Abstract
Static cold preservation remains the cornerstone for storing donor livers following procurement; however, the choice between University of Wisconsin solution (UW) and histidine‐tryptophan‐ketoglutarate solution (HTK) remains controversial. Recent International Liver Transplantation Society (ILTS) guidelines have recommended avoiding HTK for donation after circulatory death (DCD) grafts based on older reports. We studied the latest US adult graft outcomes in three recent eras (2006–2010, 2011–2015, 2016–2020) comparing HTK and UW among 5956 DCD LTs: 3873 (65.0%) used UW and 1944 (32.7%) used HTK. In a total of 82,679 donation after brain death (DBD) liver transplantations (LTs), 63,511 (76.8%) used UW and 15,855 (19.2%) used HTK. The HTK group had higher 1‐year and 5‐year graft survival rates of 89.7% and 74.3%, respectively, compared with 85.9% and 70.8% in the UW group in the 2016–2020 era (p = 0.005). This difference remained when adjusted for important potential confounders (hazard ratio, 0.78; 95% confidence interval: 0.60, 0.99). There were no differences between groups among DCD LTs in the earlier eras or among DBD LTs in all eras (all p values > 0.05). The latest US data suggest that HTK is at least noninferior to UW for preserving DCD livers. These data support HTK use in DCD LT and contradict ILTS guidance.
Abbreviations
- BMI
body mass index
- CI
confidence interval
- CIT
cold ischemia time
- DBD
donation after brain death
- DCD
donation after circulatory death
- ECD
expanded criteria donor
- fDWIT
functional donor warm ischemia time
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HTK
histidine‐tryptophan‐ketoglutarate solution
- HR
hazard ratio
- ICU
intensive care unit
- ILTS
International Liver Transplantation Society
- LOS
length of stay
- LT
liver transplantation
- MAP
mean arterial pressure
- MELD
Model for End‐Stage Liver Disease
- OPTN
Organ Procurement and Transplantation Network
- PVT
portal vein thrombosis
- SLKT
simultaneous liver–kidney transplantation
- SpO2
oxygen saturation
- UNOS
United Network for Organ Sharing
- UW
University of Wisconsin solution
INTRODUCTION
Although efforts continue to optimize donor liver quality to mitigate ischemia/reperfusion injury and by extension improve graft longevity,[ 1 , 2 ] static cold preservation solutions remain the mainstay of management given their ease of use and time‐tested efficacy.[ 3 ] The evolution of the composition of static cold preservation solutions have focused on ionic composition (including buffers), osmotic and oncotic agents, and antioxidants aimed to reduce intracellular and interstitial edema and the consequential ischemia/reperfusion damage. Traditionally, there are two main static cold preservation solutions for liver transplantation (LT) in the United States:
University of Wisconsin solution (UW), which is an isotonic, high‐potassium, phosphate‐buffered solution and has been in clinical use for LT since 1987; and
Histidine‐tryptophan‐ketoglutarate solution (HTK) in clinical use for LT since 1999.
Although UW remains the most widely used, the use of HTK has gradually increased in frequency buoyed by the perceived advantages that include lower cost, decreased risk of hyperkalemia, and reduced viscosity culminating in improved microvasculature perfusion.[ 4 ]
However, the perceived advantages of HTK over UW have not been demonstrated convincingly in clinical studies to date and thus remain a controversial topic. With regard to donation after circulatory death (DCD) liver procurement and cold organ preservation solutions, the recent International Liver Transplantation Society (ITLS) guidelines recommend avoiding the use of HTK in DCD livers in cases where cold ischemia is estimated to be >8 h.[ 5 ] This recommendation is based on two registry studies, one from Europe and one from the United States. The study from the European Liver Transplant Registry evaluated more than 42,000 liver recipients who received transplants over 10 years (2003–2012) and found that 3‐year graft survival rate significantly reduced with HTK at 69% compared with UW at 75%, and HTK use was an independent risk factor for increasing the probability of graft loss by 10%.[ 6 ] This observation persisted in a further analysis of the same study cohort that used propensity‐score matching and excluded living donor LTs and German centers because of their relatively increased use of HTK.[ 7 ] Of note, the proportion of DCD use was low (2.8%), and thus DCDs were not analyzed independently. The US national registry study was an analysis of >17,000 LTs performed during a 4‐year period (2004–2008) and suggested a significantly increased risk of graft loss in livers preserved with HTK compared with those preserved with UW, in particular among those arising from DCD donors.[ 8 ] This report by Stewart et al. has been criticized for potentially misrepresented analyses including a concern for selection bias given the proportion of missing data, the failure to control for center effect (impact of limited distribution of HTK and DCD centers at that time), and inadequate allowance for the inevitable learning curve of HTK use.[ 8 ]
Despite these registry studies and ILTS recommendations, there have been other studies, including more recent studies, consistently showing no difference in graft outcomes with regard to HTK versus UW use.[ 9 , 10 , 11 , 12 ] Moreover, a meta‐analysis on 16 randomized clinical trials concluded that there was no evidence of any difference in outcomes between HTK and UW, although data were acknowledged as limited.[ 13 ] Interestingly, there has been evidence that HTK is superior to UW in protection against biliary complications in expanded criteria grafts.[ 14 ] The field most definitely lacks adequately powered prospective randomized controlled trials.
