ABSTRACT
Collagen X biomarker (CXM) is suggested to be a biomarker of linear growth velocity. However, early childhood data are limited. This study examines the relationship of CXM to the linear growth rate and bone development, including the possible modifying effects of vitamin D supplementation. We analyzed a cohort of 276 term‐born children participating in the Vitamin D Intervention in Infants (VIDI) study. Infants received 10 μg/d (group‐10) or 30 μg/d (group‐30) vitamin D3 supplementation for the first 2 years of life. CXM and length were measured at 12 and 24 months of age. Tibial bone mineral content (BMC), volumetric bone mineral density (vBMD), cross‐sectional area (CSA), polar moment of inertia (PMI), and periosteal circumference (PsC) were measured using peripheral quantitative computed tomography (pQCT) at 12 and 24 months. We calculated linear growth as length velocity (cm/year) and the growth rate in length (SD unit). The mean (SD) CXM values were 40.2 (17.4) ng/mL at 12 months and 38.1 (12.0) ng/mL at 24 months of age (p = 0.12). CXM associated with linear growth during the 2‐year follow‐up (p = 0.041) but not with bone (p = 0.53). Infants in group‐30 in the highest tertile of CXM exhibited an accelerated mean growth rate in length compared with the intermediate tertile (mean difference [95% CI] −0.50 [−0.98, −0.01] SD unit, p = 0.044) but not in the group‐10 (p = 0.062) at 12 months. Linear association of CXM and growth rate until 12 months was weak, but at 24 months CXM associated with both length velocity (B for 1 increment of √CXM [95% CI] 0.32 [0.12, 0.52] cm/yr, p = 0.002) and growth rate in length (0.20 [0.08, 0.32] SD unit, p = 0.002). To conclude, CXM may not reliably reflect linear growth from birth to 12 months of age, but its correlation with growth velocity improves during the second year of life. © 2022 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).
Keywords: COLLAGEN X BIOMARKER, LINEAR GROWTH, BONE, VITAMIN D, EARLY CHILDHOOD
Introduction
Growth is carefully monitored during early childhood because child health and developmental problems can often be detected as deviations from normal growth references. Additional markers, such as circulating biomarkers reflecting longitudinal growth velocity, possibly complementing anthropometric monitoring, provide a more accurate means for the early detection of growth impairments and to potentially evaluate growth‐promoting therapies. Recently, collagen X biomarker (CXM) in blood was identified as a potential biomarker of linear growth velocity during childhood.( 1 , 2 )
Type X collagen is produced by hypertrophic chondrocytes in active growth plates during bone formation.( 3 , 4 ) CXM is a molecular component of type X collagen, representing the intact noncollagenous 1 domain (NC1).( 1 , 5 ) Type X collagen is cleaved during the ossification process by collagenase, releasing CXM into circulation as a byproduct.( 1 , 2 , 4 ) CXM has been suggested as a marker of bone growth and shown to correlate linearly with height growth velocity from birth until 20 years of age.( 2 ) However, the data available remain limited, and we know of no studies that have evaluated how CXM reflects growth and bone development during early childhood.
Vitamin D is essential for normal bone growth and both vitamin D deficiency and excess of vitamin D associate with impaired growth. In previous reports from the Vitamin D Intervention in Infants (VIDI) study, we found that a high vitamin D supplemental dose and a high vitamin D concentration (25‐hydroxy‐vitamin D [25(OH)D] may delay growth in healthy infants up to the age of 24 months.( 6 , 7 ) The underlying mechanisms are not understood but likely involve vitamin D–mediated effects on the growth plate.( 8 , 9 ) Given that growth plate mineralization and collagen production are significantly impacted by vitamin D deficiency( 10 ) and that an excess of vitamin D induces osteoclastogenesis and mineral resorption( 9 ) together with the suppression of bone mineralization,( 11 ) we hypothesized that vitamin D may also associate with CXM.
In the current study we measured the blood CXM concentration in healthy 12‐month‐old infants and 24‐month‐old toddlers participating in the VIDI study. In doing so, we aimed to study age and sex differences in CXM and to examine whether CXM associates with parameters of linear growth and bone strength during the first 2 years of life. Because the vitamin D status influences the mineralization process in the growth plate, we also explored whether vitamin D supplementation or the 25(OH)D concentration during early childhood associated with CXM.
Subjects and Methods
Subjects
Between January 2013 and June 2014, we recruited 987 families treated at Kätilöopisto Maternity Hospital in Helsinki, Finland, to participate in the VIDI study. A detailed description of the recruitment process and study protocol appears elsewhere.( 12 , 13 ) Infants were born between 37 and 42 weeks of gestation with birth weights appropriate for gestational age (standard deviation score [SDS] ±2.0).
Infants were randomized to receive daily vitamin D3 supplementation of either 10 μg (hereafter referred to as group‐10) or 30 μg (hereafter referred to as group‐30) beginning at age 2 weeks through age 24 months. Study visits, which included anthropometric measurements, were held at the age of 6, 12, and 24 months, and bone parameters were measured through peripheral quantitative computed tomography (pQCT) at ages 12 and 24 months.( 12 , 13 )
Written informed consent was obtained from the parents upon recruitment. This study was conducted according to the guidelines put forth in the Declaration of Helsinki. Ethical approval was obtained from the Research Ethics Committee of the Hospital District of Helsinki and Uusimaa (107/13/03/03/2012), and the project protocol is registered at ClinicalTrials.gov (NCT01723852).
From the 987 families recruited, we excluded 12 who did not meet the inclusion criteria. In the present study, participants for whom both pQCT scans were of an excellent, good, or moderate quality were included, while those with a sufficient, poor, and failed pQCT scan quality were excluded.( 14 ) In addition, we excluded subjects for whom the blood sample volume was insufficient for assessment of the CXM concentration. This resulted in a total sample of 276 subjects, for whom 256 had CXM values at 12 months, 270 had CXM at 24 months, and 250 (91%) had CXM concentrations measured at both time points.
Biochemical methods
Concentrations of CXM for each serum sample were determined by diluting freshly thawed serum samples and diluting to 1:200 in a CXM sample diluent. Each serum sample was run in duplicate on a CXM ELISA plate and the mean intra‐assay variation for this study was 4%. The average CXM concentration was multiplied by its dilution factor and then used for the analysis reported here. A detailed explanation of the methods involved in the CXM assay can be found in the publications.( 1 , 2 )
The concentrations of 25(OH)D, intact parathyroid hormone (PTH), and ionized calcium (iCa) were analyzed from serum samples at the age of 12 and 24 months. The concentrations of 25(OH)D were determined using the IDS‐iSYS fully automated immunoassay system with chemiluminescence detection (Immunodiagnostic Systems Ltd., Bolton, UK), with an intra‐assay variation of <7%. The PTH concentration was measured using the IDS‐iSYS immunoassay. The serum iCa (adjusted to pH 7.40) was analyzed at the Central Laboratory of Helsinki University Hospital (HUSLAB) applying the ABL 90 FLEX or ABL 835 FLEX blood gas analyzers. The details of these assessments were provided earlier.( 13 )
Anthropometrics
Birth size was measured by midwives according to standard procedures. The measurements were collected from birth records and transformed to parity‐, gestational age‐ and sex‐specific SDSs based on national newborn body size curves.( 15 ) Infant weight (kg), length (cm), and head circumference (cm) were measured during 6‐, 12‐, and 24‐month follow‐up visits by a pediatrician or a research nurse. Length was measured using a tabletop meter in the supine position, and weight was measured on an electronic scale (Seca, Hamburg, Germany). Weight, length, length‐adjusted weight, and head circumference were expressed as SDSs using age‐ and sex‐specific national references( 16 ) and considered normal when falling within ±2.0 SDSs.
Bone parameters
A single slice pQCT (XCT2000L Research+, Stratec Medizintechnik GmbH, Pforzheim, Germany) scan at a 2.0‐mm thickness, 0.40‐mm voxel size, and 25‐mm/s scan speed was used to measure the bone parameters from the left tibia. The length of the tibia was measured from the medial malleolus to the medial condyle, and the 20% distal site was marked for scanning. The bone mineral content (BMC) (mg/mm), volumetric bone mineral density (vBMD) (mg/cm3), cross‐sectional area (CSA) (mm2), polar moment of inertia (PMI) (mm4), and periosteal circumference (PsC) (mm) were assessed for the total tibial bone during 12‐ and 24‐month follow‐up visits. The method and procedures were previously reported in detail.( 14 )
Statistical analyses
The normality of the variables was visually inspected, and statistical tests were selected as appropriate. Logarithmic or square‐root transformations were applied when necessary. The difference between groups was examined using the independent samples t test, the paired samples t test, the Mann–Whitney U test, the analysis of variance (ANOVA) F test or Brown‐Forsythe‐test, the analysis of covariance (ANCOVA), and the Bonferroni post hoc test. Repeated measures were Huynh‐Feldt corrected. The Pearson's correlation and simple and multivariate linear regression analyses were applied to the analysis of continuous variables. The difference in proportions were tested using the chi‐square tests. Overall test of multiple analysis of variance (MANOVA) was applied to examine the association between repeated measurements of CXM and linear growth and bone parameters, allowing correlations of repeated measurements in a single model.
