Abstract
Aim
Hypertensive nephropathy is embodied by kidney tissue fibrosis and glomerular sclerosis, as well as renal inflammation. The Hippo/YAP (yes‐associated protein, YAP) axis has been reported to promote inflammation and fibrosis and may participate in the pathogenesis of heart, vascular and renal injuries. However, the role of the Hippo/YAP pathway in hypertensive renal injury has not been reported so far. We explored the role of the Hippo/YAP signalling pathway in hypertensive renal injury and the effect of peptide 17 on its effects.
Methods
Histopathological analyses were performed based on the Masson and Haematoxylin/eosin (HE) staining approaches. Biochemical indexes were determined and immunofluorescence and western blotting were used to detect protein expression levels. The mRNA expression levels were determined by qRT‐PCR.
Results
Our results showed that peptide 17 reduced the systolic blood pressure (SBP) and urine protein/creatinine ratio in hypertensive rats. In addition, peptide 17 reduced the histopathological damage of kidneys in spontaneously hypertensive rats (SHRs). Moreover, peptide 17 downregulated genes in the Hippo/Yap pathway in kidney tissue of SHRs and Ang II‐treated kidney cells. The expression levels of inflammatory factors TNF‐α, IL‐1β and MCP‐1 and the pro‐fibrotic factors TGF‐β1, fibronectin, and CTGF were increased in the kidney of hypertensive rats, but reversed by peptide 17 treatment. Silencing of YAP had effect similar to that of peptide 17 in vivo and in vitro.
Conclusion
Peptide 17 alleviates early renal injury in hypertension by regulating the Hippo/YAP signalling pathway. These findings may be useful in the treatment of hypertensive renal injury.
Keywords: angiotensin II, hippo/YAP pathway, hypertension, peptide 17, renal damage
Summary at a Glance
Herein, we explored the effect of peptide 17 on hypertensive renal injury and its mechanism of action. The results hinted that peptide 17 attenuated the deleterious inflammatory and fibrotic effects of hypertensive renal injury via downregulating the Hippo/YAP axis. These findings may be relevant for treating hypertensive nephropathy.
1. INTRODUCTION
Chronic kidney disease (CKD) results from the progressive destruction of the number of functioning nephrons and is associated with the structural and functional impairment of both glomeruli and tubular cells. 1 When there is a significant loss of functional nephrons, the remaining nephrons gradually adapt to the increased workload by increasing their glomerular filtration rate. Therefore, high blood pressure is a major risk factor for CKD, worsening cardiovascular morbidity and mortality. 2 However, the mechanisms of high blood pressure associated with kidney disease are still poorly defined. In chronic kidney disease, hypertension increases as the glomerular filtration rate (GFR) decreases and proteinuria increases. 3 In addition, hypertension is significantly more present in glomerular damage than in tubulointerstitial damage. High blood pressure is, therefore, a risk factor for CKD, just as CKD can lead to high blood pressure 4 ; these two pathologies contribute each to the progression and aggravation of the other, like a vicious circle. High blood pressure first causes changes in kidney function. It then generates histological arteriosclerotic lesions known as benign nephrosclerosis, threatening the patient's life. 5 Finally, exceptionally, hypertension triggers malignant nephrosclerosis. 6 Recent studies showed that arterial hypertension secondary to CKD is generally associated with dysfunction of the vascular endothelium, characterized by excessive production of vasoconstrictors such as endothelin‐1 (ET‐1) and angiotensin II (Ang II) as well as a reduction in the release of the vasodilator nitric oxide (NO). 7 , 8 Endothelial dysfunction is also associated with the increased production of cell transforming agents such as the transforming growth factor beta‐1 (TGF‐β1), affecting endothelial vasoactive factors. To define the mechanisms of hypertension associated with CKD, it is essential to better understand the interactions between the various factors derived from the endothelium and underline the signal pathways involved in treating renal lesions.
Yes‐associated protein (YAP)‐transcriptional enhancer activation domain family member (TEAD) interaction is a crucial element for YAP activity, while TEAD is essential for normal tissue growth. 9 Peptide 17 (YAP‐TEAD Inhibitor 1) is a YAP‐TEAD protein–protein interaction inhibitor with a potential role in organ size control and tumorigenesis. 10 TEAD transcription factors have been described as the main partners of YAP in both Drosophila and mammals. 11 , 12 , 13 Numerous studies have experimentally demonstrated the conservation of the structure of the Hippo signalling pathway and its role in the organ size regulation in mammals. However, these studies also revealed greater complexities. Indeed, most of the components of the pathway discovered in Drosophila have several homologues in mammals. In the same way as in Drosophila, the kinases MST and LATS form in mammals a cascade of phosphorylation leading to the phosphorylation of YAP (Yes Associated Protein). 14 Thus, the MST kinases, associated with the SAV1 protein (the homologue of Salvador), phosphorylate and activate the LATS kinases related to the co‐factors MOB Kinase Activators (MOB1A and MOB1B). 15 This step results in the phosphorylation of YAP and the inhibition of its nuclear activities. Indeed, when it is phosphorylated, the YAP protein is sequestered in the cytoplasm or degraded. The activation of Hippo pathway leads to YAP phosphorylation, which is sequestered and stained in the cytoplasm. At the same time, when the Hippo pathway is inactive, YAP translocates to the nucleus where they interact with transcription factors to activate the expression of their target genes. 16 Thus, there is a need to develop new pharmacological treatment strategies through the Hippo/YAP signalling pathway in order to alleviate kidney damage associated with hypertension while improving the prognosis of patients with CKD. Peptide 17 is an inhibitor of YAP/TEAD1 interaction and has been suggested as promising therapeutics for various conditions. 17 , 18 , 19 , 20 However, whether the Hippo/YAP signalling is involved in the hypertensive kidney injury and whether peptide 17 has therapeutic potential has to be scrutinized.
Therefore, our study examined the role of the Peptide 17 in hypertensive kidney injury using peptide 17 to clarify the function and mechanism of the Hippo/YAP pathway.
