Abstract
Background
Vitiligo, an autoimmune disorder characterised by skin depigmentation, is associated with reduced quality of life (QoL). Vitiligo may be under‐reported, in part because of misconceptions that it is a cosmetic disease.
Objectives
This survey sought to characterise vitiligo prevalence and explore the relationship between sociodemographic and clinical characteristics with QoL in a population‐based, multinational study.
Methods
Participants aged ≥18 years were recruited via an online panel in Europe, Japan and the USA to answer questions regarding skin disorders they may have experienced. Those reporting vitiligo (diagnosed or undiagnosed) or vitiligo signs (experiencing loss of skin colour but unaware of vitiligo and not diagnosed) were included in the analyses of vitiligo prevalence. Participants who self‐reported physician‐diagnosed vitiligo were given a broader survey to characterise disease progression, management and QoL (as measured with the Vitiligo‐specific QoL [VitiQoL] instrument).
Results
The total estimated vitiligo prevalence among 35 694 survey participants (Europe, n = 18 785; USA, n = 8517; Japan, n = 8392) was 1.3% (diagnosed, 0.6%; undiagnosed, 0.4%; vitiligo signs, 0.3%). Among 219 patients formally diagnosed with vitiligo (Europe, n = 150; USA, n = 48; Japan, n = 21), total VitiQoL scores were associated with age (P = 0.00017), disease extent (P < 0.0001), disease progression (P < 0.0001), disease management (P < 0.0001) and time since diagnosis (P = 0.0015). Behaviour scores varied based on skin phototype (P = 0.024) and ethnicity (P = 0.048). Higher total VitiQoL scores were reported in patients with head lesions (P = 0.027) and those with head and hand and/or wrist lesions (P = 0.018). Substantial high concern (rated 8–10 on an 11‐point Likert scale) for lesions was found across all body areas and varied with geographical region.
Conclusions
The vitiligo prevalence rate may be higher than previously reported, with a substantial proportion attributed to people who have not received a formal diagnosis. Among formally diagnosed patients with vitiligo, QoL was most severely impacted by more progressive and higher extent of disease.
Introduction
Vitiligo is a chronic autoimmune skin disease characterised by patches of depigmentation resulting from the loss of melanocytes. 1 Vitiligo occurs equally between sexes, 2 with disease onset typically by age 30, although initial manifestations later in life are also common. 3 , 4 The global prevalence of vitiligo ranges from 0.5% to 2% of the population; most studies report a prevalence of ≤0.6%. 5 Prevalence rates vary geographically and are often higher in Africa and India. 6 Vitiligo in dark‐skinned populations and/or in regions where social and cultural stigmas are common likely leads to more patients seeking healthcare, resulting in a higher reported prevalence. 2 Conversely, underreporting of vitiligo in all skin types may occur because some healthcare providers dismiss vitiligo as a cosmetic concern rather than a well‐characterised autoimmune disease. 7 , 8
Vitiligo is associated with significant quality‐of‐life (QoL) impairments in routine activities, employment and psychosocial health. 9 , 10 , 11 Studies have shown that vitiligo lesions located on visible areas (e.g. face, hands) or sensitive areas (e.g. genitalia) impact patient QoL more severely. 12 , 13 Moreover, vitiligo is associated with autoimmune comorbidities, especially thyroid‐related disorders. 14 , 15 Substantial psychological and psychosocial burdens, including depression and other mental health disorders, stigmatisation, and hopelessness, further diminish QoL in affected individuals. 10 , 11 , 16 Overall QoL is associated with the extent of disease, with greater adverse effects on QoL generally associated with larger body surface area involvement and lesion visibility. 4
To further assess the prevalence of vitiligo in a broad population, a population‐based, multinational survey was designed to estimate the overall prevalence and burden of disease in participants with self‐reported vitiligo (diagnosed and undiagnosed) or vitiligo signs (i.e. loss of skin colour or patches of pale or white skin [but unaware of association with vitiligo]). This analysis describes results from a survey conducted in Europe, Japan and the USA reporting the prevalence of vitiligo or self‐reported signs of vitiligo among participants. The relationship between sociodemographic and clinical characteristics of vitiligo (including extent and distribution of lesions) and QoL, as well as perceived concerns among patients with physician‐diagnosed vitiligo, were also assessed.
Participants and methods
Study design and participants
This was a population‐based survey that recruited adult participants aged ≥18 years via an online panel in Europe (France, Germany, Italy, Spain and the UK), Japan and the USA. Participants from these countries were recruited to the survey via an online‐based existing panel of population‐based consumers who had consented to be contacted for health‐related research. All questions were completed online and submitted through the online portal of the data collection institution. The survey questions were created to identify participants with vitiligo or vitiligo signs, irrespective of formal diagnosis. All participants provided online consent to the collection and use of information obtained from the survey. The study protocol received institutional review board exemption based on survey procedures and the use of de‐identified participant‐level data.
Survey
The survey was designed by Incyte Corporation in collaboration with key leaders in the dermatology field who specialise in vitiligo and was conducted between March and May 2019. Screening questions asked participants to identify skin disorders they experienced, either currently or in the past, from a list of 20 different dermatological conditions (Fig. S1); an option for ‘none’ was also included. If participants identified a skin disorder, they were asked if they had ever been formally diagnosed with the selected skin disorder(s) by a physician. If the response was no, participants were asked whether they had signs consistent with a skin disorder; for vitiligo, these signs were loss of skin colour or patches of pale or white skin. The screening questionnaire also included questions related to participant characteristics including age, sex, race/ethnicity (not solicited in France) and Fitzpatrick skin type.
Responses that indicated a confirmed diagnosis of vitiligo, suspected vitiligo (undiagnosed) or vitiligo signs resulted in participants being directed to a broader survey in which they were asked about the history of their condition (i.e. vitiligo extent on a 6‐point Likert scale, disease progression and time since diagnosis), previous management strategies used, lesion location and effect on QoL. Participants whose responses did not indicate vitiligo or vitiligo signs were thanked for their participation and concluded the survey. Participants who continued the survey were classified into one of three groups: (1) diagnosed vitiligo (self‐identifying as having vitiligo diagnosed by a physician); (2) undiagnosed vitiligo (self‐identifying as having vitiligo but not diagnosed by a physician); (3) vitiligo signs (experiencing loss of skin colour or patches of pale or white skin but not aware of their association with vitiligo and not diagnosed by a physician). Participants had the option to upload a photograph of their skin, which was reviewed by Dr Khaled Ezzedine, a vitiligo specialist, to confirm the accuracy of self‐reported vitiligo or vitiligo signs. Upon review, images were classified as 1 (certainly vitiligo), 2 (certainly not vitiligo) and 3 (uncertain). An independent review of all images was performed twice, with a 2‐day interval. All unresolved issues (uncertain) were classified as non‐vitiligo. This approach was used to identify the accuracy of vitiligo status in participants with undiagnosed vitiligo and vitiligo signs.
Management strategy options included in the survey were cosmetic products used to alter or conceal skin tone; sun protection; non‐prescription oral and topical medications; prescription oral and topical medications; surgery or other procedures; and light, laser or phototherapy. Survey options for lesion location(s) included the head, armpit, hand and/or wrist, top of the arm, underside of the arm, chest and/or abdomen/stomach, back, buttocks, hip area and/or genitals, front of the leg, back of the leg, and foot and/or ankle; participants could select all that applied. For each current lesion, participants were asked to assess their level of concern using an 11‐point Likert scale ranging from 0 (not at all concerned) to 10 (extremely concerned); a high degree of concern was defined as levels 8 to 10. For QoL assessment, participants with physician‐diagnosed vitiligo were asked to respond to the validated Vitiligo‐specific QoL (VitiQoL) instrument (higher scores indicate poorer QoL). 17 The stigma domain of VitiQoL assesses how patients feel about their vitiligo and their concern about what others are thinking. The participation limitation domain is designed to examine the effects of vitiligo on the patient's ability to perform daily activities and social or leisure activities, as well as emotional well‐being and overall physical health. The behaviour domain assesses the effect of the patient's skin condition on the choice of clothing, grooming practices and the use of sun protection.
