Abstract
Aims
Tapentadol, an opioid with mu‐opioid receptor agonism and noradrenaline reuptake inhibition, has been increasingly used in Australia since 2011. However, data on hospital prescribing trends and indications are scarce. This study aimed to investigate hospital prescribing trends of tapentadol, oxycodone and tramadol in a Sydney local health district (LHD) and the indications for tapentadol hospital prescriptions in an Australian tertiary hospital.
Methods
We analysed 5‐year patient dispensing for tapentadol, oxycodone and tramadol from four hospitals in a Sydney LHD with data expressed as oral morphine equivalents (OME). We also conducted a retrospective review of 140 and 54 patients prescribed tapentadol at a tertiary hospital's surgical and spinal units in 2020.
Results
Over 5 years in the Sydney LHD, there was a 19.5% reduction in total dispensing of these opioids from 1 225 210 to 986 477.5 OME milligrams. Decreases were specifically for oxycodone (−37.8% immediate‐release, −65.2% sustained‐release) and tramadol (−74.6% immediate‐release, −70.1% sustained‐release). Contrastingly, hospital prescriptions of tapentadol immediate‐release increased by 223.2% between 2018–19 and 2020–21 while sustained‐release increased by 17.9% from 2016–17 to 2020–21. By 2020–21, tapentadol overtook oxycodone to become the most prescribed opioid in the Sydney LHD (51.4%). At the hospital's surgical units, 137 (97.9%) patients were prescribed tapentadol for acute post‐operative pain with the majority (54.0%) prescribed both immediate‐release and sustained‐release tapentadol, while 71.1% were prescribed for neuropathic pain in the spinal units.
Conclusion
In a Sydney LHD, tapentadol prescriptions increased significantly to become the preferred opioid analgesic. At the hospital's surgical units, off‐label prescriptions of tapentadol sustained‐release for acute post‐operative pain were observed.
Keywords: hospital prescription, immediate‐release, sustained‐release, tapentadol
What is known about this subject?
Tapentadol is a mu‐opioid agonist and has noradrenaline re‐uptake inhibition.
Sustained‐release tapentadol has been recommended to be used for moderate chronic pain in particular for neuropathic pain.
What this study adds?
Tapentadol has replaced oxycodone and tramadol to become the most frequently prescribed oral opioid for moderate to severe pain in a Sydney local health district.
Off label prescription of sustained‐release tapentadol has been used for acute post‐operative pain in a Sydney tertiary hospital.
1. INTRODUCTION
Tapentadol is an opioid with two mechanisms of action – mu‐opioid receptor agonism and noradrenaline reuptake inhibition. 1 This dual‐action allows tapentadol to be an effective analgesic in various pain conditions, from nociceptive to neuropathic pain with reductions in frequency of opioid‐related gastrointestinal side effects, 2 , 3 contributing to improved patient compliance. Currently, there are two formulations of tapentadol: sustained‐release (SR) and immediate‐release (IR). Tapentadol IR, a short‐acting analgesic is registered in Australia in 2011 for the treatment of moderate to severe pain. 4 Meanwhile, tapentadol SR is indicated for the management of moderate to severe chronic pain for which no other non‐opioid analgesia are adequate. 5
Tapentadol has been advocated as a drug that provides analgesia through its parent compound, with no active metabolites, and that it has less potential for drug interactions 6 or genetic variability. 7 However, there is emerging data suggesting risks remain significant for dependence, 8 abuse and diversion 9 and fatal poisoning. 10 , 11 , 12 A recent study reported tapentadol was contributory to 159 fatalities with data retrieved from the National Coronial Information System in Australia over a 20 year period (2000–2020). 13 In this study, about 76% was considered unintentional and involved multiple drugs with numerous morbidities. 13
Tapentadol has been increasingly used in the Australian community. 14 In December 2017, an Australian study reported that tapentadol had the sixth largest market share of number of packs sold and third largest market share of total monthly OME sold at 10.7% behind oxycodone (31.2%) and tramadol (18.3%). 14 In particular, Western Australia recorded nearly two‐fold the annual national average unit sales of tapentadol slow‐release formulation. 14 However, this study excluded hospital sales. The increase in tapentadol's community use has raised concerns in terms of indications and burden of cost. Yet, there is a lack of information on tapentadol use within hospitals. As tapentadol is a relatively new opioid, monitoring its prescribing trends is useful in identifying the reasons for its use compared to other available opioids and establishing guidelines for the safer prescription of tapentadol in clinical practice. Hence, it is imperative to describe the hospital prescribing trend of tapentadol and relate this trend to its indications.
