Abstract
Aim
To establish treatment response biomarkers that reflect the pathophysiology of depression, it is important to use an integrated set of features. This study aimed to determine the relationship between regional brain activity at rest and blood metabolites related to treatment response to escitalopram to identify the characteristics of depression that respond to treatment.
Methods
Blood metabolite levels and resting‐state brain activity were measured in patients with moderate to severe depression (n = 65) before and after 6–8 weeks of treatment with escitalopram, and these were compared between Responders and Nonresponders to treatment. We then examined the relationship between blood metabolites and brain activity related to treatment responsiveness in patients and healthy controls (n = 36).
Results
Thirty‐two patients (49.2%) showed a clinical response (>50% reduction in the Hamilton Rating Scale for Depression score) and were classified as Responders, and the remaining 33 patients were classified as Nonresponders. The pretreatment fractional amplitude of low‐frequency fluctuation (fALFF) value of the left dorsolateral prefrontal cortex (DLPFC) and plasma kynurenine levels were lower in Responders, and the rate of increase of both after treatment was correlated with an improvement in symptoms. Moreover, the fALFF value of the left DLPFC was significantly correlated with plasma kynurenine levels in pretreatment patients with depression and healthy controls.
Conclusion
Decreased resting‐state regional activity of the left DLPFC and decreased plasma kynurenine levels may predict treatment response to escitalopram, suggesting that it may be involved in the pathophysiology of major depressive disorder in response to escitalopram treatment.
Keywords: dorsolateral prefrontal cortex, fMRI, kynurenine, major depressive disorder, metabolomics
Antidepressants are recommended as the first‐line treatment for moderate to severe depression 1 ; however, response and remission rates to antidepressant therapy remain disappointingly low. The largest pragmatic clinical trial for the treatment of depression to date, the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial, reported that only 48.6% of patients respond to initial antidepressant treatment and 36.8% of patients achieve remission. 2 To evaluate the efficacy and prognosis of treatment objectively and to select an appropriate treatment, it is necessary to assess accurately the characteristics of depression that respond to specific antidepressants and to develop biomarkers that can help predict treatment efficacy.
Several promising biomarkers for treatment response in patients with depression have been reported in the fields of genetic variation, gene expression profiling, proteomics, metabolomics, neuroendocrinology, electrophysiology, and neuroimaging, but none have been established yet. 3 To establish a treatment response biomarker that reflects the pathophysiology, it is important to clarify the relationship between each biomarker and treatment response and use them in an integrated manner.
Metabolites are the final products of interactions between gene expression, protein function, and cellular environment. 4 Thus, metabolomics holds great promise for the identification of the pathways involved in the antidepressant response and pathophysiology of depression. 5 For example, with regard to the treatment response to sertraline, studies have shown that the metabolic pathways of phenylalanine, tryptophan (TRP), purine, and tocopherol are associated 6 as well as decreases in the kynurenine (KYN)/melatonin and 3‐hydroxykynurenine/melatonin ratios. 7 Furthermore, the treatment response to escitalopram is associated with low glycine levels 8 as well as high serotonin levels and a low KYN/TRP ratio. 9 Recently, we also reported that low KYN and kynurenic acid levels were associated with the treatment response to escitalopram. 10 Several meta‐analyses have revealed the involvement of abnormalities in the KYN pathway in the pathophysiology of depression, 11 , 12 , 13 which may be useful for the development of treatment response biomarkers in metabolomics.
Conversely, in neuroimaging studies, functional magnetic resonance imaging (fMRI) has the advantage of high spatial resolution and noninvasive assessment of brain function, and the activity in several brain regions is reportedly involved in treatment response. For example, low activation of the anterior cingulate gyrus, dorsolateral prefrontal cortex (DLPFC), thalamus, and caudate nucleus in response to negative word stimuli is associated with a favorable treatment response to escitalopram, 14 while low activation of the amygdala in response to negative facial expressions is associated with a favorable treatment response to paroxetine. 15 In a study using resting‐state fMRI, a decrease in resting‐state functional connectivity in the cognitive control network was associated with non‐remission 16 and the strength of functional connectivity between the anterior insula and left DLPFC was associated with early favorable treatment response. 17 We reported that the fractional amplitude of low‐frequency fluctuations (fALFFs) in the right thalamus is increased in treatment‐resistant depression and that its activity is negatively correlated with treatment response. 18 The fALFF method has been shown to reflect pathophysiological aspects of depression 19 , 20 , 21 , 22 , 23 and has been suggested to be the most sensitive index for detecting the effects of metabolic state on resting brain activity. 24 , 25
As mentioned above, blood metabolomics and fMRI are promising methods in the development of biomarkers for predicting treatment response in patients with depression, but each approach has its advantages and disadvantages. Although it is easy to obtain blood samples for metabolomics analysis, it cannot evaluate brain function directly, which is the locus of pathology. Conversely, fMRI has the advantage of noninvasively assessing the function of each region of the whole brain, but does not provide information on metabolic mechanisms. Integrative measurement and analysis using both approaches in the same subject may help to establish biomarkers that more accurately reflect the diverse pathological features of depression. However, few studies have examined how blood metabolites and the activity of brain regions are interrelated with pathological conditions in the therapeutic effects of antidepressants and changes before and after treatment, and the therapeutic mechanisms are unknown.
We hypothesized that the differences in blood metabolite levels and spontaneous regional neural activity that are related to each other would be associated with responsiveness to escitalopram treatment in patients with major depressive disorder (MDD). We also hypothesized that these indicators would differ more in responders than in healthy subjects, and that the more these indicators changed from pre‐treatment to post‐treatment toward the values of healthy subjects then the better the response to treatment would be.
In this study, we measured blood metabolite levels and performed resting‐state fMRI before and after escitalopram administration for 6–8 weeks in depressed patients to investigate the interrelationship between metabolites related to treatment response and regional brain fALFFs related to treatment response. Regarding metabolites, we focused on KYN, as it can cross the blood–brain barrier and is reportedly associated with treatment response to escitalopram. 10
Materials and Methods
Participants
A total of 67 patients with MDD in the acute phase of the disease were recruited from Hiroshima University and local clinics according to the following inclusion criteria: (a) age between 25 and 75 years; (b) outpatient status; (c) presentation of moderate or more severe depressive symptoms, as determined by a score of 14 or more on the 17‐item Hamilton Rating Scale for Depression (HRSD) 26 ; and (d) diagnosis of non‐psychotic MDD and current depressive episode, as determined by an experienced psychiatrist according to the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM‐IV) and verified through the Mini‐International Psychiatric Structural Interview (MINI) 27 , 28 conducted by trained evaluators. The exclusion criteria were as follows: (a) diagnosis of neurological disease, current or previous psychotic disorder, current high risk of suicide, current or previous substance abuse, and serious somatic disease as determined by the MINI; (b) left‐handedness, which was defined as a score <0 on the Edinburgh handedness test 29 ; and (c) current pregnancy or nursing.
Thirty‐eight healthy control (HC) subjects were recruited from the local community, and their ages and sex were matched with the MDD patient group. They were interviewed with the MINI, and none showed a history of psychiatric disorders according to the DSM‐IV criteria.
We determined the minimum number of participants in each group based on previous metabolomics and resting‐state fMRI studies. Our previous metabolomics study 10 showed that the average effect size of metabolites identified as biomarkers predicting treatment responsiveness was r = 0.359. We calculated that statistical analysis would require a minimum of 32 participants to detect this effect with a power of 0.80 and α of 0.05. Conversely, our previous resting‐state fMRI study 18 reported that the mean fALFF in the area related to antidepressant treatment response was correlated with the percentage change in HRSD17 scores with an effect size of r = 0.519. We calculated that a minimum of 24 subjects would be needed to detect this association with a power of 0.80 and α of 0.05. Consequently, we aimed to recruit more than 32 participants in each group in the present study.