In addition to the suboptimal consideration of selection bias, center variability, and HTK learning curve, period effects have also been inadequately considered in the existing literature, including changes in LT candidate selection,[ 15 ] better patient care, and increasing LT frequency in the United States, in which donation after brain death (DBD) LTs increased from 5463 (95.0%) in 2009 to 7633 (91.5%) in 2019, whereas DCD LTs increased from 285 (5.0%) in 2009 to 712 (8.5%) in 2019.[ 16 ] Our study hypothesis was that as time has progressed, LT frequency has increased, and familiarity with HTK use has improved, HTK would have better graft outcomes, particularly among the marginal grafts such as DCD. To this end, our aims were to (1) analyze the latest trends in HTK and UW in DCD and DBD LTs in the United States and (2) ascertain short‐term and long‐term graft outcomes between HTK and UW among DCD and DBD LT recipients, adjusting for important confounders.
PATIENTS AND METHODS
Study population and data acquisition
First‐time adult (≥18 years) deceased donor LT recipients between January 1, 2006, and December 31, 2019, were identified from the United Network for Organ Sharing (UNOS)/Organ Procurement and Transplantation Network (OPTN) database within the LT recipient data set. These data are prospectively collected from transplant programs and organ procurement organizations.[ 17 ] Patients who underwent retransplantation or multiorgan transplantation, apart from simultaneous liver–kidney transplantation (SLKT), were excluded. The LT recipients were divided into two primary groups: DCD LT and DBD LT recipients.
The deceased donor data set, which contains the information on cold storage preservation solutions, was then merged with the LT recipient data set. Demographic and pertinent clinical characteristics were explored. LT recipients were further divided into either HTK or UW groups by assignment of the recorded “final flush” initially and then the “initial flush” if an LT recipient was not already assigned a group. UW recipients were identified by UNOS “flush” codes of 300, 311, and 313 and by manual inspection of any reference to UW in the free‐text entry section including “Belzer (Bridge‐to‐Life),” Viaspan® (DuPont Pharma), Static Preservation Solution ([SPS‐1], Organ Recovery Systems, Inc.), UW Machine Perfusion Solution ([MPS®], Bridge to Life), “Bridge to Life (Bridge‐to‐Life),” and Servator‐B®, (S.A.L.F. S.p.A. Pharmacological Laboratory). HTK recipients were identified by UNOS “flush” codes 312 and 308 and by manual inspection of reference to HTK (including Custodial® (Kohler Chemie) and Servator‐H®).
Statistical analysis
Continuous variables were summarized with medians and interquartile ranges or with means and standard deviations, and categorical variables were summarized with frequencies and percentages. Comparative analysis of continuous variables was based on a two‐sample Wilcoxon rank test (if observations failed the Shapiro‐Wilk normality test) or a two‐sample t‐test, and comparative analysis of categorical variables was based on the two‐sided chi‐square test. To mitigate period effects on the analysis (eg, improved medical care, LT recipient and donor selection changes, learning curve of using HTK), we divided the analysis into the following three eras: 2006–2010, 2011–2015, and 2016–2020. The eras were limited to 5‐year intervals to limit period effects of changes in LT, beginning at 2020, which had the most contemporary data available.
The primary study endpoint was graft survival, defined as being free of the occurrence of either recipient death or removal of the transplanted organ (as per the UNOS/OPTN definition). Graft survivals were computed by the Kaplan–Meier curve method, with log‐rank testing used to ascertain differences between groups. Cox proportional hazards modeling was used to adjust for important confounders in graft survival. These covariates were selected a priori as they have been consistently associated with graft survival in the medical literature and included the donor variables age, cold ischemia time (CIT), sex, race, expanded criteria donors (ECDs; UNOS definition as applied to deceased kidney donors), and the following recipient variables: age, sex, diabetes mellitus, body mass index (BMI), Model for End‐Stage Liver Disease (MELD) score, life support requirement (defined as ventilator or circulatory support at the time of transplant), ascites (mild or more), etiology of liver disease (grouped as [1] alcohol‐related liver disease, [2] nonalcoholic fatty liver disease, [3] hepatitis C virus [HCV], [4] cholestatic/autoimmune, and [5] other), hepatocellular carcinoma (HCC), and SLKT.[ 18 , 19 , 20 , 21 ] ECD livers are recorded by UNOS as per the kidney allocation classification of ECD: DCD or DBD donor and either (1) aged ≥60 years or (2) aged 50–59 years with two of either history of hypertension, creatinine ≥1.5 mg/dL, or cerebrovascular cause of death. In the DCD analysis, functional donor warm ischemia time (fDWIT) was also incorporated a priori given the importance of this parameter in DCD LT.[ 22 ] fDWIT was defined as per ILTS guidance, which defines the start of fDWIT as the time point of either/or oxygen saturation (SpO2) <80% and mean arterial pressure (MAP) <60 mm Hg through to the start of the cold flush.[ 22 ] A systolic blood pressure of <80 mm Hg was used as the cutoff in cases where SpO2 and MAP parameters were missing. Because of the high colinearity between fDWIT and total DWIT, only fDWIT was included in the modeling. After basic Cox proportional hazards models were fitted, possible clustering by individual transplant centers was assessed and adjusted for using a shared frailty random‐effects Cox model in which transplant centers were included as a random effect. The likelihood ratio test assessing the null hypothesis (that there is no difference between the models) was used to confirm that the shared frailty model was a better fit and that transplant centers did indeed confer variability to outcomes. To further explore the potential confounder of individual transplant centers, a sensitivity analysis was performed in which stratified analysis was carried out on the basis of DCD transplant volume. A further subanalysis was performed restricted to DCD LTs with CITs >8 h, in light of the recent ILTS recommendation to avoid HTK use in this clinical scenario. These results are provided in hazard ratios (HRs) with 95% confidence intervals (CIs). A p value of <0.05 was considered significant. The statistical analyses were completed using the Stata statistical package (Stata Statistical Software Release 16, StataCorp, LLC). Our study qualified for institutional review board exemption given the presence of deidentified data (IRB20‐0804).