2. MATERIALS AND METHODS
2.1. Animal and experimental design
Twenty‐week‐old Wistar‐Kyoto (WKY) rats and spontaneously hypertensive rats (SHRs), weighing 120 g were purchased from Beijing Weitongli Huashi Laboratory of Animal Technology Co., Ltd. The rats were raised in a specific pathogen‐free (SPF) animal room. All the experiments were conformed to the international standards for the use of experimental animals and were approved by the Animal Experiment Ethics Committee of Xinhua Hospital Chongming Branch Affiliated to Shanghai Jiao Tong University School of Medicine.
Systolic blood pressure (SBP) was detected by tail artery manometry using RBP‐1B rat tail artery manometer (Nanjing Debao Biochemical factory). Conscious rats were kept in a plastic holder put on a pad with temperature adjusted at 37°C during the monitoring. Measurements were successively taken five times for each rat after acclimation to the environment. After measuring and recording SBP, we randomly divided the rats into six groups (n = 10/group): WKY group, WKY + peptide 17 group, SHR group, SHR + peptide 17 group, SHR group + ctrl siRNA, SHR + YAP siRNA group.
After 21 days of the experiment, urine collection was done using metabolic cages over 24 h to measure proteinuria and urine creatinine. Urine specimens were kept at −220°C after centrifugation pending analysis. A protein assay kit (MicroTP‐test, Wako Co. Ltd., Osaka, Japan) was employed for analysis of protein in urine. The animals were then anaesthetised and exsanguinated by a puncture in the aorta. Abdominal blood was saved for serum creatinine measurements. The left kidney was removed and stored at −80°C. A longitudinal section of the remaining kidney from animals was fixed in a 4% formaldehyde solution and stored for Masson trichrome staining and H&E staining.
2.2. YAP silencing in rats by tail vein injection
The specific YAP siRNA or control siRNA (ctrl siRNA) were obtained from GenePharm (Shanghai, China). The sequence of siRNA targeting rat YAP was 5′‐GCGTCTAGAGCCGAAGTTTCT‐3′. The rats in the SHR + YAP siRNA group were injected with YAP siRNA lentivirus at the tail vein. The SHR + ctrl siRNA received the scramble vector at the tail veil.
2.3. Histological evaluation
Two pathologists unaware of the experimental conditions were chosen to perform the analysis. A piece of kidney tissue was fixed in 10% formalin, dehydrated, and inserted in paraffin for preparing 4 μm thin sections. The sections were placed on slides and subsequently subjected to H&E staining in order to evaluate renal histological damage. We analysed 10–15 fields for each slide under a Zeiss fluorescence microscope (Germany). Cross‐sections were photographed and stained areas were analysed. The size of the glomeruli was estimated as the average of the diameters of the glomeruli in the midcortex and the juxtamedullary zone in a field of view; calculations were performed on 5–15 glomeruli per kidney. Tubular damage is characterized by tubular atrophy and dilation, desquamation and degeneration of tubular epithelial cells, vacuolation, or thickening of the basement membrane of the tubules. 21 Histological changes were assessed based on the number of tubules with loss of brush border, palpable tubular cell necrosis, tubular dilation, and plaster formation, as follows: 0 = none, 1 = <10%, 2 = 11%–25%, 3 = 26%–45%, 4 = 46%–75% and 5 = > 76%.
The degree of collagen fibre accumulation was assessed by staining kidney tissue with Masson's trichome. Forty‐five fields were randomly selected from different sections. The ratio of stained area to total area of histological sections (positive ratio) was assessed as interstitial collagen deposition (fibrosis). ImageJ software was used to evaluate the area of positive areas stained by Masson.
2.4. Biochemical analysis
The serum was obtained following the centrifugation of 1 ml of blood taken during the sacrifices, primarily incubated for 1 h at room temperature. Serum creatinine, proteinuria, urine creatinine, and neutrophil gelatinase‐associated lipocalin (NGAL) were measured using an automated biochemical analysis system (Ilab 1800; Instrumentation Laboratory, Lexington, MA, USA).
2.5. Measurement of renal Ang II content
To determine the Ang II content, a commercial enzyme immunoassay kit was used (Westang Biotech, Shanghai, China) following the experimental protocols promulgated by the manufacturer. A standard curve of Ang II (Sigma‐Aldrich) was established for determining the concentration of Ang II.
2.6. Silencing of YAP in NRK‐52E cells
To inhibit YAP expression, YAP siRNA was transfected into NRK‐52E cells obtained from China Center for Type Culture Collection (CCTC, Shanghai, China). Approximately 2 × 105 NRK‐52E cells/well of a microtiter plate was cultured in Dulbecco's modified Eagle's medium (DMEM) (Gibco, Gaithersburg, MD, USA) added with 100 U/ml penicillin, 100 μg/mL streptomycin and 10% fetal bovine serum (Gibco) for 24 h before transfection. Cell culture was performed in a CO2 incubator at 37°C until 75%–80% confluence and transfected with YAP siRNA or the control siRNA using the lipofectamine reagent and further cultured for 48 h at 37°C.
2.7. Cellular model of hypertension and renal injury induced by Ang II
The cellular model of renal damage by hypertension was established by treatment of normal rat kidney epithelial cells (NRK‐52E cells) with Ang II. These cells were seeded in DMEM supplemented with 10% BCS at 37°C in a 5% CO2 95% air incubator. After the cells reached 80% confluence, they were serum starved and subsequently pretreated with 5 μmol/L of vehicle or peptide 17 for 30 min and then treated with 1 μmol/L of Ang II. Cells transfected with YAP siRNA or control siRNA were also subjected to ANG II treatment.
