Abstract
Background
This study identifies populations who may benefit most from expanded cancer screening.
Methods
Two American Cancer Society prospective cohort studies, Cancer Prevention Study‐II Nutrition Cohort and Cancer Prevention Study‐3, were used to identify the risk factors associated with a > 2% absolute risk of any cancer within 5 years. In total, 429,991 participants with no prior personal history of cancer were followed for cancer for up to 5 years. Multivariable Cox proportional hazards models were used to estimate hazard ratios and 95% confidence intervals for association. By using these hazard ratios, individualized coherent absolute risk estimation was used to calculate absolute risks by age.
Results
Overall, 15,226 invasive cancers were diagnosed among participants within 5 years of enrollment. The multivariable‐adjusted relative risk of any cancer was strongest for current smokers compared with never‐smokers. In men, alcohol intake, family history of cancer, red meat consumption, and physical inactivity were also associated with risk (p < .05). In women, body mass index, type 2 diabetes, hysterectomy, parity, family history of cancer, hypertension, tubal ligation, and physical inactivity were associated (p < .05). The absolute 5‐year risk exceeded 2% among nearly all participants older than 50 years and among some participants younger than 50 years, including current or former smokers (<30 years since quitting) and long‐term nonsmokers with a body mass index >25 kg/m2 or a first‐degree family history of cancer. The absolute 5‐year risk was as high as 29% in men and 25% in women.
Conclusions
Older age and smoking were the two most important risk factors associated with the relative and absolute 5‐year risk of developing any cancer.
Keywords: benefit–risk assessment, cancer prevention, cancer screening, epidemiologic factors, risk factors
Short abstract
Older age and smoking are the two most important risk factors associated with the relative and absolute 5‐year risk of developing any cancer. These results quantified the absolute risks for population subgroups to identify those who may benefit most from expanded cancer screening and prevention.
INTRODUCTION
Screening of asymptomatic persons for cancer represents a bedrock of cancer control because it increases the probability of successful treatment. Currently, screening is recommended for breast, cervix, colorectal, and prostate cancers in the general population and for lung cancers in smokers. However, these cancer types represent only approximately one third of all cancer deaths. Innovations in characterizing circulating tumor DNA can enable early detection of multiple cancers simultaneously from a single blood draw, and multiple groups have described blood‐based multicancer early detection (MCED) tests 1 , 2 , 3 , 4 , 5 , 6 for detecting a range of cancer types with no other screening option. However, an important gap in identifying populations who will benefit from enhanced cancer screening using MCED tests (along with continued screening with traditional guideline‐recommended screening tests) involves understanding the key risk factors associated with and the spectrum of the absolute risk of developing any cancer.
Previous studies have examined risk factors for single cancer types to improve etiologic understanding or to assess risk as part of established single‐cancer screening. Cancer registry data routinely report absolute incidence rates for all cancers combined, but only across sociodemographic factors (e.g., age, sex, race/ethnicity, geography) because of the lack of additional risk factor information. Large prospective cohort studies with detailed lifestyle and environmental risk factors offer unique possibilities to understand variability in age‐specific absolute risk. To that end, we took advantage of the American Cancer Society (ACS)’s long‐term prospective cohort studies to assess major risk factors for developing at least one invasive cancer within 5 years and to quantify the absolute risks associated with such factors. Excluding known high‐risk populations (e.g., those with familial hereditary syndromes, history of transplantation, or cancer survivors), the goal was to define subgroups in the general population who could benefit from enhanced cancer screening and prevention because these types of data are not widely available but are necessary to inform future multicancer screening tests.
MATERIALS AND METHODS
Cohort design
We included persons participating in two ACS prospective observational cohort studies: the Cancer Prevention Study‐II Nutrition Cohort (CPS‐IINC; n = 184,183) and the Cancer Prevention Study‐3 (CPS‐3; n = 303,682), which were established in 1992/1993 and between 2006 and 2013, respectively. Detailed study descriptions have been published elsewhere. 7 , 8 CPS‐IINC participants were invited from a larger 1982 CPS‐II mortalitycohort 7 if they were between ages of 50 and 74 years at enrollment in 1992/1993 and resided in one of 21 US states with population‐based cancer registries that ascertained at least 90% of incident cancers by 1990. Participants completed a 10‐page self‐administered survey that included questions on demographic, lifestyle, medical, and behavioral factors.
CPS‐3 participants were enrolled across 35 US states, the District of Columbia, and Puerto Rico at various community events between 2006 and 2013. Eligibility criteria included being ages 30–65 years and having no prior personal history of cancer, although a small number of participants who did not meet these criteria enrolled. Participants completed a brief enrollment survey that included sociodemographic and key cancer‐related risk factor information and provided a blood sample at enrollment. Most participants also completed at home a second, more extensive survey (24 pages for men, 28 pages for women).
Participants were excluded from this analysis if they were lost to follow‐up (n = 6192), had a reported but unverified cancer or missing diagnosis date (n = 952), were missing sex or age (n = 133), were a revoked or duplicate record (n = 209), died before their survey return date (n = 7), or, for CPS‐3 participants, lived in a state where cancer registry linkages have yet to be completed (n = 24,287). We also excluded participants who self‐reported prevalent cancer before enrollment (n = 26,094) because of the lack of detail on cancer type or time since diagnosis and because the risk factors associated with a second cancer differ from those associated with the first cancer. After exclusions, 137,334 men and 292,657 women from the combined cohorts were available for analysis.
Outcomes and covariates
Exposure assessment
All exposures were assessed at time of enrollment. Because there is not a comprehensive list of potential risk factors associated with the risk of developing any cancer, we identified candidate exposures based on risk factors for single cancer types described in the Washington University Your Disease Risk resource (https://publichealthsciences.wustl.edu/community‐focus/your‐disease‐risk‐assessment‐tool/, accessed July 18, 2022). 9
From this full list of candidate exposures, we included only those exposures that were queried in both cohorts. The exposures examined and the survey questions used to capture them are detailed in Table S1 and include the following: age (single year), sex, race (non‐Hispanic White, non‐Hispanic Black, other/mixed race), family history of cancer (yes, no, missing), body mass index (BMI [in kg/m2]; <18.5/missing, from 18.5 to <25.0, from 25.0 to <27.5, from 27.5 to <30.0, from 30.0 to <35.0, ≥35.0), current alcohol use (in drinks per day: none, <1, 1–2, >2, missing), smoking (never, former [<10, from 10 to <20, from 20 to <30, and ≥ 30 years since quitting], current, missing); moderate‐to‐vigorous physical activity (metabolic equivalent hours per week: 0.0, from >0.0 to <7.5, from 7.5 to <15.0, ≥15.0, missing), ≥5 daily servings of fruits or vegetables (yes, no, missing), limiting red meat consumption (yes, no, missing), history of type 2 diabetes (yes, no, missing), history of hypertension (yes, no, missing), and daily multivitamin use (yes, no, missing). In addition, in women only, we examined parity (0, 1–2, ≥3 live births, missing), hysterectomy (no, yes, missing), tubal ligation (no, yes, missing), ≥5 years of oral contraceptive use (no, yes, missing), and exogenous postmenopausal hormone use (never, current estrogen only, current combined estrogen and progesterone, former estrogen only, former combined use, missing/unknown).
Because CPS‐3 participants completed two different enrollment surveys, responses from the later survey were used unless the variable was missing. Physical activity and family history of cancer were only asked on the at‐home survey, thus participants who did not complete that survey were considered to have missing information.
