Abstract
Background
Fractional flow reserve (FFR) and instantaneous wave‐free ratio (iFR) disagree in about 20% of intermediate coronary lesions. As the physiological pattern of coronary artery disease has a significant influence on FFR‐iFR discordance, we sought to assess it may impact on the diagnostic accuracy of quantitative flow reserve (QFR).
Methods
One hundred and ninety‐four patients with 224 intermediate coronary lesions were investigated with iFR, FFR, and QFR. The physiological pattern of disease was assessed with iFR Scout pullback and QFR virtual pullback in all the cases.
Results
A predominantly physiologically focal pattern was observed in 81 (36.2%) lesions, whereas a predominantly physiologically diffuse was observed in 143 (63.8%) cases. QFR demonstrated a significant correlation (r = 0.581, p < 0.001) and a substantial agreement with iFR, both in diffuse (AUC = 0.798) and in focal (AUC = 0.812) pattern of disease. Discordance between QFR and iFR was observed in 51 (22.8%) lesions, consisting of iFR+/QFR− (64.7%) and iFR−/QFR+ (35.3%). Notably, the physiological pattern of disease was the only variable significantly associated with iFR/QFR discordance. QFR virtual pullback demonstrated an excellent agreement (83.9%) with iFR Scout pullback in classifying the physiological pattern of disease.
Conclusions
QFR has a good diagnostic accuracy in assessing myocardial ischemia independently of the pattern of coronary disease. However, the physiological pattern of disease has an influence on the QFR/iFR discordance, which occurs in ~20% of the cases. The QFR virtual pullback correctly defined the physiological pattern of disease in the majority of the cases using the iFR pullback as reference.
Keywords: coronary artery disease, fractional flow reserve, quantitative coronary angiography
Abbreviations
- ACS
acute coronary syndrome
- FFR
fractional flow reserve
- iFR
instantaneous wave‐free ratio
- MLD
minimum luminal diameter
- PCI
percutaneous coronary intervention
- QFR
quantitative flow reserve
- RVD
reference vessel diameter
- TIMI
thrombolysis in myocardial infarction
1. INTRODUCTION
Coronary physiology has a crucial role in the assessment of coronary artery disease (CAD) with intermediate angiographic significance.
Fractional flow reserve (FFR) is considered the reference standard technique for detecting myocardial ischemia in the catheterization laboratory. In recent years, instantaneous wave‐free ratio (iFR) was proved to be non‐inferior compared with FFR in guiding myocardial revascularization at mid‐term follow‐up. 1 Comparisons between these two methods showed a 20% degree of discordance in evaluating epicardial stenosis. The disease pattern (focal vs. diffuse) and the lesion location demonstrated a significant impact on FFR/iFR agreement. 2 , 3 , 4 , 5 , 6
Despite the favorable clinical outcome of physiology‐guided revascularization, the adoption of physiology in the clinical practice is heterogeneous and globally remains low, with large areas performing less than 15% of eligible procedures with physiological guidance. 7
Costs, additional procedural time, and technical difficulties related to the use of the pressure‐wire are probably responsible for the low penetration of physiology guidance in the clinical practice.
Novel indices of coronary physiology have been recently developed. In particular, quantitative flow reserve (QFR) is a novel angiography‐based index that promises to simplify the assessment of coronary flow reserve by providing an angiography‐derived adenosine‐free FFR and obviating the need for pressure‐wire. 8 QFR has been validated against FFR in the clinical setting. 9 The physiological pattern of CAD is an important influencing factor for FFR/iFR discordance. 4 However, no data is available on the impact of the physiological pattern of disease (focal vs. diffuse) on the QFR accuracy. In this study, we aimed to assess the agreement of QFR with FFR and iFR in patients with different patterns of coronary disease defined according to the iFR scout pullback. Moreover, we sought to assess if QFR virtual pullback may provide an agreement of the disease pattern as compared with the iFR pullback.
2. METHODS
2.1. Study design
Between March 2015 and May 2021, 208 patients with 259 intermediate coronary lesions underwent pressure‐wire functional assessment with iFR Scout pullback system (Philips Medical Systems, Best, The Netherlands) at Verona University Hospital (Verona, Italy) and entered in a prospective clinical registry. The pressure‐wire traces were then retrospectively reviewed to be included in the present analysis. QFR analysis was performed off‐line using the QAngio XA 3D software (Medis Medical Imaging Systems, Leiden, The Netherlands). Clinical decisions were based on pressure‐wire assessment and operators' decisions. Inclusion criteria were lesion severity 50–90% in at least 2 mm vessel, as defined by visual estimation.
Exclusion criteria were lesion severity >90% by visual estimation, culprit lesions of acute coronary syndrome (ACS) or non‐culprit lesions of ACS during index percutaneous coronary intervention (PCI), previous coronary artery bypass grafting to the target vessel, vessels with angiographically identifiable myocardial bridging or collaterals. Other exclusion criteria were clinical or angiographic features limiting QFR computation (ostial left main or ostial right coronary artery, ongoing ventricular arrhythmias or significant and persistent tachycardia, poor angiography image quality, and severe tortuosity or overlapping limiting an optimal 3D reconstruction of the target vessel).
The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and it was approved by our institutional ethical board. All the patients included provided their written consent for the anonymous collection of the data.
2.2. Coronary angiography and physiology
Coronary angiography was performed by a standard percutaneous radial or femoral approach using 6Fr‐guiding catheters. The decision to perform iFR and FFR assessment was clinically oriented and thus left to operator's discretion. Intracoronary nitrates (100–300 mcg) were administered in all cases.