2.8. Immunofluorescence staining analysis
The antibodies against YAP (anti‐YAP mAB [cat. no. 14074; Cell Signalling Technology, Shanghai, China]) and heparanase (anti‐Heparanase 1 antibody [cat. no. GTX57224; GeneTex Inc., Alton Pkwy Irvine, CA 92606 USA]) were used for detecting the expression of YAP and heparanase in the glomerular tissue. Briefly, 7 μm tissue sections were mounted on slides and fixed in 100% acetone for 10 minutes at −20°C. The tissues were washed in a solution of 0.2% triton (in PBS 1X) for 5 minutes, then washed two more times only in PBS 1X at room temperature. The slides were then incubated in a blocking solution (10% bovine serum fetal [BSF] in 1X PBS) for 30 min, then at 4°C for 16 h with the primary antibodies previously diluted in 1X PBS (TGF‐β1 1: 200). The binding of the primary antibodies was detected following incubation with a secondary antibody coupled with Alexa fluor 594 (1: 1000; Molecular Probes). We used the DAPI (4′,6‐diamidino‐2‐phenylindole) to stain the nucleus (dilution 1:1000). YAP or heparanase fluorescence was determined by confocal microscopy (MRC‐1024 Confocal System, Bio‐Rad, CA, USA) and quantified using the Metamorph program (Molecular Devices Corporation, Orleans Drive Sunnyvale, CA, USA).
2.9. Co‐immunoprecipitation analysis of YAP‐TEAD interaction
To analyse the effect of peptide 17 on the YAP‐TEAD interaction, NRK‐52E cells were transiently transfected with the vectors pRK5‐myc‐TEAD1 (Addgene #33109) or pEGFP‐C3‐YAP1 (Addgene #17843). Briefly, pelleted nuclei were isolated in 10 mM Tris pH 7.6, 10 mM KCl, 450 mM NaCl, 0.5 mM dithiothreitol and 0.5 mM ethylenediaminetetraacetic acid (EDTA) and mixed with the cytosolic isolates prepared in 10 mM Tris pH 7.6, 10 mM KCl, 0.5 mM EDTA and 0.2% NP‐40. After incubation of the isolates comprising Myc‐TEAD with 200 μM of peptide 17 for half an hour on ice, the addition of the lysate containing GFP‐YAP was carried out, followed by an additional incubation of 30 min on ice. GFP‐Trap or Myc‐Trap beads (Chromotek) were then used for immunoprecipitation of GFP‐YAP or myc‐TEAD1, followed by washing and elution by boiling the immunoprecipitated proteins in sodium dodecyl sulfate buffer (SDS) (50 mM Tris pH 6.8, 20% glycerol, 2% SDS). Finally, the proteins were analysed using Western blot as indicated below.
2.10. Western blotting
Protein expression in kidney homogenates was analysed by Western blot analysis. Proteins were extracted from prepared homogenates, quantified by BCA assay and purified by electrophoresis. Next, we transferred proteins to a nitrocellulose membrane, blocked non‐specific sites with 3% BSA, then incubated the membrane with primary antibodies directed against our proteins of interest. The primary antibodies used were YAP Antibody#4912 (Cell Signalling Technology), LATS1 Antibody#9153 (Cell Signalling Technology), Phospho‐LATS1 (Ser909) Antibody#9157 (Cell Signalling Technology), TNF‐α Antibody#3707 (Cell Signalling Technology), anti‐TGF beta 1 antibody (ab92486, Abcam), IL‐1β antibody (B122) SCBT (Santa Cruz Biotechnology), anti‐MCP1 antibody (ab9899, Abcam), anti‐fibronectin antibody (ab2413, Abcam), anti‐CTGF antibody (ab6992, Abcam) and anti‐beta Actin antibody (ab8227, Abcam), Recombinant Anti‐Collagen I antibody (cat.no.ab270993; Abcam), Collagen II Monoclonal Antibody (cat.no.MA1‐40065; Invitrogen), Angiotensin II antibody (cat.no. orb500967; Biorbyt). The dilution of antibodies was 1:1000. The membranes were then incubated with secondary antibodies anti‐rabbit (926‐68 071, Li‐Cor Biosciences, NE, USA), anti‐goat (926‐32 214, Li‐Cor Biosciences), or anti‐mouse (926‐32 210, Li‐Cor Biosciences) diluted to 1/5000th. The revelation was performed with an Odyssey infrared scanner (Li‐Cor Biosciences, NE, USA). The densitometry quantitation was achieved using the ImageJ software. The internal control for normalization was β‐actin to which was normalized the relative expression of the above proteins.
2.11. RNA extraction and quantitative RT‐PCR analysis of mRNA levels in kidney tissue
According to the manufacturer's recommendations, total RNAs were extracted from samples by the TRIzol/chloroform method (Invitrogen) and stored in an RNA Later solution (QIAGEN). The quality of the RNAs was verified by measuring their optical density with a spectrophotometer. Complementary DNAs were synthesized from 1 μg of RNA by reverse transcriptase (Superscript II, Invitrogen) then amplified by a thermocycler (Roche LightCycler 480) for 40 cycles (95°C, 20 s; 60°C, 15 s; 72°C, 15 s) in the presence of SYBR Green (Roche) and specific primers (2.5 pmol) of genes of interest. The primers used are summarized in Table 1. The relative expression was computed according to the formula 2−ΔΔCT with β‐Actin as endogenous reference gene.
TABLE 1.
The primers used in this study
| Forward sequence (5′→3′) | Reverse sequence (5′→3′) | |
|---|---|---|
| YAP | ACCCCTCATCCCTAACACCTT | ACCACTATGATTGGCTCCGT |
| MCP1/ccl2 | AGGGCTTGGGTTGGTTCATT | CCCCATTGGGAACCACTTGAT |
| LATS1 | CCTGCATGAAAAATCCTCAGCC | ATTCCAAGTCACGCAGAGCA |
| TGF‐β1 | GGTTGGGAGAGAATGAGAGGC | TTGCGACCCACGTAGTAGAC |
| TNF‐α | AAGGGTCTCATCTCCGCCTT | CCCAGAGCCACAATTCCCTT |
| Fibronectin | TCCAGCATCGGTCATCAGTG | GCTGTTCCTCTCAGCAGGTT |
| IL‐1β | CAGTTCCCACTGACCTGTCG | TGCCCTGAACTAGAGCTGAGA |
| CTGF | TCCCAAAATCTCCAAGCCTA | GTAATGGCAGGCACAGGTCT |
| β‐Actin | AGGGAAATCGTGCGTGACAT | ACAACTACAGGGCTGACCAC |
2.12. ELISA detection of inflammatory markers in cell supernatant
To determine the levels of TNF‐α, IL‐1β and MCP1, the supernatants were collected from the culures of NRK‐52E cells and subjected to ELISA analysis using specific kits. The kits used were TNF alpha Rat ELISA Kit (Invitrogen), IL‐1 beta Rat ELISA Kit (Invitrogen), and Rat MCP‐1 ELISA Kit (RayBiotech). The protocols were in accordance with the manufacturer‐provided manual.
