Abstract
Background and Aim
Approximately 30% of inflammatory bowel disease (IBD) patients develop depression. Conversely, several studies reported increased IBD risk among patients with depression. Such bidirectional relationship has not been reported within one representative cohort, nor investigated among patients' family members. These associations may further implicate the gut–brain axis in IBD.
Methods
We conducted parallel retrospective cohort analyses to investigate depression risk among IBD patients and their unaffected siblings, and IBD risk among patients with depression and their unaffected siblings using the Taiwanese National Health Insurance Research Database. Individuals were followed up to 11 years for new‐onset depression or IBD. Controls were matched to unaffected siblings based on predefined characteristics.
Results
To investigate depression risk among IBD ‐ 422 IBD patients, 537 unaffected siblings, and 2148 controls were enrolled. During follow‐up, 78 (18.5%) IBD patients, 26 (4.8%) unaffected siblings, and 54 (2.5%) controls developed depression. Adjusted odds ratios (ORs) for depression among IBD patients and unaffected siblings were 9.43 (95% CI 6.43–13.81; P < 0.001) and 1.82 (95% CI 1.14–2.91; P = 0.013), respectively. To investigate IBD risk among depression ‐ 25 552 patients with depression, 26 147 unaffected siblings, and 104 588 controls were enrolled. During follow‐up, 18 (0.70/1000) depression patients, 25 (0.96/1000) unaffected siblings, and 58 (0.55/1000) controls developed IBD. ORs for IBD among depression patients and unaffected siblings were 1.87 (95% CI 1.07–3.26; P = 0.028) and 1.69 (95% CI 1.05–2.69; P = 0.029), respectively.
Conclusions
This population‐based study elucidates bidirectional association between IBD and depression. Elevated risks for either disease among patients and their unaffected siblings suggest shared etiologic contributors, offering novel insight into the gut–brain axis' influence in IBD pathophysiology.
Keywords: Crohn's disease, depression, gut–brain axis, IBD, ulcerative colitis
Introduction
Patients with inflammatory bowel disease (IBD), a chronic relapsing–remitting inflammatory condition postulated to develop from dysregulated immune response to intestinal microbiome in a genetically susceptible individual, have increased risks for psychiatric disorders including depression and anxiety, Parkinson's disease, and dementia. 1 , 2 , 3 , 4 Neuropsychiatric involvement in Crohn's disease (CD) and ulcerative colitis (UC), two predominant types of IBD, likely reflect factors including symptom burden, chronic inflammation, and gut dysbiosis associated with these enteric diseases. This relationship may partially reflect the “gut–brain axis,” which describes the bidirectional influence between the gastrointestinal and central nervous systems in health and disease. 5 , 6 Factors contributing to this intimate connection include shared genetic underpinnings between gastrointestinal and neurologic and psychiatric disorders, gut microbiome–metabolome composition and neuronal signaling via the autonomous nervous system, and cerebral and systemic inflammatory burden. 7 , 8 , 9
Among psychiatric disorders associated with IBD, the association with depression is most established. 10 Nearly 30% of IBD patients meet criteria for lifetime depression, and up to 60% experience depressive symptoms. 11 Poorly controlled depression among IBD patients is linked to increased treatment noncompliance and worse disease outcomes including higher relapse rates, more hospitalizations, and increased likelihood of surgery. 12 , 13 , 14 Conversely, patients with depression have greater risk of developing IBD, and the use of select antidepressants may protect against the development of CD and UC. 15 , 16 Taken together, these findings suggest an underlying bidirectional relationship, which previous longitudinal study could not observe. 17
Familial studies elucidated higher IBD risk among blood relatives of UC or CD patients. 18 , 19 , 20 Similarly, genetic and environmental factors contribute to familial aggregation of depression. 21 , 22 Given the comorbidity and bidirectional clinical influence between IBD and depression, it is plausible that the two diseases share aspects of underlying pathogenesis contributing to their development. Investigating the familial aggregation of these diseases may heighten diagnostic vigilance, improve clinical management, and contribute to etiologic research.
While previous studies demonstrated unidirectional associations between IBD and depression and vice versa, none have satisfactorily evaluated the bidirectional relationship between the two diseases within the same representative cohort. 15 , 16 Furthermore, efforts to examine the association between IBD and depression among the unaffected siblings of either disease, which provides additional evidence for the linkage between these two diseases, have not been attempted. The current study constructs two parallel retrospective cohorts within one population‐based health‐care database to investigate the bidirectional association between IBD and depression among patients with either disease and their unaffected siblings.
Materials and methods
Data source
The Taiwan National Health Insurance (NHI) program was established in 1995 and provides compulsory medical coverage to approximately 99.6% of the 23 million Taiwanese residents at the end of 2010. The NHI Research Database (NHIRD), curated by the NHI administration for research purposes, contains comprehensive information on all insured individuals including demographics (sex, birthdate, residential location, and income status) and claims data (medical diagnoses, outpatient and hospitalization visits, prescriptions, procedures and surgeries). The NHIRD has been widely used for epidemiologic research. 23 , 24 , 25 , 26 , 27 To protect privacy, each beneficiary is assigned a unique identifier upon NHI enrollment, allowing researchers to link their entire medical record. All data underlying this article were provided by the NHIRD and are available upon request and approval by the organization.
Following previously described methods of genealogy reconstruction using familial relationships recorded in the NHIRD, kinship was established from birth certificates and insurance dependency, and employment status. 23 , 25 , 26 , 27 Diagnoses were coded using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM). This study was approved by the Institutional Review Board of the Taipei Veterans General Hospital (2018‐07‐016AC).
