Abstract
Background
This study aimed to assess the diagnostic utility and associated cost of oral liquid‐based brush cytology (OLBC) in the diagnosis of oral cancer and oral potentially malignant disorders (OPMDs).
Methods
A total of 284 patients with oral mucosal lesions were included. OLBC samples were collected from all patients immediately before undergoing surgical biopsies. A liquid‐based cytology slide was prepared from each OLBC sample for cytological evaluation using the modified 2014 Bethesda cytology system. The results and the cost were compared with the histopathological outcomes.
Results
The level of agreement between the two approaches was very good (weighted kappa = 0.824). The accuracy of OLBC in differentiating between the different diagnostic groups was 91.69%, whereas the associated sensitivity and specificity were 79.23% and 94.81%, respectively. The estimated cost of each OLBC sample was at least 26% less than the cost of a single biopsy and more than 42% less in cases of multiple biopsied lesions.
Conclusions
The proposed modifications of the Bethesda system can be adopted as a standardized system for oral cytological assessment. Our findings support OLBC as a reliable adjunct to surgical biopsy in the diagnosis of OPMDs. This tool has potential for oral cancer–finding and surveillance programs.
Keywords: brush biopsy, cost analysis, oral liquid‐based brush cytology, oral potentially malignant disorders
Short abstract
Oral brush liquid‐based cytology is a reliable adjunct to surgical biopsy in the diagnosis of oral potentially malignant disorders. It has potential for oral cancer‐finding and surveillance programs.
INTRODUCTION
Oral potentially malignant disorders (OPMDs) are a group of miscellaneous mucosal lesions that share a relative risk of malignancy. 1 Otherwise, these disorders are dissimilar at both clinical and pathological levels. The malignant transformation rate of these lesions varies significantly from 33% for oral erythroplakia to less than 1% for lesions such as oral lichen planus (OLP). 2 , 3 The diagnosis and management of OPMDs falls short of ideal even with present‐day standards of care. 4
The current gold standard for diagnosis of an OPMD is by histopathological evaluation of surgical biopsies. This provides a relatively objective tool for diagnosis in addition to its prognostic utility in stratifying the potential risk of malignancy through identifying and grading specific microscopic features known as oral epithelial dysplasia (OED). 2 , 5
Surgical biopsy is an invasive process associated with some morbidity 4 , 6 ; therefore, many clinicians perform biopsies only for lesions that clinically display suspicious features of malignancy. 6 A recent study in the United States found that the clinician’s decision of selecting suspicious oral lesions for biopsy was associated with low sensitivity and specificity of approximately 60% each, so that approximately 40% of oral cancers would have been missed. 7 A further potential sampling bias is selecting the representative biopsy site, and this issue becomes more complex in cases of large or multiple lesions. 8 A previous study found that 29.5% of single‐site biopsied OPMDs and oral cancers were underdiagnosed, which was significantly higher than multiple‐site biopsied lesions. 8 This may contribute to the finding that more than 50% of oral squamous cell carcinomas (OSCCs) are diagnosed in late stages, negatively affecting patient survival rates. 9
The current approach of patient care is to detect those with OPMDs at early stages to improve patient prognosis. General dentists and allied dental practitioners provide a unique opportunity in this area by conducting visual and tactile oral examinations. However, the sensitivity and specificity of this approach is variable because it relies more on individuals’ knowledge, training, and competency. 7 Reports have found that the ability of general dentists to differentiate between benign and suspicious oral lesions was closer to being random guessing, rather than being scientifically based. 10 , 11
In this context, it has been reported that early detection of OSCC reduces mortality and enhances the 5‐year survival rate to approximately 94%. 12 , 13 It has been proposed that screening for oral cancer in high‐risk individuals would lead to an obvious increase in the quality‐adjusted life years saved and a significant decrease in the intervention cost. 14 Despite many attempts to evaluate oral cancer screening methodologies, there is insufficient evidence to support the benefits and cost‐effectiveness of these programs. 15 , 16 , 17 Overall, these studies suffered from major drawbacks, including considerable heterogeneity, unpredictable sensitivity, and inappropriate design. 13 Although most of the studies were observational or case‐control studies, the only randomized controlled study was criticized because of several methodological weaknesses. 15 , 18 Taken together, there is no robust evidence to support the adoption of national screening programs for oral cancer. 17
Several noninvasive diagnostic adjunctive tools have been proposed to outweigh the diagnostic accuracy limitations but are yet to demonstrate certain utility. 19 Among many tools, oral cytology showed higher sensitivity and specificity in detecting OPMDs and oral cancers compared with other techniques. 20 Oral liquid‐based brush cytology (OLBC) has gained significant attention in the past decade as a promising and minimally invasive tool to harvest diagnostic transepithelial cells. 21 More importantly, this technique overcomes the historical shortcomings of exfoliative cytology by consistently producing high‐quality slides with adequate staining. 21 Previous studies in this area showed contradictory results in terms of accuracy. 21 This would be expected because of an absence of consensus protocols for collecting samples and reporting results, with the vast majority of studies adopting criteria designed for cervical pathology.
