Abstract
The neurochemical mechanisms underlying motor memory consolidation remain largely unknown. Based on converging work showing that ethyl alcohol retrogradely enhances declarative memory consolidation, this work tested the hypothesis that post‐learning alcohol ingestion would enhance motor memory consolidation. In a within‐subject and fully counterbalanced design, participants (n = 24; 12M; 12F) adapted to a gradually introduced visual deviation and ingested, immediately after adaptation, a placebo (PBO), a medium (MED) or high (HIGH) dose of alcohol. The alcohol doses were bodyweight‐ and gender‐controlled to yield peak breath alcohol concentrations of 0.00% in the PBO, ~0.05% in the MED and ~0.095% in the HIGH condition. Retention was evaluated 24 h later through reach aftereffects when participants were sober. The results revealed that retention levels were neither significantly nor meaningfully different in both the MED and HIGH conditions as compared to PBO (all absolute Cohen's dz values < ~0.2; small to negligible effects), indicating that post‐learning alcohol ingestion did not alter motor memory consolidation. Given alcohol's known pharmacological GABAergic agonist and NMDA antagonist properties, one possibility is that these neurochemical mechanisms do not decisively contribute to motor memory consolidation. As converging work demonstrated alcohol's retrograde enhancement of declarative memory, the present results suggest that distinct neurochemical mechanisms underlie declarative and motor memory consolidation. Elucidating the neurochemical mechanisms underlying the consolidation of different memory systems may yield insights into the effects of over‐the‐counter drugs on everyday learning and memory but also inform the development of pharmacological interventions seeking to alter human memory consolidation.
Keywords: alcohol, consolidation, motor learning, motor memory, visuomotor adaptation
Post‐learning ingestion of alcohol did not enhance motor memory consolidation of a gradual visuomotor adaptation task learned with the dominant hand as compared to placebo. Moreover, despite greatly differing breath alcohol concentrations, alcohol neither interfered with learning nor retention capabilities as compared to placebo, as assessed via visuomotor adaptation of the non‐dominant hand 1 h following alcohol ingestion.

List of abbreviations
- ABV
alcohol by volume
- AUDIT
Alcohol Use Disorder Identification Test
- BrAC
breath alcohol concentration
- GABA
Gamma‐aminobutyric acid
- HIGH
high
- M1
primary motor cortex
- MED
medium
- MRS
magnetic resonance spectroscopy
- NMDA
N‐methyl‐d‐aspartate
- OCT
opportunistic consolidation theory
- PBO
placebo
- PV
peak velocity
- RT
reaction time
- MT
movement time
- MAD
median absolute deviation
- MLM
mixed linear model
- AIC
Akaike information criterion
- SD
standard deviation
1. INTRODUCTION
Motor memories are known to undergo consolidation (Brashers‐Krug et al., 1996), but the neurochemical mechanisms underlying this process remain largely unknown. At the moment, human evidence indicates that gamma‐aminobutyric acid (GABA)ergic activity (Donchin et al., 2002; Floyer‐Lea et al., 2006; Kolasinski et al., 2019; Mooney et al., 2021; Shibata et al., 2017; van Vugt et al., 2020) and N‐methyl‐D‐Aspartate (NMDA) receptor activity (Cherry et al., 2014; Günthner et al., 2016; Hadj Tahar et al., 2004; Kuriyama et al., 2011) contribute to motor learning, suggesting they could also contribute to motor memory consolidation. On the one hand, by recording magnetic resonance spectroscopy (MRS) data, Kolasinski et al. (2019) showed that motor sequence learning reduces GABA concentrations in the primary motor cortex (M1) while van Vugt et al. (2020) rather showed that M1 GABA concentrations increase during the learning of a novel auditory‐motor mapping. Although conflicting, this evidence indicates that GABAergic activity changes accompany the learning of novel motor behaviours and could thus mechanistically contribute to motor memory consolidation. On the other hand, through pharmacological interventions, Kuriyama et al. (2011) showed that administering D‐cycloserine (an NMDA receptor agonist) before learning a sequential finger‐tapping task enhanced learning and consolidation while both Cherry et al. (2014) and Günthner et al. (2016) found that the same treatment had no effect on a balance and serial reaction time task, respectively. Overall, based on the above and further conflictual work on sleep‐dependent memory consolidation (Feld, Lange, et al., 2013; Feld, Wilhelm, et al., 2013; Gais et al., 2008; Hadj Tahar et al., 2005; Kuriyama et al., 2011; Morgan et al., 2010; Smith & Smith, 2003), it remains unclear whether and how GABA and NMDA receptor‐mediated activity mechanistically contribute to motor memory consolidation.
One insightful framework to gain insight into this issue is the Opportunistic Consolidation Theory (OCT) (Mednick et al., 2011). Namely, the OCT posits that enhancing GABAergic inhibition and inhibiting NMDA receptor activity immediately following learning retrogradely enhances memory consolidation by preventing the interfering influences of additional learning (Mednick et al., 2011). Four decades of human studies investigating the influence of post‐learning ethyl alcohol ingestion—a GABAergic agonist and NMDA antagonist pharmacological agent (Abrahao et al., 2017; Grant & Lovinger, 2018)—on declarative memory consolidation directly support the OCT (Bruce et al., 1999; Bruce et al., 1999; Bruce & Pihl, 1997; Carlyle et al., 2017; Doss et al., 2018; Lamberty et al., 1990; Mueller et al., 1983; Parker et al., 1980, 1981; Tyson & Schirmuly, 1994; Weafer et al., 2016). For instance, seminal work from Parker et al. (1980) showed that post‐learning alcohol ingestion enhanced visual and verbal memory consolidation as compared to placebo (Parker et al., 1980). One year later, Parker et al. (1981) showed that this relationship was dose‐dependent; the greater the amount of alcohol ingested following learning, the greater the memory consolidation enhancements (Parker et al., 1981) Interestingly, separate lines of evidence showed that post‐learning alcohol ingestion also enhances kinesthetic memory consolidation (Hewitt et al., 1996; Scholey & Fowles, 2002), evaluated as the capacity to proprioceptively trace shapes. This indicates that alcohol‐induced retrograde enhancements of consolidation are not restricted to declarative memories but could also be extended to other memory systems. Given the well‐known effects of alcohol on mammalian brains (Abrahao et al., 2017; Grant & Lovinger, 2018), its use as a pharmacological agent will allow substantiating if GABA and NMDA receptor activity is part of the neurochemical mechanisms underlying motor memory consolidation.
The objective of this work was to test the hypothesis that ingesting alcohol immediately following learning would enhance motor memory consolidation. In a fully within‐subject and placebo‐controlled design, participants used their dominant hand to undergo gradual visuomotor adaptation and—immediately after—ingested a beverage (see Figure 1). Namely, participants either ingested a placebo (PBO), a medium (MED) or high dose (HIGH) dose of alcohol, designed to induce maximal breath alcohol concentrations (BrAC) of 0.00%, ~0.05% and ~0.095%, respectively. To assess consolidation, retention was evaluated through reach aftereffects 24 h later (Hamel et al., 2017; Hamel et al., 2019; Hamel et al., 2021) when participants were sober again. Based on previous work on declarative memories (Carlyle et al., 2017; Parker et al., 1981), it was hypothesized that alcohol would enhance motor memory consolidation in a dose‐dependent manner: HIGH would yield greater retention than MED and MED would yield greater retention levels than PBO. To determine if alcohol retrogradely enhances consolidation by preventing the interfering influence of additional learning (Doss et al., 2018; Mednick et al., 2011; Mueller et al., 1983), participants took part in a second gradual visuomotor adaptation with their non‐dominant hand 60 min following complete ingestion of the beverages (see Figure 1).
FIGURE 1.

Overview of the within‐subject placebo‐controlled procedures. (a) Timeline of the experimental acquisition and retention visits (separated by 24 h). Condition order was fully counterbalanced across participants. Participants arrived drug‐free and on an empty stomach. Breathalyser measurements were taken at several time points to ensure sobriety and measure BrACs. Immediately following dominant hand acquisition, participants ingested their placebo or alcohol‐containing beverage. Memory consolidation of the dominant hand acquisition block was evaluated 24 h later. (b) Dominant hand acquisition block. Participants reached visual targets while adapting to a gradually introduced visual deviation in an alcohol‐free state. The beverage was ingested immediately after this block. (c) Non‐dominant hand session. To evaluate the effects of alcohol on learning, participants used their non‐dominant hand to reach visual targets while adapting to a visual deviation introduced in a stepwise manner. Immediately after adaptation, reach aftereffects without corrective visual feedback were evaluated (50 NoVision trials). This block occurred 60 min after the complete beverage ingestion. (d) Procedures of the dominant hand retention block. In an alcohol‐free state, memory consolidation of the dominant hand acquisition was evaluated 24 h later through reach aftereffects first without (50 NoVision trials) and then with corrective visual feedback (50 Washout trials). (e) Bodyweight‐ and gender‐controlled alcohol dosages. Females ingested 15% less alcohol than their male counterparts in both the MED and HIGH conditions.
2. METHODS
2.1. Participants
A total of 24 medication‐free, non‐smoking and neurologically healthy participants took part in this experiment (gender‐controlled experiment; 12 males, 12 females; 23.25 ± 2.45 years old [Mean ± SD]). Two participants were self‐reported left‐handed, while every other participant was self‐reported right‐handed. All participants were of legal drinking age for alcohol consumption and had a normal or corrected‐to‐normal vision. Participants reported drinking an average of 2.5 ± 2 standardized alcohol units (13.5 g of ethanol/unit) at an average frequency of 2 ± 1 occasions per month. They also reported not using cannabis (available legally in Canada) as well as other substances of abuse. Participants were not eligible for the study if they scored above 8 on the Alcohol Use Disorders Identification Test (Bohn et al., 1995; Saunders et al., 1993), indicative of symptoms of alcohol use disorders. Specifically, participants had a total average AUDIT score of 4.7 ± 1.8. Female participants took an over‐the‐counter pregnancy test before each experimental visit to ensure non‐pregnancy at the moment of testing. Ethics approval was obtained from the local institutional ethics review board (project ID: 2021‐4081) and conformed to the Declaration of Helsinki.
2.2. Apparatus and procedures
This work used a randomized within‐subject placebo‐controlled design to control for individual differences in subjective (Morean & Corbin, 2010) and physiological responses to alcohol (Brunelle et al., 2007; Mundt et al., 1997), as well as environmental, biological and genetics individual differences in alcohol metabolism (Wall et al., 2016). Experimental visits were carried out in pairs; participants took part in an acquisition session (lasting ~3 h), followed by a retention session 24 h later. During the acquisition session, participants had to reach visual targets while learning to compensate for a gradually introduced visual deviation (Hamel et al., 2017, 2019; Hamel, Dallaire‐Jean, et al., 2021; Hamel, de la Fontaine, et al., 2021). During the retention session, the persistence of reach aftereffects was evaluated to infer the extent of motor memory consolidation. (Hamel et al., 2017, 2019; Hamel, Dallaire‐Jean, et al., 2021; Hamel, de la Fontaine, et al., 2021). The experimental visit pairs differed based on the beverage's content: one pair of experimental visits for each of the PBO, MED and HIGH conditions. Participants thus took part in a total of six experimental visits. To minimize carryover effects between experimental visit pairs, condition ordering was fully counterbalanced (Brooks, 2012) and at least 7 days separated each visit pair. Importantly, every experimental visit occurred at the same time of day to minimize the effects of circadian rhythms on memory consolidation (Hartsock & Spencer, 2020) and alcohol metabolism (Wasielewski & Holloway, 2001). Details of the procedures are provided in Figure 1 and below.
