Abstract
Background and purpose
Although disabling fatigue is common in Parkinson disease (PD), available consensus‐based diagnostic criteria have not yet been empirically validated. The aim of this study was to evaluate the clinimetric properties of the criteria.
Methods
A sample of outpatients with PD was evaluated for demographic, clinical, behavioral, and cognitive features. Fatigue was diagnosed according to the new diagnostic criteria and was rated by means of the Parkinson Fatigue Scale (PFS) and Fatigue Severity Scale (FSS). Acceptability, concurrent and discriminant validity, and interrater reliability were evaluated with binary logistic regression analyses and Cohen kappa (κ).
Results
Of 241 included patients, 17 (7.1%) met the diagnostic criteria for PD‐related fatigue. Eight of nine symptoms described in Section A of the diagnostic criteria occurred in >50% of patients with fatigue. Acceptability (missing data = 0.8%) of the criteria was good, as was their concurrent validity with the PFS (odds ratio = 3.65) and FSS (odds ratio = 3.63). The discriminant validity of fatigue criteria with other PD‐related behavioral and cognitive features was good (odds ratio < 1.68). The interrater reliability was excellent (κ = 0.92).
Conclusions
This is the first study to test the clinimetric properties of case definition diagnostic criteria for PD‐related fatigue. Our results suggest that current diagnostic criteria may be useful in both clinical practice and research. Future longitudinal studies should examine their long‐term stability.
Keywords: fatigability, fatigue, nonmotor symptoms, Parkinson disease, validation
We evaluated the clinimetric properties of consensus‐based, Kluger et al. (2016) diagnostic criteria for fatigue in Parkinson disease (PD) in a sample of outpatients with mild to moderate symptoms. The criteria identified “pure” fatigue in 17 of 241 patients (7.1%), and had moderate to high acceptability, concurrent and discriminant validity, and interrater reliability. The criteria allowed identification of “secondary” fatigue in 38 patients (13.7%), related to depression, apathy, sleep disorders, beta‐blocker medication, or, less frequently, heart failure, hypothyroidism, or anemia (i.e., based on Section D of the PD‐related fatigue criteria).
![]()
INTRODUCTION
Fatigue is one of the most disabling and common nonmotor feature in Parkinson disease (PD) [1]. At the phenomenological level, patients describe fatigue as an overwhelming lack of energy or a need for increased effort during daily activities [2]. Fatigue affects from 44% to 56% of patients with PD [3], tends to develop early, persists over time, and impacts patients' quality of life [4, 5, 6, 7].
Despite several epidemiological studies linking fatigue to PD, little is known about its pathophysiology [7, 8, 9], and there are currently no evidence‐based treatment options [9, 10, 11]. Converging evidence supported the involvement of nondopaminergic pathways (particularly serotonergic) and nonmotor networks in fatigue in PD [1, 12, 13], but key mechanisms are still not fully understood. This knowledge gap could be partially ascribed to the lack of validated diagnostic criteria capable of distinguishing the fatigue specifically related to the disease from the fatigue that may be associated with concurrent motor and nonmotor aspects of PD [1, 2, 14].
In 2016, an international work group convened by the Parkinson Foundation developed case definition criteria for PD‐related fatigue on the basis of expert consensus (Table 1) [1], yet no validation study has been conducted to support their use in clinical and research contexts. The main aims of these criteria were: (i) to promote a consistent case definition of PD‐related fatigue similar to clinically significant fatigue in other serious illnesses like cancer [15], (ii) to differentiate fatigue from other potentially similar neuropsychiatric symptoms (e.g., depression, apathy), (iii) to distinguish clinically relevant fatigue as a durable syndrome from normal physiologic fatigue or temporary (state) fatigue, and (iv) to distinguish fatigue related to PD pathology from fatigue arising from other causes (e.g., anemia, medications) [1, 2, 16]. A recent qualitative study [17] explored the lived experiences of 22 patients with PD suffering from fatigue and suggested that the fatigue criteria proposed by Kluger et al. [1] are ecologically valid but need to be optimized and tested. Despite their face validity and potential utility, the clinimetric properties of the new criteria for PD‐related fatigue have not yet been assessed.
TABLE 1.
Criteria for diagnosis of PD‐related fatigue
| Patients must report significantly diminished energy levels or increased perceptions of effort that are disproportionate to attempted activities or general activity level. Symptoms must be present for most of the day, every day, or nearly every day during the previous month. In addition, patients must have four or more of the symptoms from Section A and meet criteria in Sections B, C, and D. |
|---|
| A. Symptoms |
| 1. Symptoms may be induced by routine activities of daily living |
| 2. Symptoms may occur with little or no exertion |
| 3. Symptoms limit the type, intensity, or duration of activities performed by the patient |
| 4. Symptoms are not reliably relieved by rest or may require prolonged periods of rest |
| 5. Symptoms may be brought on by cognitive tasks or situations requiring sustained attention including social interactions |
| 6. Patients avoid rigorous activities because of fear of experiencing worsening of symptoms |
| 7. Mild to moderate exertion may induce a worsening of symptoms lasting hours to days |
| 8. Symptoms have a predictable diurnal pattern regardless of activities performed (e.g., worsening in the afternoon) |
| 9. Symptoms are unpredictable and may have a sudden onset |
| B. The patient experiences clinically significant distress or impairment in social, occupational, or other important areas of function as a result of fatigue |
| C. There is evidence from the history and physical examination suggesting fatigue is a consequence of PD |
| D. The symptoms are not primarily a consequence of comorbid psychiatric disorders (e.g., depression), sleep disorders (e.g., obstructive sleep apnea), or medical conditions (e.g., anemia, congestive heart failure) |
Abbreviation: PD, Parkinson disease.