In 2018, our research group developed modified criteria adopted from the 2014 Bethesda System for Reporting Cervical Cytology for clinical application in the oral cavity. 22 The accuracy of this approach was 75% with similar percentages for sensitivity and specificity. 22 The present study was designed as a continuum to our previous research to further validate the utility of using OLBC as a reliable and minimally invasive diagnostic tool for OPMDs and OSCCs by expanding the patient cohort and including additional diagnostic categories.
MATERIALS AND METHODS
Study design
This semiquantitative cross‐sectional prospective study was conducted following the principles of the Declaration of Helsinki and reported in accordance with the Standards for Reporting Diagnostic Accuracy Studies (STARD 2015). 23 The study was granted ethics approval from the University of Western Australia Human Ethics Committee (RA/4/20/4530 and RA/4/1/8682).
Focused questions
This study was designed to answer the following questions. (1) What is the diagnostic utility of using OLBC as an adjunctive tool to surgical biopsies? (2) What is the level of agreement between the histopathological diagnosis and the cytological assessment? (3) What are the limitations (if present) of applying OLBC in daily clinical practice? (4) What is the estimated cost of the OLBC test?
Inclusion and exclusion criteria
The study included patients presenting with clinical features suggestive of OPMDs of either oral leukoplakia/oral erythroplakia, clinical OLPs/oral lichenoid lesions (OLLs), or OSCCs between 2017 and 2021. All cases were adult patients aged 18 years and above who provided written informed consent. Patients with a history of chemotherapy and/or radiotherapy were excluded.
Sample collection and preparation
OLBC using Orcellex brush (Rovers Medical Devices, The Netherlands) was performed on each patient under local anesthesia before undergoing surgical biopsy as described previously. 22 Brush heads were preserved immediately into vials of methanol‐based buffered preservative (ThinPrep PreservCyt, Hologic Inc, MA, USA) and transferred to a laboratory for processing. A liquid‐based cytology (LBC) slide was automatically prepared for each OLBC sample using the standard protocol of the ThinPrep 2000 processor (Hologic Inc). LBC slides were Papanicolaou stained by applying ThinPrep staining reagents (Hologic Inc) according to the manufacturer's protocol for cytological assessment.
Histopathological assessment
Histopathological assessment is considered the gold standard for diagnosis. Clinicopathological correlations were performed by one author (M.I.) to diagnose OLP and OLL cases based on OLP diagnostic criteria. 24 OED grading was undertaken using the binary grading system when applicable. 25 The patients were categorized into the following five diagnostic groups: (1) keratosis with no dysplasia, (2) genuine OLP and OLL without dysplasia, (3) low‐risk OED, (4) high‐risk OED, and (5) OSCC.
Cytological assessment
All Papanicolaou‐stained LBC slides were blindly and independently assessed by three assessors (M.I., O.K., P.S.) using the modified 2014 Bethesda system. 22 Any discrepancy in the assessment was settled by consensus. The LBC slides were categorized into five groups (Fig. 1) according to the cytological findings as follows: (1) atypical squamous cells of undetermined significance (undetermined significance); (2) atypical squamous cells suggestive of oral lichen planus or lichenoid reaction (cyto OLP/OLL); (3) low‐grade squamous intraepithelial lesion; (4) high‐grade squamous intraepithelial lesion; and (5) OSCC.
FIGURE 1.
