ABSTRACT
Patients with osteoporosis and chronic kidney disease (CKD) are at increased risk of fracture and associated negative outcomes, including increased mortality. The present post hoc analysis of two randomized, multicenter, phase 3 clinical trials—Fracture Study in Postmenopausal Women with Osteoporosis (FRAME) and Active‐Controlled Fracture Study in Postmenopausal Women with Osteoporosis at High Risk (ARCH)—investigated the efficacy and safety of romosozumab in postmenopausal women with osteoporosis and mild‐to‐moderate CKD. The analysis included data from 7147 patients from FRAME and 4077 from ARCH. Eighty‐one percent of patients from FRAME and 85% from ARCH had mild or moderate reduction in estimated glomerular filtration rate (eGFR) at baseline, and part of this reduction is likely age related. During the 1‐year double‐blind phases of the trials, patients received romosozumab 210 mg sc or placebo monthly in FRAME and romosozumab 210 mg sc monthly or alendronate 70 mg po weekly in ARCH. Bone mineral density (BMD) at the lumbar spine, total hip, and femoral neck and vertebral and nonvertebral fractures were assessed at baseline and month 12. In both trials, the least‐square mean percent change from baseline BMD was significantly greater in the romosozumab groups versus controls across all kidney function categories at month 12. Romosozumab reduced the relative risk of new vertebral fractures at month 12 among patients with eGFR of 30–59, 60–89, and ≥90 mL/min by 72% (95% confidence interval [CI] 14–91; p = 0.017), 70% (40–85; p < 0.001), and 84% (30–96; p = 0.005), respectively, in FRAME versus placebo, and by 51% (5–75; p = 0.04), 19% (−28 to 49; p = 0.39), and 57% (1–81, p = 0.04), respectively, in ARCH versus alendronate. Incidences of adverse events, asymptomatic decreases in serum calcium, and evolution of kidney function during the studies were similar across all baseline kidney function groups. Romosozumab is an effective treatment option for postmenopausal women with osteoporosis and mild‐to‐moderate reduction in kidney function, with a similar safety profile across different levels of kidney function. © 2022 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).
Keywords: ANABOLICS, CHRONIC KIDNEY DISEASE, MENOPAUSE, OSTEOPOROSIS, ROMOSOZUMAB
Introduction
A substantial portion of postmenopausal women have the comorbidities of reduction in kidney function and osteoporosis. The prevalence of chronic kidney disease (CKD) in females ≥65 years in the United States is estimated to be 12% to 16%.( 1 , 2 ) Moderate‐to‐severe reduction in kidney function is present in approximately 85% of women with osteoporosis.( 3 ) CKD predisposes patients to metabolic bone disease that causes quantitative and qualitative changes in bone tissue, leading to increased risk of fracture.( 4 , 5 , 6 ) Results from the Third National Health and Nutrition Examination Survey (NHANES III) indicated a twofold increase in the prevalence of hip fracture among participants with an estimated glomerular filtration rate (eGFR) ≤60 mL/min compared with participants with normal kidney function.( 7 )
Given the increased risk of fracture observed in conjunction with CKD, it is important to evaluate the efficacy and safety of osteoporosis treatments in this patient population.( 8 ) Bisphosphonates are often first‐line therapy for osteoporosis( 9 ) and are generally well tolerated in patients with mild‐to‐moderate reduction in kidney function. However, they are not recommended for patients with severe reduction in kidney function (creatinine clearance <30 mL/min for risedronate and ibandronate; <35 mL/min for alendronate and zoledronic acid).( 6 , 10 , 11 ) Bisphosphonates undergo renal clearance and may accumulate in patients with CKD, theoretically leading to an increase in adverse effects. Clinical trials in osteoporosis have excluded patients with severe reduction in kidney function or end‐stage renal disease (ESRD).( 12 ) In a post hoc analysis, denosumab, an antiresorptive agent that is not cleared by kidneys,( 4 ) was found to reduce fracture risk in women with osteoporosis and impaired kidney function.( 13 ) Patients with severe CKD are at increased risk for developing hypocalcemia while on denosumab.( 14 , 15 ) A limited number of post hoc analyses assessing the use of anabolic agents (teriparatide, abaloparatide) suggest no clinically meaningful difference in bone mineral density (BMD) increase, fracture risk reduction, or treatment‐emergent adverse events (TEAE) between patients with normal or impaired kidney function.( 16 , 17 , 18 )
Romosozumab has been shown to be superior to placebo and oral alendronate in reducing fractures in two large phase 3 clinical trials. In the pivotal Fracture Study in Postmenopausal Women with Osteoporosis (FRAME), 1 year of romosozumab treatment, 210 mg subcutaneously monthly, was compared with placebo.( 19 ) Romosozumab treatment resulted in significantly greater BMD gains of 13.3% at the lumbar spine, 6.9% at total hip, and 5.9% at the femoral neck compared with placebo. Patients treated with romosozumab also had a 73% lower relative risk of new vertebral fractures and a 36% lower relative risk of clinical fractures at 1 year.
In the Active‐Controlled Fracture Study in Postmenopausal Women with Osteoporosis at High Risk (ARCH), 1 year of romosozumab treatment was compared with ≥1 year of alendronate treatment.( 20 ) Compared with alendronate, romosozumab treatment resulted in significantly greater gains in BMD from baseline at the lumbar spine (13.7% versus 5.0%, p < 0.001), total hip (6.2% versus 2.8%, p < 0.001), and femoral neck (4.9% versus 1.7%, p < 0.001). Patients receiving romosozumab for 12 months experienced a significant 37% and 28% reduction in the relative risk of vertebral and clinical fractures, respectively, as well as a 26% reduction in nonvertebral fractures and a 36% reduction in hip fractures compared with alendronate.
We performed a post hoc analysis of 1‐year data from these two studies to determine the efficacy and safety of romosozumab versus control among women with osteoporosis and different levels of kidney function.