In the present study, we recruited a large sample of patients with PD to assess the clinimetric properties of the criteria in terms of acceptability (whether a clinical measure is acceptable to the patients), validity (whether a clinical measure actually measures what it set out to measure), and reliability (whether a clinical measure can be interpreted consistently across different situations).
METHODS
Patients and procedure
The study sample was recruited consecutively from the movement disorders outpatient clinic of the First Division of Neurology at the University of Campania “Luigi Vanvitelli” (Naples, Italy). The diagnosis of PD was made according to the modified diagnostic criteria of the UK Parkinson's Disease Society Brain Bank [18, 19].
Exclusion criteria were (i) neurodegenerative disorders other than PD; (ii) current or past cerebrovascular disorders or any major or unstable medical disease; and (iii) PD‐related dementia, following the Level I testing procedures proposed by the Movement Disorders Society Task Force [20], or severe global cognitive impairment or language deficits, defined on the basis of an age‐ and education‐adjusted Montreal Cognitive Assessment (MoCA) total or language domain score below the Italian cutoff scores (15.5 or 3.08 points, respectively), to avoid any bias in responding to self‐report scales [21].
All patients were assessed in their “ON” state with their routine dopaminergic medication [22].
The local ethical committee supervised and approved all procedures, following the Declaration of Helsinki. All participants gave their written informed consent before their inclusion in the study.
Demographics and clinical features
We recorded patients' demographic characteristics (age, education, and sex), and assessed severity of motor symptoms via the Unified Parkinson's Disease Rating Scale (UPDRS) [23] and the modified Hoehn and Yahr staging system (HY) [24]. Daily levodopa equivalent dosage, daily dopamine agonist equivalent dosage, and the total amount of dopaminergic medication were determined using Tomlinson et al.'s algorithm [25].
Behavioral and cognitive measures
We used the Beck Depression Inventory (BDI) [26] for assessing depressive symptoms and the Diagnostic and Statistical Manual‐based Mini International Neuropsychiatric Inventory (Module A) for diagnosing major depressive episodes [27]. The self‐rated version of the Apathy Evaluation Scale (AES) [28], the Epworth Sleepiness Scale (ESS) [29], and the Parkinson's Disease Sleep Scale (PDSS) [30] were also collected.
The MoCA was employed to measure cognitive functioning [21] and to support the diagnosis of PD‐related dementia according to the Level I testing procedures [20].
Clinical diagnosis and scales of fatigue
A movement disorder specialist (R.D.M.) classified the patients as having (f‐PD) or not having (nf‐PD) fatigue via a semistructured clinical interview (Supplementary Material 1 in Appendix S1) based on the new diagnostic criteria for PD‐related fatigue (Table 1) [1]. To operationalize Section D of the diagnostic criteria, we excluded the diagnosis of fatigue in the presence of major depressive episodes [27], apathy (AES ≥ 37 [28]), or sleep disorders (ESS > 10 [29] and/or PDSS Z‐score < −2 [30] and/or obstructive sleep apnea [31]), as well as heart failure, hypothyroidism, anemia, or beta‐blocker medication; presence of these last conditions was inferred from medical records.
Additionally, patients completed two self‐rated fatigue scales: the Parkinson Fatigue Scale (PFS [32]) and the Fatigue Severity Scale (FSS [33]).
Statistical analyses
We provided descriptive statistics for demographic and clinical features of f‐PD and nf‐PD. Moreover, we employed multiple binary logistic regression analysis to explore the association between these features and fatigue (coded as 1 = present, 0 = absent; see Chen et al. [34] for odds ratio interpretation).
Then, the diagnostic criteria for PD‐related fatigue were assessed for acceptability, concurrent and discriminant validity, and interrater reliability.
Acceptability relates to the facility of administration and was evaluated by the rate of missing data and incomplete evaluations; values up to 5% are considered acceptable [35, 36].
Concurrent validity refers to the association between the proposed set of criteria and other scales assessing the same construct. We assessed it by two simple binary logistic regression analyses, evaluating the association of the diagnostic criteria for PD‐related fatigue with the PFS and the FSS, and we used the area under the curve (AUC; see Hosmer and Lemeshow [37] for effect size interpretation) to assess the accuracy of PFS and FSS in classifying the presence of fatigue independently of specific cutoff scores. Moreover, we employed Cohen kappa (κ; see Landis and Koch [38] for effect size interpretation) as a measure of the agreement between the diagnostic criteria for PD‐related fatigue and available cutoff scores for PFS (i.e., ≥2.95 and ≥3.30 [32]) and FSS (i.e., ≥4 and >5 [33, 39]).
Discriminant validity refers to the ability to discriminate the construct of interest from potential confounds. As we did not consider as affected by fatigue the patients with concomitant abnormal scores on the BDI, AES, ESS, PDSS, or MoCA, we assessed discriminant validity via binary multiple logistic regression analysis between the diagnostic criteria (coded as 1 = present, 0 = absent) and the subthreshold measures of the distinct constructs.
Interrater reliability was evaluated by computing Cohen κ in each clinical section between diagnosis of fatigue made by the main rater (R.D.M.) and by a second movement disorder specialist (A.T.), who was blind to clinical and diagnostic information.
All statistics were performed by the Statistical Package for Social Science version 20, using a Benjamini–Hochberg corrected p < 0.05.