Cancer ascertainment
In CPS‐IINC, cancers were self‐reported on the 1997 follow‐up survey (approximately 5 years after baseline) and subsequently verified through medical records or linkage with state cancer registries. A previous study 10 linking CPS‐IINC participants with state cancer registries showed 93% sensitivity in self‐reports of cancer diagnoses. A small number of cancers were identified as interval deaths (occurring between baseline and the end of 5 years of follow‐up) through routine automated linkage of the entire cohort with the National Death Index in which an invasive cancer was listed as a cause of death. For most of these interval deaths, additional information was obtained through linkage with the state cancer registries; otherwise, the date of death was used as a proxy. There were 10,574 invasive cancers (6589 in men and 3985 in women) between the date of enrollment and end of follow‐up at 5 years.
In CPS‐3, all participants were linked with state cancer registries to identify all reportable cancer diagnoses through December 31, 2015. The sensitivity of this method for ascertaining incident cancers in a multistate cohort is described elsewhere. 11 We categorized cancers with an established screening paradigm as breast, colorectal, prostate, or cervical cancer and cancers without an established screening paradigm as all other cancers, including lung cancer. Lung cancer was not considered as having an established screening paradigm because of its restricted eligibility to heavy smokers, low utilization, and because lung cancer screening was not first recommended until after the enrollment of both cohorts concluded. 12 In total, 50% of cancers in women and 57% of cancers in men were those with an established screening paradigm.
Statistical analysis
Cox proportional hazards models estimated hazard ratios (HRs) and 95% confidence intervals (CIs) for associations between potential risk factors and the subsequent risk of any cancer. Only statistically significant (p < .05) variables were retained in the final multivariable‐adjusted models. The end of follow‐up was defined as the date of a cancer diagnosis, death, or 5 years after baseline (unless 5 years was after December 31, 2015), whichever came first. Participants were also censored if they were diagnosed with an in situ cancer (except in situ bladder cancer, which was included as an incident invasive cancer). All analyses were stratified by sex, age at enrollment, and cohort and were adjusted for race.
Five‐year absolute risks were estimated using the Individualized Coherent Absolute Risk Estimator (iCARE) software packaged in R. 13 , 14 In addition to HRs from the Cox models, other model inputs included risk factor prevalence from CPS and single year, age‐specific incidence rates from the Surveillance, Epidemiology, and End Results (SEER) Program. For some risk factors, categories were collapsed when relative risk estimates were similar. We considered a cutoff point of 2% per 5 years as meaningful based on SEER data showing this as the absolute risk of any cancer at age 50 years, when most established screenings begin. 15
Several secondary analyses were conducted. First, we ran risk models separately for cancer types with and without established screening. Second, because prior observations in CPS‐II and other studies document interaction between smoking and other lifestyle risk factors, especially BMI, 16 , 17 we stratified analyses by smoking history (current and former smokers who quit within the past 30 years; never‐smokers and former smokers who quit ≥30 years ago). For parsimonious reduced absolute risk models, we re‐ran models for men and women including only the three risk factors with the largest relative risks from the multivariable‐adjusted Cox models. Finally, we re‐ran analyses stratified by cohort to internally validate our findings. SAS version 10 (SAS Institute Inc) was used to conduct all statistical analyses other than iCARE.
RESULTS
We included 429,991 participants (CPS‐IINC, 81,280 women and 73,056 men; CPS‐3, 211,377 women and 64,278 men). CPS‐IINC participants were older, enrolled about 20 years earlier and, accordingly, had different distributions of certain risk factors (Table 1). For example, CPS‐IINC had lower proportions of lifelong nonsmokers (32% of men and 54% of women vs 65% of men and 68% of women in CPS‐3) and obesity (BMI ≥30 kg/m2; 14% of men and 15% of women vs 31% of men and 30% of women in CPS‐3).
Table 1.
Distribution of Demographic and Cancer Risk Factor Baseline Characteristics by Cohort and Participant Sex, American Cancer Society Cancer Prevention Study Cohorts, 1992–2013
| No. of participants (%) | ||||
|---|---|---|---|---|
| CPS‐II, N = 154,336 | CPS‐3, N = 275,655 | |||
| Variable | Male, N = 73,056 | Female, N = 81,280 | Male, N = 64,278 | Female, N = 211,377 |
| Follow‐up: Mean ± SD, years | 4.7 ± 0.99 | 4.8 ± 0.7 | 3.6 ± 1.17 | 3.5 ± 1.14 |
| Baseline year: Median [range] | 1992 [1992–1993] | 1992 [1992–1993] | 2012 [2006–2015] | 2012 [2006–2015] |
| Age at start of follow‐up: Median (range), years | 64 [41–93] | 62 [40–90] | 48 [20–82] | 48 [19–80] |
| Diagnosed with at least one invasive cancer within 5 years | ||||
| No | 66,467 (91.0) | 77,295 (95.1) | 63,137 (98.2) | 207,866 (98.3) |
| Breast, prostate, colorectal, or cervical cancer | 3826 (5.2) | 2004 (2.5) | 546 (0.8) | 1714 (0.8) |
| All other cancers | 2763 (3.8) | 1981 (2.4) | 595 (0.9) | 1797 (0.9) |
| Race | ||||
| White | 71,145 (97.4) | 79,047 (97.3) | 53,066 (82.6) | 176,090 (83.3) |
| Black | 902 (1.2) | 1256 (1.5) | 2557 (4.0) | 10,090 (4.8) |
| Other | 1009 (1.4) | 977 (1.2) | 8655 (13.5) | 25,197 (11.9) |
| First‐degree family history of cancer | ||||
| No | 44,265 (60.6) | 47,015 (57.8) | 22,193 (34.5) | 75,566 (35.7) |
| Yes | 28,791 (39.4) | 34,265 (42.2) | 27,561 (42.9) | 100,895 (47.7) |
| Missing | 0 (0.0) | 0 (0.0) | 14,524 (22.6) | 34,916 (16.5) |
| Body mass index, kg/m2 | ||||
| 18.5 to <25 | 25,578 (35.0) | 40,739 (50.1) | 15,022 (23.4) | 82,497 (39.0) |
