Abstract
T cell activation and function depend on Ca2+ signals mediated by store-operated Ca2+ entry (SOCE) through Ca2+ release–activated Ca2+ (CRAC) channels formed by ORAI1 proteins. We here investigated how SOCE controls T cell function in pulmonary inflammation during a T helper 1 (TH1) cell–mediated response to influenza A virus (IAV) infection and TH2 cell–mediated allergic airway inflammation. T cell–specific deletion of Orai1 did not exacerbate pulmonary inflammation and viral burdens following IAV infection but protected mice from house dust mite–induced allergic airway inflammation. ORAI1 controlled the expression of genes including p53 and E2F transcription factors that regulate the cell cycle in TH2 cells in response to allergen stimulation and the expression of transcription factors and cytokines that regulate TH2 cell function. Systemic application of a CRAC channel blocker suppressed allergic airway inflammation without compromising immunity to IAV infection, suggesting that inhibition of SOCE is a potential treatment for allergic airway disease.
Deletion of ORAI1 in T cells suppresses allergic airway inflammation but preserves antiviral immunity to influenza infection.
INTRODUCTION
T cell activation depends on Ca2+ signals following T cell receptor (TCR) engagement. Ca2+ signals regulate many aspects of T cell function including gene expression, cytokine production, metabolism, and proliferation (1, 2). Ca2+ influx after antigen binding to the TCR is mediated by the highly Ca2+-selective Ca2+ release–activated Ca2+ (CRAC) channel in the plasma membrane (3, 4). In T cells, CRAC channels are formed by ORAI1 and its homolog ORAI2, which are tetraspanning membrane proteins that assemble as hexameric complexes and form the pore of the channel (5). CRAC channels are bound and opened by stromal interaction molecule 1 (STIM1) and STIM2, two single-pass membrane protein in the endoplasmic reticulum (ER). The activation of STIM proteins follows the release of Ca2+ from the ER through inositol 1,4,5-trisphosphate (IP3) receptors, which are Ca2+-permeable channels in the ER membrane that open upon TCR stimulation, activation of phospholipase C–γ, and production of IP3. Because the activation of STIM and ORAI proteins is regulated by the Ca2+ concentration in the ER, this form of Ca2+ influx is called store-operated Ca2+ entry (SOCE). Complete lack of SOCE due to inherited mutations in ORAI1 and STIM1 genes causes combined immunodeficiency (CID) defined by recurrent infections with viral, bacterial, and fungal pathogens in human patients (6). In mice, combined deletion of both Orai1 and Orai2 or Stim1 and Stim2 genes in T cells is necessary to abolish SOCE and to impair immunity to infection with viral and fungal pathogens (7–9). T cell–specific deletion of Orai1 or Stim1 alone, by contrast, reduces SOCE only partially and does not impair immunity to infection with lymphocytic choriomeningitis virus (LCMV) (8, 10). Deletion of Orai1 or Stim1 in T cells protects mice from T cell–mediated autoimmunity in rodent models of inflammatory bowel disease (IBD), experimental autoimmune encephalomyelitis (EAE), graft-versus-host disease (GvHD), and psoriasiform skin inflammation (8, 11–16). Protection is due to impaired production of T helper 1 (TH1) and TH17 cytokines including interferon-γ (IFN-γ), interleukin-17A (IL-17A), and granulocyte-macrophage colony-stimulating factor; attenuated expression of the TH17 cell–specific transcription factor RAR-related orphan receptor gamma t (RORγt); and defects in the metabolic function of TH17 cells. The role of SOCE in the differentiation and function of TH2 cells is not well understood. The Ca2+-regulated transcription factor nuclear factor of activated T cells (NFAT), however, is well known to control the expression of the TH2 cytokines IL-4, IL-5, and IL-13, by binding to the promoters of all three genes (17).
Allergic asthma (AA) is a chronic inflammatory airway disease, which, in 50 to 70% of patients, is characterized by type 2 inflammation with increased levels of TH2 cytokines, enhanced immunoglobulin E (IgE) production, and eosinophilia (18). Airway inflammation in AA is triggered by a plethora of aeroallergens, including house dust mite (HDM) feces, which stimulate the release of cytokines such as IL-33, IL-25, and thymic stromal lymphopoietin (TSLP) from airway epithelial cells (AECs) (19). These mediators recruit TH2 cells that produce cytokines including IL-4, which triggers B cell maturation and IgE production, and IL-5, which enhances IgE production and mobilizes eosinophils from the bone marrow. TH2 cells are characterized by the expression of the transcription factor GATA-binding protein 3 (GATA3) and the type 2 cytokines IL-4, IL-5, and IL-13 (20). Little is known about the role of SOCE in TH2 cells and type 2 immune responses. The expression of IL-4 is reduced in SOCE-deficient murine T cells (8, 21–23) and in T cells of patients with loss-of-function mutations in STIM1 and ORAI1 (24). By contrast, ectopic expression of STIM1 or STIM2 elevated IL-4 production in murine CD4+ T cells in vitro (23). Treatment of mice with two different CRAC channel inhibitors prevented ovalbumin (OVA)–induced, late-phase asthmatic responses in animal models (25–27). However, whether these effects were due to altered T cell function remains unknown because CRAC channels and SOCE were also shown to mediate cytokine production in AECs treated with HDM allergens (28, 29).
Influenza A viruses (IAVs) cause annual epidemics with high mortality especially in infants and elderly (30, 31). Immunity against IAV is mediated by CD4+ and CD8+ T cells as well as neutralizing antibodies. CD4+ T cells differentiate into TH1 cells that express proinflammatory cytokines such as IFN-γ and tumor necrosis factor–α (TNF-α) and may differentiate into lung-resident memory T cells that control influenza infection (32–34). CD4+ T cell help is also required for an effective CD8+ T cell memory response during IAV infection (35). In addition, CD4+ T follicular helper (Tfh) cells promote humoral immunity by providing costimulation to B cells in germinal centers (GCs) and helping them mature into antibody-producing long-lived plasma cells or memory B cells (36). This function of Tfh cells is mediated by molecules such as CD40L and ICOS (inducible T cell costimulator) and cytokines such as IL-4 and IL-21. Tfh cell differentiation requires CRAC channels because mice with T cell–specific deletion of Stim1 and Stim2 and abolished SOCE have strongly decreased numbers of Tfh cells and GC B cells and lack class-switched, antigen-specific antibodies after viral infection (9). SOCE controls the differentiation of Tfh cells and the expression of CD40L, ICOS, IL-4, IL-21, and other factors required for Tfh cell function. Only complete deletion of SOCE in mice with T cell–specific deletion of either Orai1 and Orai2 or Stim1 and Stim2 compromises Tfh cell function and antiviral immunity (8, 9), whereas no defects are observed after deletion of Orai1 or Stim1 alone (8, 10). The quantitative requirements of SOCE for adaptive immunity to IAV infection are not known.
Given the SOCE-dependent role of T cells in adaptive immunity to viral and other infections on one hand and the protective effects of SOCE inhibition in autoimmune disease models on the other, the question arises whether CRAC channel inhibition is a possible treatment for autoimmune and allergic inflammation without compromising immunity to infection. To address this question, we compared how genetic and pharmacological suppression of CRAC channel function affects immunity to IAV infection and inflammation in HDM-induced AA. We found that T cell–specific deletion of Orai1 leaves immunity to the H3N2 strain of IAV largely intact because viral burdens and pulmonary inflammation were comparable to wild-type (WT) controls. By contrast, deletion of Orai1 protected mice from HDM-induced allergic airway inflammation, which was due to impaired antigen-specific expansion, differentiation, and function of TH2 cells. These defects, in turn, were associated with altered expression of genes that regulate cell cycle progression and are required for establishing and maintaining the TH2 cell differentiation program including the transcription factors GATA3, interferon regulatory factor 4 (IRF4), and basic leucine zipper ATF-like transcription factor (BATF) and cytokines IL-2 and IL-4. Treatment of mice with the CRAC channel inhibitor CM4620 suppressed allergic airway inflammation but did not compromise immunity to IAV infection. Collectively, our data suggest that TH2 cell–mediated airway inflammation is more dependent on CRAC channels than TH1 cell–mediated antiviral immunity. These findings may have important implications for the treatment of AA and other T cell–dependent inflammatory diseases.
RESULTS
T cell–specific deletion of Orai1 does not exacerbate IAV infection
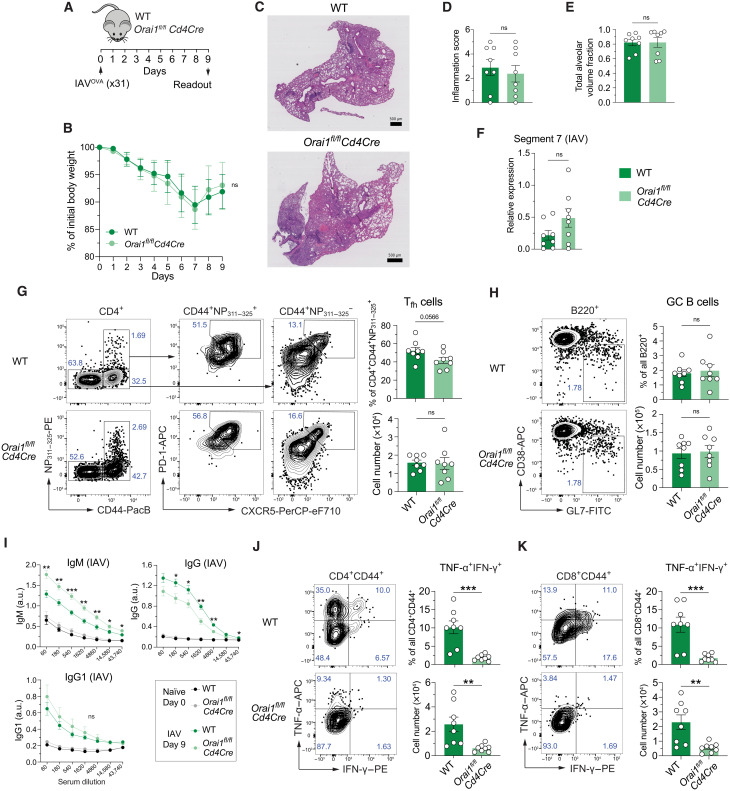
Given the important role of SOCE in T cell–mediated immunity to infection in humans and mice (1), we speculated that deletion of Orai1, the dominant CRAC channel homolog in T cells, may result in compromised immune responses to IAV infection. To test this hypothesis, we infected Orai1fl/flCd4Cre and WT control mice intranasally with a single dose of the laboratory strain A/HK/x31 (Hkx31, H3N2) of IAV that was engineered to express OVA323–339 (IAVOVA) (Fig. 1A) (37). Note that both CD4+ and CD8+ T cells of Orai1fl/flCd4Cre mice lack ORAI1 protein (fig. S1A) and have reduced (but not abolished) SOCE (fig. S1B) because of Cd4Cre expression at the CD4+ CD8+ double-positive stage of T cell development in the thymus. All infected WT and Orai1fl/flCd4Cre mice survived and experienced a similar ~10% reduction in body weight at day 7 before starting to recover (Fig. 1B). The histological analysis of lungs from mice at day 9 postinfection (p.i.) showed comparable pulmonary inflammation and total alveolar volume fraction between Orai1-deficient and WT mice (Fig. 1, C to E). IAV burdens in the lungs of mice were analyzed by quantitative real-time polymerase chain reaction (qRT-PCR) of viral genome segments as previously described (38, 39) and showed no significant differences between WT and Orai1fl/flCd4Cre mice at day 9 p.i. (Fig. 1F and fig. S1C).
Fig. 1. Deletion of Orai1 in T cells does not attenuate pulmonary inflammation and antibody production after infection with IAV.
(A) Mouse model of x31 (H3N2) IAV infection. (B) Body weight of WT versus Orai1fl/flCd4Cre mice after infection with IAV, shown as weight normalized to day 0. (C) Representative hematoxylin and eosin (H&E) stains of lungs from WT and Orai1fl/flCd4Cre mice at day 9 after infection with x31 influenza A. Scale bar, 500 μm. (D) Inflammation score calculated from the lungs of WT and Orai1fl/flCd4Cre mice at day 9 following IAV infection. (E) Total alveolar volume fraction of lungs from WT and Orai1fl/flCd4Cre mice. (F) qRT-PCR analysis of IAV (segment 7) levels in total lung tissue from WT versus Orai1fl/flCd4Cre mice at day 9 after infection. (G) Representative flow cytometry plots of IAV-specific CXCR5+PD-1+ Tfh cells, their frequencies (%), and absolute numbers in mediastinal lymph nodes (mLNs) from WT and Orai1fl/flCd4Cre mice 9 days after IAV infection. (H) Representative flow cytometry plots of B220+CD38−GL7+ GC B cells, their frequencies (%), and absolute numbers in mLNs from WT and Orai1fl/flCd4Cre mice 9 days after IAV infection. FITC, fluorescein isothiocyanate. (I) IAV (x31)–specific IgM, IgG, and IgG1 levels in the serum of WT and Orai1fl/flCd4Cre mice before (day 0) and after (day 9) IAV infection. a.u., arbitrary units. (J and K) Analysis of TNF-α– and IFN-γ–producing CD4+CD44+ (J) and CD8+CD44+ (K) T cells from mLNs of WT and Orai1fl/flCd4Cre mice at day 9 after IAV infection. Representative flow cytometry plots, frequencies (%), and total numbers of T cells. Data in (A) to (K) are the means ± SEM of eight WT and eight Orai1fl/flCd4Cre mice and two independent experiments. Statistical analysis is performed by unpaired Student’s t test with the following significance levels: ***P < 0.001; **P < 0.01; *P < 0.05. ns, not significant.
Normal humoral immune response in IAV infected Orai1-deficient mice
Immunity to pulmonary infection with IAV is mediated by several T cell subsets including CD8+ cytotoxic T lymphocytes and CD4+ TH1 and Tfh cells (40). To understand the effects of Orai1 deletion on T cells in the lung during IAV infection, we analyzed the mediastinal lymph nodes (mLNs) and lungs of Orai1fl/flCd4Cre and WT littermate mice after infection with IAVOVA. We observed moderately higher frequencies but normal total numbers of CD4+ T cells in the lungs of Orai1fl/flCd4Cre mice at day 9 p.i. compared to WT littermates, whereas frequencies of Orai1-deficient CD8+ T cells were normal (fig. S1D). The frequencies and total numbers of all CD4+CD44+CD62L− effector T cells in the lungs of Orai1-deficient and WT mice were comparable, with moderate but significant reduction in the frequencies of IAV-specific CD4+CD44+CD62L−NP311–325+ effector T cells in Orai1fl/flCd4Cre mice (fig. S1E). Moreover, the frequencies and total numbers of CD8+ naïve, effector, and effector memory T cells were similar in both cohorts of mice (fig. S1F). Likewise, the frequencies and numbers of CD4+ and CD8+ tissue-resident memory T cells in the lungs of Orai1fl/flCd4Cre and WT mice were comparable. Overall, these data show that lack of ORAI1 and reduced SOCE in T cells does not grossly affect the composition of the IAV-specific T cell compartment.
We had previously reported that complete abolition of SOCE in Orai1/Orai2- or Stim1/Stim2-deficient T cells impairs Tfh cell differentiation and function and thus humoral immunity to LCMV infection (8, 9). We therefore analyzed the numbers of IAV-specific Tfh cells (CD4+CD44+PD-1+CXCR5+NP311–325+) in the mLNs of Orai1fl/flCd4Cre mice. Whereas the frequencies of IAV-specific Tfh cells were slightly reduced in the absence of ORAI1, their total numbers were comparable to WT mice (Fig. 1G). The frequencies and numbers of B220+CD38−GL7+ GC B cells were similar in IAV-infected WT and Orai1fl/flCd4Cre mice (Fig. 1H). In line with slightly reduced Tfh cell frequencies in mLNs, we observed moderately lower levels of IAV-specific IgG antibodies in the serum of Orai1fl/flCd4Cre mice, whereas IAV-specific IgG1 antibodies were comparable to WT mice. Conversely, IAV-specific IgM titers were higher in Orai1-deficient mice, suggesting that lack of ORAI1 in T cells results in a moderate decrease in Ig class switching or differentiation of plasma cells (Fig. 1I). IAV infection induces the production of IFN-γ and TNF-α by T cells, which contribute to immunity to IAV and cause tissue damage, respectively (41–43). We measured the numbers of effector CD4+ and CD8+ T cells producing both cytokines 9 days after IAV infection. The percentages and absolute numbers of TNF-α+IFN-γ+ double-producing CD4+CD44+ and CD8+CD44+ effector T cells were significantly reduced in Orai1fl/flCd4Cre compared to WT mice (Fig. 1, J and K). To further validate our findings, we tested the effects of Orai1 deletion in T cells in another viral infection model by inoculating Orai1fl/flCd4Cre and WT mice with the Armstrong strain of LCMV. Ten days after LCMV infection, the frequencies of Tfh cells and GC B cells in the spleen and mesenteric lymph nodes of WT and Orai1-deficient mice were comparable, and robust GC formation was observed in both strains (fig. S2, A to C). Furthermore, the numbers of CD4+CXCR5hiPD-1hiCD44hiFoxp3+ T follicular regulatory cells, which are involved in controlling the GC reaction, were similar in LCMV-infected WT and Orai1fl/flCd4Cre mice (fig. S2D). Consistent with the above observations, the levels of IgM and class-switched Igs were largely normal, although a moderate reduction in total IgG and IgE levels was found in the serum of Orai1-deficient mice (fig. S2E). Together, our findings show that T cell–specific deletion of Orai1 does not significantly impair immune responses to viral infection, notably IAV.