2.13. Statistical analysis
The experimental data obtained above are all statistically analysed by SPSS 22.0 software, and the results are based on the mean ± standard. Thus, the difference is expressed, and the comparison between multiple groups was based on one‐way analysis of variance (ANOVA) for significant differences; p < .05 was used as the standard of statistically significant difference.
3. RESULTS
3.1. Peptide 17 inhibits YAP/TEAD1 interaction in renal cells
To confirm the inhibitory effect of peptide 17 on YAP/TEAD1 interaction, we performed co‐immunoprecipitation assays using cell lysates prepared from NRK‐52E cells transfected with myc‐TEAD1 and GFP‐YAP constructs. The results indicated that peptide 17 inhibited the interaction of myc‐TEAD1 to immunoprecipitated GFP‐YAP (Figure 1A). Likewise, peptide 17 also inhibited the interaction of GFP‐YAP to myc‐TEAD1 immune complexes (Figure 1B). These results signposted that peptide 17 is an inhibitor of the interaction of YAP with TEAD1 in renal cells.
FIGURE 1.

Peptide 17 inhibits the interaction of YAP/TEAD1 in the renal cells. The effect of peptide 17 on the interaction of YAP/TEAD1 was analysed by co‐immunoprecipitation. (A) Inhibition of the binding of myc‐TEAD1 to affinity‐purified GFP–YAP by peptide 17. (B) Inhibition of the binding of GFP–YAP to immunoprecipitated myc‐TEAD1 by Peptide 17. The experiments were repeated thrice
3.2. Peptide 17 reduces SBP and modulates biochemical parameters of kidney injury in hypertensive rats
Compared with the WKY group, the tail artery systolic blood pressure (SBP), urine albumin, urine NGAL, plasma NGAL and plasma creatinine in the SHR group were significantly increased while no significant difference in body weight and in the ratio of kidney weight (KW)/body weight (BW) was observed (Figure 2A–I). Administration of peptide 17 significantly reversed the above observations, suggesting that peptide 17 may reduce kidney injury in hypertensive rats. Moreover, treatment of WKY rats with peptide 17 did not significantly change the above variables (Figure 2A–I). In addition, silencing of YAP siRNA mimicked the effect of peptide 17 on SBP, urine albumin, urine NGAL, plasma NGAL and plasma creatinine in the SHR group (Figure 2A–I).
FIGURE 2.

Peptide 17 reduces SBP and modulates biochemical parameters of kidney injury in hypertensive rats. (A) Body weight. (B) Ratio of kidney weight/body weight. (C) Creatine content in plasma. (D) NGAL in plasma. (E) Urine volume. (F) Albumin content in urine. (G) Albumin content in urine. (H) NGAL in urine. (I) SBP in different groups. The one‐way ANOVA followed by Tukey's multiple comparison posttests was performed. For A‐H, *p < .05 compared with WKY group; the significance difference for other group comparisons is reported in the figure panels. For I, * in orange indicates p < .05 in the comparison of SHR + ctrl siRNA with WKY, * in red indicates p < .05 in the comparison of SHR with WKY, * in green indicates p < .05 in the comparison of SHR+ peptide 17 with SHR, * in black indicates p < .05 in the comparison of SHR+ YAP siRNA with SHR. The results were obtained from six rats/group
3.3. Peptide 17 reduces the histopathological damage of kidney in hypertensive rats through Hippo/YAP signalling pathway
To evaluate the histological damages, H&E staining experiments were achieved (Figure 3A and B). Compared to the untreated WKY rats with complete glomerulus structure and unobstructed capillary lumen, treatment of these rats with peptide 17 had no significant effect on the kidney tissue (Figure 3A and B). In addition, compared to the WKY rats, H&E staining photomicrographs (Figure 3A) showed that the glomerulus of the SHRs group was reduced, and the renal tubular epithelial cells were swollen and shedding. Peptide 17 treatment of SHRs significantly decreased the tissue damage (Figure 3A and B). The pathological changes of the SHRs group treated with YAP siRNA were all significantly relieved to a certain extent and these changes were similar to that of the peptide 17 (Figure 3A and B). In Masson staining, the Masson‐stained blue‐positive area of the renal interstitium of the SHRs group increased significantly compared to the WKY group, indicating an increase in the degree of renal fibrosis (Figure 3C and D). The blue‐stained area in the SHRs treated with peptide 17 or YAP siRNA was significantly reduced (Figure 3C and D). The treatment of WKY with peptide 17 did not significantly affect tissue structure (Figure 3A–D).
FIGURE 3.

