 8.7% | 0.0% | 13.0% | 2.4% | |||||
| Papilla index (mesial) | 1 | 63.0% | 55.8% | .438 | 65.2% | 73.2% | .420 | 1.000 | .146 |
| 2 | 37.0% | 41.9% | 34.8% | 26.8% | |||||
| 3 | 0.0% | 2.3% | 0.0% | 0.0% | |||||
| Papilla index (distal) | 1 | 82.2% | 97.6% | .013 | 93.3% | 100% | .073 | .063 | .882 |
| 2 | 17.8% | 2.4% | 6.7% | 0.0% | |||||
| Level of the margin | 1 | 69.6% | 86.4% | .049 | 69.6% | 83.3% | .112 | .882 | 1.000 |
| 2 | 30.4% | 13.6% | 28.3% | 16.7% | |||||
| 3 | 0.0% | 0.0% | 2.2% | 0.0% | |||||
| Soft tissue texture | 1 | 65.2% | 84.1% | .035 | 84.8% | 92.9% | .223 | .022 | .125 |
| 2 | 34.8% | 15.9% | 15.2% | 7.1% | |||||
| Soft tissue curvature | 1 | 84.8% | 95.5% | .083 | 93.5% | 95.2% | .720 | .219 | 1.000 |
| 2 | 15.2% | 4.5% | 6.5% | 4.8% | |||||
| Alveolar process deficiency | 1 | 71.7% | 59.1% | .182 | 58.7% | 50.0% | .240 | .031 | .030 |
| 2 | 28.3% | 38.6% | 41.3% | 40.5% | |||||
| 3 | 0.0% | 2.3% | 0.0% | 9.5% | |||||
| Marginal adaptation score | 1 | 89.1% | 93.2% | .492 | 87.0% | 95.2% | .153 | .280 | .870 |
| 2 | 6.5% | 4.5% | 8.7% | 4.8% | |||||
| 3 | 4.3% | 2.3% | 4.3% | 0% | |||||
| Cement excess (0/1) | No | 91.3% | 95.5% | .677 | 97.8% | 97.6% | 1.000 | .250 | 1.000 |
| Yes | 8.7% | 4.5% | 2.2% | 2.4% | |||||
Note: Symmetry/harmony: assessment according to facial midline, tooth axis, contralateral tooth and smile line. Score 1: excellent; score 2: suboptimal but satisfactory; score 3: moderate; score 4: poor symmetry and harmony.
Crown morphology: assessment in relation to anatomy, surface texture, contour, prominence, contact points, crown length and crown width in relation to neighboring teeth. Score 1: excellent; score 2: satisfactory, but suboptimal in one or two of the subparameters; score 3: moderate with suboptimal for several subpara‐meters; score 4: was poor concerning most of the subparameters.
Crown color: assessment according to the hue value, chroma and translucency of the implant‐supported crown compared with neighboring teeth. Score 1 excellent color and not easy to distinguish from the natural, neighboring teeth; score 2 was satisfactory, almost optimal but the reconstruction differed from the natural, neighboring teeth; score 3 was moderate, suboptimal color, and score 4 was poor color match.
Soft tissue score: Score 1: no discoloration, score 2: light grayish discoloration, score 3: distinguishable grayish discoloration, score 4: metal or abutment visible.
Papilla index: Score1: papilla filling the entire proximal space; score 2: papilla filling at least half of the entire proximal space; score 3: papilla filling less than half of the proximal space, score 4: no papilla.
Level of the margin: assessment of the apically or incisally position of the buccal marginal peri‐implant mucosa in the middle of the implant crown compared to the contralateral tooth or the neighboring teeth. Score 1: match; score 2: slight mismatch; score 3: moderate mismatch; score 4: mismatch.
Soft tissue texture: assessment related to the smoother or rougher surface texture of the buccal peri‐implant mucosa compared to natural gingiva at the contralateral tooth or the neighboring teeth. Score1: match; score 2: slight mismatch; score 3: moderate mismatch; score 4: distinct mismatch.
Soft tissue curvature: assessment according to the over‐contoured or under‐ contoured buccal marginal peri‐implant mucosa compared to natural gingiva at the contralateral tooth or the neighboring teeth. Score1: match; score 2: slight mismatch; score 3: moderate mismatch; score 4: distinct mismatch.
Alveolar process deficiency: assessment related to the concavity or convexity of the buccal peri‐implant mucosa compared to the natural contour of the buccal gingiva at the contralateral tooth or the neighboring teeth. Score1: match; score 2: slight mismatch; score 3: moderate mismatch; score 4: distinct mismatch.
Marginal adaptation score: radiological assessment of fit or any gap between the implant crown and the abutment mesially and/or distally. Score 1: excellent fit; score 2: distinguishable misfit; score 3: distinct misfit; score 4: unacceptable misfit.
Cement excess: radiographic presence (score 1) or absence (score 0) of cement in relation to the implant crowns.
Kendall's Tau‐b was used for comparisons between groups.
McNemar's test was used for comparisons intra‐groups.
Bold values indicate statistical significance of p‐value.
More specifically, the following parameters were assessed:
Symmetry/harmony: assessment according to facial midline, tooth axis, contralateral tooth and smile line. Score 1: excellent; score 2: suboptimal but satisfactory; score 3: moderate; and score 4: poor symmetry and harmony
Crown morphology: assessment in relation to anatomy, surface texture, contour, prominence, contact points, crown length and crown width in relation to neighboring teeth. Score 1: excellent; score 2: satisfactory, but suboptimal in one or two of the sub‐parameters; score 3: moderate with suboptimal for several sub‐parameters; and score 4: was poor concerning most of the sub‐parameters
Crown color: assessment according to the hue value, chroma and translucency of the implant‐supported crown compared with neighboring teeth. Score 1 excellent color and not easy to distinguish from the natural, neighboring teeth; score 2 was satisfactory, almost optimal but the reconstruction differed from the natural, neighboring teeth; score 3 was moderate, suboptimal color, and score 4 was poor color match.
Soft tissue score: Score 1: no discoloration, score 2: light grayish discoloration, score 3: distinguishable grayish discoloration, and score 4: metal or abutment visible.
Papilla index: Score1: papilla filling the entire proximal space; score 2: papilla filling at least half of the entire proximal space; score 3: papilla filling less than half of the proximal space, and score 4: no papilla.
Level of the margin: assessment of the apically or incisal position of the buccal marginal peri‐implant mucosa in the middle of the implant crown compared with the contralateral tooth or the neighboring teeth. Score 1: match; score 2: slight mismatch; score 3: moderate mismatch; and score 4: mismatch.
