Abstract
The growing number of people living with dementia will result in increased costs of dementia worldwide. The e‐Health intervention ‘Exergaming’ may improve health and quality of life of people with dementia, but the cost‐effectiveness is unknown. We assessed the cost‐effectiveness of exergaming compared to regular activities from a societal perspective in day‐care centres (DCC) for people with dementia and their informal caregivers (IC) alongside a cluster randomised controlled trial. We included 112 dyads (person with dementia and IC) from 20 psychogeriatric DCCs (11 exergaming, 9 control) across the Netherlands. Exergaming consisted of interactive cycling at least twice a week for 6 months. Measurements were conducted at baseline (T0), after 3 (T1) and 6 (T2) months. Primary outcomes were minutes of physical activity, mobility of the participants with dementia (Short Physical Performances Battery, SPPB), and Quality‐Adjusted Life‐Years (QALYs) of participants with dementia and ICs. ICs filled out cost diaries to measure healthcare and informal care utilisation during the study. There were no statistically significant differences in outcomes or costs between the groups at the level of participants with dementia, the ICs or the dyad. With regard to QALYs and SPPB, the probability that exergaming is cost‐effective compared to control was low for all possible willingness‐to‐pay (WTP) thresholds. However, for physical activity at WTP thresholds of 0, 50 and 250 Euros per additional minute of physical activity, the probability of cost‐effectiveness is 0.46, 0.84 and 0.87, respectively. Exergaming in DCC was not cost‐effective compared to usual activities. However, considering the small sample size and the large number of missing observations, findings should be interpreted with caution. Future studies with larger samples are recommended to obtain definitive answers on the cost‐effectiveness of exergaming. This trial was registered in the Netherlands Trial Register (NTR5537/NL5420).
Keywords: costs and cost analysis, dementia, exercise, healthcare costs, physical fitness, quality of life, randomised controlled trial
What is known about this topic?
Physical exercise and exergaming offer benefits to people with dementia.
Exergaming is feasible for people with dementia.
To date, there is no research into the cost‐effectiveness of exergaming for people with dementia and informal caregivers.
What does this paper add?
Exergaming in day‐care centres was not cost‐effective compared to activities as usual regarding physical activity and mobility for people with dementia and Quality‐Adjusted Life‐Years (QALYs) for people with dementia and informal caregivers.
The results should be interpreted with caution because of the small sample and many missing observations.
We recommend improvements in future exergaming research, e.g., regarding intervention frequency and choice of outcome measures for quality of life.
1. INTRODUCTION
The number of people with dementia worldwide has risen from 29 million in 2005 to 50 million in 2019 (Wimo et al., 2007; World Health Organization, 2019). This number is predicted to climb to 82 million in 2030 and 152 million in 2050 (World Health Organization, 2019). As a result, the worldwide costs of dementia increased from €258 billion ($315 billion) in 2005 to €669 billion ($818 billion) in 2015, and are predicted to increase to up to €1.64 trillion ($2 trillion) in 2030 (Wimo et al., 2007; World Health Organization, 2017, 2019).
In the Netherlands, the number of people with dementia has increased from 50,000 in 1950 to 280,000 in 2019 and is predicted to rise to 520,000 by 2040 (Alzheimer Nederland, 2019) due to the ageing of the population and increased life expectancy. Dementia care costs in the Netherlands are expected to rise by 2.7% annually (Alzheimer Nederland, 2019). As such, dementia is the most expensive disease for the population in the Netherlands (Winkelhof et al., 2019).
Physical exercise is known to have potential benefits, both for people with and without dementia, on physical, cognitive, emotional and social functioning, as well as on quality of life (QoL) (Blondell et al., 2014; Heyn et al., 2004; Penedo & Dahn, 2005; Pitkälä et al., 2013; Potter et al., 2011; Taylor et al., 2004). However, symptoms accompanying dementia, such as increased apathy and a decrease of motivation and interest, may make it hard for people with dementia to engage in physical exercise, (Clarke et al., 2008; Crombie et al., 2004). Additionally, they might feel unsafe when being physically active (outdoors) due to fear of falling and risk of becoming lost (van Alphen et al., 2016).
An innovative form of physical exercise is exergaming, which we define as physical exercise that is interactively combined with cognitive stimulation in a gaming environment. Sensors register the player's movements, which influences a game on a connected screen (Heuvelink et al., 2014). Exergaming is fun and engaging and can contribute to a decrease of apathy and promote social contact. This in turn can motivate participation (Heuvelink et al., 2014; Meekes & Stanmore, 2017). Moreover, exergaming can make it easier for people with dementia to engage in physical exercise, because they do not have to worry about becoming lost. An example of exergaming is interactive cycling: players cycle on a stationary bicycle and can select a route on a screen. The speed of the film is determined by the cycling pace (van Santen et al., 2018).
Previous research demonstrated that exergaming had positive effects on walking capacity, functional mobility and balance performance in older adults without dementia (Corregidor‐Sánchez et al., 2020; Donath et al., 2016). For people living with dementia and MCI, exergaming can improve physical, cognitive and social functioning (van Santen et al., 2020; Zhao et al., 2020).
Although physical exercise has the potential to be cost‐effective (Saha et al., 2018), the cost‐effectiveness of exergaming for people with dementia has not been evaluated before. In a recently conducted randomised controlled trial (RCT), we compared exergaming (interactive cycling) to usual activities in day‐care centres (DCC). In our RCT, we did find positive effects on the secondary outcomes of cognition and social functioning of participants with dementia, as well as on distress in informal caregivers caused by the neuropsychiatric symptoms of the person with dementia and the sense of competence of informal caregivers (van Santen et al., 2020). However, this was not accompanied by improvements in mobility (SPPB scores) and/or physical activity, which were our primary study outcomes. The current paper reports on the outcomes of the accompanying cost‐effectiveness analysis from a societal perspective for physical activity and mobility of the participants with dementia, and Quality‐Adjusted Life‐Years (QALYs) of persons with dementia and informal caregivers.
Informal caregivers were included in the cost‐effectiveness analysis, because informal care costs make up a large portion of the total societal costs of people with dementia. Informal caregivers often spend the equivalent of a full‐time working week caring for the person with dementia (Alzheimer Nederland, 2019; Joling et al., 2015; World Health Organization, 2017, 2019). As a consequence, informal caregivers of people with dementia are at increased risk of reduced psychological well‐being and QoL, cognitive dysfunction and depressive disorders, and physical health issues compared to informal caregivers caring for someone with a different (mental) health disorder (Pinquart & Sörensen, 2007; Vitaliano et al., 2003). There are some first indications that suggest exergaming by the person with dementia can positively influence the QoL of the informal caregiver as well, which could result in lower healthcare use and costs (Lin et al., 2020; Stowell et al., 2019; Unbehaun et al., 2018; van Santen et al., 2020).
