1. INTRODUCTION
Chronic rhinosinusitis with nasal polyps (CRSwNP) is an inflammatory disease of the nasal cavity and paranasal sinuses characterized by nasal obstruction/blockage, loss of sense of smell, and rhinorrhea, and imparts substantial burden on health‐related quality of life. 1 , 2 CRSwNP displays a predominantly type 2 inflammatory signature with interleukin (IL)‐4, IL‐13, and IL‐5 as key cytokines. 1 , 3 , 4 Several inflammatory biomarkers are potentially associated with CRSwNP pathophysiology, including eosinophils, IgE, periostin, eotaxin‐3, thymus activation‐regulated chemokine (TARC), and leukotriene E4 (LTE4). 5 Dupilumab is a fully human VelocImmune‐derived monoclonal antibody that blocks IL‐4Rα, the shared receptor component for IL‐4/IL‐13. 6 , 7 In SINUS‐24 (A Controlled Clinical Study of Dupilumab in Patients With Bilateral Nasal Polyps; NCT02912468) and SINUS‐52 (Controlled Clinical Study of Dupilumab in Patients With Nasal Polyps; NCT02898454), dupilumab significantly improved measures of disease, symptom severity, and health‐related quality of life in patients with severe CRSwNP versus placebo. 8 There is limited understanding of the potential role of biomarkers of type 2 inflammation in CRSwNP treatment outcomes, with currently available data suggesting no strong correlations between change in measures of disease severity and inflammatory biomarkers. 9 However, investigating associations between biomarkers and treatment response through the correlation between changes in biomarker levels and measures of disease over time may elucidate the role of biomarkers in disease processes and treatment mechanisms. This post hoc analysis investigated associations between change in nasal polyp score (NPS), an objective, physician‐assessed measure of disease severity in CRSwNP, and local and systemic inflammatory biomarker levels in dupilumab‐treated patients in SINUS‐24 and SINUS‐52.
2. METHODS
2.1. Study design
Details of the phase 3, randomized, double‐blind, placebo‐controlled SINUS‐24/SINUS‐52 designs have been previously published. 1 Patients were randomized 1:1 to subcutaneous (SC) dupilumab 300 mg/placebo (n = 143/133) every 2 weeks (q2w) for 24 weeks (SINUS‐24), or 1:1:1 to SC dupilumab 300 mg/placebo q2w for 52 weeks (n = 150/153), or SC dupilumab 300 mg q2w for 24 weeks, then every 4 weeks for 28 weeks (n = 145) (SINUS‐52). Patients provided written informed consent before enrollment.
NPS (range, 0 to 8) scoring has been previously reported. 8 NPS is a physician‐evaluated score of the severity and extent of nasal polyps, providing an objective measure of disease severity in CRSwNP. Serum periostin, eotaxin‐3, IgE, TARC, and urine LTE4 were assessed at weeks 0 and 24 (both studies) and week 52 (SINUS‐52). Periostin, IL‐5, and eotaxin‐3 in nasal secretions were assessed at weeks 0 and 24 (SINUS‐52). 9
2.2. Statistical analysis
Post hoc analyses were performed in the intent‐to‐treat population and patient subgroups with and without history of asthma, nonsteroidal anti‐inflammatory drug‐exacerbated respiratory disease (NSAID‐ERD), prior surgery, and systemic corticosteroid (SCS) use within the previous 2 years. Pearson correlation coefficients were determined to evaluate the strength of any associations between baseline NPS and baseline biomarker levels, and between changes from baseline in NPS and biomarkers (assessed at weeks 24 and 52). Correlations were interpreted as very weak (0.00−0.19), weak (0.20−0.39), moderate (0.40−0.59), or strong (0.60−0.79). 10
3. RESULTS
3.1. Baseline characteristics
Baseline demographics, disease characteristics, and biomarkers (Supplementary Table 1) were balanced among treatment groups and were consistent with a population with severe CRSwNP. There were weak to very weak correlations between baseline NPS and biomarker levels (Supplementary Table 2).