Soft tissue texture: assessment related to the smoother or rougher surface texture of the buccal peri‐implant mucosa compared to natural gingiva at the contralateral tooth or the neighboring teeth. Score 1: match; score 2: slight mismatch; score 3: moderate mismatch; and score 4: distinct mismatch.
Soft tissue curvature: assessment according to the over‐contoured or under‐contoured buccal marginal peri‐implant mucosa compared with natural gingiva at the contralateral tooth or the neighboring teeth. Score1: match; score 2: slight mismatch; score 3: moderate mismatch; and score 4: distinct mismatch.
Alveolar process deficiency: assessment related to the concavity or convexity of the buccal peri‐implant mucosa compared with the natural contour of the buccal gingiva at the contralateral tooth or the neighboring teeth. Score 1: match; score 2: slight mismatch; score 3: moderate mismatch; and score 4: distinct mismatch.
Marginal adaptation score: radiological assessment of fit or any gap between the implant crown and the abutment mesially and/or distally. Score 1: excellent fit; score 2: distinguishable misfit; score 3: distinct misfit; and score 4: unacceptable misfit.
Cement excess: radiographic presence (score 1) or the absence (score 0) of cement in relation to the implant crowns
A periodontist (A.R.) and a prosthodontist (M.H.) not involved in any part of the treatments assessed the esthetic parameters. In cases of disagreement, the senior author (S.S.J.) was asked to provide his assessment and consensus was reached following discussion. All assessments were blinded regarding the implant diameters and were recorded as frequencies.
2.12. Patient‐reported outcomes assessment
The impact on oral health‐related quality of life was evaluated using a validated Danish version of the OHIP‐49 questionnaire before prosthetic treatment (i.e., before impression taking for the temporary crown) and at the 1‐year follow‐up examination. Each patient was asked to score each question with a Likert response scale from 0 (never experienced problem) to 4 (problem experienced very often). The summary of questions 3, 4, 20, 22, 31, and 38 was used to describe the patient‐reported “esthetic outcome” (Dueled et al., 2009), while the “masticatory function” was expressed by the summary scores of questions 1, 28, 29, and 32 (Goshima et al., 2010). The overall oral health‐related impact on quality of life was described by the sum of scores from all 49 OHIP questions before prosthetic treatment, and one year after loading.
2.13. Statistical analysis
In cases with bilateral MLIs, where implants with identical diameters were placed, only one implant was randomly selected for the statistical analysis (www.randomization.com).
2.13.1. Sample size calculation
Sample size calculation was performed on the secondary outcome parameter: peri‐implant crestal bone resorption according to Hosseini et al., 2013. More in detail, a mean crestal bone loss of 0.6 mm in the test group and 0.2 mm in the control group were considered significant. Therefore, to detect a difference of 0.4 mm and a standard deviation of 0.6, 49 patients per group were needed with an alpha (type I error) = 0.05 and a pf power = 0.9. Using an independent sample t‐test, a group size of N = 49 was calculated. Patients number per group was rounded to 50.
2.13.2. Data analysis
Each patient contributed with one dental implant only and was, therefore, considered as the statistical unit. Descriptive analysis was performed providing absolute and relative frequencies for categorical variables and mean, standard deviation, for continuous variables. Normal distribution of the quantitative measures was checked by Kolmogorov–Smirnov test. Two‐sample t‐test was used to compare mean CBL between both implant groups. The calculated inter‐examiner agreement with Dahlberg's d test was in the range 0.09–0.14 mm and the intra‐class correlation coefficient (ICC) was in the range 0.95–0.98 providing a very high level of reproducibility of the performed measurements.
Chi2 independence, Kendall's Tau‐b, and Fisher's exact test were used to assess the association between categorical/ordinal esthetic variables and group. Similar tests were used to compare collected parameters (i.e., width of the alveolar process (WAP), width of the alveolar ridge (WAR), and thickness of the facial bone (TFB) after osteotomy) and need of bone regeneration between groups. Paired t‐test was used for intra‐group comparisons of the OHIP scores over time. All the tests were two‐tailed and the level of significance was set at 5%. The statistical analysis was performed with a commercially available dedicated software (spss 15.0).
3. RESULTS
3.1. Study sample characteristics
The investigated population included 100 patients rehabilitated with 100 dental implants Ø2.9 mm (n = 50) or Ø3.3 mm (n = 50). All 100 patients underwent preoperative orthodontic treatment to allow implant placement. At the time of implant placement, the mean age was 21.5 ± 2.6 years (range: 18–31) and did not statistically significant differ between groups (p = .258). The gender distribution (male: female) in the whole sample was 1:1.44 and in the two groups Ø2.9 mm: 1:1.94 vs. Ø3.3 mm: 1:1.08 (p = .155). During the implant placement procedure, 34 implants with Ø3.3 mm and 22 with a Ø2.9 mm needed a bone augmentation procedure (p = .017). No soft tissue augmentation procedures were performed simultaneous with implant placement. Additional data on the population have been reported in Roccuzzo et al., 2021. At the 1‐year follow‐up examination, a total of seven dropout patients ((Ø2.9 mm (n = 3); Ø3.3 mm (n = 4)) were recorded.
Details of the patients' characteristic and reasons for dropouts are listed in Tables 1 and 2. The overall study flow is presented in Figure 1.
TABLE 1.
Patients and implants characteristics within the two groups (2.9 diameter; 3.3 diameter). Mean (SD)/number
| 2.9 Ø | 3.3 Ø | p | |
|---|---|---|---|
| Number of patients/implants | 50 | 50 | |
| Age § | 21.2 ± 2.5 | 21.8 ± 2.8 | .258 |
| Sex (M/F) | 17 M, 33 F | 24 M, 26 F | .155 |
|
Congenital missing lateral incisor (12; 22) |
29 (1.2) 21 (2.2) |
23 (1.2) 27 (2.2) |
.230 |
|
Length of the implants placed (10; 12 mm) |
16 (10 mm) 34 (12 mm) |
17 (10 mm) 33 (12 mm) |
.832 |
Abbreviations: F: female, M: male.
Note: Two sample t‐test for age and Chi2 independence test.
TABLE 2.
Reasons for dropout
| Ø2.9 mm | Ø3.3 mm | Total | |
|---|---|---|---|
| Deceased | 0 | 1 | 1 |
| Moved abroad | 2 | 1 | 3 |
| Withdraw acceptance to participate | 1 | 2 | 3 |
| Total | 3 | 4 | 7 |
FIGURE 1.

Study flow chart
3.2. Biological outcome parameters
Of the 93 patients available for follow‐up, one patient with a Ø3.3 mm experienced implant loss before loading (i.e., early implant failure) leading to an implant survival rate of 100% in the Ø2.9 mm group and 98% in the Ø3.3 mm group with no statistically significant difference between the two groups (p = 1.000; 95% CI: 94.6%–99.9%).