Effect of peptide 17 on the histopathological damages of kidney in kidney tissue of rats. (A) Representative images of H&E staining. (B) Tissue damage rate from HE staining. (C) Representative images of H&E staining. (D) Masson‐positive staining areas. *p < .05, **p < .01 compared to the WKY group; the significance of the difference among the other compared groups is indicated in the figure. The results were obtained from six rats/group
3.4. Peptide 17 inhibits inflammation and fibrosis in kidney tissue of hypertensive rats via inhibiting the Hippo/YAP axis
The immunofluorescence staining showed that compared with the WKY group, the expression of YAP was significantly increased in the mesangial region of the kidney tissue (green fluorescence) of SHRs (Figure 4A and B). At the same time, peptide 17 as well as YAP siRNA reduced YAP expression in kidney tissue of SHR rats (p < .05) (Figure 4A and B). In addition, immunofluorescence analysis indicated that heparanase (red fluorescence) was upregulated in the SHR rats compared to the WKY rats. However, peptide 17 as well as YAP siRNA decreased heparanase expression in SHR rats (Figure 4A and C). Furthermore, qRT‐PCR experiments indicated that the mRNA expression levels of YAP, TNF‐α, IL‐1β, MCP1, TGF‐β1, Fibronectin as well as CTGF were increased in SHRs comparatively to WKY group (Figure 5A). Moreover, peptide 17 or YAP siRNA treatment of SHRs significantly decreased the mRNA expression of these genes compared to the untreated SHRs (Figure 5A). On the contrary, LATS1 mRNA expression was decreased in SHRs compared to the WKY group, but this trend was reversed by the treatment of SHRs with peptide 17 as well as YAP silencing (Figure 5A). Western blotting indicated that, in addition to the above trends, the protein levels of p‐LATs was decreased in the SHRs compared to the WKY group, but increased by treatment of SHRs with peptide 17 or YAP silencing (Figure 5B and C). Moreover, the protein expression levels of Collagen I, Collagen II and Ang II were increased in the SHR group compared to the WKY group, but this trend was reversed following the treatments of SHRs with peptide 17 or YAP silencing (Figure 5B and C). The determination of Ang II indicated that the concentration of Ang II was increased in renal tissues of SHRs compared to the WKY rats (Figure 5D). These effects were reversed by the treatment with peptide 17 or YAP siRNA (Figure 5D). The treatment of WKY rats with peptide 17 did not affect the protein and mRNA expression levels of genes indicated above as well as the content of Ang II (Figures 5A–D). These data demonstrated that peptide 17 inhibits inflammation and fibrosis in kidney tissue of hypertensive rats via inhibiting the Hippo/YAP axis via the activation of LATS1.
FIGURE 4.

Immunofluoresence analysis of YAP and heparanase in kidney tissue. (A) Representative images of immunofluorescence experimental results. (B) Ratio of immunofluorescence intensity of YAP in different groups over WKY group. (C) Ratio of immunofluorescence intensity of heparanase in different groups over WKY group. **p < .01 compared to the WKY group; the significance of the difference among the other compared groups is indicated in the figure. The results were obtained from six rats/group
FIGURE 5.

Peptide 17 regulates Hippo/YAP signalling, inflammatory markers. Fibrosis markers and Ang II in kidney tissue of SHRs. (A) mRNA levels of YAP, LATS1, TNF‐α, MPC1, TGF‐β1, IL‐1β, Fibronectin, and CTGF in the kidney tissue of rats from different groups. (B) Bands of western blot analysis of protein expression. (C) Densitometry analysis of bands from the western blot analysis showing quantitative representation of gene expression. (D) Determination of Ang II content in the kidney tissue of different rat groups. *p < .05, **p < .01 compared with the WKY group; the significance of the comparisons between other groups are also indicated in the figure. The results were obtained from six rats/group
3.5. Peptide 17 counteracts Ang II‐induced activation of kidney cells through Hippo/YAP signalling pathway
As a peptide involved in vasoconstriction, Ang II can regulate the homeostasis of blood pressure and instigate hypertensive renal inflammation. Therefore, in the present study, the Ang II‐induced injury model using NRK‐52E cells, was used to determine the function of peptide 17 in vitro. To investigate whether Hippo/YAP signalling Ang II are a target for peptide 17 in kidney cells, the NRK‐52E cells were subjected to different treatment. As shown in Figure 6A and B, the Ang II treatment significantly up‐regulated the expression level of YAP as demonstrated by the immunofluorescence assay. In addition, silencing of YAP downregulated YAP expression and counteracted the effect of ANG II (Figure 6A and B). The mRNA expression levels of YAP, TNF‐α, IL‐1β, MCP1, TGF‐β1, Fibronectin as well as CTGF were significantly increased while that of LATS1 was significantly decreased by Ang‐II treatment (Figure 7A). These effects were reversed by the treatment of ANG II‐induced NRK‐52E cells with peptide 17 or YAP siRNA (Figure 7A). Similar results were observed in western blot analysis and the level of YAP, Collagen I, Collagen II and ANG II were increased while that of p‐LATS was significantly decreased in ANG II‐treated cells (Figure 7B and C). The trends of these protein levels in ANG II‐treated cells were reversed by the treatment with peptide 17 or YAP siRNA (Figures 7B–C). ELISA analysis of cell lysates indicated that the levels of MCP1, TNF‐α and IL‐1β were increased by Ang II treatment, but this trend was reversed by the treatment with peptide 17 or YAP siRNA (Figure 7D).
FIGURE 6.

Immunofluorescence analysis of the expression of YAP in NRK‐52E cells subjected to different treatments. (A) Immuno‐stained cell photomicrographs; (B) YAP immunofluorescence intensity in cell. *p < .05, **p < .01 compared with the Control group; the significance of the comparisons between other groups are also indicated in the figure. The results were obtained from six repeated experiments
FIGURE 7.
