Abstract
Borrelia burgdorferi spends a significant proportion of its life cycle within an ixodid tick, which has a cuticle containing chitin, a polymer of N-acetylglucosamine (GlcNAc). The B. burgdorferi celA, celB, and celC genes encode products homologous to transporters for cellobiose and chitobiose (the dimer subunit of chitin) in other bacteria, which could be useful for bacterial nutrient acquisition during growth within ticks. We found that chitobiose efficiently substituted for GlcNAc during bacterial growth in culture medium. We inactivated the celB gene, which encodes the putative membrane-spanning component of the transporter, and compared growth of the mutant in various media to that of its isogenic parent. The mutant was no longer able to utilize chitobiose, while neither the mutant nor the wild type can utilize cellobiose. We propose renaming the three genes chbA, chbB, and chbC, since they probably encode a chitobiose transporter. We also found that the chbC gene was regulated in response to growth temperature and during growth in medium lacking GlcNAc.
Borrelia burgdorferi, a Lyme disease agent, resides in the midgut of an ixodid tick for a significant part of its natural life cycle (17). The bacteria are acquired from an infected small mammal when a larval tick takes its first blood meal. The spirochetes multiply within the tick as the meal is digested, and then their numbers decline precipitously when the tick molts to the nymphal stage (8, 21). When a nymph feeds again on a mammal (which can be months after the larval feeding), some bacteria move to the tick salivary glands and are transmitted to the mammal, causing a mammalian infection.
The arthropod vector undergoes a number of physiological and metabolic changes to which resident bacteria are exposed. These changes include blood feeding and digestion, cuticle synthesis and degradation that are required to accommodate the blood meal and carry out the molt (27), and tick adaptation to the resting state between blood digestion and development of the next metamorphic stage. The ability of B. burgdorferi to adapt to this changing environment, and to the vastly different mammalian environment, is likely to be essential to the successful completion of an infectious cycle.
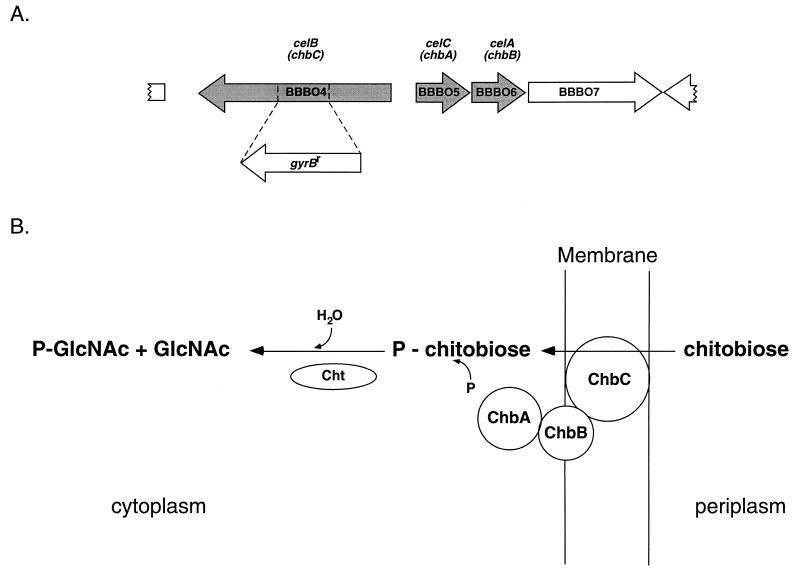
Ixodid tick integument expansion during feeding and preparation for molting requires the synthesis of new cuticle, of which chitin, a polymer of N-acetylglucosamine (GlcNAc), is a component (27). These ticks also have chitinous peritrophic matrices that surround the blood meal within the midgut (24, 32). Chitin components available during cuticle remodeling may serve as nutrients for bacteria growing in ticks. Spirochetes require GlcNAc to reach high densities in culture (1, 13). The genome sequence of B. burgdorferi (12) revealed several genes likely to facilitate chitin by-product utilization by the bacteria. Among these are the celA, celB, and celC genes (Fig. 1A), which encode a phosphotransferase system (PTS) predicted to recognize the substrate(s) chitobiose (the dimer subunit of chitin, two β-1,4-linked GlcNAc molecules) and/or cellobiose (the dimer subunit of cellulose, two β-1,4-linked glucose molecules) (Fig. 1B). The genome also includes a paralogous pair of genes whose products were predicted to have β-N-acetyl-glucosaminidase (chitobiase) and/or β-glucosidase activities, which would cleave chitobiose or cellobiose into two molecules of GlcNAc or glucose, respectively.
FIG. 1.
Arrangement of chb genes and mechanism of chitobiose transport by a PTS transporter. (A) Relative orientation of the celB (chbC), celC (chbA), and celA (chbB) genes on a portion of cp26, with arrows indicating direction of transcription. The orientation and approximate position of the insertion of gyrBr and deletion of chbC constructed in the chbC72 mutation are also shown. The gyrBr gene is not drawn to scale. (B) Expected arrangement of Chb (or Cel) proteins and mechanism of chitobiose transport and utilization. The phosphate group is predicted to be donated by proteins common to all PTS systems, encoded by the chromosomal BB558, BB557, and BB448 genes (12). Cht, chitobiase. Modified from reference 16.
The celA, celB, and celC genes (BBB06, BBB04, and BBB05, respectively) reside on cp26 (the 26-kbp circular plasmid), whereas the genes encoding the putative chitobiase (BB0002) and β-glucosidase (BB0620) are located on the linear chromosome (12). Further analysis of the genome sequence predicts that B. burgdorferi can funnel free GlcNAc-6-P (resulting from transport via a PTS system) either into glycolysis, using a putative GlcNAc-6-P deacetylase and glucosamine-6-P isomerase, or into cell wall biosynthesis, using a putative phosphoglucomutase (12).
Mammals contain no chitin. However, tick cuticle, which contains chitin, is synthesized and degraded during tick development. Bacterial numbers within ticks fluctuate during this process (21). Therefore, genes for chitobiose transport and cleavage most likely play key roles while the bacteria reside in the tick. We have begun a study to determine if the B. burgdorferi cel products actually facilitate chitobiose utilization and, by extension, might help bacteria grow in ticks. We found that chitobiose efficiently substitutes for GlcNAc in allowing B. burgdorferi to grow to high densities in culture. In contrast, celB mutant bacteria were unable to utilize chitobiose. We therefore propose that the cel genes be renamed chb genes, to reflect the sugar specificity of the encoded transporter. We also propose that celB be renamed chbC, with celC and celA renamed chbA and chbB, respectively, based on the previously used rationale in which the gene name correlates with the subunit of the PTS system encoded thereby (14). We use these names throughout this communication.
