SUMMARY
B vitamins are a group of water‐soluble micronutrients that are required in all life forms. With the lack of biosynthetic pathways, humans depend on dietary uptake of these compounds, either directly or indirectly, from plant sources. B vitamins are frequently given little consideration beyond their role as enzyme accessory factors and are assumed not to limit metabolism. However, it should be recognized that each individual B vitamin is a family of compounds (vitamers), the regulation of which has dedicated pathways. Moreover, it is becoming increasingly evident that individual family members have physiological relevance and should not be sidelined. Here, we elaborate on the known forms of vitamins B1, B6 and B9, their distinct functions and importance to metabolism, in both human and plant health, and highlight the relevance of vitamer homeostasis. Research on B vitamin metabolism over the past several years indicates that not only the total level of vitamins but also the oft‐neglected homeostasis of the various vitamers of each B vitamin is essential to human and plant health. We briefly discuss the potential of plant biology studies in supporting human health regarding these B vitamins as essential micronutrients. Based on the findings of the past few years we conclude that research should focus on the significance of vitamer homeostasis – at the organ, tissue and subcellular levels – which could improve the health of not only humans but also plants, benefiting from cross‐disciplinary approaches and novel technologies.
Keywords: coenzymes, B vitamins, plant health, human health, vitamer, homeostasis
Significance Statement
We provide an up‐to‐date review on the metabolism of selected B vitamins in humans and plants in the context of the health of both. We emphasize the significance of vitamer homeostasis, which could improve the health of not only humans but also plants, benefiting from cross‐disciplinary approaches and novel technologies.
INTRODUCTION
Plants, as outstanding natural chemists, offer a vast set of bioactive chemicals that fulfil their own purposes for survival. Human health has benefitted from many of these resources, and discoveries in plants continue to provide the potential to prevent and treat human diseases. Among the repository of plant chemicals are the micronutrients comprising mineral elements and vitamins that are essential in the human diet, and for which plants serve as a major source (Ross et al., 2012). By now, it is well recognized that limited access to nutritious plant resources or equivalent food products compromise human health (Borelli et al., 2020). Moreover, plants themselves require access to adequate resources for healthy growth and survival to facilitate and optimize their ‘in‐house’ chemical pantry, including the autotrophic synthesis of vitamin compounds that we focus upon here.
By definition, vitamins are essential organic compounds, required in small quantities, that humans cannot biosynthesize de novo. Although plants also require vitamin compounds for growth and development, they can biosynthesize these molecules de novo (and so these are not technically vitamins for plants) and serve as a dietary source for humans, either directly or indirectly (through animals that feed on plants) (Fitzpatrick et al., 2012). Based on solubility, vitamins are classified as either fat‐soluble (A, D, E and K) or water‐soluble (B complex and C). In humans, it is well established that vitamin deficiencies result in serious health issues globally, particularly among vulnerable groups such as pregnant females and children (Black et al., 2013; Ruel‐Bergeron et al., 2015; Tulchinsky, 2010). Insufficient vitamin intake (as well as any of the essential mineral elements) is referred to as hidden hunger because it often goes unnoticed and symptoms may not appear until there is a severe deficiency (Black et al., 2013). According to recent reports at least two billion people are estimated to suffer from hidden hunger, representing over a quarter of the global population (Bailey et al., 2015). Among the vitamins, deficiencies related to vitamin A and B9 (folates) are some of the most widespread, but those of other B vitamins (such as B1 (thiamine) and B6 (pyridoxine)) also frequently occur (Bailey et al., 2015; Strobbe & Van Der Straeten, 2018). Furthermore, deficiencies of multiple micronutrients often transpire concurrently because of the lack of diversity in food sources and foods with low nutrient levels (Nair et al., 2016). The damage can have lifelong consequences on human health. Even mild to moderate deficiencies can lead to physical and cognitive defects in infants, children and adults (Nair et al., 2016; Plecko et al., 2017). Other health issues attributed specifically to deficiencies of B vitamins are related to brain development, kidney diseases, cancers, mental health and cognitive function (Parletta et al., 2013).
In this review, given the breadth of the literature on vitamins, we restrict our focus to three of the B vitamins (B1, B6 and B9). Over the past years our increasing knowledge on the metabolism of these B vitamins has been paving the way for strategies to improve their levels in staple crop species, aiming towards alleviating deficiencies. Although these studies have shown potential in fighting against deficiencies in these vitamins, they also raise new challenges. In some cases, the engineering of certain genes did not increase levels as expected, raising the possibility of interplay among certain regulatory pathways that is yet to be investigated. Further, as B vitamins in general play a pivotal role in plant metabolism, it is essential to monitor the response to changes in their levels, keeping the overall health of the plants in the foreground (Titcomb & Tanumihardjo, 2019). Of considerable significance is the emerging evidence indicating that balance within these vitamin families is essential for proper growth and development (Colinas et al., 2016; Fitzpatrick & Chapman, 2020; Gonzalez & Vera, 2019; Gorelova et al., 2022; Gorelova et al., 2017a; Hanson et al., 2016). As a consequence, vitamin homeostasis is likely to affect yield and agricultural productivity and, in turn, human nutrition and health (Box 1). To complement previous reviews on vitamin metabolism, here we consolidate the work on both plants and humans, as well as knowledge on coenzyme versus non‐coenzyme, and provide our opinion on the importance of considering vitamer homeostasis.
Box 1: Summary.
Coenzymes derived from B vitamins are essential for both human and plant health.
Each individual B vitamin is a family of compounds from which the coenzyme form can be derived.
Individual members of the B vitamin family (with B1, B6 and B9 focused upon here) known as vitamers can have distinct functions.
Balance between the individual vitamers (vitamer homeostasis) is important for both human and plant health.
Vitamer homeostasis should be taken into account in the alleviation of hidden hunger.
THE VITAMIN B1 FAMILY
Vitamin B1 metabolism in plants and humans
Vitamin B1 is a family of water‐soluble sulfur‐containing compounds. The basic unit of vitamin B1 consists of a pyrimidine and thiazole heterocycle, bridged by a methylene group and is referred to as thiamine (Figure 1a). The thiamine unit can carry between one and three phosphate moieties, thiamine monophosphate (TMP), thiamine diphosphate (TDP) or thiamine triphosphate (TTP), respectively, which can in turn be adenosylated. However, only adenosine TDP (ATDP) and adenosine TTP (ATTP) have been reported (Bettendorff, 2021).
Figure 1.
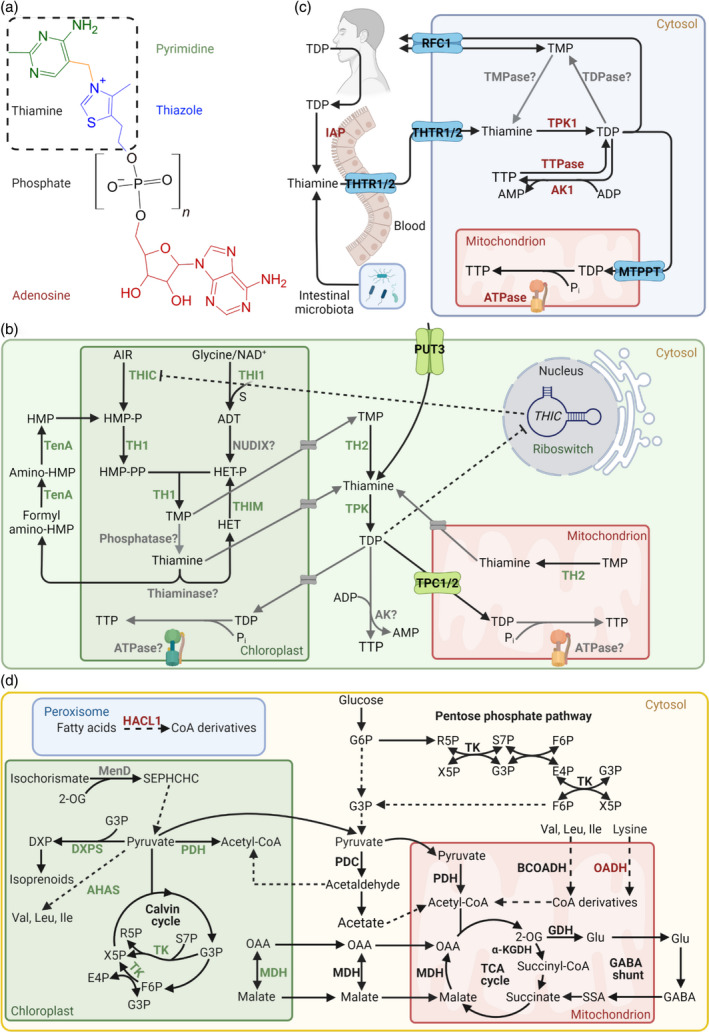
Vitamin B1 metabolism in plants and humans. (a) Generalized chemical structure of vitamin B1. The basic unit thiamine (hashed box) consists of a pyrimidine (green) and thiazole (blue) heterocycle, bridged by a methylene group (yellow). Thiamine derivatives vary in phosphorylation states (black, n = 1–3), as thiamine monophosphate (TMP), thiamine diphosphate (TDP), thiamine triphosphate (TTP), and in adenosylation states (red), as adenosine TDP (ATDP) and adenosine TTP (ATTP). (b) Vitamin B1 metabolism in plants. Biosynthesis de novo takes place in the chloroplast, where each heterocycle is separately synthesized. 5‐Aminoimidazole ribonucleotide (AIR) is used by THIC to generate 4‐amino‐5‐hydroxymethyl‐2‐methylpyrimidine phosphate (HMP‐P) and is further phosphorylated by TH1 to generate 4‐amino‐5‐hydroxymethyl‐2‐methylpyrimidine pyrophosphate (HMP‐PP). Glycine, nicotinamide adenine dinucleotide (NAD+) and a sulfur from a cysteine residue in the THI1 backbone is used to generate the adenylated thiazole intermediate (ADT) and is hydrolyzed into 4‐methyl‐5‐(2‐hydroxyethyl)thiazole phosphate HET‐P by a nucleoside diphosphate hydrolase (NUDIX, gray), but remains to be characterized. The two heterocycles are condensed by TH1 into TMP, which is dephosphorylated to thiamine by a phosphatase, although it is not clear if this can take place in the chloroplast. In any case, TH2 in the cytosol and mitochondrion can dephosphorylate TMP to thiamine, which is then pyrophosphorylated to the coenzyme form TDP by thiamine pyrophosphokinase (TPK) in the cytosol. Cytosolic TDP must be transported back into the chloroplast and mitochondrion for use in enzyme reactions (d). Although most vitamin B1 transporters remain to be identified (putative transporters depicted in gray), polyamine uptake transporter 3 (PUT3, green) facilitates thiamine transport through the plasma membrane and the thiamine phosphate carriers (TPC1/2, green) facilitate TDP transport into the mitochondrion. The biosynthesis of TTP in plants remains to be fully elucidated but may be derived from TDP by adenylate kinase (AK) in the cytosol or through ATP synthase (ATPase) in the chloroplast and mitochondrion. Regulation of TDP biosynthesis occurs through TDP binding to the THIC riboswitch in the nucleus, which in turn downregulates THIC expression (dashed lines). Thiamine can also be catabolized to derivatives of its respective heterocycles by thiaminase (and needs further investigation in plants), which can be recycled through TenA and THIM. In the pyrimidine branch, TenA can hydrolyze both formylamino‐HMP and amino‐HMP into HMP. In the thiazole branch, THIM has kinase activity and phosphorylates HET into HET‐P. (c) Metabolism of vitamin B1 in humans. Humans depend on dietary uptake and absorption of vitamin B1, the most abundant form of which is TDP and is converted into thiamine by the intestinal alkaline phosphatase (IAP) for uptake by thiamine transporter 1/2 (THTR1/2). Intestinal microbiota can also produce thiamine for human usage. THTR1/2 facilitate cellular thiamine uptake, which is pyrophosphorylated in the cytosol to TDP by TPK1. The reduced folate carrier 1 (RFC1) can facilitate the transport of TDP and TMP across the plasma membrane and the mitochondrial thiamine pyrophosphate transporter (MTPPT) can transport TDP into the mitochondrion. The dephosphorylation of TDP by a thiamine pyrophosphatase (TDPase) and TMP by a thiamine monophosphatase (TMPase) occurs inside the cell, but the precise genes are not known. TTP biosynthesis is well characterized in humans and is postulated to occur through adenylate kinase 1 (AK1) in the cytosol using TDP and adenosine diphosphate (ADP) as substrates, and through the mitochondrial ATPase using TDP and inorganic phosphate (Pi) as substrates. Cytosolic thiamine triphosphatase (TTPase) is responsible for TTP hydrolysis to TDP. (d) An overview of enzymatic reactions that either use TDP as a coenzyme or are allosterically regulated by B1 vitamers. Enzymes specific for plants and humans are highlighted in green and red, respectively, and those common to both are presented in black. All enzymes shown here (bold) are TDP‐dependent except GDH (allosterically regulated by TTP and ATTP) and MDH (allosterically regulated by TDP and thiamine). MenD (gray) has a TDP‐dependent feature in microorganisms and its plant homolog has a TDP‐binding domain but has not undergone investigation. Abbreviations: Acetyl‐CoA, acetyl coenzyme A; AHAS, acetohydroxyacid synthase; BCOADH, branched chain oxy‐acid dehydrogenase; DXP, 1‐deoxy‐d‐xylulose‐5‐phosphate; DXPS, DXP synthase; E4P, erythrose‐4‐phosphate; F6P, fructose‐6‐phosphate; GABA, γ‐amino‐butyric acid; Glu, glutamate; G3P, glyceraldehyde 3‐phosphate; G6P, glucose 6‐phosphate; HACL1, 2‐hydroxyacyl‐CoA lyase 1; α‐KGDH, α‐ketoglutarate dehydrogenase; MDH, malate dehydrogenase; MenD, SEPHCHC synthase; OAA, oxaloacetate; OADH, 2‐oxoadipate dehydrogenase; 2‐OG, α‐ketoglutarate or 2‐oxoglutarate; PDC, pyruvate decarboxylase; PDH, pyruvate dehydrogenase; R5P, ribose 5‐phosphate; SEPHCHC, 5‐enolpyruvoyl‐6‐hydroxy‐2‐succinyl‐cyclohex‐3‐ene‐1‐carboxylate; S7P, sedoheptulose‐7‐phosphate; SSA, succinic semialdehyde; TK, transketolase; X5P, xylulose‐5‐phosphate. [Colour figure can be viewed at wileyonlinelibrary.com]
The most renowned and frequently the most abundant member of the vitamin B1 family is TDP, the coenzyme form. In contrast to humans, biosynthesis de novo of TDP can take place in plants and we refer the reader to recent detailed descriptions of this pathway (Fitzpatrick & Chapman, 2020; Strobbe & Van Der Straeten, 2018). Briefly, based on findings in Arabidopsis, the pyrimidine moiety 4‐amino‐5‐hydroxymethyl‐2‐methylpyrimidine pyrophosphate (HMP‐PP) and the thiazole moiety 4‐methyl‐5‐(2‐hydroxyethyl)thiazole phosphate (HET‐P) are separately synthesized in the chloroplast, involving THIC and THI1, respectively (Figure 1b). The two heterocycles are then condensed into TMP by TH1, also in the chloroplast, after which TMP is dephosphorylated into thiamine by phosphatases, although the precise cellular location(s) are not clear (Figure 1b). Recently, TH2 was shown to specifically catalyze this reaction and is reported to be found in mitochondria and the cytosol (Mimura et al., 2016) (Figure 1b). Plastid TMP phosphatases might also exist, but they have yet to be validated in vivo (Hasnain et al., 2016; Tannert et al., 2021). Pyrophosphorylation is catalyzed by two functionally redundant thiamine pyrophosphokinases (TPKs) that are localized to the cytosol in Arabidopsis (Ajjawi et al., 2007) (Figure 1b). However, the requirement for TDP, a polar molecule, by enzymes localized to the cellular organelles implicates facilitated transport processes. Indeed, cellular transporters for TDP, thiamine and intermediate products have been found in the past decade (Beaudoin et al., 2018; Frelin et al., 2012; Guan et al., 2014; Martinis et al., 2016; Noordally et al., 2020) (Figure 1b).
Humans mainly rely on the dietary uptake of vitamin B1 (1.1–1.5 mg/day for a typical adult) to satisfy the need for TDP (Dhir et al., 2019; Fitzpatrick et al., 2012; Martin et al., 2003). Although TDP itself can be a major form of vitamin B1 in the diet, only dephosphorylated thiamine is taken up through the intestine (Figure 1c). This means that dietary TDP needs to be hydrolyzed into thiamine (by intestinal alkaline phosphatases) before absorption and transportation (Dhir et al., 2019; Schaller & Holler, 1975) (Figure 1c). Transport is facilitated in the jejunum and ileum by two thiamine transporters, THTR1 and THTR2, which are also responsible for thiamine uptake from the blood (Rindi & Laforenza, 2000) (Figure 1c). TPK1 converts thiamine back into TDP in the cytosol, which in turn can be dephosphorylated into TMP by acid phosphatases (Zylka et al., 2008) (Figure 1c). Several studies have shown that intestinal microbiota can also produce thiamine that can be used by humans (Said, 2011; Said, 2013).
