Abstract
Background and Aim
Hypermobile Ehlers–Danlos syndrome (hEDS) and the hypermobility spectrum disorders (HSD) can be challenging to diagnose and manage. Gastrointestinal symptoms and disorders of gut‐brain interaction are common in this cohort and multifactorial in origin. The primary aim of this review is to arm the gastroenterologist with a clinically useful understanding of HSD/hEDS, by exploring the association of gastrointestinal disorders with HSD/hEDS, highlighting current pathophysiological understanding and providing a pragmatic approach to managing these patients.
Methods
Literature relevant to the gastrointestinal system and hypermobile Ehlers–Danlos syndrome was systematically searched, critically appraised, and summarized.
Results
Diagnosis is based upon clinical criteria and a genetic basis is yet to be defined. The prevalence of many gut symptoms, including abdominal pain (69% vs 27%, P < 0.0001), postprandial fullness (34% vs 16%, P = 0.01), constipation (73% vs 16%, P < 0.001), and diarrhea (47% vs 9%, P < 0.001) are significantly higher in HSD/hEDS compared with non‐HSD/hEDS individuals. Disorders of gut‐brain interaction are also common, particularly functional dyspepsia. The pathophysiology of gut symptoms is poorly understood but may involve effects of connective tissue laxity and its functional consequences, and the influence of autonomic dysfunction, medication and comorbid mental health disorders. Awareness is the key to early diagnosis. Management is limited in evidence‐base but ideally should include an integrated multidisciplinary approach.
Conclusions
HSD/hEDS is a multisystemic disorder in which gastrointestinal symptoms, particularly related to disorders of gut‐brain interaction are common. Deficiencies in knowledge regarding the pathophysiological processes limit evidence‐based interventions and remain important areas for future research.
Keywords: autonomic dysfunction, disorders of gut‐brain interaction, functional dyspepsia, hypermobile Ehlers–Danlos syndrome, integrated care, pelvic floor dysfunction
Introduction
Ehlers–Danlos syndrome (EDS) was first recognized in the time of Hippocrates in the fourth century BC. Appreciation of its heterogeneity continues to evolve to this day. EDS is the most common non‐inflammatory connective tissue disorder featuring joint hypermobility, with the hypermobile EDS (hEDS) subtype representing 80–90% of the burden of disease. 1 , 2 hEDS is now recognized as part of the “hypermobility spectrum disorders” (HSD), which are characterized by varying articular and extra‐articular involvement and impact on quality of life. The vast majority of those affected are female, gastrointestinal symptoms are very common and healthcare utilization is high. 3 , 4 , 5 Many patients meet diagnostic criteria for disorders of gut‐brain interaction (DGBI), but the pathophysiological link between DGBI and HSD/hEDS is yet to be established beyond association. The primary aim of this review is to arm the gastroenterologist with a clinically useful understanding of HSD/hEDS, by exploring the association of gastrointestinal disorders with HSD/hEDS, highlighting current pathophysiological understanding and providing a pragmatic approach to managing these patients.
Methodology
In order to perform this narrative review, the published literature was systematically searched via PubMed, ProQuest and OVID using key words that included hypermobile Ehlers–Danlos syndrome, hEDS, joint hypermobility syndrome, gastrointestinal disorders, functional gut disorders, disorders gut‐brain interaction, functional dyspepsia, irritable bowel syndrome, constipation, diarrhea, rectal evacuatory dysfunction, autonomic function, and motility. Each subsection was additionally explored using targeted searching, for example, “eating disorder” and hypermobile Ehlers–Danlos Syndrome. Abstracts were appraised and relevant articles were then reviewed and analyzed in full. Additional studies were located via cross‐referencing. Studies in pediatric cohorts were not included.
Terminology and diagnostic criteria
A major hindrance to the general understanding of EDS has been its heterogeneity, in part related to the multiple classification systems used over the years. The current nosology and diagnostic criteria are defined by the 2017 International Classification of the Ehlers–Danlos Syndromes in which 13 variants are recognized (Table S1) although an additional subtype was added provisionally in 2018 and is referred to as classical‐like type 2 EDS. 6 , 7 Recognized genetic mutations simplify the diagnosis for nearly all subtypes. The exception is hEDS, where the genetic basis has not been established. 8 As a result, the diagnosis of hEDS relies on clinical features (Table 1). Central to the diagnosis of hEDS is the Beighton score, which evaluates joint hypermobility using established criteria (Fig. 1, Table 1). 9
Table 1.
New diagnostic criteria for hypermobile Ehlers–Danlos syndrome (hEDS) 7
|
Criterion 1: Must be met |
The Beighton score for generalized joint hypermobility |
|
|
Criterion 2: Two or more of the following features |
Feature A: Systemic manifestations of a more generalized connective tissue disorder |
At least five of the following must be present:
|
|
Feature B: Positive family history |
|
|
| Feature C: Musculoskeletal Complications | One of the following:
|
|
|
Criterion 3: All must be met |
|
Figure 1.
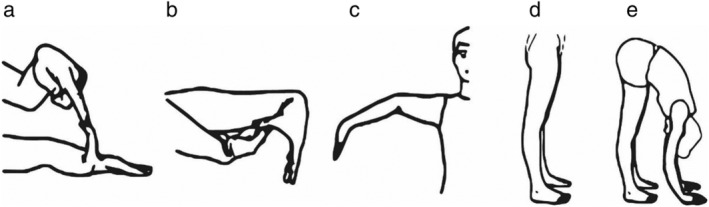
Beighton scoring system measures joint hypermobility on a 9‐point scale. Joints assessed (left to right) include (a) passive dorsiflexion of fifth finger ≥ 90° (one point per side); (b) passive apposition of the thumb to ipsilateral forearm (one point per side); (c) hyperextension of the elbow ≥ 10° (one point per side); (d) hyperextension of the knee ≥ 10° (one point per side); and (e) spinal assessment (one point if both palms reach the floor when bending over with knees locked in extension and feet together). Redrawn from Malfait et al. (2017) with permission.
Various terms have been used historically to describe this group of hypermobile patients, the most common being “joint hypermobility syndrome” (JHS), which includes patients who meet the current hEDS criteria, but also some who do not. To declutter the confusing nomenclature, the term “hypermobility spectrum disorder” (HSD) is now used as an umbrella term (Fig. 2). hEDS is considered to sit on the more severe end of the spectrum as it is associated with significant somatic complaints related to musculoskeletal manifestations such fibromyalgia (40%), chronic fatigue (38%), and pain (almost 100%) (acute and chronic, nociceptive, neuropathic, and nociplastic). 2 , 5 , 11 , 12 , 13 In addition, various non‐musculoskeletal manifestations can be present including, neurological (e.g. headaches), psychiatric and neurodevelopmental (e.g. mood disorders, anxiety, and sleep disturbances), cardiorespiratory (e.g. palpitations, chest pain, and dyspnea), autonomic (e.g. syncope, postural instability, and thermoregulatory difficulties), urogynecological (e.g. prolapse, urinary incontinence, and dyspareunia), and gastroenterological. 2 , 5 , 12 , 14 , 15 , 16 , 17 , 18 Inflammatory and systemic manifestations, postulated to relate to mast cell activation, are also reported. 19 Given the complexity of the historical nomenclature and in order to incorporate published data that have utilized previous terminology, the disorder will be referred to as “HSD/hEDS” (unless specifically describing hEDS) for this review. It is acknowledged that this will introduce some phenotypic and genotypic heterogeneity. Using this definition, it is estimated that the prevalence of HSD/hEDS is greater than 1:500. 20
Figure 2.
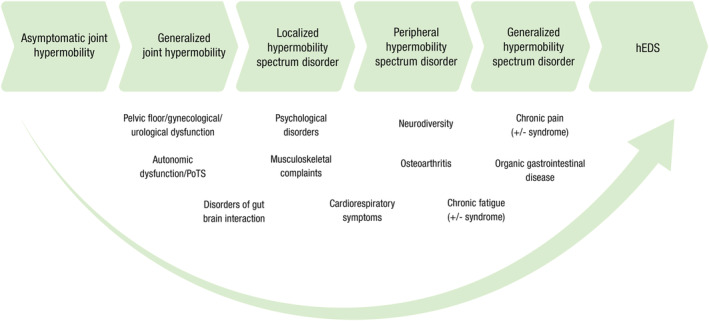
Spectrum of joint hypermobility. The horizontal arrows depict the spectrum of joint disease, ranging from asymptomatic, non‐syndromic joint hypermobility, progressing through the newly recognized hypermobility spectrum disorder with various combinations of musculoskeletal and non‐musculoskeletal manifestations (insufficient to meet the criteria for hEDS). Also depicted are the common manifestations contributing to the somatic complaints described. PoTS, postural orthostatic tachycardia syndrome.
Hypermobility spectrum disorders/hypermobile Ehlers‐Danlos syndrome and the gastrointestinal system
The association between gastrointestinal symptoms and HSD/hEDS was first described 15 years ago. 15 A vast array of symptoms occur significantly more often in HSD/hEDS compared with non‐HSD/hEDS patients (Fig. 3). Commonly, more than one gastrointestinal symptom is present. 5 , 21 , 22 A greater severity and extent of gastrointestinal involvement has been described in patients referred to gastroenterology clinics with a pre‐existing diagnosis of HSD/hEDS, compared with patients with features of HSD/hEDS but without a prior diagnosis, followed by those without any features of HSD/hEDS. 4
Figure 3.