1.1. Aims
This study aimed to investigate the five‐year hospital prescribing trend of tapentadol compared to other commonly prescribed opioids (tramadol and oxycodone) in a Sydney local health district; and review the indications of tapentadol prescription in an Australian tertiary hospital.
2. METHODS
2.1. Study design
This was a retrospective observational study conducted in Sydney, Australia using two data sources described below. The study was approved by the South‐Eastern Sydney Local Health District Human Research Ethics Committee (HREC00134; 2020/ETH01256).
2.2. Data source
2.2.1. Opioid prescribing data
Inpatient dispensing claims for tapentadol, tramadol and oxycodone from four hospitals: Prince of Wales Hospital (POWH), Sydney and Sydney Eye Hospital (SYD), St George Hospital (STG) and Sutherland Hospital (TSH) in the South‐Eastern Sydney Local Health District (SESLHD) were collected from August 2016 to July 2021. Tapentadol, tramadol and oxycodone were all available in two formulations: SR and IR. Tapentadol IR was available in the pharmacy formulary at TSH and SGH since 2017 and at all four hospitals since 2018. This study presents data on hospital dispensing of the three opioids; community sales were excluded.
2.2.2. Tapentadol individual patient data
We further investigated indications for tapentadol prescription at POWH by performing a retrospective chart review of 20% of patients that were prescribed tapentadol in the surgical unit. This is performed by making a random selection of patients each month from January to December in 2020. We also included all patients in spinal rehabilitation and acute spinal medicine that were prescribed tapentadol in 2020. Individual patient data were retrieved from electronic medical records and entered into a pre‐formatted Excel spreadsheet. Data collected included demographic data (gender and age), indication of use (instance.g., post‐operative pain, neuropathic pain, etc.), formulation of tapentadol prescribed (SR, IR or both) and concurrent use of other pain medications (in particular other neuropathic pain medications).
2.3. Data analysis
Opioid prescribing data were analysed over 5 years from August 2016 to July 2021 to evaluate tapentadol's annual prescribing trend compared with oxycodone and tramadol in the SESLHD and to provide an overview on the distribution of tapentadol prescription at POWH's specialty units. Results were presented graphically by each opioid type and formulation with data expressed as total oral morphine equivalent (OME) to adjust for opioid potency. In this study, OME measurement for each opioid was calculated as follows: the number of tablets dispensed × the number of milligrams per tablet × the OME conversion factor that was adapted from the guidelines developed by the National Drug and Alcohol Research Centre (Table 1). 15 In this analysis, we used the terms opioid prescription, dispensing and use interchangeably.
TABLE 1.
Recommended oral morphine equivalent (OME) conversion factor, adapted from National Drug and Alcohol Research Centre 10
| Oral preparations | Recommended OME conversion factor National Drugs and Alcohol Research Centre guidelines |
|---|---|
| Morphine | 1 |
| Oxycodone | 1.5 |
| Tapentadol | 0.4 |
| Tramadol | 0.2 |
| Hydromorphone | 5 |
| Codeine | 0.13 |
| Dextropropoxyphene | 0.1 |
| Methadone | 4.7 |
Descriptive statistics were used to describe the indications of tapentadol prescription among the 2020 individual patient data sample from POWH. Continuous variables were reported as medians and interquartile ranges (IQR). Categorical data were reported as frequencies and percentages.
Data analyses in the study were performed using Microsoft Excel (version 16.51) and GraphPad Prism (version 9.2.0); graphs were generated using GraphPad Prism (version 9.2.0).
2.4. Nomenclature of Targets and Ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, and are permanently archived in the Concise Guide to PHARMACOLOGY 2021/22. 16 , 17
3. RESULTS
3.1. Trends of opioid prescribing
Over 5 years in SESLHD, there was a 19.5% reduction in the total annual hospital prescription of the three opioids (tapentadol, oxycodone and tramadol) from 1 225 210 in 2016–17 to 986 477.5 OME milligrams in 2020–21. Specifically, there were decreases in OME prescription of oxycodone (−37.8% IR and −65.2% SR) and tramadol (−74.6% IR and −70.1 SR) (Figure 1A). Contrastingly, hospital prescriptions of tapentadol increased (Figure 1A). Tapentadol IR's prescription increased dramatically by 223.2% from 82 620 in 2018–19 to 267 040 OME milligrams in 2020–21 across the SESLHD. Meanwhile, the prescription of tapentadol SR increased at a smaller percentage of 17.9% from 203 460 in 2016–17 to 239 980 OME milligrams in 2020–21 (Figure 1A).
