Abstract
Schizophrenia is a complex, chronic mental health disorder whose heterogeneous genetic and neurobiological background influences early brain development, and whose precise etiology is still poorly understood. Schizophrenia is not characterized by gross brain pathology, but involves subtle pathological changes in neuronal populations and glial cells. Among the latter, astrocytes critically contribute to the regulation of early neurodevelopmental processes, and any dysfunctions in their morphological and functional maturation may lead to aberrant neurodevelopmental processes involved in the pathogenesis of schizophrenia, such as mitochondrial biogenesis, synaptogenesis, and glutamatergic and dopaminergic transmission. Studies of the mechanisms regulating astrocyte maturation may therefore improve our understanding of the cellular and molecular mechanisms underlying the pathogenesis of schizophrenia.
Keywords: astrocytes, dopamine, gliotransmitter, mitochondria, schizophrenia
Main Points
Recent studies provided evidence that impaired maturation of astrocytes maybe involved in the pathogenesis of schizophrenia.

1. INTRODUCTION
Schizophrenia (SCZ) is a chronic and debilitating psychiatric disorder affecting 1% of the population with complex aberrations in the structure, wiring and chemistry of multiple neuronal systems that lead to a reduction in brain volume, neuron size and spine density and the abnormal distribution of neurons in the prefrontal cortex (PFC) and hippocampus (Tandon et al., 2008; Wong & Van Tol, 2003). Neuropharmacological studies have also found that the dopaminergic (DAergic), GABAergic, glutamatergic, and serotonergic systems are involved in abnormal neurotransmission (Carlsson & Carlsson, 2006; Javitt et al., 2008; Wong & Van Tol, 2003).
As might be expected in the case of such a complex phenotype that has not yet been fully described, its etiology is still largely unknown. The progression has been divided into three phases: a prodromal (prepsychotic) phase; the initial onset of psychosis; and chronic illness. The core symptoms of SCZ cover the social, emotional, perceptive and cognitive spheres, and its clinical phenotype can be subdivided into positive psychosis‐related symptoms, negative symptoms associated with a loss of functions (a lack of motivation and social withdrawal), and PFC‐related cognitive impairments such as deficits in memory, attention, and executive functions (working memory and behavioral flexibility).
It is now widely recognized that SCZ has a substantial genetic component, and a neurodevelopmental hypothesis postulates that the interactions of multiple genes trigger a cascade of neuropathological events during the embryonic and postnatal development of the brain that may be initiated and directed by environmental factors (Fatemi & Folsom, 2009; Green & Glausier, 2016; Hilker et al., 2018; Rapoport et al., 2012; Sun et al., 2010; Thompson et al., 2004) such as maternal infections or to infectious agents associated with the onset of inflammatory responses (Russo et al., 2014). The current mainstream theory of the development of the disorder is that genetic predisposition to SCZ is pronounced during embryonic and early postnatal development and environmental factors trigger symptoms in early adolescence (Davis et al., 2016) and lead to the emergence of psychosis at the time of the transition from late adolescence to young adulthood. This has given rise to the hypothesis that SCZ may need to be studied in the context of developmental processes, and that interventions aimed at promoting normal brain development during late adolescence may prevent the pathological process (Gomes et al., 2019; Hadar et al., 2018; Millan et al., 2016; Owen et al., 2011; Tamura et al., 2016; Uhlhaas & Singer, 2011).
Linkage analysis and gene expression studies suggest that a network of 160 genes contribute to the etiology of the disease (Hjelm et al., 2015; Lam et al., 2019; J. Li et al., 2021; Li et al., 2017; Pardiñas et al., 2018; Periyasamy et al., 2019; Ripke et al., 2013; Rodriguez‐Murillo et al., 2012; Schulmann et al., 2019; Singh et al., 2022; J. Sun et al., 2010; Vawter et al., 2021) by mediating a wide range of neurodevelopmental events that include progenitor cell proliferation and differentiation, cell migration, the formation of functioning synapses, axonal connectivity, the patterning of brain structures, inflammation, and the development of glial cell functions (Ayalew et al., 2012; Jaaro‐Peled et al., 2010; Moskvina et al., 2009; Potkin et al., 2010; Sun et al., 2010). One of the more robust findings regarding the pathology of SCZ is that many candidate genes encode synaptic proteins. Over the last decade, accumulating proteomic, transcriptomic, neurophysiological and histological evidence indicates that a hallmark of SCZ pathophysiology is widespread synaptic dysfunction and loss, also known as synaptopathy (Adams et al., 2022; Berkel et al., 2010; Gauthier et al., 2010; Glessner et al., 2010; Hall & Bray, 2021; Osimo et al., 2019; Trubetskoy et al., 2022). However, understanding the initiation of synaptic dysfunction and its contribution to the development of neurodevelopmental disorders such as SCZ is challenging because alterations in the neuronal component do not fully explain the synaptic alterations or the behavioral and cognitive deficits. For example, strategies focused on restoring neuronal dendritic abnormalities, impaired synaptic plasticity, and neurotransmitter imbalance only lead to partial recovery of the neurodevelopmental deficits observed in mouse models (Catuara‐Solarz et al., 2016; De la Torre et al., 2014) and humans (De la Torre et al., 2014; de la Torre et al., 2016; Jacquemont et al., 2014), thus underlining the need to consider alternative mechanisms that may contribute to cognitive pathology.
Over the last decade, the concept of the tri‐partite synaptic assembly of pre and postsynaptic neuronal elements and peri‐synaptic astrocytes has evolved as it became clearer that complex multidirectional relationships between neuronal and astroglial components emerge as early as in the postnatal developmental phase (Figure 1) (Araque et al., 2014; Dityatev & Rusakov, 2011; Farhy‐Tselnicker & Allen, 2018; Halassa, Fellin, & Haydon, 2007; Pannasch & Rouach, 2013; Petrelli & Bezzi, 2016; Petrelli & Bezzi, 2018).
FIGURE 1.
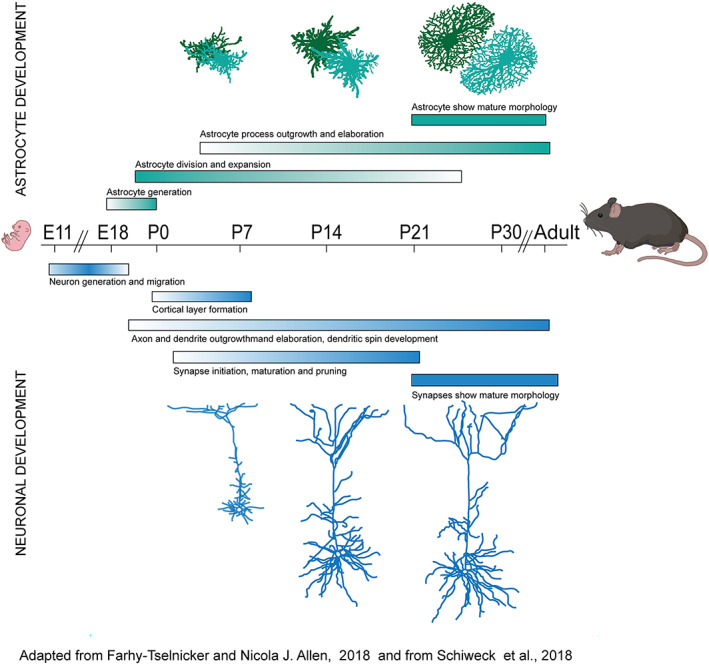
Schematic of prominent processes occurring during different periods of mouse fetal and postnatal brain development. Developmental processes as occur in astrocytes (green, above), and neurons (blue, below) are shown.
Excellent reviews have recently explored the role of astrocytes in neurodevelopmental disorders (Cresto et al., 2019; Allen & Eroglu, 2017; Petrelli & Bezzi, 2018, Petrelli & Bezzi, 2016), therefore the aim of this review is to describe the evidence supporting the role of astrocytes in SCZ, and propose the intriguing hypothesis that early defects in astrocyte function may even trigger the pathogenesis of the disorder.
2. EVIDENCES FOR ASTROCYTES ALTERATIONS IN SCHIZOPHRENIA
It is now widely accepted that astrocytes play important role both during postnatal development and in the adulthood by regulating the formation and development of neuronal circuits (Eroglu & Barres, 2010) and by regulating multiple homeostatic functions such as the buffering of extracellular potassium or the modulation of synaptic activity (Hu et al., 2015) as well as of functional hyperaemia (Verkhratsky & Nedergaard, 2018), respectively, (Figure 2). They not only provide metabolic support for synaptic activity, but are also necessary for synapse formation and maintenance (Chung et al., 2015). Consistent with the crucial roles of astrocytes in the physiology of neuronal circuits, changes in astrocytic numbers have been shown to trigger cognitive dysfunction. For example, selective elimination of astrocytes located in the prefrontal cortex (PFC) using an astrocyte specific toxin L‐α‐aminoadipate (L‐AA) (Lima et al., 2014), or in a transgenic mouse line with inducible and selective tetanus neurotoxin (TeNT) expression in astrocytes (Lee et al., 2014) results in mice with deficits in attentional setshifting, working memory, reversal learning (Lima et al., 2014), recognition memory, and abnormal cortical gamma oscillations (Lee et al., 2014). Similarly, decreased expression of the astrocytic glutamate transporter GLT‐1, reduces prepulse inhibition of the acoustic startle response (Bellesi et al., 2009), a well‐established feature of SCZ.
FIGURE 2.
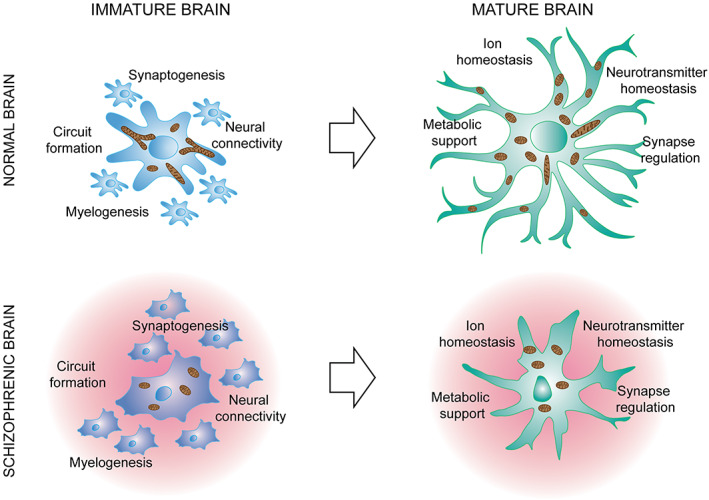
Astrocytes in normal brain and in brain affected of schizophrenia. It is now widely recognized that astrocytes play crucial roles both during postnatal development and in the adulthood because they are necessary for development of neuronal circuits and for the maintenance of multiple homeostatic functions such as the buffering of extracellular ions or the modulation of synaptic activity. Several recent studies have point out that schizophrenia induces molecular changes in astrocytes (including modifications in the rate of mitochondrial biogenesis) that can results in an aberrant postnatal maturation of astrocytes. As the formation and maturation of neuronal circuits occurs concomitantly to astrocytes maturation, it is evident that any dysfunctions in their morphological and functional maturation may have a direct impact to the formation of neuronal circuits that ultimately may lead to aberrant neurodevelopmental processes involved in the pathogenesis of schizophrenia.
The involvement of astrocytes in SCZ has long been suspected (Bernstein et al., 2015; Birnbaum & Weinberger, 2020; Kelly et al., 2018; Kerns et al., 2010; Trépanier et al., 2016; Van Kesteren et al., 2017; Wang et al., 2015), and it has been shown that changes in astrocytic cell density and morphology in rodent PFC trigger SCZ‐related cognitive dysfunctions (Lee et al., 2014; Lima et al., 2014). The evidence concerning altered astrocyte status in SCZ originated from the findings of postmortem studies that identified significant changes in astrocyte density and morphology, as well as the deregulated expression of some common astrocyte markers such as glial fibrillary acidic proteins (GFAP), aquaporin 4 (AQ‐4), S100β, glutaminase, thrombospondin (TSB‐1), and excitatory amino acid transporter 2 (EAAT2) (Katsel et al., 2011; Kim et al., 2018; Tarasov et al., 2020; Trépanier et al., 2016). However, there were quite significant differences in the findings as some studies indicated a decrease in marker levels and the number of astroglial cells in comparison with controls, and others an increase. Therefore, the nature of their contribution in humans has not yet been defined, probably because of confounding factors related to the use of postmortem tissues, the brain regions analyzed, the severity of the disease in the subjects studied, differences in pharmacological treatments, and so on. Many studies have not actually investigated the status of astroglial cells during postnatal brain development, but were limited to the postmortem examination of adult brain tissues where dysregulation evident in developing astroglial cells are hidden, whereas this may have profound effects on the formation and maturation of neuronal networks. In line with this hypothesis, recent studies of in vitro cultures of human glial progenitors taken from SCZ patients has shown that the genes associated with glial differentiation in particular those associated with early oligodendroglial and astroglial lineage progression and those encoding for glia‐derived metalloproteinases, were disrupted, thus indicating the existence of a cell autonomous glial pathology (Szabo et al., 2021; Windrem et al., 2017).