RESULTS
Frequency of UW and HTK use among DCD and DBD LTs
Among 92,462 adult LTs who met the study selection criteria for 2006 through 2020 (Figure 1), 3827 (4.1%) were missing deceased donor type and were thus excluded from the analysis. Of the remaining 88,635, there were 5956 (6.7%) DCD and 82,679 (93.3%) DBD LTs. Among the 5956 DCD LTs, 3873 (65.0%) used UW, 1944 (32.7%) used HTK solution, and 139 (2.3%) used other types of organ preservation strategies. Among the 82,679 DBD LTs, 63,511 (76.8%) used UW; 15,855 (19.2%) used HTK; 2473 (3.0%) used other types of organ preservation strategies; and 840 (1.0%) were missing static cold preservation information.
FIGURE 1.
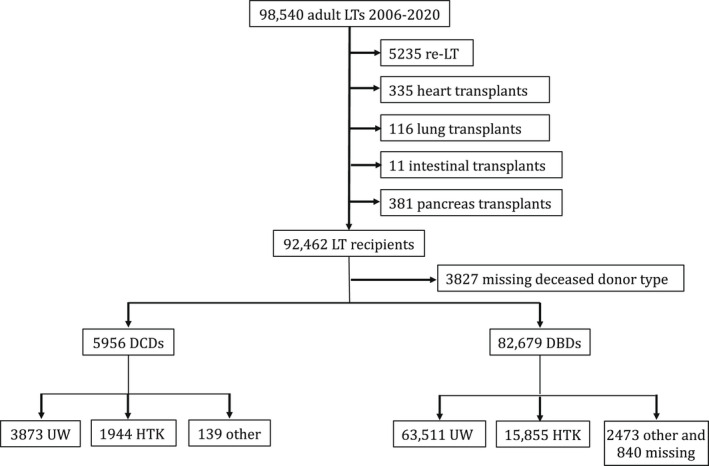
Study flow diagram outlines the inclusions and exclusions of the study cohort
The use of DCD LT increased threefold during the study time period, with the annual number increasing from 269 in 2006 to 828 in 2020 (Figure 2A). The corresponding increase in DBD LT during the study time period was less pronounced with the annual number increasing from 5044 in 2006 to 6711 in 2020 (Figure 2A). Among DCD LTs, the annual proportion using UW increased from 56.1% in 2006 to 64.9% in 2020, whereas in contrast, the annual proportion using HTK decreased from 43.5% in 2006 to 31.6% in 2020 (Figure 2A). Among DBD LTs, the annual proportion using UW increased from 67.0% in 2006 to 80.8% in 2020, whereas in contrast, the annual proportion using HTK decreased from 31.6% in 2006 to 14.0% in 2020 (Figure 2B).
FIGURE 2.
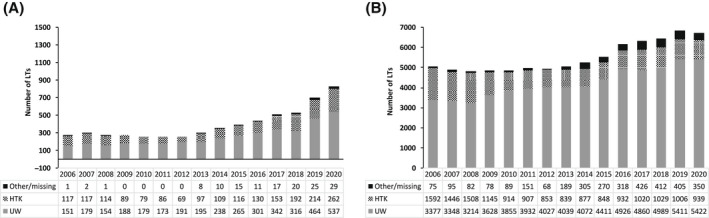
Annual number of (A) DCD and (B) DBD LTs in the United States from 2006 to 2020 stratified by static cold storage solution (UW vs. HTK vs. other/missing)
Comparative demographics between UW and HTK recipients
Among DCD LTs, HTK recipients had lower rates of diabetes mellitus (15% vs. 30%) and received older donors (median 36 vs. 34 years) with decreased CITs (median 5.3 vs. 5.7 h) compared with UW recipients (all p values <0.05; Table 1). Among DBD LTs, HTK recipients had lower MELD scores (median 21 vs. 22), lower rates of pretransplant life support requirements (5.5% vs. 9.0%), and lower rates of pretransplant ICU admission (10.2% vs. 15.1%) and received donor livers with decreased CITs (median 5.9 vs. 6.1 h) compared with UW recipients (all p values <0.05; Table 1).
