Abstract
Background
Locally advanced cervical cancer (CC) remains lethal in the United States. We investigate the effect of receiving care at an National Cancer Institute–designated cancer center (NCICC) on survival.
Methods
Data for women diagnosed with CC from 2004 to 2016 who received radiation treatment were extracted from the California Cancer Registry (n = 4250). Cox proportional hazards regression models assessed whether (1) receiving care at NCICCs was associated with risk of CC‐specific death, (2) this association remained after multivariable adjustment for age, race/ethnicity, and insurance status, and (3) this association was explained by receipt of guideline‐concordant treatment.
Results
Median age was 50 years (interquartile range [IQR] 41–61 years), with median follow‐up of 2.7 years (IQR 1.3–6.0 years). One‐third of patients were seen at an NCICC, and 29% died of CC. The hazard of CC‐specific death was reduced by 20% for those receiving care at NCICCs compared with patients receiving care elsewhere (HR = .80; 95% CI, 0.70–0.90). Adjustment for guideline‐concordant treatment and other covariates minimally attenuated the association to 0.83 (95% CI, 0.74–0.95), suggesting that the survival advantage associated with care at NCICCs may not be due to receipt of guideline‐concordant treatment.
Conclusions
This study demonstrates survival benefit for patients receiving care at NCICCs compared with those receiving care elsewhere that is not explained by differences in guideline‐concordant care. Structural, organizational, or provider characteristics and differences in patients receiving care at centers with and without NCI designation could explain observed associations. Further understanding of these factors will promote equality across oncology care facilities and survival equity for patients with CC.
Keywords: brachytherapy, California, cancer center, cervical cancer, treatment outcome, uterine cervical neoplasms
Short abstract
This study demonstrates survival benefit for patients receiving care for cervical cancer at National Cancer Institute–designated cancer centers that is not explained by receipt of guideline‐concordant treatment. Further understanding of these factors will promote equality across oncology care facilities resulting in survival equity for patients with cervical cancer.
INTRODUCTION
Cervical cancer remains a leading cause of cancer mortality for women in the United States, with an estimated 13,800 new cases and 4290 deaths in 2020. 1 Standard‐of‐care therapy for locally advanced cervical cancer consists of concurrent external beam radiation (EBRT) and chemotherapy followed by brachytherapy (BT). BT is necessary to deliver high doses of radiation to the tumor while minimizing the dose to surrounding tissues and is associated with both improved cancer‐specific and overall survival. 2 , 3 , 4 The Commission on Cancer Accreditation quality of care metric for cervical cancer includes the use of chemotherapy added to radiation, and the use of BT in women treated with primary radiation with curative intent in any stage of cervical cancer. Despite the clear evidence of benefit, use of BT in the United States has declined, and this decline has been linked to decreased cervical cancer survival. 5 , 6 Certain populations of patients, including Black women, patients of lower socioeconomic status, older patients, those with public or no insurance, those with earlier stage disease, and those with greater comorbidities, are less likely to receive BT. 5 , 7 For some, a correlation between the lack of BT and lower survival rates has been observed. 5 , 7
Several studies have examined additional treatment factors that affect both BT and cervical cancer outcomes. In terms of oncologic surgery, patients receiving care from high‐volume providers and in high‐volume centers have the best outcomes. 8 , 9 , 10 , 11 , 12 , 13 Two National Cancer Database studies of hospital volume during similar timeframes found that, although hospital volume was associated with receipt of BT, survival was not consistently affected. 14 , 15 Another study of the Taiwan Cancer Registry found that greater hospital patient load, as defined by the number of definitive radiotherapy procedures annually, increased use of BT, cancer‐specific survival, and overall survival. 16
National Cancer Institute (NCI) designation is a rigorous process by which a cancer center demonstrates comprehensive cancer care and quality. In lung, breast, gastrointestinal, ovarian, and bladder cancers, treatment at an NCI‐designated cancer center (NCICC) has been shown to improve survival. 17 , 18 , 19 , 20 , 21 It is unknown whether that survival advantage extends to cervical cancer and what factors might account for this advantage, if any. This is particularly of interest given that access to NCICCs is limited; only 64 cancer centers are currently designated as NCI cancer centers across the United States (excluding basic laboratory cancer centers), with 14 states having none. 22 Despite NCICC catchment areas extending to more than 77% of US counties, 23 a recent report characterized 72% of US counties as undercovered by members of the Association of American of Cancer Institutes (the vast majority of whose members are NCICCs). 24 Although there were 1.76 million new cases of cancer reported in the United States in 2019, 25 newly registered patients at NCICCs accounted for only 22% (387,415) of these individuals. 26
Given the declining use of cervical cancer BT and its effect on cervical cancer treatment and outcomes, we sought to evaluate whether receiving care at an NCICC was associated with improved cancer‐specific survival among cervical cancer patients in California receiving definitive radiation therapy and whether guideline‐concordant treatment, specifically concurrent chemotherapy and radiation with a BT boost, explained that association.