Patients with MDD provided blood samples and underwent MRI during the acute phase of the disease (T1) and after approximately 6–8 weeks of treatment with escitalopram (T2). The HRSD was administered to measure the severity of depression of each patient at each session. HC participants provided blood samples and underwent MRI once. The verbal intelligence quotient (IQ) equivalent of all participants was assessed using the Japanese version of the Adult Reading Test. 30 Thirty‐two patients and 24 HCs were participants in our previous study. 10
The study was conducted in compliance with relevant guidelines and regulations and the latest version of the World Medical Association's Declaration of Helsinki. The current study protocol was approved by the Ethics Committee of Hiroshima University and the Institutional Ethics Committee of the University of Tokushima Graduate School. Before the administration of any experimental procedure, written informed consent was obtained from all participants.
MRI data acquisition, preprocessing, and analysis
Functional brain images were acquired using 3T MRI scanners from Siemens and GE Healthcare at four different sites; details of the MRI acquisition sequences are summarized in Supplementary Table S1. All scans were acquired with an echo‐planar imaging sequence. Baseline (T1) and second (T2) MRI scans of the MDD patients were performed at an average of 7.52 (range: 1–14) days and 49.65 (range: 38–63) days after the start of escitalopram administration, respectively. MRI scans of HC subjects were performed on the same day as consent to participate in the study was obtained. All subjects were instructed to look at a central fixation point, lie still, stay awake, and not think of anything specific.
The first eight images were discarded to ensure steady‐state fMRI signals during acclimation of the subjects. Then, the images were preprocessed using CONN toolbox (version 20.b). 31 After slice timing correction, realignment, normalization, and smoothing (6‐mm FWHM Gaussian filter), the images were co‐registered to the structural data using linear transformation and normalized to Montreal Neurological Institute space using nonlinear transformation. The Artifact Detection Tools (ART) in CONN identified outlier images if head motion in the x, y, or z direction was greater than 1 mm or if the global mean intensity in the image was greater than three standard deviations from the mean image intensity of all images. Individual T1‐weighted images were segmented into gray matter, white matter, and cerebrospinal fluid to generate three masks. Linear regression was performed to remove the confounding effects of ART‐based scrubbing parameters containing invalid scans and BOLD signals from the white matter and cerebrospinal fluid, which were used for aCompCor, head motion confounding defined by six rigid body motion parameters and six first‐order time derivatives. Then, band‐pass filtering (0.008–0.09 Hz) and linear detrending were applied to the resulting residual BOLD time series. Subsequently, using the CONN toolbox option, voxel‐wise maps of fALFFs were calculated for each subject. For standardization purposes, the individual fALFF map was transformed to z‐scores by subtracting its mean and dividing by the standard deviation. fALFF values describe the level of spontaneous activity of each individual element in the resting state from all aspects of energy and provide information on the magnitude of activity of each brain region within a network of interest. 32 It has been reported that fALFF values have high temporal stability 33 and test–retest reliability, 34 are robust to non‐specific signal components such as physiological noise, 35 and have high specificity in detecting regional spontaneous brain activity, especially in gray matter. 32 , 35
The image quality of the raw MRI data of all participants was checked by a diagnostic radiologist to exclude obvious anatomical abnormalities and artifacts. All normalized images were checked for alignment errors during data preprocessing, and data with alignment errors were excluded. Finally, subjects with head movements greater than 2.0 mm or rotations greater than 2.0° in the x, y, or z direction were excluded. As a result, two MDD patients and two HC subjects were excluded. Thus, 65 MDD patients and 36 HC subjects were included in further analysis.
As mentioned above, we acquired functional brain images at four different sites. Multisite MRI data are known to have differences in measurements across sites due to systematic bias and nonbiological variability resulting from the use of different scanners and different imaging parameters. 36 , 37 , 38 , 39 Therefore, we applied the ComBat harmonization method 40 , 41 to each fALFF value to eliminate the effects of these scanner and site differences; see Supplementary Method for details of the ComBat harmonization method.
Quantitative metabolome analysis
The baseline (T1) blood samples of MDD patients were collected on the day they visited a local clinic and started escitalopram. The second (T2) blood samples were collected when they visited for the second MRI scan. Blood samples were collected from the HC subjects on the same day at the facility where MRI was performed. All blood samples were sent from Hiroshima University to Tokushima University, where 50 μL sample was mixed with 450 μL methanol containing internal standards (10 μM) and vortexed. Chloroform (500 μL) and Milli‐Q water (200 μL) were added, mixed thoroughly, and centrifuged (2,300 × g, 4°C, 5 min). Then, 375 μL of the aqueous layer was filtered through a 5‐kDa cutoff filter (EMD Millipore, Billerica, MA, USA) to remove macromolecules. The filtrate was lyophilized and dissolved in 50 μL Milli‐Q water containing the reference compound before mass spectrometry analysis.
Metabolome measurements were conducted at Tokushima University. Plasma metabolite profiling and a mixture of 110 standard metabolites (50 μM each; HMT, Tsuruoka, Japan) were analyzed using a capillary electrophoresis electrospray ionization time‐of‐flight mass spectrometry (CE‐ESI‐TOFMS) system (Agilent 7100 CE ‐ 6230 TOFMS; Agilent Technologies, Palo Alto, CA, USA) in cation and anion modes, with a mass range of 50–1,000 m/z. Metabolites in the samples were analyzed using a fused silica capillary column (50 μm i.d. × 80 cm length) filled with electrolyte buffer solution (HMT, Tsuruoka, Japan) with the applied voltage or cation and anion modes set at 27 kV and 30 kV, respectively.
Peak information, such as the mass‐to‐charge ratio (m/z), migration time, and peak area, was extracted from the peaks detected by CE‐TOFMS using MassHunter integration software (Agilent Technologies). The peaks were annotated to the metabolites inferred from the standard metabolites based on their migration time and m/z values. The tolerance range of peak annotation was ±0.2 min for migration time and ± 10 ppm for m/z. The peak areas were normalized to the internal standard.
The quality of KYN measurements by CE‐TOFMS was checked by measuring a standard concentration of KYN (10 μM) at 10 sample intervals and checking the migration time of KYN in plasma samples and standard compounds in extracted ion chromatograms and the mass‐to‐charge ratio in the mass spectrum of each target peak region. For the other metabolites, we used a commercial metabolite mixture (HMT) as reference metabolites. The qualities of sample preparation were checked and calibrated by the recovery rates of internal standards.
We performed metabolomics analysis of plasma metabolites at two time points, baseline (T1) and post‐treatment (T2), in 65 patients with MDD. We also performed metabolomics analysis of plasma metabolites at one time point in 36 HC subjects. As a result, 34 metabolites were identified in the metabolomic analysis. Among these metabolites, we focused our analysis on KYN. This was based on the results of our previous metabolomics study using LC–MS, which showed that low KYN levels were associated with the treatment response of escitalopram.
Statistical analyses
Demographic and clinical data were compared using the chi‐square test for categorical variables and a two‐sample t‐test for quantitative variables. Follow‐up HRSD scores were calculated for all 65 MDD patients, and HRSD scores at baseline and follow‐up were compared by a paired sample t‐test.