MATERIALS AND METHODS
Bacterial strains and mutant construction.
Experiments were performed with B31-A (4), a clone derived from high-passage noninfectious B31 (1), or B31-4A, a reisolate derived from mouse infection with a clone of low-passage B31 (7, 9). The chbC mutant was constructed by allelic exchange using a plasmid (pKK80) in which part of the chbC gene had been removed and replaced with the gyrBr gene, encoding a mutant B subunit of gyrase that confers coumermycin A1 resistance (23) (Fig. 1A). PKK80 was made by: (i) PCR amplifying a 4.3-kb sequence including the chbC gene and flanking sequences (using primers cp26-24974 and cp26-20651 [Table 1]); (ii) cloning that fragment into pCR2.1 (Invitrogen; Carlsbad, Calif.); (iii) recloning a SpeI-XbaI fragment containing those sequences with part of the polylinker of pCR2.1 into SpeI-digested pOK12 (30); and (iv) replacing a 389-bp KpnI-NcoI fragment from the chbC gene with the gyrBr gene, which had been cloned into pCR2.1 after amplification with primers that provided those restriction enzyme sites (U178F-KpnI and 1905R-NcoI). The gyrBr gene is inserted in the same orientation as the chbC gene. Recloning into pOK12 was necessary to avoid introducing an ampicillin resistance marker into B. burgdorferi. Twenty-five micrograms of pKK80 DNA was used to transform electrocompetent B31-A (25). The 552 resulting coumermycin A1-resistant colonies were screened for the presence of allelic exchange by PCR using the primers chbC-F and cp26-24116 (Table 1), and two mutants were obtained. Restriction enzymes and T4 DNA ligase were purchased from New England Biolabs (Beverly, Mass.).
TABLE 1.
Oligonucleotide primers used in this study
| Primer | Sequence (5′→3′) | Use |
|---|---|---|
| cp26-24974 | CAGGACGACGTCCTATTGCC | chb region amplification |
| cp26-20651 | CCATCGATAAGAAACTTTTTATTAGTGC | chb region amplification |
| cp26-24116 | CCAAGCTTAAGTTTTGCAATAGCAATTC | Allelic exchange; screening |
| gyrB-U178F-KpnI | GGGGTACCTGTTGGTTTTAGCACTATA | gyrBr amplification |
| gyrB-1905R-NcoI | TGCCATGGTTACACATCAAGATTAATTAC | gyrBr amplification |
| chbC-F | TTAATTGCTTTAAGAGATGGC | chbC probe; screening |
| chbC-R | TACCATGAAGACCACAAAACC | chbC probe |
| FL-6 | TTCAGGGTCTCAAGCGTCTTGGACT | flaB probe |
| FL-7 | GCATTTTCAATTTTAGCAAGTGATG | flaB probe |
GlcNAc assay.
The modified Morgan-Elson assay that we used (22) is linear in the range from 0.1 to 1.6 mM GlcNAc (where BSKII medium contains 1.8 mM GlcNAc). To assay GlcNAc, 0.1 ml of sample was mixed with 0.1 ml of 0.8 M sodium borate and boiled for 12 min. After cooling to room temperature, 0.55 ml of 10% Ehrlich reagent was added and the tubes were incubated for 20 min at 37°C. After cooling again to room temperature, the absorbance at 585 nm was compared to that of a standard curve derived using known concentrations of GlcNAc. Ehrlich reagent is 10% (wt/vol) p-dimethylaminobenzaldehyde in a mixture of 9 ml of glacial acetic acid and 1 ml of concentrated HCl. Ehrlich reagent (10%) was prepared by diluting the above stock with 9 volumes of glacial acetic acid. BSKII medium and derivatives were assayed after passing the sample through a Centricon 10 filter (Amicon, Inc., Beverly, Mass.). Without this step, the protein in the medium precipitated and the samples became solid after boiling. Of the free GlcNAc found in BSKII, 50 to 80% remained in an assayable form after this procedure.
Growth curves.
For analyzing growth in various media, bacteria were diluted from stationary phase (2 × 108 to 4 × 108 bacteria/ml) to 105 bacteria/ml and counted daily using a Petroff-Hausser counting chamber and a dark-field microscope. The lowest concentration of bacteria accurately enumerated by this method is about 105 bacteria/ml. Typical dilutions inoculated less than 5 μl of culture into 5 ml of fresh deficient medium, so only insignificant amounts of nutrients were transferred with the inoculum. BSKII medium without gelatin or, in some cases, BSK-H medium (Sigma, St. Louis, Mo.) was used. Because of batch-to-batch medium variation and different times at which bacteria were enumerated, experimental data could not be pooled, and representative growth curves are shown. Experiments were repeated a minimum of two times and often were repeated more than 10 times. In several experiments, bacterial viability was confirmed by concentrating the bacteria 5- to 10-fold and staining with the LIVE/DEAD BacLight bacterial viability kit (Molecular Probes, Wilsonville, Oreg.), according to the manufacturer's instructions. Stained cells were visualized with a fluorescence microscope (Zeiss, Jena, Germany) and photographed with a Nikon camera (9). On one occasion, the numbers were also confirmed by directly assessing CFU within the culture. In general, bacteria were enumerated until no change was detected. In various experiments, chitobiose was a gift from S. Roseman and N. Keyhani (Johns Hopkins University, Baltimore, Md.) or purchased from Sigma or Seikagaku (Tokyo, Japan). The source made no discernible difference in the results.
Northern blot analysis.
RNA was prepared and Northern blotting was performed by previously described methods (5). RNA was isolated from B31-A grown in medium without GlcNAc at the peak of the first exponential phase (50 h), during the death phase (100 h), and at the peak of the second exponential phase (190 h). RNA was also isolated from B31-4A grown to late exponential phase at 23°C, or from a 23°C culture diluted 100-fold, shifted to 34°C, and grown to late exponential phase (2 to 4 days). We also prepared RNA from a culture of B31-A that had been grown to late exponential phase in BSKII lacking GlcNAc but supplemented with 0.9 mM chitobiose. Probes were derived by PCR (primers described in Table 1), were internal to the genes, and were labeled with [32P]dATP by random priming (Gibco BRL, Gaithersburg, Md.).
Southern blot analysis.
B. burgdorferi plasmid DNA was prepared using Qiagen columns (Qiagen, Chatsworth, Calif.). Restriction enzyme-digested or undigested DNA was separated by electrophoresis through a 0.3% agarose gel, blotted to nylon membranes, and hybridized as described previously (28, 29). The chbC probe was prepared by PCR (primers described in Table 1), the gyrB probe was the fragment used for insertional inactivation, and both were labeled with [32P]dATP by random priming (Gibco BRL).