Methods
Patients with cervical cancer who were treated with radiation were identified using the California Cancer Registry (CCR). The CCR is the largest population‐based state cancer registry in the United States and participates in the NCI's Surveillance, Epidemiology, and End Results program. The CCR contains demographic, diagnostic, treatment, and outcome information on cancers diagnosed in patients residing in California.
We queried the CCR for adult women diagnosed with a first and only primary invasive cervical cancer from January 1, 2004, through December 31, 2016 (n = 16,560). Women were excluded from the analysis hierarchically as follows: diagnosed by death certificate or autopsy only (n = 90); tumor not microscopically confirmed (n = 261); tumor not International Federation of Gynecology and Obstetrics (FIGO) stage IB2‐IVA (n = 9996); did not receive radiation as part of the first course of treatment (n = 885); were treated with regional radiation other than EBRT or boost radiation other than EBRT or BT (n = 567); started radiation therapy after the study end date (December 31, 2016) (n = 84); were missing month and day of diagnosis or radiation start date (n = 66); had less than 35 days of follow‐up after the start of radiation (to give women time to receive a boost course of radiation) (n = 69); had an uncertain or ungeocodable residential address at diagnosis (n = 151); or had missing data for one or more of the confounders (n = 141), yielding 4250 individuals. Human subjects' approval was obtained from the University of California San Francisco institutional review board, as a part of the Greater Bay Area Cancer Registry protocol for operating a population‐based cancer registry and conducting surveillance and related analyses with the data. The data were anonymized before analysis.
Cervical cancer was identified as International Classification of Disease for Oncology, third edition, site code C53.0–C53.9, excluding histology 9050–9055, 9140, and 9590–9992. Because FIGO stage was not directly available, stage at diagnosis was based on the Surveillance, Epidemiology, and End Results modification of the American Joint Committee on Cancer staging system, which is closely aligned with the FIGO staging structure. 27 , 28 However, because American Joint Committee on Cancer stage does not distinguish between IB1 and IB2 (having IB only), we classified those with stage IB and tumor size >4 cm as IB2. Neighborhood‐level socioeconomic status (nSES) and urbanicity were determined using data from Census 2000 (for those diagnosed 2004–2005) and the American Community Survey 2007–2011 (for those diagnosed 2006–2016) at the census block group level. nSES uses an established composite index based on educational attainment, employment rate, occupation type, median household income, median rent, house values, and poverty level, and was categorized into statewide quintiles. 29 Urbanicity measured urban/rural status using census defined Urbanized Areas (population ≥ 50,000) and Urban Clusters (population between 2500 and 50,000). The CCR collects information on radiation therapy used as the first course of treatment. Radiation therapy is often given in two or more phases, which are all considered part of the initial treatment and are collapsed and coded as regional (larger field) and boost (more targeted field) radiation therapy. CCR data include the start date of the initial radiation treatment and the types of regional and boost radiation therapy. Standard of care for cervical cancer consists of concurrent regional EBRT with chemotherapy followed by BT boost, typically approximately 1 month later. We required women to have at least 35 days of follow‐up after the start of radiation to allow women time to start boost radiation.
Those eligible for analysis (n = 4250) were categorized by whether they received care from an NCICC (yes, no, based on any admission for diagnosis and/or treatment of the cancer), age (18–49, 50–59, 60–69, 70+ years), race/ethnicity (non‐Hispanic White, non‐Hispanic Black, Hispanic, Asian/Pacific Islander, non‐Hispanic other or unknown race), insurance status (no insurance, had insurance), nSES quintile (low [Q1–Q3], high [Q4–Q5]), urbanicity (rural/town, suburb, metro/city), FIGO stage (IB2, II, III, IVA), and guideline‐concordant treatment (yes [chemotherapy and brachytherapy boost], no). The outcome of interest was cervical cancer–specific mortality. Follow‐up time was calculated as the number of days between cervical cancer diagnosis and the earliest of death from cervical cancer (ICD‐10 C53), death from another cause, last follow‐up (i.e., date of last known contact), or study end (December 31, 2016). Statistical modeling was based on a conceptual model and directed acyclic graph (Fig. 1).