Pressure‐wire assessment was performed in a standard fashion using a pressure‐monitoring guidewire (VerrataPlus, Volcano Therapeutics, Rancho Cordova, CA). Hyperemia was induced using an intracoronary bolus of adenosine (100 μg for the right coronary artery and 200 μg for the left coronary artery). Routine cut‐off values of hemodynamic significance (FFR ≤ 0.80 and iFR < 0.89) were used to classify stenoses.
2.3. Three‐dimensional QCA and QFR
The 3D quantitative coronary angiographic analysis and QFR computation were performed by experienced, trained, and certified investigators, blinded to both FFR and iFR values, iFR pullback, and clinical decisions about coronary revascularization. A validated software (QAngio XA 3D version 1.0.28.4, Medis Medical Imaging Systems, Leiden, The Netherlands) was used for the analysis. QFR computation was performed in agreement with the step‐by‐step procedure validated in previous studies. 9 , 10 Angiographic pattern of CAD was classified as focal visually based on angiographic lesion length <20 mm at the quantitative coronary analysis (QCA) as previously reported. 11
Routine cutoff value of hemodynamic significance (QFR ≤ 0.80) was used to classify stenoses into four groups: QFR+/iFR+ (QFR ≤ 0.80 and iFR < 0.89), QFR−/iFR+ (QFR > 0.80 and iFR < 0.89), QFR+/iFR− (QFR ≤ 0.80 and iFR ≥ 0.89), and QFR−/iFR− (QFR > 0.80 and iFR ≥ 0.89).
2.4. iFR pullback and physiological pattern of disease definition
iFR Scout pullback recordings were performed manually at a pullback speed of ≈0.5–1.0 mm/s. The presence of a significant pressure‐wire drift was excluded as the pressure sensor reached the ostium of left main or right coronary artery. Cases were included exclusively when pullback started at an adequately distal point of the vessel or if fluoroscopy was provided to confirm the distal wire position.
All iFR‐pullback traces were evaluated independently by two expert interventional cardiologists (R.S. and M.P.) who were blinded to the clinical presentation, patient characteristics, coronary angiography, and QFR results. The physiological pattern of disease was classified based on the iFR pullback as follows: (i) predominantly physiologically focal (presence of an abrupt drop‐down in the iFR curve with ΔiFR ≥ 0.03 in < 15 mm); (ii) predominantly physiologically diffuse (progressive and constant iFR decrease without significant drop‐down), as recently recommended. 12 For each case, the consensus opinion for the physiological pattern of disease was generated by the unanimous agreement of the two experts.
2.5. QFR virtual pullback and physiological pattern of disease definition
All QFR pullback traces were evaluated by the expert operators (R.S. and M.P) blinded to the clinical presentation, patient characteristics, coronary angiography, iFR/FFR, and iFR pullback results. The physiological pattern of disease was classified as based on the QFR virtual pullback as follows: (i) predominantly physiologically focal (presence of an abrupt drop‐down in the QFR virtual curve with ΔQFR ≥ 0.05 in < 10 mm); (ii) predominantly physiologically diffuse (progressive and constant QFR decrease without significant drop‐down), as recently recommended. 12 , 13 For each case, the consensus opinion for the physiological pattern of disease was generated by the unanimous agreement of the two experts (Figure S1).
2.6. Study endpoints
The primary endpoint of this study was to determine whether the physiological pattern of CAD, as assessed by iFR pullback, may have an impact on the diagnostic accuracy of QFR defined according to iFR < 0.89.
Secondary endpoints of this analysis were: (1) The agreement between physiological patterns of disease defined according to iFR pullback and QFR virtual pullback. (2) The agreement between iFR‐FFR and QFR‐FFR in coronary lesions with predominantly diffuse versus the focal pattern of disease is defined according to the iFR pullback. (3) Clinical predictors of predominantly diffuse versus focal CAD.
2.7. Statistical analysis
Continuous variables are presented as mean and standard deviation if normally distributed and compared with unpaired t‐test. Categorical data are reported as a percentage and compared with the χ 2 test or Fisher exact test as appropriate. Correlation among variables was determined by Pearson or Spearman correlation tests as appropriate and expressed as r value.
The agreement between physiology indices including iFR, FFR, and QFR was assessed using the Bland Altman analysis. Sensitivity, specificity, diagnostic accuracy, and optimal cut‐off value were defined from the calculated receiver operator characteristic (ROC) curve. Predictors of physiological pattern of disease were investigated using logistic regression. Inter‐observer classification agreement was defined using the Kappa coefficient.
A p‐value <0.05 was considered significant. All analyses were performed with IBM® SPSS® Statistics (Version 26, SPSS Inc., Chicago, IL). Graphics were realized with GraphPad Prism 7.0.
3. RESULTS
3.1. Study population
During the study period, 208 patients with 259 intermediate coronary lesions underwent pressure‐wire functional assessment with iFR Scout pullback system (Philips Medical Systems, Best, The Netherlands) at Verona University Hospital (Verona, Italy). Fourteen patients (n = 35 lesions) were excluded from the analysis because of the suboptimal quality of the pressure‐wire traces quality or of the angiographic views. Ultimately, QFR analysis was performed in 224 coronary vessels (194 patients), that were included in the final analysis. iFR and iFR Scout pullback were available in 100% of the cases. FFR was available in 94% of the cases. A detailed flowchart of the study is provided in Figure S1. The mean age was 67.5 ± 11.1 years and 80.4% were men. The most frequently assessed vessel was the left anterior descending artery (66.7%). Mean percentage diameter stenosis and lesion length were 47.84 ± 9.53% and 23.04 ± 10.91 mm, respectively. Full description of baseline, vessel, and stenosis characteristics is provided in Table 1.
TABLE 1.
