Abstract
Aims
To report data from EMPEROR‐Preserved according to prespecified endpoints of DELIVER.
Methods and results
In order to assess the impact of DELIVER‐like definition on EMPEROR‐Preserved outcomes, the following differences were reconciled: (1) the primary outcome in DELIVER added urgent heart failure (HF) visits to cardiovascular death or HF hospitalizations; (2) the EMPEROR‐Preserved trial did not require documentation of physical findings or laboratory tests for confirming a HF hospitalization and it included events of 12–24 h if intensification of treatment was not only oral diuretics; (3) DELIVER excluded undetermined causes of deaths from the primary endpoint; (4) the composite renal endpoint in DELIVER included a sustained ≥50% decline in estimated glomerular filtration rate and incorporated renal death; and (5) DELIVER will assess outcomes in the overall population and in patients with ejection fraction (EF) <60% separately. Using the endpoint definitions from DELIVER, the primary outcome overall occurred in 13.1% in the empagliflozin and 16.8% in the placebo group (hazard ratio [HR] 0.76, 95% confidence interval [CI] 0.67–0.87; p < 0.0001). The relative risk reduction (RRR) changed from 21% to 24% when urgent HF visits were added, and undetermined death was eliminated. Compared to overall population RRR of 24%, it was 28% in patients with EF <60%. Death from cardiovascular causes excluding undetermined causes occurred in 6.2% in the empagliflozin and in 7.1% in the placebo group (HR 0.88, 95% CI 0.73–1.07). The RRR for the composite renal endpoint changed from 22% in the overall population (HR 0.78, 95% CI 0.54–1.13) to 40% when patients with EF <60% were assessed (p = 0.037).
Conclusion
Findings from EMPEROR‐Preserved were modestly altered when analysed using cardiovascular trial endpoint definitions of the DELIVER trial. For the composite renal endpoint, the effect of empagliflozin became statistically significant in patients with EF <60% using the DELIVER definition.
Keywords: EMPEROR‐Preserved, Heart Failure with preserved ejection fraction, Empagliflozin, Outcomes, DELIVER
Introduction
The EMPEROR‐Preserved (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Preserved Ejection Fraction) trial was the first published outcome trial specifically designed to assess the effect of sodium–glucose co‐transporter 2 (SGLT2) inhibitors on cardiovascular and renal outcomes in patients with heart failure (HF) and preserved ejection fraction (HFpEF). 1 It showed that empagliflozin significantly reduced the risk of cardiovascular death or hospitalization for HF in these patients. The ongoing DELIVER (Dapagliflozin Evaluation to Improve the LIVEs of Patients with Preserved Ejection Fraction Heart Failure) trial was designed to study the effect of dapagliflozin in the same target population. 2 Taken together, the results of these two trials may increase our understanding of the effect of SGLT2 inhibition in patients with HFpEF. However, the definition of endpoints in both trials have several meaningful differences, potentially making comparison of the results difficult. Thus, we report data from the EMPEROR‐Preserved trial according to prespecified endpoints of the DELIVER trial.
Methods
Study design and patient population
The design for the EMPEROR‐Preserved trial has been described previously. 3 In brief, the EMPEROR‐Preserved trial was a phase 3, placebo‐controlled trial that enrolled adult patients who have chronic HF with New York Heart Association (NYHA) functional class II–IV symptoms and a left ventricular ejection fraction (LVEF) of >40% with no prior measurement of ≤40%. Patients were also required to have elevated N‐terminal pro‐hormone B‐type natriuretic peptide (NT‐proBNP) levels (>900 or >300 pg/ml in patients with and without atrial fibrillation, respectively) and a documented hospitalization for HF within 12 months prior to enrolment or evidence of structural heart disease. Patients were randomized to receive either placebo or empagliflozin 10 mg once daily.
Trial outcomes
The outcomes of the EMPEROR‐Preserved trial have been described and reported in detail previously. 1 , 3 In brief, the primary outcome was the composite of cardiovascular death or hospitalization for HF. Secondary outcomes included a composite renal endpoint (sustained ≥40% decline in estimated glomerular filtration rate [eGFR] or end‐stage kidney disease or need for renal replacement therapy). The investigation conforms with the principles outlined in the Declaration of Helsinki. The ethics committee at each participating centre approved the trial, and all patients provided written informed consent.
Using the endpoint definitions similar to DELIVER, the outcomes for this analysis included the primary outcome of cardiovascular death or worsening HF events (consisting of adjudicated hospitalization for HF [following Hicks criteria] 4 or investigator‐reported urgent care or emergency room visit for HF requiring intravenous therapy) and secondary outcomes included time to worsening HF event, cardiovascular death, and composite renal endpoint (sustained ≥50% decline in eGFR or end‐stage kidney disease or renal death or need for renal replacement therapy).