Although thiamine and TMP are generated as intermediates along the route to TDP, the biosynthesis of the triphosphorylated and adenosylated forms of vitamin B1 are less well established. In the case of TTP, two separate biosynthetic pathways have been proposed in bacteria and mammals: one catalyzed by adenylate kinase 1 (AK1) in the cytosol (Shikata et al., 1989) and the other by mitochondrial ATP synthase (Gangolf et al., 2010) (Figure 1c). It is noteworthy that AK1 displays relatively low activity in the cytosol, as mice with the corresponding gene knocked out maintain normal TTP levels (Gangolf et al., 2010). This also suggests that the cytosolic AK1 is not the major contributor for TTP biosynthesis or that mitochondrial TTP biosynthesis can compensate for a lack of TTP in the cytosol. In plants, TTP is thought to accumulate in both plastids and mitochondria, but the biosynthesis route remains to be elucidated (Hofmann et al., 2020). Although the vitamer form ATTP has been reported to exist across life domains, including plants (Bettendorff et al., 2007), a recent study could not reproduce the presence of ATTP in plants yet (Hofmann et al., 2020). Furthermore, the enzymes involved in the biosynthesis of ATTP (or ATDP) have not yet been identified in any organism.
Catabolic pathways of vitamin B1 exist, with one of the best‐characterized enzymes being thiaminase (Figure 1b). Thiaminase cleaves thiamine into its thiazole and pyrimidine moieties, and although not present in humans it is abundant in fish and certain plants, which if consumed raw, in large quantities, can lead to vitamin B1 deficiency (Jurgenson et al., 2009). In mammalian cells, the hydrolysis of TTP is also well characterized and is catalyzed by a highly efficient thiamine triphosphatase (Makarchikov et al., 2003) (Figure 1c), although to date there is no report of a corresponding enzyme in plants. Nonetheless, TTP is detectable in plants particularly during light periods and in shoot tissues (Hofmann et al., 2020), but how and when it is catabolized is not yet known. Excepting the work discussed above, the degradation of vitamin B1 has not been extensively studied in plant and human cells.
Pathways that salvage vitamin B1 intermediates have also been characterized. For example, TenA regenerates pyrimidine moiety derivatives resulting from degradation or damage of thiamine itself (Jenkins et al., 2008; Zallot et al., 2014) and THIM contributes to regeneration of the thiazole moiety (Yazdani et al., 2013), both of which can be used to rebuild the thiamine molecule (Figure 1b). Furthermore, oxidative damage of TDP during catalytic cycles can be countered by NUDIX hydrolases (Goyer et al., 2013). These enzymes are important contributors to the vitamin B1 pool but necessitate further study to realize their impact in humans and plants.
Biochemical and physiological roles of vitamin B1
One of the major functions of vitamin B1 is to serve as a coenzyme in its TDP form for energy metabolism in both plants and animals (Figure 1d). For example, enzymes that require TDP include α‐ketoglutarate dehydrogenase (α‐KGDH), within the TCA cycle, as well as pyruvate dehydrogenase and branched‐chain α‐ketoacid dehydrogenase that contribute to acetyl‐CoA provision (Fitzpatrick & Chapman, 2020) (Figure 1d). Further, pyruvate decarboxylase that catalyzes the decarboxylation of pyruvate to acetaldehyde, contributing to acetyl‐CoA biosynthesis, firmly cements the role of TDP in energy metabolism (Joshi et al., 2019) (Figure 1d). In plants, TDP is also required for transketolase, to facilitate photosynthesis and the non‐oxidative phase of the pentose phosphate pathway, as well as for deoxyxylulose 5′‐phosphate (DXP) synthase, to supply the precursor to chlorophyll and several phytohormones (Estévez et al., 2000) (Figure 1d). Beyond the coenzyme role in energy metabolism, additional roles for B1 vitamers have been proposed. In the nervous system of animals, TTP has been implicated in chloride channel regulation, membrane excitability, nerve conduction and neurotransmitter release (Bettendorff, 2021). Furthermore, TTP was also described to phosphorylate rapsyn (a membrane protein that binds acetylcholine receptors); however, the physiological significance of this process remains unknown (Nghiem et al., 2000). In plants, the physiological role of TTP or ATTP is not yet known. Interestingly, though, glutamate dehydrogenase (GDH) has been reported to be allosterically affected by TTP and ATTP in animal cells (Mezhenska et al., 2020; Mkrtchyan et al., 2015) and should also be tested in plants. This observation could suggest the modulation of carbon/nitrogen balance via α‐ketoglutarate (α‐KG), a substrate for carbon metabolism through the TCA cycle and nitrogen metabolism through GDH. In animal cells, malate dehydrogenase involved in the malate–aspartate shuttle, providing malate to the TCA cycle, is reported to be allosterically affected by thiamine and TDP (Mezhenska et al., 2020, Mkrtchyan et al., 2015). In plant cells, B1 vitamers have been suggested to play important roles in responses to biotic and abiotic stress conditions, and to trigger defense responses in plants, but further experimentation is required to describe these processes mechanistically (Fitzpatrick & Chapman, 2020).
The importance of vitamin B1 homeostasis in human and plant health
Several disease states in humans and plants linked to alterations in vitamin B1 can be accounted for by the essential role of TDP as a coenzyme in energy metabolism. This may be direct or indirect, through genetic aberrations in biosynthesis, salvage or degradation pathways, as well as the transport of intermediates or precursors to facilitate the biosynthesis of TDP. For example, mutations in the thiamine transporters THTR1 and THTR2 in humans, necessary for cellular TDP biosynthesis (Figure 1c), cause thiamine‐responsive megaloblastic anaemia (Brown, 2014; Marce‐Grau et al., 2019). A mutation in TPK1 was associated with episodic encephalopathy concomitant with a deficiency of TDP, and at the molecular level manifested in lactic acidosis and enhanced α‐KG excretion (Marce‐Grau et al., 2019). Indeed, a common phenomenon is mitochondrial dysfunction because there is insufficient TDP to fuel the enzymes of energy metabolism that, in turn, can lead to a drop in ATP biosynthesis along with cell damage through oxidative stress (Belsky et al., 2018). Further, the decreased activity of α‐KGDH resulting from a limitation in TDP can lead to increased metabolic flux towards glutamate synthesis, which can cause abnormalities of the glutamatergic and γ‐aminobutyric acid (GABA) systems, and subsequently result in a toxic state of neurotransmission and neuroexcitation (de Freitas‐Silva et al., 2010; Todd & Butterworth, 1999). This state is often linked to age‐related neurodegenerative diseases, including Alzheimer's disease (Gibson et al., 2005), as well as diabetes (Pácal et al., 2014). Recently, vitamin B1 deficiency has also been found in critically ill patients with COVID‐19 and complications like sepsis and encephalopathy, whose survival rates were improved when thiamine adjunctive therapy was administered (Al Sulaiman et al., 2021; Branco de Oliveira et al., 2021). There are no well‐established toxic effects of too much thiamine intake in humans.
In plants, insufficient TDP manifests in mitochondrial as well as plastidial dysfunction, and commonly such plants are chlorotic and severely impaired in growth and fitness (Colinas & Fitzpatrick, 2015). Conversely, the oversupply of TDP impacts plant health as it fuels an increase in respiration rates that depletes the carbohydrate substrate derived from starch too early during dark periods, and the plants suffer starvation before the end of the night (Bocobza et al., 2013). Moreover, the overexpression of enzymes dependent on TDP has the capacity to divest other enzymes dependent on this coenzyme and can severely impact plant growth, as recently shown by the overexpression of plastidic transketolase (Khozaei et al., 2015). Thus, regulation of coenzyme supply is critical as it may dictate enzymatic flux, resulting in the over‐ or under‐activation of a pathway that can lead to negative effects.
Given the disperse cellular pattern of vitamin B1 metabolic components and its essential functions, a complex network of control nodes can be anticipated. In the case of TDP, there are several checkpoints that allow the management of its metabolism. Cellular biosynthesis can be controlled by regulating thiamine entry through transporters (akin to gatekeepers) and by the activity of TPK (Sambon et al., 2022). Equally, the degradation or turnover of TDP may trigger any of the salvage pathways as a function of the availability of their corresponding substrate, which can happen under stress conditions. However, it is imperative that a balance is achieved such that the supply, at least of the coenzyme form, meets cellular demands for the reasons outlined above. In contrast to what has been found in humans, plants harbor a TDP‐sensing mechanism in the nucleus in the form of a riboswitch (Figure 1b). The riboswitch operates an alternative splicing mechanism in the 3′ untranslated region (3′‐UTR) of the biosynthesis de novo gene THIC that alters transcript abundance as a function of TDP levels (Bocobza et al., 2007; Mangel et al., 2017; Wachter et al., 2007). Interestingly, mutants that can no longer sense TDP levels through the riboswitch are chlorotic and cannot acclimate to a change in photoperiod (Bocobza et al., 2013; Rosado‐Souza et al., 2019). The mechanism behind this is not clear but chlorosis has been assigned to an overaccumulation of TDP that leads to an increase in respiration rates and carbon starvation. Further, in plants, several of the genes involved in vitamin B1 metabolism are under control of the circadian clock, thus anticipating the needs of the cell whilst integrating environmental cues (Noordally et al., 2020). Whether the vitamin B1 metabolic pathways are under circadian control in humans has not been investigated. Nonetheless, diel control of TDP supply with demand will also be expected to influence metabolic flux and the control of downstream metabolites. It has recently been postulated that TDP could play a role in the coordination of metabolic processes and, in particular, the allocation of the carbon budget (Joshi et al., 2019). This may have enormous implications for plant and animal fitness and deserves further investigation. Among the other vitamers, the appearance of ATTP and TTP under amino acid starvation and a glucose source, respectively, at least in bacteria (Gigliobianco et al., 2010), provides a link between these non‐coenzyme forms and specific nutritional states, and may be a requirement in vitamer homeostasis. On the other hand, there is evidence that accumulation of vitamin B1 biosynthesis intermediates is also not favorable, and need to be dealt with. For example, although the chlorotic phenotype resulting from the overexpression of THIC is attributed to a TDP level that is too high (Bocobza et al., 2013), more recent work demonstrates that there is an inappropriate build‐up of HMP that might also account for the phenotypic observations (Strobbe et al., 2021b).
THE VITAMIN B6 FAMILY
Vitamin B6 metabolism in plants and humans
The vitamin B6 family consists of six vitamers: pyridoxal (PL), pyridoxamine (PM), pyridoxine (PN) and their phosphorylated derivatives, pyridoxal 5′‐phosphate (PLP), pyridoxamine 5′‐phosphate (PMP) and pyridoxine 5′‐phosphate (PNP) (Figure 2a). Each of these vitamers has a pyridine‐based core that may be phosphorylated at carbon 5 and carries a formyl (PL/PLP), an aminomethyl (PM/PMP) or a hydroxymethyl (PN/PNP) group at carbon 4. Glycosylated derivatives also exist, notably for PN, with the glucose moiety attached to the hydroxmethyl group of carbon 5 (Figure 2a).
Figure 2.
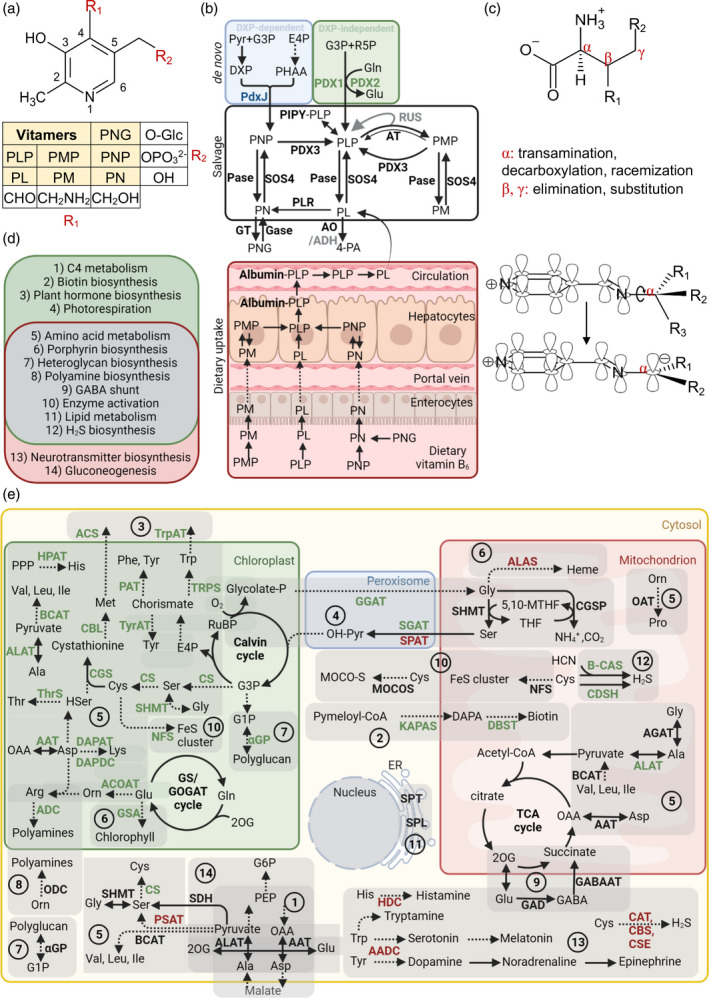
Vitamin B6 metabolism in plants and humans. (a) Generalized chemical structure of vitamin B6. Functional groups at C4 (R1) and C5 (R2) corresponding to the different vitamers are indicated in the box below. Vitamers are pyridoxal (PL), pyridoxamine (PM), pyridoxine (PN) and their phosphorylated derivatives, pyridoxal 5′‐phosphate (PLP), pyridoxamine 5′‐phosphate (PMP), pyridoxine 5′‐phosphate (PNP) and pyridoxine‐5′‐β‐d‐glucoside (PNG). (b) Vitamin B6 can be biosynthesized de novo via two pathways. The DXP‐dependent pathway (blue background) used by certain bacteria generates PNP from deoxyxylulose 5‐phosphate (DXP) and 3‐phosphohydroxy‐1‐aminoacetone (PHAA). DXP is formed from pyruvate and glyceraldehyde 3‐phosphate (G3P), and PHAA is generated through a chain of reactions from erythrose‐4‐phosphate (E4P). In the DXP‐independent pathway (green background), used by plants, PDX1 generates PLP directly from G3P and ribose 5‐phosphate (R5P), with PDX2 providing ammonia from glutamine. The salvage pathway regulates the balance between the different vitamer forms and contributes to vitamin B6 uptake in animals (bottom panel). Phosphatases (Pase) and the pyridoxal kinase (SOS4) are responsible for the interconversion of the phosphorylated and dephosphorylated vitamers, respectively. Pyridoxal reductase (PLR) can reduce the PL pool by converting it to PN. The storage forms of vitamin B6 in plants are glucosylated derivatives of PN, such as PNG, generated by glycosyltransferases (GT), and the remobilization of PNG into PN is catalyzed by glucosidases (Gase). Aminotransferases (AT) are responsible for the generation of PMP from PLP by a mechanism in which PMP dissociates from the enzyme after the first transamination half‐reaction (represented by a thicker arrow). This free PMP can be converted back to PLP by the aminotransferases (represented by a thinner arrow) or by the PMP/PNP oxidase (PDX3). Certain aminotransferases are proposed to regulate free PLP levels by sequestering PLP, which is reversed by interactions with RUS1 and RUS2 (RUS). The PLP homeostasis protein (PIPY) binds PLP covalently and is involved in balancing the different vitamer forms by unknown mechanisms. The degradation of vitamin B6 is catalyzed by aldehyde oxidases (AO) that generate 4‐pyridoxic acid (4‐PA) from PL. NAD‐dependent aldehyde dehydrogenases (ADH) are also implicated in this process. Factors in gray deserve broader investigation. (c) Type of reactions at given positions on an amino acid substrate that are catalyzed by PLP. Dunathan's hypothesis showing a simplified structure of PLP in a Schiff‐base link that enables the deprotonation or decarboxylation of the α‐carbon of the substrate amino acid by providing a delocalization path to the electron of the resulting carbanion is represented below. (d) Physiological roles of PLP as a coenzyme in plants (green) and humans (pink), with overlapping roles listed in the middle (gray). (e) An overview of enzymatic reactions that use PLP as a coenzyme in plants and humans, with their subcellular localization and the physiological processes that they are involved in labeled using the numbering from panel (d) and grouped with a light‐gray shadow. Enzymes specific for plants and humans are highlighted in green and red, respectively, and enzymes common to both are in black. Abbreviations: AADC, aromatic amino acid decarboxylase; AAT, aspartate aminotransferase; ACOAT, acetylornithine aminotransferase; ACS, 1‐aminocyclopropane‐1‐carboxylate (ACC) synthase; ADC, arginine decarboxylase; ALAS, 5‐aminolevulinate synthase; ALAT, alanine aminotransferase; B‐CAS, β‐cyanoalanine synthase; BCAT, branched‐chain amino acid aminotransferase; CAT, cysteine aminotransferase; CBL, cystathionine‐β‐lyase; CBS, cystathionine‐β‐synthase; CDSH, cysteine desulfhydrase; CGS, cystathionine‐γ‐synthase; CS, cysteine synthase; CSE, cystathionine‐γ‐lyase; DAPAT, diaminopimelate aminotransferase; DAPDC, diaminopimelate decarboxylase; DTBS, dethiobiotin synthetase; GABA‐AT, γ‐amino‐butyric acid aminotransferase; GAD, glutamate decarboxylase; GCSP, glycine cleavage system P protein; GGAT, glutamate‐glyoxalate aminotransferase; αGP, α‐glucan phosphorylase; GSA, glutamate‐1‐semialdehyde 2,1‐aminomutase; HDC, histidine decarboxylase; HPAT, histidinolphosphate aminotransferase; KAPAS, 7‐keto‐8‐amino‐pelargonic acid synthase; MOCOS, molybdenum cofactor sulphurase; NFS, cysteine desulphurase; OAT, ornithine aminotransferase; ODC, ornithine decarboxylase; PAT, prephenate aminotransferase; PSAT, phosphoserine aminotransferase; SDH, serine dehydratase; SGAT, serine‐glyoxalate aminotransferase; SHMT, serine hydroxamethyltransferase; SPAT, serine‐pyruvate aminotransferase; SPL, sphingosine‐1‐phosphate lyase; SPT, serine palmitoyltransferase; ThrS, threonine synthase; TrpAT, tryptophan aminotransferase; TRPS, tryptophan synthase; TyrAT, tyrosine aminotransferase. [Colour figure can be viewed at wileyonlinelibrary.com]
Vitamin B6 can be biosynthesized de novo in microbes and plants via one of two reported pathways (Fitzpatrick et al., 2007) (Figure 2b). Escherichia coli and a few other eubacteria (mainly in the γ‐division of proteobacteria) use a pathway involving seven enzymes, during which 3‐phosphohydroxy‐1‐aminoacetone and DXP are generated and used by PNP synthase (PdxJ) to form PNP (Figure 2b). The coenzyme form PLP is then biosynthesized by a PNP oxidase (PdxH, annotated as PDX3 in Arabidopsis) (Figure 2b). This pathway is often referred to as the DXP‐dependent pathway (Tambasco‐Studart et al., 2005). However, there is no report of vitamin B6 biosynthesis via this pathway in plants. Instead, plants use a simpler pathway involving just two enzymes, PDX1 and PDX2, by which PLP is produced from ribose 5‐phosphate, glyceraldehyde 3‐phosphate and glutamine (Tambasco‐Studart et al., 2005) (Figure 2b). This pathway is often referred to as the DXP‐independent pathway. The different vitamers can also be interconverted by pathways that are present in both plants and humans (di Salvo et al., 2011; Fitzpatrick, 2011) (Figure 2b). For example, PL, PM and PN can be phosphorylated by pyridoxal kinase (PLK, annotated as SOS4 in Arabidopsis) to yield PLP, PMP and PNP, which in turn can be dephosphorylated by broad substrate specificity alkaline phosphatases and acid phosphatases (Figure 2b), although a PLP‐specific phosphatase has been described in both humans and plants (Altensell et al., 2022; Fonda, 1992; ShuoHao et al., 2019). Further, PMP and PNP can be converted into PLP by the PNP oxidase PDX3 (PNPO in humans) (di Salvo et al., 2011). In addition, a pyridoxal reductase (PLR) is responsible for the conversion of PL into PN (Herrero et al., 2011; Ramos et al., 2019) (Figure 2b).