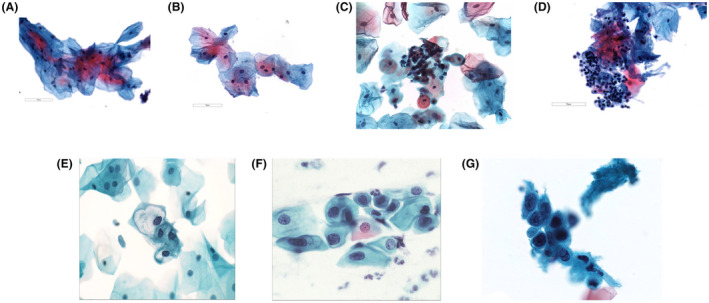
(A) Normal superficial and intermediate squamous cells. (B) A sheet of atypical squamous cells of undetermined significance (note there is a slight increase in N/C ratio ≤ 10% with even chromatin distribution) (×400). (C) Sheets of immature squamous cells exhibiting slightly enlarged nuclei with dense chromatin suggestive of changes consistent with oral lichen planus/lichenoid lesion (×400). (D) Sheets of degenerative squamous cells associated with lymphocytes suggestive of changes consistent with oral lichen planus/lichenoid lesion. Note there is a slight increase in N/C ratio ≤ 10% with even chromatin distribution (×400). (E) Squamous intraepithelial lesion (low grade). Note the increase in N/C ratio ≤ 50% (×500). (F) Squamous intraepithelial lesion (high grade). Note the increase in N/C ratio > 50% and ≤ 75% (×500). (G) Squamous cells suggestive of OSCC. Note the massive increase in N/C ratio ≥ 75% (×500). N/C indicates nuclear/cytoplasmic ratio; OSCC, oral squamous cell carcinoma
The estimated cost of OLBC and surgical biopsy procedures
The estimated costs of OLBC and surgical biopsies were calculated based on the Medicare Benefits Schedule for both dental specialists and general dental practitioners. 26 Medicare is the public health scheme of Australia that provides medical services for beneficiaries at no cost or low cost, whereas the schedule clarifies the fees owed to medical providers for providing covered medical services. Because the schedule does not specify cytology procedures for the oral cavity, the fee schedule of the closest procedure for cervical pathology was considered. The total cost was detailed as (1) specialist consultation for surgical biopsy and general dental practitioner consultation for OLBC, (2) the cost of sample collection, and (3) the cost of microscopic examination. All values were reported in Australian dollars (AUD).
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics software version 28 (IBM Corporation, NY, USA). The level of significance was set as p < .05. The Pearson χ2 test of independence was used to analyze the associations between nominal variables. Cramer's V was used to assess the strength of association for the χ2 analyses. 27 One‐way analysis of variance was used to assess the association between nominal and continuous variables. Post hoc analyses of pairwise comparisons were performed wherever an overall statistically significant difference was observed among χ2 results. For post hoc analyses, the p value was adjusted by dividing 0.05 by the total number of pairwise comparisons.
Weighted kappa (κѡ) was used for measuring the interobserver agreement in the diagnosis between the histopathological diagnosis and the cytological diagnosis. The κѡ were graded as follows: κѡ < 0.4 = poor agreement, κѡ ≥ 0.4 and < 0.6 = moderate agreement, κѡ ≥ 0.6 and < 0.8 = good agreement, and κѡ ≥ 0.8 = very good agreement. 28 Standard formulas were used to calculate the accuracy, sensitivity, specificity, positive predictive value, and negative predictive value, whereas the histopathological diagnosis was considered the gold standard for comparisons. A multinomial logistic regression model was run to assess whether the histopathological diagnosis was influenced by patient variables.
RESULTS
General characteristics of included cases
Of the initial cohort of 315 patients, 31 (9.8%) were excluded because of inadequate cellularity. There were no statistically significant associations between obtaining inadequate cellularity samples and patient age (p = .787), sex (p = .674), lesion site (p = .252), or histopathological diagnosis (p = .778).
Of the included 284 patients, there were 149 females (52.5%) and 135 males (47.5%). The patients’ ages ranged from 30 to 92 years (mean age, 63.24; SD ±13.3 years), whereas the association between age and sex was not significant (p = .275). The most common site of sampling was labial/buccal mucosa, accounting for 34.2% of all cases followed by the lateral surface of the tongue (27.1%) (Table 1). Neither patient age nor sex was statistically significantly associated with lesion site, p = .968 and p = .145, respectively.
TABLE 1.