Study population, exposure, and outcome
Risk of depression
Parallel retrospective cohorts were employed to investigate the longitudinal association between IBD and depression. Subjects born before 2000 were included. In Analysis 1, we included subjects with IBD and their siblings without a diagnosis of IBD (unaffected siblings). The primary outcome was new‐onset depression among IBD patients and their unaffected siblings. The diagnosis of IBD was identified by the ICD‐9‐CM codes 555 and 556, and depression was captured by the ICD‐9‐CM codes 296.2, 296.3, and 300.4. Each respective diagnosis was made at least twice by board‐certified gastroenterologists, colorectal surgeons, or psychiatrists; the date of the first diagnosis was recorded as initiation of the condition. In Taiwan, psychiatrists are responsible for the longitudinal management of depression. To identify the incidence of depression following IBD development, subjects with depression diagnosed prior were excluded. Both subjects and matched controls were followed from January 1, 2001 until a diagnosis of depression, death, or study termination on December 31, 2011.
Risk of inflammatory bowel disease
In Analysis 2, we included subjects with depression and their unaffected siblings. Similar to Analysis 1, we excluded subjects with the diagnosis of IBD prior to the development of depression. The cohort and its matched controls were followed from January 1, 2001 to December 31, 2011 until a diagnosis of IBD is made or death.
Matched controls
Each unaffected sibling of the patient with either IBD or depression was matched to four controls randomly selected from the NHIRD based on the premises of age (±1 year), sex, enrollment time, monthly income (≤15 840 NTD (New Taiwanese Dollars) or 528 USD (United States Dollars), 15 841–25 000 NTD or 528–833 USD, and ≥25 000 NTD or ≥833 USD), and residence urbanization level (proxy for health‐care availability; levels 1–5, most to least urbanized). 28 Monthly income and residence location were used to represent socioeconomic status. Charlson comorbidity index (CCI) was used for comorbidity adjustment and clinical prognosis.
Statistical analysis
For between‐group comparisons, F‐test was applied to continuous variables, and Pearson test was used for categorical variables. Standardized mean differences were used to compare baseline characteristics between the study groups. Given equal follow‐up duration between subjects in each matched set, logistic regression was performed to calculate the odds ratio (OR) and 95% confidence interval (CI) of IBD/depression between patients and their unaffected siblings. Adjusted ORs were computed after controlling for potential confounders including age, sex, income, residence urbanization status, and CCI. Statistical significance was set to two‐sided P value < 0.05. Data processing and computation were performed with SAS (Version 9.1, SAS Institute, Cary, NC, USA) and SPSS (Version 17, SPSS Inc., Chicago, IL, USA).
Results
Risk of depression
Analysis 1 included 422 patients with IBD, 537 unaffected siblings, and 2148 matched controls (Table 1). The all‐cause clinical visits per year, surgery, IBD‐related hospitalizations, and disease severity as defined previously were also included. 4 No significant differences were observed between the IBD patients versus their unaffected siblings and controls for sex, income level, and residence urbanization level. During follow‐up, depression developed in 18.5% of IBD patients (n = 78) and 4.8% of their unaffected siblings (n = 26), compared with 2.5% of controls (n = 54). The rates of developing depression were statistically different among the three groups. After adjustments for potential confounders (age, sex, income level, residence urbanization level, and CCI), the ORs for depression among IBD patients and their unaffected siblings were 9.43 (95% CI 6.43–13.81; P < 0.001) and 1.82 (95% CI 1.14–2.91; P = 0.013), respectively, compared with controls (Table 2). Kaplan–Meier survival curve with log‐rank test showed that patients with IBD and their unaffected siblings had significantly higher risk of developing depression during follow‐up (Fig. 1).
Table 1.
Demographic data of probands with IBD, unaffected siblings, and controls
| IBD probands, (A) n = 422 | Unaffected siblings (B), n = 537 | Controls (C), n = 2148 | Pvalue | Post‐hoc | ||||
|---|---|---|---|---|---|---|---|---|
| Age (years, SD) | 26.98 | 9.47 | 21.12 | 9.97 | 21.11 | 9.97 | < 0.001 | A > B ~ C |
| Sex, n (%) | 0.051 | |||||||
| Male | 186 | 44.1 | 271 | 50.5 | 10 634 | 50.5 | ||
| Female | 236 | 55.9 | 266 | 49.5 | 1084 | 49.5 | ||
| IBD diagnosis (n, %) | ||||||||
| UC | 175 | |||||||
| CD | 247 | |||||||
| Unaffected siblings (n, %) | ||||||||
| Of UC probands | 201 | 37.4 | ||||||
| Of CD probands | 336 | 62.6 | ||||||
| IBD status (n, %) | ||||||||
| Mild | 338 | 80.1 | ||||||
| Moderate–severe | 78 | 18.5 | ||||||
| Surgery | 6 | 1.4 | ||||||
| IBD‐related hospitalization | 42 | 10.0 | ||||||
| Income (n, %) | 0.556 | |||||||
| ≤ 15 840 NTD/month | 160 | 37.9 | 181 | 33.7 | 724 | 33.7 | ||
| 15 841 ~ 25 000 NTD/month | 121 | 28.7 | 169 | 31.5 | 676 | 31.5 | ||
| ≥ 25 001 NTD/month | 141 | 33.4 | 187 | 34.8 | 748 | 34.8 | ||
| Residence (n, %) | 0.980 | |||||||
| 1 (urbanized) | 113 | 26.8 | 155 | 28.9 | 620 | 28.9 | ||
| 2 | 135 | 32.0 | 178 | 33.1 | 712 | 33.1 | ||
| 3 | 75 | 17.8 | 87 | 16.2 | 348 | 16.2 | ||
| 4 | 31 | 7.3 | 40 | 7.4 | 160 | 7.4 | ||
| 5 (rural) | 68 | 16.1 | 77 | 14.3 | 308 | 14.3 | ||
| Charlson comorbidity index | 1.33 | 1.36 | 0.77 | 1.03 | 0.65 | 1.05 | < 0.001 | A > B > C |
| Medical comorbidities (n, %) | ||||||||
| Hypertension | 25 | 5.9 | 24 | 4.5 | 83 | 3.9 | 0.153 | |
| Dyslipidemia | 28 | 6.6 | 19 | 3.5 | 71 | 3.3 | 0.004 | A > B ~ C |
| Type 2 diabetes | 6 | 1.4 | 8 | 1.5 | 45 | 2.1 | 0.486 | |
| Obesity | 12 | 2.8 | 16 | 3.0 | 23 | 1.1 | 0.001 | A ~ B > C |
| Smoking | 18 | 4.3 | 12 | 2.2 | 40 | 1.9 | 0.010 | A > B ~ C |
| Depressive disorder (n, %) | 78 | 18.5 | 26 | 4.8 | 54 | 2.5 | < 0.001 | A > B > C |
| Age at diagnosis (SD) | 27.63 | 7.16 | 26.99 | 9.51 | 29.83 | 10.08 | 0.256 | |
| All‐cause clinical visits per year (SD) | 11.30 | 9.47 | 6.17 | 5.65 | 5.09 | 4.73 | < 0.001 | A > B > C |
CD: Crohn's disease; IBD, inflammatory bowel disease; NTD, New Taiwan Dollar; SD, standard deviation; UC: ulcerative colitis.