T cell–specific deletion of Orai1 protects mice from HDM-induced allergic airway inflammation
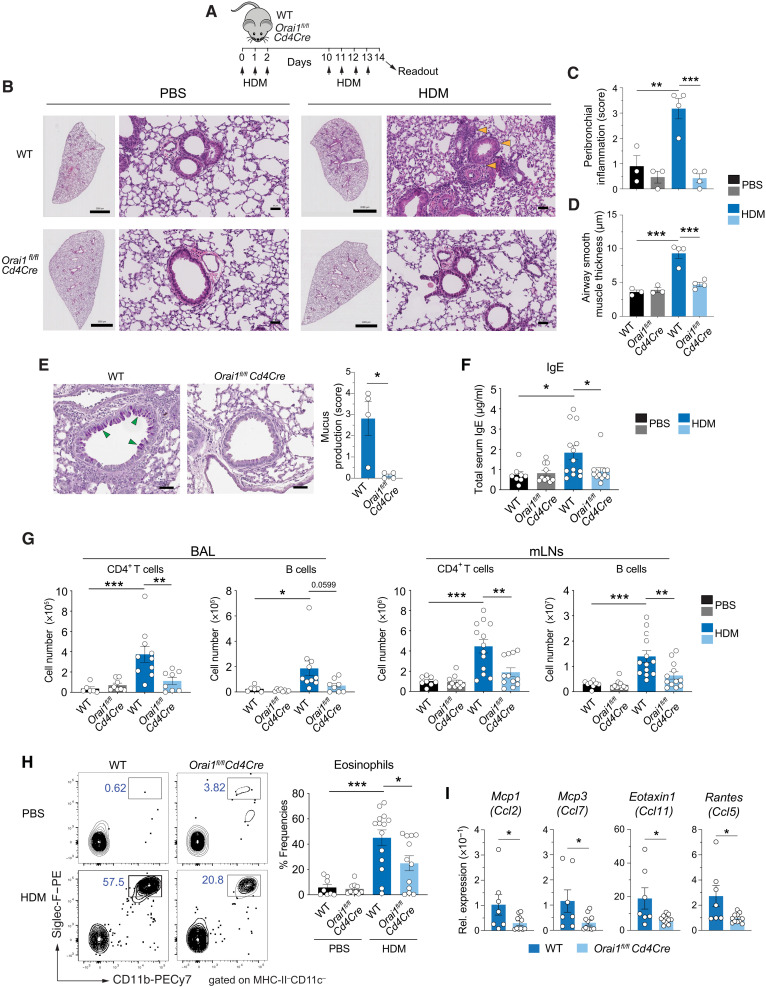
We had previously reported that deletion of Orai1 in T cells significantly attenuates the severity of T cell–mediated inflammation in the EAE model of multiple sclerosis (12) and GvHD (8). To directly compare the role of ORAI1 in T cell–mediated immunity to IAV infection and inflammation in the same organ, we chose to induce allergic airway inflammation in Orai1-deficient mice. To this end, we sensitized WT and Orai1fl/flCd4Cre mice intranasally with HDM extract on three consecutive days and rechallenged them with HDM 8 days later (Fig. 2A). Inflammation and cellular and humoral immune responses were investigated 1 day after the last rechallenge by analyzing the lungs, mLNs, bronchoalveolar lavage (BAL) fluid, and blood of mice. To characterize airway inflammation, we stained the lungs of WT and Orai1fl/flCd4Cre mice treated with HDM or phosphate-buffered saline (PBS) as control with hematoxylin and eosin (H&E). HDM-challenged WT mice showed severe pulmonary inflammation, which was characterized by peribronchial inflammation, a hallmark of AA, and moderate perivascular cellular infiltrates (Fig. 2, B and C). Peribronchial inflammation was significantly attenuated in HDM-challenged Orai1fl/flCd4Cre mice compared to WT littermates. In addition, we found that the increased airway smooth muscle thickness in HDM-treated WT mice was significantly attenuated in HDM-challenged Orai1fl/flCd4Cre mice (Fig. 2D). Whereas HDM-induced airway inflammation in WT mice was accompanied by strong mucus production by bronchial epithelial cells identified by periodic acid–Schiff (PAS) staining, a similar increase in mucus production was absent in Orai1-deficient mice (Fig. 2E). A common feature of AA is elevated serum IgE levels. We detected significantly lower levels of IgE in the serum of Orai1fl/flCd4Cre than WT mice after HDM sensitization and rechallenge (Fig. 2F). Consistent with the pulmonary inflammation in HDM-treated WT mice, we observed significantly elevated numbers of CD4+ T cells as well as B cells in their BAL fluid, which were absent in the BAL of HDM-challenged Orai1fl/flCd4Cre mice (Fig. 2G). Similarly, the numbers of CD4+ T cells and B cells in the mLNs of HDM-treated Orai1fl/flCd4Cre mice were decreased compared to HDM-treated WT littermates (Fig. 2G). A hallmark of AA is the recruitment of eosinophils to the airways where they play important roles in asthma exacerbation (44, 45). We detected significantly increased frequencies of eosinophils in the BAL fluid of WT mice after HDM immunization, whereas eosinophil frequencies in HDM-treated Orai1fl/flCd4Cre mice were significantly lower than those in WT mice (Fig. 2H). Eosinophils express the C-C motif chemokine 3 (CCR3), which is required for their recruitment to the lung (46). CCR3 responds to a variety of chemokines, including eotaxin [C-C motif chemokine ligand 11 (CCO11)], eotaxin-3 (CCL26), monocyte-chemotactic protein 3 [MCP-3 (CCL7)], MCP-4 (CCL13), and RANTES [regulated upon activation, normal T cell expressed and secreted (CCL5)] whose production is stimulated by TH2 cytokines (47, 48). We found that mRNA levels of Eotaxin1, Rantes, Mcp3, and Mcp1 were significantly lower in the lungs of HDM-treated Orai1fl/flCd4Cre mice compared to WT littermates (Fig. 2I), providing a likely explanation for reduced eosinophil frequencies in the absence of ORAI1. Together, our findings demonstrate that the deletion of Orai1 in T cells significantly attenuates allergic airway inflammation following sensitization and rechallenge with HDM allergen.
Fig. 2. ORAI1 is essential for T cell–dependent allergic airway inflammation in response to HDM exposure.
(A) Mouse model of sensitization and rechallenge of mice with HDM extract to induce allergic airway inflammation. (B) Representative H&E stains of lungs from WT and Orai1fl/flCd4Cre mice at day 14. Scale bars, 2000 μm (right) and 50 μm (left). (C and D) Analysis of peribronchial inflammation (C) and airway smooth muscle layer thickness (D) of WT and Orai1fl/flCd4Cre mice at day 14. (E) Representative PAS stains of lungs and quantitative analysis of mucus production of WT and Orai1fl/flCd4Cre mice at day 14. Scale bars, 50 μm. (F) Total IgE levels in the serum of WT and Orai1fl/flCd4Cre mice at day 14. (G) Numbers of CD4+ and B220+ B cells in the BAL fluid (left) and mLNs (right) of mice at day 14. (H) Representative flow cytometry plots and summary bar graphs showing the frequencies of Cd11b+Siglec-F+ eosinophils within MHC-II−CD11c− cells in the BAL fluid of WT and Orai1fl/flCd4Cre mice at day 14. (I) Relative mRNA expression of chemokines in whole lung tissue (qRT-PCR). Data in (C) to (E) are the means ± SEM of three to four mice per group. Data in (F) are the means ± SEM from 8 to 13 mice per cohort. Data in (G) are the means ± SEM from 6 to 13 mice per cohort. Data in (H) are the means ± SEM of 8 to 14 mice per cohort. Data in (I) are the means ± SEM of 7 to 11 mice per cohort. Data are from at least two independent experiments; statistical analysis in (C), (D), and (F) to (H) is performed by one-way analysis of variance (ANOVA) (Tukey’s multiple comparisons test) and that in (E) and (I) is performed by unpaired Student’s t test. ***P < 0.001; **P < 0.01; *P < 0.05.
Lack of ORAI1 in OVA-specific T cells protects from HDM/OVA-induced airway inflammation without impairing immunity to IAVOVA infection
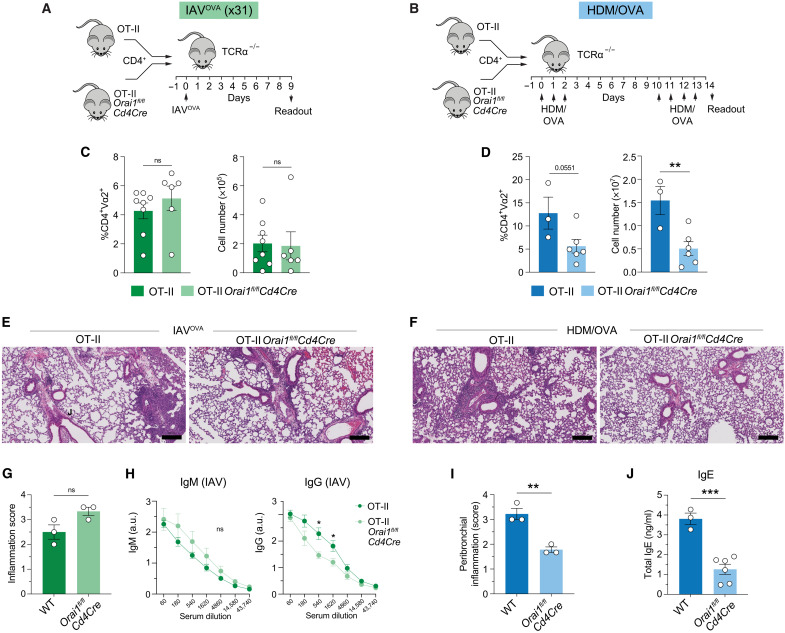
Because deletion of Orai1 in T cells protected mice from HDM-induced allergic airway inflammation while maintaining immunity to IAV infection, we investigated the mechanisms underlying this dichotomy. To rule out that the difference is due to different strengths of TCR signaling induced by HDM- and IAV-derived antigens, we generated Orai1fl/flCd4Cre OT-II mice whose CD4+ T cells express a transgenic TCR that is specific for OVA. Naïve CD4+ T cells from Orai1fl/flCd4Cre OT-II and WT OT-II mice were injected into T cell–deficient TCRα−/− host mice, which were either infected with IAVOVA (Fig. 3A) or immunized with OVA and HDM extract (HDM/OVA) (Fig. 3B). In this model, only the TCR-transgenic, transferred T cells with fixed antigen specificity mediate antiviral and allergic immunity. Following infection with IAVOVA, the lungs of host mice showed similar frequencies and total numbers of Orai1fl/flCd4Cre OT-II and WT OT-II donor T cells (Fig. 3C). By contrast, the numbers of Orai1fl/flCd4Cre OT-II cells in the lungs of host mice after HDM/OVA immunization and rechallenge were significantly reduced compared to control OT-II cells (Fig. 3D). Pulmonary inflammation after x31 IAVOVA infection was similar in host mice that had received WT OT-II or Orai1fl/flCd4Cre OT-II donor T cells (Fig. 3, E and G). By contrast, the lungs of host mice transferred with Orai1fl/flCd4Cre OT-II donor cells showed significantly less peribronchial inflammation than recipients of WT OT-II cells after immunization and rechallenge with HDM/OVA (Fig. 3, F and I). The analysis of serum antibodies following x31 IAVOVA infection showed similar titers of IAVOVA-specific IgM and a slight decrease in IAVOVA-specific IgG in mice injected with Orai1fl/flCd4Cre OT-II donor cells compared to WT OT-II (Fig. 3H). This was in contrast to the significantly reduced total IgE levels in the serum of HDM/OVA-challenged TCRα−/− host mice that had been injected with Orai1fl/flCd4Cre OT-II compared to WT OT-II T cells (Fig. 3J). Together, these data show that deletion of Orai1 in T cells expressing the same TCR has distinct effects on their ability to mount an antiviral immune response to IAVOVA and to mediate airway inflammation against HDM/OVA.
Fig. 3. Deletion of Orai1in antigen-specific T cells protects from allergic airway inflammation without compromising immunity to IAV infection.
(A and B) Experimental protocols to study antigen-specific T cell responses to pulmonary infection with 105 median tissue culture infectious dose (TCID50) IAVOVA (x31) (A) and allergic airway inflammation after immunization and rechallenge with HDM/OVA (B) following adoptive transfer of OVA-specific CD4+ T cells from WT OT-II and Orai1fl/flCd4Cre OT-II mice into T cell–deficient TCRα−/− mice. (C and D) Frequencies and total numbers of donor OT-II T cells in the lungs of TCRα−/− recipient mice at day 9 after IAV infection (C) and at day 14 after HDM/OVA immunization and rechallenge (D). (E and F) Representative H&E stains of lungs of mice at day 9 after IAVOVA infection (E) and at day 14 after sensitization and rechallenge with HDM/OVA (F). Scale bars, 200 μm. (G and I) Quantification of lung inflammation of three TCRα−/− recipient mice per cohort at day 9 after IAVOVA infection (G) and at day 14 after sensitization and rechallenge with HDM/OVA (I). (H) IAV-specific IgM and IgG titers in the serum of TCRα−/− mice at day 9 after transfer of WT and Orai1fl/flCd4Cre OT-II T cells and IAVOVA infection. (J) Total IgE levels in the serum of the serum of TCRα−/− mice at day 14 after transfer of WT and Orai1fl/flCd4Cre OT-II T cells and sensitization and rechallenge with HDM/OVA. Data in (C) and (D) are the means ± SEM of six to eight (C) and three to six (D) TCRα−/− recipient mice per cohort. Data in (G) and (I) are the means ± SEM of three TCRα−/− recipient mice per cohort. Data in (H) are from five to six TCRα−/− recipient mice per cohort. Data in (J) are from three to six TCRα−/− recipient mice per cohort. Statistical analysis is performed by unpaired Student’s t test. ***P < 0.001; **P < 0.01; *P < 0.05.
Differential regulation of cell cycle pathways in T cells after HDM-induced allergic airway inflammation compared to IAV infection
To understand why T cell–specific Orai1 deletion attenuates allergic airway inflammation but not immunity to IAV infection, we compared T cell activation and gene expression in donor WT OT-II and Orai1fl/flCd4Cre OT-II cells isolated from the lungs of TCRα−/− host mice after IAVOVA infection or HDM/OVA challenge (Fig. 3, A and B). Principal components analysis (PCA) of RNA sequencing (RNA-seq) data showed that WT OT-II T cells isolated from HDM/OVA-immunized and IAVOVA-infected mice clearly segregated, suggesting that both treatments induce a different T cell response despite the shared TCR specificity (fig. S3, A and B). To elucidate specific differences in gene expression in WT OT-II T cells following HDM immunization and IAV infection, we analyzed differentially expressed genes (DEGs) and conducted Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis. Several pathways were differentially regulated after HDM/OVA challenge and IAVOVA infection including TCR signaling, influenza A, and TH1/TH2 differentiation (fig. S3, C and D). Whereas several negative regulators of T cell activation such as Pdcd1, Ctla4, and Il-10 were differentially up-regulated in HDM/OVA-stimulated T cells, proinflammatory genes such as Ifng and positive regulators of T cell signaling such as Jun, Nfatc3, and Ppp3ca were differentially up-regulated after IAVOVA infection. In the influenza A pathway, most of the genes were significantly up-regulated in OT-II cells only after IAVOVA infection. In addition, as expected, several TH1-associated genes were significantly increased only after IAVOVA infection, but not HDM/OVA stimulation, including Ifng, Il12rb2, and Stat4 (fig. S3C). Other pathways that were differentially regulated in HDM/OVA compared to IAVOVA-treated WT OT-II T cells were cell cycle, FoxO signaling, and p53 signaling (fig. S3D). The differential regulation of the KEGG cell cycle pathway was confirmed using BioCarta and Gene Ontology, which both revealed G2-M and cell cycle G2-M among the top identified pathways (fig. S3E). Within these pathways, 65 to 100% of genes were specifically up-regulated in HDM/OVA-stimulated compared to IAVOVA-stimulated OT-II T cells. Among the up-regulated genes were key regulators of the cell cycle including Cdk1, Mcm6, and Bub1 (fig. S3F). Many of these genes are also part of the p53 signaling pathway. Together, the comparison of differentially regulated KEGG pathways in OT-II T cells suggests that HDM/OVA stimulation results in stronger induction of cell cycle regulatory genes than IAVOVA infection.