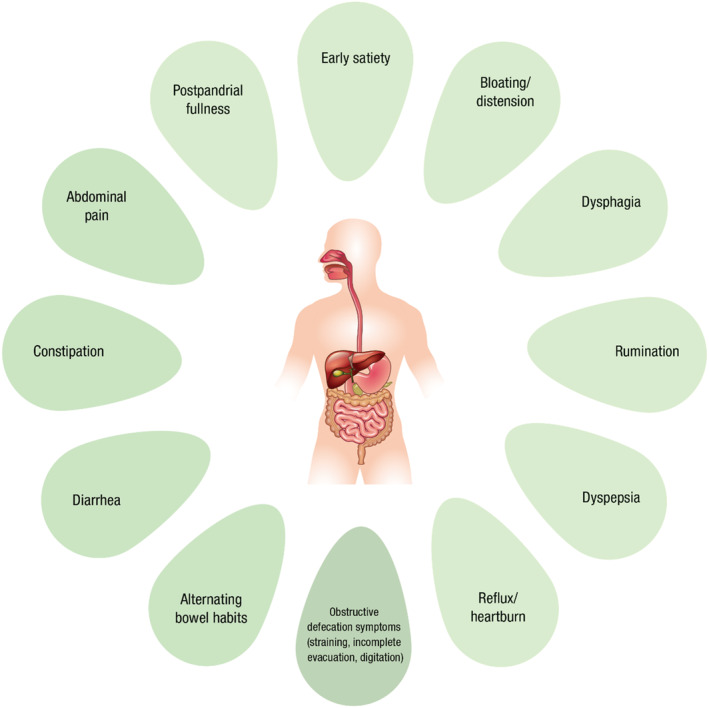
Various gastrointestinal symptoms have been reported to occur significantly more often in patients with HSD/hEDS compared with non‐HSD/hEDS controls. See Tables 2 and 3.
Despite many studies, the true prevalence of gastrointestinal disorders in this cohort is difficult to assess due to varying nomenclature and methodological biases, particularly selection bias, in the studies published. Moreover, the reported prevalence of gastrointestinal symptoms varies widely depending on whether it is derived from population‐based studies, support groups, non‐gastroenterological specialist (genetics, cardiology and rheumatology) clinics (Table 2) and gastroenterology clinics (Table 3). 4 , 14 , 23 , 24 , 25 , 26 , 27 , 28 Nevertheless, gastrointestinal symptoms have been associated with impairment of quality of life in patients with HSD/hEDS in each of the study settings. 3 , 4 , 5 , 22 , 27
Table 2.
Overview of gastrointestinal symptoms observed in studies of patients with hypermobilility spectrum disorder/hypermobile Ehlers–Danlos Syndrome (HSD/hEDS) (excluding studies in gastroenterology specialist clinics)
| Author, year | Classification utilized | Study type | Study setting and number of patients | Symptom prevalence |
|---|---|---|---|---|
| Castori et al., 2010 3 | Villefranche criteria + Brighton criteria | Observational cross‐sectional |
General genetics outpatients; Italy n = 21 (18 female) |
Reflux/heartburn (57%); dyspepsia (67%); abdominal pain (62%); constipation/diarrhea (33%); hernias (abdominal) (5%) |
| Castori et al., 2011 25 | Villefranche criteria + Brighton criteria | Observational cross‐sectional |
Multidisciplinary joint hypermobility clinic; Italy Cumulative prevalence of symptoms according to age reported—based on patient recall n = 50 (44 female) |
By age ≥ 40 years: reflux/heartburn (74%); abdominal pain (68%); chronic gastritis (48%); alternating bowel habits (72%); hernias (abdominal) (20%) |
| Mastoroudes et al., 2013 31 | Revised 1998 Brighton criteria | Observational case–control |
Hypermobility clinic; UK n = 60 HSD/hEDS; 60 age‐matched and sex‐matched controls from medical staff |
Obstructive defecation symptoms: 23% vs 5% controls (P = 0.007); straining: 62% (P < 0.001); incomplete evacuation: 63% (P < 0.001); digitation: 33% (P = 0.001); constipation: 72% (P < 0.001) |
| Zeitoun et al., 2013 28 | Villefranche criteria | Observational cross‐sectional |
EDS patient support group; France n = 134 (122 female); 108 HSD/hEDS 64% survey response rate |
Nausea (71%); reflux/heartburn (69%); dysphagia (63%); regurgitation (69%); postprandial fullness (67%); belching (71%); epigastric pain (71%); constipation (36%); IBS‐like symptoms (48%) |
| Castori et al., 2014 24 | Villefranche criteria | Observational cross‐sectional | Pedigrees were selected from two Italian outpatient clinics for EDS and inherited connective tissue disorders. 23 families with HSD/hEDS (n = 82) | Reflux/heartburn (34%); chronic gastritis (23%); abdominal pain (20%); constipation (28%) |
| Nelson et al., 2015 14 | Villefranche criteria + Brighton criteria | Observational retrospective |
Medical Genetics Clinic (1994–2013) 687 EDS patients (n = 471 HSD/hEDS) No control group included |
HSD/hEDS vs other EDS: constipation: 42% vs 29% (P = 0.02); nausea: 44% vs 37%; reflux/heartburn: 38% vs 36%; vomiting: 25% vs 22%; waterbrash: 1% vs 2%; dysphagia: 11% vs 12%; regurgitation: 4% vs 6%; postprandial fullness: 7% vs 3%; bloating: 17% vs 10%; dyspepsia: 11% vs 7%; abdominal pain: 56% vs 56%; diarrhea: 23% vs 17%; fecal urgency: 1.5% vs 2.8% |
| Fikree et al., 2017 21 | Villefranche criteria + Brighton criteria | Cross‐sectional, double‐blinded, case–control |
University students (without prior diagnosis of HSD/hEDS); UK HSD/hEDS: n = 74 (48 female) Controls: n = 88 |
HSD/hEDS vs controls: postprandial fullness: 34% vs 16% (P = 0.01); early satiety: 32% vs 17% (P = 0.03); bloating: 26% vs 23% (P = 0.59); functional dyspepsia: 39% vs 23% controls (P = 0.02); No differences in lower gastrointestinal symptoms (IBS, constipation, diarrhea, alternating bowel habit, ≤ 4 bowel motions/week). |
| Inayet et al., 2018 27 | Not specified | Observational cross‐sectional, case–control |
Cardiology and rheumatology clinics; UK 45 Marfan syndrome and 45 HSD/hEDS (33 female) 90 age‐matched and sex‐matched controls |
HSD/hEDS vs controls: functional abdominal pain: 69% vs 27% (P < 0.001); functional constipation: 73% vs 16% (P < 0.001); functional diarrhea: 47% vs 9% (P < 0.001); IBS: 33% vs 7% (P = 0.0014); functional heartburn: 47% vs 13% (P = 0.0011); functional dyspepsia: 38% vs 9% control (P = 0.029); functional bloating/distension: 31% vs 7% (P = 0.006) |
| Nee et al., 2019 23 | Not specified (Villefranche and Berlin nomenclature accepted) | Observational, cross‐sectional |
Members of local and national Marfan and EDS societies; US EDS: n = 1804 HSD/hEDS, n = 1325); MFS: n = 600); 94% female |
HSD/hEDS vs other subtypes of EDS: aerophagia: 24% vs 26% (P = 0.35); bloating: 13% 12%; heartburn: 32% vs 37% (P = 0.04); dysphagia: 29% vs 28%; IBS: 58% vs 56%; functional constipation: 8% vs 7%; diarrhea: 0.5% vs 1.3%; functional dyspepsia: 55% vs 56% |
| Alomari et al., 2020 36 | 2017 International classification of EDS | Observational retrospective |
Genetics clinic; US n = 218 (198 female) |
63% gastrointestinal symptoms at hEDS diagnosis (63%); abdominal pain (50%); nausea (50%); constipation (45%); diarrhea (38%); heartburn (36%); belching/bloating (27%); vomiting (26%); IBS‐like symptoms (22%); dysphagia (14%); fecal incontinence (6%) |
| Lam et al., 2020 5 | Not specified | Case–control |
EDS support group; UK HSD/hEDS: n = 603 Age‐matched and sex‐matched controls: n = 1994 Mean age: 39 years, 96% female 20% survey response rate |
HSD/hEDS vs control: functional dyspepsia: 57% vs 9% (P < 0.0001); IBS: 54% vs 8% (P < 0.001); functional dysphagia: 42% vs 4% (P < 0.001); rumination: 31% vs 5% (P < 0.001); functional constipation: 12% vs 10%; functional diarrhea: 5% vs 4.6%; functional anorectal disorders: 53% vs 9% (P < 0.001) |
| Tai et al., 2020 71 | Not specified | Observational cross‐sectional, case–control |
EDS support group: UK Established HSD/hEDS and hypermobility spectrum disorder: n = 616 (573 female); mean age 39 years PoTS n = 231 20% survey response |
PoTS vs non‐PoTS: functional esophageal disorders: 66% vs 50% (P < 0.001); functional heartburn: 31% vs 21% (P = 0.007); functional dysphagia: 51% vs 37% (P = 0.001); functional gastroduodenal disorders: 75% vs 67% (P = 0.04); functional dyspepsia: 68% vs 50% (P < 0.001); postprandial distress syndrome: 63% vs 42% (P < 0.001); epigastric pain syndrome: 40% vs 28% (P = 0.002); functional bowel disorders: 89% vs 91% (P = 0.5); IBS: 59% vs 51%; functional diarrhea: 3% vs 7% (P = 0.01); functional anorectal disorders: 60% vs 49% (P = 0.01) |
Villefranche criteria. 1
Table 3.