2. MATERIALS AND METHODS
2.1. Study design
We performed an economic evaluation alongside a cluster randomised controlled trial (RCT) evaluating the effectiveness of exergaming (van Santen et al., 2019). In summary, an independent researcher used Random Allocation Software to randomise DCCs to the experimental (exergaming) group or the control (traditional, non‐exergaming activities) group (Saghaei, 2004). Measurements were conducted at baseline (T0), after 3 (T1) and 6 months (T2). The RCT was approved by the Medical Ethics Committee (METc) of the Amsterdam University Medical Centers (UMC), location VUmc (VUmc; NL58227.029.16) and registered in the Netherlands Trial Register (NTR5537/NL5420). The study protocol of the complete RCT was published elsewhere but is summarised here (van Santen et al., 2019).
2.2. Participants and setting
We recruited a convenience sample of psychogeriatric DCCs across the Netherlands. Inclusion criteria for participants with dementia were: a diagnosis of any type of dementia, all ages, community‐dwelling and not expected to be admitted into residential care in the next 6 months, visiting the DCC at least twice per week, and an informal caregiver willing to participate. If it was impossible for a person with dementia to participate in the exergaming activity (according to DCC staff) due to a severe physical disorder or (terminal) disease (other than dementia), they were not invited to participate in the study. The investigator instructed DCC staff in a one‐hour meeting about the research study procedures and, for the exergaming group, the intervention (this could also be done by the provider of the equipment). Dyads (participant with dementia, informal caregiver) for the study were recruited by DCC staff. All participants with dementia and ICs gave written informed consent. Informal caregivers co‐signed the informed consent form of the participant with dementia. People with dementia who were unable to give informed consent were not included.
2.3. Intervention
Participants in the exergaming group attended the regular activity programme at the DCC. In addition, DCC staff encouraged them to participate in the exergaming intervention (interactive cycling) at least twice a week for 6 months. If the DCC did not have an interactive cycling system, they had to buy one of the following systems, which were offered at a discount: DiFiets, Fietslabyrint, PraxFit, or SilverFit Mile (van Santen et al., 2019). While cycling on a stationary bicycle (i.e., home trainer), the participant sees a previously selected route on a connected screen. This offers simultaneous physical and cognitive stimulation and mimics the experience of outdoor cycling.
In the control group, participants with dementia attended the regular DCC activity programme and were not offered exergaming activities. The regular activities usually consist of activities such as listening to music, singing, arts and crafts, cooking and physical exercise, such as (chair) gymnastics and outdoor walking.
2.4. Outcome measures
The primary outcome of the economic evaluation was QALYs. The three‐level version of the EuroQol questionnaire (EQ‐5D‐3L) was used to measure QoL of the participant with dementia him‐/herself, of the participant with dementia through his/her informal caregiver (proxy), and of the informal caregiver him‐/herself at T0, T1 and T2 (Brooks & Group, 1996).
The Dutch EQ‐5D‐3L tariff was used to convert the EQ‐5D‐3L health states to utility scores (Lamers et al., 2005). QALYs were calculated using the area‐under‐the‐curve method in which the time spent in a health state was multiplied by the utility score of that health state. Linear interpolation was used to calculate changes between health states. QALYs were estimated for the participant with dementia and informal caregiver separately and were also summed for the participant with dementia and informal caregiver together.
In addition, the cost‐effectiveness was also evaluated for the primary RCT outcome measures of physical activity (in minutes) and mobility, using the Short Physical Performances Battery (SPPB) (Guralnik et al., 1994). DCC staff and informal caregivers registered all physical activities in minutes at DCC and at home over a period of 7 days using a specifically developed form at each measurement (T0, T1, and T2). Mobility was measured with the SPPB by evaluating lower extremity functioning, which strongly correlates with the risk of mobility disability in older adults (Vasunilashorn et al., 2009). There are three subtests: balance, gait speed, and chair stands. Total scores of the SPPB range between 0 and 12 and are categorised into: loss of mobility already present (0–3), elevated risk of developing mobility disability (4–9), and good functioning with no risk of developing mobility disability (10–12). The interviewers administered the SPPB at DCC to measure mobility at T0, T1, and T2 (Guralnik et al., 1994).
2.5. Cost measures
We measured costs from a societal perspective including costs of healthcare utilisation (primary care, secondary care, home care and medication) of both the participant with dementia and the informal caregiver, informal care, additional expenses, and lost productivity costs [absenteeism from (un)paid work] for the IC. Costs were measured using two consecutive cost diaries each covering a period of 3 months. Costs were calculated using standard prices from the Dutch guidelines for economic evaluations (Zorginstituut Nederland, 2016). Lost productivity costs related to absenteeism from paid work were calculated using the human capital approach (Drummond et al., 2015). At the end of the study, DCC staff in the experimental group was asked to fill out the form 'Costs and benefits of applied games' to enable calculation of the costs of the (implementation of the) exergaming intervention (Heuvelink et al., 2014). We calculated total societal costs by summing all costs over the 6‐month follow‐up period for both participants with dementia and ICs.
2.6. Statistical analyses
All analyses were conducted according to the intention‐to‐treat principle. Missing data were imputed using multiple imputations by chained equations (MICE). To account for the skewed distribution of costs, predictive mean matching was used in the MICE procedure. The number of datasets was increased until the loss of efficiency was less than 5% (White et al., 2011). Analyses as described below were performed on each dataset separately, after which results were pooled using Rubin's rules (Rubin, 2004).
Bivariate regression analyses were performed to estimate the differences in costs and effects between groups, while accounting for the correlation between costs and effects in estimating the standard errors. In the main analysis, costs and effects were adjusted for baseline MMSE‐scores. Incremental cost‐effectiveness ratios (ICERs) were calculated by dividing the difference in total societal costs between the two groups by the difference in effect. Bias‐corrected accelerated bootstrapping with 5,000 samples was used to estimate statistical uncertainty surrounding the ICERs. The bootstrapped cost‐effect pairs were plotted in a cost‐effectiveness (CE) plane. In a CE plane, the difference in effects between the treatment groups is plotted on the x‐axis and the difference in costs on the y‐axis, resulting in four quadrants. The northeast (NE) quadrant indicates that the intervention is more expensive and more effective than control, the southeast (SE) quadrant that the intervention is less expensive and more effective than control, the southwest (SW) quadrant that the intervention is less expensive and less effective than control, and the northwest (NW) quadrant that the intervention is more expensive and less effective than control. In addition, ‘cost‐effectiveness acceptability curves’ were estimated in which the probability of cost‐effectiveness is plotted against the willingness‐to‐pay (WTP) threshold. This WTP threshold indicates the maximum amount of money society is willing to pay to gain one additional unit of effect.