Transmission electron microscopy.
Samples were pelleted and fixed 1 h with 4% paraformaldehyde–2.5% glutaraldehyde–0.1 M sodium cacodylate buffer, pH 7.4, and then postfixed 1 h with 0.5% osmium tetroxide–0.8% potassium ferricyanide, and then 1% tannic acid. Samples were then stained overnight, en bloc, with 1% uranyl acetate. Samples were washed with water and then dehydrated with a graded ethanol series and embedded in Spurr's resin. Thin sections were cut with an RMC MT-7000 ultramicrotome (Ventana, Tucson, Ariz.) and stained with 1% uranyl acetate and Reynold's lead citrate prior to viewing at 80 kV on a Philips CM-10 transmission electron microscope (FEI, Hillsboro, Oreg.). Digital images were acquired with a digital camera system (Amount, Chazy, New York).
Scanning electron microscopy.
Bacterial suspension (50 μl) was settled on 0.1% poly-l-lysine-coated Thermanox coverslips for 30 min. Samples were fixed as described above through dehydration. Samples were then critical point dried under CO2 in a Bal-Tec model cpd 030 drier (Bal-Tec, Middlebury, Conn.), mounted on aluminum studs, and sputter coated with 150 Å of iridium in a model IBS/TM200S ion beam sputterer (VCR Group, South San Francisco, Calif.) prior to examination at 5 kV in a Hitachi S-4500 field emission scanning electron microscope (Hitachi, Tokyo, Japan)
RESULTS
B. burgdorferi utilization of chitobiose.
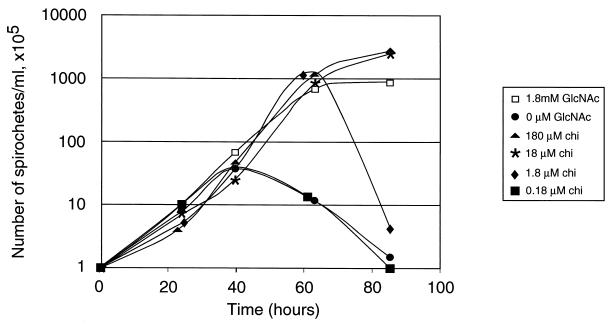
In order to assess the significance of the chb genes for B. burgdorferi growth, we tested whether B. burgdorferi could use chitobiose in place of GlcNAc. To do this, we grew clone B31-A in medium lacking GlcNAc (normally present at 1.8 mM) but supplemented with various amounts of chitobiose (Fig. 2). As previously described (1, 13), the bacteria were unable to grow to typical high density (2 × 108 to 4 × 108 bacteria per ml) in medium lacking GlcNAc. Bacterial growth was defective when the GlcNAc concentration was reduced to 180 μM, and lowering the concentration to 18 μM resulted in growth indistinguishable from that found in the absence of any added GlcNAc (data not shown). In contrast, supplementation with as little as 18 μM chitobiose restored normal growth and 1.8 μM chitobiose allowed growth to near normal numbers, but 0.18 μM chitobiose had no positive growth effect (Fig. 2). These results indicate that chitobiose concentrations 50 to 100 times lower than those of GlcNAc supported B. burgdorferi growth.
FIG. 2.
Growth of B31-A in medium containing various amounts of chitobiose (chi) substituting for GlcNAc. Complete BSKII medium contains 1.8 mM GlcNAc. Bacteria were diluted to 105/ml, grown at 35°C in the indicated media, and enumerated daily using a Petroff-Hausser chamber and dark-field microscope.
Temperature-shift effects on chb gene expression.
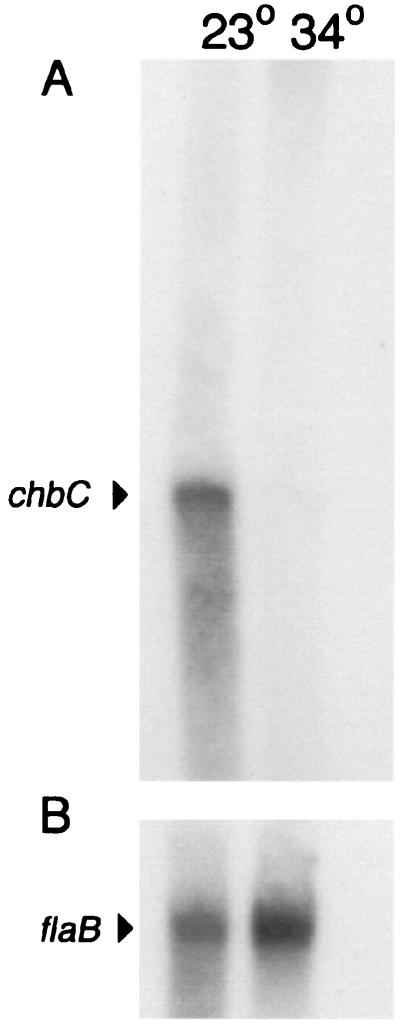
Although mammals contain free GlcNAc and GlcNAc monomers and oligomers as components of modifications on proteins and other molecules (e.g., see reference 11), they do not appear to have free chitobiose. Since chitobiose is likely to be present in ticks, we determined if the chbC gene conformed to the previously established correlation between temperature regulation in vitro and gene expression within ticks or mammals (26, 31). RNA was prepared from infectious clone B31-4A after growth at 23°C, simulating the ambient temperature found inside a tick, or after a shift to 34°C, more closely resembling a mammalian environment. Northern blot analysis showed that chbC RNA is present at a higher level at 23°C than after the shift to 34°C (Fig. 3). Probes to the chbB and chbA genes, which are predicted, by sequence analysis, to be cotranscribed together with a downstream gene (Fig. 1), hybridized to a broad band of RNA whose level was unaffected by temperature shift (data not shown). The flaB transcript, which was used for normalization of RNA loading (Fig. 3B), has been previously shown to be unaltered by temperature shift (5).
FIG. 3.
Northern blot analysis of B31-4A RNA from bacteria before and after a temperature upshift. (A) chbC probe; (B) flaB probe. Arrowheads indicate the 1.4-kb chbC and 1-kb flaB mRNA positions; growth temperatures (degrees Celsius) of cultures from which RNA was prepared are indicated above the lanes. The exposure for the chbC probe was approximately 10 times longer than that for the flaB probe.
chbC gene inactivation.