FIGURE 1.
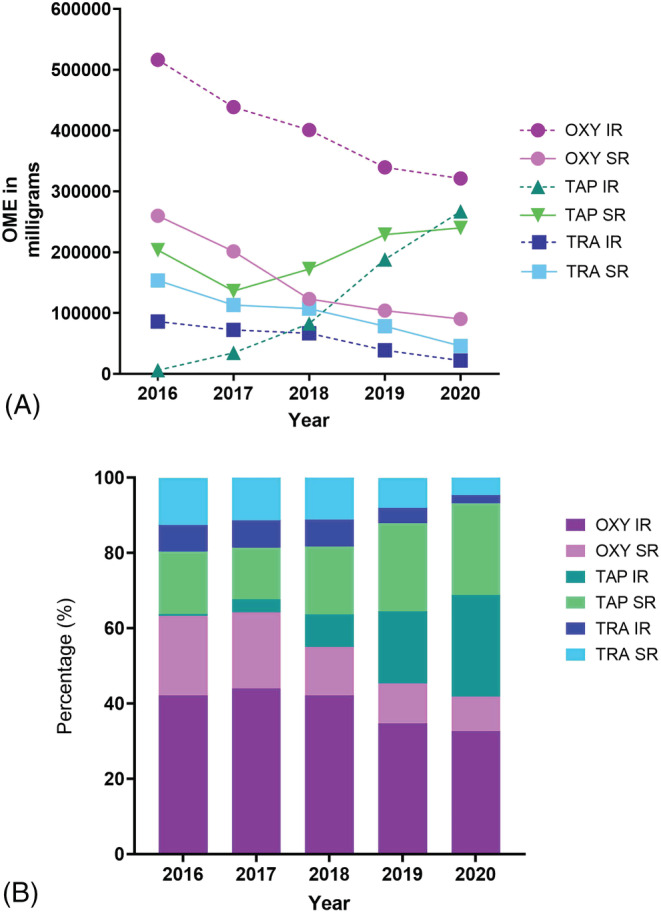
Annual hospital prescribing trends in the South‐Eastern Sydney Local Health District from August 2016 to July 2021 by opioid type and formulation: (A) Total OME milligrams dispensed; (B) Percentage of total OME milligrams dispensed.OME: oral morphine equivalent, OXY: oxycodone, TAP: tapentadol, TRA: tramadol, SR: sustained‐release, IR: immediate‐release
In 2016–17, oxycodone was the most prescribed opioid (63.3% of total OME prescriptions), followed by tramadol (19.5%) and tapentadol (17.1%) (Figure 1B). By 2020–21, however, amongst the three opioids studied, tapentadol was the most prescribed opioid, accounting for 51.4% of total OME prescriptions, followed by oxycodone at 41.8% and tramadol 6.9%.
3.2. Pattern of tapentadol prescribing
The pattern of tapentadol prescription in the different specialty units at POWH is presented in Figure 2A and B. Over 5 years, the surgical, neurology and spinal rehabilitation units consistently accounted for the three largest percentage of tapentadol prescriptions at POWH. During this period, the surgical unit was responsible for the majority of tapentadol prescriptions, accounting for 55.4% and 41.1% of all tapentadol IR and SR prescriptions, respectively. In the surgical unit, there was a 3.0‐fold and 2.2‐fold increase in tapentadol IR and SR dispensing respectively.
FIGURE 2.
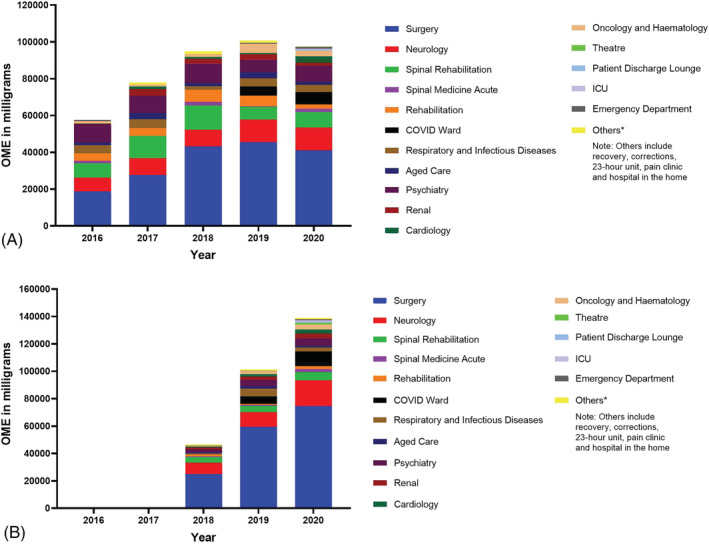
Pattern of tapentadol prescribing at Prince of Wales Hospital: (A) tapentadol SR; (B) tapentadol IR
Meanwhile, over 5 years, the neurology unit accounted for 13.2% and 11.7% of all tapentadol IR and SR prescriptions, respectively. Contrastingly, during the same period, the spinal rehabilitation unit was responsible for only 5.2% and 11.4% of all tapentadol IR and SR prescriptions, respectively.