Statistical analyses
There was no prespecified statistical hypothesis for vitiligo prevalence; therefore, prevalence was analysed using descriptive statistics only. Total vitiligo prevalence was calculated using the sum of participants who self‐reported physician‐diagnosed vitiligo, undiagnosed vitiligo and vitiligo signs with all survey participants used as the denominator. The prevalence of vitiligo among participants with vitiligo signs was adjusted based on confirmation of vitiligo through a review of uploaded photographs.
Analysis of QoL only included survey participants with physician‐diagnosed vitiligo. Participant demographic and vitiligo disease characteristic data are reported using descriptive statistics intended to characterise the population with vitiligo. Patients were stratified by demographic and disease characteristic variables; the VitiQoL domains (stigma, participation limitation and behaviour) were assessed independently and summarized by the total score within each subgroup. QoL scores were also compared for patients with routinely visible (i.e. head, hand and/or wrist) and non‐visible lesions. Statistical significance was assessed using the non‐parametric Kruskal–Wallis test. Vitiligo lesion location (multiple selections possible) and level of concern were analysed using descriptive statistics.
Results
Participant characteristics
A total of 35 694 respondents participated in the survey (Europe, n = 18 785; USA, n = 8517; Japan, n = 8392; Table 1). Among all participants, 55.7% were women and 57.9% were Caucasian (race was not solicited in France). Uploaded participant photographs were reviewed, and high consistency was found among participants who self‐reported vitiligo (diagnosed and undiagnosed). Among participants who reported vitiligo signs, self‐reports for 13% who uploaded photographs (3/24) were considered accurate.
Table 1.
Vitiligo prevalence by country
| Region | N | Total vitiligo prevalence | Diagnosed* | Undiagnosed† | Vitiligo signs‡ | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | n § | % (95% CI) | ||
| Total | 35 694 | 471 | 1.3 (1.2–1.4) | 219 | 0.6 (0.5–0.7) | 133 | 0.4 (0.3–0.4) | 119 | 0.3 (0.3–0.4) |
| Europe | 18 785 | 308 | 1.6 (1.5–1.8) | 150 | 0.8 (0.7–0.9) | 88 | 0.5 (0.4–0.6) | 70 | 0.4 (0.3–0.5) |
| France | 3678 | 46 | 1.2 (0.9–1.6) | 30 | 0.8 (0.5–1.1) | 5 | 0.1 (0.0–0.3) | 11 | 0.3 (0.1–0.5) |
| Germany | 6590 | 75 | 1.1 (0.9–1.4) | 30 | 0.5 (0.3–0.6) | 26 | 0.4 (0.2–0.5) | 19 | 0.3 (0.2–0.4) |
| Italy | 2025 | 64 | 3.1 (2.4–3.9) | 30 | 1.5 (1.0–2.0) | 27 | 1.3 (0.8–1.8) | 7 | 0.3 (0.1–0.6) |
| Spain | 3794 | 74 | 1.9 (1.5–2.4) | 30 | 0.8 (0.5–1.1) | 17 | 0.4 (0.2–0.7) | 27 | 0.7 (0.4–1.0) |
| UK | 2698 | 50 | 1.9 (1.3–2.4) | 30 | 1.1 (0.7–1.5) | 13 | 0.5 (0.2–0.7) | 7 | 0.3 (0.1–0.5) |
| USA | 8517 | 118 | 1.4 (1.1–1.6) | 48 | 0.6 (0.4–0.7) | 32 | 0.4 (0.2–0.5) | 38 | 0.4 (0.3–0.6) |
| Japan | 8392 | 45 | 0.5 (0.4–0.7) | 21 | 0.3 (0.1–0.4) | 13 | 0.2 (0.1–0.2) | 11 | 0.1 (0.1–0.2) |
Self‐reporting vitiligo and diagnosed by a physician.
Self‐reporting vitiligo but not diagnosed.
Experiencing signs associated with vitiligo but not diagnosed.
The total number of participants with vitiligo signs was rounded for each region/country because they are based on the algorithm used to account for the 13% adjustment in reporting accuracy.
Vitiligo prevalence
Among all 35 694 survey participants, the total estimated vitiligo prevalence across groups was 1.3% (diagnosed, 0.6%; undiagnosed, 0.4%; vitiligo signs, 0.3%; Table 1). Prevalence in the vitiligo signs group was adjusted to represent 13% of participants who were originally placed in this group, excluding those who likely did not have vitiligo (based on photographs). Prevalence was highest in Europe (1.6%), followed by the USA (1.4%) and Japan (0.5%). Vitiligo prevalence was highest among participants with type III (light brown; 0.5%) and type IV (moderate brown; 0.4%) skin phototypes per the Fitzpatrick scale (Table 2).
Table 2.
Vitiligo prevalence by Fitzpatrick scale
| Fitzpatrick Scale† | Total (N = 35 694) | Europe* (n = 18 785) | USA (n = 8517) | Japan (n = 8392) | ||||
|---|---|---|---|---|---|---|---|---|
| N | % (95% CI) | n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | |
| Any‡ | 471 | 1.3 (1.2–1.4) | 308 | 1.6 (1.5–1.8) | 118 | 1.4 (1.1–1.6) | 45 | 0.5 (0.4–0.7) |
| I | 16 | 0.0 (0.0–0.1) | 9 | 0.0 (0.0–0.1) | 7 | 0.1 (0.0–0.1) | 0 | 0.0 (0.0–0.0) |
| II | 92 | 0.3 (0.2–0.3) | 64 | 0.3 (0.3–0.4) | 27 | 0.3 (0.2–0.4) | 1 | 0.0 (0.0–0.0) |
| III | 195 | 0.5 (0.5–0.6) | 135 | 0.7 (0.6–0.8) | 51 | 0.6 (0.4–0.8) | 9 | 0.1 (0.0–0.2) |
| IV | 147 | 0.4 (0.3–0.5) | 88 | 0.5 (0.4–0.6) | 28 | 0.3 (0.2–0.5) | 31 | 0.4 (0.2–0.5) |
| V | 21 | 0.1 (0.0–0.1) | 12 | 0.1 (0.0–0.1) | 5 | 0.1 (0.0–0.1) | 4 | 0.1 (0.0–0.1) |
European countries included in the survey were France, Germany, Italy, Spain and the UK.
Skin phototype per the Fitzpatrick scale.
Fitzpatrick scale numbers are defined as follows: type I, pale white skin; type II, white skin; type III, light brown skin; type IV, moderate brown skin; type V, dark brown skin; type VI, deeply pigmented dark brown to black skin. No participants had deeply pigmented dark brown to black skin.
Vitiligo diagnosis and lesion location
Among 352 participants self‐reporting vitiligo (diagnosed and undiagnosed), mean (SD) age upon first noticing vitiligo signs was 24.1 (13.7) years. Of the 352 participants self‐reporting vitiligo, 219 (62.2%) reported formal diagnosis by a physician and participated in the complete survey. Characteristics of survey participants with confirmed vitiligo diagnosis (i.e. patients with vitiligo) are shown in Table 3. Of 219 patients diagnosed with vitiligo, 150 (68.5%) were from Europe, 48 (21.9%) from the USA and 21 (9.6%) from Japan. A majority of patients were <45 years old (68.9%), female (54.1%), Caucasian (77.7%), and had skin types III–V (73.5%). Most patients (63.9%) described their extent of vitiligo as low (1–2 on a 6‐point Likert scale) with some degree of progression. Vitiligo was diagnosed by a dermatologist in 63.0% of patients (Table 4). Dermatologists were the foremost diagnosing physicians across populations (Japan, 90.5%; Europe, 62.7%; USA, 52.1%), followed by primary care physicians (PCPs) in the USA (27.1%) and Europe (23.3%), and paediatricians or rheumatologists in Japan (each 4.8%). The first presentation of vitiligo lesions most commonly occurred on the back (13.7%), hands and/or wrists (11.4%), and chest and/or abdomen/stomach (11.0%). Among the reported sites with currently present vitiligo lesions, the hands and/or wrists (39.0%), feet and/or ankles (31.0%), top of arms (30.5%) and back of legs (30.5%) were most commonly affected (Fig. S2). In general, most patients reported current lesions on both sides of the body (Fig. S3).