FIGURE 1.
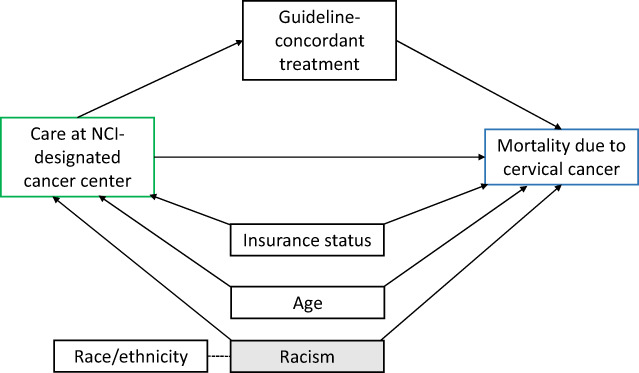
Hypothesized directed acyclic graph of the association between care at a National Cancer Institute–designated cancer center and reduced mortality from cervical cancer.
Patient characteristics were summarized overall and by whether care was received at an NCICC center using χ 2 tests. A Kaplan–Meier plot was used to compare cervical cancer–specific survival by care at an NCICC using a log‐rank test. Cox proportional hazards regression models with time from diagnosis as the time scale and left truncation (patients entered the model 35 days after the start of radiation) were used to assess whether (1) care at an NCICC was associated with risk of cervical cancer‐specific death, (2) this association remained after adjusting for hypothesized confounders, and (3) the association could be explained by the receipt of guideline‐concordant treatment. An additional 39 patients with unknown cause of death were excluded from Cox regression analyses. Adjusted and unadjusted hazard rate ratios (HR) with 95% CIs were evaluated. Model 1 was adjusted for age. Based on the a priori directed acyclic graph (Fig. 1), model 2 was adjusted for factors hypothesized to be associated with both care at an NCICC and disease outcome, including age, race/ethnicity, and insurance status. Model 3 additionally adjusted for guideline‐concordant treatment. The proportional hazards assumption was tested by examining the correlation between time and scaled Schoenfeld residuals for all confounders. Because the proportional hazards assumption was violated for guideline‐concordant treatment, it was included as an underlying stratification variable in model 3 to allow the baseline hazard to vary by receipt of guideline‐concordant treatment. All analyses were performed using SAS, version 9.4 (SAS Institute, Cary, North Carolina).
RESULTS
The median age of the sample was 50 years (interquartile range [IQR] 41–61 years; range 21–98 years). Median follow‐up time was 2.7 years (IQR 1.3–6.0 years). Of the 4250 patients in the sample, 33% received care at an NCICC, and 29% died of cervical cancer during the follow‐up period (Table 1).
TABLE 1.
Sample Characteristics of People Diagnosed with Ib2‐Iva Cervical Cancer in California Between 2004 and 2016 by Whether Received Care at an Nci‐Designated Cancer Center (n = 4250)
| Characteristic | NCI cancer center (n = 1395) | Other location (n = 2855) | All (n = 4250) | p |
|---|---|---|---|---|
| Survival outcome | <.0001 | |||
| Alive | 966 (69%) | 1675 (59%) | 2641 (62%) | |
| Died of cervical cancer | 346 (25%) | 882 (31%) | 1228 (29%) | |
| Died of another cause | 72 (5%) | 270 (10%) | 342 (8%) | |
| Died of unknown cause | 11 (1%) | 28 (1%) | 39 (1%) | |
| Age, y | <.0001 | |||
| 18–49 | 698 (50%) | 1357 (48%) | 2055 (48%) | |