| 25 to <27.5 | 22,729 (31.1) | 16,518 (20.3) | 15,870 (24.7) | 35,663 (16.9) |
| 27.5 to <30 | 12,951 (17.7) | 8715 (10.7) | 12,419 (19.3) | 24,365 (11.5) |
| 30 to <35 | 8601 (11.8) | 8905 (11.0) | 13,499 (21) | 34,919 (16.5) |
| ≥35 | 1779 (2.4) | 3613 (4.4) | 6733 (10.5) | 30,114 (14.2) |
| Other/missing | 1418 (1.9) | 2790 (3.4) | 735 (1.1) | 3819 (1.8) |
| Current alcohol use, drinks per day | ||||
| None | 23,892 (32.7) | 36,935 (45.4) | 12,161 (18.9) | 45,250 (21.4) |
| <1 | 28,054 (38.4) | 30,860 (38.0) | 39,147 (60.9) | 138,339 (65.4) |
| 1–2 | 9138 (12.5) | 6156 (7.6) | 6826 (10.6) | 13,205 (6.2) |
| >2 | 8949 (12.2) | 3809 (4.7) | 2781 (4.3) | 2540 (1.2) |
| Missing | 3023 (4.1) | 3520 (4.3) | 3363 (5.2) | 12,043 (5.7) |
| Smoking history | ||||
| Never smoker | 23,481 (32.1) | 44,256 (54.4) | 41,973 (65.3) | 144,337 (68.3) |
| ≥30 years since quitting | 11,623 (15.9) | 6831 (8.4) | 3366 (5.2) | 8927 (4.2) |
| 20 to <30 years since quitting | 12,225 (16.7) | 7979 (9.8) | 3641 (5.7) | 13,030 (6.2) |
| 10 to <20 years since quitting | 9744 (13.3) | 6744 (8.3) | 4434 (6.9) | 14,249 (6.7) |
| <10 years since quitting | 8614 (11.8) | 7242 (8.9) | 5385 (8.4) | 16,070 (7.6) |
| Current smoker | 6687 (9.2) | 6888 (8.5) | 4246 (6.6) | 12,114 (5.7) |
| Missing | 682 (0.9) | 1340 (1.6) | 1233 (1.9) | 2650 (1.3) |
| Physical activity, METs | ||||
| None/sedentary | 8871 (12.1) | 7457 (9.2) | 2421 (3.8) | 9418 (4.5) |
| >0.0 to <7.5 | 21,536 (29.5) | 27,413 (33.7) | 8050 (12.5) | 38,720 (18.3) |
| 7.5 to <15.0 | 19,097 (26.1) | 23,943 (29.5) | 6829 (10.6) | 26,443 (12.5) |
| ≥15.0 | 22,435 (30.7) | 21,216 (26.1) | 29,490 (45.9) | 86,932 (41.1) |
| Missingb | 1117 (1.5) | 1251 (1.5) | 17,488 (27.2) | 49,864 (23.6) |
| Diet: ≥5 servings of F and V per day | ||||
| No | 56,490 (77.3) | 61,330 (75.5) | 50,670 (78.8) | 154,524 (73.1) |
| Yes | 9410 (12.9) | 12,448 (15.3) | 8319 (12.9) | 41,890 (19.8) |
| Missing | 7156 (9.8) | 7502 (9.2) | 5289 (8.2) | 14,963 (7.1) |
| Diet: Limited red meat consumption | ||||
| No | 48,455 (66.3) | 38,833 (47.8) | 43,808 (68.2) | 118,211 (55.9) |
| Yes | 17,445 (23.9) | 34,945 (43.0) | 15,060 (23.4) | 77,470 (36.7) |
| Missing | 7156 (9.8) | 7502 (9.2) | 5410 (8.4) | 15,696 (7.4) |
| Had mammogram in last 3 yearsa | ||||
| No | 0 (0.0) | 9729 (12.0) | 0 (0.0) | 4697 (2.2) |
| Yes | 0 (0.0) | 69,497 (85.5) | 0 (0.0) | 95,111 (45.0) |
| Missing | 73,056 (100.0) | 2054 (2.5) | 64,278 (100.0) | 111,569 (52.8) |
| Parity | ||||
| Nulliparous | 0 (0.0) | 6089 (7.5) | 0 (0.0) | 43,824 (20.7) |
| 1–2 Births | 0 (0.0) | 27,422 (33.7) | 0 (0.0) | 113,046 (53.5) |
| ≥3 Births | 0 (0.0) | 46,199 (56.8) | 0 (0.0) | 47,975 (22.7) |
| Missing | 73,056 (100.0) | 1570 (1.9) | 64,278 (100.0) | 6532 (3.1) |
| Diabetes | ||||
| No | 57,011 (78.0) | 65,054 (80.0) | 59,349 (92.3) | 199,494 (94.4) |
| Yes | 6516 (8.9) | 4821 (5.9) | 3458 (5.4) | 8446 (4.0) |
| Missing | 9529 (13.0) | 11,405 (14) | 1471 (2.3) | 3437 (1.6) |
| Hypertension | ||||
| No | 39,825 (54.5) | 48,731 (60.0) | 45,172 (70.3) | 162,430 (76.8) |
| Yes | 28,585 (39.1) | 27,138 (33.4) | 18,275 (28.4) | 46,899 (22.2) |
| Missing | 4646 (6.4) | 5411 (6.7) | 831 (1.3) | 2048 (1.0) |
| Uses multivitamin daily | ||||
| No | 52,945 (72.5) | 53,303 (65.6) | 7699 (12.0) | 35,462 (16.8) |
| Yes | 18,479 (25.3) | 25,812 (31.8) | 16,742 (26.0) | 59,389 (28.1) |
| Missingb | 1632 (2.2) | 2165 (2.7) | 39,837 (62.0) | 116,526 (55.1) |
| Hysterectomy | ||||
| No | 0 (0.0) | 49,718 (61.2) | 0 (0.0) | 156,523 (74.0) |
| Yes | 0 (0.0) | 28,404 (34.9) | 0 (0.0) | 36,679 (17.4) |
| Missing | 73,056 (100.0) | 3158 (3.9) | 64,278 (100.0) | 18,175 (8.6) |
| Tubal ligation | ||||
| No | 0 (0.0) | 73,566 (90.5) | 0 (0.0) | 140,478 (66.5) |
| Yes | 0 (0.0) | 7714 (9.5) | 0 (0.0) | 39,178 (18.5) |
| Missing | 73,056 (100.0) | 0 (0.0) | 64,278 (100.0) | 31,721 (15.0) |
| Oral contraceptive use >5 years | ||||
| No | 0 (0.0) | 65,627 (80.7) | 0 (0.0) | 108,662 (51.4) |
| Yes | 0 (0.0) | 13,314 (16.4) | 0 (0.0) | 102,715 (48.6) |
| Missing | 73,056 (100.0) | 2339 (2.9) | 64,278 (100.0) | 0 (0.0) |
| Used hormone‐replacement therapy | ||||
| Never | 0 (0.0) | 34,554 (42.5) | 0 (0.0) | 166,787 (78.9) |
| Currently uses estrogen | 0 (0.0) | 14,909 (18.3) | 0 (0.0) | 7181 (3.4) |
| Formerly used estrogen | 0 (0.0) | 11,991 (14.8) | 0 (0.0) | 10,306 (4.9) |
| Currently used estrogen and progestin | 0 (0.0) | 7182 (8.8) | 0 (0.0) | 8038 (3.8) |
| Formerly used estrogen and progestin | 0 (0.0) | 1824 (2.2) | 0 (0.0) | 9958 (4.7) |
| Unknown | 73,056 (100.0) | 10,820 (13.3) | 64,278 (100.0) | 9107 (4.3) |
| Had cervical cancer screening in past 3 years | ||||
| No | 0 (0.0) | 7171 (8.8) | 0 (0.0) | 15,947 (7.5) |
| Yes | 0 (0.0) | 46,539 (57.3) | 0 (0.0) | 191,644 (90.7) |
| Missing | 73,056 (100.0) | 27,570 (33.9) | 64,278 (100.0) | 3786 (1.8) |
Abbreviations: CPS, Cancer Prevention Study; F, fruits; METs, metabolic energy equivalent hours; SD, standard deviation; V, vegetables.
The value was set to missing for those participants who were under the age limit for eligibility to be screened.
Participants who did not complete the more detailed survey in CPS‐3 have missing data.
In total, 15,226 invasive cancers (men, 7730; women, 7496) were diagnosed within 5 years of enrollment. Among these cancers, 57% in men and 50% in women were cancer types with an established screening paradigm, with breast cancer being the most commonly diagnosed cancer in women (Fig. 1A) and prostate cancer in men (Fig. 1B). Among the cancers without established screening at the time of enrollment of both cohorts, the most commonly diagnosed cancers were lung cancer in men and uterine cancer in women.
FIGURE 1.
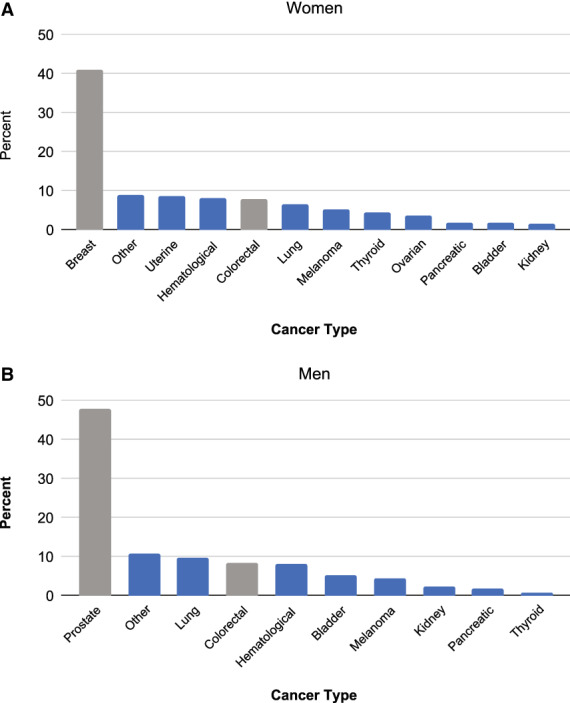
The most commonly diagnosed cancers in American Cancer Society Cancer Prevention Studies II and 3 by sex are represented as percentages among (A) women and (B) men. Gray shading indicates cancers with existing screening tests, and blue indicates cancers without existing screening tests.