A sensitivity analysis was performed in which costs and effects were adjusted for BMI of the participant with dementia, and sex, age and level of education of both the participant with dementia and informal caregiver, in addition to baseline MMSE‐score. Due to the small sample and the high rate of missing data, it was not possible to impute data for these additional confounding variables.
3. RESULTS
3.1. Participant flow and baseline measures of the study population
Figure 1 shows the process of enrolment and allocation of DCCs and participants to experimental group and control group, and the reasons for drop‐out (van Santen et al., 2020). In the exergaming group, 11 DCCs participated, and in the control group, 9 DCCs. In total, 112 dyads (73 exergaming, 39 control) were included. The study drop‐out in the exergaming group was 29% for participants with dementia and 42% for informal caregivers, resulting in 52 people with dementia and 42 informal caregivers participating at T2. In the control group, 18% of participants with dementia and 41% of informal caregivers dropped out of the study, leading to 32 people with dementia and 23 (41%) informal caregivers participating at T2. No statistically significant differences were found between participants with dementia and informal caregivers who dropped out and those who continued with the study.
FIGURE 1.
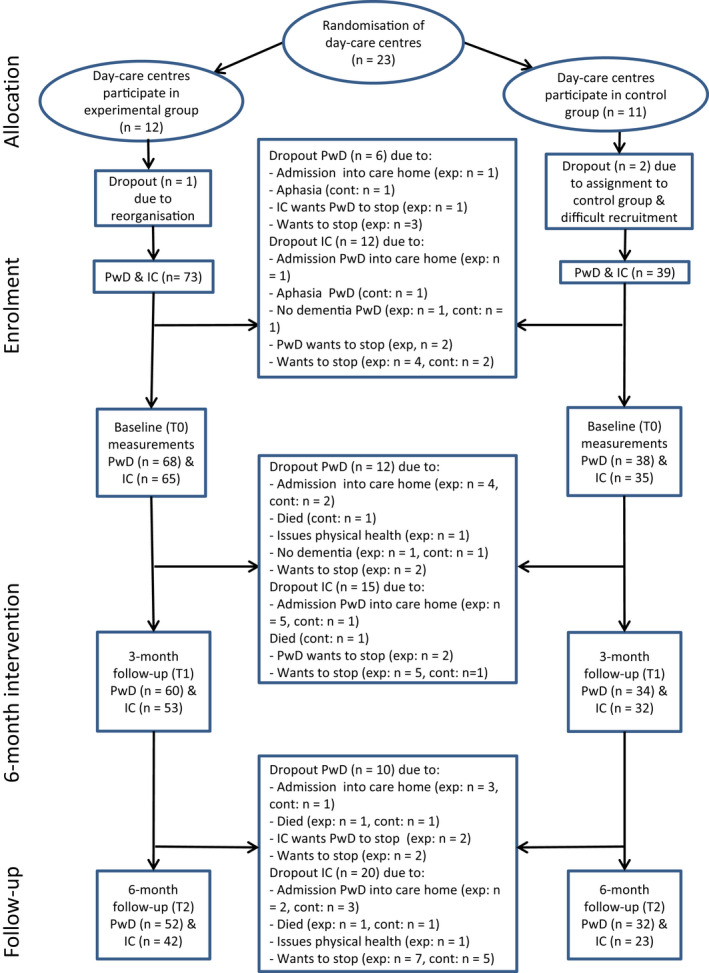
Flow chart of day‐care centres and participants. Note: From ‟Effects of Exergaming on Cognitive and Social Functioning of People with Dementia: A Randomised Controlled Trial,” by J. van Santen, R.‐M. Dröes, J. W. R. Twisk, O. A. Blanson Henkemans, A. van Straten and F. J. M. Meiland, 2020, Journal of the American Medical Director Association, 21:12, p. 1958–1967.” Abbreviations: cont, control group; exp, exergaming group; IC, informal caregiver; PwD, people with dementia [Colour figure can be viewed at wileyonlinelibrary.com]
The baseline characteristics of the study population are presented in Table 1. There were no statistically significant differences in baseline characteristics between the exergaming group and the control group. In the exergaming group, the mean (SD) of minutes of exergaming during one week by participants with dementia at T0 was 32.4 (39.2), at T1 22.8 (24.7) and at T2 29.8 (32.0).
TABLE 1.
Baseline characteristics of the study population
| Variables | Exergaming group (n = 73) | Control group (n = 39) | Difference between groups | |
|---|---|---|---|---|
| Test statistic (df) | p value | |||
| Participants with dementia (PwD) | ||||
| Age in years, mean (SD) | 79.0 (6.0) | 79.0 (7.0) | t (104) = −0.19 | 0.99 |
| Gender | χ 2 (1) = 0.70 | 0.40 | ||
| Male, n (%) | 37 (51) | 23 (59) | ||
| Female, n (%) | 36 (49) | 16 (41) | ||
| Body mass index, mean (SD) a | 28.0 (4.7) | 29.0 (5.5) | t (98) = 0.96 | 0.34 |
| Mini‐Mental State Examination, mean (SD) b | 18.1 (6.7) | 19.4 (6.5) | t (100) = 0.92 | 0.36 |
| Dementia type, n (%) | χ 2 (4) = 2.76 | 0.60 | ||
| Alzheimer's | 25 (34) | 12 (31) | ||
| Vascular | 6 (8) | 3 (8) | ||
| Mixed | 3 (4) | 4 (10) | ||
| Other | 7 (10) | 6 (15) | ||
| Unknown | 32 (44) | 14 (36) | ||
| Living situation, n (%) | χ 2 (3) = 0.63 | 0.89 | ||
| Independent, alone | 15 (21) | 9 (23) | ||
| Independent, with others | 50 (68) | 26 (67) | ||
| Unknown | 8 (11) | 4 (10) | ||
| Level of education, n (%) | χ 2 (3) = 1.79 | 0.62 | ||
| Primary education or less | 15 (21) | 5 (13) | ||
| Secondary education | 34 (47) | 17 (44) | ||
| Higher education | 13 (18) | 10 (26) | ||
| Unknown | 11 (15) | 7 (18) | ||
| Civil status, n (%) | χ 2 (4) = 5.67 | 0.23 | ||
| Married/long‐term cohabitation | 52 (71) | 26 (67) | ||
| Divorced/unmarried | 3 (4) | 2 (5) | ||
| Widow(er)/partner deceased | 12 (16) | 8 (21) | ||
| Unknown | 6 (8) | 3 (8) | ||
| Experience with sports, n (%) | 46 (63) | 24 (62) | χ 2 (1) = 0.00 | 0.98 |
| Experience with cycling, n (%) | 57 (78) | 33 (85) | χ 2 (1) = 3.35 | 0.07 |
| Experience with technology, n (%) | 27 (37) | 18 (46) | χ 2 (1) = 1.19 | 0.28 |
| Experience with computer games, n (%) | 12 (16) | 6 (15) | χ 2 (1) = 0.00 | 0.97 |
| Informal caregivers | ||||
| Age in years, mean (SD) | 65.0 (13.0) | 67.0 (12.0) | t (97) = 0.91 | 0.37 |
| Gender | χ 2 (1) = 0.01 | 0.94 | ||
| Male, n (%) | 18 (25) | 10 (26) | ||
| Female, n (%) | 54 (74) | 29 (74) | ||
| Unknown | 1 (1) | 0 (0) | ||
| Level of education, n (%) | χ 2 (3) = 3.58 | 0.31 | ||
| Primary education or less | 5 (7) | 0 (0) | ||
| Secondary education | 32 (44) | 19 (49) | ||
| Higher education | 24 (33) | 11 (28) | ||
| Unknown | 12 (16) | 9 (23) | ||
| Marital status, n (%) | χ 2 (3) = 0.66 | 0.88 | ||
| Married/long‐term cohabitation | 62 (85) | 33 (85) | ||
| Unmarried | 4 (5) | 2 (5) | ||
| Widow(er)/partner deceased | 1 (1) | 0 (0) | ||
| Unknown | 6 (8) | 4 (0) | ||
| Living together with PwD | 42 (58) | 26 (67) | χ 2 (1) = 1.62 | 0.20 |
| Relationship with PwD, n (%) | χ 2 (3) = 3.65 | 0.30 | ||