In the examination of KYN levels, the Wilcoxon rank‐sum test was used to compare Responders and Nonresponders, Responders and HCs, and Nonresponders and HCs. The Holm‐Bonferroni method was used for multiple comparison correction. Changes in KYN levels before and after treatment with escitalopram were compared using the Wilcoxon signed‐rank test. We also compared the percentage change ([{T2 – T1} / T1] × 100) in KYN levels between Responders and Nonresponders using the Wilcoxon rank‐sum test. A significance test of Spearman's rank correlation coefficient was used to examine the relationship between escitalopram treatment response and change of KYN levels in patients with MDD.
A two‐sample t‐test was performed on the fALFF values for each voxel using Statistical Parametric Mapping (SPM12) software (Wellcome Department of Cognitive Neurology, London, UK). The significance level was set at a familywise error‐corrected P‐value <0.05 for cluster level inference.
The Wilcoxon rank‐sum test was used to compare left DLPFC activity in Responders and Nonresponders, Responders and HCs, and Nonresponders and HCs. The Holm‐Bonferroni method was used for multiple comparison correction. The changes in left DLPFC activity before and after treatment with escitalopram were compared using the Wilcoxon signed‐rank test. We also compared the percentage change ([{T2 – T1}/T1] × 100) in left DLPFC activity between Responders and Nonresponders using the Wilcoxon rank‐sum test. Correlation analysis of left DLPFC activity to treatment response and KYN levels was performed using the significance test of Spearman's rank correlation coefficient. We also compared the relationship between plasma KYN levels and left DLPFC activity at baseline between MDD patients and HCs using Fisher's r‐to‐z transformation.
Statistical analyses were conducted using R version 4.1.1 software, with the significance level defined as P < 0.05 (two‐tailed).
Results
Demographics and clinical characteristics
Demographic information, including sex, age, body mass index, smoking status, verbal IQ, age at illness onset, duration of illness, and first or recurrent episode, escitalopram dose, and HRSD score, are shown in Table 1. There was a significant difference in the HRSD scores recorded at baseline and follow‐up (P < 0.05). Thirty‐two patients (49.2%) showed a clinical response (>50% reduction in the HRSD score) and were classified as Responders; the remaining 33 patients (50.7%) were classified as Nonresponders. Furthermore, 25 patients (38.5%) achieved remission (follow‐up HRSD score <8). Responders and Nonresponders did not differ significantly in sex, age, and average baseline HRSD score.
Table 1.
Demographics of the study participants
| MDD N = 65 | Statistical analysis | ||||
|---|---|---|---|---|---|
| Variable (Mean ± SD) | Responders N = 32 | Nonresponders N = 33 | HCN = 36 | Responders vs. Nonresponders | HC vs. MDD |
| Sex (male/female) | 16/16 | 15/18 | 19/17 | χ2 (1) = 0.14, P = 0.71 | χ2 (1) = 0.24, P = 0.62 |
| Age (years) | 38.5 ± 9.8 | 41.9 ± 11.6 | 41.3 ± 11.0 | t (63) = 1.28, P = 0.21 | t(99) = −0.50, P = 0.62 |
| BMI | 22.2 ± 3.6 | 23.5 ± 5.1 | 22.4 ± 3.5 | t (63) = 1.17, P = 0.25 | t(99) = 0.55, P = 0.59 |
| Smoking (yes / no) | 7 / 25 | 9 / 24 | 7 / 29 | χ2 (1) = 0.26, P = 0.61 | χ2 (1) = 0.35, P = 0.55 |
| Verbal IQ (JART) | 109.2 ± 10.4 | 112.1 ± 10.9 | 111.4 ± 8.4 | t (63) = 1.12, P = 0.27 | t(99) = −0.37, P = 0.71 |
| Age at illness onset (years) | 35.5 ± 11.1 | 35.4 ± 13.5 | ‐ | t (63) = −0.03, P = 0.97 | ‐ |
| Duration of illness (month) | 2.98 ± 2.13 | 3.80 ± 2.39 | ‐ | t (63) = 1.42, P = 0.16 | ‐ |
| Episode (single/ recurrent) | 19 / 13 | 13 / 20 | ‐ | χ2 (1) = 2.60, P = 0.11 | ‐ |
| Use of benzodiazepines (yes/no) | 10 / 22 | 16 / 17 | ‐ | χ2 (1) = 2.01, P = 0.16 | ‐ |
| Starting dose of escitalopram (mg) | 8.91 ± 2.07 | 9.09 ± 1.93 | ‐ | t (63) = 0.37, P = 0.72 | ‐ |
| Dose of escitalopram at 6–8 weeks (mg) | 10.16 ± 5.37 | 12.88 ± 4.93 | ‐ | t (63) = 2.10, P = 0.04 | ‐ |
| HRSD17 score at baseline (T1) | 19.1 ± 4.5 | 20.8 ± 5.0 | ‐ | t (63) = 1.45, P = 0.15 | ‐ |
| HRSD17 score after 6–8 weeks (T2) | 5.4 ± 3.5 | 15.2 ± 4.3 | ‐ | t (63) = 10.18, P < 0.001 | ‐ |
BMI, body mass index; HC, healthy control subjects; HRSD17, Hamilton Rating Scale for Depression 17‐item; MDD, patients with major depressive disorder; SD, standard deviation; Verbal IQ, Verbal Intelligence Quotient; JART, The Japanese version of the Adult Reading Test.
Whole brain comparison of fALFF values between Responders and Nonresponders at baseline
To identify the brain regions related to treatment response, we compared Responders and Nonresponders at baseline (T1) for each voxel's fALFF value. As a result, Responders had significantly lower fALFF values than Nonresponders in the left DLPFC (familywise error‐corrected P = 0.001 for cluster level inference, 117 voxels) (Fig. 1).
Fig. 1.
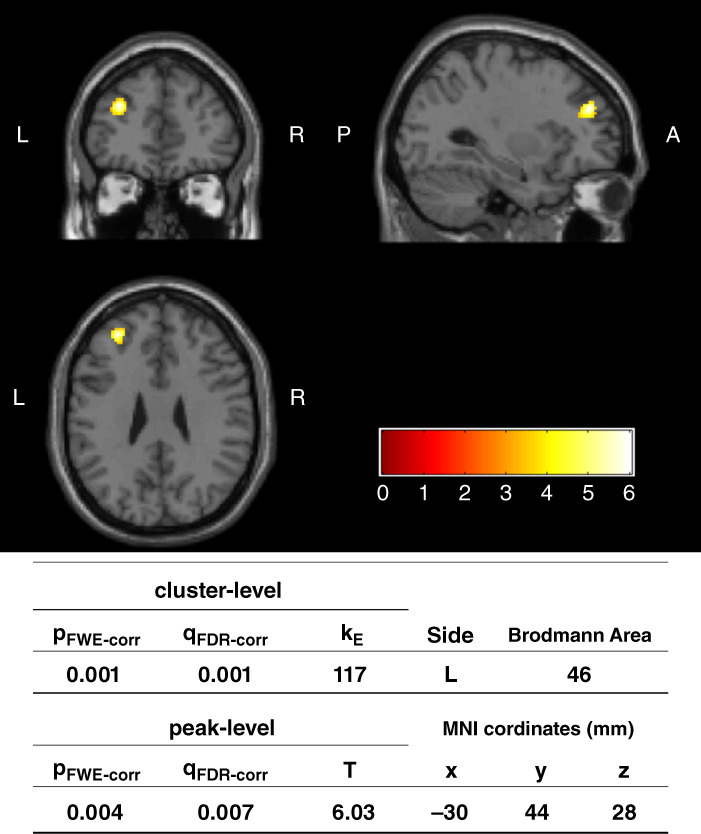
Whole brain comparison of fractional amplitude of low‐frequency fluctuation values between Responders and Nonresponders at baseline (T1). The region where Responders (T1) were significantly lower than Nonresponders (T1) in cluster‐level inference is indicated. Voxel level threshold was P (uncorrected) < 0.001, and cluster size threshold was P (FWE corrected) < 0.05. L, left; PFWE‐corr, familywise error corrected P‐value; T, T value of peak activation within the cluster. Coordinates for the peak voxel are listed as Montreal Neurological Institute (MNI) coordinates. The color scale represents t‐values from 0 to 6.