RESULTS
Of 336 eligible patients, 67 refused to participate in the study (main reasons: personal reasons and no interest in the research), five were excluded due to PD‐related dementia, and 21 were excluded because of severe cognitive impairment or language deficits. On average, the patients who agreed to participate in the study had lower motor burden (UPDRS‐III mean = 24.98 ± 10.60 vs. 32.94 ± 12.27 points) and disease duration (3.44 ± 2.91 vs. 7.85 ± 4.88 years) than the patients who declined (see Supplementary Material 2 in Appendix S1 for further details). Among the 243 participants meeting our selection criteria, two participants who started the assessment refused to complete it and the structured clinical interview; thus, complete data were available for 241 participants. Seventeen (7.1%) of them met the diagnostic criteria for PD‐related fatigue (i.e., f‐PD). Of the 224 nf‐PD subjects, 159 (66.0%) patients did not endorse the two screening questions exploring the core features of the PD‐related fatigue diagnosis, and <8% patients did not meet the criteria on the basis of Section A, Section B, or Section C (Table 2). Thirty‐three (13.7%) patients did not meet the criteria of Section D, with depression, apathy, sleep disorders, and beta‐blocker medication being the most frequent causes of secondary fatigue (see Figure 1 and Supplementary Material 3 Appendix S1 for further details).
TABLE 2.
Frequency and percentage of patients meeting PD‐related fatigue diagnostic criteria, and agreement between diagnostic criteria and rating scales in defining presence of fatigue
| Overall sample, n = 241 | Patients with PFS ≥ 2.95, n = 70/241; 29.0% | Patients with PFS ≥ 3.30, n = 54/241; 22.4% | Patients with FSS ≥ 4.00, n = 112/241; 46.5% | Patients with FSS > 5.00, n = 43/241; 17.8% | |
|---|---|---|---|---|---|
| Not meeting screening Question 1 | 126 (52.3%) | 14 (20.0%) | 8 (14.8%) | 30 (26.8%) | 6 (13.9%) |
| Not meeting screening Question 2 | 33 (13.7%) | 6 (8.6%) | 5 (9.3%) | 13 (11.7%) | 1 (2.4%) |
| Not meeting Section A | 19 (7.9%) | 4 (5.7%) | 2 (3.7%) | 10 (8.9%) | 1 (2.4%) |
| Not meeting Section B | 10 (4.1%) | 3 (4.3%) | 2 (3.7%) | 8 (7.1%) | 2 (4.6%) |
| Not meeting Section C | 3 (1.2%) | 1 (1.4%) | 1 (1.8%) | 2 (1.9%) | 1 (2.4%) |
| Not meeting Section D | 33 (13.7%) | 26 (37.1%) | 22 (40.7%) | 30 (26.8%) | 15 (34.8%) |
| Meeting fatigue criteria | 17 (7.1%) | 16 (22.9%) | 14 (26.0%) | 19 (16.8%) | 17 (39.5%) |
Note: Screening Question 1: "Have you experienced diminished energy levels or increased perception of effort that are disproportionate to attempted activities or general activity level?" Screening Question 2: "Have these symptoms been present for most of the day every day or nearly every day during the previous month?" Section A: Patients must have four or more of the symptoms from Section A. Section B: The patient experiences clinically significant distress or impairment in social, occupational, or other important areas of function as a result of fatigue. Section C: There is evidence from the history and physical examination suggesting fatigue is a consequence of PD. Section D: The symptoms are not primarily a consequence of comorbid psychiatric disorders (e.g., depression), sleep disorders (e.g., obstructive sleep apnea), or medical conditions (e.g., anemia, congestive heart failure).
Abbreviations: FSS, Fatigue Severity Scale; PD, Parkinson disease; PFS, Parkinson Fatigue Scale.
FIGURE 1.
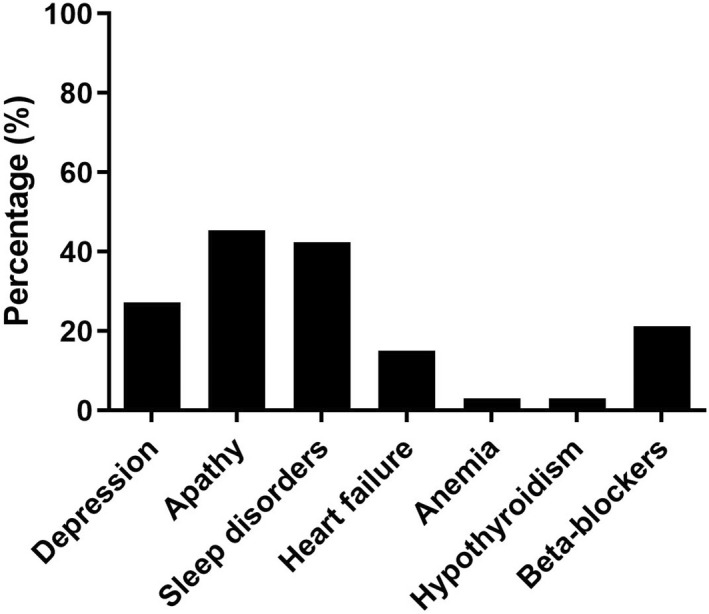
Percentage of comorbid conditions for patients not meeting the Section D criteria of the Parkinson disease‐related fatigue diagnostic criteria (n = 38)
In f‐PD subjects (n = 17), all symptoms described in Section A were present in >50%, except Symptom A6 (“Patients avoid rigorous activities because of fear of experiencing worsening of symptoms,” 41.2%; Figure 2). As for nf‐PD subjects who affirmatively answered at least the two screening questions (n = 65), Symptoms A4 (i.e., “Symptoms are not reliably relieved by rest or may require prolonged periods of rest”), A5 (i.e., “Symptoms may be brought on by cognitive tasks or situations requiring sustained attention including social interactions”), A7 (i.e., “Mild to moderate exertion may induce a worsening of symptoms lasting hours to days”), and A9 (i.e., “Symptoms are unpredictable and may have a sudden onset”) were less frequently recorded, whereas the remaining symptoms in Section A were present in >50% (Figure 2).
FIGURE 2.
