Abstract
Aim
Macroecological studies that require habitat suitability data for many species often derive this information from expert opinion. However, expert‐based information is inherently subjective and thus prone to errors. The increasing availability of GPS tracking data offers opportunities to evaluate and supplement expert‐based information with detailed empirical evidence. Here, we compared expert‐based habitat suitability information from the International Union for Conservation of Nature (IUCN) with habitat suitability information derived from GPS‐tracking data of 1,498 individuals from 49 mammal species.
Location
Worldwide.
Time period
1998–2021.
Major taxa studied
Forty‐nine terrestrial mammal species.
Methods
Using GPS data, we estimated two measures of habitat suitability for each individual animal: proportional habitat use (proportion of GPS locations within a habitat type), and selection ratio (habitat use relative to its availability). For each individual we then evaluated whether the GPS‐based habitat suitability measures were in agreement with the IUCN data. To that end, we calculated the probability that the ranking of empirical habitat suitability measures was in agreement with IUCN's classification into suitable, marginal and unsuitable habitat types.
Results
IUCN habitat suitability data were in accordance with the GPS data (> 95% probability of agreement) for 33 out of 49 species based on proportional habitat use estimates and for 25 out of 49 species based on selection ratios. In addition, 37 and 34 species had a > 50% probability of agreement based on proportional habitat use and selection ratios, respectively.
Main conclusions
We show how GPS‐tracking data can be used to evaluate IUCN habitat suitability data. Our findings indicate that for the majority of species included in this study, it is appropriate to use IUCN habitat suitability data in macroecological studies. Furthermore, we show that GPS‐tracking data can be used to identify and prioritize species and habitat types for re‐evaluation of IUCN habitat suitability data.
Keywords: expert opinion, GPS, habitat suitability, habitat type, habitat use, IUCN, mammals, movement, selection ratio, telemetry
1. INTRODUCTION
Habitat is broadly defined as the entirety of environmental conditions that enable a species to survive and reproduce (Doligez et al., 2008; Stamps, 2008). Hence, information on species' habitats is crucial for evaluating the effects of environmental change on species and for designing and planning conservation (e.g., Brooks et al., 2019; Manly et al., 2002; Rondinini et al., 2011). Large‐scale and multi‐species assessments typically employ expert‐based habitat suitability information, for example from the International Union for Conservation of Nature (IUCN; e.g., Crooks et al., 2017; Di Marco et al., 2017; Powers & Jetz, 2019; Santini et al., 2019). The IUCN created an internationally recognized habitat classification scheme that distinguishes 54 terrestrial habitat types (https://www.iucnredlist.org/resources/habitat‐classification‐scheme; IUCN, 2020). Knowledge from more than 1,700 experts was used to identify suitable habitat types (i.e., habitat type in which a species occurs regularly or frequently) for as many species as possible (IUCN, 2020; Schipper et al., 2008). For some species marginal habitat types are also identified (i.e., habitat type in which a species only occurs irregularly or infrequently, or in which only a small proportion of individuals are found). All other habitat types are considered unsuitable and do not constitute habitat for a species. As the IUCN habitat suitability data are based solely on expert judgment, without direct empirical data input, the information is inherently subjective. This may bias the results of studies using these expert‐based data and misinform conservation decisions (Campbell et al., 2020; Johnson & Gillingham, 2004).
An alternative to expert opinion is to generate information on habitat suitability directly from empirical data. For example, species distribution models or resource selection functions can be used to identify habitat for a species (e.g., Dellinger et al., 2020; Peterson et al., 2018). However, using expert‐based data on habitat suitability is often the only option, particularly for large‐scale analyses, as empirical data are typically scarce and spatially restricted for many species (Amano & Sutherland, 2013; Boakes et al., 2010). This points towards a clear need to evaluate the accuracy of expert‐based habitat suitability information where possible, for example by comparing it with empirical data. A few studies have evaluated expert‐based habitat suitability data by calculating the number of occurrences of species in suitable and unsuitable habitat types, using point locality data from, for example, the Global Biodiversity Information Facility (e.g., Ficetola et al., 2015; Jung et al., 2020; Rondinini et al., 2011). These studies found that 55–94% of the point locations occurred in suitable habitat types. However, the number of locations for each species in point locality datasets is often small and does not include information on the identity of the individual. It is therefore unknown whether observations in unsuitable habitat types come from individuals that consistently use unsuitable habitat types (i.e., IUCN habitat suitability is misclassified), or individuals that only traverse unsuitable habitat types transiently to reach suitable habitat types (Beyer et al., 2010).
Tracking individuals fitted with GPS tags generates a large number of individual‐specific po location data, which enables distinguishing between habitat types that are used consistently or only transiently. The recent increasing use of GPS‐tracking data therefore offers opportunities to better evaluate expert‐based habitat suitability data. To our knowledge, no study has yet systematically evaluated expert‐based data on habitat suitability with GPS‐tracking data for a large number of species.
In this study, we use GPS‐tracking data from 1,498 individuals of 49 terrestrial mammal species to estimate two empirical measures of habitat suitability and compare these measures with expert‐based habitat suitability data recorded by the IUCN. One measure is proportional habitat use, which is defined as the proportion of locations in each habitat type (Lele et al., 2013; Manly et al., 2002). The other measure is the selection ratio, which relates proportional habitat use to its availability (i.e., the proportion of a given habitat type that is accessible for the species; Johnson, 1980; Lele et al., 2013; Manly et al., 2002). As each measure has its strengths and limitations, they provide complementary information. For example, according to the IUCN definition of suitable habitat types, we would expect that suitable habitat types are characterized by a greater proportional use. However, a low availability of a habitat type might lead to a low proportional use, even when the habitat type is in fact suitable. The selection ratio accounts for habitat availability and thus does not have this problem. A disadvantage of studying the selection ratio is that there can be a functional response of the selection ratio to the availability of a habitat type, which in some cases could lead to a decrease in the selection ratio when the availability of this habitat type increases (Aarts et al., 2013; Holbrook et al., 2019; Mysterud & Ims, 1998; van Beest et al., 2016). There are several reasons that could explain the presence of functional responses. One of these reasons is that species may use a habitat type only up to a level sufficient to meet its requirements (Aarts et al., 2013; Johnson, 1980), and once this requirement is fulfilled, a higher availability of that habitat type does not necessarily lead to higher proportional use. As a consequence, suitable habitat types with a high proportional use may have a low selection ratio (but see Van Moorter et al., 2013). Because the proportional habitat use and selection ratio provide complementary information, we studied both metrics in our study.
We also explored whether the probability of agreement between our empirical measures and the IUCN data was related to the species' body mass, habitat specialization, or IUCN Red List category. Because larger‐bodied species are generally better studied than smaller species (dos Santos et al., 2020), they may have more accurate IUCN habitat suitability data, and hence we expect a greater probability of agreement between empirical measures and IUCN data on habitat suitability for larger‐bodied species. We further expect that habitat specialists (i.e., species that only occur in a few habitat types) would have a greater probability of agreement than habitat generalists (i.e., species that occur in many different habitat types), because it is easier for experts to identify suitable habitat types when species only occur in a few habitat types. Finally, the IUCN Red List category may impact the probability of agreement between the IUCN data and empirical measures. First of all, threatened species may have a greater research prioritization (Rodrigues et al., 2006), which could lead to a greater probability of agreement. Secondly, a threatened species might have been forced to move to suboptimal habitat, due to high population densities of species (McLoughlin et al., 2010; van Beest et al., 2014) or high anthropogenic disturbances (Kerley et al., 2020), which makes it more difficult for experts to identify the suitable habitat types, potentially leading to a lower probability of agreement. This could be the case for non‐threatened species, as these species are generally more abundant and thus often have higher population densities. However, this could also be the case for threatened species, which have more restricted distributions due to anthropogenic disturbances and might thus only occur in suboptimal habitat. Identifying the role of species characteristics might help to identify species not included in our analysis for which expert‐based suitability of some habitat types is misclassified. Research on habitat suitability could then be directed towards these groups of species.