Basal characteristics
| Patients characteristics (n = 194) | |
|---|---|
| Age | 67.5 ± 11.1 |
| Sex male | 156 (80.4) |
| Hypertension | 152 (78.4) |
| Dyslipidaemia | 119 (61.3) |
| Diabetes mellitus | 57 (29.4) |
| Current smoker | 38 (19.6) |
| Former smoker | 72 (37.1) |
| Peripheral artery disease | 60 (30.9) |
| Previous PCI | 76 (39.2) |
| Chronic kidney disease | 44 (22.7) |
| Clinical presentation | |
| Chronic coronary syndrome | 108 (55.6) |
| Acute coronary syndrome | 86 (44.4) |
| Vessels | |
|---|---|
| Left anterior descending | 149 (66.7) |
| Left circumflex | 38 (16.9) |
| Right coronary artery | 21 (9.3) |
| Left Main | 13 (5.8) |
| Ramus | 3 (1.3) |
| Quantitative coronary angiography | |
| Diameter stenosis, % | 47.84 ± 9.53 |
| Minimum lumen diameter, mm | 1.35 ± 0.36 |
| Reference diameter, mm | 2.58 ± 0.55 |
| Lesion length, mm | 23.04 ± 10.91 |
| Physiological indices | |
| iFR | 0.88 [0.80;0.94] |
| QFR | 0.81 [0.72;0.88] |
| FFR | 0.82 [0.76;0.88] |
Abbreviations: FFR, Fractional flow reserve; iFR, instantaneous wave‐free ratio; PCI, Percutaneous coronary intervention; QFR, quantitative flow reserve.
Median FFR and iFR values were 0.82 (interquartile range, 0.76–0.88) and 0.88 (interquartile range, 0.80–0.94), respectively. Median QFR value was 0.81 (interquartile range, 0.72–0.88).
3.2. Pattern of disease at iFR pullback analysis
At the iFR pullback analysis, the physiological pattern of disease, was classified as predominantly physiologically focal in 81 (36.2%) coronary lesions and as predominantly physiologically diffuse in 143 (63.8%) lesions. The inter‐observer Kappa coefficients for iFR pullback defined focal versus diffuse pattern of disease were 0.822 (CI 95% 0.765–0.865; p < 0.001). Physiological classification of coronary lesions with iFR‐QFR and iFR‐FFR agreement is provided in Table 2. Clinical and angiographic predictors of physiologically diffuse coronary disease are presented in Table S1. At multivariate logistic regression only the presence of diabetes (OR 2.22, 95% CI 1.15–4.29, p = 0.018) was significantly associated with diffuse disease.
TABLE 2.
Lesion characteristics
| Physiological classification (n = 224) | |
|---|---|
| QFR/iFR agreement | |
| QFR+/iFR+ | 91 (40.6) |
| QFR−/iFR+ | 33 (14.6) |
| QFR+/iFR− | 18 (8.0) |
| QFR−/iFR− | 82 (36.6) |
| iFR/FFR agreement | |
| iFR+/FFR+ | 78 (37.2) |
| iFR+/FFR− | 37 (17.6) |
| iFR−/FFR+ | 15 (7.1) |
| iFR−/FFR− | 80 (38.1) |
| Physiological pattern of disease | |
| iFR predominantly physiological focal | 81 (36.2) |
| iFR predominantly physiological diffuse | 143 (63.8) |
| QFR predominantly physiological focal | 95 (42.4) |
| QFR predominantly physiological diffuse | 129 (57.6) |
Abbreviations: FFR, fractional flow reserve; iFR, instantaneous wave‐free ratio; QFR, quantitative flow reserve.
3.3. Agreement between QFR and iFR in diffuse versus focal disease
Overall, QFR demonstrated a significant correlation (r = 0.581, p < 0.001) and a substantial agreement with iFR at the Bland Altman analysis (Figure 1 and Figure S2). The estimated bias was −0.06, with 95% limits of agreement −0.019 to 0.07. At ROC curve analysis the AUC of QFR in predicting iFR < 0.89 was 0.813 [95% CI 0.755–0.871] with a sensitivity of 73.4%, a specificity of 82.0%, NPV of 71.3%, and a PPV of 83.5%. The overall classification agreement between QFR and iFR was 77.2% (173 out of 224 lesion), consisting of iFR+/QFR+ (n = 91; 40.6%) and iFR−/QFR− (n = 82; 36.6%). Discordance between QFR and iFR was observed in 51 (22.8%) lesions, consisting of iFR+/QFR− (n = 33; 14.7%) and iFR−/QFR+ (n = 18; 8.0%).
FIGURE 1.
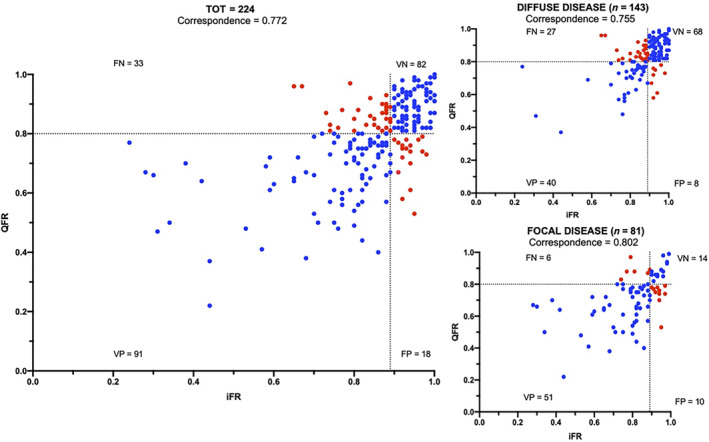
Scatter plot showing the relationship between quantitative flow reserve (QFR) and instantaneous wave‐free ratio (iFR) values according to the physiological pattern of coronary disease [Color figure can be viewed at wileyonlinelibrary.com]
In physiological predominantly focal disease, the agreement between QFR and iFR was 80.2%. In this subgroup QFR predicted an iFR < 0.89 with an AUC of 0.812 (95% CI 0.710–0.914), sensitivity of 89.5%, specificity of 58.3%, NPV 70.0%, and PPV 83.6%. (Figures 1 and 2).
