Abstract
Introduction
Atrial fibrillation (AF) is a growing health problem and is associated with increased risk of stroke. The Cox‐Maze surgical procedure has offered the highest success rate, but utilization of this technique is low due to procedure invasiveness and complexity. Advances in catheter ablation and minimally invasive surgical techniques offer new options for AF treatment.
Methods
In this review, we describe current trends and outcomes of minimally invasive treatment of persistent and long‐standing persistent AF.
Results
Treatment of persistent and long‐standing persistent AF can be successfully treated using a team approach combining cardiac surgery and electrophysiology procedures. With this approach, the 1‐year freedom from AF off antiarrhythmic drugs was 85%.
Discussion
There are a variety of techniques and approaches used around the world as technology evolves to help develop new treatment strategies for AF. Our report will focus on a hybrid treatment approach using surgical and electrophysiology approaches providing enhanced treatment options by replicating Cox‐Maze IV lesions using skills from each specialty. Closure of the left atrial appendage as part of these procedures enhances protection from late stroke. A team approach provides a cohesive evaluation, treatment, and monitoring plan for patients. Development of successful, less invasive treatment options will help address the growing population of patients with AF.
Keywords: atrial fibrillation, catheter ablation, thoracoscopic hybrid maze procedure
1. INTRODUCTION
Atrial fibrillation (AF) is the most common arrhythmia and is associated with stroke, heart failure, dementia, and premature death. The prevalence of AF in the United States alone is projected to reach up to 16 million by 2050 and is currently 3–6 million. 1 In the Medicare population, 5‐year mortality after diagnosis of AF is almost 50%. 2 In a recent randomized clinical trial for patients with persistent and long‐standing persistent AF treatment with endocardial catheter ablation resulted in 1‐year freedom from AF off antiarrhythmics of 32% at 1 year in a randomized clinical trial. 3
A small percentage of the patients with AF have other cardiac conditions requiring surgical intervention (concomitant AF). The majority of patients have stand‐alone AF which is frequently associated with multiple comorbidities such as diabetes, heart failure, vascular problems, and hypertension and are at increased risk for transient ischemic attacks and stroke. Major societies (American Association for Thoracic Surgery, Society of Thoracic Surgeons, and Heart Rhythm Society) involved in the management of AF patients give a Class I recommendation for concomitant ablation. Unfortunately, utilization of concomitant surgical ablation of AF remains as low as 16%, especially for Medicare‐age patients requiring nonmitral surgery. 4 , 5 , 6 , 7 Stand‐alone symptomatic AF that has failed antiarrhythmic drugs currently has a Class IIb recommendation for surgical ablation of paroxysmal AF, and IIa recommendation for nonparoxysmal AF by the Heart Rhythm Society. 8
Left atrial appendage (LAA) closure is an important part of any AF ablation. Currently, the Society of Thoracic Surgeons gives a Class IIa recommendation for closure of LAA with concomitant AF surgery, but this was before the publication of the landmark LAAOS III Trial. 9 Several publications have shown safety and improved outcomes with LAA closure, 10 , 11 including a recent randomized clinical trial. 9
There are several options for the surgical treatment of stand‐alone AF. The Cox‐Maze procedure can be performed via median sternotomy or minimally invasively via right mini‐thoracotomy with 79% and 66% freedom from AF off antiarrhythmic medication at 1 and 5 years, respectively. 12 Using a standalone mini‐thoracotomy approach, 1‐ and 5‐year freedom from AF off antiarrhythmics was 88% and 73%, respectively, in a population that included 78% of patients who had long‐standing persistent AF. 13 While multiple studies have documented the safety and efficacy of this procedure, it is utilized in few centers because of its undesirable level of invasiveness, including the use of cardiopulmonary bypass. Unfortunately, it is impossible to replicate the Cox‐Maze III or IV lesion sets (Figure 1) with either endocardial catheter ablation alone or with epicardial thoracoscopic surgical ablation alone. However, by combining thoracoscopic epicardial ablation and endocardial catheter ablation into a single or staged procedure, it is possible to replicate most of the lesions of the Cox‐Maze III and IV without using cardiopulmonary bypass. Obviously, these “off‐pump hybrid procedures” are more appealing to patients and referring physicians.
Figure 1.