3.2. Overall population
At week 24 in SINUS‐52, changes in NPS with dupilumab were moderately to weakly correlated with changes in nasal biomarkers (correlation coefficients, 0.2895−0.4035) (Fig. 1, Supplementary Fig. 1), and at week 52 were very weakly correlated with changes in serum biomarkers (correlation coefficients, 0.1171−0.1850) (Fig. 1, Supplementary Fig. 1) and weakly correlated with changes in urinary LTE4 (correlation coefficient, 0.2070) (Fig. 1C).
FIGURE 1.
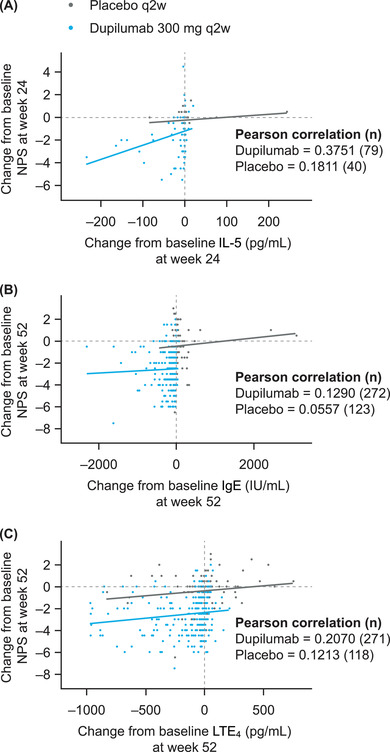
Scatterplots of the relationship between change from baseline in nasal polyp score (NPS) and change in (A) nasal interleukin (IL)‐5 at week 24 in SINUS‐52 (Controlled Clinical Study of Dupilumab in Patients With Nasal Polyps), and (B) serum total IgE and (C) urinary leukotriene E4 (LTE4) at week 52 in SINUS‐52 (observed values; intent‐to‐treat [ITT] population). n indicates the number of patients with available data for biomarker assessment; and q2w, every 2 weeks.
3.3. Patient subgroups
Generally weak or very weak correlations were observed for changes from baseline in NPS and changes in serum and urine biomarkers with dupilumab in the subgroups of patients with and without asthma, NSAID‐ERD, prior surgery, or SCS use within the previous 2 years; similar results were seen with nasal biomarkers (Table).
TABLE 1.
Correlations between change from baseline in NPS and change in local and systemic type 2 biomarker levels following dupilumab treatment in subgroups of patients with asthma, NSAID‐ERD, prior surgery, or SCS use within the previous 2 years (pooled ITT population)
| Pearson correlation for change in NPS | ||||||||
|---|---|---|---|---|---|---|---|---|
| Correlation coefficient (n) a | ||||||||
| Biomarker | Asthma | Without asthma | NSAID‐ERD | Without NSAID‐ERD | Prior surgery | Without prior surgery | SCS use in previous 2 y | Without SCS use in previous 2 y |
| (n = 258) | (n = 180) | (n = 122) | (n = 316) | (n = 272) | (n = 166) | (n = 329) | (n = 109) | |
| Nasal secretion (week 24) b | ||||||||
| IL‐5 | 0.2241 (42) | 0.3500 (37) | 0.2177 (20) | 0.3135 (59) | 0.3232 (46) | 0.2205 (33) | 0.2873 (67) | 0.1400 (12) |
| Periostin | 0.1536 (43) | 0.2825 (36) | 0.2114 (20) | 0.2579 (59) | 0.2960 (46) | 0.0899 (33) | 0.2362 (68) | 0.1357 (11) |
| Eotaxin‐3 | 0.4575 (26) | 0.2628 (24) | 0.3268 (13) | 0.2808 (37) | 0.2711 (31) | 0.2653 (19) | 0.2457 (42) | 0.6938 (8) |
| Serum (week 52) | ||||||||
| Periostin | 0.0891 (157) | 0.2476 (100) | 0.1036 (69) | 0.1796 (188) | 0.0777 (151) | 0.2855 (106) | 0.1971 (206) | 0.1084 (51) |
| Eotaxin‐3 | 0.0880 (165) | 0.1341 (103) | 0.1684 (71) | 0.0948 (197) | 0.0466 (155) | 0.1463 (113) | 0.1164 (218) | 0.1886 (50) |
| Total IgE | 0.0695 (168) | −0.0491 (104) | −0.1753 (73) | 0.0773 (199) | 0.0450 (159) | −0.0064 (113) | 0.0274 (221) | 0.0512 (51) |
| TARC | 0.0329 (165) | 0.0870 (105) | −0.0084 (72) | 0.0901 (198) | 0.0168 (157) | 0.0993 (113) | 0.1043 (219) | −0.0512 (51) |
| Urine (week 52) | ||||||||
| LTE4 | 0.0425 (166) | 0.2342 (105) | −0.0346 (72) | 0.2133 (199) | 0.1426 (158) | 0.0836 (113) | 0.0980 (219) | 0.3180 (52) |
Abbreviations: IL, interleukin; IT, intent‐to‐treat; LTE4, leukotriene E4; NPS, nasal polyp score; NSAID‐ERD, nonsteroidal anti‐inflammatory drug‐exacerbated respiratory disease; SCS, systemic corticosteroid; TARC, thymus activation‐regulated chemokine; y, years.