| Spouse | 39 (53) | 26 (67) | ||
| Son/daughter | 22 (30) | 6 (15) | ||
| Other | 11 (15) | 7 (18) | ||
| Unknown | 1 (1) | 0 (0) | ||
From ‟Effects of Exergaming on Cognitive and Social Functioning of People with Dementia: A Randomised Controlled Trial,” by J. van Santen, R.M. Dröes, J.W.R. Twisk, O.A. Blanson Henkemans, A. van Straten and F.J.M. Meiland, 2020, Journal of the American Medical Directors Association, 21:12, p. 1958–1967.
Abbreviation: PwD, Participant(s) with dementia.
For people of 70 years or older a Body Mass Index between 22–27.9 is considered healthy.
Mini‐Mental State Examination ranges from 0 to 30 (higher score stands for better cognitive functioning; ≥25 indicates normal functioning).
3.2. Costs
The form 'Costs and benefits of applied games' was only filled out by a few DCCs, mostly incompletely. We therefore did not include costs of the (implementation of the) exergaming intervention in our analysis. Table 2 shows mean costs stratified by treatment group over the study period of 6 months. For participants with dementia, the difference in total societal costs between the exergaming group and the control group was not statistically significant in the first 3‐month period, but over the second 3‐month period of the study costs were statistically significantly higher for the exergaming group (mean difference 733, 95% CI: 301 to 2,006). The mean difference in total societal costs between the exergaming group and the control group for the two periods together (6 months) was not statistically significant (207, 95% CI: −2,585 to 2,203).
TABLE 2.
Mean societal costs stratified by treatment group and the mean difference in societal costs between treatment groups adjusted for MMSE‐score at baseline
| Societal costs (€) | Intervention, mean (SE) | Control, mean (SE) | Difference, mean (95% CI) |
|---|---|---|---|
| Person with dementia | |||
| Month 1–3 | 1,613 (369) | 2,213 (1,072) | −526 (−3,581 to 924) |
| Month 4–6 | 1,265 (319) | 537 (130) | 733 (301 to 2,006) |
| Month 1–6 | 2,877 (640) | 2,750 (1,071) | 207 (−2,585 to 2,203) |
| Informal caregiver | |||
| Month 1–3 | 935 (279) | 575 (143) | 387 (−46 to 1,155) |
| Month 4–6 | 315 (56) | 881 (436) | −553 (−1,122 to 187) |
| Month 1–6 | 1,250 (292) | 1,456 (485) | −166 (−872 to 635) |
| Total societal costs | 4,127 (756) | 4,206 (1,300) | 41 (−2,893 to 2,308) |
Abbreviation: MMSE, Mini‐Mental State Examination.
For informal caregivers, the differences in total societal costs between the exergaming group and the control group over the first 3 months of the study, the second 3 months, and the two periods together (6 months) were not statistically significant (mean difference −166, 95% CI: −872 to 635).
After summing of the total societal costs for participants with dementia and informal caregivers, mean total costs in the exergaming group were €4,127 and in the control group €4,206. The total costs when adjusted for baseline MMSE‐score of the participants with dementia were €41 higher for exergaming, which was not statistically significant (95% CI: −2,893 to 2,308).
3.3. Clinical effects
Clinical effect outcomes are presented in Table 3. The difference between the exergaming group and the control group in number of QALYs experienced over the 6‐month follow‐up period by participants with dementia as assessed by both the participant with dementia and the informal caregiver was not statistically significant. Also, the difference in QALYs experienced by the informal caregiver over 6 months between the exergaming group and the control group was not statistically significant. There were no statistically significant differences in SPPB score or the average number of minutes of physical activity per week of the participant with dementia at 6 months after baseline between the exergaming group and the control group.
TABLE 3.
Clinical effect outcomes by treatment group adjusted for MMSE‐scores at baseline
| Outcome measure | Intervention, mean (SE) | Control, mean (SE) | Difference, mean (95% CI) |
|---|---|---|---|
| QALYs PwD | 0.36 (0.017) | 0.42 (0.015) | −0.053 (−0.097 to 0.009) |
| QALYs PwD (IC) | 0.22 (0.017) | 0.24 (0.026) | −0.014 (−0.076 to 0.048) |
| QALYs IC | 0.42 (0.011) | 0.39 (0.026) | 0.032 (−0.024 to 0.087) |
| SPPB | 7.4 (0.30) | 7.8 (0.40) | 0.077 (−0.87 to 1.02) |
| Physical activity PwD (in minutes) | 278 (50) | 184 (29) | 59 (−44 to 162) |
Abbreviations: IC, informal caregiver; MMSE, Mini‐Mental State Examination; PwD, participant with dementia; QALYs, Quality‐Adjusted Life‐Years; SPPB, Short Physical Performance Battery.
3.4. Cost‐effectiveness
Table 4 shows the cost‐effectiveness outcomes for exergaming compared to control. The ICERs for QALYs experienced by the participant with dementia were negative, indicating that exergaming was dominated by control (i.e., societal costs were higher and effects smaller in the exergaming group than in the control group). Consequently, most bootstrapped cost‐effect pairs were located in the NW quadrant of the CE plane.