ORAI1 controls the expression of cell cycle regulators and T cell proliferation during allergic airway inflammation
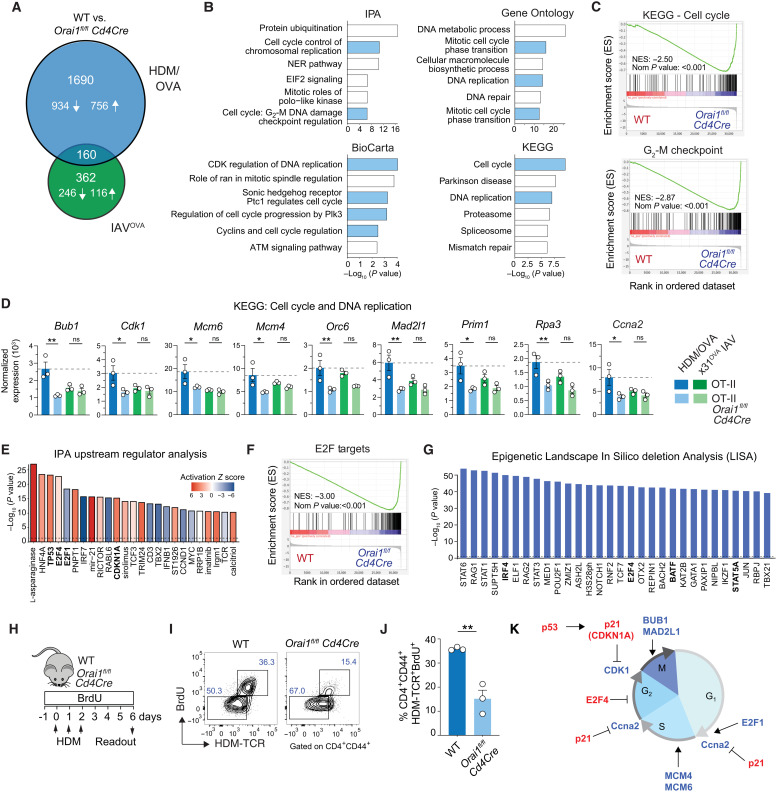
We next investigated how ORAI1 affects gene expression in OT-II T cells after HDM/OVA immunization compared to IAVOVA infection to understand why Orai1fl/flCd4Cre mice are protected from allergic airway inflammation but have normal T cell responses to IAV infection. To elucidate the molecular mechanisms behind this dichotomy, we compared DEG and pathways in CD4+ T cells from Orai1fl/flCd4Cre OT-II and WT OT-II mice that had been adoptively transferred to host mice immunized and rechallenged with HDM/OVA or infected with IAVOVA. A total of 362 genes were differentially up- or down-regulated in WT versus Orai1-deficient T cells after IAVOVA infection compared to 1690 genes after HDM/OVA immunization and rechallenge (Fig. 4A). Of these 1690 genes, 934 were down-regulated and 756 were up-regulated in the absence of ORAI1. Further assessment of these DEGs using Ingenuity Pathway Analysis (IPA), BioCarta, Gene Ontology, and KEGG pathway analysis identified a preponderance of deregulated pathways in Orai1-deficient T cells that are related to cell cycle regulation, cell division, and DNA replication (Fig. 4B). Gene set enrichment analysis (GSEA) confirmed these results and showed that expression of cell cycle and G2-M checkpoint–related genes was reduced in Orai1fl/flCd4Cre OT-II cells (Fig. 4C). In-depth analysis of the KEGG pathways cell cycle and DNA replication revealed significantly reduced expression of genes that promote cell cycle progression, DNA synthesis, and replication in Orai1-deficient compared to WT T cells after HDM/OVA immunization. Among these genes were Bub1, Cdk1, Mcm6, Mcm4, Orc6, Mad2l1, Prim1, Rpa3, and Ccna2 (Fig. 4D). By contrast, no difference in expression of these genes was observed between Orai1fl/flCd4Cre OT-II and WT OT-II cells after infection with IAVOVA. These data suggest that ORAI1 is required for the regulation of cell cycle progression in the context of allergen challenge but not viral infection.
Fig. 4. ORAI1 controls the expression of cell cycle regulators and T cell proliferation during allergic airway inflammation.
(A) Numbers of up-regulated (↑) or down-regulated (↓) DEGs in OT-II T cells of WT and Orai1fl/flCd4Cre mice at days 9 and 14 after IAVOVA (x31) infection and HDM/OVA treatment, respectively (Fig. 3, A and B). (B and C) Analysis of WT and Orai1fl/flCd4Cre T cells at day 14 of HDM/OVA treatment (Fig. 3B). (B) Top six dysregulated pathways obtained by IPA, BioCarta, Gene Ontology, and KEGG, ranked by P values. Cell cycle–related pathways are shown in blue. ATM, ataxia telangiectasia mutated; NER, nucleotide excision repair; NES, normalized enrichment score; Plk3, Polo-like kinase 3. (C) GSEA of KEGG cell cycle (M7963) and hallmark G2-M checkpoint (M5901). (D) Normalized RNA-seq–based gene expression from the KEGG pathways cell cycle and DNA replication in T cells after IAVOVA infection and HDM/OVA treatment. (E to G) Analysis of WT and Orai1fl/flCd4Cre T cells at day 14 of HDM/OVA treatment. (E) IPA upstream regulator analysis of DEG ranked by P value. Colors indicate the activation Z score. Key regulators are highlighted in bold. (F) GSEA of E2F targets (M5925). (G) Epigenetic Landscape In Silico deletion Analysis (LISA) of transcriptional regulators of DEG ranked by P value. Key regulators are highlighted in bold. (H to J) Antigen-specific T cell proliferation after HDM treatment. (H) Experimental protocol. (I) Representative flow cytometry plot of CD4+CD44+ T cells after BrdU incorporation and staining with I-Ab tetramers (HDM-TCR+). (J) Frequencies of CD4+CD44+BrdU+HDM-TCR+ T cells from three WT and three Orai1fl/flCd4Cre mice. (K) Control of cell cycle progression by ORAI1. Inhibitors are highlighted in red, activators in blue. Statistical analysis in (B) and (E) is performed by Fisher’s exact test: P < 0.1; that in (D) is performed by ANOVA (Sidak’s multiple comparisons); that in (F) is performed by a Wilcoxon rank sum test; and that in (I) is performed by unpaired Student’s t test. **P < 0.01; *P < 0.05.
To identify ORAI1-dependent regulators of DEGs after HDM/OVA immunization, we used two independent approaches, the IPA upstream regulator analysis and epigenetic Landscape In Silico deletion Analysis (LISA) (49). Of all upstream regulators detected by IPA, a number of transcription factors, kinases, and other proteins regulating cell cycle progression such as cyclin-dependent kinase (CDK) inhibitor 1A (CDKN1A), tumor suppressor protein TP53, and the E2F transcription factor 4 (E2F4) and E2F1 were among those detected at the highest level of significance (Fig. 4E). Whereas activation Z scores of TP53, its downstream target CDKN1A and E2F4, which mediate cell cycle arrest, were up in Orai1-deficient OT-II T cells, those for E2F1, which promotes proliferation, were down. Accordingly, almost all CDKN1A target genes were suppressed in the absence of ORAI1 (fig. S4A). Moreover, GSEA further revealed that the expression of E2F target genes was strongly depleted in Orai1fl/flCd4Cre compared to WT OT-II T cells (Fig. 4F and fig. S4, B and C), which was consistent with impaired proliferation of Orai1-deficient T cells. IPA’s definition of upstream transcriptional regulator is broad and includes transcription factors, kinases, and signaling molecules that can affect gene expression. By contrast, LISA compares lists of genes with chromatin immunoprecipitation sequencing datasets to identify transcription factors and chromatin regulators that are directly responsible for the perturbation of a DEG set (49). Comparison of DEG in Orai1fl/flCd4Cre and WT OT-II T cells by LISA identified E2F4 as a potential direct regulator of cell cycle progression in the context of HDM/OVA-induced allergic airway inflammation (Fig. 4G). Other ORAI1-dependent transcription factors identified by LISA included signal transducer and activator of transcription 6 (STAT6) and STAT5A, which mediate IL-4 and IL-2 receptor signaling, respectively, and thus TH2 differentiation. LISA also identified IRF4 and BATF as regulators of DEGs in Orai1-deficient T cells, which was intriguing because both transcription factors are required for efficient TH2 differentiation (50–53). To test whether dysregulation of cell cycle gene expression in Orai1-deficient T cells indeed affects T cell proliferation, we immunized WT and Orai1fl/flCd4Cre mice with HDM/OVA and measured proliferation by 5-bromo-2′-deoxyuridine (BrdU) incorporation (Fig. 4, H to J). We observed significantly reduced frequencies of HDM-specific, proliferative (BrdU+) effector CD4+CD44+ T cells in the mLNs of Orai1fl/flCd4Cre mice compared to their WT littermates. These results strongly suggest that ORAI1 regulates the expression of genes regulating cell cycle progression and proliferation in antigen-specific T cells during allergic airway inflammation (Fig. 4K).
To investigate the effects of Orai1 deletion on the proliferation of TH2 cells further, we differentiated CD4+ T cells from WT and Orai1fl/flCd4Cre mice in TH2 cells in vitro. Orai1-deficient TH2 cells had significantly reduced SOCE compared to WT TH2 cells following passive store depletion with thapsigargin or TCR cross-linking with anti-CD3 antibodies (fig. S5, A and B). The extent of the SOCE defect in the absence of ORAI1 was comparable in TH2 and TH1 cells. TH2, but not TH1, cells showed significantly reduced expression of regulators of cell cycle progression including Cdk1, Mcm6, Bub1, and Ccna2 (fig. S5C). When we stimulated TH2 cells by anti-CD3/CD28 cross-linking in vitro, we observed significantly reduced frequencies of cells in the G2-M phase (fig. S5D) and a moderate but significant proliferation defect (fig. S5E) in Orai1-deficient TH2 cells compared to WT controls. These data are consistent with the proliferation defect and dysregulated cell cycle gene expression of Orai1fl/flCd4Cre CD4+ T cells in vivo after HDM immunization (Fig. 4).
By contrast, we did not observe differential expression of genes or pathways related to cell cycle regulation in Orai1fl/flCd4Cre OT-II T cells in the context of IAVOVA infection (fig. S6, A and B). Neither did an IPA upstream regulator analysis reveal genes controlling the cell cycle to be dysregulated in the absence of ORAI1 (fig. S6C). Instead, KEGG pathway analysis showed aberrant regulation of several other pathways in Orai1fl/flCd4Cre OT-II T cells following IAVOVA infection. These included influenza A, TH1 and TH2 cell differentiation, and retinoic acid-inducible gene I (RIG-1)–like signaling pathways (fig. S6, A and B), which was consistent with the reported roles of TH1 cells and RIG-I in antiviral immunity to influenza infection (54, 55). Furthermore, the IPA upstream regulator analysis showed strongly reduced activation scores for the TH1-associated cytokines IL-12 and IFN-γ (fig. S6C). Overall, however, the deletion of Orai1 did not have a strong impact on the expression of TH1 genes because only 5% of known TH1 signature genes were either up- or down-regulated in Orai1fl/flCd4Cre OT-II T cells after IAV infection (fig. S6D). Collectively, our data demonstrate that ORAI1 plays a specific role in regulating cell cycle progression in TH2 cells in vitro and during allergic airway inflammation in vivo.
ORAI1 promotes TH2 cell effector functions in HDM-induced allergic airway inflammation
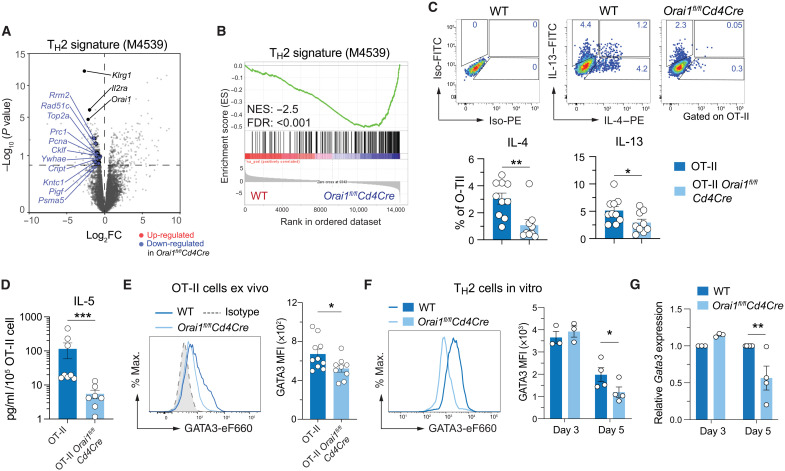
AA is characterized by type 2 inflammation that is associated with increased IgE production, eosinophilia, and increased levels of TH2 cytokines. When we compared DEGs in OT-II T cells of WT and Orai1fl/flCd4Cre mice following HDM/OVA immunization to previously reported TH2 gene signatures, we found that expression of all genes belonging to a TH2 signature gene set was down-regulated in Orai1-deficient compared to WT T cells (Fig. 5A). GSEA confirmed that most TH2 signature genes were depleted in Orai1fl/flCd4Cre compared to WT OT-II T cells after HDM/OVA immunization (Fig. 5B). Given the role of ORAI1 in promoting gene expression in TH2 cells, we investigated the production of TH2 cytokines by CD4+ T cells in the lungs of TCRα−/− mice that had been injected with T cells from WT OT-II or Orai1fl/flCd4Cre OT-II mice and immunized with HDM extract using the same protocol as shown in Fig. 3B. We found significantly reduced numbers of Orai1-deficient CD4+ T cells in the lungs of mice that are able to produce IL-4 and IL-13 compared to CD4+ WT T cells (Fig. 5C). Similarly, Il4 and Il13 mRNA levels were reduced in lung tissue of Orai1fl/flCd4Cre mice after HDM rechallenge (fig. S7A). To further investigate the role of ORAI1 for TH2 cell function, we differentiated CD4+ T cells of WT and Orai1fl/flCd4Cre mice into TH2 cells in vitro. Stimulation of TH2 cells with anti-CD3/CD28 resulted in the production of IL-4 and IL-13 protein, which was significantly impaired in Orai1-deficient TH2 cells (fig. S7, B and C). IL-5 expression by human TH2 cells requires several rounds of antigen stimulation in vitro and is a hallmark of differentiated TH2 cells (56). IL-5–producing CD4+ T cells were undetectable even in HDM-immunized WT mice by intracellular cytokine staining, but a more sensitive analysis by cytometric bead array showed that IL-5 production by Orai1-deficient CD4+ T cells isolated from the lungs of HDM-treated mice was significantly reduced compared to WT CD4+ T cells (Fig. 5D). As expected, CD4+ T cells polarized into TH1 cells for comparison did not produce IL-4 or IL-13 but expressed IFN-γ, whose levels were strongly reduced in Orai1-deficient TH1 cells (fig. S8, A and B). Reduced cytokine production was not limited to TH2 cytokines as Tnfa mRNA levels were moderately reduced in the lungs of HDM-treated Orai1fl/flCd4Cre mice compared to WT controls (fig. S7A); moreover, protein levels of TNF-α, IL-10, and IL-2 were reduced in both TH1 and TH2 cells of Orai1fl/flCd4Cre mice (fig. S8, A and B). Collectively, these findings demonstrate that ORAI1 is an important regulator of TH2 responses in allergic airway inflammation.
Fig. 5. ORAI1 regulates TH2 cell differentiation and function.
(A to E) Analysis of RNA-seq data from T cells of WT and Orai1fl/flCd4Cre mice at day 14 of HDM/OVA treatment (Fig. 3B). (A and B) Volcano plot (A) and GSEA plot (B) of DEGs in Orai1-deficient versus WT CD4+ T cells that belong to the gene set naïve CD4 T cell versus 48h act TH2 down (M4529) (115). FC, fold change. (C) Representative flow cytometry plots and frequencies of IL-4– and IL-13–producing WT and Orai1fl/flCd4Cre donor OT-II CD4+ T cells isolated from the lungs of TCRα−/− host mice day 14 after HDM/OVA treatment. Cells were stimulated with phorbol 12-myristate 13-acetate (PMA)/ionomycin for 4 hours in vitro. (D) IL-5 production by donor OT-II T cells isolated from the lungs of TCRα−/− host mice at day 14 after HDM/OVA treatment and restimulated in vitro with anti-CD3 for 24 hours. IL-5 concentration was measured in the cell culture supernatants by cytometric bead array and normalized to T cell numbers. (E) Representative flow cytometry plots and quantification of GATA3 mean fluorescence intensity (MFI) in OT-II cells isolated from lungs of mice at day 14 after HDM/OVA treatment. (F and G) GATA3 expression by CD4+ T cells polarized into TH2 cells for 3 to 5 days in vitro. GATA3 protein (F) and mRNA (G) expression measured by flow cytometry and qRT-PCR, respectively. mRNA levels were normalized to Rpl32 housekeeping gene and WT T cells. Data in (C) to (E) are the means ± SEM of 9 to 10 mice from two independent experiments. Data in (F) and (G) are the means ± SEM of three to four mice from at least two independent experiments. Statistical analysis in (C) is performed by unpaired Student’s t test; that in (D) and (E) is performed by Mann-Whitney test; that in (F) is performed by paired Student’s t test; and that in (G) is performed by two-way ANOVA (Sidak’s multiple comparison). ***P < 0.001; **P < 0.01; *P < 0.05.