Overview of the key findings generated from studies based on patients attending gastroenterology clinics
| Author | Study type, clinic setting | Patient cohort | Assessment | Key findings |
|---|---|---|---|---|
| Mohammed, et al., 2010 50 | Retrospective cohort Gastroenterology clinic | Intractable constipation and rectal evacuatory dysfunction: n = 200 (joint hypermobile n = 65; 179 female, median age 53 years) |
|
Cases vs controls:
Hypermobile vs non‐hypermobile group:
|
| Zarate et al., 2010 26 | Retrospective neuro‐gastroenterology clinic | 129 consecutive newly referred patients stratified by joint hypermobility status; subset of 21 patients confirmed with HSD/hEDS |
|
49% (63/129) had generalized joint hypermobility:
|
| Fikree et al., 2014 4 |
Prospective cross‐sectional General gastroenterology clinic |
Consecutive new referrals (16–70 years) stratified by HSD/hEDS status (Brighton criteria) (Total n = 552; HSD/hEDS = 372 Non‐HSD/hEDS: n = 80 HSD/hEDS patients referred from rheumatology clinic (positive control): n = 44 |
|
Undiagnosed HSD/hEDS 33% (n = 180/552):
Non‐HSD/hEDS vs new HSD/hEDS vs previously diagnosed HSD/hEDS:
|
| Fikree et al., 2015 22 |
Prospective case–control (functional and organic diagnosis) Secondary gastroenterology clinic |
Consecutive referrals of patients with gastrointestinal symptoms, no prior HSD/hEDS diagnosis Total n = 641 (Organic disease controls n = 306 vs DBI cases n = 336; 378 female; mean age 42 years) |
|
DGBI vs organic disease controls:
Adjusted OR (age, gender) for HSD/hEDS:
DGBI‐HSD/hEDS vs non‐HSD/EDS:
|
| Fikree et al., 2017 43 |
Retrospective, observational Neuro‐gastroenterology clinic |
Consecutive HSD/hEDS patients referred to gastrointestinal physiology unit for assessment of reflux or dysphagia HSD/hEDS: n = 30 (28 female; median age 30 years)—further stratified by PoTS status; non‐HSD/hEDS dysphagia: n = 98 (56 female) Reflux controls: n = 108 (61 female) |
|
HSD/hEDS vs non‐HSD/hEDS:
PoTS vs non‐PoTS‐HSD/hEDS:
|
| Menys et al., 2017 54 |
Pilot feasibility Tertiary neuro‐gastroenterology clinic |
HSD/hEDS with Postprandial distress (Rome III): n = 9 Healthy controls: n = 9 |
|
HSD/hEDS vs control:
|
| Zweig et al., 2018 30 | Retrospective review of prospectively collected data at neuro‐gastroenterology clinic | 228 IBS (Rome III) patients (67% female); stratified by joint hypermobility status |
|
Joint hypermobility
|
| Carbone et al., 2021 55 |
Prospective case–control University hospital clinic |
Functional dyspepsia (Rome III): n = 39 stratified by HSD/hEDS status using Brighton classification Healthy controls: n = 15 |
|
HSD/hEDS vs controls
|
| Carbone, et al. 2022 61 |
Retrospective recruitment, prospective evaluation of joint hypermobility Gastroenterology clinic |
62 patients with preexisting functional dyspepsia n = 62 (68% female, age 44 years, BMI 22 kg/m2) |
|
55% HSD/hEDS criteria met vs 39% no joint disease/syndrome vs 6% “other” joint disorder HSD/hEDS vs non‐HSD/hEDS
|
Association with disorders of gut‐brain interaction
Criteria for DGBI are met frequently in patients with HSD/hEDS, in both the community and hospital settings. For example, 94% in HSD/hEDS survey respondents from the UK EDS support group fulfilled criteria for DGBI compared with 47% of the control population (P < 0.0001) and 91% in rheumatology‐referred HSD/hEDS patients compared with 48% of non‐HSD/hEDS patient referrals. 4 , 5 Moreover, patients seeking gastroenterological review for DGBI were more likely to meet diagnostic criteria for HSD/hEDS than those presenting with organic disorders (39% vs 28%, P = 0.002). 22
Dyspeptic symptoms are common and the diagnosis of functional dyspepsia by both Rome III and IV criteria appears to be more common in HSD/hEDS compared with controls in both gastroenterology (OR 2.08, CI 1.25–3.46 for functional gastroduodenal disorders, P = 0.005), and non‐gastroenterology hospital clinics (38% vs 9%, P = 0.029), support groups (57% vs 9%, P < 0.0001) and the general population (39% vs 23%, P = 0.02). 5 , 21 , 22 , 27 There appears to be no difference in the type or patterns of symptoms in patients with functional dyspepsia with or without HSD/hEDS. 29
The prevalence of other DGBI in the HSD/hEDS population have been inconsistently assessed (Tables 2 and 3). Irritable bowel syndrome (IBS) has a similar prevalence in HSD/hEDS patients referred to gastroenterology clinics (OR 1.34, CI 0.90–2.00, P = 0.15) 22 and in the general population examined for gut symptoms and hypermobility, 21 although studies conducted through support groups and non‐gastroenterology clinics have found IBS to be generally more common than in the non‐HSD/hEDS population (54% vs 8%, P = 0.0001 in support group; 73% vs 16%, P = 0.001 in non‐gastroenterology clinic setting). 5 , 27 Zweig et al. reported a higher prevalence of joint hypermobility (but not HSD/hEDS) in a cohort of constipation‐predominant IBS compared with diarrhea‐predominant IBS (58% vs 35%, P = 0.008) and found those with IBS and joint‐hypermobility (not HSD/hEDS) were more likely to have concomitant postprandial distress (72% vs 49%, P = 0.007). 30 The data are even less clear when considering other functional bowel disorders, including functional constipation and functional diarrhea. 5 , 21 , 22 , 25 Similarly, the prevalence of functional anorectal disorders is variably reported 5 , 22 , 27 , 31 with, for example, no differences in patients referred to gastroenterology clinics with gastrointestinal symptoms (OR 1.79, CI 0.97–3.30, P = 0.06) 22 through to a much greater prevalence using Rome IV criteria in the UK support group (53% vs 9%, P < 0.0001). 5
Importantly, the presence of HSD/hEDS with DGBI appears to be associated with greater overall impact in terms of healthcare utilization, quality of life, somatic symptoms and the extent of gastrointestinal involvement, compared with respondents meeting criteria for DGBI alone. 5 , 22 Individuals with comorbid HSD/hEDS and DGBI report more frequent experiences of pain (23% vs 12%, P = 0.01), worse pain‐related quality of life scores (45 vs 63.5, P = 0.004), comorbid diagnosis of fibromyalgia (11% vs 3%, P = 0.01), higher somatization scores (13 vs 10, P < 0.001) and higher anxiety scores (0.50 vs 0.30, P = 0.01) compared with non‐HSD/hEDS DGBI patients. 22
Organic gastrointestinal disease
There is a paucity of studies exploring associations between HSD/hEDS with organic gastrointestinal conditions although potential links have been identified.
Celiac disease
The potential association between celiac disease and HSD/hEDS was initially proposed after 5 of 31 Italian patients with HSD/hEDS (16%) had celiac disease, considerably more than might be anticipated from the background prevalence of about 1%. 32 Subsequent interrogation of a large population‐based Swedish registry identified a significant association of HSD/hEDS with histologically proven celiac disease (14 vs 9 per 100 000 person‐years with a hazard ratio of 1.49, 95% CI, 1.07–2.07, P = 0.018). 33
Crohn's disease
Joint hypermobility (using the Beighton score alone) has been observed more frequently in patients with Crohn's disease with 29 of the 41 Crohn's patients (71%) having joint hypermobility compared with 10 of the 28 patients (36%) with ulcerative colitis (P = 0.006) and 17 of the 67 age‐matched and sex‐matched healthy controls (25%) (P < 0.0001). 34 Furthermore, the prevalence of HSD/hEDS (using the Brighton criteria) followed similar trends (12% in Crohn's disease vs 4% in ulcerative colitis [OR 3.75, 95% CI: 0.41–34.0]). 34 In another cohort, HSD/hEDS was present in 8 of 25 patients with Crohn's disease (32%) and 8 of 38 patients (21%) with ulcerative colitis. 22
Other gastrointestinal diseases
There are no signals for an increased risk of gastric or colorectal neoplasia or of complications related to diverticular disease in patients with HSD/hEDS although formal studies are lacking. The prevalence of diverticular disease, similarly, has not been systematically assessed. In the only relevant report, a study of issues associated with colonoscopy such as safety and post procedure pain, 22 of 200 patients met criteria for HSD and that sub‐group had a similar prevalence of polyps (27% vs 41%, respectively; P = 0.2) and diverticulosis (39% vs 36%, P = 0.7) as those without HSD. 35 Other studies have reported rates of diverticular disease of 10–13% and rates of polyps of 8–23%. However, these data are drawn from retrospective chart reviews of patients who were not systematically evaluated and so the generalizability of these figures is uncertain. 14 , 36
Liver disease
While “at‐risk” alcohol consumption has been reported to occur more often in patients with joint hypermobility (not hEDS specifically), there have been no data suggesting a higher incidence of chronic liver disease in the HSD/hEDS population. 37 , 38 One case–control study conducted from a rheumatology clinic reported an association between unconjugated hyperbilirubinemia from Gilbert's syndrome and hypermobile joints. 39 However, the reason these patients were referred to the rheumatology clinic in the first instance was not examined and the source of recruitment of the control group was not reported, which may limit the generalizability of the conclusions.