In summary, outcomes in the EMPEROR‐Preserved and DELIVER trials differ in five ways. First, the primary composite outcome in EMPEROR‐Preserved was cardiovascular death or hospitalizations for HF, whereas DELIVER also included urgent HF visits requiring evidence of HF therapy other than only use of oral diuretics. Second, because it was based on the Hicks criteria, DELIVER required patients to have an unscheduled hospital admission for a primary diagnosis of HF with a length of stay that exceeds 24 h and have signs, symptoms, and diagnostic testing results consistent with the diagnosis of HF to fulfil the criteria for hospitalization for HF. 4 In addition, patients were required to receive treatment specifically directed at HF. In contrast, EMPEROR‐Preserved did not require documentation of worsening physical findings or worsening laboratory tests for confirming a HF hospitalization and it included events of 12–24 h if intensification of treatment was not only based on the use of oral diuretics. Third, EMPEROR‐Preserved included undetermined causes of deaths in the category of cardiovascular death, while DELIVER will not. Fourth, as for the renal composite endpoints, EMPEROR‐Preserved included a sustained ≥40% decline in eGFR and did not include renal death, while DELIVER will include a sustained ≥50% decline in eGFR and incorporate renal death; both definitions included the development of end‐stage kidney disease as a renal event. Lastly, DELIVER will assess outcomes in the total population and in those with ejection fraction <60% separately.
Statistical analysis
Like DELIVER, the primary endpoint was assessed in a dual primary analysis – the full population and in those with left ventricular ejection fraction (LVEF) <60%. This was also done for the other prespecified endpoints. All endpoints were analysed using Cox regression model. Models were adjusted for baseline covariates of sex, age, eGFR, LVEF, region and diabetes.
Results
Patient characteristics
From 27 March 2017 to13 April 2020, a total of 5988 patients were randomly assigned to receive either empagliflozin or matching placebo at 622 centres in 23 countries. Baseline characteristics and HF therapies were well balanced between the two treatment groups and have been reported in detail previously. The median duration of follow‐up was 26.2 months (interquartile range 18.1–33.1).
Outcomes
Using the endpoint definitions in DELIVER, the primary composite outcome of cardiovascular death or worsening HF event occurred in 394 of 2997 patients (13.1%) in the empagliflozin group and in 502 of 2991 patients (16.8%) in the placebo group (hazard ratio [HR] 0.76, 95% confidence interval [CI] 0.67–0.87; p < 0.0001) (Figure 1 and Table 1 ). Compared to the overall population (relative risk reduction [RRR] 24%), the RRR in patients with LVEF <60% changed to 28% (HR 0.72, 95% CI 0.61–0.84; p < 0.0001). A worsening HF event occurred in 263 patients (8.8%) in the empagliflozin group and in 366 (12.2%) in the placebo group (HR 0.69, 95% CI 0.59–0.81; p < 0.0001) (Figure 2A ). Death from cardiovascular causes occurred in 186 patients (6.2%) who received empagliflozin and in 213 (7.1%) who received placebo (HR 0.88, 95% CI 0.73–1.07) (Figure 2B ). When only patients with LVEF <60% were considered, the RRR increased from 31% to 37% for worsening HF events (HR 0.63, 95% CI 0.51–0.76) and from 12% to 15% for death from cardiovascular cause (HR 0.85, 95% CI 0.67–1.07).
Figure 1.
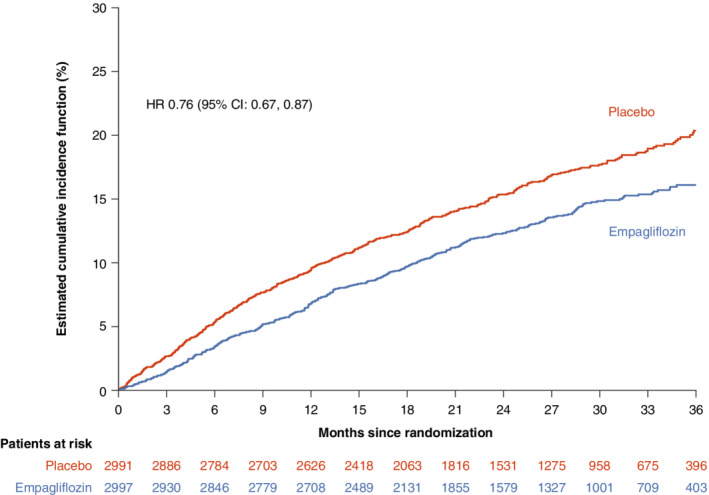
Cumulative incidence plots for the primary composite of cardiovascular death and worsening heart failure event. CI, confidence interval; HR, hazard ratio.