TABLE 1.
Clinical features at the time of transplant of DCD and DBD LT recipients in the United States stratified by UW versus HTK, 2006–2020
| Variables | DCD a | DBD a | ||
|---|---|---|---|---|
| UW (n = 3873) | HTK (n = 1944) | UW (n = 63,511) | HTK (n = 15,855) | |
| Donor | ||||
| Age, years | 34.0 (24.0–46.0) | 36.0 (24.0–47.0)** | 43.0 (28.0–55.0) | 43.0 (29.0–55.0)** |
| BMI, kg/m2 | 26.1 (22.8–30.2) | 26.1 (22.8–30.5) | 26.7 (23.4–31.0) | 27.0 (23.5–31.5)*** |
| Male sex | 2646 (68.3) | 1300 (66.9) | 37,999 (59.8) | 9324 (58.8)* |
| White race | 3029 (78.2) | 1589 (81.7)** | 39,287 (61.9) | 11,313 (71.4)*** |
| HCV | 125 (3.2) | 88 (4.5)* | 3841 (6.0) | 1172 (7.4)*** |
| ECD | 228 (5.9) | 121 (6.2) | 16,709 (26.3) | 4133 (26.1) |
| CIT, h | 5.7 (4.7–7.0) | 5.3 (4.3–6.9)*** | 6.1 (4.9–7.8) | 5.9 (4.5–7.3)*** |
| Recipient | ||||
| Age, years | 58.0 (52.0–63.0) | 58.0 (52.0–63.0) | 57.0 (50.0–63.0) | 57.0 (50.0–62.0)** |
| Male sex | 2670 (68.9) | 1314 (67.6) | 42,060 (66.2) | 10,710 (67.5)** |
| White race | 2816 (72.7) | 1500 (77.2)*** | 43,718 (68.8) | 11,941 (75.3)*** |
| BMI, kg/m2 | 27.9 (24.5–32.1) | 28.2 (24.8–32.5) | 28.0 (24.5–32.3) | 28.2 (24.6–32.4)* |
| Posttransplant LOS, days | 9.0 (6.0–15.0) | 9.0 (7.0–15.0)** | 10.0 (7.0–17.0) | 10.0 (7.0–16.0) |
| Waiting list time, days | 113.0 (29.0–294.0) | 101.0 (29.5–277.0) | 84.0 (14.0–287.0) | 86.0 (17.0–258.0) |
| Diabetes mellitus | 1168 (30.2) | 11 (14.9)* | 17,305 (27.2) | 4257 (26.8) |
| MELD score | 18.0 (13.0–24.0) | 18.0 (13.0–24.0) | 22.0 (14.0–32.0) | 21.0 (14.0–29.0)*** |
| Life support requirement | 166 (4.3) | 53 (2.7) | 5730 (9.0) | 865 (5.5)*** |
| ICU | 217 (5.6) | 86 (4.4) | 9581 (15.1) | 1610 (10.2)*** |
| Dialysis requirement | 314 (8.1) | 131 (6.7) | 10,124 (15.9) | 2000 (12.6)*** |
| Ascites, mild or worse | 2789 (72.0) | 1484 (76.3)*** | 47,183 (74.3) | 12,420 (78.3)*** |
| Hepatic encephalopathy, grade 1 or worse | 2406 (62.1) | 1257 (64.7) | 39,688 (62.5) | 10,691 (67.4)*** |
| PVT | 447 (11.5) | 239 (12.3) | 7380 (11.6) | 1796 (11.3) |
| HCC | 1330 (34.3) | 637 (32.8) | 18,773 (29.6) | 4666 (29.4) |
| SLKT | 279 (7.2) | 110 (5.7)* | 5256 (8.3) | 1293 (8.2) |
Values are n (%) or median (interquartile range).
Abbreviations: BMI, body mass index; CIT, cold ischemia time; DBD, donation after brain death; DCD, donation after circulatory death; ECD, expanded criteria donor; HCV, hepatitis C virus; HTK, histidine‐tryptophan‐ketoglutarate solution; ICU, intensive care unit; LOS, length of stay; LT, liver transplantation; MELD, Model for End‐Stage Liver Disease; PVT, portal vein thrombosis; SLKT, simultaneous liver–kidney transplantation; UW, University of Wisconsin solution.
The UW and HTK groups are compared with each other within respective deceased donor categories (DCD or DBD) by pairwise comparisons via the chi‐square test for categorical variables and the two‐sample Wilcoxon rank test for continuous variables (all continuous variables failed the Shapiro–Wilk normality test). The variables contained <1% of missing data: missing values of continuous variables were ignored, whereas missing values of categorical variables were assumed to be negative.
p < 0.001
0.001 < p ≤ 0.01
0.01 < p ≤ 0.05 (all other p values > 0.05).