2.2.1. Dominant hand acquisition (alcohol‐free)
The motor task to be learned was a gradual visuomotor adaptation protocol. The present apparatus and single‐trial procedures used for every visuomotor adaptation block of the present work are similar to previous work (Hamel, Dallaire‐Jean, et al., 2021; Hamel, de la Fontaine, et al., 2021; Hamel, Lepage, & Bernier, et al., 2021). Briefly, participants performed centre‐out reaching movements towards one of five targets located around a circular array (10 cm radius) from the centre of the virtual environment. The targets were located in every workspace quadrant at the following angles: 0°, 36°, 72°, 108°, 144°, 180°, 216°, 252°, 288° and 324°. Their order of appearance was pseudorandomized so that every target would appear once every 10‐trial cycle. Participants were instructed to react as fast as possible to an auditory Go Cue, to produce straight movements with minimal online corrections in a target movement time of ~300 ms and to end their movement by landing the cursor within the presented target's boundaries. A typical trial lasted about 5 s.
The purpose of the dominant hand acquisition was to induce learning and then manipulate memory consolidation through alcohol ingestion (see Figure 1). The motor task to be learned consisted of compensating for a gradually introduced visual deviation (see Figure 1b), which was to prevent participants from gaining awareness of the deviation, therefore allowing to carry out a fully within‐subject learning design (Hamel, Dallaire‐Jean, et al., 2021; Hamel, de la Fontaine, et al., 2021; Hamel, Lepage, & Bernier, et al., 2021). Using a gradual rather than abrupt deviation was also to minimize the possible anterograde interference with the subsequent learning in the non‐dominant hand session, as adaptation through a gradually introduced sensorimotor perturbation does not transfer between limbs (Hamel, Lepage, & Bernier, et al., 2021; Malfait & Ostry, 2004; Werner et al., 2019). Importantly, participants were sober during dominant hand acquisition (see Figures 1 and 2). Verbal reports confirmed that none of the participants perceived the deviation.
FIGURE 2.
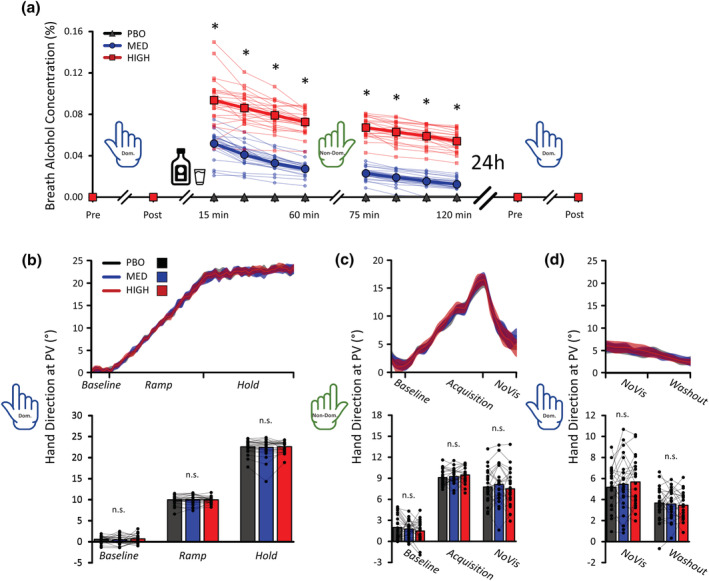
BrAC and hand direction at PV results. (a) BrAC results. All participants were sober during acquisition (Day 1) and retention (Day 2) when using their dominant hand. The ingestion of alcohol elevated BrAC values in a dose‐dependent manner, confirming that participants were under the influence of alcohol in the MED and HIGH conditions. The average and individual data of each condition are shown. (b) dominant hand acquisition. Top panel: the time‐course of hand direction at PV is shown. Bottom panel: The average and individual data of each condition for each phase are shown. (c) Non‐dominant hand session. Top panel: The time‐course of hand direction at PV is shown. Bottom panel: The average and individual data of each condition for each phase are shown. Note the lack of difference in hand direction at PV despite the greatly differing BrAC values. (d) Dominant hand retention. Top panel: The time‐course of hand direction at PV is shown. Bottom panel: The average and individual data of each condition for each phase are shown. Post‐learning alcohol ingestion did not enhance memory consolidation as compared to placebo. Asterisks (*) indicate significant differences, and ‘n.s.’ means ‘non‐significant’. The data represent the mean ± 1 SD (middle row only). For each depicted condition, n = 24.
Participants first executed 100 familiarization (non‐deviated) trials with their dominant and then non‐dominant hands (total of 200 trials; not shown in Figure 1). This was done at the start of every dominant hand acquisition to wash out any residual adaptation from previous visits (Hamel, de la Fontaine, et al., 2021) and therefore prevent any carryover effect between conditions (Brooks, 2012). Subsequently, participants blew in the breathalyser and then, with their dominant hand, executed 50 trials of non‐deviated reaches (baseline). A gradually introduced visual deviation (+1° every 10‐trial cycle) was implemented over the subsequent 250 trials (Ramp) and was then maintained constant at 25° for the following 250 trials (hold). Immediately following the end of this block, participants blew in the breathalyser and ingested their beverage.
2.2.2. Non‐dominant hand session (under alcohol's influence in the MED and HIGH conditions)
The purpose of this block was to determine if post‐learning alcohol ingestion retrogradely enhanced consolidation of memories formed during dominant hand acquisition by interfering with subsequent learning capabilities, as declarative memory work has shown (Doss et al., 2018; Mednick et al., 2011). With their non‐dominant hand, 60 min following the complete beverage ingestion, participants first executed 30 trials of non‐deviated reaches (Baseline), followed by four incremental steps of 30 trials each. In each of these steps, the visual deviation was increased by 5° to reach a maximum of 20° (acquisition). This introduction rate was to strike a balance between inducing abrupt learning rates and preventing participants' awareness of the ongoing visual deviation. Then, immediate retention was evaluated through 50 trials of reach aftereffects in the absence of corrective visual feedback (NoVision).
The rationale behind allowing 60 min to elapse between the complete beverage ingestion and non‐dominant hand session was twofold. First, it was to minimize retrograde interference with memory consolidation of the dominant hand acquisition, as an inter‐session interval of 60 min was shown sufficient for memory stabilization to occur (Hamel, Dallaire‐Jean, et al., 2021) and to minimize interference between distinct memories (Lerner et al., 2020). Second, it was to ensure that participants would still be under the influence of alcohol. As the results confirmed a posteriori (see Figure 2), 60 min was insufficient time to fully metabolize alcohol (Cederbaum, 2012; Holford, 1987), indicating that non‐dominant hand session was performed under the effects of alcohol in the MED and HIGH conditions.
2.2.3. Dominant hand retention (alcohol‐free)
The purpose of this block was to evaluate the retrograde effects of post‐learning alcohol ingestion on memory consolidation of the dominant hand acquisition 24 h later. With their dominant hand, participants first executed 50 trials without (NoVision trials) and then 50 trials with corrective visual feedback (washout trials). This was to evaluate memory consolidation through reach aftereffects, taken here as a measure of the extent of memory consolidation (Hamel et al., 2017, 2019; Hamel, Dallaire‐Jean, et al., 2021; Hamel, de la Fontaine, et al., 2021). Participants were sober during this block (see Figure 2).
2.3. Alcohol administration and beverage ingestion
Participants were weighed on a medical‐grade personal scale, which was used to adjust the quantity of alcohol and volume of liquids to be ingested. To induce maximal BrAC readings of ~0.05% and ~0.095% in the MED and HIGH conditions, respectively, 94% alcohol by volume (ABV) ethyl alcohol was dosed using a validated equation (Andersson et al., 2009). The doses were also bodyweight‐ and gender‐controlled. Namely, in the MED and HIGH conditions, male participants, respectively, ingested 0.5 and 1 ml/kg, while female participants, respectively, ingested 0.425 and 0.85 ml/kg of 94% ABV alcohol (see Figure 1e). Females ingested 15% less alcohol than males because they reach higher BrAC readings even after correcting for body weight (Dubowski, 1985; Frezza et al., 1990; Mumenthaler et al., 1999). These BrAC values were chosen based on previous studies (see Bruce & Pihl, 1997; Parker et al., 1980, 1981; Weafer et al., 2016) to approximate intoxication levels reached in social settings while minimizing the adverse reactions to alcohol, which loosely appear above BrACs of ~0.15% (Jones, 2019; Pohorecky & Brick, 1988).
To normalize participants' absorption of the beverage, participants arrived in a 3 h‐fasted state to ensure an empty stomach (Goyal et al., 2019). Also, the beverage was halved, and participants had 5 min to ingest each half (total of 10 min). Across conditions, beverages had identical volumes for a given participant (total volume; male: 4 ml/kg; female: 3.4 ml/kg). The alcohol content of the following beverages is identical to previous work (Parker et al., 1981). Concerning the MED condition, the 94% ABV alcohol part was first diluted in water in a 1:1 ratio to equate the volume of alcohol administered in the HIGH condition. The resulting solution was then mixed with three parts of orange juice. Concerning the HIGH condition, one part 94% ABV alcohol was mixed with three parts of orange juice. Concerning the placebo condition (PBO), the beverage contained four parts of orange juice. For every beverage, pulp‐free commercial orange juice containing ~11.5 g of sugar/100 ml was used. To improve taste, a liquid sugar‐free Mango Peach‐flavoured commercial syrup was added to both the alcohol‐containing and placebo beverages. Given that participants arrived in a fasted state, they ate ad libitum pieces of toast (with a complementary choice of peanut butter and/or strawberry jam) while drinking their beverage. This was to minimize any gastric discomfort during the experiment. For a given participant, the number of pieces of toast and spreading eaten was identical across all conditions. Participants watched emotionally neutral nature documentaries (Planet Earth, British Broadcasting Corporation ®) to normalize their psychomotor activity while at the laboratory (see Figure 1).