| 50–59 | 356 (26%) | 624 (22%) | 980 (23%) | |
| 60–69 | 201 (14%) | 471 (17%) | 672 (16%) | |
| 70+ | 140 (10%) | 403 (14%) | 543 (13%) | |
| Race/ethnicity | .0042 | |||
| Non‐Hispanic White | 459 (33%) | 1039 (36%) | 1498 (35%) | |
| Non‐Hispanic Black | 72 (5%) | 198 (7%) | 270 (6%) | |
| Hispanic | 586 (42%) | 1150 (40%) | 1736 (41%) | |
| Asian/Pacific Islander | 264 (19%) | 445 (16%) | 709 (17%) | |
| Non‐Hispanic Other/unknown | 14 (1%) | 23 (1%) | 37 (1%) | |
| Insurance status | 0.0405 | |||
| Insured | 1366 (98%) | 2764 (97%) | 4130 (97%) | |
| Not insured | 29 (2%) | 91 (3%) | 120 (3%) | |
| Neighborhood socioeconomic status quintile | .44 | |||
| Q1–Q3 (low) | 1012 (73%) | 2039 (71%) | 3051 (72%) | |
| Q4–Q5 (high) | 383 (28%) | 816 (29%) | 1199 (28%) | |
| Urbanicity | .29 | |||
| Rural/town | 134 (10%) | 250 (9%) | 384 (9%) | |
| Suburb | 608 (44%) | 1314 (46%) | 1922 (45%) | |
| Metro/city | 653 (47%) | 1291 (45%) | 1944 (46%) | |
| FIGO stage | .11 | |||
| IB2 | 163 (12%) | 276 (10%) | 439 (10%) | |
| II | 413 (30%) | 920 (32%) | 1333 (31%) | |
| III | 768 (55%) | 1546 (54%) | 2314 (54%) | |
| IVA | 51 (4%) | 113 (4%) | 164 (4%) | |
| Chemotherapy | <.0001 | |||
| Yes | 1294 (93%) | 2357 (83%) | 3651 (86%) | |
| No | 101 (7%) | 498 (17%) | 599 (14%) | |
| Brachytherapy boost | <.0001 | |||
| Yes | 815 (58%) | 1291 (45%) | 2106 (50%) | |
| No | 580 (42%) | 1564 (55%) | 2144 (50%) | |
| Guideline‐concordant treatment | <.0001 | |||
| Yes | 769 (55%) | 1148 (40%) | 1917 (45%) | |
| No | 626 (45%) | 1707 (60%) | 2333 (55%) |
Abbreviations: FIGO, International Federation of Gynecology and Obstetrics; NCI, National Cancer Institute
Bivariate analyses showed no significant differences in nSES quintile, urbanicity, or FIGO stage between patients receiving care at NCICCs and those seen elsewhere. However, those receiving care at NCICCs were more likely to be alive, tended to be younger, were less likely to be Black and more likely to be Asian/Pacific Islander, and were more likely to receive chemotherapy, brachytherapy boost, and the combination of both (referred to as guideline‐concordant treatment) compared with those receiving care elsewhere (Table 1).
In the Kaplan–Meier plot (Fig. 2) and age‐adjusted Cox proportional hazards model, patients with cervical cancer who received care at an NCICC had a statistically significant survival benefit over those seen at a non‐NCICC. The hazard of death from cervical cancer was reduced by 20% for those receiving care at NCICCs vs. those seen elsewhere (HR = .80; 95% CI, 0.70–0.90; Table 2, model 1).
FIGURE 2.
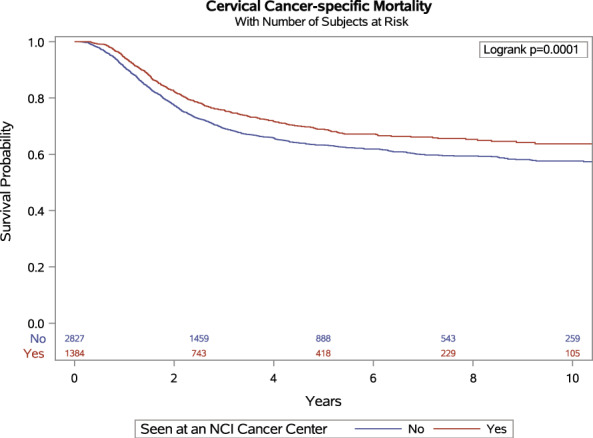
Cervical cancer–specific survival among patients diagnosed with IB2‐IVA cervical cancer in California between 2004 and 2016 by whether received care at a National Cancer Institute–designated cancer center (n = 4250).
TABLE 2.