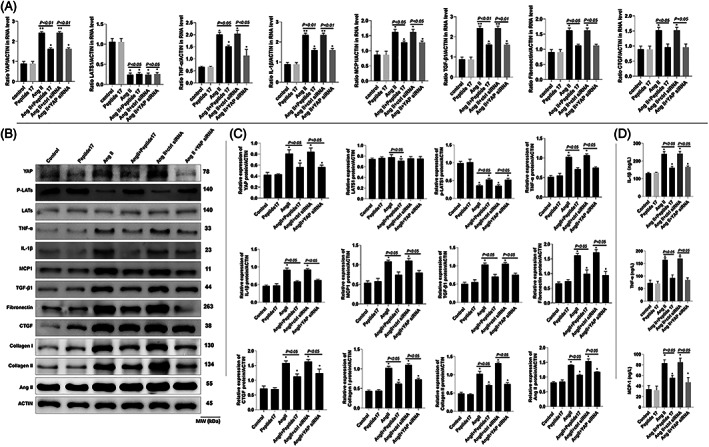
Peptide 17 regulates Hippo/YAP signalling, inflammatory markers. Fibrosis markers and Ang II in NRK‐52E cells. (A) mRNA levels of YAP, LATS1, TNF‐α, MPC1, TGF‐β1, IL‐1β, Fibronectin, and CTGF in NRK‐52E cells from different treatment groups. (B) Bands of western blot analysis of protein expression. (C) Densitometry analysis of bands from the western blot analysis showing quantitative representation of gene expression. (D) Determination of MCP‐1, TNF‐α, and IL‐1β by ELISA. *p < .05, **p < .01 compared with the control group; the significance of the comparisons between other groups are also indicated in the figure. The results were obtained from six repeated experiments
4. DISCUSSION
Hypertension is one of the most common causes of chronic renal disease. The pathogenesis of hypertensive nephropathy has not been fully clarified. 22 This study focused on the kidney injury induced by hypertension in SHRs and the involvement of the Hippo/YAP signalling pathway and its modulation by the peptide 17. After treatment with the YAP inhibitor (peptide 17), the arterial SBP and the renal structural damage of SHRs were significantly alleviated. Peptide 17 also counteracted the expression trends of inflammatory and fibrotic markers in the kidney of hypertensive rats and this effect was through inhibitory regulation of the Hippo/YAP signalling pathway.
The pathological changes such as white urine may reduce inflammatory cytokines and pro‐fibrotic factors in the kidney tissue. The Hippo pathway is mainly composed of upstream regulatory elements (such as NF2, Mer), three core components (such as Mst1/2 and Lats1/2), and downstream effect molecules (YAP). 23 The Hippo/YAP signalling pathway is highly conserved in cell evolution 24 and plays a role in many physiological processes. Studies have shown that Hippo/YAP can regulate cell division, proliferation, differentiation, and apoptosis and play an important role in growth and development, and regulation of organ size. 12 , 25 , 26 As an important effector downstream of this pathway, YAP can activate the Hippo pathway‐related genes which play an important role in the biological process of Hippo pathway activation. In the normal body, the activated Mst1/2 can phosphorylate and activate its substrate Lats1/2. Therefore, phosphorylating its downstream effector YAP makes it unable to enter the nucleus and losing its role as a transcriptional regulator. 27 In some pathological conditions, YAP will dephosphorylate and enter the nucleus. Studies have shown that the Hippo pathway under physiological conditions can be negatively affected by phosphorylation of this series of effector proteins. Regulating the transcriptional activity of its downstream effector molecule YAP seems important to limit the overgrowth of tissues, thereby maintaining cell growth and playing an important role in proliferation and apoptosis. 28 More evidence shows that the Hippo/YAP pathway in vascular remodelling and related cardiovascular diseases plays an important role in pathological processes. 29 In addition, Hippo/YAP Pathway can also affect the production of extracellular matrix and the proliferation and migration of vascular smooth muscle cells and endothelial cells involved in cardiovascular diseases such as pulmonary hypertension, atherosclerosis, aortic aneurysm, and angiogenesis vascular remodelling. 30 , 31 In this study, in SHRs, the expression level of YAP proteins in kidney tissue was significantly upregulated while the activation level of its upstream negative regulator LATS was downregulated. Peptide 17 treatment reversed the above changes. This suggests that the activation of the YAP signalling pathway in renal tissue may be involved in the pathogenesis of hypertensive nephropathy. Hypertensive nephropathy is often accompanied by increased expression of inflammatory factors in the kidney. Studies have shown that TNF‐α, an important inflammatory factor in the reaction, is involved in the occurrence and development of hypertensive renal injury. 32 In Ang II hypertensive mice, liposomal clodronate disodium was used as a removal chemical of mouse macrophages to significantly reduce renal inflammatory factors, including the expression of TNF‐α and IL‐1β, and reverse Ang II‐induced kidney damage in mice. 33
Our results also suggested that renal YAP signalling was activated in hypertensive rats. In addition, we found that peptide 17 significantly decreased blood pressure by inhibiting the YAP expression and the interaction of YAP and TEAD1. Furthermore, peptide 17 as well as the silencing of YAP both downregulated Ang II. Thus, we anticipated that the renoprotective effects of Peptide 17 were ascribed to decreased blood pressure by decreased expression of Ang II through YAP inhibition in the kidney. It was suggested that the renal inflammation caused by the infiltration of macrophages in the kidney tissue plays an important role in the renal injury of Ang II hypertension. In this study, peptide 17 treatment inhibited Ang II‐induced TNF‐α and the expression of various inflammatory factors, including IL‐1β and MCP‐1. Another important pathological feature of Ang II hypertensive kidney injury is renal fibrosis and its mechanism. It is related to the up‐regulation of the expression of the pro‐fibrosis factor TGF‐β1. A previous study found that YAP can be activated by the TGF‐β/Smad signalling pathway and further promote the expression of a series of matrix protein molecules. 28 Some studies found that, in animal models (such as diabetic nephropathy, polycystic kidney disease, and renal fibrosis induced by renal, ureteral obstruction), verteporfin has a significant inhibitory effect on renal fibrosis. In addition, the Pro‐fibrosis factor CTGF is the target gene of YAP, and the YAP protein can regulate its transcription and expression. 34 , 35 , 36 , 37 , 38 Ang II can promote CTGF expression and promotes the fibrosis process through CTGF. Our research showed that Peptide 17 significantly improves renal fibrosis pathological state in hypertensive rats through the inhibition of YAP pathway. At the same time significant reduction in the pathological state of renal fibrosis in hypertensive rats was recorded. Low renal tissue pro‐fibrosis molecules such as TGF‐β1, fibronectin, and CTGF expression suggest that Ang II may increase the above pro‐fibrotic factors by activating the LATS1/YAP pathway, eventually promoting the high pathological process of renal fibrosis caused by blood pressure. In contrast, the renal blood flow dynamic changes are an important mechanism in hypertensive kidney injury. Therefore, we cannot exclude peptide 17 participation in improving hypertensive kidney damage by lowering blood pressure.