With respect to mean CBL changes, no statistically significant differences were detected at any time points (T1‐T0; T2‐T0; T2‐T1) within the 2 groups: more specifically, in the first year after loading (i.e., T2‐T1) a CBL of −0.19 ± 0.25 mm in the Ø2.9 mm and − 0.25 ± 0.31 mm Ø3.3 mm was detected, with no statistically significant difference between the groups (p = .342). Details of CBL changes over time are provided in Figure 3.
FIGURE 3.

Crestal bone level changes over time within the two groups
Mean peri‐implant probing depth (PPD) value was 2.55 ± 0.41 mm in the Ø2.9 mm group and 2.50 ± 0.45 mm in Ø3.3 mm group, with no statistically significant difference between groups (p = .576).
Plaque scores did not statistically significant differ between the 2 groups: 15% in the Ø2.9 mm group and 12% in the in Ø3.3 mm group (p = .631).
The presence of a buccal fistula was recorded in four cases at the 1‐year follow‐up visit (two per group) (Figure 4a), while suppuration after probing was detected around two implants (1x per group). None of the neighboring teeth displayed radiographic nor clinical signs of endodontic complications.
FIGURE 4.
Clinical and radiographic presentation of biological and prosthetic complications recorded at the 1‐year follow‐up: Presence of a buccal fistula at the border between keratinized and non‐keratinized mucosa (a) and ceramic chipping of the distal incisal edge (b)
3.3. Technical outcome parameters
One event of a loosening abutment screw was detected in the Ø3.3 mm group, while loss of retention of the reconstruction occurred twice in the Ø2.9 mm and once in the Ø3.3 mm group. None of the restorations had to be remade due to unacceptable marginal adaptation (i.e., Score 4). Finally, radiographic evidence of cement excesses was observed twice at the 1‐year examination (1x Ø2.9 mm; 1x Ø3.3 mm group).
The biological and technical complications are reported in Table 3a–b.
TABLE 3.
Biologic (a) and technical (b) complications within the Ø2.9 mm and Ø3.3 mm groups at baseline (T1) and 1‐year follow‐up examination (T2)
| a | T1 | T2 | ||
|---|---|---|---|---|
| Ø2.9 mm | Ø3.3 mm | Ø2.9 mm | Ø3.3 mm | |
| n = 47 | n = 45 | n = 47 | n = 45 | |
| Fistula | 0 (0%) | 0 (0%) | 2 (4%) | 2 (8%) |
| Exudation/ suppuration on probing | 0 (0%) | 0 (0%) | 1 (2%) | 1 (2%) |
| Pain | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Necrosis of neighboring teeth | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| b | T1 | T2 | ||
| Ø2.9 mm | Ø3.3 mm | Ø2.9 mm | Ø3.3 mm | |
| n = 47 | n = 45 | n = 47 | n = 45 | |
| Loosening or fracture of abutment screw | 0 (0%) | 0 (0%) | 0 (0%) | 1 (2%) |
| Loss of retention | 0 (0%) | 0 (0%) | 2 (4%) | 1 (2%) |
| Fracture or chipping of veneering ceramic | 0 (0%) | 0 (0%) | 1 (0%) | 1 (2%) |
3.4. Esthetic outcome parameters
The assessment of the esthetic presentation of the implant‐supported single‐crowns showed good to optimal results (i.e., scores 1 and 2) at T2, irrespective of the implant diameter, in the majority of the cases (i.e., variables: Symmetry; Soft tissue color; Soft tissue curvature; Alveolar process deficiency) (p > .05). Nevertheless, peri‐implant soft tissue score 3 (i.e., presence of discoloration) was detected in 13% of the Ø2.9 mm and 2.4% in the Ø3.3 mm (p = .747). With respect to papilla fill, no statistically significant differences were identified between the groups at T2 in terms of mesial (p = .420) and distal (p = .073) papilla index score.
Details of the esthetic outcomes are listed in Table 4.
3.5. Patient‐reported outcome parameters
Table 5 summarizes the patient‐reported esthetic outcome, masticatory function and overall oral health‐related impact on quality of life in all patients from before delivery of the final reconstruction until the 1‐year control. During the observation period, a decrease in all the scores with respect to the both the esthetic and the masticatory functions analysis were detected indicating subjective improvement without a statistically significant difference between the groups (p > .05). Moreover, the summary scores of the 49 OHIP questions decreased through the study period indicating an overall improvement in oral health‐related impact on quality of life with no statistically significant difference between the two groups (p > .05).
TABLE 5.
Patient‐reported outcome means of sum scores of questions related to esthetic outcome, masticatory function, and overall oral health impact on quality of prosthetic treatment, before prosthetic treatment and at 1‐year follow‐up (T2)
| Before prosthetic treatment | p‐value | T2 | p‐value | |||
|---|---|---|---|---|---|---|
| Ø2.9 mm | Ø3.3 mm | Ø2.9 mm | Ø3.3 mm | |||
| Esthetic outcome a | 10.4 ± 6.00 | 9.24 ± 6.38 | .382 | 3.51 ± 5.33 | 1.76 ± 3.74 | .079 |
| Masticatory function b | 3.02 ± 2.76 | 4.10 ± 3.04 | .093 | 1.04 ± 1.94 | 0.86 ± 1.49 | .617 |
| Overall health impact of OHQoL | 36.6 ± 25.0 | 37.9 ± 22.3 | .798 | 16.9 ± 23.1 | 10.3 ± 14.2 | .110 |
Note: Two sample t‐test.
Esthetic outcome: summary scores of OHIP questions 3, 4, 20, 22, 31, 38.
Functional outcome: summary scores of OHIP questions 1, 28, 29, 32.
4. DISCUSSION
The present study could not demonstrate any statistical differences in terms of implant survival, crestal bone loss, esthetic outcome, or patient‐reported outcome measures between narrow‐diameter implants with a diameter of 2.9 or 3.3 mm for replacement of MLIs after one year of loading. The null hypothesis of no difference in peri‐implant marginal bone level changes after one year of loading could therefore not be rejected.
With respect to the outcome implant survival, high percentages within both groups were recorded: Our results are in accordance with those reported by (Schiegnitz & Al‐Nawas, 2018) who have calculated an implant survival rate of 94.7 ± 5% for implants with a diameter <3.0 mm and 97.7 ± 2.3% with a diameter of 3.3–3.5 mm for single unit‐gap tooth replacement, respectively.