Breath alcohol concentration (BrAC) values were assessed using the BACtrack mobile device (Riordan et al., 2017) (Breathalyzer.ca®) before and every 15 min for a total of 120 min following the ingestion of the beverage. The BACtrack device uses an electrochemical fuel‐cell sensor, a widespread and reliable technology to assess BrACs (Ozoemena et al., 2018; Sorbello et al., 2018) that has accuracy levels similar to roadside law enforcement devices (Riordan et al., 2017). The reported BACtrack fuel‐cell sensory accuracy is ±0.005% when at 0.05% of blood alcohol content. Here, importantly, evaluating BrAC was to confirm sobriety during dominant‐hand acquisition and retention as well as PBO condition. This was also to measure the extent of BrAC changes in the MED and HIGH conditions. Participants were allowed to leave the laboratory 120 min after beverage ingestion if their BrAC value was below 0.06%.
2.4. Kinematic data reduction
A custom‐made MATLAB script was used to display and acquire kinematic data during the experiment. The cursor position data were acquired at 100 Hz. The primary variable of interest was hand direction at peak tangential velocity (PV), which was used to evaluate performance. This early kinematic marker was chosen because it is considered a reflection of the movement planning process (Carlton, 1992). Additionally, reaction time (RT; defined as the temporal difference in milliseconds between the auditory Go cue and movement onset), movement time (MT; defined as the temporal difference in milliseconds between movement onset and movement end) and endpoint accuracy (the absolute distance in centimetres between the cursor and target centroids at movement end) were also analysed.
Outlying trials were detected based on RT, MT and accuracy at movement endpoint data. Namely, individual trials were excluded from all analyses if RTs were below 100 ms or above 3 median absolute deviations (MAD) (Leys et al., 2013) or if MTs were ±3 MAD from each participant's median. Accuracy at movement endpoint greater than 10 cm also resulted in trial rejection. Then, data were individually averaged across phases (Dominant Hand Acquisition: Baseline, Ramp and Hold; Non‐Dominant Hand Session: Baseline, Acquisition and NoVision; Dominant Hand Retention: NoVision and Washout) for each beverage condition to perform subsequent analyses.
2.5. Adverse reactions
None of the participants reported adverse reactions to the present procedures.
2.6. Statistical analyses
In this work, mixed linear models (MLMs) were used (Boisgontier & Cheval, 2016; Koerner & Zhang, 2017; Magezi, 2015). The random coefficients included in the models were determined based on the most recent recommendations (see Harrison et al., 2018, for a comprehensive review). Namely, the manipulated factors were always included in the model as fixed effects. Regarding the random coefficients, maximally complex models (random intercepts for participants and random slopes for all of the fixed effects and interactions, wherever the data allowed their inclusion) were built. As recommended by Harrison et al. (2018), the random coefficients that best minimized the model's information loss, as determined via the model‐specific lowest relative Akaike Information Criterion (AIC) value, were chosen to analyse the data and report the results. The random coefficients included in the models are reported below every statistics table. The Benjamini‐Hochberg procedure was used to correct for multiple comparisons (Benjamini & Hochberg, 1995). Where possible, effect sizes (β regression coefficients or Cohen's dz) and their confidence intervals are also reported to provide a nuanced result interpretation that does not solely rely on p values (Amrhein et al., 2019; Lytsy, 2018). This was to provide a straightforward assessment of the direction, size and plausibility of the discovered effects, as focusing solely on significant p values has been argued to offer poor support for scientific conclusions and to yield low replication rates (Lytsy, 2018). In the event of null effects, equivalence testing was performed (Lakens, 2017; Lakens et al., 2020). Briefly, equivalence testing allows to statistically reject the presence of effects large enough to be considered theoretically meaningful (see Lakens, 2017; Lakens et al., 2020, for comprehensive reviews), deemed to be a Cohen's d z value of 0.8 in the present work. To test for equivalence, the 2 one‐sided t tests procedure was used (Lakens, 2017). All of the reported descriptive statistics represent the Mean ± SD. Every statistical comparison reported below involves 24 participants (n = 24), unless when comparing males (n = 12) and females (n = 12).
3. RESULTS
3.1. Breath alcohol concentrations
3.1.1. Condition‐dependent increases in BrAC
Concerning BrAC data, a 3 Conditions * 12 Times (Pre Acquisition, Post Acquisition, 15, 30, 45, 60, 75, 90, 105 and 120 min, Pre Retention, Post Retention) * 2 Genders (Male, Female) MLM was conducted. The results revealed a Conditions * Times * Genders interaction (p = .0066; see Table 1), which suggests that Genders did not behave similar through Times in each Condition. This three‐way interaction was decomposed by conducting separate Times * Genders MLMs for each condition. Since the PBO condition selectively contains BrAC values of zeros, it could not be included in the subsequent analyses. BrAC data per condition and per gender are shown in Figure 2a and Table 2, respectively.
TABLE 1.
Breath alcohol concentration (BrAC)
| Model info | AIC | R 2 marginal | R 2 conditional | ICC |
|---|---|---|---|---|
| −6,366.498 | 0.945 | 0.969 | 0.276 |
| Model results | F | Num df | Den df | P value |
|---|---|---|---|---|
| Conditions | 474.068 | 2 | 22.40 | <0.0001 |
| Times | 783.146 | 11 | 748.00 | <0.0001 |
| Genders | 1.677 | 1 | 22.06 | 0.2087 |
| Conditions * Times | 292.057 | 22 | 748.00 | <0.0001 |
| Conditions * Genders | 4.908 | 2 | 748.00 | 0.0171 |
| Times * Genders | 1.642 | 11 | 22.40 | 0.0826 |
| Conditions * Times * Genders | 1.928 | 22 | 748.00 | 0.0066 |
Note: The random effects that minimized the relative AIC value were the inclusion of participants as random intercepts and conditions as a random slope coefficient. For each level of each factor, n = 24.
TABLE 2.
BrAC values: Descriptive statistics
| PBO | MED | HIGH | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Males | Females | P value | Males | Females | P value | Males | Females | P value | |
| Pre‐dominant hand acquisition | 0.000 ± 0.000 | 0.000 ± 0.000 | 1.0000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 1.0000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 1.0000 |
| Post‐dominant hand acquisition | 0.000 ± 0.000 | 0.000 ± 0.000 | 1.0000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 1.0000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 1.0000 |
| Beverage ingestion | |||||||||
| 15 min | 0.000 ± 0.000 | 0.000 ± 0.000 | 1.0000 | 0.056 ± 0.007 | 0.047 ± 0.015 | 0.0006 | 0.091 ± 0.007 | 0.097 ± 0.015 | 1.0000 |
| 30 min | 0.000 ± 0.000 | 0.000 ± 0.000 | 1.0000 | 0.047 ± 0.006 | 0.035 ± 0.009 | <0.0001 | 0.085 ± 0.006 | 0.087 ± 0.009 | 1.0000 |
| 45 min | 0.000 ± 0.000 | 0.000 ± 0.000 | 1.0000 | 0.037 ± 0.005 | 0.029 ± 0.006 | 0.0033 | 0.078 ± 0.005 | 0.080 ± 0.006 | 1.0000 |
| 60 min | 0.000 ± 0.000 | 0.000 ± 0.000 | 1.0000 | 0.030 ± 0.005 | 0.024 ± 0.005 | 0.0327 | 0.073 ± 0.005 | 0.073 ± 0.005 | 1.0000 |
| Non‐dominant hand session | |||||||||
| 75 min | 0.000 ± 0.000 | 0.000 ± 0.000 | 1.0000 | 0.026 ± 0.004 | 0.020 ± 0.005 | 0.0300 | 0.068 ± 0.004 | 0.067 ± 0.005 | 1.0000 |
| 90 min | 0.000 ± 0.000 | 0.000 ± 0.000 | 1.0000 | 0.022 ± 0.003 | 0.016 ± 0.005 | 0.0122 | 0.065 ± 0.003 | 0.061 ± 0.005 | 1.0000 |
| 105 min | 0.000 ± 0.000 | 0.000 ± 0.000 | 1.0000 | 0.019 ± 0.003 | 0.012 ± 0.004 | 0.0092 | 0.061 ± 0.003 | 0.057 ± 0.004 | 1.0000 |
| 120 min | 0.000 ± 0.000 | 0.000 ± 0.000 | 1.0000 | 0.016 ± 0.003 | 0.009 ± 0.004 | 0.0134 | 0.057 ± 0.003 | 0.051 ± 0.004 | 1.0000 |
| 24 h | |||||||||
| Pre‐dominant hand retention | 0.000 ± 0.000 | 0.000 ± 0.000 | 1.0000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 1.0000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 1.0000 |
| Post‐dominant hand retention | 0.000 ± 0.000 | 0.000 ± 0.000 | 1.0000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 1.0000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 1.0000 |
Note: In each comparison, n = 12 for both males and females. The data represent the mean ± 1 SD of BrAC values (%). Exact p values are provided for each comparison. The contrasts were conducted with MLMs.
Concerning the MED condition, the results revealed a Times * Genders interaction (F [11, 242] = 4.626, p < .0001), which indicated that BrAC values were significantly higher in males than in females at each time point following alcohol ingestion in the MED condition only (from 15 to 120 min; see Table 2 for statistical details). To further determine if BrAC time‐curves were parallel, the differences in BrAC values with respect to the 15 min timepoint were calculated to estimate gender differences in metabolism. The results revealed that differences in BrAC values did not differ between males and females (all F [1, 26.63] < 0.763, all p > .6707), suggesting parallel and similar alcohol metabolism between genders despite higher BrAC values in males than females. Concerning the HIGH condition, the results revealed no Times * Genders interaction (F [11, 242.00] = 0.929, p = .5127) and no effect of Genders (F [1, 22.00] = 0.0226, p = .8818), suggesting that males did not differ from females in HIGH. Overall, these results indicate that participants were sober during both dominant hand acquisition and retention. They also confirm the presence of alcohol on the breath of participants in the MED and HIGH conditions and sobriety in the PBO condition during the non‐dominant hand session.
3.2. Dominant hand acquisition
3.2.1. Hand direction at PV: No difference between PBO, MED, and HIGH during acquisition
Concerning hand direction at PV during dominant hand acquisition, the results revealed no Conditions * Phases * Genders interaction (p = .9242), no Conditions * Phases (p = .7491) or Conditions * Genders (p = .9954), or Phases * Genders interaction (p = .2759), and no effect of Conditions (p = .8460) or effect of Genders (p = .3612; see Figure 2b and Table 3 for details). This indicates similar adaptation levels across the PBO (Baseline: 0.563 ± 0.547°; Ramp: 9.939 ± 0.367°; Hold: 22.542 ± 0.445°), MED (Baseline: 0.420 ± 0.708°; Ramp: 10.014 ± 0.523°; Hold: 22.402 ± 0.764°) and HIGH conditions (Baseline: 0.661 ± 0.688°; Ramp: 9.947 ± 0.607°; Hold: 22.575 ± 0.784°) during dominant hand acquisition that is before participants ingested their beverage.
TABLE 3.