Materials and Methods
Study design and patient population
This post hoc analysis was performed using results from the double‐blind portions of the phase 3 FRAME (NCT01575834) and ARCH (NCT01631214) studies (Supplemental Table S1).( 19 , 20 ) The FRAME trial enrolled postmenopausal women with osteoporosis (BMD T‐score ≤ −2.5 at the total hip or femoral neck),( 19 ) whereas the active‐controlled ARCH trial enrolled postmenopausal women with osteoporosis and a fragility fracture.( 20 )
Patients were stratified according to degree of kidney function at baseline, as determined by eGFR. Age‐related reductions in eGFR would be expected in this population. eGFR was calculated using the Modification of Diet in Renal Disease (MDRD) study equation and normalized to body surface area (mL/min/1.73 m2).( 21 ) Kidney function was categorized as: normal (eGFR ≥90 mL/min), mild reduction in kidney function (eGFR 60–89 mL/min), or moderate reduction in kidney function (eGFR 30–59 mL/min).
Patients with severe reduction in kidney function (eGFR 15–29 mL/min) were not included in this efficacy analysis owing to the small number enrolled in the studies. Among the 33 patients enrolled in FRAME and not included in this analysis, 18 met eGFR criteria for severe reduction in kidney function, and baseline eGFR information was unavailable for 15 patients. Although the ARCH protocol called for subjects with eGFR <35 mL/min to be excluded—in line with alendronate label—a small number of patients with eGFR below this threshold were nonetheless enrolled in the study, and these patients were still included in that study based on intent‐to‐treat analysis. Among the 16 patients enrolled in ARCH and not included in this analysis, 11 had severe CKD and 5 had no baseline eGFR information available. Therefore, this post hoc analysis included data from 7147 (99.5%) of the 7180 patients enrolled in FRAME and 4077 (99.6%) of the 4093 patients enrolled in ARCH (Supplemental Fig. S1).
Study outcomes
Efficacy outcomes included percent change in BMD from baseline to month 12 at the lumbar spine, total hip, and femoral neck and incidence of new vertebral fractures at month 12. BMD was measured by dual‐energy X‐ray absorptiometry (DXA) performed at baseline, month 6, and month 12. Vertebral fractures were assessed by lateral radiographs of the spine obtained at baseline, month 6, and month 12 during the double‐blind period in FRAME and at baseline and month 12 during the double‐blind period in ARCH. Vertebral and nonvertebral fracture radiographs were assessed at a central imaging center.
Adverse events (AEs) were reported by individual trial sites. Events related to calcium homeostasis were graded based on the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0.
Statistical methods
BMD analysis included all randomized subjects with a baseline BMD and ≥1 post‐baseline evaluation at or before month 12. Least squares (LS) mean percent change from baseline in BMD at the lumbar spine, total hip, and femoral neck was estimated by analysis of variance (ANCOVA) model, adjusted for treatment, baseline value, machine type, and baseline value‐by‐machine type interaction. In ARCH, BMD results were additionally adjusted for age strata and prevalence of severe vertebral fracture at baseline, whereas in FRAME, results were additionally adjusted for age and prevalent vertebral fracture stratification variables. Analyses of new vertebral fractures included all randomized patients with a baseline and ≥1 post‐baseline evaluation of vertebral fracture at or before month 12. Relative risk of new vertebral fracture was estimated based on the Mantel–Haenszel method, and odds ratio (OR) was calculated based on logistic regression model. New vertebral fracture results were adjusted for age and prevalent vertebral fracture stratification variables in FRAME and adjusted for age strata, baseline total hip BMD T‐score, and presence of severe vertebral fracture at baseline in ARCH. Missing values were imputed by the last observation carried forward (LOCF) method. Safety analysis included all patients who received ≥1 dose of investigational product in the double‐blind period, and results were summarized according to baseline kidney function as groups of normal, mild, and moderate.
Results
Patient population
At baseline, most patients had mild (4939 [69%] in FRAME and 2478 [61%] in ARCH) or moderate (1360 [19%] in FRAME and 993 [24%] in ARCH) decreases in kidney function. Within each trial, baseline and clinical characteristics were similar across treatment groups and kidney function categories (Table 1). Patients in ARCH were approximately 4 to 5 years older than patients in FRAME; thus, the higher percentage of patients in ARCH with a moderate decrease in kidney function is likely due to age‐related reduction in eGFR. The median age of patients with a moderate reduction in kidney function was ≥5 years older than for patients with normal or mildly impaired kidney function for both trials. Baseline BMD was lower and the proportion of patients with prior osteoporotic fracture and prevalent vertebral fracture was greater in ARCH than in FRAME.
Table 1.
