Abstract
Objectives
To challenge a vapour fast freezing (VFF) cryopreservation procedure (conventional VFF) with several vitrification protocols and VFF conducted with small semen volumes (10 μl, microVFF), in order to implement a procedure for sperm banking in subjects with small sperm number.
Materials and methods
Conventional VFF was conducted with test yolk buffer (TYB) as freezing medium and 500 μl straws as carriers. MicroVFF was conducted with TYB and using tips or cell sleepers as carriers. Vitrification was performed with TYB or SpermFreeze as freezing medium and with microspheres and tips as carriers. The effect of different procedures on progressive and total motility, viability, oxidative stress and DNA fragmentation of spermatozoa (sDF) was determined. Fresh and thawed samples, the latter after adequate washing/centrifuging, were evaluated. In some experiments, motility and viability recovery was determined in thawed samples, omitting the washing/centrifuging step.
Results
All the cryopreservation procedures blunted sperm motility and viability and induced increase of oxidative stress and sDF. However, VFF better preserved sperm motility and viability and less induced oxidative stress and sDF than vitrification, independently from the freezing medium and the carriers used in the latter. MicroVFF with cell sleepers resulted in a percentage increase of 57.58 ± 63.63%, 48.82 ± 74.96% and 24.55 ± 39.20% of, respectively, progressive and total motility and viability compared to the conventional VFF. Further, when tips were used, microVFF resulted in a percentage decrease of 15.77 ± 20.77% of sDF with respect to conventional VFF. Finally, omission of washing/centrifuging in post thawed samples, resulted in a much lower negative effect on motility and viability.
Discussion and conclusion
VFF, and in particular microVFF, better prevents sperm cryodamage than vitrification. Washing/centrifuging step after sample thawing seems to be responsible for a relevant fraction of damage to sperm motility and viability. Overall, our results are promising for developing a novel strategy of sperm banking in subjects with small sperm number, where low semen volumes are mandatory.
Keywords: oxidative stress, sperm cryopreservation, sperm DNA fragmentation, vapour fast freezing, vitrification
1. INTRODUCTION
Sperm cryopreservation is an essential procedure for assisted reproductive techniques (ARTs) and male fertility preservation. Indeed, this procedure gives a chance to preserve fertility to subjects who are about to undergo gonadotoxic therapies, like cancer patients. In addition, sperm cryopreservation offers the azoospermic and oligozoospermic patients undergoing in vitro fertilization (IVF) cycles a way to avoid repeated biopsies or aspirations to recover spermatozoa from testis, 1 which are necessary in case of treatment failure.
Unfortunately, sperm cryopreservation provokes several damages, due to the intra‐ and extracellular formation of ice and induction of osmotic stress. 2 Such damages include loss of motility and viability, 3 alterations of acrosome with loss of the outer acrosomal membrane and depletion of acrosomal content, 4 and induction of DNA fragmentation. 5 Oxidative stress, occurring when high concentrations of Reactive Oxygen Species (ROS) overwhelm available antioxidant defences, is believed to be one of the main mechanisms responsible for damage induced by freezing/thawing spermatozoa. 6 On one hand, it is known that ROS production increases during cell freezing, 7 and on the other hand, ROS attack is potentially able to target all cell components. 8 Spermatozoa are particularly vulnerable to oxidative damage due to their high amount of poly‐unsaturated fatty acids 9 and limited antioxidant defences. 10 These features make spermatozoa very susceptible to lipid peroxidation which, in turn, alters both structure and permeability properties of the membrane. 6 Another consequence of cryopreservation is sperm DNA fragmentation (sDF), a relevant damage due to its negative impact 11 , 12 on outcomes of ARTs, used with cryopreserved semen samples. This type of DNA damage can be provoked by three known mechanisms: abortive apoptosis, defects in chromatin maturation and ROS attack, the latter being the only one acting in in vitro condition, including freezing and thawing of spermatozoa. 13
To reduce sperm cryodamage, permeable cryoprotectants (CPAs) have been used to lower the melting point and decrease the likelihood of ice nucleation in freezing media. 14 The addition and removal of these CPAs, however, induce dramatic osmotic changes, in turn provoking mechanical damage to cell membrane and even cell death. Thus, non‐permeant CPAs have been introduced to protect cells from excessive osmotic changes occurring during their post‐thaw CPAs removal. 15
Slow/fast freezing and vitrification are the two main approaches to cryopreserve spermatozoa. Fast freezing is also called vapour fast freezing (VFF) as samples are first placed into liquid nitrogen vapours. Vitrification is a process solidifying liquid into an amorphous, glassy state and consists in placing the sample directly into liquid nitrogen. It has been suggested that vitrification is less detrimental than conventional freezing since it prevents the intra‐ and extracellular ice formation. 16 Moreover, vitrification is also considered more feasible for sperm cryopreservation in samples with extremely low number of spermatozoa, such as those retrieved from surgical testicular sperm extraction or extended semen search in azoospermic subjects.
Our laboratory, since 1998, has been using a VFF procedure with test yolk buffer (TYB) as the freezing medium and 500 μl straws as carriers to cryopreserve semen samples (from herein indicated as conventional VFF). In the effort to optimise sperm banking procedures, especially in subjects with a very low number of spermatozoa, we conducted a pilot study to compare conventional VFF with different vitrification protocols (i.e., with different freezing media and carriers) and to VFF conducted with small semen volumes (microVFF) and with different carriers. To this aim, we determined motility, viability, levels of oxidative stress and sDF in semen samples before and after cryopreservation with the different procedures. Finally, we assessed how two thawing protocols affected recovery of motility and viability after cryopreservation with conventional VFF and vitrification procedures.
2. MATERIAL AND METHODS
2.1. Reagents and media
Halosperm kit was from Halotech DNA (Madrid, Spain). Cell sleepers were from Nipro (Osaka, Japan). Around 500 μl straws were from Cryo Bio System (L'Aigle, France). TYB and SpermFreeze (SF) medium were purchased by Fujifilm, Irvine Scientific (Rome, Italy) and FertiPro (Beernem, Belgium), respectively. MitoSOX Red and LIVE/DEAD Fixable Green Dead Cell Stain (LD‐G) were from Thermo Fisher Scientific (Waltham, MA USA). All the other reagents were from Merck Life Science, Milan, Italy.