Historically, reduced peri‐implant crestal bone resorption has been reported around standard diameter implants (SDIs) compared with NDIs, which has mainly been ascribed to a better distribution of occlusal forces around a wider implant (Qian et al., 2009). During recent years, several clinical studies have reported good clinical outcomes and limited radiographic CBL changes (<1 mm) for NDIs short‐term (Degidi et al., 2009; Maiorana et al., 2015; Reddy et al., 2008) as well as long‐term (Branzén et al., 2015; Galindo‐Moreno et al., 2017). In accordance with previous studies, the present study identified the major part of crestal remodeling to take place between implant placement and prosthetic loading. This process might be explained as the physiologic bone remodeling following sub‐crestal implant placement with the concomitant establishment of the peri‐implant soft tissue seal (Berglundh et al., 2007; Cardaropoli et al., 2006). After prosthetic loading, only minimal crestal bone loss was observed which is compatible with generalized healthy peri‐implant conditions as expected in a young healthy population (Santing et al., 2013).
With respect to the recorded biological complications, two implants per group developed a buccal fistula without suppuration not associated with an increasing peri‐implant probing depth nor in the CBL changes (Figure 4a). Similar findings have previously been correlated with the presence of subgingival remnants of cement (Bonde et al., 2010) or suboptimal marginal adaptation of the crowns (Gotfredsen, 2004). However, no such problems could be identified around the four implants, and it was decided to observe the conditions at the following control visits.
The use of NDIs has been correlated with an increased risk of technical complications due to their weaker structure (Shi et al., 2018). Nevertheless, in the present study, only minor complications (i.e., 1x loosing of the abutment screw and chipping of veneering ceramic [Figure 4b]) were recorded and could be handled without negatively affecting the survival of the restoration. More specifically, it should be underlined that at the time of crown delivery, a careful check of the occlusal contacts and lateral guidance has been performed to minimize the risks of premature contacts which could have had a detrimental effect of the implant‐supported restoration. One of the most frequently reported technical complications is decementation of the reconstruction (Woelber et al., 2016). In the investigated cohort, two events in the Ø2.9 mm group and one in the Ø3.3 mm group were recorded. These results are consistent with those reported in the literature (King et al., 2016; Lee et al., 2013).
Esthetics is considered as one of the most important aspect of oral rehabilitation in the maxillary anterior region. In the present study, especially due to the young age of the included patients, every effort was made to maximize the esthetic outcomes (Figure 5a–b) as demonstrated by the high percentage of good to optimal crown morphology and colors. Nevertheless, it must be underlined that differences at the time of the crown delivery between the groups with respect to the crown morphology and crown color were detected (<0.001). These differences might be speculated to be related primary to the prosthetic manufacturing phase than to the implant diameter. In addition, due to the high number of included patients, slight differences in the esthetic assessment (i.e., Score 1 vs. 2) can be easier detected, as within the present cohort.
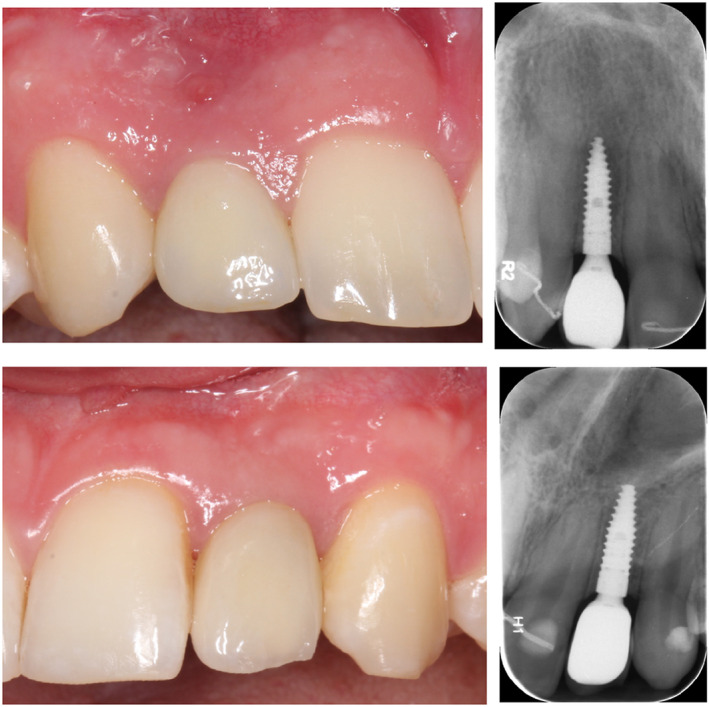
FIGURE 5.

Clinical and radiographic presentation of a test (a) and control (b) implant at the 1‐year follow‐up
When focusing on the peri‐implant soft tissue conditions, it has to be underlined that at the 1‐year follow‐up, a consistent number of patients exhibited a soft tissue color mismatch (i.e., grayness or redness). One possible explanation of this color mismatch might be the use of a titanium abutments. Zirconia abutments may be preferable over metal abutments to reduce the risk of peri‐implant mucosal discoloration (Bidra & Rungruanganunt, 2013; Linkevicius & Vaitelis, 2015; Totou et al., 2021). However, other studies indicated no differences in the peri‐implant mucosa discoloration between the zirconia and metal abutments (Hosseini et al., 2011, 2013). Moreover, as zirconia abutments are not available for the Ø2.9 mm implants, titanium‐fabricated abutments had to be used to have only one type of abutment with no difference between test and control groups.
One of the interesting findings from the present study is that more than 60% of cases scored as “optimal” (i.e., Score 1) with respect to the alveolar process deficiency evaluation have received a bone augmentation procedure either with DBBM alone or with DBBM in combination with autogenous bone, providing an indirect evidence of the need for grafting procedure at the time of implant placement to allow an optimal peri‐implant soft tissue architecture (Table 6). On the contrary, it has to be underlined that a minimum of 1.7 mm of facial bone wall was not able to avoid the development of alveolar process deficiency as demonstrated by the 40% of cases at T2 that scored 2 (i.e., slight deficiency). One possible explanation is that, at the time of implant placement, no soft tissue grafting procedures were performed to limit the intraoperative morbidity as most of the implants already required a bone regenerative procedure. Nevertheless, during recent years, soft tissue grafting procedures in the esthetic zone have shown promising results and gained increased popularity and have become standard of care (Thoma et al., 2021).
TABLE 6.