Cox Proportional Hazards Models of the Effect of Receiving Care at an Nci‐Designated Cancer Center on Cervical Cancer–Specific Mortality
| Model | Purpose | HR (95% CI) | p |
|---|---|---|---|
| 1. Age‐adjusted model for receiving care at NCI‐designated cancer center | Determine age‐adjusted hazard of death associated with receiving care at an NCI‐designated cancer center | 0.80 (0.70–0.90) | .0003 |
| 2. Adjusted for confounders (age, race/ethnicity, and insurance) | Adjust hazard for known and hypothesized confounders | 0.81 (0.72–0.92) | .0011 |
| 3. Model 2 + guideline‐concordant treatment in a STRATA statement | Determine whether guideline‐concordant care accounts for the association between receiving care at an NCI‐designated cancer center and mortality | 0.83 (0.74–0.95) | .0050 |
Abbreviations: HR, hazard ratio; NCI, National Cancer Institute
Adjustment for known and hypothesized confounders (Fig. 1) in the association between receiving care at an NCICC and cervical cancer–specific mortality only minimally attenuated the HR ratio; those receiving care at NCICCs had 19% reduced hazard of death from cervical cancer compared with those seen elsewhere (HR = .81; 95% CI, 0.72–0.92; Table 2, model 2). To investigate whether guideline‐concordant treatment accounts for the association between receiving care at NCICCs and cervical cancer–specific mortality, model 2 was further adjusted for guideline‐concordant treatment. The inclusion of guideline‐concordant treatment only minimally attenuated the HR to 0.83 (95% CI, 0.74–0.95; Table 2, model 3), indicating that guideline‐concordant treatment (i.e., treatment with both chemotherapy and a brachytherapy boost in a cohort who received radiation as part of the first course of treatment) does not explain the association between receiving care at NCICCs and reduced cervical cancer–specific mortality.
Discussion
This study explored the association between patient factors and receipt of cervical cancer care at an NCICC in California and the potential influence of care at an NCICC on cervical cancer–specific survival. We found that patients who received care at an NCICC had lower risk of cervical cancer–specific mortality compared with those receiving care in non‐NCICCs, despite controlling for known confounders. Because it is known that the addition of concurrent chemotherapy and a brachytherapy boost to primary radiation improves survival from locally advanced cervical cancer, 2 , 3 , 4 , 30 we included this treatment protocol in our fully adjusted model. Contrary to our hypotheses, inclusion of adherence to guideline concordant care did not account for the association between care at NCICCs and cervical cancer mortality. After our robust analysis and modeling, none of the clinicopathologic nor demographic factors we examined contributed to this survival benefit seen at an NCICC. Therefore, our results suggest that other factors in care and/or social determinants of patients who are able to access an NCICC may be contributing to the association between care at NCICCs and cervical cancer survival.
Prior research studying the effect of care at NCICCs for other solid tumors have shown improvement in guideline‐concordant care, 18 , 31 surgical outcome, 17 , 21 and inpatient mortality. 32 A population‐based study of adults in Los Angeles County showed a trend toward increased risk of mortality in patients treated at non‐NCICCs, 33 with the authors hypothesizing that statistical significance was not reached because of small numbers of patients treated an NCICC.
Much of the emphasis on cervical cancer therapy quality has been on receipt of concurrent chemotherapy during radiation, total treatment time, and the addition of brachytherapy. 34 However, there may be center‐level differences in treatment quality, affecting survival. These center‐level factors may include patient population, more accurate staging from pretreatment imaging, the use of more appropriate or higher quality radiation, the greater availability of and participation in clinical trials at NCICCs, the broader experience of physician providers, and the intangible benefits to physicians of working in a research active environment. Our research group has recently examined the impact of time to treatment on outcomes. 35 We found that Hispanic women were more likely to undergo delayed time to treatment than non‐Hispanic White women. However, delayed time to treatment was not associated with inferior overall survival and locoregional failure. 35 In addition, NCICCs often use a multidisciplinary team approach with discussion of patient cases at tumor boards or a team with expertise in radiation therapy with real‐time peer review 36 ; these practices might also contribute to improved survival. The multidisciplinary team approach often includes senior experts in the field with dedicated knowledge about cervical cancer treatment decision and clinical trial opportunities.
Another consideration is whether the survival benefit seen at NCICCs is a result of receiving care at a high‐volume center. Lin and colleagues found that the receipt of care at a high‐volume center was associated with increased likelihood of receiving BT and chemotherapy and shorter time to radiotherapy completion. 14 In a subsequent study, Lin et al. found that this greater use of chemotherapy and BT were major contributors to the improved survival of patients treated at large‐patient‐load hospitals. Larger loads were also an independent prognostic variable for survival in certain patient populations, 16 whereas another study found that hospital volume had little impact on outcome for patients with locally advanced cervical cancer. 15
Aspects of highly specialized oncologic care may be superior at centers of excellence, such as those receiving an NCI designation. Although our study showed a similar distribution of race/ethnicity, insurance status, and nSES between patients seen at NCICCs and non‐NCICCs, there is heterogeneity within California. Access to an NCICC remains out of reach for many patients; in a population‐based study of adults in Los Angeles County, patients with cervical cancer who were Hispanic, uninsured, of low SES, or lived more than 9 miles from an NCICC were significantly less likely to receive care at an NCICC. 33 In our study, in a state with eight NCI‐designated comprehensive cancer centers, only 33% of patients with locally advanced cervical cancer received care at an NCICC. It is imperative, therefore, that the factors underlying the survival benefit for those receiving care at an NCICC be further identified to best serve the entire population of patients with cervical cancer. Once identified, all oncologic institutions will be informed of optimal practices and consider their implementation to improve patient outcomes and quality of care and thereby reduce survival disparities for patients with cervical cancer. Until this survival disparity is reduced, however, access to NCICCs is an equity issue, and we must ensure meaningful access across sociodemographic groups.