Baseline Demographics and Clinical Characteristics in the FRAME and ARCH Studies
| FRAME study | eGFR ≥90 mL/min/1.73 m2 | eGFR 60–89 mL/min/1.73 m2 | eGFR 30–59 mL/min/1.73 m2 | |||
|---|---|---|---|---|---|---|
| Normal kidney function | Mild reduction in kidney function | Moderate reduction in kidney function | ||||
| Placebo | Romosozumab | Placebo | Romosozumab | Placebo | Romosozumab | |
| n = 417 | n = 431 | n = 2531 | n = 2408 | n = 627 | n = 733 | |
| Age, median (range), years | 69 (55–88) | 68 (55–88) | 70 (55–90) | 69 (55–90) | 75 (56–89) | 75 (57–90) |
| Ethnicity, n (%) | ||||||
| Hispanic | 146 (35) | 153 (36) | 997 (39) | 959 (40) | 269 (43) | 306 (42) |
| Non‐Hispanic | 271 (65) | 278 (64) | 1534 (61) | 1449 (60) | 358 (57) | 427 (58) |
| BMI (kg/m2), mean (SD) | 23.4 (4.0) | 23.5 (4.0) | 24.7 (4.3) | 24.5 (4.2) | 25.7 (4.8) | 25.8 (4.5) |
| eGFR (mL/min/1.73 m2), mean (SD) | 102.7 (9.5) | 102.7 (9.3) | 73.1 (8.2) | 73.5 (8.2) | 50.4 (6.5) | 50.2 (6.5) |
| BMD T‐score, mean (SD) | ||||||
| Lumbar spine | −2.8 (1.0) | −2.9 (1.0) | −2.8 (1.0) | −2.8 (1.0) | −2.5 (1.1) | −2.5 (1.1) |
| Total hip | −2.5 (0.5) | −2.6 (0.4) | −2.5 (0.5) | −2.5 (0.5) | −2.5 (0.5) | −2.5 (0.5) |
| Femoral neck | −2.8 (0.3) | −2.8 (0.3) | −2.7 (0.3) | −2.8 (0.3) | −2.8 (0.3) | −2.8 (0.3) |
| Prevalent vertebral fractures, n (%) | 65 (16) | 83 (19) | 470 (19) | 440 (18) | 107 (17) | 145 (20) |
| Prior osteoporotic fracture, n (%) a | 134 (32) | 151 (35) | 916 (36) | 832 (35) | 201 (32) | 281 (38) |
| Severe vertebral fracture, n (%) | 0 (0.0) | 0 (0.0) | 3 (0.1) | 0 (0.0) | 0 (0.0) | 1 (0.1) |
| FRAX score, median (IQR) b | n = 416 | n = 430 | n = 2528 | n = 2402 | n = 627 | n = 731 |
| 11.4 | 11.3 | 10.9 | 10.6 | 10.5 | 11.3 | |
| (7.5, 16.5) | (7.5, 17.4) | (7.1, 16.7) | (7.0, 16.8) | (6.9, 16.4) | (7.2, 17.4) | |
| Median (IQR) 25‐hydroxyvitamin D, ng/mL | 28.0 | 28.0 | 27.1 | 27.3 | 27.2 | 26.6 |
| (24.2, 34.4) | (24.0, 32.9) | (23.3, 32.0) | (23.6, 32.7) | (23.6, 32.0) | (23.2, 31.6) | |
| ARCH Study | eGFR ≥90 mL/min/1.73 m2 | eGFR 60–89 mL/min/1.73 m2 | eGFR 30–59 mL/min/1.73 m2 | |||
|---|---|---|---|---|---|---|
| Alendronate | Romosozumab | Alendronate | Romosozumab | Alendronate | Romosozumab | |
| n = 336 | n = 270 | n = 1217 | n = 1261 | n = 485 | n = 508 | |
| Age, years | ||||||
| median (range) | 73 (55–90) | 74 (55–89) | 74 (55–90) | 74 (55–90) | 79 (56–89) | 79 (56–90) |
| Ethnicity, n (%) | ||||||
| Hispanic | 129 (38) | 102 (38) | 392 (32) | 386 (31) | 140 (29) | 140 (28) |
| Non‐Hispanic | 207 (62) | 168 (62) | 825 (68) | 875 (69) | 345 (71) | 368 (72) |
| BMI (kg/m2), mean (SD) | 24.8 (4.4) | 25.1 (4.8) | 25.3 (4.4) | 25.3 (4.3) | 26.0 (4.5) | 26.0 (4.5) |
| eGFR (mL/min/1.73 m2), mean (SD) | 103.0 (10.4) | 103.6 (13.2) | 73.0 (8.1) | 72.9 (7.9) | 50.7 (6.8) | 49.5 (6.9) |
| BMD T‐score, mean (SD) | ||||||
| Lumbar spine | −3.2 (1.3) | −3.2 (1.3) | −3.0 (1.2) | −3.0 (1.2) | −2.7 (1.3) | −2.7 (1.3) |
| Total hip | −2.9 (0.7) | −2.9 (0.7) | −2.8 (0.6) | −2.7 (0.7) | −2.8 (0.7) | −2.8 (0.7) |
| Femoral neck | −3.0 (0.6) | −3.0 (0.5) | −2.9 (0.5) | −2.9 (0.5) | −2.9 (0.5) | −2.9 (0.5) |
| Prevalent vertebral fractures, n (%) | 317 (94) | 256 (95) | 1172 (96) | 1213 (96) | 467 (96) | 494 (97) |
| Prior osteoporotic fracture, n (%) a | 333 (99) | 263 (97) | 1207 (99) | 1248 (99) | 482 (99) | 504 (99) |
| Severe vertebral fracture, n (%) | 219 (65) | 181 (67) | 784 (64) | 826 (66) | 313 (65) | 360 (71) |
| FRAX score, median (IQR) b | n = 335 | n = 269 | n = 1214 | n = 1259 | n = 483 | n = 508 |
| 18.8 | 17.9 | 17.3 | 17.8 | 18.2 | 18.7 | |
| (13.3, 26.2) | (13.7, 24.2) | (12.3, 24.3) | (12.3, 24.9) | (12.7, 25.4) | (13.4, 27.1) | |
| Median (IQR) 25‐hydroxyvitamin D, ng/mL | 28.3 | 29.4 | 27.4 | 28.2 | 28.0 | 28.4 |
| (24.0, 35.6) | (24.4, 38.0) | (23.6, 33.9) | (24.0, 34.8) | (24.4, 34.4) | (24.0, 34.9) | |
eGFR = estimated glomerular filtration rate; BMI = body mass index; BMD = bone mineral density; FRAX = Fracture Risk Assessment Tool version 3.9; IQR = interquartile range; SD = standard deviation.
At or after age 45 years.
Indicates 10‐year probability of major osteoporotic fracture, expressed as percentage and calculated with BMD. FRAX version 3.9 includes adjustment for country‐based differences in fracture risk.
Change from baseline BMD
In both ARCH and FRAME, significant BMD increases were observed across all kidney function categories and across each of three different anatomical BMD samples after 1 year of romosozumab treatment (Fig. 1). Similar trends were found for both least‐square means (LSM) percent differences in BMD from baseline and absolute LSM differences in BMD (data not shown).
Fig. 1.