TABLE 4.
Incremental cost‐effectiveness estimates for the intervention in comparison with control
| Outcome | ΔC (95% CI) | ΔE (95% CI) | ICER | Distribution on CE plane | |||
|---|---|---|---|---|---|---|---|
| NE | SE | SW | NW | ||||
| Adjustment for MMSE only (n = 112) | |||||||
| QALY PwD | 41 (−2,825 to 2,297) | −0.053 (−0.09 to 0.009) | −781 | 1% | 0% | 46% | 53% |
| QALY PwD (IC) | 41 (−2,825 to 2,297) | −0.014 (−0.076 to 0.048) | −2,951 | 16% | 17% | 29% | 38% |
| QALY IC | 41 (−2,825 to 2,297) | 0.032 (−0.024 to 0.087) | 1,316 | 48% | 39% | 7% | 6% |
| SPPB | 41 (−2,825 to 2,297) | 0.077 (−0.87 to 1.02) | 533 | 31% | 26% | 20% | 23% |
| Physical activity (minutes) | 41 (−2,825 to 2,297) | 59 (−44 to 162) | 0.70 | 47% | 40% | 6% | 7% |
| Full adjustment a (n = 82) | |||||||
| QALY PwD | 75 (−3,996 to 3,012) | −0.055 (−0.11 to −0.0029) | −1,350 | 2% | 1% | 44% | 53% |
| QALY PwD (IC) | 75 (−3,996 to 3,012) | −0.0039 (−0.070 to 0.062) | −19,285 | 22% | 20% | 25% | 32% |
| QALY IC | 75 (−3,996 to 3,012) | 0.046 (−0.026 to 0.12) | 1,626 | 48% | 41% | 4% | 6% |
| SPPB | 75 (−3,996 to 3,012) | 0.28 (−0.74 to 1.30) | 266 | 40% | 32% | 13% | 14% |
| Physical activity (minutes) | 75 (−3,996 to 3,012) | 89 (−58 to 237) | 0.84 | 49% | 41% | 4% | 5% |
Abbreviations: CE plane, cost‐effectiveness plane; IC, informal caregiver; ICER, incremental cost‐effectiveness ratio; MMSE, Mini‐Mental State Examination; NE, northeast; NW, northwest; PwD, participant with dementia; QALY, Quality‐Adjusted Life‐Year; SE, southeast; SPPB, Short Physical Performances Battery; SW, southwest; ΔC, difference in costs; ΔE, difference in effects.
Adjustment for Body Mass Index of the PwD, and sex, age and level of education of both the PwD and the IC.
For QALYs experienced by the informal caregiver, the ICER was 1,316, indicating that, compared to the control group, €1,316 more needs to be invested to gain 1 QALY in the exergaming group. Thus, most cost‐effect pairs were located in the NE quadrant of the CE plane. The cost‐effectiveness acceptability curve (Figure 2) shows that if society is not willing to invest any money to gain one QALY, that is a ceiling ratio of €0 per QALY gained, the probability that exergaming is cost‐effective compared to the control group is 0.46. At willingness‐to‐pay (WTP) thresholds of €10,000 and €20,000 per QALY gained, this probability was 0.57 and 0.64, respectively.
FIGURE 2.
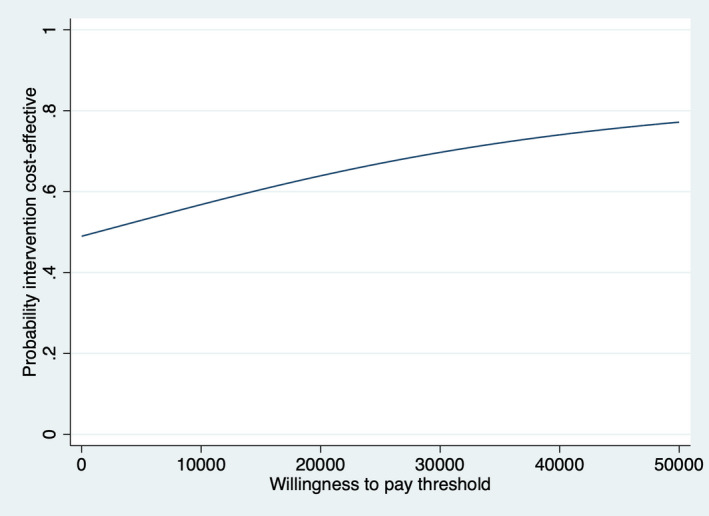
Cost‐effectiveness acceptability curve for Quality‐Adjusted Life‐Years experienced over the 6‐month follow‐up period by the informal caregiver (exergaming versus control) [Colour figure can be viewed at wileyonlinelibrary.com]
The ICER for SPPB (outcome to measure mobility) was 533, which means that to gain one point on the SPPB €553 needs to be invested in the exergaming group in comparison with control (most cost‐effect pairs in the NE quadrant). The cost‐effectiveness curve (figure not shown) indicates that if society is willing to pay €0, 1,000 or 5,000 per point gained on the SPPB, the probability of cost‐effectiveness of exergaming compared to control is 0.46, 0.51 and 0.55 respectively.
To gain one additional minute of physical activity €0.70 needs to be invested in the exergaming group compared to the control group (most cost‐effect pairs in the NE quadrant). The cost‐effectiveness acceptability curve (figure not presented) shows that the probability that exergaming is cost‐effective compared to control is 0.46, 0.84 and 0.87 at WTP thresholds of €0, 50 and 250 per additional minute of physical activity, respectively.
3.5. Sensitivity analysis
Due to missing values in the confounders, the fully adjusted cost‐effectiveness analysis included only 82 dyads (53 in the exergaming group and 29 in the control group). Table 4 shows the results of this adjusted analysis. The difference in societal costs between exergaming and control in the fully adjusted analysis is comparable to the cost difference in the main analysis. Again, uncertainty was large and the difference was not statistically significant. In this adjusted analysis, the number of QALYs according to the participants with dementia themselves in the exergaming group was statistically significantly lower than in the control group. ICERs indicate that exergaming was more expensive and less effective than the control group for QALYs experienced by the participants with dementia, both according to themselves and their informal caregivers. For QALYs experienced by the informal caregivers, for SPPB scores and minutes of physical activity, exergaming was also more expensive, but also more effective than control. However, uncertainty was still considerable as shown by the distribution of the bootstrapped cost‐effect pairs across the CE plane. Moreover, probabilities of the adjusted cost‐effectiveness were similar to the main analysis.
4. DISCUSSION
We found that exergaming by participants with dementia in DCC was not effective nor cost‐effective compared to care as usual for our primary outcome measures: QALYs, physical activity and mobility (SPPB).