Pathophysiological contributors to gastrointestinal symptoms in HSD/hEDS
An understanding of the current status of the pathophysiological basis for gastrointestinal and other symptoms in patients with HSD/hEDS is valuable in counseling the patients. There are multiple hypotheses and potential explanations that largely fall into three categories—the anatomical effects of connective tissue laxity and weakness per se, their functional consequences, and the influence of non‐gastrointestinal issues that include autonomic dysfunction, medication effects or comorbid mental health disorders. The current evidence base supporting these proposed hypotheses remains limited.
Anatomical variation/abnormalities
A variety of anatomical abnormalities related to increased connective tissue laxity and weakness have been observed. 40 On first principles, it seems reasonable that such abnormalities might be associated with symptoms. Specific abnormalities include the following:
Hiatus hernia: The prevalence of hiatus hernia in HSD/hEDS has been variably reported, as high as 58%. 41 Other studies, however, have shown it to be similar to the background population (8–26% in HSD/hEDS vs 2–22% general population) suggesting hiatus hernia may not be the main mechanism responsible for the commonly reported symptom of reflux. 14 , 28 , 42 , 43 , 44 It has been postulated that there is laxity of the gastro‐hepatic and phreno‐esophageal ligaments in patients with HSD/hEDS, providing a basis for the reported association. This hypothesis is supported by the observed depletion of elastic fibers in those ligaments of patients with gastroesophageal reflux disease and hiatus hernia, although these patients were not assessed for underlying HSD/hEDS. 45
Visceroptosis : Abnormal connective tissue leading to altered fixation of viscera to the peritoneum has been implicated in the development of visceroptosis (defined as sinking of an organ below its normal position) of various organs in case reports of patients with HSD/hEDS. 46 , 47 , 48 , 49 The relationship between the structural change and clinical presentation has not been well‐defined.
Pelvic organ prolapse : This occurs about twice as often in patients with HSD/hEDS compared with non‐HSD/hEDS individuals. For example, in a case–control study of 60 females referred to a tertiary hypermobility clinic, 73% had clinically‐significant prolapse compared with 35% of the age‐matched and sex‐matched healthy controls (P < 0.001). 31 These individuals were also more likely to experience symptoms of obstructive defecation on questioning (23% vs 5%, P = 0.007). 31 Likewise, more patients with rectal evacuatory dysfunction reported a history of pelvic organ prolapse (with or without surgery) in the HSD/hEDS cohort compared with the non‐HSD/hEDS group (31% vs 17%, P = 0.04). 50 Objectively, anorectal anatomical abnormalities seen on proctography were more common in the HSD/hEDS group compared with the non‐HSD/hEDS cohort (86% vs 64%, P = 0.001), specifically for large functional rectoceles (28% vs 14%, P = 0.03) and extrinsic compression of the anterior rectal wall from an enterocele or the uterus (11% vs 1%, P = 0.006). Higher frequencies of reduced squeeze increment pressures (32% vs 19%, P = 0.05) and incomplete rectal evacuation (80% vs 59%, P = 0.004) compared with the non‐HSD/hEDS controls were also seen. No differences in rectal sensation or frequency of reduced anal resting tone were noted. 50 Fikree et al. also described a higher incidence of organ prolapse in their study, which increased statistically significantly by HSD/hEDS status. 4 Despite this, the clinical significance of these findings is difficult to interpret given the lack of control data and the relatively common finding of anatomical variations like prolapse in asymptomatic subjects. 50
Short‐segment intussusception: This is postulated to arise secondarily to altered tensile strength of hollow viscera leading to excessive visceral distension in combination with altered fixation of the viscera to the peritoneum in patients with HSD/hEDS. However, the prevalence of intussusception in both the HSD/hEDS and general populations remains unknown and the mechanistic relationship of short‐segment intussusception to gastrointestinal symptoms, such as abdominal pain and bloating, is poorly understood. There was no observed difference in the prevalence of rectal intussusception, which presumably has similar pathophysiological mechanisms, in the study of rectal evacuatory dysfunction in patients with and without HSD/hEDS (41% vs 39%, P = 0.76). 51
Dolichocolon (elongation or redundancy of the colon) : Such an anatomical variant may predispose patients to volvulus, abdominal pain or constipation. To date this has not been substantiated by evidence as a potential mechanism in HSD/hEDS. 40 , 52
Altered gastrointestinal tract function
There are several interrelated aspects to potential alteration of gastrointestinal function:
Altered compliance of the gastrointestinal tract wall and changes in mechanoreceptor function : Increased elasticity (compliance) of the gastrointestinal wall will manifest as increased distension from a given intraluminal force. Thus, theoretically, a given amount of luminal gas in a patient with altered connective tissue arising from HSD/hEDS may yield greater intestinal distension and subsequent mechanoreceptor stimulation than a person without HSD/hEDS. Because luminal wall stretch is a major stimulus to inducing pain and bloating, it might be anticipated that patients with HSD/hEDS will be more susceptible to symptom induction following gaseous distension. 53 Neither colonic compliance nor gut mechanoreceptor function in the hEDS population have been measured to test this hypothesis although no changes in gastric accommodation have been observed in HSD/hEDS studies of functional dyspepsia using MRI or intragastric barostat measures, arguing against this hypothesis. 54 , 55
Dysmotility : Following on from the aforementioned hypothesis, altered wall compliance/elasticity and mechanoreceptor function also influences gastrointestinal motility. Studies in murine and guinea pig models show that enteric neurons are activated or inhibited by luminal stretch with resultant motility changes. 56 This hypothesis is supported by studies in the IBS population following luminal gas infusion, in which objective abdominal distension and subjective reporting of abdominal symptoms appear to be more related to an altered motility response (poor gas transit) than to increased gas volume. 57 , 58 It is possible a similar process predominates in hEDS. In support of this, gastric MRI revealed altered motility in response to water ingestion in HSD/hEDS patients with functional dyspepsia compared with healthy controls. 54 Other studies of gastric sensorimotor function, compliance and emptying in small cohorts of patients with HSD/hEDS have otherwise revealed few specific abnormalities. 14 , 26 , 29 , 36 , 43 , 54 , 55 Similarly, colonic transit studies have also revealed no specific abnormalities in HSD/hEDS patients. 14 , 26 , 36 , 50
Visceral hypersensitivity: Direct alterations in neuronal function leading to visceral hypersensitivity have also been proposed as a contributor for symptoms in hEDS. A number of hypotheses have been proposed. First, tenascin‐X, a glycoprotein component of extracellular matrix for which a genetically‐driven deficiency, has been rarely linked with HSD/hEDS, plays a role in the neural control of colonic sensory and motor function. This has also recently been shown to play a role in upper gastrointestinal function. 59 , 60 TNX deficiency in mice has been shown to correlate with increased sensitivity of vagal afferent nerves to gastric distension and associated with accelerated gastric emptying. 60 Nevertheless, the correlation between gastric emptying and symptoms in functional dyspepsia has not been borne out in studies in non‐HSD/hEDS cohorts, which limits the conclusions that can be drawn. 61 Secondly, α‐2 adrenergic activity plays a role in visceral sensitivity in healthy volunteers and could potentially play a role in connective tissue disorders, although this has not been specifically studied in the HSD/hEDS population. 62 Thirdly, central sensitization, as seen with generalized, chronic widespread pain, will secondarily promote visceral hypersensitivity. 13 This hypothesis is supported by a greater prevalence of reflux hypersensitivity (high‐resolution manometry and pH manometry) in patients with upper gastrointestinal symptoms in those with and without HSD/hEDS (21% vs 5%, P = 0.01). 43
Altered vascular compliance: Venous pooling in the lower limbs has been described in HSD/hEDS related to altered connective tissue of blood vessels and has been proposed to contribute to cardiovascular and autonomic symptoms present in HSD/hEDS patients. Alterations in splanchnic circulation may also be expected and could contribute to the gastrointestinal symptoms experienced.
Non‐gastrointestinal mechanisms
HSD/hEDS is characterized by a number of non‐musculoskeletal manifestations (Figs 2 and 3) which can contribute to the patient's presentation to a gastroenterologist. Three important factors are proposed to contribute: autonomic dysfunction, effects of medications, and mental health disorders, including eating disorders.