The growth rate in length was calculated as the residuals from the linear regression models in which length in SDS at each successive age was regressed corresponding to the length SDS at all earlier ages.( 6 , 7 , 17 ) These residuals indicated how much the measurement of body size at each time point differs from that predicted by the corresponding measurements at earlier time points (in SD units). In addition, we applied a conventional length velocity of an absolute length increment for each year (cm/yr). Both are referred to as measures of the linear growth rate. We also calculated the change in all bone markers, such as bone mineral content increment, between measures at 12 and 24 months of age (referred to with Δ).
We categorized the CXM values according to previously published reference data derived from regression models of generalized additive models for location, scale, and shape (GAMLSS).( 2 ) For 12‐month‐old girls and boys, normal CXM variations were identified as within 25–75 ng/mL and 35–95 ng/L, respectively. For 24‐month‐old girls and boys, the corresponding normal variations were identified as 25–55 ng/L and 20–75 ng/mL, respectively. In addition, we applied sex‐specific tertiles of CXM to examine the differences in growth and bone parameters, and we present these results separately for group‐10 and group‐30 because of the observed interaction with the intervention group. Group differences for CSA, PMI, and PsC were adjusted for the tibial length (mm, as a proxy for the growth plate size), and linear associations for the linear growth rate were adjusted for length (cm, as a proxy for the growth plate size) at the corresponding time point.( 18 ) Because very few children had 25(OH)D values below 50 nmol/L,( 7 , 13 ) we used additional cut‐offs of 75 nmol/L( 19 ) and 125 nmol/L( 20 , 21 ) for categorization.
The interaction of sex and intervention group was examined using MANOVA and stratification, and if an interaction was observed in both analyses (p < 0.05), we presented the results stratified by group assuming the number of participants was adequate. A small and inconsistent interaction was observed in the analyses comparing CXM reference groups. Thus these analyses were adjusted for sex and intervention group at 12 and 24 months (Table 2). Sensitivity analyses were conducted in the linear models adjusting for the duration of breastfeeding, for which we reported any significant findings.
Table 2.
Linear Growth Parameters in CXM Sex‐Specific Reference Groups in 12‐Month‐Old Infants
| CXM below normal reference | CXM within normal reference | CXM above normal reference | p Value | |
|---|---|---|---|---|
| n (%) | 70 (27) | 179 (70) | 7 (3) | |
| At 12 months of age | Adjusted mean (95% CI) | |||
| Length (SDS) | −0.73 (−0.99, −0.48) | −0.64 (−0.79, −0.48) | −0.31 (−1.37, 0.75) | 0.54 |
| Length (cm) | 74.8 (74.3, 75.4) | 75.0 (74.6, 75.3) | 75.6 (73.9, 77.4) | 0.69 |
| Growth rate in length (SD unit) | −0.11 (−0.35, 0.13) | 0.01 (−0.14, 0.16) | 0.49 (−0.24, 1.21) | 0.28 |
| Length velocity (cm/yr) | 24.2 (23.6, 24.9) | 24.8 (24.4, 25.2) | 25.5 (23.6, 27.3) | 0.23 |
| Tibial length (mm) | 137 (135, 139) | 137 (136, 138) | 137 (132, 143) | 0.98 |
CXM = collagen X biomarker; CI = confidence interval; SDS = standard deviation score (based on Finnish sex‐ and age‐specific normative data of child size).
Differences between reference groups tested using ANCOVA adjusted for sex and intervention group.
Normal CXM variations were identified as 25–75 ng/mL for girls and 35–95 ng/mL for boys at this age group.( 2 )
Two values missing for the tibial length, and one bone mineral content outlier value was excluded.
We considered p < 0.05 as statistically significant. All statistical analyses were performed using the IBM's SPSS program for Windows, versions 25 and 27 (IBM, Chicago, IL, USA).
Results
Cohort characteristics
Table 1 summarizes the cohort characteristics by sex. More girls than boys participated in this study, whereby 57% (n = 146/256, p = 0.024) were girls at 12 months of age, and 58% (n = 157/270, p = 0.007) were girls at 24 months. A higher proportion of children belonged to group‐30 than group‐10: 59% (n = 151/256, p = 0.004) at 12 months of age and 57% (n = 154/270, p = 0.021) at 24 months were in group‐30. Boys were larger and had a higher BMC and vBMD than girls at both ages, although the growth rate in length was lower in boys compared with girls at 24 months (Table 1).
Table 1.
Descriptive Data on Collagen X Biomarker (CXM) and Parameters of Growth and Bone Mineralization, Strength, and Volume
| At 12 months of age | All (n = 256) | Girls (n = 146) | Boys (n = 110) |
|---|---|---|---|
| Mean (SD) | |||
| CXM (ng/mL) [range] | 40.2 (17.4) [1.6–136.6] | 38.7 (13.9) [3.5–92.0] | 42.1 (21.1) [1.6–136.6] |
| PTH (pg/mL) | 26.1 (15.8) | 26.1 (15.4) | 26.1 (16.3) |
| ICa (mmol/L) | 1.33 (0.04) | 1.34 (0.04) | 1.33 (0.03) |
| Length (cm) | 75.0 (2.6) | 74.1 (2.4) | 76.1 (2.4)*** |
| Length (SDS) | −0.64 (1.03) | −0.72 (1.01) | −0.53 (1.05) |
| Weight (kg) | 9.7 (1.1) | 9.3 (0.9) | 10.2 (1.1)*** |
| Weight (SDS) | −0.31 (0.98) | −0.39 (0.95) | −0.20 (1.02) |
| Growth rate in length (SD unit) | −0.01 (0.97) | −0.06 (1.03) | 0.06 (0.89) |
| Length velocity (cm/yr) | 24.7 (2.6) | 24.2 (2.5) | 25.3 (2.5)*** |
| Tibial length (mm) | 137 (7) | 136 (7) | 138 (6)* |
| BMC (mg/mm) | 35.8 (8.2) | 34.1 (8.0) | 38.0 (8.0)*** |
| vBMD (mg/cm3) | 303 (75) | 288 (70) | 322 (77)*** |
| CSA (mm2) | 123 (34) | 123 (34) | 123 (35) |
| PMI (mm4) | 2656 (1675) | 2666 (1626) | 2644 (1745) |
| PsC (mm) | 39.0 (5.2) | 39.0 (5.3) | 39.0 (5.1) |
| At 24 months of age | All (N = 270) | Girls (n = 157) | Boys (n = 113) |
|---|---|---|---|
| CXM (ng/mL) [range] | 38.1 (12.0) [15.7–93.5] | 38.9 (12.9) [15.7–93.51] | 37.1 (10.6) [17.1–73.0] |
| PTH (pg/mL) | 17.7 (8.9) | 18.5 (9.2) | 16.6 (8.5) |
| ICa (mmol/L) | 1.31 (0.04) | 1.32 (0.03) | 1.31 (0.04) |
| Length (cm) | 87.4 (3.2) | 86.6 (3.2) | 88.4 (3.0)*** |
| Length (SDS) | −0.35 (1.08) | −0.40 (1.10) | −0.28 (1.05) |
| Weight (kg) | 12.4 (1.4) | 12.1 (1.3) | 12.8 (1.3)*** |
| Weight (SDS) | −0.25 (1.00) | −0.30 (0.99) | −0.19 (1.01) |
| Growth rate in length (SD unit) | −0.04 (0.97) | 0.07 (1.01) | −0.19 (0.91)* |
| Length velocity (cm/yr) | 12.4 (1.6) | 12.6 (1.7) | 12.1 (1.4)* |
| Tibial length (mm) | 166 (9) | 166 (9) | 167 (9) |
| BMC (mg/mm) | 54.5 (8.2) | 52.8 (7.8) | 57.0 (8.1)*** |
| vBMD (mg/cm3) | 376 (75) | 365 (70) | 392 (79)** |
| CSA (mm2) | 149 (27) | 148 (27) | 149 (27) |
| PMI (mm4) | 3731 (1406) | 3713 (1423) | 3757 (1388) |
| PsC (mm) | 43.0 (3.9) | 43.0 (3.9) | 43.1 (3.9) |
CXM = collagen X biomarker; SDS = standard deviation score (based on Finnish sex‐ and age‐specific norms for child size); BMC = total bone mineral content; vBMD = total volumetric bone mineral density; CSA = total cross‐sectional area; PMI = total polar moment of inertia; PsC = periosteal circumference; PTH = parathyroid hormone; iCa = ionized calcium.