Left DLPFC activity at baseline and its pre‐ and post‐treatment changes
The 117 voxels of the left DLPFC that were significant in whole brain comparisons of fALFF values between Responders and Nonresponders at baseline (T1) were defined as the region of interest (ROI). The activity of the left DLPFC of each subject was assessed by the mean fALFF value in the ROI. At baseline, left DLPFC activity was significantly lower in Responders than in Nonresponders (W = 156, adjusted P < 0.001, r = 0.66) and HCs (W = 228, adjusted P < 0.001, r = 0.47) after multiple comparison correction. There was no significant difference between Nonresponders and HCs (W = 439, adjusted P = 0.064, r = 0.18). The activity of the left DLPFC of Responders increased significantly after escitalopram treatment (V = 150, 95% CI: [−0.43–0.016], P = 0.032, r = 0.38). Conversely, Nonresponders did not show a significant change in the ROI before and after treatment (V = 353, 95% CI: [−0.067–0.39], P = 0.20, r = 0.22) (Fig. 2a). The percentage change in the mean fALFF value of the left DLPFC ROI was significantly greater in Responders than in Nonresponders (W = 680, 95% CI: [0.017 1.27], P = 0.046, r = 0.25). There was a significant positive correlation (rho = 0.26, T = 2.16, df = 63, 95% CI: [0.021 0.47], P = 0.034) between the change in left DLPFC activity and treatment response (Fig. 2b).
Fig. 2.
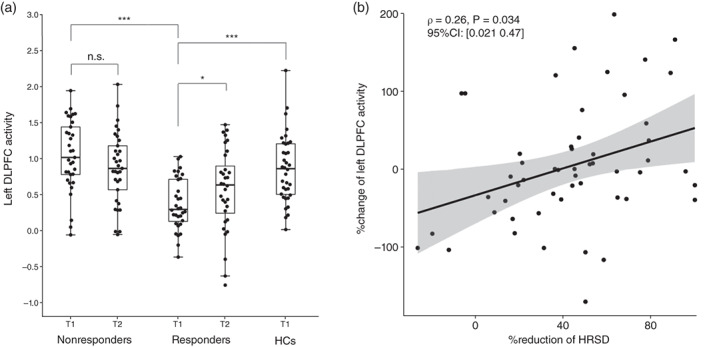
Left dorsolateral prefrontal cortex (DLPFC) activity at baseline, and their treatment changes. (a) Boxplots and beeswarm plots of the mean fractional amplitude of low‐frequency fluctuation values of the left DLPFC region of interest for each group. Responders had significantly lower left DLPFC activity than Nonresponders (Z = 5.29, adjusted P < 0.001, r = 0.66) and healthy control (HC) subjects (Z = −3.91, adjusted P < 0.001, r = 0.47). On the other hand, a comparison of baseline (T1) and post‐treatment (T2) activity showed a significant increase in left DLPFC activity in Responders (V = 150, 95% CI: [−0.43–0.016], P = 0.032) but no significant change in Nonresponders. n.s. P > 0.05, *P < 0.05, ***P < 0.001. (b) Percentage change of left DLPFC activity showed a significant positive correlation with the percentage reduction of the Hamilton Rating Scale for Depression (HRSD) score.
KYN levels at baseline and their pre‐ and post‐treatment changes
To investigate the role of KYN in treatment response, we compared the blood levels of KYN in Responders and Nonresponders at baseline (T1). See Supplementary Table S2 for the results of 34 metabolites. KYN levels were significantly lower in Responders than in Nonresponders (W = 356, 95% CI: [−0.46–0.035], P = 0.024, r = 0.28). At baseline, KYN levels were significantly lower in Responders than in Nonresponders (W = 356, adjusted P = 0.047, r = 0.32) and HCs (W = 233, adjusted P < 0.001, r = 0.48) after multiple comparison correction. There was no significant difference between Nonresponders and HCs (W = 683, adjusted P = 0.29, r = 0.16). KYN levels were significantly increased in Responders after treatment (V = 57, 95% CI: [−0.40–0.16], P < 0.001, r = 0.74) (Fig. 3a). Conversely, Nonresponders did not show a significant change in plasma KYN levels between before and after treatment (V = 353, 95% CI: [−0.068 0.40], P = 0.20, r = 0.23) (Fig. 3a). The percentage change of plasma KYN levels was significantly greater in Responders than in Nonresponders (W = 690, 95% CI: [0.014 0.29], P = 0.033, r = 0.26). There was a significant positive correlation (rho = 0.25, T = 2.06, df = 63, 95% CI: [0.0079 0.46], P = 0.043) between the change in KYN levels and treatment response (Fig. 3b).
Fig. 3.
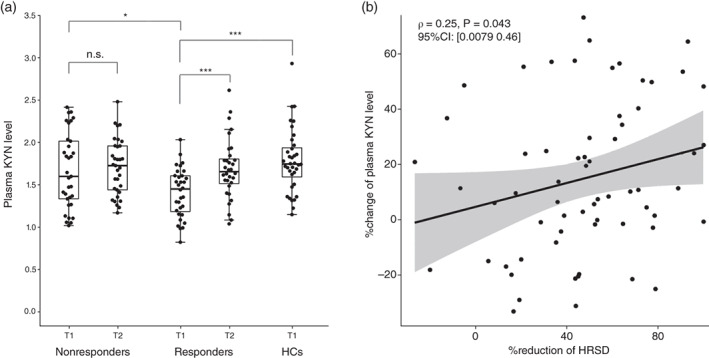
Plasma kynurenine (KYN) levels at baseline, and their treatment changes. (a) Boxplots and beeswarm plots of plasma KYN levels in each group. At baseline, Responders had significantly lower KYN levels than Nonresponders (Z = 2.56, adjusted P = 0.031, r = 0.32) and healthy control (HC) subjects (Z = −3.95, adjusted P < 0.001, r = 0.48). Comparison of Plasma KYN levels at baseline (T1) and post‐treatment (T2) showed a significant increase (V = 57, 95% CI: [−0.40–0.16], P < 0.001) in Plasma KYN levels in Responders. However, no significant change was observed in Nonresponders. n.s. P > 0.05, *P < 0.05, ***P < 0.001. (b) Percentage change of plasma KYN levels showed a significant positive correlation with the percentage reduction of the Hamilton Rating Scale for Depression (HRSD) score.
Relationship between plasma KYN levels and left DLPFC activity
We examined the relationship between baseline (T1) plasma KYN levels and left DLPFC activity in patients with MDD and HCs. The results showed that there was a significant positive correlation between left DLPFC activity and plasma KYN levels in MDD patients (rho = 0.43, T = 3.74, df = 63, 95% CI: [0.20 0.61], P = 0.0004) (Fig. 4a) and HCs (rho = 0.42, T = 2.75, df = 34, 95% CI: [0.11 0.66], P = 0.01) (Fig. 4b). When correlations between MDD patients and HCs were compared, no significant differences were found (Z = 0.06, P = 0.95).
Fig. 4.