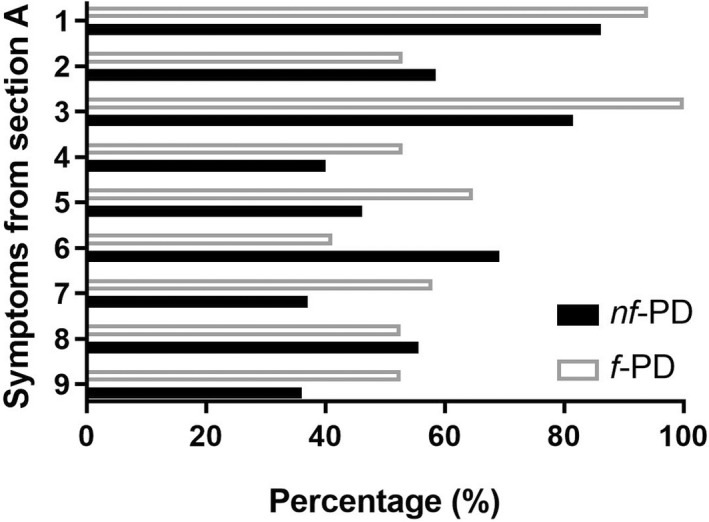
Percentage of presence of symptoms described in Section A for patients with (f‐PD) and without (nf‐PD) Parkinson disease‐related fatigue diagnosis
We also checked that if the number of symptoms required from Section A was lowered from 4 to 3 or 2, none of the nf‐PD subjects met the criteria in Sections B, C, or D.
Demographic and clinical features were not significantly associated with diagnosis of PD‐related fatigue (Table 3).
TABLE 3.
Descriptive statistics and binary multiple logistic regression analysis assessing demographics or clinical features that distinguished Parkinson disease patients with (f‐PD) and without (nf‐PD) fatigue
| Variable | Overall sample, n = 241 | f‐PD, n = 17 | nf‐PD, n = 224 | Wald test | p a | OR [95% CI] |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age, years | 65.36 ± 9.36 | 63.12 ± 9.38 | 65.53 ± 9.35 | 1.03 | 0.307 | 0.97 [0.92–1.02] |
| Education, years | 10.82 ± 4.61 | 10.94 ± 3.63 | 10.81 ± 4.69 | 0.01 | 0.912 | 1.00 [0.90–1.12] |
| Sex, male b | 142 (58.9%) | 11 (64.7%) | 131 (58.5%) | 0.25 | 0.616 | 0.76 [0.27–2.15] |
| Clinical features | ||||||
| Disease duration, years | 3.44 ± 2.91 | 3.28 ± 3.50 | 3.45 ± 2.87 | 0.05 | 0.818 | 0.97 [0.81–1.17] |
| UPDRS‐III | 24.98 ± 10.60 | 24.88 ± 13.82 | 24.99 ± 10.36 | 0.02 | 0.968 | 0.99 [0.95–1.04] |
| Modified HY scale | 1.92 ± 0.47 | 1.81 ± 0.54 | 1.92 ± 0.46 | 0.05 | 0.813 | 0.89 [0.35–2.26] |
| Modified HY stage | ||||||
| 1.0 | 34 (14.1%) | 4 (23.5%) | 30 (13.4%) | ‐ | ‐ | ‐ |
| 1.5 | 7 (2.9%) | 1 (5.9%) | 6 (2.7%) | ‐ | ‐ | ‐ |
| 2.0 | 146 (60.6%) | 5 (29.4%) | 141 (62.9%) | ‐ | ‐ | ‐ |
| 2.5 | 37 (15.4%) | 6 (35.3%) | 31 (13.8%) | ‐ | ‐ | ‐ |
| 3.0 | 15 (6.2%) | 1 (5.9%) | 14 (6.3%) | ‐ | ‐ | ‐ |
| 4.0 | 2 (0.8%) | 0 (0.0%) | 2 (0.9%) | ‐ | ‐ | ‐ |
| LEDD total, mg/day | 472.86 ± 246.10 | 592.73 ± 317.27 | 462.47 ± 237.70 | 2.72 | 0.099 | 1.00 [0.99–1.00] |
| LEDDDA, mg/day | 86.55 ± 117.85 | 142.00 ± 141.24 | 82.19 ± 115.33 | 2.27 | 0.132 | 1.00 [0.99–1.00] |
| LEDDL‐DOPA, mg/day | 353.52 ± 246.26 | 427.27 ± 325.85 | 347.13 ± 238.74 | 1.06 | 0.303 | 1.00 [0.99–1.00] |
| Antidepressants | 29 (12.0%) | 3 (17.6%) | 26 (11.6%) | 0.53 | 0.465 | 1.63 [0.43–6.06] |
| Anxiolytics | 10 (4.1%) | 0 (0.0%) | 10 (4.5%) | 0.00 | 0.999 | 0.00 [0.00–0.00] |
| Sleeping drugs | 11 (4.6%) | 0 (0.0%) | 11 (4.9%) | 0.00 | 0.999 | 0.00 [0.00–0.00] |
Note: Data are shown as mean ± SD or n (%). Model χ2 (12) = 9.63, p = 0.648, R 2 = 0.18 (Nagelkerke).
Abbreviations: CI, confidence interval; HY, Hoehn and Yahr; LEDDDA, dopamine agonists equivalent daily dosage; LEDDL‐DOPA, levodopa equivalent daily dosage; OR, odds ratio; UPDRS, Unified Parkinson's Disease Rating Scale.
Probability value related to unstandardized beta coefficient via Wald statistic.
Coded as 0 = male, 1 = female.
Acceptability of the criteria was high; as reported above, percentage of missing data was 0.8%.