Independent samples t test used to determine statistical significance between sexes, where * indicates p < 0.05, ** indicates p < 0.01, and *** indicates p < 0.001.
Two values missing for the tibial length at 12 months, 10 values missing for PTH at 12 months, 4 values for iCa at 12 months, 24 values missing for iCa at 24 months.
CXM characteristics
CXM as well as growth and bone characteristics appear in Table 1. CXM at 12 months correlated with CXM at 24 months (r = 0.45, p < 0.001) (Fig. 1). The mean CXM values were similar between ages (p = 0.12) and sexes (p > 0.21) across the entire cohort. However, when analyzing boys and girls separately, CXM concentrations were higher at 12 months than at 24 months in boys (p = 0.018) but not in girls (p = 0.75) (Table 1).
Fig. 1.
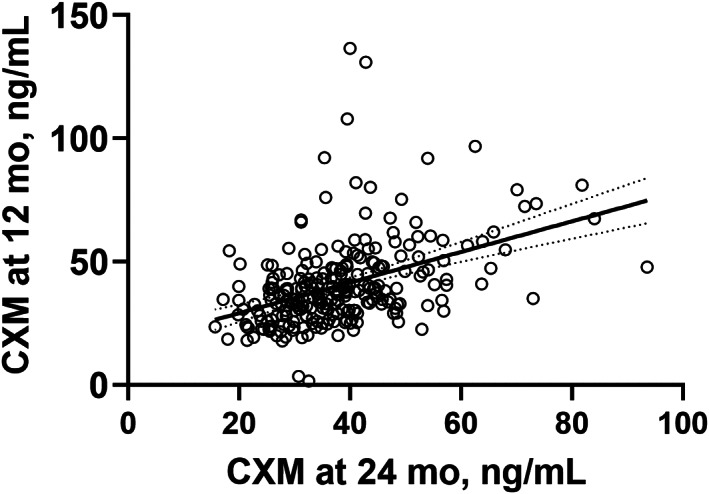
Scatter plot of CXM values (ng/mL) at two time points: child age 12 and 24 months (Pearson's correlation coefficient 0.45, p < 0.001, n = 250). Line represents the simple linear regression with 95% confidence bands of the best‐fit line. CXM = collagen X biomarker.
At 12 months, the reference norms were identified as falling within 25–75 ng/mL for girls and 35–95 ng/mL for boys; correspondingly, these values at 24 months were 25–55 ng/mL for girls and 20–75 ng/mL for boys.( 2 ) Using these reference values, we found that at 12 and 24 months of age, 27% (70/256) and 7% (18/270), respectively, had CXM values below the reference range, 70% (179/256) and 87% (236/270) fell within the normal reference range, and 3% (7/256) and 6% (16/270) exceeded the reference range. These deviations suggest that the reference range at 12 months was not appropriate for our cohort.
To identify the impact of a potential diurnal variation, we categorized the sampling time as morning (7:00–11:45) or afternoon (12:00–15:15). We observed minimal differences between the morning and afternoon values with mean (SD) CXM values at 12 months of 41.6 (18.6) ng/mL (n = 137) in the morning versus 38.5 (16.0) ng/mL (n = 115) in the afternoon, and at 24 months of 38.4 (11.6) ng/mL (n = 110) in the morning versus 35.9 (11.9) ng/mL (n = 69) in the afternoon (p > 0.12).
Vitamin D intervention and CXM
We hypothesized that vitamin D associated with CXM. The vitamin D intervention had a small effect on CXM values. Longitudinally, group‐10 exhibited a lower mean (95% confidence interval [CI]) CXM concentration compared with group‐30 (group‐10: n = 105, 37.8 [35.4, 40.3] ng/mL; group‐30: n = 151: 40.4 [38.3, 42.4] ng/mL, p = 0.045). The CXM concentrations were similar between 25(OH)D categories of <75 nmol/L, 75–125 nmol/L and >125 nmol/L at 12 and 24 months (p > 0.16) with no correlation between CXM and 25(OH)D at 12 or 24 months (p > 0.09).
CXM and linear growth
We also hypothesized that CXM associated with the linear growth rate and bone development in early childhood. Based on the overall test of MANOVA with all linear growth markers serving as dependent variable (length in cm and SDS at birth, 6, 12, and 24 months, growth rate in length, length velocity, the tibial length at 12 and 24 months, and the tibial length velocity at 24 months) and both CXM measures serving as predictors, we found that CXM associated with linear growth during the first 2 years of life (Wilks' exact lambda, p = 0.041). Adjusting for sex and intervention group did not change the results (p = 0.036).
At 12 months of age
When using the previously reported CXM reference values, we observed no differences in linear growth between the reference groups considered normal or falling below or above the reference values after adjusting for intervention group and sex (Table 2). We next divided the CXM values into age‐ and sex‐specific tertiles stratified by intervention group and compared the mean differences in growth and bone parameters between these tertiles. At 12 months of age, infants in group‐30 in the highest tertile of CXM exhibited an accelerated mean linear growth (measured in length [SDS and cm], growth rate in length [SD unit], and length velocity [cm/yr]) compared with the intermediate or lowest tertiles, whereas no such differences were observed in group‐10. Furthermore, we observed a sex interaction between the CXM tertiles and length velocity: this association emerged only among girls (Table 3). The PTH concentrations differed between the CXM tertiles in group‐30. Specifically, at 12 months of age, infants in the lowest tertile exhibited a lower mean PTH than infants in the intermediate tertile, although no differences were observed in the 25(OH)D concentration. In addition, no linear association was found between CXM and the linear growth measures from birth to 12 months of age for the entire group (Table 4). However, in group‐10, we identified a linear association between CXM and length velocity at 12 months, although this was attenuated after adjusting for size in length (cm) and after adjusting for the duration of breastfeeding in the sensitivity analysis (Table 4). At 12 months, infants with a normal‐length SDS had similar mean values for CXM (mean 40.3 [SD 17.8] ng/mL, n = 233) compared with those with length below −2.0 SDS (39.1 [13.0] ng/mL, n = 23) (p > 0.90). Figs. 2 and 3 provide the scatter plots between CXM and linear growth measurements stratified by age, sex, and intervention group.
Table 3.
Growth and Bone Parameters in Sex‐Specific Tertiles of CXM in 12‐Month‐Old Infants According to Vitamin D Intervention Group
| Lowest (first tertile) | Intermediate (second tertile) | Highest (third tertile) | p Value | |
|---|---|---|---|---|
| Mean (95% CI) | ||||
| Group‐10 | ||||
| n | 38 | 40 | 27 | |
| Length (SDS) | −0.73 (−1.03, −0.43) | −0.45 (−0.76, −0.13) | −0.34 (−0.85, 0.16) | 0.28 |
| Length (cm) | 75.0 (74.3, 75.8) | 75.3 (74.5, 76.2) | 75.7 (74.4, 76.9) | 0.65 |
| Growth rate in length (SD unit) | −0.15 (−0.46, 0.15) | 0.18 (−0.10, 0.46) | 0.34 (0.04, 0.65) | 0.062 |
| Length velocity (cm/yr) | 24.5 (23.7, 25.4) | 25.1 (24.3, 25.9) | 25.7 (24.6, 26.8) | 0.22 |
| Tibial length (mm) | 138 (136, 140) | 137 (135, 139) | 138 (135, 141) | 0.77 |
| BMC (mg/mm) | 35.1 (32.6, 37.6) | 35.6 (33.3, 37.8) | 36.7 (33.6, 39.8) | 0.70 |
| vBMD (mg/cm3) | 298 (269, 327) | 309 (282, 336) | 309 (278, 340) | 0.81 |
| CSA (mm2)a | 124 (113, 136) | 121 (110, 132) | 124 (111, 138) | 0.89 |
| PMI (mm4)a | 2737 (2169, 3306) | 2541 (1994, 3089) | 2746 (2080, 3411) | 0.85 |
| PsC (mm) a | 39.1 (37.4, 40.9) | 38.7 (37.0, 40.4) | 39.2 (37.0, 41.2) | 0.91 |
| PTH (pg/mL) | 26.9 (21.3, 32.6) | 27.3 (22.9, 31.7) | 31.2 (26.6, 35.8) | 0.14 |
| iCa (mmol/L) | 1.33 (1.33, 1.34) | 1.33 (1.32, 1.34) | 1.33 (1.32, 1.34) | 0.88 |
| 25(OH)D (nmol/L) | 82.4 (76.3, 88.6) | 81.3 (75.3, 87.3) | 79.2 (71.7, 86.7) | 0.79 |
| Group‐30 | ||||
| n | 48 | 46 | 57 | |
| Length (SDS) | −0.85 (−1.10, −0.60) | −1.00 (−1.30, −0.69) | −0.37 (−0.64, −0.10) | 0.003 b |
| Length (cm) e | 74.4 (73.7, 75.1) | 74.1 (73.3, 74.8) | 75.5 (74.8, 76.1) | 0.012 c |
| Growth rate in length (SD unit) | −0.15 (−0.40, 0.10) | −0.33 (−0.71, 0.05) | 0.16 (−0.07, 0.40) | 0.044 d |
| Length velocity (cm/yr) | 24.1 (23.4, 24.7) | 23.7 (23.0, 24.5) | 25.2 (24.5, 25.8) | 0.008 e |
| Tibial length (mm) | 136 (134, 138) | 136 (134, 138) | 138 (136, 140) | 0.24 |
| BMC (mg/mm) | 34.4 (32.2, 36.6) | 35.0 (32.4, 37.6) | 37.0 (35.0, 39.1) | 0.20 |
| vBMD (mg/cm3) | 306 (283, 328) | 306 (286, 325) | 293 (276, 310) | 0.54 |
| CSA (mm2)a | 117 (107, 126) | 120 (110, 130) | 132 (123, 141) | 0.067 |
| PMI (mm4)a | 2370 (1899, 2841) | 2549 (2069, 3030) | 2997 (2560, 3435) | 0.14 |
| PsC (mm) a | 38.0 (36.6, 40.4) | 38.5 (37.0, 40.0) | 40.3 (39.0, 41.7) | 0.054 |
| PTH (pg/mL) | 20.6 (16.9, 24.3) | 29.2 (23.3, 35.1) | 24.3 (19.8, 28.8) | 0.048 f |
| iCa (mmol/L) | 1.34 (1.33, 1.35) | 1.34 (1.33, 1.35) | 1.33 (1.32, 1.34) | 0.66 |
| 25(OH)D (nmol/L) | 122.0 (112.8, 131.3) | 114.7 (107.1, 122.4) | 117.2 (110.1, 124.3) | 0.44 |
CXM = collagen X biomarker; CI = confidence interval; group‐10 = vitamin D intervention group receiving 10 μg/d; group‐30 = vitamin D intervention group receiving 30 μg/d; SDS = standard deviation score (based on Finnish sex‐ and age‐specific normative data of child size); BMC = total bone mineral content; vBMD = total volumetric bone mineral density; CSA = total cross‐sectional area; PMI = total polar moment of inertia; PsC = periosteal circumference; PTH = parathyroid hormone; iCa = ionized calcium; 25(OH)D = 25‐hydroxy‐vitamin D.