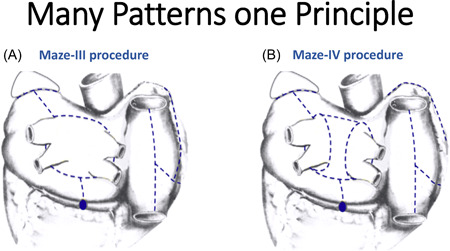
Lesion sets of (A) Cox‐Maze III and (B) Cox‐Maze IV procedures. The Cox‐Maze lesion set has historically provided the highest success rate for treatment of atrial fibrillation. Modifications of the original procedure need to replicate these original lesions regardless of the approach or energy source used.
Hybrid ablations include the Convergent procedure 3 , 14 and full plethora of other thoracoscopic approaches. Some of the surgical procedures mirror the full Cox‐Maze III or IV approach 15 , 16 while others lack the complete set of Maze lesions.
2. HYBRID TOTALLY THORACOSCOPIC PROCEDURES
Several devices are designed to be utilized during thoracoscopic procedures. The Cobra Fusion device (Atricure Inc.) creates a box lesion using RF energy around all four pulmonary vein and the posterior left atrial wall (Figure 2A). The device can also be used to create a lesion from the superior vena cava (SVC) to the inferior vena cava (IVC). This procedure is performed through three small ports in the right chest. A follow‐up electrophysiology study is performed approximately 3 months later to identify and ablate any gaps in the endocardial aspect of the surgical lesions. Any additional ablation lines that might be required based on the interval history of arrhythmias and on intraoperative mapping are also completed (Figure 2B). This method has been popularized by Italian group from Brescia, Italy. 17 In their prospective, single‐arm HISTORIC‐AF trial of long‐standing persistent AF of less than 5 years duration, the 30‐day freedom from major adverse events was 94% (one bleeding requiring revision, two strokes, and three pacemaker implants) and the 1‐year success rate for this hybrid procedure was 88% but thus far, those results have not been reproduced by others. 18 However, the Cobra Fusion device is no longer commercially available due to increased stroke risk.
Figure 2.
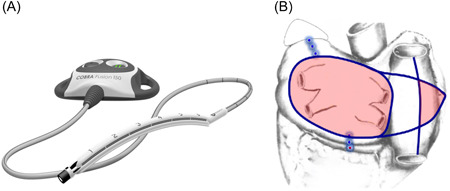
Cobra Fusion device and Muneretto hybrid lesions set. The Cobra Fusion device (A) delivers radiofrequency energy to create a box lesion around the pulmonary veins and perform additional lesions to the right atrium (B) (fusion image used with permission from Atricure Inc.).
Groups from Brussels and Amsterdam use intraprocedural mapping to guide their ablation strategy. 19 Bilateral pulmonary vein isolation (PVI) is performed with a bipolar clamp. In the Brussel's group approach, the lesion pattern for each patient is based on the intraoperative mapping findings in that specific patient, and therefore, the lesion patterns vary from one patient to another. 19 In their subsequent paper, it was reported that in 72 patients with persistent AF, 79% were free of atrial arrhythmias at 1‐year. 20 Recently, a unilateral approach to create epicardial lesions has been reported using a hybrid technique in which the entire thoracoscopic procedure is performed through the left chest. 21 A success rate of 68.8% at a mean follow‐up of 24.9 + 11.8 months in 51 patients was achieved.