Efforts should also be made to standardize care between all levels of oncologic institutes through the dissemination of best practices using common benchmarks and guidelines. Despite efforts at standardization, some technologies and procedures, such as BT, 37 , 38 require image guidance or detailed coordination with the health care team for quality delivery, which may not be widely available outside of centers with NCI designation. 38 In these circumstances, coordination of care between oncologic centers would allow patients access to these technologies and procedures, although maintaining the bulk of their care at their chosen oncology practice.
Outcome differences that are linked to structural changes in the institution are more difficult to target because resources vary among oncology care facilities. Our results indicating a link between NCI designation and improved outcomes suggests the need for a deeper investigation into core drivers of quality care beyond what we already know regarding cervical cancer: the addition of chemotherapy to radiation, treatment care time within 56 days, and the addition of BT. In addition, there could be specific medical comorbidities that limit the ability to receive the aforementioned factors. Organizational and provider level factors, structure, and processes require further investigation to determine whether they are associated with improved outcomes. The identification of factors that drive outcome improvement will enable institutions to implement interventions that improve patient outcomes and quality of care without overburdening NCICCs.
The use of the CCR in our analyses provides both strengths and limitations. One strength is the comprehensiveness of the data; every case of cervical cancer in the state of California during the study period is available for analysis, which allows for robust analysis. However, the accuracy and completeness of registry data represent a limitation, particularly in complete and accurate reporting of treatment(s) received, 39 , 40 , 41 including duration of treatment. Furthermore, documentation of radiation therapy receipt is variable across data sets, 40 , 41 which could affect our conclusions that are based on guideline‐concordant treatment. In addition, variable availability is limited. For example, locoregional failure is not included in CCR data, which, if known, would provide important information about whether brachytherapy may have been beneficial to patients.
Our study findings show a survival benefit among patients receiving care at an NCICC relative to those seen elsewhere that is not explained by receipt of guideline‐concordant care. We found that there are unmeasured variables that may account for survival outcomes, perhaps based on differences in structural, organizational, or provider characteristics. Further understanding of these factors will facilitate increased equality among institutions and oncology care facilities and increased health equity for patients.
AUTHOR CONTRIBUTIONS
Corinne McDaniels‐Davidson: Conceptualization, funding acquisition, investigation, supervision, and writing – original draft. Christine H. Feng: Conceptualization, investigation, and writing – original draft. Maria Elena Martinez: Funding acquisition and writing – review and editing. Alison J. Canchola: Formal analysis, methodology, and writing – review and editing. Scarlett Lin Gomez: Data curation, investigation, supervision, and writing – review and editing. Jesse N. Nodora: Writing – review and editing. Sandip P. Patel: Writing – review and editing. Arno J. Mundt: Conceptualization and writing – review and editing. Jyoti S. Mayadev: Conceptualization, funding acquisition, investigation, supervision, and writing – original draft.
CONFLICTS OF INTEREST
Jyoti S. Mayadev reports consulting honoraria from Varian Medical Systems, Merck, Astra Zeneca, and Primmune Bio and grants from NCI, NRG Oncology, and GOG Foundation. The other authors made no disclosure.
FUNDING INFORMATION
National Cancer Institute at the National Institutes of Health grant numbers U54CA132379, U54CA132384, and 5P30CA023100.