-
Autonomic dysfunction: The autonomic nervous system plays a key role in maintaining homeostasis in the body, with roles in fluid balance, temperature regulation and blood pressure. Symptoms of autonomic dysfunction can include presyncope, orthostatic intolerance, chest pain, palpitations, thermoregulatory difficulties, and gastrointestinal complaints and are commonly reported in patients with HSD/hEDS. 63 , 64 , 65 For example, they are almost three times more likely than healthy controls to experience presyncopal symptoms (41% vs 15%) and experience orthostatic intolerance frequently (94% of HSD/hEDS in one study). 15 , 63 , 65
Cardiovascular autonomic dysfunction can include orthostatic hypotension, orthostatic intolerance, neurally‐mediated hypotension and postural orthostatic tachycardia syndrome (PoTS), the latter of which is commonly associated with HSD/hEDS. 66 PoTS is a heterogeneous syndrome that is manifested by a rapid increase in heart rate (> 30 bpm in adults) within 10 minutes of changing from recumbent to upright position without orthostatic hypotension. 67 Multisystemic involvement is common, with gastrointestinal symptoms (including nausea, irregular bowel movements, abdominal pain, bloating and constipation) the most frequent non‐cardiovascular symptoms reported. 68 , 69 The cause for these symptoms is likely to be multifactorial, but may be related to changes in splanchnic circulation, presence of small fiber neuropathy or altered vascular compliance related to the generalized tissue laxity in hEDS. 68 , 69 The prevalence of PoTS in HSD/hEDS cohorts ranges from 15% to 41%. 36 , 63 , 64 , 70 , 71 , 72 The prevalence of hEDS also appears to be higher in PoTS cohorts compared with the general population, with 31% of PoTS patients meeting the 2017 diagnositc criteria for hEDS. 72
The clinical association between HSD/hEDS and PoTS is noted, but their relationship remains unclear. There is some evidence that the copresence of the two disorders may represent a more severe disease phenotype. 36 , 43 , 70 , 71 For example, studies have shown that symptoms of gastroesophageal reflux and dysphagia are worse, esophageal hypomotility more marked and pathological gastroesophageal reflux disease more severe in those with comorbid HSD/hEDS and PoTS compared with HSD/hEDS alone. 43 Abnormal gastrointestinal motility is also more than five times likely, 36 and the burden of DGBI is greater. 71 Cohorts of patients with comorbid PoTS and HSD/hEDS appear to be younger than either alone, suggesting that the symptoms become apparent at an earlier age or perhaps present more severely leading to earlier diagnosis. 67 , 71
Medication effects: Patients with HSD/hEDS report high regular medication use, with opiates being potentially the most troublesome from a gastrointestinal perspective. Up to 92% of HSD/hEDS respondents in one study reported regular medication use of which analgesics were the most common. 12 Polypharmacy is also prevalent, with an average of three medications per patient reported and chronic opiate use seen in over one third of HSD/hEDS patients. 12 While Fikree et al.'s studies did not find any association between gastrointestinal symptoms or esophageal dysmotility and opiate use, the widespread actions of opiates on multiple aspects of gastrointestinal function and their established side effect profile including constipation, nausea, vomiting and pain sensitization means they cannot be ignored. 4 , 43 , 73 Other potential pharmaceutical contributors such as antidepressants, are used by 15–27% of HSD/hEDS patients. These medications also have vasoactive properties, effects on autonomic function and a range of gastrointestinal side effects. 4 , 65 , 74
Mental health contributors: There is a recognized increased prevalence of mental health disorders in patients with HSD/hEDS, including anxiety and depression. 38 , 75 , 76 , 77 In a retrospective survey of 391 patients with a diagnosis of mostly HSD (80%) or EDS (notably no hEDS), almost half of the respondents were affected by a psychiatric disorder and almost 30% described two or more simultaneous psychiatric diagnoses. 75 Significant associations were noted between gastrointestinal dysfunction and mood disorders (OR 2.07, 95% CI 1.33–3.25, P = 0.001), depression (OR 1.68, 95% CI 1.07–2.66, P = 0.026), somatoform disorders (OR 2.61, 95% CI 1.62–4.19, P < 0.001) and anxiety (OR 2.26, 95% CI 1.39–3.67, P < 0.001). In a long term population cohort study with 15 years of follow up, those patients with HSD/hEDS defined by Brighton criteria performed at the time of recruitment (29 of the 137 subjects) had a relative risk of panic disorder or agoraphobia 22 times greater than that of the non‐HSD/hEDS patients. 18 There is also increasing interest in the association between HSD and neurodevelopmental disorders, including attention‐deficit/hyperactivity‐disorder and autism spectrum disorder. 17
-
Disordered eating and HSD/hEDS: There are multiple reasons patients with HSD/hEDS might modify their eating patterns. Oral mucosal fragility, temporomandibular dysfunction, masticatory muscular problems and dental issues, such as poor dentition or overcrowding of the teeth may lead to altered oral intake. 2 , 78 , 79 Patients with hEDS may also experience enhanced interoception (heightened awareness of bodily information and stimuli), somatosensory amplification and have underlying chemosensory disorders (involving smell and taste changes), influencing oral intake and the development of food aversions. 79 Finally, and unrelated to HSD/hEDS itself, is that modification of diet is common in patients with DGBI, where food type and quantity is altered by perceived food intolerances as a strategy to minimize symptoms such as bloating. 80
Differentiating a primary eating disorder from disordered eating patterns related to the aforementioned factors can be challenging in patients with HSD/hEDS. 81 A weak association of eating disorders with EDS, based upon theory and case reports, is noted but formal studies are limited. 79 Awareness of such diagnostic challenges is critical to managing these patients who are often young females, with anorexia, low body weight and significant gastrointestinal symptoms. The consequences of weight loss and poor nutrition should also not been overlooked as these may worsen the natural history of hEDS by contributing to physical deconditioning, reduced bone mass, fatigue, and poor quality of life. 79 , 82
Management considerations
There is a general lack of evidence to guide therapeutic approaches for patients with HSD/hEDS. Indeed there is also no single approach that will fit all HSD/hEDS patients, given the various combinations of manifestations that may be present. Management is largely supportive in nature and symptom‐focused, and often recommended on the basis of prior clinical experience or using strategies adapted from management of “similar” patient groups. We have proposed a suggested “hEDS checklist” that could be followed in defining therapeutic strategies in patients with hEDS who are presenting with gastrointestinal symptoms (Table 4). Awareness of the multiple pathways by which such symptoms can arise is important in order to provide the best individualized treatment. If standard therapies fail, alternative etiologies for the symptoms should be considered.
Table 4.
Checklist for a patient presenting with gastrointestinal symptoms potentially associated with HSD/hEDS
| Actions | Notes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Screen patients for hEDS with 5‐point questionnaire |
|
||||||||||
| Exclusion of organic gastrointestinal conditions |
|
||||||||||
| Institute integrated management for DGBI symptoms |
Evidence for management specifically in HSD/hEDS lacking–attention to:
|
||||||||||
| Consider nutritional and dietary issues |
|
||||||||||
| Address extra‐intestinal manifestations—consider referral to appropriate healthcare professional |
|
||||||||||
| Pharmacological considerations |
|
||||||||||
| Support |
|
||||||||||
| Professional education and training |
|
Awareness of HSD/hEDS
The presentation of HSD/hEDS varies greatly between individuals and thus the diagnosis is often delayed, referred to as the “diagnostic odyssey.” 83 Greater awareness of HSD/hEDS among physicians may enable an earlier diagnosis and more timely uptake of integrated, multidisciplinary care providing holistic and expert support for the various systems potentially affected. This process is particularly important given that few centers offer multi‐specialty HSD/hEDS clinics. Additional benefits of this approach might include reduced healthcare utilization and costs associated with numerous medical consultations, investigations, unnecessary surgeries and other interventions, treatment regimens and time off work. It is also important to avoid the incorrect use of the label “hEDS.” Clear diagnostic criteria should be used and patient education facilitated by a sound working knowledge of the hypermobility spectrum disorders and their diagnostic limitations.
Integrated care
While evidence for timely, collaborative care is limited in the HSD/hEDS population, integrated care of patients with irritable bowel syndrome and other DGBI have been shown to improve health‐related quality of life, patient psychological wellbeing and other outcomes. 84 , 85 Given the complexity of the multisystemic issues that often present in patients with HSD/hEDS, care of highly symptomatic patients may require collaborative input from various health professionals including (but not limited to) general practitioners, physicians (e.g. gastroenterology, cardiology, and rheumatology), psychiatrists and psychologists, chronic pain specialists, dietitians, physiotherapists/exercise physiologists, and dentists.