Clinical preoperative and intraoperative parameters and alveolar process deficiency at T2 in the entire cohort Mean ± (SD)/number (%). Some of the number are mm +/− SD others are n and the parenthesis % please specify
| Alveolar process deficiency | p‐value | |||
|---|---|---|---|---|
| Score 1 | Score 2 | Score 3 | ||
| Number of implants | 48 | 36 | 4 | |
| Width of the alveolar process | 8.02 ± 0.94 | 8.35 ± 1.19 | 7.23 ± 1.56 | .161 |
| Width of the alveolar ridge | 5.51 ± 1.02 | 5.63 ± 0.87 | 5.15 ± 0.74 | .555 |
| Thickness facial bone after osteotomy§ | 1.58 ± 0.64 | 1.76 ± 0.60 | 1.50 ± 0.22 | .195 |
| Bone augmentation procedure (yes) | 30 (62.5) | 14 (38.9) | 3 (75.0) | .032 |
Note: Score 3 subgroup was excluded from the statistical analysis due to small sample size.
Two sample t‐test for quantitative variables.
Chi2 independence test for bone augmentation procedure.
Bold value indicates statistical significance of p‐value.
Since their introduction in 2008 (Lang et al., 2012), PROMs have received increasing focus for the evaluation of implant‐supported rehabilitations (Duong et al., 2022). The results from the present study have shown a general improvement in patient‐reported outcomes irrespective of the implant diameter, which may be explained by the overall high quality of the provided treatments. Indeed, it is well known that most of the patients undergoing implant placement for the replacement MLIs have reported lower baseline‐scores as consequence of dissatisfaction with the temporary removable restorations and the long‐term orthodontic pretreatment (Gotfredsen, 2012). With respect to the esthetic and masticatory function scores, our results are consistent with previous publications (Feine et al., 2018; Gotfredsen, 2012; Pjetursson et al., 2005).
This study presents some limitations: First, despite the large sample size, it has to be pointed out that the selection of implant diameter should be based on the accurate analysis of several anatomical (i.e., available amount of bone) and prosthetic factors (i.e., expected functional load and on the emergence profile) and not only depending on mesio‐distal gap dimension. On this point, it has to be mentioned that the functional load in the region of the MLI is in considered low. However, for esthetic reasons, it was not considered ethical to place a Ø2.9 mm implant when the mesio‐distal space allowed placement of a Ø3.3 mm implant. Indeed, narrow‐diameter implants placed in wide tooth gaps need to be placed vertically deeper to allow a harmonious emergence profile which may be accompanied by a risk of increased peri‐implant pocket depth. Consequently, the present study could not be planned as a randomized controlled study and direct comparison between the two groups should therefore be made with caution. Moreover, even though it is widely accepted that implant‐supported single‐crowns in the esthetic area should be screw‐retained (Wittneben et al., 2017), all the reconstructions of the present studies were cemented. The reason for this was that at the time of the study initiation (i.e., 2016), original components to support a screw‐retained implant‐supported single‐crowns were not available for the Ø2.9 mm implants. To have only one type of crown retention without differences between test and control, all the definitive crowns were cemented. Furthermore, it has to be mentioned that this study has been designed with the focus on the hard‐tissue conditions and that of a precise assessment of the peri‐implant soft tissue dehiscence is lacking. Finally, due to the limited follow‐up period (i.e., 12 months), the presented clinical and radiographic outcomes should be interpreted with caution and additional long‐term studies (i.e., 3‐ and 5‐years) including the assessment of mechanical stability, infraposition, the presence of missing contact points, and peri‐implant soft tissue margin changes will provide relevant information.
In conclusion, within the limitations of the present short‐term study, the use of an implant with a diameter of 2.9 and 3.3 mm showed equivalent performance in terms of survival rate, CBL changes, esthetic, and patients‐reported outcomes. Consequently, clinicians should consider the use of such NDIs in case of replacement of maxillary lateral incisors especially when limited mesio‐distal space otherwise would challenge the possibility implant treatment.
AUTHORS’ CONTRIBUTIONS
A.R. and S.S.J. conceived the idea and led the writing, S.S.J performed the surgeries, J.L. performed the prosthetics, A.R., J.C.I., and M.H. collected, analyzed, and interpreted the data, M.H. and J.C.I. contributed to the writing.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest with respect to this study. A.R. was the recipient of a 1‐year scholarship from the Italian Society of Osseointegration (SIO) during the planning of the study. A.R. was the recipient of a 3‐year scholarship from the Clinical Research Foundation (CFR) for the Promotion of Oral Health, Brienz, Switzerland. A.R. is the recipient of a 1‐year scholarship from the International Team of Implantology (ITI).
ACKNOWLEDGMENTS
The authors thank Mr. Juan Luis Gómez Martínez (stHalley Statistics) for his valuable help in statistical analysis and Ms. Vibeke Nielsen (RDH) for her thorough assistance with the follow‐up visits. Open access funding provided by Universitat Bern. Open access funding provided by Universitat Bern.
Roccuzzo, A. , Imber, J‐C , Lempert, J. , Hosseini, M. , & Jensen, S. S. (2022). Narrow diameter implants to replace congenital missing maxillary lateral incisors: A 1‐year prospective, controlled, clinical study. Clinical Oral Implants Research, 33, 844–857. 10.1111/clr.13966
Funding information
The study was funded by a large grant of the ITI Foundation (n 1151_2016). Implants, healing abutments, impression posts, implant analogs, and standard temporary TAN abutments were provided free of charge from Straumann AG, Basel, Switzerland.
