Abstract
The clinical translation of FLASH radiotherapy (RT) requires challenges related to dosimetry and beam monitoring of ultra‐high dose rate (UHDR) beams to be addressed. Detectors currently in use suffer from saturation effects under UHDR regimes, requiring the introduction of correction factors. There is significant interest from the scientific community to identify the most reliable solutions and suitable experimental approaches for UHDR dosimetry. This interest is manifested through the increasing number of national and international projects recently proposed concerning UHDR dosimetry. Attaining the desired solutions and approaches requires further optimization of already established technologies as well as the investigation of novel radiation detection and dosimetry methods. New knowledge will also emerge to fill the gap in terms of validated protocols, assessing new dosimetric procedures and standardized methods. In this paper, we discuss the main challenges coming from the peculiar beam parameters characterizing UHDR beams for FLASH RT. These challenges vary considerably depending on the accelerator type and technique used to produce the relevant UHDR radiation environment. We also introduce some general considerations on how the different time structure in the production of the radiation beams, as well as the dose and dose‐rate per pulse, can affect the detector response. Finally, we discuss the requirements that must characterize any proposed dosimeters for use in UDHR radiation environments. A detailed status of the current technology is provided, with the aim of discussing the detector features and their performance characteristics and/or limitations in UHDR regimes. We report on further developments for established detectors and novel approaches currently under investigation with a view to predict future directions in terms of dosimetry approaches, practical procedures, and protocols. Due to several on‐going detector and dosimetry developments associated with UHDR radiation environment for FLASH RT it is not possible to provide a simple list of recommendations for the most suitable detectors for FLASH RT dosimetry. However, this article does provide the reader with a detailed description of the most up‐to‐date dosimetric approaches, and describes the behavior of the detectors operated under UHDR irradiation conditions and offers expert discussion on the current challenges which we believe are important and still need to be addressed in the clinical translation of FLASH RT.
Keywords: dosimetry, FLASH, proton therapy, radiotherapy, ultra‐high dose rate
1. INTRODUCTION
The main goal of radiation therapy (RT) is to kill cancerous cells while minimizing any radiation damage to the adjacent healthy cells/tissues. Current literature indicates that RT delivered at an ultra‐high dose rate (UHDR) is where the dose rate is greater than 40 Gy/s compared to ∼0.1 Gy/s for conventional dose rate RT. Here‐in we refer to this RT delivery technique as FLASH RT, and several published studies indicate that it is a promising technique with great potential to better achieve the above RT goal. 1 , 2 , 3 Preclinical studies have shown that FLASH RT may substantially improve normal tissue sparing while maintaining high tumor control probability compared to conventional dose rate RT. 4 , 5 , 6 , 7 , 8 , 9 , 10 The FLASH effect, in sparing normal tissues, is not fully understood. A series of hypotheses 11 , 12 , 13 , 14 , 15 , 16 based on the fundamentals of radiophysics, radiobiology, and radiochemistry connecting it to reactive oxygen species has been proposed in the literature. 11 , 12 , 13 , 14 , 15 , 16 These hypotheses continue to be met with vigorous and exciting debate in the scientific community. Various platforms for FLASH RT have been demonstrated using electrons generated by linear accelerators, 4 , 5 , 6 , 17 , 18 X‐rays generated in a synchrotron facility, 7 , 19 protons using an isochronous cyclotron 20 , 21 , 22 , 23 and a synchrocyclotron, 24 , 25 as well as helium 26 and carbon 27 ion beams generated using a synchrotron (see Table 1).
TABLE 1.
Realization of FLASH radiation beams in recent literature (adapted with addition from Ref. 24 )
| Year | Radiation type | Machine | Energy (MeV) | Average dose rate (Gy/s) | Dose per pulse (Gy/pulse) | Pulse repetition rate (Hz) | Field size | Purpose | Dosimetry method |
|---|---|---|---|---|---|---|---|---|---|
| 1995 | Photon 30 | Brookhaven National Laboratory (USA) | 0.08 mean | 310–620 | Not provided | 52 MHz |
4 × 0.02/0.04 mm 0.075/0.2 × 7 mm |
Rat neuro‐study | IC, RCF, TLD |
| 2014 | Electron 4 | Kinetron Linac 37 (Switzerland) | 4.5 | 60 | 5 × 106 | 19 |
Ø 1.2 cm 1.8 cm × 2.0 cm |
Mouse study (bilateral thorax irradiation) | Chemical dosimetry with blue methyl viologen |
| 2017 | Electron 5 | Oriatron 6e Linac (Switzerland) | 6 | 100 | 5 × 106 | 100 | Ø 1.7 cm | Mouse study (brain irradiation) | TLD |
| 2017 | Electron 17 | Varian 21EX (USA) | 9 and 20 | 35–210 | 1.7 × 106 | 182 | 1–5 cm @ 90% | Feasibility study | EBT2 RCF |
| 2018 | Photon 7 | European Synchrotron Radiation Facility (France) | 0.102 mean | 37 | 1.2 × 104 Gy/s instantaneous | Continuous | 2 × 2 cm (reference size) | Mouse study (brain irradiation) | IC 39 |
| 2018 | Proton 20 | IBA isochronous cyclotron (France) | 138–198 | 40 | N/A | 106.14 MHz (quasi‐continuous) | ∼1.2 cm @ 90% | Feasibility study | Cylindrical IC, EBT3 RCF |
| 2019 | Electron 18 | ELEKTA Precise Linac (Sweden) | 8 | 30–300 | Not provided | 200 | Ø 2 cm (at the highest dose rate) | Feasibility study | EBT3 RCF |
| 2019 | Electron 6 | Kinetron Linac and Oriatron 6e (Switzerland) | 4.5 and 6 | 300 | 5 × 106 | Not provided | Ø 2.6 cm or 1.8–4.5 cm rectangular | Mini‐pig (skin) and cat (nasal tumor) study | TLD, alanine pellets, EBT3 RCF |
| 2019 | Electron 31 | Oriatron ERT6 Linac (Switzerland) | 5.6 | 150 | 1 × 106 | 100 |
Ø 3.5 cm 1.3 depth @ 90% |
Human patient treatment (skin) | Alanine pellets, EBT3 RCF |
| 2019 | Proton 21 | Varian isochronous cyclotron (USA) | 245 | 40 | N/A | Quasi‐continuous | 1 cm × 3 cm | Mouse study (whole thorax irradiation) | Not provided |
| 2020 | Proton 22 | IBA isochronous cyclotron (USA) | 230 | 80 | N/A | 106.14 MHz (quasi‐continuous) | ∼2 cm FWHM | Mouse study (abdomen irradiation) | Plane‐parallel IC |
| 2020 | Proton 24 | Mevion synchrocyclotron (USA) | 70 | 100–200 | 0.16–0.32 Gy/pulse (8–16 × 103 Gy/s instantaneous) | 648 | ∼1.2 cm FWHM (5 mm @ 90% isodose) | Feasibility study | Plane‐parallel IC, FC, MC simulation, and RCF |
| 2020 | Proton 32 | IBA isochronous cyclotron (USA) | 227.5 | 130 | N/A | 106 MHz (quasi‐continuous) | 1.6 × 1.2 cm2 ellipse | Mouse (partial abdomen irradiation) | Plane‐parallel IC, FC, MC simulation, EBT3 RCF |
| 2020 | Photon 33 | ANSTO Australian Synchrotron | 0.07 and 0.09 mean | 40–350 (at treatment depth and filtration) | 200 (at 20 mm reference depth and filtration) | Continuous | 2 × 2 cm (reference dosimetry size) | Rat study (brain cancer irradiation) | Pinpoint IC (reference), silicon semiconductor, and MC |
| 2021 | Proton 25 | Mevion synchrocyclotron (USA) | 60 | 120–160 | 0.22 Gy/pulse (9.3 × 103 Gy/s instantaneous) | 750 | Ø 1.1 cm FWHM (5 mm @ 90% isodose) | Feasibility of SOBP beam using a synchrocyclotron | IC, FC, MC simulation, and EBT‐XD RCF |
| 2021 | Electron 34 | Varian Clinac 2100 C/D (USA) | 10 | 240–260 | 0.81 Gy/pulse | 360 | Ø 1–1.5 cm | Feasibility of UHDR at the machine's isocenter | EBT‐XD RCF |
| 2021 | Proton 35 | Research isochronous cyclotron (Germany) | 68 | 75 | N/A | 20 MHz | Ø 1.3 cm | Preclinical setup for mouse irradiation | IC and RC |
| 2021 | Proton 36 | COMET 38 isochronous cyclotron (Switzerland) | 170—250 | 9000 (for a single spot) | N/A | 72.85 MHz | ∼2.3–5 mm (16 × 1.2 cm2 by scanning) | Feasibility study | FC |
| 2021 | Helium ion 26 |
Synchrotron (Germany) |
145.74 MeV/u | 185 | N/A | Quasi‐continuous | 1 cm2 (by spot scanning) | In vitro study of dose, LET, and O2 concentration | Parallel‐plate IC |
| 2021 | Carbon ion 27 | Synchrotron (Germany) | 280 MeV/u | 70 | N/A | Quasi‐continuous | 1 cm2 (by spot scanning) | Dosimetry and in vitro study | IC and EBT3 RCF |
Abbreviations: FC, Faraday cup; FWHM, full‐width at half‐maximum; IC, ion chamber; LET, linear energy transfer; MC, Monte Carlo; RCF, radiochromic film; TLD, thermoluminescent dosimeters; UHDR, ultra‐high dose rate.
To support a reliable clinical translation of FLASH RT, the challenges related to accurate dosimetry and real‐time beam monitoring of UHDR must be properly addressed. Although several preclinical studies have been carried out to investigate and confirm the FLASH effect, results may still be partially affected by the lack of established methods for dose measurements and beam monitoring. These methods currently used in conventional RT are employing detectors which can be heavily affected by the dose rates and dose per pulse characterizing UHDR beams. In particular, the lack of real‐time in‐beam transmission detectors able to accurately monitor the dose delivery, can partially limit the full exploitation of recently installed facilities, introducing non‐negligible uncertainties in the obtained results. Unlike dosimetry of conventional RT for which professional societies' recommendations are available (e.g., American Association of Physicists in Medicine's [AAPM's] Task Group [TG]‐51, 28 International Atomic Energy Agency's TRS‐398 29 ), currently there is no such recommendation available for FLASH RT dosimetry. This special issue of Medical Physics aims to highlight aspects of established and emerging dosimeters used in UHDR dosimetry and relevant for FLASH RT. In this paper, our aim is therefore to critically assess these dosimeters to highlight limiting characteristics that could affect their performance when used for FLASH RT dosimetry. Various available dosimeters are assessed and the considerations associated with them for use in FLASH dosimetry are mentioned.
2. CONSIDERATIONS BASED ON THE BEAM TIME STRUCTURE
Table 1 indicates the different technologies able to deliver UHDR beams for FLASH RT studies. The average dose rates of the related acceleration systems satisfy the main physical requirement to enable the FLASH effect (≥40 Gy/s). However, the deliverable treatment dose range, dose‐rate per pulse, and repetition frequency, as well as, pulse duration, characterize each individual irradiation facility, depending on the specific accelerator utilized. This list of characteristics is crucial, from the point of view of active dosimeters and real‐time detectors for beam monitoring. The saturation effects on the detector response and the required temporal resolution can drastically change depending on the beam time structure, which can vary from continuous to pulsed beams.
Examples of typical beam time structures are schematically illustrated in Figure 1. The radiation output structure for an isochronous cyclotron (Figure 1a) is quasi‐continuous, with nanosecond pulses spaced by tens of nanoseconds (i.e., with pulse frequency of the order of tens or hundreds of MHz). Figures 1b and 1d‐e show that in a synchrocyclotron or a particle linear accelerator (i.e., clinical linacs), the output has a distinct and very different pulse duration. Pulses are of the order of 1–10 µs wide and are delivered every few milliseconds, with a consequent repetition rate ranging between 100 and 1000 Hz. Strictly speaking, the time structure of pulses produced by accelerators is characterized by "micro‐pulses" of few nanoseconds and ∼100 MHz in repetition frequency. 40 However, this sub‐structure is effectively not detectable by ionization chambers, the recommended detectors for reference dosimetry, and also by most of the active dosimeters. Common ionization chambers used for clinical radiation dosimetry have ion collection times varying between tens of microseconds and 200 µs. As such, all the mentioned beams can be considered as being pulsed radiation sources, with the exception of the output of isochronous cyclotrons (and of course synchrotrons), which from this perspective can be considered as being continuous radiation sources.
FIGURE 1.