As the increasing use of tapentadol at POWH was primarily driven by prescriptions in the surgical unit, a retrospective chart review of 140 patients who were prescribed tapentadol at POWH's surgical unit in 2020 was analysed. Eighty‐seven (62.1%) were males, median age of 63 years old (IQR: 47–74). Tapentadol was prescribed to 137 (97.9%) patients for acute post‐operative pain with 74 (54.0%) patients prescribed both tapentadol IR and SR, 46 (33.6%) patients prescribed tapentadol IR and 17 (12.4%) patients prescribed tapentadol SR.
In 2020, 54 patients prescribed tapentadol at POWH's spinal rehabilitation and spinal medicine acute units were analysed. Thirty‐one (57.4%) were males, with a median age of 53 years old (IQR: 38–68). Of the 54 patients, 45 (83.3%) were prescribed tapentadol for neuropathic pain with 32 (71.1%) prescribed both tapentadol IR and SR, five (11.1%) with tapentadol IR and eight (17.8%) with tapentadol SR. Additionally, of the 45 patients prescribed tapentadol for neuropathic pain, 42 (93.3%) were concurrently taking “anti‐neuropathic” drugs (gabapentin, pregabalin, tricyclic antidepressants, topical capsaicin cream and lignocaine).
4. DISCUSSION
4.1. Trends of opioid prescribing
Our study reported a 19.5% reduction over 5 years in the total dispensing of tapentadol, oxycodone and tramadol OMEs across the four hospitals in the SESLHD. In contrast, there was an increase of tapentadol IR (223.2%) and tapentadol SR (17.9%) dispensing in the SESLHD. This has led to tapentadol becoming the most prescribed opioid amongst the three opioids in the SESLHD by 2020–21.
Our findings of increasing hospital prescribing of tapentadol are consistent with published literature that documents an increase in community sales of tapentadol. Alarmingly, by December 2019, tapentadol had the seventh largest market share of number of units sold and become the largest market share of total OME sold at 1540.1 OME kilograms. Highest sales were observed in Western Australia with 12.9 packs sold per 100 people followed by Tasmania (8.4), then Queensland (6.1), New South Wales came sixth (4.8) and Northern Territory (1.2) was lowest in 2019. 18 Increasing trends in tapentadol prescribing were also reported in published studies in other countries. An Irish study reported an 8.8‐fold increase (from 0.2% to 1.6%) in tapentadol's national prescribing prevalence between 2012 and 2019. 19 Similarly, dramatic increases in tapentadol prescribing were demonstrated in a United Kingdom study since 2011, more by OME than by number of packets, suggesting there was a tendency towards high‐dose prescribing. 20 Increasing tapentadol prescription rates may be driven by the perception of tapentadol having lower incidences of gastrointestinal side effects and tapentadol SR exerting comparable analgesic effectiveness as oxycodone SR in the treatment of moderate to severe chronic pain. 2 , 21 Yet, tapentadol IR's effectiveness in acute pain relief is uncertain. A meta‐analysis 22 involving 14 studies suggested that an equianalgesic dose of tapentadol IR was significantly less effective than oxycodone IR in the management of moderate to severe acute pain. Higher doses of tapentadol IR, however, is as effective as other opioids in acute pain relief. 22 This highlights the need for caution when prescribing tapentadol IR for pain relief as a higher dose of tapentadol is required, which may lead to higher incidences of adverse effects. 23 , 24 Furthermore, tapentadol is a relatively new opioid with an abuse potential that is theoretically comparable to other strong opioids. 25 There are new published case series on dependence, abuse and diversion of tapentadol. 8 , 9 The dramatic increase in hospital prescribing is a cause for concern, hence consumption trends need to be continuously monitored.