As animals lack pathways of vitamin B6 biosynthesis de novo, they must obtain it from their diet. The recommended daily intake of vitamin B6 is 1.3 mg for a typical adult. Food sources can contain all six vitamers (Ollilainen, 1999). In meat, PLP is the primary B6 vitamer form (Albersen et al., 2013), whereas plant‐derived foods contain a variety of vitamin B6‐related compounds, including glycosylated derivatives such as PN‐5′‐β‐d‐glucoside, that can make up to 70% of the total vitamin B6 content (Gregory, 1998). PN‐5'‐β‐D‐glucoside derived from plants is limited by bioavailability (the proportion that enters the bloodstream to have an active effect), because glycosidases are inefficient in cleaving the glucose group that allows uptake in the gut (Gregory, 1998; Gregory & Ink, 1987). Notably, plants can also contain antagonistic forms of PN, such as the antivitamin ginkgotoxin (4‐(methoxymethyl)‐PN), which can inhibit enzymes dependent on PLP as a coenzyme (Kästner et al., 2007). The non‐phosphorylated forms of vitamin B6 can be taken up directly in the jejunum (Stover & Field, 2015; Yaman & Mizrak, 2019) (Figure 2b). However, upon consumption of phosphorylated or glucosylated forms they must first be dephosphorylated or hydrolyzed by intestinal phosphatases and glucosidases, respectively, before uptake (Parra et al., 2018) (Figure 2b). After uptake, the vitamers are phosphorylated in the liver by PLK, and PMP and PNP are subsequently oxidized to PLP by PNPO (Figure 2b). PLP enters the circulation bound to lysine residues of proteins, mostly serum albumin, but needs to dissociate from albumin and be dephosphorylated by an alkaline phosphatase to enter cells (Figure 2b). Once inside cells, the different vitamers can be phosphorylated again by PLK to yield PNP, PMP and PLP, with PNP and PMP being converted into PLP when necessary by PNPO (Merrill & Henderson, 1990) (Figure 2b).
The catabolism of vitamin B6 also takes place, the best‐characterized mechanism of which is degradation to 4‐pyridoxic acid (Figure 2b). 4‐Pyridoxic acid is produced through the oxidation of PL by non‐specific aldehyde oxidases (Schwartz & Kjeldgaard, 1951), although a non‐specific NAD‐dependent aldehyde dehydrogenase has also been proposed to catalyze this reaction (Stanulovic et al., 1976).
Biochemical and physiological roles of vitamin B6
Vitamin B6 in its form as PLP is a versatile coenzyme that catalyzes over 200 documented enzymatic reactions involving decarboxylation, transamination, racemization, and β‐ and γ‐elimination, as well as β‐ and γ‐substitution in most organisms (Percudani & Peracchi, 2003) (Figure 2c). Indeed, the range of PLP‐dependent enzymes covers five of the six classes of enzymes: oxidoreductases, transferases, hydrolases, lyases and isomerases (Fitzpatrick, 2011). In contrast to other coenzymes, the versatility of PLP is the result of its ability to form a covalent bond with the enzyme substrate through a Schiff‐base link, which creates a bridge between the α‐carbon of the substrate and the pyridine ring of PLP that allows the delocalization of the α‐carbon electron, thereby stabilizing different types of reaction intermediates (Figure 2c). Almost 60 years ago, Harmon Dunathan proposed that the bond to be cleaved is held perpendicular to the plane of the PLP‐imine π bond system (parallel with the p orbitals), which weakens the substrate bond to be broken thus acheiving reaction specificity (Dunathan, 1966) (Figure 2c). Thus, to utilize PLP and separate its many functions, enzymes have evolved active sites with binding pockets that tend to favor one specific type of PLP‐catalyzed reaction. Aminotransferases constitute the largest group of PLP‐dependent enzymes, covering almost half of all such annotated activities (Percudani & Peracchi, 2003). Consequently, PLP has a pivotal role in amino acid biosynthesis and catabolic processes remobilizing intermediates from proteins and amino acids in both plants and humans. It should be noted that during transamination reactions (interconversion of amino/keto acids), both PLP and PMP act as coenzymes, although PMP does not form a covalent bond with the enzyme (unlike PLP) and can easily dissociate from it. The released PMP can be converted to PLP by PNPO/PDX3 (Figure 2b). PLP‐dependent enzymes are also required for lipid metabolism (Han et al., 2009; Nishikawa et al., 2008; Van Veldhoven et al., 2000), as well as heteroglycan (e.g. glycogen or starch) metabolism, but as an acid/base catalyst, in both plants and humans (Zeeman et al., 2004; Zhang et al., 2012b) (Figure 2d,e). PLP also assists in the biosynthesis of heme, polyamines, 1‐carbon (1C) units and hydrogen sulfide in both taxa (Fitzpatrick, 2011). Furthermore, PLP also indirectly regulates the activity of certain enzymes as it is required for the generation of iron–sulfur clusters and the sulfuration of the molybdenum cofactor in both plants and humans (Cory et al., 2017; Schwarz et al., 2009) (Figure 2d,e). In plants, PLP is involved in photorespiration, as it is required as a coenzyme for the glycine cleavage system P‐protein, serine hydroxymethyltransferase and aminotransferases involved in the process (Fitzpatrick, 2011) (Figure 2d,e). It is also required for enzymes of the GABA shunt, important in carbon/nitrogen balance (Michaeli & Fromm, 2015) (Figure 2d,e). In contrast to humans, plants possess biosynthetic pathways for all proteinogenic amino acids, which all include steps that require PLP, such as aminotransferases and lyases (Hildebrandt et al., 2015). Certain reactions in the biosynthesis of biotin (vitamin B7) (Patton et al., 1996), chlorophyll and the plant hormones ethylene and auxin, and in the detoxification of cyanide, also require PLP as a coenzyme (Ilag et al., 1994; Liang et al., 1992; Stepanova et al., 2008) (Figure 2d,e). Aside from amino/keto acid metabolism, a main physiological role of PLP‐dependent enzymes in humans is the biosynthesis and regulation of neurotransmitters. Production of GABA, histamine, serotonin, melatonin, dopamine, epinephrine, norepinephrine and tryptamine all require PLP‐dependent enzymes (Hellmann & Mooney, 2010). Also, aminotransferases regulate glutamate levels in the brain, and along with serine dehydratase, catalyze reactions of gluconeogenesis in humans (Cooper & Jeitner, 2016) (Figure 2d,e).
Several of the B6 vitamers have been reported to have potent antioxidant activity. In humans, PM, PN and PL were shown to be involved in responses to radical‐mediated oxidative damage and UV‐A‐mediated damage (Endo et al., 2007; Jain & Lim, 2001). In plants, PL, PN and PM have also been shown to have antioxidant activity, quenching reactive oxygen species (ROS) such as superoxide and singlet oxygen (Bilski et al., 2000; Danon et al., 2005; Ehrenshaft et al., 1999; Titiz et al., 2006). Furthermore, PN was shown to mitigate ROS production upon infection with Pseudomonas syringae, raising the possibility of a role in modulating redox status during pathogen defense responses (Denslow et al., 2005). Vitamin B6 has also been implicated in responses and acclimation to UV‐B (Czegeny et al., 2019). The precise antioxidant mechanism of vitamin B6 has not yet been confirmed in vivo, and whether its role is direct, and thereby scavenges radicals, or whether it plays an indirect antioxidant role by serving as a coenzyme in antioxidant defense systems, e.g. glutathione biosynthesis, is not yet established. Nonetheless, these properties have implicated vitamin B6 as stress protectants in both humans and plants (Vanderschuren et al., 2013).
The importance of vitamin B6 homeostasis in human and plant health
Several disease states are associated with a deficiency of vitamin B6, which can develop from suboptimal vitamin content in dietary foodstuffs or through an insufficient uptake capacity that can result from underlying diseases, such as coeliac disease or genetic disorders of enzymes responsible for vitamin B6 metabolism. Symptoms of vitamin B6 deficiency vary and include depression, confusion, glossitis, microcytic anaemia and dermatitis, affecting mental health and cognitive abilities (Stach et al., 2021). Further, vitamin B6 deficiency causes elevated glucose levels, which has been linked to the accumulation of advanced glycation end products and chromosome aberrations, providing a link between type‐II diabetes and cancer (Marzio et al., 2014; Merigliano et al., 2018). Severe deficiency in infancy can lead to epileptic seizures, which if not treated can result in death (Baumgart et al., 2014). Functional vitamin B6 deficiency in plants has been described, making them susceptible to salt and high‐light stress, concomitant with a reduction in the maximum quantum efficiency of photosystem II photochemistry (F v/F m) (Hanson et al., 2016; Titiz et al., 2006). The reduction in F v/F m was ascribed to the overaccumulation of singlet oxygen causing a decrease in the steady‐state levels of the D1 protein, which could be alleviated by the exogenous application of PN (Titiz et al., 2006). For a long time, one of the leading theories explaining the presence of different vitamers and pathways interconverting them was to provide an insurance of PLP supply for the enzymes that are dependent on it as a coenzyme, which is estimated to cover 4% of all catalytic activities (Percudani & Peracchi, 2003). At the same time, free PLP concentrations need to be kept low, avoiding this reactive aldehyde from forming harmful covalent bonds with primary or secondary amine and thiol groups on off‐target proteins (Hanson et al., 2016). However, with emerging evidence on other vitamers having distinct functions, such as antioxidants, the importance of balancing the different vitamers is becoming clearer. Recently, factors involved in balancing vitamin B6 in plants as well as in humans have been identified. Tong et al. (2021) reported on a mechanism in which ROOT UV‐B SENSITIVE 1 and 2 (RUS1 and RUS2) and certain aminotransferases function together to maintain vitamin B6 homeostasis in Arabidopsis. They identified three aspartate aminotransferases and one alanine aminotransferase that sequester PLP in order to maintain low cellular levels, which is reversed upon interaction with RUS1/2. In several studies, a PLP binding protein called PROSC/PLPBP in humans, PIPY in cyanobacteria and YggS in E. coli has been described as a novel regulator of vitamin B6 homeostasis (Ito et al., 2019; Johnstone et al., 2019; Plecko et al., 2017; Prunetti et al., 2016; Vu et al., 2020). In particular, when mutated a consistent increase in PNP was observed that correlates with alterations in amino and keto acid levels and compromised PLP‐dependent enzyme activity, and leads to vitamin B6‐dependent epilepsy in humans. Functional conservation of the role of PROSC/PLPBP/PIPY/YggS is implicated among different kingdoms, as orthologs from human, Arabidopsis, Bacillus subtilis, Saccharomyces cerevisiae and Zea mays complemented the PN‐sensitive growth phenotype of the yggS mutant of E. coli (Ito et al., 2013; Prunetti et al., 2016). Interestingly, in yggS the elevated levels of PNP inhibited the glycine cleavage system and, similarly, in an experiment in vitro PNP inhibited the activity of an E. coli aspartate aminotransferase (Ito et al., 2019; Vu et al., 2020; Vu & Downs, 2021), suggesting a specific role for PNP as a competitive inhibitor of certain PLP‐dependent enzymes. In this context, it is interesting that symptoms similar to vitamin B6 deficiency in humans – after taking relatively high doses of vitamin supplements – are believed to be the consequence of PN being converted to PNP and inhibiting the activity of the coenzyme form PLP (Vrolijk et al., 2017).
The importance of balancing vitamin B6 levels is highlighted in studies of salvage pathway mutants. Enzyme activity via PNPO/PDX3 is a source of PLP in both humans and plants, whereas its mutation leads to the accumulation of PMP/PNP and a decrease in PLP levels, at least in plants (Colinas et al., 2016). Plants have a severe phenotype upon PDX3 mutation; defective shoot development, decreased nitrate reductase activity and activated defense responses were observed in Arabidopsis (Colinas et al., 2016). In PDX3‐deficient Arabidopsis and zebra fish the accumulation of PMP correlated with increased nitrogen assimilation (Ciapaite et al., 2020; Colinas et al., 2016), which could be explained by increased flux through transamination second half‐reactions; however, the exact mechanism is unclear. In humans, mutations in PNPO and PROSC both lead to decreased PLP levels in the brain, accompanied by vitamin B6‐dependent epilepsy and neonatal epileptic encephalopathy (Barile et al., 2020; Jaeger et al., 2016). However, several disease‐causing variants of PLK, which lead to decreased PLP levels, are described as a source of peripheral neuropathy with normal central nervous system function, and are associated with elevated glucose levels and chromosomal aberrations (Chelban et al., 2019; Marzio et al., 2014). Similarly, in plants, mutations of the PLK homolog SOS4 resulted in decreased PLP levels (Gorelova et al., 2022), although accompanied by phenotypes distinct from that of PDX3. The sos4 mutants displayed impaired root hair formation, increased suberization and lignification in the endodermis, and accumulated transition metals and ROS (Gorelova et al., 2022). These observations imply distinct roles for PDX3 and SOS4. This could be a spatiotemporal division of tasks in providing PLP to enzymes that are dependent on it, and maintaining a balance with the help of factors such as PROSC and RUS1/2 between the different B6 vitamers based on requirements dictated by the environment.
Cellular phosphatases have the ability to convert the phosphorylated forms of vitamin B6 to the corresponding non‐phosphorylated derivatives, maintaining a balance between these forms, but more research is needed to define the spatiotemporal mode of action in line with cellular health status. Recently, Barile et al. (2020) described a negative feedback loop by which PLP allosterically inhibits PdxH in E. coli, which implies that this enzyme might have a role in monitoring free PLP levels, providing a regulatory step in maintaining the concentration below certain toxic levels. Furthermore, to go beyond the coenzyme role, PLP has been described as a modulator of steroid hormone receptor and transcription factor activity (Oka et al., 2001; Tully et al., 1994), and has also been implicated in the protein folding of PLP‐dependent enzymes (Parra et al., 2018), thus acting as a type of chaperone. This latter aspect deserves further study and could be extended to all coenzymes because thus far the folding of proteins as a function of their corresponding coenzymes has received little attention.