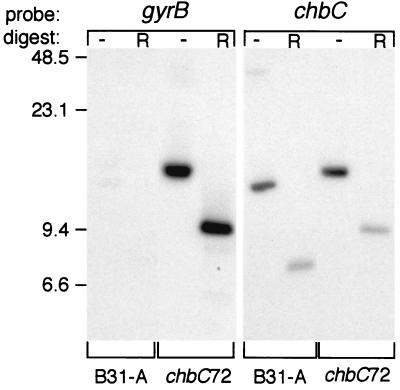
For several reasons, we chose to further analyze the importance of the chbC gene product for utilization of chitobiose and other medium components. First, the gene encodes the predicted membrane-spanning component of a chitobiose transporter (Fig. 1B), without which the other components would be ineffective. Second, the chbC gene was temperature regulated (Fig. 3), suggesting that it might also be regulated during bacterial growth within ticks. Third, the chbC gene has a monocistronic transcript, so a mutation should not have polar effects. Consequently, we mutated the gene by partial deletion and insertion of the gyrBr gene (Fig. 1A; see Materials and Methods). One of the two mutants obtained, chbC72, was selected for further characterization. Southern blot (Fig. 4 and data not shown) and PCR analysis (data not shown) confirmed that the mutant had the expected insertion-deletion on cp26. In particular, a chbC probe hybridized to bands in uncut or EcoRI-digested chbC72 plasmid DNA that were appropriately larger than those in B31-A DNA (Fig. 4, right panel). Also, a gyrBr probe hybridized to the same bands in chbC72 plasmid DNA and did not hybridize to B31-A plasmid DNA (Fig. 4, left panel). To confirm the insertion site, we amplified the chbC-gyrBr junctions from the mutant and found that they had the expected sequences (data not shown). The chbC mutant grew normally in complete BSKII medium (see below).
FIG. 4.
Southern blot analysis of the chbC region of wild-type and chbC72 bacteria. Undigested (−) or EcoRI-digested (R) plasmid DNA from B31-A or chbC72 was probed with gyrB or chbC PCR products. The wild-type gyrB gene is chromosomal, so it is not present in these DNA preparations. The chbC probe contains a single EcoRI site, yielding 1.3-kbp (no longer present on the gel) and 7.5-kbp fragments in B31-A. The insertion-deletion event leads to a net increase of 1.7 kbp in the sizes of cp26 and of the larger EcoRI fragment (which becomes 9.2 kbp). Sizes (in kilobase pairs) corresponding to migration positions of DNA standards are indicated on the left.
Growth in various derivatives of BSKII medium.
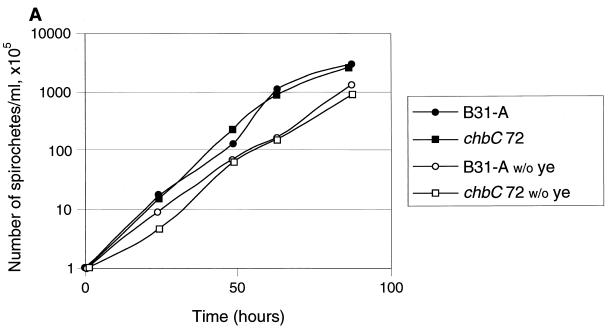
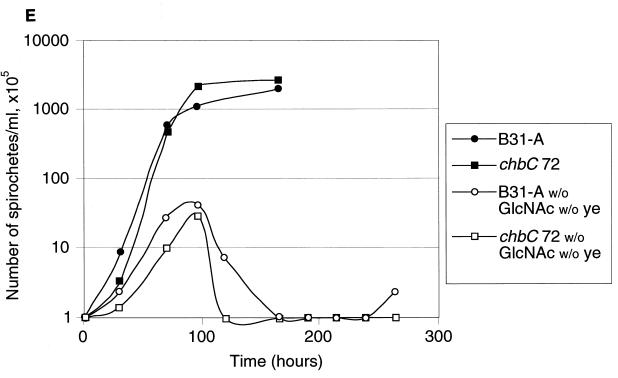
Although GlcNAc is an essential component of BSKII medium (1, 13), the medium also contains several other potential sources of complexed GlcNAc. Yeastolate, an enzymatic digest of yeast (which has a chitinous cell wall) probably contains chito-oligomers. Serum includes glycosylated proteins with GlcNAc-containing modifications (e.g., see reference 11), glycolipids, and possibly other GlcNAc-containing molecules, which could supply GlcNAc. Using the Morgan-Elson assay for GlcNAc (22), we found that neither serum nor yeastolate contributes significant free GlcNAc (data not shown). Gelatin is a nonessential ill-defined product derived from animals and, therefore, was omitted in most experiments. In an attempt to separate the contributions of various GlcNAc sources, we assayed the growth of the B. burgdorferi wild type and chbC mutant in BSKII medium lacking GlcNAc, yeastolate, and both (Fig. 5). In medium lacking yeastolate, wild-type and chbC mutant bacteria both grew more slowly than in complete medium, but to almost the same density (Fig. 5A).
FIG. 5.
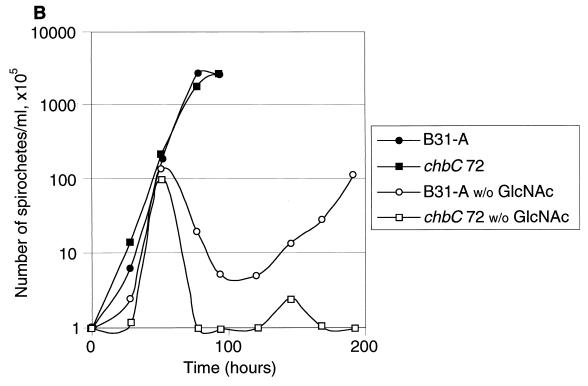
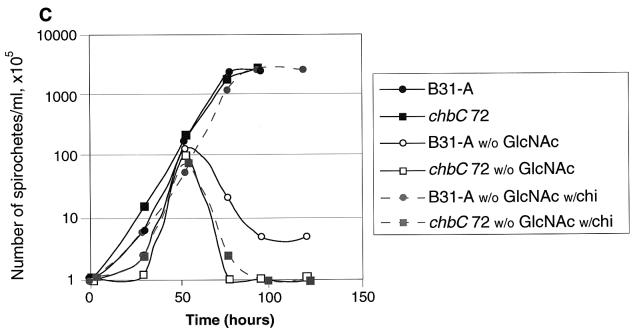
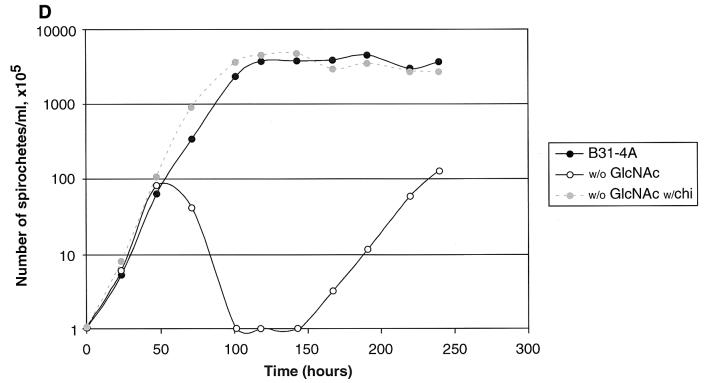
Growth of B31-A and the chbC72 mutant in various media. (A) Growth in BSKII with and without yeastolate (ye). (B) Growth in BSKII with and without GlcNAc. (C) Growth in BSKII with and without GlcNAc, and with the substitution of chitobiose (chi) (1.8 mM) for GlcNAc. (D) Growth of low-passage B31-4A in BSKII with and without GlcNAc, and with the substitution of chitobiose (chi) (1.8 mM) for GlcNAc. E. Growth in BSKII with and without both yeastolate (ye) and GlcNAc. Bacteria were enumerated as for Fig. 2. Representative experiments are shown.