In terms of validity, we found moderate associations between the diagnostic criteria for PD‐related fatigue and the PFS (AUC = 0.90, 95% confidence interval [CI] = 0.85–0.94) and the FSS (AUC = 0.94, 95% CI = 0.91–0.97), independently of specific cutoff scores (concurrent validity; Table 4). The prevalence of fatigue diagnosed according to PFS and FSS cutoff scores (Table 2) ranged from 17.8% to 46.5%. Moreover, 22.9% of patients with PFS ≥ 2.95 (κ = 0.29), 26.0% of those with PFS ≥ 3.30 (κ = 0.32), 16.8% of those with FSS ≥ 4 (κ = 0.17), and 39.5% of patients with FSS > 5 (κ = 0.48) had fatigue according to diagnostic criteria (concurrent validity). For most patients with fatigue according to rating‐scale cutoff scores, the diagnosis was not confirmed by diagnostic criteria (particularly screening Questions 1 and 2, and Section D; Table 2).
TABLE 4.
Concurrent and discriminant validity of PD‐related fatigue diagnosis (coded as 1 = present, 0 = absent) explored by simple and multiple binary logistic regression analyses
| Variable | Overall sample, n = 241 | f‐PD, n = 17 | nf‐PD, n = 224 | Wald test | p a | OR [CI 95%] |
|---|---|---|---|---|---|---|
| Concurrent validity | ||||||
| Fatigue Severity Scale | 3.04 (1.76) | 6.09 (0.81) | 2.78 (1.56) | 24.63 | <0.001 | 3.63 [2.18–6.05] |
| Parkinson Fatigue Scale | 2.35 (1.19) | 4.08 (0.75) | 2.21 (1.11) | 24.08 | <0.001 | 3.65 [2.17–6.12] |
| Discriminant validity b | ||||||
| Beck Depression Inventory | 7.25 ± 7.21 | 10.53 ± 7.85 | 7.00 ± 7.12 | 3.27 | 0.070 | 1.06 [0.99–1.14] |
| Apathy Evaluation Scale | 31.28 ± 8.63 | 31.47 ± 5.43 | 31.27 ± 8.83 | 0.54 | 0.459 | 0.97 [0.90–1.04] |
| Epworth Sleepiness Scale | 4.73 ± 3.99 | 5.18 ± 2.60 | 4.70 ± 4.07 | 0.04 | 0.835 | 0.98 [0.87–1.11] |
| Parkinson's Disease Sleep Scale | 118.28 ± 23.65 | 109.56 ± 35.44 | 118.91 ± 22.55 | 1.95 | 0.162 | 0.98 [0.97–1.00] |
| Montreal Cognitive Assessment | 21.27 ± 3.80 | 22.65 ± 4.93 | 21.17 ± 3.69 | 3.08 | 0.079 | 1.14 [0.98–1.32] |
Note: Data are shown as mean ± SD or n (%). Statistically significant variables are shown in bold.
Abbreviations: CI, confidence interval; f‐PD, PD with fatigue; nf‐PD, PD without fatigue; OR, odds ratio; PD, Parkinson disease.
Probability value related to unstandardized beta coefficient via Wald statistic.
Model χ2 (5) = 7.74, p = 0.171, R 2 = 0.08 (Nagelkerke).
Diagnosis of PD‐related fatigue was not significantly associated with subthreshold measures on the BDI, AES, ESS, PDSS, MoCA (discriminant validity; Table 4).
Interrater reliability was excellent, with an almost perfect agreement between the two independent raters (κ = 0.92, p < 0.001).
DISCUSSION
This is the first study to investigate the clinimetric properties of the recently proposed diagnostic criteria for PD‐related fatigue in a large sample of patients with PD.
In our cohort, fatigue occurred in approximately 7% of patients. This prevalence rate is lower than that reported in a recent meta‐analysis (44%–56%), summarizing the prevalence estimates from more than 40 primary studies using self‐rating scales [3]. The discrepancy between ours and previous prevalence rates may depend on the way the diagnosis is established, the time frame in which fatigue is assessed, and the clinical features of the study samples. As for the first of these aspects, in our study up to 46% of the patients were affected by fatigue according to rating scales (i.e., PFS and FSS cutoff scores), whereas only 7% met the diagnostic criteria for fatigue diagnosis. In PD, it has been demonstrated that diagnostic criteria are typically more conservative than self‐rating scales. For example, the Diagnostic and Statical Manual for Mental Disorders criteria for depression generated markedly lower prevalence estimates than rating scales (7.3% vs. 76%) [40] and were not sensitive enough to detect the subthreshold or subsyndromal forms of depression [41]. A similar scenario may apply to fatigue. Notably, on the basis of Kluger et al.'s diagnostic criteria [1] we excluded from diagnosis patients suffering from secondary fatigue (i.e., on the basis of Section D for comorbid depression, apathy, sleep disorders, beta‐blocker medication, or, less frequently, heart failure, hypothyroidism, or anemia) [42]. The percentage of patients with fatigue according to rating scales who did not meet the Section D of diagnostic criteria (i.e., who were probably affected by secondary fatigue) was quite high in our sample (27.1%–39.3%). This evidence may suggest that fatigue as a primary nonmotor disorder is rare, but it is often secondary to other disorders.
As regards the time frame in which fatigue is assessed, according to Kluger et al.'s diagnostic criteria, symptoms must be present for most of the day, every day, or nearly every day during the previous month (Question 2 of the semistructured clinical interview), whereas the time frame for the commonly used self‐rating scales is from “2 weeks” to “today” [14]. In the present study, 6.2%–13.2% of patients with fatigue according to rating scales did not conform to Question 2 and were likely affected by a condition similar to “state fatigue,” as they reported increased perception of effort disproportionate to attempted activities but not as a persistent symptom. Therefore, self‐rating scales may bias the assessment toward momentary perception of fatigue or state fatigue, often observed in general health care practice, also in relation with unspecific health care‐seeking behaviors [43]. A more stringent definition of PD‐related fatigue, such as the one operationalized in Kluger et al.'s diagnostic criteria [1], enables a more nuanced characterization of fatigue in PD and could offer better insights into its pathophysiology.