THE VITAMIN B9 FAMILY
Vitamin B9 metabolism in plants and humans
Folates, or vitamin B9, is a generic term referring to tetrahydrofolate (THF) and its 1C‐substituted derivatives, comprising a pterin ring, p‐aminobenzoate (p‐ABA) and one or more glutamate moieties (Glu(n)) (Figure 3a). The different folate forms are distinguished by the 1C units carried at the N5 and/or N10 position of the pterin ring, with distinctive oxidation states ranging from fully reduced to oxidized (Figure 3a).
Figure 3.
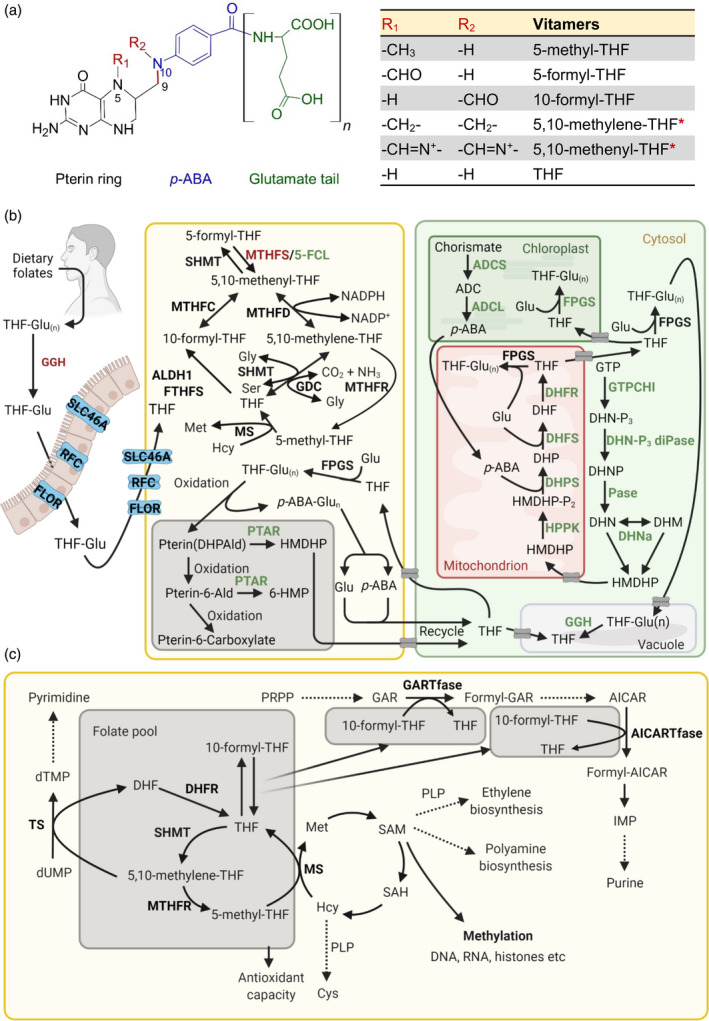
Vitamin B9 metabolism in plants and humans. (a) Generalized chemical structure of the vitamin B9 family, consisting of a pterin ring (black), p‐aminobenzoic acid (p‐ABA, blue) and one or more glutamate residues (glutamate tail, green). Vitamers differ at N5/N10, as indicated by R1 and R2, where 1C units could be attached. Vitamers listed in the table are the major coenzyme forms. The asterisks indicate linkage of the R1 and R2 groups attached at N5/N10. The C9–N10 bond (red) indicates where folate breakdown occurs, yielding pterin and p‐ABA‐glutamate (p‐ABA‐Glu(n)). (b) Biosynthesis, salvage and catabolism of vitamin B9 in plants and humans. Left: humans depend on the dietary uptake and absorption of folates, of which the tetrahydrofolate (THF) polyglutamated forms (THF‐Glu(n)) need to be converted into monoglutamate forms (THF‐Glu) by γ‐glutamyl hydrolase (GGH). Uptake, absorption and transport depend on carriers, as indicated: solute carrier family 46 member 1 (SLC46A1), reduced folate carrier (RFC) and folate receptor (FLOR), before being metabolized. Right panel: folate biosynthesis de novo in plants. pABA is biosynthesized in the plastid from chorismate via aminodeoxychorismate (ADC) by ADC synthase (ADCS) and ADC lyase (ADCL). It is then shuttled into the mitochondria for folate assembly. The pterin moiety 6‐hydroxymethyldihydropterin (HMDHP) is biosynthesized in the cytosol from guanosine triphosphate (GTP) via dihydroneopterin triphosphate (DHN‐P3), DHN phosphate (DHNP) and dihydroneopterin (DHN), catalyzed by GTP cyclohydrolase I (GTPCHI), DHN triphosphate diphosphatase (DHN‐P3 diPase) and a phosphatase (Pase). DHN can be interconverted with dihydromonapterin (DHM) by DHN aldolase (DHNa). HMDHP is then translocated into the mitochondrion and is diphosphorylated (HMDHP‐P2) by HMDHP pyrophosphokinase (HPPK). It is then conjugated with p‐ABA to form dihydropteroate (DHP), catalyzed by DHP synthase (DHPS) and then Glu to form dihydrofolate (DHF), catalyzed by DHF synthase (DHFS) and reduced to THF by DHF reductase (DHFR). Once made, folates need to be translocated into various organelles or neighbor cells to implement their respective functions. Gray boxes indicate transporters that need further investigation. Folylpolyglutamate synthase (FPGS) adds additional Glu residues in the mitochondrion, plastid or cytosol. Middle panel: salvage pathways and breakdown of folates in human and/or plants. Methylene THF reductase (MTHFR) catalyzes the conversion of 5,10‐methylene‐THF to 5‐methyl‐THF, and 5‐methyl‐THF can then be converted into THF by methionine synthase (MS), simultaneously with the conversion of homocysteine (Hcy) to methionine (Met). THF can generate 10‐formyl‐THF catalyzed by aldehyde dehydrogenase 1(ALDH1)/formyl‐THF synthetase (FTHFS). Meanwhile, THF interconverts with 5,10‐methylene‐THF, with assistance from serine hydroxymethyltransferase (SHMT) and the glycine decarboxylase complex (GDC). Additionally, conversion from 5,10‐methenyl‐THF into 10‐formyl‐THF and 5,10‐methylene‐THF requires methylene THF cyclohydrolase (MTHFC) and methylene THF dehydrogenase (MTHFD), respectively. Interconversion between 5,10‐methenyl‐THF and 5‐formyl‐THF involves SHMT, 5,10‐methenyltetrahydrofolate synthetase (MTHFS) and 5‐formyl‐THF cycloligase (5‐FCL). Folate is generally subject to oxidative cleavage in both humans and plants, yielding a pterin moiety DHP‐6‐aldehyde (DHPAld) and p‐ABA‐Glu(n). In plants, DHPAld can be converted to HMDHP by pterin aldehyde reductase (PATR) or oxidized to pterin aldehyde (pterin 6‐Ald), which can be converted to 6‐hydroxymethylpterin (6‐HMP) by PTAR or further oxidized to pterin‐6‐carboxylate. In plants (but not in humans) Glu, p‐ABA and pterin products can be recycled to assemble folate again. Also in plants THF can be stored in the vacuole in monoglutamate and polyglutamate forms, and the polyglutamate forms can be converted into THF by GGH. (c) Biochemical and physiological roles of vitamin B9. 5‐Methyl‐THF (within the folate cycle) provides the methyl group for the biosynthesis of Met from Hcy by MS (as also indicated in panel b), yielding S‐adenosyl methionine (SAM), and facilitating subsequent roles in metabolic pathways including polyamine and ethylene biosynthesis (in plants; also requires pyridoxal 5′‐phosphate (PLP)), as well as epigenetic regulation such as methylation reactions of DNA, RNA and histones. SAM can also be transformed to Hcy via S‐adenosyl‐homocysteine (SAH) to regenerate Met. Hcy is also the precursor to cysteine (Cys) via a PLP‐dependent reaction. Conversion of 10‐formyl‐THF to THF donates formyl groups for two steps in purine biosynthesis from phosphoribosylpyrophosphate (PRPP) via glycinamide ribonucleotide (GAR) transformylase (GARTfase) and 5‐aminoimidazole‐4‐carboxamide‐1‐β‐d‐ribofuranosyl 5′‐monophosphate (AICAR) transformylase (AICARTfase). In pyrimidine biosynthesis, thymidylate synthase (TS)‐catalyzed reductive methylation of deoxyuridine monophosphate (dUMP) into deoxythymidine monophosphate (dTMP) is dependent on the conversion of 5,10‐methylene‐THF to DHF. DHF can be reduced to THF by DHFR for various purposes, e.g. generating 5,10‐methylene‐THF by SHMT and subsequently 5‐methyl‐THF by MTHFR, as described in Figure 3(b). Additionally, homeostasis of the folate pool contributes to cellular redox homeostasis. Enzymes specific for plants and humans are highlighted in green and red, respectively, whereas enzymes common to both are in presented in black. The dashed lines represent sequences of multiple reactions. IMP refers to inosine monophosphate. [Colour figure can be viewed at wileyonlinelibrary.com]
The biosynthesis de novo of folates in plants operates across cellular compartments (Figure 3b) (Gorelova et al., 2017a; Hanson & Gregory, 2011; Ravanel et al., 2011). Translocation of the two precursors, pterin and p‐ABA, that are made in the cytosol and plastids, respectively, into mitochondria primarily determines the total folate pool. The initial step of pterin synthesis is the conversion of guanosine triphosphate (GTP) into dihydroneopterin (DHN) triphosphate (P3), catalyzed by GTP cyclohydrolase I (GTPCHI). Homologs of the latter have been identified not only in plants, but also in mammals that cannot biosynthesize folate themselves (Hossain et al., 2004; Yoneyama & Hatakeyama, 1998). DHN‐P3 is then subject to two rounds of dephosphorylation before being converted into glycolaldehyde and 6‐hydroxymethyldihydropterin (HMDHP), catalyzed by DHN aldolase (Goyer et al., 2004; Klaus, Wegkamp, et al., 2005; Suzuki & Brown, 1974). The enzyme is also accountable for converting DHN into dihydromonapterin as another source of HMDHP (Figure 3b). HMDHP is then transported into mitochondria for the subsequent assembly of folates. The other component, p‐ABA, is synthesized in a two‐step process localized in plastids starting from chorismate (Figure 3b). Chorismate (derived through the shikimate pathway) is first converted to aminodeoxychorismate (ADC) by ADC synthase (ADCS), then converted to p‐ABA by ADC lyase. It is thought that p‐ABA is translocated into the mitochondria by passive diffusion, where it is condensed with the pyrophosphorylated HMDHP to yield dihydropteroate, which together with glutamate produces dihydrofolate (DHF) catalyzed by DHF synthase before being reduced into THF to derive various forms of folates (Figure 3b).
Folate transport, including the shuttling of the two precursors, largely affects the availability of various B9 vitamers within humans and plants. Humans absorb folates in a transporter‐based manner, primarily in the jejunum. Various transporters have been identified as responsible for this process (Figure 3b), and include SLC46A1, a proton‐coupled folate transporter (PCFT) that facilitates efficient import at low pH levels (Qiu et al., 2006; Zhao et al., 2007; Zhao et al., 2011). A similarity shared by these transporters is that their major substrate is the monoglutamated vitamer, as those with more than three glutamate residues are unable to cross the cellular membrane. This is in line with 5‐methyl‐THF monoglutamate being identified as the major circulating vitamer in human serum (Ratanasthien et al., 1974). Similarly, transporters are also required for intra‐ and intercellular folate exchange in plants (Hanson & Gregory, 2011; Ravanel et al., 2011) (Figure 3b). Even though more studies are required to explore the carriers in plants, evidence suggests a potential influence of these carriers on folate content and vitamer homeostasis. For example, in Arabidopsis, loss‐of‐function mutants of the plastid folate transporter at locus At2g32040 showed increased total folates alongside reduced 5‐methyl‐THF (Klaus, Kunji, et al., 2005). Additionally, shuttling of the two precursors, p‐ABA and pterin, primarily determines biosynthesis de novo in plants (Quinlivan et al., 2003). Although p‐ABA can diffuse through membranes, pterin needs assistance from transporters that largely remain to be discovered.
In addition to biosynthesis de novo (in plants) or uptake (in humans and plants), other aspects of folate metabolism, including salvage routes, degradation and conversion between the different forms, also largely influence vitamer homeostasis. Studies suggest a daily breakdown rate of approximately 10% in plant leaves and fruits, whereas only about 0.5% occurs in mammals (Gregory & Quinlivan, 2002; Orsomando et al., 2006; Stralsjo et al., 2003). Folates are sensitive to oxidative or photooxidative degradation at the C9–N10 bond (Figure 3a), which cleaves the molecule into pterin and p‐ABA‐Glu(n), and is the main form of folate degradation in all organisms (Suh et al., 2001) (Figure 3b). Salvage approaches to counter folate breakdown occur in almost all organisms, including humans and plants. The cleaved p‐ABA‐Glu(n) in plants can be recycled through the hydrolysis of the polyglutamate chain catalyzed by vacuolar γ‐glutamyl hydrolase, releasing free p‐ABA for folate synthesis (Akhtar et al., 2010) (Figure 3b). The recycling of the pterin ring relies on the conversion of dihydropterin‐6‐aldehyde into HMDHP before dihydropterin‐6‐aldehyde is oxidized into pterin‐6‐aldehyde, which cannot be recycled (Figure 3b). This conversion is catalyzed by NADPH‐dependent pterin aldehyde reductases (Noiriel et al., 2007a; Noiriel et al., 2007b). In humans, folates are polyglutamylated by folylpolyglutamate synthase from the circulating monoglutamylated forms (Osborne et al., 1993). It was recently discovered that the oxidative breakdown of folates could be repaired by quinoid dihydropteridine reductase, an enzyme in mammalian cells (Zheng et al., 2018). Dietary folic acid is reduced into THF by DHF reductase before being methylated into circulating forms.
Biochemical and physiological roles of vitamin B9
The most significant biochemical role of folates is carrying 1C units at various oxidation states attached to the N5 and/or N10 of the pterin ring (Figure 3a). The pterin ring of folates exists naturally in dihydro‐ and tetrahydro‐ forms, with only the tetrahydro‐ form possessing coenzyme activity, whereas the dihydro‐ form is incapable of carrying 1C units. The three major coenzyme forms include 10‐formyl‐THF, 5,10‐methylene‐THF and 5‐methyl‐THF (Hanson & Gregory, 2011; Ravanel et al., 2011; Ross et al., 2012). The biosynthesis of purine and thymine bases, both being critical for nucleic acid metabolism, requires folates as coenzymes in donating 1C units (Huennekens, 1968). During purine biosynthesis, the conversion of 5′‐phosphoribosyl‐glycinamide (GAR) into formyl‐GAR and the conversion of 5′‐phosphoribosyl‐5‐aminoimidazole‐4‐carboxamide (AICAR) into formyl‐AICAR requires two transformylases, respectively, that both rely on 10‐formyl‐THF as a formyl donor (Figure 3c). These steps are critical for the generation of inosine monophosphate as the purine precursor (Huennekens, 1968). Thymidylate synthase is responsible for converting deoxyuridine monophosphate into deoxythymidine monophosphate, with 5,10‐methylene‐THF as a 1C donor, which serves as the sole source of thymine supporting DNA synthesis (Figure 3c). The significance of folates is also reflected in the metabolism of other molecular components that involve the 1C cycle, such as amino acids, including glycine, serine and methionine (Met) (Clare et al., 2019; Ross et al., 2012). S‐Adenosylmethionine (SAM), an essential methyl donor in many biological processes, is biosynthesized from Met, which is generated by Met synthase from homocysteine (Hcy) receiving a methyl group from 5‐methyl‐THF, which concurrently is converted into THF (Figure 3c). SAM in turn can be subsequently used either in polyamine synthesis or can be transmethylated back to Hcy to maintain Met homeostasis (Ouyang et al., 2020). Notably, SAM is also the primary substrate for the biosynthesis of the gaseous plant hormone ethylene (Pattyn et al., 2021) (Figure 3c), which functions either independently or together with other hormones such as auxin, jasmonate and abscisic acid throughout the life cycle of plants (Van de Poel et al., 2015). Putatively, folate homeostasis could thus be impactful for ethylene production and subsequential phytohormone responses. As folates function as coenzymes to donate and/or receive 1C units, their homeostasis in turn can be influenced by 1C metabolism. Total folate content, with 5‐methyl‐THF as the representative vitamer, was proposed to be tightly regulated through the methyl cycle and involved in the elevated chlorophyll content in high‐folate accessions of Brassica rapa subsp. chinensis (pak choi), compared with those with low folate levels (Shohag et al., 2020). Additionally, 5‐formyl‐THF was recently described to directly bind regulatory factors that are involved in various pathways, including nitrogen and carbohydrate metabolism, photosynthesis and proteostasis (Li et al., 2021). For example, direct binding of 5‐formyl‐THF to the bifunctional DHF reductase‐thymidylate synthase (DHFR‐TS), serine hydroxymethyltransferase (SHMT) and glutamine synthetase in plants inhibits their function and consequently influences folate biosynthesis and nitrogen metabolism, respectively (Goyer et al., 2006; Li et al., 2021).