In medium lacking GlcNAc, both strains had complex growth patterns (Fig. 5B). After dilution to 105 bacteria/ml, both grew to ∼107 bacteria/ml, and then the numbers of viable bacteria dropped precipitously (death phase). After the death phase, the wild-type bacteria began a second exponential phase, at a somewhat lower growth rate, and finally achieved bacterial numbers comparable to or greater than those found in the first exponential phase. In contrast, the chbC mutant did not grow further. Although chitobiose efficiently substituted for GlcNAc for wild-type bacterial growth (Fig. 2 and Fig. 5C), growth of the chbC72 mutant was not restored by the addition of chitobiose (Fig. 5C). Cellobiose (at 1.8 mM) did not restore the growth of either the wild-type or the chbC mutant bacteria (data not shown). The low-passage infectious clone B31-4A had the same growth pattern as high-passage B31-A during growth in these media (Fig. 5D), as did BL224, a clinical isolate from a patient in the early stages of Lyme disease (data not shown).
Although previous studies had shown 90% lower bacterial yield in medium lacking GlcNAc (1, 13), these authors did not describe the complex growth pattern that we observed. The initial growth, death, and second exponential growth phases were all unanticipated, and our subsequent experiments were designed to help understand what bacterial genes and medium components contributed to these phases.
In medium lacking both GlcNAc and yeastolate (Fig. 5E), both strains grew to about 107 bacteria/ml, died, and did not grow further. This growth pattern was the same as that found for the chbC mutant in medium lacking only GlcNAc. We conclude that the second exponential phase requires the chbC gene product and involves utilization of a component of yeastolate (perhaps chitobiose).
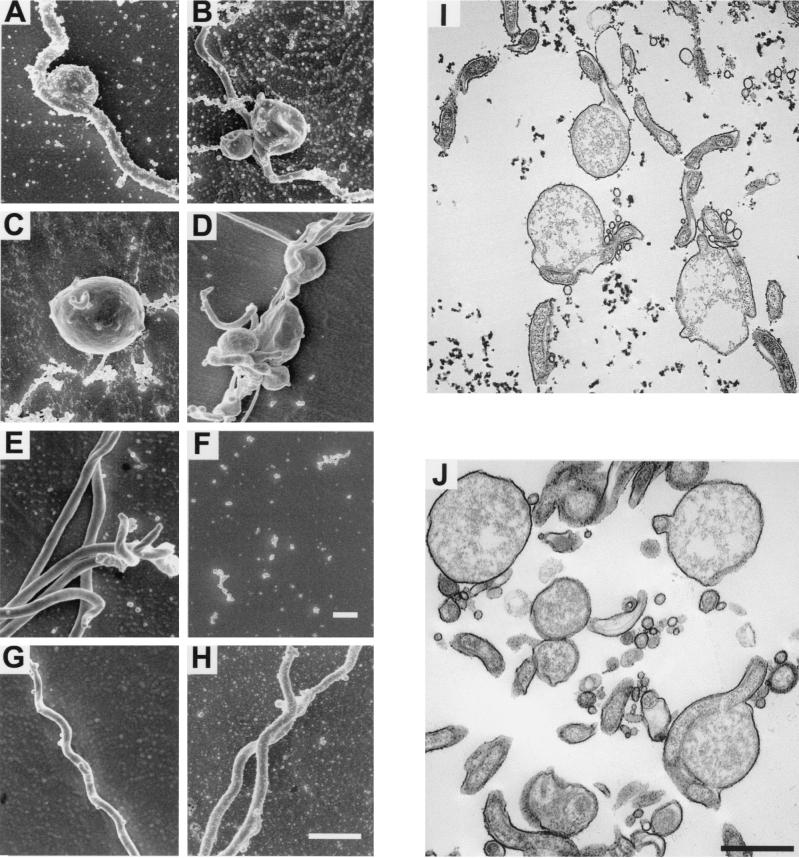
Morphological analysis of bacteria by dark-field microscopy and scanning and transmission electron microscopy revealed the dramatic changes that both the wild-type and chbC mutant bacteria undergo in medium lacking GlcNAc. At the peak of the first exponential phase, most wild-type and chbC mutant bacteria had round membrane-bound masses near their midpoints (Fig. 6A, B, I, and J). Transmission electron microscopy (Fig. 6I and J) showed that the membrane surrounding the masses was contiguous with that surrounding the rest of the spirochetes and that the cytosolic contents of the masses were less electron dense than in the rest of the spirochete. In some cases, membranes with discontinuities appeared to partially separate the masses from the bodies of the spirochetes and disrupted flagellar bundles were detected. These masses differ from previously described gemmae (2), which have well-defined contents, and spheroplasts (6, 10), which aggregate and have numerous extended flagella. They do resemble, however, structures also called spheroplasts that were formed after treating Borrelia hermsii or B. burgdorferi with penicillin (3, 8a). During the death phase, all bacteria had a progressively more amorphous structure and assumed a less refractile appearance, and most eventually disappeared and presumably lysed (Fig. 6C and D). Wild-type bacteria in the second exponential phase looked normal by microscopic examination (Fig. 6E). By dark-field examination, few if any viable spirochetes were found at the equivalent time in cultures of the chbC mutant, and particulate structures observed by scanning electron microscopy (Fig. 6F) were probably medium components, since preparations of BSKII medium without spirochetes looked the same (data not shown). Wild-type or chbC mutant bacteria grown for the same amount of time in complete medium retained typical morphology (Fig. 6G and H), although their motility diminished with time.
FIG. 6.