Finally, the clinical features of the study sample also may affect the observed prevalence rate of fatigue. A growing amount of evidence suggests that the fatigue in PD persists and increases over the disease course [44, 45, 46]. In a community‐based study on 233 patients with PD, fatigue (diagnosed by FSS) increased from 32.1% to 38.9% during an 8‐year follow‐up [45]. As our study sample had a mean disease duration of approximately 4 years and included 76.8% of patients at an early stage of PD (i.e., modified HY Stage 1 or 2), our study may underestimate prevalence of fatigue in the general population of patients with PD.
Eight of the nine symptoms described in Section A of the diagnostic criteria were present in >50% of patients who received the diagnosis of fatigue (f‐PD). At a phenomenological level, our results suggested that in f‐PD subjects, the experience of fatigue (i) rarely induces avoidance of doing something due to the fear of worsening symptoms (Symptom A6, the least frequently reported); and (ii) is induced by daily routine activities and thus limits the type, intensity, or duration of such activities (Symptom A1). Consistently, George et al. [17] observed that patients with PD suffering from fatigue never avoided doing something due to fear of worsening symptoms but were frequently frustrated by the hindering effect of fatigue on their ability to initiate and complete important activities. When the number of symptoms required from Section A was lowered from four to three or two, none of the nf‐PD subjects met the criteria in Section B, C, or D (i.e., changed to f‐PD). This means that these criteria and the minimum number of symptoms required from Section A are ecologically valid, as they well describe the experience of patients with fatigue diagnosis.
Demographic aspects or clinical features did not distinguish patients with fatigue diagnosis from those without it, in line with previous literature [3], and consistent with the idea that fatigue in PD might be related to impairments of nonmotor/nondopaminergic networks [47].
After the Movement Disorder Society recommendation [14], we considered the PFS and FSS as “proxy gold standards” of fatigue and found moderate associations between the diagnostic criteria [1] and the scores on these scales, which implies an overall good concurrent validity of the diagnostic criteria. By the same token, we found a substantial accuracy of PFS and FSS in classifying the presence of fatigue diagnosis independently of specific cutoff scores. Nevertheless, when specific cutoff scores were considered, a fair‐to‐moderate agreement was found. Overall, these findings support the results of a recent clinimetric validation study of PFS and FSS, which found that both scales and the available cutoff scores were useful but not sufficient measurements in detecting fatigue according to Kluger et al.'s diagnostic criteria [1, 48]. Moreover, the criteria had good validity in distinguishing fatigue from subthreshold depressive, apathetic, and sleep disorder symptoms, and cognitive functioning (discriminant validity). This adds robustness to the process of discriminating fatigue from co‐occurring and potentially confounding nonmotor features [1, 42].
As validity is a necessary but not sufficient property to support the appropriateness of a measure, we also checked acceptability and interrater reliability of PD‐related fatigue diagnostic criteria and found that both were high. These findings suggest that these criteria are suitable in clinical practice and research, and could be employed in different settings, where there is a need of consistency in clinical measures [49].
Our study has limitations that offer opportunities for further research. First, this is a monocentric study where patients with advanced stages of PD are underrepresented, and this limits the generalizability of our results. Probably, the patients with higher motor burden and disease duration refused to participate in our study due to poor motivation or for logistic reasons, and this contributed to the overrepresentation of patients with early/mild stages of disease in our sample, as in previous validation studies on PD (e.g., Chaudhuri et al. [22]). Second, the lack of a comparison group of healthy participants or patients affected by a different disease associated with chronic fatigue (e.g., multiple sclerosis, chronic fatigue syndrome) did not allow exploration of possible differences in fatigue diagnosis between patients with PD and other populations. Moreover, we did not longitudinally evaluate other potential clinimetric properties of diagnostic criteria (e.g., test–retest reliability). Finally, 13.7% of our sample was excluded from the fatigue diagnosis because of comorbid disorders (e.g., apathy and sleep disorders). We conservatively excluded these patients, but it is possible that apathy and sleep were concurrent but not causally related to PD‐related fatigue. In future research, it will be important to address the issue of the possible causal relationships between fatigue and comorbid conditions. In other terms, to ascertain whether comorbidities (such as apathy and sleep disorders) could drive fatigue, or the opposite, could even provide clues for a revision of definition criteria for PD‐related fatigue.
In conclusion, our study supported the soundness of the clinimetric properties of Kluger et al.'s recently proposed diagnostic criteria for PD‐related fatigue, in terms of acceptability, validity, and reliability. Further validation studies of Kluger et al.'s criteria [1] on samples representative of the total population of PD are needed to support their use as a gold standard in clinical practice and research as well as an “external marker” to validate (screening and diagnostic) cutoffs on the existing scales (e.g., FSS, PFS). Based on their promising clinimetric properties, Kluger et al.'s criteria [1] may also help to understand the pathophysiology of “pure” PD‐related fatigue. Moreover, future studies are warranted to examine the long‐term stability of these criteria, their role as outcome measures in clinical trials, and their consistency in comparison with other diseases associated with chronic fatigue (e.g., multiple sclerosis, chronic fatigue syndrome).
AUTHOR CONTRIBUTIONS
Mattia Siciliano: Conceptualization (lead); formal analysis (lead); writing – original draft (lead); writing – review and editing (lead). Benzi Kluger: Conceptualization (supporting); formal analysis (supporting); writing – original draft (supporting); writing – review and editing (supporting). Rosa De Micco: Methodology (supporting); writing – review and editing (supporting). Carlo Chiorri: Methodology (supporting); writing – review and editing (supporting). Valeria Sant'Elia: Writing – review and editing (supporting). Marcello Silvestro: Writing – review and editing (supporting). Alfonso Giordano: Writing – review and editing (supporting). Gioacchino Tedeschi: Writing – review and editing (supporting). Luca Passamonti: Writing – review and editing (supporting). Luigi Trojano: Conceptualization (supporting); writing – original draft (supporting); writing – review and editing (equal). Alessandro Tessitore: Conceptualization (supporting); writing – original draft (supporting); writing – review and editing (equal).