Moreover, the increase in hospital prescription of tapentadol is concerning from a cost perspective. As tapentadol is a new drug, it is more expensive than tramadol and oxycodone. 26 The increasing hospital prescribing trend of tapentadol in SESLHD creates a larger burden of cost to these hospitals. While tapentadol SR has been subsidised under the Pharmaceutical Benefits Scheme (PBS) in Australia since 2014, tapentadol IR is not. Hence, this makes tapentadol IR even more expensive for patients who are required to continue taking it upon hospital discharge. The lack of PBS subsidy for IR preparation demonstrates the uncertainty on whether reductions in incidences of gastrointestinal side effects would justify the higher cost. However, tapentadol may be the preferred choice of opioid in patients with gastrointestinal intolerability or neuropathic pain complaints. 26
Our study showed that there is a shift in opioid utilisation with prominence moving from oxycodone to tapentadol within hospitals. Hence, it is crucial to strictly assess the indications of tapentadol prescriptions in Australia (including private scripts) as inappropriate opioid prescriptions may lead to worse quality of life and increased risk of negative outcomes such as overdose, death, mental health disorders and illicit opioid use. 27 , 28
4.2. Pattern of tapentadol prescribing
This study demonstrated that the increase in tapentadol prescription at POWH was primarily driven by prescription in surgical units. Nearly all the surgical patients (97.9%) were prescribed tapentadol for acute post‐operative pain with the majority (54.0%) prescribed both tapentadol IR and SR. This finding is in contrast to recommendations by Therapeutic Guidelines Australia, which state that tapentadol SR should only be used for the treatment of moderate to severe chronic pain unresponsive to other non‐opioid analgesic. 5 Additionally, in 2018, the Australian and New Zealand College of Anaesthetists stated that ‘slow‐release opioids are not recommended for use in the management of patients with acute pain’ due to the significant risk of respiratory depression, leading to severe adverse events and deaths. 29 Furthermore, there have been growing concerns on long‐term use of opioids following post‐operative opioid prescribing intended for short‐term management of acute pain. 30 An Australian study reported about 4% of post‐surgical patients who were previously opioid naive became chronic users of opioid (defined by >90 days use of opioid) post discharge. 31 In this study, the most frequently prescribed opioids were oxycodone with or without naloxone (51%). 31 In addition, another Australian study showed that orthopaedic post‐operative use of tapentadol IR was more likely to be associated with opioid changing upon discharge when compared with oxycodone IR (odds raio [OR] = 16.5). 32 This practice could have potential patient safety implications with accidental overdose and misuse. On the other hand, several US and Australian studies showed that both tapentadol IR and extended release (ER) might have lower abuse liability in the community than oxycodone. 33 , 34 , 35 Hence, use of a lower abuse liability drug in the hospital setting could potentially reduce the risk of chronic use but care needs to be taken on the choice of opioid upon hospital discharge. Finally, initiatives to reduce inappropriate prescription, including prescriber education regarding the risks of prescribing SR opioids for acute pain should be encouraged.
At POWH's spinal rehabilitation and spinal medicine acute units, our study revealed that neuropathic pain was the main indication for tapentadol prescription in these units. It has been agreed that classical opioids are not usually the mainstay for the treatment of neuropathic pain as they are less effective. 26 , 36 As noradrenaline plays an important role in the inhibition of neuropathic pain, 37 medications with combined mechanisms of action, especially those with noradrenaline reuptake inhibition such as tapentadol are potentially effective in neuropathic pain relief. 21 , 26 , 37 , 38 Further research is needed to support tapentadol's analgesic efficacy in neuropathic pain relief. 36
4.3. Limitations
The study has several limitations. First, although inpatient opioid dispensing claims provided a good indication of opioid utilisation, it is not a direct measure of opioid consumption, that is whether the opioid was taken by the patient as prescribed or taken at all. Second, the OME metric was used as a measure of opioid prescribing trends. While it is a useful and reliable metric for data standardisation, there is an absence of a universally accepted OME conversion factor. 15 , 39 Hence, national and international comparisons of opioid use may be difficult to achieve. Third, the study only analysed the prescription trend of tapentadol compared to two other opioids, excluding other oral opioids that are available for use in Australian hospitals (e.g., hydromorphone and morphine). Lastly, our study only collected data from a single health district. Hence, the applicability of our results to other hospitals in Australia or even outside Australia should be approached with great caution as prescription patterns may vary depending on other factors. Therefore, larger studies involving multiple hospitals across Australia to examine the pattern of tapentadol prescribing compared to other pharmaceutical opioids available for use in Australian hospitals should be conducted.