Previous research showed that physical activity and exergaming can benefit people with dementia and their informal caregivers on various outcomes, such as physical, cognitive, emotional and social functioning and QoL (Blondell et al., 2014; Corregidor‐Sánchez et al., 2020; Donath et al., 2016; Heyn et al., 2004; Pitkälä et al., 2013; Potter et al., 2011; Stowell et al., 2019; Unbehaun et al., 2018; van Santen et al., 2018, 2020; Zhao et al., 2020).
The fact that exergaming could not be considered cost‐effective compared to regular day‐care activities in our study may have been caused by the small sample size and perhaps by participants not performing exergaming activities often enough to benefit with regard to our primary outcome measures. In our study, the average number of minutes of physical activity of participants per week was less than the recommended amount of physical activity, even with exergaming. Nevertheless, on average, the amount of physical activity per week by participants with dementia in the exergaming group was higher than by participants with dementia in the control group. Although the difference was not statistically significant, this seems to indicate a positive trend in favour of exergaming. In future studies, participants should preferably engage in the exergaming intervention more than our minimum of twice per week, although we are aware that a frequency of more than twice per week will have consequences for the recruitment of DCCs and participants, as part of the participants with dementia only visit the DCC twice a week.
Previous research has shown that adherence can be an issue for physical exercise interventions, but exergaming seemed to motivate people to exercise longer (Meekes & Stanmore, 2017; Unbehaun et al., 2018; Windle et al., 2010). In our sample, 24% from the exergaming group dropped out of the research study between T0 and T2, compared to 16% in the control group. However, only four persons in the intervention group reported the reason was that they wanted to stop exergaming (6%). We can therefore conclude that adherence to exergaming was not an issue in our study.
As far as we know, no previous studies have evaluated the cost‐effectiveness of exergaming for people with dementia and informal caregivers. We did find one study demonstrating that exergaming was cost‐effective compared to standard care (physiotherapy advice and leaflet) as a fall prevention strategy for people aged 55 years or older (Stanmore et al., 2019). This suggests that exergaming can potentially be cost‐effective compared to usual care on different outcomes. The body of research needs to be expanded to enable definitive conclusions.
This study had several limitations. The small sample size and high amount of missing data mean the study is underpowered. Consequently, there was large uncertainty surrounding the results. Reasons for the small sample size were difficulties in recruiting participants, which was often (partly) related to informal caregiver burden. In addition, there was a substantial percentage of drop‐out, which was higher than we expected for this target group during a 6‐month intervention period. Reasons for drop‐out were, for example, admission into a residential care setting, physical illness and death. In addition, the measurement instruments were sometimes burdensome for participants, more specifically the informal caregivers. For example, keeping cost diaries on paper can be time consuming, is easily forgotten, and prone to errors. The diaries were sometimes also got lost at home or in the mail. This resulted in many missing data. All of this may also have led to data bias.
To measure QoL both for participants with dementia and informal caregivers, we used the EQ‐5D‐3L and the EQ‐5D‐3L proxy version using informal caregivers as proxies (Brooks & Group, 1996). Previous research showed that the use of the EQ‐5D can be ambiguous for people with dementia and informal caregiver proxies, as large differences are found in self‐report ratings of people with dementia and proxy‐ratings, as well as between different informal caregivers as proxies (i.e. between children and spouses of people with dementia) (Hounsome et al., 2011; Orgeta et al., 2015).
Costs of the (implementation of the) exergaming intervention were not included in our analysis. However, as the exergaming equipment is used by many people over several years, the costs per participant are expected to be low. Therefore, the inclusion of these costs would most probably not have changed the overall results.
Despite these limitations, this study contributes to a base for further research into the cost‐effectiveness of exergaming for people living with dementia. The described limitations offer suggestions for improvement in future research, for example with regard to the frequency of engaging people in the exergaming intervention or the choice of outcome measures to assess the quality of life in this target group.
In conclusion, this study found that exergaming in DCC was not cost‐effective compared to treatment as usual for participants with dementia and informal caregivers regarding the primary outcomes of this study, i.e., QALYs, physical activity and mobility (based on SPPB scores). However, considering the large uncertainty surrounding the results due to the small sample size and the high rate of missing data, we cannot draw a definitive conclusion on the cost‐effectiveness of exergaming based on this study. We recommend future studies with larger samples specifically set up to further explore the cost‐effectiveness of exergaming for this target group, possibly also in different settings such as at home and residential care settings. Relevant for resource allocation in healthcare is not only effects but also factors such as cost‐effectiveness. Therefore, more in‐depth investigation of which physical, emotional and social outcomes exergaming may be cost‐effective for could be worthwhile.
CONFLICTS OF INTEREST
All authors declare that they have no competing interests.
AUTHORS CONTRIBUTION
RMD, FJMM, JEB and JvS designed the study. JvS, RMD, and FJMM conducted the study and were responsible for data collection. JvS and JEB performed the data analyses and wrote the manuscript with input from all authors. JvS was supervised by RMD, FJMM, and AvS throughout the process.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Olivier Blanson Henkemans (TNO), Sjef van Bommel (Sjef van Bommel Management & Support), Esther Hakvoort (Evean), Marije Holstege (GRZ Plus), Carla Scholten (Embedded Fitness), Marian Schoone (TNO), Marjolein Smit (SilverFit), Ronald Valk (HilverZorg), and Joris Wiersinga (SilverFit) for their contributions to the study as project group members. Additionally, we would like to thank all the persons with dementia, informal caregivers, staff of day‐care centres and care organisations who participated, and all other people who contributed to the research study.