Models for associations between all potential risk factors and subsequent cancer risk are shown in Table S2. Associations were strongest for current smoking compared with never smoking (HR, 1.63; 95% CI, 1.51–1.77 in men; HR, 1.55; 95% CI, 1.43–1.68 in women). In men, statistically significant associations were also observed for family history of cancer, alcohol intake, red meat consumption, and physical activity (Table 2). In women, statistically significant associations were observed for BMI, type 2 diabetes, family history of cancer, current estrogen and progesterone exogenous postmenopausal hormone use, hypertension, hysterectomy, parity, and tubal ligation (Table 2). In models stratified by cancers with or without established screening (Table 3), relative risk associations were generally similar. Two notable exceptions were current smoking and BMI, in which associations were stronger for cancers without established screening. Relative risk associations were not meaningfully different when models were stratified by cohort; 95% CIs overlapped but were wider in CPS‐3 because of smaller case numbers (data not shown).
TABLE 2.
Risk Factors for Developing any Invasive Cancer in 5 Years that were Deemed Significant in Multivariate Modeling, by Sex: American Cancer Society Cancer Prevention Study Cohorts, 1992–2013
| Male | Female | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Person‐years | No. of cancers | HR adjusted minimallya | HR adjusted multivariately | Person‐years | No. of cancers | HR adjusted minimallya | HR adjusted multivariately |
| Smoking history | ||||||||
| Never smoker | 259,880 | 2456 | 1.00 (Ref) | 1.00 (Ref) | 712,502 | 4189 | 1.00 (Ref) | 1.00 (Ref) |
| ≥30 years since quitting | 65,902 | 1189 | 1.06 (0.98–1.13) | 1.05 (0.98–1.13) | 62,025 | 542 | 1.03 (0.94–1.13) | 1.04 (0.95–1.14) |
| 20 to <30 years since quitting | 70,374 | 1150 | 1.10 (1.03–1.18)b | 1.09 (1.02–1.18)b | 83,954 | 661 | 1.11 (1.02–1.20)b | 1.11 (1.02–1.20)b |
| 10 to <20 years since quitting | 61,055 | 1006 | 1.24 (1.15–1.34)b | 1.23 (1.14–1.32)b | 81,877 | 580 | 1.15 (1.05–1.25)b | 1.14 (1.04–1.24)b |
| <10 years since quitting | 58,766 | 981 | 1.41 (1.31–1.52)b | 1.38 (1.28–1.49)b | 90,557 | 706 | 1.39 (1.28–1.51)b | 1.37 (1.27–1.49)b |
| Current smoker | 46,205 | 871 | 1.69 (1.56–1.83)b | 1.63 (1.51–1.77)b | 77,303 | 698 | 1.55 (1.43–1.68)b | 1.55 (1.43–1.68)b |
| Current alcohol use, drinks per day | ||||||||
| None | 152,892 | 2383 | 1.00 (Ref) | 1.00 (Ref) | 333,105 | 2629 | 1.00 (Ref) | 1.00 (Ref) |
| <1 | 271,604 | 3095 | 0.99 (0.93–1.04) | 0.98 (0.93–1.04) | 627,891 | 3636 | 0.95 (0.90–1.00)b | 0.94 (0.89–0.99)b |
| 1–2 | 67,049 | 922 | 0.94 (0.87–1.01) | 0.93 (0.86–1.01) | 74,072 | 535 | 0.95 (0.86–1.04) | 0.93 (0.84–1.02) |
| >2 | 51,520 | 973 | 1.17 (1.08–1.26)b | 1.08 (1.00–1.17)b | 27,089 | 286 | 1.22 (1.08–1.37)b | 1.09 (0.96–1.23) |
| Family history of cancer | ||||||||
| No | 284,208 | 4040 | 1.00 (Ref) | 1.00 (Ref) | 483,710 | 3232 | 1.00 (Ref) | 1.00 (Ref) |
| Yes | 229,763 | 3476 | 1.15 (1.10–1.20)b | 1.15 (1.10–1.20)b | 508,694 | 3668 | 1.08 (1.03–1.13)b | 1.08 (1.03–1.13)b |
| Body mass index, kg/m2 | ||||||||
| 18.5 to <25.0 | — | — | — | — | 480,240 | 3205 | 1.00 (Ref) | 1.00 (Ref) |
| 25.0 to <27.5 | — | — | — | — | 204,412 | 1325 | 0.96 (0.90–1.03) | 0.96 (0.90–1.03) |
| 27.5 to <30.0 | — | — | — | — | 127,370 | 858 | 1.06 (0.98–1.14) | 1.06 (0.98–1.14) |
| 30.0 to <35.0 | — | — | — | — | 164,989 | 1116 | 1.15 (1.07–1.23)b | 1.14 (1.06–1.22)b |
| ≥35.0 | — | — | — | — | 122,481 | 794 | 1.31 (1.21–1.42)b | 1.25 (1.15–1.36)b |
| Diabetes | ||||||||
| No | — | — | — | — | 1,004,460 | 6300 | 1.00 (Ref) | 1.00 (Ref) |
| Yes | — | — | — | — | 51,570 | 481 | 1.21 (1.10–1.32)b | 1.10 (1.00–1.21)b |
| Diet: Limited red meat consumption | ||||||||
| No | 379,577 | 5200 | 1.00 (Ref) | 1.00 (Ref) | — | — | — | — |
| Yes | 132,185 | 1727 | 0.90 (0.85–0.95)b | 0.95 (0.90–1.00)b | — | — | — | — |
| Physical activity, METs | ||||||||
| None/sedentary | 48,058 | 926 | 1.00 (Ref) | 1.00 (Ref) | — | — | — | — |
| >0.0 to <7.5 | 128,051 | 2084 | 0.86 (0.80–0.93)b | 0.91 (0.84–0.98)b | — | — | — | — |
| 7.5 to <15.0 | 113,231 | 1827 | 0.83 (0.77–0.90)b | 0.89 (0.82–0.97)b | — | — | — | — |
| ≥15.0 | 208,102 | 2478 | 0.81 (0.75–0.87)b | 0.87 (0.81–0.94)b | — | — | — | — |
| Hypertension | ||||||||
| No | — | — | — | — | 799,294 | 4657 | 1.00 (Ref) | 1.00 (Ref) |
| Yes | — | — | — | — | 290,978 | 2518 | 1.14 (1.09–1.20)b | 1.09 (1.03–1.15)b |
| Parity | ||||||||
| Nulliparous | NA | NA | NA | NA | 175,765 | 1046 | 1.00 (Ref) | 1.00 (Ref) |
| 1–2 Births | NA | NA | NA | NA | 523,083 | 3294 | 0.90 (0.84–0.97)b | 0.90 (0.84–0.97)b |
| ≥3 Births | NA | NA | NA | NA | 392,458 | 2955 | 0.82 (0.76–0.88)b | 0.82 (0.76–0.88)b |
| Hysterectomy | ||||||||
| No | NA | NA | NA | NA | 774,137 | 5130 | 1.00 (Ref) | 1.00 (Ref) |
| Yes | NA | NA | NA | NA | 265,516 | 1918 | 0.84 (0.80–0.88)b | 0.83 (0.78–0.88)b |
| Used hormone‐replacement therapy | ||||||||
| Never | NA | NA | NA | NA | 741,859 | 4251 | 1.00 (Ref) | 1.00 (Ref) |
| Currently uses estrogen | NA | NA | NA | NA | 96,734 | 770 | 0.88 (0.81–0.95)b | 1.01 (0.92–1.10) |
| Formerly used estrogen | NA | NA | NA | NA | 94,109 | 878 | 0.93 (0.86–1.01) | 0.96 (0.89–1.04) |
| Currently used estrogen and progestin | NA | NA | NA | NA | 62,812 | 513 | 1.08 (0.98–1.19) | 1.13 (1.03–1.24)b |
| Formerly used estrogen and progestin | NA | NA | NA | NA | 43,210 | 297 | 0.93 (0.82–1.04) | 0.95 (0.84–1.08) |
| Unknown | NA | NA | NA | NA | 87,730 | 787 | 1.10 (1.02–1.19)b | 1.11 (1.03–1.21) b |
| Tubal ligation | ||||||||
| No | NA | NA | NA | NA | 830,777 | 5822 | 1.00 (Ref) | 1.00 (Ref) |
| Yes | NA | NA | NA | NA | 172,093 | 1129 | 1.07 (1.00–1.14)b | 1.07 (1.00–1.15)b |
Note: Some frequencies may not add up to 100 because of missing data for that exposure.