FIGURE 2.
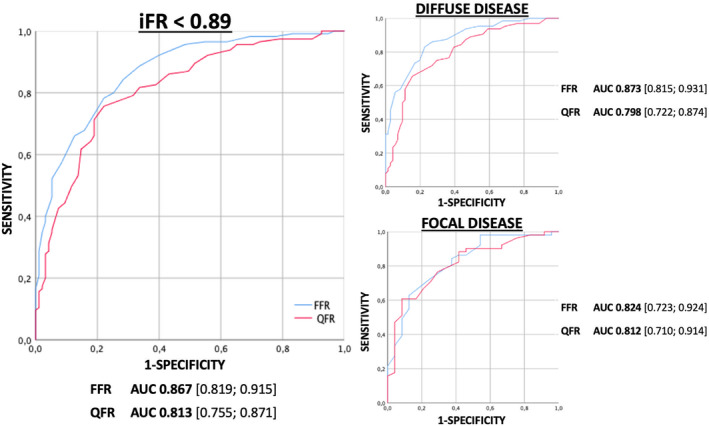
ROC curves analysis. Diagnostic performance of QFR in predicting iFR < 0.89 in the overall population and according to iFR pullback defined pattern of CAD. CAD, coronary artery disease; iFR, instantaneous wave‐free ratio; QFR, quantitative flow reserve; ROC, receiver operator characteristic [Color figure can be viewed at wileyonlinelibrary.com]
In physiological predominantly diffuse disease, the agreement between QFR and iFR was 75.5%. In this subgroup of lesions QFR predicted an iFR < 0.89 with an AUC of 0.798 (0.722–0.874), sensitivity of 59.7%, specificity of 89.5%, NPV 71.6% and PPV 83.3% (Figures 1 and 2).
3.4. Coronary lesions with iFR/QFR discordance
In the subgroup with iFR/QFR discordance (iFR+/QFR− and iFR−/QFR+), median iFR and QFR were 0.88 (interquartile range, 0.80–0.94) and 0.81 (interquartile range, 0.72–0.88) respectively.
Clinical and lesion characteristics between concordant (iFR+/QFR+ and iFR−/QFR−) and discordant (iFR+/QFR− and iFR−/QFR+) groups are displayed in Table S2. The left anterior descending artery was associated with a trend toward a less frequent iFR/QFR discordance. Notably, the physiological pattern of disease was the only variable significantly associated with iFR/QFR discordance (Table S2). In particular, coronary lesions with iFR+/QFR− demonstrated a significantly higher prevalence of physiologically diffuse pattern of disease compared with the subgroup with iFR−/QFR+ (81.2% [26 of 32] vs. 44.4% [8 of 18]; p = 0.012; Figure 3). Conversely, the angiographic defined pattern of disease was not associated with iFR/QFR discordance (Table 3 and Figure 3).
FIGURE 3.
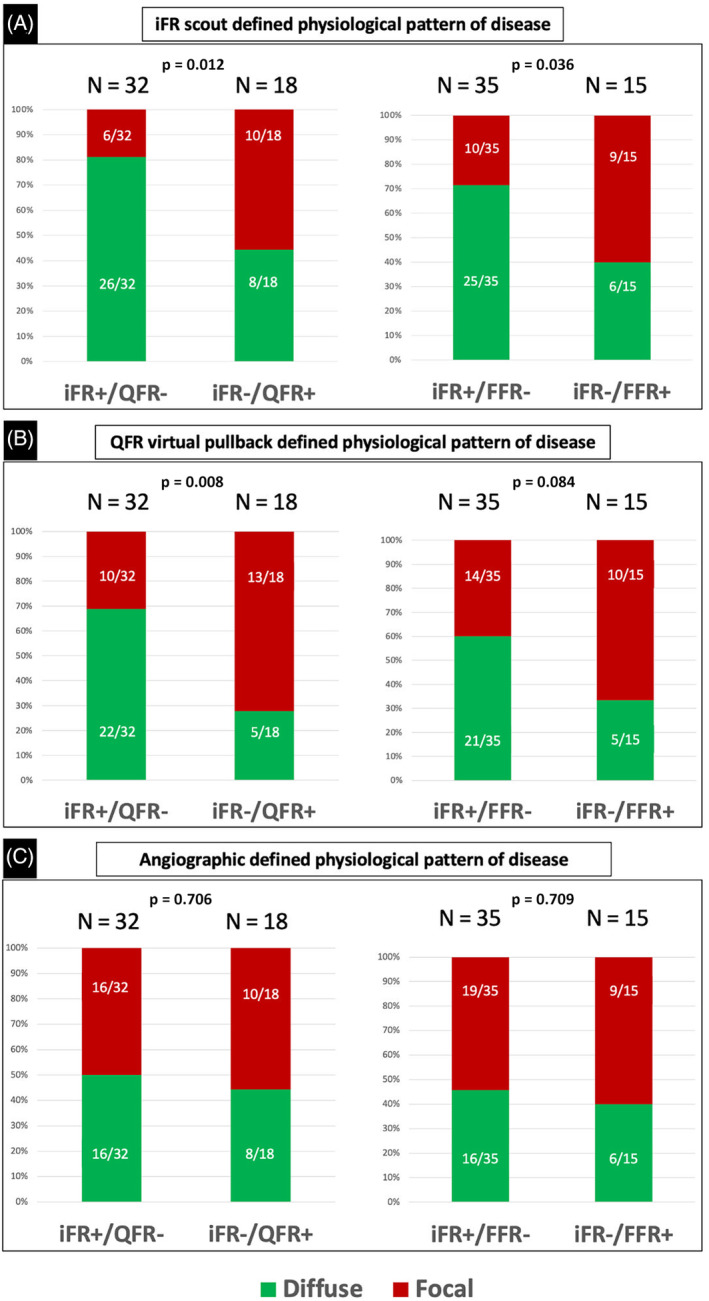
Association between the physiological pattern of disease and iFR/QFR and iFR/FFR discordance based on the iFR scout pullback system (Panel A), the QFR virtual pullback (Panel B), or angiography (Panel C). FFR, fractional flow reserve; iFR, instantaneous wave‐free ratio; QFR, quantitative flow reserve [Color figure can be viewed at wileyonlinelibrary.com]
TABLE 3.