ACKNOWLEDGEMENT
Open Access Funding provided by Universita degli Studi della Campania Luigi Vanvitelli within the CRUI‐CARE Agreement.
CONFLICT OF INTEREST
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
Supporting information
APPENDIX S1
Siciliano M, Kluger B, De Micco R, et al. Validation of new diagnostic criteria for fatigue in patients with Parkinson disease. Eur J Neurol. 2022;29:2631‐2638. doi: 10.1111/ene.15411
DATA AVAILABILITY STATEMENT
Research data are not shared.
REFERENCES
- 1. Kluger BM, Herlofson K, Chou KL, et al. Parkinson's disease‐related fatigue: a case definition and recommendations for clinical research. Mov Disord. 2016;31:625‐631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kluger BM, Krupp LB, Enoka RM. Fatigue and fatigability in neurologic illnesses: proposal for a unified taxonomy. Neurology. 2013;80:409‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siciliano M, Trojano L, Santangelo G, De Micco R, Tedeschi G, Tessitore A. Fatigue in Parkinson's disease: a systematic review and meta‐analysis. Mov Disord. 2018;33:1712‐1723. [DOI] [PubMed] [Google Scholar]
- 4. Gallagher DA, Lees AJ, Schrag A. What are the most important nonmotor symptoms in patients with Parkinson's disease and are we missing them? Mov Disord. 2010;25:2493‐2500. [DOI] [PubMed] [Google Scholar]
- 5. Ongre SO, Larsen JP, Tysnes OB, Herlofson K. Fatigue in early Parkinson's disease: the Norwegian ParkWest study. Eur J Neurol. 2017;24:105‐111. [DOI] [PubMed] [Google Scholar]
- 6. Herlofson K, Kluger BM. Fatigue in Parkinson's disease. J Neurol Sci. 2017;374:38‐41. [DOI] [PubMed] [Google Scholar]
- 7. Chaudhuri A, Behan PO. Fatigue and basal ganglia. J Neurol Sci. 2000;179:34‐42. [DOI] [PubMed] [Google Scholar]
- 8. Chaudhuri A, Behan PO. Fatigue in neurological disorders. Lancet. 2004;363:978‐988. [DOI] [PubMed] [Google Scholar]
- 9. Lazcano‐Ocampo C, Wan YM, van Wamelen DJ, et al. Identifying and responding to fatigue and apathy in Parkinson's disease: a review of current practice. Expert Rev Neurother. 2020;20:477‐495. [DOI] [PubMed] [Google Scholar]
- 10. Franssen M, Winward C, Collett J, Wade D, Dawes H. Interventions for fatigue in Parkinson's disease: a systematic review and meta‐analysis. Mov Disord. 2014;29:1675‐1678. [DOI] [PubMed] [Google Scholar]
- 11. Elbers RG, Verhoef J, van Wegen EE, Berendse HW, Kwakkel G. Interventions for fatigue in Parkinson's disease. Cochrane Database Syst Rev. 2015;(10):CD010925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pavese N, Metta V, Bose SK, Chaudhuri KR, Brooks DJ. Fatigue in Parkinson's disease is linked to striatal and limbic serotonergic dysfunction. Brain. 2010;133:3434‐3443. [DOI] [PubMed] [Google Scholar]
- 13. Tessitore A, Giordano A, De Micco R, et al. Functional connectivity underpinnings of fatigue in "drug‐Naïve" patients with Parkinson's disease. Mov Disord. 2016;31:1497‐1505. [DOI] [PubMed] [Google Scholar]
- 14. Friedman JH, Alves G, Hagell P, et al. Fatigue rating scales critique and recommendations by the movement disorders society task force on rating scales for Parkinson's disease. Mov Disord. 2010;25:805‐822. [DOI] [PubMed] [Google Scholar]
- 15. Bower JE. Cancer‐related fatigue—mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014;11:597‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kluger BM. Fatigue in Parkinson's disease. Int Rev Neurobiol. 2017;133:743‐768. [DOI] [PubMed] [Google Scholar]
- 17. George DD, Baer NK, Berliner JM, Jones J, Kluger BM. What fatigue means to persons living with Parkinson's disease? A qualitative study. Mov Disord Clin Pract. 2021;8:919‐924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gibb WR, Lees AJ. A comparison of clinical and pathological features of young‐ and old‐onset Parkinson's disease. Neurology. 1988;38:1402‐1406. [DOI] [PubMed] [Google Scholar]
- 19. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord. 2015;30:1591‐1601. [DOI] [PubMed] [Google Scholar]
- 20. Dubois B, Burn D, Goetz C, et al. Diagnostic procedures for Parkinson's disease dementia: recommendations from the movement disorder society task force. Mov Disord. 2007;22:2314‐2324. [DOI] [PubMed] [Google Scholar]
- 21. Santangelo G, Siciliano M, Pedone R, et al. Normative data for the Montreal cognitive assessment in an Italian population sample. Neurol Sci. 2015;36:585‐591. [DOI] [PubMed] [Google Scholar]
- 22. Chaudhuri KR, Rizos A, Trenkwalder C, et al. EUROPAR and the IPMDS non motor PD study group. King's Parkinson's disease pain scale, the first scale for pain in PD: an international validation. Mov Disord. 2015;30:1623‐1631. [DOI] [PubMed] [Google Scholar]
- 23. Fahn S, Elton RL. UPDRS development committee. The unified Parkinson's disease rating scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, eds. Recent Developments in Parkinson'sDisease. 2nd ed. Macmillan Healthcare Information; 1987:153‐163. [Google Scholar]