Hand direction at PV (baseline, ramp and hold)
| Model info | AIC | R 2 marginal | R 2 conditional | ICC |
|---|---|---|---|---|
| 567.039 | 0.980 | 0.996 | 0.710 |
| Model results | F | Num df | Den df | P value |
|---|---|---|---|---|
| Conditions | 0.168 | 2 | 34.23 | 0.8460 |
| Phases | 3,853.681 | 2 | 31.19 | <0.0001 |
| Genders | 0.870 | 1 | 22.00 | 0.3612 |
| Conditions * Phases | 0.482 | 4 | 131.67 | 0.7491 |
| Conditions * Genders | 0.005 | 2 | 34.23 | 0.9954 |
| Phases * Genders | 1.343 | 2 | 31.19 | 0.2759 |
| Conditions * Phases * Genders | 0.225 | 4 | 131.67 | 0.9242 |
Note: The random coefficients that minimized the AIC value were the inclusion of participants as random intercepts and both conditions and phases as random slope coefficients. For each level of each factor, n = 24.
Additional analyses on RT, MT and Endpoint Accuracy data revealed no meaningful or systematic differences between conditions during Dominant Hand Acquisition (see Tables S1–S3 for statistical details).
3.3. Non‐dominant hand session
3.3.1. Hand direction at PV: No effect of alcohol on motor adaptation
Concerning Hand Direction at PV during the Non‐Dominant Hand Session, the results selectively revealed a trend for a Conditions * Phases interaction (p = .0656; see Figure 2c and Table 4). Subsequent analyses revealed no simple effect of Conditions during the Baseline (F [2, 80.61] = 1.626, p = .3045), Acquisition (F [2, 80.61] = .929, p = .3991) and NoVision phases (F [2, 80.61] = 2.180, p = .3591). This indicates similar adaptation levels across the PBO (Baseline: 1.980 ± 0.872°; Acquisition: 9.103 ± 0.547°; NoVision: 7.758 ± 0.849°), MED (Baseline: 1.756 ± 0.953°; Acquisition: 9.232 ± 0.539°; NoVision: 8.113 ± 1.264°) and HIGH conditions (Baseline: 1.481 ± 0.756°; Acquisition: 9.475 ± 0.570°; NoVision: 7.497 ± 0.927°) during non‐dominant hand session that is 60 min after participants ingested their beverage.
TABLE 4.
Hand direction at PV (baseline, acquisition and NoVision)
| Model info | AIC | R 2 marginal | R 2 conditional | ICC |
|---|---|---|---|---|
| 765.244 | 0.769 | 0.941 | 0.623 |
| Model results | F | Num df | Den df | P value |
|---|---|---|---|---|
| Conditions | 0.647 | 2 | 25.08 | 0.5319 |
| Phases | 508.740 | 2 | 22.39 | <0.0001 |
| Genders | 1.406 | 1 | 22.00 | 0.2483 |
| Conditions * Phases | 2.276 | 4 | 110.00 | 0.0656 |
| Conditions * Genders | 0.909 | 2 | 25.08 | 0.4158 |
| Phases * Genders | 1.228 | 2 | 22.39 | 0.3118 |
| Conditions * Phases * Genders | 1.402 | 4 | 110.00 | 0.2382 |
Note: The random coefficients that minimized the AIC value were the inclusion of participants as random intercepts and both conditions and phases as random slope coefficients. For each level of each factor, n = 24.
The lack of difference between conditions was surprising since motor coordination impairments are a well‐documented sign of alcohol intoxication (Hanchar et al., 2005; Sullivan et al., 1995). To reliably support the absence of a meaningful effect, the effect size of the between‐condition differences was estimated and compared to a theoretical smallest Cohen's d z of interest of ±0.8 (large effect size) using equivalence testing (Lakens, 2017; Lakens et al., 2020). Overall, the results revealed that all of the comparison's effect sizes were all significantly smaller and greater than Cohen's d z of 0.8 and −0.8, respectively (see Table 5 for statistical details). This confirms that alcohol did not meaningfully influence adaptation as compared to placebo.
TABLE 5.
Effect size and equivalence testing: Hand Dir. at PV (baseline, acquisition and NoVision)
| Baseline | Acquisition | NoVision | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PBO vs. MED | PBO vs. HIGH | MED vs. HIGH | PBO vs. MED | PBO vs. HIGH | MED vs. HIGH | PBO vs. MED | PBO vs. High | MED vs. HIGH | |
| Point and SD estimate of the between‐condition standardized effect sizes | |||||||||
| Cohen's d z [1 SD] | 0.135 [1.001] | 0.377 [1.027] | 0.186 [1.004] | −0.139 [1.001] | −0.379 [1.027] | −0.252 [1.010] | −0.183 [1.004] | 0.208 [1.006] | 0.301 [1.015] |
| Equivalence testing against upper and lower bounds of Cohen's dz values of 0.8 and −0.8, respectively | |||||||||
| T (23) upper bound | −3.259 | −2.074 | −3.010 | −4.599 | −5.778 | −5.153 | −4.815 | −2.900 | −2.446 |
| P value upper bound | 0.0017 | 0.0247 | 0.0031 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0040 | 0.0113 |
| T (23) lower bound | 4.759 | 5.765 | 4.829 | 3.240 | 2.061 | 2.685 | 3.023 | 4.938 | 5.393 |
| P value lower bound | <0.0001 | <0.0001 | <0.0001 | 0.0018 | 0.0254 | 0.0066 | 0.0030 | <0.0001 | <0.0001 |
Note: These results indicate that effect sizes between conditions were small to negligible and that all were significantly different from Cohen's d z values of ± 0.8. This suggests that if a true effect was to be reported significant, it would be of small magnitude (Cohen's d z of ~0.3), limiting its theoretical meaningfulness. For each comparison, n = 24.
Additional analyses on RT data revealed no Conditions * Phases * Genders interaction (p = .5164), no Conditions * Phases (p = .8922) or Conditions * Genders (p = .2499), or Phases * Genders interaction (p = 0.4755), and no effect of Conditions (p = .1379) or effect of Genders (p = .7174; see Table S4). These results indicate that RTs did not differ between the PBO (Non‐Dominant Hand Session: 310 ± 14 ms; Baseline: 313 ± 17 ms; Acquisition: 302 ± 14 ms; NoVision: 314 ± 22 ms), MED (Non‐Dominant Hand Session: 317 ± 13 ms; Baseline: 321 ± 22 ms; Acquisition: 310 ± 15 ms; NoVision: 321 ± 18 ms) and HIGH conditions (Non‐Dominant Hand Session: 320 ± 17 ms; Baseline: 326 ± 21 ms; Acquisition: 309 ± 16 ms; NoVision: 326 ± 24 ms), suggesting that alcohol did not influence reaction time.
Concerning MT data, the results selectively revealed an effect of Conditions (p = .0127; see Table S5 for statistical details), which indicated that MT were faster in the HIGH condition (Non‐Dominant Hand Session: 326 ± 18 ms) as compared to PBO (Non‐Dominant Hand Session: 344 ± 16 ms; β = −0.018 ± 0.028; p = .0126). The MED condition (Non‐Dominant Hand Session: 333 ± 20 ms) neither differed from the HIGH (β = −0.007 ± 0.035; p = .3424) nor PBO conditions (β = −0.011 ± 0.032; p = .1437). Overall, these results indicate that the high dose of alcohol invigorated movement speed as compared to placebo.
Concerning Endpoint Accuracy data, the results revealed a trend for a Conditions * Phases interaction (p = 0.0591; see Table S6 for statistical details). Further analyses revealed no simple effects of Conditions during both Baseline (F [2, 50.17] = 2.833; p = .1025) and Acquisition (F [2, 50.17] = 8.250; p = .4329). However, the results revealed a simple effect of Conditions during NoVision (F [2, 50.17] = 8.250; p = .0024), which indicated greater accuracy in the PBO (2.612 ± 0.444 cm) as compared to both the MED (2.862 ± 0.458 cm; β = 0.249 ± 0.454; p = .0135) and HIGH conditions (3.049 ± 0.442 cm; β = 0.437 ± 0.535; p = .0003). The HIGH condition also tended to show impaired accuracy as compared to MED (β = 0.188 ± 0.489; p = .0656). Overall, these results suggest that alcohol tended to dose dependently impair accuracy at movement endpoint as compared to placebo, but only during NoVision.
3.4. Dominant hand retention
3.4.1. Hand direction at PV: No effect of alcohol on retention
Concerning Hand Direction at PV during Dominant Hand Retention, the results revealed no Conditions * Phases * Genders interaction (p = .5186), no Conditions * Phases (p = .0940), or Conditions * Genders (p = .9096), or Phases * Genders interaction (p = .8873), and no effect of Conditions (p = .8958) or effect of Genders (p = .7660; see Figure 2d and Table 6). Altogether, these results indicate similar retention levels across the PBO (NoVision: 5.169 ± 1.214°; Washout: 3.665 ± 0.765°), MED (NoVision: 5.448 ± 0.956°; Washout: 3.579 ± 0.625°) and HIGH conditions (NoVision: 5.679 ± 0.947°; Washout: 3.456 ± 0.729°), suggesting that post‐learning alcohol ingestion did not alter motor memory consolidation as compared to placebo.
TABLE 6.
Retention (NoVision and washout)
| Model info | AIC | R 2 marginal | R 2 conditional | ICC |
|---|---|---|---|---|
| 528.958 | 0.196 | 0.869 | 0.771 |
| Model results | F | Num df | Den df | P value |
|---|---|---|---|---|
| Conditions | 0.111 | 2 | 22.00 | 0.896 |
| Phases | 27.307 | 1 | 22.00 | <0.0001 |
| Genders | 0.091 | 1 | 22.00 | 0.7660 |
| Conditions * Phases | 2.496 | 2 | 44.00 | 0.0940 |
| Conditions * Genders | 0.095 | 2 | 22.00 | 0.9096 |
| Phases * Genders | 0.021 | 1 | 22.00 | 0.8873 |
| Conditions * Phases * Genders | 0.666 | 2 | 44.00 | 0.5186 |
Note: The random coefficients that minimized the AIC value were the inclusion of participants as random intercepts and both conditions and phases as random slope coefficients. For each level of each factor, n = 24.
To reliably support the absence of a meaningful effect, the effect sizes of the between‐condition differences were estimated and compared to a theoretical smallest Cohen's d z of interest of ± 0.8 (large effect size) using equivalence testing (Lakens, 2017; Lakens et al., 2020). Overall, the results revealed that all of the comparisons' effect sizes were all significantly smaller and greater than a Cohen's d z of 0.8 and −0.8, respectively (see Table 7 for statistical details). This confirms that alcohol did not meaningfully influence consolidation as compared to placebo.
TABLE 7.