Statistical significance between tertiles analyzed using ANOVA and ANCOVA.
CXM tertiles in girls: lowest, 3.45–31.17 ng/mL; intermediate, 31.97–40.85 ng/mL; highest, 40.99–91.99 ng/mL; and in boys: lowest, 1.59–32.81 ng/mL; intermediate, 32.82–43.22 ng/mL; highest, 43.37–136.62 ng/mL.
Two values missing for tibial length; one BMC outlier excluded; and five values missing for 25(OH)D.
Adjusted for the tibial length (mm) at 12 months.
Significance between first and third tertile (mean difference [95% CI] –0.48 [−0.95, −0.02], p = 0.039) and between second and third tertile (mean difference [95% CI] –0.63 [−1.10, −0.16], p = 0.004).
Significance between second and third tertile (mean difference [95% CI] –1.42 [−2.60, −0.23], p = 0.014).
Significance between second and third tertile (mean difference [95% CI] –0.50 [−0.98, −0.01], p = 0.044).
Significance between second and third tertile (mean difference [95% CI] –1.43 [−2.58, −0.27], p = 0.010); sex‐interaction (MANOVA, p < 0.001); significant differences observed in girls in group‐30 (p = 0.008) and not in group‐10 (p = 0.83) and no differences in boys in either intervention groups (p > 0.12).
Significance between first and second tertile (mean difference in logarithmic scale [95% CI] –0.15 [−0.30, −0.00], p = 0.043).
Table 4.
Linear Association Between CXM a and Linear Growth Parameters in 12‐Month‐ and 24‐Month‐Old Children
| At 12 months | All | Group‐10 | Group‐30 | Girls | Boys |
|---|---|---|---|---|---|
| B (95% CI) | |||||
| n | 256 | 105 | 151 | 146 | 110 |
| Length (SDS) | 0.09 (−0.01, 0.19) p = 0.088 | 0.10 (−0.08, 0.27) p = 0.28 | 0.09 (−0.03, 0.21) p = 0.14 | 0.14 (−0.01, 0.29) p = 0.064 | 0.04 (−1.00, 0.17) p = 0.60 |
| Length (cm) | 0.20 (−0.05, 0.45) p = 0.11 | 0.26 (−0.19, 0.70) p = 0.25 | 0.21 (−0.10, 0.51) p = 0.18 | 0.24 (−0.11, 0.59) p = 0.18 | 0.07 (−0.25, 0.38) p = 0.68 |
| Growth rate in length (SD unit) | 0.07 (−0.02, 0.17) p = 0.14 | 0.10 (−0.05, 0.24) p = 0.21 | 0.07 (−0.05, 0.19) p = 0.27 | 0.14 (−0.01, 0.29) p = 0.073 | 0.02 (−0.10, 0.13) p = 0.80 |
| Adjusted growth rate in length (SD unit) b | 0.03 (−0.05, 0.12) p = 0.42 | 0.05 (−0.08, 0.18) p = 0.44 | 0.03 (−0.08, 0.14) p = 0.59 | 0.09 (−0.05, 0.22) p = 0.20 | (−0.10, 0.10) p = 0.97 |
| Length velocity (cm/yr) | 0.23 (−0.02, 0.48) p = 0.068 | 0.45 (0.02, 0.89) p = 0.042 c | 0.15 (−0.15, 0.45) p = 0.32 | 0.30 (−0.06, 0.67) p = 0.10 | 0.11 (−0.21, 0.44) p = 0.49 |
| Adjusted length velocity (cm/yr) b | 0.07 (−0.08, 0.23) p = 0.35 | 0.25 (−0.02, 0.52) p = 0.067 | −0.01 (−0.20, 0.19) p = 0.96 | 0.10 (−0.12, 0.33) p = 0.37 | 0.06 (−0.16, 0.28) p = 0.57 |
| At 24 months | |||||
| n | 270 | 116 | 154 | 157 | 113 |
| Length (SDS) | 0.15 (0.02, 0.29) p = 0.029 | 0.17 (−0.08, 0.42) p = 0.17 | 0.15 (−0.02, 0.32) p = 0.084 | 0.18 (0.00, 0.35) p = 0.050 d | 0.13 (−0.10, 0.36) p = 0.27 |
| Length (cm) | 0.38 (−0.03, 0.79) p = 0.071 | 0.44 (−0.33, 1.22) p = 0.26 | 0.37 (−0.13, 0.86) p = 0.14 | 0.49 (−0.02, 0.99) p = 0.059 | 0.37 (−0.29, 1.04) p = 0.27 |
| Growth rate in length (SD unit) | 0.20 (0.08, 0.32) p = 0.002 | 0.25 (0.04, 0.45) p = 0.019 | 0.16 (0.01, 0.32) p = 0.040 | 0.23 (0.07, 0.39) p = 0.005 | 0.12 (−0.08, 0.32) p = 0.23 |
| Adjusted growth rate in length (SD unit) b | 0.15 (0.04, 0.25) p = 0.010 | 0.20 (0.01, 0.39) p = 0.040 | 0.10 (−0.03, 0.23) p = 0.14 | 0.14 (0.01, 0.27) p = 0.038 | 0.07 (−0.11, 0.25) p = 0.43 |
| Length velocity (cm/yr) | 0.32 (0.12, 0.52) p = 0.002 | 0.35 (−0.00, 0.71) p = 0.051 e | 0.28 (0.03, 0.53) p = 0.027 | 0.35 (0.09, 0.61) p = 0.008 | 0.20 (−0.11, 0.52) p = 0.21 |
| Adjusted length velocity (cm/yr) b | 0.21 (0.05, 0.38) p = 0.013 | 0.25 (−0.06, 0.55) p = 0.11 | 0.17 (−0.03, 0.37) p = 0.096 | 0.19 (−0.01, 0.38) p = 0.065 | 0.10 (−0.16, 0.36) p = 0.45 |
| From birth until 24 months f | |||||
| n | 256 | 105 | 151 | 146 | 110 |
| Growth rate in length (SD unit) | 0.09 (−0.01, 0.19) p = 0.091 | 0.11 (−0.07, 0.29) p = 0.25 | 0.08 (−0.05, 0.21) p = 0.21 | 0.17 (0.00, 0.33) p = 0.002 | 0.03 (−1.0, 0.17) p = 0.61 |
| Length velocity (cm) | 0.27 (−0.03, 0.57) p = 0.073 | 0.39 (−0.13, 0.91) p = 0.14 | 0.22 (−0.15, 0.59) p = 0.24 | 0.46 (−0.01, 0.92) p = 0.055 g | 0.10 (−0.28, 0.48) p = 0.59 |
CXM = collagen X biomarker; CI = confidence interval; group‐10 = vitamin D intervention group of 10 μg/d; group‐30 = vitamin D intervention group of 30 μg/d; SDS = standard deviation score (based on Finnish sex‐ and age‐specific norms for child size).