Further supporting the hypothesis of astrocytes involvement in the pathophysiology of SCZ, the same study showed that the transplantation of the glial cell precursors into a normal mouse induced abnormal behaviors, such as increased anxiety, deficits in social behavior, and problems with prepulse inhibition, a hallmark of SCZ patients (Mena et al., 2016). Interestingly, it is thought that decreased prepulse inhibition in SCZ patients reflects an alteration in DAergic and glutamatergic neurotransmission in which it is likely that astrocytes play an important functional role (see next paragraphs).
Despite these indications, defining the extent to which the astroglial changes are causal or merely secondary to neuronal pathology is still difficult. Some help has come from genetic association and transcriptome studies that have provided additional evidence of astroglial abnormalities. The involvement of astrocytes in SCZ is supported by RNA sequencing data (Gandal, Haney, et al., 2018; Jaffe et al., 2018) and the findings of genome‐wide association studies (Gandal, Zhang, et al., 2018; Schork et al., 2019) showing that SCZ risk loci are enriched in genes related to astrocytes specification and maturation such as SOX9 (astrocyte specific nuclear marker), GJA1 (encoding for Cx43), SPON1 and NOTCH2 (involved in proliferation, cell fate decisions and survival of astroglial lineage) and, more particularly, suggesting the dysregulation of neuro‐inflammatory pathways and the up‐regulation of astrocyte‐related genes. It is widely accepted that inflammatory conditions can trigger reactive astrogliosis and, although this can lead to a number of significant benefits, it can also have harmful effects such as the production of toxic molecules (Baev et al., 2022; Chavda et al., 2022; De Sousa, 2022; Liddelow et al., 2020; Sofroniew, 2014, 2015, 2020). Reactive astrocytes have different functional properties: they lose spontaneous Ca2+ oscillation (Buscemi et al., 2017; Escartin et al., 2021; Santello et al., 2019; Shigetomi et al., 2019), which has recently been shown to cause the onset of repetitive behavior (Yu et al., 2018), and changes in the release of neuroactive compounds (gliotransmitters) compared to physiological conditions (Agulhon et al., 2012; Bezzi & Volterra, 2001; Cali et al., 2014; Habbas et al., 2015; Petrelli & Bezzi, 2016; Rossi et al., 2005; Santello et al., 2019; Halassa, Fellin, & Haydon, 2007), which correlates with neuronal damage (Santello et al., 2012; Santello & Volterra, 2011).
Interestingly, in a different study, RNA sequencing of glial progenitor cells from SCZ patients has revealed the down‐regulation of a host of genes associated with astroglial differentiation, in particular those associated with early oligodendroglial and astroglial lineage progression including a coherent set of the key human glial progenitor cells (hGPC) lineage transcription factors OLIG1, OLIG2, SOX10, and ZNF488 which give rise to astrocytes as well as oligodendrocytes. These data suggest that failed oligodendrocyte and astrocyte differentiation may be an initial event in SCZ and that astrogliosis may be a secondary event occurring later during the course of disease progression (Dietz et al., 2020; Windrem et al., 2017). The defective astrocytic maturation might have profound effects on developmental synaptogenesis and circuit formation as well as on myelinogenesis (Figure 2) and indeed, the importance of postnatal astrocytes maturation has been recently highlighted by several studies showing that neural connectivity and synaptic development are both intimately dependent on astrocytic guidance (Allen et al., 2012; Allen & Lyons, 2018; Clarke & Barres, 2013; Farhy‐Tselnicker et al., 2017; Jo et al., 2021) and, hence, on the appropriate timing of astrocytic maturation. As a result, any disruption in astrocytic maturation by SCZ hGPC, might be expected to significantly confound the construction and functional architecture of neural networks (Dietz et al., 2020).
The processes governing astrocyte maturation are still being investigated, and little is known about the molecular mechanisms underlying the ways in which they are specified and grow to take on their complex morphologies, or how they interact with and sculpt developing neuronal circuits. However, recent studies have given us a basic understanding of the intrinsic and extrinsic mechanisms governing their origin from precursor cells and the generation of their diversity (Freeman, 2010), and revealed that morphologically highly complex mature astrocytes interact with as many as ~100,000 individual synapses and cluster with other astrocytes to occupy unique spatial domains in the brain (Bushong et al., 2002; Halassa, Fellin, Takano, et al., 2007; Schiweck et al., 2018). This complexity is even greater in humans as a single astrocyte occupies a brain volume that is almost 30 times that occupied in rodent brain and interacts with ~2,000,000 synapses (Oberheim et al., 2006).
Astrocyte maturation requires a number of morphological and molecular re‐arrangements (Figure 1). During the first week of postnatal development, when they are still replicating, astrocytes send out their long processes well beyond their still quite ragged borders and, in the following two weeks, continue to expand in a manner similar to the growth and expansion of neuronal protrusions, and elaborate the fine processes that come into contact with developing synapses (Allen & Eroglu, 2017; Bandeira et al., 2009; Bushong et al., 2004; Farhy‐Tselnicker & Allen, 2018; Freeman, 2010; Ge et al., 2012; Petrelli & Bezzi, 2018; Silbereis et al., 2016; Schiweck et al., 2018; Zehnder et al., 2021). Consequently, while growing in number and overall density, the morphology of maturing astrocytes changes (Bushong et al., 2004; Stogsdill et al., 2017; Schiweck et al., 2018) from that of small cells with a few long processes to that of mature cells whose complex architecture is characterized by numerous very thin processes (peripheral astrocytic processes, PAPs) (Calì et al., 2019; Derouiche & Frotscher, 2001) reaching out to contact many thousands of synapses (Halassa, Fellin, Takano, et al., 2007; Reichenbach et al., 2010; Ventura & Harris, 1999).
Molecularly, recent transcriptome analyzes have shown that many of the genes regulating proliferation are progressively down‐regulated during postnatal astrocyte maturation, whereas those encoding glutamate and GABA transporters, connexin (Cx) 30 and Cx43, and the inwardly rectifying potassium channel Kir 4.1 are progressively up‐regulated (Cahoy et al., 2008; Zhang et al., 2016). The genes that are progressively up‐regulated encode the proteins involved in maintaining synaptic homeostasis (glutamate and GABA transporters, and Kir 4.1) and creating a functional interconnected syncytium to spread nutrients and ions (such as Cx30 and Cx43), thus indicating that astrocyte maturation parallels the gradually increasing strength of synaptic activity. In particular, the larger syncytium of mature astrocytes indicates that the delivery of metabolites to active synapses is greater than that in less mature brains (Rouach et al., 2008) and, as the expression of Cx30 can also regulate the spatial proximity of PAPs to glutamatergic synapses, the gradual increase in connexin levels can modulate the efficacy of the astrocytic glutamate uptake of K+ buffering (Pannasch et al., 2014; Pannasch & Rouach, 2013). Interestingly, astrocytes in the context of SCZ may show a morphological phenotype reminiscent immature cells and may express decreased levels of K+ channels, CX30 and CX43, or glutamate transporter GLT‐1 (i.e., proteins that are progressively up‐regulated during postnatal maturation), and all of these conditions have been associated with features of SCZ in transgenic mice (Bellesi et al., 2009) (Figure 2). Thus, the understanding of molecular mechanisms underlying the generation and maturation of astrocytes during the first postnatal week, is a key factor for cracking the pathophysiology of SCZ.
3. MITOCHONDRIA DYSFUNCTIONS IN SCHIZOPHRENIA: IS THERE A ROLE FOR ASTROCYTIC MITOCHONDRIA?
Among the most important risk factors for SCZ, abnormalities in energy mitochondrial metabolism have been found in functional assays and in magnetic resonance spectroscopy studies on SCZ patients (Jensen et al., 2006; Maurer et al., 2001; Öngür et al., 2009; Regenold et al., 2009, Adams et al., 2022) and an analysis of several published studies on genomic, transcriptomic, and proteomic factors associated with SCZ revealed 295 genes that mediate mitochondrial structure or function (Hjelm et al., 2015; Lam et al., 2019; Li et al., 2021; Schulmann et al., 2019; Vawter et al., 2021). Moreover, 22 genes encoding mitochondrial proteins have been mapped within the 108 risk loci (encompassing more than 300 genes) identified by the largest SCZ genome‐wide association studies (GWAS) to date (Hjelm et al., 2015; Lam et al., 2019; Li et al., 2021; Ripke et al., 2013; Schulmann et al., 2019; Vawter et al., 2021) and further studies show a decrease of factors and enzymes involved in the production of energy (i.e., adenosine triphosphate–ATP‐generation and storage) (Iwamoto et al., 2005; Karry et al., 2004; Klushnik et al., 1991; Middleton et al., 2002; Pennington et al., 2008; Washizuka et al., 2009). Alterations in mitochondrial activity have been consistently reported in SCZ patients: for example, reduced energy metabolism has been reported in patients with psychosis (Regenold et al., 2012) and phosphorous magnetic resonance studies (31P‐MRS) reported lower levels of ATP and phosphocreatine in brain of patients with SCZ (Volz et al., 2000). Further studies reported mitochondrial defects in neural progenitors, cerebral organoids, and cortical interneurons derived from induced pluripotent stem cells (iPSCs) from patients with SCZ compared with healthy control individuals (Brennand et al., 2015; Kathuria et al., 2020; Ni et al., 2020). Mitochondria are the main intracellular location for generating adenosine triphosphate (ATP) but they are far more than just power suppliers. They are also involved in many other cellular functions, including calcium signaling (Courchet et al., 2013; Lewis et al., 2018; Rangaraju et al., 2019), proliferation (Iwata et al., 2020) or apoptosis, production of free radical species, redox homeostasis maintenance (Friedman & Nunnari, 2014; Rizzuto et al., 2012) and neuronal development and synaptic plasticity (Iwata et al., 2020; Iwata & Vanderhaeghen, 2021; Lee et al., 2018) thus suggesting that compromised mitochondrial function can alter critical neuronal processes underlying abnormal brain development and cognitive impairment in psychosis.
This preponderant association of mitochondrial gene with SCZ have been also demonstrated for 22q11 deletion syndrome(DS), a common microdeletion syndrome in humans that represent one of the strongest genetic risk factors for SCZ (Chow et al., 2006; Pulver et al., 2000). 22q11 DS is caused by a hemizygous 1.5–3 Mb microdeletion on the long arm of chromosome 22 that affects approximately 35–60 known genes (Karayiorgou et al., 2010) and occurs in about 1/4000 live births (Meechan et al., 2015). It is estimated that patients with 22q11DS have about 30% risk of developing SCZ in a manner that is not grossly distinguishable from nonsyndromic SCZ (Chow et al., 2006; Sun et al., 2020). The psychotic symptoms and related cognitive deficits emerge at the time of the transition from late adolescence to adulthood, which has led to the hypotheses that 22q11DS, similar to SCZ, should be studied in the context of developmental processes and that preadolescent interventions aimed at promoting normal brain development may prevent its occurrence. Altered brain energy metabolism and mitochondrial dysfunction have been implicated in the etiology of SCZ symptoms (Prabakaran et al., 2004), and the fact that six of the 45 genes deleted in 22q11DS (Prodh, Slc25a1, Mrpl40, Zdhhc8, T10, and Txnrd2) encode mitochondrial proteins (Devaraju & Zakhareko, 2017; Maynard et al., 2008) suggest that mitochondria may be key organelles in its pathophysiology. One study has found evidence for mitochondrial dysfunction in lymphocytes and plasma samples from children with 22q11DS, (Napoli et al., 2015) while another using a mouse model of 22q11DS has found the dysregulated expression of many genes related to mitochondrial functions specifically in the PFC (Stark et al., 2008). Furthermore, recent transcriptomic, proteomic and metabolomic studies of mouse models and 22q11DS patients have identified multiple deregulated mitochondrial pathways (Carrera et al., 2012; Fernandez et al., 2019; Gokhale et al., 2019; Napoli et al., 2015; Wesseling et al., 2017) and shown that neuronal Txnrd2 and Mrpl40 genes are important players in the psychosis‐related cognitive deficits of transgenic mice (Carrera et al., 2012; Devaraju et al., 2017; Fernandez et al., 2019). Finally mitochondrial dysfunctions have been found in iPSC‐derived neurons from individuals with 22q11DS with SCZ versus healthy control individuals (Li et al., 2021) and reduced ATP levels and reduced activity of complexes I and IV of the ETC have been found in patient‐derived neurons. Overall, these results suggest that mitochondrial deficits may contribute to the development of SCZ in the context of 22q11DS but still no studies to date have indicated whether mitochondrial dysfunctions mediate the altered developmental mechanisms of the PFC as a result of the diminished number of 22q11 genes. This lack of information possibly originates from the fact that mitochondrial dysfunctions associated with 22q11DS or with SCZ have only been investigated from a neuronal perspective.