Scanning (A to H) and transmission (I and J) electron microscopic appearance of B31-A (A, C, E, G, and I) and chbC72 (B, D, F, H, and J) bacteria at various times during growth in medium with or without GlcNAc. Bacteria are shown at 50 h, representing the first exponential phase (A, B, I, and J); at 100 h, representing the death phase (C and D); and at 215 h, representing the second exponential phase for wild type bacteria (E and F). (G and H) Spirochetes from cultures grown in complete BSKII for 215 h. Scale bars = 1 μm.
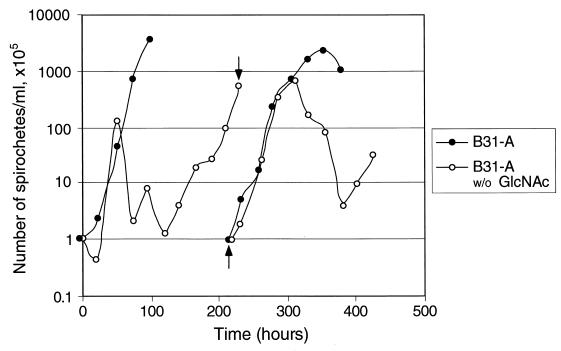
One possible explanation for the second exponential phase was that the bacteria able to grow had acquired an undefined mutation, selected by growth in the absence of GlcNAc. To address this possibility, we diluted a culture from this phase back into medium with or without GlcNAc (Fig. 7). In medium lacking GlcNAc, the bacteria exhibited the same growth pattern as previously (i.e., with first exponential, death, and second exponential phases), excluding the possibility of a mutant population (Fig. 7). This experiment also shows that the bacterial adaptation that leads to the second exponential phase is lost after growth in fresh BSKII lacking GlcNAc. Finally, the bacterial number achieved in the second exponential phase in this and many other experiments is higher than in the first exponential phase. This higher density would not be achieved if scavenging of dead bacteria from the first exponential phase were the only source of an essential nutrient for growth.
FIG. 7.
Growth during first and second passages in BSKII without GlcNAc. B31-A bacteria were diluted to 105/ml in BSKII without GlcNAc, grown for 214 h (to the second exponential phase), and then diluted back to 105/ml in BSKII without GlcNAc (arrows). The same culture was also diluted into medium with GlcNAc, both at the t = 0 time point and at 214 h. Bacteria were enumerated as for Fig. 2.
chbC expression during growth without GlcNAc.
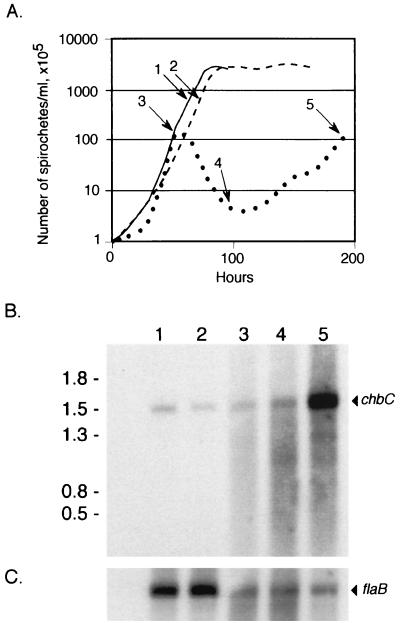
Induction of the chbC gene (or another gene required for chitobiose utilization) in response to the absence of GlcNAc during growth in BSKII lacking GlcNAc might explain why the wild-type bacteria exhibit a complex growth pattern in this medium. To address this possibility, we used Northern blot analysis to compare chbC expression at various times during growth without GlcNAc to expression in BSKII complete medium (Fig. 8). RNA was prepared from B31-A at the peak of the first exponential phase (50 h), during the death phase (100 h), and at the peak of the second exponential phase (190 h) (indicated on growth curves in Fig. 8A). chbC transcript was present at higher levels in bacteria from the second exponential phase (Fig. 8B, lane 5), as expected from the growth characteristics of wild-type and mutant bacteria in various depleted media. After normalizing to the constitutively expressed flaB transcript level (Fig. 8C), induction of chbC expression during the second exponential phase was about 60-fold over that found in bacteria grown in BSKII complete medium (Fig. 8B, lane 1). When B31-A was grown without GlcNAc but with the addition of enough chitobiose (0.9 mM) to contribute an equivalent molar amount of GlcNAc (Fig. 8B, lane 2), chbC transcript was present at a level similar to that found during growth in normal BSKII medium (Fig. 8B, lane 1).
FIG. 8.
Northern blot analysis of RNA prepared from B31-A bacteria grown in BSKII medium lacking GlcNAc, with and without chitobiose supplementation. (A) Growth curves, with numbers (corresponding to lanes in B and C) and arrows indicating times at which RNA was prepared. The solid line indicates B31-A grown in complete BSKII medium, the dotted line indicates growth in BSKII without GlcNAc, and the dashed line indicates growth in BSKII lacking GlcNAc but supplemented with chitobiose. (B and C) Northern blot analysis of RNA from the indicated time points. Lanes: 1, bacteria grown in complete BSKII; 2, bacteria grown in BSKII without GlcNAc supplemented with 0.9 mM chitobiose; 3 to 5, bacteria grown in BSKII without GlcNAc, with RNA isolated from the first exponential phase, the death phase, and the second exponential phase, respectively. The blot was hybridized first with a chbC probe (arrowhead in panel B) and then rehybridized with a flaB probe (arrowhead in panel C). The exposure for the chbC probe was approximately 10 times longer than that for the flaB probe. Densitometric comparison of the flaB hybridization signals indicated that lanes 1 and 2 contained threefold more RNA than lanes 3 to 5.
DISCUSSION
We have undertaken an analysis of chbC gene function because we suspect that its product, along with those of the chbA and chbB genes, is important for bacterial growth within ticks. The studies described here support that idea, since the chbC gene product is required for utilization of chitobiose, a compound likely to be present in ticks but not found free in mammalian tissues. In other bacteria, chitobiose transporters are encoded in combination with secreted and periplasmic chitinases that can release chitobiose as a degradation product (15). In marine bacteria, which live in environments replete in chitin-containing material, the transporters are used for nutrient acquisition. B. burgdorferi does not contain a homolog of known chitinases but may instead rely on release of chitobiose by its tick host for a nutrient source.
Our model for explaining the unusual growth of B. burgdorferi in medium lacking GlcNAc is the following. The first exponential phase growth involves utilization of GlcNAc recycled from nonessential bacterial components, which become depleted. Despite the loss of available GlcNAc for cell wall biosynthesis, the bacteria continue to grow, and most eventually lyse because of compromised cell wall integrity (see, e.g., reference 19). Some of the bacteria survive, however, through increased expression of the chbC gene so that they can now utilize chito-oligomers, present at low concentration in yeastolate, to supply GlcNAc.