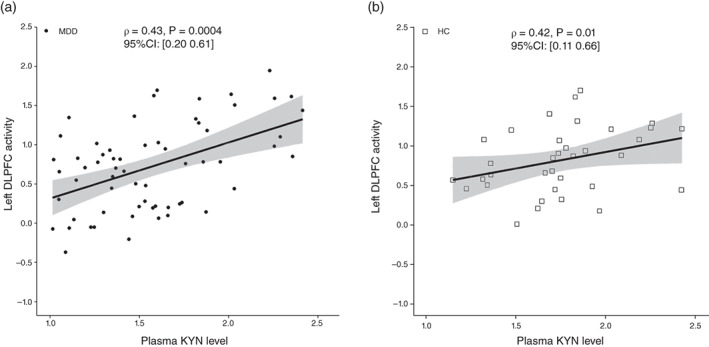
Relationship between plasma kynurenine (KYN) levels and left dorsolateral prefrontal cortex (DLPFC) activity at baseline (T1). (a) The mean fractional amplitude of low‐frequency fluctuation (fALFF) values of the left DLPFC region of interest (ROI) was correlated with plasma KYN levels in patients with major depressive disorder. (b) Mean fALFF values of the left DLPFC ROI were also correlated with plasma KYN levels in healthy control (HC) subjects.
Finally, we examined the relationship between the percentage change in KYN levels and the percentage change in left DLPFC activity with treatment, but there was no significant correlation and no direct relationship between the two (rho = 0.09, T = 0.76, df = 63, 95% CI: [−0.15 0.33], P = 0.45).
Discussion
This study revealed that patients with depression who respond well to escitalopram are characterized by lower plasma KYN levels and resting‐state regional activity in the left DLPFC. Both were significantly lower in Responders than in Nonresponders and HCs. The increase in left DLPFC activity and plasma KYN levels from before to after treatment reflected a favorable treatment response, suggesting an association with a condition that improves with escitalopram. Furthermore, there was a significant correlation between individual differences in pretreatment regional activity of the left DLPFC and plasma KYN levels in MDD patients and HCs, suggesting a relationship between them. However, there was no significant correlation between the percentage increase in KYN levels and the percentage increase in regional activity of the left DLPFC after treatment, suggesting that the increase in KYN levels did not lead directly to the increased activity of the left DLPFC. A visual summary of the main findings of the present study is depicted in Fig. 5.
Fig. 5.
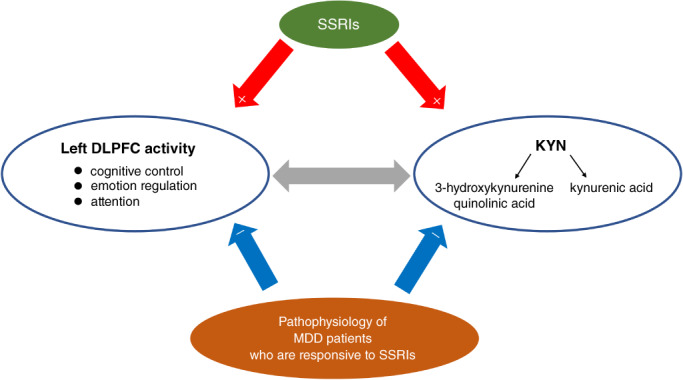
Summary figure describing the main findings of the current paper. The findings of this study suggest that decrease in kynurenine (KYN) and decrease in left dorsolateral prefrontal cortex (DLPFC) activity are involved in the pathophysiology of depression who are responsive to SSRI (blue arrow), that SSRI improve these pathologies (red arrow), and that there is a correlation between KYN and left DLPFC activity (gray arrow), but further studies are needed to elucidate the complex relationship between the two.
The DLPFC is a hub of cognitive control and emotion regulation 42 , 43 , 44 , 45 , 46 , 47 and is a key brain region comprising the cognitive control network. The function of the cognitive control network, mainly the DLPFC, is known to be impaired in MDD, and abnormal function and hypoactivity of the cognitive control network are thought to be associated with cognitive impairment and consequent emotional dysregulation. 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 Previous studies have shown that hypoactivity of the DLPFC and cognitive control network in MDD improves after treatment with psychological, pharmacological, or transcranial magnetic stimulation, 56 , 57 , 58 , 59 , 60 leading to a clinical improvement in symptoms. 56 , 61 , 62 In this study, we found that MDD patients with low fALFF values in the left DLPFC responded well to treatment with escitalopram and their fALFF values increased after treatment, and that the rate of increase in fALFF values in the left DLPFC correlated significantly with treatment response. These results are consistent with those of previous studies.
Pretreatment plasma KYN/TRP and plasma KYN levels are reportedly negatively correlated with treatment response to antidepressants, 9 , 63 , 64 and we have demonstrated that the plasma levels of KYN are negatively correlated with the reduction rate of the HRSD score. 10 In the present study, KYN levels were significantly lower in Responders than in Nonresponders, and we confirmed the previously reported finding that KYN can be a predictive biomarker for treatment with escitalopram. Although 32 (49.2%) patients overlapped with our previous study 10 in metabolome analysis by LC–MS, excluding these subjects did not change the result that KYN levels were significantly lower in Responders than in Nonresponders (Supplementary Fig. S1). Kocki et al. (2012) reported that treatment with selective serotonin reuptake inhibitors increased KYN levels, 65 and in the present study, plasma KYN levels increased significantly from before to after treatment with escitalopram. Moreover, the increase in KYN levels from before to after treatment correlated positively with treatment response.
KYN is a metabolite that crosses the blood–brain barrier, 66 , 67 and in the central nervous system, approximately 60–80% of KYN is supplied from the periphery, 68 , 69 , 70 and plasma and cerebrospinal fluid KYN levels are correlated to some extent. 64 , 71 Although the role of KYN in the brain is not understood well, it is reportedly associated with cognitive function. Zhou et al. (2019) demonstrated that learning function and processing speed of female patients with MDD were associated with serum KYN levels and the KYN/TRP ratio. 72 Solvang et al. (2019) also indicated that KYN was non‐linearly and quadratically associated with cognitive function test performance. 73 KYN and metabolites downstream of the KYN pathway are thought to play an important role in some aspects of cognitive function and mediate environmental influences on cognition. 74 Although KYN itself does not excite or inhibit neural activity directly, downstream metabolites of KYN, e.g., quinolinic acid and 3‐hydroxykynurenine, have agonist effects on N‐methyl‐D‐aspartate (NMDA) receptors, while kynurenic acid has antagonistic effects on NMDA receptors. 11 The balance of both affects neural activity via NMDA receptors, 75 and therefore, it may be related to brain functions, such as attention and working memory, in which NMDA receptors play an important role. 76 , 77 , 78 , 79 In the DLPFC, NMDA receptors are involved in attention and working memory by activating pyramidal cells to fire synapses persistently. 80 , 81 Therefore, it is presumed that DLPFC activity is influenced indirectly by the action of downstream metabolites of KYN on NMDA receptors. In the present study, we found a positive correlation between plasma KYN levels and fALFF values of the left DLPFC in HCs and MDD patients. These results suggest that KYN may have an indirect effect on regional activity in the left DLPFC, which is related to the treatment response to escitalopram.
The major strength of this study is the use of metabolomics and fMRI in the same subjects and their longitudinal assessment. This study determined the relationship between blood metabolites associated with treatment response to escitalopram and regional brain activity and identified the characteristics of depression that responded to treatment, which we believe will make a significant contribution to the future development of biomarkers for predicting treatment response.