Table 3.
Characteristics of survey participants diagnosed with vitiligo
| Characteristic, n (%) | Europe* (n = 150) | USA (n = 48) | Japan (n = 21) | All patients (N = 219) |
|---|---|---|---|---|
| Aged <45 years | 106 (70.6) | 29 (60.4) | 16 (76.2) | 151 (68.9) |
| Women † | 81 (54.0) | 29 (60.4) | 8 (38.1) | 118 (54.1) |
| Ethnicity ‡ | ||||
| Caucasian | 109 (90.8) | 37 (78.7) | 0 | 146 (77.7) |
| Hispanic | 5 (4.2) | 4 (8.5) | 0 | 9 (4.8) |
| Black | 1 (0.8) | 4 (8.5) | 0 | 5 (2.7) |
| Japanese | 2 (1.7) | 0 | 21 (100) | 23 (12.2) |
| Other | 3 (2.5) | 2 (4.3) | 0 | 5 (2.7) |
| Fitzpatrick scale § | ||||
| I | 7 (4.7) | 3 (6.3) | 0 | 10 (4.6) |
| II | 35 (23.3) | 12 (25.0) | 1 (4.8) | 48 (21.9) |
| III | 66 (44.0) | 18 (37.5) | 4 (19.0) | 88 (40.2) |
| IV | 38 (25.3) | 13 (27.1) | 14 (66.7) | 65 (29.7) |
| V | 4 (2.7) | 2 (4.2) | 2 (9.5) | 8 (3.7) |
| VI | 0 | 0 | 0 | 0 |
| Vitiligo extent ¦¦ | ||||
| Low | 99 (66.0) | 29 (60.4) | 12 (57.1) | 140 (63.9) |
| Medium | 37 (24.7) | 13 (27.1) | 6 (28.6) | 56 (25.6) |
| High | 14 (9.3) | 6 (12.5) | 3 (14.3) | 23 (10.5) |
| Early‐stage progression | ||||
| No progression | 17 (11.3) | 6 (12.5) | 2 (9.5) | 25 (11.4) |
| Slow progression | 47 (31.3) | 11 (22.9) | 7 (33.3) | 65 (29.7) |
| Stable, then rapid | 40 (26.7) | 18 (37.5) | 4 (19.0) | 62 (28.3) |
| Rapid, no stabilisation | 13 (8.7) | 6 (12.5) | 2 (9.5) | 21 (9.6) |
| Rapid at first, then stabilised | 16 (10.7) | 4 (8.3) | 5 (23.8) | 25 (11.4) |
| Rapid, short bursts separated by stabilisation | 15 (10.0) | 2 (4.2) | 1 (4.8) | 18 (8.2) |
| Other | 2 (1.3) | 1 (2.1) | 0 | 3 (1.4) |
| Reported previous management strategy | 134 (89.3) | 41 (85.4) | 20 (95.2) | 195 (89.0) |
| Time since diagnosis, y | ||||
| <5 | 58 (38.7) | 24 (50.0) | 5 (23.8) | 87 (39.7) |
| 5–10 | 34 (22.7) | 9 (18.8) | 8 (38.1) | 51 (23.3) |
| >10 | 58 (38.7) | 15 (31.2) | 8 (38.1) | 81 (37.0) |
European countries included in the survey were France, Germany, Italy, Spain and the UK.
Sex data were available for 218 patients.
Ethnicity data were available for 188 patients (Europe, n = 120; USA, n = 47; Japan, n = 21); ethnicity data were not solicited in France.
Fitzpatrick scale numbers are defined as follows: type I, pale white skin; type II, white skin; type III, light brown skin; type IV, moderate brown skin; type V, dark brown skin; type VI, deeply pigmented dark brown to black skin.
Vitiligo extent was rated using a 6‐point Likert scale, where low vitiligo extent was based on a score of 1–2, medium on a score of 3–4, and high on a score of 5–6.
Table 4.
Vitiligo diagnosis by physician specialty
| Region | Self‐reported vitiligo,* N | Diagnosed, n (%) | Top diagnosing physicians | ||
|---|---|---|---|---|---|
| Dermatologist, n (%) | PCP, n (%) | Other, n (%) | |||
| Total | 352 | 219 (62.2) | 138 (63.0) | 48 (21.9) | 33 (15.1) |
| Europe | 238 | 150 (63.0) | 94 (62.7) | 35 (23.3) | 21 (14.0) |
| France | 35 | 30 (85.7) | 16 (53.3) | 10 (33.3) | 4 (13.3) |
| Germany | 56 | 30 (53.6) | 23 (76.7) | 6 (20.0) | 1 (3.3) |
| Italy | 57 | 30 (52.6) | 22 (73.3) | 5 (16.7) | 3 (10.0) |
| Spain | 47 | 30 (63.8) | 21 (70.0) | 5 (16.7) | 4 (13.3) |
| UK | 43 | 30 (69.8) | 12 (40.0) | 9 (30.0) | 9 (30.0) |
| USA | 80 | 48 (60.0) | 25 (52.1) | 13 (27.1) | 10 (20.8) |
| Japan | 34 | 21 (61.8) | 19 (90.5) | 0 | 2 (9.5) |
PCP, primary care physician.
Includes participants with and without a formal diagnosis of vitiligo.
Quality of life in patients with vitiligo
Total VitiQoL scores
Total VitiQoL scores were highest (indicating poorer QoL) among patients aged 25 to 44 years and generally declined with age (P = 0.00017; Table 5). No significant differences in total VitiQoL scores were observed for other patient demographics including sex, Fitzpatrick scale, ethnicity or geographical region. Analysis of VitiQoL total scores indicated worse QoL among patients with a greater extent of disease (median, 79; P < 0.0001) and in patients who started with stable disease that quickly spread later in life (median, 74; P < 0.0001; Fig. 1). Patients who reported ever using ≥1 management strategy (i.e. cosmetics, sun protection, prescription and non‐prescription products, surgery, phototherapy) reported higher VitiQoL scores (median, 51; P < 0.0001) than those with no previous management (median, 11).
Table 5.