General characteristics of the study subjects
| Variables | Histopathological diagnosis | Total (%) | p | ||||
|---|---|---|---|---|---|---|---|
| Oral epithelial dysplasia | |||||||
| Keratosis | OLP/OLL | Low risk | High risk | OSCC | |||
| Sex | .827 | ||||||
| Male | 60 | 30 | 22 | 16 | 7 | 135 (47.5) | |
| Female | 58 | 35 | 26 | 18 | 12 | 149 (52.5) | |
| Lesion site | <.005 | ||||||
| Labia/buccal mucosae | 41 | 39* | 13 | 2* | 2 | 97 (34.2) | |
| Lateral tongue | 26 | 12 | 16 | 13 | 10 | 77 (27.1) | |
| Floor of the mouth | 16 | 4 | 7 | 14* | 4 | 45 (15.8) | |
| Alveolar ridge/palate | 35 | 10 | 12 | 5 | 3 | 65 (22.9) | |
| OLBC (2014 Bethesda System) | |||||||
| Undetermined significance | 95** | 10** | 6** | 0** | 1 | 112 (39.4) | <.005 |
| Cyto OLP/OLL | 5** | 49** | 3 | 0 | 0 | 57 (20.1) | |
| Low‐grade SIL | 15 | 5 | 36** | 4 | 0 | 60 (21.1) | |
| High‐grade SIL | 3** | 1 | 3 | 28** | 1 | 36 (12.7) | |
| OSCC | 0** | 0 | 0 | 2 | 17** | 19 (6.7) | |
| Total (%) | 118 (41.5) | 65 (22.9) | 48 (16.9) | 34 (12) | 19 (6.7) | 284 (100) | |
Abbreviations: OLBC, oral liquid‐based brush cytology; OLL, oral lichenoid lesion; OLP, oral lichen planus; OSCC, oral squamous cell carcinoma; SIL, squamous intraepithelial lesion
Post hoc adjusted p < .0025
Post hoc adjusted p < .002
Histopathological diagnosis and its associated variables
The majority of OPMDs were histopathologically diagnosed as either orthokeratosis or parakeratosis without dysplasia (41.5%) followed by OLP/OLL (22.9%) (Table 1). The histopathological diagnosis was not influenced by patient age or sex. However, a χ2 test of independence showed a statistically significant association between the histopathological diagnosis and lesion site, χ2 (12) 58.1, p < .0005; the strength of this association was moderate, Cramer's V = 0.261. Post hoc analysis revealed that the histopathological diagnosis of OLP/OLL was significantly greater on the labial and buccal mucosae. Similarly, lesions diagnosed with high‐risk OED were significantly greater on the floor of the mouth. This was contrary to labial/buccal mucosae where high‐risk OED cases were significantly low. No other pairwise comparisons were statistically significantly different (Table 1).
Accuracy of OLBC in predicting the histopathological diagnosis
The association between the cytological assessment of OLBC and the histopathological diagnosis was statistically significant according to the χ2 test of independence (χ2 [16] 656.4, p < .0005), whereas the associated strength of association was strong (Cramer's V = 0.76). Eleven of 25 pairwise comparisons were statistically significant in this association (adjusted p < .002) as shown in Table 1.
The κѡ was run to further validate the utility of OLBC in predicting the histopathological diagnosis. The level of agreement among the two approaches of diagnosis was very good, κѡ = 0.824 (95% CI, 0.761–0.887), p < .0005.
The accuracy of OLBC in predicting the histopathological diagnosis was 91.69%, as shown in Table 2. Furthermore, the accuracy of OLBC in detecting specific oral conditions against others was assessed for keratosis, OLP/OLL, OED, OSCC, and OED/OSCC (Table 2).
TABLE 2.
The accuracy of implementing OLBC in the diagnosis of oral lesions
| Sensitivity, % | Specificity, % | PPV, % | NPV, % | Accuracy, % | |
|---|---|---|---|---|---|
| OLBC (all diagnostic categories) | 79.23 | 94.81 | 79.23 | 94.81 | 91.69 |
| Keratosis vs. others | 80.51 | 89.76 | 84.82 | 86.63 | 85.92 |
| OLP/OLL vs. others | 75.38 | 96.35 | 85.96 | 92.95 | 91.55 |
| OED vs. others | 86.59 | 87.62 | 73.96 | 94.15 | 87.32 |
| OSCC vs. others | 89.47 | 99.25 | 89.47 | 99.25 | 98.59 |
| OED/OSCC vs. others | 90.1 | 86.89 | 79.13 | 94.08 | 88.03 |
Abbreviations: NPV, negative predictive value; OED, oral epithelial dysplasia; OLBC, oral liquid‐based brush cytology; OLL, oral lichenoid lesion; OLP, oral lichen planus; OSCC, oral squamous cell carcinoma; PPV, positive predictive value.
Finally, a multiple regression model was run to predict the histopathological diagnosis from age, sex, lesion site, and cytological assessment. The model was statistically significant, F(4.2) = 151.969, p < .0005. However, only cytological assessment added statistically significant value to this prediction, p < .0005.
Estimate cost of OLBC versus surgical biopsies
The estimated cost of OLBC was less than 26% of the estimated cost of surgical biopsy in dental speciality clinics ($224.45 AUD vs. $305.8 AUD). This gap is 36% for cases attending a general dental practice. Likewise, the cost of a surgical biopsy procedure is greatly increased in cases of large lesions with multiple biopsy sites, as shown in Table 3.
TABLE 3.