Table 2.
Multivariable analysis for risk of major depressive disorder and IBD among probands and unaffected siblings
| Risk of depression | Risk of IBD | |||||
|---|---|---|---|---|---|---|
| Adjusted HR † | (95% CI) | P | Adjusted HR † | (95% CI) | P | |
| Probands and their siblings | ||||||
| Unaffected siblings vs. control | 1.82 | 1.14–2.91 | 0.013 | 1.69 | 1.05–2.69 | 0.029 |
| Probands vs. control | 9.43 | 6.43–13.81 | < 0.001 | 1.87 | 1.07–3.26 | 0.028 |
| Male sample | ||||||
| Unaffected siblings vs. control | 1.67 | 0.77–3.63 | 0.199 | 1.47 | 0.81–2.66 | 0.206 |
| Probands vs. control | 14.43 | 8.16–25.51 | < 0.001 | 2.17 | 1.07–4.38 | 0.032 |
| Female sample | ||||||
| Unaffected siblings vs. control | 1.87 | 1.02–3.40 | 0.042 | 2.17 | 1.00–4.70 | 0.050 |
| Probands vs. control | 6.92 | 4.08–11.74 | < 0.001 | 1.61 | 0.65–3.97 | 0.306 |
| Probands and younger siblings | ||||||
| Unaffected siblings vs. control | 1.58 | 0.84–2.97 | 0.156 | 2.02 | 1.16–3.53 | 0.013 |
| Probands vs. control | 9.29 | 6.14–14.05 | < 0.001 | 1.54 | 0.78–3.53 | 0.210 |
| Probands and older siblings | ||||||
| Unaffected siblings vs. control | 2.10 | 1.16–3.86 | 0.015 | 1.47 | 0.75–2.89 | 0.265 |
| Probands vs. control | 9.03 | 5.83–13.97 | < 0.001 | 3.17 | 1.75–5.75 | < 0.001 |
| Sex, male vs. female | 0.86 | 0.62–1.19 | 0.358 | 1.76 | 1.16–2.68 | 0.008 |
| Charlson comorbidity index | 1.19 | 1.08–1.30 | < 0.001 | 1.31 | 1.15–1.50 | < 0.001 |
Adjusted for age, sex, monthly income, urbanization, Charlson comorbidity index, medical comorbidities, and all‐cause clinical visits.
Bold indicates the statistical significance.
CI, confidence interval; HR, hazard ratio; IBD, inflammatory bowel disease.
Figure 1.
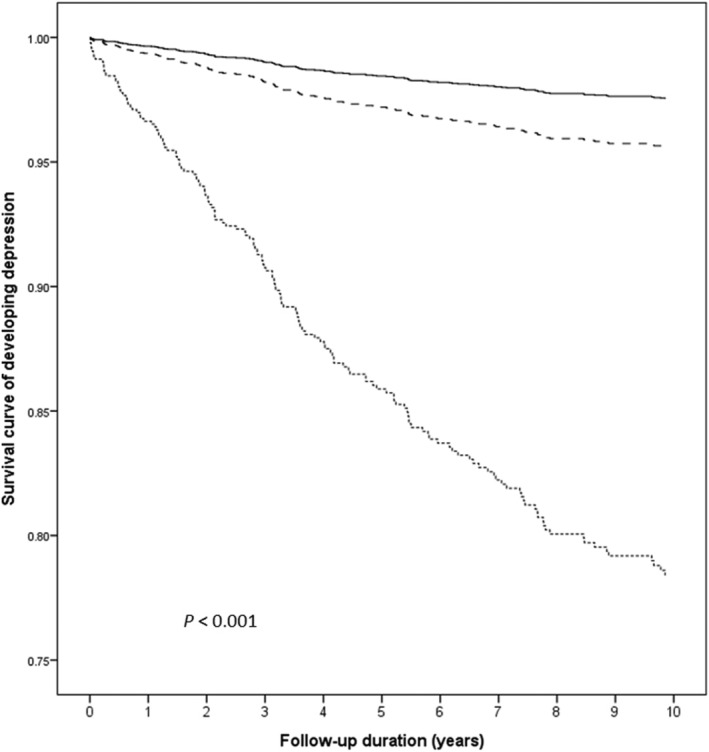
Kaplan–Meier survival curve of developing depression among patients with inflammatory bowel disease, their unaffected siblings, and control group.  , Control group;
, Control group;  , unaffected siblings;
, unaffected siblings;  , inflammatory bowel disease probands.
, inflammatory bowel disease probands.