Table 1.
Primary and secondary cardiovascular outcomes according to different definitions
| Definition from trial | Endpoint | Empagliflozin (n = 2997) | Placebo (n = 2991) | Hazard ratio (95% CI) | p‐value | ||
|---|---|---|---|---|---|---|---|
| N (%) | Events/100 patient‐years | N (%) | Events/100 patient‐years | ||||
| EMPEROR‐Preserved | Cardiovascular death or HHF | 415 (13.8) | 6.9 | 511 (17.1) | 8.7 | 0.79 (0.69–0.90) | 0.0003 |
| Cardiovascular death or HHF a | 394 (13.1) | 6.5 | 485 (16.2) | 8.2 | 0.79 (0.69–0.90) | 0.0005 | |
| Cardiovascular death b or HHF a | 369 (12.3) | 6.1 | 461 (15.4) | 7.8 | 0.78 (0.68–0.89) | 0.0003 | |
| DELIVER definitions applied to EMPEROR‐Preserved | Cardiovascular death b or worsening HF c in the overall population | 394 (13.1) | 6.5 | 502 (16.8) | 8.5 | 0.76 (0.67–0.87) | <0.0001 |
| DELIVER definitions applied to EMPEROR‐Preserved | Cardiovascular death b or worsening HF c in patients with LVEF <60% | 263/2023 (13.0) | 6.4 | 357/2018 (17.7) | 9.0 | 0.72 (0.61–0.84) | <0.0001 |
| EMPEROR‐Preserved | Time to first HHF | 259 (8.6) | 4.3 | 352 (11.8) | 6.0 | 0.71 (0.60–0.83) | <0.0001 |
| DELIVER definitions applied to EMPEROR‐Preserved | Time to worsening HF c in the overall population | 263 (8.8) | 4.4 | 366 (12.2) | 6.2 | 0.69 (0.59–0.81) | <0.0001 |
| DELIVER definitions applied to EMPEROR‐Preserved | Time to worsening HF c in patients with LVEF <60% | 165/2023 (8.2) | 4.0 | 257/2018 (12.7) | 6.5 | 0.63 (0.51–0.76) | <0.0001 |
| EMPEROR‐Preserved | Cardiovascular death | 219 (7.3) | 3.4 | 244 (8.2) | 3.8 | 0.91 (0.76–1.09) | 0.295 |
| DELIVER definitions applied to EMPEROR‐Preserved | Cardiovascular death b in the overall population | 186 (6.2) | 2.9 | 213 (7.1) | 3.3 | 0.88 (0.73–1.07) | 0.214 |
| DELIVER definitions applied to EMPEROR‐Preserved | Cardiovascular death b in patients with LVEF <60% | 132/2023 (6.5) | 3.1 | 158/2018 (7.8) | 3.6 | 0.85 (0.67–1.07) | 0.163 |
| EMPEROR‐Preserved | Composite renal endpoint (sustained ≥40% decline in eGFR and ESKD) | 108 (3.6) | 2.1 | 112 (3.7) | 2.2 | 0.95 (0.73–1.24) | 0.724 |
| DELIVER definitions applied to EMPEROR‐Preserved | Composite renal endpoint (sustained ≥50% decline in eGFR, ESKD and renal death) in the overall population | 50 (1.7) | 1.0 | 62 (2.1) | 1.2 | 0.78 (0.54–1.13) | 0.193 |
| DELIVER definitions applied to EMPEROR‐Preserved | Composite renal endpoint (sustained ≥50% decline in eGFR, ESKD and renal death) in patients with LVEF <60% | 27 (1.3) | 0.8 | 45 (2.2) | 1.3 | 0.60 (0.37–0.97) | 0.0371 |
CI, confidence interval; DELIVER, Dapagliflozin Evaluation to Improve the LIVEs of Patients with Preserved Ejection Fraction Heart Failure; eGFR, estimated glomerular filtration rate; EMPEROR‐Preserved, Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Preserved Ejection Fraction; ESKD, end‐stage kidney disease (sustained eGFR <15 ml/min/1.73 m2 [for patients with baseline eGFR ≥30 ml/min/1.73 m2] or sustained eGFR <10 ml/min/1.73 m2 [for patients with baseline eGFR <30 ml/min/1.73 m2] or chronic dialysis or renal transplant); HF, heart failure; HHF, hospitalization for heart failure; LVEF, left ventricular ejection fraction.