ORAI1 regulates TH2 cell differentiation and function
TH2 responses are dependent on the transcription factor GATA3, which is essential for TH2 cell differentiation, proliferation, and function (57). GATA3 directly binds to several regions in the TH2 cytokine gene locus and regulates expression of the TH2 signature cytokines IL-4, IL-5, and IL-13 (58). Given the reduced expression of IL-4 and IL-13 and attenuated TH2 responses in vivo after HDM sensitization and rechallenge, we analyzed the expression of GATA3 in CD4+ T cells isolated from mLNs of WT and Orai1fl/flCd4Cre mice that were sensitized and rechallenged with HDM. We observed reduced numbers of GATA3+CD4+ T cells in Orai1-deficient mice compared to WT littermates (Fig. 5E). We next investigated the effects of Orai1 deletion on the expression levels of GATA3 in CD4+ T cells that were polarized into TH2 cells in the presence of IL-4 in vitro. Whereas TH2 cells from WT mice showed robust GATA3 expression, GATA3 mRNA and protein levels were significantly attenuated in Orai1-deficient TH2 cells at day 5 of differentiation (Fig. 5, F and G, and fig. S8C). Besides GATA3, we also observed reduced expression of IRF4 protein, and Irf4 and Batf mRNA in Orai1-deficient TH2 cells in vitro (fig. S9, A and B). Both transcription factors are expressed by various CD4+ T cell subsets and induced after TCR stimulation and IL-4 receptor (IL-4R) signaling (59). IRF4 and BATF are considered to be pioneer factors that mediate the remodeling and accessibility of chromatin (60). IRF4 is required for TH2 differentiation by promoting the expression of GATA3 (50) and IL-4 (51). Note that the levels of GATA3, IRF4, and BATF were reduced in Orai1-deficient T cells only at day 5, and not yet at day 3, of TH2 differentiation. These findings suggest that deletion of Orai1 and partial reduction of SOCE do not interfere with the induction of IRF4 and BATF but their maintenance in TH2 cells.
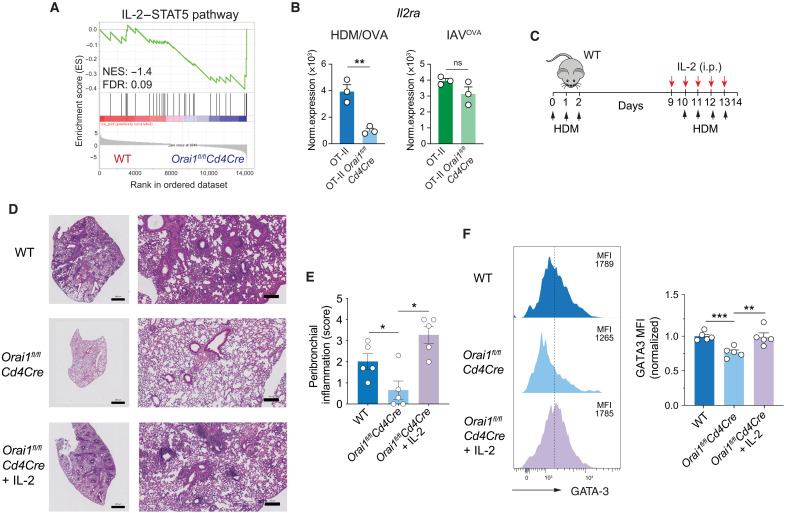
ORAI1 regulates HDM-induced airway inflammation through IL-2R expression and IL-2 signaling
The expression of GATA3 requires IL-4 signaling, which is mediated by the IL-4R and the transcription factor STAT6 (61, 62). The expression of IL-4 and IL-4R, in turn, depends on IL-2 signaling via STAT5 (fig. S9C) (63). We therefore hypothesized that impaired IL-2 and IL-4 production by Orai1-deficient CD4+ T cells may contribute to the impaired maintenance of GATA3 expression and TH2 differentiation. Further analysis of DEG and signaling pathways that are dysregulated in Orai1-deficient T cells after HDM/OVA immunization revealed a strong depletion of genes belonging to the IL-2/STAT5A signaling pathway (Fig. 6A). STAT5A and its homolog STAT5B are critical mediators of IL-2R signaling, which controls the differentiation and homeostasis of many T cell subsets (64). One of the most significantly reduced genes in Orai1fl/flCd4Cre compared to WT OT-II T cells was Il2ra, which encodes the α chain of the IL-2 receptor (CD25) (Fig. 6B). A similar reduction was not observed in Orai1-deficient OT-II T cells after IAVOVA infection. IL-2 has been shown to enhance airway inflammation in response to inhaled allergen (65, 66). To assess the significance of reduced IL-2/STAT5 pathway expression in Orai1-deficient T cells for allergic airway inflammation, we tested whether exogenous IL-2 restores lung inflammation in Orai1fl/flCd4Cre mice. To this end, Orai1fl/flCd4Cre mice were sensitized with HDM and injected with exogenous IL-2 at the time of HDM rechallenge (Fig. 6C). Whereas Orai1fl/flCd4Cre mice had significantly reduced peribronchial inflammation compared to WT mice, Orai1fl/flCd4Cre mice treated with IL-2 showed a similar degree of pulmonary inflammation as WT mice (Fig. 6, D and E). Given the reduced GATA3 expression in Orai1-deficient T cells (Fig. 5, E to G), we measured GATA3 levels in T cells isolated from the lungs of Orai1fl/flCd4Cre mice that were injected with IL-2 at the time of HDM challenge. GATA3 expression was significantly increased in Orai1-deficient CD4+ T cells following IL-2 injection compared to untreated Orai1-deficient mice (Fig. 6F). Collectively, these data show that ORAI1 regulates IL-2/STAT5 signaling in CD4+ T cells, which is required to maintain TH2 cell differentiation and allergic airway inflammation.
Fig. 6. ORAI1 regulates HDM-induced airway inflammation through IL-2R expression and IL-2 signaling.
(A) GSEA plot of DEGs that belong to the gene set IL-2/STAT5 pathway (M234) (116) in OT-II CD4+ T cells from WT and Orai1fl/flCd4Cre at day 14 after HDM/OVA treatment (Fig. 3B). (B) Normalized Il2ra mRNA expression in CD4+ OT-II T cells from mice after IAVOVA infection or HDM/OVA treatment from RNA-seq (Fig. 3, A and B). (C to F) IL-2 treatment restores HDM-induced airway inflammation in Orai1-deficient mice. (C) Experimental protocol. WT and Orai1fl/flCd4Cre mice were immunized and challenged with HDM; some Orai1-deficient mice received recombinant human IL-2 (1.5 × 105 IU/day, i.p.) during HDM challenge from days 9 to 13. (D) Representative H&E stains of lungs. Scale bars, 1000 μm (left) and 50 μm (right). (E) Peribronchial inflammation scores. (F) Representative flow cytometry plots and MFI of GATA3 expression in CD4+ T cells isolated from the lungs normalized to levels in WT mice. Data in (B) are the means ± SEM of three mice per cohort. Data in (E) and (F) are the means ± SEM of five mice per cohort from two independent experiments. Statistical analysis in (B) and (F) is performed by unpaired Student’s t test; that in (E) is performed by Mann-Whitney test. ***P < 0.001; **P < 0.01; *P < 0.05.
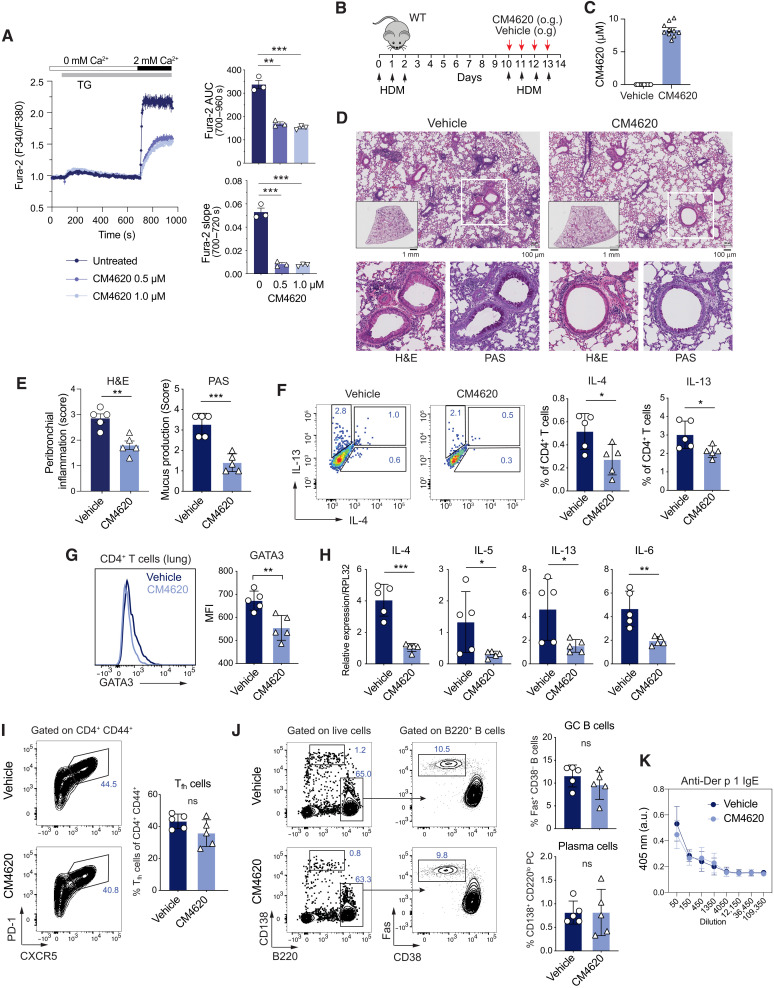
Pharmacological CRAC channel inhibition reduces asthmatic airway inflammation without compromising immunity to IAV infection
To test whether pharmacological inhibition of CRAC channels could be an effective approach to treating allergic airway inflammation, we used the selective CRAC channel blocker CM4620 (67). CM4620 has passed two phase 2 clinical trials for COVID-19 (coronavirus disease 2019)–associated pulmonary inflammation (NCT04661540) and acute pancreatitis (NCT03401190). Treatment of CD4+ T cells from WT mice with 0.5 or 1 μM CM4620 resulted in significant inhibition of the rate of increase of [Ca2+]i after thapsigargin stimulation (which is an indirect readout for CRAC channel function) and the integrated Ca2+ signal over time (Fig. 7A). To test the effects of systemic SOCE inhibition on allergic airway inflammation, we sensitized WT mice with HDM and treated them orally with CM4620 at the time of HDM rechallenge (Fig. 7B). Serum concentrations of CM4620 were confirmed by liquid chromatography–mass spectrometry (LC-MS) at the end of the treatment period and ranged from 6.76 to 10.17 μM, equivalent to 25.7 to 38.7 nM free compound levels (Fig. 7C). Mice treated with vehicle alone showed severe peribronchial inflammation and mucus production, whereas allergic airway inflammation was significantly reduced in mice that had been treated with CM4620 (Fig. 7, D and E). Reduced airway inflammation was associated with lower frequencies of IL-4– and IL-13–producing CD4+ T cells in the lungs of CM4620-treated mice compared to controls (Fig. 7F) and reduced expression of GATA3 (Fig. 7G). In addition, mRNA levels of proinflammatory cytokines including Il4, Il5, Il13, and Il6 were reduced in the lungs of mice treated with the CRAC channel inhibitor compared to vehicle injected mice (Fig. 7H). Notably, the administration of CM4620 at the time of HDM rechallenge had no effects on the frequencies of Tfh cells and GC B cells in the mLNs (Fig. 7, I and J). Accordingly, the titers of Der p 1-specific IgE antibodies were comparable in mice treated with the CRAC inhibitor and vehicle alone (Fig. 7K). The anti-inflammatory effects of CRAC channel inhibition on HDM-induced allergic airway inflammation are consistent with the attenuated inflammation observed in Orai1fl/flCd4Cre compared to WT mice.
Fig. 7. Pharmacological CRAC channel inhibition alleviates asthmatic airway inflammation.
(A) Intracellular Ca2+ levels ([Ca2+]i) in WT naïve CD4+ T cells treated with 0.5 or 1 μM CM4620. Cells were stimulated with 1 μM thapsigargin in Ca2+-free Ringer solution, followed by perfusion with 2 mM extracellular Ca2+. Quantification of the area under the curve (AUC) and slope following Ca2+ readdition. (B) Experimental protocol for HDM sensitization/rechallenge. WT mice were treated with CM4620 (25 mg/kg) or vehicle by oral gavage (o.g.) on days 10 to 13, analyzed at day 14. (C) Concentrations of CM4620 in the serum of mice measured by LC-MS. (D and E) Representative H&E and PAS stains (D) and peribronchial inflammation and mucus production (E) in the lungs. (F) Representative flow cytometry plots and frequencies of IL-4– and IL-13–producing CD4+ T cells isolated from the lungs of mice treated with vehicle or CM4620 and stimulated with PMA/ionomycin for 4 hours in vitro. (G) GATA3 expression in CD4+ T cells from the lungs of vehicle or CM4620-treated mice by flow cytometry. (H) Cytokine mRNA levels in total lung tissues of mice by qRT-PCR. (I) Representative flow cytometry plots and quantification of CXCR5+PD-1+ Tfh cells in mLNs. (J) Representative flow cytometry plots and quantification of Fas+CD38− GC B cells and CD138+B220lo plasma cells in mLNs of mice treated with vehicle or CM4620. (K) Der p 1-specific IgE levels in the serum of mice analyzed by enzyme-linked immunosorbent assay (ELISA). Data in (A) are the means ± SEM from three mice and independent experiments. Data in (C) to (K) are the means ± SEM from five mice per cohort. Statistical analysis is performed by unpaired Student’s t test. ***P < 0.001; **P < 0.01; *P < 0.05.
To investigate whether CRAC channel inhibition is associated with an increased susceptibility to IAV infection because of impaired antiviral immunity, we infected WT mice with the x31 strain of IAV and treated them with CM4620 on four consecutive days after infection (fig. S10A). Serum concentrations of CM4620 in the median inhibitory concentration range were confirmed by LC-MS at the end of the treatment period and ranged from 7.1 to 13.0 μM (free compound levels, 26.9 to 49.5 nM) (fig. S10B). IAV infection resulted in similar degrees of pulmonary inflammation (fig. S10, C and D) and had no effect on the viral burdens detected in the lungs of CM4620-treated mice compared to littermates injected with vehicle only (fig. S10E). However, the numbers of CD4+ T cells isolated from the lungs of CM4620-treated mice at day 14 of IAV infection producing the cytokines IL-2, IFN-γ, and TNF-α were reduced compared to controls (fig. S10F), indicating that the CRAC channel inhibitor treatment indeed suppresses T cell function. Because humoral immunity is required to control IAV infection, we measured the frequencies and total numbers of CXCR5+PD-1+ Tfh cells, Fas+GL-7+ GC B cells, and CD138+ plasma cells in mLNs. Treatment of IAV-infected mice with CM4620 had no effect on the frequencies of either cell population compared to the vehicle control group (fig. S10, G and H). Accordingly, the levels of IAV-specific IgM and IgG antibodies in the serum of CM4620 and vehicle-treated mice were comparable (fig. S10I). Collectively, our data demonstrate that pharmacological CRAC channel inhibition suppresses the airway inflammation associated with HDM allergen challenge without dampening antiviral immunity to IAV infection.
DISCUSSION
We here investigated the role of ORAI1 and SOCE in allergic airway inflammation and pulmonary infection with IAV. Deletion of Orai1 in T cells strongly attenuated HDM-induced type 2 airway inflammation evident from reduced peribronchial immune cell infiltration, eosinophils in the BAL, production of TH2 cytokines, and IgE levels. The effects of Orai1 deletion were intrinsic to T cells because the adoptive transfer of allergen-specific CD4+ T cells to lymphopenic host mice had similar protective effects against HDM-induced allergic airway inflammation. Moreover, pharmacological inhibition of SOCE with a selective CRAC channel inhibitor attenuated HDM-induced allergic airway inflammation and TH2 responses. At the molecular level, protection from AA was associated with impaired IL-2–dependent maintenance of TH2 differentiation and a TH2 cell–specific defect in cell cycle progression and proliferation.
By contrast, Orai1 deletion in T cells did not significantly impair T cell–mediated antiviral immunity to IAV infection because pulmonary inflammation, viral titers, IAV-specific antibody levels, and the frequencies of IAV-specific Tfh cells and GC B cells were comparable in Orai1fl/flCd4Cre and WT mice. The only strong defect we were able to detect following IAV infection was a reduction in the frequencies of IFN-γ– and TNF-α–producing CD4+ T cells in Orai1fl/flCd4Cre mice or WT mice treated with the CRAC channel blocker CM4620. The role of IFN-γ and TNF-α in immunity to IAV infection is complex. TNF-α has mostly an immune regulatory role in immunity to IAV and its reduction might enhance, rather than impair, CD8+ T cell responses to IAV (68, 69). IFN-γ was shown by some studies (70, 71) to be critical for antiviral immunity to IAV, whereas others observed no protective role or reported increased susceptibility to IAV infection (72, 73). In addition, note that T cells are not the only producers of IFN-γ because natural killer cells and type 1 innate lymphoid cells are also able to secrete IFN-γ. Because only T cells lack IFN-γ in Orai1fl/flCd4Cre mice, this defect may be compensated by other immune cells. We conclude that partially reduced SOCE following deletion on Orai1 in T cells or pharmacological CRAC channel inhibition does not compromise immunity to IAV infection despite reduced TNF-α and IFN-γ production by T cells.