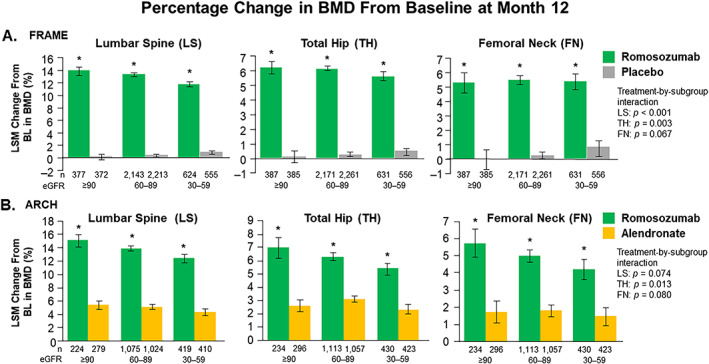
Percent change in bone mineral density (BMD) from baseline (BL) at month 12 by estimated glomerular filtration rate (eGFR) subgroup. *Denotes statistical significance. Error bars = 95% confidence intervals (CI). LSM = least‐square means.
In FRAME, the between‐group difference in LS mean (95% confidence interval [CI]) change from baseline BMD at month 12 at the lumbar spine was 13.7% (13.1–14.4; p < 0.001) in patients with normal kidney function, 13.0% (12.7–13.3; p < 0.001) in patients with mild CKD, and 10.9% (10.4–11.4; p < 0.001) in patients with moderate CKD. LS mean (95% CI) differences between romosozumab and placebo in total hip BMD were 6.1% (5.6–6.6; p < 0.001), 5.9% (5.7–6.1; p < 0.001), and 5.2% (4.7–5.6; p < 0.001) in patients with normal kidney function, mild CKD, and moderate CKD, respectively. LS mean (95% CI) differences between treatment groups in femoral neck BMD were 5.3% (4.6–5.9; p < 0.001), 5.3% (5.0–5.5; p < 0.001), and 4.6% (4.1–5.1; p < 0.001) in patients with normal kidney function, mild CKD, and moderate CKD, respectively. The BMD increase at the femoral neck was similar for patients with normal kidney function compared with those with CKD; however, the BMD increase appears to be slightly different at the lumbar spine (subgroup interaction p < 0.001) and total hip (subgroup interaction p = 0.003) according to kidney function status.
In ARCH, BMD LS mean (95% CI) differences between treatment groups at month 12 at the lumbar spine were 9.6% (8.5–10.7; p < 0.001) in patients with normal kidney function, 8.8% (8.3–9.3; p < 0.001) mild CKD, and 8.1% (7.3–8.9; p < 0.001) moderate CKD; for total hip BMD, they were 4.3% (3.5–5.2; p < 0.001), 3.2% (2.9–3.6; p < 0.001), and 3.0% (2.5–3.6; p < 0.001), respectively; differences at the femoral neck were 4.0% (3.1–5.0; p < 0.001), 3.2% (2.8–3.6; p < 0.001), and 2.7% (2.1–3.4; p < 0.001) in patients with normal kidney function, mild CKD, and moderate CKD, respectively. The BMD increases at the lumbar spine and femoral neck were similar among women with normal kidney function compared with those with CKD but varied slightly at the total hip according to kidney function status (subgroup interaction p = 0.013); these results differ from those summarized above for the FRAME study.
Incidence of new vertebral fracture
In FRAME, patients treated with romosozumab had significantly lower risk for new vertebral fractures compared with placebo regardless of kidney function status (Fig. 2A ). The relative risk of new vertebral fractures at month 12 diminished by 84% (95% CI 30–96, p = 0.005), 70% (95% CI 40–85, p < 0.001), and 72% (95% CI 14–91, p = 0.017), in patients with normal kidney function, mild CKD, and moderate CKD, respectively, compared with placebo. There was no treatment‐by‐subgroup interaction effect (p = 0.75).
Fig. 2.
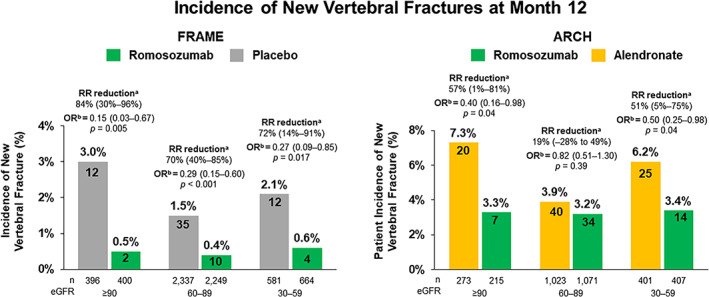
Incidence of new vertebral fractures at month 12 by eGFR subgroup. Relative risk (RR) reduction was calculated as 1 ‐ ratio risk x 100 and expressed as a precentage with 95% confidence intervals (CIs) in parentheses. Analyses included all randomized patients with a baseline and ≥1 postbaseline radiograph. aBased on Mantel–Haenszel method. bBased on logistic‐regression model. FRAME results adjusted for age and prevalent vertebral fracture stratification variables; ARCH results adjusted for age strata, baseline total hip BMD T‐score, and presence of severe vertebral fracture at baseline; p value based on score test. eGFR = estimated glomerular filtration rate; RR = relative risk; OR = odds ratio.
In ARCH, the relative risk of new vertebral fractures at month 12 was significantly reduced in patients with normal kidney function and those with moderate CKD who were treated with romosozumab compared with patients treated with alendronate (relative risk reduction: 57% [95% CI 1–81; p = 0.04] and 51% [95% CI 5–75, p = 0.04], respectively). Patients with mild CKD had a 19% (95% CI −28 to 49) relative risk reduction in new vertebral fractures at month 12 (p = 0.39; Fig. 2B ). There was no treatment‐by‐subgroup interaction effect (p = 0.28).
Incidence of nonvertebral fractures
The risk of nonvertebral fractures was reduced by 26% at month 12 in both FRAME and ARCH (p = 0.08 and p = 0.06, respectively). Subgroup analyses showed a consistent treatment effect across eGFR groups as evidenced by overlapping 95% CIs and lack of treatment‐by‐subgroup interactions (Supplemental Table S2).