Subanalyses to explore the influence of sex on depression were performed (Table 2). Among male probands with IBD, there was increased risk for depression (14.43, 95% CI 8.16–25.51; P < 0.001). This increased risk was not seen among the unaffected siblings. Among female probands, there was increased risk for depression (6.92, 95% CI 4.08–11.74; P < 0.001). Female unaffected siblings also demonstrated increased risk for depression (1.87, 95% CI 1.02–3.40; P = 0.042).
Additional subanalyses to explore the influence of sibling age on depression risk were performed (Table 2). Among older probands with IBD, there was elevated risk for depression (9.92, 95% CI 6.14–14.05; P < 0.001). Among younger probands with IBD, there was also elevated risk for depression (9.03, 95% CI 5.83–13.97; P < 0.001). Among younger unaffected siblings, there was no increased risk for depression; among older unaffected siblings, there was increased risk for depression (2.10, 95% CI 1.16–3.86; P = 0.015).
To evaluate the risk of depression among probands with CD or UC and their unaffected siblings, another group of subanalyses was performed (Table 3). Higher depression risks were found among both CD and UC probands. Unaffected siblings of CD probands had higher risk for depression; this was not statistically significant among unaffected siblings of UC probands, which may reflect inadequate power.
Table 4.
Demographic data of probands with depression, unaffected siblings, and controls
| Depression probands (A), n = 25 552 | Unaffected siblings (B), n = 26 147 | Controls (C), n = 104 588 | Pvalue | Post‐hoc | ||||
|---|---|---|---|---|---|---|---|---|
| Age (years, SD) | 26.23 | 8.49 | 19.43 | 9.55 | 19.46 | 9.57 | < 0.001 | A > B ~ C |
| Sex, n (%) | < 0.001 | |||||||
| Male | 9664 | 37.8 | 13 929 | 53.3 | 55 716 | 53.3 | ||
| Female | 15 888 | 62.2 | 12 218 | 46.7 | 48 872 | 46.7 | ||
| Income (n, %) | < 0.001 | |||||||
| ≤ 15 840 NTD/month | 10 101 | 39.5 | 10 120 | 38.7 | 40 480 | 38.7 | ||
| 15 841 ~ 25 000 NTD/month | 8744 | 34.2 | 7668 | 29.3 | 30 672 | 29.3 | ||
| ≥ 25 001 NTD/month | 6706 | 26.3 | 8359 | 32.0 | 33 436 | 32.0 | ||
| Residence, n (%) | < 0.001 | |||||||
| 1 (urbanized) | 6940 | 27.1 | 6923 | 26.5 | 27 692 | 26.5 | ||
| 2 | 8553 | 33.5 | 8834 | 33.8 | 35 336 | 33.8 | ||
| 3 | 3555 | 13.9 | 3503 | 13.4 | 14 012 | 13.4 | ||
| 4 | 2598 | 10.2 | 2457 | 9.4 | 9828 | 9.4 | ||
| 5 (rural) | 3906 | 15.3 | 4430 | 16.9 | 17 720 | 16.9 | ||
| Charlson comorbidity index | 0.92 | 1.16 | 0.57 | 0.89 | 0.51 | 0.83 | < 0.001 | A > B > C |
| Medical comorbidities (n, %) | ||||||||
| Hypertension | 1098 | 4.3 | 605 | 2.3 | 2358 | 2.3 | < 0.001 | A > B ~ C |
| Dyslipidemia | 1079 | 4.2 | 608 | 2.3 | 2281 | 2.2 | < 0.001 | A > B ~ C |
| Type 2 diabetes | 521 | 2.0 | 343 | 1.3 | 1295 | 1.2 | < 0.001 | A > B ~ C |
| Obesity | 754 | 3.0 | 401 | 1.5 | 1315 | 1.3 | < 0.001 | A > B > C |
| Smoking | 1103 | 4.3 | 475 | 1.8 | 1457 | 1.4 | < 0.001 | A > B > C |
| IBD (n, per 1000) | 18 | 0.70 | 25 | 0.96 | 58 | 0.55 | 0.068 | |
| UC | 11 | 0.43 | 13 | 0.50 | 23 | 0.22 | 0.029 | A ~ B > C |
| CD | 7 | 0.27 | 12 | 0.46 | 35 | 0.33 | 0.499 | |
| Age at diagnosis (SD) | 32.61 | 11.80 | 28.02 | 9.79 | 28.15 | 10.37 | 0.262 | |
| All‐cause clinical visits per year (SD) | 8.52 | 7.45 | 4.81 | 4.83 | 4.77 | 10.16 | < 0.001 | A > B ~ C |
CD, Crohn's disease; IBD, inflammatory bowel disease; NTD, New Taiwan Dollar; SD, standard deviation; UC, ulcerative colitis.
Risk of inflammatory bowel disease
Analysis 2 included 25 552 patients with depression, 26 147 unaffected siblings, and 104 588 matched controls (Table 4). These patients were more likely to be older than their unaffected siblings, female, made lower income, and had higher CCI. During follow‐up, 18 depression patients developed IBD (n = 0.70 per 1000), compared with 25 unaffected siblings (n = 0.96 per 1000) and 58 controls (n = 0.55 per 1000). After adjustments for potential confounders, the ORs for IBD among depression patients and their unaffected siblings were 1.87 (95% CI 1.07–3.26; P = 0.028) and 1.69 (95% CI 1.05–2.69; P = 0.029), respectively, compared with controls (Table 2). Kaplan–Meier survival curve with log‐rank test showed that patients with depression and their unaffected siblings had significantly higher risk of developing IBD during follow‐up (Fig. 2).
Table 3.
Multivariable analysis for risk of depression among IBD probands and unaffected siblings
| Events (n, %) | Risk of depression, adjusted HR † (95% CI) | |
|---|---|---|
| CD proband or trait | ||
| Control group | 37 (2.8) | 1 (ref.) |
| Unaffected siblings vs. control | 18 (5.4) | 1.79 (1.01–3.16) |
| CD probands vs. control | 42 (17.0) | 7.41 (4.55–12.05) |
| UC proband or trait | ||
| Control group | 17 (2.1) | 1 (ref.) |
| Unaffected siblings vs. control | 8 (4.0) | 1.84 (0.79–4.29) |
| UC probands vs. control | 36 (20.6) | 14.98 (7.73–29.02) |
Adjusted for age, sex, monthly income, urbanization, Charlson comorbidity index, medical comorbidities, and all‐cause clinical visits.