One challenge facing researchers trying to elucidate the mechanisms underlying the 22q11DS and SCZ is that the processes of brain maturation from childhood to adolescence and adulthood are still unclear. The PFC is one of the last brain regions to acquire adult‐like features between late adolescence and early adulthood and, as PFC dysfunction has been related to defined features of SCZ (e.g., deficits in working memory) (Meyer‐Lindenberg et al., 2005; Millan et al., 2016; Uhlhaas & Singer, 2015), it is possible that SCZ may be linked to alterations in PFC maturation. Many studies have shown morphological and molecular alterations in the PFC of SCZ patients, including decreased spine and synapses density and truncated dendritic trees, and specific transcriptome alterations in layers 3 and 5 that are not detected in other cortical areas (Black et al., 2004; Garey et al., 1998; Kolluri et al., 2005; Rajkowska et al., 1998), and some of these molecular alterations seem to be mainly related to mitochondrial functions (Arion et al., 2017). Interestingly enough, astrocytes are important regulators of synapse formation and function during development. While neurogenesis precedes astrogenesis in the cortex, synaptogenesis only begins after astrocytes have been generated, concurrent with neuronal branching and process elaboration. Over the last few decades, it has become increasingly clear that brain maturation and function depend on the efficient cooperation between neurons and astrocytes in shaping neural circuits and appropriate astrocyte maturation during postnatal development may be crucial for the formation of neuronal circuitry. Each of these steps requires astrocyte‐derived factors and the astrocytic control of neuronal homeostasis, which raises the question as to whether astrocyte dysfunction during this critical period contributes to the alterations in the PFC maturations and consequently to the pathophysiology of cognitive deficits associated to of 22q11DS and of SCZ. The findings of some recent studies have further strengthened this hypothesis. A recent transcriptomic analysis (Boisvert et al., 2018; Cahoy et al., 2008; Zhang et al., 2016) has identified all of the six genes deleted in 22q11DS and coding for mitochondrial proteins in astrocytes. It has also been shown that Prodh (a gene encoding a mitochondrial enzyme proline dehydrogenase or PRODH involved in regulating oxidative stress) is among the 30 genes that are highly expressed by astrocytes (Boisvert et al., 2018; Cahoy et al., 2008; Zhang et al., 2016) and PRODH‐deficient mice, which carry a mutation in the mouse orthologue of the human Prodh gene, that introduces a premature termination (E453X) and leads to an ~60% reduction in enzymatic expression and an ~90% reduction in enzymatic activity, has been implicated in deficient sensorimotor gating (Paterlini et al., 2005). Furthermore, a recent study has highlighted that those mitochondrial dysfunctions in developing astrocytes can affect their postnatal maturation. Indeed, the conditional deletion of the metabolic regulator PPARγ co‐activator 1α (PGC‐1α) and the consequent perturbation of the mitochondrial network in developing astrocytes impairs their morphological maturation (Zehnder et al., 2021), thus highlighting the importance of mitochondria to astrocyte morphogenesis. Recent studies have highlighted the importance of the postnatal morphogenesis of astrocytes by showing that their morphological maturation takes place in tune with the growth and activity of synaptic circuits and can actually control synaptogenesis and circuit functions (Stogsdill et al., 2017; Stogsdill & Eroglu, 2017) and consistent with these studies, the deletion of PGC‐1α in astrocytes decreases the formation and function of excitatory synapses in the PFC (Zehnder et al., 2021).
Interestingly, the PGC‐1α‐dependent pathway is critically controlled by the mGluR5, a metabotropic glutamate receptor that has been shown to be highly expressed in developing astrocytes (Buscemi et al., 2017; Sun et al., 2010). Indeed, the temporal relationship between postnatal synapse and astrocyte maturation suggests that bi‐directional interactions orchestrate both in order to fine‐tune the maturation of functional circuits (Allen & Eroglu, 2017; Petrelli & Bezzi, 2018). The mGluR5 gene (i.e., GRM5) as well as the genes regulating glutamatergic signaling in astrocytes are also developmentally regulated: for example, the expression of Homer and Shank, which respectively encode Homer1b/c and Shank2 or 3 scaffold proteins, is particularly high when astrocytes are immature (Boisvert et al., 2018; Cahoy et al., 2008; Zhang et al., 2016), thus indicating that immature astrocytes sense glutamatergic activity from the early phase of postnatal development, even before the major wave of synaptogenesis occurs. In line with its high expression level in immature astrocytes, mGluR5 plays a crucial role in regulating postnatal astrocyte growth and arborization by inducing mitochondrial biogenesis in immature astrocytes (Morel et al., 2014; Zehnder et al., 2021), thus suggesting that mGluR5 signaling oversees glutamatergic synaptic activity and adapts postnatal astrocytic energy demands in such a way as to support the large number of fine processes typically seen in adult astroglia. The events coupling the monitoring of synaptic activity by mGluR5 and increasing mitochondrial biogenesis seem to be temporally correlated in developing astrocytes, in which the levels of mGluR5 abruptly decrease when those of PGC‐1α reach their peak during the third postnatal week.
It is known that cellular energy metabolism requires the rapid activation of the mitochondrial biogenesis program orchestrated by PGC‐1α (Wu et al., 1999), and that the consequent increase in the number of mitochondria is crucial to the differentiation and maturation of many cell types, including neurons (Cheng et al., 2012; Li et al., 2004). Immature astrocytes rely on a PGC‐1α‐dependent network of mitochondria to stop their replication and reach postnatal maturation, and the dysfunctional mitochondrial network induced by the selective deletion of astrocyte PGC‐1α impairs correct postnatal astrocyte maturation and correct synaptogenesis (Zehnder et al., 2021), thus affecting postnatal PFC development, a situation similar to those in SCZ and 22q11DS.
Consistent with a possible role of PGC‐1α in the 22q11DS, recent results obtained from iPSC derived neurons of 22q11patients with or without SCZ have shown that variable penetrance to the development of SCZ is influenced by compensatory mitochondrial biogenesis that occurs in patients with 22q11DS without SCZ (Li et al., 2021). Indeed, in the 22q11DS without SCZ, expression of nuclear genes encoding ETC subunits, and of multiple mitochondrial encoded genes, were upregulated, as was mitochondrial DNA content and the expression of genes affecting mitochondrial biogenesis and function, such as PGC‐1α and PPARα, in human induced pluripotent stem cells (hiPSC) from the 22q11DS without SCZ, thus suggesting that the 22q11DS group without SCZ is characterized by effective compensation for the various mitochondrial abnormalities present in the 22q11DS group with SCZ. Moreover they also found that stimulation of mitochondrial biogenesis in hiPSC‐derived neurons from 22q11DS patients with SCZ, normalizes the ATP deficit seen in this group of neurons (Li et al., 2021). These results are suggesting that enhanced mitochondrial biogenesis and functions are associated with the absence of SCZ in hiPSC‐derived neurons from patients with 22q11DS and that deficits in mitochondrial function in the 22q11DS group with SCZ can be reversible by activating PGC‐1α and mitochondrial biogenesis.
4. FUNCTIONAL ROLE OF ASTROCYTES IN THE REGULATION OF GLUTAMATERGIC AND DOPAMINERGIC TRANSMISSION AND ITS POSSIBLE IMPLICATIONS IN SCHIZOPHRENIA
The contribution of glutamate to the pathology of SCZ has been studied extensively. The glutamate hypothesis of SCZ is mainly based on the fact that hypofunctioning N‐methyl‐d‐aspartate (NMDA) receptors decrease PFC functions (Herédi et al., 2017) together with the ability of NMDA antagonists such as ketamine or phencyclidine to induce SCZ‐like psychosis (Javitt & Zukin, 1991; Moghaddam & Javitt, 2012; Steeds et al., 2015). This hypothesis is supported by studies showing lower mRNA and protein expression of some NMDA receptor subunits, changes in the postsynaptic density of glutamatergic synapses (Balu, 2016; Banerjee et al., 2015), and significant levels of anti‐NMDA antibodies in patients experiencing a first episode of SCZ (Levite, 2014).
The activation of NMDA receptors requires the binding of glutamate and endogenous co‐agonists D‐serine or glycine (Oliet & Mothet, 2009). A number of studies have shown that deficits in the availability of D‐serine may contribute to the NMDA hypofunction associated with SCZ (Hu et al., 2015; Labrie et al., 2012; Mei et al., 2018). The effects of D‐serine on synaptic plasticity and NMDA receptor currents have mainly been attributed to astrocyte D‐serine release (Fossat et al., 2012; Henneberger et al., 2010; Mothet et al., 2006; Panatier et al., 2006), and this hypothesis is supported by the finding that that the inhibition of astrocyte‐mediated D‐serine release by clamping the increase in intra‐cellular calcium levels or poisoning astrocytes with fluoroacetate impairs the plasticity of nearby synapses (Fossat et al., 2012; Henneberger et al., 2010).
The finding that D‐serine levels in mammalian brain increase during early development suggests that it may play a role in normal brain development and circuit refinement, and recent evidence indicating that the synaptogenesis induced by transforming growth factor β‐1 (TGF‐β1) is dependent on D‐serine is consistent with the idea that D‐serine plays a role in promoting the formation of functional synapses (Diniz et al., 2012; Packard et al., 2003). Furthermore, it has been found that, like TGF‐β1 treatment, the application of D‐serine alone is sufficient to induce the formation of synapses and that the synaptogenic property of TGF‐ß1 is eliminated when D‐serine levels are reduced by D‐amino acid oxidase (DAAO) treatment, which suggests that D‐serine release may be responsible for TGF‐β1‐mediated synaptogenesis.
The subunit composition of NMDA receptors changes during development and by brain region, which suggests that the developmental roles of the various NMDA receptor subtypes may be different (Bellone & Nicoll, 2007): for example, GluN2B expression in the hippocampus peaks early in development whereas GluN2A is expressed later, and this difference correlates with the maturation of neuronal circuits and the control of a number of important developmental events (van Zundert et al., 2004; Yashiro & Philpot, 2008). Recent findings have shown that the availability of D‐serine and glycine preferentially favors the diffusion of NR2B over NR2A subunits (Burnet et al., 2011; Papouin et al., 2012), thus suggesting that D‐serine may play a role in the development of neuronal circuits, possibly by influencing the subunit make‐up of glutamatergic synapses.
The initial therapeutic use of D‐serine to alleviate SCZ was based on the hypothesis that NMDA receptor hypofunction was partially responsible for the disease, and was supported by the subsequent hypothesis that a deficiency in the availability of endogenous D‐serine was an underlying cause of NMDA receptor hypofunction, and the finding that SCZ patients have reduced plasma and cerebrospinal fluid D‐serine levels (Bendikov et al., 2007; Calcia et al., 2012; Hashimoto et al., 2003). This reduction may be partially explained by excessive D‐serine degradation, a hypothesis that is supported by the finding of high levels of highly active DAAO (an enzyme with the molecular function of oxidizing D‐amino acids) in postmortem brain samples taken from SCZ patients (Habl et al., 2009; Madeira et al., 2008). Moreover, association studies have identified several mutations in human D‐serine metabolic enzymes as risk factors for SCZ, including single‐nucleotide polymorphism (SNP) variants of serine racemase (Morita et al., 2007), DAAO (Boks et al., 2007; Caldinelli et al., 2013), and the DAAO interacting protein G72 (Müller et al., 2011). It is particularly worth noting that the SCZ susceptibility gene DISC1 seems to bind serine racemase directly, thus protecting it from ubiquitin‐mediated degradation (Ma et al., 2013).
The glutamate hypothesis of SCZ is also based on analyzes of postmortem tissues which indicate layer‐specific alterations in glutamatergic neurotransmission and receptors, as well as in the glutamate metabolic pathways (Spangaro et al., 2012). The changes in glutamate homeostasis, include reductions that are associated with SCZ in the expression of the astrocytic glutamate transporters (Hu et al., 2015), glutamine synthase (Hu et al., 2015), glutamate dehydrogenase (Burbaeva et al., 2003), glutaminase (Hu et al., 2015), and D‐serine (Steffek et al., 2006) however, the extent to which these changes occur autonomously in astrocytes, rather than being downstream of neuronal dysregulations, remains unclear.
The DA hypothesis of SCZ is based on the presence of hyperactive DA projections in the mesolimbic system and reduced DA projections in the mesocortical system (Tarasov et al., 2020). The pharmacotherapy based on the DA theory of SCZ takes advantage of antipsychotic agents: the first‐generation antipsychotics work mainly by inhibiting DAergic neuromodulation (D2 antagonists) or other receptors such as noradrenergic, cholinergic, and histaminergic receptors while the second‐generation antipsychotics work by blocking both D2 receptors and serotonin receptor (5HT2A subtype). However, there are a number of significant limitations when using antipsychotics that prevalently act on D2 receptors because they mainly improve only positive symptoms (e.g., sensory hallucinations, thought disorders, schizophasia) and approximately 25% of SCZ patients are treatment resistant (Remington et al., 2017), which suggests that excess DA neuron recruitment from mid‐brain areas is not the only cause of the disorder. For example, hyperactivity in the PFC is a characteristic pathophysiological feature of patients with SCZ (Grace & Gomes, 2019; Heckers & Konradi, 2015; Tseng et al., 2007), and may account for the hyperactivity of DA projections. It has indeed recently been shown that chronic treatment of the PFC with a D2 antagonist during the sensitive adolescent period can prevent a decrease in the functions of inhibitory parvalbumin (PV) neurons and cognitive dysfunctions in a mouse model of SCZ (Mukherjee et al., 2019), which suggests that antipsychotic drugs acting on D2 receptors may attenuate psychosis by means of mechanisms of network activity that influence the recruitment of PV neurons. Possible direct mechanisms may involve the functions of D2 receptors expressed by adult PV neurons (Tomasella et al., 2018; Tseng & O'Donnell, 2007) or DAergic terminals in the mPFC (Petrie et al., 2005) as neurodevelopmental alterations in the frontal and PFC (which are greatly involved in cognition, memory and learning) are probably involved in the etiology of SCZ (Jonas et al., 2014; Langen et al., 2012; Simpson et al., 2010). These cortical regions receive DA innervations and express DA receptors (Björklund & Dunnett, 2007; Roeper, 2013; Tritsch & Sabatini, 2012) from the embryonic phase, and DAergic maturation of the PFC continues affecting postnatal synaptogenesis and connectivity until they become stable in young adulthood (Bhide, 2009; Hamilton et al., 2010; Lu et al., 2009; Mameli et al., 2011; McCarthy et al., 2011; Spencer et al., 1998; Stanwood et al., 2001, 2005; Stanwood & Levitt, 2007; Zhang et al., 2010). This protracted phase of development provides ample time for the development and maturation of the functional properties of the PFC, which may be strongly dependent on potential disruptors of DA innervations. Alterations in DAergic pathways have been found in SCZ patients, including fewer DAergic axons in the deep layers of the medial PFC and decreased D1 receptor binding (Akil et al., 1999; Arnsten & Shansky, 2004; Gibbs & D'Esposito, 2005; Goldman‐Rakic & Selemon, 1997; Okubo et al., 1997).