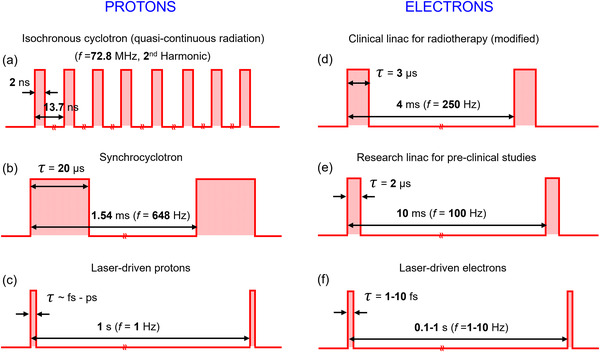
(a–f) Beam time structure and pulse duration (τ) for various accelerators delivering ultra‐high dose rate (UHDR) beams. Adapted with addition from Ref. 24
A quite different and peculiar beam time structure characterizes laser‐driven beams (Figures 1c and 1f), for which temporal pulses of less than 1 ns are achieved with single‐shot irradiations or with repetition rates ranging between 1 and 10 Hz.43 However, for these emerging technologies, additional challenges related to the beam stability and uniformity and energy selection must be considered. Investigations of the FLASH effect through these modalities are considered less mature relative to the studies carried out using radiofrequency accelerators.
It is evident from Figure 1 and the associated discussion above, that we must distinguish between average dose rate, instantaneous dose rate, and dose per pulse, as each of these parameters may play a role in the radiobiological aspects of the FLASH protective effects, in addition to the expected impact on the radiation detector response. In order to fully understand and exploit FLASH RT it may well be necessary to unfold the relative contribution of each of the above parameters in each radiation environment separately.
For a (quasi‐) continuous radiation, the (average) dose rate is defined according to Equation (1):
| (1) |
where D is the total delivered dose over a time period t.
For a pulse output, however, the average dose rate is defined according to
| (2) |
in which N is the number of delivered pulses and f is the pulse repetition rate. The instantaneous dose rate is therefore obtained from Equation (3):
| (3) |
in which τ is the pulse duration.
Calculation of dose rate for scanning beams is not straightforward due to the spatial heterogeneity of the dose rate. In such dose delivery scenarios, a voxel‐specific metric can more accurately specify the dose rate. 41 , 42
The FLASH effect may depends on the instantaneous dose rate (dose‐per‐pulse and number of pulses), the average dose rate, or a combination of the two. Ideally, a real‐time dosimeter capable of instantaneous dose rate measurement is needed in order to investigate the role of instantaneous dose rate versus average dose rate in the FLASH irradiation protective effect. Such dosimeters will need to incorporate a very wide dynamic range in their response to accurately characterize the temporal and spatial components of FLASH RT.
3. CONSIDERATIONS ON BEAM MONITORING, DOSIMETRY, AND DETECTOR FEATURES
Real‐time beam monitoring and reference dosimetry often share similar radiation detection challenges for dealing with UHDR beams, but are usually characterized by different requirements. Some radiation detection technologies can, however, be used for both purposes, if properly designed. A typical example is provided by gas‐based detectors. Ionization chambers are clinically the detectors for reference dosimetry, and recommended by international protocols. At the same time, a different design of ionization chambers is used in transmission for real‐time beam monitoring. The challenges coming from high and ultra‐high dose rates require major modifications of the currently used ion chambers and their readout systems. This is especially the case for extremely pulsed radiation beams, where the exploitation of alternative techniques, relying on different technologies for the two different purposes, is necessary.
In particular, the main additional requirements for a beam monitoring detector with respect to the ones used for reference dosimetry are: (i) high spatial resolution for beam flatness and symmetry measurements; (ii) high temporal resolution, as these detectors are typically providing the feedback signal to the control system of the accelerator for the beam stop; (iii) high level of beam transparency, as monitors must perturb the beam transport as little as possible; (iv) large response dynamic range, as demanded by modern treatment regimes with complex fields; (v) reduced footprint, as they are typically installed at the exit of the acceleration system; (vi) a large area to cover the whole beam spot cross‐section that, depending on the acceleration and beam delivery systems, can sensibly vary between a few centimeters and several centimeters; (vii) radiation hardness, since the detectors are a permanent fixture integral to the delivery system and always exposed to radiation. All the above‐mentioned requirements must be satisfied, as is the case of beam monitors for conventional RT, but the high dose rates characterizing FLASH RT makes the task even more challenging to satisfy. The response saturation issues affecting transmission detectors are similar to those for reference dosimetry detectors. However, specific to transmission detectors, is the required fast‐feedback signal, which can become an issue given the timing structures of UHDR beams. As described in the previous section, the beam timing structures can vary considerably, depending on the type of machine being used. The timing response of ionization chambers is typically quite slow (up to 300 µs of ion collection time for about half centimeter air gap) and these chambers are also affected by ion recombination in FLASH RT regimes. Novel prototypes of thin two‐dimensional (2D) in‐transmission ionization chambers have been developed and investigated for FLASH proton beams accelerated by cyclotrons, where the timing structure and dose per pulse still allow the use of suitably modified, yet robust technology. One such modification is a reduction of the air gap to 1 mm, which has been implemented to allow for a reduction in the ion recombination effects (<0.5% charge recombination) at the proton beam current relevant for FLASH irradiations, while still providing high spatial resolution (<100 µm) thanks to the strip electrodes. 44
Another approach for beam monitoring through in‐transmission ionization chambers was recently explored and tested with UHDR carbon ion beams accelerated by a synchrotron. Modeling the ion recombination to try to correct for it is not feasible especially when dealing with non‐reproducible intensity fluctuations from the synchrotron, and, if pencil beam irradiation must be implemented, due to the high local dose rate. Minimizing as much as possible the recombination in the in‐transmission chamber is, therefore, the only possible approach. This has been done by replacing the Ar–CO2 gas mixture present in the chamber gap with helium, which produces collected currents much smaller than that for Ar–CO2. 27 Tinganelli et al. 27 show that at UHDRs, the saturation curve for helium reaches the plateau at 500 V, providing a linear response at 1000 V, differently from the case of Ar–CO2 curve, which is still increasing at 2000 V. This plateauing is due to the much lower density of the electron‐ion pairs produced, and the much faster drifting time for helium ions. They found that the monitored dose values at UHDR and conventional rate during the irradiation of a series of biological samples were in agreement, with a constancy of better than ±1%. An interesting alternative approach to be further investigated for real‐time monitoring through gas‐based detectors is the use of double‐ or multi‐gap in‐transmission ionization chambers, as proposed by Giordanengo and Palmans. 45 They developed a device composed by two or three parallel‐plate ionization chambers with independent anodes and cathodes separated by gaps of different thickness to have different charge recombination effects and therefore different charge collection efficiencies. Due to recombination effects, the charge collected by each chamber is expected to deviate from the gap width proportionality and this can be modeled through a phenomenological approach that can finally correct for the collection efficiency.
Alternative technologies must be and are being investigated, especially for pulsed beams, such as the particle linear accelerators for RT, because the lower pulse repetition frequency implies larger doses per pulse to achieve similar average dose rates. In‐transmission ionization chamber would suffer large ion recombination effects under these irradiation conditions, and one alternative is solid‐state detectors, which are being proposed to address this limitation. In particular, ultra‐thin (<10 µm thickness) silicon detectors are explored, thanks to their high sensitivity and excellent spatial resolution, although thin planar silicon devices have never been used so far as online monitoring systems on clinical therapeutic beam lines. 46 Other solid‐state detectors that show promising features are being developed for UHDR applications based on diamond and silicon carbide (SiC) technology, although a systematic characterization under full clinical conditions with UHDR beams is yet to be carried out. Indeed, electron–hole pair recombination effects and other sources of response saturation in solid‐state detectors need more systematic studies at the dose and dose‐rate per pulse of interest for FLASH RT. 47
Other emerging alternative and robust technologies include the integrating current transformers (ICT), which provide charge measurements with high level of accuracy and reproducibility, as shown in Ref. 48 However, they cannot provide any information on beam flatness and symmetry as the final output is basically a beam fluence measurement.
A detector is considered a radiation dosimeter if it can provide a reading that is a measure of the delivered dose by ionizing radiation in its sensitive volume. If the dose can be determined without calibrating it in a known irradiation field, the device is considered an absolute dosimeter, otherwise it is referred as a relative dosimeter. Measuring the dose in water under specific reference conditions provides the output calibration of a clinical beam and is referred to as reference dosimetry. An absolute dosimeter can be used independently, that is, relying on its own accuracy. However, it is more common and safe in clinics to calibrate them in a known radiation field to have traceability to a Standards Laboratory. An ideal dosimeter for reference dosimetry should be dose, dose rate, energy, and angular independent, with high radiation hardness, linearity, accuracy, and precision. It is important to note that any possible UHDR dosimeter must also provide precise and accurate measurements under conventional dose rates. Ionization chambers are recommended by the international protocols for reference dosimetry in RT. 29 However, at very high dose rates their response is heavily affected by the ion recombination, as highlighted in the previous section, and demands major modifications of current commercially available ion chambers and the assessment of novel alternative dosimetry techniques. Some passive detectors may offer high accuracy and reproducibility, but active detectors provide less complicated and time‐consuming measurements, although the accuracy and dose‐rate independence require further improvement. 49 It is worth noting that although accurate real‐time dose‐rate independent absorbed dose‐to‐water measurements are required for reference dosimetry for FLASH RT, no specific requirement in terms of temporal resolution, is needed, unlike that for beam monitoring detectors.
In the immediate future, many radiobiological, chemical, and preclinical studies will be carried out, and are necessary to investigate the basis of the FLASH effect. In addition, the clinical transfer of FLASH RT will necessitate numerous clinical trials as well. In this decisive context, it will be necessary to compare the treatment efficacy under UHDR irradiation with conventional irradiation conditions. Therefore, it would be very beneficial, if not essential, to use detectors that have an adequate dynamic range, covering the broad range of dose‐per‐pulse and dose rates utilized in these studies. Such a requirement makes it not only possible to use the same device for conventional and UHDR irradiations, but also to carefully extrapolate the calibration performed under conventional conditions to nonstandard fields. When National Metrology Institute (NMI) will be able to provide reference UHDR beams, this requirement will be obsolete, and more routine‐like dosimetry will be possible.
Table 2 summarizes the characteristics of different detectors used for dosimetry and beam monitoring, their main performances and limitations.
TABLE 2.
Characteristics of different types of dosimeters
| Dosimeter | Real time | In vivo dosimetry | Absolute/reference dosimetry | Beam monitoring | Spatial resolution | Temporal resolution | 2D dosimetry | Accuracy at conventional dose rates a | Other considerations |
|---|---|---|---|---|---|---|---|---|---|
| Ion chamber | Yes | No | Yes | Yes | Several mm | 10–200 µs | Array | 1%–2% | Significant ion recombination at UHDRs |
| Semiconductor | Yes | Yes | No | Yes | Sub‐mm (or µm) | 1–10 ns | Yes | 2%–5% | Angular dependency, radiation damage, LET dependence |
| TLD | No | Yes | Yes | No | Several mm | N/A | No | 3%–10% | Energy dependence, time consuming, LET dependence |
| OSLD | No | Yes | Yes | No | Sub‐mm to mm | N/A | Array | 3%–5% | Energy dependence, quenching in high LET fields |
| Scintillator | Yes | Yes | Potentially | Potentially | Sub‐mm to mm | ns to µs | Array and sheet | 3%–5% | Quenching in high LET fields, Cherenkov radiation |
| Gas scintillator | Yes | No | No | Yes | Sub‐mm | N/A | Yes | 1% | Beam centroid measurement |
| Calorimeter | Yes | No | Yes | No | cm to several mm | ms–10 ms | No | <1% at the primary standard level |
Bulky, not easy to use, correction factors, time consuming |
| Film | No b | Yes | Potentially | No | Tens of µm | N/A | Yes | 3%–5% | Quenching in high LET fields |
| Fricke | No | No | Yes | No | cm to sub‐mm | N/A | Potentially | <1% at primary standard level | Time consuming, complexity |
| Faraday cup | Yes (for charge measurements) | No | Yes | No | N/A | <µs | No | 2%–5% for commercial devices; 1%–2% for dedicated equipment c | Measures the total collected charge (other detectors are required for dose determination) |
| Nuclear track detector | No | Yes | No | No | mm; sub‐mm with specialized equipment | N/A | Yes | 5%–7% | Time consuming, energy dependence, LET dependence |
| Alanine | No | Yes | Yes | No | mm | N/A | No | 2%–7% for doses larger than 10 Gy | Decreased accuracy for doses less than 10 Gy (minimum 2 Gy) |
| Integrated current transformer | Yes | Potentially | No | Yes | N/A | sub‐µs | No | <1% for charge measurements | Lack of 2D measurements, only charge measurements |
Abbreviations: LET, linear energy transfer; OSLD, optically stimulated luminescence dosimeter; TLD, thermoluminescent dosimeter; UHDR, ultra‐high dose rate.
Only accuracy for conventional dose rates has been indicated, as systematic studies for UHDR beams are still on‐going and final uncertainty budget for them is not yet fully established.