5. CONCLUSION
There was a rapid increase in the annual hospital prescription of tapentadol between August 2016 and July 2021 with tapentadol overtaking both oxycodone and tramadol as the preferred opioid analgesic across the four hospitals in the SESLHD. At POWH's surgical unit, tapentadol SR was largely prescribed for off‐label purposes. On the other hand, tapentadol SR was primarily prescribed for neuropathic pain in the spinal rehabilitation and acute spinal medicine unit.
COMPETING INTERESTS
At the time of submission, Dr. Angela Chiew is an editor for the British Journal of Clinical Pharmacology.
CONTRIBUTORS
J.M. was responsible for data entry, analysis and writing up of the manuscript. D.R. was responsible for providing data from the pharmacy. A.C. was responsible for planning and reviewing the protocol. N.B. contributed towards data analysis. B.C. was the Principal Investigator, responsible for planning, reviewing the data. All five authors were responsible for writing and revising the paper.
ACKNOWLEDGEMENT
The authors would like to thank Simon Nguyen, iPharmacy Application Manager at Health Information and Communication Technologies for his assistance in providing the pharmacy dispensing data. There was no funding provided for this study. Open access publishing facilitated by University of New South Wales, as part of the Wiley ‐ University of New South Wales agreement via the Council of Australian University Librarians.
Mirabella J, Ravi D, Chiew AL, Buckley NA, Chan BS. Prescribing trend of tapentadol in a Sydney local health district. Br J Clin Pharmacol. 2022;88(9):3929‐3935. doi: 10.1111/bcp.15448
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author, subject to ethical approval. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Tzschentke TM, Christoph T, Kogel BY. The mu‐opioid receptor agonist/noradrenaline reuptake inhibition (MOR‐NRI) concept in analgesia: the case of tapentadol. CNS Drugs. 2014;28(4):319‐329. doi: 10.1007/s40263-014-0151-9 [DOI] [PubMed] [Google Scholar]
- 2. Lange B, Kuperwasser B, Okamoto A, et al. Efficacy and safety of tapentadol prolonged release for chronic osteoarthritis pain and low back pain. Adv Ther. 2010;27(6):381‐399. doi: 10.1007/s12325-010-0036-3 [DOI] [PubMed] [Google Scholar]
- 3. Wild JE, Grond S, Kuperwasser B, et al. Long‐term safety and tolerability of tapentadol extended release for the management of chronic low back pain or osteoarthritis pain. Pain Pract. 2010;10(5):416‐427. doi: 10.1111/j.1533-2500.2010.00397.x [DOI] [PubMed] [Google Scholar]
- 4. Therapeutic Goods Administration . Australian Public Assessment Report for Tapentadol, Proprietary Product Name: Palexia IR. 2011;2021. Accessed July 1, 2022. https://www.tga.gov.au/sites/default/files/auspar-palexia.pdf
- 5. Therapeutic Goods Administration . Australian Public Assessment Report for Tapentadol, Proprietary Product Name: Palexia SR 2011;2021. Accessed July 1, 2022. https://www.tga.gov.au/file/1393/download
- 6. Tzschentke TM, Christoph T, Kögel B, et al. (−)‐(1R,2R)‐3‐(3‐dimethylamino‐1‐ethyl‐2‐methyl‐propyl)‐phenol hydrochloride (tapentadol HCl): a novel mu‐opioid receptor agonist/norepinephrine reuptake inhibitor with broad‐spectrum analgesic properties. J Pharmacol Exp Ther. 2007;323(1):265‐276. doi: 10.1124/jpet.107.126052 [DOI] [PubMed] [Google Scholar]
- 7. Romualdi P, Grilli M, Canonico PL, Collino M, Dickenson AH. Pharmacological rationale for tapentadol therapy: a review of new evidence. J Pain Res. 2019;12:1513‐1520. doi: 10.2147/JPR.S190160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kathiresan P, Pakhre A, Kattula D, Sarkar S. Tapentadol dependence: a case series. Prim Care Companion CNS Disord. 2019;21(5):19l02444. doi: 10.4088/PCC.19l02444 [DOI] [PubMed] [Google Scholar]
- 9. Mukherjee D, Shukla L, Saha P, et al. Tapentadol abuse and dependence in India. Asian J Psychiatr. 2020;49:101978. doi: 10.1016/j.ajp.2020.101978 [DOI] [PubMed] [Google Scholar]