Over the last few years, increasing efforts have been made to clarify whether astrocytes are involved in the brain's DAergic system. It has been widely reported that they express DAminergic receptors (Corkrum & Araque, 2021; Jennings & Rusakov, 2016) and key enzymes for DA metabolism, such as the mitochondrial enzymes monoamine oxidase B (MAO‐B) and cathecholamine‐O‐methyl transferase (COMT) (Cahoy et al., 2008; Youdim et al., 2006). Furthermore, recent transcriptome analyzes have also shown the presence of genes encoding for proteins involved in monoamines transport and storage such as plasma membrane organic cation transporter 3 (OCT3) (Cahoy et al., 2008; Cui et al., 2009; Yoshikawa et al., 2013; Zhang et al., 2016), and vesicular monoamine transporter 2 (VMAT2) (Cahoy et al., 2008; Zhang et al., 2016), an integral vesicular membrane protein that, in neurosecretory cells, directly controls the efficient uptake of cytosolic monoamines into intra‐cellular vesicles (Edwards, 2007).
A novel mRNA splice variant of Drosophila VMAT (DVMAT‐B) has been found in a small subset of glia in the lamina of the fly's optic lobe (Romero‐Calderón et al., 2008), but it is only very recently that an immunohistochemistry survey of various brain areas has revealed that VMAT2 immunolabeling is particularly enriched in the astrocytes of the frontal cortex (including the PFC) in rodents and humans (Petrelli et al., 2020, 2021). The rodent immunosignal is visible in astrocytes from the second postnatal week, but almost absent in other DAergic areas such as the striatum and ventral tegmental area, which suggests that astrocytic VMAT2 plays a specific functional role in frontal cortical regions. In line with this hypothesis, the deletion of astrocytic VMAT2 causes an imbalance of DA homeostasis, a concomitant decrease in the extra‐cellular levels of DA, and tonic DAergic modulation of excitatory transmission specifically in the PFC (Petrelli et al., 2020). The absence of the inhibitory action of DArgic modulation increases basal synaptic activity, hinders synaptic plasticity, and ultimately affects the proper development of the cognitive processes associated with the PFC, such as working memory and cognitive flexibility (something frequently observed in SCZ patients). A relatively small decrease in extracellular DA levels in the PFC can therefore have significant effects on cognitive performance, which underlines the importance of maintaining correct DAergic tone during the postnatal development of the PFC (Paterlini et al., 2005).
The ability of astrocytes to control extra‐cellular DA levels depends on VMAT2, which works in tandem with the degradation enzymes MAO‐B and COMT to maintain the correct balance between vesicular and free cytosolic DA levels and prevent abnormal DA metabolism (Fon et al., 1997). Unlike other DAergic areas, the PFC expresses low levels of the high‐affinity neuronal DA transporter (DAT), and relies on secondary mechanisms such as those of COMT and MAO‐B to maintain appropriate DA levels (Yoshikawa et al., 2013).
The COMT gene is considered an attractive candidate for inducing susceptibility to SCZ (Glatt et al., 2003; Weinberger et al., 2001), and the activity of the COMT enzyme seems to be particularly relevant in controlling PFC DA levels (Egan et al., 2001) and influencing PFC‐dependent cognitive processes such as attention, working memory, and executive functions (Bilder et al., 2002; Egan et al., 2001; Malhotra et al., 2002). COMT gene contain numerous polymorphisms, the most frequently studied of which is the functional single nucleotide polymorphism (SNP) rs4680 (G/A or Val/Met substitution) that affects enzymatic activity (Tunbridge et al., 2019), and the rs4818 polymorphism (C/G or Leu/Leu substitution) that affects COMT expression (Roussos et al., 2008). The COMT rs4680 polymorphism causes alterations in PFC DA levels, and it has been reported that its G allele is associated with SCZ (González‐Castro et al., 2016) and alterations in cognition and neuronal functions in SCZ patients (Egan et al., 2001). The rs4818 polymorphism is often transmitted with COMT rs4680 polymorphisms in a haploblock (Hirasawa‐Fujitaa et al., 2018; Perkovic et al., 2020), and it has been reported that a haplotype of COMT rs4818 and rs740603 polymorphisms is associated with negative SCZ symptoms (Li et al., 2012). Finally, as COMT maps to a region of chromosome 22 that is frequently deleted in patients with DiGeorge/velocardiofacial syndrome (22q11.2 deletion syndrome, or 22q11.2DS) (Drew et al., 2011; Karayiorgou et al., 2010), COMT deficiency and abnormal PFC DA levels may be involved in the cognitive deficits and behavioral abnormalities observed in 22q11.2DS patients.
The two biochemically distinct forms of the MAO mitochondrial enzyme (MAO‐A and MAO‐B) are encoded by different genes (Grimsby et al., 1991) and, despite being predominantly expressed in astrocytes (Duarte et al., 2021) play an important role in regulating DA levels. According to the DAergic theory of SCZ, low MAO activity may be a risk factor for the development of the disorder (Gassó et al., 2008) and, in addition to its general role in SCZ, MAO‐B plays a major role in the development of negative SCZ symptoms, as has been shown in a study of the positive effects of selegiline, a selective MAO‐B inhibitor, on the treatment of such symptoms (Amiri et al., 2008). It has been suggested that the rs1799836 polymorphism of the MAO‐B gene increases MAO‐B protein expression (Jakubauskiene et al., 2012), and that it is associated with the etiology of SCZ (Wei et al., 2011); it has also been found that another MAO‐B polymorphism, rs6651806, is associated with higher or lower levels of 3‐methoxy‐4‐hydroxyphenylglycol, one of the major monoamine metabolites measured in the cerebrospinal fluid of psychotic patients (Andreou et al., 2014). However, although the studies mentioned above have demonstrated links between the COMT rs4680 and rs4818 and MAO‐B rs1799836 polymorphisms in SCZ and its negative symptoms (Madzarac et al., 2021), their possible associations with astrocytic dysfunctions have not been investigated.
It has not yet been determined whether the ability of astrocytes to control DA in the PFC is involved in the pathophysiology of SCZ. However, on the basis of its crucial importance in regulating amines, some of the very rare human variants of VMAT2 have been associated with SCZ (Chu & Liu, 2010) and others with protection against alcohol neurotoxicity (Lin et al., 2005). Furthermore, recently described pathogenic variants in the SLC18A2 gene encoding VMAT2 cause severe forms of brain DA/serotonin vesicular transport disease and symptoms such as hypotonia, parkinsonism, tremor, developmental disability, and depression (Jacobsen et al., 2016; Rath et al., 2017; Rilstone et al., 2013).
5. DISCUSSION
Astrocytes, as an active component of the synapse since the early phase of postnatal development, not only promote synaptogenesis and regulate synaptic connectivity by influencing synapse formation, and maturation, but also maintain synaptic homeostasis and influence synaptic activity and plasticity by releasing neuroactive substances. Synaptic dysfunction is a prominent feature of various neurodevelopmental disorders, including SCZ, therefore, the importance of astrocytes is evident. As the synaptic connectivity occurs concomitantly to astrocytes maturation, it is also obvious that the cellular and molecular mechanisms regulating the transition toward the mature phenotype of astroglial cells play a crucial role for postnatal brain maturation and may be implicated in the pathogenesis of neurodevelopmental disorders and SCZ. The combination of data obtained from postmortem analyzes, genetic association studies and transcriptome investigations, induced pluripotent stem cell (iPSC) technologies as well as from studies of astrocytes in animal models have provided an unprecedented opportunity to establish first insights on the cell autonomous astroglial pathology associated to SCZ thus supporting the hypothesis that astrocytes have the capacity to be part of the cellular mechanisms involved in the pathogenesis of SCZ. The future perspective will involve a more in‐depth analysis and description of astrocytes dysfunctions in SCZ by using the combination of the above approaches in order to explore new therapeutic strategies for SCZ.
AUTHOR CONTRIBUTION
Paola Bezzi wrote the manuscript, Eva Cristina de Oliveira Figueiredo, Corrado Calì, Francesco Petrelli critically read and revised the manuscript.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
ACKNOWLEDGMENTS
We would like to thank Vigna Graphic Design for assistance in designing, developing, and reviewing graphics and artwork. This study was supported by grants from the Swiss National Foundation NCCR Switzerland “Synapsy” (51NF40‐158776), “Transcure” (51NF6240‐16) (to P.B.), Swiss National Science Foundation SNSF (26074366) (to P.B.) and Telethon Italy (GGP20037) (to P.B.). Open access funding provided by Universite de Lausanne.