Some investigations 50 have been done on real‐time film dosimetry.
Main source of uncertainty coming from effective area and energy spectrum measurements, required for the dose determination, as described in Section 3.5.
3.1. Ionization chambers
Ionization chambers are considered as the "gold standard" for absolute and reference dosimetry in RT. 51 However, at UHDRs, they must be used with extreme caution; the user must evaluate the validity of the Boag's two‐voltage method or other empirical methods in handling the ion recombination in a given radiation field. 52 , 53
For electron FLASH beams, large correction factors were reported in multiple studies to account for recombination in the sensitive volume. 52 , 54 , 55 , 56 , 57 , 58 The Advanced Markus was the ionization chamber mainly studied in the context of electron beams thanks to its small sensitive volume (5 mm surface diameter and 1 mm spacing between electrodes) and large maximum dose per pulse with respect to cylindrical ionization chambers. For example, a Farmer ionization chamber is able to measure accurately doses per pulse up to 0.91 mGy without saturating whereas the Advanced Markus ionization chamber can be used for up to a dose per pulse of 5.56 mGy (99% collection). 52 However, recombination factors larger than 3, with associated large uncertainties, were reported for very high dose per pulses commonly used in FLASH studies. Additionally, the lack of universally accepted models is still an issue and empirical models are still used. 52 Despite these large recombination factors, ionization chambers remain the instrument favored for the clinical translation of FLASH RT and recent studies tried to overcome these limitations. For example, a study investigated the ion recombination for plane‐parallel ionization chambers with electron beams at 200 MeV and dose per pulses up to 5 Gy, retrieving the related correction factor through direct comparison with absolute dose measured through a graphite calorimeter and comparing it with the one obtained using different models. 54 A collection efficiency lower than 10% was found at the highest dose per pulse values for a PTW Roos chamber at the recommended voltage (200 V). In the same study, the authors proposed to optimize charge collection by increasing the polarizing voltage in the sensitive volume and by redesigning the chamber using different chamber geometries with smaller electrode spacing or cylindrical cavity shape. Another reported study investigated the impact of a change in the sensitive volume and created ionization chambers with a reduced distance between the electrodes of a parallel‐plate chamber. 56 That study showed that the ion collection efficiency was significantly improved when the distance between electrodes was reduced to 0.5 mm thanks to a 95% collection efficiency with a dose per pulse of about 1 Gy, which was significantly better than with the Advanced Markus (95% collection efficiency with a dose per pulse of about 0.2 Gy). 52 However, this reduction of sensitive volume is limited by mechanical constraints where small fluctuations in the chamber geometry from day‐to‐day could impact significantly the ionization chamber response.
Another feasibility study has been recently presented, where ultra‐thin plane‐parallel chambers prototypes with a 0.27 mm gap polarized at ‐250 V have been realized and characterized with low‐energy electron beams (up to 9 MeV) and at large doses per pulse (up to 12 Gy/pulse). A linear response was found as a function of the dose per pulse, demonstrating how ultra‐thin gap plane‐parallel ionization chambers are a promising option as secondary standard dosimeters at UHDRs for the FLASH RT quality assurance (QA). 59 An alternative approach was also investigated by Di Martino et al., 60 who found a method for experimentally determining the saturation correction factor k sat for a commercially available ionization chamber (a PTW Advanced Markus in the considered case). Starting from an approach developed and reported in Ref., 53 they retrieved the free electron fraction contribution and were able to accurately determine k sat for doses per pulse up to 0.5 Gy. 60 For higher doses per pulses, the large charge density generated, modifies the electric field inside it, so that in some points it assumes very high values (causing discharges) and in others null values (totally recombining). Di Martino et al. 61 have developed a theory that allows the realization of a gas chamber prototype that solves the problems described above, allowing to perform accurate measurements (within 3%) of absolute dose up to values of 40 Gy/pulse. The theory has been patented and the chamber prototype is in under construction.
The response of four different chambers (IBA PPC05, PTW Advanced Markus, IBA CC04, and IBA CC13) has been characterized at a proton FLASH (dose rate ∼150 Gy/s) radiation field generated by a synchrocyclotron. 25 The authors used Equations (4)–(6) to calculate the ion recombination factors. It was found that the cylindrical chambers (IBA CC04 and CC13) show significantly high ion recombination which cannot be correctly addressed using the standard two‐voltage method. For the plane‐parallel chambers studied in that work, the PPC05 showed the smallest correction factor (1.01) while that of the Advanced Markus chamber was 1.05 calculated using the two‐voltage model and quadratic fitting (see Figure 2). Using an empirical method, by delivering the same dose but at a low and FLASH dose rate, the authors showed that the ion recombination correction for the PPC05 was very close to that obtained through the two‐voltage method, however, for the Advanced Markus chamber it was ∼2% lower than that obtained through the two‐voltage method. This observation was attributed to different electrode separation in these chambers, that is, 0.6 mm in the PPC05 versus 1 mm in the Advanced Markus chamber. It should be mentioned that the Advanced Markus chamber has been reliably used for dosimetry of a FLASH proton field generated by an isochronous cyclotron, indicating the importance of the influence of the instantaneous dose rate and pulse structure on the response of ionization chambers. 22 The polarity correction factor was ∼1 for the plane‐parallel chambers; however, it was ∼1.1–1.2 (see Figure 2) for the cylindrical chambers studied in Ref.25 Patriarca et al. 20 have reported recombination correction factors ∼1% for the IBA CC01 cylindrical chamber in a FLASH proton field (∼80 Gy/s) generated by an isochronous cyclotron. This different behavior can be attributed to the different pulse structure of an isochronous and a synchrocyclotron. The former with a quasi‐continuous output has a significantly lower dose per pulse compared to the latter with a pulsed output (cf. Figure 1).
FIGURE 2.
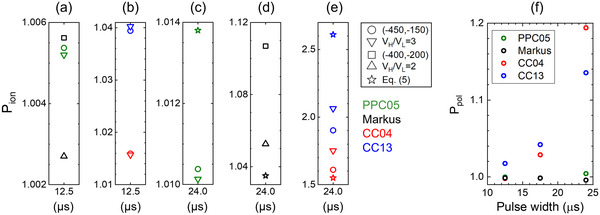
(a–e) Ion recombination correction factors calculated for different chambers for the spread‐out Bragg peak (SOBP) beam. Circle and square symbols were calculated using Equation (4), triangle symbols were calculated using Equation (5), and star symbols were calculated using Equation (6). V H/V L = 2 in the legend indicates that Equation (5) was used with (‐400, ‐200) voltage pair for the Advanced Markus chamber. V H/V L = 3 in the legend indicates that Equation (5) was used with (‐450, ‐150) voltage pair. 25 (f) Polarity correction factor for four different chambers irradiated by a proton beam, at 2–150 Gy/s dose rate, generated by a synchrocyclotron 25
Equations (4) and (5) which rely on measurement of a given beam at two different bias voltages of ion chambers are conveniently used to measure the ion recombination correction factor at conventional dose rates.
| (4) |
| (5) |
in which a 0, a 1, and a 3, are the fitting parameter provided in Ref. 62
Additionally, one can use a different method. When the same dose is delivered at two different dose rates (low and high), the measured dose at the low dose rate is considered as the ground truth since it can be reliably measured at the low dose rate. Then, the P ion at the high dose rate can be calculated according to Equation (6).
| (6) |
in which subscripts F and C represent FLASH and conventional dose rate, respectively, and:
| (7) |
Volume averaging in dosimetry of spatially nonuniform fields is an important issue in FLASH RT studies as well as in conventional RT. Indeed, most of preclinical studies carried out so far are performed with small fields, often characterized by poor uniformity. The implications of this effect on dosimetry have been investigated through Monte Carlo simulations, using a nonuniform proton beam in combination of commonly used ionization chambers for reference dosimetry, such as PTW Advanced Markus and IBA PPC05. 25
3.2. Radiochromic film
Radiochromic films (RCFs) provide 2D dosimetry over a large area with high spatial resolution which make them convenient dosimeters for beam profile measurement. Furthermore, tissue equivalency and ease‐of‐use are other appealing features of RCFs. 63 , 64 , 65 , 66 However, standard practice of RCF dosimetry does not provide real‐time measurement. Currently, most commonly used RCF models are EBT3 and EBT‐XD (GAFchromic, Ashland Inc., Bridgewater, NJ, USA) 64 , 65 with different dynamic ranges, up to 10 Gy (40 Gy) for the EBT3 (EBT‐XD). Since FLASH RT involves radiation doses between 2 and 30 Gy, the EBT‐XD model with high dynamic range would most likely be the natural choice. At conventional dose rates, the response of these films to megavoltage photon, electron, and proton beams were found to be dose‐rate independent. 62 , 64 , 65 , 67 In the context of FLASH dosimetry, overall 5% uncertainty in RCF dosimetry at high dose rates has been reported in the literature for electron film dosimetry using EBT3 film model. 68 Recently, the response of proton‐irradiated EBT3 films were reported to be dose‐rate independent (see Figure 3) when irradiated under 5 and 40 Gy/s using an isochronous cyclotron. 20 However, an important issue associated with film dosimetry in proton and other high linear energy transfer (LET) fields is the "quenching" effect manifested as under‐response of the films. The LET dependency of the response of the film can be manifested as an under‐response (∼10%) in dose in conventional dose rates. 62 , 67 Its impact on proton FLASH fields generated by systems with different pulse structures requires further investigation.
FIGURE 3.
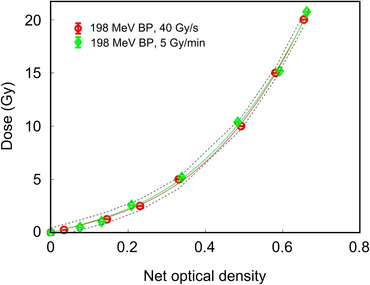
Dose–response curves obtained from the red channel of the EBT3 films irradiated by 198 MeV proton beams at 40 Gy/s and 5 Gy/s dose rates. Error bars are within the symbol size and the dashed curves represent 1‐sigma confidence level 20
In practice, RCF dosimetry is a cost‐effective solution. To improve film overall uncertainty, a high‐quality scanner providing stable and isotropic illumination is needed. Batch‐specific calibrations and care about film orientation are also typical improvements required for a 5% standard uncertainty. In addition, good care must be taken with the image formatting and filtering, in order to reproduce perfectly the calibration procedure.
3.3. Semiconductor electronic dosimeters
Dosimetry in FLASH RT presents an excellent developmental opportunity for semiconductor electronic dosimeters (SEDs) as these therapies incorporate dose rates that can be challenging for traditional SED systems. The impact of charge recombination is usually characterized by investigating their response per pulse of delivered dose. 47 , 69 The variation of the charge recombination effects at ultra‐high dose rates are analogous to those in ionization chambers, but are accentuated by the smaller work‐function. The introduction of guard ring structures sometimes utilized in lower dose rate environments to improve the statistical uncertainty in these systems requires careful design consideration in FLASH RT environments.
The radiation response of SEDs relative to tissue (or water equivalence) compounds the UHDR challenges for these systems in some radiation environments (e.g., microbeam RT). The higher dose rate environment does however allow for the reduction in the sensitive volume of the SED without compromising dose measurement uncertainty. The reduction also reduces the relative contribution from photoelectric interactions within the sensitive volume and take advantage of the stopping power ratio relative to water. Totally isolating the sensitive volume through chemical/laser etching techniques may yet also prove to play an important role in UHDR dosimetry using SEDs as the surrounding material removed can be replaced with a more tissue equivalent substitute. The introduction of alternate semiconductor technologies such as wide bandgap SEDs relative to silicon (e.g., diamond) or organic‐based semiconducting materials with an atomic number close to that of water or tissue also present future opportunities for dosimeter developments specifically for FLASH RT. In particular, diamond Schottky diode prototypes, produced at Rome Tor Vergata University in cooperation with PTW Freiburg, were realized varying a few relevant design and electronic parameters, in order to tune the overall device performance to meet the stringent requirements of ultra‐high dose per pulse irradiation. The resulting prototypes were tested by using an ElectronFlash Linac (SIT S.P.A.), by using a 9 MeV electron beam and doses per pulse up to 12.5 Gy/pulse. 70 Results show a response linearity up to a dose per pulse of 12.5 Gy/pulse in the best case, clearly demonstrating the feasibility of a diamond‐based detector at UHDRs for FLASH RT and paving the way for further related developments. Transmission‐type SEDs play an important role in real‐time in vivo QA for both photon 71 and particle therapies. 72 The introduction of FLASH RT treatment delivery techniques may necessitate the redesign of such transmission SEDs so they are application specific. The recent developments in back‐etched SED arrays and 3D p–n junction technology may well play an important role where speed is the essence in the ultra‐high dose rate environments associated with FLASH RT. SiC detectors could be a promising alternative, especially for beam monitoring at FLASH regimes. They can be considered a good compromise between the industrial maturity of silicon detectors and the robustness of diamonds, allowing for large areas and high applied voltages. Moreover, the possibility of realizing ultra‐thin SiC (1–10 µm of thickness) without the presence of substrate and to increase the applied electric field, potentially reduces possible hole–electron recombination effects. Although some preliminary not yet published investigations are on‐going, a systematic study of this novel technology at FLASH regimes is still missing. 73 The response of novel ultra‐thin SiC detectors was studied for the first time, through simulations and experimental characterization with low‐energy UHDR electron beams. Measurements with the electrons at 7–9 MeV at conventional and UHDR regimes with the SIT‐Sordina ElectronFlash Linac have been carried out by Romano et al. 74 and the response of SiCs was compared with a commercial silicon diode. A characterization of the SiCs was previously done to select the applied voltage for which the produced charge was properly collected (V = 480 V). Preliminary results showed that SiC detectors have a linear trend up to about a few Gy/pulse, differently from the silicon diode, which started saturating at less than 0.5 Gy/pulse. The authors state that the linearity range can be further extended using a dedicated electrometer able to cope with the instantaneous produced currents in the detector. These first promising results demonstrate the feasibility of using SiC detectors at UHDRs, paving the way for further future developments, including arrays or pixel configurations to be implemented for real‐time beam monitoring or relative dosimetry.