The estimated cost of OLBC and surgical biopsy based on Medicare Benefits Schedule
| Procedure | Surgical biopsy | OLBC | ||
|---|---|---|---|---|
| (Item no. a ) description |
Cost AUD |
(Item no. a ) description |
Cost AUD |
|
| Consultation | (110) dental specialist | 155.6 | (110) dental specialist | 155.6 |
| OR | OR | |||
| (36) GDP | 73.95 | (36) GDP | 73.95 | |
| Sampling | (30072) cost per site | 53.05 | (73043) cost per lesion | 22.85 |
| Microscopic examination | (72823) 1 specimen | 97.15 | (73076) | 46 |
| (72824) 2–4 specimens | 141.35 | |||
| (72825) 5–7 specimens | 180.25 | |||
| Estimated total | At a specialist clinic | ≥ 305.8 | At a specialist clinic | 224.45 |
| At a GDP clinic | ≥224.15 | At a GDP clinic | 142.8 | |
Abbreviations: AUD; Australia dollar, GDP; general dental practitioner; OLBC, oral liquid‐based brush cytology
Item number according to the Medicare Benefits Schedule (2020)
DISCUSSION
The results of this study demonstrate that OLBC assessment can be reliably used for OPMD diagnosis. This study uses OLBC as a minimally invasive diagnostic tool that has several advantages such as simplicity, low cost, and high reliability. Overall, the following specifications are required for any diagnostic tool to be considered as ideal for use in assessing oral mucosal lesions: (1) being minimally invasive for patients and can be easily performed by general practitioners; (2) helps stratify the potential risk of malignancy of OPMDs with a reasonable level of accuracy; (3) assists with a biopsy site guidance for selection of the most representative site; (4) helps follow patients longitudinally with a high risk of malignancy to detect any evolving epithelial changes; (5) provides archived materials for ancillary tests; and (6) shows utility to be adopted as a screening tool on a large epidemiological scale.
Although we previously compared OLBC results with surgical biopsies in 101 patients, the current study was expanded to include more representative OPMDs for a larger cohort of 284 patients. The associated accuracy of using our proposed modified 2014 Bethesda system for OPMD diagnosis was increased to 91.69% in comparison to 75% in our previous report. 22 This improvement can be attributed to adjusting the study design to be more consistent with cytological terminology. Primarily, the modified 2014 Bethesda system includes five diagnostic categories. 22 Unlike our previous study in 2018, 22 the patients in this study were grouped according to their histopathological findings into five groups instead of six, each of them with a counterpart in the cytological categories. The binary system of OED grading was adopted in this study instead of the previously used grading of epithelial dysplasia as mild, moderate, and severe, which in turn makes the comparison between dysplastic cases more reliable. The term carcinoma in situ is no longer used and was merged with high‐risk OED because these grades cannot be differentiated microscopically and share a high risk of malignancy. 5 Finally, the modified Bethesda system assigns a separate group for OLP and OLL cases. We previously applied this system to assess a cohort lacking OLP and OLL cases; therefore, it was not surprising to find that out of 13 false‐positive cases, six were associated with OLP. Although this did not affect the sensitivity, the specificity and the overall accuracy were affected. Thus, patients with OLP and OLL were recruited to this study to assess the utility of using this approach in a larger clinical setting.
Although the strength of association between the cytological and the histopathological assessment was strong, our proposed technique may find greatest application for excluding the presence of any condition rather than in confirming its presence because the specificity was much higher than the sensitivity. The literature includes highly variable results in terms of sensitivity and specificity of oral brush cytology. 21 This clearly reflects the lack of standard protocols for oral brush cytology reporting, which complicates comparison between studies. Goodson et al. in 2017 reported similar results in terms of higher specificity 29 ; however, the sensitivity in that study was lower than in the current study. Sciubba in 1999 assessed the utility of a commercially available brush coupled with computer‐assisted screening in the detection of oral cancerous lesions and found a sensitivity and a specificity of 100% each. 6 Not all clinically benign lesions in that study underwent surgical biopsy, and, as such, there is a possibility that some false‐negative results were missed.
The associated sensitivity of detecting OLP and OLL in this study was the lowest among the diagnostic categories. This was not surprising because the diagnosis of these lesions has been historically associated with high observer variability. 30 This also can be attributed to some cytological features of OLP that are not specific and can be seen in other conditions. 22 However, the specificity of this category was high, at 96.35%, which provides a valuable tool to limit misdiagnosing any lesion as OLP or OLL, especially because cases that are misdiagnosed are implicated in falsely elevating the malignant transformation rate of OLP and OLL. 3
From a clinical perspective, we used the Orcellex brush to sample mucosal lesions, which has a suitable design to easily adapt to all oral sites. 22 , 29 Although approximately 10% of OLBC samples were excluded as a result of inadequate cellularity, this finding was not associated statistically with any patient factors. However, this finding did not affect the quality of our results because all samples were associated with pinpoint bleeding to ensure harvested cells were obtained from all epithelial layers. In this respect, our previous study in 2018 found that lip and gingiva were among the most common anatomical sites for inadequate cellularity. 22 Likewise, the presence of lesion keratosis was proposed as a major factor that may hinder the ability to collect adequate cells. 22 Further optimization of OLBC sampling techniques based on the anatomical site and clinical appearance may be required to set a standardized protocol for high‐quality sampling.