de Oliveira Figueiredo, E. C. , Calì, C. , Petrelli, F. , & Bezzi, P. (2022). Emerging evidence for astrocyte dysfunction in schizophrenia. Glia, 70(9), 1585–1604. 10.1002/glia.24221
Funding information Fondazione Telethon, Grant/Award Number: GGP20037; Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung, Grant/Award Numbers: 26074366, 51NF40‐158776, 51NF6240‐16
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study
REFERENCES
- Adams, R. A., Pinotsis, D., Tsirlis, K., Unruh, L., Mahajan, A., Horas, A. M., Convertino, L., Summerfelt, A., Sampath, H., Du, X. M., Kochunov, P., Ji, J. L., Repovs, G., Murray, J. D., Friston, K. J., Hong, L. E., & Anticevic, A. (2022). Computational Modeling of Electroencephalography and Functional Magnetic Resonance Imaging Paradigms Indicates a Consistent Loss of Pyramidal Cell Synaptic Gain in Schizophrenia. Biological psychiatry, 91(2), 202–215. 10.1016/j.biopsych.2021.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agulhon, C. , Sun, M. Y. , Murphy, T. , Myers, T. , Lauderdale, K. , & Fiacco, T. A. (2012). Calcium signaling and gliotransmission in normal vs. Reactive astrocytes. Frontiers in Pharmacology, 3, 1–16. 10.3389/fphar.2012.00139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akil, M. , Pierri, J. N. , Whitehead, R. E. , Edgar, C. L. , Mohila, C. , Sampson, A. R. , & Lewis, D. A. (1999). Lamina‐specific alterations in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. American Journal of Psychiatry, 156(10), 1580–1589. 10.1176/ajp.156.10.1580 [DOI] [PubMed] [Google Scholar]
- Allen, N. J. , & Lyons, D. A. (2018). Glia as architects of central nervous system formation and function. Science, 362(6411), 181‐+. 10.1126/science.aat0473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, N. J. , Bennett, M. L. , Foo, L. C. , Wang, G. X. , Chakraborty, C. , Smith, S. J. , & Barres, B. A. (2012). Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature, 486(7403), 410–414. 10.1038/nature11059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, N. J. , & Eroglu, C. (2017). Cell biology of astrocyte‐synapse interactions. Neuron, 96(3), 697–708. 10.1016/j.neuron.2017.09.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiri, A. , Noorbala, A.‐A. , Nejatisafa, A.‐A. , Ghoreishi, A. , Derakhshan, M.‐K. , Khodaie‐Ardakani, M.‐R. , Hajiazim, M. , Raznahan, M. , & Akhondzadeh, S. (2008). Efficacy of selegiline add on therapy to risperidone in the treatment of the negative symptoms of schizophrenia: A double‐blind randomized placebo‐controlled study. Human Psychopharmacology: Clinical and Experimental, 23, 79–86. 10.1002/hup [DOI] [PubMed] [Google Scholar]
- Andreou, D. , Söderman, E. , Axelsson, T. , Sedvall, G. C. , Terenius, L. , Agartz, I. , & Jönsson, E. G. (2014). Polymorphisms in genes implicated in dopamine, serotonin and noradrenalin metabolism suggest association with cerebrospinal fluid monoamine metabolite concentrations in psychosis. Behavioral and Brain Functions, 10(1), 1–10. 10.1186/1744-9081-10-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque, A. , Carmignoto, G. , Haydon, P. G. , Oliet, S. H. R. , Robitaille, R. , & Volterra, A. (2014). Gliotransmitters travel in time and space. Neuron, 81(4), 728–739. 10.1016/j.neuron.2014.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arion, D. , Huo, Z. , Enwright, J. F. , Corradi, J. P. , Tseng, G. , & Lewis, D. A. (2017). Transcriptome alterations in prefrontal pyramidal cells distinguish schizophrenia from bipolar and major depressive disorders. Biological Psychiatry, 82(8), 594–600. 10.1016/j.biopsych.2017.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten, A. F. T. , & Shansky, R. M. (2004). Adolescence: Vulnerable period for stress‐induced prefrontal cortical function? Introduction to part IV. Annals of the New York Academy of Sciences, 1021, 143–147. 10.1196/annals.1308.017 [DOI] [PubMed] [Google Scholar]
- Ayalew, M. , Le‐Niculescu, H. , Levey, D. F. , Jain, N. , Changala, B. , Patel, S. D. , Winiger, E. , Breier, A. , Shekhar, A. , Amdur, R. , Koller, D. , Nurnberger, J. I. , Corvin, A. , Geyer, M. , Tsuang, M. T. , Salomon, D. , Schork, N. J. , Fanous, A. H. , O'Donovan, M. C. , & Niculescu, A. B. (2012). Convergent functional genomics of schizophrenia: From comprehensive understanding to genetic risk prediction. Molecular Psychiatry, 17(9), 887–905. 10.1038/mp.2012.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baev, A. Y. , Vinokurov, A. Y. , Novikova, I. N. , Dremin, V. V. , Potapova, E. V. , & Abramov, A. Y. (2022). Interaction of mitochondrial calcium and ROS in neurodegeneration. Cell, 11(4), 1–17. 10.3390/cells11040706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balu, D. T. (2016). The NMDA receptor and schizophrenia. From pathophysiology to treatment. In Advances in pharmacology (Vol. 76, 1st ed., pp. 351–382). Elsevier Inc. 10.1016/bs.apha.2016.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandeira, F. , Lent, R. , & Herculano‐Houzel, S. (2009). Changing numbers of neuronal and non‐neuronal cells underlie postnatal brain growth in the rat. Proceedings of the National Academy of Sciences of the United States of America, 106(33), 14108–14113. 10.1073/pnas.0804650106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee, J. , Banerjee Dixit, A. , Tripathi, M. , Sarkar, C. , Gupta, Y. K. , & Chandra, P. S. (2015). Enhanced endogenous activation of NMDA receptors in pyramidal neurons of hippocampal tissues from patients with mesial temporal lobe epilepsy: A mechanism of hyper excitation. Epilepsy Research, 117, 11–16. 10.1016/j.eplepsyres.2015.08.007 [DOI] [PubMed] [Google Scholar]
- Bellesi, M. , Melone, M. , Gubbini, A. , Battistacci, S. , & Conti, F. (2009). GLT‐1 upregulation impairs prepulse inhibition of the startle reflex in adult rats. Glia, 57(7), 703–713. 10.1002/glia.20798 [DOI] [PubMed] [Google Scholar]
- Bellone, C. , & Nicoll, R. A. (2007). Rapid bidirectional switching of synaptic NMDA receptors. Neuron, 55(5), 779–785. 10.1016/j.neuron.2007.07.035 [DOI] [PubMed] [Google Scholar]
- Bendikov, I. , Nadri, C. , Amar, S. , Panizzutti, R. , De Miranda, J. , Wolosker, H. , & Agam, G. (2007). A CSF and postmortem brain study of d‐serine metabolic parameters in schizophrenia. Schizophrenia Research, 90(1–3), 41–51. 10.1016/j.schres.2006.10.010 [DOI] [PubMed] [Google Scholar]
- Berkel, S. , Marshall, C. R. , Weiss, B. , Howe, J. , Roeth, R. , Moog, U. , Endris, V. , Roberts, W. , Szatmari, P. , Pinto, D. , Bonin, M. , Riess, A. , Engels, H. , Sprengel, R. , Scherer, S. W. , & Rappold, G. A. (2010). Mutations in the SHANK2 synaptic scaffolding gene in autism spectrum disorder and mental retardation. Nature Genetics, 42(6), 489–491. 10.1038/ng.589 [DOI] [PubMed] [Google Scholar]
- Bernstein, H. G. , Steiner, J. , Guest, P. C. , Dobrowolny, H. , & Bogerts, B. (2015). Glial cells as key players in schizophrenia pathology: Recent insights and concepts of therapy. Schizophrenia Research, 161(1), 4–18. 10.1016/j.schres.2014.03.035 [DOI] [PubMed] [Google Scholar]
- Bezzi, P. , & Volterra, A. (2001). A neuron‐glia signalling network in the active brain. Current Opinion in Neurobiology, 11(3), 387–394. 10.1016/s0959-4388(00)00223-3 [DOI] [PubMed] [Google Scholar]
- Bhide, P. G. (2009). Dopamine, cocaine and the development of cerebral cortical cytoarchitecture: A review of current concepts. Seminars in Cell and Developmental Biology, 20(4), 395–402. 10.1016/j.semcdb.2009.01.006 [DOI] [PubMed] [Google Scholar]
- Bilder, R. M. , Volavka, J. , Czobor, P. , Malhotra, A. K. , Kennedy, J. L. , Ni, X. , Goldman, R. S. , Hoptman, M. J. , Sheitman, B. , Lindenmayer, J. P. , Citrome, L. , McEvoy, J. P. , Kunz, M. , Chakos, M. , Cooper, T. B. , & Lieberman, J. A. (2002). Neurocognitive correlates of the COMT Val158Met polymorphism in chronic schizophrenia. Biological Psychiatry, 52(7), 701–707. 10.1016/S0006-3223(02)01416-6 [DOI] [PubMed] [Google Scholar]
- Birnbaum, R. , & Weinberger, D. R. (2020). A genetics perspective on the role of the (neuro)immune system in schizophrenia. Schizophrenia Research, 217, 105–113. 10.1016/j.schres.2019.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björklund, A. , & Dunnett, S. B. (2007). Dopamine neuron systems in the brain: An update. Trends in Neurosciences, 30(5), 194–202. 10.1016/j.tins.2007.03.006 [DOI] [PubMed] [Google Scholar]
- Black, J. E. , Kodish, I. M. , Grossman, A. W. , Klintsova, A. Y. , Ph, D. , Orlovskaya, D. , Ph, D. , Sci, D. M. , Vostrikov, V. , Ph, D. , Uranova, N. , Ph, D. , Sci, D. M. , Greenough, W. T. , & Ph, D. (2004). Pathology of layer V pyramidal neurons in the prefrontal cortex of patients with schizophrenia. American Journal of Psychiatry, 161, 742–744. [DOI] [PubMed] [Google Scholar]
- Boisvert, M. M. , Erikson, G. A. , Shokhirev, M. N. , & Allen, N. J. (2018). The aging astrocyte transcriptome from multiple regions of the mouse brain. Cell Reports, 22(1), 269–285. 10.1016/j.celrep.2017.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boks, M. P. M. , Rietkerk, T. , van de Beek, M. H. , Sommer, I. E. , de Koning, T. J. , & Kahn, R. S. (2007). Reviewing the role of the genes G72 and DAAO in glutamate neurotransmission in schizophrenia. European Neuropsychopharmacology, 17(9), 567–572. 10.1016/j.euroneuro.2006.12.003 [DOI] [PubMed] [Google Scholar]
- Brennand, K. , Savas, J. N. , Kim, Y. , Tran, N. , Simone, A. , Hashimoto‐Torii, K. , Beaumont, K. G. , Kim, H. J. , Topol, A. , Ladran, I. , Abdelrahim, M. , Matikainen‐Ankney, B. , Chao, S. H. , Mrksich, M. , Rakic, P. , Fang, G. , Zhang, B. , Yates, J. R. , & Gage, F. H. (2015). Phenotypic differences in hiPSC NPCs derived from patients with schizophrenia. Molecular Psychiatry, 20(3), 361–368. 10.1038/mp.2014.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbaeva, G. S. , Boksha, I. S. , Turishcheva, M. S. , Vorobyeva, E. A. , Savushkina, O. K. , & Tereshkina, E. B. (2003). Glutamine synthetase and glutamate dehydrogenase in the prefrontal cortex of patients with schizophrenia. Progress in Neuro‐Psychopharmacology and Biological Psychiatry, 27(4), 675–680. 10.1016/S0278-5846(03)00078-2 [DOI] [PubMed] [Google Scholar]
- Burnet, P. W. J. , Anderson, P. N. , Chen, L. , Nikiforova, N. , Harrison, P. J. , & Wood, M. J. A. (2011). D‐amino acid oxidase knockdown in the mouse cerebellum reduces NR2A mRNA. Molecular and Cellular Neuroscience, 46(1), 167–175. 10.1016/j.mcn.2010.08.018 [DOI] [PubMed] [Google Scholar]
- Buscemi, L. , Ginet, V. , Lopatar, J. , Montana, V. , Pucci, L. , Spagnuolo, P. , Zehnder, T. , Grubišić, V. , Truttman, A. , Sala, C. , Hirt, L. , Parpura, V. , Puyal, J. , & Bezzi, P. (2017). Homer1 scaffold proteins govern Ca2+ dynamics in normal and reactive astrocytes. Cerebral Cortex, 27(3), 2365–2384. 10.1093/cercor/bhw078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushong, E. A. , Martone, M. E. , & Ellisman, M. H. (2004). Maturation of astrocyte morphology and the establishment of astrocyte domains during postnatal hippocampal development. International Journal of Developmental Neuroscience, 22(2), 73–86. 10.1016/j.ijdevneu.2003.12.008 [DOI] [PubMed] [Google Scholar]
- Bushong, E. A. , Martone, M. E. , Jones, Y. Z. , & Ellisman, M. H. (2002). Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. The Journal of Neuroscience, 22(1), 183–192. 10.1523/jneurosci.22-01-00183.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahoy, J. D. , Emery, B. , Kaushal, A. , Foo, L. C. , Zamanian, J. L. , Christopherson, K. S. , Xing, Y. , Lubischer, J. L. , Krieg, P. A. , Krupenko, S. A. , Thompson, W. J. , & Barres, B. A. (2008). A transcriptome database for astrocytes, neurons, and oligodendrocytes: A new resource for understanding brain development and function. Journal of Neuroscience, 28(1), 264–278. 10.1523/JNEUROSCI.4178-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcia, M. A. , Madeira, C. , Alheira, F. V. , Silva, T. C. S. , Tannos, F. M. , Vargas‐Lopes, C. , Goldenstein, N. , Brasil, M. A. , Ferreira, S. T. , & Panizzutti, R. (2012). Plasma levels of D‐serine in Brazilian individuals with schizophrenia. Schizophrenia Research, 142(1–3), 83–87. 10.1016/j.schres.2012.09.014 [DOI] [PubMed] [Google Scholar]