Quality‐of‐life scores stratified by baseline patient demographics
| Demographic | N | Total VitiQoL, median (IQR) | Stigma, median (IQR) | Participation limitation, median (IQR) | Behaviour, median (IQR) |
|---|---|---|---|---|---|
| All patients | 219 | 48 (18–70) | 19 (10–25) | 19 (4–33) | 10 (3–16) |
| Age group, y | |||||
| 19–24 | 19 | 30 (8–57) | 11 (4–20) | 10 (2–27) | 7 (2–14) |
| 25–29 | 29 | 66 (40–74) | 22 (14–25) | 30 (13–34) | 13 (8–16) |
| 30–34 | 32 | 56 (30–69) | 20 (12–25) | 24 (11–33) | 12 (5–15) |
| 35–39 | 42 | 64 (37–79) | 24 (14–27) | 29 (14–36) | 13 (5–17) |
| 40–44 | 29 | 56 (43–77) | 21 (15–24) | 25 (16–37) | 12 (7–16) |
| 45–49 | 12 | 51 (20–62) | 20 (13–24) | 15 (2–24) | 14 (4–18) |
| 50–54 | 11 | 40 (30–61) | 18 (13–26) | 16 (6–23) | 10 (6–15) |
| 55–59 | 13 | 16 (8–45) | 11 (4–16) | 3 (0–21) | 2 (0–6) |
| 60–64 | 12 | 29 (15–54) | 14 (7–19) | 10 (3–22) | 8 (4–12) |
| 65–69 | 13 | 16 (11–31) | 9 (7–15) | 4 (0–8) | 5 (0–9) |
| 70–74 | 7 | 3 (0–85) | 2 (0–27) | 0 (0–40) | 1 (0–18) |
| P value for association* | 0.00017 | 0.0013 | <0.0001 | 0.0037 | |
| Sex † | |||||
| Male | 100 | 50 (17–75) | 19 (11–26) | 22 (4–35) | 10 (5–16) |
| Female | 118 | 46 (22–67) | 19 (10–24) | 16 (5–31) | 10 (3–15) |
| P value for association* | 0.29 | 0.63 | 0.12 | 0.81 | |
| Fitzpatrick scale | |||||
| I | 10 | 47 (30–77) | 17 (13–24) | 17 (11–37) | 14 (4–18) |
| II | 48 | 49 (32–70) | 19 (13–26) | 20 (14–33) | 12 (7–17) |
| III | 88 | 55 (28–73) | 20 (11–26) | 21 (7–34) | 11 (6–16) |
| IV | 65 | 35 (8–65) | 18 (4–23) | 11 (0–29) | 5 (1–14) |
| V | 8 | 60 (19–64) | 22 (13–23) | 26 (6–29) | 9 (4–16) |
| P value for association* | 0.17 | 0.34 | 0.21 | 0.024 | |
| Race/Ethnicity ‡ | |||||
| Caucasian | 146 | 48 (16–72) | 19 (10–25) | 19 (4–34) | 10 (3–16) |
| Japanese | 23 | 54 (28–66) | 19 (11–25) | 22 (12–30) | 9 (5–15) |
| Hispanic | 9 | 60 (50–82) | 20 (18–29) | 24 (16–36) | 14 (14–17) |
| Black | 5 | 8 (0–31) | 6 (0–21) | 1 (0–5) | 1 (0–5) |
| Other | 5 | 67 (57–86) | 23 (18–30) | 27 (27–39) | 17 (12–17) |
| P value for association* | 0.10 | 0.26 | 0.11 | 0.048 | |
| Geographical region | |||||
| Europe | 150 | 45 (22–66) | 18 (10–24) | 18 (5–31) | 10 (3–15) |
| USA | 48 | 56 (14–77) | 22 (8–26) | 22 (2–35) | 12 (4–17) |
| Japan | 21 | 55 (30–66) | 20 (12–25) | 26 (12–30) | 9 (6–15) |
| P value for association* | 0.48 | 0.41 | 0.45 | 0.77 | |
IQR, interquartile range (quartile 1–quartile 3); VitiQoL, Vitiligo‐specific Quality of Life.
P values for subgroup associations were derived from the non‐parametric Kruskal–Wallis test. Significant P values are in bold.
Sex data were available for 218 patients.
Ethnicity data were available for 188 patients; ethnicity data were not solicited in France.
Figure 1.
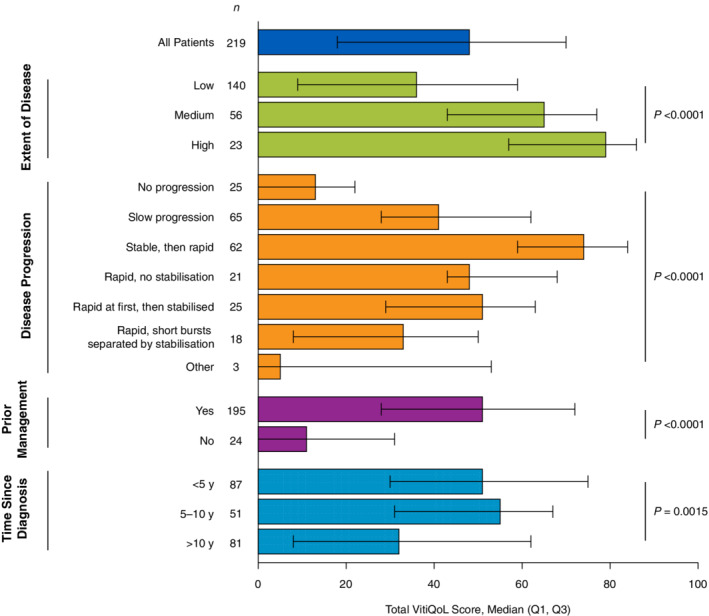
Total VitiQoL score stratified by vitiligo disease characteristics. Q1, quartile 1; Q3, quartile 3; VitiQoL, Vitiligo‐specific Quality of Life. Higher scores indicate poorer quality of life. P values were derived from the non‐parametric Kruskal–Wallis test.
Stigma scores
Stigma scores were highest among patients aged 25 to 49 years and then declined with age (P = 0.0013; Table 5). No significant differences in stigma scores were observed when stratified by other patient demographics. When stratified by disease characteristics, the highest stigma scores were observed in patients with a greater extent of disease (median, 26; P < 0.0001) and those who started with stable disease that quickly spread later in life (median, 26; P < 0.0001; Fig. 2). Patients who reported using ≥1 management strategy reported higher stigma scores (median, 20; P < 0.0001) than those with no management (median, 7).
Figure 2.
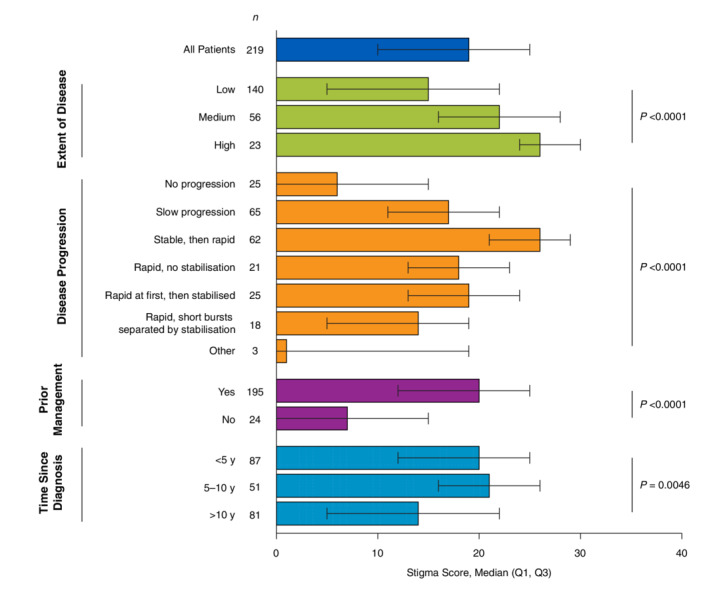
Stigma score stratified by vitiligo disease characteristics. Q1, quartile 1; Q3, quartile 3. Higher scores indicate poorer quality of life. P values were derived from the non‐parametric Kruskal–Wallis test.
Participation limitation scores
Similar to total VitiQoL and stigma scores, participation limitation scores were highest among patients aged 25 to 44 years and then generally declined with age (P < 0.0001; Table 5). No significant differences in participation limitation scores were observed when stratified by other patient demographics. The highest participation limitation scores were observed in patients with a greater extent of disease (median, 37; P < 0.0001) and those who started with stable disease that quickly spread later in life (median, 34; P < 0.0001; Fig. 3). Participation limitation scores were higher in patients who reported using ≥1 management strategy (median, 21; P < 0.0001) than those with no prior management (median, 2).