Safety with surgery and endoscopy
Patients and doctors alike may express concern regarding the safety of endoscopic and surgical procedures in patients with connective tissue and autonomic problems. Indeed orthopedic complications are known to be higher in HSD/hEDS. However, in general, the risks of severe adverse procedural outcomes are low in HSD/hEDS. 40 General considerations, however, may include: anesthetic risks (including circulatory management in patients with autonomic dysfunction, temporomandibular joint subluxation/dislocation or cervical spine instability); history of bruising and tissue fragility; hyperextension/force on joints at risk of dislocation or subluxation when the patient is being mobilized; and the procedural risks themselves. 40 , 86
Symptoms will dictate the need for a colonoscopy and/or upper gastrointestinal endsocopy in many patients. The risk of perforation does not appear to be increased. This is in contrast to patients with vascular EDS in whom vascular and visceral perforation risk is high. 87 Procedural difficulty has been theorized to be more challenging due to the presence of hernias and increased laxity of the colon, but has not been verified in studies. 35 Indeed endoscopist‐reported difficulty and cecal intubation rates are not dissimilar between HSD and non‐HSD cohorts. 35 There does not appear to be a significantly greater rate of post‐procedural pain. 35 Increased risk of bleeding in association with colonoscopy has not been reported but should be assessed in the context of the patient's personal history of bleeding (which may be increased) and the planned procedure, particularly in those with mast cell activation syndrome. 88 Laboratory results are usually within normal range. 88
Familial screening
There are currently no formal guidelines on familial screening. However, family history is a component of the new diagnostic criteria and the syndrome is believed to be inherited in an autosomal dominant manner with incomplete penetrance, which makes screening family members a relevant consideration. The spectrum of the disorder means that family members may present with their own unique manifestations and remain undiagnosed despite meeting the criteria. As there is no diagnostic molecular marker known, referrals to genetics clinics are likely to be managed variably according to local practices and based on waitlists and availability. Recommendations regarding familial echocardiographic screening are best determined by the treating cardiologist as the understanding of the natural history of cardiovascular abnormalities in HSD/hEDS continues to evolve.
Future research directions
The key impediment to progress in improving the diagnosis and understanding of the clinical manifestations of this spectrum of disorders is the identification of the genetic basis (es) to HSD/hEDS. In the absence of such objective markers, evaluation of the more stringent 2017 International Classification of the EDS is needed in order to clarify the many areas of imprecision and to minimize inaccurate and somewhat emotive attribution of many illnesses to the underlying connective tissue disorder. The same applies to how gastrointestinal anatomy and physiology are altered in hEDS, and how (and if) these relate to the intestinal and extra‐intestinal manifestations observed in hEDS. Consideration of the complex interaction between the gut, brain, other organs and the environment (including medication), and how these may alter the susceptibility of a patient to the development of abdominal symptoms, also needs further consideration. Greater understanding of pathophysiological processes will then allow more targeted treatment strategies with integrated care to be studied and implemented.
Conclusions
All general gastroenterologists will encounter patients with (diagnosed or undiagnosed) HSD/hEDS. Recognition of such patients and a general understanding of the implication of such a disorder will provide the opportunity for timely and reassuring explanation, for arranging multidisciplinary care as required, and minimizing inappropriate investigations and therapies. In this way, gastroenterologists have the opportunity to improve the long term outcomes of these patients.
Supporting information
Table S1 2017 International classification of the Ehlers‐Danlos syndromes including key clinical features, prevalence, genetic basis and gastrointestinal involvement. 7 , 91
Acknowledgment
Open access publishing facilitated by Monash University, as part of the Wiley ‐ Monash University agreement via the Council of Australian University Librarians.
Thwaites, P. A. , Gibson, P. R. , and Burgell, R. E. (2022) Hypermobile Ehlers–Danlos syndrome and disorders of the gastrointestinal tract: What the gastroenterologist needs to know. Journal of Gastroenterology and Hepatology, 37: 1693–1709. 10.1111/jgh.15927.
Declaration of conflict of interest: PAT: Has received educational support from Pfizer and Orphan Australia for conference attendance. PRG: Consultant or advisory board member for Anatara, Atmo Biosciences, Immunic Therapeutics, Novozymes, Novoviah and Comvita. He has received research grants for investigator‐driven studies from Atmo Biosciences. He holds shares in Atmo Biosciences. His Department financially benefits from the sales of a digital application, booklets and online courses on the FODMAP diet. REB: Consultant or advisory board member for Allergan, Atmo Biosciences, Antara. She has received speaking honoraria from Bayer.
Financial support: PAT is in receipt of a Postgraduate Scholarship from the National Health and Medical Research Council of Australia. This work was supported by funding from The Alfred Foundation.
REFERENCES
- 1. Beighton P, De Paepe A, Steinmann B et al. Ehlers‐Danlos syndromes: revised nosology, Villefranche, 1997. Ehlers‐Danlos National Foundation (USA) and Ehlers‐Danlos Support Group (UK). Am. J. Med. Genet. 1998; 77: 31–37. [DOI] [PubMed] [Google Scholar]
- 2. Tinkle B, Castori M, Berglund B et al. Hypermobile Ehlers‐Danlos syndrome (a.k.a. Ehlers‐Danlos syndrome Type III and Ehlers‐Danlos syndrome hypermobility type): Clinical description and natural history. Am. J. Med. Genet. C Semin. Med. Genet. 2017; 175: 48–69. [DOI] [PubMed] [Google Scholar]
- 3. Castori M, Camerota F, Celletti C et al. Natural history and manifestations of the hypermobility type Ehlers‐Danlos syndrome: a pilot study on 21 patients. Am. J. Med. Genet. A 2010; 152A: 556–564. [DOI] [PubMed] [Google Scholar]
- 4. Fikree A, Grahame R, Aktar R et al. A prospective evaluation of undiagnosed joint hypermobility syndrome in patients with gastrointestinal symptoms. Clin. Gastroenterol. Hepatol. 2014; 12: 1680–1687 e1682. [DOI] [PubMed] [Google Scholar]
- 5. Lam CY, Palsson OS, Whitehead WE et al. Rome IV Functional Gastrointestinal Disorders and Health Impairment in Subjects With Hypermobility Spectrum Disorders or Hypermobile Ehlers‐Danlos Syndrome. Clin. Gastroenterol. Hepatol. 2020. [DOI] [PubMed] [Google Scholar]
- 6. Malfait F, Castori M, Francomano CA et al. The Ehlers‐Danlos syndromes. Nat. Rev. Dis. Primers. 2020; 6: 64. [DOI] [PubMed] [Google Scholar]
- 7. Malfait F, Francomano C, Byers P et al. The 2017 international classification of the Ehlers‐Danlos syndromes. Am. J. Med. Genet. C Semin. Med. Genet. 2017; 175: 8–26. [DOI] [PubMed] [Google Scholar]
- 8. Scicluna K, Formosa MM, Farrugia R et al. Hypermobile Ehlers–Danlos syndrome: A review and a critical appraisal of published genetic research to date. Clin. Genet. 2022; 101: 20–31. [DOI] [PubMed] [Google Scholar]
- 9. Beighton P, Solomon L, Soskolne CL. Articular mobility in an African population. Ann. Rheum. Dis. 1973; 32: 413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hakim AJ, Grahame R. A simple questionnaire to detect hypermobility: an adjunct to the assessment of patients with diffuse musculoskeletal pain. Int. J. Clin. Pract. 2003; 57: 163–166. [PubMed] [Google Scholar]
- 11. Voermans NC, Knoop H, Bleijenberg G et al. Pain in ehlers‐danlos syndrome is common, severe, and associated with functional impairment. J. Pain Symptom Manage. 2010; 40: 370–378. [DOI] [PubMed] [Google Scholar]
- 12. De Wandele I, Rombaut L, Malfait F et al. Clinical heterogeneity in patients with the hypermobility type of Ehlers‐Danlos syndrome. Res. Dev. Disabil. 2013; 34: 873–881. [DOI] [PubMed] [Google Scholar]
- 13. Malfait F, Colman M, Vroman R et al. Pain in the Ehlers‐Danlos syndromes: Mechanisms, models, and challenges. Am. J. Med. Genet. C Semin. Med. Genet. 2021; 187: 429–445. [DOI] [PubMed] [Google Scholar]
- 14. Nelson AD, Mouchli MA, Valentin N et al. Ehlers Danlos syndrome and gastrointestinal manifestations: a 20‐year experience at Mayo Clinic. Neurogastroenterol. Motil. 2015; 27: 1657–1666. [DOI] [PubMed] [Google Scholar]