Bold indicates the statistical significance.
CD, Crohn's disease; CI, confidence interval; HR, hazard ratio; IBD, inflammatory bowel disease; UC, ulcerative colitis.
Figure 2.
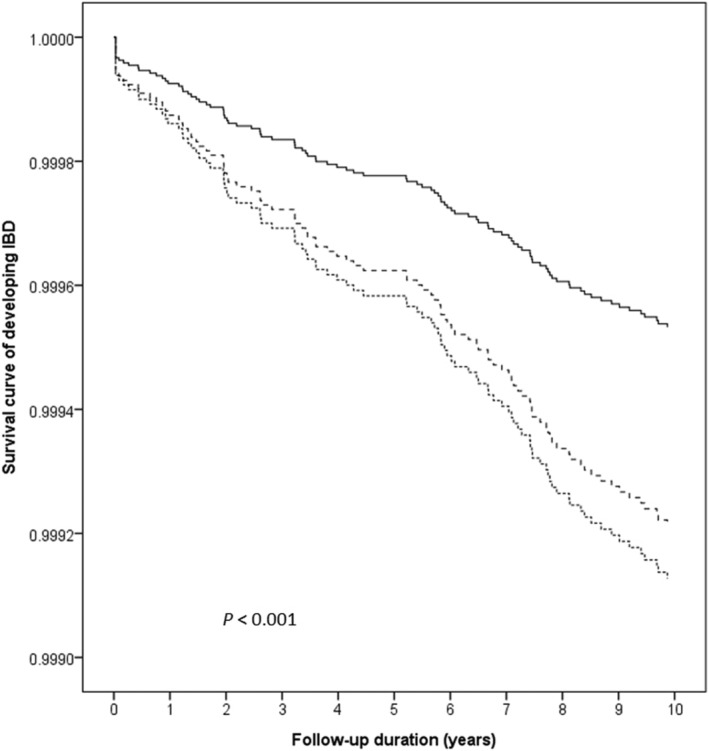
Kaplan–Meier survival curve of developing IBD among patients with depression, their unaffected siblings, and control group. IBD, inflammatory bowel disease.  , Control group;
, Control group;  , unaffected siblings;
, unaffected siblings;  , depression probands.
, depression probands.
Subanalyses to explore the influence of sex on IBD risk were performed (Table 2). Among male probands with depression, there was increased risk for IBD (2.17, 95% CI 1.07–4.38; P = 0.032). This increased risk was not seen among the unaffected siblings. Among female probands, there was no increased risk for IBD. However, female unaffected siblings demonstrated increased risk for IBD (2.17, 95% CI 1.00–4.70; P = 0.050).
Additional subanalyses to explore the influence of sibling age on IBD risk were performed (Table 2). Among older probands with depression, there was no increased risk for IBD. Among younger probands with depression, there was increased risk for IBD (3.17, 95% CI 1.75–5.75; P < 0.001). Among younger unaffected siblings, there was increased risk for IBD (2.02, 95% CI 1.16–3.53; P = 0.013). Among older unaffected siblings, there was no increased risk for IBD.
Discussion
In this population‐based study, we performed parallel retrospective cohort analyses to validate the association between IBD and depression utilizing a bidirectional design. This relationship was found to not only impact the patients of either disease but also significantly influence the clinical outcomes of their unaffected siblings. Unexpectedly, the OR of developing depression among IBD patients differed greatly from the OR of their unaffected siblings. The difference in OR of developing IBD among depression patients and their unaffected siblings was more subdued, likely reflecting the relative scarcity of the IBD diagnosis compared with depression and the symptom burden associated with IBD.
There were no sex differences in the risk of depression among IBD probands, likely reflecting the high prevalence of depression among IBD patients. Among the unaffected siblings, female siblings were likely to develop depression compared with male siblings, which may reflect sex‐related differences in phenotype and environmental contributors. Male probands with depression demonstrated higher OR for IBD, while female unaffected siblings of depressive probands showed higher OR for IBD. These again may reflect sex‐based differences in disease penetration and phenotype in addition to social and environmental factors, which are areas of active research. 29 , 30 , 31 It may also be possible that these subanalyses were not adequately powered to detect smaller differences. Furthermore, CCI was positively associated with the development of both IBD and depression. The former reflects higher CCI in the context of IBD, which is associated with higher depression risk, and the latter likely demonstrates chronic systemic illnesses including hypertension and cardiovascular diseases, which accompany depression, some of which are highlighted in Table 4. 32 , 33
Several genetic, environmental, and physiologic risk factors may contribute to the pathology of IBD and depression. Both diseases are associated with a host of genetic predispositions. 34 , 35 Their clinical comorbidity and familial co‐aggregation suggest shared underlying genetic and environmental predilections. As hallmark of IBD, inflammation also plays a critical role in depression, and repurposing of anti‐tumor necrosis factor α as a therapeutic option has been considered. 36 , 37 Gut dysbiosis, a potential cause for IBD, may also contribute to depression onset. Intestinal flora harbors the capacity to synthesize and release a variety of neurotransmitters and neuromodulator such as dopamine, serotonin, and gamma aminobyturic acid. 5 Passage of signaling molecules is possible through the autonomous nervous system vagus nerve and the blood–brain barrier, components of the gut–brain axis. 38 , 39 Recent study reported the capacity of chronic stress to alter gut microbiota, providing evidence on the capability of mental health to influence intestinal inflammation. 40 Finally, gut microbiome dysbiosis has been found in other disease conditions comorbid with depression, most prominently in inflammatory illnesses including rheumatoid arthritis and systemic lupus erythematosus. 41 , 42
Among the associations explored in this study, the greatest OR was found for the subsequent development of depression among IBD patients. This finding was not unexpected, given the psychological toll and worse quality of life from IBD disease burden. 43 Elevated depression risk among the unaffected siblings of IBD may reflect environmental stressors such as caregiver fatigue in addition to shared aspects of pathogenesis such as underlying genetic predisposition. 44 , 45 Interestingly, the pattern of IBD risk correlated strongly with depression, with the highest adjusted OR among depression patients, followed by their unaffected siblings and controls. The gradation of risk for developing IBD among patients with depression and their unaffected siblings compared with matched controls, and vice versa, further infer shared pathogenesis by these disorders. While the correlation between irritable bowel syndrome with depression has been well‐described, our findings highlight the need to ensure absence of gut inflammation among this patient population and their relatives, given the significant overlap in clinical presentation between IBD and functional bowel disorders. 46 Furthermore, the findings provide additional support for the importance of treating depression in IBD management and possibly even prevention.