Figure 3.
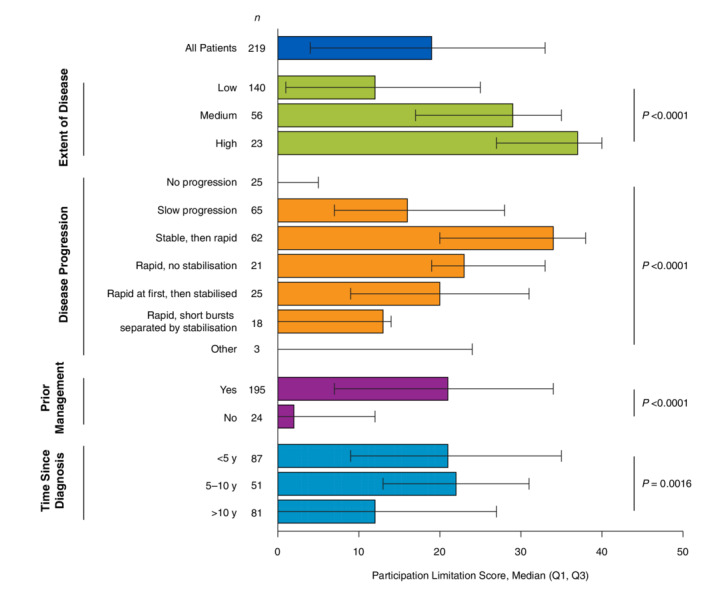
Participation limitation score stratified by vitiligo disease characteristics. Q1, quartile 1; Q3, quartile 3. Higher scores indicate poorer quality of life. P values were derived from the non‐parametric Kruskal–Wallis test.
Behaviour scores
Behaviour scores were highest among patients aged 25 to 49 years and then generally declined with age (P = 0.0037; Table 5). Patients with skin phototype I (median, 14; P = 0.024) had the highest scores, whereas those with skin phototype IV (median, 5) had the lowest scores. Among respondents who identified their ethnicity, behaviour scores were highest among Hispanic patients (median, 14; P = 0.048) and lowest among black patients (median, 1). When stratified by disease characteristics, the highest behaviour scores were observed in patients with a greater extent of disease (median, 17; P < 0.0001) and those who started with stable disease that quickly spread later in life (median, 16; P < 0.0001; Fig. 4). Patients who reported using ≥1 management strategy had higher behaviour scores (median, 11; P = 0.00031) than those with no prior management (median, 2).
Figure 4.
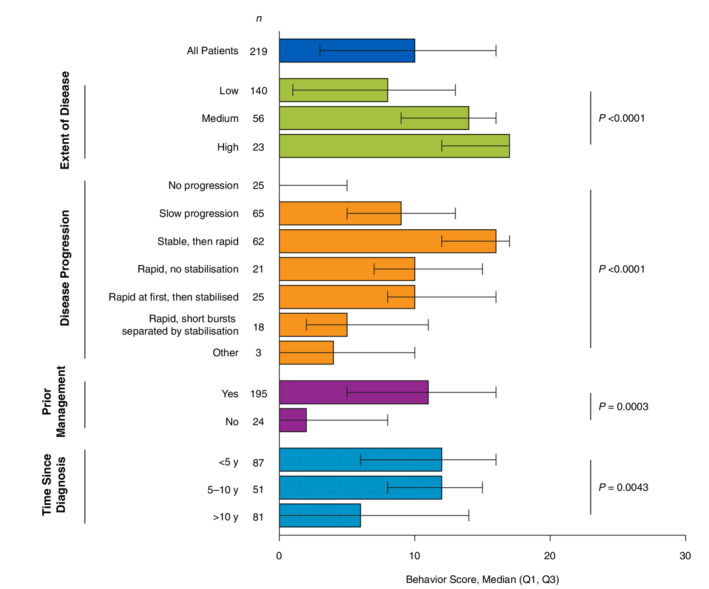
Behaviour score stratified by vitiligo disease characteristics. Q1, quartile 1; Q3, quartile 3. Higher scores indicate poorer quality of life. P values were derived from the non‐parametric Kruskal–Wallis test.
Quality of life by lesion location
When stratified by the presence or absence of lesions in routinely visible areas, total VitiQoL scores were significantly higher among patients with lesions on the head vs. those without head lesions (median, 58 vs. 44; P = 0.027) and those with head and hand and/or wrist lesions vs. those without lesions in these areas (median, 63 vs. 45; P = 0.018; Table 6). Similar findings were observed for participation limitation scores. Stigma scores were significantly higher only among patients with head and hand and/or wrist lesions compared to those without lesions in these areas.
Table 6.
Quality‐of‐life scores by visible vs. non‐visible lesions
| Site of lesions | N | Total VitiQoL, median (IQR) | Stigma, median (IQR) | Participation limitation, median (IQR) | Behaviour, median (IQR) |
|---|---|---|---|---|---|
| Head | 58 | 58 (33–77) | 21 (14–26) | 25 (12–36) | 12 (6–17) |
| No Head | 161 | 44 (15–66) | 18 (9–24) | 17 (3–30) | 10 (2–15) |
| P value for association* | 0.027 | 0.077 | 0.014 | 0.078 | |
| Hand and/or wrist | 78 | 54 (26–77) | 22 (10–26) | 20 (6–36) | 11 (5–16) |
| No hand and/or wrist | 141 | 46 (16–66) | 18 (10–24) | 18 (4–31) | 10 (3–15) |
| P value for association* | 0.18 | 0.098 | 0.15 | 0.44 | |
| Head and hand and/or wrist | 35 | 63 (30–83) | 23 (14–28) | 30 (13–38) | 12 (6–17) |
| No head and no hand and/or wrist | 184 | 45 (16–67) | 18 (9–24) | 18 (4–31) | 10 (3–15) |
| P value for association* | 0.018 | 0.039 | 0.0079 | 0.097 |
IQR, interquartile range; VitiQoL, Vitiligo‐specific Quality of Life.
P values for subgroup associations were derived from the non‐parametric Kruskal–Wallis test. Significant P values are in bold.
There was substantial high concern (scores of 8–10 on an 11‐point Likert scale) for vitiligo lesions across all body areas (Fig. 5a). Body areas associated with high concern in the largest proportion of patients were the buttocks (63.5%), underside of arms (50.9%) and back of legs (49.2%). Among body areas that are visible under normal circumstances, the hands and/or wrists were associated with a high level of concern in 42.3% of patients. Areas of high concern varied among the geographical regions surveyed (Fig. 5b); the largest proportion of Europeans reported high concern for buttock lesions (65.6%), Americans for armpit lesions (72.7%) and Japanese participants for back and hand and/or wrist lesions (both 50.0%).
Figure 5.