- 24. Goetz CG, Poewe W, Rascol O, et al. Movement Disorder Society task force on rating scales for Parkinson's disease. Movement Disorder Society task force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord. 2004;19:1020‐1028. [DOI] [PubMed] [Google Scholar]
- 25. Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord. 2010;25:2649‐2653. [DOI] [PubMed] [Google Scholar]
- 26. Leentjens AF, Verhey FR, Luijckx GJ, Troost J. The validity of the Beck depression inventory as a screening and diagnostic instrument for depression in patients with Parkinson's disease. Mov Disord. 2000;15:1221‐1224. [DOI] [PubMed] [Google Scholar]
- 27. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The mini‐international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. J Clin Psychiatry. 1998;59(Suppl 20):22‐33. [PubMed] [Google Scholar]
- 28. Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the apathy evaluation scale. Psychiatry Res. 1991;38:143‐162. [DOI] [PubMed] [Google Scholar]
- 29. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540‐545. [DOI] [PubMed] [Google Scholar]
- 30. Chaudhuri KR, Pal S, DiMarco A, et al. The Parkinson's disease sleep scale: a new instrument for assessing sleep and nocturnal disability in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2002;73:629‐635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Epstein LJ, Kristo D, Strollo PJ Jr, et al. Adult obstructive sleep apnea task force of the American Academy of sleep medicine. Clinical guideline for the evaluation, management and long‐term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263‐276. [PMC free article] [PubMed] [Google Scholar]
- 32. Brown RG, Dittner A, Findley L, Wessely SC. The Parkinson fatigue scale. Parkinsonism Relat Disord. 2005;11:49‐55. [DOI] [PubMed] [Google Scholar]
- 33. Krupp LB, LaRocca NG, Muir‐Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121‐1123. [DOI] [PubMed] [Google Scholar]
- 34. Chen H, Cohen P, Chen S. How big is a big odds ratio? Interpreting the magnitudes of odds ratios in epidemiological studies. Commun Stat Simul Comput. 2010;39:860‐864. [Google Scholar]
- 35. Drijgers RL, Dujardin K, Reijnders JS, Defebvre L, Leentjens AF. Validation of diagnostic criteria for apathy in Parkinson's disease. Parkinsonism Relat Disord. 2010;16:656‐660. [DOI] [PubMed] [Google Scholar]
- 36. Smith SC, Lamping DL, Banerjee S, et al. Measurement of health‐related quality of life for people with dementia: development of a new instrument (DEMQOL) and an evaluation of current methodology. Health Technol Assess. 2005;9:1‐93. [DOI] [PubMed] [Google Scholar]
- 37. Hosmer DG, Lemeshow S. Applied Logistic Regression. 2nd en ed. Wiley; 2000:162. [Google Scholar]
- 38. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159‐174. [PubMed] [Google Scholar]
- 39. Lerdal A, Wahl A, Rustøen T, Hanestad BR, Moum T. Fatigue in the general population: a translation and test of the psychometric properties of the Norwegian version of the fatigue severity scale. Scand J Public Health. 2005;33:123‐130. [DOI] [PubMed] [Google Scholar]
- 40. Reijnders JS, Ehrt U, Weber WE, Aarsland D, Leentjens AF. A systematic review of prevalence studies of depression in Parkinson's disease. Mov Disord. 2008;23:183‐189. [DOI] [PubMed] [Google Scholar]
- 41. Reiff J, Schmidt N, Riebe B, et al. Subthreshold depression in Parkinson's disease. Mov Disord. 2011;26:1741‐1744. [DOI] [PubMed] [Google Scholar]
- 42. Skorvanek M, Gdovinova Z, Rosenberger J, et al. The associations between fatigue, apathy, and depression in Parkinson's disease. Acta Neurol Scand. 2015;131:80‐87. [DOI] [PubMed] [Google Scholar]
- 43. de Rijk AE, Schreurs KM, Bensing JM. Patient factors related to the presentation of fatigue complaints: results from a women's general health care practice. Women Health. 2000;30:121‐136. [DOI] [PubMed] [Google Scholar]
- 44. Friedman JH, Friedman H. Fatigue in Parkinson's disease: a nine‐year follow‐up. Mov Disord. 2001;16:1120‐1122. [DOI] [PubMed] [Google Scholar]
- 45. Alves G, Wentzel‐Larsen T, Larsen JP. Is fatigue an independent and persistent symptom in patients with Parkinson disease? Neurology. 2004;63:1908‐1911. [DOI] [PubMed] [Google Scholar]
- 46. Siciliano M, Trojano L, De Micco R, et al. Predictors of fatigue severity in early, de novo Parkinson disease patients: a 1‐year longitudinal study. Parkinsonism Relat Disord. 2020;79:3‐8. [DOI] [PubMed] [Google Scholar]
- 47. Friedman JH, Brown RG, Comella C, et al. Fatigue in Parkinson's disease: a review. Mov Disord. 2007;22:297‐308. [DOI] [PubMed] [Google Scholar]
- 48. Siciliano M, Chiorri C, De Micco R, et al. Fatigue in Parkinson's disease: Italian validation of the Parkinson fatigue scale and the fatigue severity scale using a Rasch analysis approach. Parkinsonism Relat Disord. 2019;65:105‐110. [DOI] [PubMed] [Google Scholar]
- 49. Pritchard M, Hilari K, Cocks N, Dipper L. Psychometric properties of discourse measures in aphasia: acceptability, reliability, and validity. Int J Lang Commun Dis. 2018;53:1078‐1093. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
APPENDIX S1
Data Availability Statement
Research data are not shared.