2. METHODS
2.1. GPS‐tracking data and habitat data
We compiled GPS‐tracking data of 1,498 individuals from 49 species of terrestrial mammals tracked between 1998 and 2021 (see Supporting Information Appendices [Link], [Link], [Link]). Captures and handling of all these individuals were approved by the appropriate national or regional authorities (Supporting Information Appendix S4). The individuals come from 114 populations, here defined as groups of individuals that occur in the same geographic area, and occurred on all continents (except Antarctica) and most terrestrial biomes (Figure 1). The species included belong to 19 families, have body masses ranging from 0.5 to 4,000 kg, include species from each IUCN Red List category (Least Concern, Near Threatened, Vulnerable, Endangered, Critically Endangered), and include both habitat specialists and generalists (Faurby et al., 2018, 2020; IUCN, 2021; Wilman et al., 2014).
FIGURE 1.
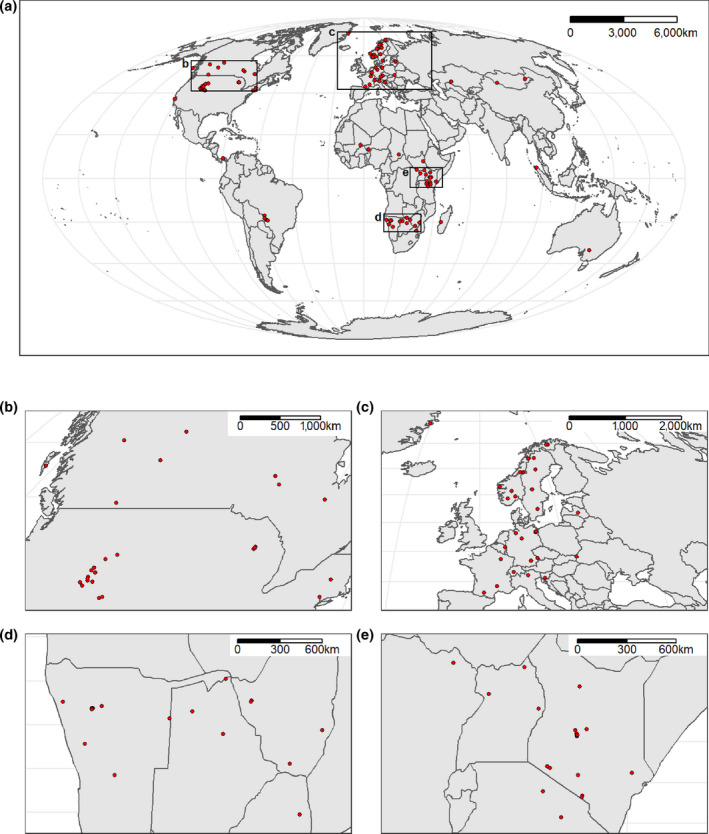
Distribution of locations of (a) all 114 studied populations, (b) populations in North America, (c) Europe, (d) southern Africa, and (e) eastern Africa. The projections of the maps are Mollweide equal‐area projections
We obtained habitat data from a global map of terrestrial IUCN habitat types in 2015 at a ~ 100‐m resolution (Jung et al., 2020). Estimates of habitat suitability could be affected by a temporal mismatch between the habitat map based on data from 2015, and the individual GPS data collected between 1998–2021. Therefore, we only included individuals for which we estimated that less than 5% of the available habitat types had changed between the year in which an individual was tracked and the year 2015 (see Supporting Information Appendix S5).
For each individual, we removed locations from the first 7 days of tracking after capture (on average 3.9% of the data), to avoid possible effects of capture and handling on the individual's locations (Bergvall et al., 2021; Gese et al., 2019; Mayer et al., 2019; Morellet et al., 2009). Furthermore, we removed locations that could be considered outliers based on unrealistic distances or speed between successive locations (on average 0.5% of the data, see Supporting Information Appendix S5 for a detailed description of the data cleaning). All individuals had at least 100 locations (mean: 6,436 locations), were tracked for a duration of at least 30 days (mean: 405 days) and had a median number of 11 locations per day after data cleaning (Supporting Information Appendix S3).
2.2. Proportional habitat use and selection ratios
We used the GPS‐tracking data and the IUCN habitat map to estimate the proportional habitat use and selection ratio of each habitat type for each individual. We calculated the proportional habitat use as the proportion of GPS locations of an individual within each habitat type (Lele et al., 2013) and the selection ratio as proportional habitat use divided by the relative amount of a habitat type available to that individual (Manly et al., 2002). We calculated the relative amount of a habitat type available to each individual as the proportional area of habitat types within an individual's 100% Minimum Convex Polygon (MCP) (Mohr, 1947), a commonly used approach to measure habitat selection at the home range scale (i.e., third‐order selection sensu Johnson, 1980). When there were distinct clusters of locations for an individual (e.g., winter and summer ranges), we fitted a separate MCP for each cluster of locations, with clusters delineated based on a species‐specific threshold distance (see Supporting Information Appendix S5). We applied this multi‐MCP approach when the locations of an individual occurred in multiple clusters, because a single MCP would lead to large areas being considered as available that do not contain any locations. A single MCP would thus not accurately define available habitat for that individual.
2.3. Probability of agreement between IUCN and GPS‐tracking data
For each species, we used the estimated proportional habitat use and selection ratios to calculate a probability of agreement between the IUCN habitat suitability data and each habitat suitability measure. To calculate the probability of agreement between the IUCN habitat suitability data and the proportional habitat use estimates, we compared the proportional use of each suitable habitat type with the proportional use of every unsuitable and marginal habitat type for each individual. Next, we calculated the log10‐transformed ratios of these proportional habitat use estimates to measure to what degree a suitable habitat type is used, compared to an unsuitable or marginal habitat type, using:
| (1) |
in which indicates the proportional use of a suitable habitat type, indicates the proportional use of a marginal or unsuitable habitat type, and R use indicates the log‐transformed ratio of these proportional use estimates. For each individual, the number of ratios depends on the number of suitable and unsuitable habitat types. For example, if an individual has two suitable habitat types and three unsuitable habitat types, six comparisons can be made between a suitable and an unsuitable habitat and six ratios are thus calculated.
We only included habitat types with a proportional use larger than zero. Individuals of some species only occurred in suitable habitat types or only in marginal and unsuitable habitat types. For these individuals no comparison could be made between suitable and unsuitable habitat types. To retain these individuals in the analyses, we compared their proportional habitat use estimates with the theoretical minimum proportional habitat use of the individual: the inverse of the individual's number of GPS locations. This proportion is equal to the proportional habitat use when there is only one location in a habitat type.
We combined the ratios of all individuals of the same species and fitted a linear mixed‐effect model:
| (2) |
In this mo del, suitability indicates whether the ratio of a suitable and an unsuitable habitat type was calculated, or the ratio of a suitable and marginal habitat type. Nested random effects of population and individual were included to account for repeat sampling of the same population and individual. The intercept of this model indicates the species‐specific log10‐transformed ratio of the proportional use of suitable compared to marginal habitat types, and the coefficient for suitability indicates the difference between this ratio and the species‐specific log10‐transformed ratio of the proportional use of suitable compared to unsuitable habitat types.
We applied parametric bootstrapping using 1,000 simulations to obtain 1,000 estimates of the intercept and suitability coefficient of this model. These bootstrap results were used to calculate the probability of agreement between the IUCN habitat suitability data and the proportional habitat use estimates. This probability of agreement is the proportion of bootstrap simulations in which the model coefficients indicate . The above condition is satisfied when the intercept estimate is larger than zero, indicating a higher proportional habitat use for suitable than for marginal habitat types. In addition, the suitability coefficient needs to be larger than zero, indicating a larger difference in proportional habitat use between suitable and unsuitable habitat types than between suitable and marginal habitat types. For 17 species that did not occur in any marginal habitat type, we fitted an intercept‐only model and calculated the probability of agreement as the proportion of bootstrap simulations in which the intercept is larger than zero, indicating .