Posttransplant outcomes
There was no difference in 1‐year or 5‐year graft survival rates between UW and HTK groups among DCD LTs in the 2006–2010 and 2011–2015 eras (p > 0.05; Figure 3A,B). There remained no difference in these graft survival rates when adjusted for several important confounders, including donor age, CIT, fDWIT, recipient age, race, MELD score, and individual transplant centers (all p values >0.05). The HTK group had higher 1‐year and 5‐year graft survival rates of 89.7% and 74.3%, respectively, compared with 85.9% and 70.8% in the UW group in the 2016–2020 era (p = 0.005; Figure 3C). This difference remained when adjusted for the confounders outlined previously (HR, 0.78, 95% CI: 0.60–0.99; p = 0.048; Table 2). Although the estimates were similar between the basic Cox model and the shared frailty Cox model, the latter model, which included transplant center as a random effect, was a better fit (likelihood ratio test of θ = 0: p = 0.003) and was thus selected as the final model.
FIGURE 3.
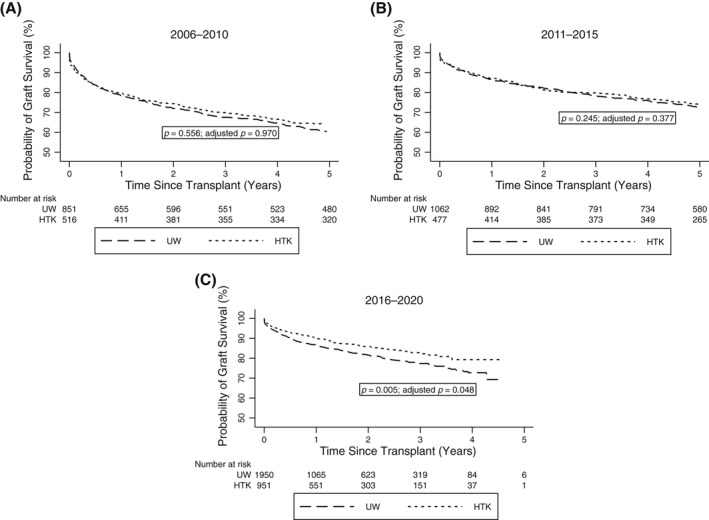
Graft survival rates among DCD LT recipients in the United States who received UW compared with HTK during static cold preservation stratified by eras ([A] 2006–2010, [B] 2011–2015, [C] 2016–2020)
TABLE 2.
Multivariate a Cox proportional hazards regression of graft failure in DCD recipients (N = 2901), 2016–2020
| Variables | HR (95% CI) | p value |
|---|---|---|
| Donor | ||
| HTK (reference UW) | 0.73 (0.55–0.98) | 0.037 |
| Age | 1.01 (1.00–1.02) | 0.018 |
| ECD | 1.41 (0.89–2.21) | 0.140 |
| fDWIT | 1.02 (1.01–1.04) | 0.005 |
| White race | 0.93 (0.71–1.21) | 0.577 |
| Male sex | 0.92 (0.72–1.18) | 0.514 |
| CIT | 1.06 (0.99–1.13) | 0.072 |
| Recipient | ||
| Age | 1.01 (0.99–1.02) | 0.601 |
| Male sex | 1.31 (1.01–1.70) | 0.045 |
| White race | 0.79 (0.60–1.03) | 0.084 |
| Diabetes mellitus | 1.16 (0.91–1.47) | 0.226 |
| BMI | 1.00 (0.99–1.03) | 0.575 |
| MELD score | 1.01 (0.99–1.02) | 0.467 |
| Pretransplant life support requirement | 2.13 (1.18–3.82) | 0.011 |
| Ascites (mild or worse) | 1.20 (0.89–1.61) | 0.240 |
| HCC | 1.24 (0.93–1.64) | 0.133 |
| SLKT | 0.95 (0.63–1.45) | 0.826 |
| Diagnosis | 0.96 (0.88–1.04) | 0.325 |
Diagnosis were the six UNOS categories for liver disease (Noncholestatic cirrhosis, Cholestatic liver diseases, Biliary atresia, Acute hepatic failure, Metabolic diseases, and Malignant neoplasms or benign tumors) – there were no overall differences in the categories of liver disease between the two groups.
Abbreviations: BMI, body mass index; CI, confidence interval; CIT, cold ischemia time; DCD, donation after circulatory death; ECD, expanded criteria donor; fDWIT, functional donor warm ischemia time; HCC, hepatocellular carcinoma; HR, hazard ratio; HTK, histidine‐tryptophan‐ketoglutarate solution; MELD, Model for End‐Stage Liver Disease; SLK, simultaneous liver–kidney transplantation; UW, University of Wisconsin solution.
Individual transplant centers were included as a random effect (θ = 0.091, likelihood ratio test of θ = 0: p = 0.006) in a shared frailty model. Of the fWDIT data, 14% was missing and was ignored in this analysis.