Similar to other B vitamins, folates have been claimed to display antioxidant capacity (Gliszczyńska‐Świgło, 2006; Joshi et al., 2001). However, it is yet to be elucidated whether folate could contribute to adaptive responses to adverse conditions that result in an imbalance of ROS.
The importance of vitamin B9 homeostasis in human and plant health
As humans depend on dietary folate sources (0.4 mg per day for a typical adult), either insufficient uptake or insufficient absorption could affect the total folate pool, thereby resulting in folate deficiency followed by subsequent health issues, such as megaloblastic anaemia and birth defects. Malfunction of the PCFT was found in patients with a genetic disorder termed as hereditary folate malabsorption (Zhao et al., 2007). With insufficient absorption and impaired transport, consequences could be manifold, including immunologic and neurologic dysfunctions (Kronn & Goldman, 2008). Methylene tetrahydrofolate reductase (MTHFR), which converts 5,10‐methylene‐THF to 5‐methyl‐THF (Figure 3b), contributes to Met generation from Hcy and thus plays a crucial role in maintaining cellular folate homeostasis and the 1C pool (Raghubeer & Matsha, 2021). Gene variants of MTHFR, particularly the C677T polymorphism, are quite common among human populations and are thought to be related to certain diseases, such as cardiovascular diseases (CVDs), and pose a higher risk of neural tube defects (NTDs) (Tsang et al., 2015). Malfunction of the enzyme causes an imbalance between 5,10‐methylene‐THF and 5‐methyl‐THF, accompanied by decreased Met and increased Hcy levels, which is considered to be largely relevant for CVDs. Synthetic folic acid, as the major supplement form, is thought to be beneficial for preventing NTDs. However, adverse effects could occur with high dosages. For example, oral folic acid uptake was considered to increase the possibility of prostate cancer (Wien et al., 2012), and high dosage has the potential of disguising vitamin B12 deficiency because of similar symptoms. This remains to be further investigated given the limitations in experimental designs discussed in a previous review (Mills et al., 2018).
Folate homeostasis is critical not only to human health but is also required for proper plant growth and development. Various environmental factors have great influence on folate homeostasis in planta (Kolton et al., 2022). For example, folate biosynthesis is largely affected by the availability of nutrients such as nitrogen and phosphorus (Mozafar, 2017). In turn, folate content and vitamer homeostasis could influence the adaptation responses of plants to the challenges of their environments. Overexpression of Arabidopsis DHFR‐TS led to a decrease in total folate abundance, and in particular 5‐methyl‐THF, 5,10‐methenyl‐THF and 10‐formyl‐THF, along with an increase in THF and 5,10‐methylene‐THF (Gorelova et al., 2017b). These changes were accompanied by delayed flowering and ROS accumulation, supporting the above‐mentioned antioxidant capability of folates. In this instance it was proposed that redox homeostasis is mediated through 5‐methyl‐THF dehydrogenase‐catalyzed NADPH production, which is in line with a previous quantitative analysis on folate‐dependent NADPH generation (Fan et al., 2014; Gorelova et al., 2017b). As discussed above, folate homeostasis largely influences the 1C cycle, and thus the level of key elements in 1C metabolism, such as SAM, which is a universal methyl donor utilized by many methyltransferases in DNA and histone methylation, affecting epigenetic modifications (Zhang et al., 2012a). Similarly, Met, another 1C molecule, is greatly affected by folate metabolism and is linked to plant immune status, possibly through DNA methylation (Gonzalez & Vera, 2019).
Interplay among the B1 , B6 and B9 vitamin families
The importance of the B vitamins in general is reflected not only by their individual homeostasis and respective functions, but also by their mutual interdependence with respect to precursors, intermediates, products or allosteric effectors. For example, interconversions among the folate family rely on PLP‐dependent enzymes, e.g. SHMT (Figure 3b,c). Although not discussed here, several steps in the biosynthesis of vitamin B1, B6 or the B9 family also rely on FMN and NAD(P)H, derived from vitamin B2 and B3, respectively (Fitzpatrick et al., 2012). It has also been reported that the vitamin B1 derivatives TTP/ATTP allosterically affect bovine PLK activity, and thus may influence PLP homeostasis via the salvage pathway in animal cells (Mkrtchyan et al., 2015), but this remains to be investigated in vivo as well as in plants. Further, the uptake of B vitamins in mammalian cells may be influenced by the respective levels in food sources because, as an example, the reduced folate carrier (RFC1) in the intestine also possesses vitamin B1 transport function (Zhao et al., 2002). Furthermore, the flux through the metabolic reactions in which these coenzymes participate may be influenced by the level of the respective coenzyme (Fitzpatrick & Noordally, 2021). As an example, in animal cells Hcy is a substrate for both cysteine and Met biosynthesis, which are processes that depend upon vitamin B6 and B9, respectively. High levels of Hcy are often considered indicative of atherosclerotic diseases (Graham et al., 1997), and thus it would be informative to evaluate whether blood Hcy levels and the abundance of these two B vitamins are directly linked (Marti‐Carvajal et al., 2017). Therefore, in general it will be important/ideal to develop full metabolome libraries that can be used to diagnose B vitamin (importantly including all individual vitamers) and metabolic status, which in turn can be used as a predictor of plant and human health levels with respect to these micronutrients (Figure 4). It will also be important to establish the influence of the environment on plant B vitamin health, e.g. the impact of rhizosphere and phyllosphere interactions, as well as abiotic stress challenges (Box 2).
Box 2: Open questions and perspectives.
The importance of vitamer homeostasis at the organ, tissue and subcellular level should be recognized in both plants and humans.
How important is the interaction of various B vitamins (intra‐ and inter‐family) in terms of aiming for integrated homeostasis in biofortified crops and humans?
What is the impact of the rhizosphere on B vitamin metabolism in crop species, and hence plant and human health?
It will be important to develop detection/quantification techniques to assist in the precise diagnosis of vitamin status in plants for agriculture as well as in humans.
Figure 4.
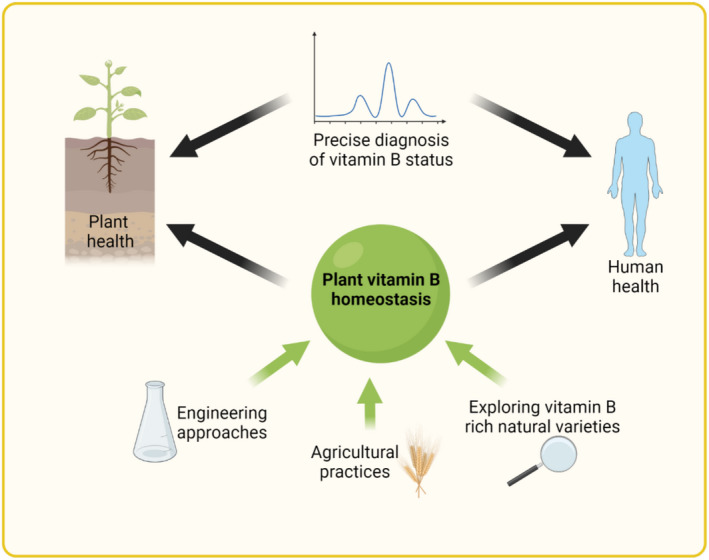
Plant vitamin B homeostasis for human and plant health. Vitamin B homeostasis of plants is essential for plant health and is also important for human health because plants serve as a primary vitamin B source for humans. State‐of‐the‐art engineering approaches and agricultural practices, as well as the exploration of natural varieties of food crops and edible plants, may provide plant‐based food sources with enhanced and balanced B vitamin content to support plant and human health. Developments in analytical technologies could provide a more precise diagnosis of vitamin B status in plants and humans, which will in turn improve our understanding of the regulation of B vitamin metabolism and assist in providing vitamin B‐rich sources in the right proportions. [Colour figure can be viewed at wileyonlinelibrary.com]
Consideration of B vitamin homeostasis in the alleviation of hidden hunger
There are various strategies towards the alleviation of hidden hunger, ranging from education to state‐of‐the‐art (bio)fortification methods (Rautiainen et al., 2016). However, some of these strategies work on time scales that are not ideal in poor areas with the most urgent demands (Blancquaert et al., 2017). In addition, limited resources can hinder significant progress in developing countries (Blancquaert et al., 2017; Reinbott et al., 2016). Similar limitations apply to solutions involving supplementation in the form of vitamin pills or fortified foodstuffs (often from synthetic sources). On the other hand, biofortification is the process of increasing the micronutrient content of a food crop through selective breeding, genetic modification or the use of enriched fertilizers (https://www.harvestplus.org). The latter approaches (alone or in combination) could serve as more sustainable solutions for humans all over the globe, as they could improve the quality of preferred food sources within the daily diet without the need to source and supply synthetically derived vitamins or fortified foods.
The metabolism and function of B vitamins will be appreciated from the detailed description of vitamin B1, B6 and B9 above, highlighting their importance in metabolic pathways that underlie proper growth and development alongside disease prevention in plants and humans. Unfortunately, as mentioned at the outset, deficiency in B vitamins is all too common in humans, as a result of insufficient access to appropriate food sources, as well as in plants, partly as a result of damage through increasing environmental stress and millennia of selective breeding that unintentionally disregarded micronutrient content (Díaz et al., 2019). We hope it is recognized upon reading this article that it is of high importance to take into consideration the homeostasis of B vitamins when designing strategies to alleviate hidden hunger (Box 1). Indeed, over the past years numerous studies have been carried out to increase the abundance of each of the three B vitamins discussed here in various staple crops. In most cases, this has involved engineering the expression of the biosynthesis pathway genes. For example, overexpression of THIC/THI1/TH1 increased the overall thiamine content in seeds of Oryza sativa (rice), but was largely eliminated upon grain polishing (Dong et al., 2016; Strobbe et al., 2021a), and would need this problem to be resolved to be of use for consumers. The expression of Arabidopsis PDX1.1 and PDX2 in Manihot esculenta (cassava) resulted in an almost six‐fold increase of total vitamin B6 in storage roots, which would reach the recommended daily intake in average food portions (Li et al., 2015). However, the same approach in rice did not lead to an accumulation in polished grains, although the leaf tissue had a 30‐fold increase (Mangel et al., 2019). By contrast, engineering GTPCHI and ADCS for pterin and p‐ABA, respectively, in combination with a synthetic folate binding protein (sFBP) in rice resulted in a 150‐fold increase in the folate content of polished raw grains (Blancquaert et al., 2015; Storozhenko et al., 2007). Similarly, engineering GTPCHI and ADCS in Zea mays (maize), Triticum aestivum (wheat) and Solanum lycopersicum (tomato) achieved great improvements in folate levels (Diaz de la Garza et al., 2007; Liang et al., 2019). However, populations are generally deficient in several micronutrients simultaneously. Given the bottlenecks in increasing the micronutrient content of the consumed tissue, often combined with yield penalties, especially in the case of the mineral nutrients (Stangoulis & Knez, 2022), further research on the diverse regulation and interaction of micronutrients, including the B vitamins, as well as their involvement in plant growth and development is required. Enhancing vitamin B1 content is particularly challenging because of its tight regulation, as mentioned above, and is also a process with high energy cost as the enzymes are limited in turnover number (1 for THI1 and 3 or 4 for THIC) (Hanson et al., 2018). The engineering of more catalytically efficient versions of these proteins, capable of multiple turnovers, has been suggested as a potential strategy to alleviate some of these issues (Amthor et al., 2019; Garcia‐Garcia et al., 2020; Minhas et al., 2018). Crops biofortified in these B vitamins may also have the added benefit of stress resistance (Vanderschuren et al., 2013). Recently, transgenic Solanum tuberosum (potato) engineered for increased vitamin B6 content also displayed increased fitness and higher salinity resistance (Bagri et al., 2018). The stability of B vitamins also needs to be taken into account because of their vulnerability to degradation and damage (Hanson et al., 2016). This problem was managed in the folate engineering strategies by modifying the biosynthesis enzymes or by including an sFBP (Blancquaert et al., 2015; De Lepeleire et al., 2018). However, despite all the efforts made to enhance B vitamin contents in biofortified crops, in many cases little attention has been paid to the individual vitamers. The significance of vitamer homeostasis as well as its relevance for plant performance will be an important consideration, as outlined above. Studying natural variation in the target crop species may provide knowledge to help further improve biofortification strategies (Figure 4). Very recently, a study of 59 rice varieties for vitamin B1 and B6 content showed a four‐fold difference across polished seeds (Mangel et al., 2022), and could be complemented by exploring an even larger diversity to determine whether there is ample divergence in vitamin content that can be exploited. Several studies have revealed the natural genetic diversity of folate abundance among various crop germplasms, including potato, Spinacia oleracea (spinach) and pak choi (Goyer & Navarre, 2007; Shohag et al., 2011; Shohag et al., 2020). Significantly, there may be a lot to learn from a recent report that revealed the great potential of 1044 edible plant species to contribute as a source of B vitamins in particular, to combat the global malnutrition issue (Cantwell‐Jones et al., 2022). The authors predict the B vitamin content of edible foods, which remains to be validated experimentally. In addition, current exploratory strategies such as nano‐biofortification (nanoparticle‐based controlled fertilization) and increasing nutrient content with the aid of microorganisms may prove fruitful (Guo et al., 2018; Ye et al., 2020).
FINAL REMARKS
B vitamins are essential micronutrients for both human and plant health. Here we emphasize that although we have a considerable wealth of information on vitamin metabolism, knowledge on vitamer homeostasis, distribution and interaction will help predict the importance of all of these aspects to plant performance under rapidly changing environmental conditions (Box 1). This is emphasized by the crucial role that B vitamins play in metabolic processes and their potential to have a major impact on the flux of certain reactions, which can alleviate or cause disease if not in the right balance. It will be critical to develop advanced detection and quantification techniques, on a high‐throughput and even automated scale, for a better understanding of the contribution of B vitamins to the metabolic profile of plants, starting with model species (Box 2). Eventually, this could lead to biomarker maps that might increase the precision of diagnosing both plant and human health (Figure 4). Although it has become evident that the current use of staple crops will not provide the micronutrient levels required for humans, the ability to devise informed strategies for improving human health, such as biofortification, will provide benefit, as will tapping into the diverse palette of edible plants, although their nutrient status will need to be examined to validate them as human food sources.
AUTHOR CONTRIBUTIONS
All authors contributed sections to this article. TBF contributed ideas and text to all sections, merged and consolidated the sections, and edited the whole review.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest associated with this work.
ACKNOWLEDGEMENTS
Financial support for research in the Fitzpatrick lab is gratefully acknowledged from the Swiss National Science Foundation (grants 31003A‐141117/1, 31003A_162555 and 310030‐192466 to TBF) as well as the University of Geneva. Open access funding provided by Universite de Geneve.