2.2. Sample collection and semen analysis
Semen samples were collected according to World Health Organization (WHO) criteria 17 from 49 male partners of infertile couples undergoing routine semen analysis in the Semen Cryopreservation and Andrology Laboratory of Careggi Hospital and after obtainment of written informed consent. Semen analysis was conducted following the WHO guidelines 17 and consisted in determination of: (i) sperm number and concentration, (ii) sperm progressive and total motility and (iii) sperm morphology. Briefly, after proper dilution with formalin, sperm concentration was determined using a Neubauer improved cell counting chamber and multiplied by semen volume to obtain sperm number/ejaculate. Semen volume was evaluated by weighting the sample. Sperm motility was determined by distinguishing progressive, non‐progressive and immotile spermatozoa, by scoring at least 200 cells. Sperm morphology was evaluated after Diff‐Quick staining in at least 200 spermatozoa. Semen pH was determined by spreading a drop of sample on a pH paper and comparing the obtained colour with the calibration strip. Inclusion criteria were: sperm concentration ≥50 million/ml and total motility ≥ 50%. Sperm viability was evaluated by using eosin test: the sample was mixed with eosin solution (1:1), spread on a slide and examined by light microscopy. 17 We calculated the intra‐assay coefficient of variation (CV) for eosin test, by processing three aliquots of the same semen sample (n = 5, Table S1). To assess the reliability of eosin test in our hands, we prepared a positive control. After heating five semen samples in a bath at 70°C (20 min), we found that viability decreases, on average, from 78.77 ± 6.69% to 19.20 ± 2.79%. Samples with detectable leukocytes were excluded. To detect leukocytes, we deposed 10 μl of semen on pre‐stained slides Testsimplets® (AB Analitica, Padua, Italy), then evaluated at microscope with a 100× objective. Semen Cryopreservation and Andrology Laboratory of Careggi Hospital participates in the external quality control programs: United Kingdom National External Quality Assessment Service (NEQAS) and Verifica Esterna di Qualità of Tuscany. The average values of age, abstinence and standard semen parameters in the recruited subjects are reported in Table 1.
TABLE 1.
Characteristics and semen parameters of subjects recruited for I, II, III experimental sets and for experiments comparing the two thawing methods. Data are expressed as mean ± SD or median [IQR], (n = 49)
| Age (years) | Abstinence (d) | Volume (ml) | pH | Sperm number (106/ejaculate) | Concentration (106/ml) | Total motility (%) | Progressive motility (%) | Morphology (%) |
|---|---|---|---|---|---|---|---|---|
| 35.04 ± 5.88 | 4.00 | 4.20 | 7.60 | 332.80 | 87.00 | 64.37 ± 8.06 | 56.35 ± 9.67 | 5.00 |
| [3.00–6.00] | [3.15–5.50] | [7.50–7.80] | [230.20–407.00] | [51.55–136.00] | [4.00–7.00] |
After completing semen analysis (1 h from sample collection), samples were split into several aliquots, subsequently cryopreserved with different procedures as indicated in the experimental design. Samples were kept at 37°C during semen analysis and till starting cryopreservation procedures. The study has been performed according to the Declaration of Helsinki and was approved by the ethical committee of AOU Careggi (protocol no. 15554_bio).
2.3. Experimental design
The part of the study comparing different cryopreservation procedures consists in three experimental sets (Figure 1). In the first set, we compared conventional VFF (see below for description) with two vitrification (VIT) protocols conducted by forming microspheres (M) 18 and using TYB or SF as freezing medium: VIT M/TYB and VIT M/SF, respectively. The two media differ for the contained CPAs: egg yolk and glycerol (TYB) and glycerol, sucrose and human serum albumin (SF).
FIGURE 1.
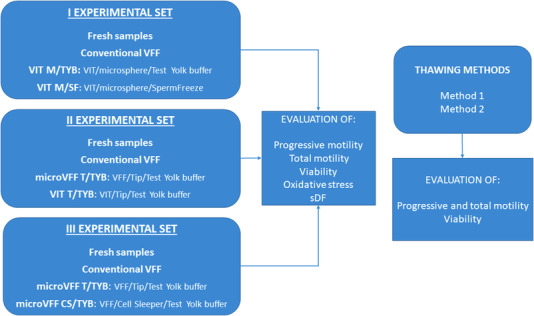
Scheme of the study design. VFF, vapour fast freezing; VIT, vitrification. Method1: thawing, washing, centrifugation and evaluation; Method 2: thawing, evaluation
The second set compared conventional VFF with: (i) microVFF using 10 μl tips as carrier and TYB as freezing medium (microVFF T/TYB) and (ii) vitrification with 10 μl tips as carrier and TYB as freezing medium (VIT T/TYB). The third set compared conventional VFF with: (i) microVFF T/TYB, as in the second set, and (ii) microVFF using cell sleepers (CS) as carrier and TYB as freezing medium (microVFF CS/TYB). In all the experimental sets, progressive and total motility, viability, oxidative stress and sDF were compared between before (fresh samples) and after cryopreservation. Finally, we compared two different methods for sample thawing (see below for description) in terms of sperm motility and viability recovery after thawing. For these last experiments, conventional VFF and the vitrification protocols of the first experimental set were used (Figure 1).
2.4. Cryopreservation procedures
Conventional VFF. Semen aliquots were diluted (1:1, vol:vol) by dropwise addition of TYB. After 15 min equilibration at 37°C, the mixture was aspirated into 500 μl high security sperm straws. The straws were sealed and frozen in liquid nitrogen vapour by 8 min exposure with a cooling rate of 15.6°C/min and then plunged into liquid nitrogen (−196°C) for storage. 19 In the second and third experimental sets, for microVFF T/TYB we followed the same procedure but using 10 μl tips as carrier (Figure 2A). Similarly, the same procedure was followed for microVFF CS/TYB but using CS as carriers. In this case, three 10 μl drops were placed in the carrier (Figure 2B). Both tips and cell sleepers were subsequently placed into cryovials that were closed and stored into liquid nitrogen.
FIGURE 2.
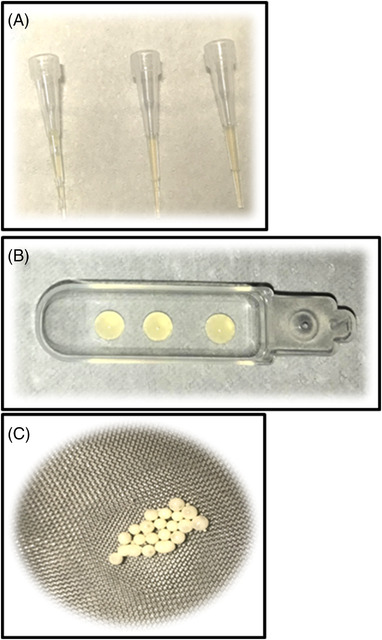
Carriers used for cryopreservation. (A) 10 μl tips; (B) cell sleepers; (C) 10 μl microspheres
Vitrification. After 1:1 dilution with TYB or 1:0.7 with SF, the mixture was plunged drop by drop (10 μl) into liquid nitrogen so that small solid microspheres were formed 18 (Figure 2C). The microspheres were then recovered and placed into cryovials for storage. In the second experimental set, for VIT T/TYB we followed the same procedure but using 10 μl tips as carrier.