In this study, we also found that peptide 17 can inhibit the interaction of YAP with TEAD in kidney cells. In addition, we found that peptide 17 inhibits YAP expression at protein and mRNA levels. Though peptide 17 as an inhibitor of the interaction of YAP and TEAD1, 17 , 18 , 19 its effect on the expression of YAP has not been reported so far. Thus, our study is the first, to the best of our knowledge, to demonstrate the inhibitory effect of peptide 17 on the expression of YAP. The decreased expression of YAP by peptide 17 may be due to the upregulation effect of peptide 17 on the expression of LATS1, an upstream negative regulator of YAP. It may be also due to the disturbance of YAP interaction with TEAD1 by peptide 17, which needs further verifications in future studies.
5. CONCLUSION
In conclusion, hypertension can significantly activate the Hippo/YAP pathway in kidney tissue and up‐regulates the protein expression levels of inflammatory factors TNF‐α, IL‐1β, and MCP‐1 and pro‐fibrosis factors TGF‐β1, Fibronectin, and CTGF, eventually causing renal structural damage and dysfunction. Moreover, peptide 17 can reverse the hypertension‐induced damage of the structure and function of the kidney via decreasing blood pressure via Ang II inhibition and/or inhibition of Hippo/YAP pathway via interfering the YAP/TEAD interaction or activating LAST1. In summary, our study revealed the role of the Hippo/YAP signalling pathway in the mechanism of action of peptide 17 on hypertensive kidney injury. Further modulation of the related mechanisms will provide a new theoretical basis for further understanding the pathogenesis of hypertensive nephropathy and development of adequate therapies.
AUTHOR CONTRIBUTIONS
San‐Bin Xu, Bin Xu, Zhi‐Sheng Gao and Jian‐Li Ni wrote the manuscript and interpreted the data. Zhi‐Heng Ma and Mei‐Qin Huang contributed to the drafting of the manuscript. Zhi‐Sheng Gao and Jian‐Li Ni designed and supervised the work. All the authors have read and approved the final version of the manuscript to submit.
FUNDING INFORMATION
This work was supported by the Three‐year Action Plan for the Development of Shanghai Traditional Chinese Medicine [grant number ZY‐FWTX‐6031], Shanghai TCM Treatment Service Capacity Building Project [grant number ZY‐ZWB‐1001] and Traditional Chinese Medicine Specialty Construction Project of Shanghai Municipal Health and Family Planning Commission [grant number ZYJX‐2017031].
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Xu S‐B, Xu B, Ma Z‐H, Huang M‐Q, Gao Z‐S, Ni J‐L. Peptide 17 alleviates early hypertensive renal injury by regulating the Hippo/YAP signalling pathway. Nephrology. 2022;27(8):712‐723. doi: 10.1111/nep.14066
San‐Bin Xu and Bin Xu have contributed equally to this work and shared first authorship.
Funding information Shanghai TCM Treatment Service Capacity Building Project, Grant/Award Number: ZY‐ZWB‐1001; Three‐year Action Plan for the Development of Shanghai Traditional Chinese Medicine, Grant/Award Number: ZY‐FWTX‐6031; Traditional Chinese Medicine Specialty Construction Project of Shanghai Municipal Health and Family Planning Commission, Grant/Award Number: ZYJX‐2017031
Contributor Information
Zhi‐Sheng Gao, Email: gaozhishen93@163.com.
Jian‐Li Ni, Email: nijianli123456@126.com, Email: jianli_Ni@yeah.net.
REFERENCES
- 1. Ruiz‐Ortega M, Rayego‐Mateos S, Lamas S, Ortiz A, Rodrigues‐Diez RR. Targeting the progression of chronic kidney disease. Nat Rev Nephrol. 2020;16:269‐288. [DOI] [PubMed] [Google Scholar]
- 2. Hamrahian SM, Falkner B. Hypertension in chronic kidney disease. Adv Exp Med Biol. 2017;956:307‐325. [DOI] [PubMed] [Google Scholar]
- 3. Hirsch S, Hirsch J, Bhatt U, Rovin BH. Tolerating increases in the serum creatinine following aggressive treatment of chronic kidney disease, hypertension and proteinuria: pre‐renal success. Am J Nephrol. 2012;36:430‐437. [DOI] [PubMed] [Google Scholar]
- 4. Udani S, Lazich I, Bakris GL. Epidemiology of hypertensive kidney disease. Nat Rev Nephrol. 2011;7:11‐21. [DOI] [PubMed] [Google Scholar]
- 5. Hall JE, Granger JP, do Carmo JM, et al. Hypertension: physiology and pathophysiology. Comprehensive Phys Ther. 2012;2:2393‐2442. [DOI] [PubMed] [Google Scholar]
- 6. Meyrier A. Nephrosclerosis: update on a centenarian. Nephrol Dialy Transplant. 2015;30:1833‐1841. [DOI] [PubMed] [Google Scholar]
- 7. Dhaun N, Goddard J, Webb D. The endothelin system and its antagonism in chronic kidney disease. J Am Soc Nephrol. 2006;17:943‐955. [DOI] [PubMed] [Google Scholar]
- 8. Rossi GP, Seccia TM, Barton M, et al. Endothelial factors in the pathogenesis and treatment of chronic kidney disease part I: general mechanisms: a joint consensus statement from the European Society of Hypertension Working Group on endothelin and endothelial factors and the Japanese Society of Hypertension. J Hypertens. 2018;36:451‐461. [DOI] [PubMed] [Google Scholar]