Our study has several limitations that should be mentioned. First, the sample size of the recruited participants was relatively small. Larger‐scale studies are needed for reproduction and validation. Second, all participants were of the same ethnicity. To verify whether our findings are replicated across different regions and ethnic groups, international studies are needed. Third, there was no active or passive treatment control group to distinguish the escitalopram‐specific effects. Fourth, this study did not consider the potential effects of factors, such as diet, exercise, renal function, and smoking status, that may affect the concentrations of metabolites in the KYN pathway. 82 , 83 Finally, the levels of metabolites downstream of the KYN pathway were not measured, e.g., kynurenic acid and quinolinic acid, which have antagonist and agonist effects on NMDA receptors, as described above. Although these metabolites do not cross the blood–brain barrier, it seems necessary to include their measurement in future studies to promote our understanding of the pathophysiology of depression.
Despite the abovementioned limitations, this is the first study to use metabolomics and fMRI to measure blood metabolites and regional brain activity related to the therapeutic effects of escitalopram in the same group of subjects and to examine the relationship between the two. The findings of this study suggest that decreased KYN levels and decreased resting‐state regional activity of the left DLPFC may be associated with treatment response to escitalopram. There was a correlation between individual differences in plasma KYN levels and regional activity of the left DLPFC, suggesting an association between them, but there was no correlation between increased KYN levels and increased left DLPFC activity before and after treatment. DLPFC activity might be mediated by downstream metabolites of KYN that exert neural effects on NMDA receptors. Further studies are needed to elucidate the complex relationship between the two.
Disclosure statement
GO received lecture fees from Otsuka Pharmaceutical Co. Ltd., Pfizer Japan, and Sumitomo Dainippon Pharmaceutical Co., LTD. SN received grants, lecture fees, and/or honoraria from Eisai Co. Ltd., Sumitomo Dainippon Pharmaceutical Co., LTD., Mochida Pharmaceutical Co. Ltd., Eli Lilly Japan K.K., Otsuka Pharmaceutical Co. Ltd. TO received grants, lecture fees, and/or honoraria from Takeda Pharmaceutical, Tanabe Mitsubishi Pharmaceutical, Pfizer Japan, Mochida Pharmaceutical, Yoshitomi Pharmaceutical, Eli Lilly Japan, and MSD. OY received grants, lecture fees, and/or honoraria from Sumitomo Dainippon Pharmaceutical Co., LTD, Eisai Co., LTD., Ono Pharmaceutical Co., LTD., Daiichi Sankyou Co., LTD, Takeda Pharmaceutical Co., LTD, Sumitomo Dainippon Pharmaceutical Co., LTD., Mochida Pharmaceutical Co., LTD., Otsuka Pharmaceutical Co., LTD. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author contributions
T.K. conducted the data analysis and drafted the manuscript. T.K., G.O., E.I., Y.M., M.T., S.Y., A.Y., and Y.O. were involved in the experimental design and data collection. K.M., S.N., A.T., and T.O. performed the metabolite analysis. T.K., G.O., and Y.O. discussed the interpretation of the data. All authors discussed the results and commented on the final manuscript. All authors read and approved the final manuscript.
Supporting information
Appendix S1. Supplementary Method ComBat harmonization method
Supplementary Table 1. MRI acquisition sequences
Supplementary Table 2. Comparison of Responders and Nonresponders for 34 metabolites
Supplementary Fig. 1. Comparison in MDD patients not included in our previous study
Supplementary References
Acknowledgments
This research was supported by AMED under Grant Numbers JP18dm0307002, JP18dm0307008, JP18dk0307076, and JP20pc0101061. The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the manuscript.
References
- 1. Kennedy SH, Lam RW, McIntyre RS et al. Canadian network for mood and anxiety treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: Section 3. Pharmacological treatments. Can. J. Psychiatry 2016; 61: 540–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rush JA, Trivedi MH, Wisniewski SR et al. STAR‐D (2006; AjPsych) tiered approach for depression. Am. J. Psychiatry 2006; 163: 1905–1917.17074942 [Google Scholar]
- 3. Breitenstein B, Scheuer S, Holsboer F. Are there meaningful biomarkers of treatment response for depression? Drug Discov. Today 2014; 19: 539–561. [DOI] [PubMed] [Google Scholar]
- 4. Fernie AR, Trethewey RN, Krotzky AJ et al. Metabolite profiling: From diagnostics to systems biology. Nat. Rev. Mol. Cell Biol. 2004; 5: 763–769. [DOI] [PubMed] [Google Scholar]
- 5. Kaddurah‐Daouk R, Krishnan KRR. Metabolomics: A global biochemical approach to the study of central nervous system diseases. Neuropsychopharmacology 2009; 34: 173–186. [DOI] [PubMed] [Google Scholar]
- 6. Kaddurah‐Daouk R, Boyle SH, Matson W et al. Pretreatment metabotype as a predictor of response to sertraline or placebo in depressed outpatients: A proof of concept. Transl. Psychiatry 2011; 1: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhu H, Bogdanov MB, Boyle SH et al. Pharmacometabolomics of response to sertraline and to placebo in major depressive disorder ‐ possible role for Methoxyindole pathway. PLoS One 2013; 8: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ji Y, Hebbring S, Zhu H et al. Glycine and a glycine dehydrogenase (GLDC) SNP as citalopram/escitalopram response biomarkers in depression: Pharmacometabolomics‐informed pharmacogenomics. Clin. Pharmacol. Ther. 2011; 89: 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sun Y, Drevets W, Turecki G et al. The relationship between plasma serotonin and kynurenine pathway metabolite levels and the treatment response to escitalopram and desvenlafaxine. Brain Behav. Immun. 2020; 87: 404–412. [DOI] [PubMed] [Google Scholar]
- 10. Erabi H, Okada G, Shibasaki C et al. Kynurenic acid is a potential overlapped biomarker between diagnosis and treatment response for depression from metabolome analysis. Sci. Rep. 2020; 10: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ogyu K, Kubo K, Noda Y et al. Kynurenine pathway in depression: A systematic review and meta‐analysis. Neurosci. Biobehav. Rev. 2018; 90: 16–25. [DOI] [PubMed] [Google Scholar]
- 12. Arnone D, Saraykar S, Salem H et al. Role of kynurenine pathway and its metabolites in mood disorders: A systematic review and meta‐analysis of clinical studies. Neurosci. Biobehav. Rev. 2018; 92: 477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marx W, McGuinness AJ, Rocks T et al. The kynurenine pathway in major depressive disorder, bipolar disorder, and schizophrenia: A meta‐analysis of 101 studies. Mol. Psychiatry 2021; 26: 4158–4178. [DOI] [PubMed] [Google Scholar]
- 14. Miller JM, Schneck N, Siegle GJ et al. FMRI response to negative words and SSRI treatment outcome in major depressive disorder: A preliminary study. Psychiatry Res ‐ Neuroimaging 2013; 214: 296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ruhé HG, Booij J, Veltman DJ et al. Successful pharmacologic treatment of major depressive disorder attenuates amygdala activation to negative facial expressions: A functional magnetic resonance imaging study. J. Clin. Psychiatry 2012; 73: 451–459. [DOI] [PubMed] [Google Scholar]