2.5. Thawing
Thawing was carried out by transferring the carriers at room temperature for 15 min. Then, samples were washed with an equal volume of Human Tubal Fluid, centrifuged and resuspended in 100 μl of the same medium for subsequent evaluations. In 15 experiments, this thawing procedure was compared to a procedure where washing and centrifugation were omitted and the samples were directly evaluated for motility and viability (Figure 1).
2.6. Double staining with MitoSOX Red and LD‐G
For MitoSOX Red/LD‐G double staining, 20 fresh or thawed samples (2–4 million of spermatozoa) were stained with LD‐G (diluted 1:10,000) for 1 h at RT in the dark in 500 μl of PBS. Hence, samples were double washed with 200 μl of PBS and split into two aliquots that were incubated with (test sample) and without (negative control) MitoSOX Red 2 μM in 100 μl of Phosphate Buffer Saline (PBS) for 15 min at RT in the dark. Then, samples were washed with PBS and resuspended in 400 μl of PBS for flow cytometry acquisition. We calculated the intra‐assay CV for MitoSOX Red/LD‐G double staining by processing three aliquots of the same semen sample (n = 5, Table S1). To assess the reliability of MitoSOX Red/LD‐G double staining, we prepared a positive control. After treating five semen samples with the superoxide inducer, menadione (30 min at RT), we found an average increase of oxidative stress from 17.85 ± 5.00% to 76.99 ± 20.20%.
2.7. Flow cytometry
MitoSOX Red/LD‐G double‐stained samples were acquired with a flow cytometer FACScan (BD Biosciences, San Jose, CA, USA) equipped with a 15‐mW argon‐ion laser for excitation. The BD CellQuest Pro software (BD Biosciences, Franklin Lakes, NJ, USA) was used for acquisition and data analysis. Preliminary experiments were conducted to set fluorescence compensation by using single‐stained cellular control (with MitoSOX Red and LD‐G). Green fluorescence of LD‐G was revealed by an FL‐1 detector (515–555 nm wavelength band), whereas red fluorescence of MitoSOX Red was detected by an FL‐2 detector (563–607 nm wavelength band). For each sample, we recorded 5,000 viable spermatozoa, gating LD‐G negative events within the FSC/SSC flame‐shaped region (FR), containing spermatozoa and apoptotic bodies 21 and excluding debris and all non‐sperm cells. 21 For data analysis, in the MitoSOX Red/LD‐G dot plot of negative control, quadrants were set to include about 99% of the viable spermatozoa (LD‐G negative events) and copied into the dot plot of the corresponding test sample. Oxidative stress was calculated as a percentage of viable spermatozoa with MitoSOX Red staining on total viable spermatozoa.
2.8. Sperm chromatin dispersion Assay
sDF was detected with sperm chromatin dispersion (SCD) Assay using Halosperm kit. We followed manufacturer's instructions with some modifications. Briefly, 50,000 spermatozoa were resuspended with 1% low melting point agarose and layered on pre‐coated agarose slides. Then slides were covered with a coverslip and placed at 4°C until solidification. Then, samples were submerged with the acid denaturation solution and then with the lysing solution, both provided by the kit. Hence, slides were dehydrated with ethanol (70% and then 100%) and stained successively with eosin (15 min at RT) and thiazine (15 min at RT) solutions. After drying, slides were examined by bright field microscope for halos. A minimum of 200 spermatozoa per sample was scored and sDF was expressed as the percentage of spermatozoa without or with small halo on total spermatozoa. 22 We calculated the intra‐assay CV for SCD test by processing three aliquots of the same semen sample (n = 5, Table S1). For SCD we could calculate also the inter‐assay CV, by processing −80°C frozen aliquots of the same semen sample (n = 5) in three different days (Table S1). To assess the reliability of SCD test, we prepared a positive control. After treating five semen samples with DNAse I (2 IU, 20 min at 37°C), we found an average increase of sDF from 11.60 ± 6.07% to 85.20 ± 6.94%.
2.9. Statistical analyses
Data were analysed with Statistical Package for the Social Sciences (SPSS 25) for Windows (SPSS, Inc. Chicago, IL, USA). Normal distribution of the variables was checked with the Kolmogorov–Smirnov test. Data were expressed as mean ± SD or median (interquartile range [IQR]). After proper logarithmic transformation, paired t‐test was used to assess statistically significant differences between the different cryopreservation protocols and between the thawing methods. The study was sized considering results of motility as primary endpoint. To have a power of 0.80 and an alpha error of 0.05, assuming a large effect size Cohen's d = 0.8 23 , the number of subjects required resulted 15.
3. RESULTS
-
I.
Experimental set: comparing conventional VFF to VIT M/TYB and VIT M/SF.
In the first experimental set (n = 15), we compared conventional VFF with two vitrification protocols, conducted with microspheres but using different freezing media: VIT M/TYB and VIT M/SF (Figure 1). As expected, all cryopreservation procedures blunted total and progressive sperm motility as compared to fresh samples, however conventional VFF better prevented the reduction of these sperm parameters than vitrification protocols (Table 2A). In addition, total motility was higher after VIT M/TYB than after VIT M/SF, and a similar trend was observed also for progressive motility (Table 2A). Results of sperm viability showed a similar pattern as motility, after the different procedures (Table 2A). Induction of oxidative stress was present during all cryopreservation procedures. Typical MitoSOX Red/LD‐G dot plots of samples processed for oxidative stress 20 detection before and after freezing/thawing with the indicated procedures are reported in Figure 3, whereas average values as obtained in 15 semen samples are shown in Figure 4A. As shown, cryopreservation induced a dramatic increase of oxidative stress with higher values for vitrification (Figure 4A). Levels of sDF increased after cryopreservation as well, with the higher levels for vitrification (Figure 4B).
TABLE 2A.