van Santen, J. , Meiland, F. J. M. , Dröes, R.‐M. , van Straten, A. , & Bosmans, J. E. (2022). Cost‐effectiveness of exergaming compared to regular day‐care activities in dementia: Results of a randomised controlled trial in The Netherlands. Health & Social Care in the Community, 30, e1794–e1804. 10.1111/hsc.13608
Funding information
The research project received funding from ZonMw‐Memorabel programme/Alzheimer Nederland (project number 733050609), Stichting Dioraphte (project number 16 02 04 03) and the EU. This trial was carried out as part of the Marie Skłodowska Curie funded INDUCT Innovative Training Network (ITN) (Interdisciplinary Network for Dementia Using Current Technology (INDUCT)), H2020‐MSCA‐ITN‐2015, under grant agreement number 676265. Additional funding was received from Association of Support VCVGZ.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Alzheimer Nederland . (2019). Factsheet cijfers en feiten over dementie. Retrieved from https://www.alzheimer‐nederland.nl/factsheet‐cijfers‐en‐feiten‐over‐dementie [Google Scholar]
- Blondell, S. J. , Hammersley‐Mather, R. , & Veerman, J. L. (2014). Does physical activity prevent cognitive decline and dementia?: A systematic review and meta‐analysis of longitudinal studies. BMC Public Health, 14(1), 510. 10.1186/1471-2458-14-510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks, R. , & Group, E . (1996). EuroQol: The current state of play. Health Policy, 37(1), 53–72. 10.1016/0168-8510(96)00822-6 [DOI] [PubMed] [Google Scholar]
- Clarke, D. E. , van Reekum, R. , Simard, M. , Streiner, D. L. , Conn, D. , Cohen, T. , & Freedman, M. (2008). Apathy in dementia: Clinical and sociodemographic correlates. The Journal of Neuropsychiatry and Clinical Neurosciences, 20(3), 337–347. 10.1176/jnp.2008.20.3.337 [DOI] [PubMed] [Google Scholar]
- Corregidor‐Sánchez, A. I. , Segura‐Fragoso, A. , Rodríguez‐Hernández, M. , Criado‐Alvarez, J. J. , González‐Gonzalez, J. , & Polonio‐López, B. (2020). Can exergames contribute to improving walking capacity in older adults? A systematic review and meta‐analysis. Maturitas, 132, 40–48. 10.1016/j.maturitas.2019.12.006 [DOI] [PubMed] [Google Scholar]
- Crombie, I. K. , Irvine, L. , Williams, B. , McGinnis, A. R. , Slane, P. W. , Alder, E. M. , & McMurdo, M. E. (2004). Why older people do not participate in leisure time physical activity: A survey of activity levels, beliefs and deterrents. Age and Ageing, 33(3), 287–292. 10.1093/ageing/afh089 [DOI] [PubMed] [Google Scholar]
- Donath, L. , Rössler, R. , & Faude, O. (2016). Effects of virtual reality training (exergaming) compared to alternative exercise training and passive control on standing balance and functional mobility in healthy community‐dwelling seniors: A meta‐analytical review. Sports Medicine, 46(9), 1293–1309. 10.1007/s40279-016-0485-1 [DOI] [PubMed] [Google Scholar]
- Drummond, M. F. , Sculpher, M. J. , Claxton, K. , Stoddart, G. L. , & Torrance, G. W. (2015). Methods for the economic evaluation of health care programmes. Oxford University Press. [Google Scholar]
- Guralnik, J. M. , Simonsick, E. M. , Ferrucci, L. , Glynn, R. J. , Berkman, L. F. , Blazer, D. G. , Scherr, P. A. , & Wallace, R. B. (1994). A short physical performance battery assessing lower extremity function: Association with self‐reported disability and prediction of mortality and nursing home admission. Journals of Gerontology, 49(2), M85–M94. 10.1093/geronj/49.2.M85 [DOI] [PubMed] [Google Scholar]
- Heuvelink, A. , Groot, J. , & Hofstede‐Kleyweg, C. (2014). Let's play: Ouderen stimuleren tot bewegen met applied games. Retrieved from Ede. [Google Scholar]
- Heyn, P. , Abreu, B. C. , & Ottenbacher, K. J. (2004). The effects of exercise training on elderly persons with cognitive impairment and dementia: A meta‐analysis. Archives of Physical Medicine and Rehabilitation, 85(10), 1694–1704. 10.1016/j.apmr.2004.03.019 [DOI] [PubMed] [Google Scholar]
- Hounsome, N. , Orrell, M. , & Edwards, R. T. (2011). EQ‐5D as a quality of life measure in people with dementia and their carers: Evidence and key issues. Value in Health, 14(2), 390–399. 10.1016/j.jval.2010.08.002 [DOI] [PubMed] [Google Scholar]
- Joling, K. J. , Schöpe, J. , van Hout, H. P. J. , van Marwijk, H. W. J. , van der Horst, H. E. , & Bosmans, J. E. (2015). Predictors of societal costs in dementia patients and their informal caregivers: A two‐year prospective cohort study. The American Journal of Geriatric Psychiatry, 23(11), 1193–1203. 10.1016/j.jagp.2015.06.008 [DOI] [PubMed] [Google Scholar]
- Lamers, L. , Stalmeier, P. , McDonnell, J. , & Krabbe, P. (2005). Measuring the quality of life in economic evaluations: The Dutch EQ‐5D tariff. Nederlands Tijdschrift Voor Geneeskunde, 149(28), 1574–1578. [PubMed] [Google Scholar]
- Lin, X. Y. , Saksono, H. , Stowell, E. , Lachman, M. E. , Castaneda‐Sceppa, C. , & Parker, A. G. (2020). Go&Grow: An evaluation of a pervasive social exergame for caregivers of loved ones with dementia. Proceedings of the ACM on Human‐Computer Interaction, 4(CSCW2), 1–28. 10.1145/3415222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meekes, W. , & Stanmore, E. K. (2017). Motivational determinants of exergame participation for older people in assisted living facilities: Mixed‐methods study. Journal of Medical Internet Research, 19(7), e238. 10.2196/jmir.6841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orgeta, V. , Edwards, R. T. , Hounsome, B. , Orrell, M. , & Woods, B. (2015). The use of the EQ‐5D as a measure of health‐related quality of life in people with dementia and their carers. Quality of Life Research, 24(2), 315–324. 10.1007/s11136-014-0770-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penedo, F. J. , & Dahn, J. R. (2005). Exercise and well‐being: A review of mental and physical health benefits associated with physical activity. Current Opinion in Psychiatry, 18(2), 189–193. 10.1097/00001504-200503000-00013 [DOI] [PubMed] [Google Scholar]
- Pinquart, M. , & Sörensen, S. (2007). Correlates of physical health of informal caregivers: A meta‐analysis. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 62(2), P126–P137. [DOI] [PubMed] [Google Scholar]
- Pitkälä, K. , Savikko, N. , Poysti, M. , Strandberg, T. , & Laakkonen, M.‐L. (2013). Efficacy of physical exercise intervention on mobility and physical functioning in older people with dementia: A systematic review. Experimental Gerontology, 48(1), 85–93. 10.1016/j.exger.2012.08.008 [DOI] [PubMed] [Google Scholar]