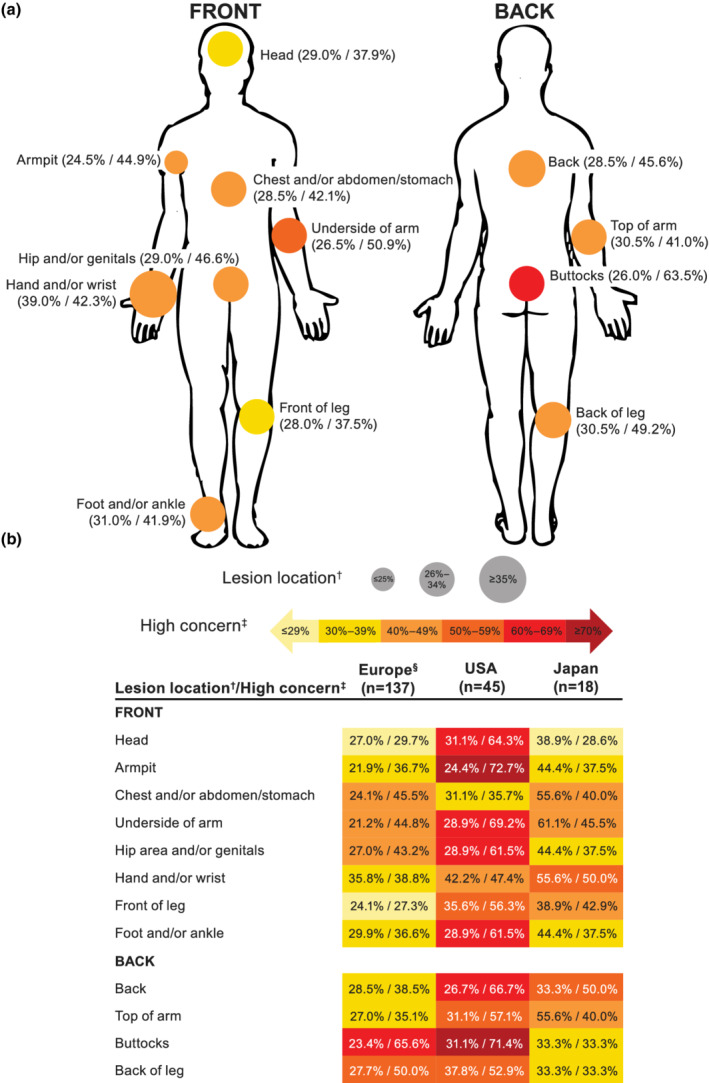
Lesion location and corresponding high level of concern among (a) all patients diagnosed with vitiligo (n = 200)* and (b) by geographical region. Percentages are shown as patients reporting lesion location/high level of concern among those reporting lesions in each location. * Data for 19 patients who did not have current lesions are not shown (Europe, n = 13; USA, n = 3; Japan, n = 3). † Patients could report lesions on multiple body regions. ‡ High levels of concern were noted for patients responding with 8, 9 or 10 on an 11‐point Likert scale ranging from 0 (not at all concerned) to 10 (extremely concerned). § European countries included France, Germany, Italy, Spain and the UK.
Discussion
To our knowledge, this is the first study to evaluate vitiligo prevalence across three large populations in a self‐reported survey and examine the correlation between vitiligo disease characteristics and QoL in several geographical regions, providing additional evidence of the burden of vitiligo. This study also included participants without a formal diagnosis of vitiligo (but confirmed by an expert review of uploaded photographs) to allow for a broader assessment of vitiligo prevalence. The prevalence of vitiligo in this population‐based survey of 35 694 participants was 1.3%, which is higher than most general population studies examined in a 2012 review (the majority were ≤0.6%; range, 0.06–2.28%) 5 and substantially greater than the 0.2% reported in a meta‐analysis of pooled population‐based studies from 1964 to 2015. 6 These results suggest that the historical prevalence of vitiligo is underreported and may be higher than current estimates.
In this study, the prevalence of vitiligo in the USA and Europe was similar (1.4% and 1.6%, respectively) and higher than previously reported in population‐based studies in the USA 18 , 19 and the five European countries 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 included in our study. Prevalence rates were much lower in Japan (0.5%) compared with a previous hospital‐based study (1.68%), 28 although methodology differences (population vs. hospital based) likely contributed to the lower prevalence in our study. Of note is that an overwhelming majority of patients in Japan, where the healthcare system allows for free access to specialists without referral from a PCP, 29 were diagnosed by a dermatologist (91%). By comparison, dermatologists diagnosed 63% of European patients and 52% of patients from the USA, with PCPs diagnosing approximately one‐quarter of patients in each region.
Results from this study also suggest that a substantial number of individuals who may have vitiligo do not seek medical attention for the condition. The prevalence of participants who had a diagnosis of vitiligo in this study (0.6%) conforms with previous estimates. 5 However, 0.4% of those surveyed self‐identified as having vitiligo without a diagnosis, and 0.3% reported vitiligo signs (following adjustment to account for 13% accuracy in reporting signs consistent with vitiligo). Recent evidence from a German study also shows a substantial discrepancy between vitiligo prevalence from primary clinical data and medical claims for the treatment of vitiligo, indicating that many individuals do not seek medical attention. 30 These findings suggest that increased awareness and education about vitiligo are necessary. Some individuals, especially in Middle Eastern countries, may choose to refrain from seeking a formal diagnosis owing to their personal beliefs that vitiligo is incurable or largely dependent on their behaviour. 31 , 32 In addition, vitiligo specialists are scarce within the field of dermatology, and no medical treatment has yet been approved for the repigmentation of vitiligo. 33
Among 219 patients formally diagnosed with vitiligo, the proportion with initially vs. currently present lesions increased for all body areas, although 19 patients (8.7%) reported no current lesions, which may be attributed to repigmentation following treatment. Patients with longstanding vitiligo may have had difficulty recalling the site(s) of their initial lesions; however, these data suggest that most patients experienced spreading of vitiligo during the disease. The most common sites of current lesions were the hands and/or wrists and feet and/or ankles, both regions that are particularly resistant to repigmentation. 34
Among patients with diagnosed vitiligo who responded to this survey, significant differences in QoL scores were observed based on several disease characteristics including the extent of disease, disease progression, prior management and time since diagnosis. In general, no significant differences by demographic variables were observed with the exception of age, wherein older adults experienced less burden, consistent with previous findings. 10 , 35 , 36 Results are consistent with previous studies that demonstrated greater QoL impairment with a higher extent of disease 4 , 10 , 13 and disease progression. 37 Disease duration has also been reported to be associated with impaired QoL. 4 , 38 In our study, QoL was better in patients with >10 years of disease activity, consistent with another study that used VitiQoL, 35 and may be related to acceptance of vitiligo over time. 39 Patients with vitiligo often experience feelings of stigmatization, which may lead to social avoidance. 40 , 41 Behavioural strategies to cope with the disease include the use of concealing clothing and camouflaging of lesions. 40 Similarly, our data indicate that stigma, participation limitation, and behavioural domains of the VitiQoL are affected by the extent of disease, disease progression, and time since diagnosis. Interestingly, our data indicated markedly lower behaviour scores among black patients and those with Fitzpatrick skin types IV–V. Because responses may not be unilateral, patients with fairer skin who routinely cover up and use sun protection to avoid burning in addition to concealing lesions could have potentially higher behaviour scores than counterparts with darker skin.
Vitiligo lesions on visible areas are associated with self‐consciousness. 10 In our study, patients with head lesions had significantly worse VitiQoL scores than those without head lesions, consistent with similar findings using the Dermatology Life Quality Index. 37 Hand/wrist vs. no hand/wrist involvement did not result in significantly different VitiQoL scores in our study, in contrast to a previous study in which a significant difference was reported. 42 Nevertheless, hand and/or wrist involvement was associated with a high level of concern for 42% of patients in our study, potentially due to visibility and propensity for resistance to repigmentation. 34 The largest proportion of patients noted high levels of concern for lesions in sensitive skin areas (e.g. buttocks), followed by areas that can generally be covered with clothing (e.g. the underside of arms and back of legs), indicating that the influence of unexposed lesions on QoL should not be disregarded. Furthermore, differences in high levels of concern among geographical regions highlight the potential impact of cultural and climatic factors on psychosocial QoL among patients with vitiligo.