Comparison of QFR/iFR discordant groups
| QFR−/iFR + group (n = 32) | QFR+/iFR− group (n = 18) | p value | |
|---|---|---|---|
| Patient characteristics | |||
| Age | 68.7 ± 9.9 | 64.3 ± 9.9 | 0.144 |
| Sex male | 25 (92.6) | 15 (88.2) | 0.715 |
| Hypertension | 28 (87.5) | 12 (66.7) | 0.244 |
| Dyslipidaemia | 20 (64.5) | 9 (50.0) | 0.753 |
| Diabetes mellitus | 15 (46.9) | 6 (33.3) | 0.388 |
| Smoking | 7 (21.9) | 3 (16.7) | 0.142 |
| Peripheral artery disease | 12 (37.5) | 4 (22.2) | 0.514 |
| Previous PCI | 15 (46.9) | 7 (38.9) | 0.762 |
| CKD | 11 (34.4) | 4 (22.2) | 0.520 |
| LAD | 16 (50.0) | 12 (64.7) | 0.374 |
| Non‐LAD | 16 (50.0) | 16 (45.3) | |
| Proximal lesions | 13 (40.6) | 3 (16.7) | 0.081 |
| Physiological indices | |||
| FFR | 0.84 ± 0.05 | 0.81 ± 0.06 | 0.113 |
| QFR | 0.86 ± 0.05 | 0.72 ± 0.07 | 0.001 |
| iFR | 0.83 ± 0.07 | 0.94 ± 0.02 | 0.001 |
| Pattern of coronary artery disease | |||
| iFR pullback physiologically focal | 6 (18.8) | 10 (55.6) | 0.012 |
| iFR pullback physiologically diffuse | 26 (81.2) | 8 (44.4) | |
| QFR pullback physiologically focal | 10 (31.3) | 13 (72.2) | 0.008 |
| QFR pullback physiologically diffuse | 22 (68.7) | 5 (28.8) | |
| Angiographically focal | 16 (50.0) | 10 (55.6) | 0.706 |
| Angiographically diffuse | 16 (50.0) | 8 (44.4) | |
Abbreviations: CKD, chronic kidney disease; FFR, fractional flow reserve; iFR, instantaneous wave‐free ratio; LAD, left anterior descending artery; PCI, percutaneous coronary intervention; QFR, quantitative flow reserve.
3.5. Agreement between FFR and iFR in diffuse versus focal disease
The correlation between FFR and iFR values is displayed in Figure S3 (R = 0.690; p < 0.001). FFR and iFR showed a significant agreement at the Bland Altman analysis (Figure S2). iFR+/FFR− group showed a significantly higher prevalence of predominantly physiologically diffuse disease (71.4% vs. 40.0%, p = 0.036).
The agreement between QFR and FFR in diffuse versus focal disease (defined according to the iFR pullback) is provided in Data S1
Pattern of disease classification agreement between iFR pullback and QFR virtual pullback. The physiological pattern of disease according to QFR virtual pullback was classified as predominantly physiologically focal in 95 (42.4%) coronary lesions and as predominantly physiologically diffuse in 129 (57.6%) lesions. The inter‐observer Kappa coefficients for QFR virtual pullback defined focal versus diffuse pattern of disease were 0.729 (CI 95% 0.647–0.792; p < 0.001).
The classification (focal vs. diffuse) agreement between QFR virtual pullback and iFR pullback was 83.9% (Figure S4). In particular, QFR and iFR were concordant in 188 out of 224 coronary lesions, in which the pattern was interpreted as predominantly diffuse in 116 (51.8%) and predominantly focal in 72 (32.1%) by both indices. Conversely, discordance between QFR virtual pullback and iFR pullback was observed in 36 (16.1%) lesions. In case of discordance, in the majority of the cases (27 out of 36, 75.0%), the pattern of disease was interpreted as predominantly focal at QFR virtual pullback and as predominantly diffuse at iFR pullback.
The physiological pattern of disease as determined by the QFR pullback was significantly different between iFR+/QFR− and iFR−/QFR+ groups. In particular, the iFR−/QFR+ subgroup demonstrated a significantly higher prevalence of predominantly physiologically focal disease compared with the iFR+/QFR− subgroup (72.2% [13 of 18] vs. 31.3% [10 of 32]; p = 0.008; Figure 3).
4. DISCUSSION
This study sought to investigate the interplay between physiological pattern of CAD and QFR diagnostic accuracy based on its agreement with the most commonly used pressure‐wire indices of myocardial ischemia. A representative central illustration is provided in Figure 4.
FIGURE 4.