Following Hicks criteria. 4
Excluding undetermined death.
Worsening HF means HHF (Hicks criteria) or urgent visit for HF requiring intravenous therapy.
Figure 2.
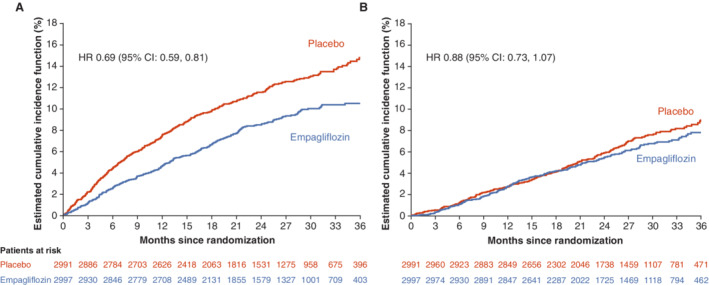
Cumulative incidence plots for time to worsening heart failure events (A) and cardiovascular death (B). CI, confidence interval; HR, hazard ratio.
In the overall population, there was a total of 50 and 62 composite renal endpoints (HR 0.78, 95% CI 0.54–1.13) (Table 1 ) in the empagliflozin and placebo groups, respectively. In patients with LVEF <60%, the RRR in patients with LVEF <60% increased to 40% (HR 0.60, 95% CI 0.37–0.97; p = 0.0371).
Discussion
In this analysis we evaluated data from the EMPEROR‐Preserved trial using endpoint definitions from DELIVER and show several key findings. The risk of the primary composite outcome of cardiovascular death or worsening HF event (hospitalization for HF or an urgent HF visit requiring intravenous therapy) was significantly lower in the empagliflozin group than in the placebo group. This benefit numerically increased when only patients with LVEF <60% were analysed. The effects on the primary endpoint were seen consistently across all prespecified subgroups, including patients with and without diabetes. Empagliflozin also reduced the time to first worsening HF event. This pattern of benefit was similar to those reported using the original endpoint definitions of the EMPEROR‐Preserved trial.
When urgent HF visits were added and undetermined death as part of cardiovascular death was eliminated from the primary composite endpoint, there was a modest impact on the effect size, with the RRR changing from 21% to 24%. Although the event rates decreased for cardiovascular death after excluding undetermined causes of death, a similar increase in RRR was noticed (i.e. from 9% to 12%). Undetermined causes of death are widely assumed to be due to sudden cardiac death and are generally incorporated into the definition of cardiovascular death; however, in HFpEF, undetermined causes of death are not well defined. Compared to the overall population, the RRR for the primary endpoint in patients with LVEF <60% increased from 24% to 28%.
It is particularly noteworthy that the RRR for the composite renal endpoint increased considerably (to a 40% reduction in risk) and became nominally significant when a threshold change of a ≥50% decrease in eGFR and renal death were included in the analysis and when the analysis was confined to patients with ejection fraction <60%. These findings are the first to suggest a significant treatment benefit on hard kidney outcomes in patients with HFpEF with LVEF <60%. This observation extends our previous finding of a 50% reduction in the risk of a renal event with empagliflozin in patients with HF and an ejection fraction of ≤40%, which was reported in the EMPEROR‐Reduced trial.
The EMPEROR‐Preserved trial varied from the strict Hicks criteria of defining HF hospitalizations since, in many global trials, the documentation of physical findings or testing for laboratory abnormalities that are required to meet all criteria are often missing. In EMPEROR‐Preserved, the clinical events committees were instructed to use their best judgement about the occurrence of a HF hospitalization if patients had worsening symptoms and required intensification of treatment for HF.
The findings of this study should be interpreted in the context of potential limitations. This analysis was performed using endpoints not prespecified for EMPEROR‐Preserved. The statistical model applied to the DELIVER trial were those prespecified in the EMPEROR trials. Emergency room and urgent care visits for HF were not adjudicated. DELIVER will also include patients recently hospitalized and those with improved LVEF, while EMPEROR did not. Of note, compared to EMPEROR‐Preserved, DELIVER had more patients at baseline with history of HF hospitalization (23% within the prior 12 months in EMPEROR‐Preserved vs. 26% in DELIVER), more patients with coronary artery disease (35% vs. 50.5%), and fewer patients in NYHA class II (82% vs. 75%), suggesting that patients enrolled in DELIVER were possibly somewhat sicker. A recent press release on DELIVER highlighted that dapagliflozin achieved the primary endpoint in patients with HFpEF. 5 A detailed outline of its findings will help us to understand the effect of dapagliflozin on clinical outcomes in this population.