Protection from HDM-induced allergic airway inflammation in mice with T cell–specific deletion of Orai1 or WT mice treated with a CRAC channel inhibitor was accompanied by reduced frequencies and numbers of IL-4– and IL-13–producing effector CD4+ T cells in the lung after sensitization and rechallenge with HDM allergen. Similar defects were observed in vitro in Orai1-deficient CD4+ T cells after polarization into TH2 cells. Moreover, the expression of a variety of chemokines known to be induced by IL-4 and IL-13 such as Mcp1, Mcp3, Eotaxin1, and Rantes was reduced in Orai1fl/flCd4Cre mice after HDM sensitization and rechallenge, providing an explanation for the attenuated pulmonary eosinophil infiltration, a hallmark of asthmatic airway inflammation in humans and mice (74). Consistent with the attenuated type 2 inflammation, RNA-seq of CD4+ T cells isolated from the lungs of HDM-immunized Orai1fl/flCd4Cre mice identified a significant down-regulation of TH2 signature genes (75). Moreover, we observed reduced expression of the TH2 transcription factor GATA3 in Orai1-deficient CD4+ T cells isolated from the lungs of HDM-sensitized mice and in Orai1-deficient CD4+ T cells polarized into TH2 cells in vitro. GATA3 cooperates with other transcription factors, including the Ca2+-regulated NFAT to promote IL-4 expression and TH2 differentiation (76). Note that naïve Orai1-deficient CD4+ T cells were initially able to induce GATA3 after TCR stimulation and polarization under TH2 conditions but failed to maintain GATA3 expression at day 5 after polarization. Besides GATA3, the expression of two other transcription factors required for TH2 function, IRF4 and BATF, was impaired in Orai1-deficient T cells. RNA-seq and LISA showed that both factors regulate gene expression in T cells of HDM-immunized mice in an ORAI1-dependent manner. Although IRF4 is not selectively expressed in TH2 cells, it is necessary for proper TH2 differentiation through the induction of GATA3 and IL-4 (50, 51). Likewise, BATF is required for TH2 differentiation (52), and together with IRF4 and IL-4, it is part of a positive feedback loop during TH2 cell polarization (53).
The fact that GATA3, IRF4, and BATF were induced at similar levels in Orai1-deficient and WT T cells suggested that their initial expression in TH2 cells, although reported to be reduced after strong suppression of SOCE (9, 16), is not sensitive to the partial reduction of SOCE found in T cells of Orai1fl/flCd4Cre mice. By contrast, the maintenance of GATA3 expression and that of IRF4 and BATF was impaired in Orai1-deficient TH2 cells in vitro. We reasoned that sustained GATA3 expression indirectly requires the Ca2+-dependent production of cytokines and cytokine receptors. It is well established that IL-4 induces TH2 polarization via IL-4R and STAT6 signaling and the induction of GATA3 expression (77). LISA of RNA-seq data from Orai1-deficient T cells of HDM-immunized mice identified STAT6 as a potential regulator of DEGs. TH2 differentiation also depends (besides IL-4) on IL-2 and IL-2R signaling via STAT5, which regulate the expression of IL-4 and the IL-4R (63, 78). Moreover, IL-2 signals directly induce the expression of IRF4 in TH9 cells (79). We hypothesized that impaired IL-2 production may be responsible for the failed maintenance of GATA3 expression and TH2 differentiation in the absence of ORAI1 for the following reasons: (i) IL-2 expression depends on SOCE (8, 14, 80); (ii) the IL-2/STAT5 signaling pathway was strongly deregulated in T cells isolated from HDM-immunized Orai1fl/flCd4Cre mice; and (iii) the expression of Il2ra encoding the α chain of the IL-2R (CD25) was strongly decreased in CD4+ T cells of Orai1fl/flCd4Cre OT-II mice following HDM immunization. Restoring IL-2 levels in Orai1fl/flCd4Cre mice by injection of IL-2 during HDM rechallenge resulted in a significantly more pronounced peribroncheal inflammation compared to untreated Orai1fl/flCd4Cre mice. IL-2 injection also normalized the expression of GATA3 in Orai1-deficient T cells to levels comparable to those in WT T cells. These results indicate that IL-2 is required for sustaining TH2 cell differentiation and their proinflammatory function in the context of AA.
Immunity to IAV infection is mediated in large part by neutralizing IgG antibodies against hemagglutinin that block the interaction between the virus and host cells. In AA, IgE antibodies mediate the activation of mast cells and airway inflammation. We reasoned that protection from HDM-induced airway inflammation may be due to reduced IgE levels, whereas preserved immunity to IAV infection may in part be explained by normal levels of flu-specific IgG. The production of IAV-specific IgG antibodies was not significantly impaired in IAV infected Orai1fl/flCd4Cre mice or WT mice treated with the CRAC channel blocker CM4620. Moreover, we observed comparable numbers of Tfh cells in Orai1fl/flCd4Cre mice or CM4620-treated WT mice following infection with IAV or LCMV. These observations are in line with our previous findings that the differentiation and function of Tfh cells following LCMV infection are only decreased when SOCE is abolished completely in Orai1/Orai2-deficient or Stim1/Stim2-deficient T cells but not by a partial reduction of SOCE in Orai1- or Stim1-deficient T cells (8, 9). The preserved differentiation of Tfh cells and production of virus-specific IgG antibodies in IAV-infected Orai1fl/flCd4Cre mice provide a compelling explanation why viral titers and pulmonary inflammation were not increased compared to WT mice.
By contrast, ORAI1 appears to have a slightly different function with regard to the ability of T cells to promote IgE production in response to allergen exposure. We observed reduced serum IgE levels in HDM-sensitized and rechallenged Orai1fl/flCd4Cre mice compared to WT controls. Similarly, IgE levels were decreased in TCRα−/− mice injected with CD4+ T cells from Orai1fl/flCd4Cre OT-II mice compared to mice that had received WT OT-II T cells. Recently, IL-13–producing Tfh cells were identified to be critical for allergen-specific IgE production and allergic airway inflammation (81). IL-13 expression was reduced in Orai1-deficient CD4+ T cells isolated from HDM immunized mice, which provides a potential explanation for decreased IgE levels. In contrast to Orai1fl/flCd4Cre mice, however, the treatment of HDM-immunized WT mice with the CRAC channel blocker CM4620 at the time of HDM rechallenge had no effects on the levels of HDM-specific IgE antibodies and the numbers of Tfh cells despite reduced IL-13 production. A likely explanation for the different effects of genetic deletion and pharmacological inhibition of ORAI1 on IgE levels and Tfh cell numbers is the timing of SOCE suppression. Whereas CD4+ T cells from Orai1fl/flCd4Cre mice lack SOCE during both the immunization and rechallenge with HDM, CD4+ T cells from CM4620-treated WT mice had attenuated SOCE only during rechallenge with HDM. These data suggest that ORAI1 and SOCE are required for the differentiation of allergen-specific Tfh cells and IgE production upon first allergen encounter. It is presently unclear why Orai1 deletion impairs the production of IgE but not IgG antibodies. IL-4 produced by Tfh cells promotes class switching to IgE, whereas IL-21 fosters class switching to IgG subtypes (82, 83) and inhibits class switching to IgE (84, 85). SOCE is required, however, for the production of both IL-4 and IL-21 (9).
Besides impaired production of TH2 cytokines, we observed a significant reduction of Orai1-deficient CD4+ T cells in the lungs, BAL, and mLNs of mice after HDM immunization. The analysis of T cell proliferation by BrdU incorporation in vivo following HDM immunization revealed a pronounced decrease in the numbers of HDM-specific, proliferating T cells in the absence of Orai1. A similar proliferation defect was observed in Orai1-deficient TH2 cells in vitro, which was associated with a reduction of TH2 cells in the G2-M phase. These findings suggested that Ca2+ influx through ORAI1 promotes cell cycle progression of TH2 cells. RNA-seq of Orai1-deficient T cells showed that some of the most significantly deregulated genes and pathways were those related to cell cycle regulation. Numerous genes and pathways positively regulating the cell cycle and G2-M checkpoint were decreased in Orai1-deficient CD4+ T cells from HDM immunized (but not IAV infected) mice including Bub1, Cdk1, Mcm6, Mcm4, Orc6, Mad2l1, Prim1, Rpa3, and Ccna2.
To identify ORAI1- and Ca2+-regulated transcription factors that control cell cycle gene expression, we used IPA upstream regulator analysis and LISA, which showed a down-regulation of several transcription factors and cyclins that promote cell cycle progression such as E2F1, MYC, and Cyclin D1 in Orai1-deficient CD4+ T cells. Conversely, transcription factors and CDK inhibitors that suppress cell cycle progression such as E2F4, p53, and p21 (CDKN1A) were up-regulated. Accordingly, the expression of p21, E2F4, and E2F1 target genes was strongly and almost uniformly down-regulated in Orai1-deficient T cells. Whereas regulation of E2F4 by Ca2+ has not been reported, the expression and phosphorylation of E2F1 were shown to be regulated by extracellular Ca2+ levels in keratinocytes (86). The Ca2+ dependence of E2F4 and E2F1 in Orai1-deficient T cells from HDM immunized mice is reminiscent of the down-regulation of other E2F family members (E2F2, E2F3) in SOCE-deficient regulatory T (Treg) cells of Stim1fl/fStim2fl/flCd4Cre mice reported earlier (87).
In addition to E2F4, the expression of two other inhibitors of cell cycle progression, p53 and p21, was up-regulated in Orai1-deficient T cells. p21 inhibits the activity of CDK1 at the G2-M transition and that of CDK2 at the G1-S and S-G2 transition (88). The function of p21 is controlled by the tumor suppressor p53, whose effects on cell cycle arrest are dependent on p21. TCR signaling results in down-regulation of p53, thereby enabling the antigen-specific, cytokine-driven proliferation of T cells (89). How Ca2+ regulates the expression of p53 and p21 is not well understood. A general role of Ca2+ in controlling cell cycle progression has been reported and occurs at several transition points, for instance, at the G0-G1 checkpoint after mitogenic stimulation and at the G1-S boundary (90). Cell cycle regulation by Ca2+ is mediated by the Ca2+ binding protein calmodulin (CaM), which, in turn, regulates CaM kinases and the phosphatase calcineurin. Inhibition of calcineurin in human T cells was shown to lead to cell cycle arrest in G1, which was associated with increased expression of transforming growth factor–β and an accumulation of p21 (91). Because ORAI1 is the main source of Ca2+ influx in T cells, it is likely that at least some of its effects on cell cycle progression are mediated through calcineurin.
Our study shows that Ca2+ influx via ORAI1 channels is critical for the differentiation, proliferation, and function of TH2 cells. As a consequence, Orai1fl/flCd4Cre mice are protected from allergic airway inflammation. Orai1fl/flCd4Cre mice have preserved immunity to infection with IAV, suggesting that SOCE in T cells is of particular importance for type 2 inflammation in allergic airway disease. What explains these different requirements for ORAI1 in T cell–mediated immune responses in the same organ? One explanation is that a single infection with IAVOVA results in weaker TCR signaling than repeated HDM exposure during sensitization and rechallenge with HDM. This explanation seems unlikely, however, because the transcriptomic analysis of OVA-specific WT T cells showed a robust up-regulation of TCR signaling genes, indicating that IAV infection is associated with sustained TCR signaling even 9 days after IAV infection. This conclusion is consistent with the prolonged presentation of influenza antigens weeks after viral clearance, which was sufficient to generate highly differentiated CD4+ effector T cells (92). Another potential explanation is that deletion of Orai1 has different effects on Ca2+ influx in TH1 and TH2 cells. We did, however, observe similar defects in SOCE in TH1 and TH2 cells from Orai1fl/flCd4Cre mice. We favor instead a model in which TH2 cells are more sensitive to a partial reduction of SOCE than TH1 cells because the expression of IL-2 and IL-4 is Ca2+ dependent, which, in turn, is required for the maintenance of GATA3, IRF4, and BATF expression. In this context, note that SOCE is not only important for the differentiation of TH2 cells but also important for the differentiation of other CD4+ T cell subsets including Tfh, TH17, and induced Treg cells (9, 16, 93), although their Ca2+ signaling requirements differ. All CD4+ TH cell subsets have in common that their ability to produce signature cytokines depends on SOCE, including IFN-γ (TH1), IL-4, IL-13 (TH2), and IL-17A (TH17), which explains, at least in part, the protection of mice with T cell–specific deletion of Orai1 and Stim1 from autoimmune, inflammatory diseases such as EAE, IBD, GvHD, and psoriasiform skin inflammation (8, 11–14). Our findings in this study indicate that SOCE has a comparable role in TH2 cells and allergic airway inflammation.
The role of ORAI1 and SOCE in human asthma is not well understood. A study in Japanese and Taiwanese populations has identified single-nucleotide polymorphisms (SNPs) in ORAI1 that are associated with susceptibility of atopic dermatitis (AD) (94). One of these SNPs (rs3741596) results in a missense mutation (ORAI1 p.S218G), which, when overexpressed, was later shown to disrupt Ca2+ influx and Ca2+-dependent gene expression (95). AD is one of the most common pediatric inflammatory skin disorders that is characterized by a TH2-dominant immune response. Many clinical studies have reinforced the concept of an “atopic march” during which the occurrence of AD in infancy is followed by an increased risk of developing one or more disorders characterized by type 2 inflammation including AA (96). It is unclear at present how the ORAI1 p.S218G polymorphism and decreased Ca2+ influx contribute to increased AD susceptibility and if this or other polymorphisms are also associated with an increased risk for developing AA.
Several studies have explored the effects of SOCE inhibition on T cells from asthma patients. The CRAC channel blocker Synta66 inhibited anti-CD2/CD3/CD28–induced production of IL-2, IL-7, IL-13, and IFN-γ in a concentration-dependent manner in healthy and severe asthma donors (26). Using an OVA-driven rat model of AA, the same study found a reduction of inflammatory cytokines in the lungs and eosinophils in the BAL of Synta66-treated rats, which was associated with attenuated airway obstruction. Similar observations were made using three other CRAC channel inhibitors, YM-58483 (BTP2), 3-fluoropyridine-4-carboxylic acid, and SKF 96365, which reduced cytokine production, airway inflammation, and airway remodeling in a guinea pig model of AA (25, 27, 97). The anti-inflammatory effects of these CRAC channel inhibitors on AA are not limited to TH2 cells. Mast cells are critical mediators of airway hyperresponsiveness in AA, and their proinflammatory function is dependent on CRAC channels. Studies of mast cells isolated from nasal polyps of patients have shown that CRAC channel blockers, alone or in combination with a leukotriene receptor antagonist, potently suppress CRAC channel activation (98). AECs are potent producers of inflammatory mediators that regulate bronchomotor tone and immune cell recruitment following stimulation by airway allergens. Ca2+ influx in AECs is mediated by ORAI1 channels, and their inhibition was shown to suppress the production of TSLP, IL-6, IL-8, and prostaglandin E2 (29). Collectively, these studies demonstrate that CRAC channels are required for the function of numerous cell types involved in the pathogenesis of AA, and their inhibition may be a feasible approach for AA therapy (99). Our finding that deletion of Orai1 in T cells attenuates AA without compromising adaptive immunity to IAV infection suggests that suppression of type 2 inflammation in AA by inhibition of CRAC channels is not associated with an increased risk or severity of viral infection. Given the still rising incidence of AA worldwide, identifying safe targets for AA therapy without compromising immunity to infection is critical.
METHODS
Mice
The generation of Orai1fl/fl mice has been described before (100). Mice were crossed to Cd4-Cre mice [The Jackson Laboratory (JAX) strain 017336] and further to OT-II mice [B6.Cg-Tg(TcraTcrb)425Cbn/J, JAX strain 004194]. TCRα−/− mice (B6.129S2-Tcratm1Mom/J) were purchased from JAX (strain 002116). Sex-matched male and female mice were used between 6 and 12 weeks of age. Mice were maintained under specific pathogen–free conditions. All experiments were conducted in accordance with protocols approved by the Institutional Animal Care and Use Committee of at New York University School of Medicine.
HDM-induced airway inflammation
To induce allergic airway inflammation, Orai1fl/flCd4Cre mice and Cre-negative Orai1fl/fl littermate controls (WT) were anesthetized with isoflurane and intranasally sensitized with 15 μg of crude extract from HDM Dermatophagoides pteronyssinus (Greer Laboratories, NC9756554) on three consecutive days (days 0 to 2), followed by rechallenge with 15 μg of HDM extract for 4 days (days 10 to 13). Control mice received PBS intranasally on days 0 to 2 and days 10 to 13. On day 14, serum and BAL fluid were collected. Following cervical dislocation, mice were perfused intracardially with 10 ml of ice-cold PBS containing 2 mM EDTA. mLNs and lungs were harvested and processed for histology and cell isolation. For IL-2 rescue experiments, Orai1fl/flCd4Cre mice were injected intraperitoneally (i.p.) with 1.5 × 105 IU of recombinant human IL-2 on days 9 to 13. Both male and female mice were used for the HDM-induced airway inflammation as sex-dependent differences in lung inflammation were reported in the HDM model (101, 102).