Abbreviations: HR, hazard ratio; METs, metabolic energy equivalents; NA, not applicable; Ref, reference category.
Adjusted for age, race, and cohort.
These values indicate a significant association.
TABLE 3.
Risk Factors for Developing any Invasive Cancer in 5 Years that were Deemed Significant in Multivariate Modeling for Cancers with and without Existing Screens: American Cancer Society Cancer Prevention Study Cohorts, 1992–2013
| Male | Female | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancers with screening tests | Cancers without screening tests | Cancers with screening tests | Cancers without screening tests | |||||||||
| Variable | No. of cancers | HR adjusted minimallya | HR adjusted multivariately | No. of cancers | HR adjusted minimallya | HR adjusted multivariately | No. of cancers | HR adjusted minimallya | HR adjusted multivariately | No. of cancers | HR adjusted minimallya | HR adjusted multivariately |
| Smoking history | ||||||||||||
| Never smoker | 1624 | 1.00 (Ref) | 1.00 (Ref) | 832 | 1.00 (Ref) | 1.00 (Ref) | 2163 | 1.00 (Ref) | 1.00 (Ref) | 2026 | 1.00 (Ref) | 1.00 (Ref) |
| ≥30 years since quitting | 769 | 0.99 (0.90–1.08) | 0.98 (0.90–1.07) | 420 | 1.18 (1.04–1.33)b | 1.18 (1.05–1.33)b | 287 | 1.08 (0.95–1.22) | 1.08 (0.95–1.22) | 255 | 0.99 (0.86–1.13) | 1.01 (0.88–1.15) |
| 20 to <30 years since quitting | 657 | 0.90 (0.82–0.99)b | 0.89 (0.81–0.97)b | 493 | 1.51 (1.35–1.69)b | 1.51 (1.35–1.69)b | 337 | 1.08 (0.96–1.21) | 1.06 (0.94–1.19) | 324 | 1.14 (1.01–1.28)b | 1.16 (1.03–1.30)b |
| 10 to <20 years since quitting | 536 | 0.95 (0.86–1.05) | 0.93 (0.85–1.03) | 470 | 1.84 (1.64–2.07)b | 1.83 (1.63–2.06)b | 293 | 1.11 (0.98–1.26) | 1.08 (0.96–1.23) | 287 | 1.19 (1.05–1.35)b | 1.19 (1.05–1.35)b |
| <10 years since quitting | 425 | 0.88 (0.79–0.97)b | 0.86 (0.77–0.96)b | 556 | 2.54 (2.28–2.83)b | 2.49 (2.23–2.78)b | 303 | 1.15 (1.02–1.30)b | 1.12 (0.99–1.26) | 403 | 1.65 (1.48–1.84)b | 1.65 (1.48–1.84)b |
| Current smoker | 322 | 0.90 (0.80–1.01) | 0.87 (0.77–0.98)b | 549 | 3.38 (3.03–3.77)b | 3.24 (2.90–3.63)b | 275 | 1.16 (1.02–1.31)b | 1.15 (1.01–1.30)b | 423 | 1.98 (1.78–2.20)b | 2.00 (1.79–2.22)b |
| Current alcohol use, drinks per day | ||||||||||||
| None | 1359 | 1.00 (Ref) | 1.00 (Ref) | 1024 | 1.00 (Ref) | 1.00 (Ref) | 1261 | 1.00 (Ref) | 1.00 (Ref) | 1368 | 1.00 (Ref) | 1.00 (Ref) |
| <1 | 1744 | 1.00 (0.93–1.07) | 1.00 (0.93–1.08) | 1351 | 0.97 (0.89–1.05) | 0.96 (0.88–1.04) | 1846 | 1.02 (0.94–1.10) | 1.02 (0.94–1.10) | 1790 | 0.88 (0.82–0.95)b | 0.87 (0.81–0.94)b |
| 1–2 | 533 | 0.96 (0.87–1.06) | 0.97 (0.88–1.07) | 389 | 0.92 (0.81–1.03) | 0.89 (0.79–1.00)b | 281 | 1.05 (0.92–1.20) | 1.05 (0.92–1.20) | 254 | 0.85 (0.75–0.98)b | 0.82 (0.71–0.94)b |
| >2 | 538 | 1.12 (1.02–1.24)b | 1.15 (1.04–1.27)b | 435 | 1.23 (1.10–1.37)b | 1.02 (0.91–1.14) | 138 | 1.22 (1.02–1.45)b | 1.16 (0.97–1.39) | 148 | 1.21 (1.02–1.44)b | 1.02 (0.86–1.21) |
| Family history of cancer | ||||||||||||
| No | 2295 | 1.00 (Ref) | 1.00 (Ref) | 1745 | 1.00 (Ref) | 1.00 (Ref) | 1612 | 1.00 (Ref) | 1.00 (Ref) | 1620 | 1.00 (Ref) | 1.00 (Ref) |
| Yes | 1985 | 1.17 (1.10–1.24)b | 1.17 (1.10–1.24)b | 1491 | 1.12 (1.04–1.20)b | 1.12 (1.05–1.21)b | 1821 | 1.09 (1.01–1.16)b | 1.09 (1.02–1.17)b | 1847 | 1.08 (1.00–1.15)b | 1.07 (1.00–1.15)b |
| Body mass index, kg/m2 | ||||||||||||
| 18.5 to <25.0 | — | — | — | — | — | — | 1604 | 1.00 (Ref) | 1.00 (Ref) | 1601 | 1.00 (Ref) | 1.00 (Ref) |
| 25.0 to <27.5 | — | — | — | — | — | — | 674 | 0.98 (0.90–1.07) | 0.98 (0.90–1.08) | 651 | 0.95 (0.87–1.04) | 0.95 (0.86–1.04) |
| 27.5 to <30.0 | — | — | — | — | — | — | 449 | 1.11 (1.00–1.23)b | 1.11 (1.00–1.23)b | 409 | 1.01 (0.91–1.13) | 1.01 (0.90–1.12) |
| 30.0 to <35.0 | — | — | — | — | — | — | 570 | 1.17 (1.06–1.29)b | 1.16 (1.05–1.28)b | 546 | 1.13 (1.03–1.25)b | 1.11 (1.01–1.23)b |
| ≥35.0 | — | — | — | — | — | — | 338 | 1.10 (0.98–1.24) | 1.07 (0.94–1.21) | 456 | 1.51 (1.36–1.68)b | 1.44 (1.29–1.61)b |
| Diabetes | ||||||||||||
| No | — | — | — | — | — | — | 3129 | 1.00 (Ref) | 1.00 (Ref) | 3171 | 1.00 (Ref) | 1.00 (Ref) |
| Yes | — | — | — | — | — | — | 229 | 1.16 (1.01–1.32)b | 1.09 (0.95–1.26) | 252 | 1.25 (1.10–1.43)b | 1.11 (0.97–1.27) |
| Diet: Limited red meat consumption | ||||||||||||
| No | 2900 | 1.00 (Ref) | 1.00 (Ref) | 2300 | 1.00 (Ref) | 1.00 (Ref) | — | — | — | — | — | — |
| Yes | 1033 | 0.96 (0.90–1.04) | 0.96 (0.90–1.04) | 694 | 0.82 (0.75–0.89)b | 0.92 (0.85–1.00)b | — | — | — | — | — | — |
| Physical activity, METs | ||||||||||||
| None/sedentary | 485 | 1.00 (Ref) | 1.00 (Ref) | 441 | 1.00 (Ref) | 1.00 (Ref) | — | — | — | — | — | — |