Incidence of adverse events
In both studies, the incidences of treatment‐emergent adverse events (TEAEs) and serious AEs (SAEs) were comparable in both treatment groups within and across eGFR categories (Table 2). The proportion of patients who discontinued treatment due to a TEAE ranged from 2.2% to 3.8% and was also comparable across all eGFR categories in both studies.
Table 2.
Adverse Events Through Month 12 (Safety Population a )
| FRAME study | Baseline eGFR (mL/min/1.73 m2) | |||||
|---|---|---|---|---|---|---|
| eGFR ≥90 | eGFR 60–89 | eGFR 30–59 | ||||
| Placebo | Romosozumab | Placebo | Romosozumab | Placebo | Romosozumab | |
| n = 416 | n = 431 | n = 2526 | n = 2406 | n = 625 | n = 734 | |
| AEs, n (%) | ||||||
| TEAEs | 332 (79.8) | 353 (81.9) | 2015 (79.8) | 1881 (78.2) | 507 (81.1) | 569 (77.5) |
| SAEs | 24 (5.8) | 38 (8.8) | 220 (8.7) | 211 (8.8) | 68 (10.9) | 93 (12.7) |
| TEAEs leading to treatment discontinuation | 9 (2.2) | 16 (3.7) | 63 (2.5) | 64 (2.7) | 22 (3.5) | 26 (3.5) |
| Fatal AEs | 0 (0.0) | 1 (0.2) | 19 (0.8) | 18 (0.7) | 5 (0.8) | 8 (1.1) |
| Hypocalcemia | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (<0.1) | 0 (0.0) | 0 (0.0) |
| Hypersensitivity | 34 (8.2) | 37 (8.6) | 170 (6.7) | 169 (7.0) | 41 (6.6) | 36 (4.9) |
| Injection‐site reactions | 14 (3.4) | 22 (5.1) | 73 (2.9) | 137 (5.7) | 15 (2.4) | 29 (4.0) |
| Malignancy | 2 (0.5) | 4 (0.9) | 43 (1.7) | 33 (1.4) | 10 (1.6) | 12 (1.6) |
| Hyperostosis | 5 (1.2) | 5 (1.2) | 19 (0.8) | 11 (0.5) | 4 (0.6) | 2 (0.3) |
| Osteoarthritis | 34 (8.2) | 41 (9.5) | 216 (8.6) | 185 (7.7) | 66 (10.6) | 57 (7.8) |
| Positively adjudicated CV events b | 1 (0.2) | 1 (0.2) | 18 (0.7) | 16 (0.7) | 10 (1.6) | 12 (1.6) |
| ARCH study | Baseline eGFR (mL/min/1.73 m2) | |||||
|---|---|---|---|---|---|---|
| eGFR ≥90 | eGFR 60–89 | eGFR 30–59 | ||||
| Alendronate | Romosozumab | Alendronate | Romosozumab | Alendronate | Romosozumab | |
| n = 333 | n = 267 | n = 1195 | n = 1259 | n = 479 | n = 509 | |
| AEs, n (%) | ||||||
| TEAEs | 269 (80.8) | 215 (80.5) | 936 (78.3) | 941 (74.7) | 374 (78.1) | 385 (75.6) |
| SAEs | 34 (10.2) | 37 (13.9) | 166 (13.9) | 149 (11.8) | 75 (15.7) | 76 (14.9) |
| TEAEs leading to treatment discontinuation | 9 (2.7) | 10 (3.7) | 37 (3.1) | 47 (3.7) | 18 (3.8) | 14 (2.8) |
| Fatal AEs | 1 (0.3) | 6 (2.2) | 12 (1.0) | 12 (1.0) | 9 (1.9) | 12 (2.4) |
| Hypocalcemia | 1 (0.3) | 0 (0.0) | 0 (0.0) | 1 (<0.1) | 0 (0.0) | 0 (0.0) |
| Hypersensitivity | 17 (5.1) | 14 (5.2) | 71 (5.9) | 82 (6.5) | 28 (5.8) | 26 (5.1) |
| Injection‐site reactions | 15 (4.5) | 15 (5.6) | 28 (2.3) | 55 (4.4) | 10 (2.1) | 20 (3.9) |
| Malignancy | 4 (1.2) | 6 (2.2) | 16 (1.3) | 16 (1.3) | 8 (1.7) | 10 (2.0) |
| Hyperostosis | 2 (0.6) | 0 (0.0) | 5 (0.4) | 1 (<0.1) | 5 (1.0) | 1 (0.2) |
| Osteoarthritis | 30 (9.0) | 25 (9.4) | 85 (7.1) | 74 (5.9) | 33 (6.9) | 39 (7.7) |
| Positively adjudicated CV events b | 2 (0.6) | 5 (1.9) | 12 (1.0) | 22 (1.7) | 8 (1.7) | 14 (2.8) |
eGFR = estimated glomerular filtration rate; AEs = adverse events; TEAEs = treatment‐emergent AEs; SAEs = serious AEs; CV = cardiovascular.
Includes all patients who received ≥1 dose of study treatment.
Defined as CV events leading to death, serious myocardial infarction, or stroke.
Investigator‐reported mild‐to‐moderate AEs of hypocalcemia occurred in 3 patients across both trials. One patient with mild CKD receiving romosozumab in FRAME had a moderate (grade 2) common terminology criteria for adverse events (CTCAE) TEAE of hypocalcemia that occurred in the setting of chronic obstructive pulmonary disease, congestive heart failure, fall, and acute encephalopathy where calcium supplementation was withheld during the patient's hospitalization. In ARCH, one patient with eGFR ≥90 receiving alendronate and one patient with eGFR 60–89 receiving romosozumab developed hypocalcemia. All events were non‐serious, mild, and resolved with increase of oral calcium supplementation. Laboratory analyses revealed that 19 patients (14 in the romosozumab and 5 in the placebo groups) in FRAME and 5 patients (all in the alendronate group) in ARCH had decreases in albumin‐adjusted serum calcium levels. No AEs of hypocalcemia were reported by the investigator in association with these findings. All these findings were of mild (<lower limit of normal–8.0 mg/dL) or moderate (7.0–<8.0 mg/dL) severity based on CTCAE grading, except for one patient receiving placebo in FRAME who had a severe (grade 4) low albumin‐corrected serum calcium level that resolved without treatment.