The simplicity of obtaining OLBC samples raises the prospect for considering this approach for cancer screening protocols where members of the general public could collect samples for themselves and send them for processing through couriers or a postal service, although feasibility would need to be assessed. Samples can be preserved in vials at room temperature for several weeks. Although there is no debate that OLBC as a minimally invasive technique is associated with less morbidity compared with conventional surgical biopsies, 22 , 29 , 31 the decision in our study was to administer local anesthesia before performing brush cytology because the patients were already anxious about the surgical biopsies. A study to measure the level of discomfort and pain among patients receiving brush cytology is recommended to conclusively clarify this point.
Using the Medicare Benefits Schedule, the estimated cost of OLBC was at least 26% less than the cost of a surgical biopsy, and this figure would be even greater in cases of large lesions when multiple biopsies are required, or in cases in which general dental practitioners collect samples. Despite these cost savings, it is acknowledged that private practitioners would charge more for their consultations and surgical procedures. Nonetheless, the potential cost‐effectiveness of adopting an OLBC approach in managing suspicious oral lesions highlights the possible role of general practitioners in the early detection of OPMDs.
Considering that the accuracy of OLBC in this study was comparable with surgical biopsy, 32 this opens new horizons for considering OLBC in cancer‐screening protocols. Previous literature does not include a robust study to assess the cost‐effectiveness of implementing oral brush biopsy in oral cancer screening. 15 However, because conducting randomized clinical trials are difficult in communities where the prevalence of the disease of interest is low, constructing computerized simulation models for cost‐utility analysis are warranted in this area to provide robust evidence for practice.
Unlike other cytological preparations, OLBC provides a unique opportunity to archive cellular yields for further molecular testing by embedding the cytological materials in formalin‐fixed, paraffin‐embedded blocks known as cell blocks. 33 , 34 This technique shows promising utility in studying immunoreactive protein expression with results proximate to surgical pathology. 35 , 36 The efficacy of cell‐block immunocytochemistry has been successfully validated in predicting the grade of OED. 37
Several limitations are potentially associated with this study. The absence of a group of healthy volunteers with normal oral mucosa limits the ability to assess the utility of this approach in differentiating between healthy and diseased individuals. However, this group was not included because of ethical considerations. Detailed clinical features of some lesions were not recorded, meaning it was not possible to link the clinical appearance of lesions with cytological outcomes. The lack of follow‐up data limited the ability to build prognostic statistical models based on the cytology findings. Finally, the number of cases in each diagnostic group was relatively low in comparison to keratosis. Although these numbers were adequate from a statistical perspective, this could be a potential source of bias.
In conclusion, this study showed the reliability of implementing OLBC in clinical diagnosis to stratify patients according to their risk of malignancy. This approach can meet the required standards of being an ideal diagnostic tool in terms of objectivity, accuracy, simplicity, and multifunctional application. Although further refining of our modified Bethesda system is recommended to improve the associated sensitivity, we encourage following this system in reporting oral brush findings to standardize the oral cytology results.
AUTHOR CONTRIBUTIONS
Majdy Idrees: Data curation, formal analysis, investigation, writing – original draft, writing review and editing. Camile S. Farah: Clinical data collection, data curation, validation, resources, writing – original draft, writing – review and editing. Philip Sloan: Diagnosis confirmation, validation, writing – original draft, writing – review and editing. Omar Kujan: Conceptualization, data curation, project administration and coordination, supervision, validation, writing – original draft, writing – review and editing.
FUNDING INFORMATION
We acknowledge funding support from the University of Western Australia. We also acknowledge the Australian Dental Research Foundation Inc and the Australian Dental Association (Western Australia branch) for the clinical dentistry research grant awarded to support this study.
CONFLICTS OF INTEREST
The author made no disclosures.
ACKNOWLEDGMENTS
We acknowledge funding support from the University of Western Australia. We also acknowledge the Australian Dental Research Foundation Inc and the Australian Dental Association (Western Australia Branch) for the clinical dentistry research grant awarded to support this study. We extend our gratitude to Rovers Medical Devices B.V., The Netherlands, for providing the Orcellex brushes and Hologic Inc, Australia, for supplying ThinPrep consumables. Open access publishing facilitated by The University of Western Australia, as part of the Wiley ‐ The University of Western Australia agreement via the Council of Australian University Librarians.