In the sensitivity analysis to further assess the effect of individual transplant centers, DCD LT volume was considered in a stratified analysis in the three eras. Transplant centers were divided into higher (≥50 DCD LTs) and lower volume (<50 DCD LTs) LT DCD centers on the basis of optimal group balancing. In the 2016–2020 era, there were 1562 DCD LT recipients in the higher volume group, of whom 664 (42.6%) and 898 (57.4%) received HTK and UW, respectively. There were 1349 DCD LT recipients in the lower volume group, of whom 287 (21.3%) and 1062 (78.7%) received HTK and UW, respectively. Among the higher volume centers, the HTK group (compared with the UW group) had an adjusted HR of 0.89 (95% CI: 0.63–1.26; p = 0.513). Among the lower volume centers, the HTK group (compared with the UW group) had an adjusted HR of 0.69 (95% CI: 0.46–1.05; p = 0.085). The highest volume DCD LT center in the 2016–2020 era performed 240 DCD LTs, of whom 146 (60.1%) used HTK. There were no differences in graft failure outcomes with regard to HTK versus UW at this single center (p = 0.292), although only 19 events (graft failures) occurred during the analysis period. The results of the sensitivity analysis were similar to the primary analysis for DCD LTs in the 2006–2010 and 2011–2015 eras.
In the Cox analysis restricted to DCD LTs and CITs > 8 h incorporating the same covariates as the final model and including transplant center as a random effect, the HTK group (compared with the UW group) had HRs of 0.78 (95% CI: 0.57–1.06; p = 0.115), 1.73 (95% CI: 0.85–3.49; p = 0.128), and 0.47 (95% CI: 0.09–2.50; p = 0.378) in the 2006–2010 (n = 359), 2011–2015 (n = 152), and 2016–2020 (n = 155) eras, respectively.
There were no differences in 1‐year or 5‐year graft survival rates between UW and HTK groups among DBD LTs in any era in both the unadjusted and adjusted analyses (all p values >0.05; Figure 4A–C).
FIGURE 4.
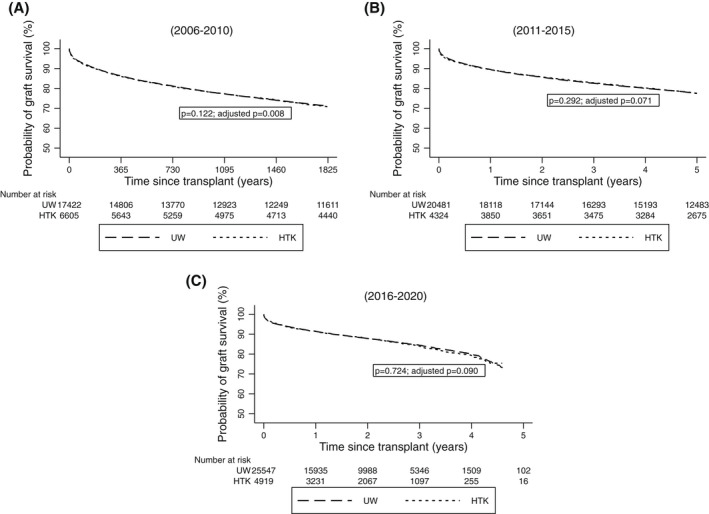
Graft survival rates among DBD LT recipients in the United States who received UW compared with HTK during static cold preservation stratified by eras ([A] 2006–2010, [B] 2011–2015, [C] 2016–2020)
DISCUSSION
In this longitudinal database analysis, we sought to define temporal trends in use and efficacy of the two primary cold preservation solutions used for liver grafts: UW and HTK. Our analysis yielded several key results. The proportion of donor livers preserved with HTK declined over time for both DCD and DBD liver donors. This finding may be a reflection of perceived inferiority of HTK as a result of earlier database studies concluding that HTK was a risk factor for graft loss.[ 6 , 7 , 8 ] Although these studies ultimately contributed to the expert consensus 2021 ILTS guidelines recommending against HTK for cold preservation in DCD transplantation particularly when prolonged CIT is anticipated, these recommendations were based on studies with significant flaws and data more than a decade old.[ 5 ]
Notably, our outcomes analysis contradicts these previous database analyses. In the earlier periods included in our study (from 2006 through 2015), there was no meaningful difference in graft failure between donor livers preserved with UW or HTK. These findings have also been reported in subsequent analysis of UNOS data,[ 9 , 10 ] contradicting the report by Stewart et al.[ 8 ] This was also true in the most recent period (2016–2020) in recipients of DBD. Interestingly, there appeared to be a significant improvement in graft survival for DCD grafts preserved with HTK compared with UW in our model, which accounted for transplant center variation. Although recipients of grafts preserved in HTK were marginally healthier and received organs with slightly shorter CITs, the improved graft survival with HTK remained even after controlling for these potential confounders. When a sensitivity analysis examining DCD LT center volume with graft survival was done, trends for improved outcomes with HTK were seen in both low and high volume DCD LT programs, although the statistical significance was lost, due to loss of power, nonetheless, it is prudent not to overinterpret these results until a rigorous prospective controlled trial is undertaken in the contemporary era. Taken together, these data indicate that HTK use is now associated with at least noninferior graft outcomes compared with UW. Importantly, HTK use was also associated with noninferior graft outcomes in DCD grafts with a CIT of >8 h. This finding should prompt ILTS to reconsider their recent guidance statement that advised against HTK in this clinical scenario.