I experimental set. Recovery of progressive, total motility and viability after the indicated cryopreservation procedures. Data are mean ± SD or median [IQR]
| Variable, n = 15 | Fresh | Conventional VFF | p a | VIT M/TYB | p a | p b | VIT M/SF | p a | p b | p c |
|---|---|---|---|---|---|---|---|---|---|---|
| Progressive motility (%) | 58.40 ± 8.75 | 21.67 ± 12.16 | <0.001 | 10.20 ± 9.65 | <0.001 | <0.001 | 5.00 ± 5.74 | <0.001 | <0.001 | >0.05 |
| Total motility (%) | 65.13 ± 9.33 | 29.33 ± 11.87 | <0.001 | 13.93 ± 11.03 | <0.001 | <0.001 | 8.27 ± 6.23 | <0.001 | <0.001 | <0.05 |
| Viability (%) | 75.33 ± 14.01 | 36.60 ± 13.42 | <0.001 | 22.93 ± 9.12 | <0.001 | <0.01 | 18.33 ± 7.38 | <0.001 | <0.001 | <0.05 |
vs. Fresh.
vs. Conventional VFF.
vs. VIT M/TYB; t‐test, paired data.
FIGURE 3.
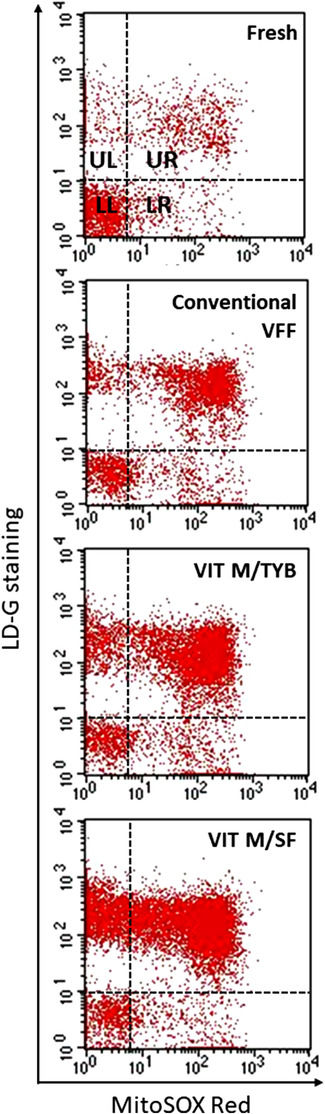
Oxidative stress in semen samples before and after the indicated procedures of cryopreservation. Representative dot plots of double staining with MitoSOX Red and LD‐G. Quadrant setting was established on the corresponding negative controls. In quadrants UL and UR, there are apoptotic bodies and non‐viable spermatozoa, respectively, whereas LL and LR quadrants contain viable spermatozoa without (LL) and with (LR) oxidative stress, respectively. 20 Oxidative stress was calculated as percentage of viable spermatozoa with MitoSOX Red staining (quadrants LR) on total viable spermatozoa (quadrants LR+LL). VVF, vapour fast freezing; VIT M/TYB, vitrification with microspheres and test yolk buffer; VIT M/SF, vitrification with microspheres and SpermFreeze
FIGURE 4.
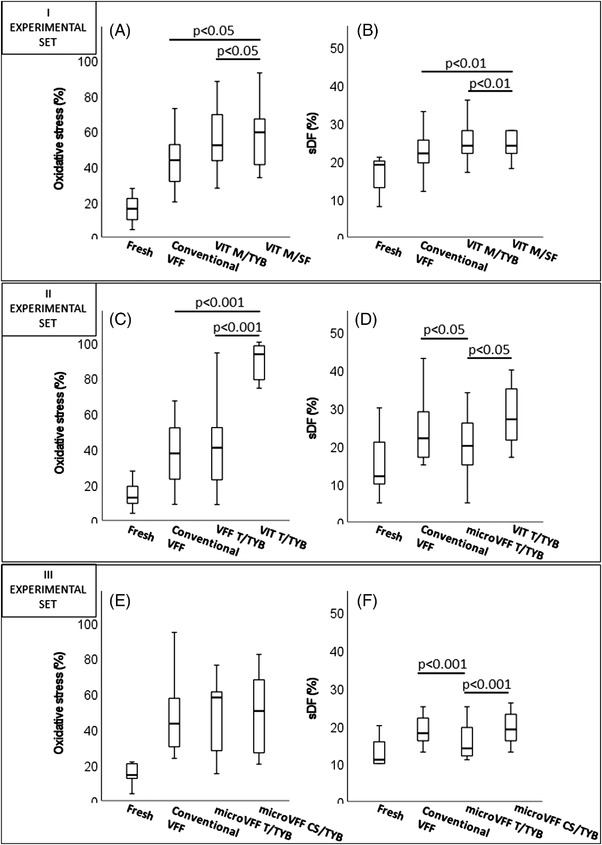
Induction of oxidative stress and sDF after the indicated procedures of cryopreservation. I experimental set: (A) average levels of oxidative stress as detected in 15 semen samples; p‐value vs. Fresh: <0.001 for all procedures. (B) Average levels of sDF as detected in 15 semen samples; p‐value vs. fresh: conventional VFF, p < 0.05; VIT M/TYB, p < 0.001; VIT M/SF, p < 0.01. II Experimental set: (C) average levels of oxidative stress as detected in 15 semen samples; p‐value vs. fresh < 0.001 for all procedures. (D) Average levels of sDF as detected in 15 semen samples; p‐value vs. fresh: conventional VFF and VFF T/TYB, p < 0.001; VIT T/TYB, p < 0.01. III Experimental set: (E) average levels of oxidative stress as detected in 15 semen samples; p‐value vs. fresh: <0.001 for all procedures. (F) Average levels of sDF as detected in 15 semen samples; p‐value vs. fresh <0.001 for all procedures. VVF, vapour fast freezing; VIT M/TYB, vitrification with microspheres and test yolk buffer; VIT M/SF, vitrification with microspheres and SpermFreeze; microVFF T/TYB, vapour fast freezing with tips and test yolk buffer; VIT T/TYB, vitrification with tips and test yolk buffer; microVFF CS/TYB, vapour fast freezing with cell sleepers and test yolk buffer
-
II.
Experimental set: comparing conventional VFF to microVFF T/TYB and VIT T/TYB.
In the second experimental set (n = 15), conventional VFF was compared with a microVFF and a vitrification procedure, respectively, microVFF T/TYB and VIT T/TYB, both using TYB as freezing medium and 10 μl tip as carrier. Whatever the carrier (i.e., straws or tips), VFF showed significantly higher motility and viability parameters (Table 2B) and lower ROS and sDF levels than vitrification, after freezing/thawing (Figure 4C,D). Interestingly, induction of sperm DNA breakage was reduced when VFF was conducted with low semen volume (10 μl vs. 500 μl, i.e., with microVFF vs. conventional VFF, Figure 4D).
TABLE 2B.