REFERENCES
- Ajjawi, I. , Milla, M.A.R. , Cushman, J. & Shintani, D.K. (2007) Thiamin pyrophosphokinase is required for thiamin cofactor activation in Arabidopsis. Plant Molecular Biology, 65, 151–162. [DOI] [PubMed] [Google Scholar]
- Akhtar, T.A. , Orsomando, G. , Mehrshahi, P. , Lara‐Nunez, A. , Bennett, M.J. , Gregory, J.F., 3rd et al. (2010) A central role for gamma‐glutamyl hydrolases in plant folate homeostasis. The Plant Journal, 64, 256–266. [DOI] [PubMed] [Google Scholar]
- Al Sulaiman, K. , Aljuhani, O. , Al Dossari, M. , Alshahrani, A. , Alharbi, A. , Algarni, R. et al. (2021) Evaluation of thiamine as adjunctive therapy in COVID‐19 critically ill patients: a two‐center propensity score matched study. Critical Care, 25, 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albersen, M. , Bosma, M. , Knoers, N.V. , de Ruiter, B.H. , Diekman, E.F. , de Ruijter, J. et al. (2013) The intestine plays a substantial role in human vitamin B6 metabolism: a Caco‐2 cell model. PLoS One, 8, e54113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altensell, J. , Wartenberg, R. , Haferkamp, I. , Hassler, S. , Scherer, V. , Steensma, P. et al. (2022) Loss of a pyridoxal‐phosphate phosphatase rescues Arabidopsis lacking an endoplasmic reticulum ATP carrier. Plant Physiology, 189, 49–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amthor, J.S. , Bar‐Even, A. , Hanson, A.D. , Millar, A.H. , Stitt, M. , Sweetlove, L.J. et al. (2019) Engineering strategies to boost crop productivity by cutting respiratory carbon loss. Plant Cell, 31, 297–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagri, D.S. , Upadhyaya, D.C. , Kumar, A. & Upadhyaya, C.P. (2018) Overexpression of PDX‐II gene in potato (Solanum tuberosum L.) leads to the enhanced accumulation of vitamin B6 in tuber tissues and tolerance to abiotic stresses. Plant Science, 272, 267–275. [DOI] [PubMed] [Google Scholar]
- Bailey, R.L. , West, K.P., Jr. & Black, R.E. (2015) The epidemiology of global micronutrient deficiencies. Annals of Nutrition & Metabolism, 66, 22–33. [DOI] [PubMed] [Google Scholar]
- Barile, A. , Nogues, I. , di Salvo, M.L. , Bunik, V. , Contestabile, R. & Tramonti, A. (2020) Molecular characterization of pyridoxine 5′‐phosphate oxidase and its pathogenic forms associated with neonatal epileptic encephalopathy. Scientific Reports, 10, 13621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgart, A. , Spiczak, S. , Verhoeven‐Duif, N.M. , Moller, R.S. , Boor, R. , Muhle, H. et al. (2014) Atypical vitamin B6 deficiency: a rare cause of unexplained neonatal and infantile epilepsies. Journal of Child Neurology, 29, 704–707. [DOI] [PubMed] [Google Scholar]
- Beaudoin, G.A.W. , Johnson, T.S. & Hanson, A.D. (2018) The PLUTO plastidial nucleobase transporter also transports the thiamin precursor hydroxymethylpyrimidine. Bioscience Reports, 38, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky, J.B. , Wira, C.R. , Jacob, V. , Sather, J.E. & Lee, P.J. (2018) A review of micronutrients in sepsis: the role of thiamine, L‐carnitine, vitamin C, selenium and vitamin D. Nutrition Research Reviews, 31, 281–290. [DOI] [PubMed] [Google Scholar]
- Bettendorff, L. (2021) Update on thiamine triphosphorylated derivatives and metabolizing enzymatic complexes. Biomolecules, 11, 1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettendorff, L. , Wirtzfeld, B. , Makarchikov, A.F. , Mazzucchelli, G. , Frédérich, M. , Gigliobianco, T. et al. (2007) Discovery of a natural thiamine adenine nucleotide. Nature Chemical Biology, 3, 211–212. [DOI] [PubMed] [Google Scholar]
- Bilski, P. , Li, M.Y. , Ehrenshaft, M. , Daub, M.E. & Chignell, C.F. (2000) Vitamin B6 (pyridoxine) and its derivatives are efficient singlet oxygen quenchers and potential fungal antioxidants. Photochemistry and Photobiology, 71, 129–134. [DOI] [PubMed] [Google Scholar]
- Black, R.E. , Victora, C.G. , Walker, S.P. , Bhutta, Z.A. , Christian, P. , de Onis, M. et al. (2013) Maternal and child undernutrition and overweight in low‐income and middle‐income countries. Lancet, 382, 427–451. [DOI] [PubMed] [Google Scholar]
- Blancquaert, D. , De Steur, H. , Gellynck, X. & Van Der Straeten, D. (2017) Metabolic engineering of micronutrients in crop plants. Annals of the New York Academy of Sciences, 1390, 59–73. [DOI] [PubMed] [Google Scholar]
- Blancquaert, D. , Van Daele, J. , Strobbe, S. , Kiekens, F. , Storozhenko, S. , De Steur, H. et al. (2015) Improving folate (vitamin B9) stability in biofortified rice through metabolic engineering. Nature Biotechnology, 33, 1076–1078. [DOI] [PubMed] [Google Scholar]
- Bocobza, S. , Adato, A. , Mandel, T. , Shapira, M. , Nudler, E. & Aharoni, A. (2007) Riboswitch‐dependent gene regulation and its evolution in the plant kingdom. Genes & Development, 21, 2874–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocobza, S.E. , Malitsky, S. , Araujo, W.L. , Nunes‐Nesi, A. , Meir, S. , Shapira, M. et al. (2013) Orchestration of thiamin biosynthesis and central metabolism by combined action of the thiamin pyrophosphate riboswitch and the circadian clock in Arabidopsis. Plant Cell, 25, 288–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borelli, T. , Hunter, D. , Powell, B. , Ulian, T. , Mattana, E. , Termote, C. et al. (2020) Born to eat wild: an integrated conservation approach to secure wild food plants for food security and nutrition. Plants (Basel), 9, 1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco de Oliveira, M.V. , Irikura, S. , Lourenco, F.H.B. , Shinsato, M. , Irikura, T.C.D.B. , Irikura, R.B. et al. (2021) Encephalopathy responsive to thiamine in severe COVID‐19 patients. Brain, Behavior, & Immunity ‐ Health, 14, 100252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, G. (2014) Defects of thiamine transport and metabolism. Journal of Inherited Metabolic Disease, 37, 577–585. [DOI] [PubMed] [Google Scholar]
- Cantwell‐Jones, A. , Ball, J. , Collar, D. , Diazgranados, M. , Douglas, R. , Forest, F. et al. (2022) Global plant diversity as a reservoir of micronutrients for humanity. Nature Plants, 8, 225–232. [DOI] [PubMed] [Google Scholar]
- Chelban, V. , Wilson, M.P. , Chardon, J.W. , Vandrovcova, J. , Zanetti, M.N. , Zamba‐Papanicolaou, E. et al. (2019) PDXK mutations cause polyneuropathy responsive to pyridoxal 5 '‐phosphate supplementation. Annals of Neurology, 86, 225–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciapaite, J. , Albersen, M. , Savelberg, S.M.C. , Bosma, M. , Tessadori, F. , Gerrits, J. et al. (2020) Pyridox(am)ine 5 '‐phosphate oxidase (PNPO) deficiency in zebrafish results in fatal seizures and metabolic aberrations. Biochimica et Biophysica Acta, 1866, 165607. [DOI] [PubMed] [Google Scholar]
- Clare, C.E. , Brassington, A.H. , Kwong, W.Y. & Sinclair, K.D. (2019) One‐carbon metabolism: Linking nutritional biochemistry to epigenetic programming of long‐term development. Annual Review of Animal Biosciences, 7, 263–287. [DOI] [PubMed] [Google Scholar]
- Colinas, M. , Eisenhut, M. , Tohge, T. , Pesquera, M. , Fernie, A.R. , Weber, A.P.M. et al. (2016) Balancing of B‐6 vitamers is essential for plant development and metabolism in Arabidopsis. Plant Cell, 28, 439–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colinas, M. & Fitzpatrick, T.B. (2015) Natures balancing act: examining biosynthesis de novo, recycling and processing damaged vitamin B metabolites. Current Opinion in Plant Biology, 25, 98–106. [DOI] [PubMed] [Google Scholar]
- Cooper, A.J. & Jeitner, T.M. (2016) Central role of glutamate metabolism in the maintenance of nitrogen homeostasis in normal and hyperammonemic brain. Biomolecules, 6, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory, S.A. , Van Vranken, J.G. , Brignole, E.J. , Patra, S. , Winge, D.R. , Drennan, C.L. et al. (2017) Structure of human Fe‐S assembly subcomplex reveals unexpected cysteine desulfurase architecture and acyl‐ACP‐ISD11 interactions. Proceedings of the National Academy of Sciences of the United States of America, 114, E5325–E5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czegeny, G. , Korosi, L. , Strid, A. & Hideg, E. (2019) Multiple roles for vitamin B6 in plant acclimation to UV‐B. Scientific Reports, 9, 1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danon, A. , Miersch, O. , Felix, G. , op den Camp, R.G.L. & Apel, K. (2005) Concurrent activation of cell death‐regulating signalling pathways by singlet oxygen in Arabidopsis thaliana . The Plant Journal, 41, 68–80. [DOI] [PubMed] [Google Scholar]
- de Freitas‐Silva, D.M. , Resende Lde, S. , Pereira, S.R. , Franco, G.C. & Ribeiro, A.M. (2010) Maternal thiamine restriction during lactation induces cognitive impairments and changes in glutamate and GABA concentrations in brain of rat offspring. Behavioural Brain Research, 211, 33–40. [DOI] [PubMed] [Google Scholar]
- De Lepeleire, J. , Strobbe, S. , Verstraete, J. , Blancquaert, D. , Ambach, L. , Visser, R.G.F. et al. (2018) Folate biofortification of potato by tuber‐specific expression of four folate biosynthesis genes. Molecular Plant, 11, 175–188. [DOI] [PubMed] [Google Scholar]
- Denslow, S.A. , Walls, A.A. & Daub, M.E. (2005) Regulation of biosynthetic genes and antioxidant properties of vitamin B‐6 vitamers during plant defense responses. Physiological and Molecular Plant Pathology, 66, 244–255. [Google Scholar]
- Dhir, S. , Tarasenko, M. , Napoli, E. & Giulivi, C. (2019) Neurological, psychiatric, and biochemical aspects of thiamine deficiency in children and adults. Frontiers in Psychiatry, 10, 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Salvo, M.L. , Contestabile, R. & Safo, M.K. (2011) Vitamin B(6) salvage enzymes: mechanism, structure and regulation. Biochimica et Biophysica Acta, 1814, 1597–1608. [DOI] [PubMed] [Google Scholar]
- Diaz de la Garza, R.I. , Gregory, J.F., 3rd & Hanson, A.D. (2007) Folate biofortification of tomato fruit. Proceedings of the National Academy of Sciences of the United States of America, 104, 4218–4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz, S. , Settele, J. , Brondízio, E.S. , Ngo, H.T. , Agard, J. , Arneth, A. et al. (2019) Pervasive human‐driven decline of life on Earth points to the need for transformative change. Science, 366, eaax3100. [DOI] [PubMed] [Google Scholar]
- Dong, W. , Thomas, N. , Ronald, P.C. & Goyer, A. (2016) Overexpression of thiamin biosynthesis genes in rice increases leaf and unpolished grain thiamin content but not resistance to Xanthomonas oryzae pv. oryzae . Frontiers in Plant Science, 7, 616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunathan, H. (1966) Conformation and reaction specificity in pyridoxal phosphate enzymes. Proceedings of the National Academy of Sciences of the United States of America, 55, 712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenshaft, M. , Bilski, P. , Li, M.Y. , Chignell, C.F. & Daub, M.E. (1999) A highly conserved sequence is a novel gene involved in de novo vitamin B6 biosynthesis. Proceedings of the National Academy of Sciences of the United States of America, 96, 9374–9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo, N. , Nishiyama, K. , Okabe, M. , Matsumoto, M. , Kanouchi, H. & Oka, T. (2007) Vitamin B6 suppresses apoptosis of NM‐1 bovine endothelial cells induced by homocysteine and copper. Biochimica et Biophysica Acta, 1770, 571–577. [DOI] [PubMed] [Google Scholar]
- Estévez, J.M. , Cantero, A. , Romero, C. , Kawaide, H. , Jiménez, L.F. , Kuzuyama, T. et al. (2000) Analysis of the expression of CLA1, a gene that encodes the 1‐deoxyxylulose 5‐phosphate synthase of the 2‐C‐methyl‐D‐erythritol‐4‐phosphate pathway in Arabidopsis. Plant Physiology, 124, 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, J. , Ye, J. , Kamphorst, J.J. , Shlomi, T. , Thompson, C.B. & Rabinowitz, J.D. (2014) Quantitative flux analysis reveals folate‐dependent NADPH production. Nature, 510, 298–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick, T.B. (2011) Vitamin B6 in plants: more than meets the eye. Advances in Botanical Research, 59, 1–38. [Google Scholar]
- Fitzpatrick, T.B. , Amrhein, N. , Kappes, B. , Macheroux, P. , Tews, I. & Raschle, T. (2007) Two independent routes of de novo vitamin B6 biosynthesis: not that different after all. The Biochemical Journal, 407, 1–13. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick, T.B. , Basset, G.J.C. , Borel, P. , Carrari, F. , DellaPenna, D. , Fraser, P.D. et al. (2012) Vitamin deficiencies in humans: can plant science help? Plant Cell, 24, 395–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick, T.B. & Chapman, L.M. (2020) The importance of thiamine (vitamin B‐1) in plant health: From crop yield to biofortification. The Journal of Biological Chemistry, 295, 12002–12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick, T.B. & Noordally, Z. (2021) Of clocks and coenzymes in plants: intimately connected cycles guiding central metabolism? The New Phytologist, 230, 416–432. [DOI] [PubMed] [Google Scholar]
- Fonda, M.L. (1992) Purification and characterization of vitamin‐B(6)‐phosphate phosphatase from human erythrocytes. The Journal of Biological Chemistry, 267, 15978–15983. [PubMed] [Google Scholar]
- Frelin, O. , Agrimi, G. , Laera, V.L. , Castegna, A. , Richardson, L.G.L. , Mullen, R.T. et al. (2012) Identification of mitochondrial thiamin diphosphate carriers from Arabidopsis and maize. Functional & Integrative Genomics, 12, 317–326. [DOI] [PubMed] [Google Scholar]
- Gangolf, M. , Wins, P. , Thiry, M. , El Moualij, B. & Bettendorff, L. (2010) Thiamine triphosphate synthesis in rat brain occurs in mitochondria and is coupled to the respiratory chain. The Journal of Biological Chemistry, 285, 583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Garcia, J.D. , Joshi, J. , Patterson, J.A. , Trujillo‐Rodriguez, L. , Reisch, C.R. , Javanpour, A.A. et al. (2020) Potential for applying continuous directed evolution to plant enzymes: An exploratory study. Life (Basel), 10, 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson, G.E. , Blass, J.P. , Beal, M.F. & Bunik, V. (2005) The alpha‐ketoglutarate‐dehydrogenase complex: a mediator between mitochondria and oxidative stress in neurodegeneration. Molecular Neurobiology, 31, 43–63. [DOI] [PubMed] [Google Scholar]
- Gigliobianco, T. , Lakaye, B. , Wins, P. , El Moualij, B. , Zorzi, W. & Bettendorff, L. (2010) Adenosine thiamine triphosphate accumulates in Escherichia coli cells in response to specific conditions of metabolic stress. BMC Microbiology, 10, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliszczyńska‐Świgło, A. (2006) Antioxidant activity of water soluble vitamins in the TEAC (trolox equivalent antioxidant capacity) and the FRAP (ferric reducing antioxidant power) assays. Food Chemistry, 96, 131–136. [Google Scholar]
- Gonzalez, B. & Vera, P. (2019) Folate metabolism interferes with plant immunity through 1C methionine synthase‐directed genome‐wide DNA methylation enhancement. Molecular Plant, 12, 1227–1242. [DOI] [PubMed] [Google Scholar]
- Gorelova, V. , Ambach, L. , Rebeille, F. , Stove, C. & Van Der Straeten, D. (2017a) Folates in plants: Research advances and progress in crop biofortification. Frontiers in Chemistry, 5, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelova, V. , Colinas, M. , Dell'Aglio, E. , Flis, P. , Salt, D.E. & Fitzpatrick, T.B. (2022) Phosphorylated B6 vitamer deficiency in SALT OVERLY SENSITIVE 4 mutants compromises shoot and root development. Plant Physiology, 188, 220–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelova, V. , De Lepeleire, J. , Van Daele, J. , Pluim, D. , Mei, C. , Cuypers, A. et al. (2017b) Dihydrofolate reductase/thymidylate synthase fine‐tunes the folate status and controls redox homeostasis in plants. Plant Cell, 29, 2831–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyer, A. , Collakova, E. , Díaz de la Garza, R. , Quinlivan, E.P. , Williamson, J. , Gregory, J.F., 3rd et al. (2006) 5‐Formyltetrahydrofolate is an inhibitory but well tolerated metabolite in Arabidopsis leaves. The Journal of Biological Chemistry, 280, 26137–26142. [DOI] [PubMed] [Google Scholar]
- Goyer, A. , Hasnain, G. , Frelin, O. , Ralat, M.A. , Gregory, J.F., 3rd & Hanson, A.D. (2013) A cross‐kingdom Nudix enzyme that pre‐empts damage in thiamin metabolism. The Biochemical Journal, 454, 533–542. [DOI] [PubMed] [Google Scholar]