The strengths of our study include the large number of LTs analyzed (a total of 88,635) for a span of 14 years and the inclusion of both DCD and DBD donors. Our sample size is large, with more than twice as many transplant recipients as the next largest analysis.[ 6 ] Moreover, our inclusion of multiple time periods allowed an analysis of trends over time. From our temporal analysis, we infer that after a likely early learning curve with using HTK (as more centers began using HTK as well as adopting DCD into their programs), HTK is now associated with at least noninferior outcomes when used in DCD livers. A limitation of our study is that it is a retrospective database analysis and therefore lacks some granular patient details. Future prospective randomized control trials will be needed to ultimately answer this question. As with any study that contains data from multiple centers, it is difficult to control for the center bias and its impact on graft survival, which could still skew the results despite our methodology. This is particularly challenging in DCD LT in which there are a core of specialist centers, many of which have implemented multiple simultaneous measures including DCD donor selection modification (limiting donor age and warm ischemia times) and changed DCD procedures such as thrombolytic use in certain settings based on the theory that microvascular thrombi form during withdrawal of life support therapy and cardiac arrest. The simultaneous application of multiple different measures may also impact the true association between preservation solutions and DCD graft outcomes.
In conclusion, our large database analysis reveals that HTK use is associated with noninferior liver graft outcomes in DCD and DBD LTs in the current era, including in DCD LTs with CITs of >8 h. This analysis should prompt reconsideration of the ILTS recommendations against HTK use in DCD transplantation.
CONFLICT OF INTEREST
Thomas G. Cotter, Matthew A. Odenwald, Angelica Perez‐Gutierrez, Kumar Jayant, Diego DiSabato, and John Fung have no relevant disclosures. Michael Charlton has received grant/research support and consultant fees from Gilead, received grant/research support from Conatus and Galectin and consultant fees from Metacrine, Enterome, Novartis, AbbVie, Intercept, and NGM Biopharmaceuticals.
AUTHOR CONTRIBUTIONS
Thomas G. Cotter contributed to the study design and acquisition, analysis, and interpretation of the data and drafted and critically revised the manuscript for important intellectual content. Matthew A. Odenwald, Angelica Perez‐Gutierrez, Kumar Jayant, Diego DiSabato, and Michael Charlton critically revised the manuscript for important intellectual content. John Fung contributed to the study concept and design and critically revised the manuscript for important intellectual content. All authors approved the final version to be published.
Cotter TG, Odenwald MA, Perez‐Gutierrez A, Jayant K, DiSabato D, Charlton M, et al. Preservation solutions for static cold storage in donation after circulatory death and donation after brain death liver transplantation in the United States. Liver Transpl. 2022;28:1454–1462. 10.1002/lt.26457
Funding information
Thomas G. Cotter is supported by National Institutes of Health (NIH) Grants STU 2019‐0472 and STU‐2019‐1368 (Alcoholic Hepatitis Network [AlcHepNet] Consortium coinvestigator). Matthew A. Odenwald is supported by NIH Grant 2T32DK007074‐47.
SEE EDITORIAL ON PAGE 1423
REFERENCES
- 1. van Rijn R, Schurink IJ, de Vries Y, van den Berg AP, Cortes Cerisuelo M, Darwish Murad S, et al. Hypothermic machine perfusion in liver transplantation—a randomized trial. N Engl J Med. 2021;384:1391–401. [DOI] [PubMed] [Google Scholar]
- 2. Dar WA, Sullivan E, Bynon JS, Eltzschig H, Ju C. Ischaemia reperfusion injury in liver transplantation: cellular and molecular mechanisms. Liver Int. 2019;39:788–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burra P, Zanetto A, Russo FP, Germani G. Organ preservation in liver transplantation. Semin Liver Dis. 2018;38:260–9. [DOI] [PubMed] [Google Scholar]
- 4. Feng LI, Zhao NA, Yao X, Sun X, Du L, Diao X, et al. Histidine‐tryptophan‐ketoglutarate solution vs. University of Wisconsin solution for liver transplantation: a systematic review. Liver Transpl. 2007;13:1125–36. [DOI] [PubMed] [Google Scholar]
- 5. Hessheimer AJ, Polak W, Antoine C, Dondero Pozzo F, Maluf D, Monbaliu D, et al. Regulations and procurement surgery in DCD liver transplantation: expert consensus guidance from the International Liver Transplantation Society. Transplantation. 2021;105:945–51. [DOI] [PubMed] [Google Scholar]