The number in parentheses is the number of patients with available data for biomarker assessment.
SINUS‐52 (Controlled Clinical Study of Dupilumab in Patients With Nasal Polyps) only.
4. DISCUSSION
In pivotal phase 3 trials, dupilumab showed efficacy in all primary and secondary outcomes of disease measures in CRSwNP, and demonstrated suppression of type 2 biomarkers in blood and nasal secretions. 8 In the current analysis, correlations between biomarker levels and NPS at baseline were weak to very weak. Improvements in NPS, an objective physician‐assessed measure of disease severity in CRSwNP, with dupilumab were moderately to weakly correlated with changes in nasal biomarkers of type 2 inflammation at week 24, but only weakly correlated with a urinary biomarker and very weakly correlated with serum biomarkers at week 52. Similar correlations were observed in subgroups of patients with comorbid asthma or NSAID‐ERD, which are associated with more refractory disease, 1 and with and without prior surgery or SCS use.
Overall, there is a general lack of studies that assess associations between change in biomarkers and change in clinical outcomes. Changes in the type 2 inflammatory biomarker fraction of exhaled nitric oxide (FeNO) have been demonstrated to correlate with improvement in asthma outcome (forced expiratory volume in 1 second) in patients with asthma treated with dupilumab. 11 However, similar to the results reported here, all other biomarkers assessed showed no correlation. FeNO was not assessed in these dupilumab CRSwNP studies, and therefore cannot be evaluated in the current analysis. Thus, in accordance with the research question posed, and to the authors’ knowledge, consistent and strong correlations between changes in biomarkers and clinical outcomes in CRSwNP are yet to be demonstrated. The current post hoc analysis therefore provides important insights into the strength of correlations between biomarkers of type 2 inflammation and clinical improvement, as measured here through NPS.
While the correlations between clinical outcomes and biomarkers of type 2 inflammation in this analysis were generally weak or very weak, this may be attributable to a variety of reasons such as heterogeneity in baseline values, small substudy sample size, or assay sensitivity. The very wide interindividual variability in biomarker levels also makes correlation analysis with NPS challenging, given the nonlinearity of this measure, especially in the score range from 3 to 4 encountered in this cohort of patients with severe CRSwNP. Despite the expected inter‐individual variability in biomarker levels, correlations of moderate strength were noted for some measures. It is possible that other outcome measures besides NPS may serve as better surrogates for clinical improvement. These associations were also determined in a population with severe CRSwNP and may therefore be different in populations with milder disease. Other limitations include the post hoc nature of the analysis, limited number of patients with nasal secretion samples, and that nasal secretion biomarker levels were only assessed to week 24. Analyses using other objective clinical end points, eg, sinus opacification, and patient‐reported end points, would be of interest to further explore these findings.