The Amsterdam group reported on a series of 66 patients with persistent AF, 88% of which were in sinus rhythm at 5 years, though only 55% of them had not experienced any recurrences of AF during the 5‐year follow‐up. 22
Our surgical approach is very similar to those performed by the high‐volume groups in Utrecht, the Netherlands, and St. Helena, California using biatrial ablation with LAA closure via bilateral thoracoscopy. The group from Utrecht reported on 82 consecutive patients with a success rate of 60% at 4 years. 16 Half of the patients were persistent and long‐standing persistent AF. The group from California recently published their results analyzing over 450 patients. 15 Almost all of the patients had nonparoxysmal AF. At 3 years, freedom from AF was 72%. Total complications (6.1%) included permanent pacemakers (1.5%), strokes (0.4%), procedure conversion (0.4%), phrenic nerve palsy (1.3%) reintubation (0.2%), and death in (1.3%). A systematic safety analysis of hybrid procedures from Vos et al. reported low 30‐day postoperative major complications of 3.4% including pericardial effusion (0.2%), hemothorax (1.3%), pacemaker (1.8%), stroke (0.2%), and death (0.2%). 23
At our institution, we perform biatrial epicardial lesions via bilateral thoracoscopy. After 3 months, endocardial catheter mapping and ablation are performed. The ultimate goal of this two‐stage approach is to create the Cox‐Maze IV lesion set (Figure 3A). We start the procedure with a right‐sided thoracoscopy. Typically, two 5 mm and one 12 mm thoracoscopic ports are placed. Occasionally, there is a need for an additional port for better exposure and assistance. The pericardium is opened longitudinally anterior to the phrenic nerve. The oblique sinus is then entered between the inferior vena cava and the right inferior pulmonary vein. The transverse sinus is also dissected between the right superior pulmonary vein and the right pulmonary artery behind the superior vena cava. This allows for right‐sided PVI on the antrum of the left atrium with a bipolar radiofrequency (RF) clamp. The creation of the PVI lesion on the antrum of the left atrium decreases the risk of developing pulmonary vein stenosis. Partial roof and floor lesions are then created through the transverse and oblique sinuses with a linear unipolar RF ablation device. Subsequently, linear lesions from the SVC to IVC are created in addition to a curvilinear lesion toward the apex of the right atrial appendage (Figure 3B). Left thoracoscopy is then performed using three thoracoscopic ports (two 5 mm and one 12 mm). Left‐sided PVI is performed with a bipolar radiofrequency ablation clamp. Roof and floor lesions are completed with a linear monopolar RF device to connect to the right‐sided lesions. This creates a “box” lesion set around pulmonary veins with entry and exit block assessed from sensing and pacing within the isolated posterior wall. Subsequently, a linear lesion from the “box” toward the LAA is created (Figure 3A). The LAA is closed with an epicardial clip. Bilateral chest tubes are placed in the pleural spaces.
Figure 3.
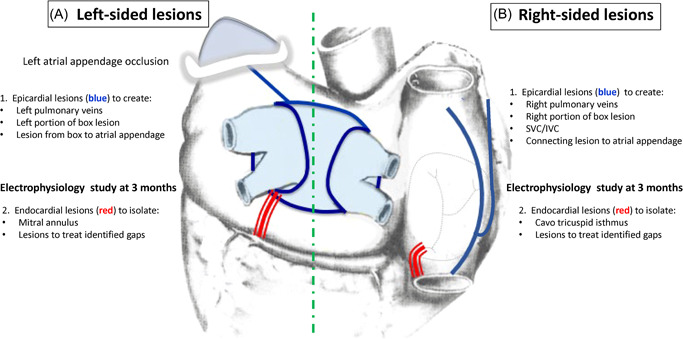
Final lesion set of our hybrid modified totally thoracoscopic maze approach with second stage catheter ablation. The lesions performed thoracoscopically in the first stage are shown in blue ((A) Left‐sided and (B) Right‐sided). The right‐sided lesions are placed first to isolate the pulmonary veins and create superior and inferior portions of a box lesion. Inferior and superior vena cave lesions are placed with a connecting lesion to the right atrial appendage. The left sided‐lesions are then placed to isolate the pulmonary lesions and connect box lesions to the right sided‐box lesions. A lesion is placed from the left portion of the superior box lesion to the left atrial appendage. The appendage is then occluded with a clip device. After 3 months, a catheter ablation is performed to add the endocardial lesions shown in red. Mapping is also performed and any additional ablation lesions are performed to close identified gaps in the surgically placed lesions.
Typically, patients are monitored for 1 day in the ICU before transfer to the stepdown unit.
The second stage of the procedure at 3 months includes endocardial mapping and ablation with creation of a cavotricuspid isthmus lesion, and a mitral annular to left inferior pulmonary vein line (and coronary sinus ablation when necessary) or an anterior mitral valve lesion roof line to prevent development of peri‐mitral flutter (Figure 3A,B). If necessary, “touch‐up” of the endocardial aspect of the epicardially placed lesions is performed.