Support for this model is as follows: the lack of the second exponential phase in the chbC mutant suggests that the ChbC protein (and presumably the ChbA and ChbB proteins) is required for this growth. Consistent with this idea is the increased chbC transcript observed at that time. The absence of a second exponential phase in medium lacking both GlcNAc and yeastolate suggests that yeastolate contributes a nutrient essential for this growth, presumably chito-oligomers. The ability of chitobiose to substitute for GlcNAc in the wild type, but not the chbC mutant, suggests that the Chb products are essential for chitobiose utilization.
We have found that lowering the level of GlcNAc in BSKII medium to 10% of normal led to poor bacterial growth in culture (data not shown). In contrast, chitobiose levels equivalent to 100-fold-lower GlcNAc concentrations sufficed for normal bacterial growth (Fig. 1), perhaps because the bacteria have a transporter specific for chitobiose but not for GlcNAc. If the cellular GlcNAc content of B. burgdorferi is similar to that of E. coli, then this low amount of chitobiose would be almost completely consumed in the process of bacterial multiplication from 105 to 2 × 108 cells per ml, suggesting that chitobiose is very efficiently utilized by B. burgdorferi. Cellobiose did not support bacterial growth, probably because it is not a source of GlcNAc, even if successfully transported and cleaved.
The abnormal morphology of the bacteria during the death phase (Fig. 6 and data not shown) is consistent with loss of cell wall integrity coupled with continuing membrane synthesis and suggests that a critical function of GlcNAc is as a cell wall precursor. The clustering of the gene encoding a putative chitobiase (BB0002), the enzyme that cleaves chitobiose to free GlcNAc, with other genes encoding homologs of products involved in cell wall biosynthesis (see the introduction) is also consistent with this interpretation. B. burgdorferi also has a paralogous gene (BB0620) whose product may be involved in chitobiose utilization (12). Although the levels of sequence similarity between these genes and those of other bacteria do not permit us to assign functions to their products, our preliminary results (data not shown) suggest that the BB0002 product is not required for chitobiose utilization, either because it recognizes a different substrate or because a redundant activity is encoded elsewhere.
B. burgdorferi within ticks exhibit a growth pattern parallel to the one that we describe in medium lacking GlcNAc (21). After Ixodes scapularis larvae feed on infected mice, the bacterial load in the tick midgut increases to several thousand spirochetes per tick and then drops to prefeeding levels as the ticks molt. When the infected ticks next feed as nymphs, the number of spirochetes in the midgut increases over 100-fold and then plummets once again when the nymphs molt to adult ticks. Piesman et al. (21) suggested that this growth pattern may be caused by limited GlcNAc availability when the ticks are actively synthesizing new chitinous cuticle during the molting process. Bacteria with membrane-bound masses resembling those seen during GlcNAc limitation (Fig. 6) have been observed in unfed nymphal ticks that were infected as larvae (T. Schwan, personal communication). These bacteria have survived the stress that led to their population decline during the molt, and their structure may reflect a response to that stress.
B. burgdorferi growth characteristics within ticks directly affect successful maintenance of a bacterial infectious cycle. The number of spirochetes within ticks (18, 20, 21) as well as surface protein phenotype (e.g., see reference 18) affects transmission to mammals. Here, we initiate a study into factors affecting spirochete growth by studying genes whose products are reasonably expected to play roles in bacterial metabolism within ticks. We investigated bacterial growth in the presence of limiting chitobiose or GlcNAc, culture conditions that mimic possible stresses encountered by the bacteria during life within the tick vector. Our results confirm that bacterial utilization of chitobiose as a source of GlcNAc is dependent on the activity of the plasmid-borne chbC gene, which encodes a component of a putative PTS transporter. Studying the physiological and regulatory consequences of chitin component metabolism will increase our understanding of the dynamic interactions between tick and spirochete that are pertinent to transmission of bacteria to a mammal.
ACKNOWLEDGMENTS
We thank Janice McClory for preparing BSKII medium lacking various components. Tom Schwan provided help with fluorescence microscopy, and we also thank him for drawing our attention to and permitting us to describe similarities between spirochetes in flat ticks and spirochetes grown in medium lacking GlcNAc. Daniel Hogan helped with DNA sequencing. G. Somerville, M. Chaussee, J. M. Musser, T. Schwan, S. Kustu, and J. Hinnebusch provided helpful comments on the manuscript. Gary Hettrick, Anita Golden, and Asher Siegelman expertly prepared figures. We also thank Nemat Keyhani and Saul Roseman for a gift of chitobiose and advice on GlcNAc assays. The participation of Caroline Ojaimi in some aspects of the transcription studies is acknowledged. This work was supported in part by grants AR41511 and AI45801 from the National Institutes of Health (to I.S.).
REFERENCES
- 1.Barbour A G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- 2.Barbour A G, Hayes S F. Biology of Borrelia species. Microbiol Rev. 1986;50:381–400. doi: 10.1128/mr.50.4.381-400.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbour A G, Todd W J, Stoenner H G. Action of penicillin on Borrelia hermsii. Antimicrob Agents Chemother. 1982;21:823–829. doi: 10.1128/aac.21.5.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bono J L, Elias A F, Kupko III J J, Stevenson B, Tilly K, Rosa P. Efficient targeted mutagenesis in Borrelia burgdorferi. J Bacteriol. 2000;182:2445–2452. doi: 10.1128/jb.182.9.2445-2452.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bono J L, Tilly K, Stevenson B, Hogan D, Rosa P. Oligopeptide permease in Borrelia burgdorferi: putative peptide-binding components encoded by both chromosomal and plasmid loci. Microbiology. 1998;144:1033–1044. doi: 10.1099/00221287-144-4-1033. [DOI] [PubMed] [Google Scholar]
- 6.Bruck D K, Talbot M L, Cluss R G, Boothby J T. Ultrastructural characterization of the stages of spheroplast preparation of Borrelia burgdorferi. J Microbiol Methods. 1995;23:219–228. [Google Scholar]