Strengths of the study originate from its design. We applied bidirectional analysis to evaluate the association between IBD and depression utilizing one representative population cohort. Positive findings from the inclusion of the patients' unaffected siblings reinforce the correlation between the two illnesses. To our knowledge, this is the first study to encompass these unique approaches. Observations made from this comprehensive study design further validate the outcomes previously reported. 15 , 16 Furthermore, we performed additional subanalyses to delineate the impacts of sex and sibling age on the risk for IBD and depression. We surmise that sex hormones, sex‐related genetic loading, and environmental conditions including familial roles may influence the phenotypes for these illnesses. Given these are subanalyses, the findings may also reflect lack of adequate power.
Study limitations are consistent with other registry‐based analyses. The findings cannot elucidate shared mechanistic pathways between the diseases, which require further research. While influence of socioeconomic status was explored, other potential environmental and lifestyle confounders could not be evaluated due to their exclusion from the NHIRD. Medication utilization and severity of IBD and depression are unavailable. Finally, misclassification of diagnoses is possible; however, diagnoses are assigned by physicians, potentially yielding better diagnostic validity relative to participant‐reported questionnaires. Furthermore, accurate diagnostic coding is mandated by the Taiwanese government for medical reimbursement. 47
In conclusion, this study observed a bidirectional association between IBD and depression among patients with either disease, which permeates to their unaffected siblings. Increased awareness of such association and understanding of mutual influence may improve the diagnostic yield and management of IBD and psychiatric disorders among IBD patients leading to better clinical outcomes. Further research on gut–brain axis is warranted to identify shared aspects of pathogenesis between the diseases, which may reveal novel therapeutic approaches leveraging the gut–brain axis.
Acknowledgments
The authors thank I‐Fan Hu for his friendship and support.
Zhang, B. , Wang, H.‐H. E. , Bai, Y.‐M. , Tsai, S.‐J. , Su, T.‐P. , Chen, T.‐J. , Wang, Y.‐P. , and Chen, M.‐H. (2022) Bidirectional association between inflammatory bowel disease and depression among patients and their unaffected siblings. Journal of Gastroenterology and Hepatology, 37: 1307–1315. 10.1111/jgh.15855.
Declaration of conflict of interest: The authors declare that they have no conflict of interest.
Ethics approval: This study was approved by the Institutional Review Board of the Taipei Veterans General Hospital.
Informed consent: Not applicable.
Financial support: This work was supported by grants from the Taipei Veterans General Hospital (grant V106B‐020, V107B‐010, V107C‐181, and V108B‐012) and Ministry of Science and Technology, Taiwan (grant 107‐2314‐B‐075‐063‐MY3 and 108‐2314‐B‐075‐037). The funding sources did not participate nor influence the study design, data collection, statistical analysis, interpretation of results, or reporting of the current study.
Statement of previous publication: Not applicable.
Contributor Information
Bing Zhang, Email: bing.zhang@med.usc.edu.
Yen‐Po Wang, Email: ulnafu@gmail.com.
Mu‐Hong Chen, Email: kremer7119@gmail.com.
Data availability statement
All data are available upon request and approval from the Taiwan National Health Insurance Research Database.