- Caldinelli, L. , Sacchi, S. , Molla, G. , Nardini, M. , & Pollegioni, L. (2013). Characterization of human DAAO variants potentially related to an increased risk of schizophrenia. Biochimica et Biophysica Acta ‐ Molecular Basis of Disease, 1832(3), 400–410. 10.1016/j.bbadis.2012.11.019 [DOI] [PubMed] [Google Scholar]
- Calì, C. , Agus, M. , Kare, K. , Boges, D. J. , Lehväslaiho, H. , Hadwiger, M. , & Magistretti, P. J. (2019). 3D cellular reconstruction of cortical glia and parenchymal morphometric analysis from serial block‐face electron microscopy of juvenile rat. Progress in Neurobiology, 183(September), 101696. 10.1016/j.pneurobio.2019.101696 [DOI] [PubMed] [Google Scholar]
- Cali, C. , Lopatar, J. , Petrelli, F. , Pucci, L. , & Bezzi, P. (2014). G‐protein coupled receptor‐evoked glutamate exocytosis from astrocytes: Role of prostaglandins. Neural Plasticity, 2014, 1–11. 10.1155/2014/254574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calì, C. , Marchaland, J. , Spagnuolo, P. , Gremion, J. , & Bezzi, P. (2009). Regulated exocytosis from astrocytes: Physiological and pathological related aspects. International Review of Neurobiology, 85(09), 261–293. 10.1016/S0074-7742(09)85020-4 [DOI] [PubMed] [Google Scholar]
- Carlsson, A. , & Carlsson, M. L. (2006). A dopaminergic deficit hypothesis of schizophrenia: The path to discovery. Dialogues in Clinical Neuroscience, 8(1), 137–142. 10.31887/dcns.2006.8.1/acarlsson [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera, N. , Arrojo, M. , Sanjuán, J. , Ramos‐Ríos, R. , Paz, E. , Suárez‐Rama, J. J. , Páramo, M. , Agra, S. , Brenlla, J. , Martínez, S. , Rivero, O. , Collier, D. A. , Palotie, A. , Cichon, S. , Nöthen, M. M. , Rietschel, M. , Rujescu, D. , Stefansson, H. , Steinberg, S. , … Costas, J. (2012). Association study of nonsynonymous single nucleotide polymorphisms in schizophrenia. Biological Psychiatry, 71(2), 169–177. 10.1016/j.biopsych.2011.09.032 [DOI] [PubMed] [Google Scholar]
- Catuara‐Solarz, S. , Espinosa‐Carrasco, J. , Erb, I. , Langohr, K. , Gonzalez, J. R. , Notredame, C. , & Dierssen, M. (2016). Combined treatment with environmental enrichment and (−)‐Epigallocatechin‐3‐Gallate ameliorates learning deficits and hippocampal alterations in a mouse model of down syndrome. ENeuro, 3(5), 1–18. 10.1523/ENEURO.0103-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavda, V. , Singh, K. , Patel, V. , Mishra, M. , & Mishra, A. K. (2022). Neuronal glial crosstalk: Specific and shared mechanisms in Alzheimer's disease. Brain Sciences, 12(1), 75. 10.3390/brainsci12010075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, A. , Wan, R. , Yang, J. , Kamimura, N. , Son, T. G. , Ouyang, X. , Luo, Y. , Okun, E. , & Mattson, M. P. (2012). Involvement of PGC‐1a in the formation and maintenance of neuronal dendritic spines. Nature Communications, 3, 1212–1250. 10.1038/ncomms2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow, E. W. C. , Watson, M. , Young, D. A. , & Bassett, A. S. (2006). Neurocognitive profile in 22q11 deletion syndrome and schizophrenia. Schizophrenia Research, 87(1–3), 270–278. 10.1016/j.schres.2006.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, T. , & Liu, Y . An integrated genomic analysis of gene‐function correlation on schizophrenia susceptibility genes. Journal of Human Genetics, 55, 285–292 (2010). 10.1038/jhg.2010.24 [DOI] [PubMed] [Google Scholar]
- Chung, W. S. , Allen, N. J. , & Eroglu, C. (2015). Astrocytes control synapse formation, function, and elimination. Cold Spring Harbor Perspectives in Biology, 7(9), a020370. 10.1101/cshperspect.a020370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke, L. E. , & Barres, B. A. (2013). Emerging roles of astrocytes in neural circuit development. Nature Reviews Neuroscience, 14(5), 311–321. 10.1038/nrn3484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corkrum, M. , & Araque, A. (2021). Astrocyte‐neuron signaling in the mesolimbic dopamine system: The hidden stars of dopamine signaling. Neuropsychopharmacology, 46(11), 1864–1872. 10.1038/s41386-021-01090-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchet, J. , Lewis, T. L. , Lee, S. , Courchet, V. , Liou, D. Y. , Aizawa, S. , & Polleux, F. (2013). Terminal axon branching is regulated by the LKB1‐NUAK1 kinase pathway via presynaptic mitochondrial capture. Cell, 153(7), 1510–1525. 10.1016/j.cell.2013.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresto, N. , Pillet, L. E. , Billuart, P. , & Rouach, N. (2019). Do astrocytes play a role in intellectual disabilities? Trends in Neurosciences, 42(8), 518–527. 10.1016/j.tins.2019.05.011 [DOI] [PubMed] [Google Scholar]
- Cui, M. , Aras, R. , Christian, W. V. , Rappold, P. M. , Hatwar, M. , Panza, J. , Jackson‐Lewis, V. , Javitch, J. A. , Ballatori, N. , Przedborski, S. , & Tieu, K. (2009). The organic cation transporter‐3 is a pivotal modulator of neurodegeneration in the nigrostriatal dopaminergic pathway. Proceedings of the National Academy of Sciences of the United States of America, 106(19), 8043–8048. 10.1073/pnas.0900358106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, J. , Eyre, H. , Jacka, F. N. , Dodd, S. , Dean, O. , McEwen, S. , Debnath, M. , McGrath, J. , Maes, M. , Amminger, P. , McGorry, P. D. , Pantelis, C. , & Berk, M. (2016). A review of vulnerability and risks for schizophrenia: Beyond the two hit hypothesis. Neuroscience and Biobehavioral Reviews, 65, 185–194. 10.1016/j.neubiorev.2016.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre, R. , de Sola, S. , Hernandez, G. , Farré, M. , Pujol, J. , Rodriguez, J. , Espadaler, J. M. , Langohr, K. , Cuenca‐Royo, A. , Principe, A. , Xicota, L. , Janel, N. , Catuara‐Solarz, S. , Sanchez‐Benavides, G. , Bléhaut, H. , del Hoyo, L. , Benejam, B. , Blanco‐Hinojo, L. , Videla, S. , … Freixas, R. (2016). Safety and efficacy of cognitive training plus epigallocatechin‐3‐gallate in young adults with Down's syndrome (TESDAD): A double‐blind, randomised, placebo‐controlled, phase 2 trial. The Lancet Neurology, 15(8), 801–810. 10.1016/S1474-4422(16)30034-5 [DOI] [PubMed] [Google Scholar]
- de la Torre, R. , De Sola, S. , Pons, M. , Duchon, A. , de Lagran, M. M. , Farré, M. , Fitó, M. , Benejam, B. , Langohr, K. , Rodriguez, J. , Pujadas, M. , Bizot, J. C. , Cuenca, A. , Janel, N. , Catuara, S. , Covas, M. I. , Blehaut, H. , Herault, Y. , Delabar, J. M. , & Dierssen, M. (2014). Epigallocatechin‐3‐gallate, a DYRK1A inhibitor, rescues cognitive deficits in down syndrome mouse models and in humans. Molecular Nutrition and Food Research, 58(2), 278–288. 10.1002/mnfr.201300325 [DOI] [PubMed] [Google Scholar]
- De Sousa, R. A. L. (2022). Reactive gliosis in Alzheimer's disease: A crucial role for cognitive impairment and memory loss. Metabolic Brain Disease, 851–857, 851–857. 10.1007/s11011-022-00953-2 [DOI] [PubMed] [Google Scholar]
- Derouiche, A. , & Frotscher, M. (2001). Peripheral astrocyte processes: Monitoring by selective immunostaining for the actin‐binding ERM proteins. Glia, 36(3), 330–341. 10.1002/glia.1120 [DOI] [PubMed] [Google Scholar]
- Devaraju, P. , Yu, J. , Eddins, D. , Mellado‐Lagarde, M. M. , Earls, L. R. , Westmoreland, J. J. , Quarato, G. , Green, D. R. , & Zakharenko, S. S. (2017). Haploinsufficiency of the 22q11.2 microdeletion gene Mrpl40 disrupts short‐term synaptic plasticity and working memory through dysregulation of mitochondrial calcium. Molecular Psychiatry, 22(9), 1313–1326. 10.1038/mp.2016.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraju, P. , & Zakhareko, S. S. (2017). Mitochondria in complex psychiatric disorders: Lessons from mouse models of 22q11.2 deletion syndrome: Hemizygous deletion of several mitochondrial genes in the 22q11.2 genomic region can lead to symptoms associated with neuropsychiatric disease. BioEssays, 39(2), 1–11. 10.1002/bies.201600177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz, A. G. , Goldman, S. A. , & Nedergaard, M. (2020). Glial cells in schizophrenia: A unified hypothesis. Lancet Psychiatry, 7(3), 272–281. 10.1016/S2215-0366(19)30302-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz, L. P. , Almeida, J. C. , Tortelli, V. , Lopes, C. V. , Setti‐Perdigão, P. , Stipursky, J. , Kahn, S. A. , Romão, L. F. , De Miranda, J. , Alves‐Leon, S. V. , De Souza, J. M. , Castro, N. G. , Panizzutti, R. , & Gomes, F. C. A. (2012). Astrocyte‐induced synaptogenesis is mediated by transforming growth factor β signaling through modulation of d‐serine levels in cerebral cortex neurons. Journal of Biological Chemistry, 287(49), 41432–41445. 10.1074/jbc.M112.380824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dityatev, A. , & Rusakov, D. A. (2011). Molecular signals of plasticity at the tetrapartite synapse. Current Opinion in Neurobiology, 21(2), 353–359. 10.1016/j.conb.2010.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew, L. J. , Crabtree, G. W. , Markx, S. , Stark, K. L. , Chaverneff, F. , Xu, B. , Mukai, J. , Fenelon, K. , Hsu, P. K. , Gogos, J. A. , & Karayiorgou, M. (2011). The 22q11.2 microdeletion: Fifteen years of insights into the genetic and neural complexity of psychiatric disorders. International Journal of Developmental Neuroscience, 29(3), 259–281. 10.1016/j.ijdevneu.2010.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte, P. , Cuadrado, A. , & León, R. (2021). Monoamine oxidase inhibitors: From classic to new clinical approaches. Handbook of Experimental Pharmacology, 264, 229–259. 10.1007/164_2020_384 [DOI] [PubMed] [Google Scholar]
- Edwards, R. H. (2007). The neurotransmitter cycle and quantal size. Neuron, 55(6), 835–858. 10.1016/j.neuron.2007.09.001 [DOI] [PubMed] [Google Scholar]
- Egan, M. F. , Goldberg, T. E. , Kolachana, B. S. , Callicott, J. H. , Mazzanti, C. M. , Straub, R. E. , Goldman, D. , & Weinberger, D. R. (2001). Effect of COMT Val108/158 met genotype on frontal lobe function and risk for schizophrenia. Proceedings of the National Academy of Sciences of the United States of America, 98(12), 6917–6922. 10.1073/pnas.111134598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu, C. , & Barres, B. A. (2010). Regulation of synaptic connectivity by glia. Nature, 468(7321), 223–231. 10.1038/nature09612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escartin, C. , Galea, E. , Lakatos, A. , O'Callaghan, J. P. , Petzold, G. C. , Serrano‐Pozo, A. , Steinhäuser, C. , Volterra, A. , Carmignoto, G. , Agarwal, A. , Allen, N. J. , Araque, A. , Barbeito, L. , Barzilai, A. , Bergles, D. E. , Bonvento, G. , Butt, A. M. , Chen, W. T. , Cohen‐Salmon, M. , … Verkhratsky, A. (2021). Reactive astrocyte nomenclature, definitions, and future directions. Nature Neuroscience, 24(3), 312–325. 10.1038/s41593-020-00783-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhy‐Tselnicker, I. , van Casteren, A. C. M. , Lee, A. , Chang, V. T. , Aricescu, A. R. , & Allen, N. J. (2017). Astrocyte‐secreted Glypican 4 regulates release of neuronal Pentraxin 1 from axons to induce functional synapse formation. Neuron, 96(2), 428‐+. 10.1016/j.neuron.2017.09.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhy‐Tselnicker, I. , & Allen, N. J. (2018). Astrocytes, neurons, synapses: A tripartite view on cortical circuit development. Neural Development, 13(1), 1–12. 10.1186/s13064-018-0104-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi, S. H. , & Folsom, T. D. (2009). The neurodevelopmental hypothesis of schizophrenia, revisited. Schizophrenia Bulletin, 35(3), 528–548. 10.1093/schbul/sbn187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez, A. , Meechan, D. W. , Karpinski, B. A. , Paronett, E. M. , Bryan, C. A. , Rutz, H. L. , Radin, E. A. , Lubin, N. , Bonner, E. R. , Popratiloff, A. , Rothblat, L. A. , Maynard, T. M. , & LaMantia, A. S. (2019). Mitochondrial dysfunction leads to cortical under‐connectivity and cognitive impairment. Neuron, 102(6), 1127–1142.e3. 10.1016/j.neuron.2019.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fon, E. A. , Pothos, E. N. , Sun, B. C. , Killeen, N. , Sulzer, D. , & Edwards, R. H. (1997). Vesicular transport regulates monoamine storage and release but is not essential for amphetamine action. Neuron, 19(6), 1271–1283. 10.1016/S0896-6273(00)80418-3 [DOI] [PubMed] [Google Scholar]