- 15. Hakim AJ, Grahame R. Non‐musculoskeletal symptoms in joint hypermobility syndrome. Indirect evidence for autonomic dysfunction? Rheumatology (Oxford) 2004; 43: 1194–1195. [DOI] [PubMed] [Google Scholar]
- 16. Chohan K, Mittal N, McGillis L et al. A review of respiratory manifestations and their management in Ehlers‐Danlos syndromes and hypermobility spectrum disorders. Chron. Respir. Dis. 2021; 18: 14799731211025313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kindgren E, Quiñones Perez A, Knez R. Prevalence of ADHD and Autism Spectrum Disorder in Children with Hypermobility Spectrum Disorders or Hypermobile Ehlers‐Danlos Syndrome: A Retrospective Study. Neuropsychiatr. Dis. Treat. 2021; 17: 379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bulbena A, Gago J, Pailhez G et al. Joint hypermobility syndrome is a risk factor trait for anxiety disorders: a 15‐year follow‐up cohort study. Gen. Hosp. Psychiatry 2011; 33: 363–370. [DOI] [PubMed] [Google Scholar]
- 19. Monaco A, Choi D, Uzun et al. Association of mast‐cell‐related conditions with hypermobile syndromes: a review of the literature. Immunol. Res. 2022: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hakim AJ, Tinkle BT, Francomano CA. Ehlers‐Danlos syndromes, hypermobility spectrum disorders, and associated co‐morbidities: Reports from EDS ECHO. Am. J. Med. Genet. C Semin. Med. Genet. 2021; 187: 413–415. [DOI] [PubMed] [Google Scholar]
- 21. Fikree A, Aktar R, Morris JK et al. The association between Ehlers‐Danlos syndrome‐hypermobility type and gastrointestinal symptoms in university students: a cross‐sectional study. Neurogastroenterol. Motil. 2017; 29. [DOI] [PubMed] [Google Scholar]
- 22. Fikree A, Aktar R, Grahame R et al. Functional gastrointestinal disorders are associated with the joint hypermobility syndrome in secondary care: a case‐control study. Neurogastroenterol. Motil. 2015; 27: 569–579. [DOI] [PubMed] [Google Scholar]
- 23. Nee J, Kilaru S, Kelley J et al. Prevalence of Functional GI Diseases and Pelvic Floor Symptoms in Marfan Syndrome and Ehlers‐Danlos Syndrome: A National Cohort Study. J. Clin. Gastroenterol. 2019; 53: 653–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Castori M, Dordoni C, Valiante M et al. Nosology and inheritance pattern(s) of joint hypermobility syndrome and Ehlers‐Danlos syndrome, hypermobility type: a study of intrafamilial and interfamilial variability in 23 Italian pedigrees. Am. J. Med. Genet. A 2014; 164A: 3010–3020. [DOI] [PubMed] [Google Scholar]
- 25. Castori M, Sperduti I, Celletti C et al. Symptom and joint mobility progression in the joint hypermobility syndrome (Ehlers‐Danlos syndrome, hypermobility type). Clin. Exp. Rheumatol. 2011; 29: 998–1005. [PubMed] [Google Scholar]
- 26. Zarate N, Farmer AD, Grahame R et al. Unexplained gastrointestinal symptoms and joint hypermobility: is connective tissue the missing link? Neurogastroenterol. Motil. 2010; 22: 252–e278. [DOI] [PubMed] [Google Scholar]
- 27. Inayet N, Hayat JO, Kaul A et al. Gastrointestinal Symptoms in Marfan Syndrome and Hypermobile Ehlers‐Danlos Syndrome. Gastroenterol. Res. Pract. 2018; 2018: 4854701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zeitoun JD, Lefevre JH, de Parades V et al. Functional digestive symptoms and quality of life in patients with Ehlers‐Danlos syndromes: results of a national cohort study on 134 patients. PLoS ONE 2013; 8: e80321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carbone F, Fikree A, Aziz Q et al. Joint Hypermobility Syndrome in Patients With Functional Dyspepsia. Clin. Transl. Gastroenterol. 2020; 11: e00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zweig A, Schindler V, Becker AS et al. Higher prevalence of joint hypermobility in constipation predominant irritable bowel syndrome. Neurogastroenterol. Motil. 2018; 30: e13353. [DOI] [PubMed] [Google Scholar]
- 31. Mastoroudes H, Giarenis I, Cardozo L et al. Prolapse and sexual function in women with benign joint hypermobility syndrome. BJOG 2013; 120: 187–192. [DOI] [PubMed] [Google Scholar]
- 32. Danese C, Castori M, Celletti C et al. Screening for celiac disease in the joint hypermobility syndrome/Ehlers‐Danlos syndrome hypermobility type. Am. J. Med. Genet. A 2011; 155A: 2314–2316. [DOI] [PubMed] [Google Scholar]
- 33. Laszkowska M, Roy A, Lebwohl B et al. Nationwide population‐based cohort study of celiac disease and risk of Ehlers‐Danlos syndrome and joint hypermobility syndrome. Dig. Liver Dis. 2016; 48: 1030–1034. [DOI] [PubMed] [Google Scholar]
- 34. Vounotrypidis P, Efremidou E, Zezos P et al. Prevalence of joint hypermobility and patterns of articular manifestations in patients with inflammatory bowel disease. Gastroenterol. Res. Pract. 2009; 2009: 924138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Beckers AB, Vork L, Fikree A et al. Colonoscopy is safe and not associated with higher pain scores in patients with hypermobility spectrum disorder: results from an exploratory prospective study. Therap. Adv. Gastroenterol. 2020; 13: 1756284820927310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Alomari M, Hitawala A, Chadalavada P et al. Prevalence and Predictors of Gastrointestinal Dysmotility in Patients with Hypermobile Ehlers‐Danlos Syndrome: A Tertiary Care Center Experience. Cureus. 2020; 12: e7881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Baeza‐Velasco C, Stoebner‐Delbarre A, Cousson‐Gelie F et al. Increased tobacco and alcohol use among women with joint hypermobility: a way to cope with anxiety? Rheumatol. Int. 2015; 35: 177–181. [DOI] [PubMed] [Google Scholar]
- 38. Bulbena A, Baeza‐Velasco C, Bulbena‐Cabre A et al. Psychiatric and psychological aspects in the Ehlers‐Danlos syndromes. Am. J. Med. Genet. C Semin. Med. Genet. 2017; 175: 237–245. [DOI] [PubMed] [Google Scholar]
- 39. Çınar M, Çakar M, Öztürk K et al. Investigation of joint hypermobility in individuals with hyperbilirubinemia. Eur. J. Rheumatol. 2017; 4: 36–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Castori M. Joint hypermobility syndrome (a.k.a. Ehlers‐Danlos Syndrome, Hypermobility Type): an updated critique. G. Ital. Dermatol. Venereol. 2013; 148: 13–36. [PubMed] [Google Scholar]
- 41. Al‐Rawi ZS, Al‐Dubaikel KY, Al‐Sikafi H. Joint mobility in people with hiatus hernia. Rheumatology (Oxford) 2004; 43: 574–576. [DOI] [PubMed] [Google Scholar]
- 42. Gordon C, Kang JY, Neild PJ et al. The role of the hiatus hernia in gastro‐oesophageal reflux disease. Aliment. Pharmacol. Ther. 2004; 20: 719–732. [DOI] [PubMed] [Google Scholar]
- 43. Fikree A, Aziz Q, Sifrim D. Mechanisms underlying reflux symptoms and dysphagia in patients with joint hypermobility syndrome, with and without postural tachycardia syndrome. Neurogastroenterol. Motil. 2017; 29. [DOI] [PubMed] [Google Scholar]
- 44. Savarino E, Gemignani L, Pohl D et al. Oesophageal motility and bolus transit abnormalities increase in parallel with the severity of gastro‐oesophageal reflux disease. Aliment. Pharmacol. Ther. 2011; 34: 476–486. [DOI] [PubMed] [Google Scholar]
- 45. Curci JA, Melman LM, Thompson RW et al. Elastic fiber depletion in the supporting ligaments of the gastroesophageal junction: a structural basis for the development of hiatal hernia. J. Am. Coll. Surg. 2008; 207: 191–196. [DOI] [PubMed] [Google Scholar]
- 46. Reinstein E, Pimentel M, Pariani M et al. Visceroptosis of the bowel in the hypermobility type of Ehlers‐Danlos syndrome: presentation of a rare manifestation and review of the literature. Eur. J. Med. Genet. 2012; 55: 548–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fukuda Y, Higuchi Y, Shinozaki K et al. Mobile Cecum in a Young Woman with Ehlers‐Danlos Syndrome Hypermobility type: A Case Report and Review of the Literature. Intern. Med. 2017; 56: 2791–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dordoni C, Ritelli M, Venturini M et al. Recurring and generalized visceroptosis in Ehlers‐Danlos syndrome hypermobility type. Am. J. Med. Genet. A 2013; 161A: 1143–1147. [DOI] [PubMed] [Google Scholar]
- 49. Kucera S, Sullivan SN. Visceroptosis and the Ehlers‐Danlos Syndrome. Cureus 2017; 9: e1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mohammed SD, Lunniss PJ, Zarate N et al. Joint hypermobility and rectal evacuatory dysfunction: an etiological link in abnormal connective tissue? Neurogastroenterol. Motil. 2010; 22: 1085–e1283. [DOI] [PubMed] [Google Scholar]
- 51. Haase AM, Gregersen T, Schlageter V et al. Pilot study trialling a new ambulatory method for the clinical assessment of regional gastrointestinal transit using multiple electromagnetic capsules. Neurogastroenterol. Motil. 2014; 26: 1783–1791. [DOI] [PubMed] [Google Scholar]
- 52. Raahave D. Dolichocolon revisited: An inborn anatomic variant with redundancies causing constipation and volvulus. World J. Gastrointest Surg. 2018; 10: 6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Grundy D, Schemann M. Enteric nervous system. Curr. Opin. Gastroenterol. 2006; 22: 102–110. [DOI] [PubMed] [Google Scholar]