IAV infection
Orai1fl/flCd4Cre and WT control mice were anesthetized and infected intranasally with the laboratory strain A/HK/x31 (x31-IAV) of the IAV subtype H3N2. The OVA323–339 peptide that binds H2I-Ab was engineered into the globular head of the hemagglutinin molecule of x31-IAV (IAVOVA) as previously described (37). The viral titers of the stocks were determined via median tissue culture infectious dose (TCID50) assay in Madin-Darby canine kidney cells as previously described (103). The virus infectious dose used in all experiments was 105 TCID50 per mouse. The body weight of mice was monitored daily, and 9 days p.i., lungs were isolated for histology after intracardial perfusion. Lungs and mLNs were used to prepare single-cell suspensions, followed by flow cytometric analysis. Viral burdens in the lungs were measured by qRT-PCR using oligonucleotide primers against different regions of IAV isolated from total lung mRNA (primer sequences are listed in table S1).
Adoptive T cell transfer
For some IAV infection and HDM immunization experiments, naïve CD4+ T cells were isolated from WT OT-II or Orai1fl/fl Cd4Cre OT-II mice, followed by retro-orbital injection of 106 cells into TCRα−/− recipient mice. One day later, mice were infected intranasally with 105 TCID50 of IAVOVA. Alternatively, mice were sensitized intranasally with a mixture of 15 μg of HDM extract and 25 μg of OVA (Sigma-Aldrich, A5503) for three consecutive days, followed on days 10 to 13 by intranasal rechallenge of mice with the same HDM and OVA mixture according to published protocols (104).
Histology
Lungs of the HDM-immunized and IAV-infected mice were fixed with 4% paraformaldehyde, embedded in paraffin, and cut at 5 μm. Sample slides were stained with H&E or PAS in 95% ethanol using standard methods. Images were acquired using an AT2 (Leica) or Nanozoomer (Hamamatsu) whole slide scanner and visualized using the OMERO Plus imaging software (Glencoe Software). Inflammation in the lungs of IAV-infected mice was quantified using the following scoring system: 0, no inflammation; 1, only moderate peribronchial Inflammation; 2, <10% inflamed lung tissue; 3, 10 to 25% inflamed lung tissue; 4, 25 to 50% inflamed lung tissue; 5, >50% inflamed lung tissue according to a scoring system reported in (105). Scoring was performed independently by two investigators, whose scores were averaged. The total alveolar volume fraction was determined using the following procedure: (i) Regions representing empty space were acquired by setting a color threshold in ImageJ (106); (ii) to calculate the total alveolar volume fraction, the area of empty space was divided by the total lung area using MATLAB (v2018a). Peribronchial inflammation in the lungs of mice that had been sensitized and rechallenged with HDM was determined using the following scoring system as previously reported (107): 0, normal; 1, few cells; 2, a ring of inflammatory cells of one cell layer deep; 3, a ring of inflammatory cells of two to four cell layers deep; 4, a ring of inflammatory cells of >4 cell layers deep. The scores of three representative bronchiole sections per mouse were averaged. Mucus production in the airways of HDM-treated mice was determined using the following method as previously reported (107, 108): 0, no stained cells per bronchiole; 1, <25% stained cells; 2, 25 to 50% stained cells; 3, 50 to 75% stained cells; 4, >75% stained cells. Peribronchial inflammation was determined in a blinded manner by a pulmonary pathologist.
Bronchioalveolar lavage
Anesthetized mice were intubated with a tracheal cannula, and BAL fluid was recovered by gently flushing the airways three times with 200 μl of Hanks’ balanced salt solution/EDTA buffer upon thoracic massage. The three BAL fractions from each mouse were pooled and analyzed by antibody staining and flow cytometry for immune cell populations.
Single-cell preparation of lung tissue and mLNs
Lungs were cut into 8 to 10 pieces and incubated for 45 min at 37°C with Liberase TL (15 mg/ml; Roche, 5401020001) in complete RPMI1640 medium supplemented with 10% fetal bovine serum (FBS). Following enzymatic digestion, samples were passed through a 70-μm cell strainer, which was flushed twice with PBS supplemented with 2% FBS. Cells were spun at 800g for 5 min, resuspended in complete RPMI1640 medium, and stained with antibodies as described below. Single-cell suspensions of mLNs prepared similar to lung tissue but without prior enzymatic digestion. Cells isolated from mLNs, lungs, and BAL were counted using trypan blue or precision counting beads (BioLegend, catalog no. 424902) and prepared for either flow cytometric analysis or restimulation to measure cytokine production.
T cell culture in vitro
Mouse CD4+ T cells were isolated from spleens using the MagniSort Mouse CD4+ T cell enrichment kit (Invitrogen, 8804-6821-74) following the manufacturer’s instructions. Cells were maintained in complete RPMI1640 medium (Corning, 10-040-CV) containing 10% FBS, 2 mM l-glutamine, 50 U/ml penicillin-streptomycin, and 5.5 mM β-mercaptoethanol. For TH2 cell differentiation, naïve CD4+ T cells isolated from WT and Orai1fl/flCd4Cre mice were stimulated with anti-CD3 (2.5 ng/ml; clone 2C11) and anti-CD28 antibodies (1 μg/ml; clone 37.51) (both Bio X Cell) on flat-bottom plates coated with rabbit anti-hamster IgG (25 μg/ml; MP Biomedicals, catalog no. MP0855398) in the presence of IL-4 (50 ng/ml; PeproTech) and anti-IFN-γ (5 μg/ml; eBioscience, clone 11B11) for 2 days. For TH1 differentiation, CD4+ T cells were stimulated with anti-CD3 (1 μg/ml) and anti-CD28 (1 μg/ml) antibodies on IgG-coated plates as described above in the presence of IL-12 (20 ng/ml; PeproTech) and anti-IL-4 (5 μg/ml; eBioscience, clone 11B11). On day 2, cells were detached and expanded in RPMI1640 medium supplemented or not with TH1 or TH2 cytokines as described above and hIL-2 (20 U/ml; PeproTech, 200-02). Cells were analyzed on days 3 to 5.
T cell proliferation, cell cycle, and apoptosis in vitro
Naïve CD4+ T cells were stained with CellTrace Violet (Invitrogen, C34557) according to the manufacturer’s instructions and stimulated with anti-CD3/28 Dynabeads (Gibco, 11456D) at a 1:4 ratio (Dynabead:T cell) under TH2-polarizing conditions as described above for 3 and 4 days. Cells were then stained for surface markers and annexin V–Alexa Fluor 647 (BioLegend, 640912) in annexin V staining buffer (BioLegend, 422201) for 10 min and analyzed by flow cytometry. For cell cycle analysis, cells were fixed with 70% ethanol at −20°C. Cells were then washed in PBS, treated with ribonuclease cocktail (1:500; Invitrogen) for 15 min, and stained with propidium iodide (0.5 mg/ml; Sigma-Aldrich) for 30 min. Cells were resuspended in PBS and analyzed by flow cytometry.
Flow cytometry
Cells isolated from lungs and lymphoid organs or T cells cultured in vitro were washed and prepared in PBS containing 3% FBS and 2 mM EDTA. To prevent unspecific antibody binding, cells were first incubated with anti-CD16/CD32 antibodies (Fc block) for 10 min. For staining of surface markers, cells were incubated with fluorescently labeled antibodies on ice for 15 min in the dark. For intracellular cytokine staining, cells were stimulated with 1 μM ionomycin (Sigma-Aldrich, 407952) and 20 nM phorbol 12-myristate 13-acetate (PMA; Calbiochem, 524400) for 4 hours in the presence of 5 μM brefeldin A (eBioscience, 00-4506-51). Cells were then fixed with an intracellular fixation kit (Invitrogen, 88-8824-00) for 30 min, permeabilized with Permeabilization Buffer (eBioscience, 00-8333-56), and incubated with anti-cytokine antibodies for 30 min. For staining of transcription factors, cells were incubated with antibodies against surface antigens, labeled with LIVE/DEAD Fixable Blue Dead Cell Stain (Thermo Fisher Scientific, L23105) following the manufacturer’s instructions, and fixed/permeabilized with Foxp3 Transcription Factor Staining Buffer Set (eBioscience, 00-5523-00). Cells were subsequently incubated with antibodies against intracellular antigens for 30 min. The specificity of antibody binding was tested using isotype control antibodies and fluorescence minus one controls to correct for the spread of fluorophores into other detection channels (figs. S7 and S8). A complete list of antibodies and their fluorophore conjugates can be found in table S2. For the detection of IAV and HDM-specific T cells, we incubated cells with an IAV nucleocapsid protein-specific I-Ab tetramer (NP311–325, QVYSLIRPNENPAHK) conjugated with allophycocyanin (APC) and an HDM-specific I-Ab tetramer (Der p 1217–227; CQIYPPNVNKI) conjugated with phycoerythrin (PE) or APC. All tetramers were obtained from the National Institutes of Health (NIH) Tetramer Core Facility (Emory University, Atlanta, GA). A complete list of tetramers and their respective fluorescent conjugates can be found in table S3. Samples were acquired on an LSR II or LSRFortessa flow cytometer (BD Biosciences) and analyzed using FlowJo software (TreeStar, versions 9.3.2 and 10.5.3.).
Cytometric bead array
To measure IL-5 production by pulmonary T cells, total lymphocytes isolated from the lungs of HDM-treated mice were stimulated with anti-CD3 (0.5 μg/ml; Bio X Cell, clone 145-2C11) in complete RPMI1640 medium containing 10% FBS for 24 hours, followed by the collection of cell culture supernatant. IL-5 levels were measured by cytometric bead array using the Mouse IL-5 Enhanced Sensitivity Flex Set (BD Biosciences, catalog no. 562234) and Mouse Enhanced Sensitivity Master Buffer Kit (BD Biosciences, catalog no. 562248) following the manufacturer’s instructions. The concentrations of IL-5 were normalized to the number of T cells to evaluate the cytokine production capacity.
BrdU analysis
For the duration of 7 days, BrdU (0.8 mg/ml) was provided into the drinking water, to which experimental mice had access ad libitum. BrdU-supplemented drinking water was renewed daily. One day after starting BrdU treatment, mice were sensitized intranasally for 3 days with 15 μg of HDM extract. On day 3, mice were euthanized, and HDM-specific T cells isolated from their lungs and mLNs were analyzed for BrdU incorporation. Specifically, single-cell suspensions of the lungs and mLNs were washed with PBS and 2% FBS and fixed and permeabilized for 45 min at 4°C using the Foxp3 staining buffer kit (eBioscience, 00-5523-00). Cells were treated with 30 μg of deoxyribonuclease (DNase) for 45 min at 37°C; incubated with anti-CD16/CD32 antibody mix (eBioscience, 2.4G2) for 20 min at room temperature; stained with anti-BrdU antibody (eBioscience, catalog no. 11-5071-42), HDM-specific I-A(b) tetramer conjugated to PE or APC (as described above), and antibodies against surface and intracellular proteins; and subsequently analyzed by flow cytometry.
Enzyme-linked immunosorbent assay
To measure IAV-specific antibodies, high-binding enzyme-linked immunosorbent assay (ELISA) plates (Corning, 9018) were coated with heat-inactivated x31-IAV overnight at 4°C. ELISA plates were blocked with 20% FBS in PBS at 37°C for 1 hour, followed by washing three times with wash buffer [0.05% (v/v) Tween-20 in PBS]. Plates were incubated with serial dilutions of sera from WT and Orai1fl/flCd4Cre mice for 1 hour at 37°C, followed by incubation with alkaline phosphatase goat anti-mouse IgM, IgG, and IgG1 secondary antibodies (SouthernBiotech, 1021-04, 1036-04, and 1071-04, respectively) at 37°C for 2 hours. After addition of substrate solution, absorption was measured at 405 nm using a FlexStation 3 plate reader (Molecular Devices). Measurements of total serum IgE levels were conducted using the IgE Mouse Uncoated ELISA Kit (Invitrogen, 88-50460-88) according to the manufacturer’s protocol.
Synthesis of cDNA and qRT-PCR
Total RNA was extracted from cells using TRIzol reagent (Invitrogen, 15596026) as previously described (109). cDNA was synthesized using the Superscript II RT kit (Invitrogen, 18080-044) or the iScript kit (Bio-Rad, 1708891BUN) following the manufacturer’s instructions. qRT-PCR was performed using the Maxima SYBR Green Master Mix (Applied Biosystems, 4309155) and the QuantStudio5 RT-PCR system (Applied Biosystems). Transcript levels were normalized to the expression of housekeeping genes using the 2−ΔCt method. A complete list of primers used in this study can be found in table S1.
RNA sequencing
For RNA-seq, we prepared single-cell suspensions of lungs from TCRα−/− recipient mice that had been injected with CD4+ T cells from WT OT-II or Orai1fl/fl Cd4Cre OT-II mice and subsequently infected with IAVOVA or sensitized and challenged with HDM/OVA. CD4+Vα2+ donor T cells were enriched to at least 83% purity by flow cytometric cell sorting using a FACSAriaII (BD Biosciences). Total RNA was extracted using the RNeasy Mini RNA Isolation Kit (QIAGEN, 74104), and RNA quality and quantity were analyzed using a Bioanalyzer 2100 (Agilent) and PICO chips. RNA libraries were prepared using the TruSeq RNA sample prep v2 kit (Illumina) using 100 ng of total RNA treated with DNase I (QIAGEN) following the manufacturer’s protocol and 15 cycles of PCR amplification. The libraries were purified with AMPure beads (Beckman Coulter), quantified using a Qubit 2.0 fluorometer (Life Technologies), and visualized with an Agilent Tapestation 2200. Libraries prepared from different samples were pooled equimolarly and run on the HiSeq 4000 sequencing system to generate 50–base pair paired-end reads. Reads per sample FASTQ files were generated using the bcl2fastq2 Conversion software (v2.20). Adaptor sequences were trimmed using the Trimmomatic (v0.39) tool (110), and resulting reads were mapped to the mouse reference genome mm10 using STAR (v2.7.3a) (111). The read count matrix for the genomic features was generated using the R package Subread (Bioconductor v2.0.0). The DESeq2 package (Bioconductor v3.10) was used for differential gene expression analysis among groups of samples with three replicates each and for PCA. A statistical cutoff of false discovery rate (FDR) of <0.10 was used to determine statistically significant differences in gene expression. The RNA-seq has been deposited in the Gene Expression Omnibus database under accession number GSE184228.
Bioinformatic pathway analysis
GSEA was performed using GSEA software (Broad Institute, v4.0.0) (112). Pathway analyses were performed using IPA (QIAGEN) and the EnrichR Bioinformatics tool (113). A statistical cutoff of P < 0.10 was used to determine significantly expressed pathways. The open-source software LISA was used to identify upstream regulators of individual DEGs (http://lisa.cistrome.org/). An upstream regulator analysis was conducted using IPA, with a statistical significance cutoff of P (adjusted) < 0.05. Activation Z score values were calculated using an asymptotic Gaussian approximation (114).
Treatment with CRAC channel inhibitor
The selective CRAC channel inhibitor CM4620 was provided by CalciMedica (La Jolla, CA) (67). The inhibitor was solubilized in vehicle composed of 0.5% methylcellulose (w/w) with a viscosity of 400 cP (Sigma-Aldrich, M0262-250G) and 1% (w/w) Tween 80 (Thermo Fisher Scientific, BP338-500) in double-distilled H2O. The active form of CM4620 was administered by oral gavage at 25 mg/kg of body weight (25% loading) and 75 mg/kg of body weight of hypromellose acetate succinate (HPMCAS) bead carrier formulated in vehicle for four consecutive days. Control mice were treated with HPMCAS carrier alone. CM4620 concentrations in mouse plasma were determined using a high-sensitivity method developed by BioAgilytix, San Diego (formerly MicroConstants). The method uses high-performance liquid chromatography with tandem MS detection and is suitable for measuring concentrations ranging from 1 to 1000 ng/ml using 25.0 μl of mouse plasma extracted using a liquid-liquid approach.
Intracellular Ca2+ measurements
Naïve CD4+ T cells as well as TH1 and TH2 cells from WT and Orai1fl/flCd4Cre mice were loaded with 1 μM Fura-2-AM (Molecular Probes, F1221) for 30 min at room temperature, washed, and attached to 96-well plates (BD Falcon, 353219) coated with 0.01% (v/v) poly-l-Lysine (Sigma-Aldrich, P8920). At the beginning of each experiment, cells were kept in Ca2+-free Ringer solution [155 mM NaCl, 4.5 mM KCl, 3 mM MgCl2, 10 mM d-glucose, and 5 mM Na-Hepes (pH 7.4)], followed by treatment with 1 μM thapsigargin (Sigma-Aldrich, 586005) to deplete ER Ca2+ stores. Cells were subsequently perfused with Ca2+-containing Ringer solution [155 mM NaCl, 4.5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM d-glucose, and 5 mM Na-Hepes (pH 7.4)] at the indicated time points to induce SOCE. Alternatively, cells were stimulated with anti-CD3 (1 μg/ml; clone 2C11) for 30 min during the loading with Fura-2-AM. Cells were then washed and kept in Ca2+-free Ringer solution at the beginning of the recording, followed by CD3 cross-linking with rabbit anti-hamster IgG (1 μg/ml; MP Biomedicals, MP0855398), and addition of Ca2+-containing Ringer solution. Fura-2 fluorescence was measured at an emission wavelength of 510 nm after excitation at 340 nm and 380 nm using FlexStation 3 (Molecular Devices) and plotted as baseline-normalized F340/F380 emission ratio.