In FRAME, the incidence of positively adjudicated cardiovascular (CV) events, defined as CV events leading to death, serious myocardial infarction, or stroke, was similar across patients with different degrees of reduced kidney function at baseline. Events were reported in 0.2%, 0.7%, and 1.6% of patients with normal kidney function, mild CKD, and moderate CKD, respectively, in both the romosozumab and placebo arms. In ARCH, the incidence of positively adjudicated CV events among romosozumab‐treated patients was 1.9%, 1.7%, and 2.8% in patients with normal kidney function, mild CKD, and moderate CKD, respectively, compared with 0.6%, 1.0%, and 1.7% of patients receiving alendronate (Table 2).
Injection site reactions occurred more frequently among romosozumab‐treated patients, but the incidence was comparable across eGFR categories. No differences were observed between treatment groups in the proportion of patients reporting other AEs of interest nor were there differences across eGFR groups (Table 2).
Change in kidney function
Kidney function remained stable during the 12‐month treatment period in both studies. Overall, 72% to 85% of patients with mild‐to‐moderate CKD at baseline remained in the same eGFR category at month 12. Among patients with normal kidney function at baseline, 30% to 42% changed to mild CKD similarly in both treatment groups of each trial (Fig. 3). The mean (SD) change from baseline eGFR at month 12 was −0.7 (10.3) mL/min/1.73m2 and −1.2 (10.1) mL/min/1.73 m2 in romosozumab‐ and placebo‐treated patients in FRAME, and 0.7 (11.8) mL/min/1.73 m2 and 0.1 (11.9) mL/min/1.73m2 in romosozumab‐ and alendronate‐treated patients in ARCH.
Fig. 3.
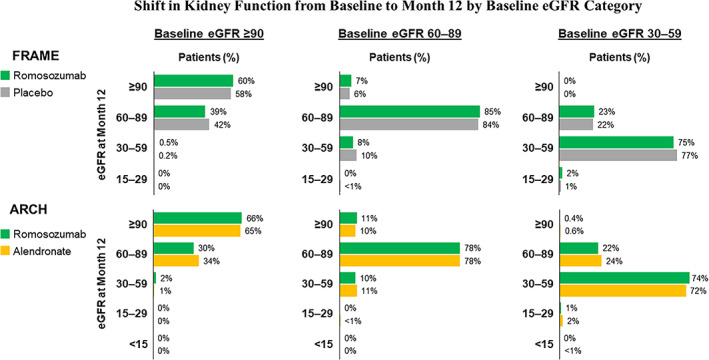
Shift in kidney function from baseline to month 12 by baseline estimated glomerular filtration rate (eGFR) category. Analysis includes patients with baseline eGFR ≥30 mL/min/1.73 m2.
Patients with severe reduction in kidney function
Twenty‐nine patients enrolled in FRAME and ARCH met the criteria for severe CKD and were not included in the overall analysis due to the small sample size. No TEAEs of hypocalcemia were reported in this subgroup of patients in either study. In ARCH, there were no positively adjudicated CV events in patients with eGFR <30 mL/min (n = 11) in any treatment group. In FRAME, among patients with eGFR <30 mL/min (n = 18), one patient in the romosozumab group had a positively adjudicated CV event. AE data were overall comparable among treatment groups, although the sample size was too low to enable meaningful conclusions.
Discussion
There is an urgent need to effectively treat osteoporosis in patients with CKD, as patients with these combined comorbidities have more than double the risk for hip fracture than the general population, and mortality rates after fracture are threefold higher for patients with CKD compared with age‐matched individuals.( 8 ) The evolving paradigm for osteoporosis treatment in the setting of CKD requires large‐scale safety data from studies on emerging treatments for patients at different levels of CKD function.( 8 , 12 )
This post hoc analysis demonstrated that, among patients with CKD stages 1 to 3, romosozumab 210 mg, administered subcutaneously once monthly for 12 months, is an effective treatment for osteoporosis compared with placebo or alendronate. Significant gains in BMD at the lumbar spine, total hip, and femoral neck were achieved with romosozumab versus alendronate or placebo across three levels of kidney function, ranging from normal to moderate reduction in function. Although data were provided for all three anatomical sites, guidelines recommend use of lumbar spine and total hip BMD for diagnosis and to guide treatment decisions.( 10 , 11 ) In both the FRAME and ARCH total study populations, clinically meaningful increases in BMD at the lumbar spine and total hip were observed in patients treated with romosozumab versus either placebo or alendronate.
In FRAME, the relative risk of new vertebral fractures significantly decreased with romosozumab compared with placebo with similar extent of reduction in all eGFR subgroups. In ARCH, significant reductions in the relative risk of new vertebral fracture were observed in romosozumab‐treated patients with moderately impaired or normal kidney function compared with alendronate‐treated patients. However, the reduction found in patients with mild CKD appeared to be less and not significant compared with alendronate. The underlying reasons for the fluctuation in fracture rate among CKD groups receiving alendronate is unknown. A secondary analysis of postmenopausal women enrolled in the placebo‐controlled Fracture Intervention Trial (FIT) found fracture risk reduction to be consistent in patients with and without reduced kidney function (eGFR <45 mL/min) treated with alendronate.( 22 ) Notably, in our analysis, the rate of vertebral fracture was lowest among alendronate‐treated patients with a mild reduction in kidney function and may have prevented detecting a significant difference between groups.