- 54. Menys A, Keszthelyi D, Fitzke H et al. A magnetic resonance imaging study of gastric motor function in patients with dyspepsia associated with Ehlers‐Danlos Syndrome‐Hypermobility Type: A feasibility study. Neurogastroenterol. Motil. 2017; 29. [DOI] [PubMed] [Google Scholar]
- 55. Carbone F, Goelen N, Fikree A et al. Impact of joint hypermobility syndrome on gastric accommodation and nutrient tolerance in functional dyspepsia. Neurogastroenterol. Motil. 2021: e14086. [DOI] [PubMed] [Google Scholar]
- 56. Won KJ, Sanders KM, Ward SM. Stretch‐dependent sensitization of post‐junctional neural effectors in colonic muscles. Neurogastroenterol. Motil. 2013; 25: e101–e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Serra J, Azpiroz F, Malagelada JR. Mechanisms of intestinal gas retention in humans: impaired propulsion versus obstructed evacuation. Am. J. Physiol. Gastrointest. Liver Physiol. 2001; 281: G138–G143. [DOI] [PubMed] [Google Scholar]
- 58. Serra J, Azpiroz F, Malagelada JR. Impaired transit and tolerance of intestinal gas in the irritable bowel syndrome. Gut 2001; 48: 14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Aktar R, Peiris M, Fikree A et al. The extracellular matrix glycoprotein tenascin‐X regulates peripheral sensory and motor neurones. J. Physiol. 2018; 596: 4237–4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Aktar R, Peiris M, Fikree A et al. A novel role for the extracellular matrix glycoprotein‐Tenascin‐X in gastric function. J. Physiol. 2019; 597: 1503–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Carbone F, De Buysscher R, Van den Houte K et al. Relationship Between Gastric Emptying Rate and Simultaneously Assessed Symptoms in Functional Dyspepsia. Clin. Gastroenterol. Hepatol. 2022; 20: e429–e437. [DOI] [PubMed] [Google Scholar]
- 62. Malcolm A, Camilleri M, Kost L et al. Towards identifying optimal doses for alpha‐2 adrenergic modulation of colonic and rectal motor and sensory function. Aliment. Pharmacol. Ther. 2000; 14: 783–793. [DOI] [PubMed] [Google Scholar]
- 63. Gazit Y, Nahir AM, Grahame R et al. Dysautonomia in the joint hypermobility syndrome. Am. J. Med. 2003; 115: 33–40. [DOI] [PubMed] [Google Scholar]
- 64. De Wandele I, Rombaut L, Leybaert L et al. Dysautonomia and its underlying mechanisms in the hypermobility type of Ehlers‐Danlos syndrome. Semin. Arthritis Rheum. 2014; 44: 93–100. [DOI] [PubMed] [Google Scholar]
- 65. De Wandele I, Calders P, Peersman W et al. Autonomic symptom burden in the hypermobility type of Ehlers‐Danlos syndrome: a comparative study with two other EDS types, fibromyalgia, and healthy controls. Semin. Arthritis Rheum. 2014; 44: 353–361. [DOI] [PubMed] [Google Scholar]
- 66. Hakim A, O'Callaghan C, De Wandele I et al. Cardiovascular autonomic dysfunction in Ehlers‐Danlos syndrome‐Hypermobile type. Am. J. Med. Genet. C Semin. Med. Genet. 2017; 175: 168–174. [DOI] [PubMed] [Google Scholar]
- 67. Kanjwal K, Saeed B, Karabin B et al. Comparative clinical profile of postural orthostatic tachycardia patients with and without joint hypermobility syndrome. Indian Pacing Electrophysiol. J. 2010; 10: 173–178. [PMC free article] [PubMed] [Google Scholar]
- 68. Mehr SE, Barbul A, Shibao CA. Gastrointestinal symptoms in postural tachycardia syndrome: a systematic review. Clin. Auton. Res. 2018; 28: 411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wang LB, Culbertson CJ, Deb A et al. Gastrointestinal dysfunction in postural tachycardia syndrome. J. Neurol. Sci. 2015; 359: 193–196. [DOI] [PubMed] [Google Scholar]
- 70. Miglis MG, Schultz B, Muppidi S. Postural tachycardia in hypermobile Ehlers‐Danlos syndrome: A distinct subtype? Auton. Neurosci. 2017; 208: 146–149. [DOI] [PubMed] [Google Scholar]
- 71. Tai FWD, Palsson OS, Lam CY et al. Functional gastrointestinal disorders are increased in joint hypermobility‐related disorders with concomitant postural orthostatic tachycardia syndrome. Neurogastroenterol. Motil. 2020: e13975. [DOI] [PubMed] [Google Scholar]
- 72. Miller AJ, Stiles LE, Sheehan T et al. Prevalence of hypermobile Ehlers‐Danlos syndrome in postural orthostatic tachycardia syndrome. Auton. Neurosci. 2020; 224: 102637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wood JD, Galligan JJ. Function of opioids in the enteric nervous system. Neurogastroenterol. Motil. 2004; 16: 17–28. [DOI] [PubMed] [Google Scholar]
- 74. Grover M, Camilleri M. Effects on gastrointestinal functions and symptoms of serotonergic psychoactive agents used in functional gastrointestinal diseases. J. Gastroenterol. 2013; 48: 177–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wasim S, Suddaby JS, Parikh M et al. Pain and gastrointestinal dysfunction are significant associations with psychiatric disorders in patients with Ehlers‐Danlos syndrome and hypermobility spectrum disorders: a retrospective study. Rheumatol. Int. 2019; 39: 1241–1248. [DOI] [PubMed] [Google Scholar]
- 76. Rocchetti M, Bassotti A, Corradi J et al. Is the Pain Just Physical? The Role of Psychological Distress, Quality of Life, and Autistic Traits in Ehlers‐Danlos Syndrome, an Internet‐Based Survey in Italy. Healthcare (Basel). 2021; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ishiguro H, Yagasaki H, Horiuchi Y. Ehlers‐Danlos Syndrome in the Field of Psychiatry: A Review. Front. Psych. 2021; 12: 803898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Castori M. Ehlers‐danlos syndrome, hypermobility type: an underdiagnosed hereditary connective tissue disorder with mucocutaneous, articular, and systemic manifestations. ISRN Dermatol. 2012; 2012: 751768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Baeza‐Velasco C, Van den Bossche T, Grossin D et al. Difficulty eating and significant weight loss in joint hypermobility syndrome/Ehlers‐Danlos syndrome, hypermobility type. Eat. Weight Disord. 2016; 21: 175–183. [DOI] [PubMed] [Google Scholar]
- 80. Halmos EP, Gibson PR. Controversies and reality of the FODMAP diet for patients with irritable bowel syndrome. J. Gastroenterol. Hepatol. 2019; 34: 1134–1142. [DOI] [PubMed] [Google Scholar]
- 81. Sato Y, Fukudo S. Gastrointestinal symptoms and disorders in patients with eating disorders. Clin. J. Gastroenterol. 2015; 8: 255–263. [DOI] [PubMed] [Google Scholar]
- 82. Castori M, Morlino S, Pascolini G et al. Gastrointestinal and nutritional issues in joint hypermobility syndrome/Ehlers‐Danlos syndrome, hypermobility type. Am. J. Med. Genet. C Semin. Med. Genet. 2015; 169C: 54–75. [DOI] [PubMed] [Google Scholar]
- 83. Halverson CME, Clayton EW, Garcia Sierra A et al. Patients with Ehlers–Danlos syndrome on the diagnostic odyssey: Rethinking complexity and difficulty as a hero's journey. Am. J. Med. Genet. C Semin. Med. Genet. 2021; 187: 416–424. [DOI] [PubMed] [Google Scholar]
- 84. Krahe AM, Adams RD, Nicholson LL. Features that exacerbate fatigue severity in joint hypermobility syndrome/Ehlers–Danlos syndrome – hypermobility type. Disabil. Rehabil. 2018; 40: 1989–1996. [DOI] [PubMed] [Google Scholar]
- 85. Chey WD, Keefer L, Whelan K et al. Behavioral and Diet Therapies in Integrated Care for Patients With Irritable Bowel Syndrome. Gastroenterology 2021; 160: 47–62. [DOI] [PubMed] [Google Scholar]
- 86. Wiesmann T, Castori M, Malfait F et al. Recommendations for anesthesia and perioperative management in patients with Ehlers‐Danlos syndrome(s). Orphanet J. Rare Dis. 2014; 9: 109–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Fikree A, Chelimsky G, Collins H et al. Gastrointestinal involvement in the Ehlers‐Danlos syndromes. Am. J. Med. Genet. C Semin. Med. Genet. 2017; 175: 181–187. [DOI] [PubMed] [Google Scholar]
- 88. Aubry‐Rozier B, Schwitzguebel A, Valerio F et al. Are patients with hypermobile Ehlers‐Danlos syndrome or hypermobility spectrum disorder so different? Rheumatol. Int. 2021; 41: 1785–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Fragkos KC, Keetarut K, Cox A et al. Joint Hypermobility Syndrome Affects Response to a Low Fermentable Oligosaccharide, Disaccharide, Monosaccharide and Polyol Diet in Irritable Bowel Syndrome Patients: A Retrospective Study. Gastroenterology Res. 2019; 12: 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Rashed ER, Ruiz Maya T, Black J et al. Cardiovascular manifestations of hypermobile Ehlers–Danlos syndrome and hypermobility spectrum disorders. Vasc. Med. 2022;0(0): 1358863X211067566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Brady AF, Demirdas S, Fournel‐Gigleux S et al. The Ehlers‐Danlos syndromes, rare types. Am. J. Med. Genet. C Semin. Med. Genet. 2017; 175: 70–115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 2017 International classification of the Ehlers‐Danlos syndromes including key clinical features, prevalence, genetic basis and gastrointestinal involvement. 7 , 91