In conclusion, using the alternative endpoint definitions of the DELIVER trial, treatment with empagliflozin reduced the risk for cardiovascular death or worsening HF events in patients with HFpEF and this benefit was consistent across pre‐specified subgroups. For cardiovascular events, the pattern of benefit was like that reported using the original endpoint definitions of the EMPEROR‐Preserved trial. However, using the DELIVER criteria, the magnitude of the effect of empagliflozin to reduce the risk of a major renal event increased meaningfully and became statistically significant when patients with LVEF ≥60% were removed from the analysis.
Acknowledgements
Graphical assistance, supported financially by Boehringer Ingelheim, was provided by 7.4 Limited.
Funding
The EMPEROR‐Preserved trial was funded by the Boehringer Ingelheim & Eli Lilly and Company Diabetes Alliance.
To ensure independent interpretation of clinical study results and enable authors to fulfil their role and obligations under the ICMJE criteria, Boehringer Ingelheim grants all external authors access to relevant clinical study data. In adherence with the Boehringer Ingelheim Policy on Transparency and Publication of Clinical Study Data, scientific and medical researchers can request access to clinical study data after publication of the primary manuscript in a peer‐reviewed journal, regulatory activities are complete and other criteria are met. Researchers should use the https://vivli.org/ link to request access to study data and visit https://www.mystudywindow.com/msw/datasharing for further information.
Conflict of interest: S.D.A. reports grants and personal fees from Vifor Int. and Abbott Vascular, and personal fees from AstraZeneca, Bayer, Brahms, Boehringer Ingelheim, Cardiac Dimensions, Novartis, Occlutech, Servier, and Vifor Int. T.J.S. has no conflicts of interest to declare. G.F. reports lecture fees and/or committee member contributions in clinical trials sponsored by Bayer, Medtronic, Vifor, Servier, Novartis, Amgen, and Boehringer Ingelheim, and research support from the European Union. F.Z. has recently received steering committee or advisory board fees from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Cardior, CVRx, Janssen, Livanova, Merck, Mundipharma, Novartis, Novo Nordisk, and Vifor Fresenius. J.P.F. reports consultancy fees from Boehringer Ingelheim. S.J.P. reports consultancy fees from Boehringer Ingelheim. M.B. and C.Z. are employees of Boehringer Ingelheim. M.P. reports personal fees from Boehringer Ingelheim during the conduct of the study; personal fees from Abbvie, Actavis, Amarin, Amgen, AstraZeneca, Boehringer Ingelheim, Caladrius, Casana, CSL Behring, Cytokinetics, Imara, Lilly, Moderna, Novartis, Reata, Relypsa, and Salamandra outside the submitted work. J.B. reports consulting fees from BI, Cardior, CVRx, Foundry, G3 Pharma, Imbria, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, NovoNordisk, Relypsa, Roche, Sanofi, Sequana Medical, V‐Wave Ltd., and Vifor.
References
- 1. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al.; EMPEROR‐Preserved Trial Investigators . Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–61. [DOI] [PubMed] [Google Scholar]
- 2. Solomon SD, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, et al. Dapagliflozin in heart failure with preserved and mildly reduced ejection fraction: rationale and design of the DELIVER trial. Eur J Heart Fail. 2021;23:1217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anker SD, Butler J, Filippatos GS, Jamal W, Salsali A, Schnee J, et al.; EMPEROR‐Preserved Trial Committees and Investigators . Evaluation of the effects of sodium‐glucose co‐transporter 2 inhibition with empagliflozin on morbidity and mortality in patients with chronic heart failure and a preserved ejection fraction: rationale for and design of the EMPEROR‐Preserved trial. Eur J Heart Fail. 2019;21:1279–87. [DOI] [PubMed] [Google Scholar]
- 4. Hicks KA, Mahaffey KW, Mehran R, Nissen SE, Wiviott SD, Dunn B, et al.; Standardized Data Collection for Cardiovascular Trials Initiative (SCTI) . 2017 Cardiovascular and stroke endpoint definitions for clinical trials. Circulation. 2018;71: 961–72. [DOI] [PubMed] [Google Scholar]
- 5. AstraZeneca. Farxiga met primary endpoint in DELIVER Phase III trial, reducing risk of cardiovascular death or worsening heart failurein patients with preserved ejection fraction. https://www.astrazeneca.com/media‐centre/press‐releases/2022/farxiga‐hfpef‐phase‐iii‐trial‐met‐primary‐endpoint.html [accessed on 16 May 2022].