3.4. Fricke dosimeter
Fricke, ferrous ammonium sulfate, detectors are chemical dosimeters based on the oxidation of ferrous ions (Fe2+) to ferric ions (Fe3+) after interaction with ionizing radiation. One of the advantages of the Fricke dosimeters relies on the fact that they are 96% water by weight, which means their dosimetric properties are very similar to those of water. Fricke dosimeters can be used as absorbed dose‐to‐water primary standard for high‐energy electron beams through a two‐step procedure 75 , 76 : first, the energy imparted to the solution is derived from measurements of basic quantities, and second, calibrated Fricke solutions are irradiated in a water phantom to determine the calibration coefficient of secondary standard ionization chambers. As any other detector to be used at dose rate regimes typical of FLASH RT, the response on the key parameters must be investigated. In particular, the dependence of Fricke solutions of different compositions on the dose‐per‐pulse has been investigated in a few works. Using a standard solution an independence on the dose‐per‐pulse within the experimental uncertainties has been found up to about 2 Gy/pulse, starting to show some saturation effects (of the order of few percent) when 10 Gy pulses are used, 77 , 78 , 79 which is typically expected by UHDR studies. To obtain independent response at larger dose per pulse (at least up to 20 Gy) super‐Fricke solutions have been realized, where actually the solution is saturated with O2 solution and the Fe2+ solution is an order of magnitude higher than the standard one. The process for which their response start saturating for large dose‐per‐pulses is related to the oxygen depletion, which contributes to decrease the radiation chemical yield. Although Fricke dosimeters show promising features for their use at very high dose rates, still a systematic characterization has to be carried out, especially also looking at other relevant parameters for FLASH RT, such as the instantaneous dose rate and the pulse frequency that, depending on the irradiation modality, can vary considerably. In addition, metrology institute will need UHDR reference beams to improve the accuracy of the chain of traceability.
3.5. Faraday cup
Faraday cups (FCs) measure the total charge of a particle beam. Converting the integrated charge to dose requires accurate knowledge of energy spectrum of the beam. A vacuum‐less design of FC, designed by Cascio and Gottschalk, 80 with accuracy between –1% and –5% in charge collection, is portable and has been used in several FLASH RT investigations.
In recent studies, 25 an FC was placed immediately at the exit of a synchrocyclotron, with a pulsed output, to find the combination of pulse widths and number of pulses that would deliver the same dose but at different dose rates. By doing so, the authors were able to study the response of different ionization chambers at conventional and FLASH dose rates. Also, the results of the FC measurements were fed to the Monte Carlo simulation as the number of initial protons for absolute dosimetry.
The effect of secondary electrons in the total charge collected by the FC can be minimized by applying a magnetic field. Application of FCs, inspired by the design of Verhey et al., 81 for FLASH commissioning and QA has been studied by Winterhalter et al. 82
FCs have been also proposed for ultra‐high dose rates typical of laser‐driven proton beams. 83 , 84 , 85 Although the response in terms of collected charge is not affected by the very high dose rates, the measurement of the energy spectrum and effective area must to be carried out independently to determine the absolute dose, as the collected charge provides only a measurement of the proton fluence. The uncertainties related to the two mentioned quantities is typically having the largest contribution on the overall uncertainty on the absorbed dose‐to‐water, which can range between 2% and 5%, depending on the used technology. This can be improved using dedicated devices including specifically designed electron suppressors for the collected charge.
3.6. Radioluminescence and Cherenkov radiation dosimetry
Radioluminescence (RL) refers to phenomena that lead to generation of light as a result of interaction of radiation with materials. Utilizing scintillation properties of materials is one of the oldest techniques for ionizing radiation detection. Scintillation fiber optic dosimeters have drawn significant attention thanks to their unique features including capability of performing in vivo and real‐time measurements with high spatial and temporal resolutions. These features make them suitable candidates for various dosimetry scenarios including in vivo and small‐field dosimetry. 86 RL signal from the scintillating sensing tip of a fiber dosimeter is 86 proportional to the absorbed dose. However, the main challenge with scintillating fiber optic dosimetry in photon and electron beams is that the signal received by the detector through the fiber is "contaminated" with Cherenkov radiation, which may not be directly proportional to the dose. Hence, the total signal must be corrected to subtract the contribution of Cherenkov radiation in order to accurately measure the absorbed dose in the scintillator. Several solutions have been proposed in the literature to deal with the Cherenkov radiation in fiber optic dosimetry. 87 , 88 In proton therapy scintillation fiber optic dosimetry, the effect of Cherenkov radiation as a contaminating signal is not as significant compared to that in photon and electron fields due to the fact that the threshold energy for the protons to induce Cherenkov radiation is ∼1830 times greater than that of electrons (E min,p = 484 MeV vs. E min,e = 0.264 MeV in water). This energy is well above the proton energies used in proton therapy; however, electrons in the medium can acquire sufficient energy from the incident protons through different types of interactions to induce Cherenkov radiation. Nevertheless, it has been shown that the Cherenkov radiation produced by such secondary electrons is mostly limited to the shallow depths and its intensity is significantly weaker than that from the scintillator. 89 , 90
Another significant issue related to scintillation dosimetry that occurs in high LET radiation fields is the non‐proportionality between the collected light from the scintillator and the proton dose. 91 , 92 , 93 , 94 , 95 , 96 At low stopping powers, the scintillation signal is linear with respect to the energy deposition, however, the scintillation signal "saturates" at high stopping powers. This nonlinearity effect, manifested as under‐response of the optical signal to the radiation absorbed dose, is mainly due to the ionization quenching phenomenon resulting from non‐radiative de‐excitations occurring at high‐density energy deposition. 97 , 98 , 99 , 100
RL‐based devices can in principle provide a high‐resolution 2D measurement (e.g., arrays of optical fibers); however, their use must be verified in UHDR radiation fields. 101 Scintillation‐based dosimeters due to their prompt response can potentially be used for measuring both the instantaneous (dose per pulse) and average dose rates. Prototypes of LYSO scintillating crystals have been recently tested at doses per pulses up to 2.5 Gy with electron beams produced by a modified IORT Novac accelerator, showing an overall linear trend at the investigated values. 102 The obtained results demonstrate how inorganic scintillators are also good candidates for FLASH RT active dosimetry.
Novel approaches based on gas scintillators are a promising alternative for beam monitoring at UHDRs, thanks to the high level of transparency and fast response. A xenon gas scintillator coupled to large photomultiplier tubes (PMTs) for real‐time 2D beam monitoring for pulsed and pencil beam scanning proton RT treatments was used by Vigdor et al. 103 A spatial resolution of a few hundred micrometers was achieved, with a linear response within 1% up to a dose rate of 300 Gy/s. An alternative approach was investigated using air as a medium in which fluorescence is developed. Fluorescence in air provides a signal unsaturated by the high number of particles per pulse, typical for FLASH, with a very wide dynamic range. 104 Preliminary tests with electron UHDR beams are promising and further systematic characterization will be carried out.
Designs based on fiber arrays, due to the small (sub‐millimeter to millimeter) diameters of the fibers have potential to serve as beam monitors as well. Dose‐rate independence has been reported at conventional dose rates. Although some preliminary tests have been performed at UHDRs, further systematic investigations are needed to verify their response at FLASH beams generated by different types of accelerators (linac, isochronous cyclotron, and synchrocyclotron).
In the past decade, it has been shown that Cherenkov and scintillation imaging can in principle provide real‐time 2D dosimetry of conventional radiation therapy beams. 105 A Cherenkov detector was used for real‐time dose monitoring by Favaudon et al. 106 with electron beams up to 5 MeV with UHDR pulses of 1 µs. Results show that the integral Cherenkov emission was not affected by saturation effects, demonstrating the potential to be used at these extreme dose rate regimes. Their application in FLASH RT looks promising but requires further investigation.
3.7. Thermoluminescent dosimeter and optically stimulated luminescence dosimeter
Thermoluminescent dosimeters (TLDs) have been used for several decades in conventional RT. 107 The detection principle of these dosimeters is based on the trapping of electrons and holes between the valence and conduction band thanks to the presence of doped impurities. During irradiation, electrons move from the valence to the conduction band, which traps the electrons and holes at the level of impurities. For dose reading, electron–hole recombination is triggered with heat that acts as an external stimulus and results in the emission of visible photons. Dose can be extracted from the emitted light intensity.
TLDs have a long history of use with UHDR and their dose‐rate independency was demonstrated for electrons and photons up to dose rates within the pulse of about 109 Gy/s. 108 , 109 , 110 More recently, TLDs have been used as in vivo dosimeters to confirm dosimetry in FLASH preclinical studies such as mice whole brain irradiation. 111 TLDs were also used to investigate the dose‐rate dependency of RCFs 112 and their dose‐rate dependency was also compared to alanine and RCFs. 49
A drawback of the TLD is that they are read hours after the irradiation, typically 24 h, to avoid low‐energy trap release. In addition, accurate TLD dosimetry necessitate well‐established procedures and dedicated personnel to lower the uncertainties under 5%.
Optically stimulated luminescence (OSL) is an interesting technique that can be potentially used for both dosimetry and beam monitoring in FLASH irradiation environments. OSL dosimeters (OSLDs) are similar to TLDs in that they effectively integrate their irradiation response via internal trapping of the irradiation‐generated charge in the conduction band. 113 Unlike TLDs, the trapped charge is released to charge recombination centers when the system is illuminated by a visible light, leading to an OSL signal that is ideally, directly proportional to the radiation absorbed dose. 114 , 115 , 116
OSLDs are traditionally passive and readout post‐irradiation, which is quite suitable for dosimetry applications. More recently, active readout methods have emerged, which may find use in both dosimetry and beam monitoring applications. 117 , 118 , 119 , 120 , 121 While the dose rate response of OSLDs relevant to FLASH RT is yet to be fully tested, 114 , 122 fast readout and reliable dose measurement after repeat bleaching could alleviate dose rate effects. 123
The lower sensitivity of OSLDs to radiation was considered a disadvantage, however this may be useful in FLASH radiation environments. OSLDs have been shown to be linear over five orders of magnitude in radiation dose response up to 10 Gy in megavoltage photon fields, 117 , 124 with some supralinearity reported in kilovoltage X‐ray fields that is more pronounced as the photon energy is lowered. 125 , 126 OSLDs have also been utilized in particle therapy with encouraging results. 114 , 127 , 128
Recently, the response of OSLD in a pencil beam proton field for a wide range of dose rates up to 9000 Gy/s has been studied by Christensen et al. 129 It was found that the OSLD response is dose‐rate independent (see Figure 4).
FIGURE 4.