Several limitations should be considered when interpreting the results of this study. There is potential for selection bias and errors in measurement inherent in patient‐reported outcomes studies. Although efforts were made to ensure that the survey population was representative of the general population, the online nature of the survey does not account for those without internet access. In addition, although the spectrum of skin phototypes for the participants may not reflect the racial or ethnic diversity of the population studied, it should be recognized that self‐assessed Fitzpatrick skin type may be difficult for some participants to report accurately. This study also used uploaded photographs for adjustment of vitiligo prevalence among participants without a formal diagnosis. Although this approach was useful to confirm the accuracy of self‐reported vitiligo or vitiligo signs, it was also limited by poor image quality in some cases, especially in participants with light skin where a Wood's lamp is often needed on physical examination. 43 In addition, not all participants with undiagnosed vitiligo or vitiligo signs uploaded images, which may affect the prevalence estimates. Inaccuracies with respect to the location of the first vitiligo lesion are also possible because of recall bias (i.e. some participants had their first lesions many years ago). The survey also included the use of sunscreen and cosmetic camouflage as management strategies for vitiligo, which, in contrast to medical therapy, does not aim to repigment lesions. Lastly, because participants in the survey were predominantly Caucasian, the data may not be representative of a global population, particularly because many patients with vitiligo in Middle Eastern 44 , 45 , 46 and South Asian 47 , 48 countries experience high QoL impairment.
In conclusion, this online population‐based survey demonstrated that the prevalence of vitiligo was 1.3% across three regions (USA, Europe, Japan), which is higher than previously estimated in population‐based studies. A sizable proportion of the prevalence rate was attributed to participants who reported having undiagnosed vitiligo or vitiligo signs. These participants represent a subgroup of non‐healthcare‐seeking individuals who could benefit from educational initiatives about the characteristics of vitiligo and available treatment options. Among patients with formally diagnosed vitiligo, those with progressive disease and high extent of disease had the worst QoL overall, felt the most stigmatized, and had the worst participation limitation and behaviour scores, suggesting that these patients could benefit from early intervention. Patients with diagnosed vitiligo experienced a high degree of concern regarding their lesion location in both exposed and unexposed areas. Further research is warranted to examine the relationship of QoL in relation to the location of vitiligo lesions.
Supporting information
Figure S1. Survey flow diagram.
Figure S2. Lesion site distribution among patients diagnosed with vitiligo.
Figure S3. Current location of lesions within body regions.
Acknowledgements
The patients in this article have given written informed consent to publication of their case details. This study was funded by Incyte Corporation (Wilmington, DE, USA). Writing assistance was provided by Joshua Solomon, PhD, an employee of ICON (Blue Bell, PA, USA), and was funded by Incyte Corporation.
Conflicts of interest
KB, JG and AL are employees and shareholders of Incyte Corporation. HJ was an employee and shareholder of Incyte Corporation at the time the study was conducted. AGP has served as an investigator for Aclaris Therapeutics, Immune Tolerance Network, Incyte Corporation and Pfizer; a consultant for AbbVie, Arcutis, Avita Medical, Chromaderm, Immune Tolerance Network, Incyte Corporation, Pfizer, TWi, Viela Bio and Villaris; and holds stock options for Tara Medical and Zerigo Health. KE is a consultant for AbbVie, Incyte Corporation, La Roche‐Posay, Pfizer, Pierre Fabre, Sanofi and Viela Bio. JEH has served as a consultant for AbbVie, Aclaris Therapeutics, BiologicsMD, EMD Serono, Genzyme/Sanofi, Janssen, Pfizer, Rheos Medicines, Sun Pharmaceuticals, TeVido BioDevices, The Expert Institute, 3rd Rock Ventures and Villaris Therapeutics; has served as an investigator for Aclaris Therapeutics, Celgene, Dermira, EMD Serono, Genzyme/Sanofi, Incyte Corporation, LEO Pharma, Pfizer, Rheos Medicines, Stiefel/GlaxoSmithKline, Sun Pharmaceuticals, TeVido BioDevices and Villaris Therapeutics; holds equity in Rheos Medicines, TeVido BioDevices and Villaris Therapeutics; is a scientific founder of Villaris Therapeutics and NIRA Biosciences; and has patents pending for IL‐15 blockade for the treatment of vitiligo, JAK inhibition with light therapy for vitiligo, and CXCR3 antibody depletion for the treatment of vitiligo.
Funding sources
This study was funded by Incyte Corporation (Wilmington, DE, USA), which was involved in study design, data collection, data analysis, manuscript preparation and publication decisions in collaboration with the authors.
Data availability statement
Access to individual participant‐level data is not publicly available for this study.
References
- 1. Rodrigues M, Ezzedine K, Hamzavi I et al. New discoveries in the pathogenesis and classification of vitiligo. J Am Acad Dermatol 2017; 77: 1–13. [DOI] [PubMed] [Google Scholar]
- 2. Ezzedine K, Eleftheriadou V, Whitton M et al. Vitiligo. Lancet 2015; 386: 74–84. [DOI] [PubMed] [Google Scholar]
- 3. Talsania N, Lamb B, Bewley A. Vitiligo is more than skin deep: a survey of members of the vitiligo society. Clin Exp Dermatol 2010; 35: 736–739. [DOI] [PubMed] [Google Scholar]
- 4. Radtke MA, Schafer I, Gajur A et al. Willingness‐to‐pay and quality of life in patients with vitiligo. Br J Dermatol 2009; 161: 134–139. [DOI] [PubMed] [Google Scholar]
- 5. Kruger C, Schallreuter KU. A review of the worldwide prevalence of vitiligo in children/adolescents and adults. Int J Dermatol 2012; 51: 1206–1212. [DOI] [PubMed] [Google Scholar]
- 6. Zhang Y, Cai Y, Shi M et al. The prevalence of vitiligo: a meta‐analysis. PLoS One 2016; 11: e0163806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Teasdale E, Muller I, Abdullah Sani A et al. Views and experiences of seeking information and help for vitiligo: a qualitative study of written accounts. BMJ Open 2018; 8: 10.1136/bmjopen‐2017‐018652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ezzedine K, Sheth V, Rodrigues M et al. Vitiligo is not a cosmetic disease. J Am Acad Dermatol 2015; 73: 883–885. [DOI] [PubMed] [Google Scholar]
- 9. Morrison B, Burden‐Teh E, Batchelor JM et al. Quality of life in people with vitiligo: a systematic review and meta‐analysis. Br J Dermatol 2017; 177: e338–e339. [DOI] [PubMed] [Google Scholar]
- 10. Silverberg JI, Silverberg NB. Association between vitiligo extent and distribution and quality‐of‐life impairment. JAMA Dermatol 2013; 149: 159–164. [DOI] [PubMed] [Google Scholar]
- 11. Osinubi O, Grainge MJ, Hong L et al. The prevalence of psychological comorbidity in people with vitiligo: a systematic review and meta‐analysis. Br J Dermatol 2018; 178: 863–878. [DOI] [PubMed] [Google Scholar]
- 12. Grimes PE, Miller MM. Vitiligo: patient stories, self‐esteem, and the psychological burden of disease. Int J Womens Dermatol 2018; 4: 32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen D, Tuan H, Zhou EY et al. Quality of life of adult vitiligo patients using camouflage: a survey in a Chinese vitiligo community. PLoS One 2019; 14: e0210581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sheth VM, Guo Y, Qureshi AA. Comorbidities associated with vitiligo: a ten‐year retrospective study. Dermatology 2013; 227: 311–315. [DOI] [PubMed] [Google Scholar]