Simple and multivariate linear regression applied.
CXM square root transformed.
Adjusted for length (cm); acceptable level of multicollinearity.
In sensitivity analysis adjusted for the duration of breastfeeding, the association attenuated (p = 0.075).
In sensitivity analysis adjusted for the duration of breastfeeding, the association enhanced (p = 0.049).
In sensitivity analysis adjusted for the duration of breastfeeding, the association enhanced (p = 0.040).
CXM at 12 months and linear growth rate parameters calculated from birth until 24 months.
In sensitivity analysis adjusted for the duration of breastfeeding, the association enhanced (p = 0.049).
Fig. 2.
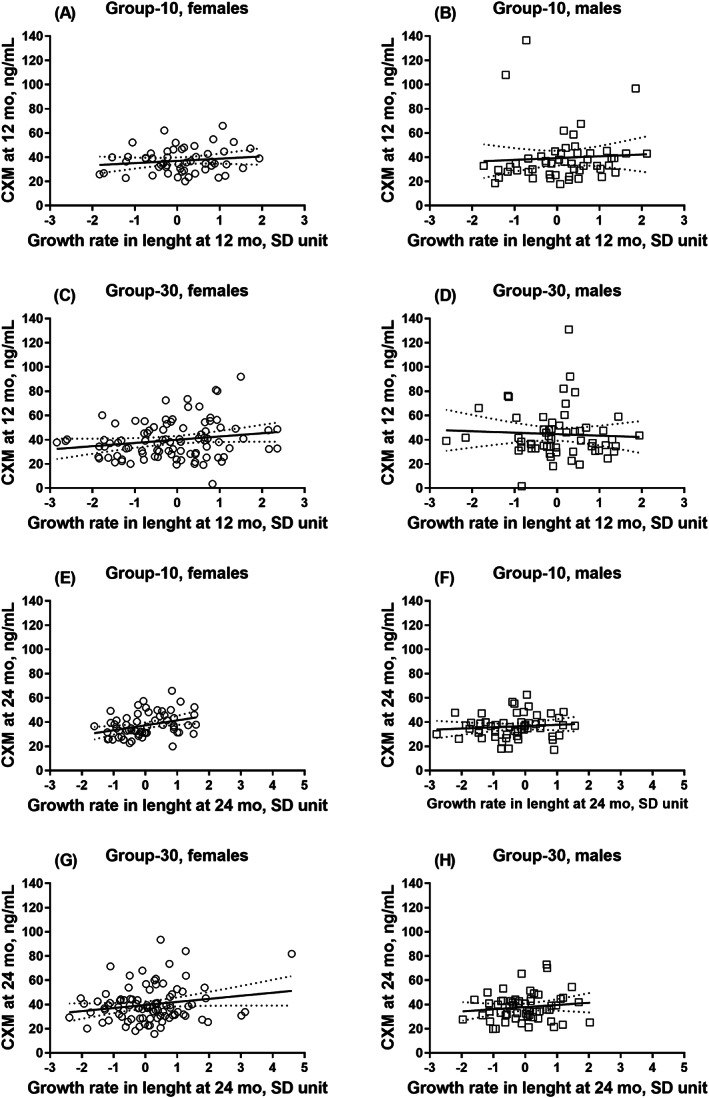
Multipanel figure of eight scatter plots of CXM (ng/mL) and growth rate in length (SD unit) stratified by sex and intervention group (circle = female; square = male): (A, B) at 12 months of age in group‐10; (C, D) at 12 months of age in group‐30; (E, F) at 24 months of age in group‐10; and (G, H) at 24 months of age in group‐30. Lines represent the simple linear regression with 95% confidence bands of the best‐fit line. CXM = collagen X biomarker; group‐10 = vitamin D intervention group receiving 10 μg/d; group‐30 = vitamin D intervention group receiving 30 μg/d.
Fig. 3.
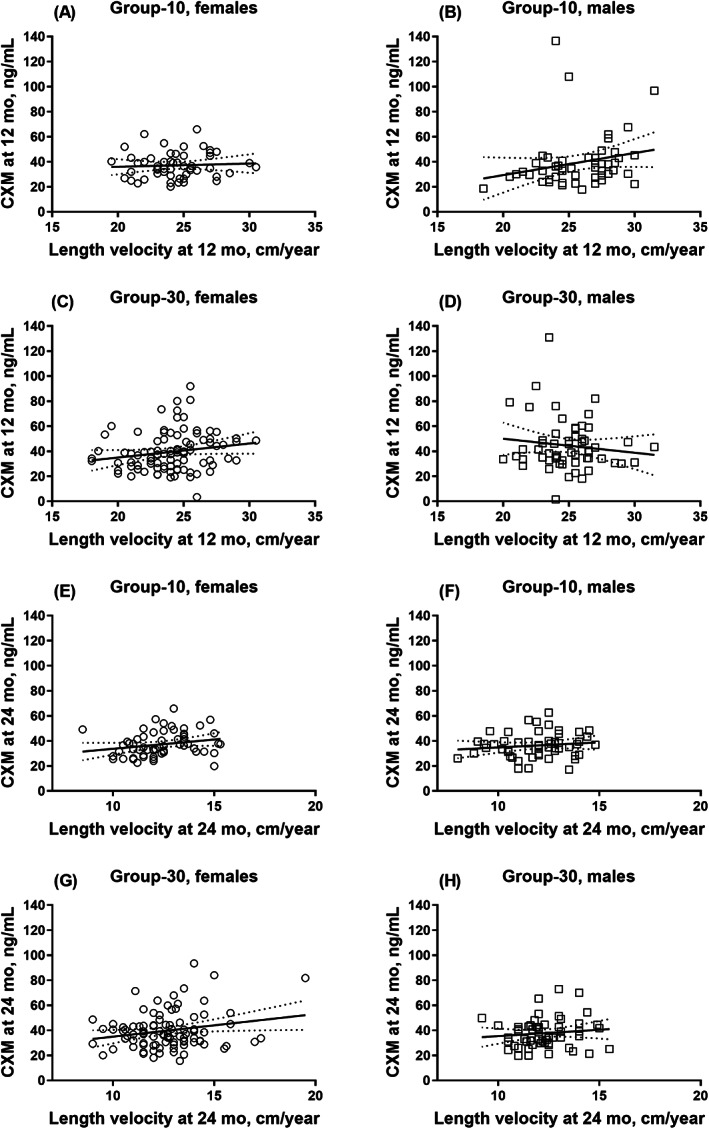
Multipanel figure of eight scatter plots of CXM (ng/mL) and length velocity (cm/yr) stratified by sex and intervention group (circle = female; square = male): (A, B) at 12 months of age in group‐10; (C, D) at 12 months of age in group‐30; (E, F) at 24 months of age in group‐10; and (G, H) at 24 months of age in group‐30. Lines represent the simple linear regression with 95% confidence bands of the best‐fit line. CXM = collagen X biomarker; group‐10 = vitamin D intervention group receiving 10 μg/d; group‐30 = vitamin D intervention group receiving 30 μg/d.
At 24 months of age
At 24 months of age, we observed a faster growth rate in length among the highest tertile of CXM compared with the lowest tertile in intervention group‐10, but this difference did not persist in the group‐30 (Table 5). CXM at 24 months exhibited a linear association with linear growth parameters in both intervention groups (measured as length [SDS and cm], growth rate in length [SD unit], and length velocity [cm/yr], with a partial attenuation of the association in the subgroups when adjusted for size in length (cm) (Table 4). Among boys, no linear association was observed between CXM and growth rate (Table 4). Children with a normal‐length SDS at 24 months exhibited similar mean values for CXM (mean [SD], 38.0 [12.0] ng/mL, n = 251) as those with length below −2.0 SDS (35.2 [7.0] ng/mL, n = 13) (p > 0.46). In addition, we examined if CXM at 12 months as the midpoint during follow‐up associated with the linear growth rate calculated for the entire 24‐month period, identifying an association only among girls (Table 4).
Table 5.