- 16. Alexopoulos GS, Hoptman MJ, Kanellopoulos D et al. Functional connectivity in the cognitive control network and the default mode network in late‐life depression. J. Affect. Disord. 2012; 139: 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yuan H, Zhu X, Tang W et al. Connectivity between the anterior insula and dorsolateral prefrontal cortex links early symptom improvement to treatment response. J. Affect. Disord. 2020; 260: 490–497. [DOI] [PubMed] [Google Scholar]
- 18. Yamamura T, Okamoto Y, Okada G et al. Association of thalamic hyperactivity with treatment‐resistant depression and poor response in early treatment for major depression: A resting‐state fMRI study using fractional amplitude of low‐frequency fluctuations. Transl. Psychiatry 2016; 6: e754–e754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gong J, Wang J, Qiu S et al. Common and distinct patterns of intrinsic brain activity alterations in major depression and bipolar disorder: Voxel‐based meta‐analysis. Transl. Psychiatry. 2020; 10: 353. 10.1038/s41398-020-01036-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Argyelan M, Lencz T, Kaliora S et al. Subgenual cingulate cortical activity predicts the efficacy of electroconvulsive therapy. Transl. Psychiatry 2016; 6: 4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Egorova N, Veldsman M, Cumming T et al. Fractional amplitude of low‐frequency fluctuations (fALFF) in post‐stroke depression. NeuroImage Clin 2017; 16: 116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu CH, Ma X, Song LP et al. Abnormal spontaneous neural activity in the anterior insular and anterior cingulate cortices in anxious depression. Behav. Brain Res. 2015; 281: 339–347. [DOI] [PubMed] [Google Scholar]
- 23. Guo W, Liu F, Zhang J et al. Dissociation of regional activity in the default mode network in first‐episode, drug‐naive major depressive disorder at rest. J. Affect. Disord. 2013; 151: 1097–1101. [DOI] [PubMed] [Google Scholar]
- 24. Al‐Zubaidi A, Heldmann M, Mertins A et al. Influences of hunger, satiety and Oral glucose on functional brain connectivity: A multimethod resting‐state fMRI study. Neuroscience 2018; 382: 80–92. [DOI] [PubMed] [Google Scholar]
- 25. Al‐Zubaidi A, Mertins A, Heldmann M et al. Machine learning based classification of resting‐state fMRI features exemplified by metabolic state (hunger/satiety). Front. Hum. Neurosci. 2019; 13. Epub ahead of print. 10.3389/fnhum.2019.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hamilton M. Development of a rating scale for primary depressive illness. Br. J. Soc. Clin. Psychol. 1967; 6: 278–296. [DOI] [PubMed] [Google Scholar]
- 27. Otsubo T, Tanaka K, Koda R et al. Reliability and validity of Japanese version of the Mini‐international neuropsychiatric interview. Psychiatry Clin. Neurosci. 2005; 59: 517–526. [DOI] [PubMed] [Google Scholar]
- 28. Sheehan DV, Lecrubier Y, Sheehan KH et al. The Mini‐international neuropsychiatric interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. J. Clin. Psychiatry 1998; 59: 22–33. [PubMed] [Google Scholar]
- 29. Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971; 9: 97–113. [DOI] [PubMed] [Google Scholar]
- 30. Matsuoka K, Uno M, Kasai K et al. Estimation of premorbid IQ in individuals with Alzheimer's disease using Japanese ideographic script (kanji) compound words: Japanese version of National Adult Reading Test. Psychiatry Clin. Neurosci. 2006; 60: 332–339. [DOI] [PubMed] [Google Scholar]
- 31. Whitfield‐Gabrieli S, Nieto‐Castanon A. Conn: A functional connectivity toolbox for correlated and Anticorrelated brain networks. Brain Connect. 2012; 2: 125–141. [DOI] [PubMed] [Google Scholar]
- 32. Zou QH, Zhu CZ, Yang Y et al. An improved approach to detection of amplitude of low‐frequency fluctuation (ALFF) for resting‐state fMRI: Fractional ALFF. J. Neurosci. Methods 2008; 172: 137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Küblböck M, Woletz M, Höflich A et al. Stability of low‐frequency fluctuation amplitudes in prolonged resting‐state fMRI. Neuroimage 2014; 103: 249–257. [DOI] [PubMed] [Google Scholar]
- 34. Zuo XN, Xing XX. Test‐retest reliabilities of resting‐state FMRI measurements in human brain functional connectomics: A systems neuroscience perspective. Neurosci. Biobehav. Rev. 2014; 45: 100–118. [DOI] [PubMed] [Google Scholar]
- 35. Zuo XN, Di Martino A, Kelly C et al. The oscillating brain: Complex and reliable. Neuroimage 2010; 49: 1432–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Friedman L, Stern H, Brown GG et al. Test‐retest and between‐site reliability in a multicenter fMRI study. Hum. Brain Mapp. 2008; 29: 958–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Feis RA, Smith SM, Filippini N et al. ICA‐based artifact removal diminishes scan site differences in multi‐center resting‐state fMRI. Front Neurosci 2015; 9. Epub ahead of print. 10.3389/fnins.2015.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rath J, Wurnig M, Fischmeister F et al. Between‐ and within‐site variability of fMRI localizations. Hum. Brain Mapp. 2016; 37: 2151–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dansereau C, Benhajali Y, Risterucci C et al. Statistical power and prediction accuracy in multisite resting‐state fMRI connectivity. Neuroimage 2017; 149: 220–232. [DOI] [PubMed] [Google Scholar]
- 40. Fortin JP, Parker D, Tunç B et al. Harmonization of multi‐site diffusion tensor imaging data. Neuroimage 2017; 161: 149–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fortin J‐P, Cullen N, Sheline YI et al. Harmonization of cortical thickness measurements across scanners and sites. Neuroimage 2018; 167: 104–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yu L, Long Q, Tang Y et al. Improving Emotion Regulation through Real‐Time Neurofeedback Training on the Right Dorsolateral Prefrontal Cortex: Evidence from Behavioral and Brain Network Analyses. Front. Hum. Neurosci. 2021; 15: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ochsner K, Gross J. The cognitive control of emotion. Trends Cogn. Sci. 2005; 9: 242–249. [DOI] [PubMed] [Google Scholar]
- 44. Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Ann. N. Y. Acad. Sci. 2012; 1251: E1–E24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Buhle JT, Silvers JA, Wage TD et al. Cognitive reappraisal of emotion: A meta‐analysis of human neuroimaging studies. Cereb. Cortex 2014; 24: 2981–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kohn N, Eickhoff SB, Scheller M et al. Neural network of cognitive emotion regulation ‐ an ALE meta‐analysis and MACM analysis. Neuroimage 2014; 87: 345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. MacDonald AW. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science (80‐ ) 2000; 288: 1835–1838. [DOI] [PubMed] [Google Scholar]
- 48. Vanderhasselt MA, De Raedt R, Leyman L et al. Acute effects of repetitive transcranial magnetic stimulation on attentional control are related to antidepressant outcomes. J. Psychiatry Neurosci. 2009; 34: 119–126. [PMC free article] [PubMed] [Google Scholar]
- 49. Menon V. Large‐scale brain networks and psychopathology: A unifying triple network model. Trends Cogn. Sci. 2011; 15: 483–506. [DOI] [PubMed] [Google Scholar]
- 50. Wang Y, Yang S, Sun W et al. Altered functional interaction hub between affective network and cognitive control network in patients with major depressive disorder. Behav. Brain Res. 2016; 298: 301–309. [DOI] [PubMed] [Google Scholar]
- 51. Rayner G, Jackson G, Wilson S. Cognition‐related brain networks underpin the symptoms of unipolar depression: Evidence from a systematic review. Neurosci. Biobehav. Rev. 2016; 61: 53–65. [DOI] [PubMed] [Google Scholar]