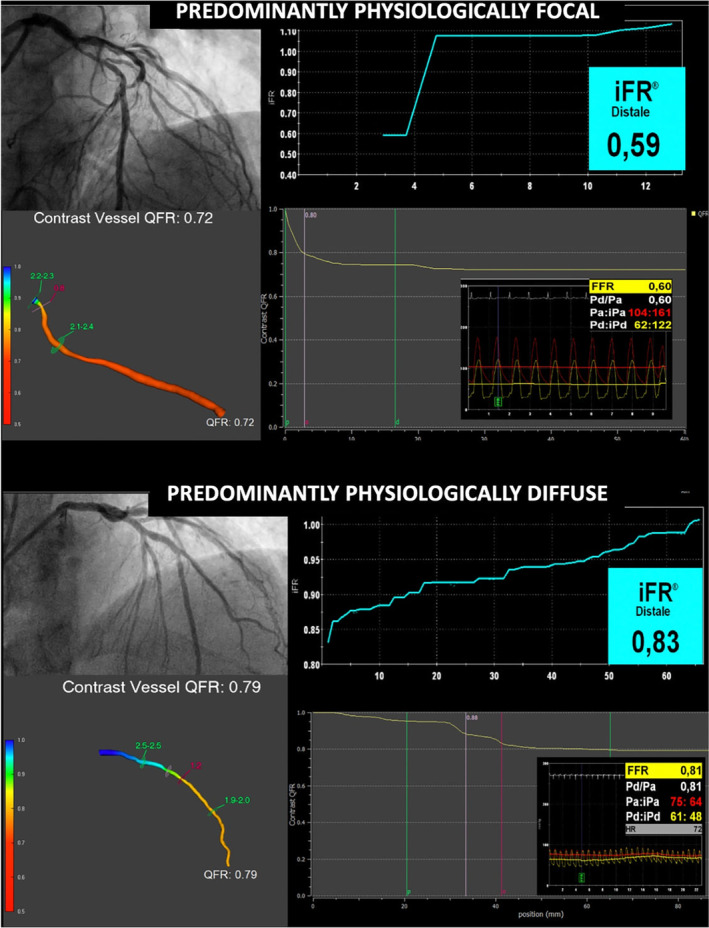
Impact of physiological pattern of disease on QFR diagnostic accuracy. QFR, quantitative flow reserve [Color figure can be viewed at wileyonlinelibrary.com]
The most relevant results of this analysis can be summarized as follows:
QFR assessment of myocardial ischemia was accurate both in physiological predominantly focal and diffuse patterns of CAD.
In case of iFR/QFR discordance, the physiological pattern of disease significantly influenced the iFR/QFR discordance; In particular, diffuse coronary disease was more frequently associated with abnormal iFR, whereas focal disease was more frequently associated with abnormal QFR values.
QFR virtual pullback effectively defined the physiological pattern of disease (focal vs. diffuse) and it was in agreement with the iFR scout pullback in the majority (83.9%) of the cases.
Functional assessment of inducible myocardial ischemia is recommended with the highest level of evidence by the most recent international guidelines. Despite the amount of evidence supporting the role of FFR and iFR in guiding coronary revascularization, their utilization in clinical practice remains limited, with only a small increase in FFR usage from 14.8% in 2009 to 18.5% in 2017. 14 Additional costs, prolonged procedural time, invasive instrumentation of the culprit artery, and, for hyperemic indices, use of vasodilator agents, which can cause uncomfortable side effects are among the factors that may explain the low penetration of coronary physiology into the standard clinical practice. In order to address these limitations, pressure‐wire‐free and adenosine‐free alternatives to invasive‐FFR have been developed and validated. 8 , 15
QFR is a novel angiography‐based and adenosine‐free physiology alternative to FFR. QFR is based on a 3D reconstruction of the stenotic coronary artery created from the lumen contours of two standard angiographic projections and on contrast flow velocity estimated by frame count in the vessel at resting state.
QFR demonstrated a significant correlation and substantial agreement with invasive iFR and FFR. 8 Similarly, in a previous work by Hwang et al., QFR showed an excellent correlation with FFR and a good correlation with iFR, regardless of the clinical presentation. 16
As previously observed, the diagnostic performance and discriminant function of QFR is better with FFR than with iFR as reported in Figure 1 and Figure S3.
In fact, QFR is derived based on a computation of the hyperemic status of coronary circulation. 9 , 10 Therefore, a better diagnostic agreement of QFR with FFR than with iFR is expected. 16 Nevertheless, in this study, iFR < 0.89 was used to define the diagnostic performance of QFR since resting physiology is less influenced by the presence of diffuse disease or tandem lesions compared with hyperemic indices. 17 Notably, QFR identified an abnormal iFR with good accuracy (AUC 0.81) regardless the physiological pattern of disease (Figure 2).
Our study builds up on the body of literature that supports the accuracy of angiography‐derived FFR in different clinical settings. The ongoing FAVOR III China (ClinicalTrial.gov Identifier: NCT03656848) and FAVOR III Europe‐Japan Study (ClinicalTrial.gov Identifier: NCT03729739) will shed definitive light on QFR value in the clinical practice, comparing QFR‐guided PCI with angio‐guided PCI and with invasive‐FFR‐guided revascularization respectively.
In this study, the discordance rate (24.8%) between FFR and iFR was consistent with that previously observed. 3 Notably, the best management of patients with FFR/iFR discordance is an important gap in knowledge that still awaits to be filled. As expected, the majority of the discordance occurs in coronary stenoses close to the FFR/iFR ischemic cut‐off. Similarly, the median values of coronary lesions with QFR/iFR discordance were close to the cut‐off and on lesion located on the LAD. 18 Importantly, further studies on the clinical significance of discordance between pressure‐wire and angiography‐derived indices are warranted to advise on the best clinical management.