- 7.Casjens S, van Vugt R, Tilly K, Rosa P A, Stevenson B. Homology throughout the multiple 32-kilobase circular plasmids present in Lyme disease spirochetes. J Bacteriol. 1997;179:217–227. doi: 10.1128/jb.179.1.217-227.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Silva A M, Fikrig E. Growth and migration of Borrelia burgdorferi in Ixodes ticks during blood feeding. Am J Trop Med Hyg. 1995;53:397–404. doi: 10.4269/ajtmh.1995.53.397. [DOI] [PubMed] [Google Scholar]
- 8a.Dever L L, Jorgensen J L, Barbour A G. In vitro activity of vancomycin against the spirochete Borrelia burgdorferi. Antimicrob Agents Chemother. 1993;37:1115–1121. doi: 10.1128/aac.37.5.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elias A F, Bono J L, Carroll J A, Stewart P, Tilly K, Rosa P. Altered stationary phase response in a Borrelia burgdorferi rpoS mutant. J Bacteriol. 2000;182:2909–2918. doi: 10.1128/jb.182.10.2909-2918.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Escudero R, Halluska M L, Backenson P B, Coleman J L, Benach J L. Characterization of the physiological requirements for the bactericidal effects of a monoclonal antibody to OspB of Borrelia burgdorferi by confocal microscopy. Infect Immun. 1997;65:1908–1915. doi: 10.1128/iai.65.5.1908-1915.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fournier T, Medjoubi N N, Porquet D. Alpha-1-acid glycoprotein. Biochim Biophys Acta. 2000;1482:157–171. doi: 10.1016/s0167-4838(00)00153-9. [DOI] [PubMed] [Google Scholar]
- 12.Fraser C M, Casjens S, Huang W M, Sutton G G, Clayton R, Lathigra R, White O, Ketchum K A, Dodson R, Hickey E K, Gwinn M, Dougherty B, Tomb J-F, Fleischmann R D, Richardson D, Peterson J, Kerlavage A R, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams M D, Gocayne J, Weidmann J, Utterback T, Watthey L, McDonald L, Artiach P, Bowman C, Garland S, Fujii C, Cotton M D, Horst K, Roberts K, Hatch B, Smith H O, Venter J C. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 13.Kelly R. Cultivation of Borrelia hermsii. Science. 1971;173:443–444. doi: 10.1126/science.173.3995.443. [DOI] [PubMed] [Google Scholar]
- 14.Keyhani N O, Roseman S. Wild-type Escherichia coli grows on the chitin disaccharide, N,N′-diacetylchitobiose, by expressing the cel operon. Proc Natl Acad Sci USA. 1997;94:14367–14371. doi: 10.1073/pnas.94.26.14367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keyhani N O, Wang L X, Lee Y C, Roseman S. The chitin catabolic cascade in the marine bacterium Vibrio furnissii. Characterization of an N,N′-diacetyl-chitobiose transport system. J Biol Chem. 1996;271:33409–33413. doi: 10.1074/jbc.271.52.33409. [DOI] [PubMed] [Google Scholar]
- 16.Lai X, Ingram L O. Cloning and sequencing of a cellobiose phosphotransferase system operon from Bacillus stearothermophilus XL-65-6 and functional expression in Escherichia coli. J Bacteriol. 1993;175:6441–6450. doi: 10.1128/jb.175.20.6441-6450.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lane R S, Piesman J, Burgdorfer W. Lyme borreliosis: relation of its causative agent to its vectors and hosts in North America and Europe. Annu Rev Entomol. 1991;36:587–609. doi: 10.1146/annurev.en.36.010191.003103. [DOI] [PubMed] [Google Scholar]
- 18.Ohnishi J, Piesman J, de Silva A M. Antigenic and genetic heterogeneity of Borrelia burgdorferi populations transmitted by ticks. Proc Natl Acad Sci USA. 2001;98:670–675. doi: 10.1073/pnas.98.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pavelka M S, Jr, Jacobs W R., Jr Biosynthesis of diaminopimelate, the precursor of lysine and a component of peptidoglycan, is an essential function of Mycobacterium smegmatis. J Bacteriol. 1996;178:6496–6507. doi: 10.1128/jb.178.22.6496-6507.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piesman J. Dynamics of Borrelia burgdorferi transmission by nymphal Ixodes dammini ticks. J Infect Dis. 1993;167:1082–1085. doi: 10.1093/infdis/167.5.1082. [DOI] [PubMed] [Google Scholar]
- 21.Piesman J, Oliver J R, Sinsky R J. Growth kinetics of the Lyme disease spirochete (Borrelia burgdorferi) in vector ticks (Ixodes dammini) Am J Trop Med Hyg. 1990;42:352–357. doi: 10.4269/ajtmh.1990.42.352. [DOI] [PubMed] [Google Scholar]
- 22.Reissig J L, Strominger J L, Leloir L F. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955;217:959–966. [PubMed] [Google Scholar]
- 23.Rosa P, Samuels D S, Hogan D, Stevenson B, Casjens S, Tilly K. Directed insertion of a selectable marker into a circular plasmid of Borrelia burgdorferi. J Bacteriol. 1996;178:5946–5953. doi: 10.1128/jb.178.20.5946-5953.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rudzinska M A, Spielman A, Lewengrub S, Piesman J, Karakashian S. Penetration of the peritrophic membrane of the tick by Babesia microti. Cell Tissue Res. 1982;221:471–481. doi: 10.1007/BF00215696. [DOI] [PubMed] [Google Scholar]
- 25.Samuels D S. Electrotransformation of the spirochete Borrelia burgdorferi. In: Nickoloff J A, editor. Methods in molecular biology. Totowa, N.J: Humana Press, Inc.; 1995. pp. 253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwan T G, Piesman J, Golde W T, Dolan M C, Rosa P A. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci USA. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sonenshine D E. Biology of ticks. New York, N.Y: Oxford University Press; 1991. pp. 95–97. [Google Scholar]
- 28.Tilly K, Casjens S, Stevenson B, Bono J L, Samuels D S, Hogan D, Rosa P. The Borrelia burgdorferi circular plasmid cp26: conservation of plasmid structure and targeted inactivation of the ospC gene. Mol Microbiol. 1997;25:361–373. doi: 10.1046/j.1365-2958.1997.4711838.x. [DOI] [PubMed] [Google Scholar]
- 29.Tilly K, Lubke L, Rosa P. Characterization of circular plasmid dimers in Borrelia burgdorferi. J Bacteriol. 1998;180:5676–5681. doi: 10.1128/jb.180.21.5676-5681.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vieira J, Messing J. New pUC-derived cloning vectors with different selectable markers and DNA replication origins. Gene. 1991;100:189–194. doi: 10.1016/0378-1119(91)90365-i. [DOI] [PubMed] [Google Scholar]
- 31.Yang X, Goldberg M S, Popova T G, Schoeler G B, Wikel S K, Hagman K E, Norgard M V. Interdependence of environmental factors influencing reciprocal patterns of gene expression in virulent Borrelia burgdorferi. Mol Microbiol. 2000;37:1470–1479. doi: 10.1046/j.1365-2958.2000.02104.x. [DOI] [PubMed] [Google Scholar]
- 32.Zhu Z, Gern L, Aeschlimann A. The peritrophic membrane of Ixodes ricinus. Parasitol Res. 1991;77:635–641. doi: 10.1007/BF00931028. [DOI] [PubMed] [Google Scholar]