DATA AVAILABILITY STATEMENT
‐The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions
REFERENCES
- Berglundh, T. , Abrahamsson, I. , Welander, M. , Lang, N. P. , & Lindhe, J. (2007). Morphogenesis of the peri‐implant mucosa: An experimental study in dogs. Clinical Oral Implants Research, 18(1), 1–8. 10.1111/j.1600-0501.2006.01380.x [DOI] [PubMed] [Google Scholar]
- Beyer, A. , Tausche, E. , Boening, K. , & Harzer, W. (2007). Orthodontic space opening in patients with congenitally missing lateral incisors. The Angle Orthodontist, 77(3), 404–409. 10.2319/0003-3219(2007)077[0404:Osoipw]2.0.Co;2 [DOI] [PubMed] [Google Scholar]
- Bidra, A. S. , & Rungruanganunt, P. (2013). Clinical outcomes of implant abutments in the anterior region: A systematic review. Journal of Esthetic and Restorative Dentistry, 25(3), 159–176. 10.1111/jerd.12031 [DOI] [PubMed] [Google Scholar]
- Bonde, M. J. , Stokholm, R. , Isidor, F. , & Schou, S. (2010). Outcome of implant‐supported single‐tooth replacements performed by dental students. A 10‐year clinical and radiographic retrospective study. Eur . The Journal of Oral Implantology, 3(1), 37–46. [PubMed] [Google Scholar]
- Branzén, M. , Eliasson, A. , Arnrup, K. , & Bazargani, F. (2015). Implant‐supported single crowns replacing congenitally missing maxillary lateral incisors: A 5‐year follow‐up. Clinical Implant Dentistry and Related Research, 17(6), 1134–1140. 10.1111/cid.12233 [DOI] [PubMed] [Google Scholar]
- Buser, D. , Halbritter, S. , Hart, C. , Bornstein, M. M. , Grutter, L. , Chappuis, V. , & Belser, U. C. (2009). Early implant placement with simultaneous guided bone regeneration following single‐tooth extraction in the esthetic zone: 12‐month results of a prospective study with 20 consecutive patients. Journal of Periodontology, 80(1), 152–162. 10.1902/jop.2009.080360 [DOI] [PubMed] [Google Scholar]
- Cardaropoli, G. , Lekholm, U. , & Wennström, J. L. (2006). Tissue alterations at implant‐supported single‐tooth replacements: A 1‐year prospective clinical study. Clinical Oral Implants Research, 17(2), 165–171. 10.1111/j.1600-0501.2005.01210.x [DOI] [PubMed] [Google Scholar]
- Chiapasco, M. , Casentini, P. , Zaniboni, M. , Corsi, E. , & Anello, T. (2012). Titanium‐zirconium alloy narrow‐diameter implants (Straumann Roxolid[®]) for the rehabilitation of horizontally deficient edentulous ridges: Prospective study on 18 consecutive patients. Clinical Oral Implants Research, 23(10), 1136–1141. 10.1111/j.1600-0501.2011.02296.x [DOI] [PubMed] [Google Scholar]
- Degidi, M. , Nardi, D. , & Piattelli, A. (2009). Immediate restoration of small‐diameter implants in cases of partial posterior edentulism: A 4‐year case series. Journal of Periodontology, 80(6), 1006–1012. 10.1902/jop.2009.080649 [DOI] [PubMed] [Google Scholar]
- Dueled, E. , Gotfredsen, K. , Trab Damsgaard, M. , & Hede, B. (2009). Professional and patient‐based evaluation of oral rehabilitation in patients with tooth agenesis. Clinical Oral Implants Research, 20(7), 729–736. 10.1111/j.1600-0501.2008.01698.x [DOI] [PubMed] [Google Scholar]
- Duong, H. Y. , Roccuzzo, A. , Stähli, A. , Salvi, G. E. , Lang, N. P. , & Sculean, A. (2022). Oral health‐related quality of life of patients rehabilitated with fixed and removable implant‐supported dental prostheses. Periodontology 2000, 88, 201–237. 10.1111/prd.12419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feine, J. , Abou‐Ayash, S. , al Mardini, M. , de Santana, R. B. , Bjelke‐Holtermann, T. , Bornstein, M. M. , … Zubiria, J. P. V. (2018). Group 3 ITI consensus report: Patient‐reported outcome measures associated with implant dentistry. Clinical Oral Implants Research, 29(Suppl 16), 270–275. 10.1111/clr.13299 [DOI] [PubMed] [Google Scholar]
- Galindo‐Moreno, P. , Nilsson, P. , King, P. , Worsaae, N. , Schramm, A. , Padial‐Molina, M. , & Maiorana, C. (2017). Clinical and radiographic evaluation of early loaded narrow‐diameter implants: 5‐year follow‐up of a multicenter prospective clinical study. Clinical Oral Implants Research, 28(12), 1584–1591. 10.1111/clr.13029 [DOI] [PubMed] [Google Scholar]
- Gallucci, G. O. , Hamilton, A. , Zhou, W. , Buser, D. , & Chen, S. (2018). Implant placement and loading protocols in partially edentulous patients: A systematic review. Clinical Oral Implants Research, 29(S16), 106–134. 10.1111/clr.13276 [DOI] [PubMed] [Google Scholar]
- Goshima, K. , Lexner, M. O. , Thomsen, C. E. , Miura, H. , Gotfredsen, K. , & Bakke, M. (2010). Functional aspects of treatment with implant‐supported single crowns: A quality control study in subjects with tooth agenesis. Clinical Oral Implants Research, 21(1), 108–114. 10.1111/j.1600-0501.2009.01809.x [DOI] [PubMed] [Google Scholar]
- Gotfredsen, K. (2004). A 5‐year prospective study of single‐tooth replacements supported by the Astra tech implant: A pilot study. Clinical Implant Dentistry and Related Research, 6(1), 1–8. 10.1111/j.1708-8208.2004.tb00021.x [DOI] [PubMed] [Google Scholar]
- Gotfredsen, K. (2012). A 10‐year prospective study of single tooth implants placed in the anterior maxilla. Clinical Implant Dentistry and Related Research, 14(1), 80–87. 10.1111/j.1708-8208.2009.00231.x [DOI] [PubMed] [Google Scholar]
- Hosseini, M. , & Gotfredsen, K. (2012). A feasible, esthetic quality evaluation of implant‐supported single crowns: An analysis of validity and reliability. Clinical Oral Implants Research, 23(4), 453–458. 10.1111/j.1600-0501.2011.02162.x [DOI] [PubMed] [Google Scholar]
- Hosseini, M. , Worsaae, N. , Schiodt, M. , & Gotfredsen, K. (2011). A 1‐year randomised controlled trial comparing zirconia versus metal‐ceramic implant supported single‐tooth restorations. European Journal of Oral Implantology, 4(4), 347–361. [PubMed] [Google Scholar]
- Hosseini, M. , Worsaae, N. , Schiødt, M. , & Gotfredsen, K. (2013). A 3‐year prospective study of implant‐supported, single‐tooth restorations of all‐ceramic and metal‐ceramic materials in patients with tooth agenesis. Clinical Oral Implants Research, 24(10), 1078–1087. 10.1111/j.1600-0501.2012.02514.x [DOI] [PubMed] [Google Scholar]