- 6. Adam R, Delvart V, Karam V, Ducerf C, Navarro F, Letoublon C, et al. Compared efficacy of preservation solutions in liver transplantation: a long‐term graft outcome study from the European Liver Transplant Registry. Am J Transplant. 2015;15:395–406. [DOI] [PubMed] [Google Scholar]
- 7. Adam R, Cailliez V, Karam V. Evaluation of HTK preservation solutions in liver transplantation: a long‐term propensity‐based analysis of outcome from the European Liver Transplant Registry. Am J Transplant. 2017;17:585–6. [DOI] [PubMed] [Google Scholar]
- 8. Stewart ZA, Cameron AM, Singer AL, Montgomery RA, Segev DL. Histidine‐Tryptophan‐Ketoglutarate (HTK) is associated with reduced graft survival in deceased donor livers, especially those donated after cardiac death. Am J Transplant. 2009;9:286–93. [DOI] [PubMed] [Google Scholar]
- 9. Klein AS, Messersmith EE, Ratner LE, Kochik R, Baliga PK, Ojo AO. Organ donation and utilization in the United States, 1999–2008. Am J Transplant. 2010;10(Pt 2):973–86. [DOI] [PubMed] [Google Scholar]
- 10. Thuluvath PJ, Guidinger MK, Fung JJ, Johnson LB, Rayhill SC, Pelletier SJ. Liver transplantation in the United States, 1999–2008. Am J Transplant. 2010;10(Pt 2):1003–19. [DOI] [PubMed] [Google Scholar]
- 11. de Boer JD, Strelniece A, van Rosmalen M, de Vries E, Ysebaert D, Guba M, et al. The effect of histidine‐tryptophan‐ketoglutarate solution and University of Wisconsin solution: an analysis of the Eurotransplant registry. Transplantation. 2018;102:1870–7. [DOI] [PubMed] [Google Scholar]
- 12. Kaltenborn A, Gwiasda J, Amelung V, Krauth C, Lehner F, Braun F, et al. Comparable outcome of liver transplantation with histidine‐tryptophan‐ketoglutarate vs. University of Wisconsin preservation solution: a retrospective observational double‐center trial. BMC Gastroenterol. 2014;14:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. O'Callaghan JM, Morgan RD, Knight SR, Morris PJ. The effect of preservation solutions for storage of liver allografts on transplant outcomes: a systematic review and meta‐analysis. Ann Surg. 2014;260:46–55. [DOI] [PubMed] [Google Scholar]
- 14. Mangus RS, Fridell JA, Vianna RM, Milgrom MA, Chestovich P, Chihara RK, et al. Comparison of histidine‐tryptophan‐ketoglutarate solution and University of Wisconsin solution in extended criteria liver donors. Liver Transpl. 2008;14:365–73. [DOI] [PubMed] [Google Scholar]
- 15. Cotter TG, Sandıkçı B, Paul S, Gampa A, Wang J, Te H, et al. Liver transplantation for alcoholic hepatitis in the United States: excellent outcomes with profound temporal and geographic variation in frequency. Am J Transplant. 2021;21:1039–55. [DOI] [PubMed] [Google Scholar]
- 16. Kwong AJ, Kim WR, Lake JR, Smith JM, Schladt DP, Skeans MA, et al. OPTN/SRTR 2019 annual data report: liver. Am J Transplant. 2021;21(Suppl 2):208–315. [DOI] [PubMed] [Google Scholar]
- 17. Organ for Procurement and Transplantation Network: Scientific Registry of Transplant Recipients . The SRTR database: overview. [cited 2021 Jul 14]. Available from: https://optn.transplant.hrsa.gov [Google Scholar]
- 18. Cotter TG, Paul S, Sandıkçı B, Couri T, Bodzin AS, Little EC, et al. Improved graft survival after liver transplantation for recipients with hepatitis C virus in the direct‐acting antiviral era. Liver Transpl. 2019;25:598–609. [DOI] [PubMed] [Google Scholar]
- 19. Cotter TG, Paul S, Sandıkçı B, Couri T, Bodzin AS, Little EC, et al. Increasing utilization and excellent initial outcomes following liver transplant of hepatitis C virus (HCV)‐viremic donors into HCV‐negative recipients: outcomes following liver transplant of HCV‐viremic donors. Hepatology. 2019;69:2381–95. [DOI] [PubMed] [Google Scholar]
- 20. Feng S, Goodrich NP, Bragg‐Gresham JL, Dykstra DM, Punch JD, DebRoy MA, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783–90. [DOI] [PubMed] [Google Scholar]
- 21. Haddad L, Cassenote AJF, Andraus W, de Martino RB, Ortega NRdS, Abe JM, et al. Factors associated with mortality and graft failure in liver transplants: a hierarchical approach. PLoS One. 2015;10:e0134874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kalisvaart M, Croome KP, Hernandez‐Alejandro R, Pirenne J, Cortés‐Cerisuelo M, Miñambres E, et al. Donor warm ischemia time in DCD liver transplantation‐working group report from the ILTS DCD, liver preservation, and machine perfusion consensus conference. Transplantation. 2021;105:1156–64. [DOI] [PubMed] [Google Scholar]