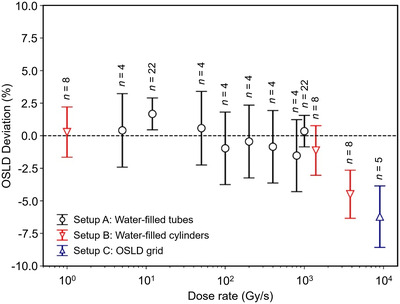
The optically stimulated luminescence dosimeters (OSLDs) dose measurement relative to the nominal dose as a function of the dose rate for the three setups studied by Christensen et al. 129 The number of aggregated data points for each dose rate is given above each marker. The appearance of an under‐response above 1000 Gy/s is due to signal averaging of the narrow pencil beam over the OSLDs and is not manifestation of a dose‐rate dependency
3.8. Alanine
Alanine is an amino acid and chemical dosimeter where stable radicals form after irradiation. The concentration of these stable radicals can be determined using electron paramagnetic resonance technique and is proportional to the delivered dose. This dosimeter is commonly used in the industrial context with high doses and is appreciated thanks to its low energy‐dependence. 130 The dose range of this dosimeter was extended and doses from 1 up to 105 Gy can be measured in electron and photon beams up to 30 MeV. 131
However, the accuracy for low doses decreases as the signal‐to‐noise ratio decreases too, leading to uncertainties of several percents. In that context, reading procedures have been optimized in this low dose range to provide fast and accurate measurement. 132 A dose‐rate dependency was highlighted for doses above 5 kGy but below that dose, these dosimeters have shown to be suitable for dose rates within the pulse up to about 1010 Gy/s and mean dose rate greater than 1 kGy/s, making them suitable for FLASH studies. 133 , 134 , 135 In that context, alanine has been used as an in vivo dosimeter for the first treatment of a human patient with FLASH RT. 136
3.9. Calorimeter
Calorimetry is the main method used for absorbed dose primary standards. 137 , 138 , 139 It provides a direct measurement of the dose‐to‐water, differently from other methods for which a reading must be typically converted to absorbed dose‐to‐water by multiplying by calibration coefficients and conversion factors. It is basically the only fundamental method of measuring the absorbed dose according to its definition. Calorimetry measures the temperature rise resulting from irradiation in an absorber—assuming all the energy deposited in a material appears as heat (i.e., there is no change in physical or chemical state of the absorber and all the ionizations eventually dissipate to heat). 140 If the specific heat capacity of the absorber is known then the energy deposited can be measured using the following equation:
| (8) |
where ΔE is the increase in energy, m is the mass of the absorber, c is the specific heat capacity of the absorber, and ΔT is the radiation‐induced temperature increase. To give some numbers, a delivered dose to water of 1 Gy leads to a temperature rise of approximately 0.2 mK. Therefore, obtaining an uncertainty lower than 1% requires the temperature to be measured with an uncertainty of 2 µK, which means that measurements at the room temperature are challenging.
The materials most widely used for absorbed dose‐to‐water calorimetry are water and graphite. Water has several advantages as a material for absorbed dose calorimetry, the most apparent of which is that using water eliminates the need for a dose conversion factor of the calorimeter material to water. However, practically, there are several drawbacks in comparison with using graphite. The presence of impurities in the water needs to be avoided, as they can lead to absorption and scattering of the radiation, whereas a graphite calorimeter can be made almost entirely of graphite. The specific heat capacity of water is higher than that of graphite, meaning the temperature rise in water is approximately six times less than the temperature rise in graphite for the same dose.
Absorbed dose calorimetry has been widely used for all the common clinical beam modalities covered in reference dosimetry codes of practice, as well as for small and nonstandard fields, and brachytherapy. Although originally developed at primary standard laboratories, absorbed dose calorimetry is not restricted to primary standard instruments and several developments are on‐going on this regard. 141 A few prototypes of smaller portable calorimeters have been designed and realized with the aim of making them suitable for measurements at the clinical facilities. 142 , 143 , 144 , 145
Regardless of the material, calorimeters have the advantage of having a response in terms of temperature rise which is independent of the radiation dose rate, indicating that they are good candidates for absorbed dose measurements at FLASH regimes, although some practical aspects need to be addressed. First experimental measurements with electron and proton beams at UHDRs have demonstrated the feasibility of using portable calorimeters at these extreme regimes, providing a reliable measurement of the absorbed dose‐to‐water with a reduced uncertainty compared to the ionization chambers which may be affected by large ion recombination effects. 54 Moreover, the first proof‐of‐principle measurement with laser‐driven proton beams at UHDRs has been recently carried out, demonstrating for the first time the possibility of using this promising technology in a laser environment. 146
Portable calorimeters are being further developed, employing the expertise on calorimetry acquired at some NMIs for a translation of these devises to a possible usage in a clinical environment. Absorbed dose calorimetry is a promising approach for UHDR beam dosimetry and new developments are expected on this regard.
3.10. Integrated current transformer
ICTs are devices designed to measure accurately the bunch charge of charged particle beams. ICTs have been reported to accurately measure the beam current and demonstrated their ability to be used as a beam monitoring system with absolute dosimetry at conventional dose rates. 147 ICTs also recently proved to be reliable tools to perform charge measurements of electron pulses on a linac traceable to primary standards at a national metrology institute. 48 In the context of FLASH RT, conventional transmission ionization chambers are not suitable tools to monitor the beam due to the high ion recombination effects occurring 148 and ICTs were cited as an alternative for monitoring FLASH beams. 149 They are planned to be tested on facilities providing FLASH beams in the context of the international project UHDpulse. 150
The main difference between ICTs relative to transmission ionization chambers commonly used in a clinical environment, is that ICTs are not able to provide information about the beam spatial distribution (flatness and symmetry) as requested by international standards to ensure the patient safety during delivery. However, thanks to their high accuracy in measuring the irradiation‐induced current and their prompt reaction, ICTs could be reliable instruments to quantify the linac output and improve dose accuracy in FLASH RT studies. Additionally, ICTs allow non‐perturbative measurements and provide information about the beam temporal structure, which might bring valuable information in the context of FLASH RT.
3.11. Nuclear track detectors
The application of nuclear track detectors (NTDs) 151 in FLASH therapies is buoyed by their ability to be used in high‐irradiation dose rate environments with successful testing up (but not limited) to 108 Gy/s quoted in the literature. 152 The linear dose response range of 0.5–5000 cGy is adequate for the QA of many clinical treatment scenarios and small (stereotactic) 2D treatment fields can be investigated by NTD wafers that are available up to 60 mm in diameter. The sub‐micrometer spatial resolution is excellent and ability to be reused after thermal annealing or optical bleaching makes them convenient to use. As they are an integrating passive dosimeter they are not able to be read out in real time, but can be used in vivo similar to OSLDs, TLDs, RCFs, etc. These detectors have been demonstrated as suitable for use in both particle therapies and intense photon therapies (e.g., microbeam RT), so represent an excellent candidate for benchmarking treatment plans, radiation transport simulation data, and QA in FLASH RT.
4. PRACTICAL CONSIDERATIONS: COMMISSIONING AND NEEDS FOR PROTOCOLS
In conventional RT, the international code of practice recommends performing and documenting the acceptance and commissioning procedure when validating a new RT machine, for example, a linear accelerator for clinical use. Such procedures define the reference absorbed dose‐to‐water for the reference geometry and provide reference values for any future QA program. This framework is based on the available calibrated instruments and adequate monitoring of the beam. As explained previously, the reference dosimetry of UHDR beams is challenging, which has direct consequences on commissioning of new facilities. The reference values of absorbed dose‐to‐water are certainly the first challenge, and will be solved when adequate reference beam qualities are available at the appropriate reference laboratories. On this regard, there are intense collaborative efforts worldwide to try to address the challenges related to dosimetry and beam monitoring of UHDR beams. In particular, the European project "UHDpulse" (metrology for advanced RT using particle beams with ultra‐high pulse dose rates) is a metrology focused program with the aim of developing a measurement framework, including traceable reference standards and validated reference methods for dose measurements with UHDR beams. 150 The UHDpulse project brings together leading European NMIs in the field of radiation dosimetry with leading universities, research institutes and academic hospitals in the field of RT and radiation detector developments. The project has also the aim of developing traceable and validated methods for relative dosimetry, characterising stray radiation, and contributing to international codes of practice.
Given the current status of detector development, it is safe to assume the challenges related to reference dosimetry and beam monitoring in FLASH RT environments will be addressed in the near future. However, there are still correction factors for the actual irradiation conditions, such as depth, energy, or dose rate from the reference conditions that remain a very significant challenge and will require guidance from an adequate code of practice as well as appropriate treatment planning systems. This is especially the case for users of linacs, because the UHDRs are far from conventional treatment dose rates. However, proton beams operating at UHDR are not that far from the already challenging requirements of proton therapy, especially in the case of pencil beam scanning systems. A few examples of commissioning of FLASH RT systems are reported in the literature, 22 , 153 , 154 but in the next years, many more centers will use UHDRs and will develop strategies to insure safe use of UHDR beams.
5. CONCLUSIONS AND FUTURE DIRECTIONS
Currently, there are neither guidelines for FLASH RT dosimetry nor recommendations for the choice of dosimeters. It signifies that there is a need for novel dosimeters that can be used as a ground truth in such ultra‐high dose rate environments. However, there is an unprecedented effort by the scientific community for the assessment of accurate dosimetric methods and standardized procedures, which are paving the way for the establishment of future reliable international protocols. The issues related to UHDR beams and the large doses per pulse typically characterizing FLASH RT studies, pose challenges which are being addressed following two different, but complimentary, approaches: (i) trying to address ion recombination issues in ionization chambers, which are considered the "gold standard" for absolute dosimetry in RT, by improving the collection efficiency through the optimization of the chamber design; (ii) investigating alternative approaches relying on active dosimeters, as much as possible dose‐rate independent. Passive detectors are also currently employed in several facilities for preclinical FLASH studies, although the establishment of dosimetric procedures through active detectors is unavoidable for clinical trials and future patient treatments. Fricke‐type dosimeters and calorimeters have shown dose‐rate independent behavior. 37 However, the implementation of these two dosimeters is not very straightforward and requires sophisticated equipment and a highly trained operator. Compact and more easy‐to‐handle calorimeters are being developed. A real‐time beam monitoring system is very desirable for FLASH RT QA, as well as to evaluate the beam flatness and symmetry. Traditional ionization chambers and diode‐based dosimeters are not the optimum choice for FLASH dosimetry due to their poor spatial resolution and significant dose‐rate dependency, respectively. RL‐based devices can provide a high‐resolution 2D measurement and other solid‐state detectors currently under development, such as SiC and amorphous silicon, can be considered promising alternatives. However, their use must be rigorously verified in these UHDR conditions.
It must be said that, according to the discussed challenges, a rather different impact on the approaches to be used for absolute and relative dosimetry and beam monitoring is found depending on the specific particle accelerator. Indeed, as shown previously, the different pulse duration, dose‐per‐pulse, and "instantaneous" dose rate imply drastically different levels of saturation effects on the considered detectors. Therefore, a combination of radiation quality and dose/dose‐rate per pulse will basically determine the dosimetric procedures to be followed, the latter strictly dependent of the specific beam time structure.
Finally, there are not yet validated and standardized protocols for UHDR beam dosimetry for FLASH RT and detectors for dosimetry and beam monitoring have not been fully established. However, a relevant number of national and international projects are being proposed with the aim of addressing the related challenges, such as the European UHDpulse project, dedicated to the metrology of UHDR beams and the assessment of traceable and validated methods for absolute and relative dosimetry. Moreover, a joint TG‐359 has been recently formed by the AAPM, the European Society for Radiotherapy and Oncology, and the European Federation of Organizations for Medical Physics charged with dosimetry aspects of FLASH RT.
All these initiatives are sensibly contributing, in a complimentary way, to support the clinical translation of FLASH RT, allowing for the delivery of robust dosimetric procedures relying on validated methods and established technologies.
CONFLICTS OF INTEREST
All the authors have no relevant conflicts of interest to disclose. The authors wish to acknowledge that in the interest of moving this exciting field forward, some mentioned detectors in the text are often used outside of the manufacturer's recommended operating conditions. The authors therefore would like to point out that there is no specific recommendation for any of the mentioned detectors, as the purpose of this manuscript is to provide a general description of the features, performances, and limitations of the discussed technologies for measurements at UHDR regimes. What is included in this manuscript is not an endorsement nor a criticism of any of the described technologies.
ACKNOWLEDGMENTS
The authors wish to thank their colleagues who contributed in this manuscript through informal discussions and exchange of views. CB and PGJ received support from the project 18HLT04 UHDpulse, which has received funding from the EMPIR programme co‐financed by the Participating States and from the European Union’s Horizon 2020 research and innovation programme.
Romano F, Bailat C, Jorge PG, Lerch MLF, Darafsheh A. Ultra‐high dose rate dosimetry: Challenges and opportunities for FLASH radiation therapy. Med Phys. 2022;49:4912‐4932. 10.1002/mp.15649
Funding information
The authors received no funding for this work.