Growth and Bone Parameters in Sex‐Specific Tertiles of CXM in 24‐Month‐Old Infants According to Vitamin D Intervention Group
| CXM | Lowest (first tertile) | Intermediate (second tertile) | Highest (third tertile) | p Value |
|---|---|---|---|---|
| Mean (95% CI) | ||||
| Group‐10 | ||||
| n | 39 | 44 | 33 | |
| Length (SDS) | −0.59 (−0.92, −0.26) | −0.23 (−0.58, 0.13) | −0.19 (−0.54, 0.16) | 0.19 |
| Length (cm) | 86.7 (85.7, 87.7) | 87.9 (86.8, 89.0) | 87.7 (86.6, 88.8) | 0.23 |
| Growth rate in length (SD unit) | −0.37 (−0.66, −0.07) | −0.27 (−0.54, 0.00) | 0.17 (−0.13, 0.46) | 0.028 a |
| Length velocity (cm/yr) | 11.9 (11.4, 12.4) | 12.1 (11.6, 12.6) | 12.7 (12.1, 13.2) | 0.086 |
| Tibial length (mm) | 165 (162, 168) | 168 (165, 170) | 169 (166, 172) | 0.11 |
| Tibial length velocity (mm/yr) | 28.1 (25.5, 30.8) | 30.1 (27.6, 32.6) | 31.6 (28.5, 34.6) | 0.22 |
| BMC (mg/mm) | 52.7 (50.3, 55.2) | 55.2 (52.7, 57.7) | 54.2 (51.5, 57.0) | 0.35 |
| ΔBMC (mg/mm/yr) | 17.8 (15.6, 20.0) | 19.4 (17.0, 21.9) | 19.5 (17.4, 21.7) | 0.50 |
| vBMD (mg/cm3) | 378 (357, 400) | 374 (348, 400) | 357 (230, 385) | 0.49 |
| ΔvBMD (mg/cm3/yr) | 83.8 (52.7, 115.0) | 66.1 (42.1, 90.2) | 65.3 (29.4, 101.2) | 0.61 |
| CSA (mm2)b | 145 (136, 154) | 152 (144, 161) | 155 (145, 165) | 0.35 |
| ΔCSA (mm2/yr) | 16.8 (6.0, 27.7) | 31.6 (19.7, 43.5) | 33.1 (18.1, 48.1) | 0.13 |
| PMI (mm4)b | 3561 (3067, 4054) | 3943 (3482, 4403) | 4102 (3567, 4638) | 0.32 |
| ΔPMI (mm4/yr) | 660 (166, 1154) | 1355 (681, 2029) | 1591 (849, 2332) | 0.11 |
| PsC (mm) b | 42.5 (41.2, 43.8) | 43.5 (42.3, 44.7) | 43.8 (42.4, 45.2) | 0.37 |
| ΔPsC (mm/yr) | 2.78 (1.11, 4.46) | 4.94 (3.30, 6.59) | 4.96 (2.73, 7.18) | 0.15 |
| PTH (pg/mL) | 18.4 (14.8, 22.0) | 20.6 (17.4, 23.9) | 18.0 (15.8, 20.2) | 0.31 |
| iCa (mmol/L) | 1.31 (1.30, 1.32) | 1.31 (1.29, 1.32) | 1.31 (1.30, 1.32) | 0.83 |
| 25(OH)D (nmol/L) | 86.5 (80.4, 92.7) | 87.5 (80.7, 94.3) | 81.3 (76.6, 86.0) | 0.34 |
| Group‐30 | ||||
| n | 52 | 45 | 57 | |
| Length (SDS) | −0.39 (−0.65, −0.12) | −0.49 (−0.83, −0.15) | −0.22 (−0.53, 0.09) | 0.43 |
| Length (cm5) | 87.2 (86.4, 88.0) | 86.9 (85.8, 87.9) | 87.7 (86.8, 88.6) | 0.39 |
| Growth rate in length (SD unit) | 0.05 (−0.24, 0.34) | 0.05 (−0.23, 0.32) | 0.10 (−0.18, 0.39) | 0.95 |
| Length velocity (cm/yr) | 12.5 (12.0, 12.9) | 12.5 (12.1, 13.0) | 12.6 (12.1, 13.1) | 0.94 |
| Tibial length (mm) | 166 (163, 168) | 165 (163, 168) | 166 (164, 169) | 0.87 |
| Tibial length velocity (mm/yr) | 29.3 (27.3, 31.4) | 29.0 (26.6, 31.4) | 29.7 (27.4, 32.0) | 0.90 |
| BMC (mg/mm) | 55.1 (52.7, 57.4) | 54.2 (51.6, 56.8) | 55.2 (53.0, 57.5) | 0.82 |
| ΔBMC (mg/mm/yr) | 19.1 (16.1, 22.0) | 18.5 (16.3, 20.7) | 19.6 (17.4, 21.7) | 0.82 |
| vBMD (mg/cm3) | 381 (359, 402) | 374 (353, 395) | 385 (366, 405) | 0.74 |
| ΔvBMD (mg/cm3/yr) | 84.3 (58.6, 110.0) | 62.5 (31.8, 93.3) | 91.3 (68.1, 114.5) | 0.29 |
| CSA (mm2)b | 149 (142, 155) | 148 (141, 154) | 145 (139, 151) | 0.76 |
| ΔCSA (mm2/yr) | 23.3 (12.6, 34.0) | 28.7 (17.2, 40.1) | 21.2 (13.9, 28.4) | 0.55 |
| PMI (mm4)b | 3731 (3411, 4050) | 3642 (3299, 3986) | 3542 (3237, 3847) | 0.70 |
| ΔPMI (mm4/yr) | 922 (363, 1480) | 1169 (616, 1722) | 915 (573, 1258) | 0.71 |
| PsC (mm) b | 43.0 (42.1, 43.9) | 42.9 (42.0, 43.9) | 42.6 (41.8, 43.5) | 0.80 |
| ΔPsC (mm/yr) | 3.79 (2.23, 5.34) | 4.63 (2.92, 6.33) | 3.34 (2.26, 4.43) | 0.45 |
| PTH (pg/mL) | 15.9 (13.9, 17.9) | 17.6 (15.1, 20.0) | 16.7 (14.3, 19.0) | 0.69 |
| iCa (mmol/L) | 1.31 (1.30, 1.32) | 1.31 (1.30, 1.33) | 1.31 (1.31, 1.32) | 0.99 |
| 25(OH)D (nmol/L) | 114.3 (106.4, 122.2) | 113.1 (106.1, 120.1) | 121.6 (115.0, 128.1) | 0.19 |
CXM = collagen X biomarker; CI = confidence interval; group‐10 = vitamin D intervention group receiving 10 μg/d; group‐30 = vitamin D intervention group receiving 30 μg/d; SDS = standard deviation score; BMC = total bone mineral content; vBMD = total volumetric bone mineral density; CSA = total cross‐sectional area; PMI = total polar moment of inertia; PsC = periosteal circumference; Δ = change between measurements at 12 and 24 months; PTH = parathyroid hormone; iCa = ionized calcium; 25(OH)D = 25‐hydroxy‐vitamin D.
Statistical significance between tertiles analyzed using ANOVA and ANCOVA.
CXM tertiles among girls: lowest, 15.65–32.03 ng/mL; intermediate, 32.19–40.88 ng/mL; highest, 41.27–93.51 ng/mL; and among boys: lowest, 17.09–32.15 ng/mL; intermediate, 32.50–39.87 ng/mL; highest, 39.99–72.99 ng/mL.
Significance between first and third tertile (mean difference [95% CI] –0.53 [−1.04, −0.03], p = 0.034).
Adjusted for tibial length (mm) at 24 months.
CXM and bone parameters
We used the pQCT‐derived parameters of tibial BMC, vBMD, CSA, PMI, PsC, and tibial length to determine if CXM reflects bone mass and bone growth. The overall test of MANOVA revealed no association between CXM and bone parameters (BMC, vBMD, CSA, PMI, PsC, and tibial length at 12 and 24 months and tibial length velocity at 24 months) during the 2‐year follow‐up period (Wilks' exact lambda, p = 0.53; adjustment with sex and intervention group, p = 0.44). Furthermore, we observed no difference in the tibial bone parameters for BMC and vBMD at 12 (Table 3) and 24 months of age (Table 5) between CXM tertiles in either of the intervention groups. In addition, the mean values for CSA and PMI at 12 (Table 3) and 24 months of age (Table 5) were similar in the CXM tertiles in both intervention groups. Furthermore, we observed no differences in the mineral increment from 12 to 24 months of age (measured in ΔBMC, ΔvBMD, ΔCSA, ΔPMI, and ΔPsC). A tendency toward a higher PsC with negligible significance in the highest CXM tertile than in the lower tertile at 12 months of age in group‐30 was observed (Table 3). Finally, the mean tibial length was similar in the CXM tertiles at 12 and 24 months of age (Tables 3 and 5). CXM exhibited no linear association with any of the pQCT‐derived bone measures (p > 0.11).