Overall, these findings suggest that dupilumab efficacy, as evaluated by reduction in NPS, in patients with severe CRSwNP is associated with a reduction in some biomarkers of local nasal type 2 inflammation. The absence of stronger correlations with serum/urine measurements suggests that inflammatory events in CRSwNP and the attendant effects of dupilumab on this inflammation are not accurately reflected in the systemic circulation. However, several interesting correlations were observed between change in NPS and change in nasal biomarkers of type 2 inflammation with dupilumab treatment. Further research is needed to determine which biomarkers of type 2 inflammation best correlate with disease‐specific objective measures of severity and subjective patient‐reported outcomes.
CONFLICTS OF INTEREST
Claus Bachert: ALK, AstraZeneca, GlaxoSmithKline, Mylan, Novartis, Sanofi, and Stallergenes Greer (advisory board member and speakers’ fees). Jonathan Corren: AstraZeneca, Genentech, Inc., Novartis, Regeneron Pharmaceuticals, Inc., and Sanofi (consultancy fees); AstraZeneca and Genentech, Inc. (member of speakers’ bureaus); and Genentech, Inc., Regeneron Pharmaceuticals, Inc., and Sanofi (institutional grant funding). Stella E. Lee: AstraZeneca, GlaxoSmithKline, Regeneron Pharmaceuticals, Inc., and Sanofi (clinical trial funding); and AstraZeneca, Genzyme, GlaxoSmithKline, Novartis, Regeneron Pharmaceuticals, Inc., and Sanofi (advisory board member). Danen Cunoosamy, Asif H. Khan, Juby A. Jacob‐Nara, and Paul J. Rowe: Sanofi (employees, may hold stock and/or stock options). Haixin Zhang, Scott Nash, Sivan Harel, Shahid Siddiqui, and Yamo Deniz: Regeneron Pharmaceuticals, Inc. (employees and shareholders).
AUTHOR CONTRIBUTIONS
All authors provided critical review and revision and final approval of the publication, and accept accountability for the accuracy and integrity of the content. Claus Bachert contributed to the study design, acquired data, and provided interpretation/analysis of the data. Jonathan Corren and Stella E. Lee acquired data and provided data interpretation/analysis. Danen Cunoosamy, Asif H. Khan, Juby A. Jacob‐Nara, Haixin Zhang, Scott Nash, Sivan Harel, Shahid Siddiqui, Paul J. Rowe, and Yamo Deniz contributed to the study concept or design and provided data interpretation/analysis.
Supporting information
SUPPLEMENTARY TABLE 1. Demographics and baseline disease characteristics and biomarkers (SINUS‐24/SINUS‐52; pooled ITT population)
SUPPLEMENTARY TABLE 2. Correlations between NPS and type 2 biomarker levels at baseline
SUPPLEMENTARY FIGURE 1. Scatterplots of the relationship between change from baseline in nasal polyp score (NPS) and change in (A) nasal periostin and (B) nasal eotaxin‐3 at week 24 (SINUS‐52 [Controlled Clinical Study of Dupilumab in Patients With Nasal Polyps]), (C) serum periostin, (D) serum eotaxin‐3, and (E) serum thymus activation‐regulated chemokine (TARC) at week 52 in SINUS‐52 (observed values; intent‐to‐treat [ITT] population)
ACKNOWLEDGMENTS
Medical writing/editorial assistance was provided by Joseph Hodgson, PhD, of Adelphi Group (Macclesfield, UK); and funded by Sanofi Genzyme and Regeneron Pharmaceuticals, Inc., according to the Good Publication Practice guideline. Research was sponsored by Sanofi and Regeneron Pharmaceuticals, Inc. ClinicalTrials.gov identifiers: SINUS‐24 (NCT02912468) and SINUS‐52 (NCT02898454).