Vertebral fractures are associated with an increased risk of mortality.( 23 , 24 ) The Dubbo Osteoporosis Epidemiology study found that the standardized mortality rate was elevated in men and women diagnosed with a vertebral fracture, particularly among patients 60–74 years of age (standardized mortality ratio [SMR] 3.77; 95% CI 2.45–5.81 in women and 4.19; 95% CI 2.42–7.27 in men).( 23 ) The clinical significance of these findings is of importance because bone health in the setting of CKD is complex and the effect of treatments on BMD may differ in this population.( 4 ) Thus, it is reassuring to find effectiveness is maintained across various levels of kidney function. The reductions in nonvertebral fractures observed at 12 months were not statistically significant. However, the relative risk of nonvertebral fracture was reduced significantly by 19% at the later time of the primary analysis (median time of 33 months) in ARCH.( 20 )
The safety profile of romosozumab was similar across different levels of kidney function. Monoclonal antibodies are large molecules not suitable for glomerular filtration such that elimination occurs via proteolysis by the liver or reticuloendothelial system.( 25 , 26 ) Thus, renal elimination of romosozumab is minimal. This is supported by results from a phase 1 study in subjects with CKD stage 4/stage 5 requiring hemodialysis, which showed that renal impairment has no clinically significant effect on romosozumab pharmacokinetics.( 27 ) In ARCH, the incidence of positively adjudicated CV events was higher in romosozumab than in alendronate‐treated patients.( 20 ) In this post hoc analysis, this numeric imbalance between treatment groups was not associated with baseline kidney function (p = 0.92 treatment‐by‐subgroup interaction).
One limitation of this analysis is that classification of kidney function reduction was determined using the MDRD equation for eGFR, and using calculations to estimate GFR can potentially result in misclassification of kidney function categories,( 28 , 29 ) especially in older people.
We did, however, undertake a preliminary subanalysis of ARCH and FRAME patients in the CKD stage 3a and 3b subgroups. The median eGFR values at baseline for ARCH CKD stage 3a and 3b patients, respectively, were 40.3 and 53.8 mL/min/1.73 m2 for the alendronate treatment group and 40.0 and 53.9 mL/min/1.73 m2 for the romosozumab treatment group. The median eGFR values at baseline for FRAME CKD stage 3a and 3b patients, respectively, were 40.6 and 53.8 mL/min/1.73 m2 for the placebo treatment group and 41.1 and 53.5 mL/min/1.73 m2 for the romosozumab treatment group. The 95% CIs for LSM difference in BMD between treatment groups overlapped for the FRAME and ARCH stage 3a and 3b subgroups at the lumbar spine, total hip, and femoral neck. Safety profiles were overall similar between treatment groups for both the stage 3a and 3b subgroups in both studies. In ARCH, the treatment‐emergent SAE and fatal AE percentages were numerically greater for the stage 3b subgroup compared with stage 3a for both the alendronate and romosozumab treatment groups. Similarly, in FRAME, the treatment‐emergent SAE and fatal AE percentages were numerically greater for patients with stage 3b versus stage 3a for both the placebo and romosozumab treatment groups.
In clinical practice, it has been suggested eGFR be complemented with other objective findings (eg, urinary albumin) to improve kidney function assessment and better predict the risk for progression to ESRD.( 30 ) There were too few patients enrolled in FRAME and ARCH with a severe reduction in kidney function to permit assessment in this subgroup. Finally, the limitations of subgroup and post hoc analyses have been well described and should be taken into consideration.( 31 )
The results of this analysis suggest that romosozumab is an effective treatment for postmenopausal women with osteoporosis and mild‐to‐moderate reduction in kidney function. Romosozumab did not impact kidney function nor was it associated with an increase in AEs in this population. Additional studies are needed to assess efficacy in patients with severe reduction of kidney function.
Disclosures
PDM has received consulting and advisory fees from Amgen and Radius Health and has received grants from Amgen, Radius Health, and Ultragenyx. JDA is a consultant and speaker for Amgen and has conducted clinical trials for Amgen and Radius Health. B‐HA has received speaking and consultation fees from Eli Lilly and Amgen. AMC has received consulting fees from Amgen and Ipsen, and research grants to institution from Amgen, Ipsen, Regeneron, and NovoNordisk Foundation. AAC, MO, NRS, ZW, and ZY are employees and stockholders of Amgen; EG has received consultancy, speaker, and/or travel fees from Alexion, Amgen, Sandoz, Takeda, and UCB Pharma; BLL has served as advisor for Amgen, UCB Pharma, Eli Lilly, Gedeon‐Richter, and Gilead and has received research grants from Amgen and Novo Nordisk. AM has received consulting fees from Amgen and Teijin Pharma. IRR has received speaking and consultancy fees from Novartis and Sandoz. MV is an employee and stockholder of UCB Pharma.
Authors’ Roles
All authors participated in the interpretation of analysis data and drafting of the manuscript, and all authors provided final approval for submission of the manuscript for publication.
Supporting information
Appendix S1.
Acknowledgments
This study was funded by Amgen Inc., Astellas Pharma Inc., and UCB, Inc. The funding source was involved in the study design and statistical analysis of the data.
Writing and editorial assistance was provided by Alexandra Stirling, PharmD, and Kathryn Miles, PhD, of BioScience Communications, funded by Amgen Inc., and Lisa Humphries, PhD, of Amgen.
Data Availability Statement
Qualified researchers may request data from Amgen clinical studies. Complete details are available at the following: https://wwwext.amgen.com/science/clinical-trials/clinical-data-transparency-practices/clinical-trial-data-sharing-request/
References
- 1. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases . Kidney disease statistics for the United States. Available at: https://www.niddk.nih.gov/health-information/health-statistics/kidney-disease. Accessed April 1, 2020.
- 2. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases . 2019. USRDS annual data report: epidemiology of kidney disease in the United States. Available at: https://www.usrds.org/media/2371/2019-executive-summary.pdf. Accessed April 1, 2020.