| >0.0 to <7.5 | 1149 | 0.91 (0.82–1.02) | 0.91 (0.82–1.01) | 935 | 0.81 (0.72–0.90)b | 0.90 (0.81–1.01) | — | — | — | — | — | — |
| 7.5 to <15.0 | 1081 | 0.94 (0.85–1.05) | 0.94 (0.84–1.05) | 746 | 0.71 (0.63–0.80)b | 0.84 (0.74–0.94)b | — | — | — | — | — | — |
| ≥15.0 | 1455 | 0.94 (0.84–1.04) | 0.93 (0.83–1.03) | 1023 | 0.67 (0.60–0.75)b | 0.80 (0.72–0.90)b | — | — | — | — | — | — |
| Hypertension | ||||||||||||
| No | — | — | — | — | — | — | 2299 | 1.00 (Ref) | 1.00 (Ref) | 2358 | 1.00 (Ref) | 1.00 (Ref) |
| Yes | — | — | — | — | — | — | 1252 | 1.16 (1.08–1.24)b | 1.13 (1.05–1.22)b | 1266 | 1.13 (1.05–1.21)b | 1.05 (0.97–1.13) |
| Parity | ||||||||||||
| Nulliparous | NA | NA | NA | NA | NA | NA | 502 | 1.00 (Ref) | 1.00 (Ref) | 544 | 1.00 (Ref) | 1.00 (Ref) |
| 1–2 Births | NA | NA | NA | NA | NA | NA | 1651 | 0.93 (0.84–1.02) | 0.92 (0.83–1.02) | 1643 | 0.88 (0.80–0.97)b | 0.89 (0.80–0.98)b |
| ≥3 Births | NA | NA | NA | NA | NA | NA | 1482 | 0.84 (0.75–0.93)b | 0.82 (0.74–0.92)b | 1473 | 0.80 (0.73–0.89)b | 0.82 (0.73–0.91)b |
| Hysterectomy | ||||||||||||
| No | NA | NA | NA | NA | NA | NA | 2571 | 1.00 (Ref) | 1.00 (Ref) | 2559 | 1.00 (Ref) | 1.00 (Ref) |
| Yes | NA | NA | NA | NA | NA | NA | 964 | 0.84 (0.78–0.91)b | 0.84 (0.77–0.91)b | 954 | 0.84 (0.78–0.90)b | 0.83 (0.76–0.90)b |
| Used hormone‐replacement therapy | ||||||||||||
| Never | NA | NA | NA | NA | NA | NA | 2143 | 1.00 (Ref) | 1.00 (Ref) | 2108 | 1.00 (Ref) | 1.00 (Ref) |
| Currently uses estrogen | NA | NA | NA | NA | NA | NA | 402 | 0.89 (0.80–0.99)b | 1.00 (0.88–1.13) | 368 | 0.86 (0.77–0.97)b | 1.01 (0.89–1.15) |
| Formerly used estrogen | NA | NA | NA | NA | NA | NA | 396 | 0.85 (0.76–0.95)b | 0.87 (0.78–0.98)b | 482 | 1.02 (0.92–1.13) | 1.06 (0.95–1.17) |
| Currently used estrogen and progestin | NA | NA | NA | NA | NA | NA | 272 | 1.10 (0.97–1.25) | 1.13 (0.99–1.28) | 241 | 1.06 (0.93–1.22) | 1.13 (0.99–1.30) |
| Formerly used estrogen and progestin | NA | NA | NA | NA | NA | NA | 132 | 0.82 (0.69–0.99)b | 0.84 (0.70–1.00)b | 165 | 1.03 (0.87–1.21) | 1.07 (0.91–1.26) |
| Unknown | NA | NA | NA | NA | NA | NA | 373 | 1.02 (0.91–1.14) | 1.05 (0.93–1.18) | 414 | 1.18 (1.06–1.32)b | 1.18 (1.06–1.32)b |
| Tubal ligation | ||||||||||||
| No | NA | NA | NA | NA | NA | NA | 2883 | 1.00 (Ref) | 1.00 (Ref) | 2939 | 1.00 (Ref) | 1.00 (Ref) |
| Yes | NA | NA | NA | NA | NA | NA | 576 | 1.09 (0.99–1.19) | 1.11 (1.01–1.22)b | 553 | 1.04 (0.95–1.15) | 1.04 (0.94–1.14) |
Note: Some frequencies may not add up to 100 because of missing data for that exposure.
Abbreviations: HR, hazard ratio; METs, metabolic energy equivalents; NA, not applicable; Ref, reference category.
Adjusted for age, race, and cohort.
These values indicate a significant association.
The absolute risk of developing any cancer within 5 years was ≥2% regardless of risk factor profile for nearly all men and women aged 50 years or older and was as high as 29% in men and 25% in women for some risk factor profiles at the oldest ages (see Table S3 for men and Table S4 for women). In addition, within any given 5‐year age range, absolute rates differed by about 2.5‐fold in men and 4‐fold in women based on risk factor profiles. In men younger than 50 years, absolute risks were only higher than 2% among current or former smokers (<30 years since quitting) at age 45 to <50 years (Table S3), with the highest risk (2.7%) for male current smokers who were also exposed to all other risk factors included (i.e., heavy drinkers with a family history of cancer who did not limit red meat intake and were physically inactive). In women, beginning at age 35 to <40 years, absolute risks were ≥ 2% for current or recent former smokers with various other risk factor combinations (Table S4). In addition, in some women who were current or recent former smokers, based on other risk factors, absolute risks between ages 40 and < 45 years were as high as 3.8% and as high as 5.8% between ages 45 and < 50 years. In Figure 2, age‐specific incidence curves are shown for the highest risk groups (current smoker, BMI >30 kg/m2, first‐degree family history of cancer), the lowest risk groups (never‐smoker, BMI <25 kg/m2, no family history of cancer) and the general population (SEER).
FIGURE 2.
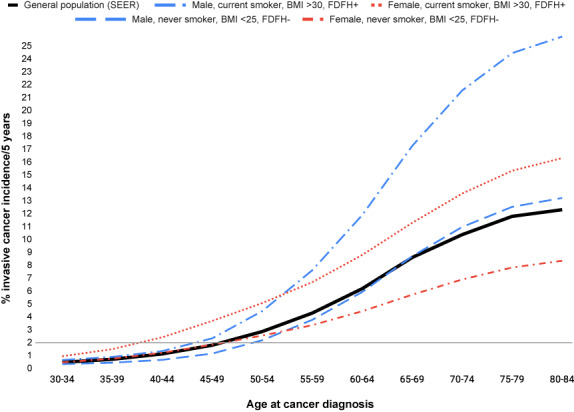
Age‐specific incidence curves describing the absolute risk for the highest risk groups (current smoker, body mass index [BMI] >30 kg/m2, first‐degree family history of cancer [FDFH+]) and the lowest risk groups (never‐smoker, BMI ≤25 kg/m2, no first‐degree family history of cancer [FDFH−]) compared with the general population, American Cancer Society Cancer Prevention Studies II and 3. SEER indicates the Surveillance, Epidemiology, and End Results Program.