Effect size and equivalence testing: Hand Dir. at PV (NoVision and washout)
| NoVision | Washout | |||||
|---|---|---|---|---|---|---|
| PBO vs. MED | PBO vs. HIGH | MED vs. HIGH | PBO vs. MED | PBO vs. HIGH | MED vs. HIGH | |
| Point and SD estimate of the between‐condition standardized effect sizes | ||||||
| Cohen's d z [1 SD] | −0.142 [1.002] | −0.259 [1.011] | −0.155 [1.002] | 0.072 [0.999] | 0.154 [1.002] | 0.109 [1.000] |
| Equivalence testing against upper and lower bounds of Cohen's dz values of 0.8 and −0.8, respectively | ||||||
| T (23) upper bound | −4.614 | −5.189 | −4.681 | −3.566 | −3.167 | −3.385 |
| P value upper bound | <0.0001 | <0.0001 | <0.0001 | 0.0008 | 0.0022 | 0.0013 |
| T (23) lower bound | 3.224 | 2.650 | 3.158 | 4.272 | 4.672 | 4.454 |
| P value lower bound | 0.0019 | 0.0072 | 0.0022 | 0.0001 | <0.0001 | <0.0001 |
Note: These results indicate that effect sizes between conditions were small to negligible and all were significantly different from Cohen's d z values of ± 0.8. This suggests that if a true effect was to be reported significant, it would be of small magnitude (Cohen's d z of ~0.2), limiting its theoretical meaningfulness. For each comparison, n = 24.
Additional analyses on RT, MT and Endpoint Accuracy data revealed no meaningful or systematic differences between conditions during Dominant Hand Retention (see Tables S4–S6 for statistical details).
4. DISCUSSION
This work sought to investigate the neurochemical mechanisms of motor memory consolidation. Informed by converging lines of human work on declarative memories (Bruce, Pihl, et al., 1999; Bruce, Shestowsky, et al., 1999; Bruce & Pihl, 1997; Carlyle et al., 2017; Doss et al., 2018; Lamberty et al., 1990; Mueller et al., 1983; Parker et al., 1980, 1981; Tyson & Schirmuly, 1994; Weafer et al., 2016), this work tested the hypothesis that post‐learning alcohol ingestion—through its GABAergic agonist and NMDA antagonist properties (Abrahao et al., 2017; Grant & Lovinger, 2018)—would retrogradely enhance motor memory consolidation. The novelty of this work is that the results disconfirmed this hypothesis. Specifically, the results revealed that reach aftereffect levels during Dominant Hand Retention did not differ between the PBO, MED and HIGH conditions, suggesting that ingesting medium (peak BrAC of ~0.052%) or high (peak BrAC of ~0.094%) alcohol doses following visuomotor adaptation did not enhance motor memory consolidation. Moreover, the results revealed that adaptation levels did not differ between conditions during Non‐Dominant Hand Session, that is, when participants were under the influence of alcohol in the MED (BrAC of ~0.027%) and HIGH conditions (BrAC of ~0.073%). This suggests that alcohol did not disrupt motor adaptation capabilities, as assessed 60 min following its ingestion. Given alcohol's known pharmacological GABAergic agonist and NMDA antagonist properties, one possibility is that these neurochemical mechanisms do not contribute to motor memory consolidation. As decades of work demonstrated alcohol's retrograde declarative memory enhancements (Bruce, Pihl, et al., 1999; Bruce, Shestowsky, et al., 1999; Bruce & Pihl, 1997; Carlyle et al., 2017; Doss et al., 2018; Lamberty et al., 1990; Mueller et al., 1983; Parker et al., 1980, 1981; Tyson & Schirmuly, 1994; Weafer et al., 2016), the present results suggest that distinct neurochemical mechanisms underly declarative and motor memory consolidation.
4.1. No effect of post‐learning alcohol ingestion on motor memory consolidation
This work's main novel finding is that moderate and high alcohol doses neither significantly nor meaningfully altered motor memory consolidation as compared to placebo treatment. Namely, results from equivalence testing revealed that all between‐condition effect sizes were negligible (absolute Cohen's d z < ~0.2; see Table 6) and significantly greater and smaller than Cohen's d z values of −0.8 and 0.8, respectively, confirming that alcohol did not alter consolidation. Given alcohol's pharmacological properties (Abrahao et al., 2017; Grant & Lovinger, 2018), the present results further indicate that increasing GABAergic and decreasing NMDA receptor activity does not contribute to motor memory consolidation. Why these results differ from declarative memory work (Bruce, Pihl, et al., 1999; Bruce, Shestowsky, et al., 1999; Bruce & Pihl, 1997; Carlyle et al., 2017; Doss et al., 2018; Lamberty et al., 1990; Mueller et al., 1983; Parker et al., 1980, 1981; Tyson & Schirmuly, 1994; Weafer et al., 2016) remains, however, unclear. A first possibility is that, despite behavioural evidence showing interactions between motor and declarative memories (Feldman et al., 1995; Keisler & Shadmehr, 2010; Kim, 2020), their consolidation may be supported by distinct neurochemical mechanisms (Feld, Lange, et al., 2013; Feld, Wilhelm, et al., 2013; Kuriyama et al., 2011; Smith & Smith, 2003). For instance, Smith and Smith (2003) showed that alcohol ingestion immediately before sleeping prevented overnight improvements of motor but not declarative memories (see Feld, Wilhelm, et al., 2013, for similar results). Similarly, Feld, Lange, et al. (2013) found that D‐Cycloserine (an NMDA receptor agonist) administration before sleeping enhanced declarative but not motor memory consolidation (although see Kuriyama et al., 2011). In further indirect support, earlier work showed inhomogeneous distributions of GABAA (Bowery et al., 1987; Young & Chu, 1990), GABAB (Bowery et al., 1987; Young & Chu, 1990) and NMDA (Petralia et al., 1994) receptors across mammalian cortical and subcortical structures, suggesting that alcohol does not similarly affect processes of memory consolidation located in distinct neural structures. The above and the present results suggest that pharmacologically altering GABAergic and NMDA activity does not similarly affect declarative and motor memory consolidation. One possibility is thus that distinct neurochemical processes underlie the consolidation of declarative and motor memories.
A second possibility is that reaching asymptotic performance levels during the present Hold phase initiated memory consolidation (Hamel et al., 2017; Orban de Xivry et al., 2011; Yin & Kitazawa, 2001), which prevented alcohol from altering it. In support, Hadj Tahar et al. (2005) had participants ingest Amantadine (an NMDA receptor antagonist) after asymptotic performance levels were reached during a joystick motor adaptation task. Their results revealed no significant performance difference 24 h later between Amantadine and placebo treatment, suggesting that reaching performance asymptote prevented Amantadine from altering memory consolidation. Moreover, Shibata et al. (2017) recorded MRS data and showed that overlearning a visual orientation detection task shifted excitatory‐dominant to inhibitory‐dominant glutamate and GABA concentrations in visual areas, suggesting that reaching performance asymptote initiates consolidation by increasing endogenous inhibition of neural activity. One possibility is thus that pharmacological agents inhibiting neural activity effectively enhance consolidation when inhibition has not already been endogenously increased by reaching performance asymptote.
A third possibility is that alcohol doses previously used to enhance declarative (e.g., Parker et al., 1981) memories—also used in the present work—are insufficient to enhance motor memory consolidation. In support, Hernández et al. (2006, 2007) showed that aspects of cognitive but not motor performance were impaired by reaching BrAC values of ~0.07%, suggesting that greater alcohol doses are required to perturb the motor system. This evidence also echoes alcohol's effects on human behaviours, which are primarily cognitive at doses yielding BrAC values up to ~0.12% (e.g., euphoria, talkativeness and impaired attention) and broaden to motor impairments at doses yielding BrAC values of ~0.15% and above (e.g., impaired balance, coordination and gait) (Jones, 2019; Pohorecky & Brick, 1988). One possibility is thus that alcohol doses greater than those used to enhance declarative memories are required to influence motor memory consolidation.
Perplexingly, while alcohol appears to ubiquitously enhance declarative memory consolidation in humans (Bruce, Pihl, et al., 1999; Bruce, Shestowsky, et al., 1999; Bruce & Pihl, 1997; Carlyle et al., 2017; Doss et al., 2018; Lamberty et al., 1990; Mueller et al., 1983; Parker et al., 1980, 1981; Tyson & Schirmuly, 1994; Weafer et al., 2016), its effects on consolidation are not as consensual in animal work (Alkana & Parker, 1979; Aversano et al., 2002; Castellano & Pavone, 1983; Castellano & Pavone, 1988; Colbern et al., 1986; de Carvalho et al., 1978). Namely, using passive avoidance tasks and alcohol doses that are twofold to fivefold those used in human work, early rodent work showed that post‐learning alcohol injection enhances (Alkana & Parker, 1979; Colbern et al., 1986), disrupts (Aversano et al., 2002; Castellano & Pavone, 1983, 1988) or does not alter memory consolidation (de Carvalho et al., 1978). As this evidence suggests that alcohol heterogeneously affects passive avoidance memory consolidation in animals, its relationship to previous (Bruce, Pihl, et al., 1999; Bruce, Shestowsky, et al., 1999; Bruce & Pihl, 1997; Carlyle et al., 2017; Doss et al., 2018; Hewitt et al., 1996; Lamberty et al., 1990; Mueller et al., 1983; Parker et al., 1980, 1981; Scholey & Fowles, 2002; Tyson & Schirmuly, 1994; Weafer et al., 2016) and present work remains, however, unclear. Finally, although classified as a central nervous depressant, it should be noted that alcohol has stimulating properties (Hendler et al., 2013); has dopaminergic, noradrenergic and serotoninergic agonist properties (Abrahao et al., 2017) and has widespread molecular targets (e.g., opioid and endocannabinoid receptors, neuropeptides such as corticotropin‐releasing factor and intracellular signaling molecules such as protein kinase C, respectively) (Abrahao et al., 2017). How these additional properties of alcohol relate to human motor memory consolidation remains a query for future work.
4.2. Alcohol did not alter learning during adaptation with the non‐dominant hand
Another novel finding is that alcohol did not impair adaptation during Non‐Dominant Hand Session, evidenced by the lack of significant difference across the PBO, MED and HIGH conditions. As above, results from equivalence testing revealed that all between‐condition effect sizes were small (absolute Cohen's d z < ~ 0.3; see Table 4) and significantly greater and smaller than Cohen's d z values of −0.8 and 0.8, respectively, confirming that alcohol did not acutely alter adaptation capabilities. However, the results also revealed that movements were faster and accuracy was lesser in the HIGH as compared to the PBO condition, confirming that the effects of alcohol on motor performance were not completely absent. While resonating with its null result on consolidation, the null effect of alcohol on adaptation opposes work showing that alcohol acutely impairs cerebellar‐dependent motor coordination and learning in animals (He et al., 2013; Sullivan et al., 1995; Valenzuela et al., 2010; Zorumski et al., 2014). However, as GABA and NMDA receptors were reported to increase or decrease their activity during motor learning in humans (Donchin et al., 2002; Floyer‐Lea et al., 2006; Hadj Tahar et al., 2004; Kolasinski et al., 2019; van Vugt et al., 2020), the implications of this evidence remain unclear. For instance, Donchin et al. (2002) showed that administering lorazepam (a GABAA agonist) or dextromethorphan (an NMDA antagonist) before learning impaired force‐field adaptation as compared to placebo, suggesting that pharmacological agents with properties similar to those of alcohol impair sensorimotor adaptation. However, human MRS studies have shown that M1 GABA concentrations either decrease (Floyer‐Lea et al., 2006; Kolasinski et al., 2019) or increase (van Vugt et al., 2020) during learning of motor tasks, making the contribution of GABAergic activity to motor learning unclear. Furthermore, Hadj Tahar et al. (2004) showed that Amantadine ingestion before joystick motor adaptation did not significantly impair learning capabilities, questioning if intact NMDA receptor activity is necessary for motor adaptation. Overall, the above and present results suggest that the contributions of GABAergic and NMDA activity to human sensorimotor adaptation remain unclear, warranting further investigations.