- 15. Yuan J, Sun C, Jiang S et al. The prevalence of thyroid disorders in patients with vitiligo: a systematic review and meta‐analysis. Front Endocrinol (Lausanne) 2018; 9: 803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang G, Qiu D, Yang H et al. The prevalence and odds of depression in patients with vitiligo: a meta‐analysis. J Eur Acad Dermatol Venereol 2018; 32: 1343–1351. [DOI] [PubMed] [Google Scholar]
- 17. Lilly E, Lu PD, Borovicka JH et al. Development and validation of a vitiligo‐specific quality‐of‐life instrument (VitiQoL). J Am Acad Dermatol 2013; 69: e11–e18. [DOI] [PubMed] [Google Scholar]
- 18. Prahalad S, Shear ES, Thompson SD et al. Increased prevalence of familial autoimmunity in simplex and multiplex families with juvenile rheumatoid arthritis. Arthritis Rheum 2002; 46: 1851–1856. [DOI] [PubMed] [Google Scholar]
- 19. Vanderhooft SL, Francis JS, Pagon RA et al. Prevalence of hypopigmented macules in a healthy population. J Pediatr 1996; 129: 355–361. [DOI] [PubMed] [Google Scholar]
- 20. Cellini A, Offidani A. An epidemiological study on cutaneous diseases of agricultural workers authorized to use pesticides. Dermatology 1994; 189: 129–132. [DOI] [PubMed] [Google Scholar]
- 21. Wolkenstein P, Grob JJ, Bastuji‐Garin S et al. French people and skin diseases: results of a survey using a representative sample. Arch Dermatol 2003; 139: 1614–1619 discussion 1619. [DOI] [PubMed] [Google Scholar]
- 22. Naldi L, Colombo P, Placchesi EB et al. Study design and preliminary results from the pilot phase of the PraKtis study: self‐reported diagnoses of selected skin diseases in a representative sample of the Italian population. Dermatology 2004; 208: 38–42. [DOI] [PubMed] [Google Scholar]
- 23. Ingordo V, Gentile C, Iannazzone SS et al. Vitiligo and autoimmunity: an epidemiological study in a representative sample of young Italian males. J Eur Acad Dermatol Venereol 2011; 25: 105–109. [DOI] [PubMed] [Google Scholar]
- 24. Ingordo V, Gentile C, Iannazzone SS et al. The 'EpiEnlist' project: a dermo‐epidemiologic study on a representative sample of young Italian males. Prevalence of selected pigmentary lesions. J Eur Acad Dermatol Venereol 2007; 21: 1091–1096. [DOI] [PubMed] [Google Scholar]
- 25. Richard MA, Corgibet F, Beylot‐Barry M et al. Sex‐ and age‐adjusted prevalence estimates of five chronic inflammatory skin diseases in France: results of the << OBJECTIFS PEAU >> study. J Eur Acad Dermatol Venereol 2018; 32: 1967–1971. [DOI] [PubMed] [Google Scholar]
- 26. Svensson A, Ofenloch RF, Bruze M et al. Prevalence of skin disease in a population‐based sample of adults from five European countries. Br J Dermatol 2018; 178: 1111–1118. [DOI] [PubMed] [Google Scholar]
- 27. Schallreuter KU, Levenig C, Berger J. Vitiligo and cutaneous melanoma. A case study. Dermatologica 1991; 183: 239–245. [DOI] [PubMed] [Google Scholar]
- 28. Furue M, Yamazaki S, Jimbow K et al. Prevalence of dermatological disorders in Japan: a nationwide, cross‐sectional, seasonal, multicenter, hospital‐based study. J Dermatol 2011; 38: 310–320. [DOI] [PubMed] [Google Scholar]
- 29. Nomura H, Nakayama T. The Japanese healthcare system. BMJ 2005; 331: 648–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mohr N, Petersen J, Kirsten N et al. Epidemiology of vitiligo ‐ a dual population‐based approach. Clin Epidemiol 2021; 13: 373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. AlGhamdi KM. Beliefs and perceptions of Arab vitiligo patients regarding their condition. Int J Dermatol 2010; 49: 1141–1145. [DOI] [PubMed] [Google Scholar]
- 32. Firooz A, Bouzari N, Fallah N et al. What patients with vitiligo believe about their condition. Int J Dermatol 2004; 43: 811–814. [DOI] [PubMed] [Google Scholar]
- 33. Bergqvist C, Ezzedine K. Vitiligo: a focus on pathogenesis and its therapeutic implications. J Dermatol 2021; 48: 252–270. [DOI] [PubMed] [Google Scholar]
- 34. Esmat SM, El‐Tawdy AM, Hafez GA et al. Acral lesions of vitiligo: why are they resistant to photochemotherapy? J Eur Acad Dermatol Venereol 2012; 26: 1097–1104. [DOI] [PubMed] [Google Scholar]
- 35. Hedayat K, Karbakhsh M, Ghiasi M et al. Quality of life in patients with vitiligo: a cross‐sectional study based on Vitiligo Quality of Life index (VitiQoL). Health Qual Life Outcomes 2016; 14: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Al‐Mubarak L, Al‐Mohanna H, Al‐Issa A et al. Quality of life in Saudi vitiligo patients. J Cutan Aesthet Surg 2011; 4: 33–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ingordo V, Cazzaniga S, Gentile C et al. Dermatology Life Quality Index score in vitiligo patients: a pilot study among young Italian males. G Ital Dermatol Venereol 2012; 147: 83–90. [PubMed] [Google Scholar]
- 38. Linthorst Homan MW, Spuls PI, de Korte J et al. The burden of vitiligo: patient characteristics associated with quality of life. J Am Acad Dermatol 2009; 61: 411–420. [DOI] [PubMed] [Google Scholar]
- 39. Ahmed A, Steed L, Burden‐Teh E et al. Identifying key components for a psychological intervention for people with vitiligo ‐ a quantitative and qualitative study in the United Kingdom using web‐based questionnaires of people with vitiligo and healthcare professionals. J Eur Acad Dermatol Venereol 2018; 32: 2275–2283. [DOI] [PubMed] [Google Scholar]
- 40. Kruger C, Schallreuter KU. Stigmatisation, avoidance behaviour and difficulties in coping are common among adult patients with vitiligo. Acta Derm Venereol 2015; 95: 553–558. [DOI] [PubMed] [Google Scholar]
- 41. Thompson AR, Clarke SA, Newell RJ et al. Vitiligo linked to stigmatization in British south Asian women: a qualitative study of the experiences of living with vitiligo. Br J Dermatol 2010; 163: 481–486. [DOI] [PubMed] [Google Scholar]
- 42. Florez‐Pollack S, Jia G, Zapata L Jr et al. Association of quality of life and location of lesions in patients with vitiligo. JAMA Dermatol 2017; 153: 341–342. [DOI] [PubMed] [Google Scholar]
- 43. Alghamdi KM, Kumar A, Taieb A et al. Assessment methods for the evaluation of vitiligo. J Eur Acad Dermatol Venereol 2012; 26: 1463–1471. [DOI] [PubMed] [Google Scholar]
- 44. Ghaderi R, Saadatjoo A. Evaluating of life quality in Iranian patients with vitiligo using generic and special questionnaires. Shiraz E‐Med J 2014; 15: e22359. [Google Scholar]
- 45. Ghajarzadeh M, Ghiasi M, Kheirkhah S. Associations between skin diseases and quality of life: a comparison of psoriasis, vitiligo, and alopecia areata. Acta Med Iran 2012; 50: 511–515. [PubMed] [Google Scholar]
- 46. Salman A, Kurt E, Topcuoglu V et al. Social anxiety and quality of life in vitiligo and acne patients with facial involvement: a cross‐sectional controlled study. Am J Clin Dermatol 2016; 17: 305–311. [DOI] [PubMed] [Google Scholar]
- 47. Noor SM, Khurshid K, Mahmood T et al. Quality of life in vitiligo patients. J Pakistan Assoc Dermatol 2004; 14: 55–58. [Google Scholar]
- 48. Mishra N, Rastogi MK, Gahalaut P et al. Dermatology specific quality of life in vitiligo patients and its relation with various variables: a hospital based cross‐sectional study. J Clin Diagn Res 2014; 8: YC01–YC03. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Survey flow diagram.
Figure S2. Lesion site distribution among patients diagnosed with vitiligo.
Figure S3. Current location of lesions within body regions.
Data Availability Statement
Access to individual participant‐level data is not publicly available for this study.