Discussion
The current study represents one of only a few to examine the association between the recently identified height velocity biomarker CXM and linear growth and bone parameters during the first 2 years of life. In doing so, we relied on a unique sample of healthy young children aged 12 and 24 months who participated in a vitamin D supplementation trial. We found that a higher CXM associated with an accelerated linear growth but not with bone mineralization or other bone parameters during early childhood. Vitamin D supplementation modified the association between CXM and growth. At 12 months of age, infants in the highest tertile of CXM exhibited a greater linear growth compared with infants in the lower tertile in the group receiving 30 μg/d vitamin D. The linear association between CXM and the growth rate was weak until 12 months of age, but at 24 months of age, a positive consistently linear association was observed in both vitamin D intervention groups. These results indicate that while CXM may not reliably reflect linear growth from birth to 12 months of age, its correlation with the growth velocity improves during the second year of life.
The reference values provided by Coghlan and colleagues may not be applicable during the rapid growth phase during infancy.( 2 ) The basis for this assumption is that we found no differences in the linear growth rate according to the reference values, and a linear association of the growth rate was not observed at 12 months across the entire study population. Repeated and more frequent measurements of CXM may be necessary to reliably evaluate the relevance of this biomarker during periods of intense growth. In this study, we relied on single serum samples drawn at two distinct time periods; further sampling and averaging of the CXM concentration during these time points may lead to stronger correlations with growth. Growth during infancy is rapid and also affected by prenatal factors. A weaker association between CXM and linear growth at 12 months compared with 24 months may reflect greater variability in the growth rate during infancy than during later childhood. Thus, CXM does not consistently reflect the growth rate for the entire 12‐month period but rather the growth plate activity at the time of blood sampling.( 1 )
At 24 months, CXM linearly associated with all growth parameters. The growth rate during the second year of life becomes more stable and variability in dietary factors, motor skill milestones, and other external factors are likely smaller than during the first year of life. Interestingly, the association between CXM and linear growth was weaker in this study compared with previous reports,( 2 , 22 , 23 ) especially among infants. The repeated measurement of both CXM and height velocity in the same individuals over a longer period of time during childhood performed in previous studies most likely explains the stronger associations reported elsewhere. In our study, however, the variation in CXM values was larger (2–137 ng/mL) compared with previous studies by Coghlan and colleagues (20–95 ng/mL),( 2 ) by Nicol and colleagues (10–95 ng/mL)( 22 ) in subjects of comparable ages, and by Welborn and colleagues (4–47 ng/mL)( 23 ) among subjects with ages 7 to 16 years of age. The numbers of normal‐growth children aged 12 and 24 months from this study are higher than those among children in the same age range in the reference study by Coghlan and colleagues. Therefore, although the data variation in this study is greater than the reference range, this might result from the larger sampling in our study population. Furthermore, the original reference range included a variety of races and ethnicities among children in the United States, whereas this study consisted of Finnish children characterized by a similar maternal ethnicity, possibly playing a role in these reference values. Coghlan and colleagues mentioned that the reference range for CXM should be updated as more normal‐growth children are analyzed,( 2 ) such that the data from this study could be included in an updated reference range for future analyses. In general, in the few studies conducted, CXM concentrations followed a pattern of a higher concentration during early childhood, subsequently declining but peaking again at the age of puberty.( 2 , 22 , 23 )
A higher vitamin D supplementation dose slightly increased CXM and modified the association between CXM and linear growth. However, the clinical importance of these findings remains unclear. At 12 months of age, the increase in linear growth was greatest in the highest tertile of CXM in group‐30, although not in the group‐10. No linear association between CXM and linear growth measurements was observed at 12 months, except for length velocity in the group‐10. By contrast, at 24 months of age, the growth rate in length was most accelerated in toddlers in the highest CXM tertile in group‐10 but not in group‐30. These contrasting findings might be explained by the impact of vitamin D on growth in this cohort, since growth in length was delayed from birth to 12 months and growth in weight was accelerated from 12 to 24 months in group‐30 but not in group‐10.( 7 ) Nevertheless, in this study, a similar trend of CXM and linear growth was observed in both intervention groups. The possible mechanisms of CXM and growth in relation to vitamin D remain unclear. In an experimental study, 1,25‐dihydroxyvitamin D (1,25(OH)2D) inhibited type X collagen expression in the presence of the thyroid hormone but not when the thyroid hormone was missing from growth plate chondrocytes,( 24 ) indicating an interaction between steroid hormones.( 24 , 25 ) Furthermore, in rats, very high doses of 1,25(OH)2D resulted in a decreased zone of hypertrophic chondrocytes in the growth plate and stunted growth.( 26 ) Yet, vitamin D deficiency rickets causes chondrocyte hypertrophy and impairs growth.( 27 ) Data on the 1,25(OH)2D concentration were not available in this study. However, among infants receiving a higher dose of 30 μg vitamin D, PTH decreased in the lowest CXM tertile, suggesting a possible hormonal interaction. In our study cohort, PTH was lower in group‐30 than in group‐10 at both ages.( 13 ) Because vitamin D carries a delayed effect on the growth rate and appears to increase CXM, which in turn associates positively with linear growth, it is likely that the mechanism of vitamin D on growth is not directly related to CXM.
CXM did not correlate with the tibial total bone parameters at 12 or 24 months of age or with increments of these parameters from 12 to 24 months of age. It seems likely that in the growth plate during chondrocyte proliferation, type X collagen is among the final collagen types produced before endochondral ossification.( 5 ) In animal models, the expression of type X collagen has been observed specifically in the mineralized zone of the hypertrophic chondrocytes.( 28 ) Thus, it is possible that during the process of growth plate mineralization, type X collagen and its byproduct CXM correlate with linear growth, indicating acute activity in the hypertrophic cartilage, rather than with the total bone mineralization markers available in this study, which reflect the final stage of ossification.
The usability of CXM in the clinical setting requires further studies with repeated measures and a comparison of healthy children with children experiencing stunted growth. In children with osteogenesis imperfecta, the association between CXM and growth showed a weaker correlation than that among healthy children, indicating less accuracy as a biomarker of osteogenesis imperfecta as a skeletal abnormality.( 22 ) However, an interesting potential exists particularly in later childhood or during puberty when an individual decline in CXM may indicate growth cessation and provide a tool for patient monitoring.( 23 )
Our rare sample of healthy infants and toddlers experiencing rapid growth phase provides important knowledge on the usability of CXM as an indicator of early childhood linear growth and bone development. One limitation to our study, however, is that we only had two measures of CXM. Possibly including repeated monthly measurements, by collecting samples at least around 12 and 24 months of age, would have accounted for individual variation in these distinct growth periods. Welborn and colleagues suggested three repeated measurements per month to create a more reliable average value for CXM.( 23 ) That said, performing repeated blood sampling in small children on a large scale might prove challenging. Furthermore, because CXM is thought to reflect the acute linear growth process, it might not correlate with the length growth velocity calculated for a period of 1 year rather than for shorter intervals.( 2 ) We did not have direct measure of growth plate size, which might modify the relationship between CXM and linear growth;( 18 ) however, we used size in length (cm) as a proxy of this in adjustments. Our study applied pQCT scanning to derive bone parameters. Currently, there are no validated reference values available from pQCT parameters for this age group and therefore dual‐energy X‐ray absorptiometry (DXA) remains the recommended method in clinical settings.( 14 , 29 ) Thus, the bone mineralization results obtained using pQCT should be evaluated with caution. However, the possible effects on the associations between CXM and bone are likely minimal because we measured children and evaluated the scans in a standardized manner in a large cohort of healthy young children.( 14 ) Furthermore, in the current study, we included only children with at least a moderate scan quality in pQCT. One advantage to our study was that we applied a diverse set of outcomes indicating bone growth in order to identify any possible associations.
In this study, CXM associated with the linear growth rate but not with the tibial bone mineralization parameters during early childhood. A weak linear association emerged between CXM and linear growth during the first year of life when growth is very rapid but strengthens during the second year of life. Vitamin D supplementation appears to carry an inconsistent modifying impact on the relationship between CXM and linear growth. Further studies are required to definitely determine the reference values during infancy.
Disclosures
All authors state that they have no conflicts of interest.
Author Contributions
Helena Henrietta Hauta‐alus: Conceptualization; formal analysis; funding acquisition; investigation; methodology; visualization; writing – original draft; writing – review and editing. Elisa Holmlund‐Suila: Conceptualization; investigation; writing – review and editing. Saara Valkama: Conceptualization; investigation; writing – review and editing. Maria Enlund‐Cerullo: Conceptualization; investigation; writing – review and editing. Jenni Rosendahl: Conceptualization; investigation; writing – review and editing. Ryan F Coghlan: Methodology; validation; writing – review and editing. Sture Andersson: Conceptualization; funding acquisition; project administration; resources; supervision; writing – review and editing. Outi Mäkitie: Conceptualization; funding acquisition; methodology; project administration; resources; supervision; writing – review and editing.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1002/jbmr.4650.