4.3. Limitations
One limitation is that the HIGH, MED and PBO beverages were all mixed with orange juice, which contained glucose. Although the effect of glucose on motor memory consolidation remains unknown, it could have influenced motor memory consolidation similar to alcohol, thus concealing its effect (Scholey & Fowles, 2002). In support, Scholey and Fowles (2002) found that post‐learning ingestion of alcohol (0.38 g/kg) and glucose (25 g) beverages similarly enhanced early consolidation of kinesthetic memories as compared to a saccharin PBO. Future work should replicate the present results using sugar‐free beverage solutions. Another limitation is that sleep quality was not objectively measured, suggesting that alcohol could have failed to enhance motor memory consolidation by interfering with sleep patterns (see Smith & Smith, 2003, for support). The relationship between post‐learning alcohol ingestion, sleep patterns and motor memory consolidation remains a query for future work.
5. CONCLUSION
This study's objective was to investigate the neurochemical mechanisms of human motor memory consolidation. Informed by converging work showing that alcohol retrogradely enhances declarative memory consolidation, this work tested the hypothesis that post‐learning alcohol ingestion would enhance motor memory consolidation as compared to placebo treatment. However, the results disconfirmed this hypothesis by showing that neither medium nor high doses of alcohol enhanced motor memory consolidation as compared to placebo. As this directly opposes studies on human declarative memories, the present results suggest that GABA and NMDA receptor activity distinctly contribute to declarative and motor memory consolidation. Elucidating the neurochemical mechanisms underlying the consolidation of different memory systems may yield insights into the effects of over‐the‐counter drugs on everyday learning and memory, but also inform pharmacological interventions seeking to alter human memory consolidation (e.g., reconsolidation of pathogenic memories; see Diergaarde et al., 2008; Elsey et al., 2018; Walsh et al., 2018).
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
AUTHOR CONTRIBUTIONS
R.H. designed the experiment, collected the data, conducted the analyses, prepared the figures and wrote the manuscript. O.D. helped to design the experiment, collected the data, helped to conduct the analyses, helped to prepare the figures and helped to write the manuscript. J.F.L. and P.M.B. helped to design the experiment and revised the manuscript.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/ejn.15772.
Supporting information
Table S1. RT (Baseline, Ramp, Hold)
Table S2. MT (Baseline, Ramp, Hold)
Table S3. Endpoint Accuracy (Baseline, Ramp, Hold)
Table S4. RT (Baseline, Acquisition, NoVision)
Table S5. MT (Baseline, Acquisition, NoVision)
Table S6. Endpoint Accuracy (Baseline, Acquisition, NoVision)
Table S7. RT (NoVision, Washout)
Table S8. MT (NoVision, Washout)
Table S9. Endpoint Accuracy (NoVision, Washout)
ACKNOWLEDGEMENT
This work was funded by the Natural Sciences and Engineering Research Council of Canada (Grant number: 418589).
Hamel, R. , Demers, O. , Lepage, J.‐F. , & Bernier, P.‐M. (2022). The effects of post‐learning alcohol ingestion on human motor memory consolidation. European Journal of Neuroscience, 56(5), 4600–4618. 10.1111/ejn.15772
Edited by: John Foxe
Funding information Natural Sciences and Engineering Research Council of Canada, Grant/Award Number: 418589
DATA AVAILABILITY STATEMENT
The full data set can be freely accessed through the following URL: (https://docs.google.com/spreadsheets/d/1Zr0JkMQCkvX_Wfme24GhN2nuQVUxjLvL/edit?usp=sharing&ouid=115650053125584404489&rtpof=true&sd=true).
REFERENCES
- Abrahao, K. P. , Salinas, A. G. , & Lovinger, D. M. (2017). Alcohol and the brain: Neuronal molecular targets, synapses, and circuits. Neuron, 96, 1223–1238. 10.1016/j.neuron.2017.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkana, R. L. , & Parker, E. S. (1979). Memory facilitation by post‐training injection of ethanol. Psychopharmacology, 66, 117–119. 10.1007/BF00427617 [DOI] [PubMed] [Google Scholar]
- Amrhein, V. , Greenland, S. , & McShane, B. (2019). Scientists rise up against statistical significance. Nature, 567, 305–307. 10.1038/d41586-019-00857-9 [DOI] [PubMed] [Google Scholar]
- Andersson, A. , Wiréhn, A.‐B. , Ölvander, C. , Ekman, D. S. , & Bendtsen, P. (2009). Alcohol use among university students in Sweden measured by an electronic screening instrument. BMC Public Health, 9(1), 229. 10.1186/1471-2458-9-229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aversano, M. , Ciamei, A. , Cestari, V. , Passino, E. , Middei, S. , & Castellano, C. (2002). Effects of MK‐801 and ethanol combinations on memory consolidation in CD1 mice: Involvement of GABAergic mechanisms. Neurobiology of Learning and Memory, 77, 327–337. 10.1006/nlme.2001.4029 [DOI] [PubMed] [Google Scholar]
- Benjamini, Y. , & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B: Methodological, 57, 289–300. [Google Scholar]
- Bohn, M. J. , Babor, T. F. , & Kranzler, H. R. (1995). The alcohol use disorders identification test (AUDIT): Validation of a screening instrument for use in medical settings. Journal of Studies on Alcohol, 56, 423–432. 10.15288/jsa.1995.56.423 [DOI] [PubMed] [Google Scholar]
- Boisgontier, M. P. , & Cheval, B. (2016). The anova to mixed model transition. Neuroscience and Biobehavioral Reviews, 68, 1004–1005. 10.1016/j.neubiorev.2016.05.034 [DOI] [PubMed] [Google Scholar]
- Bowery, N. G. , Hudson, A. L. , & Price, G. W. (1987). GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience, 20, 365–383. 10.1016/0306-4522(87)90098-4 [DOI] [PubMed] [Google Scholar]
- Brashers‐Krug, T. , Shadmehr, R. , & Bizzi, E. (1996). Consolidation in human motor memory. Nature, 382, 252–255. 10.1038/382252a0 [DOI] [PubMed] [Google Scholar]
- Brooks, J. L. (2012). Counterbalancing for serial order carryover effects in experimental condition orders. Psychological Methods, 17, 600–614. 10.1037/a0029310 [DOI] [PubMed] [Google Scholar]
- Bruce, K. R. , & Pihl, R. O. (1997). Forget ‘drinking to forget’: Enhanced consolidation of emotionally charged memory by alcohol. Experimental and Clinical Psychopharmacology, 5, 242–250. 10.1037/1064-1297.5.3.242 [DOI] [PubMed] [Google Scholar]
- Bruce, K. R. , Pihl, R. O. , Mayerovitch, J. I. , & Shestowsky, J. S. (1999). Alcohol and retrograde memory effects: Role of individual differences. Journal of Studies on Alcohol, 60, 130–136. 10.15288/jsa.1999.60.130 [DOI] [PubMed] [Google Scholar]
- Bruce, K. R. , Shestowsky, J. S. , Mayerovitch, J. I. , & Pihl, R. O. (1999). Motivational effects of alcohol on memory consolidation and heart rate in social drinkers. Alcoholism, Clinical and Experimental Research, 23, 693–701. 10.1111/j.1530-0277.1999.tb04171.x [DOI] [PubMed] [Google Scholar]
- Brunelle, C. , Barrett, S. P. , & Pihl, R. O. (2007). Relationship between the cardiac response to acute intoxication and alcohol‐induced subjective effects throughout the blood alcohol concentration curve. Human Psychopharmacology, 22, 437–443. 10.1002/hup.866 [DOI] [PubMed] [Google Scholar]
- Carlton, L. G. (1992). Chapter 1 Visual processing time and the control of movement. In Proteau L. & Elliott D. (Eds.), Advances in psychology (Vol. 85) (pp. 3–31). Elsevier. [Google Scholar]
- Carlyle, M. , Dumay, N. , Roberts, K. , McAndrew, A. , Stevens, T. , Lawn, W. , & Morgan, C. J. A. (2017). Improved memory for information learnt before alcohol use in social drinkers tested in a naturalistic setting. Scientific Reports, 7(1), 6213. 10.1038/s41598-017-06305-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano, C. , & Pavone, F. (1983). Naloxone‐reversible effects of ethanol on passive avoidance behavior in mice. Physiological Psychology, 11, 291–295. 10.3758/BF03326810 [DOI] [Google Scholar]
- Castellano, C. , & Pavone, F. (1988). Effects of ethanol on passive avoidance behavior in the mouse: Involvement of GABAergic mechanisms. Pharmacology, Biochemistry, and Behavior, 29, 321–324. 10.1016/0091-3057(88)90163-3 [DOI] [PubMed] [Google Scholar]
- Cederbaum, A. I. (2012). Alcohol metabolism. Clinics in Liver Disease, 16, 667–685. 10.1016/j.cld.2012.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry, K. M. , Lenze, E. J. , & Lang, C. E. (2014). Combining d‐cycloserine with motor training does not result in improved general motor learning in neurologically intact people or in people with stroke. Journal of Neurophysiology, 111, 2516–2524. 10.1152/jn.00882.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbern, D. L. , Sharek, P. , & Zimmermann, E. G. (1986). The effect of home or novel environment on the facilitation of passive avoidance by post‐training ethanol. Behavioral and Neural Biology, 46, 1–12. 10.1016/S0163-1047(86)90850-2 [DOI] [PubMed] [Google Scholar]
- de Carvalho, L. P. , Vendite, D. A. , & Izquierdo, I. (1978). A near‐lethal dose of ethanol, given intraperitoneally, does not affect memory consolidation of two different avoidance tasks. Psychopharmacology, 59, 71–74. 10.1007/BF00428033 [DOI] [PubMed] [Google Scholar]
- Diergaarde, L. , Schoffelmeer, A. N. M. , & de Vries, T. J. (2008). Pharmacological manipulation of memory reconsolidation: Towards a novel treatment of pathogenic memories. European Journal of Pharmacology, 585, 453–457. 10.1016/j.ejphar.2008.03.010 [DOI] [PubMed] [Google Scholar]
- Donchin, O. , Sawaki, L. , Madupu, G. , Cohen, L. G. , & Shadmehr, R. (2002). Mechanisms influencing acquisition and recall of motor memories. Journal of Neurophysiology, 88, 2114–2123. 10.1152/jn.2002.88.4.2114 [DOI] [PubMed] [Google Scholar]