- Fossat, P. , Turpin, F. R. , Sacchi, S. , Dulong, J. , Shi, T. , Rivet, J. M. , Sweedler, J. V. , Pollegioni, L. , Millan, M. J. , Oliet, S. H. R. , & Mothet, J. P. (2012). Glial D‐serine gates NMDA receptors at excitatory synapses in prefrontal cortex. Cerebral Cortex, 22(3), 595–606. 10.1093/cercor/bhr130 [DOI] [PubMed] [Google Scholar]
- Freeman, M. R. (2010). Specification and morphogenesis of astrocytes. Science, 330(6005), 774–778. 10.1126/science.1190928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman, J. R. , & Nunnari, J. (2014). Mitochondrial form and function. Nature, 505(7483), 335–343. 10.1038/nature12985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandal, M. J. , Haney, J. R. , Parikshak, N. N. , Leppa, V. , Ramaswami, G. , Hartl, C. , Schork, A. J. , Appadurai, V. , Buil, A. , Werge, T. M. , Liu, C. , White, K. P. , Horvath, S. , & Geschwind, D. H. (2018). Shared molecular neuropathology across major psychiatric disorders parallels polygenic overlap. Science, 359(6376), 693–697. 10.1126/science.aad6469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandal, M. J. , Zhang, P. , Hadjimichael, E. , Walker, R. L. , Chen, C. , Liu, S. , Won, H. , Van Bakel, H. , Varghese, M. , Wang, Y. , Shieh, A. W. , Haney, J. , Parhami, S. , Belmont, J. , Kim, M. , Losada, P. M. , Khan, Z. , Mleczko, J. , Xia, Y. , … Geschwind, D. H. (2018). Transcriptome‐wide isoform‐level dysregulation in ASD, schizophrenia, and bipolar disorder. Science, 362(6420), eaat8127. 10.1126/science.aat8127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garey, L. J. , Ong, W. Y. , Patel, T. S. , Kanani, M. , Davis, A. , Mortimer, A. M. , Barnes, T. R. E. , & Hirsch, S. R. (1998). Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. Journal of Neurology Neurosurgery and Psychiatry, 65(4), 446–453. 10.1136/jnnp.65.4.446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassó, P. , Bernardo, M. , Mas, S. , Crescenti, A. , Garcia, C. , Parellada, E. , & Lafuente, A. (2008). Association of a/G polymorphism in intron 13 of the monoamine oxidase B gene with schizophrenia in a Spanish population. Neuropsychobiology, 58(2), 65–70. 10.1159/000159774 [DOI] [PubMed] [Google Scholar]
- Gauthier, J. , Champagne, N. , Lafrenière, R. G. , Xiong, L. , Spiegelman, D. , Brustein, E. , Lapointe, M. , Peng, H. , Côté, M. , Noreau, A. , Hamdan, F. F. , Addington, A. M. , Rapoport, J. L. , DeLisi, L. E. , Krebs, M. O. , Joober, R. , Fathalli, F. , Mouaffak, F. , Haghighi, A. P. , … Gourion, D. (2010). De novo mutations in the gene encoding the synaptic scaffolding protein SHANK3 in patients ascertained for schizophrenia. Proceedings of the National Academy of Sciences of the United States of America, 107(17), 7863–7868. 10.1073/pnas.0906232107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge, W. P. , Miyawaki, A. , Gage, F. H. , Jan, Y. N. , & Jan, L. Y. (2012). Local generation of glia is a major astrocyte source in postnatal cortex. Nature, 484(7394), 376–380. 10.1038/nature10959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs, S. E. B. , & D'Esposito, M. (2005). Individual capacity differences predict working memory performance and prefrontal activity following dopamine receptor stimulation. Cognitive, Affective, & Behavioral Neuroscience, 5(2), 212–221. 10.3758/CABN.5.2.212 [DOI] [PubMed] [Google Scholar]
- Glatt, S. J. , Faraone, S. V. , & Tsuang, M. T. (2003). Meta‐analysis identifies an association between the dopamine D2 receptor gene and schizophrenia. Molecular Psychiatry, 8(11), 911–915. 10.1038/sj.mp.4001321 [DOI] [PubMed] [Google Scholar]
- Glessner, J. T. , Reilly, M. P. , Kim, C. E. , Takahashi, N. , Albano, A. , Hou, C. , Bradfield, J. P. , Zhang, H. , Sleiman, P. M. A. , Flory, J. H. , Imielinski, M. , Frackelton, E. C. , Chiavacci, R. , Thomas, K. A. , Garris, M. , Otieno, F. G. , Davidson, M. , Weiser, M. , Reichenberg, A. , … Hakonarson, H. (2010). Strong synaptic transmission impact by copy number variations in schizophrenia. Proceedings of the National Academy of Sciences of the United States of America, 107(23), 10584–10589. 10.1073/pnas.1000274107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokhale, A. , Freeman, A. A. H. , Hartwig, C. , Bassell, J. L. , Zlatic, S. A. , Sapp, C. , Vadlamudi, T. , Abudulai, F. , Crocker, A. , Werner, E. , Wen, Z. , Repetto, G. M. , Gogos, J. A. , Claypool, S. M. , Forsyth, J. K. , Bearden, C. , Gausier, J. , Lewis, D. A. , Seyfried, N. T. , & Faundez, V. (2019). Systems analysis of the 22q11.2 microdeletion syndrome converges on a mitochondrial interactome necessary for synapse function and behavior. The Journal of Neuroscience, 39(18), 3561–3581. 10.1101/315143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman‐Rakic, P. S. , & Selemon, L. D. (1997). Functional and anatomical aspects of prefrontal pathology in schizophrenia. Schizophrenia Bulletin, 23(3), 437–458. 10.1093/schbul/23.3.437 [DOI] [PubMed] [Google Scholar]
- Gomes, F. V. , Zhu, X. , & Grace, A. A. (2019). Stress during critical periods of development and risk for schizophrenia. Schizophrenia Research, 213, 107–113. 10.1016/j.schres.2019.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González‐Castro, T. B. , Hernández‐Díaz, Y. , Juárez‐Rojop, I. E. , López‐Narváez, M. L. , Tovilla‐Zárate, C. A. , & Fresan, A. (2016). The role of a catechol‐O‐Methyltransferase (COMT) Val158Met genetic polymorphism in schizophrenia: A systematic review and updated meta‐analysis on 32,816 subjects. Neuromolecular Medicine, 18(2), 216–231. 10.1007/s12017-016-8392-z [DOI] [PubMed] [Google Scholar]
- Grace, A. A. , & Gomes, F. V. (2019). The circuitry of dopamine system regulation and its disruption in schizophrenia: Insights into treatment and prevention. Schizophrenia Bulletin, 45(1), 148–157. 10.1093/schbul/sbx199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, I. W. , & Glausier, J. R. (2016). Different paths to core pathology: The equifinal model of the schizophrenia syndrome. Schizophrenia Bulletin, 42(3), 542–549. 10.1093/schbul/sbv136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsby, J. , Chen, K. , Wang, L. J. , Lan, N. C. , & Shih, J. C. (1991). Human monoamine oxidase a and B genes exhibit identical exon‐intron organization. Proceedings of the National Academy of Sciences of the United States of America, 88(9), 3637–3641. 10.1073/pnas.88.9.3637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habbas, S. , Santello, M. , Becker, D. , Stubbe, H. , Zappia, G. , Liaudet, N. , Klaus, F. R. , Kollias, G. , Fontana, A. , Pryce, C. R. , Suter, T. , & Volterra, A. (2015). Neuroinflammatory TNFα impairs memory via astrocyte signaling. Cell, 163(7), 1730–1741. 10.1016/j.cell.2015.11.023 [DOI] [PubMed] [Google Scholar]
- Habl, G. , Zink, M. , Petroianu, G. , Bauer, M. , Schneider‐Axmann, T. , Von Wilmsdorff, M. , Falkai, P. , Henn, F. A. , & Schmitt, A. (2009). Increased d‐amino acid oxidase expression in the bilateral hippocampal CA4 of schizophrenic patients: A post‐mortem study. Journal of Neural Transmission, 116(12), 1657–1665. 10.1007/s00702-009-0312-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadar, R. , Bikovski, L. , Soto‐Montenegro, M. L. , Schimke, J. , Maier, P. , Ewing, S. , Voget, M. , Wieske, F. , Götz, T. , Desco, M. , Hamani, C. , Pascau, J. , Weiner, I. , & Winter, C. (2018). Early neuromodulation prevents the development of brain and behavioral abnormalities in a rodent model of schizophrenia. Molecular Psychiatry, 23(4), 943–951. 10.1038/mp.2017.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa, M. M. , Fellin, T. , & Haydon, P. G. (2007). The tripartite synapse: Roles for gliotransmission in health and disease. Trends in Molecular Medicine, 13(2), 54–63. 10.1016/j.molmed.2006.12.005 [DOI] [PubMed] [Google Scholar]
- Halassa, M. M. , Fellin, T. , Takano, H. , Dong, J. H. , & Haydon, P. G. (2007). Synaptic islands defined by the territory of a single astrocyte. Journal of Neuroscience, 27(24), 6473–6477. 10.1523/JNEUROSCI.1419-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, J. , & Bray, N. J. (2021). Schizophrenia genomics: Convergence on synaptic development, adult synaptic plasticity, or both? Biological Psychiatry, 91, 709–717. 10.1016/j.biopsych.2021.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton, T. J. , Wheatley, B. M. , Sinclair, D. B. , Bachmann, M. , Larkum, M. E. , & Colmers, W. F. (2010). Dopamine modulates synaptic plasticity in dendrites of rat and human dentate granule cells. Proceedings of the National Academy of Sciences of the United States of America, 107(42), 18185–18190. 10.1073/pnas.1011558107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto, T. , Volk, D. W. , Eggan, S. M. , Mirnics, K. , Pierri, J. N. , Sun, Z. , Sampson, A. R. , & Lewis, D. A. (2003). Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. Journal of Neuroscience, 23(15), 6315–6326. 10.1523/jneurosci.23-15-06315.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers, S. , & Konradi, C. (2015). GABAergic mechanisms of hippocampal hyperactivity in schizophrenia. Schizophrenia Research, 167(1–3), 4–11. 10.1016/j.schres.2014.09.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneberger, C. , Papouin, T. , Oliet, S. H. R. , & Rusakov, D. A. (2010). Long‐term potentiation depends on release of d‐serine from astrocytes. Nature, 463(7278), 232–236. 10.1038/nature08673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herédi, J. , Berkó, A. M. , Jankovics, F. , Iwamori, T. , Iwamori, N. , Ono, E. , Horváth, S. , Kis, Z. , Toldi, J. , Vécsei, L. , & Gellért, L. (2017). Astrocytic and neuronal localization of kynurenine aminotransferase‐2 in the adult mouse brain. Brain Structure and Function, 222(4), 1663–1672. 10.1007/s00429-016-1299-5 [DOI] [PubMed] [Google Scholar]
- Hilker, R. , Helenius, D. , Fagerlund, B. , Skytthe, A. , Christensen, K. , Werge, T. M. , Nordentoft, M. , & Glenthøj, B. (2018). Heritability of schizophrenia and schizophrenia spectrum based on the nationwide danish twin register. Biological Psychiatry, 83(6), 492–498. 10.1016/j.biopsych.2017.08.017 [DOI] [PubMed] [Google Scholar]
- Hirasawa‐Fujitaa, M. , Tudor, L. , Nikolac Pekovic, M. , Kozumplik, O. , Nedic Erjavec, G. , Uzun, S. , Svob Strac, D. , Konjevod, M. , Mimica, N. , Domino, E. , & Pivac, N. (2018). Genotypic and haplotypic associations of catechol‐O‐methyltransferase (COMT) rs4680 and rs4818 with salivary cortisol in patients with schizophrenia. Psychiatry Research, 259(October 2017), 262–264. 10.1016/j.psychres.2017.10.037 [DOI] [PubMed] [Google Scholar]
- Hjelm, B. E. , Rollins, B. , Mamdani, F. , Lauterborn, J. C. , Kirov, G. , Lynch, G. , Gall, C. M. , Sequeira, A. , & Vawter, M. P. (2015). Evidence of mitochondrial dysfunction within the complex genetic etiology of schizophrenia. Molecular Neuropsychiatry, 1(4), 201–219. 10.1159/000441252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, W. , Macdonald, M. L. , Elswick, D. E. , & Sweet, R. A. (2015). The glutamate hypothesis of schizophrenia: Evidence from human brain tissue studies. Annals of the New York Academy of Sciences, 1338(1), 38–57. 10.1111/nyas.12547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto, K. , Bundo, M. , & Kato, T. (2005). Altered expression of mitochondria‐related genes in postmortem brains of patients with bipolar disorder or schizophrenia, as revealed by large‐scale DNA microarray analysis. Human Molecular Genetics, 14(2), 241–253. 10.1093/hmg/ddi022 [DOI] [PubMed] [Google Scholar]
- Iwata, R. , Casimir, P. , & Vanderhaeghen, P. (2020). Mitochondrial dynamics in postmitotic cells regulate neurogenesis. Science, 369(6505), 858–862. 10.1126/science.aba9760 [DOI] [PubMed] [Google Scholar]