REFERENCES
- 1. Durante M, Bräuer‐Krisch E, Hill M. Faster and safer? FLASH ultra‐high dose rate in radiotherapy. Br J Radiother. 2017;91(1082):20170628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harrington KJ. Ultrahigh dose‐rate radiotherapy: next steps for FLASH‐RT. Clin Cancer Res. 2018;25(1):3‐5. [DOI] [PubMed] [Google Scholar]
- 3. Maxim PG, Keall P, Cai J. FLASH radiotherapy: newsflash or flash in the pan? Med Phys. 2019;46(10):4287‐4290. [DOI] [PubMed] [Google Scholar]
- 4. Favaudon V, Caplier L, Monceau V, et al. Ultrahigh dose‐rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci Transl Med. 2014;6(245):245ra93. [DOI] [PubMed] [Google Scholar]
- 5. Montay‐Gruel P, Petersson K, Jaccard M, et al. Irradiation in a flash: unique sparing of memory in mice after whole brain irradiation with dose rates above 100 Gy/s. Radiother Oncol. 2017;124(3):365‐369. [DOI] [PubMed] [Google Scholar]
- 6. Vozenin M‐C, De Fornel P, Petersson K, et al. The advantage of FLASH radiotherapy confirmed in mini‐pig and cat‐cancer patients. Clin Cancer Res. 2019;25(1):35‐42. [DOI] [PubMed] [Google Scholar]
- 7. Montay‐Gruel P, Bouchet A, Jaccard M, et al. X‐rays can trigger the FLASH effect: ultra‐high dose‐rate synchrotron light source prevents normal brain injury after whole brain irradiation in mice. Radiother Oncol. 2018;129(3):582‐588. [DOI] [PubMed] [Google Scholar]
- 8. Pratx G, Kapp DS. Ultra‐high‐dose‐rate FLASH irradiation may spare hypoxic stem cell niches in normal tissues. Int J Radiat Oncol Biol Phys. 2019;105(1):190‐192. [DOI] [PubMed] [Google Scholar]
- 9. Vozenin MC, Hendry JH, Limoli CL. Biological benefits of ultra‐high dose rate FLASH radiotherapy: sleeping beauty awoken. Clin Oncol. 2019;31:407‐415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bourhis J, Montay‐Gruel P, Jorge PG, et al. Clinical translation of FLASH radiotherapy: why and how? Radiother Oncol. 2019;139:11‐17. [DOI] [PubMed] [Google Scholar]
- 11. Spitz DR, Buettner GR, Petronek MS, et al. An integrated physico‐chemical approach for explaining the differential impact of FLASH versus conventional dose rate irradiation on cancer and normal tissue responses. Radiother Oncol. 2019;139:23‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koch CJ. Re: differential impact of FLASH versus conventional dose rate irradiation: Spitz et al. Radiother Oncol. 2019;139:62‐63. [DOI] [PubMed] [Google Scholar]
- 13. Montay‐Gruel P, Acharya MM, Petersson K, et al. Long‐term neurocognitive benefits of FLASH radiotherapy driven by reduced reactive oxygen species. Proc Natl Acad Sci USA. 2019;116(22):10943‐10951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Simmons DA, Lartey FM, Schuler E, et al. Reduced cognitive deficits after FLASH irradiation of whole mouse brain are associated with less hippocampal dendritic spine loss and neuroinflammation. Radiother Oncol. 2019;139:4‐10. [DOI] [PubMed] [Google Scholar]
- 15. Jansen J, Knoll J, Beyreuther E, et al. Does FLASH deplete oxygen? Experimental evaluation for photons, protons, and carbon ions. Med Phys. 2021;48:3982‐3990. 10.1002/mp.14917 [DOI] [PubMed] [Google Scholar]
- 16. Abolfath R, Grosshans D, Mohan R. Oxygen depletion in FLASH ultra‐high‐dose‐rate radiotherapy: a molecular dynamics simulation. 2020;47(12):6551‐6561. 10.1002/mp.1454 [DOI] [PubMed] [Google Scholar]
- 17. Schuler E, Trovati S, King G, et al. Experimental platform for ultra‐high dose rate FLASH irradiation of small animals using a clinical linear accelerator. Int J Radiat Oncol Biol Phys. 2017;97(1):195‐203. [DOI] [PubMed] [Google Scholar]
- 18. Lempart M, Blad B, Adrian G, et al. Modifying a clinical linear accelerator for delivery of ultra‐high dose rate irradiation. Radiother Oncol. 2019;139:40‐45. [DOI] [PubMed] [Google Scholar]
- 19. Crosbie JC, Fournier P, Bartzsch S, et al. Energy spectra considerations for synchrotron radiotherapy trials on the ID17 bio‐medical beamline at the European Synchrotron Radiation Facility. J Synchrotron Radiat. 2015;22:1035‐1041. [DOI] [PubMed] [Google Scholar]
- 20. Patriarca A, Fouillade C, Auger M, et al. Experimental set‐up for FLASH proton irradiation of small animals using a clinical system. Int J Radiat Oncol Biol Phys. 2018;102(3):619‐626. [DOI] [PubMed] [Google Scholar]
- 21. Girdhani S, Abel E, Katsis A, et al. FLASH: a novel paradigm changing tumor irradiation platform that enhances therapeutic ratio by reducing normal tissue toxicity and activating immune pathways. In: AACR Annual Meeting 2019 Online Proceeding, Atlanta, GA. 2019.
- 22. Diffenderfer ES, Verginadis II, Kim MM, et al. Design, implementation, and in vivo validation of a novel proton FLASH radiation therapy system. Int J Radiat Oncol Biol Phys. 2020;106(2):440‐448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Beyreuther E, Brand M, Hans S, et al. Feasibility of proton FLASH effect tested by zebrafish embryo irradiation. Radiother Oncol. 2019;139:46‐50. [DOI] [PubMed] [Google Scholar]
- 24. Darafsheh A, Hao Y, Zwart T, et al. Feasibility of proton FLASH irradiation using a synchrocyclotron for preclinical studies. Med Phys. 2020;47(9):4348‐4355. 10.1002/mp.14253 [DOI] [PubMed] [Google Scholar]
- 25. Darafsheh A, Hao Y, Zhao X, et al. Spread‐out Bragg peak proton FLASH irradiation using a clinical synchrocyclotron: proof of concept and ion chamber characterization. Med Phys. 2021;48(8):4472‐4484. 10.1002/mp.15021 [DOI] [PubMed] [Google Scholar]
- 26. Tessonnier T, Mein S, Walsh DWM, et al. FLASH dose rate helium ion beams: first in vitro investigations. Int J Radiat Oncol Biol Phys. 2021;111(4):1011‐1022. 10.1016/j.ijrobp.2021.07.1703 [DOI] [PubMed] [Google Scholar]
- 27. Tinganelli W, Sokol O, Quartieri M, et al. Ultra‐high dose rate (FLASH) carbon ion irradiation: dosimetry and first cell experiments. Int J Radiat Oncol Biol Phys. 2022;112(4):1012‐1022. 10.1016/j.ijrobp.2021.11.020 [DOI] [PubMed] [Google Scholar]
- 28. Almond PR, Biggs PJ, Coursey BM, et al. AAPM's TG‐51 protocol for clinical reference dosimetry of high‐energy photon and electron beams. Med Phys. 1999;26:1847‐1870. 10.1118/1.598691 [DOI] [PubMed] [Google Scholar]
- 29. Andreo P, Burns DT, Hohlfeld K, et al. Absorbed dose determination in external beam radiotherapy: An international code of practice for dosimetry based onstandards of absorbed dose to water, IAEA Technical Reports Series No. 368, Vienna, 2006.
- 30. Slatkin DN, Spanne P, Dilmanian FA, Gebberst JO, Laissue JA. Subacute neuropathological effects of microplanar beams of x‐rays from a synchrotron wiggler. Med Sci. 1995;92:8783‐8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bourhis J, Sozzi WJ, Jorge PG, et al. Treatment of a first patient with FLASH‐radiotherapy. Radiother Oncol. 2019;139:18‐22. [DOI] [PubMed] [Google Scholar]
- 32. Zhang Q, Cascio E, Li C, et al. FLASH investigations using protons: design of delivery system, preclinical setup and confirmation of FLASH effect with protons in animal systems. Radiat Res. 2020;194(6):656‐664. 10.1667/RADE-20-00068.1 [DOI] [PubMed] [Google Scholar]
- 33. Engels E, Li N, Davis J, et al. Toward personalized synchrotron microbeam radiation therapy. Sci Rep. 2020;10:8833. 10.1038/s41598-020-65729-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rahman M, Ashraf MR, Zhang R, et al. Electron FLASH delivery at treatment room isocenter for efficient reversible conversion of a clinical Linac. Int J Radiat Oncol Biol Phys. 2021;110(3):872‐882. 10.1016/j.ijrobp.2021.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kourkafas G. FLASH proton irradiation setup with a modulator wheel for a single mouse eye. Med Phys. 2021;48(4):1839‐1845. 10.1002/mp.1473 [DOI] [PubMed] [Google Scholar]
- 36. Nesteruk KP, Togno M, Grossmann M, et al. Commissioning of a clinical pencil beam scanning proton therapy unit for ultra‐high dose rates (FLASH). Med Phys. 2021;48(7):4017‐4026. 10.1002/mp.14933 [DOI] [PubMed] [Google Scholar]
- 37. Favaudon V, Tourbez H, Houee‐Levin C, Lhoste JM. CO2 •− radical induced cleavage of disulfide bonds in proteins. A γ‐ray and pulse radiolysis mechanistic investigation. Biochemistry. 1990;29:10978‐10989. [DOI] [PubMed] [Google Scholar]
- 38. Schippers M, Dölling R, Duppich J, et al. The SC cyclotron and beam lines of PSI's new proton therapy facility PROSCAN. Nucl Instrum Meth Phys Res B . 2007;261(1‐2):773‐776 [Google Scholar]
- 39. Fournier P, Crosbie JC, Cornelius I, et al. Absorbed dose‐to‐water protocol applied to synchrotron‐generated x‐rays at very high dose rates. Phys Med Biol. 2016;61(14):N349. [DOI] [PubMed] [Google Scholar]
- 40. Kim MM, Darafsheh A, Schuemann J, et al. Development of ultra‐high dose rate (FLASH) particle therapy. IEEE Trans Radiat Plasma Med Sci. 2022;6(3): 252‐262. 10.1109/TRPMS.2021.3091406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Folkerts MM, Abel E, Busold S, Perez JR, Krishnamurthi V, Ling CC. A framework for defining FLASH dose rate for pencil beam scanning. Med Phys. 2020;47:6396‐6404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Van Marlen P, Dahele M, Folkerts M, Abel E, Slotman BJ, Verbakel W. Ultra‐high dose rate transmission beam proton therapy for conventionally fractionated head and neck cancer: treatment planning and dose rate distributions. Cancers. 2021;13:1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bolton PR, Parodi K, Schreiber J, eds. Applications of Laser‐Driven Particle Acceleration. 1st ed. CRC Press; 2020. ISBN 978042944510. 10.1201/9780429445101 [DOI] [Google Scholar]
- 44. Zou W, Diffenderfer ES, Ota K, et al. Characterization of a high‐resolution 2D transmission ion chamber for independent validation of proton pencil beam scanning of conventional and FLASH dose delivery. Med Phys. 2021;48(7):3948‐3957. 10.1002/mp.14882 [DOI] [PubMed] [Google Scholar]
- 45. Giordanengo S, Palmans H. Dose detectors, sensors, and their applications. Med Phys. 2018;45(11):e1051‐e1072. 10.1002/mp.13089 [DOI] [PubMed] [Google Scholar]
- 46. Vignati A, Giordanengo S, Fausti F, et al. Beam monitors for tomorrow: the challenges of electron and photon FLASH RT. Front Phys. 2020;8:375. 10.3389/fphy.2020.00375 [DOI] [Google Scholar]
- 47. Di Martino F, Barca P, Barone S, et al. FLASH radiotherapy with electrons: issues related to the production, monitoring and dosimetric characterization of the beam. Front Phys. 2020;8:570697. 10.3389/fphy.2020.570697 [DOI] [Google Scholar]
- 48. Schüller A, Illemann J, Renner F, Makowski C, Kapsch R‐P. Traceable charge measurement of the pulses of a 27 MeV electron beam from a linear accelerator. J Instrum. 2017;12:P03003. [Google Scholar]
- 49. Jorge PG, Jaccard M, Petersson K, et al. Dosimetric and preparation procedures for irradiating biological models with pulsed electron beam at ultra‐high dose‐rate. Radiother Oncol. 2019;139:34‐39. [DOI] [PubMed] [Google Scholar]
- 50. Casolaro P, Campajola L, Breglio G, et al. Real‐time dosimetry with radiochromic films. Sci Rep. 2019;9:5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Darafsheh A, ed. Radiation Therapy Dosimetry: A Practical Handbook. Boca Raton, FL: CRC Press; 2021. [Google Scholar]
- 52. Petersson K, Jaccard M, Germond J‐F, et al. High dose‐per‐pulse electron beam dosimetry —a model to correct for the ion recombination in the Advanced Markus ionization chamber. Med Phys. 2017;44(3):1157‐1167. [DOI] [PubMed] [Google Scholar]
- 53. Di Martino F, Giannelli M, Traino AC, Lazzeri M. Ion recombination correction for very high dose‐per‐pulse high‐energy electron beams. Med Phys. 2005;32(7):2204‐2210. [DOI] [PubMed] [Google Scholar]
- 54. Mcmanus M, Romano F, Lee ND, et al. The challenge of ionisation chamber dosimetry in ultra‐short pulsed high dose‐rate very high energy electron beams. Sci Rep. 2020;10:9089. 10.1038/s41598-020-65819-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bourgouin A, Schuller A, Hackel T, et al. Calorimeter for real‐time dosimetry of pulsed ultra‐high dose rate electron beams. Front Phys‐Lausanne. 2020;8:567340. [Google Scholar]
- 56. Kranzer R, Poppinga D, Weidner J, et al. Ion collection efficiency of ionization chambers in ultrahigh dose‐per‐pulse electron beams. Med Phys. 2020;48(2):819‐830. 10.1002/mp.14620 [DOI] [PubMed] [Google Scholar]
- 57. Poppinga D, Kranzer R, Farabolini W, et al. VHEE beam dosimetry at CERN linear electron accelerator for research under ultra‐high dose rate conditions. Biomed Phys Eng Expr. 2021;7(1). [DOI] [PubMed] [Google Scholar]
- 58. Kokurewicz K, Schuller A, Brunetti E, et al. Dosimetry for new radiation therapy approaches using high energy electron accelerators. Front Phys‐Lausanne. 2020;8:568302. [Google Scholar]
- 59. Gomez F, Paz‐Martin J, Gonzalez Castano D, et al. Ultra thin plane‐parallel ionization chambers: expanding the range of air ionization chambers into ultra‐high dose rate. Phys Med. 2022;64:S21‐S22. Conference Presentation Abstract of the Flash Radiotherapy & Particle Therapy Conference (FRPT2021).