- 9. Morice S, Mullard M, Brion R, et al. The YAP/TEAD Axis as a new therapeutic target in osteosarcoma: effect of Verteporfin and CA3 on primary tumor growth. Cancer. 2020;12:3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nouri K, Azad T, Ling M, et al. Identification of Celastrol as a novel YAP‐TEAD inhibitor for cancer therapy by high throughput screening with ultrasensitive YAP/TAZ–TEAD biosensors. Cancer. 2019;11:1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhao B, Ye X, Yu J, et al. TEAD mediates YAP‐dependent gene induction and growth control. Genes Dev. 2008;22:1962‐1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhao B, Lei Q‐Y, Guan K‐L. The Hippo–YAP pathway: new connections between regulation of organ size and cancer. Curr Opin Cell Biol. 2008;20:638‐646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Landin‐Malt A, Benhaddou A, Zider A, Flagiello D. An evolutionary, structural and functional overview of the mammalian TEAD1 and TEAD2 transcription factors. Gene. 2016;591:292‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dong J, Feldmann G, Huang J, et al. Elucidation of a universal size‐control mechanism in drosophila and mammals. Cell. 2007;130:1120‐1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Praskova M, Xia F, Avruch J. MOBKL1A/MOBKL1B phosphorylation by MST1 and MST2 inhibits cell proliferation. Curr Biol. 2008;18:311‐321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Plouffe SW, Meng Z, Lin KC, et al. Characterization of hippo pathway components by gene inactivation. Mol Cell. 2016;64:993‐1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fu J, Zheng M, Zhang X, et al. Fibulin‐5 promotes airway smooth muscle cell proliferation and migration via modulating hippo‐YAP/TAZ pathway. Biochem Biophys Res Commun. 2017;493:985‐991. [DOI] [PubMed] [Google Scholar]
- 18. Yang C, Tan J, Zhu J, Wang S, Wei G. YAP promotes tumorigenesis and cisplatin resistance in neuroblastoma. Oncotarget. 2017;8:37154‐37163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wei X, Jia Y, Lou H, et al. Targeting YAP suppresses ovarian cancer progression through regulation of the PI3K/Akt/mTOR pathway. Oncol Rep. 2019;42:2768‐2776. [DOI] [PubMed] [Google Scholar]
- 20. Fu XY, Zhou WB, Xu J. TM4SF1 facilitates non‐small cell lung cancer progression through regulating YAP‐TEAD pathway. Eur Rev Med Pharmacol Sci. 2020;24:1829‐1840. [DOI] [PubMed] [Google Scholar]
- 21. Shih J‐M, Shih Y‐M, Pai M‐H, Hou Y‐C, Yeh C‐L, Yeh S‐L. Fish oil‐based fat emulsion reduces acute kidney injury and inflammatory response in antibiotic‐treated polymicrobial septic mice. Nutrients. 2016;8:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Siasos G, Sara JD, Zaromytidou M, et al. Local low shear stress and endothelial dysfunction in patients with nonobstructive coronary atherosclerosis. J Am Coll Cardiol. 2018;71:2092‐2102. [DOI] [PubMed] [Google Scholar]
- 23. Lin KC, Park HW, Guan K‐L. Regulation of the Hippo pathway transcription factor TEAD. Trends Biochem Sci. 2017;42:862‐872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mo JS, Park HW, Guan KL. The Hippo signaling pathway in stem cell biology and cancer. EMBO Rep. 2014;15:642‐656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ehmer U, Sage J. Control of proliferation and cancer growth by the Hippo signaling pathway. Mol Cancer Res. 2016;14:127‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yu F‐X, Zhang Y, Park HW, et al. Protein kinase A activates the Hippo pathway to modulate cell proliferation and differentiation. Genes Dev. 2013;27:1223‐1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhao B, Li L, Wang L, Wang C‐Y, Yu J, Guan K‐L. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 2012;26:54‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang K‐C, Yeh Y‐T, Nguyen P, et al. Flow‐dependent YAP/TAZ activities regulate endothelial phenotypes and atherosclerosis. Proc Natl Acad Sci. 2016;113:11525‐11530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sun X, Fu Y, Gu M, et al. Activation of integrin α5 mediated by flow requires its translocation to membrane lipid rafts in vascular endothelial cells. Proc Natl Acad Sci. 2016;113:769‐774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. He J, Bao Q, Yan M, et al. The role of Hippo/yes‐associated protein signalling in vascular remodelling associated with cardiovascular disease. Br J Pharmacol. 2018;175:1354‐1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhou J. An emerging role for Hippo‐YAP signaling in cardiovascular development. J Biomed Res. 2014;28:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang L, Luo J‐Y, Li B, et al. Integrin‐YAP/TAZ‐JNK cascade mediates atheroprotective effect of unidirectional shear flow. Nature. 2016;540:579‐582. [DOI] [PubMed] [Google Scholar]
- 33. Xu S, Koroleva M, Yin M, Jin ZG. Atheroprotective laminar flow inhibits Hippo pathway effector YAP in endothelial cells. Transl Res. 2016;176(18–28):18‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hoon JL, Tan MH, Koh C‐G. The regulation of cellular responses to mechanical cues by Rho GTPases. Cell. 2016;5:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gilbert RE, Cooper ME. The tubulointerstitium in progressive diabetic kidney disease: more than an aftermath of glomerular injury? Kidney Int. 1999;56:1627‐1637. [DOI] [PubMed] [Google Scholar]
- 36. Cachofeiro V, Goicochea M, De Vinuesa SG, Oubiña P, Lahera V, Luño J. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease: new strategies to prevent cardiovascular risk in chronic kidney disease. Kidney Int. 2008;74:S4‐S9. [DOI] [PubMed] [Google Scholar]
- 37. Möhring J. Pathogenesis of malignant hypertension: experimental evidence from the renal hypertensive rat. Clin Nephrol. 1975;4:167‐174. [PubMed] [Google Scholar]
- 38. Vassilev A, Kaneko KJ, Shu H, Zhao Y, DePamphilis ML. TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes‐associated protein localized in the cytoplasm. Genes Dev. 2001;15:1229‐1241. [DOI] [PMC free article] [PubMed] [Google Scholar]