II experimental set. Recovery of progressive, total motility and viability after the indicated cryopreservation procedures. Data are mean ± SD or median [IQR]
| Variable, n = 15 | Fresh | Conventional VFF | p a | microVFF T/TYB | p a | p b | VIT T/TYB | p a | p b | p c |
|---|---|---|---|---|---|---|---|---|---|---|
| Progressive motility (%) | 53.06 ± 7.85 | 17.94 ± 9.60 | <0.001 | 17.06 ± 7.40 | <0.001 | >0.05 |
0.00 [0.00–1.00] |
<0.01 | <0.001 | <0.001 |
| Total motility (%) | 63.12 ± 6.56 | 25.53 ± 10.31 | <0.001 | 24.53 ± 10.31 | <0.001 | >0.05 |
2.00 [1.00–3.00] |
<0.001 | <0.001 | <0.001 |
| Viability (%) | 74.12 ± 9.13 | 29.64 ± 9.21 | <0.001 | 27.91 ± 8.13 | <0.001 | >0.05 |
10.00 [10.00–14.50] |
<0.001 | <0.001 | <0.001 |
vs. Fresh.
vs. Conventional VFF.
vs. microVFF T/TYB; t‐test, paired data.
-
III.
Experimental set: comparing conventional VFF to microVFF T/TYB and microVFF CS/TYB.
In the third experimental set (n = 15), VFF with TYB as freezing medium was conducted with three different carriers: straws, tips and cell sleepers. As shown in Table 2C, we found that cell sleepers much better maintained motility and viability of spermatozoa than both straws (percentage increase of progressive motility: 57.58 ± 63.63%, total motility: 48.82 ± 74.96% and viability: 24.55 ± 39.20%) and tips (percentage increase of progressive motility: 56.25 ± 53.59%, total motility: 41.28 ± 38.48% and viability: 19.59 ± 20.14%). However, the three carriers provoked a similar induction of oxidative stress (Figure 4E). Regarding sDF, cell sleepers induced levels of damage similar to straws and higher than tips (Figure 4F). The latter confirmed a reduction of induction of sDF with respect to conventional VFF (percentage decrease: 15.77 ± 20.77%, n = 30, I and II experimental sets, Figure 4D,F).
TABLE 2C.
III experimental set. Recovery of progressive, total motility and viability after the indicated cryopreservation procedures. Data are mean ± SD or median [IQR]
| Variable, n = 15 | Fresh | Conventional VFF | p a | microVFF T/TYB | p a | p b | microVFF CS/TYB | p a | p b | p c |
|---|---|---|---|---|---|---|---|---|---|---|
| Progressive motility (%) | 53.40 ± 11.31 | 24.27 ± 11.25 | <0.001 | 24.13 ± 11.14 | <0.001 | >0.05 | 33.67 ± 10.20 | <0.001 | <0.001 | <0.001 |
| Total motility (%) | 62.53 ± 9.52 | 30.53 ± 12.18 | <0.001 | 30.53 ± 12.47 | <0.001 | >0.05 | 39.60 ± 10.06 | <0.001 | <0.01 | <0.001 |
| Viability (%) |
82.00 [72.00–85.00] |
43.60 ± 13.04 | <0.001 | 43.40 ± 10.68 | <0.001 | >0.05 | 50.73 ± 10.28 | <0.001 | <0.05 | <0.001 |
vs. Fresh.
vs. Conventional VFF.
vs. microVFF T/TYB; t‐test, paired data.
3.1. Comparing two different methods of sample thawing
In this section, we compared two methods of sample thawing (n = 15), after cryopreservation with the procedures of the I experimental set (conventional VFF, VIT M/TYB and VIT M/SF). The first method (washing, centrifugation and evaluation) was used in all the above described procedures, whereas the second one consisted in immediate evaluation of motility and viability after sample thawing. Figure 5 reports the average values obtained for progressive and total motility and viability of spermatozoa with these two thawing methods. As shown, although both methods reduced all sperm parameters of fresh samples, the second one much better preserved motility and viability than the first one. Interestingly, omitting washing and centrifugation (method 2) blunted the differences between conventional VFF and vitrification protocols, in particular with TYB. Indeed, with method 2, progressive motility after conventional VFF (41.71 ± 8.04%) was higher than after VIT M/SF (31.41 ± 9.07%, p < 0.01) but similar to that after VIT M/TYB (38.65 ± 12.71%, p > 0.05), whereas no difference in total motility was present between conventional VFF (43.88 ± 8.27%) and vitrification protocols (VIT M/TYB: 42.59 ± 11.48% and VIT M/SF: 36.94 ± 11.66%, p > 0.05, Figure 5A,B). Regarding viability, with method 2, conventional VFF (55.24 ± 8.33%) yielded higher results than VIT M/SF (46.94 ± 8.05%, p < 0.001), but similar as VIT T/TYB: (52.47 ± 9.86%, p > 0.05, Figure 5C). Conversely, using thawing method 1, we confirmed results of the I experimental set (Table 2): (i) conventional VFF was better than vitrification in the recovery of both total (p < 0.001 vs. both VIT M/TYB and VIT M/SF) and progressive motility (p < 0.001 vs. both VIT M/TYB and VIT M/SF); (ii) vitrification with TYB better prevented the reduction of total (p < 0.01) and progressive motility (p < 0.01) than vitrification with SF. Similarly, viability was better after: (i) conventional VFF than vitrification (p < 0.05 vs. VIT M/TYB and p < 0.01 vs. VIT M/SF); (ii) vitrification with TYB than SF (p < 0.05, Figure 5).
FIGURE 5.
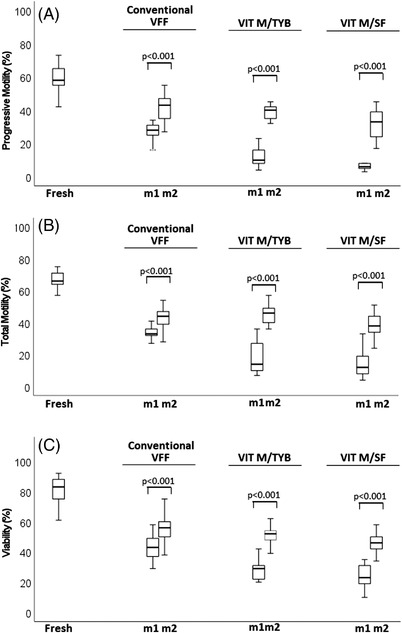
Comparison of effects of two thawing methods on recovery of motility and viability after cryopreservation with the indicated procedures. (A) progressive motility; p‐value vs. fresh <0.001 for all procedures. (B) Total motility; p‐value vs. fresh <0.001 for all procedures. (C) Viability; p‐value vs. fresh <0.001 for all procedures. m1, method 1; m2, method 2. VVF, vapour fast freezing; VIT M/TYB, vitrification with microspheres and test yolk buffer; VIT M/SF, vitrification with microspheres and SpermFreeze
4. DISCUSSION
This study showed that the conventional VFF (conducted with TYB and 500μl straws) better prevents the motility and viability reduction, and the induction of oxidative stress and sDF when compared to some tested vitrification protocols. However, when the same procedure was performed with small semen volumes (10 μl, microVFF), we obtained an even better protection of sperm DNA integrity (tips as carriers) or of motility and viability (cell sleepers as carriers) than with the procedure conducted in the conventional way. We also showed that final washing after sample thawing is crucial for induction of cryodamage to sperm motility and viability.