Statistical analyses
All results are means with or without the SEM. The statistical significance of differences between experimental groups was determined by paired or unpaired Student’s t test, Mann-Whitney test, one-way analysis of variance (ANOVA) with Tukey’s or Sidak’s multiple comparison test, or two-way ANOVA with Sidak multiple comparison test, as indicated in the figure legends. Differences were considered significant for *P < 0.05, **P < 0.01, and ***P < 0.001. The number of mice per experimental group is indicated in the respective figure legends. The statistical significance for DEGs was determined through DESeq2 using the Wald test. Differences were considered significant for an FDR of <0.10. Significantly expressed pathways through EnrichR and IPA were determined using Fisher’s exact test, with significance being defined for P < 0.10. The statistical significance for transcription factors in the LISA was determined using the one-sided Wilcoxon rank sum test statistic (49).
Acknowledgments
We acknowledge technical support from the Experimental Pathology Research Laboratory (RRID:SCR_017928), Genome Technology Center (RRID: SCR_017929), and Cytometry and Cell Sorting Laboratory (RRID: SCR_017926) at NYU Langone Health.
Funding: This study was funded by NIH grants AI097302 and AI130143 to S.F., AI150747 and AI121832 to P.G.T., and AI130343 and AI133076 to M.A.C.d.L. Additional funding was provided by postdoctoral fellowships from the German Research Foundation (DFG) to S.K. (KA 4514/1-1), M.V. (VA 882/1-1), and U.K. (KA 4083/2-1); postdoctoral fellowships from the SASS Foundation for Medical Research and the American Society of Hematology (ASH) to L.N.; and a Bernard Levine postdoctoral fellowship (NYU) to S.P.S. The Experimental Pathology Research Laboratory is partially supported by the Cancer Center Support Grant P30CA016087 at NYU Langone’s Laura and Isaac Perlmutter Cancer Center.
Author contributions: Y.-H.W., L.N., S.K., U.K., J.Z., M.M.-F., and M.V. conducted experiments. Y.-H.W., L.N., S.K., D.R., A.Y.T., U.K., M.M.-F., A.Y.T., I.S., F.Z., M.V., S.P.S., and S.F. analyzed data and interpreted the results. Y.-H.W., L.N., S.K., and S.F. designed the experiments. K.S., P.G.T., and M.A.C.d.L. provided reagents and expertise. S.K., L.N., and S.F. wrote the manuscript. All authors read and approved the final version of the manuscript.
Competing interests: S.F. is a scientific cofounder of Calcimedica. K.S. is a co-founder and CSO of CalciMedica. P.G.T. is on the scientific advisory board of Immunoscape and Cytoagents and has consulted for Johnson and Johnson and PACT Pharma in the past 3 years. The other authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. The RNA-seq data can be obtained in the Gene Expression Omnibus (GSE184228). CM4620 can be provided by CalciMedica pending scientific review and a completed material transfer agreement. Requests for the CM4620 should be submitted to: ken@calcimedica.com. A/HK/x31 can be provided by P.G.T. pending scientific review and a completed material transfer agreement. Requests for A/HK/x31 should be submitted to: paul.thomas@stjude.org.
Supplementary Materials
This PDF file includes:
Figs. S1 to S10
Tables S1 to S3
REFERENCES AND NOTES
- 1.Vaeth M., Kahlfuss S., Feske S., CRAC channels and calcium signaling in T cell-mediated immunity. Trends Immunol. 41, 878–901 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Y., Tao A., Vaeth M., Feske S., Calcium regulation of T cell metabolism. Curr. Opin. Physiol. 17, 207–223 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feske S., Calcium signalling in lymphocyte activation and disease. Nat. Rev. Immunol. 7, 690–702 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Trebak M., Kinet J. P., Calcium signalling in T cells. Nat. Rev. Immunol. 19, 154–169 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prakriya M., Lewis R. S., Store-operated calcium channels. Physiol. Rev. 95, 1383–1436 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lacruz R. S., Feske S., Diseases caused by mutations in ORAI1 and STIM1. Ann. N. Y. Acad. Sci. 1356, 45–79 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kahlfuss S., Kaufmann U., Concepcion A. R., Noyer L., Raphael D., Vaeth M., Yang J., Pancholi P., Maus M., Muller J., Kozhaya L., Khodadadi-Jamayran A., Sun Z., Shaw P., Unutmaz D., Stathopulos P. B., Feist C., Cameron S. B., Turvey S. E., Feske S., STIM1-mediated calcium influx controls antifungal immunity and the metabolic function of non-pathogenic Th17 cells. EMBO Mol. Med. 12, e11592 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaeth M., Yang J., Yamashita M., Zee I., Eckstein M., Knosp C., Kaufmann U., Karoly Jani P., Lacruz R. S., Flockerzi V., Kacskovics I., Prakriya M., Feske S., ORAI2 modulates store-operated calcium entry and T cell-mediated immunity. Nat. Commun. 8, 14714 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaeth M., Eckstein M., Shaw P. J., Kozhaya L., Yang J., Berberich-Siebelt F., Clancy R., Unutmaz D., Feske S., Store-operated Ca2+ entry in follicular T cells controls humoral immune responses and autoimmunity. Immunity 44, 1350–1364 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaw P. J., Weidinger C., Vaeth M., Luethy K., Kaech S. M., Feske S., CD4+ and CD8+ T cell-dependent antiviral immunity requires STIM1 and STIM2. J. Clin. Invest. 124, 4549–4563 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaufmann U., Kahlfuss S., Yang J., Ivanova E., Koralov S. B., Feske S., Calcium signaling controls pathogenic Th17 cell-mediated inflammation by regulating mitochondrial function. Cell Metab. 29, 1104–1118.e6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaufmann U., Shaw P. J., Kozhaya L., Subramanian R., Gaida K., Unutmaz D., McBride H. J., Feske S., Selective ORAI1 inhibition ameliorates autoimmune central nervous system inflammation by suppressing effector but not regulatory T cell function. J. Immunol. 196, +573–+585 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma J., McCarl C. A., Khalil S., Luthy K., Feske S., T-cell-specific deletion of STIM1 and STIM2 protects mice from EAE by impairing the effector functions of Th1 and Th17 cells. Eur. J. Immunol. 40, 3028–3042 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCarl C. A., Khalil S., Ma J., Oh-hora M., Yamashita M., Roether J., Kawasaki T., Jairaman A., Sasaki Y., Prakriya M., Feske S., Store-operated Ca2+ entry through ORAI1 is critical for T cell-mediated autoimmunity and allograft rejection. J. Immunol. 185, 5845–5858 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schuhmann M. K., Stegner D., Berna-Erro A., Bittner S., Braun A., Kleinschnitz C., Stoll G., Wiendl H., Meuth S. G., Nieswandt B., Stromal interaction molecules 1 and 2 are key regulators of autoreactive T cell activation in murine autoimmune central nervous system inflammation. J. Immunol. 184, 1536–1542 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Kim K. D., Srikanth S., Tan Y. V., Yee M. K., Jew M., Damoiseaux R., Jung M. E., Shimizu S., An D. S., Ribalet B., Waschek J. A., Gwack Y., Calcium signaling via Orai1 is essential for induction of the nuclear orphan receptor pathway to drive Th17 differentiation. J. Immunol. 192, 110–122 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hermann-Kleiter N., Baier G., NFAT pulls the strings during CD4+ T helper cell effector functions. Blood 115, 2989–2997 (2010). [DOI] [PubMed] [Google Scholar]
- 18.Choy D. F., Hart K. M., Borthwick L. A., Shikotra A., Nagarkar D. R., Siddiqui S., Jia G., Ohri C. M., Doran E., Vannella K. M., Butler C. A., Hargadon B., Sciurba J. C., Gieseck R. L., Thompson R. W., White S., Abbas A. R., Jackman J., Wu L. C., Egen J. G., Heaney L. G., Ramalingam T. R., Arron J. R., Wynn T. A., Bradding P., TH2 and TH17 inflammatory pathways are reciprocally regulated in asthma. Sci. Transl. Med. 7, 301ra129 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Lambrecht B. N., Hammad H., Fahy J. V., The cytokines of asthma. Immunity 50, 975–991 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Hirahara K., Poholek A., Vahedi G., Laurence A., Kanno Y., Milner J. D., O’Shea J. J., Mechanisms underlying helper T-cell plasticity: Implications for immune-mediated disease. J. Allergy Clin. Immunol. 131, 1276–1287 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gwack Y., Srikanth S., Oh-Hora M., Hogan P. G., Lamperti E. D., Yamashita M., Gelinas C., Neems D. S., Sasaki Y., Feske S., Prakriya M., Rajewsky K., Rao A., Hair loss and defective T- and B-cell function in mice lacking ORAI1. Mol. Cell. Biol. 28, 5209–5222 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oh-Hora M., Lu X., Shiokawa M., Takayanagi H., Yamasaki S., Stromal interaction molecule deficiency in T cells promotes spontaneous follicular helper T cell development and causes type 2 immune disorders. J. Immunol. 202, 2616–2627 (2019). [DOI] [PubMed] [Google Scholar]
- 23.Oh-Hora M., Yamashita M., Hogan P. G., Sharma S., Lamperti E., Chung W., Prakriya M., Feske S., Rao A., Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat. Immunol. 9, 432–443 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feske S., Draeger R., Peter H. H., Eichmann K., Rao A., The duration of nuclear residence of NFAT determines the pattern of cytokine expression in human SCID T cells. J. Immunol. 165, 297–305 (2000). [DOI] [PubMed] [Google Scholar]
- 25.Yoshino T., Ishikawa J., Ohga K., Morokata T., Takezawa R., Morio H., Okada Y., Honda K., Yamada T., YM-58483, a selective CRAC channel inhibitor, prevents antigen-induced airway eosinophilia and late phase asthmatic responses via Th2 cytokine inhibition in animal models. Eur. J. Pharmacol. 560, 225–233 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Kaur M., Birrell M. A., Dekkak B., Reynolds S., Wong S., De Alba J., Raemdonck K., Hall S., Simpson K., Begg M., Belvisi M. G., Singh D., The role of CRAC channel in asthma. Pulm. Pharmacol. Ther. 35, 67–74 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Sutovska M., Kocmalova M., Kazimierova I., Forsberg C. I. N., Joskova M., Adamkov M., Franova S., Effects of inhalation of STIM-Orai antagonist SKF 96365 on ovalbumin-induced airway remodeling in guinea pigs. Adv. Exp. Med. Biol. , (2021). [DOI] [PubMed] [Google Scholar]
- 28.Jairaman A., Maguire C. H., Schleimer R. P., Prakriya M., Allergens stimulate store-operated calcium entry and cytokine production in airway epithelial cells. Sci. Rep. 6, 32311 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jairaman A., Yamashita M., Schleimer R. P., Prakriya M., Store-operated Ca2+ release-activated Ca2+ channels regulate PAR2-activated Ca2+ signaling and cytokine production in airway epithelial cells. J. Immunol. 195, 2122–2133 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson W. W., Shay D. K., Weintraub E., Brammer L., Cox N., Anderson L. J., Fukuda K., Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 289, 179–186 (2003). [DOI] [PubMed] [Google Scholar]
- 31.Krammer F., Smith G. J. D., Fouchier R. A. M., Peiris M., Kedzierska K., Doherty P. C., Palese P., Shaw M. L., Treanor J., Webster R. G., Garcia-Sastre A., Influenza. Nat. Rev. Dis. Primers 4, 3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pizzolla A., Nguyen T. H. O., Smith J. M., Brooks A. G., Kedzieska K., Heath W. R., Reading P. C., Wakim L. M., Resident memory CD8+ T cells in the upper respiratory tract prevent pulmonary influenza virus infection. Sci. Immunol. 2, eaam6970 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Zens K. D., Chen J. K., Guyer R. S., Wu F. L., Cvetkovski F., Miron M., Farber D. L., Reduced generation of lung tissue-resident memory T cells during infancy. J. Exp. Med. 214, 2915–2932 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strutt T. M., Dhume K., Finn C. M., Hwang J. H., Castonguay C., Swain S. L., McKinstry K. K., IL-15 supports the generation of protective lung-resident memory CD4 T cells. Mucosal Immunol. 11, 668–680 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cullen J. G., McQuilten H. A., Quinn K. M., Olshansky M., Russ B. E., Morey A., Wei S., Prier J. E., La Gruta N. L., Doherty P. C., Turner S. J., CD4+ T help promotes influenza virus-specific CD8+ T cell memory by limiting metabolic dysfunction. Proc. Natl. Acad. Sci. U.S.A. 116, 4481–4488 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crotty S., T follicular helper cell differentiation, function, and roles in disease. Immunity 41, 529–542 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas P. G., Brown S. A., Yue W., So J., Webby R. J., Doherty P. C., An unexpected antibody response to an engineered influenza virus modifies CD8+ T cell responses. Proc. Natl. Acad. Sci. U.S.A. 103, 2764–2769 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davidson S., Crotta S., McCabe T. M., Wack A., Pathogenic potential of interferon αβ in acute influenza infection. Nat. Commun. 5, 3864 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fu W., Liu X., Lin X., Feng H., Sun L., Li S., Chen H., Tang H., Lu L., Jin W., Dong C., Deficiency in T follicular regulatory cells promotes autoimmunity. J. Exp. Med. 215, 815–825 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.La Gruta N. L., Turner S. J., T cell mediated immunity to influenza: Mechanisms of viral control. Trends Immunol. 35, 396–402 (2014). [DOI] [PubMed] [Google Scholar]
- 41.Turner S. J., Olivas E., Gutierrez A., Diaz G., Doherty P. C., Disregulated influenza A virus-specific CD8+ T cell homeostasis in the absence of IFN-γ signaling. J. Immunol. 178, 7616–7622 (2007). [DOI] [PubMed] [Google Scholar]
- 42.Hussell T., Pennycook A., Openshaw P. J., Inhibition of tumor necrosis factor reduces the severity of virus-specific lung immunopathology. Eur. J. Immunol. 31, 2566–2573 (2001). [DOI] [PubMed] [Google Scholar]
- 43.Peper R. L., Van Campen H., Tumor necrosis factor as a mediator of inflammation in influenza A viral pneumonia. Microb. Pathog. 19, 175–183 (1995). [DOI] [PubMed] [Google Scholar]
- 44.Denlinger L. C., Phillips B. R., Ramratnam S., Ross K., Bhakta N. R., Cardet J. C., Castro M., Peters S. P., Phipatanakul W., Aujla S., Bacharier L. B., Bleecker E. R., Comhair S. A., Coverstone A., DeBoer M., Erzurum S. C., Fain S. B., Fajt M., Fitzpatrick A. M., Gaffin J., Gaston B., Hastie A. T., Hawkins G. A., Holguin F., Irani A. M., Israel E., Levy B. D., Ly N., Meyers D. A., Moore W. C., Myers R., Opina M. T., Peters M. C., Schiebler M. L., Sorkness R. L., Teague W. G., Wenzel S. E., Woodruff P. G., Mauger D. T., Fahy J. V., Jarjour N. N.; National Heart, Lung, and Blood Institute’s Severe Asthma Research Program-3 Investigators , Inflammatory and comorbid features of patients with severe asthma and frequent exacerbations. Am. J. Respir. Crit. Care Med. 195, 302–313 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakagome K., Nagata M., Involvement and possible role of eosinophils in asthma exacerbation. Front. Immunol. 9, 2220 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fulkerson P. C., Fischetti C. A., McBride M. L., Hassman L. M., Hogan S. P., Rothenberg M. E., A central regulatory role for eosinophils and the eotaxin/CCR3 axis in chronic experimental allergic airway inflammation. Proc. Natl. Acad. Sci. U.S.A. 103, 16418–16423 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teran L. M., Mochizuki M., Bartels J., Valencia E. L., Nakajima T., Hirai K., Schroder J. M., Th1- and Th2-type cytokines regulate the expression and production of eotaxin and RANTES by human lung fibroblasts. Am. J. Respir. Cell Mol. Biol. 20, 777–786 (1999). [DOI] [PubMed] [Google Scholar]
- 48.Li L., Xia Y., Nguyen A., Lai Y. H., Feng L., Mosmann T. R., Lo D., Effects of Th2 cytokines on chemokine expression in the lung: IL-13 potently induces eotaxin expression by airway epithelial cells. J. Immunol. 162, 2477–2487 (1999). [PubMed] [Google Scholar]