- 60. Di Martino F, Barone S, Del Sarto D, et al. A novel method for determining IC saturation factor (up to 0.5 gy/p for adv. Markus). Phys Med. 2022;94:S43. Conference Presentation Abstract of the Flash Radiotherapy & Particle Therapy Conference (FRPT2021).
- 61. Di Martino F, Barone S, Del Sarto D, et al. A new model of gas chamber for UHDR range. Phys Med. 2022;94:S82. Conference Presentation Abstract of the Flash Radiotherapy & Particle Therapy Conference (FRPT2021).
- 62. Darafsheh A, León‐Marroquín EY, Mulrow DJ, Baradaran‐Ghahfarokhi M, Zhao T, Khan R. On the spectral characterization of radiochromic films irradiated with clinical proton beams. Phys Med Biol. 2019;64(13):135016. [DOI] [PubMed] [Google Scholar]
- 63. Mosahebi S, Hoshyar N, Khan R, Darafsheh A. Film dosimetry. In: Darafsheh A, ed. Radiation Therapy Dosimetry: A Practical Handbook. Chapter 5. CRC Press; 2021:61‐74. [Google Scholar]
- 64. León‐Marroquín EY, Mulrow DJ, Khan R, Darafsheh A. Spectral analysis of EBT3 radiochromic films for clinical photon and electron beams. Med Phys. 2019;46(2):973‐982. [DOI] [PubMed] [Google Scholar]
- 65. León‐Marroquín EY, Mulrow D, Darafsheh A, Khan R. Response characterization of the EBT‐XD radiochromic films in megavoltage photon and electron beams. Med Phys. 2019;46(9):4246‐4256. [DOI] [PubMed] [Google Scholar]
- 66. Darafsheh A, Hao Y, Maraghechi B, Cammin J, Reynoso FJ, Khan R. Influence of 0.35 T magnetic field on the response of EBT3 and EBT‐XD radiochromic films. Med Phys. 2020;47(9):4543‐4552. [DOI] [PubMed] [Google Scholar]
- 67. Darafsheh A, Zhao T, Khan R. Spectroscopic analysis of EBT‐XD radiochromic films irradiated with proton and photon therapy beams. Phys Med Biol. 2020;65(20):205002. [DOI] [PubMed] [Google Scholar]
- 68. Jaccard M, Petersson K, Buchillier T, et al. High dose‐per‐pulse electron beam dosimetry: usability and dose‐rate independence of EBT3 Gafchromic films. Med Phys. 2017;44(2):725‐735. [DOI] [PubMed] [Google Scholar]
- 69. Petasecca M, Cullen A, Fuduli I, et al. X‐Tream: a novel dosimetry system for synchrotron microbeam radiation therapy. JINST. 2012;7:P07022. [Google Scholar]
- 70. Verona Rinati G, Felici G, Galante F, et al. Realization and characterization of novel diamond detector prototypes for flash therapy applications. Phys Med. 2022;94:S40‐S41. Conference Presentation Abstract of the Flash Radiotherapy & Particle Therapy Conference (FRPT2021).
- 71. Alagoz E, Brauer‐Krisch E, Bravin A, et al. Multi‐strip silicon sensors for beam array monitoring in micro‐beam radiation therapy. Phys Med. 2016;32(12):1795‐1800. [DOI] [PubMed] [Google Scholar]
- 72. Schulte R, Bashkirov V, Hurley F, Penfold S, Rosenfeld A, Patyal B. TH‐D‐BRC‐09: a status update on the development of proton CT at Loma Linda University Medical Center. Med Phys. 2009;36(6):2813. [Google Scholar]
- 73. Nida S, Tsibizov A, Ziemann T, et al. Silicon carbide X‐ray beam position monitors for synchrotron applications. J Synchrotron Radiat. 2019;26(Pt 1):28‐35. 10.1107/S1600577518014248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Romano F, Del Mar Carulla Areste M, Camarda M Feasibility study of using innovative technology based on silicon carbide detectors for FLASH irradiations. Phys Med. 2022;94:S97‐S98. Conference Presentation Abstract of the Flash Radiotherapy & Particle Therapy Conference (FRPT2021).
- 75. Feist H, Muller U. Measurement of the total stopping power of 5.3 MeV electrons in polystyrene by means of electron beam absorption in ferrous sulphate solution. Phys Med Biol. 1989;34(12):1863‐1869. 10.1088/0031-9155/34/12/009 [DOI] [Google Scholar]
- 76. Vörös S, Anton M, Boillat B. Relative response of alanine dosemeters for high‐energy electrons determined using a Fricke primary standard. Phys Med Biol. 2012;57(5):1413‐1432. 10.1088/0031-9155/57/5/1413 [DOI] [PubMed] [Google Scholar]
- 77. Boag JW, Epp E, Fielden EM, Parker RP. ICRU Report 34: the dosimetry of pulsed radiation, 3. Chemical dosimetry. Reports of the International Commission on Radiation Units and Measurements os‐18. No. 1. 1982:14‐21. 10.1093/jicru_os18.1.14 [DOI] [Google Scholar]
- 78. Sehested K, Bjergbakke E, Rasmussen OL, Fricke H. Reactions of H2O3 in the pulse‐irradiated Fe(II)‐O2 system. J Chem Phys. 1969;51(8):3159‐3166. 10.1063/1.1672489 [DOI] [Google Scholar]
- 79. Thomas JK, Hart EJ. The radiolysis of aqueous solutions at high intensities. Radiat Res. 1962;17(3):408‐418. www.jstor.org/stable/3571103 [Google Scholar]
- 80. Cascio EW, Gottschalk B. A simplified vacuumless Faraday cup for the experimental beamline at the Francis H. Burr proton therapy center. In: IEEE Radiation Effects Data Workshop. 2009:161‐165. [Google Scholar]
- 81. Verhey LJ, Koehler AM, Mcdonald JC, et al. The determination of absorbed dose in a proton beam for purposes of charged‐particle radiation therapy. Radiat Res. 1979;79:34‐54. [PubMed] [Google Scholar]
- 82. Winterhalter C, Togno M, Nesteruk KP, et al. Faraday cup for commissioning and quality assurance for proton pencil beam scanning beams at conventional and ultra‐high dose rates. Phys Med Biol. 2021;66:124001. https://orcid.org/0000‐0002‐0171‐5783 [DOI] [PubMed] [Google Scholar]
- 83. Richter C, Karsch L, Dammene Y, et al. A dosimetric system for quantitative cell irradiation experiments with laser‐accelerated protons. Phys Med Biol. 2011;56:1529‐1543. 10.1088/0031-9155/56/6/002 [DOI] [PubMed] [Google Scholar]
- 84. Scuderi V, Amato A, Amico A, et al. Diagnostics and dosimetry solutions for multidisciplinary applications at the ELIMAIA beamline. Appl Sci. 2018;8:1415. 10.3390/app8091415 [DOI] [Google Scholar]
- 85. Romano F, Cirrone GAP, Cuttone G, et al. Status of the ELIMED multidisciplinary and medical beam‐line at ELI‐Beamlines. J Phys Conf Ser. 2017;777:012016. 10.1088/1742-6596/777/1/012016 [DOI] [Google Scholar]
- 86. Darafsheh A. Scintillation fiber optic dosimetry. In: Darafsheh A, ed. Radiation Therapy Dosimetry: A Practical Handbook. Chapter 9. Boca Raton, FL: CRC Press; 2021:123‐137. [Google Scholar]
- 87. Darafsheh A, Melzer JE, Harrington J, Kassaee A, Finlay JC. Fiber optic probes based on silver‐only coated hollow glass waveguides for radiation therapy dosimetry. J Biomed Opt. 2018;23(1):015006. [DOI] [PubMed] [Google Scholar]
- 88. Darafsheh A, Zhang R, Kanick SC, Pogue BW, Finlay JC. Spectroscopic separation of Čerenkov radiation in high‐resolution radiation fiber dosimeters. J Biomed Opt. 2015;20(9):095001. [DOI] [PubMed] [Google Scholar]
- 89. Darafsheh A, Taleei R, Kassaee A, Finlay JC. The visible signal responsible for proton therapy dosimetry using bare optical fibers is not Čerenkov radiation. Med Phys. 2016;43(11):5973‐5980. [DOI] [PubMed] [Google Scholar]
- 90. Darafsheh A, Taleei R, Kassaee A, Finlay JC. Proton therapy dosimetry using the scintillation of the silica fibers. Opt Lett. 2017;42(4):847‐850. [DOI] [PubMed] [Google Scholar]
- 91. Hitachi A, Doke T, Mozumder A. Luminescence quenching in liquid argon under charged‐particle impact: relative scintillation yield at different linear energy transfers. Phys Rev B. 1992;46(18):11463. [DOI] [PubMed] [Google Scholar]
- 92. Wang LLW, Perles LA, Archambault L, Sahoo N, Mirkovic D, Beddar S. Determination of the quenching correction factors for plastic scintillation detectors in therapeutic high‐energy proton beams. Phys Med Biol. 2012;57(23):7767‐7781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Robertson D, Mirkovic D, Sahoo N, Beddar S. Quenching correction for volumetric scintillation dosimetry of proton beams. Phys Med Biol. 2013;58(2):261‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Tretyak VI. Semi‐empirical calculation of quenching factors for ions in scintillators. Astropart Phys. 2010;33:40‐53. [Google Scholar]
- 95. Darafsheh A, Soldner A, Liu H, Kassaee A, Zhu T, Finlay J. Phosphor‐based fiber optic probes for proton beam characterization. Med Phys. 2015;42(6):3476. [Google Scholar]
- 96. Darafsheh A, Kassaee A, Taleei R, Dolney D, Finlay J. Fiber optic microprobes with rare‐earth‐based phosphor tips for proton beam characterization. Proc SPIE. 2016;9700:97000Q. [Google Scholar]
- 97. Birks JB. Scintillations from organic crystals: specific fluorescence and relative response to different radiations. Proc Phys Soc Lond Sect A. 1951;64(10):874‐877. [Google Scholar]
- 98. Birks JB. The Theory and Practice of Scinillation Counting. Oxford: Pergamon Press; 1964. [Google Scholar]
- 99. Gooding TJ, Pugh HG. The response of plastic scintillators to high‐energy particles. Nucl Instrum Methods. 1960;7:189‐192. [Google Scholar]
- 100. Torrisi L. Plastic scintillator investigations for relative dosimetry in proton‐therapy. Nucl Instrum Meth Phys Res B. 2000;170:523‐530. [Google Scholar]
- 101. Ashraf MR, Rahman M, Zhang R, et al. Dosimetry for FLASH radiotherapy: a review of tools and the role of radioluminescence and Cherenkov emission. Front Phys. 2020;8:328. 10.3389/fphy.2020.00328 [DOI] [Google Scholar]
- 102. Bisogni G, Ciarrocchi E, Di Martino F, et al. Inorganic scintillators for flash‐iort dosimetry: development and test of a lyso detector prototype. Phys Med. 2022;94:S41‐S42. Conference Presentation Abstract of the Flash Radiotherapy & Particle Therapy Conference (FRPT2021).
- 103. Vigdor SE, Klyachko AV, Solberg KA, Pankuch M. A gas scintillator detector for 2D dose profile monitoring in pencil beam scanning and pulsed beam proton radiotherapy treatments. Phys Med Biol. 2017;62(12):4946‐4969. 10.1088/1361-6560/aa6ce2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Toppi M, De Gregorio A, De Maria P, et al. FLASHDC project: development of a beam monitor for flash radiotherapy. Phys Med. 2022;94:S84. Conference Presentation Abstract of the Flash Radiotherapy & Particle Therapy Conference (FRPT2021).