As expected, all the tested cryopreservation procedures dramatically reduced sperm motility and viability and induced higher levels of ROS production and sDF. However, we observed a high variability among samples, possibly due to variations in many biochemical aspects impacting the response to CPAs addition. Among the tested procedures, the conventional one was used as reference as it has been long used in our laboratory for sperm banking. Noteworthy, this procedure was first proposed as the most adequate to cryopreserve semen in subjects mainly represented by oligozoospermic and cancer patients, by showing an improved recovery of motility and viability. 19 Indeed, in 2013, it was reported a median recovery for progressive and total motility and viability of 5.8%, 12.4% and 32.8%, respectively. 19 This previous study showed that samples with one basal semen parameter below the reference value presented lower percentages of motile and viable spermatozoa, after cryopreservation. Therefore, we decided to conduct the present study in subjects with sperm motility and concentration highly over the reference values. This difference of study population might explain the better values of motility (progressive motility: 22.9%; total motility: 29.6%) and viability (38.0%) that we observed on average after cryopreservation with the same procedure (n = 49).
Whatever the freezing medium (TYB or SF) or the carrier (microspheres or tips), vitrification presented a higher impact on motility, viability and DNA integrity than VFF (Table 2 and Figure 4B,D, I and II experimental sets). Similarly, other authors reported that both motility and viability 24 or only motility 25 , 26 were higher after cryopreservation with a VFF procedure than with vitrification. However, opposite results were also found 18 , 27 , 28 and some studies found no differences between the two types of procedure in terms of recovery of motility and viability. 25 , 29 , 30 , 31 In addition, few studies found no differences on sperm DNA damage between samples cryopreserved with VFF and vitrification. 25 , 29 , 32 Heterogeneity in several relevant aspects for the quality of post‐thawed spermatozoa, including type and amount of CPAs, 33 type of carrier, 34 , 35 thawing procedure, 36 sample preparation, 37 and inclusion criteria for subject recruitment 19 , 38 could underpin these conflicting results.
In the present study, we also evaluated the induction of oxidative stress during sperm cryopreservation with the tested procedures. Oxidative stress is considered one of the main causes of sperm cryodamage and also the main mechanism inducing sDF during in vitro manipulation of spermatozoa. 13 We revealed oxidative stress with a new flow cytometric technique, 20 able to detect the percentage of viable spermatozoa with excessive production of mitochondrial ROS in native semen samples. In a previous study, we showed that this technique is able to detect both induction of sperm ROS production by H2O2 and menadione, and spontaneous ROS production during in vitro short sperm incubations. 20 Similar to results on motility and viability, data on oxidative stress of the first and second experimental sets, showed that vitrification was significantly more dangerous than VFF, whatever the carrier in the latter (straws or tips, Figure 4A,C). Consistently, also induction of sDF appeared to be higher after vitrification in microspheres (Figure 4B) than after VFF in straws. However, when vitrification was conducted in tips, damage to sperm DNA was higher than VFF in tips, but similar to that observed after VFF in straws (Figure 4D), consistent with studies which found no difference between VFF and vitrification. 25 , 29 , 32
We also investigated whether VFF conducted with small semen volumes, carried by tips or cell sleepers, could ameliorated the recovery of sperm motility, viability and DNA quality (second and third experimental sets). Our results indicated that tips yielded values of motility and viability (Tables 2B, 2C) and induction of oxidative stress quite similar to straws but, interestingly, provoked less damage to sperm DNA (Figure 4D,F). On the other hand, cell sleepers showed a similar induction of oxidative stress and sDF as straws (Figure 4E,F), but a much better recovery of motility and viability (Table 2C). Overall, these results not only indicated that VFF can yield better results when conducted with small semen volumes, but also confirmed the importance of the shape/carrier of the sample. 34 , 35 Surprisingly, in the second and third experimental sets, induction of oxidative stress did not reflect sDF (when tips are used) or motility/viability levels (when cell sleepers are used). These latter results suggest that mechanisms, other than ROS induced ones, might be responsible for motility/viability loss during cryopreservation. Indeed, it has been reported a role for mitochondrial defects in the impairment of sperm motility 2 and for changes in membrane proteins and carbohydrate composition 39 in disrupting membrane structures. Conversely, it is less clear why tips better preserve sperm DNA quality than straws, albeit provoking similar percentages of oxidative stress. It is possible that, in certain conditions, not all cells with excessive mitochondrial ROS production develop in DNA fragmented ones. Alternatively, SCD test might be not sufficiently sensitive to reveal subtle DNA damage produced when VFF is conducted with tips. Considering the impact of sDF on outcomes of ARTs, where cryopreserved samples are used, this issue deserves further investigation.
Finally, we tested the effect of omitting sample washing/centrifugation after thawing on sperm motility and viability of samples frozen with the procedures of the first experimental set. Such thawing procedure highly improved recovery of both motility and viability, in all cryopreservation protocols (Figure 5). Interestingly, the improvement was above all in the vitrification protocols, so that the differences in total and progressive motility and viability were blunted between VFF and vitrification, in particular when the latter was conducted with TYB (Figure 5). Apparently, these experiments suggest that a relevant part of sperm cryodamage is due to washing after thawing, consistent with studies reporting a deleterious effect of centrifugation. 40 , 41 However, it is also possible that centrifugation simply unveils a damage already present in samples insulted by freezing/thawing. Whatever the explanation, these results confirm the importance of the thawing method for damage induction during cryopreservation. In addition, when cryopreservation is conducted in subjects with a very low available sperm number, skipping the washing step to avoid sperm loss, these results can explain the better results obtained in terms of recovery of motility and viability. 42 , 43
One limitation of the study was that sperm motility was not graded as slow and rapid, whereas the identification of rapidly progressive spermatozoa is important for both natural and assisted reproduction, 44 the latter used with frozen samples. Another limitation was that we did not assess sperm parameters immediately after addition of freezing media and before cooling. Hence, we could not determine the effect of TYB or SF independently from the used procedure and carrier.