[Correction added on July 21 2022, after first online publication: CAUL funding statement has been added.]
REFERENCES
- 1. Warnakulasuriya S, Kujan O, Aguirre‐Urizar JM, et al. Oral potentially malignant disorders: a consensus report from an international seminar on nomenclature and classification, convened by the WHO Collaborating Centre for Oral Cancer. Oral Dis. 2020;27(8):1862–1880. [DOI] [PubMed] [Google Scholar]
- 2. Iocca O, Sollecito TP, Alawi F, et al. Potentially malignant disorders of the oral cavity and oral dysplasia: a systematic review and meta‐analysis of malignant transformation rate by subtype. Head Neck. 2019;42(3):539–555. [DOI] [PubMed] [Google Scholar]
- 3. Idrees M, Kujan O, Shearston K, Farah CS. Oral lichen planus has a very low malignant transformation rate: a systematic review and meta‐analysis using strict diagnostic and inclusion criteria. J Oral Pathol Med. 2020;50(3):287–298. [DOI] [PubMed] [Google Scholar]
- 4. Yang E, Tan M, Schwarz R, Richards‐Kortum R, Gillenwater A, Vigneswaran N. Noninvasive diagnostic adjuncts for the evaluation of potentially premalignant oral epithelial lesions: current limitations and future directions. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125(6):670–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Odell E, Kujan O, Warnakulasuriya S, Sloan P. Oral epithelial dysplasia: recognition, grading and clinical significance. Oral Dis. 2021;27(8):1947–1976. [DOI] [PubMed] [Google Scholar]
- 6. Sciubba JJ. Improving detection of precancerous and cancerous oral lesions. Computer‐assisted analysis of the oral brush biopsy. U.S. Collaborative OralCDx Study Group. J Am Dent Assoc. 1999;130(10):1445–1457. [DOI] [PubMed] [Google Scholar]
- 7. Chaturvedi AK, Udaltsova N, Engels EA, et al. Oral leukoplakia and risk of progression to oral cancer: a population‐based cohort study. J Natl Cancer Inst. 2020;112(10):1047–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee J, Hung H, Cheng S, et al. Factors associated with underdiagnosis from incisional biopsy of oral leukoplakic lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104(2):217–225. [DOI] [PubMed] [Google Scholar]
- 9. McGurk M, Chan C, Jones J, O'Regan E, Sherriff M. Delay in diagnosis and its effect on outcome in head and neck cancer. Br J Oral Maxillofac Surg. 2005;43(4):281–284. [DOI] [PubMed] [Google Scholar]
- 10. Seoane J, Warnakulasuriya S, Varela‐Centelles P, Esparza G, Dios PD. Oral cancer: experiences and diagnostic abilities elicited by dentists in North‐western Spain. Oral Dis. 2006;12(5):487–492. [DOI] [PubMed] [Google Scholar]
- 11. Gaballah K, Faden A, Fakih FJ, Alsaadi AY, Noshi NF, Kujan O. Diagnostic accuracy of oral cancer and suspicious malignant mucosal changes among future dentists. Healthcare (Basel). 2021;9(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sciubba JJ, Larian B. Oral squamous cell carcinoma: early detection and improved 5‐year survival in 102 patients. Gen Dent. 2018;66(6):e11‐e16. [PubMed] [Google Scholar]
- 13. Brocklehurst P, Kujan O, O'Malley LA, Ogden G, Shepherd S, Glenny AM. Screening programmes for the early detection and prevention of oral cancer. Cochrane Database Syst Rev. 2013(11):CD004150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Speight PM, Palmer S, Moles DR, et al. The cost‐effectiveness of screening for oral cancer in primary care. Health Technol Assess. 2006;10(14):1–144, iii‐iv. [DOI] [PubMed] [Google Scholar]
- 15. Kujan O, Glenny AM, Oliver RJ, Thakker N, Sloan P. Screening programmes for the early detection and prevention of oral cancer. Cochrane Database Syst Rev. 2006(3):CD004150. [DOI] [PubMed] [Google Scholar]
- 16. Downer MC, Moles DR, Palmer S, Speight PM. A systematic review of measures of effectiveness in screening for oral cancer and precancer. Oral Oncol. 2006;42(6):551–560. [DOI] [PubMed] [Google Scholar]
- 17. Speight PM, Epstein J, Kujan O, et al. Screening for oral cancer—a perspective from the Global Oral Cancer Forum. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;123(6):680–687. [DOI] [PubMed] [Google Scholar]