- Hwang, D. , & Wang, H. L. (2006). Medical contraindications to implant therapy: Part I: Absolute contraindications. Implant Dentistry, 15(4), 353–360. 10.1097/01.id.0000247855.75691.03 [DOI] [PubMed] [Google Scholar]
- Hwang, D. , & Wang, H. L. (2007). Medical contraindications to implant therapy: Part II: Relative contraindications. Implant Dentistry, 16(1), 13–23. 10.1097/ID.0b013e31803276c8 [DOI] [PubMed] [Google Scholar]
- Ioannidis, A. , Gallucci, G. O. , Jung, R. E. , Borzangy, S. , Hammerle, C. H. , & Benic, G. I. (2015). Titanium‐zirconium narrow‐diameter versus titanium regular‐diameter implants for anterior and premolar single crowns: 3‐year results of a randomized controlled clinical study. Journal of Clinical Periodontology, 42(11), 1060–1070. 10.1111/jcpe.12468 [DOI] [PubMed] [Google Scholar]
- Jensen, S. S. (2019). Timing of implant placement after traumatic dental injury. Journal of Endodontia, 45(12s), S52–S56. 10.1016/j.joen.2019.05.013 [DOI] [PubMed] [Google Scholar]
- Jung, R. E. , al‐Nawas, B. , Araujo, M. , Avila‐Ortiz, G. , Barter, S. , Brodala, N. , … Windisch, P. (2018). Group 1 ITI consensus report: The influence of implant length and design and medications on clinical and patient‐reported outcomes. Clinical Oral Implants Research, 29 Suppl 16(Suppl 16), 69–77. 10.1111/clr.13342 [DOI] [PubMed] [Google Scholar]
- Jung, R. E. , Zembic, A. , Pjetursson, B. E. , Zwahlen, M. , & Thoma, D. S. (2012). Systematic review of the survival rate and the incidence of biological, technical, and esthetic complications of single crowns on implants reported in longitudinal studies with a mean follow‐up of 5 years. Clinical Oral Implants Research, 23(Suppl 6), 2–21. 10.1111/j.1600-0501.2012.02547.x [DOI] [PubMed] [Google Scholar]
- Kafantaris, S. N. , Tortopidis, D. , Pissiotis, A. L. , & Kafantaris, N. M. (2020). Factors affecting decision‐making for congenitally missing permanent maxillary lateral incisors: A retrospective study. The European Journal of Prosthodontics and Restorative Dentistry, 28(1), 43–52. 10.1922/EJPRD_1959Kafantaris10 [DOI] [PubMed] [Google Scholar]
- Kern, M. , Passia, N. , Sasse, M. , & Yazigi, C. (2017). Ten‐year outcome of zirconia ceramic cantilever resin‐bonded fixed dental prostheses and the influence of the reasons for missing incisors. Journal of Dentistry, 65, 51–55. 10.1016/j.jdent.2017.07.003 [DOI] [PubMed] [Google Scholar]
- Khalaf, K. , Miskelly, J. , Voge, E. , & Macfarlane, T. V. (2014). Prevalence of hypodontia and associated factors: A systematic review and meta‐analysis. Journal of Orthodontics, 41(4), 299–316. 10.1179/1465313314y.0000000116 [DOI] [PubMed] [Google Scholar]
- Kiliaridis, S. , Sidira, M. , Kirmanidou, Y. , & Michalakis, K. (2016). Treatment options for congenitally missing lateral incisors. European Journal of Oral Implantology, 9(Suppl 1), S5–S24. [PubMed] [Google Scholar]
- King, P. , Maiorana, C. , Luthardt, R. G. , Sondell, K. , Øland, J. , Galindo‐Moreno, P. , & Nilsson, P. (2016). Clinical and radiographic evaluation of a small‐diameter dental implant used for the restoration of patients with permanent tooth agenesis (hypodontia) in the maxillary lateral incisor and mandibular incisor regions: A 36‐month follow‐up. The International Journal of Prosthodontics, 29(2), 147–153. 10.11607/ijp.4444 [DOI] [PubMed] [Google Scholar]
- Lacarbonara, M. , Cazzolla, A. P. , Lacarbonara, V. , Lo Muzio, L. , Ciavarella, D. , Testa, N. F. , … Capogreco, M. (2021). Prosthetic rehabilitation of maxillary lateral incisors agenesis using dental mini‐implants: A multicenter 10‐year follow‐up. Clinical Oral Investigations, 26, 1963–1974. 10.1007/s00784-021-04176-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang, N. P. , Zitzmann, N. U. , & Working Group 3 of the VIII European Workshop on Periodontology . (2012). Clinical research in implant dentistry: Evaluation of implant‐supported restorations, aesthetic and patient‐reported outcomes. J Clin Periodontol, 39(Suppl 12), 133–138. [DOI] [PubMed] [Google Scholar]
- Lee, J. S. , Kim, H. M. , Kim, C. S. , Choi, S. H. , Chai, J. K. , & Jung, U. W. (2013). Long‐term retrospective study of narrow implants for fixed dental prostheses. Clinical Oral Implants Research, 24(8), 847–852. 10.1111/j.1600-0501.2012.02472.x [DOI] [PubMed] [Google Scholar]
- Linkevicius, T. , & Vaitelis, J. (2015). The effect of zirconia or titanium as abutment material on soft peri‐implant tissues: A systematic review and meta‐analysis. Clinical Oral Implants Research, 26(Suppl 11), 139–147. 10.1111/clr.12631 [DOI] [PubMed] [Google Scholar]
- Ma, M. , Qi, M. , Zhang, D. , & Liu, H. (2019). The clinical performance of narrow diameter implants versus regular diameter implants: A meta‐analysis. The Journal of Oral Implantology, 45(6), 503–508. 10.1563/aaid-joi-D-19-00025 [DOI] [PubMed] [Google Scholar]
- Maiorana, C. , King, P. , Quaas, S. , Sondell, K. , Worsaae, N. , & Galindo‐Moreno, P. (2015). Clinical and radiographic evaluation of early loaded narrow‐diameter implants: 3 years follow‐up. Clinical Oral Implants Research, 26(1), 77–82. 10.1111/clr.12281 [DOI] [PubMed] [Google Scholar]
- Matalova, E. , Fleischmannova, J. , Sharpe, P. T. , & Tucker, A. S. (2008). Tooth agenesis: From molecular genetics to molecular dentistry. Journal of Dental Research, 87(7), 617–623. 10.1177/154405910808700715 [DOI] [PubMed] [Google Scholar]
- McKeown, H. F. , Robinson, D. L. , Elcock, C. , al‐Sharood, M. , & Brook, A. H. (2002). Tooth dimensions in hypodontia patients, their unaffected relatives and a control group measured by a new image analysis system. European Journal of Orthodontics, 24(2), 131–141. 10.1093/ejo/24.2.131 [DOI] [PubMed] [Google Scholar]
- Nieminen, P. , Arte, S. , Pirinen, S. , Peltonen, L. , & Thesleff, I. (1995). Gene defect in hypodontia: Exclusion of MSX1 and MSX2 as candidate genes. Human Genetics, 96(3), 305–308. 10.1007/bf00210412 [DOI] [PubMed] [Google Scholar]
- Pjetursson, B. E. , Karoussis, I. , Bürgin, W. , Brägger, U. , & Lang, N. P. (2005). Patients' satisfaction following implant therapy. A 10‐year prospective cohort study. Clinical Oral Implants Research, 16(2), 185–193. 10.1111/j.1600-0501.2004.01094.x [DOI] [PubMed] [Google Scholar]