In conclusion, this study showed that VFF better preserved motility and viability than vitrification. Overall, the latter also resulted in higher oxidative stress and sDF induction. When VFF was conducted in small semen volumes, an improvement in sperm DNA quality and in sperm motility/viability was obtained, depending on the carrier, respectively, tips and cell sleepers. The worst results obtained with conventional VFF versus microVFF should be balanced by higher semen volumes (and thus sperm numbers) used by the former in subjects presenting with middle/fine sperm quality. However, these results are promising for developing a novel strategy for banking very low numbers of spermatozoa. Finally, we showed that post thawing sample washing/centrifugation is responsible for a high fraction of damage to viable and motile spermatozoa. Further studies are necessary to understand whether such damage is induced or only unveiled by centrifugation.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
FUNDING
The authors have no funding sources to report.
AUTHORS CONTRIBUTION
Valentina Arciero performed cryopreservation procedures, flow cytometric acquisition and analyses and collected data of the study. Oumaima Ammar participated to statistical analyses and to graph building. Mario Maggi conceived the project and prompted us to challenge the conventional VFF procedure. Linda Vignozzi provided resources and critically discussed the results. Monica Muratori designed the study, performed statistical analyses and drafted the paper. Sara Dabizzi trained and supervised Valentina Arciero in cryopreservation procedures and critically discussed the results. All authors critically reviewed the manuscript and gave their approval.
Supporting information
Supporting Information
ACKNOWLEDGEMENTS
We wish to thank Benjamin Steegen from Nipro Medical Europe who provided us cell sleepers. We are also grateful to all the staff of the Semen Cryopreservation and Andrology Laboratory of Careggi Hospital for providing us data on semen parameters determined by routine semen analysis.
Open Access Funding provided by Universita degli Studi di Firenze within the CRUI‐CARE Agreement.
Arciero V, Ammar O, Maggi M, Vignozzi L, Muratori M, Dabizzi S. Vapour fast freezing with low semen volumes can highly improve motility and viability or DNA quality of cryopreserved human spermatozoa. Andrology. 2022;10:1123–1133. 10.1111/andr.13208
DATA AVAILABILITY STATEMENT
The datasets generated during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Donnelly ET, Mcclure N, Lewis SE. Cryopreservation of human semen and prepared sperm: effects on motility parameters and DNA integrity. Fertil Steril. 2001;76:892‐900. [DOI] [PubMed] [Google Scholar]
- 2. Ozkavukcu S, Erdemli E, Isik A, Oztuna D, Karahuseyinoglu S. Effects of cryopreservation on sperm parameters and ultrastructural morphology of human spermatozoa. J Assist Reprod Genet. 2008;25:403‐411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hammadeh ME, Askari AS, Georg T, Rosenbaum P, Schmidt W. Effect of freeze–thawing procedure on chromatin stability, morphological alteration and membrane integrity of human spermatozoa in fertile and subfertile men. Int J Androl. 1999;22:155‐162. [DOI] [PubMed] [Google Scholar]
- 4. Hezavehei M, Sharafi M, Kouchesfahani HM, et al. Sperm cryopreservation: a review on current molecular cryobiology and advanced approaches. Reprod Biomed Online. 2018;37:327‐339. [DOI] [PubMed] [Google Scholar]
- 5. Kopeika J, Thornhill A, Khalaf Y. The effect of cryopreservation on the genome of gametes and embryos: principles of cryobiology and critical appraisal of the evidence. Hum Reprod Update. 2015;21:209‐227. [DOI] [PubMed] [Google Scholar]
- 6. Kumar A, Prasad JK, Srivastava N, Ghosh SK. Strategies to minimize various stress‐related freeze‐thaw damages during conventional cryopreservation of mammalian spermatozoa. Biopreserv Biobank. 2019;17:603‐612. [DOI] [PubMed] [Google Scholar]
- 7. Garcez ME, dos Santos Branco C, Lara LV, Pasqualotto FF, Salvador M. Effects of resveratrol supplementation on cryopreservation medium of human semen. Fertil Steril. 2010;94:2118‐2121. [DOI] [PubMed] [Google Scholar]
- 8. Agarwal A, Makker K, Sharma R. Clinical relevance of oxidative stress in male factor infertility: an update. Am J Reprod Immunol. 2008;59:2‐11. [DOI] [PubMed] [Google Scholar]
- 9. Storey BT. Biochemistry of the induction and prevention of lipoperoxidative damage in human spermatozoa. Mol Hum Reprod. 1997;3:203‐213. [DOI] [PubMed] [Google Scholar]
- 10. Bisht S, Faiq M, Tolahunase M, Dada R. Oxidative stress and male infertility. Nat Rev Urol. 2017;14:470‐485. [DOI] [PubMed] [Google Scholar]
- 11. Cissen M, Wely MV, Scholten I, et al. Measuring sperm DNA fragmentation and clinical outcomes of medically assisted reproduction: a systematic review and meta‐analysis. PLoS One. 2016;10:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Simon L, Emery B, Carrell DT. Sperm DNA fragmentation: consequences for reproduction. Adv Exp Med Biol. 2019;1166:87‐105. [DOI] [PubMed] [Google Scholar]
- 13. Muratori M, Marchiani S, Tamburrino L, Baldi E. Sperm DNA fragmentation: mechanisms of origin. Adv Exp Med Biol. 2019;1166:75‐85. [DOI] [PubMed] [Google Scholar]
- 14. Benson JD, Woods EJ, Walters EM, Critser JK. The cryobiology of spermatozoa. Theriogenology. 2012;78:1682‐1699. [DOI] [PubMed] [Google Scholar]
- 15. Woods EJ, Benson JD, Agca Y, Critser JK. Fundamental cryobiology of reproductive cells and tissues. Cryobiology. 2004;48:146‐156. [DOI] [PubMed] [Google Scholar]
- 16. Schulz M, Risopatrón J, Uribe P, Isachenko E, Isachenko V, Sánchez R. Human sperm vitrification: a scientific report. Andrology. 2020;8:1642‐1650. [DOI] [PubMed] [Google Scholar]
- 17. WHO . World Health Organization Laboratory Manual for the Examination and Processing of Human Semen. WHO Press; 2010. [Google Scholar]