- 105. Hachadorian RL, Tendler II, Pogue BW. Cherenkov and scintillation imaging dosimetry. In: Darafsheh A, ed. Radiation Therapy Dosimetry: A Practical Handbook. Chapter 10. Boca Raton, FL: CRC Press; 2021:139‐150. [Google Scholar]
- 106. Favaudon V, Lentz J‐M, Heinrich S, et al. Time‐resolved dosimetry of pulsed electron beams in very high doserate, FLASH irradiation for radiotherapy preclinical studies. Nucl Instrum Methods Phys Res. 2019;944:162537. 10.1016/j.nima.2019.162537 [DOI] [Google Scholar]
- 107. Kron T, Lonski P. Thermoluminescent dosimetry. In: Darafsheh A, ed. Radiation Therapy Dosimetry: A Practical Handbook. Chapter 6. Boca Raton, FL: CRC Press; 2021:75‐96. [Google Scholar]
- 108. Karsch L, Beyreuther E, Burris‐Mog T, et al. Dose rate dependence for different dosimeters and detectors: TLD, OSL, EBT films, and diamond detectors. Med Phys. 2012;39(5):2447‐2455. [DOI] [PubMed] [Google Scholar]
- 109. Tochilin E, Goldstein N. Dose rate and spectral measurements from pulsed X‐ray generators. Health Phys. 1966;12(12):1705. [Google Scholar]
- 110. Karzmark CJ. Some aspects of lithium‐fluoride thermoluminescence dosimetry. Phys Med Biol. 1964;9(1):102. [DOI] [PubMed] [Google Scholar]
- 111. Montay‐Gruel P, Petersson K, Jaccard M, et al. Irradiation in a flash: unique sparing of memory in mice after whole brain irradiation with dose rates above 100Gy/s. Radiother Oncol. 2017;124(3):365‐369. [DOI] [PubMed] [Google Scholar]
- 112. Jaccard M, Petersson K, Buchillier T, et al. High dose‐per‐pulse electron beam dosimetry: usability and dose‐rate independence of EBT3 Gafchromic films. Med Phys. 2017;44(2):725‐735. [DOI] [PubMed] [Google Scholar]
- 113. Kry S, O'Daniel J. Optically stimulated luminescence dosimeters in clinical practice. In: Darafsheh A, ed. Radiation Therapy Dosimetry: A Practical Handbook. Chapter 7. Boca Raton, FL: CRC Press; 2021:97‐108. [Google Scholar]
- 114. Reft CS. The energy dependence and dose response of a commercial optically stimulated luminescent detector for kilovoltage photon, megavoltage photon, and electron, proton, and carbon beams. Med Phys. 2009;36(5):1690‐1699. [DOI] [PubMed] [Google Scholar]
- 115. Yukihara EG, Gasparian PBR, Sawakuchi GO, et al. Medical applications of optically stimulated luminescence dosimeters (OSLDs). Radiat Meas. 2010;45(3‐6):658‐662. [Google Scholar]
- 116. Yuan L, Jin Y, Su Y, Wu H, Hu Y, Yang S. Optically stimulated luminescence phosphors: principles, applications, and prospects. Laser Photonics Rev. 2020;14:2000123. [Google Scholar]
- 117. Akselrod M, McKeever S. A radiation dosimetry method using pulsed optically stimulated luminescence. Radiat Prot Dosim. 1999;81:167. [Google Scholar]
- 118. Polf JC, McKeever SW, Akselrod MS, Holmstrom S. A real‐time, fibre optic dosimetry system using Al2O3 fibres. Radiat Prot Dosim. 2002;100:301‐304. [DOI] [PubMed] [Google Scholar]
- 119. Gaza R, McKeever SWS, Akselrod MS. Near‐real‐time radiotherapy dosimetry using optically stimulated luminescence of Al2O3:C: mathematical models and preliminary results. Med Phys. 2005;32(4):1094‐1102. [DOI] [PubMed] [Google Scholar]
- 120. Caraça Santos AM, Mohammadi M, Asp J, Monro TM, Afshar S. Characterisation of a real‐time fibre‐coupled beryllium oxide (BeO) luminescence dosimeter in X‐ray beams. Radiat Meas. 2013;53‐54:1‐7. [Google Scholar]
- 121. Nascimento LF, Verellen D, Goossens J, et al. Two‐dimensional real‐time quality assurance dosimetry system using μ‐Al2O3:c,Mg radioluminescence films. Phys Imaging Radiat Oncol. 2020;16:26‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Karsch L, Beyreuther E, Burris‐Mog T, et al. Dose rate dependence for different dosimeters and detectors: TLD, OSL, EBT films, and diamond detectors. Med Phys. 2012;39(5):2447‐2455. [DOI] [PubMed] [Google Scholar]
- 123. Jursinic PA. Optically stimulated luminescent dosimeters stable response to dose after repeated bleaching. Med Phys. 2020;47(7):3191‐3203. [DOI] [PubMed] [Google Scholar]
- 124. Jursinic PA. Characterization of optically stimulated luminescent dosimeters, OSLDs, for clinical dosimetric measurements: optically stimulated luminescent dosimeters for clinical dosimetric measurements. Med Phys. 2007;34:4594‐4604. [DOI] [PubMed] [Google Scholar]
- 125. Poirier Y, Kuznetsova S, Villarreal‐Barajas JE. Characterization of nanoDot optically stimulated luminescence detectors and high‐sensitivity MCP‐N thermoluminescent detectors in the 40‐300 kVp energy range. Med Phys. 2018;45:402‐413. [DOI] [PubMed] [Google Scholar]
- 126. McGrath A. Relative dosimetry measurements in kilovoltage X‐rays with OSLDs. Phys Eng Sci Med. 2020;43:289‐295. [Google Scholar]
- 127. Kerns JR, Kry SF, Sahoo N. Characteristics of optically stimulated luminescence dosimeters in the spread‐out Bragg peak region of clinical proton beams. Med Phys. 2012;39(4):1854‐1863. [DOI] [PubMed] [Google Scholar]
- 128. Santos AMC, Mohammadi M, Asp J, Monro TM, Afshar VS. Characterisation of a real‐time fibre‐coupled beryllium oxide (BeO) luminescence dosimeter in X‐ray beams. Radiat Meas. 2013;1:53‐54. [Google Scholar]
- 129. Christensen JB, Togno M, Nesteruk KP, et al. Al2O3:c optically stimulated luminescence dosimeters (OSLDs) for ultra‐high dose rate proton dosimetry. Phys Med Biol. 2021;66(8). 10.1088/1361-6560/abe554 [DOI] [PubMed] [Google Scholar]
- 130. Esplen N, Mendonca MS, Bazalova‐Carter M. Physics and biology of ultrahigh dose‐rate (FLASH) radiotherapy: a topical review. Phys Med Biol. 2020;65(23). [DOI] [PubMed] [Google Scholar]
- 131. International Standardization Organization. Standard Practice for Use of an Alanine‐EPR Dosimetry System Standard 51607. 2004.
- 132. Gondre M, Jorge PG, Vozenin MC, et al. Optimization of alanine measurements for fast and accurate dosimetry in FLASH radiation therapy. Radiat Res. 2020;194(6):573‐579. [DOI] [PubMed] [Google Scholar]
- 133. Desrosiers MF, Publ JM, Cooper SL. An absorbed‐dose/dose‐rate dependence for the alanine‐EPR dosimetry system and its implications in high‐dose ionizing radiation metrology. J Res Natl Inst Stan. 2008;113(2):79‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Desrosiers MF, Puhl JM. Absorbed‐dose/dose‐rate dependence studies for the alanine‐EPR dosimetry system. Radiat Phys Chem. 2009;78(7‐8):461‐463. [Google Scholar]
- 135. Kudoh H, Celina M, Kaye RJ, Gillen KT, Clough RL. Response of alanine dosimeters at very high dose rate. Appl Radiat Isotopes. 1997;48(4):497‐499. [Google Scholar]
- 136. Bourhis J, Sozzi WJ, Jorge PG, et al. Treatment of a first patient with FLASH‐radiotherapy. Radiother Oncol. 2019;139:18‐22. [DOI] [PubMed] [Google Scholar]
- 137. McEwen MR, DuSautoy AR. Primary standards of absorbed dose for electron beams. Metrologia. 2009;46(2):S59‐S79. 10.1088/0026-1394/46/2/S05 [DOI] [Google Scholar]
- 138. Seuntjens J, Duane S. Photon absorbed dose standards. Metrologia. 2009;46(2, SI):S39‐S58. 10.1088/0026-1394/46/2/S04 [DOI] [Google Scholar]
- 139. Pruitt JS, Domen SR, Loevinger R. The graphite calorimeter as a standard of absorbed dose for Co‐60 gamma‐radiation. J Res Nat Bur Stand. 1981;86(5):495‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. DeWerd LA, Smith BR. Calorimetry. In: Darafsheh A, ed. Radiation Therapy Dosimetry: A Practical Handbook. Chapter 3. Boca Raton, FL: CRC Press; 2021:31‐37. [Google Scholar]
- 141. Renaud J, Palmans H, Sarfehnia A, Seuntjens J. Absorbed dose calorimetry. Phys Med Biol. 2020;65(5):05TR02. 10.1088/1361-6560/ab4f29 [DOI] [PubMed] [Google Scholar]
- 142. Renaud J, Marchington D, Seuntjens J, Sarfehnia A. Development of a graphite probe calorimeter for absolute clinical dosimetry. Med Phys. 2013;40(2):020701. https://aapm.onlinelibrary.wiley.com/doi/abs/10.1118/1.4773870 [DOI] [PubMed] [Google Scholar]
- 143. Renaud J, Sarfehnia A, Bancheri J, Seuntjens J. Aerrow: a probe‐format graphite calorimeter for absolute dosimetry of high‐energy photon beams in the clinical environment. Med Phys. 2018;45(1):414‐428. 10.1002/mp.12669 [DOI] [PubMed] [Google Scholar]
- 144. Palmans H, Thomas R, Simon M, et al. A small‐body portable graphite calorimeter for dosimetry in low‐energy clinical proton beams. Phys Med Biol. 2004;49(16):3737‐3749. 10.1088/0031-9155/49/16/019 [DOI] [PubMed] [Google Scholar]
- 145. Duane S, Aldehaybes M, Bailey M, Lee N, Thomas C, Palmans H. An absorbed dose calorimeter for IMRT dosimetry. Metrologia. 2012;49. 10.1088/0026-1394/49/5/S168 [DOI] [Google Scholar]
- 146. Romano F, Subiel A, McManus M, et al. Challenges in dosimetry of particle beams with ultra‐high pulse dose rates. J Phys Conf Ser. 1662(2020):012028. 10.1088/1742-6596/1662/1/012028 [DOI] [Google Scholar]
- 147. Dunn PC. Absolute beam charge measurements with toroid monitors – experience at the bates linac. Nucl Instrum Methods. 1979;165(2):163‐167. [Google Scholar]
- 148. Konradsson E, Ceberg C, Lempart M, et al. Correction for ion recombination in a built‐in monitor chamber of a clinical linear accelerator at ultra‐high dose rates. Radiat Res. 2020;194(6):580‐586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Felici G, Barca P, Barone S, et al. Transforming an IORT linac into a FLASH research machine: procedure and dosimetric characterization. Front Phys. 2020;8:374. [Google Scholar]
- 150. Schüller A, Heinrich S, Fouillade C, et al. The European Joint Research Project UHDpulse – metrology for advanced radiotherapy using particle beams with ultra‐high pulse dose rates. Phys Med. 2020;80:134‐150. [DOI] [PubMed] [Google Scholar]
- 151. Akselrod MS, Sykora GJ. Fluorescent nuclear track detector technology – a new way to do passive solid state dosimetry. Radiat Meas. 2011;46:1671‐1679. 10.1016/j.radmeas.2011.06.018 [DOI] [Google Scholar]
- 152. Bräuer‐Krisch E, Rosenfeld A, Lerch M, et al. Potential high resolution dosimeters for MRT. AIP Conf Proc . 2010;1266:89‐97. 10.1063/1.3478205 [DOI] [Google Scholar]
- 153. Nesteruk KP, Togno M, Grossmann M, et al. Commissioning of a clinical pencil beam scanning proton therapy unit for ultra‐high dose rates (FLASH). Med Phys. 2021;48(7):4017‐4026. 10.1002/mp.14933 [DOI] [PubMed] [Google Scholar]
- 154. Jaccard M, Durán MT, Petersson K, et al. High dose‐per‐pulse electron beam dosimetry: commissioning of the Oriatron eRT6 prototype linear accelerator for preclinical use. Med Phys. 2018;45(2):863‐874. [DOI] [PubMed] [Google Scholar]