- Goyer, A. , Illarionova, V. , Roje, S. , Fischer, M. , Bacher, A. & Hanson, A.D. (2004) Folate biosynthesis in higher plants. cDNA cloning, heterologous expression and characterization of dihydroneopterin aldolases. Plant Physiology, 135, 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyer, A. & Navarre, D.A. (2007) Determination of folate concentrations in diverse potato germplasm using a trienzyme extraction and a microbiological assay. Journal of Agricultural and Food Chemistry, 55, 3523–3528. [DOI] [PubMed] [Google Scholar]
- Graham, I.M. , Daly, L.E. , Refsum, H.M. , Robinson, K. , Brattstrom, L.E. , Ueland, P.M. et al. (1997) Plasma homocysteine as a risk factor for vascular disease ‐ The European concerted action project. Journal of the American Medical Association, 277, 1775–1781. [DOI] [PubMed] [Google Scholar]
- Gregory, J.F. (1998) Nutritional properties and significance of vitamin glycosides. Annual Review of Nutrition, 18, 277–296. [DOI] [PubMed] [Google Scholar]
- Gregory, J.F. & Ink, S.L. (1987) Identification and quantification of pyridoxine‐beta‐glucoside as a major form of vitamin‐B6 in plant‐derived foods. Journal of Agricultural and Food Chemistry, 35, 76–82. [Google Scholar]
- Gregory, J.F. & Quinlivan, E.P. (2002) In vivo kinetics of folate metabolism. Annual Review of Nutrition, 22, 199–220. [DOI] [PubMed] [Google Scholar]
- Guan, J.‐C. , Hasnain, G. , Garrett, T.J. , Chase, C.D. , Gregory, J.F., 3rd , Hanson, A.D. et al. (2014) Divisions of labor in the thiamin biosynthetic pathway among organs of maize. Frontiers in Plant Science, 5, 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, H. , White, J.C. , Wang, Z. & Xing, B. (2018) Nano‐enabled fertilizers to control the release and use efficiency of nutrients. Current Opinion in Environmental Science & Health, 6, 77–83. [Google Scholar]
- Han, G. , Gupta, S.D. , Gable, K. , Niranjanakumari, S. , Moitra, P. , Eichler, F. et al. (2009) Identification of small subunits of mammalian serine palmitoyltransferase that confer distinct acyl‐CoA substrate specificities. Proceedings of the National Academy of Sciences of the United States of America, 106, 8186–8191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson, A.D. , Amthor, J.S. , Sun, J. , Niehaus, T.D. , Gregory, J.F., 3rd , Bruner, S.D. et al. (2018) Redesigning thiamin synthesis: prospects and potential payoffs. Plant Science, 273, 92–99. [DOI] [PubMed] [Google Scholar]
- Hanson, A.D. , Beaudoin, G.A. , McCarty, D.R. & Gregory, J.F., 3rd . (2016) Does abiotic stress cause functional B vitamin deficiency in plants? Plant Physiology, 172, 2082–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson, A.D. & Gregory, J.F., 3rd . (2011) Folate biosynthesis, turnover, and transport in plants. Annual Review of Plant Biology, 62, 105–125. [DOI] [PubMed] [Google Scholar]
- Hasnain, G. , Roje, S. , Sa, N. , Zallot, R. , Ziemak, M.J. , de Crecy‐Lagard, V. et al. (2016) Bacterial and plant HAD enzymes catalyse a missing phosphatase step in thiamin diphosphate biosynthesis. The Biochemical Journal, 473, 157–166. [DOI] [PubMed] [Google Scholar]
- Hellmann, H. & Mooney, S. (2010) Vitamin B6: a molecule for human health? Molecules, 15, 442–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero, S. , Gonzalez, E. , Gillikin, J.W. , Velez, H. & Daub, M.E. (2011) Identification and characterization of a pyridoxal reductase involved in the vitamin B6 salvage pathway in Arabidopsis. Plant Molecular Biology, 76, 157–169. [DOI] [PubMed] [Google Scholar]
- Hildebrandt, T.M. , Nunes Nesi, A. , Araujo, W.L. & Braun, H.P. (2015) Amino acid catabolism in plants. Molecular Plant, 8, 1563–1579. [DOI] [PubMed] [Google Scholar]
- Hofmann, M. , Loubery, S. & Fitzpatrick, T.B. (2020) On the nature of thiamine triphosphate in Arabidopsis. Plant Direct, 4, e00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain, T. , Rosenberg, I. , Selhub, J. , Kishore, G. , Beachy, R. & Schubert, K. (2004) Enhancement of folates in plants through metabolic engineering. Proceedings of the National Academy of Sciences of the United States of America, 101, 5158–5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huennekens, F.M. (1968) Folic acid coenzymes in the biosynthesis of purines and pyrimidines. Vitamins and Hormones, 26, 375–394. [DOI] [PubMed] [Google Scholar]
- Ilag, L.L. , Kumar, A.M. & Soll, D. (1994) Light regulation of chlorophyll biosynthesis at the level of 5‐aminolevulinate formation in Arabidopsis. Plant Cell, 6, 265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, T. , Iimori, J. , Takayama, S. , Moriyama, A. , Yamauchi, A. , Hemmi, H. et al. (2013) Conserved pyridoxal protein that regulates Ile and Val metabolism. Journal of Bacteriology, 195, 5439–5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, T. , Yamamoto, K. , Hori, R. , Yamauchi, A. , Downs, D.M. , Hemmi, H. et al. (2019) Conserved pyridoxal 5′‐phosphate‐binding protein YggS impacts amino acid metabolism through pyridoxine 5′‐phosphate in Escherichia coli . Applied and Environmental Microbiology, 85, e00430–e00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger, B. , Abeling, N.G. , Salomons, G.S. , Struys, E.A. , Simas‐Mendes, M. , Geukers, V.G. et al. (2016) Pyridoxine responsive epilepsy caused by a novel homozygous PNPO mutation. Molecular Genetics and Metabolism Reports, 6, 60–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain, S.K. & Lim, G. (2001) Pyridoxine and pyridoxamine inhibits superoxide radicals and prevent lipid peroxidation, protein glycosylation, and (Na+K) ATPase activity reduction in high glucose treated human erythrocytes. Free Radical Biology & Medicine, 30, 232–237. [DOI] [PubMed] [Google Scholar]
- Jenkins, A.L. , Zhang, Y. , Ealick, S.E. & Begley, T.P. (2008) Mutagenesis studies on TenA: a thiamin salvage enzyme from Bacillus subtilis . Bioorganic Chemistry, 36, 29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone, D.L. , Al‐Shekaili, H.H. , Tarailo‐Graovac, M. , Wolf, N.I. , Ivy, A.S. , Demarest, S. et al. (2019) PLPHP deficiency: clinical, genetic, biochemical, and mechanistic insights. Brain, 142, 542–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi, J. , Folz, J.S. , Gregory, J.F., 3rd , McCarty, D.R. , Fiehn, O. & Hanson, A.D. (2019) Rethinking the PDH bypass and GABA shunt as thiamin‐deficiency workarounds. Plant Physiology, 181, 389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi, R. , Adhikari, S. , Patro, B.S. , Chattopadhyay, S. & Mukherjee, T. (2001) Free radical scavenging behavior of folic acid: evidence for possible antioxidant activity. Free Radical Biology & Medicine, 30, 1390–1399. [DOI] [PubMed] [Google Scholar]
- Jurgenson, C.T. , Begley, T.P. & Ealick, S.E. (2009) The structural and biochemical foundations of thiamin biosynthesis. Annual Review of Biochemistry, 78, 569–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kästner, U. , Hallmen, C. , Wiese, M. , Leistner, E. & Drewke, C. (2007) The human pyridoxal kinase, a plausible target for ginkgotoxin from Ginkgo biloba . The FEBS Journal, 274, 1036–1045. [DOI] [PubMed] [Google Scholar]
- Khozaei, M. , Fisk, S. , Lawson, T. , Gibon, Y. , Sulpice, R. , Stitt, M. et al. (2015) Overexpression of plastid transketolase in tobacco results in a thiamine auxotrophic phenotype. Plant Cell, 27, 432–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus, S.M. , Kunji, E.R. , Bozzo, G.G. , Noiriel, A. , de la Garza, R.D. , Basset, G.J. et al. (2005) Higher plant plastids and cyanobacteria have folate carriers related to those of trypanosomatids. The Journal of Biological Chemistry, 280, 38457–38463. [DOI] [PubMed] [Google Scholar]
- Klaus, S.M. , Wegkamp, A. , Sybesma, W. , Hugenholtz, J. , Gregory, J.F., 3rd & Hanson, A.D. (2005) A nudix enzyme removes pyrophosphate from dihydroneopterin triphosphate in the folate synthesis pathway of bacteria and plants. The Journal of Biological Chemistry, 280, 5274–5280. [DOI] [PubMed] [Google Scholar]
- Kolton, A. , Dlugosz‐Grochowska, O. , Wojciechowska, R. & Czaja, M. (2022) Biosynthesis regulation of folates and phenols in plants. Scientia Horticulturae, 291, 110561. [Google Scholar]
- Kronn, D. & Goldman, D. (2008) Hereditary folate malabsorption. Medicine, 81, 51–68. [DOI] [PubMed] [Google Scholar]
- Li, K.T. , Moulin, M. , Mangel, N. , Albersen, M. , Verhoeven‐Duif, N.M. , Ma, Q. et al. (2015) Increased bioavailable vitamin B6 in field‐grown transgenic cassava for dietary sufficiency. Nature Biotechnology, 33, 1029–1032. [DOI] [PubMed] [Google Scholar]
- Li, W. , Liang, Q. , Mishra, R.C. , Sanchez‐Mu Oz, R. , Wang, H. , Chen, X. et al. (2021) The 5‐formyl‐tetrahydrofolate proteome links folates with C/N metabolism and reveals feedback regulation of folate biosynthesis. Plant Cell, 33, 3367–3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, Q. , Wang, K. , Liu, X. , Riaz, B. , Jiang, L. , Wan, X. et al. (2019) Improved folate accumulation in genetically modified maize and wheat. Journal of Experimental Botany, 70, 1539–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, X. , Abel, S. , Keller, J.A. , Shen, N.F. & Theologis, A. (1992) The 1‐aminocyclopropane‐1‐carboxylate synthase gene family of Arabidopsis thaliana . Proceedings of the National Academy of Sciences of the United States of America, 89, 11046–11050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarchikov, A.F. , Lakaye, B. , Gulyai, I.E. , Czerniecki, J. , Coumans, B. , Wins, P. et al. (2003) Thiamine triphosphate and thiamine triphosphatase activities: from bacteria to mammals. Cellular and Molecular Life Sciences, 60, 1477–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangel, N. , Fudge, J.B. , Fitzpatrick, T.B. , Gruissem, W. & Vanderschuren, H. (2017) Vitamin B1 diversity and characterization of biosynthesis genes in cassava. Journal of Experimental Botany, 68, 3351–3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangel, N. , Fudge, J.B. , Gruissem, W. , Fitzpatrick, T.B. & Vanderschuren, H. (2022) Natural variation in vitamin B1 and vitamin B6 contents in rice germplasm. Frontiers in Plant Science, 13, 856880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangel, N. , Fudge, J.B. , Li, K.T. , Wu, T.Y. , Tohge, T. , Fernie, A.R. et al. (2019) Enhancement of vitamin B6 levels in rice expressing Arabidopsis vitamin B6 biosynthesis de novo genes. The Plant Journal, 99, 1047–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marce‐Grau, A. , Marti‐Sanchez, L. , Baide‐Mairena, H. , Ortigoza‐Escobar, J.D. & Perez‐Duenas, B. (2019) Genetic defects of thiamine transport and metabolism: a review of clinical phenotypes, genetics, and functional studies. Journal of Inherited Metabolic Disease, 42, 581–597. [DOI] [PubMed] [Google Scholar]
- Marti‐Carvajal, A.J. , Sola, I. , Lathyris, D. & Dayer, M. (2017) Homocysteine‐lowering interventions for preventing cardiovascular events. Cochrane Database of Systematic Reviews, 8, CD006612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, P.R. , Singleton, C.K. & Hiller‐Sturmhofel, S. (2003) The role of thiamine deficiency in alcoholic brain disease. Alcohol Research & Health, 27, 134–142. [PMC free article] [PubMed] [Google Scholar]
- Martinis, J. , Gas‐Pascual, E. , Szydlowski, N. , Crevecoeur, M. , Gisler, A. , Burkle, L. et al. (2016) Long‐distance transport of thiamine (vitamin B1) is concomitant with that of polyamines. Plant Physiology, 171, 542–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzio, A. , Merigliano, C. , Gatti, M. & Verni, F. (2014) Sugar and chromosome stability: clastogenic effects of sugars in vitamin B6‐deficient cells. PLoS Genetics, 10, e1004199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merigliano, C. , Mascolo, E. , Burla, R. , Saggio, I. & Verni, F. (2018) The relationship between vitamin B6, diabetes and cancer. Frontiers in Genetics, 9, 388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill, A.H. & Henderson, J.M. (1990) Vitamin‐B6 metabolism by human liver. Annals of the New York Academy of Sciences, 585, 110–117. [DOI] [PubMed] [Google Scholar]
- Mezhenska, O.A. , Aleshin, V.A. , Kaehne, T. , Artiukhov, A.V. & Bunik, V.I. (2020) Regulation of malate dehydrogenases and glutamate dehydrogenase of mammalian brain by thiamine in vitro and in vivo. Biochemistry (Mosc), 85, 27–39. [DOI] [PubMed] [Google Scholar]
- Michaeli, S. & Fromm, H. (2015) Closing the loop on the GABA shunt in plants: are GABA metabolism and signaling entwined? Frontiers in Plant Science, 6, 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills, J.L. , Molloy, A.M. & Reynolds, E.H. (2018) Do the benefits of folic acid fortification outweigh the risk of masking vitamin B‐12 deficiency? British Medical Journal, 360, k724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura, M. , Zallot, R. , Niehaus, T.D. , Hasnain, G. , Gidda, S.K. , Nguyen, T.N. et al. (2016) Arabidopsis TH2 encodes the orphan enzyme thiamin monophosphate phosphatase. Plant Cell, 28, 2683–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minhas, A.P. , Tuli, R. & Puri, S. (2018) Pathway editing targets for thiamine biofortification in rice grains. Frontiers in Plant Science, 9, 975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mkrtchyan, G. , Aleshin, V. , Parkhomenko, Y. , Kaehne, T. , Di Salvo, M.L. , Parroni, A. et al. (2015) Molecular mechanisms of the non‐coenzyme action of thiamin in brain: biochemical, structural and pathway analysis. Scientific Reports, 5, 12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozafar, A. (2017) Plant vitamins: Agronomic, physiological, and nutritional aspects. Boca Raton: CRC Press. [Google Scholar]
- Nair, M.K. , Augustine, L.F. & Konapur, A. (2016) Food‐based interventions to modify diet quality and diversity to address multiple micronutrient deficiency. Frontiers in Public Health, 3, 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nghiem, H.O. , Bettendorff, L. & Changeux, J.P. (2000) Specific phosphorylation of Torpedo 43K rapsyn by endogenous kinase(s) with thiamine triphosphate as the phosphate donor. The FASEB Journal, 14, 543–554. [DOI] [PubMed] [Google Scholar]
- Nishikawa, M. , Hosokawa, K. , Ishiguro, M. , Minamioka, H. , Tamura, K. , Hara‐Nishimura, I. et al. (2008) Degradation of sphingoid long‐chain base 1‐phosphates (LCB‐1Ps): Functional characterization and expression of AtDPL1 encoding LCB‐1P lyase involved in the dehydration stress response in Arabidopsis. Plant & Cell Physiology, 49, 1758–1763. [DOI] [PubMed] [Google Scholar]
- Noiriel, A. , Naponelli, V. , Bozzo, G.G. , Gregory, J.F., 3rd & Hanson, A.D. (2007a) Folate salvage in plants: pterin aldehyde reduction is mediated by multiple non‐specific aldehyde reductases. The Plant Journal, 51, 378–389. [DOI] [PubMed] [Google Scholar]
- Noiriel, A. , Naponelli, V. , Gregory, J.F., 3rd & Hanson, A.D. (2007b) Pterin and folate salvage. Plants and Escherichia coli lack capacity to reduce oxidized pterins. Plant Physiology, 143, 1101–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordally, Z.B. , Trichtinger, C. , Dalvit, I. , Hofmann, M. , Roux, C. , Zamboni, N. et al. (2020) The coenzyme thiamine diphosphate displays a daily rhythm in the Arabidopsis nucleus. Communications Biology, 3, 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka, T. , Sugitatsu, H. , Nordin, H. , Thakur, M.K. , Aoyama, M. , Sasagawa, T. et al. (2001) Pyridoxal 5′‐phosphate inhibits DNA binding of HNF1. Biochimica et Biophysica Acta, 1568, 189–196. [DOI] [PubMed] [Google Scholar]
- Ollilainen, V.M. (1999) HPLC analysis of vitamin B6 in foods. Agricultural and Food Science, 8, 515–619. [Google Scholar]
- Orsomando, G. , Bozzo, G.G. , de la Garza, R.D. , Basset, G.J. , Quinlivan, E.P. , Naponelli, V. et al. (2006) Evidence for folate‐salvage reactions in plants. The Plant Journal, 46, 426–435. [DOI] [PubMed] [Google Scholar]
- Osborne, C.B. , Lowe, K.E. & Shane, B. (1993) Regulation of folate and one‐carbon metabolism in mammalian cells. I. Folate metabolism in Chinese hamster ovary cells expressing Escherichia coli or human folylpoly‐gamma‐glutamate synthetase activity. The Journal of Biological Chemistry, 268, 21657–21664. [PubMed] [Google Scholar]
- Ouyang, Y. , Wu, Q. , Li, J. , Sun, S. & Sun, S. (2020) S‐adenosylmethionine: A metabolite critical to the regulation of autophagy. Cell Proliferation, 53, e12891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pácal, L. , Kuricová, K. & Kaňková, K. (2014) Evidence for altered thiamine metabolism in diabetes: Is there a potential to oppose gluco‐ and lipotoxicity by rational supplementation? World Journal of Diabetes, 5, 288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parletta, N. , Milte, C.M. & Meyer, B.J. (2013) Nutritional modulation of cognitive function and mental health. The Journal of Nutritional Biochemistry, 24, 725–743. [DOI] [PubMed] [Google Scholar]