- 18. Aizpurua J, Medrano L, Enciso M, et al. New permeable cryoprotectant‐free vitrification method for native human sperm. Hum Reprod. 2017;32:2007‐2015. [DOI] [PubMed] [Google Scholar]
- 19. Degl'Innocenti S, Filimberti E, Magini A, et al. Semen cryopreservation for men banking for oligospermia, cancers, and other pathologies: prediction of post‐thaw outcome using basal semen quality. Fertil Steril. 2013;100:1555‐1563. [DOI] [PubMed] [Google Scholar]
- 20. Riley L, Ammar O, Mello T, Giovannelli L, Vignozzi L, Muratori M. Novel methods to detect ROS in viable spermatozoa of native semen samples. Reprod Toxicol. 2021;9(106):51‐60. [DOI] [PubMed] [Google Scholar]
- 21. Marchiani S, Tamburrino L, Maoggi A, et al. Characterization of M540 bodies in human semen: evidence that they are apoptotic bodies. Mol Hum Reprod. 2007;13:621‐631. [DOI] [PubMed] [Google Scholar]
- 22. Fernández JL, Muriel L, Rivero MT, Goyanes V, Vazquez R, Alvarez JG. The sperm chromatin dispersion test: a simple method for the determination of sperm DNA fragmentation. J Androl. 2003;24:59‐66. [PubMed] [Google Scholar]
- 23. Cohen J. Statistical power analysis for the behavioural sciences. 2nd ed. Lawrence Erlbaum; 1988. 567 p. [Google Scholar]
- 24. Le MT, Nguyen TTT, Nguyen TT, et al. Cryopreservation of human spermatozoa by vitrification versus conventional rapid freezing: effects on motility, viability, morphology and cellular defects. Eur J Obstet Gynecol Reprod Biol. 2019;234:14‐20. [DOI] [PubMed] [Google Scholar]
- 25. Darvishnia H, Lakpour N, Lahijani MS, et al. Effects of very rapid versus vapor phase freezing on human sperm parameters. Cell Tissue Bank. 2013;14:679‐685. [DOI] [PubMed] [Google Scholar]
- 26. Liu J, Tanrikut C, Wright DL, et al. Cryopreservation of human spermatozoa with minimal non‐permeable cryoprotectant. Cryobiology. 2016;73:162‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhu J, Jin RT, Wu LM, et al. Cryoprotectant‐free ultra‐rapid freezing of human spermatozoa in cryogenic vials. Andrologia. 2014;46:642‐649. [DOI] [PubMed] [Google Scholar]
- 28. Pabón D, Meseguer M, Sevillano G, et al. A new system of sperm cryopreservation: evaluation of survival, motility, DNA oxidation, and mitochondrial activity. Andrology. 2019;7:293‐301. [DOI] [PubMed] [Google Scholar]
- 29. Ali Mohamed MS. Slow cryopreservation is not superior to vitrification in human spermatozoa: an experimental controlled study. Iran J Reprod Med. 2015;13:633‐644. [PMC free article] [PubMed] [Google Scholar]
- 30. Saritha KR, Bongso A. Comparative evaluation of fresh and washed human sperm cryopreserved in vapor and liquid phases of liquid nitrogen. J Androl. 2001;22:857‐862. [PubMed] [Google Scholar]
- 31. Agha‐Rahimi A, Khalili MA, Nabi A, Ashourzadeh S. Vitrification is not superior to rapid freezing of normozoospermic spermatozoa: effects on sperm parameters, DNA fragmentation and hyaluronan binding. Reprod Biomed Online. 2014;28:352‐358. [DOI] [PubMed] [Google Scholar]
- 32. Satirapod C, Treetampinich C, Weerakiet S, Wongkularb A, Rattanasiri S, Choktanasiri W. Comparison of cryopreserved human sperm from solid surface vitrification and standard vapor freezing method: on motility, morphology, vitality and DNA integrity. Andrologia. 2012;44:786‐790. [DOI] [PubMed] [Google Scholar]
- 33. Du C, Zheng X, Jiang J, et al. The effects of extenders, cryoprotectants and conditions in two‐step cooling method on Varicorhinus barbatulus sperm. Cryobiology. 2021;100:133‐141. [DOI] [PubMed] [Google Scholar]
- 34. Isachenko V, Isachenko E, Montag M, et al. Clean technique for cryoprotectant‐free vitrification of human spermatozoa. Reprod Biomed Online. 2005;10:350‐354. [DOI] [PubMed] [Google Scholar]
- 35. Sharma R, Kattoor AJ, Ghulmiyyah J, Agarwal A. Effect of sperm storage and selection techniques on sperm parameters. Syst Biol Reprod Med. 2015;61:1‐12. [DOI] [PubMed] [Google Scholar]
- 36. Martínezsoto JC, García‐Vazquez FA, Gumbao D, Landeras J, Gadea J. Assessment of two thawing processes of cryopreserved human sperm in pellets. Cryobiology. 2011;63:131‐136. [DOI] [PubMed] [Google Scholar]
- 37. Counsel M, Bellinge R, Burton P. Vitality of oligozoospermic semen samples is improved by both swim‐up and density gradient centrifugation before cryopreservation. J Assist Reprod Genetics. 2004;21:137‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stanic P, Tandara M, Sonicki Z, Simunic V, Radakovic B, Suchanek E. Comparison of protective media and freezing techniques for cryopreservation of human semen. Eur J Obstet Gynecol Reprod Biol. 2000;91:65‐70. [DOI] [PubMed] [Google Scholar]
- 39. Pedersen H, Lebech PE. Ultrastructural changes in the human spermatozoon after freezing for artificial insemination. Fertil Steril. 1971;22:125‐133. [DOI] [PubMed] [Google Scholar]
- 40. Agarwal A, Durairajanayagam D, du Plessis SS. Utility of antioxidants during assisted reproductive techniques: an evidence based review. Reprod Biol Endocrinol. 2014;24:12‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Muratori M, Tarozzi N, Carpentiero F, et al. Sperm selection with density gradient centrifugation and swim up: effect on DNA fragmentation in viable spermatozoa. Sci Rep. 2019;9:7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sereni E, Bonu MA, Fava L, et al. Freezing spermatozoa obtained by testicular fine needle aspiration: a new technique. Reprod Biomed Online. 2008;16:89‐95. [DOI] [PubMed] [Google Scholar]
- 43. Berkovitz A, Miller N, Silberman M, Belenky M, Itsykson P. A novel solution for freezing small numbers of spermatozoa using a sperm vitrification device. Hum Reprod. 2018;33:1975‐1983. [DOI] [PubMed] [Google Scholar]
- 44. WHO . World Health Organization Laboratory Manual for the Examination and Processing of Human Semen. WHO Press; 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The datasets generated during the current study are available from the corresponding author on reasonable request.