References
- 1. Bernstein CN, Hitchon CA, Walld R et al. Increased burden of psychiatric disorders in inflammatory bowel disease. Inflamm. Bowel Dis. 2019; 25: 360–368. 2018/07/10. 10.1093/ibd/izy235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goodhand JR, Wahed M, Mawdsley JE, Farmer AD, Aziz Q, Rampton DS. Mood disorders in inflammatory bowel disease: relation to diagnosis, disease activity, perceived stress, and other factors. Inflamm. Bowel Dis. 2012; 18: 2301–2309. 2012/02/24. 10.1002/ibd.22916 [DOI] [PubMed] [Google Scholar]
- 3. Villumsen M, Aznar S, Pakkenberg B, Jess T, Brudek T. Inflammatory bowel disease increases the risk of Parkinsons disease: a Danish nationwide cohort study 1977‐2014. Gut 2019; 68: 18–24. 2018/05/23. 10.1136/gutjnl-2017-315666 [DOI] [PubMed] [Google Scholar]
- 4. Zhang B, Wang HE, Bai YM et al. Inflammatory bowel disease is associated with higher dementia risk: a nationwide longitudinal study. Gut 2021; 70: 85–91. 2020/06/25. 10.1136/gutjnl-2020-320789 [DOI] [PubMed] [Google Scholar]
- 5. Dinan TG, Cryan JF. The microbiome‐gut‐brain axis in health and disease. Gastroenterol. Clin. North Am. 2017; 46: 77–89. 2017/02/07. 10.1016/j.gtc.2016.09.007 [DOI] [PubMed] [Google Scholar]
- 6. Osadchiy V, Martin CR, Mayer EA. The gut‐brain axis and the microbiome: mechanisms and clinical implications. Clin. Gastroenterol. Hepatol. 2019; 17: 322–332. 2018/10/08. 10.1016/j.cgh.2018.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Voet S, Mc Guire C, Hagemeyer N et al. A20 critically controls microglia activation and inhibits inflammasome‐dependent neuroinflammation. Nat. Commun. 2018; 9: 2036. 2018/05/24. 10.1038/s41467-018-04376-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Walker KA, Gottesman RF, Wu A et al. Systemic inflammation during midlife and cognitive change over 20 years: the ARIC study. Neurology 2019; 92: e1256–e1267. 2019/02/15. 10.1212/WNL.0000000000007094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang B, Nakamura BN, Perlman A et al. Identification of functional missense single‐nucleotide polymorphisms in TNFAIP3 in a predominantly Hispanic population. J. Clin. Transl. Sci. 2018; 2: 350–355. 10.1017/cts.2019.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Keefer L, Kane SV. Considering the bidirectional pathways between depression and IBD: recommendations for comprehensive IBD care. Gastroenterol. Hepatol. (N Y) 2017; 13: 164–169. [PMC free article] [PubMed] [Google Scholar]
- 11. Mikocka‐Walus A, Knowles SR, Keefer L, Graff L. Controversies revisited: a systematic review of the comorbidity of depression and anxiety with inflammatory bowel diseases. Inflamm. Bowel Dis. 2016; 22: 752–762. 10.1097/MIB.0000000000000620 [DOI] [PubMed] [Google Scholar]
- 12. Allegretti JR, Borges L, Lucci M et al. Risk factors for rehospitalization within 90 days in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2015; 21: 2583–2589. 2015/08/06. 10.1097/MIB.0000000000000537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kochar B, Barnes EL, Long MD et al. Depression is associated with more aggressive inflammatory bowel disease. Am. J. Gastroenterol. 2018; 113: 80–85. 2017/11/15. 10.1038/ajg.2017.423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nigro G, Angelini G, Grosso SB, Caula G, Sategna‐Guidetti C. Psychiatric predictors of noncompliance in inflammatory bowel disease: psychiatry and compliance. J. Clin. Gastroenterol. 2001; 32: 66–68. 2001/01/12. 10.1097/00004836-200101000-00015 [DOI] [PubMed] [Google Scholar]
- 15. Ananthakrishnan AN, Khalili H, Pan A et al. Association between depressive symptoms and incidence of Crohns disease and ulcerative colitis: results from the Nurses Health Study. Clin. Gastroenterol. Hepatol. 2013; 11: 57–62. 2012/09/05. 10.1016/j.cgh.2012.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frolkis AD, Vallerand IA, Shaheen AA et al. Depression increases the risk of inflammatory bowel disease, which may be mitigated by the use of antidepressants in the treatment of depression. Gut 2019; 68: 1606–1612. 2018/10/20. 10.1136/gutjnl-2018-317182 [DOI] [PubMed] [Google Scholar]
- 17. Gracie DJ, Guthrie EA, Hamlin PJ, Ford AC. Bi‐directionality of brain‐gut interactions in patients with inflammatory bowel disease. Gastroenterology 2018; 154: 1635, e1633–1646.e3. 10.1053/j.gastro.2018.01.027 [DOI] [PubMed] [Google Scholar]
- 18. Childers RE, Eluri S, Vazquez C, Weise RM, Bayless TM, Hutfless S. Family history of inflammatory bowel disease among patients with ulcerative colitis: a systematic review and meta‐analysis. J. Crohns Colitis 2014; 8: 1480–1497. 2014/06/30. 10.1016/j.crohns.2014.05.008 [DOI] [PubMed] [Google Scholar]
- 19. Nunes T, Fiorino G, Danese S et al. Familial aggregation in inflammatory bowel disease: is it genes or environment? World J. Gastroenterol. 2011; 17: 2715–2722. 2011/07/08. 10.3748/wjg.v17.i22.2715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Santos MPC, Gomes C, Torres J. Familial and ethnic risk in inflammatory bowel disease. Ann. Gastroenterol. 2018; 31: 14–23. 2018/01/16. 10.20524/aog.2017.0208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gotlib IH, Joormann J, Foland‐Ross LC. Understanding familial risk for depression: a 25‐year perspective. Perspect. Psychol. Sci. 2014; 9: 94–108. 10.1177/1745691613513469 [DOI] [PubMed] [Google Scholar]
- 22. Weissman MM, Wickramaratne P, Nomura Y et al. Families at high and low risk for depression: a 3‐generation study. Arch. Gen. Psychiatry 2005; 62: 29–36. 10.1001/archpsyc.62.1.29 [DOI] [PubMed] [Google Scholar]
- 23. Chen MH, Hsu JW, Huang KL et al. Risk and coaggregation of major psychiatric disorders among first‐degree relatives of patients with bipolar disorder: a nationwide population‐based study. Psychol. Med. 2019; 49: 2397–2404. 2018/11/13. 10.1017/S003329171800332X [DOI] [PubMed] [Google Scholar]
- 24. Chen MH, Lan WH, Hsu JW et al. Risk of developing type 2 diabetes in adolescents and young adults with autism spectrum disorder: a nationwide longitudinal study. Diabetes Care 2016; 39: 788–793. 2016/03/24. 10.2337/dc15-1807 [DOI] [PubMed] [Google Scholar]