- 10. Carelli C, Freni F, Moretti M, Vignali C, Ballardini M, Morini L. A case report on fatal intoxication by tapentadol: study of distribution and metabolism. Forensic Sci Int. 2021;324:110825. doi: 10.1016/j.forsciint.2021.110825 [DOI] [PubMed] [Google Scholar]
- 11. Franco DM, Ali Z, Levine B, Middleberg RA, Fowler DR. Case report of a fatal intoxication by Nucynta. Am J Forensic Med Pathol. 2014;35(4):234‐236. doi: 10.1097/PAF.0b013e3182887804 [DOI] [PubMed] [Google Scholar]
- 12. Kemp W, Schlueter S, Smalley E. Death due to apparent intravenous injection of tapentadol. J Forensic Sci. 2013;58(1):288‐291. doi: 10.1111/j.1556-4029.2012.02299.x [DOI] [PubMed] [Google Scholar]
- 13. Darke S, Duflou J, Peacock A, Farrell M, Lappin J. Characteristics of fatal tapentadol‐related toxicity in Australia. Drug Alcohol Rev. 2022;232:109292. doi: 10.1016/j.drugalcdep.2022.109292 [DOI] [PubMed] [Google Scholar]
- 14. Peacock A, Gisev N, Memedovic S, et al. Opioid use and harms associated with a sustained‐release tapentadol formulation: a post‐marketing surveillance study. Drug Alcohol Depend. 2020;206:107697. doi: 10.1016/j.drugalcdep.2019.107697 [DOI] [PubMed] [Google Scholar]
- 15. Nielsen S, Degenhardt L, Hoban B, Gisev N. Comparing opioids: a guide to estimating oral morphine equivalents (OME) in research. Technical Report No. 329. Sydney: National Drug and Alcohol Research Centre, University of NSW; 2014. [Google Scholar]
- 16. Alexander SPH, Christopoulos A, Davenport AP, et al. THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: G protein‐coupled receptors. Br J Pharmacol. 2021;178:S27‐S156. [DOI] [PubMed] [Google Scholar]
- 17. Alexander SPH, Kelly E, Mathie A, et al. THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: Transporters. Br J Pharmacol. 2021;178(S1):S412‐S513. doi: 10.1111/bph.15543 [DOI] [PubMed] [Google Scholar]
- 18. Chrzanowska AGN, Price O, Sutherland R, et al. Tapentadol use and harms in Australia: analysis of opioid sales data and sentinel survey data from people who inject drugs. Drug Trends. 2020;1‐26. [Google Scholar]
- 19. Norris BA, Smith A, Doran S, Barry M. Trends in strong opioid prescribing in Ireland: a repeated cross‐sectional analysis of a national pharmacy claims database between 2010 and 2019. Pharmacoepidemiol Drug Saf. 2021;30(8):1003‐1011. doi: 10.1002/pds.5247 [DOI] [PubMed] [Google Scholar]
- 20. Curtis HJ, Croker R, Walker AJ, Richards GC, Quinlan J, Goldacre B. Opioid prescribing trends and geographical variation in England, 1998‐2018: a retrospective database study. Lancet Psychiatry. 2019;6(2):140‐150. doi: 10.1016/S2215-0366(18)30471-1 [DOI] [PubMed] [Google Scholar]
- 21. Baron R, Likar R, Martin‐Mola E, et al. Effectiveness of tapentadol prolonged release (PR) compared with oxycodone/naloxone PR for the management of severe chronic low back pain with a neuropathic component: a randomized, controlled, open‐label, phase 3b/4 study. Pain Pract. 2016;16(5):580‐599. doi: 10.1111/papr.12308 [DOI] [PubMed] [Google Scholar]
- 22. Wang X, Narayan SW, Penm J, Patanwala AE. Efficacy and safety of tapentadol immediate release for acute pain: a systematic review and meta‐analysis. Clin J Pain. 2020;36(5):399‐409. doi: 10.1097/AJP.0000000000000809 [DOI] [PubMed] [Google Scholar]
- 23. Daniels S, Casson E, Stegmann J‐U, et al. A randomized, double‐blind, placebo‐controlled phase 3 study of the relative efficacy and tolerability of tapentadol IR and oxycodone IR for acute pain. Curr Med Res Opin. 2009;25(6):1551‐1561. doi: 10.1185/03007990902952825 [DOI] [PubMed] [Google Scholar]
- 24. Hartrick C, Van Hove I, Stegmann J‐U, Oh C, Upmalis D. Efficacy and tolerability of tapentadol immediate release and oxycodone HCl immediate release in patients awaiting primary joint replacement surgery for end‐stage joint disease: a 10‐day, phase III, randomized, double‐blind, active‐ and placebo‐controlled study. Clin Ther. 2009;31(2):260‐271. doi: 10.1016/j.clinthera.2009.02.009 [DOI] [PubMed] [Google Scholar]