We perform extensive rhythm monitoring in our patients with implantable devices when available or 14‐day patch monitor. All patients have continuous rhythm monitors during the inpatient period. Post‐discharge, rhythm monitoring is performed at 3, 6, and 12 months and annually thereafter. In our first 20 patients, the freedom from AF at 12 months was 95% (including six patients who declined follow‐up endocardial mapping and ablation). In the 14 patients who complete both stages, freedom from AF at 1 year was 100% and freedom from AF off antiarrhythmic drugs was 85%. Complications occurred in four patients (one phrenic nerve palsy, two pericardial effusions requiring drainage, and one pleural effusion requiring drainage).
3. CONCLUSION
The hybrid totally thoracoscopic maze approach is a complex procedure but is highly effective. The results of this procedure at our institution and others are superior to catheter ablation alone. This approach is also more effective than the Convergent procedure which reported a 53.5% freedom from AF off antiarrhythmic drugs at 1 year in a randomized clinical trial. Our modified hybrid thoracoscopic maze is one of multiple minimally invasive techniques to treat AF. We believe the critical factor to these approaches is adherence to performing as many of the Cox‐Maze IV lesions as possible set. A surgery and electrophysiology team approach to AF enhances the best possible patient outcomes using a staged hybrid approach.
Churyla A, Passman R, McCarthy PM, Kislitsina ON, Kruse J, Cox JL. Staged hybrid totally thoracoscopic maze and catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2022;33:1961‐1965. 10.1111/jce.15594
Disclosure Dr. Churyla: Atricure: consultant. Dr. Passman: Abbott: Research support and Advisory Board; American Heart Association: Research support; Janssen: Advisory board; Medtronic: Advisory board; UpToDate: Royalties. Dr. McCarthy: Edwards Lifesciences: Speaker, Royalties; Atricure, Medtronic: speaker; Abbott: PI for REPAIR MR (unpaid) egnite: Advisory board. Dr. Cox: Adagio Medical: Co‐founder, Board of Directors, Stockholder, Consultant; Atricure: Stockholder, Consultant. Other authors: No disclosures.
REFERENCES
- 1. Kornej J, Börschel CS, Benjamin EJ, Schnabel RB. Epidemiology of atrial fibrillation in the 21st century. Circ Res. 2020;127(1):4‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Piccini JP, Hammill BG, Sinner MF, et al. Clinical course of atrial fibrillation in older adults: the importance of cardiovascular events beyond stroke. Eur Heart J. 2014;35(4):250‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. DeLurgio DB, Ferguson E, Gill J, et al. Convergence of epicardial and endocardial RF ablation for the treatment of symptomatic persistent AF (CONVERGE trial): rationale and design. Am Heart J. 2020;224:182‐191. [DOI] [PubMed] [Google Scholar]
- 4. Rankin JS, Lerner DJ, Braid‐Forbes MJ, McCrea MM, Badhwar V. Surgical ablation of atrial fibrillation concomitant to coronary‐artery bypass grafting provides cost‐effective mortality reduction. J Thorac Cardiovasc Surg. 2020;160(3):675‐686. [DOI] [PubMed] [Google Scholar]
- 5. Badhwar V, Rankin JS, Ad N, et al. Surgical ablation of atrial fibrillation in the United States: trends and propensity matched outcomes. Ann Thorac Surg. 2017;104(2):493‐500. [DOI] [PubMed] [Google Scholar]
- 6. Malaisrie SC, McCarthy PM, Kruse J, et al. Ablation of atrial fibrillation during coronary artery bypass grafting: late outcomes in a Medicare population. J Thorac Cardiovasc Surg. 2021;161(4):1251‐1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McCarthy PM, Davidson CJ, Kruse J, et al. Prevalence of atrial fibrillation before cardiac surgery and factors associated with concomitant ablation. J Thorac Cardiovasc Surg. 2019;159(6):2245‐2253. [DOI] [PubMed] [Google Scholar]