Acknowledgments
We gratefully acknowledge all of the children and families who participated in this study. In addition, we extend our thanks to study nurses Sirpa Nolvi, Rhea Paajanen, Päivi Turunen, and Nea Boman and technician Sari Lindén, as well as the personnel of the Kätilöopisto Maternity Hospital for their valuable contribution to this work. We are also grateful for the financial support provided by the Sigrid Jusélius Foundation, the Novo Nordisk Foundation, the Folkhälsan Research Foundation, the Academy of Finland, the Foundation for Pediatric Research, the Special Governmental Subsidy for Clinical Research, Finska Läkaresällskapet, the Päivikki and Sakari Sohlberg the Foundation, Juho Vainio Foundation, and the Finnish Medical Foundation.
Authors’ roles: HHH, SMV, EMH‐S, JR, ME‐C, SA, and OM made substantial contributions to the conception and design of the study and to the acquisition of data. RC contributed to development of the CXM assay and results. HHH was responsible for the data analysis and drafting the first version of the manuscript. HHH, EMH‐S, SA, and OM contributed to the interpretation of the data. HHH, SMV, EMH‐S, JR, ME‐C, RC, SA, and OM participated in drafting the manuscript or revising it critically for important intellectual content. HHH, SMV, EMH‐S, JR, ME‐C, RC, SA, and OM approved the final version of the manuscript submitted. HHH, SMV, EMH‐S, JR, ME‐C, RC, SA, and OM agree to be accountable for all aspects of the work and ensuring that questions related to the accuracy or integrity of any part of this manuscript are appropriately investigated and resolved.
Data Availability Statement
Data analyzed for the current study are not publicly available but are available from the corresponding author upon reasonable request.
References
- 1. Coghlan RF, Oberdorf JA, Sienko S, et al. A degradation fragment of type X collagen is a real‐time marker for bone growth velocity. Sci Transl Med. 2017;9(419):eaan4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coghlan RF, Olney RC, Boston BA, Coleman DT, Johnstone B, Horton WA. Norms for clinical use of CXM, a real‐time marker of height velocity. J Clin Endocrinol Metab. 2021;106(1):e255‐e264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Long F, Sonenshein GE, Linsenmayer TF. Multiple transcriptional elements in the avian type X collagen gene. Identification of Sp1 family proteins as regulators for high level expression in hypertrophic chondrocytes. J Biol Chem. 1998;273(11):6542‐6549. [DOI] [PubMed] [Google Scholar]
- 4. Schmid TM, Popp RG, Linsenmayer TF. Hypertrophic cartilage matrix. Type X collagen, supramolecular assembly, and calcification. Ann N Y Acad Sci. 1990;580:64‐73. [DOI] [PubMed] [Google Scholar]
- 5. Shen G. The role of type X collagen in facilitating and regulating endochondral ossification of articular cartilage. Orthod Craniofac Res. 2005;8(1):11‐17. [DOI] [PubMed] [Google Scholar]
- 6. Hauta‐alus HH, Kajantie E, Holmlund‐Suila EM, et al. High pregnancy, cord blood, and infant vitamin D concentrations may predict slower infant growth. J Clin Endocrinol Metab. 2019;104(2):397‐407. [DOI] [PubMed] [Google Scholar]
- 7. Hauta‐alus HH, Holmlund‐Suila EM, Kajantie E, et al. The effects of vitamin D supplementation during infancy on growth during the first 2 years of life. J Clin Endocrinol Metab. 2021;106(3):e1140‐e1155. [DOI] [PubMed] [Google Scholar]
- 8. St‐Arnaud R. The direct role of vitamin D on bone homeostasis. Arch Biochem Biophys. 2008;473(2):225‐230. [DOI] [PubMed] [Google Scholar]
- 9. Goltzman D. Functions of vitamin D in bone. Histochem Cell Biol. 2018;149(4):305‐312. [DOI] [PubMed] [Google Scholar]
- 10. Creo AL, Thacher TD, Pettifor JM, Strand MA, Fischer PR. Nutritional rickets around the world: an update. Paediatr Int Child Health. 2017;37(2):84‐98. [DOI] [PubMed] [Google Scholar]
- 11. Verlinden L, Carmeliet G. Integrated view on the role of vitamin D actions on bone and growth plate homeostasis. JBMR Plus. 2021;5(12):e10577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Helve O, Viljakainen H, Holmlund‐Suila E, et al. Towards evidence‐based vitamin D supplementation in infants: vitamin D intervention in infants (VIDI)—study design and methods of a randomised controlled double‐blinded intervention study. BMC Pediatr. 2017;17(1):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rosendahl J, Valkama S, Holmlund‐Suila E, et al. Effect of higher vs standard dosage of vitamin D3 supplementation on bone strength and infection in healthy infants: a randomized clinical trial. JAMA Pediatr. 2018;172(7):646‐654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Valkama S, Holmlund‐Suila E, Ireland A, et al. Peripheral quantitative computed tomography (pQCT) in 12‐ and 24‐month‐old children—practical aspects and descriptive data. Bone. 2020;141:115670. [DOI] [PubMed] [Google Scholar]
- 15. Sankilampi U, Hannila M‐L, Saari A, Gissler M, Dunkel L. New population‐based references for birth weight, length, and head circumference in singletons and twins from 23 to 43 gestation weeks. Ann Med. 2013;45(5–6):446‐454. [DOI] [PubMed] [Google Scholar]
- 16. Saari A, Sankilampi U, Hannila M‐L, Kiviniemi V, Kesseli K, Dunkel L. New Finnish growth references for children and adolescents aged 0 to 20 years: length/height‐for‐age, weight‐for‐length/height, and body mass index‐for‐age. Ann Med. 2011;43(3):235‐248. [DOI] [PubMed] [Google Scholar]
- 17. De Stavola BL, Nitsch D, dos Santos SI, et al. Statistical issues in life course epidemiology. Am J Epidemiol. 2006;163(1):84‐96. [DOI] [PubMed] [Google Scholar]
- 18. Tuchman S, Thayu M, Shults J, Zemel BS, Burnham JM, Leonard MB. Interpretation of biomarkers of bone metabolism in children: impact of growth velocity and body size in healthy children and chronic disease. J Pediatr. 2008;153(4):484‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Holick MF, Binkley NC, Bischoff‐Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911‐1930. [DOI] [PubMed] [Google Scholar]
- 20. Lips P, Cashman KD, Lamberg‐Allardt C, et al. Management of endocrine disease: current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency; a position statement of the European Calcified Tissue Society. Eur J Endocrinol. 2019;180(4):P23‐P54. [DOI] [PubMed] [Google Scholar]
- 21. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metabol. 2011;96(1):53‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nicol LE, Coghlan RF, Cuthbertson D, et al. Alterations of a serum marker of collagen X in growing children with osteogenesis imperfecta. Bone. 2021;1(149):115990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Welborn MC, Coghlan R, Sienko S, Horton W. Correlation of collagen X biomarker (CXM) with peak height velocity and radiographic measures of growth in idiopathic scoliosis. Spine Deform. 2021;9(3):645‐653. [DOI] [PubMed] [Google Scholar]
- 24. Ballock RT, Zhou X, Mink LM, Chen DH, Mita BC. Both retinoic acid and 1,25(OH)2 vitamin D3 inhibit thyroid hormone‐induced terminal differentiaton of growth plate chondrocytes. J Orthop Res. 2001;19(1):43‐49. [DOI] [PubMed] [Google Scholar]
- 25. Sanchez CP, Salusky IB, Kuizon BD, Abdella P, Jüppner H, Goodman WG. Growth of long bones in renal failure: roles of hyperparathyroidism, growth hormone and calcitriol. Kidney Int. 1998;54(6):1879‐1887. [DOI] [PubMed] [Google Scholar]
- 26. Silbermann M, Mirsky N, Levitan S, Weisman Y. The effect of 1,25‐dihydroxyvitamin D3 on cartilage growth in neonatal mice. Metab Bone Dis Relat Res. 1983;4(6):337‐345. [PubMed] [Google Scholar]
- 27. Elder CJ, Bishop NJ. Rickets. Lancet. 2014;383(9929):1665‐1676. [DOI] [PubMed] [Google Scholar]
- 28. Silbermann M, von der Mark K. An immunohistochemical study of the distribution of matrical proteins in the mandibular condyle of neonatal mice: I collagens. J Anat. 1990;170:11‐22. [PMC free article] [PubMed] [Google Scholar]
- 29. Kalkwarf HJ, Abrams SA, DiMeglio LA, et al. Bone densitometry in infants and young children: the 2013 ISCD pediatric official positions. J Clin Densitom. 2014;17(2):243‐257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data analyzed for the current study are not publicly available but are available from the corresponding author upon reasonable request.