- 25. Stoops WW, Glaser PEA, Rush CR. Miotic and subject‐rated effects of therapeutic doses of tapentadol, tramadol, and hydromorphone in occasional opioid users. Psychopharmacology (Berl). 2013;228(2):255‐262. doi: 10.1007/s00213-013-3031-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vadivelu N, Huang Y, Mirante B, et al. Patient considerations in the use of tapentadol for moderate to severe pain. Drug Healthc Patient Saf. 2013;5:151‐159. doi: 10.2147/DHPS.S28829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hurtado I, García‐Sempere A, Peiró S, Sanfélix‐Gimeno G. Increasing trends in opioid use from 2010 to 2018 in the region of Valencia, Spain: a real‐world, population‐based study. Front Pharmacol. 2020;11:612556. doi: 10.3389/fphar.2020.612556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rummans TA, Burton MC, Dawson NL. How good intentions contributed to bad outcomes: the opioid crisis. Mayo Clin Proc. 2018;93(3):344‐350. doi: 10.1016/j.mayocp.2017.12.020 [DOI] [PubMed] [Google Scholar]
- 29. Australian and New Zealand College of Anaesthetists and Faculty of Pain Medicine . Position statement on the use of slow‐release opioid preparations in the treatment of acute pain. 2018. Accessed July 1, 2022. https://www.anzca.edu.au/getattachment/d9e2a7c5‐0f17‐42d3‐bda7‐c6dae7e55ced/Position‐statement‐on‐the‐use‐of‐slow‐release‐opioid‐preparations‐in‐the‐treatment‐of‐acute‐pain
- 30. Sun EC, Darnall BD, Baker LC, Mackey S. Incidence of and risk factors for chronic opioid use among opioid‐naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286‐1293. doi: 10.1001/jamainternmed.2016.3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Roughead EE, Lim R, Ramsay E, Moffat AK, Pratt NL. Persistence with opioids post discharge from hospitalisation for surgery in Australian adults: a retrospective cohort study. BMJ Open. 2019;9(4):e023990. doi: 10.1136/bmjopen-2018-023990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang X, Tay HP, Narayan SW, Penm J, Patanwala AE. Comparison of opioid prescribing upon hospital discharge in patients receiving tapentadol versus oxycodone following orthopaedic surgery. Int J Clin Pharmacol. 2021;43(6):1602‐1608. doi: 10.1007/s11096-021-01290-7 [DOI] [PubMed] [Google Scholar]
- 33. Butler SF, McNaughton EC, Black RA. Tapentadol abuse potential: a postmarketing evaluation using a sample of individuals evaluated for substance abuse treatment. Pain Med. 2015;16(1):119‐130. doi: 10.1111/pme.12524 [DOI] [PubMed] [Google Scholar]
- 34. Vosburg SK, Beaumont J, Dailey‐Govoni ST, Butler SF, Green JL. Evaluation of abuse and route of administration of extended‐release tapentadol among treatment‐seeking individuals, as captured by the Addiction Severity Index‐Multimedia Version (ASI‐MV). Pain Med. 2020;21(9):1891‐1901. doi: 10.1093/pm/pnz250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Black J, Margolin ZR, Bau G, Olson R, Iwanicki JL, Dart RC. Web‐based discussion and illicit street sales of tapentadol and oxycodone in Australia: epidemiological surveillance study. JMIR Public Health Surveill. 2021;7(12):e29187. doi: 10.2196/29187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Smith HS. Opioids and neuropathic pain. Pain Physician. 2012;15(3S):ES93‐ES110. doi: 10.36076/ppj.2012/15/ES93 [DOI] [PubMed] [Google Scholar]
- 37. Obata H. Analgesic mechanisms of antidepressants for neuropathic pain. Int J Mol Sci. 2017;18(11):2483. doi: 10.3390/ijms18112483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schwartz S, Etropolski M, Shapiro DY, et al. Safety and efficacy of tapentadol ER in patients with painful diabetic peripheral neuropathy: results of a randomized‐withdrawal, placebo‐controlled trial. Curr Med Res Opin. 2011;27(1):151‐162. doi: 10.1185/03007995.2010.537589 [DOI] [PubMed] [Google Scholar]
- 39. Karanges EA, Blanch B, Buckley NA, Pearson SA. Twenty‐five years of prescription opioid use in Australia: a whole‐of‐population analysis using pharmaceutical claims. Br J Clin Pharmacol. 2016;82(1):255‐267. doi: 10.1111/bcp.12937 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author, subject to ethical approval. The data are not publicly available due to privacy or ethical restrictions.