- Doss, M. K. , Weafer, J. , Ruiz, N. A. , Gallo, D. A. , & de Wit, H. (2018). Alcohol and pharmacologically similar sedatives impair encoding and facilitate consolidation of both recollection and familiarity in episodic memory. Cognitive Neuroscience, 9, 89–99. 10.1080/17588928.2018.1504764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubowski, K. M. (1985). Absorption, distribution and elimination of alcohol: Highway safety aspects. Journal of Studies on Alcohol. Supplement, (10), 98–108. 10.15288/jsas.1985.s10.98 [DOI] [PubMed] [Google Scholar]
- Elsey, J. W. B. , van Ast, V. A. , & Kindt, M. (2018). Human memory reconsolidation: A guiding framework and critical review of the evidence. Psychological Bulletin, 144, 797–848. 10.1037/bul0000152 [DOI] [PubMed] [Google Scholar]
- Feld, G. B. , Lange, T. , Gais, S. , & Born, J. (2013). Sleep‐dependent declarative memory consolidation—Unaffected after blocking NMDA or AMPA receptors but enhanced by NMDA coagonist D‐cycloserine. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 38, 2688–2697. 10.1038/npp.2013.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feld, G. B. , Wilhelm, I. , Ma, Y. , Groch, S. , Binkofski, F. , Mölle, M. , & Born, J. (2013). Slow wave sleep induced by GABA agonist Tiagabine fails to benefit memory consolidation. Sleep, 36, 1317–1326. 10.5665/sleep.2954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman, J. , Kerr, B. , & Streissguth, A. P. (1995). Correlational analyses of procedural and declarative learning performance. Intelligence, 20, 87–114. 10.1016/0160-2896(95)90007-1 [DOI] [Google Scholar]
- Floyer‐Lea, A. , Wylezinska, M. , Kincses, T. , & Matthews, P. M. (2006). Rapid modulation of GABA concentration in human sensorimotor cortex during motor learning. Journal of Neurophysiology, 95, 1639–1644. 10.1152/jn.00346.2005 [DOI] [PubMed] [Google Scholar]
- Frezza, M. , di Padova, C. , Pozzato, G. , Terpin, M. , Baraona, E. , & Lieber, C. S. (1990). High blood alcohol levels in women. The role of decreased gastric alcohol dehydrogenase activity and first‐pass metabolism. The New England Journal of Medicine, 322, 95–99. 10.1056/NEJM199001113220205 [DOI] [PubMed] [Google Scholar]
- Gais, S. , Rasch, B. , Wagner, U. , & Born, J. (2008). Visual‐procedural memory consolidation during sleep blocked by glutamatergic receptor antagonists. The Journal of Neuroscience, 28, 5513–5518. 10.1523/JNEUROSCI.5374-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal, R. K. , Guo, Y. , & Mashimo, H. (2019). Advances in the physiology of gastric emptying. Neurogastroenterology and Motility, 31, e13546. 10.1111/nmo.13546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant, K. A. , & Lovinger, D. M. (Eds.) (2018). The neuropharmacology of alcohol. Springer International Publishing. 10.1007/978-3-319-96523-9 [DOI] [Google Scholar]
- Günthner, J. , Scholl, J. , Favaron, E. , Harmer, C. J. , Johansen‐Berg, H. , & Reinecke, A. (2016). The NMDA receptor partial agonist d‐cycloserine does not enhance motor learning. Journal of Psychopharmacology (Oxford, England), 30, 994–999. 10.1177/0269881116658988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadj Tahar, A. , Blanchet, P. J. , & Doyon, J. (2004). Motor‐learning impairment by amantadine in healthy volunteers. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology, 29, 187–194. 10.1038/sj.npp.1300317 [DOI] [PubMed] [Google Scholar]
- Hadj Tahar, A. , Blanchet, P. J. , & Doyon, J. (2005). Effect of amantadine on motor memory consolidation in humans. Behavioural Pharmacology, 16, 107–112. 10.1097/00008877-200503000-00006 [DOI] [PubMed] [Google Scholar]
- Hamel, R. , Côté, K. , Matte, A. , Lepage, J.‐F. , & Bernier, P.‐M. (2019). Rewards interact with repetition‐dependent learning to enhance long‐term retention of motor memories. Annals of the New York Academy of Sciences, 1452, 34–51. 10.1111/nyas.14171 [DOI] [PubMed] [Google Scholar]
- Hamel, R. , Dallaire‐Jean, L. , de la Fontaine, É. , Lepage, J. F. , & Bernier, P. M. (2021). Learning the same motor task twice impairs its retention in a time‐ and dose‐dependent manner. Proceedings of the Royal Society B: Biological Sciences, 288, 20202556. 10.1098/rspb.2020.2556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel, R. , de la Fontaine, É. , Lepage, J. F. , & Bernier, P. M. (2021). Punishments and rewards both modestly impair visuomotor memory retention. Neurobiology of Learning and Memory, 185, 107532. 10.1016/j.nlm.2021.107532 [DOI] [PubMed] [Google Scholar]
- Hamel, R. , Lepage, J.‐F. , & Bernier, P.‐M. (2021). Anterograde interference emerges along a gradient as a function of task similarity: A behavioral study. The European Journal of Neuroscience, 55, 49–66. 10.1111/ejn.15561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel, R. , Trempe, M. , & Bernier, P.‐M. (2017). Disruption of M1 activity during performance plateau impairs consolidation of motor memories. Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 37, 9197–9206. 10.1523/JNEUROSCI.3916-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanchar, H. J. , Dodson, P. D. , Olsen, R. W. , Otis, T. S. , & Wallner, M. (2005). Alcohol‐induced motor impairment caused by increased extrasynaptic GABAA receptor activity. Nature Neuroscience, 8, 339–345. 10.1038/nn1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison, X. A. , Donaldson, L. , Correa‐Cano, M. E. , Evans, J. , Fisher, D. N. , Goodwin, C. E. D. , Robinson, B. S. , Hodgson, D. J. , & Inger, R. (2018). A brief introduction to mixed effects modelling and multi‐model inference in ecology. PeerJ, 6, e4794. 10.7717/peerj.4794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartsock, M. J. , & Spencer, R. L. (2020). Memory and the circadian system: Identifying candidate mechanisms by which local clocks in the brain may regulate synaptic plasticity. Neuroscience and Biobehavioral Reviews, 118, 134–162. 10.1016/j.neubiorev.2020.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, Q. , Titley, H. , Grasselli, G. , Piochon, C. , & Hansel, C. (2013). Ethanol affects NMDA receptor signaling at climbing fiber‐Purkinje cell synapses in mice and impairs cerebellar LTD. Journal of Neurophysiology, 109, 1333–1342. 10.1152/jn.00350.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendler, R. A. , Ramchandani, V. A. , Gilman, J. , & Hommer, D. W. (2013). Stimulant and sedative effects of alcohol. Current Topics in Behavioral Neurosciences, 13, 489–509. 10.1007/7854_2011_135 [DOI] [PubMed] [Google Scholar]
- Hernández, O. H. , Vogel‐Sprott, M. , Huchín‐Ramirez, T. C. , & Aké‐Estrada, F. (2006). Acute dose of alcohol affects cognitive components of reaction time to an omitted stimulus: Differences among sensory systems. Psychopharmacology, 184, 75–81. 10.1007/s00213-005-0237-7 [DOI] [PubMed] [Google Scholar]
- Hernández, O. H. , Vogel‐Sprott, M. , & Ke‐Aznar, V. I. (2007). Alcohol impairs the cognitive component of reaction time to an omitted stimulus: A replication and an extension. Journal of Studies on Alcohol and Drugs, 68, 276–281. 10.15288/jsad.2007.68.276 [DOI] [PubMed] [Google Scholar]
- Hewitt, G. P. , Holder, M. , & Laird, J. (1996). Retrograde enhancement of human kinesthetic memory by alcohol: Consolidation or protection against interference? Neurobiology of Learning and Memory, 65, 269–277. 10.1006/nlme.1996.0032 [DOI] [PubMed] [Google Scholar]
- Holford, N. H. (1987). Clinical pharmacokinetics of ethanol. Clinical Pharmacokinetics, 13, 273–292. 10.2165/00003088-198713050-00001 [DOI] [PubMed] [Google Scholar]
- Jones, A. W. (2019). Alcohol, its analysis in blood and breath for forensic purposes, impairment effects, and acute toxicity. WIREs Forensic Science, 1, e1353. [Google Scholar]
- Keisler, A. , & Shadmehr, R. (2010). A shared resource between declarative memory and motor memory. Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 30(44), 14817–14823. 10.1523/JNEUROSCI.4160-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S. (2020). Bidirectional competitive interactions between motor memory and declarative memory during interleaved learning. Scientific Reports, 10(1), 6916. 10.1038/s41598-020-64039-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerner, T. K. , & Zhang, Y. (2017). Application of linear mixed‐effects models in human neuroscience research: A comparison with Pearson correlation in two auditory electrophysiology studies. Brain Sciences, 7(3), 1–11. 10.3390/brainsci7030026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolasinski, J. , Hinson, E. L. , Divanbeighi Zand, A. P. , Rizov, A. , Emir, U. E. , & Stagg, C. J. (2019). The dynamics of cortical GABA in human motor learning. The Journal of Physiology, 597, 271–282. 10.1113/JP276626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama, K. , Honma, M. , Koyama, S. , & Kim, Y. (2011). D‐cycloserine facilitates procedural learning but not declarative learning in healthy humans: A randomized controlled trial of the effect of d‐cycloserine and valproic acid on overnight properties in the performance of non‐emotional memory tasks. Neurobiology of Learning and Memory, 95, 505–509. 10.1016/j.nlm.2011.02.017 [DOI] [PubMed] [Google Scholar]
- Lakens, D. (2017). Equivalence tests: A practical primer for t tests, correlations, and Meta‐analyses. Social Psychological and Personality Science, 8, 355–362. 10.1177/1948550617697177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakens, D. , McLatchie, N. , Isager, P. M. , Scheel, A. M. , & Dienes, Z. (2020). Improving inferences about null effects with Bayes factors and equivalence tests. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 75, 45–57. 10.1093/geronb/gby065 [DOI] [PubMed] [Google Scholar]
- Lamberty, G. J. , Beckwith, B. E. , Petros, T. V. , & Ross, A. R. (1990). Posttrial treatment with ethanol enhances recall of prose narratives. Physiology & Behavior, 48, 653–658. 10.1016/0031-9384(90)90206-J [DOI] [PubMed] [Google Scholar]