It has been suggested that in case of physiologically predominantly diffuse disease, the most important mode of pressure energy loss is friction losses along the length of the vessel. Conversely, separation losses close to the stenosis is the predominant mode of pressure energy loss in physiologically predominantly focal disease. 4 Consequently, in physiologically diffuse disease the most common pattern of disease would be iFR+/QFR− because friction losses are more evident under resting conditions and less important under maximal hyperemic conditions. Conversely, in physiologically focal disease, the most diffuse pattern of discordance would be iFR−/QFR+ because stenosis‐related separation losses are more evident under hyperemic conditions. 4
In this study, we adopted the iFR Scout pullback system to define the physiological pattern of disease. Under resting conditions, coronary flow is thought to be more stable and predictable across multiple epicardial sequential stenoses. Therefore, iFR has the potential to perform a hemodynamic mapping of the entire vessel based on a beat‐to‐beat analysis.
QFR demonstrated an excellent diagnostic performance in both diffuse and focal physiological scenarios. Moreover, as expected, the majority of the discordant cases were close to the cut‐off. Therefore, QFR may be reasonably used for the assessment of the majority of the coronary lesions, reserving further investigation with pressure‐wire only in cases with borderline QFR values. Clearly, the validity of this approach needs to be confirmed by prospective dedicated studies.
QFR trace provides a virtual hemodynamic mapping of the coronary artery that, in this analysis demonstrated an excellent classification agreement with the iFR scout pullback. QFR trace “pullback” may guide the procedural planning and may allow the operator to perform a virtual PCI. This may represent an important step forward toward precision medicine implementation. 12 , 19
5. LIMITATIONS
This study has limitations. First, it is a single‐center retrospective study with a relatively small sample size. Nevertheless, to the best of our knowledge, this is the first study aiming to assess the influence of physiological pattern of disease on QFR.
Moreover, invasive measurements of coronary flow and coronary resistances were not available in this analysis and the possible influences of microvascular dysfunction in the physiological different pattern of disease could not be assessed.
Furthermore, there are no established validated criteria to define the physiological pattern of disease as focal or diffuse using coronary pressure‐wire pullback. Several studies proposed focal (abrupt pressure drop) pattern and diffuse (gradual pressure drop) pattern on pressure‐wire pullback. 12 , 13 , 20 However, definitions are heterogeneous and, ultimately, depend on the operator. In this study, all the pressure‐wire traces, iFR pullback, and QFR virtual pullback were reviewed independently by two different expert operators blinded of the clinical and procedural data.
Lastly, the use of iFR and QFR as dichotomous variables is inevitably a stretch since physiology is rather a continuum and reduces the statistical power of the analysis. Nevertheless, the use of ischemic cut‐off is commonly used in the clinical practice to guide the clinical decision on the myocardial revascularization.
6. CONCLUSIONS
QFR is an accurate angiography‐based tool to assess myocardial ischemia independently of the physiological pattern of CAD. Nevertheless, the physiological pattern of disease has an influence on the discordance between iFR and QFR and, in particular, physiologically diffuse disease is associated with abnormal iFR whereas physiologically focal disease is associated with abnormal QFR. The QFR virtual pullback correctly defined the physiological pattern of disease in the majority of the cases using the iFR scout pullback as reference.
7. IMPACT ON DAILY PRACTICE
Despite the favorable clinical outcome of physiology‐guided revascularization, its adoption in the clinical practice is heterogeneous and globally remains low, due to additional procedural time, costs, and technical issues. QFR is a novel angiography‐based, wire and adenosine‐free index validated against FFR in the clinical setting. According to our findings, QFR provides an accurate assessment of inducible myocardial ischemia independently of the physiological pattern of CAD. Nevertheless, the pattern of disease has an influence on the discordance between iFR and QFR and, in particular, physiologically diffuse disease is associated with abnormal iFR whereas physiologically focal disease is associated with abnormal QFR. Furthermore, QFR virtual pullback provides an accurate definition of the physiological pattern of disease using the iFR scout pullback as comparator.
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
Supporting information
Figure S1. Study Flowchart.
Figure S2. Bland–Altman plot of quantitative flow reserve (QFR), instantaneous wave‐free ratio (iFR), and fractional flow reserve (FFR) values.
Figure S3. Scatter plot showing the relationship between QFR‐FFR and FFR‐iFR values according to the iFR pullback defined physiological pattern of coronary disease.
Figure S4. Correspondence between iFR pullback and QFR virtual pullback defined physiological pattern of disease. In case of discordance the physiological pattern of disease was interpreted as predominantly focal by QFR virtual pullback and as predominantly diffuse by iFR pullback.
Data S1. Supporting information.
ACKNOWLEDGMENT
Open Access Funding provided by Universita degli Studi di Verona within the CRUI‐CARE Agreement.