ACKNOWLEDGMENTS
National Cancer Institute at the National Institutes of Health grant numbers U54CA132379, U54CA132384, and 5P30CA023100.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7‐30. [DOI] [PubMed] [Google Scholar]
- 2. Han K, Milosevic M, Fyles A, Pintilie M, Viswanathan AN. Trends in the utilization of brachytherapy in cervical cancer in the United States. Int J Radiat Oncol Biol Phys. 2013;87:111‐119. [DOI] [PubMed] [Google Scholar]
- 3. Hanks GE, Herring DF, Kramer S. Patterns of care outcome studies. Results of the national practice in cancer of the cervix. Cancer. 1983;51:959‐967. [DOI] [PubMed] [Google Scholar]
- 4. Logsdon MD, Eifel PJ. FIGO IIIB squamous cell carcinoma of the cervix: an analysis of prognostic factors emphasizing the balance between external beam and intracavitary radiation therapy. Int J Radiat Oncol Biol Phys. 1999;43:763‐775. [DOI] [PubMed] [Google Scholar]
- 5. Mayadev J, Klapheke A, Yashar C, et al. Underutilization of brachytherapy and disparities in survival for patients with cervical cancer in California. Gynecol Oncol. 2018;150:73‐78. [DOI] [PubMed] [Google Scholar]
- 6. Schad MD, Moore J, Camacho F, Anderson RT, Cantrell LA, Showalter TN. Predictors of quality of care and survival in a three‐state cohort of locally advanced cervical cancer patients and development of a predictive model to identify women at risk of incomplete treatment. Medicine. 2019;98:e16874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alimena S, Yang DD, Melamed A, et al. Racial disparities in brachytherapy administration and survival in women with locally advanced cervical cancer. Gynecol Oncol. 2019;154:595‐601. [DOI] [PubMed] [Google Scholar]
- 8. Hillner BE, Smith TJ, Desch CE. Hospital and physician volume or specialization and outcomes in cancer treatment: Importance in quality of cancer care. J Clin Oncol. 2000;18:2327‐2340. [DOI] [PubMed] [Google Scholar]
- 9. Paulsen T, Kjærheim K, Kærn J, Tretli S, Tropé C. Improved short‐term survival for advanced ovarian, tubal, and peritoneal cancer patients operated at teaching hospitals. Int J Gynecol Cancer. 2006;16:11‐17. [DOI] [PubMed] [Google Scholar]
- 10. Perera HK, Ananth CV, Richards CA, et al. Variation in ovarian conservation in women undergoing hysterectomy for benign indications. Obst Gynecol. 2013;121:717‐726. [DOI] [PubMed] [Google Scholar]
- 11. Thabut G, Christie JD, Kremers WK, Fournier M, Halpern SD. Survival differences following lung transplantation among US transplant centers. JAMA. 2010;304:53‐60. [DOI] [PubMed] [Google Scholar]
- 12. Tingulstad S, Skjeldestad FE, Hagen B. The effect of centralization of primary surgery on survival in ovarian cancer patients. Obst Gynecol. 2003;102:499‐505. [DOI] [PubMed] [Google Scholar]
- 13. Yasunaga H, Nishii O, Hirai Y, Ochiai K, Matsuyama Y, Ohe K. Impact of surgeon and hospital volumes on short‐term postoperative complications after radical hysterectomy for cervical cancer. J Obstet Gynaecol Res. 2009;35:699‐705. [DOI] [PubMed] [Google Scholar]
- 14. Lin JF, Berger JL, Krivak TC, et al. Impact of facility volume on therapy and survival for locally advanced cervical cancer. Gynecol Oncol. 2014;132:416‐422. [DOI] [PubMed] [Google Scholar]
- 15. Wright JD, Huang Y, Ananth CV, et al. Influence of treatment center and hospital volume on survival for locally advanced cervical cancer. Gynecol Oncol. 2015;139:506‐512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lin S‐M, Ku H‐Y, Chang T‐C, Liu T‐W, Chang C‐S, Hong J‐H. Outcomes for cervical cancer patients treated with radiation in high‐volume and low‐volume hospitals. Int J Radiat Oncol Biol Phys. 2018;102:184‐193. [DOI] [PubMed] [Google Scholar]
- 17. Birkmeyer NJ, Goodney PP, Stukel TA, Hillner BE, Birkmeyer JD. Do cancer centers designated by the National Cancer Institute have better surgical outcomes? Cancer. 2005;103:435‐441. [DOI] [PubMed] [Google Scholar]
- 18. Bristow RE, Chang J, Ziogas A, Campos B, Chavez LR, Anton‐Culver H. Impact of national cancer institute comprehensive cancer centers on ovarian cancer treatment and survival. J Am Coll Surgeons. 2015;220:940‐950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Etzioni DA, Young‐Fadok TM, Cima RR, et al. Patient survival after surgical treatment of rectal cancer: Impact of surgeon and hospital characteristics. Cancer. 2014;120:2472‐2481. [DOI] [PubMed] [Google Scholar]
- 20. Onega T, Duell EJ, Shi X, Demidenko E, Gottlieb D, Goodman DC. Influence of NCI cancer center attendance on mortality in lung, breast, colorectal, and prostate cancer patients. Med Care Res Rev. 2009;66:542‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Paulson EC, Mitra N, Sonnad S, et al. National cancer institute designation predicts improved outcomes in colorectal cancer surgery. Ann Surg. 2008;248:675‐685. [DOI] [PubMed] [Google Scholar]
- 22. National Cancer Institute. NCI‐designated cancer centers . https://www.cancer.gov/research/infrastructure/cancer‐centers. Accessed February 14, 2022.