- 25. Cheng CM, Chang WH, Chen MH et al. Co‐aggregation of major psychiatric disorders in individuals with first‐degree relatives with schizophrenia: a nationwide population‐based study. Mol. Psychiatry 2018; 23: 1756–1763. 2017/11/08. 10.1038/mp.2017.217 [DOI] [PubMed] [Google Scholar]
- 26. Huang MH, Cheng CM, Tsai SJ et al. Familial coaggregation of major psychiatric disorders among first‐degree relatives of patients with obsessive–compulsive disorder: a nationwide study. Psychol. Med. 2021; 51: 680–687. 2020/01/08. 10.1017/S0033291719003696 [DOI] [PubMed] [Google Scholar]
- 27. Wang HE, Cheng CM, Bai YM et al. Familial coaggregation of major psychiatric disorders in first‐degree relatives of individuals with autism spectrum disorder: a nationwide population‐based study. Psychol. Med. 2020: 1–11. 10.1017/S0033291720003207 [DOI] [PubMed] [Google Scholar]
- 28. Liu CY, Hung YT, Chuang YL, Chen YJ, Weng WS, Liu JS, Liang KY. Incorporating development stratification of Taiwan townships into sampling design of large scale health interview survey. J. Health Management (Chin) 2006; 4: 1–22. [Google Scholar]
- 29. Greuter T, Manser C, Pittet V, Vavricka SR, Biedermann L, on behalf of Swiss IBDnet, an official working group of the Swiss Society of Gastroenterology . Gender differences in inflammatory bowel disease. Digestion 2020; 101: 98–104. 10.1159/000504701 [DOI] [PubMed] [Google Scholar]
- 30. Rustgi SD, Kayal M, Shah SC. Sex‐based differences in inflammatory bowel diseases: a review. Therap. Adv. Gastroenterol. 2020; 13: 1756284820915043. 2020/06/12. 10.1177/1756284820915043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shah SC, Khalili H, Gower‐Rousseau C et al. Sex‐based differences in incidence of inflammatory bowel diseases‐pooled analysis of population‐based studies from Western countries. Gastroenterology 2018; 155: 1079, e1073–1089.e3. 10.1053/j.gastro.2018.06.043 [DOI] [PubMed] [Google Scholar]
- 32. Dhar AK, Barton DA. Depression and the link with cardiovascular disease. Front. Psych. 2016; 7: 33. 2016/04/06. 10.3389/fpsyt.2016.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gold SM, Köhler‐Forsberg O, Moss‐Morris R et al. Comorbid depression in medical diseases. Nat. Rev. Dis. Primers. 2020; 6: 69. 10.1038/s41572-020-0200-2 [DOI] [PubMed] [Google Scholar]
- 34. Howard DM, Adams MJ, Clarke TK, Hafferty JD, Gibson J, Shirali M, McIntosh AM. Genome‐wide meta‐analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat. Neurosci. 2019; 22: 343–352. 2019/02/06. 10.1038/s41593-018-0326-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Momozawa Y, Dmitrieva J, Theatre E et al. IBD risk loci are enriched in multigenic regulatory modules encompassing putative causative genes. Nat. Commun. 2018; 9: 2427. 10.1038/s41467-018-04365-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kappelmann N, Lewis G, Dantzer R et al. Antidepressant activity of anti‐cytokine treatment: a systematic review and meta‐analysis of clinical trials of chronic inflammatory conditions. Mol. Psychiatry 2018; 23: 335–343. 2016/10/19. 10.1038/mp.2016.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Raison CL, Rutherford RE, Woolwine BJ et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment‐resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiat. 2013; 70: 31–41. 2012/09/05. 10.1001/2013.jamapsychiatry.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bonaz B, Bazin T, Pellissier S. The Vagus nerve at the interface of the microbiota‐gut‐brain axis. Front. Neurosci. 2018; 12: 49. 2018/02/23. 10.3389/fnins.2018.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Logsdon AF, Erickson MA, Rhea EM, Salameh TS, Banks WA. Gut reactions: how the blood–brain barrier connects the microbiome and the brain. Exp. Biol. Med. (Maywood) 2018; 243: 159–165. 2017/11/25. 10.1177/1535370217743766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gao X, Cao Q, Cheng Y et al. Chronic stress promotes colitis by disturbing the gut microbiota and triggering immune system response. Proc. Natl. Acad. Sci. U. S. A. 2018; 115: E2960–E2969. 2018/03/14. 10.1073/pnas.1720696115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bodkhe R, Balakrishnan B, Taneja V. The role of microbiome in rheumatoid arthritis treatment. Ther. Adv. Musculoskelet. Dis. 2019; 11: 1759720X19844632. 2019/08/23. 10.1177/1759720X19844632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Luo XM, Edwards MR, Mu Q et al. Gut microbiota in human systemic lupus erythematosus and a mouse model of lupus. Appl. Environ. Microbiol. 2018; 84: e02288‐17. 10.1128/AEM.02288-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kubesch A, Boulahrout P, Filmann N, Blumenstein I, Hausmann J. Real‐world data about emotional stress, disability and need for social care in a German IBD patient cohort. PLoS ONE 2020; 15: e0227309. 10.1371/journal.pone.0227309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Parekh NK, Shah S, McMaster K et al. Effects of caregiver burden on quality of life and coping strategies utilized by caregivers of adult patients with inflammatory bowel disease. Ann. Gastroenterol. 2017; 30: 89–95. 10.20524/aog.2016.0084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shukla R, Thakur E, Bradford A et al. Caregiver burden in adults with inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 2018; 16: 7–15. 2017/05/23. 10.1016/j.cgh.2017.05.020 [DOI] [PubMed] [Google Scholar]
- 46. Masand PS, Kaplan DS, Gupta S et al. Major depression and irritable bowel syndrome: is there a relationship? J. Clin. Psychiatry 1995; 56: 363–367 1995/08/01. [PubMed] [Google Scholar]
- 47. Lin JC, Lin CS, Hsu CW, Lin CL, Kao CH. Association between Parkinsons disease and inflammatory bowel disease: a nationwide Taiwanese retrospective cohort study. Inflamm. Bowel Dis. 2016; 22: 1049–1055. 2016/02/27. 10.1097/MIB.0000000000000735 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available upon request and approval from the Taiwan National Health Insurance Research Database.