To examine associations in the absence of smoking exposure, we stratified Cox models by smoking status (Table S5). Although high BMI was not statistically significantly associated with cancer risk in men who were current and recent former smokers, BMI was associated with a 27% higher risk (HR, 1.27; 95% CI, 1.07–1.53 for BMI >35.0 vs 18.5 to <25.0 kg/m2) in never‐smokers or smokers who quit ≥30 years ago. In men who never smoked or who quit ≥30 years ago, diabetes and family history of cancer also increased cancer risk. In women who never smoked or who quit ≥30 years ago, BMI, family history of cancer, and parity were most strongly associated with cancer risk followed by hypertension, current combination postmenopausal hormone use, hysterectomy, and tubal ligation (Table S5).
Absolute risks did not exceed 2% for any risk factor profile among men younger than 50 years who were never‐smokers or long‐term former smokers (Table S6). At older ages (up to 80 to <85 years), the absolute risk in men who were never‐smokers or long‐term former smokers never exceeded 19%. In women who never smoked or who quit ≥30 years ago, the absolute risks were ≥ 2% for nearly all risk factor profiles beginning at age 45 to <50 years (Table S7), suggesting that nonsmokers assume risk from additional risk factors. At older ages (up to 80 to <85 years), the absolute risk in women who were never‐smokers or long‐term former smokers never exceeded 17%.
DISCUSSION
Understanding risk factors for, and the spectrum of, absolute risk of any cancer can help identify populations who might benefit from enhanced cancer screening and prevention, including MCED testing. 5 , 18 , 19 Here, in two large, well studied, prospective cohorts, we observed that, after age, the most important risk factor for developing any cancer in 5 years was smoking history. For never‐smokers (or those who quit a long time ago), BMI, and family history of cancer were the most important. Although several other risk factors were statistically significantly associated with a higher relative risk of any cancer, they did not substantially aid in discriminating between absolute risk groups. Our estimation of the 5‐year absolute risk of developing cancer within 5‐year age groups (e.g., ages 40 to <45 years or 50 to <55 years) showed substantial (2.5–4 fold) variation according to risk factor profile. We found that nearly all persons aged 50 years or older (regardless of risk profile), male smokers (current or quit <30 years ago) aged 45–49 years, and women aged 35–49 years with particular risk factor profiles, including smoking, had risk above a 2% threshold.
Recognizing that the utility of enhanced screening may be most beneficial for cancer types other than those for which screening paradigms existed at the time of study enrollment, we repeated analyses to exclude breast, prostate, colorectal, and cervical cancers, and generally observed similar patterns of association, although the magnitude of association with smoking history and BMI was stronger when considering only cancers without an established screening paradigm. This finding may either be because the cancers included in the group (e.g., lung, pancreas, bladder) are more strongly smoking‐related, 20 or it may reflect a detection bias in those cancers with an established screening paradigm because studies have documented that smokers are less likely to be screened. 21
Our approach emphasized both relative and absolute estimates of age‐specific risk of any invasive cancer. Absolute risks can be used by primary care physicians interested in segmenting patient populations for enhancing overall cancer screening and prevention. We observed that age remained the most important risk factor and was associated with the highest absolute risks (≥2% in those older than 50 years), but that, within a given age group (age younger than 50 years), other risk factors could increase risk to ≥2%.
Although some early CPS‐II analyses assessed risk factors associated with overall cancer mortality, to our knowledge, few prior efforts have addressed the risk factors and absolute risks of developing any type of cancer. 16 , 22 At least one team, however, has set forth a risk stratification strategy for MCED, suggesting that it might increase risk–benefit balance. Chatterjee et al. propose a sophisticated model including BMI, smoking, family history of cancer, and cancer‐specific polygenic risk scores, which culminate into an index predicting risk of at least one of eight cancer types in women. 23 Our analysis incorporated risk factor data that would be routinely available in primary care settings, as germline genetic information is unlikely to be part of routine medical care in the near future.
Limitations
This study had the strengths of large size and detailed data on a broad range of risk factors, including those included in previous assessments of population‐attributable risk. 24 Limitations include lack of data on every risk factor included in Your Disease Risk or all factors that a primary care physician might want to consider. For example, we did not have information on hereditary cancer syndromes, gene variants, or polygenic risk scores strongly associated with individual cancers; chronic viral infections (e.g., HIV) or medical conditions (e.g., solid organ transplantation) associated with multiple cancers; or some lifestyle factors (e.g., breastfeeding). 25 , 26 Related to this, the exclusion of participants with cancer before enrollment could be seen as a limitation, although detailed studies of cancer survivors may be better suited for assessing the risk of second cancers. Another limitation is the possibility of various degrees of random systematic error in measuring different exposures (e.g., diet), but we do not expect this to significantly alter the results because previous publications from the CPS cohorts have demonstrated the predictive validity of all exposures included in this analysis. Finally, there were only 860 cancers (5% of the total) among non‐White participants; thus, we were unable to examine risks stratified by individual racial/ethnic groups.
Conclusions
Although risk factor studies of cancers by single anatomic sites are essential from an etiologic perspective and to target conventional screening, it is useful to describe the epidemiology of invasive cancer as a single end point to improve the identification of populations in need of enhanced cancer screening, including MCED, and prevention efforts. These results suggest that more detailed risk assessment can identify currently nontargeted groups in the general population younger than 50 years that are at equivalent or higher risk as populations older than 50 years.
This study demonstrates that in addition to age, clinicians should consider smoking history, BMI, and family history of any cancer when helping patients determine whether they may benefit from enhanced cancer screening and prevention interventions.
AUTHOR CONTRIBUTIONS
Alpa V. Patel: Conception, methodology, supervision, writing–original draft, and writing–review and editing. Emily Deubler: Data curation, formal analysis, validation, and writing–review and editing. Lauren R. Teras: Data curation, formal analysis, and writing–review and editing. Graham A. Colditz: Conceptualization, project administration, supervision, investigation, and writing–review and editing. Cari J. Lichtman: Data curation, formal analysis, and writing–review and editing. William G. Cance: Conceptualization, project administration, supervision, investigation, writing–review and editing. Christina A. Clarke: Conceptualization, methodology, supervision, writing–original draft, and writing–review and editing.
Funding information
The American Cancer Society funds the creation, maintenance, and updating of the Cancer Prevention Study‐II and Cancer Prevention Study‐3. The American Cancer Society is a not‐for‐profit public health organization that receives support from the public through fundraising and direct contributions. The Society also receives a small portion of support from corporations and industry to support its mission programs and services. This analysis was supported by GRAIL, LLC, a subsidiary of Illumina, Inc., currently held separate from Illumina Inc., under the terms of the Interim Measures Order of the European Commission dated 29 October 2021.
CONFLICTS OF INTEREST
William G. Cance reports royalties from the University of North Carolina based on an invention from 1999; is involved in ongoing litigation regarding a patent; and has a family member involved with FAKnostics, LLC, all outside the submitted work. Christina Clarke is employed by GRAIL, LLC, a subsidiary of Illumina, Inc., currently held separate from Illumina Inc., under the terms of the Interim Measures Order of the European Commission dated 29 October 2021 with equity in Illumina, Inc, and owns equity in the company. The remaining authors are employed by their affiliated institutions and made no disclosures.
Supporting information
Table S1.
Table S2.
Table S5.
Table S6.
Table S7.
Table S3.