In an analogous way, we calculated the probability of agreement between the IUCN habitat suitability data and the selection ratio estimates by calculating the log10‐transformed ratios () of the selection ratios for a suitable habitat type (SR suitable ) and a marginal or unsuitable habitat type (SR unsuitable/marginal ) for each individual:
| (3) |
To avoid selection ratios of zero, for which the log10 is undefined, we substituted the zero selection ratios with the lowest possible selection ratios: the theoretical minimum proportional habitat use of the individual (the inverse of the individual's number of GPS locations) divided by the relative amount of available area of the habitat type. Because of this correction for zeros, we excluded habitat types for which the relative amount of available area was lower than the theoretical minimum proportional habitat use of the individual. If these habitat types were included, they would always have a selection ratio larger than one, even though they are not used. Furthermore, we also excluded these habitat types because selection ratios are unreliable for habitat types with a low availability. For habitat types with a low availability, the absence of any GPS location in this habitat type might not indicate habitat avoidance, but could just be the result of the scarcity of this habitat type. We note that some of the habitat types included in the selection ratio analyses have a proportional habitat use of zero; therefore, the number of included habitat types is larger than in the analyses of the proportional habitat use. For individuals where only suitable or only unsuitable or marginal habitat types were retained, we compared the selection ratios with the theoretical minimum selection ratio of these habitat types: the inverse of the individual's number of GPS locations, divided by the relative amount of available area of the habitat type [see Supporting Information Appendix S6 for a detailed calculation of the probability of agreement for the Impala (Aepyceros melampus)].
We note that a high probability of agreement (e.g., 100%) for a species does not necessarily imply that all suitable habitat types have a higher proportional habitat use or selection ratio than unsuitable habitat types. It is possible that the suitability of one habitat type is consistently misclassified, but because the selection ratio or proportional use of all the other habitat types is higher for suitable than unsuitable habitat types, the general probability of agreement for this species can still be high. To study whether the suitability of a habitat type might be misclassified, we also calculated a habitat type‐specific probability of agreement for a few habitat types and species for which we suspect the suitability might be misclassified by the IUCN. These habitat types were found by looking for (a) suitable habitat types that were used less or that were selected for less than unsuitable habitat types in multiple individuals, or (b) unsuitable habitat types that were used or selected more than suitable habitat types. To calculate a habitat‐type‐specific probability of agreement, we performed the same analyses as described above, but only used the log10‐transformed ratios that included the specific habitat type.
2.4. Effects of species characteristics on probability of agreement
We evaluated whether the probability of agreement between our habitat suitability measures and the IUCN data were related to the species' body mass, habitat specialization and the IUCN Red List category. We derived body mass values from the literature or used the mean weight of the tracked individuals (Supporting Information Appendices S1 and S2). To retrieve information on habitat specialization, researchers who collected the data classified the species they tracked as either habitat specialists or generalists. With regard to the IUCN Red List category, we classified species as non‐threatened (Least Concern, Near Threatened) or threatened (Vulnerable, Endangered, Critically Endangered) (IUCN, 2021). We then fitted beta regression models relating the probability of agreement based on proportional use or selection ratio to the three species characteristics. Because the response variable in beta regression models is bounded between zero and one, we transformed the probability of agreement with a Smithson–Verkuilen transformation to avoid zeros and ones (Smithson & Verkuilen, 2006):
| (4) |
in which n indicates the sample size, that is, number of species.
To account for differences in the number of tracked individuals (n individuals ), number of populations (n populations ) and median tracking duration (t median ) between species, which could constrain the effects of species characteristics, we also included these factors as predictor variables in the models:
| (5) |
We used a model selection approach based on the Akaike's information criterion corrected for small sample size (AICc) to identify which combination of the species' characteristics best explained the probability of agreement between our habitat suitability measures and the IUCN data (Burnham & Anderson, 2002). We included models containing all possible combinations of additive effects of the species' characteristics. The variance inflation factors of all predictor variables were lower than two, indicating no substantial collinearity problems (Zuur et al., 2010).
2.5. Sensitivity analyses
To assess whether our results were robust to methodological choices made in our analyses, we re‐ran our analyses in three different ways. First, we repeated our analyses using longer minimum tracking durations (60–600 days) and greater minimum number of observations (100–20,000). Secondly, we also calculated the availability of each habitat type as the proportional area within an individual‐specific buffer‐distance around each GPS location, that is, calculating habitat availability using the buffer‐approach (Montgomery et al., 2018). Finally, we repeated all analyses using GPS‐tracking data subsampled to approximately constant time intervals for each individual. (See Supporting Information Appendix S5 for more information on these different methodological approaches.)
3. RESULTS
The average probability of agreement across all species between habitat suitability data derived from the IUCN and empirical proportional use estimates was 76% (± 39% SD; Figure 2). Similarly, the average probability of agreement between IUCN habitat suitability data and selection ratio estimates across all species was 69% (± 40% SD; Figure 3). Thirty‐three (67%) and 25 (51%) species had a probability of agreement greater than 95%, based on the proportional habitat use and selection ratio, respectively. These numbers increased to 37 (76%) and 34 (69%) species when we used a probability of agreement greater than 50% as a threshold. There were also six species for the proportional habitat use and five species for the selection ratio with a very low probability of agreement (< 5%). In addition, for several species we found one or more individuals for which a suitable habitat type had a lower selection ratio or proportional use than an unsuitable or marginal habitat type (see Broekman et al., 2022). For example, for the gray wolf (Canis lupus) the suitable habitat type ‘Pastureland’ was often less used and less selected than unsuitable habitat types, such as arable land. The probability of agreement for this habitat type for C. lupus was therefore only 0.3% based on the proportional habitat use and 9.9% based on the selection ratio. Similarly, for several species we also found one or more individuals for which an unsuitable or marginal habitat was used and selected more than suitable habitat types. For example, for the jaguar (Panthera onca), the most used and selected habitat type in all four individuals was ‘Forest Subtropical‐tropical dry’, which is classified as marginal by the IUCN. The probability of agreement for this habitat type for P. onca was zero based on both the proportional habitat use and selection ratio.
FIGURE 2.
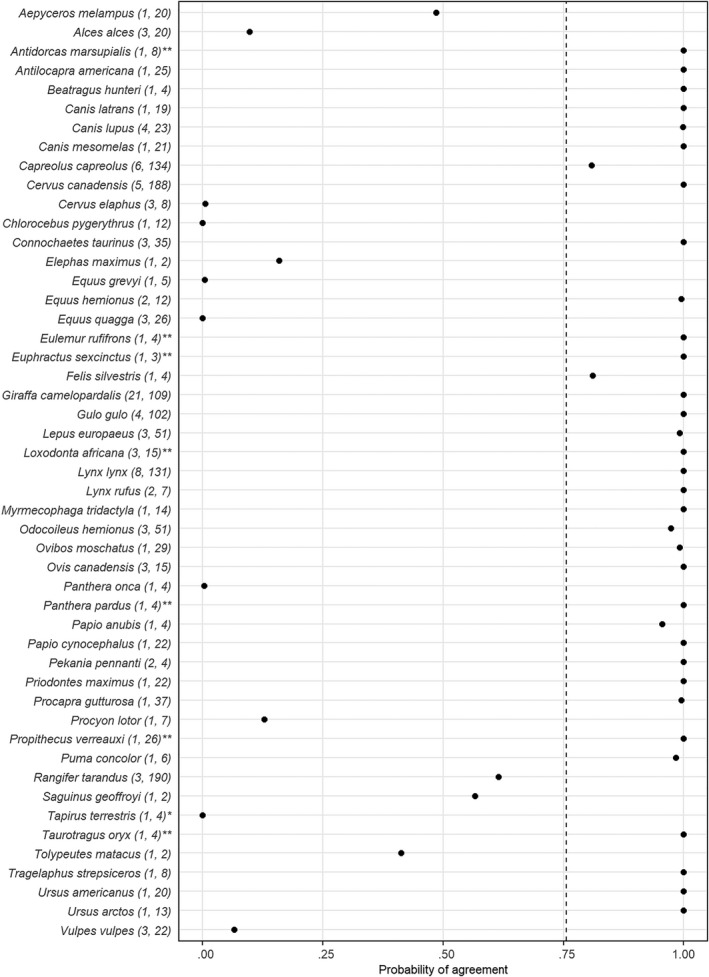
Probability of agreement between the International Union for Conservation of Nature (IUCN) habitat suitability data and the proportional habitat use estimates for each species. The dashed line indicates the average probability of agreement across all species. The numbers in parentheses indicate the number of populations and number of individuals for the species, respectively. * indicates species for which all individuals only occurred in unsuitable habitat types. ** indicates species for which all individuals only occurred in suitable habitat types
FIGURE 3.