- 49.Qin Q., Fan J., Zheng R., Wan C., Mei S., Wu Q., Sun H., Brown M., Zhang J., Meyer C. A., Liu X. S., Lisa: Inferring transcriptional regulators through integrative modeling of public chromatin accessibility and ChIP-seq data. Genome Biol. 21, 32 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lohoff M., Mittrucker H. W., Prechtl S., Bischof S., Sommer F., Kock S., Ferrick D. A., Duncan G. S., Gessner A., Mak T. W., Dysregulated T helper cell differentiation in the absence of interferon regulatory factor 4. Proc. Natl. Acad. Sci. U.S.A. 99, 11808–11812 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rengarajan J., Mowen K. A., McBride K. D., Smith E. D., Singh H., Glimcher L. H., Interferon regulatory factor 4 (IRF4) interacts with NFATc2 to modulate interleukin 4 gene expression. J. Exp. Med. 195, 1003–1012 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Betz B. C., Jordan-Williams K. L., Wang C., Kang S. G., Liao J., Logan M. R., Kim C. H., Taparowsky E. J., Batf coordinates multiple aspects of B and T cell function required for normal antibody responses. J. Exp. Med. 207, 933–942 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuwahara M., Ise W., Ochi M., Suzuki J., Kometani K., Maruyama S., Izumoto M., Matsumoto A., Takemori N., Takemori A., Shinoda K., Nakayama T., Ohara O., Yasukawa M., Sawasaki T., Kurosaki T., Yamashita M., Bach2-Batf interactions control Th2-type immune response by regulating the IL-4 amplification loop. Nat. Commun. 7, 12596 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu G., Lu Y., Thulasi Raman S. N., Xu F., Wu Q., Li Z., Brownlie R., Liu Q., Zhou Y., Nuclear-resident RIG-I senses viral replication inducing antiviral immunity. Nat. Commun. 9, 3199 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Graham M. B., Braciale V. L., Braciale T. J., Influenza virus-specific CD4+ T helper type 2 T lymphocytes do not promote recovery from experimental virus infection. J. Exp. Med. 180, 1273–1282 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Upadhyaya B., Yin Y., Hill B. J., Douek D. C., Prussin C., Hierarchical IL-5 expression defines a subpopulation of highly differentiated human Th2 cells. J. Immunol. 187, 3111–3120 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu J., Yamane H., Cote-Sierra J., Guo L., Paul W. E., GATA-3 promotes Th2 responses through three different mechanisms: Induction of Th2 cytokine production, selective growth of Th2 cells and inhibition of Th1 cell-specific factors. Cell Res. 16, 3–10 (2006). [DOI] [PubMed] [Google Scholar]
- 58.Yagi R., Zhu J., Paul W. E., An updated view on transcription factor GATA3-mediated regulation of Th1 and Th2 cell differentiation. Int. Immunol. 23, 415–420 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sahoo A., Wali S., Nurieva R., T helper 2 and T follicular helper cells: Regulation and function of interleukin-4. Cytokine Growth Factor Rev. 30, 29–37 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Murphy T. L., Tussiwand R., Murphy K. M., Specificity through cooperation: BATF-IRF interactions control immune-regulatory networks. Nat. Rev. Immunol. 13, 499–509 (2013). [DOI] [PubMed] [Google Scholar]
- 61.Kurata H., Lee H. J., O’Garra A., Arai N., Ectopic expression of activated Stat6 induces the expression of Th2-specific cytokines and transcription factors in developing Th1 cells. Immunity 11, 677–688 (1999). [DOI] [PubMed] [Google Scholar]
- 62.Zhu J., Guo L., Watson C. J., Hu-Li J., Paul W. E., Stat6 is necessary and sufficient for IL-4’s role in Th2 differentiation and cell expansion. J. Immunol. 166, 7276–7281 (2001). [DOI] [PubMed] [Google Scholar]
- 63.Cote-Sierra J., Foucras G., Guo L., Chiodetti L., Young H. A., Hu-Li J., Zhu J., Paul W. E., Interleukin 2 plays a central role in Th2 differentiation. Proc. Natl. Acad. Sci. U.S.A. 101, 3880–3885 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ross S. H., Cantrell D. A., Signaling and function of interleukin-2 in T lymphocytes. Annu. Rev. Immunol. 36, 411–433 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Renzi P. M., Sapienza S., Waserman S., Du T., Olivenstein R., Wang N. S., Martin J. G., Effect of interleukin-2 on the airway response to antigen in the rat. Am. Rev. Respir. Dis. 146, 163–169 (1992). [DOI] [PubMed] [Google Scholar]
- 66.Milne A. A., Teixeira M. M., Hellewell P. G., Piper P. J., Induction of leucocyte recruitment and bronchial hyperresponsiveness in the guinea pig by aerosol administration of interleukin-2. Int. Arch. Allergy Immunol. 108, 60–67 (1995). [DOI] [PubMed] [Google Scholar]
- 67.Waldron R. T., Chen Y., Pham H., Go A., Su H. Y., Hu C., Wen L., Husain S. Z., Sugar C. A., Roos J., Ramos S., Lugea A., Dunn M., Stauderman K., Pandol S. J., The Orai Ca2+ channel inhibitor CM4620 targets both parenchymal and immune cells to reduce inflammation in experimental acute pancreatitis. J. Physiol. 597, 3085–3105 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.DeBerge M. P., Ely K. H., Enelow R. I., Soluble, but not transmembrane, TNF-α is required during influenza infection to limit the magnitude of immune responses and the extent of immunopathology. J. Immunol. 192, 5839–5851 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Quinn K. M., Kan W. T., Watson K. A., Liddicoat B. J., Swan N. G., McQuilten H., Denton A. E., Li J., Chen W., Brown L. E., Jackson D. C., Reading P. C., Doherty P. C., Kedzierska K., Kedzierski L., Turner S. J., La Gruta N. L., Extrinsically derived TNF is primarily responsible for limiting antiviral CD8+ T cell response magnitude. PLOS ONE 12, e0184732 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baumgarth N., Kelso A., In vivo blockade of gamma interferon affects the influenza virus-induced humoral and the local cellular immune response in lung tissue. J. Virol. 70, 4411–4418 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Karupiah G., Chen J. H., Mahalingam S., Nathan C. F., MacMicking J. D., Rapid interferon γ–dependent clearance of influenza A virus and protection from consolidating pneumonitis in nitric oxide synthase 2-deficient mice. J. Exp. Med. 188, 1541–1546 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Graham M. B., Dalton D. K., Giltinan D., Braciale V. L., Stewart T. A., Braciale T. J., Response to influenza infection in mice with a targeted disruption in the interferon gamma gene. J. Exp. Med. 178, 1725–1732 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nicol M. Q., Campbell G. M., Shaw D. J., Dransfield I., Ligertwood Y., Beard P. M., Nash A. A., Dutia B. M., Lack of IFNγ signaling attenuates spread of influenza A virus in vivo and leads to reduced pathogenesis. Virology 526, 155–164 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hammad H., Lambrecht B. N., The basic immunology of asthma. Cell 184, 1469–1485 (2021). [DOI] [PubMed] [Google Scholar]
- 75.Wei G., Wei L., Zhu J., Zang C., Hu-Li J., Yao Z., Cui K., Kanno Y., Roh T. Y., Watford W. T., Schones D. E., Peng W., Sun H. W., Paul W. E., O’Shea J. J., Zhao K., Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity 30, 155–167 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kock J., Kreher S., Lehmann K., Riedel R., Bardua M., Lischke T., Jargosch M., Haftmann C., Bendfeldt H., Hatam F., Mashreghi M. F., Baumgrass R., Radbruch A., Chang H. D., Nuclear factor of activated T cells regulates the expression of interleukin-4 in Th2 cells in an all-or-none fashion. J. Biol. Chem. 289, 26752–26761 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maier E., Duschl A., Horejs-Hoeck J., STAT6-dependent and -independent mechanisms in Th2 polarization. Eur. J. Immunol. 42, 2827–2833 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liao W., Lin J. X., Wang L., Li P., Leonard W. J., Modulation of cytokine receptors by IL-2 broadly regulates differentiation into helper T cell lineages. Nat. Immunol. 12, 551–559 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gomez-Rodriguez J., Meylan F., Handon R., Hayes E. T., Anderson S. M., Kirby M. R., Siegel R. M., Schwartzberg P. L., Itk is required for Th9 differentiation via TCR-mediated induction of IL-2 and IRF4. Nat. Commun. 7, 10857 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McCarl C. A., Picard C., Khalil S., Kawasaki T., Rother J., Papolos A., Kutok J., Hivroz C., Ledeist F., Plogmann K., Ehl S., Notheis G., Albert M. H., Belohradsky B. H., Kirschner J., Rao A., Fischer A., Feske S., ORAI1 deficiency and lack of store-operated Ca2+ entry cause immunodeficiency, myopathy, and ectodermal dysplasia. J. Allergy Clin. Immunol. 124, 1311–1318.e7 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gowthaman U., Chen J. S., Zhang B., Flynn W. F., Lu Y., Song W., Joseph J., Gertie J. A., Xu L., Collet M. A., Grassmann J. D. S., Simoneau T., Chiang D., Berin M. C., Craft J. E., Weinstein J. S., Williams A., Eisenbarth S. C., Identification of a T follicular helper cell subset that drives anaphylactic IgE. Science 365, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Coffman R. L., Carty J., A T cell activity that enhances polyclonal IgE production and its inhibition by interferon-γ. J. Immunol. 136, 949–954 (1986). [PubMed] [Google Scholar]
- 83.Pene J., Gauchat J. F., Lecart S., Drouet E., Guglielmi P., Boulay V., Delwail A., Foster D., Lecron J. C., Yssel H., Cutting edge: IL-21 is a switch factor for the production of IgG1 and IgG3 by human B cells. J. Immunol. 172, 5154–5157 (2004). [DOI] [PubMed] [Google Scholar]
- 84.Ozaki K., Spolski R., Feng C. G., Qi C. F., Cheng J., Sher A., Morse H. C. III, Liu C., Schwartzberg P. L., Leonard W. J., A critical role for IL-21 in regulating immunoglobulin production. Science 298, 1630–1634 (2002). [DOI] [PubMed] [Google Scholar]
- 85.Shang X. Z., Ma K. Y., Radewonuk J., Li J., Song X. Y., Griswold D. E., Emmell E., Li L., IgE isotype switch and IgE production are enhanced in IL-21-deficient but not IFN-γ-deficient mice in a Th2-biased response. Cell. Immunol. 241, 66–74 (2006). [DOI] [PubMed] [Google Scholar]
- 86.D’Souza S. J., Pajak A., Balazsi K., Dagnino L., Ca2+ and BMP-6 signaling regulate E2F during epidermal keratinocyte differentiation. J. Biol. Chem. 276, 23531–23538 (2001). [DOI] [PubMed] [Google Scholar]
- 87.Vaeth M., Wang Y. H., Eckstein M., Yang J., Silverman G. J., Lacruz R. S., Kannan K., Feske S., Tissue resident and follicular Treg cell differentiation is regulated by CRAC channels. Nat. Commun. 10, 1183 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xiong Y., Hannon G. J., Zhang H., Casso D., Kobayashi R., Beach D., p21 is a universal inhibitor of cyclin kinases. Nature 366, 701–704 (1993). [DOI] [PubMed] [Google Scholar]
- 89.Watanabe M., Moon K. D., Vacchio M. S., Hathcock K. S., Hodes R. J., Downmodulation of tumor suppressor p53 by T cell receptor signaling is critical for antigen-specific CD4+ T cell responses. Immunity 40, 681–691 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kahl C. R., Means A. R., Regulation of cell cycle progression by calcium/calmodulin-dependent pathways. Endocr. Rev. 24, 719–736 (2003). [DOI] [PubMed] [Google Scholar]
- 91.Khanna A. K., Hosenpud J. D., Cyclosporine induces the expression of the cyclin inhibitor p21. Transplantation 67, 1262–1268 (1999). [DOI] [PubMed] [Google Scholar]
- 92.Jelley-Gibbs D. M., Brown D. M., Dibble J. P., Haynes L., Eaton S. M., Swain S. L., Unexpected prolonged presentation of influenza antigens promotes CD4 T cell memory generation. J. Exp. Med. 202, 697–706 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Desvignes L., Weidinger C., Shaw P., Vaeth M., Ribierre T., Liu M., Fergus T., Kozhaya L., McVoy L., Unutmaz D., Ernst J. D., Feske S., STIM1 controls T cell-mediated immune regulation and inflammation in chronic infection. J. Clin. Invest. 125, 2347–2362 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chang W. C., Lee C. H., Hirota T., Wang L. F., Doi S., Miyatake A., Enomoto T., Tomita K., Sakashita M., Yamada T., Fujieda S., Ebe K., Saeki H., Takeuchi S., Furue M., Chen W. C., Chiu Y. C., Chang W. P., Hong C. H., Hsi E., Juo S. H., Yu H. S., Nakamura Y., Tamari M., ORAI1 genetic polymorphisms associated with the susceptibility of atopic dermatitis in Japanese and Taiwanese populations. PLOS ONE 7, e29387 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yeh Y. C., Lin Y. P., Kramer H., Parekh A. B., Single-nucleotide polymorphisms in Orai1 associated with atopic dermatitis inhibit protein turnover, decrease calcium entry and disrupt calcium-dependent gene expression. Hum. Mol. Genet. 29, 1808–1823 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Paller A. S., Spergel J. M., Mina-Osorio P., Irvine A. D., The atopic march and atopic multimorbidity: Many trajectories, many pathways. J. Allergy Clin. Immunol. 143, 46–55 (2019). [DOI] [PubMed] [Google Scholar]
- 97.Sutovska M., Kocmalova M., Joskova M., Adamkov M., Franova S., The effect of long-term administered CRAC channels blocker on the functions of respiratory epithelium in guinea pig allergic asthma model. Gen. Physiol. Biophys. 34, 167–176 (2015). [DOI] [PubMed] [Google Scholar]
- 98.Di Capite J., Nelson C., Bates G., Parekh A. B., Targeting Ca2+ release-activated Ca2+ channel channels and leukotriene receptors provides a novel combination strategy for treating nasal polyposis. J. Allergy Clin. Immunol. 124, 1014–1021.e1–3 (2009). [DOI] [PubMed] [Google Scholar]
- 99.Di Capite J. L., Bates G. J., Parekh A. B., Mast cell CRAC channel as a novel therapeutic target in allergy. Curr. Opin. Allergy Clin. Immunol. 11, 33–38 (2011). [DOI] [PubMed] [Google Scholar]
- 100.Somasundaram A., Shum A. K., McBride H. J., Kessler J. A., Feske S., Miller R. J., Prakriya M., Store-operated CRAC channels regulate gene expression and proliferation in neural progenitor cells. J. Neurosci. 34, 9107–9123 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Laffont S., Blanquart E., Savignac M., Cenac C., Laverny G., Metzger D., Girard J. P., Belz G. T., Pelletier L., Seillet C., Guery J. C., Androgen signaling negatively controls group 2 innate lymphoid cells. J. Exp. Med. 214, 1581–1592 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Melgert B. N., Postma D. S., Kuipers I., Geerlings M., Luinge M. A., van der Strate B. W., Kerstjens H. A., Timens W., Hylkema M. N., Female mice are more susceptible to the development of allergic airway inflammation than male mice. Clin. Exp. Allergy 35, 1496–1503 (2005). [DOI] [PubMed] [Google Scholar]
- 103.Karakus U., Crameri M., Lanz C., Yanguez E., Propagation and titration of influenza viruses. Methods Mol. Biol. 1836, 59–88 (2018). [DOI] [PubMed] [Google Scholar]
- 104.Ballesteros-Tato A., Randall T. D., Lund F. E., Spolski R., Leonard W. J., Leon B., T follicular helper cell plasticity shapes pathogenic T helper 2 cell-mediated immunity to inhaled house dust mite. Immunity 44, 259–273 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kajon A. E., Gigliotti A. P., Harrod K. S., Acute inflammatory response and remodeling of airway epithelium after subspecies B1 human adenovirus infection of the mouse lower respiratory tract. J. Med. Virol. 71, 233–244 (2003). [DOI] [PubMed] [Google Scholar]
- 106.Schneider C. A., Rasband W. S., Eliceiri K. W., NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim D. I., Song M. K., Lee K., Comparison of asthma phenotypes in OVA-induced mice challenged via inhaled and intranasal routes. BMC Pulm. Med. 19, 241 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kujur W., Gurram R. K., Haleem N., Maurya S. K., Agrewala J. N., Caerulomycin A inhibits Th2 cell activity: A possible role in the management of asthma. Sci. Rep. 5, 15396 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lian J., Cuk M., Kahlfuss S., Kozhaya L., Vaeth M., Rieux-Laucat F., Picard C., Benson M. J., Jakovcevic A., Bilic K., Martinac I., Stathopulos P., Kacskovics I., Vraetz T., Speckmann C., Ehl S., Issekutz T., Unutmaz D., Feske S., ORAI1 mutations abolishing store-operated Ca2+ entry cause anhidrotic ectodermal dysplasia with immunodeficiency. J. Allergy Clin. Immunol. 142, 1297–1310.e11 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bolger A. M., Lohse M., Usadel B., Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dobin A., Davis C. A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., Gingeras T. R., STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Subramanian A., Tamayo P., Mootha V. K., Mukherjee S., Ebert B. L., Gillette M. A., Paulovich A., Pomeroy S. L., Golub T. R., Lander E. S., Mesirov J. P., Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. U.S.A. 102, 15545–15550 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen E. Y., Tan C. M., Kou Y., Duan Q., Wang Z., Meirelles G. V., Clark N. R., Ma’ayan A., Enrichr: Interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics 14, 128 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kramer A., Green J., Pollard J. Jr., Tugendreich S., Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 30, 523–530 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Abbas A. R., Baldwin D., Ma Y., Ouyang W., Gurney A., Martin F., Fong S., van Lookeren Campagne M., Godowski P., Williams P. M., Chan A. C., Clark H. F., Immune response in silico (IRIS): Immune-specific genes identified from a compendium of microarray expression data. Genes Immun. 6, 319–331 (2005). [DOI] [PubMed] [Google Scholar]
- 116.Schaefer C. F., Anthony K., Krupa S., Buchoff J., Day M., Hannay T., Buetow K. H., PID: The pathway interaction database. Nucleic Acids Res. 37, D674–D679 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S10
Tables S1 to S3