- 3. Klawansky S, Komaroff E, Cavanaugh PF Jr, Mitchell DY, Gordon MJ. Relationship between age, renal function and bone mineral density in the US population. Osteoporos Int. 2003;14(7):570‐573. [DOI] [PubMed] [Google Scholar]
- 4. Bover J, Bailone L, Lopez‐Baez V, et al. Osteoporosis, bone mineral density and CKD‐MBD: treatment considerations. J Nephrol. 2017;30(5):677‐687. [DOI] [PubMed] [Google Scholar]
- 5. Chen H, Lips P, Vervloet MG, van Schoor M, de Jongh RT. Association of renal function with bone mineral density and fracture risk in the longitudinal aging study Amsterdam. Osteoporos Int. 2018;29(9):2129‐2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miller PD. Fragility fractures in chronic kidney disease: an opinion‐based approach. Cleveland Clinic J Med. 2009;76(12):715‐723. [DOI] [PubMed] [Google Scholar]
- 7. Nikolas TL, McMahon DJ, Shane E. Relationship between moderate to severe kidney disease and hip fracture in the United States. J Am Soc Nephrol. 2006;17(11):3223‐3232. [DOI] [PubMed] [Google Scholar]
- 8. Khairallah P, Nickolas TL. Management of osteoporosis in CKD. Clin J Am Soc Nephrol. 2018;13(6):962‐969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shoback D, Rosen CJ, Black DM, Cheun AM, Murad MH, Eastell R. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society guideline update. J Clin Endocrinol Metab. 2020;105(3):587‐594. [DOI] [PubMed] [Google Scholar]
- 10. Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists/American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis – 2020 update. Endocr Pract. 2020;26(5):564‐570. [DOI] [PubMed] [Google Scholar]
- 11. Eastell R, Rosen CJ, Black DM, Cheung AM, Murah MH, Shoback D. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2019;104(5):1595‐1622. [DOI] [PubMed] [Google Scholar]
- 12. Nitta K, Yajima A, Tsuchiya K. Management of osteoporosis in chronic kidney disease. Intern Med. 2017;56(24):3271‐3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jamal SA, Ljunggren Ö, Stehman‐Breen C, et al. Effects of denosumab on fracture and bone mineral density by level of kidney function. J Bone Miner Res. 2011;26(8):1829‐1839. [DOI] [PubMed] [Google Scholar]
- 14. Jalleh R, Basu G, LeLeu R, Jesudason S. Denosumab‐induced severe hypocalcaemia in chronic kidney disease. Case Rep Nephrol. 2018;2018:7384763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Block GA, Bone HG, Fang L, Lee E, Padhi D. A single‐dose study of denosumab in patients with various degrees of renal impairment. J Bone Miner Res. 2012;27(7):1471‐1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bilezikian JP, Hattersley G, Mitlak BH, et al. Abaloparatide in patients with mild or moderate renal impairment: results from the ACTIVE phase 3 trial. Curr Med Res Opin. 2019;35(12):2097‐2102. [DOI] [PubMed] [Google Scholar]
- 17. Miller PD, Schwartz EN, Chen P, Misurski DA, Krege JH. Teriparatide in postmenopausal women with osteoporosis and mild or moderate renal impairment. Osteoporos Int. 2007;18(1):59‐68. [DOI] [PubMed] [Google Scholar]
- 18. Nishikawa A, Yoshiki F, Taketsuna M, Kajimoto K, Enomoto H. Safety and effectiveness of daily teriparatide for osteoporosis in patients with severe stages of chronic kidney disease: post hoc analysis of a postmarketing observational study. Clin Interv Aging. 2016;11:1653‐1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375(16):1532‐1543. [DOI] [PubMed] [Google Scholar]
- 20. Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377(15):1417‐1427. [DOI] [PubMed] [Google Scholar]
- 21. Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247‐254. [DOI] [PubMed] [Google Scholar]
- 22. Jamal SA, Bauer DC, Ensrud KE, et al. Alendronate treatment in women with normal to severely impaired renal function: an analysis of the Fracture Intervention Trial. J Bone Miner Res. 2007;22(4):503‐508. [DOI] [PubMed] [Google Scholar]
- 23. Bluic D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA, Center JR. Mortality risk associated with low‐trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301(5):513‐521. [DOI] [PubMed] [Google Scholar]
- 24. Cauley JA. Public health impact of osteoporosis. J Gerontol. 2013;68(10):1243‐1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lewiecki EM. Sclerostin: a novel target for intervention in the treatment of osteoporosis. Discov Med. 2011;12(65):263‐273. [PubMed] [Google Scholar]
- 26. Lim SY, Bolster MB. Profile of romosozumab and its potential in the management of osteoporosis. Drug Des Devel Ther. 2017;11:1221‐1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Evenity [package insert] . Thousand Oaks, CA: Amgen, Inc., 2019.
- 28. Alani A, Malhotra D, Rondon‐Berrios H, et al. Establishing the presence or absence of chronic kidney disease: uses and limitations of formulas estimating the glomerular filtration rate. World J Methodol. 2017;7(3):73‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Botev R, Mallié J‐P, Wetzels JFM, Couchoud C, Shück O. The clinician and estimation of glomerular filtration rate by creatinine‐based formulas: current limitations and quo vadis. Clin J Am Soc Nephrol. 2011;6(4):937‐950. [DOI] [PubMed] [Google Scholar]
- 30. Hallan SI, Ritz E, Lydersen S, Romundstad S, Kvenild K, Orth SR. Combining GFR and albuminuria to classify CKD improves prediction of ESRD. J Am Soc Nephrol. 2009;20(5):1069‐1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rothwell PM. Treating individuals 2. Subgroup analysis in randomised controlled trials: importance, indications and interpretation. Lancet. 2005;365(9454):176‐186. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.
Data Availability Statement
Qualified researchers may request data from Amgen clinical studies. Complete details are available at the following: https://wwwext.amgen.com/science/clinical-trials/clinical-data-transparency-practices/clinical-trial-data-sharing-request/