Bachert C , Corren J , Lee SE , et al. Dupilumab efficacy and biomarkers in chronic rhinosinusitis with nasal polyps: Association between dupilumab treatment effect on nasal polyp score and biomarkers of type 2 inflammation in patients with chronic rhinosinusitis with nasal polyps in the phase 3 SINUS‐24 and SINUS‐52 trials. Int Forum Allergy Rhinol. 2022;12:1191–1195. 10.1002/alr.22964
REFERENCES
- 1. Bachert C, Marple B, Schlosser RJ, et al. Adult chronic rhinosinusitis. Nat Rev Dis Primers. 2020;6:86. [DOI] [PubMed] [Google Scholar]
- 2. Khan A, Vandeplas G, Huynh TMT, et al. The Global Allergy And Asthma European Network (GALEN) rhinosinusitis cohort: a large European cross‐sectional study of chronic rhinosinusitis patients with and without nasal polyps. Rhinology. 2019;57:32–42. [DOI] [PubMed] [Google Scholar]
- 3. Hulse KE, Stevens WW, Tan BK, Schleimer RP. Pathogenesis of nasal polyposis. Clin Exp Allergy. 2015;45:328–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stevens WW, Ocampo CJ, Berdnikovs S, et al. Cytokines in chronic rhinosinusitis. Role in eosinophilia and aspirin‐exacerbated respiratory disease. Am J Respir Crit Care Med. 2015;192:682–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schleimer RP. Immunopathogenesis of chronic rhinosinusitis and nasal polyposis. Annu Rev Pathol. 2017;12:331–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Macdonald LE, Karow M, Stevens S, et al. Precise and in situ genetic humanization of 6 Mb of mouse immunoglobulin genes. Proc Natl Acad Sci U S A. 2014;111:5147–5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Murphy AJ, Macdonald LE, Stevens S, et al. Mice with megabase humanization of their immunoglobulin genes generate antibodies as efficiently as normal mice. Proc Natl Acad Sci U S A. 2014;111:5153–5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bachert C, Han JK, Desrosiers M, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS‐24 and LIBERTY NP SINUS‐52): results from two multicentre, randomized, double‐blind, placebo‐controlled, parallel‐group phase 3 trials. Lancet. 2019;394:1638–1650. [DOI] [PubMed] [Google Scholar]
- 9. Hamilton JD, Harel S, Swanson BN, et al. Dupilumab suppresses type 2 inflammatory biomarkers across multiple atopic, allergic diseases. Clin Exp Allergy. 2021;51:915–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Swinscow TD . Statistics at Square One. 9th ed. In: Campbell MJ. BMJ Publishing Group: 1997. [Google Scholar]
- 11. Wenzel S, Ford L, Pearlman D, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368:2455–2466. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTARY TABLE 1. Demographics and baseline disease characteristics and biomarkers (SINUS‐24/SINUS‐52; pooled ITT population)
SUPPLEMENTARY TABLE 2. Correlations between NPS and type 2 biomarker levels at baseline
SUPPLEMENTARY FIGURE 1. Scatterplots of the relationship between change from baseline in nasal polyp score (NPS) and change in (A) nasal periostin and (B) nasal eotaxin‐3 at week 24 (SINUS‐52 [Controlled Clinical Study of Dupilumab in Patients With Nasal Polyps]), (C) serum periostin, (D) serum eotaxin‐3, and (E) serum thymus activation‐regulated chemokine (TARC) at week 52 in SINUS‐52 (observed values; intent‐to‐treat [ITT] population)