Table S4.
ACKNOWLEDGEMENTS
The authors express sincere appreciation to all Cancer Prevention Study‐II and Cancer Prevention Study‐3 participants and to each member of the study and biospecimen management group. The authors acknowledge the contribution to this study from central cancer registries supported through the Centers for Disease Control and Prevention's National Program of Cancer Registries and cancer registries supported by the National Cancer Institute's Surveillance, Epidemiology, and End Results Program. They also thank Anuraag Kansal, Earl Hubbell, Ze Cong, Marie Coignet, and Josh Ofman, employees of GRAIL, for critical review of the article. Mia DeFino, MS, ELS, of DeFino Consulting, LLC (Chicago, IL), provided editorial support paid for by GRAIL, LLC, a subsidiary of Illumina, Inc., currently held separate from Illumina Inc., under the terms of the Interim Measures Order of the European Commission dated 29 October 2021. The American Cancer Society funds the creation, maintenance, and updating of the Cancer Prevention Study‐II and Cancer Prevention Study‐3. The Society also receives a small portion of support from corporations and industry to support its mission programs and services. This analysis was supported by GRAIL, LLC, a subsidiary of Illumina, Inc., currently held separate from Illumina Inc., under the terms of the Interim Measures Order of the European Commission dated 29 October 2021.
See editorial on pages 3443–3445, this issue.
REFERENCES
- 1. Ofman JJ, Raza A. Taking early cancer detection to the next level Scientific American; November 1, 2020. Accessed January 22, 2021. https://www.scientificamerican.com/article/taking‐early‐cancer‐detection‐to‐the‐next‐level/ [Google Scholar]
- 2. Liu MC. Transforming the landscape of early cancer detection using blood tests—commentary on current methodologies and future prospects. Br J Cancer. 2021;124(9):1475‐1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klein EA, Richards D, Cohn A, et al. Clinical validation of a targeted methylation‐based multi‐cancer early detection test using an independent validation set. Ann Oncol. 2021;32(9):1167‐1177. [DOI] [PubMed] [Google Scholar]
- 4. Liu MC, Oxnard GR, Klein EA, et al. Sensitive and specific multi‐cancer detection and localization using methylation signatures in cell‐free DNA. Ann Oncol. 2020;31(6):745‐759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lennon AM, Buchanan AH, Kinde I, Warren A, Honushefsky A, Cohain AT, Ledbetter DH, Sanfilippo F, Sheridan K, Rosica D, Adonizio CS, Hwang HJ, Lahouel K, Cohen JD, Douville C, Patel AA, Hagmann LN, Rolston DD, Malani N, Zhou S, Bettegowda C, Diehl DL, Urban B, Still CD, Kann L, Woods JI, Salvati ZM, Vadakara J, Leeming R, Bhattacharya P, Walter C, Parker A, Lengauer C, Klein A, Tomasetti C, Fishman EK, Hruban RH, Kinzler KW, Vogelstein B, Papadopoulos N Feasibility of blood testing combined with PET‐CT to screen for cancer and guide intervention. Science 2020;369(6499):eabb9601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen X, Gole J, Gore A, et al. Non‐invasive early detection of cancer four years before conventional diagnosis using a blood test. Nat Commun. 2020;11(1):3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Calle EE, Rodriguez C, Jacobs EJ, et al. The American Cancer Society Cancer Prevention Study II nutrition cohort: rationale, study design, and baseline characteristics. Cancer. 2002;94(9):2490‐2501. [DOI] [PubMed] [Google Scholar]
- 8. Patel AV, Jacobs EJ, Dudas DM, et al. The American Cancer Society's Cancer Prevention Study 3 (CPS‐3): recruitment, study design, and baseline characteristics. Cancer. 2017;123(11):2014‐2024. [DOI] [PubMed] [Google Scholar]
- 9. Colditz GA, Atwood KA, Emmons K, et al. Harvard report on cancer prevention volume 4: Harvard Cancer Risk Index. Risk Index Working Group, Harvard Center for Cancer Prevention. Cancer Causes Control. 2000;11(6):477‐488. [DOI] [PubMed] [Google Scholar]
- 10. Bergmann MM, Calle EE, Mervis CA, Miracle‐McMahill HL, Thun MJ, Heath CW. Validity of self‐reported cancers in a prospective cohort study in comparison with data from state cancer registries. Am J Epidemiol. 1998;147(6):556‐562. [DOI] [PubMed] [Google Scholar]
- 11. Jacobs EJ, Briggs PJ, Deka A, et al. Follow‐up of a large prospective cohort in the United States using linkage with multiple state cancer registries. Am J Epidemiol. 2017;186(7):876‐884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fedewa SA, Kazerooni EA, Studts JL, et al. State variation in low‐dose computed tomography scanning for lung cancer screening in the United States. J Natl Cancer Inst. 2021;113(8):1044‐1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maas P, Pal Choudhury P, Chatterjee N, Wheeler W. iCARE: A Tool for Individualized Coherent Absolute Risk Estimation (iCARE) . R package version 1.18.0.
- 14. Pal Choudhury P, Maas P, Wilcox A, et al. iCARE: an R package to build, validate and apply absolute risk models. PLoS ONE. 2020;15(2):e0228198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Surveillance, Epidemiology, and End Results (SEER) Program . SEER*Stat Databases: November 2018 Submission. Incidence‐SEER Research Data, 18 Registries, November 2019 Submission (2000–2017). Linked To County Attributes‐Time Dependent (1990–2017) Income/Rurality, 1969. –2017 Counties National Cancer Institute; 2020. Accessed April 15, 2020. www.seer.cancer.gov [Google Scholar]
- 16. Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CWJR. Body‐mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341(15):1097‐1105. [DOI] [PubMed] [Google Scholar]
- 17. Calle EE, Rodriguez C, Walker‐Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625‐1638. [DOI] [PubMed] [Google Scholar]
- 18. Cristiano S, Leal A, Phallen J, et al. Genome‐wide cell‐free DNA fragmentation in patients with cancer. Nature. 2019;570(7761):385‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shen SY, Singhania R, Fehringer G, et al. Sensitive tumour detection and classification using plasma cell‐free DNA methylomes. Nature. 2018;563(7732):579‐583. [DOI] [PubMed] [Google Scholar]
- 20. National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health . The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Centers for Disease Control and Prevention (US); 2014. Accessed September 15, 2021. https://www.ncbi.nlm.nih.gov/books/NBK294317/table/ch4.t1/ [PubMed]
- 21. Sanford NN, Sher DJ, Butler S, et al. Cancer screening patterns among current, former, and never smokers in the United States, 2010–2015. JAMA Netw Open. 2019. 17;2(5):e193759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Patel AV, Bernstein L, Deka A, et al. Leisure time spent sitting in relation to total mortality in a prospective cohort of US adults. Am J Epidemiol 2010. 15;172(4):419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chatterjee N, Kim S, Garcia‐Closas M, Scharpf R, Visvanathan K, Velculescu V. Promise of risk stratification for multicancer screening with liquid biopsy tests. doi: 10.21203/rs.3.rs-400074/v1. Accessed September 15, 2021. https://www.researchsquare.com/article/rs‐400074/v1 [DOI] [PMC free article] [PubMed]
- 24. Islami F, Goding Sauer A, Miller KD, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin. 2018;68(1):31‐54. [DOI] [PubMed] [Google Scholar]
- 25. Engels EA, Pfeiffer RM, Fraumeni JF Jr, et al. spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306(17):1891‐1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hernandez‐Ramirez RU, Shiels MS, Dubrow R, Engels EA. Cancer risk in HIV‐infected people in the USA from 1996 to 2012: a population‐based, registry‐linkage study. Lancet HIV. 2017;4(11):e495‐e504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Table S2.
Table S5.
Table S6.
Table S7.
Table S3.
Table S4.