Scarsini R, Fezzi S, Pesarini G, Del Sole PA, Venturi G, Mammone C, et al. Impact of physiologically diffuse versus focal pattern of coronary disease on quantitative flow reserve diagnostic accuracy. Catheter Cardiovasc Interv. 2021;99:736–745. 10.1002/ccd.30007
Roberto Scarsini and Simone Fezzi contributed equally to this study.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Götberg M, Christiansen EH, Gudmundsdottir IJ, et al. Instantaneous wave‐free ratio versus fractional flow reserve to guide PCI. N Engl J Med. 2017;376:1813‐1823. [DOI] [PubMed] [Google Scholar]
- 2. Davies JE, Sen S, Dehbi H‐M, et al. Use of the instantaneous wave‐free ratio or fractional flow reserve in PCI. N Engl J Med. 2017;376(19):1824‐1834. [DOI] [PubMed] [Google Scholar]
- 3. Jeremias A, Maehara A, Généreux P, et al. Multicenter core laboratory comparison of the instantaneous wave‐free ratio and resting Pd/pa with fractional flow reserve: the RESOLVE study. J Am Coll Cardiol. 2014;63(13):1253‐1261. doi:10.1016/j.jacc.2013.09.060 [DOI] [PubMed] [Google Scholar]
- 4. Warisawa T, Cook CM, Howard JP, et al. Physiological pattern of disease assessed by pressure‐wire pullback has an influence on fractional flow reserve/instantaneous wave‐free ratio discordance. Circ Cardiovasc Interv. 2019;12(5):e007494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kobayashi Y, Johnson NP, Berry C, et al. The influence of lesion location on the diagnostic accuracy of adenosine‐free coronary pressure wire measurements. JACC Cardiovasc Interv. 2016;9(23):2390‐2399. [DOI] [PubMed] [Google Scholar]
- 6. Pighi M, Gratta A, Marin F, et al. Cardiac allograft vasculopathy: pathogenesis, diagnosis and therapy. Transplant Rev. 2020;23:100569. [DOI] [PubMed] [Google Scholar]
- 7. Hennigan B, Oldroyd KG, Berry C, et al. Discordance between resting and hyperemic indices of coronary stenosis severity: the VERIFY 2 study (a comparative study of resting coronary pressure gradient, instantaneous wave‐free ratio and fractional flow reserve in an unselected population referred). Circ Cardiovasc Interv. 2016;9(11):1‐10. [DOI] [PubMed] [Google Scholar]
- 8. De Maria GL, Garcia‐Garcia HM, Scarsini R, et al. Novel indices of coronary physiology: do we need alternatives to fractional flow reserve? Circ Cardiovasc Interv. 2020;13(4):e008487. [DOI] [PubMed] [Google Scholar]
- 9. Westra J, Andersen BK, Campo G, et al. Diagnostic performance of in‐procedure angiography‐derived quantitative flow reserve compared to pressure‐derived fractional flow reserve: the FAVOR II Europe‐Japan study. J Am Heart Assoc. 2018;7(14):e009603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tu S, Westra J, Yang J, et al. Diagnostic accuracy of fast computational approaches to derive fractional flow reserve from diagnostic coronary angiography: the international multicenter FAVOR pilot study. JACC Cardiovasc Interv. 2016;9(19):2024‐2035. [DOI] [PubMed] [Google Scholar]
- 11. Collet C, Sonck J, Vandeloo B, et al. Measurement of hyperemic pullback pressure gradients to characterize patterns of coronary atherosclerosis. J Am Coll Cardiol. 2019;74(14):1772‐1784. [DOI] [PubMed] [Google Scholar]
- 12. Biscaglia S, Uretsky B, Barbato E, et al. Invasive coronary physiology after stent implantation. JACC Cardiovasc Interv. 2021;14(3):237‐246. [DOI] [PubMed] [Google Scholar]
- 13. Biscaglia S, Uretsky BF, Tebaldi M, et al. Angio‐based fractional flow reserve, functional pattern of coronary artery disease, and prediction of percutaneous coronary intervention result: a proof‐of‐concept study. Cardiovasc Drugs Ther. Published online April 8, 2021. doi: 10.1007/s10557-021-07162-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Parikh RV, Liu G, Plomondon ME, et al. Utilization and outcomes of measuring fractional flow reserve in patients with stable ischemic heart disease. J Am Coll Cardiol. 2020;75(4):409‐419. [DOI] [PubMed] [Google Scholar]
- 15. De Maria GL, Wopperer S, Kotronias R, et al. From anatomy to function and then back to anatomy: invasive assessment of myocardial ischaemia in the catheterization laboratory based on anatomy‐derived indices of coronary physiology. Minerva Cardiol Angiol. Published online March 11, 2021. doi: 10.23736/S2724-5683.20.05486-9 [DOI] [PubMed] [Google Scholar]
- 16. Hwang D, Choi KH, Lee JM, et al. Diagnostic agreement of quantitative flow ratio with fractional flow reserve and instantaneous wave‐free ratio. J Am Heart Assoc. 2019;8(8):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Modi BN, De Silva K, Rajani R, Curzen N, Perera D. Physiology‐guided management of serial coronary artery disease a review. JAMA Cardiol. 2018;3(5):432‐438. [DOI] [PubMed] [Google Scholar]
- 18. Sen S, Ahmad Y, Dehbi HM, et al. Clinical events after deferral of LAD revascularization following physiological coronary assessment. J Am Coll Cardiol. 2019;73(4):444‐453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Biscaglia S, Tebaldi M, Brugaletta S, et al. Prognostic value of QFR measured immediately after successful stent implantation: the international multicenter prospective HAWKEYE study. JACC Cardiovasc Interv. 2019;12(20):2079‐2088. [DOI] [PubMed] [Google Scholar]
- 20. Ando H, Takashima H, Suzuki A, et al. Impact of lesion characteristics on the prediction of optimal poststent fractional flow reserve. Am Heart J. 2016;182:119‐124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Study Flowchart.
Figure S2. Bland–Altman plot of quantitative flow reserve (QFR), instantaneous wave‐free ratio (iFR), and fractional flow reserve (FFR) values.
Figure S3. Scatter plot showing the relationship between QFR‐FFR and FFR‐iFR values according to the iFR pullback defined physiological pattern of coronary disease.
Figure S4. Correspondence between iFR pullback and QFR virtual pullback defined physiological pattern of disease. In case of discordance the physiological pattern of disease was interpreted as predominantly focal by QFR virtual pullback and as predominantly diffuse by iFR pullback.
Data S1. Supporting information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