- Parra, M. , Stahl, S. & Hellmann, H. (2018) Vitamin B‐6 and its role in cell metabolism and physiology. Cells‐Basel, 7, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton, D.A. , Johnson, M. & Ward, E.R. (1996) Biotin synthase from Arabidopsis thaliana ‐ cDNA isolation and characterization of gene expression. Plant Physiology, 112, 371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattyn, J. , Vaughan‐Hirsch, J. & van de Poel, B. (2021) The regulation of ethylene biosynthesis: a complex multilevel control circuitry. The New Phytologist, 229, 770–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percudani, R. & Peracchi, A. (2003) A genomic overview of pyridoxal‐phosphate‐dependent enzymes. EMBO Reports, 4, 850–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plecko, B. , Zweier, M. , Begemann, A. , Mathis, D. , Schmitt, B. , Striano, P. et al. (2017) Confirmation of mutations in PROSC as a novel cause of vitamin B‐6‐dependent epilepsy. Journal of Medical Genetics, 54, 809–814. [DOI] [PubMed] [Google Scholar]
- Prunetti, L. , El Yacoubi, B. , Schiavon, C.R. , Kirkpatrick, E. , Huang, L.L. , Bailly, M. et al. (2016) Evidence that COG0325 proteins are involved in PLP homeostasis. Microbiology, 162, 694–706. [DOI] [PubMed] [Google Scholar]
- Qiu, A.D. , Jansen, M. , Sakaris, A. , Min, S.H. , Chattopadhyay, S. , Tsai, E. et al. (2006) Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell, 127, 917–928. [DOI] [PubMed] [Google Scholar]
- Quinlivan, E.P. , Roje, S. , Basset, G. , Shachar‐Hill, Y. , Gregory, J.F. & Hanson, A.D. (2003) The folate precursor p‐aminobenzoate is reversibly converted to its glucose ester in the plant cytosol. The Journal of Biological Chemistry, 278, 20731–20737. [DOI] [PubMed] [Google Scholar]
- Raghubeer, S. & Matsha, T.E. (2021) Methylenetetrahydrofolate (MTHFR), the one‐carbon cycle, and cardiovascular risks. Nutrients, 13, 4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos, R.J. , Albersen, M. , Vringer, E. , Bosma, M. , Zwakenberg, S. , Zwartkruis, F. et al. (2019) Discovery of pyridoxal reductase activity as part of human vitamin B6 metabolism. Biochimica et Biophysica Acta, 1863, 1088–1097. [DOI] [PubMed] [Google Scholar]
- Ratanasthien, K. , Blair, J.A. , Leeming, R.J. , Cooke, W.T. & Melikian, V. (1974) Folates in human‐serum. Journal of Clinical Pathology, 27, 875–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautiainen, S. , Manson, J.E. , Lichtenstein, A.H. & Sesso, H.D. (2016) Dietary supplements and disease prevention ‐ a global overview. Nature Reviews. Endocrinology, 12, 407–420. [DOI] [PubMed] [Google Scholar]
- Ravanel, S. , Douce, R. & Rebeille, F. (2011) Metabolism of folates in plants. Advances in Botanical Research, 59, 67–106. [Google Scholar]
- Reinbott, A. , Schelling, A. , Kuchenbecker, J. , Jeremias, T. , Russell, I. , Kevanna, O. et al. (2016) Nutrition education linked to agricultural interventions improved child dietary diversity in rural Cambodia. The British Journal of Nutrition, 116, 1457–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rindi, G. & Laforenza, U. (2000) Thiamine intestinal transport and related issues: recent aspects. Proceedings of the Society for Experimental Biology and Medicine, 224, 246–255. [DOI] [PubMed] [Google Scholar]
- Rosado‐Souza, L. , Proost, S. , Moulin, M. , Bergmann, S. , Bocobza, S.E. , Aharoni, A. et al. (2019) Appropriate thiamin pyrophosphate levels are required for acclimation to changes in photoperiod. Plant Physiology, 180, 185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, C.A. , Caballero, B. , Cousins, R.J. , Tucker, K.L. & Ziegler, T.R. (2012) Modern nutrition in health and disease. Philadelphia, PA: Wolters Kluwer Health Adis (ESP). [Google Scholar]
- Ruel‐Bergeron, J.C. , Stevens, G.A. , Sugimoto, J.D. , Roos, F.F. , Ezzati, M. , Black, R.E. et al. (2015) Global update and trends of hidden hunger, 1995‐2011: The hidden hunger index. PLoS One, 10, e0143497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said, H.M. (2011) Intestinal absorption of water‐soluble vitamins in health and disease. The Biochemical Journal, 437, 357–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said, H.M. (2013) Recent advances in transport of water‐soluble vitamins in organs of the digestive system: a focus on the colon and the pancreas. American Journal of Physiology. Gastrointestinal and Liver Physiology, 305, G601–G610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambon, M. , Pavlova, O. , Alhama‐Riba, J. , Wins, P. , Brans, A. & Bettendorff, L. (2022) Product inhibition of mammalian thiamine pyrophosphokinase is an important mechanism for maintaining thiamine diphosphate homeostasis. Biochimica et Biophysica Acta, 1866, 130071. [DOI] [PubMed] [Google Scholar]
- Schaller, K. & Holler, H. (1975) Thiamine absorption in the rat. ii. intestinal alkaline phosphatase activity and thiamine absorption from rat small intestine in‐vitro and in‐vivo. International Journal for Vitamin and Nutrition Research, 45, 30–38. [PubMed] [Google Scholar]
- Schwartz, R. & Kjeldgaard, N.O. (1951) The enzymic oxidation of pyridoxal by liver aldehyde oxidase. The Biochemical Journal, 48, 333–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz, G. , Mendel, R.R. & Ribbe, M.W. (2009) Molybdenum cofactors, enzymes and pathways. Nature, 460, 839–847. [DOI] [PubMed] [Google Scholar]
- Shikata, H. , Koyama, S. , Egi, Y. , Yamada, K. & Kawasaki, T. (1989) Cytosolic adenylate kinase catalyzes the synthesis of thiamin triphosphate from thiamin diphosphate. Biochemistry International, 18, 933–941. [PubMed] [Google Scholar]
- Shohag, M.J. , Wei, Y.Y. , Yu, N. , Zhang, J. , Wang, K. , Patring, J. et al. (2011) Natural variation of folate content and composition in spinach (Spinacia oleracea) germplasm. Journal of Agricultural and Food Chemistry, 59, 12520–12526. [DOI] [PubMed] [Google Scholar]
- Shohag, M.J.I. , Wei, Y. , Zhang, J. , Feng, Y. , Rychlik, M. , He, Z. et al. (2020) Genetic and physiological regulation of folate in pak choi (Brassica rapa subsp. Chinensis) germplasm. Journal of Experimental Botany, 71, 4914–4929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ShuoHao, H. , Jing, L. , Jie, Z. , JianYun, Z. & LongQuan, H. (2019) Identification and characterization of a pyridoxal 5′‐phosphate phosphatase in tobacco plants. Plant Science, 278, 88–95. [DOI] [PubMed] [Google Scholar]
- Stach, K. , Stach, W. & Augoff, K. (2021) Vitamin B6 in health and disease. Nutrients, 13, 3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stangoulis, J.C.R. & Knez, M. (2022) Biofortification of major crop plants with iron and zinc ‐ achievements and future directions. Plant and Soil, 474, 57–76. [Google Scholar]
- Stanulovic, M. , Jeremic, V. , Leskovac, V. & Chaykin, S. (1976) New pathway of conversion of pyridoxal to 4‐pyridoxic acid. Enzyme, 21, 357–369. [DOI] [PubMed] [Google Scholar]
- Stepanova, A.N. , Robertson‐Hoyt, J. , Yun, J. , Benavente, L.M. , Xie, D.Y. , DoleZal, K. et al. (2008) TAA1‐mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell, 133, 177–191. [DOI] [PubMed] [Google Scholar]
- Storozhenko, S. , De Brouwer, V. , Volckaert, M. , Navarrete, O. , Blancquaert, D. , Zhang, G.F. et al. (2007) Folate fortification of rice by metabolic engineering. Nature Biotechnology, 25, 1277–1279. [DOI] [PubMed] [Google Scholar]
- Stover, P.J. & Field, M.S. (2015) Vitamin B‐6. Advances in Nutrition, 6, 132–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stralsjo, L.M. , Witthoft, C.M. , Sjoholm, I.M. & Jagerstad, M.I. (2003) Folate content in strawberries (Fragaria x ananassa): effects of cultivar, ripeness, year of harvest, storage, and commercial processing. Journal of Agricultural and Food Chemistry, 51, 128–133. [DOI] [PubMed] [Google Scholar]
- Strobbe, S. & Van Der Straeten, D. (2018) Toward eradication of B‐vitamin deficiencies: considerations for crop biofortification. Frontiers in Plant Science, 9, 443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobbe, S. , Verstraete, J. , Stove, C. & Van Der Straeten, D. (2021a) Metabolic engineering of rice endosperm towards higher vitamin B1 accumulation. Plant Biotechnology Journal, 19, 1253–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobbe, S. , Verstraete, J. , Stove, C. & Van Der Straeten, D. (2021b) Metabolic engineering provides insight into the regulation of thiamin biosynthesis in plants. Plant Physiology, 186, 1832–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh, J.R. , Herbig, A.K. & Stover, P.J. (2001) New perspectives on folate catabolism. Annual Review of Nutrition, 21, 255–282. [DOI] [PubMed] [Google Scholar]
- Suzuki, Y. & Brown, G.M. (1974) The biosynthesis of folic acid. XII. Purification and properties of dihydroneopterin triphosphate pyrophosphohydrolase. The Journal of Biological Chemistry, 249, 2405–2410. [PubMed] [Google Scholar]
- Tambasco‐Studart, M. , Titiz, O. , Raschle, T. , Forster, G. , Amrhein, N. & Fitzpatrick, T.B. (2005) Vitamin B6 biosynthesis in higher plants. Proceedings of the National Academy of Sciences of the United States of America, 102, 13687–13692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannert, M. , Balcke, G.U. , Tissier, A. & Kock, M. (2021) At4g29530 is a phosphoethanolamine phosphatase homologous to PECP1 with a role in flowering time regulation. The Plant Journal, 107, 1072–1083. [DOI] [PubMed] [Google Scholar]
- Titcomb, T.J. & Tanumihardjo, S.A. (2019) Global concerns with B vitamin statuses: biofortification, fortification, hidden hunger, interactions, and toxicity. Comprehensive Reviews in Food Science and Food Safety, 18, 1968–1984. [DOI] [PubMed] [Google Scholar]
- Titiz, O. , Tambasco‐Studart, M. , Warzych, E. , Apel, K. , Amrhein, N. , Laloi, C. et al. (2006) PDX1 is essential for vitamin B6 biosynthesis, development and stress tolerance in Arabidopsis. The Plant Journal, 48, 933–946. [DOI] [PubMed] [Google Scholar]
- Todd, K.G. & Butterworth, R.F. (1999) Mechanisms of selective neuronal cell death due to thiamine deficiency. Annals. New York Academy of Sciences, 893, 404–411. [DOI] [PubMed] [Google Scholar]
- Tong, H. , Leasure, C.D. , Yen, R. , Hou, X. , O'Neil, N. , Ting, D. et al. (2021) Arabidopsis ROOT UV‐B SENSITIVE 1 and 2 interact with aminotransferases to regulate vitamin B6 homeostasis. bioRxiv 2021.2003.2001.433438. [Google Scholar]
- Tsang, B.L. , Devine, O.J. , Cordero, A.M. , Marchetta, C.M. , Mulinare, J. , Mersereau, P. et al. (2015) Assessing the association between the methylenetetrahydrofolate reductase (MTHFR) 677C > T polymorphism and blood folate concentrations: a systematic review and meta‐analysis of trials and observational studies. The American Journal of Clinical Nutrition, 101, 1286–1294. [DOI] [PubMed] [Google Scholar]
- Tulchinsky, T.H. (2010) Micronutrient deficiency conditions: global health issues. Public Health Reviews, 32, 243–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully, D.B. , Allgood, V.E. & Cidlowski, J.A. (1994) Modulation of steroid receptor‐mediated gene expression by vitamin B6. The FASEB Journal, 8, 343–349. [PubMed] [Google Scholar]
- Van de Poel, B. , Smet, D. & Van Der Straeten, D. (2015) Ethylene and hormonal cross talk in vegetative growth and development. Plant Physiology, 169, 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Veldhoven, P.P. , Gijsbers, S. , Mannaerts, G.P. , Vermeesch, J.R. & Brys, V. (2000) Human sphingosine‐1‐phosphate lyase: cDNA cloning, functional expression studies and mapping to chromosome 10q22(1). Biochimica et Biophysica Acta, 1487, 128–134. [DOI] [PubMed] [Google Scholar]
- Vanderschuren, H. , Boycheva, S. , Li, K.T. , Szydlowski, N. , Gruissem, W. & Fitzpatrick, T.B. (2013) Strategies for vitamin B6 biofortification of plants: a dual role as a micronutrient and a stress protectant. Frontiers in Plant Science, 4, 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrolijk, M.F. , Opperhuizen, A. , Jansen, E.H.J.M. , Hageman, G.J. , Bast, A. & Haenen, G.R.M.M. (2017) The vitamin B6 paradox: Supplementation with high concentrations of pyridoxine leads to decreased vitamin B6 function. Toxicology In Vitro, 44, 206–212. [DOI] [PubMed] [Google Scholar]
- Vu, H.N. & Downs, D.M. (2021) Loss of YggS (COG0325) impacts aspartate metabolism in Salmonella enterica . Molecular Microbiology, 116, 1232–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu, H.N. , Ito, T. & Downs, D.M. (2020) The role of YggS in vitamin B‐6 homeostasis in Salmonella enterica is informed by heterologous expression of yeast SNZ3. Journal of Bacteriology, 202, e00383–e00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachter, A. , Tunc‐Ozdemir, M. , Grove, B.C. , Green, P.J. , Shintani, D.K. & Breaker, R.R. (2007) Riboswitch control of gene expression in plants by splicing and alternative 3′ end processing of mRNAs. Plant Cell, 19, 3437–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wien, T.N. , Pike, E. , Wisloff, T. , Staff, A. , Smeland, S. & Klemp, M. (2012) Cancer risk with folic acid supplements: a systematic review and meta‐analysis. BMJ Open, 2, e000653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaman, M. & Mizrak, O.F. (2019) Determination and evaluation of in vitro bioaccessibility of the pyridoxal, pyridoxine, and pyridoxamine forms of vitamin B6 in cereal‐based baby foods. Food Chemistry, 298, 125042. [DOI] [PubMed] [Google Scholar]
- Yazdani, M. , Zallot, R. , Tunc‐Ozdemir, M. , de Crécy‐Lagard, V. , Shintani, D.K. & Hanson, A.D. (2013) Identification of the thiamin salvage enzyme thiazole kinase in Arabidopsis and maize. Phytochemistry, 94, 68–73. [DOI] [PubMed] [Google Scholar]
- Ye, Y. , Qu, J. , Pu, Y. , Rao, S. , Xu, F. & Wu, C. (2020) Selenium biofortification of crop food by beneficial microorganisms. Journal of Fungi, 6, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama, T. & Hatakeyama, K. (1998) Decameric GTP cyclohydrolase I forms complexes with two pentameric GTP cyclohydrolase I feedback regulatory proteins in the presence of phenylalanine or of a combination of tetrahydrobiopterin and GTP. The Journal of Biological Chemistry, 273, 20102–20108. [DOI] [PubMed] [Google Scholar]
- Zallot, R. , Yazdani, M. , Goyer, A. , Ziemak, M.J. , Guan, J.‐C. , McCarty, D.R. et al. (2014) Salvage of the thiamine pyrimidine moiety by plant TenA proteins lacking an active‐site cysteine. The Biochemical Journal, 463, 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeman, S.C. , Thorneycroft, D. , Schupp, N. , Chapple, A. , Weck, M. , Dunstan, H. et al. (2004) Plastidial alpha‐glucan phosphorylase is not required for starch degradation in Arabidopsis leaves but has a role in the tolerance of abiotic stress. Plant Physiology, 135, 849–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. , Deng, X. , Miki, D. , Cutler, S. , La, H. , Hou, Y.J. et al. (2012a) Sulfamethazine suppresses epigenetic silencing in Arabidopsis by impairing folate synthesis. Plant Cell, 24, 1230–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, T. , Wang, S. , Lin, Y. , Xu, W. , Ye, D. , Xiong, Y. et al. (2012b) Acetylation negatively regulates glycogen phosphorylase by recruiting protein phosphatase 1. Cell Metabolism, 15, 75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, R. , Diop‐Bove, N. , Visentin, M. & Goldman, I.D. (2011) Mechanisms of membrane transport of folates into cells and across epithelia. Annual Review of Nutrition, 31, 177–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, R. , Gao, F. & Goldman, I.D. (2002) Reduced folate carrier transports thiamine monophosphate: an alternative route for thiamine delivery into mammalian cells. American Journal of Physiology. Cell Physiology, 282, 1512–1517. [DOI] [PubMed] [Google Scholar]
- Zhao, R. , Min, S.H. , Qiu, A. , Sakaris, A. , Goldberg, G.L. , Sandoval, C. et al. (2007) The spectrum of mutations in the PCFT gene, coding for an intestinal folate transporter, that are the basis for hereditary folate malabsorption. Blood, 110, 1147–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, Y. , Lin, T.Y. , Lee, G. , Paddock, M.N. , Momb, J. , Cheng, Z. et al. (2018) Mitochondrial one‐carbon pathway supports cytosolic folate integrity in cancer cells. Cell, 175, 1546–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylka, M.J. , Sowa, N.A. , Taylor‐Blake, B. , Twomey, M.A. , Herrala, A. , Voikar, V. et al. (2008) Prostatic acid phosphatase is an ectonucleotidase and suppresses pain by generating adenosine. Neuron, 60, 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]