- 8. Calkins H, Hindricks G, Cappato R, et al. HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. Heart Rhythm. 2017;201714(10):e445‐e494. [DOI] [PubMed] [Google Scholar]
- 9. Whitlock RP, Belley‐Cote EP, Paparella D, et al. Left atrial appendage occlusion during cardiac surgery to prevent stroke. N Engl J Med. 2021;384(22):2081‐2091. [DOI] [PubMed] [Google Scholar]
- 10. Soltesz EG, Dewan KC, Anderson LH, Ferguson MA, Gillinov AM. Improved outcomes in CABG patients with atrial fibrillation associated with surgical left atrial appendage exclusion. J Card Surg. 2021;36(4):1201‐1208. [DOI] [PubMed] [Google Scholar]
- 11. Caliskan E, Sahin A, Yilmaz M, et al. Epicardial left atrial appendage AtriClip occlusion reduces the incidence of stroke in patients with atrial fibrillation undergoing cardiac surgery. Europace. 2018;20(7):e105‐e114. [DOI] [PubMed] [Google Scholar]
- 12. Ad N, Holmes SD, Friehling T. Minimally invasive stand‐alone Cox maze procedure for persistent and long‐standing persistent atrial fibrillation: perioperative safety and 5‐year outcomes. Circ Arrhythm Electrophysiol. 2017;10(11):e005352. [DOI] [PubMed] [Google Scholar]
- 13. Henn MC, Lancaster TS, Miller JR, et al. Late outcomes after the Cox maze IV procedure for atrial fibrillation. J Thorac Cardiovasc Surg. 2015;150(5):1168‐1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. DeLurgio DB, Crossen KJ, Gill J, et al. Hybrid convergent procedure for the treatment of persistent and long‐standing persistent atrial fibrillation. Circ Arrhythm Electrophysiol. 2020;13(12):e009288. [DOI] [PubMed] [Google Scholar]
- 15. Dunnington GH, Pierce CL, Eisenberg S, et al. A heart‐team hybrid approach for atrial fibrillation: a single‐centre long‐term clinical outcome cohort study. Eur J Cardiothorac Surg. 2021;60(6):1343‐1350. [DOI] [PubMed] [Google Scholar]
- 16. Vos LM, Bentala M, Geuzebroek GS, Molhoek SG, van Putte BP. Long‐term outcome after totally thoracoscopic ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2020;31(1):40‐45. [DOI] [PubMed] [Google Scholar]
- 17. Muneretto C, Bisleri G, Bontempi L, Curnis A. Durable staged hybrid ablation with thoracoscopic and percutaneous approach for treatment of long‐standing atrial fibrillation: a 30‐month assessment with continuous monitoring. J Thorac Cardiovasc Surg. 2012;144(6):1460‐1465. [DOI] [PubMed] [Google Scholar]
- 18. Muneretto C, Bisleri G, Rosati F, et al. European prospective multicentre study of hybrid thoracoscopic and transcatheter ablation of persistent atrial fibrillation: the HISTORIC‐AF trial. Eur J Cardiothorac Surg. 2017;52(4):740‐745. [DOI] [PubMed] [Google Scholar]
- 19. Maesen B, Pison L, Vroomen M, et al. Three‐year follow‐up of hybrid ablation for atrial fibrillation. Eur J Cardiothorac Surg. 2018;53(suppl 1):i26‐i32. [DOI] [PubMed] [Google Scholar]
- 20. Krul SP, Pison L, La Meir M, et al. Epicardial and endocardial electrophysiological guided thoracoscopic surgery for atrial fibrillation: a multidisciplinary approach of atrial fibrillation ablation in challenging patients. Int J Cardiol. 2014;173(2):229‐235. [DOI] [PubMed] [Google Scholar]
- 21. de Asmundis C, Varnavas V, Sieira J, et al. Two‐year follow‐up of one‐stage left unilateral thoracoscopic epicardial and transcatheter endocardial ablation for persistent and long‐standing persistent atrial fibrillation. J Interv Card Electrophysiol. 2020;58(3):345‐346. [DOI] [PubMed] [Google Scholar]
- 22. Driessen AHG, Berger WR, Chan Pin Yin DRPP, et al. Electrophysiologically guided thoracoscopic surgery for advanced atrial fibrillation. J Am Coll Cardiol. 2017;69(13):1753‐1754. [DOI] [PubMed] [Google Scholar]
- 23. Vos LM, Kotecha D, Geuzebroek GSC, et al. Totally thoracoscopic ablation for atrial fibrillation: a systematic safety analysis. Europace. 2018;20(11):1790‐1797. [DOI] [PMC free article] [PubMed] [Google Scholar]