- Priest, G. (2019). The treatment dilemma of missing maxillary lateral incisors‐part I: Canine substitution and resin‐bonded fixed dental prostheses. Journal of Esthetic and Restorative Dentistry, 31(4), 311–318. 10.1111/jerd.12484 [DOI] [PubMed] [Google Scholar]
- Qian, L. , Todo, M. , Matsushita, Y. , & Koyano, K. (2009). Effects of implant diameter, insertion depth, and loading angle on stress/strain fields in implant/jawbone systems: Finite element analysis. The International Journal of Oral and Maxillofacial Implants, 24(5), 877–886. [PubMed] [Google Scholar]
- Rakhshan, V. , & Rakhshan, A. (2016). Systematic review and meta‐analysis of congenitally missing permanent dentition: Sex dimorphism, occurrence patterns, associated factors and biasing factors. International Orthodontics, 14(3), 273–294. 10.1016/j.ortho.2016.07.016 [DOI] [PubMed] [Google Scholar]
- Reddy, M. S. , O'Neal, S. J. , Haigh, S. , Aponte‐Wesson, R. , & Geurs, N. C. (2008). Initial clinical efficacy of 3‐mm implants immediately placed into function in conditions of limited spacing. The International Journal of Oral and Maxillofacial Implants, 23(2), 281–288. [PubMed] [Google Scholar]
- Richardson, G. , & Russell, K. A. (2001). Congenitally missing maxillary lateral incisors and orthodontic treatment considerations for the single‐tooth implant. Journal of the Canadian Dental Association, 67(1), 25–28. [PubMed] [Google Scholar]
- Roccuzzo, A. , Imber, J. C. , & Jensen, S. S. (2021). Need for lateral bone augmentation at two narrow‐diameter implants: A prospective, controlled, clinical study. Clinical Oral Implants Research, 32(4), 511–520. 10.1111/clr.13721 [DOI] [PubMed] [Google Scholar]
- Roccuzzo, A. , Jensen, S. S. , Worsaae, N. , & Gotfredsen, K. (2020). Implant‐supported 2‐unit cantilevers compared with single crowns on adjacent implants: A comparative retrospective case series. The Journal of Prosthetic Dentistry, 123(5), 717–723. 10.1016/j.prosdent.2019.04.024 [DOI] [PubMed] [Google Scholar]
- Roccuzzo, M. , Roccuzzo, A. , & Ramanuskaite, A. (2018). Papilla height in relation to the distance between bone crest and interproximal contact point at single‐tooth implants: A systematic review. Clinical Oral Implants Research, 29(Suppl 15), 50–61. 10.1111/clr.13116 [DOI] [PubMed] [Google Scholar]
- Sailer, I. , Muhlemann, S. , Zwahlen, M. , Hammerle, C. H. , & Schneider, D. (2012). Cemented and screw‐retained implant reconstructions: A systematic review of the survival and complication rates. Clinical Oral Implants Research, 23(Suppl 6), 163–201. 10.1111/j.1600-0501.2012.02538.x [DOI] [PubMed] [Google Scholar]
- Santing, H. J. , Raghoebar, G. M. , Vissink, A. , den Hartog, L. , & Meijer, H. J. (2013). Performance of the Straumann bone level implant system for anterior single‐tooth replacements in augmented and nonaugmented sites: A prospective cohort study with 60 consecutive patients. Clinical Oral Implants Research, 24(8), 941–948. 10.1111/j.1600-0501.2012.02486.x [DOI] [PubMed] [Google Scholar]
- Schiegnitz, E. , & Al‐Nawas, B. (2018). Narrow‐diameter implants: A systematic review and meta‐analysis. Clinical Oral Implants Research, 29(Suppl 16), 21–40. 10.1111/clr.13272 [DOI] [PubMed] [Google Scholar]
- Shi, J. Y. , Xu, F. Y. , Zhuang, L. F. , Gu, Y. X. , Qiao, S. C. , & Lai, H. C. (2018). Long‐term outcomes of narrow diameter implants in posterior jaws: A retrospective study with at least 8‐year follow‐up. Clinical Oral Implants Research, 29(1), 76–81. 10.1111/clr.13046 [DOI] [PubMed] [Google Scholar]
- Thoma, D. S. , Cosyn, J. , Fickl, S. , Jensen, S. S. , Jung, R. E. , Raghoebar, G. M. , Rocchietta, I. , Roccuzzo, M. , Sanz, M. , Sanz‐Sánchez, I. , Scarlat, P. , Schou, S. , Stefanini, M. , Strasding, M. , Bertl, K. , working group 2 of the 6th EAO Consensus Conference 2021 , & Bertl, K. (2021). Soft tissue management at implants: Summary and consensus statements of group 2. The 6th EAO consensus conference 2021. Clinical Oral Implants Research, 21(Suppl 21), 174–180. 10.1111/clr.13798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totou, D. , Naka, O. , Mehta, S. B. , & Banerji, S. (2021). Esthetic, mechanical, and biological outcomes of various implant abutments for single‐tooth replacement in the anterior region: A systematic review of the literature. International Journal of Implant Dentistry, 7(1), 85. 10.1186/s40729-021-00370-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vastardis, H. (2000). The genetics of human tooth agenesis: New discoveries for understanding dental anomalies. American Journal of Orthodontics and Dentofacial Orthopedics, 117(6), 650–656. [PubMed] [Google Scholar]
- Wittneben, J. G. , Buser, D. , Salvi, G. E. , Burgin, W. , Hicklin, S. , & Bragger, U. (2014). Complication and failure rates with implant‐supported fixed dental prostheses and single crowns: A 10‐year retrospective study. Clinical Implant Dentistry and Related Research, 16(3), 356–364. 10.1111/cid.12066 [DOI] [PubMed] [Google Scholar]
- Wittneben, J. G. , Joda, T. , Weber, H. P. , & Brägger, U. (2017). Screw retained vs. cement retained implant‐supported fixed dental prosthesis. Periodontology 2000, 73(1), 141–151. 10.1111/prd.12168 [DOI] [PubMed] [Google Scholar]
- Woelber, J. P. , Ratka‐Krueger, P. , Vach, K. , & Frisch, E. (2016). Decementation rates and the peri‐implant tissue status of implant‐supported fixed restorations retained via zinc oxide cement: A retrospective 10–23‐year study. Clinical Implant Dentistry and Related Research, 18(5), 917–925. 10.1111/cid.12372 [DOI] [PubMed] [Google Scholar]
- Zarone, F. , Sorrentino, R. , Vaccaro, F. , & Russo, S. (2006). Prosthetic treatment of maxillary lateral incisor agenesis with osseointegrated implants: A 24–39‐month prospective clinical study. Clinical Oral Implants Research, 17(1), 94–101. 10.1111/j.1600-0501.2005.01188.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
‐The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions