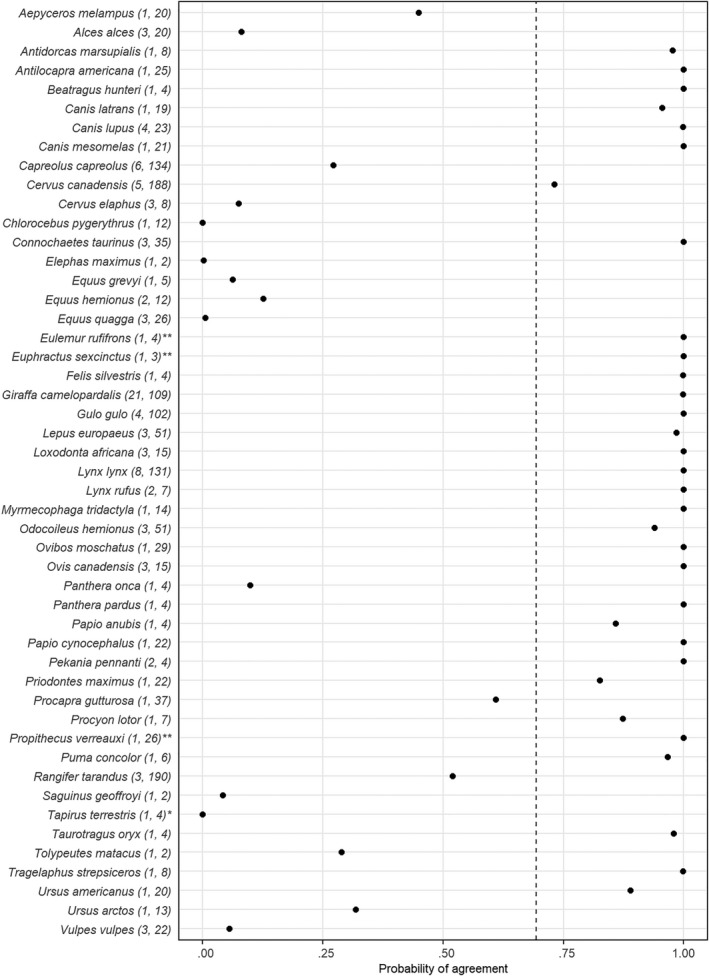
Probability of agreement between the International Union for Conservation of Nature (IUCN) habitat suitability data and the selection ratio estimates for each species. The dashed line indicates the average probability of agreement across all species. The numbers in parentheses indicate the number of populations and number of individuals for the species, respectively. * indicates species for which all individuals only have unsuitable habitat types available. ** indicates species for which all individuals only have suitable habitat types available
The most parsimonious model explaining the probability of agreement between the empirical habitat suitability measures and the IUCN habitat suitability data was an intercept‐only model for both the proportional use and selection ratio (Supporting Information Appendices S7 and S8). Two models within 2 AICc units from the most parsimonious model for proportional habitat use contained the effects of the number of individuals and number of populations, respectively. For the selection ratio, three models within 2 AICc units from the most parsimonious model contained the effects of body mass, number of populations, and median tracking duration, respectively.
All the results were robust to methodological changes, including increments in minimum tracking duration and minimum number of observations per individual, data subsampling, and using the buffer‐approach to estimate habitat availability (Supporting Information Appendix S9).
4. DISCUSSION
4.1. Interpretation
In this study, we showed how GPS‐tracking data can be used to evaluate expert‐based habitat suitability data. Our findings indicate that for the majority of species included in this study, it is appropriate to use IUCN habitat suitability data in macroecological studies. Nevertheless, caution should still be taken when using IUCN habitat suitability data, as for some species there might be a few misclassified habitat types. For example, for the vervet monkey (Chlorocebus pygerythrus) and the lowland tapir (Tapirus terrestris), the probability of agreement between IUCN habitat suitability data and our empirical habitat suitability measures was low, which may reflect misclassification of suitability for some habitat types. In addition, for some species, including several with a high probability of agreement, there were unsuitable or marginal habitat types with a greater proportional use or selection ratio than one or more suitable habitat types (see Broekman et al., 2022). Some other species had suitable habitat types with a lower proportional use or selection ratio than one or more unsuitable or marginal habitat types (see Broekman et al., 2022). These results also suggest that the suitability of these habitat types might be misclassified. We have not provided a list of habitat types that are potentially misclassified by the IUCN to avoid the use of arbitrary thresholds for identifying suitable and unsuitable habitat types. In fact, there could also be other reasons for a mismatch between the IUCN habitat suitability data and our habitat suitability measures (see below); our results might serve as a starting point to identify species and habitat types for which further research is necessary to evaluate suitability.
Our results also suggest that body mass, habitat specialization and Red List category are not informative characteristics for identifying species for which the IUCN habitat suitability data agree with inference from GPS‐tracking data. These species characteristics were not included in the most parsimonious model predicting the probability of agreement between IUCN habitat suitability data and our empirical habitat suitability measures. Whether the IUCN classified habitat suitability for a species correctly most likely depends on various species‐specific factors. For example, while larger‐bodied species are, in general, better studied than smaller‐bodied species, some large‐bodied species may be highly elusive, and their habitat use may have been poorly known before the advent of GPS telemetry (e.g., jaguar, puma). Furthermore, smaller‐bodied species often range over smaller areas, exposing them to fewer habitat types, given the spatial resolution of the IUCN habitat map (Jung et al., 2020), which may reduce the possibilities of misclassifying habitat suitability. Moreover, species living in large groups in open habitats (e.g., ungulates in grasslands) might have been studied more intensively than solitary, forest‐dependent species. Because of the complexity of factors determining the accuracy of IUCN habitat suitability data, it is difficult for the moment to draw conclusions. In addition, inferences from the analyses are limited by our relatively small sample of species given the total number of terrestrial mammal species classified by the IUCN and several other limitations described in the next section. A larger number of species and more species‐specific characteristics (e.g., home range size, diet, elusiveness of the species) should thus be investigated to identify characteristics that may explain for which species the IUCN habitat suitability data are likely to show a low probability of agreement with results from GPS‐tracking data.
4.2. Limitations
There are several limitations in the habitat maps, IUCN habitat suitability data, and GPS‐tracking data that may explain why not all species have corresponding results on habitat suitability. First of all, the IUCN habitat types are sometimes difficult to match to vegetation or habitat types used in the field. This makes it difficult for experts to determine the suitability of a habitat type and might also have led to misassignments of habitat types to grid cells of the IUCN habitat map (Jung et al., 2020). For example, the IUCN defines ‘plantations’ as plantings of trees and shrubs (IUCN, 2020), which can be, for example, coffee plantations, but also mature forest plantations. For the Eurasian lynx (Lynx lynx) ‘plantations’ are classified as an unsuitable habitat type by the IUCN, which might be correct for coffee plantations. However, the lynx in our dataset are frequently located in ‘plantations’, which covers large parts of Europe (especially southern Sweden) and are more likely mature forest plantations, that is, commercially operated forests subject to cutting and replanting. From a lynx point of view, the suitability of mature forest plantations might be similar to, for example, temperate or boreal forest, which are suitable habitat types for the lynx, according to the IUCN. Most ‘plantations’ in Europe might therefore be better represented by one of the forest habitat types. Despite these possible misassignments, we still used the IUCN map as it currently contains the best available data for global analyses.
Second, individuals have intrinsic differences in habitat suitability (e.g., Leclerc et al., 2016; Lesmerises & St‐Laurent, 2017; Montgomery et al., 2018), and the suitability of habitat types likely varies spatially and temporally for most species. Mismatches between the IUCN and GPS‐tracking data might therefore arise, because for most species we only studied a few individuals that occurred on a small subset of the entire range or that were only tracked for a short time period. For example, the Eurasian lynx data in this study came from multiple studies across the European part of their distribution, but none from Asia. Estimated habitat suitability measures for these individuals might not be representative of the species as a whole. Regional differences in habitat suitability could arise due to other abiotic and biotic factors that we did not account for, such as weather, distance to water, human disturbances, or prevalence of prey/predator/competitor species (e.g., Attias et al., 2018; Dellinger et al., 2020; Jones et al., 2019; Mayer et al., 2019; Rivrud et al., 2019; Roever et al., 2012). For example, temperate grassland is listed as suitable habitat for the pronghorn (Antilocapra americana) by the IUCN, but this species tends to avoid roads (Jones et al., 2019). This habitat type might thus be unsuitable when it is located close to roads, but suitable otherwise. Temporal variability in habitat suitability could arise due to seasonal differences, such as between summer and winter (e.g., Rivrud et al., 2019) or between wet and dry seasons (e.g., Roever et al., 2012). Nevertheless, our results did not change when using longer minimum tracking durations as a threshold to select individuals. This indicates that our results were not influenced by individuals that were only tracked during a specific season.
Third, mismatches in habitat suitability might be attributed to taxonomic classifications that diverge from more recent phylogenetic assessments. For example, four species of giraffe have been classified based on phylogenetic analyses (Coimbra et al., 2021), whereas the IUCN only identifies a single species (Giraffa camelopardalis). We followed the IUCN taxonomy, as habitat suitability data are provided for this single species. However, there might be differences in habitat suitability among these four species. The taxonomic classifications of giraffe species are relatively new, and we are only now starting to understand the ecological implications of these new taxonomic classifications in terms of habitat use and conservation (O'Connor et al., 2019).
Fourth, mismatches between the IUCN habitat suitability data and our empirical measures of habitat suitability could occur because proportional habitat use and selection ratio do not always correctly indicate habitat suitability. For example, variability in habitat availability can lead to changes in selection ratios, that is, functional responses, even when the suitability of a habitat type does not change (Aarts et al., 2013; Holbrook et al., 2019; Mysterud & Ims, 1998; van Beest et al., 2016). Furthermore, the proportional use and selection ratio of a given habitat type depends on the other habitat types available (Johnson, 1980; Lele et al., 2013). More precisely, the selection ratio and proportional use of a habitat type will be greater when all other available habitat types are unsuitable, compared to when some of the other available habitat types are also suitable (see also Beyer et al., 2010; Van Moorter et al., 2013). Moreover, a species might be forced to move to less suitable habitat types due to, for example, high population densities of species (McLoughlin et al., 2010; van Beest et al., 2014) or anthropogenic disturbances (Kerley et al., 2020), which might lead to an overestimation of the suitability for these habitat types. To limit the impact of individuals occurring only in suboptimal habitat on the calculations of the probability of agreement, we included individuals from as many different populations as possible, increasing the likelihood of including individuals that were not forced into suboptimal habitat. For example, for the giraffe, we included data from 21 different populations spread across its range. However, for some other species we had access to data from only one population. Nevertheless, by focussing on the overall rather than the species‐specific results, we tried to limit the bias that might be generated by individuals that are forced to move to less suitable habitat types.
Finally, we note that our sample size of 49 species is relatively small compared to the total number of 5,480 mammal species for which the IUCN has habitat suitability data (IUCN, 2021). Furthermore, we only focused on mammal species, so our results are not representative of all species groups (e.g., birds, reptiles and amphibians). Our data also include some well‐studied species, for which habitat suitability might be better known than for less‐studied species, and it could thus be argued that our results represent a best case scenario. However, our data include also less‐studied species (e.g., Beatragus hunteri, Priodontes maximus). In addition, for the red fox (Vulpes vulpes), a highly studied species, we found a probability of agreement of only 7% for the proportional habitat use and 6% for the selection ratio, which is well below the average. Thus, our results do not seem to be biased by unrepresentative sampling of mammal species. Given the difficulty of collecting GPS‐tracking data, our dataset includes a very large number of individuals and species and is currently the best available. Nevertheless, the lack of data for the majority of mammal species highlights the need for more GPS‐tracking studies, especially for species that are still poorly studied.
4.3. Recommendations
Although our results show that for the majority of studied species it is appropriate to use IUCN habitat suitability data in macroecological studies, there might still be several habitat types for which suitability is misclassified by the IUCN. Reducing potential misclassifications of habitat suitability by the IUCN would benefit all studies that make use of these expert judgments. For example, when allocating new protected areas to prevent local extinction of species at risk, it is vital to have reliable information on species' habitat suitability, otherwise we risk making expensive but ineffective conservation decisions. Habitat suitability data can also be used to calculate the area of habitat for each species and subsequently assess its Red List status (e.g., Santini et al., 2019). More accurate habitat suitability information will therefore contribute to more accurate assessments of progress towards international targets related to nature conservation, including the Aichi targets and the targets within the post‐2020 global biodiversity framework (SCBD, 2011, 2020).
We showed that GPS‐tracking data can be used to identify species and habitat types for which re‐evaluations of the suitability information might be necessary. In addition, GPS‐tracking data can be used to account for several factors that constrain the habitat suitability assessment. For example, because the GPS data provide individual‐specific information on habitat suitability, they can be used to derive separate habitat suitability data for different regions. Similarly, GPS‐tracking data can be used to derive season‐specific habitat suitability data. In this study, we did not calculate different habitat suitability metrics for, for example, different seasons, or regions, even though this might be appropriate for some species, to optimize comparability with the IUCN data. Nevertheless, future studies would benefit from using regional‐ and season‐specific habitat suitability data, as this would lead to more accurate estimates of, for example, the area of habitat for a species within (potential) protected areas (e.g., Di Marco et al., 2017).
GPS‐tracking data can also be used to distinguish more habitat suitability categories that may better reflect a species' habitat suitability. Currently, the IUCN only distinguishes suitable, marginal and unsuitable habitat types, and for many species marginal habitat types are not reported. However, using individual‐specific GPS‐tracking data we could rank habitat types for each individual from least to most used or selected. We could then classify habitat types as, for example, ‘highly suitable’ when they are always selected over all other habitat types, whereas we could classify habitat types as ‘partly suitable’ when they are only selected over unsuitable habitat types, but not other suitable habitat types. In this way, GPS‐tracking information might also be used to accommodate inter‐individual differences in habitat suitability, for example, ‘highly suitable’ habitat types are suitable for all individuals, and ‘partly suitable’ habitat types are only suitable for a subset of individuals, as a consequence of, for example, inherent individual differences or regional differences in habitat suitability. This will only be feasible when tracking data are available from a range of representative regions and seasons, as discussed in the previous paragraph. However, with the increasing availability of GPS‐tracking data, these limitations may soon be overcome for several species. Because of these opportunities, we recommend using GPS‐tracking data when updating IUCN habitat suitability data.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
ETHICS APPROVAL
Captures and handling of all individuals were approved by the appropriate authorities (see Supporting Information Appendix S3).
BIOSKETCH
Maarten Broekman is a PhD candidate at the Radboud University in Nijmegen, the Netherlands. His research focuses on quantifying the effects of habitat conversion on biodiversity. Specifically, he studies terrestrial mammals at a global scale.
Supporting information
Appendix S1
Appendix S2
Appendix S3
Appendix S4
Appendix S5
Appendix S6
Appendix S7
Appendix S8
Appendix S9
ACKNOWLEDGMENTS
We are grateful to M. Kauffman for contributing data. D.R. is grateful to the supporters of Australian Wildlife Conservancy and the Australian Government's National Environmental Science Program. L.A.I. thanks the Kenya Wildlife Service for local affiliation, D. Simpson, S. Ekwanga, M. Mutinda, G. Omondi, W. Longor, M. Iwata, A. Surmat, M. Snider, W. Fox and K. VanderWaal for field assistance, L. Frank for the use of their field equipment, and M. Kinnaird and T. Young for logistical support in the field. R.G.M. is grateful to the Taiamã Ecological Reserve Team for supporting the jaguar capturing and handling. Springbok, kudu and eland data acquisition was supported by Etosha Heights Private Reserve (Outjo, Namibia). Some roe deer, red deer, and Eurasian lynx datasets were collated from the Euromammals database: https://euromammals.org/. For the study of Papio cynocephalus (baboons) in Amboseli see, please visit http://amboselibaboons.nd.edu/acknowledgements/ for a complete set of acknowledgments.
Broekman, M. J. , Hilbers, J. P. , Huijbregts, M. A. , Mueller, T. , Ali, A. H. , Andrén, H. , Altmann, J. , Aronsson, M. , Attias, N. , Bartlam‐Brooks, H. L. , van Beest, F. M. , Belant, J. L. , Beyer, D. E. , Bidner, L. , Blaum, N. , Boone, R. B. , Boyce, M. S. , Brown, M. B. , Cagnacci, F. … Tucker, M. A. (2022). Evaluating expert‐based habitat suitability information of terrestrial mammals with GPS‐tracking data. Global Ecology and Biogeography, 31, 1526–1541. 10.1111/geb.13523
Handling Editor: Melodie McGeoch
Funding informationM.J.E.B., J.P.H. and M.A.J.H. were financed by a grant from the Dutch research foundation (016.Vici.170.190). M.A.T. was supported by a Radboud Excellence Initiative Fellowship. N.D. was supported by the German Federal Ministry of Education and Research (BMBF, 01LC1820A). M.S.B. was funded by Natural Sciences and Engineering Research Council of Canada, Alberta Conservation Association. Data collection at Zackenberg, NE Greenland, was supported by the 15 June Foundation. J.L.B. was supported by Federal Aid in Wildlife Restoration, Safari Club International Foundation, Safari Club International Michigan Involvement Committee. Funding to L.A.I. was provided by the National Science Foundation (grant nos. BCS 99–03949, BCS 1266389), the L.S.B. Leakey Foundation, and the Committee on Research, University of California, Davis. The study from LTSER ZA Pyrénées Garonne was funded in part by the ANR grant Mov‐It (ANR‐16‐CE02‐0010). Data on European brown hare (Ch.F., F.J., J.S., N.B., W.U.) were collected within the DFG funded research training group ‘BioMove’ (RTG 2118–1). N.A. was supported by scholarships from Capes (number 1575316) and Fundect (process 23/200.715/2013). N.S., A.S., F.Z., T.Z.K. were supported by the Polish‐Norwegian Research Programme operated by the National Centre for Research and Development in Poland under the Norwegian Financial Mechanism 2009–2014 in the frame of project contract no. POL‐NOR/198352/85/2013 (GLOBE). N.S. was also supported by the BearConnect project funded by the National Science Centre in Poland (2016/22/Z/NZ8/00121) through the BiodivERsA COFUND call for research proposals, with the national funders ANR/DLR‐PT/UEFISCDI/NCN/RCN. R.G.M. was supported by FAPESP (2014/24921–0). Data provided by S.C.J. were collected thanks to grants ANR‐2010‐BLAN‐1718, ANR‐11‐CEPS‐003 and ANR‐16‐CE02‐0001‐01 and support from the Zone Atelier Hwange CNRS program of the CNRS. Springbok, kudu and eland data are part of the ORYCS project funded by the German Federal Ministry of Education and Research (Grant FKZ 01LL1804A). For the study of Papio cynocephalus (baboons) in Amboseli, please visit http://amboselibaboons.nd.edu/acknowledgements/ for a complete set of funding sources. The data on Lynx from Slovenia were partly financed by the European Union (INTERREG IIIA Neighborhood Program Slovenia/Hungary/Croatia 2004–2006, project ‘DinaRis’) and the European Commission (LIFE16 NAT/SL/000634 project ‘LIFE Lynx’). M.K. was supported by the Slovenian Research Agency (grants no. P4‐0059 and N1‐0163). F.C. was supported by the Autonomous Province of Trento (Grant no. 3479 BECOCERWI) and by Institutional funds during the collection of roe deer data in the Alps. Funding for the GPS fisher collar data from Alberta, Canada, was provided by InnoTech Alberta, the Natural Science and Engineering Research Council of Canada, MITACS Accelerate and the Friends of Elk Island Society, the Beaver Hills Initiative, Alberta Environment and Parks, Royal Canadian Geographic Society, TD Friends of the Environment Foundation, Fur Institute of Canada, University of Victoria, and Alberta Conservation Association
DATA AVAILABILITY STATEMENT
Data is published on DANS (https://doi.org/10.17026/dans‐xq3‐6muu). These data contain the following information for each individual of each species: individual ID, population ID, species ID, proportional use of each habitat type, available proportions of each habitat type, selection ratios of each habitat type. Furthermore, the GPS‐tracking data for some individuals are published on Movebank or are published in Eurommammals (euromammals.org) (see Appendix S4). In Supporting Information Appendix S4 we indicate the Movebank details for the individuals that have their GPS‐tracking data published on Movebank. Data from Euromammals can be retrieved by logging into their website or via a contact form.
REFERENCES
- Aarts, G. , Fieberg, J. , Brasseur, S. , & Matthiopoulos, J. (2013). Quantifying the effect of habitat availability on species distributions. Journal of Animal Ecology, 82(6), 1135–1145. 10.1111/1365-2656.12061 [DOI] [PubMed] [Google Scholar]
- Amano, T. , & Sutherland, W. J. (2013). Four barriers to the global understanding of biodiversity conservation: Wealth, language, geographical location and security. Proceedings of the Royal Society B‐Biological Sciences, 280(1756), 7. 10.1098/rspb.2012.2649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attias, N. , Oliveira‐Santos, L. G. R. , Fagan, W. F. , & Mourao, G. (2018). Effects of air temperature on habitat selection and activity patterns of two tropical imperfect homeotherms. Animal Behaviour, 140, 129–140. 10.1016/j.anbehav.2018.04.011 [DOI] [Google Scholar]
- Bergvall, U. A. , Morellet, N. , Kjellander, P. , Rauset, G. R. , Groeve, J. D. , Borowik, T. , Brieger, F. , Gehr, B. , Heurich, M. , Hewison, A. J. M. , Kröschel, M. , Pellerin, M. , Saïd, S. , Soennichsen, L. , Sunde, P. , & Cagnacci, F. (2021). Settle down! Ranging behaviour responses of roe deer to different capture and release methods. Animals, 11(11), 3299. https://doi.org/10.3390/ani11113299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer, H. L. , Haydon, D. T. , Morales, J. M. , Frair, J. L. , Hebblewhite, M. , Mitchell, M. , & Matthiopoulos, J. (2010). The interpretation of habitat preference metrics under use‐availability designs. Philosophical Transactions of the Royal Society B‐Biological Sciences, 365(1550), 2245–2254. 10.1098/rstb.2010.0083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boakes, E. H. , McGowan, P. J. K. , Fuller, R. A. , Ding, C. Q. , Clark, N. E. , O'Connor, K. , & Mace, G. M. (2010). Distorted views of biodiversity: Spatial and temporal bias in species occurrence data. PLoS Biology, 8(6), 11. 10.1371/journal.pbio.1000385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekman, M. J. E. , Hilbers, J. P. , Huijbregts, M. A. J. , Mueller, T. , Ali, A. H. , Andrén, H. , Altmann, J. , Aronsson, M. , Attias, N. , Bartlam‐Brooks, H. L. A. , van Beest, F. M. , Belant, J. L. , Beyer, D. E. , Bidner, L. , Blaum, N. , Boone, R. B. , Boyce, M. S. , Brown, M. B. , Cagnacci, F. … Tucker, M. A. (2022). Data of “Evaluating expert‐based habitat suitability information of terrestrial mammals with GPS‐tracking data”. DANS EASY [Dataset]. 10.17026/dans-xq3-6muu [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks, T. M. , Pimm, S. L. , Akcakaya, H. R. , Buchanan, G. M. , Butchart, S. H. M. , Foden, W. , Hilton‐Taylor, C. , Hoffmann, M. , Jenkins, C. N. , Joppa, L. , Li, B. V. , Menon, V. , Ocampo‐Peñuela, N. , & Rondinini, C. (2019). Measuring terrestrial area of habitat (AOH) and its utility for the IUCN red list. Trends in Ecology & Evolution, 34(11), 977–986. 10.1016/j.tree.2019.06.009 [DOI] [PubMed] [Google Scholar]
- Burnham, K. P. , & Anderson, D. R. (2002). Model selection and multimodel inference: A practical information‐theoretic approach (2nd ed.). Springer‐Verlag. [Google Scholar]
- Campbell, M. , Kopach, B. , Komers, P. E. , & Ford, A. T. (2020). Quantifying the impacts of oil sands development on wildlife: Perspectives from impact assessments. Environmental Reviews, 28(2), 129–137. 10.1139/er-2018-0118 [DOI] [Google Scholar]
- Coimbra, R. T. , Winter, S. , Kumar, V. , Koepfli, K.‐P. , Gooley, R. M. , Dobrynin, P. , Fennessy, J. , & Janke, A. (2021). Whole‐genome analysis of giraffe supports four distinct species. Current Biology, 31, 2929–2938.e5. [DOI] [PubMed] [Google Scholar]
- Crooks, K. R. , Burdett, C. L. , Theobald, D. M. , King, S. R. B. , Di Marco, M. , Rondinini, C. , & Boitani, L. (2017). Quantification of habitat fragmentation reveals extinction risk in terrestrial mammals. Proceedings of the National Academy of Sciences of the United States of America, 114(29), 7635–7640. 10.1073/pnas.1705769114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellinger, J. A. , Cristescu, B. , Ewanyk, J. , Gammons, D. J. , Garcelon, D. , Johnston, P. , Martins, Q. , Thompson, C. , Vickers, T. W. , Wilmers, C. C. , Wittmer, H. U. , & Torres, S. G. (2020). Using mountain lion habitat selection in management. Journal of Wildlife Management, 84(2), 359–371. 10.1002/jwmg.21798 [DOI] [Google Scholar]
- Di Marco, M. , Watson, J. E. M. , Possingham, H. P. , & Venter, O. (2017). Limitations and trade‐offs in the use of species distribution maps for protected area planning. Journal of Applied Ecology, 54(2), 402–411. 10.1111/1365-2664.12771 [DOI] [Google Scholar]
- Doligez, B. , Boulinier, T. , & Fath, D. (2008). Habitat selection and habitat suitability preferences. Encyclopedia of Ecology, 5, 1810–1830. 10.1016/B978-008045405-4.00015-X [DOI] [Google Scholar]
- dos Santos, J. W. , Correia, R. A. , Malhado, A. C. M. , Campos‐Silva, J. V. , Teles, D. , Jepson, P. , & Ladle, R. J. (2020). Drivers of taxonomic bias in conservation research: A global analysis of terrestrial mammals. Animal Conservation, 23(6), 679–688. 10.1111/acv.12586 [DOI] [Google Scholar]
- Faurby, S. , Davis, M. , Pedersen, R. O. , Schowanek, S. D. , Antonelli, A. , & Svenning, J. C. (2018). PHYLACINE 1.2: The phylogenetic atlas of mammal macroecology. Ecology, 99(11), 2626. 10.1002/ecy.2443 [DOI] [PubMed] [Google Scholar]
- Faurby, S. , Pedersen, R. O. , Davis, M. , Schowanek, S. D. , Jarvie, S. , Antonelli, A. , & Svenning, J. C. (2020). PHYLACINE 1.2.1: An update to the phylogenetic atlas of mammal macroecology. https://doi.org/105281/zenodo.3690867 [Google Scholar]
- Ficetola, G. F. , Rondinini, C. , Bonardi, A. , Baisero, D. , & Padoa‐Schioppa, E. (2015). Habitat availability for amphibians and extinction threat: A global analysis. Diversity and Distributions, 21(3), 302–311. 10.1111/ddi.12296 [DOI] [Google Scholar]
- Gese, E. M. , Terletzky, P. A. , Erb, J. D. , Fuller, K. C. , Grabarkewitz, J. P. , Hart, J. P. , Humpal, C. , Sampson, B. A. , & Young, J. K. (2019). Injury scores and spatial responses of wolves following capture: Cable restraints versus foothold traps. Wildlife Society Bulletin, 43(1), 42–52. 10.1002/wsb.954 [DOI] [Google Scholar]
- Holbrook, J. D. , Olson, L. E. , DeCesare, N. J. , Hebblewhite, M. , Squires, J. R. , & Steenweg, R. (2019). Functional responses in habitat selection: Clarifying hypotheses and interpretations. Ecological Applications, 29(3), 15. 10.1002/eap.1852 [DOI] [PubMed] [Google Scholar]
- IUCN . (2020). Habitats classification scheme (Version 3.1). https://www.iucnredlist.org/resources/habitat‐classification‐scheme [Google Scholar]
- IUCN . (2021). The IUCN red list of threatened species (Version 2021‐3). [Google Scholar]
- Johnson, C. J. , & Gillingham, M. P. (2004). Mapping uncertainty: Sensitivity of wildlife habitat ratings to expert opinion. Journal of Applied Ecology, 41(6), 1032–1041. 10.1111/j.0021-8901.2004.00975.x [DOI] [Google Scholar]
- Johnson, D. H. (1980). The comparison of usage and availability measurements for evaluating resource preference. Ecology, 61(1), 65–71. 10.2307/1937156 [DOI] [Google Scholar]
- Jones, P. F. , Jakes, A. F. , Telander, A. C. , Sawyer, H. , Martin, B. H. , & Hebblewhite, M. (2019). Fences reduce habitat for a partially migratory ungulate in the northern sagebrush steppe. Ecosphere, 10(7), 25. 10.1002/ecs2.2782 [DOI] [Google Scholar]
- Jung, M. , Dahal, P. R. , Butchart, S. H. M. , Donald, P. F. , De Lamo, X. , Lesiv, M. , Kapos, V. , Rondinini, C. , & Visconti, P. (2020). A global map of terrestrial habitat types. Scientific Data, 7(1), 8. 10.1038/s41597-020-00599-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerley, G. I. H. , te Beest, M. , Cromsigt, J. , Pauly, D. , & Shultz, S. (2020). The protected area paradox and refugee species: The giant panda and baselines shifted towards conserving species in marginal habitats. Conservation Science and Practice, 2(6), 6. 10.1111/csp2.203 [DOI] [Google Scholar]
- Leclerc, M. , Vander Wal, E. , Zedrosser, A. , Swenson, J. E. , & Kindberg, J. (2016). Quantifying consistent individual differences in habitat selection. Oecologia, 180, 697–705. 10.1007/s00442-015-3500-6 [DOI] [PubMed] [Google Scholar]
- Lele, S. R. , Merrill, E. H. , Keim, J. , & Boyce, M. S. (2013). Selection, use, choice and occupancy: Clarifying concepts in resource selection studies. Journal of Animal Ecology, 82(6), 1183–1191. 10.1111/1365-2656.12141 [DOI] [PubMed] [Google Scholar]
- Lesmerises, R. , & St‐Laurent, M. ‐H. (2017). Not accounting for interindividual variability can mask habitat selection patterns: a case study on black bears. Oecologia, 185, 415–425. 10.1007/s00442-017-3939-8 [DOI] [PubMed] [Google Scholar]
- Manly, B. F. J. , McDonald, L. L. , Thomas, D. L. , McDonald, T. L. , & Erickson, W. P. (2002). Resource selection by animals. Statistical design and analysis for field studies (2nd ed.). Kluwer Academic Publishers. [Google Scholar]
- Mayer, M. , Ullmann, W. , Heinrich, R. , Fischer, C. , Blaum, N. , & Sunde, P. (2019). Seasonal effects of habitat structure and weather on the habitat selection and home range size of a mammal in agricultural landscapes. Landscape Ecology, 34(10), 2279–2294. 10.1007/s10980-019-00878-9 [DOI] [Google Scholar]
- McLoughlin, P. D. , Morris, D. W. , Fortin, D. , Vander Wal, E. , & Contasti, A. L. (2010). Considering ecological dynamics in resource selection functions. Journal of Animal Ecology, 79(1), 4–12. 10.1111/j.1365-2656.2009.01613.x [DOI] [PubMed] [Google Scholar]
- Mohr, C. O. (1947). Table of equivalent populations of north American small mammals. American Midland Naturalist, 37(1), 223–249. 10.2307/2421652 [DOI] [Google Scholar]
- Montgomery, R. A. , Redilla, K. M. , Ortiz‐Calo, W. , Smith, T. , Keller, B. , & Millspaugh, J. J. (2018). Evaluating the individuality of animal‐habitat relationships. Ecology and Evolution, 8(22), 10893–10901. 10.1002/ece3.4554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morellet, N. , Verheyden, H. , Angibault, J. M. , Cargnelutti, B. , Lourtet, B. , & Hewison, M. A. J. (2009). The effect of capture on ranging behaviour and activity of the European roe deer Capreolus capreolus . Wildlife Biology, 15(3), 278–287. 10.2981/08-084 [DOI] [Google Scholar]
- Mysterud, A. , & Ims, R. A. (1998). Functional responses in habitat use: Availability influences relative use in trade‐off situations. Ecology, 79(4), 1435–1441. 10.2307/176754 [DOI] [Google Scholar]
- O'Connor, D. , Stacy‐Dawes, J. , Muneza, A. , Fennessy, J. , Gobush, K. , Chase, M. J. , Brown, M. B. , Bracis, C. , Elkan, P. , Zaberirou, A. R. M. , Rabeil, T. , Rubenstein, D. , Becker, M. S. , Phillips, S. , Stabach, J. A. , Leimgruber, P. , Glikman, J. A. , Ruppert, K. , Masiaine, S. , & Mueller, T. (2019). Updated geographic range maps for giraffe, Giraffa spp., throughout sub‐Saharan Africa, and implications of changing distributions for conservation. Mammal Review, 49(4), 285–299. 10.1111/mam.12165 [DOI] [Google Scholar]
- Peterson, A. T. , Navarro‐Siguenza, A. G. , & Gordillo, A. (2018). Assumption‐versus data‐based approaches to summarizing species' ranges. Conservation Biology, 32(3), 568–575. 10.1111/cobi.12801 [DOI] [PubMed] [Google Scholar]
- Powers, R. P. , & Jetz, W. (2019). Global habitat loss and extinction risk of terrestrial vertebrates under future land‐use‐change scenarios. Nature Climate Change, 9(4), 323–329. 10.1038/s41558-019-0406-z [DOI] [Google Scholar]
- Rivrud, I. M. , Meisingset, E. L. , Loe, L. E. , & Mysterud, A. (2019). Future suitability of habitat in a migratory ungulate under climate change. Proceedings of the Royal Society B‐Biological Sciences, 286(1899), 10. 10.1098/rspb.2019.0442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues, A. S. L. , Pilgrim, J. D. , Lamoreux, J. F. , Hoffmann, M. , & Brooks, T. M. (2006). The value of the IUCN red list for conservation. Trends in Ecology & Evolution, 21(2), 71–76. 10.1016/j.tree.2005.10.010 [DOI] [PubMed] [Google Scholar]
- Roever, C. L. , van Aarde, R. J. , & Leggett, K. (2012). Functional responses in the habitat selection of a generalist mega‐herbivore, the African savannah elephant. Ecography, 35(11), 972–982. 10.1111/j.1600-0587.2012.07359.x [DOI] [Google Scholar]
- Rondinini, C. , Di Marco, M. , Chiozza, F. , Santulli, G. , Baisero, D. , Visconti, P. , Hoffmann, M. , Schipper, J. , Stuart, S. N. , Tognelli, M. F. , Amori, G. , Falcucci, A. , Maiorano, L. , & Boitani, L. (2011). Global habitat suitability models of terrestrial mammals. Philosophical Transactions of the Royal Society B‐Biological Sciences, 366(1578), 2633–2641. 10.1098/rstb.2011.0113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini, L. , Butchart, S. H. , Rondinini, C. , Benítez‐López, A. , Hilbers, J. P. , Schipper, A. M. , Cengic, M. , Tobias, J. A. , & Huijbregts, M. A. (2019). Applying habitat and population‐density models to land‐cover time series to inform IUCN red list assessments. Conservation Biology, 33(5), 1084–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCBD . (2011). Strategic plan for biodiversity 2011–2020 and the Aichi targets (Secretariat of the Convention on Biological Diversity). Secretariat of the Convention on Biological Diversity (SCBD). https://www.cbd.int/sp/ [Google Scholar]
- SCBD . (2020). Update of the zero draft of the post‐2020 global biodiversity framework. Secretariat of the Convention on Biological Diversity (SCBD). https://www.cbd.int/conferences/post2020/post2020‐prep‐01/documents [Google Scholar]
- Schipper, J. , Chanson, J. S. , Chiozza, F. , Cox, N. A. , Hoffmann, M. , Katariya, V. , Lamoreux, J. , Rodrigues, A. S. L. , Stuart, S. N. , Temple, H. J. , Baillie, J. , Boitani, L. , Lacher, T. E., Jr. , Mittermeier, R. A. , Smith, A. T. , Absolon, D. , Aguiar, J. M. , Amori, G. , Bakkour, N. , … Young, B. E. (2008). The status of the world's land and marine mammals: Diversity, threat, and knowledge. Science, 322(5899), 225–230. 10.1126/science.1165115 [DOI] [PubMed] [Google Scholar]
- Smithson, M. , & Verkuilen, J. (2006). A better lemon squeezer? Maximum‐likelihood regression with beta‐distributed dependent variables. Psychological Methods, 11(1), 54–71. 10.1037/1082-989x.11.1.54 [DOI] [PubMed] [Google Scholar]
- Stamps, J. (2008). Habitat. Encyclopedia of Ecology, 5, 1807–1810. 10.1016/B978-008045405-4.00502-4 [DOI] [Google Scholar]
- van Beest, F. M. , McLoughlin, P. D. , Mysterud, A. , & Brook, R. K. (2016). Functional responses in habitat selection are density dependent in a large herbivore. Ecography, 39(6), 515–523. 10.1111/ecog.01339 [DOI] [Google Scholar]
- van Beest, F. M. , Uzal, A. , Vander Wal, E. , Laforge, M. P. , Contasti, A. L. , Colville, D. , & McLoughlin, P. D. (2014). Increasing density leads to generalization in both coarse‐grained habitat selection and fine‐grained resource selection in a large mammal. Journal of Animal Ecology, 83(1), 147–156. 10.1111/1365-2656.12115 [DOI] [PubMed] [Google Scholar]
- Van Moorter, B. , Visscher, D. , Herfindal, I. , Basille, M. , & Mysterud, A. (2013). Inferring behavioural mechanisms in habitat selection studies getting the null‐hypothesis right for functional and familiarity responses. Ecography, 36(3), 323–330. 10.1111/j.1600-0587.2012.07291.x [DOI] [Google Scholar]
- Wilman, H. , Belmaker, J. , Simpson, J. , de la Rosa, C. , Rivadeneira, M. M. , & Jetz, W. (2014). EltonTraits 1.0: Species‐level foraging attributes of the world's birds and mammals: Ecological archives E095‐178. Ecology, 95(7), 2027. [Google Scholar]
- Zuur, A. F. , Ieno, E. N. , & Elphick, C. S. (2010). A protocol for data exploration to avoid common statistical problems. Methods in Ecology and Evolution, 1(1), 3–14. 10.1111/j.2041-210X.2009.00001.x [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Appendix S2
Appendix S3
Appendix S4
Appendix S5
Appendix S6
Appendix S7
Appendix S8
Appendix S9
Data Availability Statement
Data is published on DANS (https://doi.org/10.17026/dans‐xq3‐6muu). These data contain the following information for each individual of each species: individual ID, population ID, species ID, proportional use of each habitat type, available proportions of each habitat type, selection ratios of each habitat type. Furthermore, the GPS‐tracking data for some individuals are published on Movebank or are published in Eurommammals (euromammals.org) (see Appendix S4). In Supporting Information Appendix S4 we indicate the Movebank details for the individuals that have their GPS‐tracking data published on Movebank. Data from Euromammals can be retrieved by logging into their website or via a contact form.