- 23. DelNero PF, Buller ID, Jones RR, et al. A national map of NCI‐designated cancer center catchment areas on the 50th anniversary of the cancer centers program. Cancer Epidemiol Biomarkers Prev. 2022;31:965‐971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leader AE, McNair C, Yurick C, et al. Assessing the coverage of U.S. cancer center primary catchment areas. Cancer Epidemiol Biomarkers Prev. 2022;31:955‐964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7‐34. [DOI] [PubMed] [Google Scholar]
- 26. National Cancer Institute . Office of Cancer Centers data table 3: Reportable patients/participation in therapeutic studies. The Office of Cancer Centers at NCI. https://cancercenters.cancer.gov/DT/DT3. Accessed February 14, 2022.
- 27. Amin MB, Edge SB, Greene FL, et al. for the American College of Surgeons. AJCC cancer staging manual. 8th ed. Springer; 2017:1024. [Google Scholar]
- 28. Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynecol Obstet. 2009;105:103‐104. [DOI] [PubMed] [Google Scholar]
- 29. Yang J, Schupp C, Harrati A, Clarke C, Keegan T, Gomez S. Developing an area‐based socioeconomic measure from American Community Survey data. Cancer Prevention Institute of California; 2014. [Google Scholar]
- 30. Josefson D. Adding chemotherapy improves survival in cervical cancer. BMJ. 1999;318:623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bilimoria KY, Balch CM, Wayne JD, et al. Health care system and socioeconomic factors associated with variance in use of sentinel lymph node biopsy for melanoma in the United States. J Clin Oncol. 2009;27:1857‐1863. [DOI] [PubMed] [Google Scholar]
- 32. Ho G, Wun T, Muffly L, et al. Decreased early mortality associated with the treatment of acute myeloid leukemia at National Cancer Institute‐designated cancer centers in California. Cancer. 2018;124:1938‐1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wolfson JA, Sun CL, Wyatt LP, Hurria A, Bhatia S. Impact of care at comprehensive cancer centers on outcome: results from a population‐based study. Cancer. 2015;121:3885‐3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chino J, Annunziata CM, Beriwal S, et al. The ASTRO clinical practice guidelines in cervical cancer: optimizing radiation therapy for improved outcomes. Gynecol Oncol. 2020;159:607‐610. [DOI] [PubMed] [Google Scholar]
- 35. Kotha NV, Williamson CW, Mell LK, et al. Disparities in time to start of definitive radiation treatment for patients with locally advanced cervical cancer. Int J Gynecol Cancer. 2022;32:613‐618. [DOI] [PubMed] [Google Scholar]
- 36. Huynh‐Le MP, Simon AB, Hoopes DJ, et al. Implementation of peer‐review quality rounds for gynecologic brachytherapy in a high‐volume academic center. Brachytherapy. 2020;19:881‐888. [DOI] [PubMed] [Google Scholar]
- 37. Mayadev J, Qi L, Lentz S, et al. Implant time and process efficiency for CT‐guided high‐dose‐rate brachytherapy for cervical cancer. Brachytherapy. 2014;13:233‐239. [DOI] [PubMed] [Google Scholar]
- 38. Mayadev J, Viswanathan A, Liu Y, et al. American Brachytherapy Task Group Report: a pooled analysis of clinical outcomes for high‐dose‐rate brachytherapy for cervical cancer. Brachytherapy. 2017;16:22‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cress RD, Zaslavsky AM, West DW, Wolf RE, Felter MC, Ayanian JZ. Completeness of information on adjuvant therapies for colorectal cancer in population‐based cancer registries. Med Care. 2003;41:1006‐1012. [DOI] [PubMed] [Google Scholar]
- 40. Malin JL, Kahn KL, Adams J, Kwan L, Laouri M, Ganz PA. Validity of cancer registry data for measuring the quality of breast cancer care. J Natl Cancer Inst. 2002;94:835‐844. [DOI] [PubMed] [Google Scholar]
- 41. Walker GV, Giordano SH, Williams M, et al. Muddy water? Variation in reporting receipt of breast cancer radiation therapy by population‐based tumor registries. Int J Radiat Oncol Biol Phys. 2013;86:686‐693. [DOI] [PMC free article] [PubMed] [Google Scholar]