- 18. Kujan O, Sloan P. Dilemmas of oral cancer screening: an update. Asian Pac J Cancer Prev. 2013;14(5):3369–3373. [DOI] [PubMed] [Google Scholar]
- 19. Walsh T, Macey R, Kerr AR, Lingen MW, Ogden GR, Warnakulasuriya S. Diagnostic tests for oral cancer and potentially malignant disorders in patients presenting with clinically evident lesions. Cochrane Database Syst Rev. 2021(7):CD010276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Macey R, Walsh T, Brocklehurst P, et al. Diagnostic tests for oral cancer and potentially malignant disorders in patients presenting with clinically evident lesions. Cochrane Database Syst Rev. 2015(5):CD010276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alsarraf A, Kujan O, Farah CS. The utility of oral brush cytology in the early detection of oral cancer and oral potentially malignant disorders: a systematic review. J Oral Pathol Med. 2018;47(2):104–116. [DOI] [PubMed] [Google Scholar]
- 22. Alsarraf A, Kujan O, Farah CS. Liquid‐based oral brush cytology in the diagnosis of oral leukoplakia using a modified Bethesda Cytology system. J Oral Pathol Med. 2018;47(9):887–894. [DOI] [PubMed] [Google Scholar]
- 23. Bossuyt PM, Reitsma JB, Bruns DE, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ. 2015;351:h5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cheng Y, Gould A, Kurago Z, Fantasia J, Muller S. Diagnosis of oral lichen planus: a position paper of the American Academy of Oral and Maxillofacial Pathology. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122(3):332–354. [DOI] [PubMed] [Google Scholar]
- 25. Kujan O, Oliver RJ, Khattab A, Roberts SA, Thakker N, Sloan P. Evaluation of a new binary system of grading oral epithelial dysplasia for prediction of malignant transformation. Oral Oncol. 2006;42(10):987–993. [DOI] [PubMed] [Google Scholar]
- 26. Department of Health, Australian Government, MBS Online . January 2020. Medicare Benefits Schedule. Accessed January 10, 2022. http://www.mbsonline.gov.au/internet/mbsonline/publishing.nsf/Content/Downloads‐202001
- 27. Lee D. Alternatives to P value: confidence interval and effect size. Korean J Anesthesiol. 2016;69(6):555–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20(1):37–46. [Google Scholar]
- 29. Goodson ML, Smith DR, Thomson PJ. Efficacy of oral brush biopsy in potentially malignant disorder management. J Oral Pathol Med. 2017;46(10):896–901. [DOI] [PubMed] [Google Scholar]
- 30. Idrees M, Farah CS, Khurram SA, Firth N, Soluk‐Tekkesin M, Kujan O. Observer agreement in the diagnosis of oral lichen planus using the proposed criteria of the American Academy of Oral and Maxillofacial Pathology. J Oral Pathol Med. 2021;50(5):520–527. [DOI] [PubMed] [Google Scholar]
- 31. Naugler C. Practice tips. Brush biopsy sampling of oral lesions. Can Fam Physician. 2008;54(2):194. [PMC free article] [PubMed] [Google Scholar]
- 32. Chen S, Forman M, Sadow PM, August M. The diagnostic accuracy of incisional biopsy in the oral cavity. J Oral Maxillofac Surg. 2016;74(5):959–964. [DOI] [PubMed] [Google Scholar]
- 33. Nambirajan A, Jain D. Cell blocks in cytopathology: an update. Cytopathology. 2018;29(6):505–524. [DOI] [PubMed] [Google Scholar]
- 34. Kujan O, Desai M, Sargent A, Bailey A, Turner A, Sloan P. Potential applications of oral brush cytology with liquid‐based technology: results from a cohort of normal oral mucosa. Oral Oncol. 2006;42(8):810–818. [DOI] [PubMed] [Google Scholar]
- 35. Qin SY, Zhou Y, Li P, Jiang HX. Diagnostic efficacy of cell block immunohistochemistry, smear cytology, and liquid‐based cytology in endoscopic ultrasound‐guided fine‐needle aspiration of pancreatic lesions: a single‐institution experience. PLoS One. 2014;9(9):e108762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fetsch PA, Simsir A, Brosky K, Abati A. Comparison of three commonly used cytologic preparations in effusion immunocytochemistry. Diagn Cytopathol. 2002;26(1):61–66. [DOI] [PubMed] [Google Scholar]
- 37. Kujan O, Idrees M, Anand N, Soh B, Wong E, Farah CS. Efficacy of oral brush cytology cell block immunocytochemistry in the diagnosis of oral leukoplakia and oral squamous cell carcinoma. J Oral Pathol Med. 2020;50(5):451–458. [DOI] [PubMed] [Google Scholar]