- Potter, R. , Ellard, D. , Rees, K. , & Thorogood, M. (2011). A systematic review of the effects of physical activity on physical functioning, quality of life and depression in older people with dementia. International Journal of Geriatric Psychiatry, 26(10), 1000–1011. 10.1002/gps.2641 [DOI] [PubMed] [Google Scholar]
- Rubin, D. B. (2004). Multiple imputation for nonresponse in surveys (Vol. 81). John Wiley & Sons. [Google Scholar]
- Saghaei, M. (2004). Random allocation software for parallel group randomized trials. BMC Medical Research Methodology, 4(1), 26. 10.1186/1471-2288-4-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha, S. , Gerdtham, U.‐G. , Toresson, H. , Minthon, L. , & Jarl, J. (2018). Economic evaluation of nonpharmacological interventions for dementia patients and their caregivers—A systematic literature. Working Papers, 2018(10), 1–34. [Google Scholar]
- Stanmore, E. K. , Mavroeidi, A. , de Jong, L. D. , Skelton, D. A. , Sutton, C. J. , Benedetto, V. , Munford, L. A. , Meekes, W. , Bell, V. , & Todd, C. (2019). The effectiveness and cost‐effectiveness of strength and balance Exergames to reduce falls risk for people aged 55 years and older in UK assisted living facilities: A multi‐centre, cluster randomised controlled trial. BMC Medicine, 17(1), 49. 10.1186/s12916-019-1278-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowell, E. , Zhang, Y. , Castaneda‐Sceppa, C. , Lachman, M. , & Parker, A. G. (2019). Caring for Alzheimer's disease caregivers: A qualitative study investigating opportunities for Exergame innovation. Proceedings of the ACM on Human‐Computer Interaction, 3(CSCW), 1–27. 10.1145/3359232 34322658 [DOI] [Google Scholar]
- Taylor, A. H. , Cable, N. T. , Faulkner, G. , Hillsdon, M. , Narici, M. , & Van Der Bij, A. K. (2004). Physical activity and older adults: A review of health benefits and the effectiveness of interventions. Journal of Sports Sciences, 22(8), 703–725. 10.1080/02640410410001712421 [DOI] [PubMed] [Google Scholar]
- Unbehaun, D. , Vaziri, D. D. , Aal, K. , Wieching, R. , Tolmie, P. , & Wulf, V. (2018). Exploring the Potential of Exergames to affect the Social and Daily Life of People with Dementia and their Caregivers. Paper presented at the Proceedings of the 2018 CHI Conference on Human Factors in Computing Systems, Montreal QC, Canada [Google Scholar]
- van Alphen, H. J. M. , Hortobágyi, T. , & van Heuvelen, M. J. G. (2016). Barriers, motivators, and facilitators of physical activity in dementia patients: A systematic review. Archives of Gerontology and Geriatrics, 66, 109–118. 10.1016/j.archger.2016.05.008 [DOI] [PubMed] [Google Scholar]
- van Santen, J. , Dröes, R.‐M. , Bosmans, J. E. , Blanson Henkemans, O. A. , van Bommel, S. , Hakvoort, E. , Valk, R. , Scholten, C. , Wiersinga, J. , van Straten, A. , & Meiland, F. (2019). The (cost‐) effectiveness of exergaming in people living with dementia and their informal caregivers: Protocol for a randomized controlled trial. BMC Geriatrics, 19(1), 50. 10.1186/s12877-019-1062-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Santen, J. , Dröes, R.‐M. , Holstege, M. , Blanson Henkemans, O. A. , van Rijn, A. , de Vries, R. , & Meiland, F. (2018). Effects of exergaming in people with dementia: Results of a systematic literature review. Journal of Alzheimer's Disease, 64(2), 741–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Santen, J. , Dröes, R.‐M. , Twisk, J. W. R. , Blanson Henkemans, O. A. , van Straten, A. , & Meiland, F. J. M. (2020). Effects of exergaming on cognitive and social functioning of people with dementia: A randomized controlled trial. Journal of the American Medical Directors Association, 21(12), 1958–1967.e5. 10.1016/j.jamda.2020.04.018 [DOI] [PubMed] [Google Scholar]
- Vasunilashorn, S. , Coppin, A. K. , Patel, K. V. , Lauretani, F. , Ferrucci, L. , Bandinelli, S. , & Guralnik, J. M. (2009). Use of the Short Physical Performance Battery Score to predict loss of ability to walk 400 meters: Analysis from the InCHIANTI study. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 64(2), 223–229. 10.1093/gerona/gln022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitaliano, P. P. , Zhang, J. , & Scanlan, J. M. (2003). Is caregiving hazardous to one's physical health? A meta‐analysis. Psychological Bulletin, 129(6), 946. 10.1037/0033-2909.129.6.946 [DOI] [PubMed] [Google Scholar]
- White, I. R. , Royston, P. , & Wood, A. M. (2011). Multiple imputation using chained equations: Issues and guidance for practice. Statistics in Medicine, 30(4), 377–399. 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- Wimo, A. , Winblad, B. , & Jönsson, L. (2007). An estimate of the total worldwide societal costs of dementia in 2005. Alzheimer's & Dementia, 3(2), 81–91. 10.1016/j.jalz.2007.02.001 [DOI] [PubMed] [Google Scholar]
- Windle, G. , Hughes, D. , Linck, P. , Russell, I. , & Woods, B. (2010). Is exercise effective in promoting mental well‐being in older age? A systematic review. Aging & Mental Health, 14(6), 652–669. 10.1080/13607861003713232 [DOI] [PubMed] [Google Scholar]
- van Winkelhof, M. , Wilmink, G. , Scheltens, P. , Rikkert, M. O. , Scherder, E. , van 't Land, K. , Richard, E. , Lahuis, B. , Biessels, G. J. , & Wenselaar, R. . (2019, October 21). Laten we de duurste ziekte aanpakken – dementie. In Moerland R. (Ed.), NRC Handelsblad. NRC Media. Retrieved from https://www.nrc.nl/nieuws/2019/10/21/laten‐we‐de‐duurste‐ziekte‐aanpakken‐dementie‐a3977408 [Google Scholar]
- World Health Organization . (2017, September 20). Infographic Dementia ‐ A public health priority. Retrieved from https://www.who.int/mental_health/neurology/dementia/infographic_dementia.pdf?ua=1 [Google Scholar]
- World Health Organization . (2019, September 19). Dementia fact‐sheet. Retrieved from https://www.who.int/news‐room/fact‐sheets/detail/dementia [Google Scholar]
- Zhao, Y. , Feng, H. , Wu, X. , Du, Y. , Yang, X. , Hu, M. , Ning, H. , Liao, L. , Chen, H. , & Zhao, Y. (2020). Effectiveness of exergaming in improving cognitive and physical function in people with mild cognitive impairment or dementia: systematic review. JMIR Serious Games, 8(2), e16841. 10.2196/16841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorginstituut Nederland . (2016). Dutch guidelines for economic evaluations in healthcare (original title"Richtlijn voor het uitvoeren van economische evaluaties in de gezondheidszorg"). Retrieved from https://www.zorginstituutnederland.nl/over‐ons/publicaties/publicatie/2016/02/29/richtlijn‐voor‐het‐uitvoeren‐van‐economische‐evaluaties‐in‐de‐gezondheidszorg [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