- 52. Koenigs M, Grafman J. The functional neuroanatomy of depression: Distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav. Brain Res. 2009; 201: 239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Okada G, Okamoto Y, Morinobu S et al. Attenuated left prefrontal activation during a verbal fluency task in patients with depression. Neuropsychobiology 2003; 47: 21–26. [DOI] [PubMed] [Google Scholar]
- 54. Okada G, Okamoto Y, Yamashita H et al. Attenuated prefrontal activation during a verbal fluency task in remitted major depression. Psychiatry Clin. Neurosci. 2009; 63: 423–425. [DOI] [PubMed] [Google Scholar]
- 55. Takamura M, Okamoto Y, Okada G et al. Disrupted brain activation and deactivation pattern during semantic verbal fluency task in patients with major depression. Neuropsychobiology 2017; 74: 69–77. [DOI] [PubMed] [Google Scholar]
- 56. AL B, Saxena S, MA M et al. Brain metabolic changes associated with symptom factor improvement in major depressive disorder. Biol. Psychiatry 2001; 50: 171–178. [DOI] [PubMed] [Google Scholar]
- 57. Fitzgerald PB, Laird AR, Maller J et al. A meta‐analytic study of changes in brain activation in depression. Hum. Brain Mapp. 2008; 29: 683–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Shajahan PM, Glabus MF, Steele J et al. Left dorso‐lateral repetitive transcranial magnetic stimulation affects cortical excitability and functional connectivity, but does not impair cognition in major depression. Prog. Neuro‐Psychopharmacol. Biol. Psychiatry 2002; 26: 945–954. [DOI] [PubMed] [Google Scholar]
- 59. Walsh ND, Williams SCR, Brammer MJ et al. A longitudinal functional magnetic resonance imaging study of verbal working memory in depression after antidepressant therapy. Biol. Psychiatry 2007; 62: 1236–1243. [DOI] [PubMed] [Google Scholar]
- 60. Fales CL, Barch DM, Rundle MM et al. Antidepressant treatment normalizes hypoactivity in dorsolateral prefrontal cortex during emotional interference processing in major depression. J. Affect. Disord. 2009; 112: 206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mayberg HS, Brannan SK, Tekell JL et al. Regional metabolic effects of fluoxetine in major depression: Serial changes and relationship to clinical response. Biol. Psychiatry 2000; 48: 830–843. [DOI] [PubMed] [Google Scholar]
- 62. Kennedy SH, Evans KR, Krüger S et al. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. Am. J. Psychiatry 2001; 158: 899–905. [DOI] [PubMed] [Google Scholar]
- 63. Umehara H, Numata S, Watanabe S et al. Altered KYN/TRP, Gln/Glu, and met/methionine sulfoxide ratios in the blood plasma of medication‐free patients with major depressive disorder. Sci. Rep. 2017; 7: 4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Haroon E, Welle JR, Woolwine BJ et al. Associations among peripheral and central kynurenine pathway metabolites and inflammation in depression. Neuropsychopharmacology 2020; 45: 998–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kocki T, Wnuk S, Kloc R et al. New insight into the antidepressants action: Modulation of kynurenine pathway by increasing the kynurenic acid/3‐hydroxykynurenine ratio. J. Neural Transm. 2012; 119: 235–243. [DOI] [PubMed] [Google Scholar]
- 66. Gál EM, Sherman AD. L‐kynurenine its synthesis and possible regulatory function in brain. Neurochem. Res. 1980; 5: 223–239. [DOI] [PubMed] [Google Scholar]
- 67. Fukui S, Schwarcz R, Rapoport SI et al. Blood?Brain barrier transport of kynurenines: Implications for brain synthesis and metabolism. J. Neurochem. 1991; 56: 2007–2017. [DOI] [PubMed] [Google Scholar]
- 68. Gál EM, Sherman AD. Synthesis and metabolism of l‐kynurenine in rat brain. J. Neurochem. 1978; 30: 607–613. [DOI] [PubMed] [Google Scholar]
- 69. Kita T, Morrison PF, Heyes MP et al. Effects of systemic and central nervous system localized inflammation on the contributions of metabolic precursors to the L‐kynurenine and quinolinic acid pools in brain. J. Neurochem. 2002; 82: 258–268. [DOI] [PubMed] [Google Scholar]
- 70. Vécsei L, Szalárdy L, Fülöp F et al. Kynurenines in the CNS: Recent advances and new questions. Nat. Rev. Drug Discov. 2013; 12: 64–82. [DOI] [PubMed] [Google Scholar]
- 71. Hestad KA, Engedal K, Whist JE et al. The relationships among tryptophan, kynurenine, indoleamine 2,3‐dioxygenase, depression, and neuropsychological performance. Front. Psychol. 2017; 8: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zhou Y, Zheng W, Liu W et al. Cross‐sectional relationship between kynurenine pathway metabolites and cognitive function in major depressive disorder. Psychoneuroendocrinology 2019; 101: 72–79. [DOI] [PubMed] [Google Scholar]
- 73. Solvang SEH, Nordrehaug JE, Tell GS et al. The kynurenine pathway and cognitive performance in community‐dwelling older adults. The Hordaland health study. Brain Behav. Immun. 2019; 75: 155–162. [DOI] [PubMed] [Google Scholar]
- 74. Stone TW, Darlington LG. The kynurenine pathway as a therapeutic target in cognitive and neurodegenerative disorders. Br. J. Pharmacol. 2013; 169: 1211–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Nemeth H, Toldi J, Vecsei L. Role of kynurenines in the central and Peripherial nervous systems. Curr. Neurovasc. Res. 2005; 2: 249–260. [DOI] [PubMed] [Google Scholar]
- 76. Driesen NR, McCarthy G, Bhagwagar Z et al. Relationship of resting brain hyperconnectivity and schizophrenia‐like symptoms produced by the NMDA receptor antagonist ketamine in humans. Mol. Psychiatry 2013; 18: 1199–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Guidi M, Kumar A, Foster TC. Impaired attention and synaptic senescence of the prefrontal cortex involves redox regulation of NMDA receptors. J. Neurosci. 2015; 35: 3966–3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Rodermund P, Westendorff S, Nieder A. Blockage of NMDA‐ and GABA(A) receptors improves working memory selectivity of primate prefrontal neurons. J. Neurosci. 2020; 40: 1527–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lee J, van den Buuse M, Nithianantharajah J et al. Acute NMDA receptor antagonism impairs working memory performance but not attention in rats—Implications for the NMDAr hypofunction theory of schizophrenia. Behav. Neurosci. 2020; 134: 323–331. [DOI] [PubMed] [Google Scholar]
- 80. Wang M, Yang Y, Wang C‐J et al. NMDA receptors subserve persistent neuronal firing during working memory in dorsolateral prefrontal cortex. Neuron 2013; 77: 736–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. van Vugt B, van Kerkoerle T, Vartak D et al. The contribution of AMPA and NMDA receptors to persistent firing in the dorsolateral prefrontal cortex in working memory. J. Neurosci. 2020; 40: 2458–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zhao J. Plasma kynurenic acid/tryptophan ratio: A sensitive and reliable biomarker for the assessment of renal function. Ren. Fail. 2013; 35: 648–653. [DOI] [PubMed] [Google Scholar]
- 83. Martin KS, Azzolini M, Lira RJ. The kynurenine connection: How exercise shifts muscle tryptophan metabolism and affects energy homeostasis, the immune system, and the brain. Am. J. Physiol. 2020; 318: C818–C830. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supplementary Method ComBat harmonization method
Supplementary Table 1. MRI acquisition sequences
Supplementary Table 2. Comparison of Responders and Nonresponders for 34 metabolites
Supplementary Fig. 1. Comparison in MDD patients not included in our previous study
Supplementary References