- Lerner, G. , Albert, S. , Caffaro, P. A. , Villalta, J. I. , Jacobacci, F. , Shadmehr, R. , & Della‐Maggiore, V. (2020). The origins of anterograde interference in Visuomotor adaptation. Cerebral cortex (New York, N.Y. 1991: Online), 1991(30), 4000–4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leys, C. , Ley, C. , Klein, O. , Bernard, P. , & Licata, L. (2013). Detecting outliers: Do not use standard deviation around the mean, use absolute deviation around the median. Journal of Experimental Social Psychology, 49, 764–766. 10.1016/j.jesp.2013.03.013 [DOI] [Google Scholar]
- Lytsy, P. (2018). P in the right place: Revisiting the evidential value of P‐values. Journal of Evidence‐Based Medicine, 11, 288–291. 10.1111/jebm.12319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magezi, D. A. (2015). Linear mixed‐effects models for within‐participant psychology experiments: An introductory tutorial and free, graphical user interface (LMMgui). Frontiers in Psychology, 6(2), 1–7. 10.3389/fpsyg.2015.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfait, N. , & Ostry, D. J. (2004). Is interlimb transfer of force‐field adaptation a cognitive response to the sudden introduction of load? Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 24, 8084–8089. 10.1523/JNEUROSCI.1742-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mednick, S. C. , Cai, D. J. , Shuman, T. , Anagnostaras, S. , & Wixted, J. T. (2011). An opportunistic theory of cellular and systems consolidation. Trends in Neurosciences, 34, 504–514. 10.1016/j.tins.2011.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney, R. A. , Bastian, A. J. , & Celnik, P. A. (2021). Training at asymptote stabilizes motor memories by reducing intracortical excitation. Cortex, 143, 47–56. 10.1016/j.cortex.2021.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean, M. E. , & Corbin, W. R. (2010). Subjective response to alcohol: A critical review of the literature. Alcoholism, Clinical and Experimental Research, 34, 385–395. 10.1111/j.1530-0277.2009.01103.x [DOI] [PubMed] [Google Scholar]
- Morgan, P. T. , Kehne, J. H. , Sprenger, K. J. , & Malison, R. T. (2010). Retrograde effects of triazolam and zolpidem on sleep‐dependent motor learning in humans. Journal of Sleep Research, 19, 157–164. 10.1111/j.1365-2869.2009.00757.x [DOI] [PubMed] [Google Scholar]
- Mueller, C. W. , Lisman, S. A. , & Spear, N. E. (1983). Alcohol enhancement of human memory: Tests of consolidation and interference hypotheses. Psychopharmacology, 80, 226–230. 10.1007/BF00436158 [DOI] [PubMed] [Google Scholar]
- Mumenthaler, M. S. , Taylor, J. L. , O'Hara, R. , & Yesavage, J. A. (1999). Gender differences in moderate drinking effects. Alcohol Research & Health: The Journal of the National Institute on Alcohol Abuse and Alcoholism, 23, 55–64. [PMC free article] [PubMed] [Google Scholar]
- Mundt, J. C. , Perrine, M. W. , & Searles, J. S. (1997). Individual differences in alcohol responsivity: Physiological, psychomotor and subjective response domains. Journal of Studies on Alcohol, 58, 130–140. 10.15288/jsa.1997.58.130 [DOI] [PubMed] [Google Scholar]
- Orban de Xivry, J.‐J. , Criscimagna‐Hemminger, S. E. , & Shadmehr, R. (2011). Contributions of the motor cortex to adaptive control of reaching depend on the perturbation schedule. Cerebral Cortex, 21, 1475–1484. 10.1093/cercor/bhq192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozoemena, K. I. , Musa, S. , Modise, R. , Ipadeola, A. K. , Gaolatlhe, L. , Peteni, S. , & Kabongo, G. (2018). Fuel cell‐based breath‐alcohol sensors: Innovation‐hungry old electrochemistry. Current Opinion in Electrochemistry, 10, 82–87. 10.1016/j.coelec.2018.05.007 [DOI] [Google Scholar]
- Parker, E. S. , Birnbaum, I. M. , Weingartner, H. , Hartley, J. T. , Stillman, R. C. , & Wyatt, R. J. (1980). Retrograde enhancement of human memory with alcohol. Psychopharmacology, 69, 219–222. 10.1007/BF00427653 [DOI] [PubMed] [Google Scholar]
- Parker, E. S. , Morihisa, J. M. , Wyatt, R. J. , Schwartz, B. L. , Weingartner, H. , & Stillman, R. C. (1981). The alcohol facilitation effect on memory: A dose‐response study. Psychopharmacology, 74, 88–92. 10.1007/BF00431763 [DOI] [PubMed] [Google Scholar]
- Petralia, R. S. , Yokotani, N. , & Wenthold, R. J. (1994). Light and electron microscope distribution of the NMDA receptor subunit NMDAR1 in the rat nervous system using a selective anti‐peptide antibody. The Journal of Neuroscience, 14, 667–696. 10.1523/JNEUROSCI.14-02-00667.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohorecky, L. A. , & Brick, J. (1988). Pharmacology of ethanol. Pharmacology & Therapeutics, 36, 335–427. 10.1016/0163-7258(88)90109-X [DOI] [PubMed] [Google Scholar]
- Riordan, B. C. , Scarf, D. , Moradi, S. , Flett, J. A. M. , Carey, K. B. , & Conner, T. S. (2017). The accuracy and promise of personal breathalysers for research: Steps toward a cost‐effective reliable measure of alcohol intoxication? Digital Health, 3, 2055207617746752. 10.1177/2055207617746752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders, J. B. , Aasland, O. G. , Babor, T. F. , de la Fuente, J. R. , & Grant, M. (1993). Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption‐II. Addiction (Abingdon, England), 88, 791–804. 10.1111/j.1360-0443.1993.tb02093.x [DOI] [PubMed] [Google Scholar]
- Scholey, A. B. , & Fowles, K. A. (2002). Retrograde enhancement of kinesthetic memory by alcohol and by glucose. Neurobiology of Learning and Memory, 78, 477–483. 10.1006/nlme.2002.4065 [DOI] [PubMed] [Google Scholar]
- Shibata, K. , Sasaki, Y. , Bang, J. W. , Walsh, E. G. , Machizawa, M. G. , Tamaki, M. , Chang, L. H. , & Watanabe, T. (2017). Overlearning hyperstabilizes a skill by rapidly making neurochemical processing inhibitory‐dominant. Nature Neuroscience, 20, 470–475. 10.1038/nn.4490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, C. , & Smith, D. (2003). Ingestion of ethanol just prior to sleep onset impairs memory for procedural but not declarative tasks. Sleep, 26, 185–191. [PubMed] [Google Scholar]
- Sorbello, J. G. , Devilly, G. J. , Allen, C. , Hughes, L. R. J. , & Brown, K. (2018). Fuel‐cell breathalyser use for field research on alcohol intoxication: An independent psychometric evaluation. PeerJ, 6, e4418. 10.7717/peerj.4418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan, E. V. , Rosenbloom, M. J. , Deshmukh, A. , Desmond, J. E. , & Pfefferbaum, A. (1995). Alcohol and the cerebellum. Alcohol Health and Research World, 19, 138–141. [PMC free article] [PubMed] [Google Scholar]
- Tyson, P. D. , & Schirmuly, M. (1994). Memory enhancement after drinking ethanol: Consolidation, interference, or response bias? Physiology & Behavior, 56, 933–937. 10.1016/0031-9384(94)90326-3 [DOI] [PubMed] [Google Scholar]
- Valenzuela, C. F. , Lindquist, B. , & Zamudio‐Bulcock, P. A. (2010). A review of synaptic plasticity at Purkinje neurons with a focus on ethanol‐induced cerebellar dysfunction. In Reilly M. T. & Lovinger D. M. (Eds.), international review of neurobiology (Vol. 91) (pp. 339–372). Academic Press. 10.1016/S0074-7742(10)91011-8 [DOI] [PubMed] [Google Scholar]
- van Vugt, F. T. , Near, J. , Hennessy, T. , Doyon, J. , & Ostry, D. J. (2020). Early stages of sensorimotor map acquisition: Neurochemical signature in primary motor cortex and its relation to functional connectivity. Journal of Neurophysiology, 124, 1615–1624. 10.1152/jn.00285.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall, T. L. , Luczak, S. E. , & Hiller‐Sturmhöfel, S. (2016). Biology, genetics, and environment: Underlying factors influencing alcohol metabolism. Alcohol Research: Current Reviews, 38, 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh, K. H. , Das, R. K. , Saladin, M. E. , & Kamboj, S. K. (2018). Modulation of naturalistic maladaptive memories using behavioural and pharmacological reconsolidation‐interfering strategies: A systematic review and meta‐analysis of clinical and ‘sub‐clinical’ studies. Psychopharmacology, 235, 2507–2527. 10.1007/s00213-018-4983-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasielewski, J. A. , & Holloway, F. A. (2001). Alcohol's interactions with circadian rhythms. Alcohol Research and Health, 25(2), 94–100. PMID: https://pubs.niaaa.nih.gov/publications/arh25-2/94-100.htm [PMC free article] [PubMed] [Google Scholar]
- Weafer, J. , Gallo, D. A. , & de Wit, H. (2016). Effect of alcohol on encoding and consolidation of memory for alcohol‐related images. Alcoholism, Clinical and Experimental Research, 40, 1540–1547. 10.1111/acer.13103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner, S. , Strüder, H. K. , & Donchin, O. (2019). Intermanual transfer of visuomotor adaptation is related to awareness. PLoS ONE, 14, e0220748. 10.1371/journal.pone.0220748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, P.‐B. , & Kitazawa, S. (2001). Long‐lasting aftereffects of prism adaptation in the monkey. Experimental Brain Research, 141, 250–253. 10.1007/s002210100892 [DOI] [PubMed] [Google Scholar]
- Young, A. B. , & Chu, D. (1990). Distribution of GABAA and GABAB receptors in mammalian brain: Potential targets for drug development. Drug Development Research, 21, 161–167. 10.1002/ddr.430210303 [DOI] [Google Scholar]
- Zorumski, C. F. , Mennerick, S. , & Izumi, Y. (2014). Acute and chronic effects of ethanol on learning‐related synaptic plasticity. Alcohol, 48, 1–17. 10.1016/j.alcohol.2013.09.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. RT (Baseline, Ramp, Hold)
Table S2. MT (Baseline, Ramp, Hold)
Table S3. Endpoint Accuracy (Baseline, Ramp, Hold)
Table S4. RT (Baseline, Acquisition, NoVision)
Table S5. MT (Baseline, Acquisition, NoVision)
Table S6. Endpoint Accuracy (Baseline, Acquisition, NoVision)
Table S7. RT (NoVision, Washout)
Table S8. MT (NoVision, Washout)
Table S9. Endpoint Accuracy (NoVision, Washout)
Data Availability Statement
The full data set can be freely accessed through the following URL: (https://docs.google.com/spreadsheets/d/1Zr0JkMQCkvX_Wfme24GhN2nuQVUxjLvL/edit?usp=sharing&ouid=115650053125584404489&rtpof=true&sd=true).