- Iwata, R. , & Vanderhaeghen, P. (2021). Regulatory roles of mitochondria and metabolism in neurogenesis. Current Opinion in Neurobiology, 69, 231–240. 10.1016/j.conb.2021.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaaro‐Peled, H. , Ayhan, Y. , Pletnikov, M. V. , & Sawa, A. (2010). Review of pathological hallmarks of schizophrenia: Comparison of genetic models with patients and nongenetic models. Schizophrenia Bulletin, 36(2), 301–313. 10.1093/schbul/sbp133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen, J. C. , Wilson, C. , Cunningham, V. , Glamuzina, E. , Prosser, D. O. , Love, D. R. , Burgess, T. , Taylor, J. , Swan, B. , Hill, R. , Robertson, S. P. , Snell, R. G. , & Lehnert, K. (2016). Brain dopamine‐serotonin vesicular transport disease presenting as a severe infantile hypotonic parkinsonian disorder. Journal of Inherited Metabolic Disease, 39(2), 305–308. 10.1007/s10545-015-9897-6 [DOI] [PubMed] [Google Scholar]
- Jacquemont, S. , Berry‐Kravis, E. , Hagerman, R. , Von Raison, F. , Gasparini, F. , Apostol, G. , Ufer, M. , Des Portes, V. , & Gomez‐Mancilla, B. (2014). The challenges of clinical trials in fragile X syndrome. Psychopharmacology, 231(6), 1237–1250. 10.1007/s00213-013-3289-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe, A. E. , Straub, R. E. , Shin, J. H. , Tao, R. , Gao, Y. , Collado‐Torres, L. , Kam‐Thong, T. , Xi, H. S. , Quan, J. , Chen, Q. , Colantuoni, C. , Ulrich, W. S. , Maher, B. J. , Deep‐Soboslay, A. , Cross, A. J. , Brandon, N. J. , Leek, J. T. , Hyde, T. M. , Kleinman, J. E. , & Weinberger, D. R. (2018). Developmental and genetic regulation of the human cortex transcriptome illuminate schizophrenia pathogenesis. Nature Neuroscience, 21(8), 1117–1125. 10.1038/s41593-018-0197-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubauskiene, E. , Janaviciute, V. , Peciuliene, I. , Söderkvist, P. , & Kanopka, A. (2012). G/a polymorphism in intronic sequence affects the processing of MAO‐B gene in patients with Parkinson disease. FEBS Letters, 586(20), 3698–3704. 10.1016/j.febslet.2012.08.028 [DOI] [PubMed] [Google Scholar]
- Javitt, D. C. , Spencer, K. M. , Thaker, G. K. , Winterer, G. , & Hajos, M. (2008). Neurophysiological biomarkers for drug development in schizophrenia. Nature Reviews Drug Discovery, 7(1), 68–83. 10.1038/nrd2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt, D. C. , & Zukin, S. R. (1991). Recent advances in the phencyclidine model of schizophrenia. American Journal of Psychiatry, 148(10), 1301–1308. 10.1176/ajp.148.10.1301 [DOI] [PubMed] [Google Scholar]
- Jennings, A. , & Rusakov, D. A. (2016). Do astrocytes respond to dopamine? Opera Medica Et Physiologica, 2(1 (2)), 47–56. [Google Scholar]
- Jensen, J. E. , Miller, J. , Williamson, P. C. , Neufeld, R. W. J. , Menon, R. S. , Malla, A. , Manchanda, R. , Schaefer, B. , Densmore, M. , & Drost, D. J. (2006). Grey and white matter differences in brain energy metabolism in first episode schizophrenia: 31P‐MRS chemical shift imaging at 4 tesla. Psychiatry Research ‐ Neuroimaging, 146(2), 127–135. 10.1016/j.pscychresns.2005.11.004 [DOI] [PubMed] [Google Scholar]
- Jo, J. , Woo, J. , Cristobal, C. D. , Choi, J. M. , Wang, C. , Ye, Q. , Smith, J. A. , Ung, K. , Liu, G. , Cortes, D. , Jung, S. Y. , Arenkiel, B. R. , & Lee, H. K. (2021). Regional heterogeneity of astrocyte morphogenesis dictated by the formin protein, Daam2, modifies circuit function. EMBO Reports, 22(12), 1–17. 10.15252/embr.202153200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas, R. K. , Montojo, C. A. , & Bearden, C. E. (2014). The 22q11.2 deletion syndrome as a window into complex neuropsychiatric disorders over the lifespan. Biological Psychiatry, 75(5), 351–360. 10.1016/j.biopsych.2013.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayiorgou, M. , Simon, T. J. , & Gogos, J. A. (2010). 22q11.2 microdeletions: Linking DNA structural variation to brain dysfunction and schizophrenia. Nature Reviews Neuroscience, 11(6), 402–416. 10.1038/nrn2841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karry, R. , Klein, E. , & Shachar, D. B. (2004). Mitochondrial complex I subunits expression is altered in schizophrenia: A postmortem study. Biological Psychiatry, 55(7), 676–684. 10.1016/j.biopsych.2003.12.012 [DOI] [PubMed] [Google Scholar]
- Kathuria, A. , Lopez‐Lengowski, K. , Jagtap, S. S. , McPhie, D. , Perlis, R. H. , Cohen, B. M. , & Karmacharya, R. (2020). Transcriptomic landscape and functional characterization of induced pluripotent stem cell‐derived cerebral organoids in schizophrenia. JAMA Psychiatry, 77(7), 745–754. 10.1001/jamapsychiatry.2020.0196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsel, P. , Byne, W. , Roussos, P. , Tan, W. , Siever, L. , & Haroutunian, V. (2011). Astrocyte and glutamate markers in the superficial, deep, and white matter layers of the anterior cingulate gyrus in schizophrenia. Neuropsychopharmacology, 36(6), 1171–1177. 10.1038/npp.2010.252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, S. , Jahanshad, N. , Zalesky, A. , Kochunov, P. , Agartz, I. , Alloza, C. , Andreassen, O. A. , Arango, C. , Banaj, N. , Bouix, S. , Bousman, C. A. , Brouwer, R. M. , Bruggemann, J. , Bustillo, J. , Cahn, W. , Calhoun, V. , Cannon, D. , Carr, V. , Catts, S. , … Donohoe, G. (2018). Widespread white matter microstructural differences in schizophrenia across 4322 individuals: Results from the ENIGMA schizophrenia DTI working group. Molecular Psychiatry, 23(5), 1261–1269. 10.1038/mp.2017.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns, D. , Vong, G. S. , Barley, K. , Dracheva, S. , Katsel, P. , Casaccia, P. , Haroutunian, V. , & Byne, W. (2010). Gene expression abnormalities and oligodendrocyte deficits in the internal capsule in schizophrenia. Schizophrenia Research, 120(1–3), 150–158. 10.1016/j.schres.2010.04.012 [DOI] [PubMed] [Google Scholar]
- Kim, R. , Healey, K. L. , Sepulveda‐Orengo, M. T. , & Reissner, K. J. (2018). Astroglial correlates of neuropsychiatric disease: From astrocytopathy to astrogliosis. Progress in Neuro‐Psychopharmacology and Biological Psychiatry, 87(September 2017), 126–146. 10.1016/j.pnpbp.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klushnik, T. P. , Spunde, A. Y. , Yakovlev, A. G. , Khuchua, Z. A. , Saks, V. A. , & Vartanyan, M. E. (1991). Intracellular alterations of the creatine kinase isoforms in brains of schizophrenic patients. Molecular and Chemical Neuropathology, 15(3), 271–280. 10.1007/BF03161065 [DOI] [PubMed] [Google Scholar]
- Kolluri, N. , Sun, Z. , Sampson, A. R. , & Lewis, D. A. (2005). Lamina‐specific reductions in dendritic spine density in the prefrontal cortex of subjects with schizophrenia. American Journal of Psychiatry, 162(6), 1200–1202. 10.1176/appi.ajp.162.6.1200 [DOI] [PubMed] [Google Scholar]
- Labrie, V. , Pai, S. , & Petronis, A. (2012). Epigenetics of major psychosis: Progress, problems and perspectives. Trends in Genetics, 28(9), 427–435. 10.1016/j.tig.2012.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam, M. , Chen, C. Y. , Li, Z. , Martin, A. R. , Bryois, J. , Ma, X. , Gaspar, H. , Ikeda, M. , Benyamin, B. , Brown, B. C. , Liu, R. , Zhou, W. , Guan, L. , Kamatani, Y. , Kim, S. W. , Kubo, M. , Kusumawardhani, A. A. A. A. , Liu, C. M. , Ma, H. , … Huang, H. (2019). Comparative genetic architectures of schizophrenia in east Asian and European populations. Nature Genetics, 51(12), 1670–1678. 10.1038/s41588-019-0512-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langen, M. , Leemans, A. , Johnston, P. , Ecker, C. , Daly, E. , Murphy, C. M. , Dell'Acqua, F. , Durston, S. , & Murphy, D. G. M. (2012). Fronto‐striatal circuitry and inhibitory control in autism: Findings from diffusion tensor imaging tractography. Cortex, 48(2), 183–193. 10.1016/j.cortex.2011.05.018 [DOI] [PubMed] [Google Scholar]
- Lee, A. , Hirabayashi, Y. , Kwon, S.‐K. , & Lewis T. L., Jr. , Polleux, F. (2018). Emerging roles of mitochondria in synaptic transmission and neurodegeneration. Curr Opin Physiol., 3, 82–93. 10.1016/j.cophys.2018.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, H. S. , Ghetti, A. , Pinto‐Duarte, A. , Wang, X. , Dziewczapolski, G. , Galimi, F. , Huitron‐Resendiz, S. , Piña‐Crespo, J. C. , Roberts, A. J. , Verma, I. M. , Sejnowski, T. J. , & Heinemann, S. F. (2014). Astrocytes contribute to gamma oscillations and recognition memory. Proceedings of the National Academy of Sciences of the United States of America, 111(32), E3343‐E3352. 10.1073/pnas.1410893111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levite, M. (2014). Glutamate receptor antibodies in neurological diseases: anti‐AMPA‐GluR3 antibodies, anti‐NMDA‐NR1 antibodies, anti‐NMDA‐NR2A/B antibodies, anti‐mGluR1 antibodies or anti‐mGluR5 antibodies are present in subpopulations of patients with either: epilepsy, encephalitis, cerebellar ataxia, systemic lupus erythematosus (SLE) and neuropsychiatric SLE, Sjogren's syndrome, schizophrenia, mania or stroke. These autoimmune anti‐glutamate receptor antibodies can bind neurons in few brain regions, activate glutamate. Journal of Neural Transmission, 121(8), 1029–1075. 10.1007/s00702-014-1193-3 [DOI] [PubMed] [Google Scholar]
- Lewis, T. L. , Kwon, S. K. , Lee, A. , Shaw, R. , & Polleux, F. (2018). MFF‐dependent mitochondrial fission regulates presynaptic release and axon branching by limiting axonal mitochondria size. Nature Communications, 9(1), 1–15. 10.1038/s41467-018-07416-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Tran, O. T. , Crowley, T. B. , Moore, T. M. , Zackai, E. H. , Emanuel, B. S. , McDonald‐Mcginn, D. M. , Gur, R. E. , Wallace, D. C. , & Anderson, S. A. (2021). Association of mitochondrial biogenesis with variable penetrance of schizophrenia. JAMA Psychiatry, 78(8), 911–921. 10.1001/jamapsychiatry.2021.0762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W. J. , Kou, C. G. , Yu, Y. , Sun, S. , Zhang, X. , Kosten, T. R. , & Zhang, X. Y. (2012). Association of Catechol‐O‐methyltransferase gene polymorphisms with schizophrenia and negative symptoms in a Chinese population. American Journal of Medical Genetics, Part B: Neuropsychiatric Genetics, 159(4), 370–375. 10.1002/ajmg.b.32038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Z. , Okamoto, K. I. , Hayashi, Y. , & Sheng, M. (2004). The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell, 119(6), 873–887. 10.1016/j.cell.2004.11.003 [DOI] [PubMed] [Google Scholar]
- Li, Z. , Chen, J. , Yu, H. , He, L. , Xu, Y. , Zhang, D. , Yi, Q. , Li, C. , Li, X. , Shen, J. , Song, Z. , Ji, W. , Wang, M. , Zhou, J. , Chen, B. , Liu, Y. , Wang, J. , Wang, P. , Yang, P. , … Shi, Y. (2017). Genome‐wide association analysis identifies 30 new susceptibility loci for schizophrenia. Nature Genetics, 49(11), 1576–1583. 10.1038/ng.3973 [DOI] [PubMed] [Google Scholar]
- Liddelow, S. A. , Marsh, S. E. , & Stevens, B. (2020). Microglia and astrocytes in disease: Dynamic duo or partners in crime? Trends in Immunology, 41(9), 820–835. 10.1016/j.it.2020.07.006 [DOI] [PubMed] [Google Scholar]
- Lima, A. , Sardinha, V. M. , Oliveira, A. F. , Reis, M. , Mota, C. , Silva, M. A. , Marques, F. , Cerqueira, J. J. , Pinto, L. , Sousa, N. , & Oliveira, J. F. (2014). Astrocyte pathology in the prefrontal cortex impairs the cognitive function of rats. Molecular Psychiatry, 19(7), 834–841. 10.1038/mp.2013.182 [DOI] [PubMed] [Google Scholar]
- Lin, Z., Walther, D., Yu, X. Y., Li, S., Drgon, T., & Uhl, G. R. (2005). SLC18A2 promoter haplotypes and identification of a novel protective factor against alcoholism. Human molecular genetics, 14(10), 1393–1404. 10.1093/hmg/ddi148 [DOI] [PubMed]
- Lu, X. H. , Fleming, S. M. , Meurers, B. , Ackerson, L. C. , Mortazavi, F. , Lo, V. , Hernandez, D. , Sulzer, D. , Jackson, G. R. , Maidment, N. T. , Chesselet, M. F. , & Yang, X. W. (2009). Bacterial artificial chromosome transgenic mice expressing a truncated mutant parkin exhibit age‐dependent hypokinetic motor deficits, dopaminergic neuron degeneration, and accumulation of proteinase k‐resistant α‐Synuclein. Journal of Neuroscience, 29(7), 1962–1976. 10.1523/JNEUROSCI.5351-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, T. M. , Abazyan, S. , Abazyan, B. , Nomura, J. , Yang, C. , Seshadri, S. , Sawa, A. , Snyder, S. H. , & Pletnikov, M. V. (2013). Pathogenic disruption of DISC1‐serine racemase binding elicits schizophrenia‐like behavior via D‐serine depletion. Molecular Psychiatry, 18(5), 557–567. 10.1038/mp.2012.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira, C. , Freitas, M. E. , Vargas‐Lopes, C. , Wolosker, H. , & Panizzutti, R. (2008). Increased brain d‐amino acid oxidase (DAAO) activity in schizophrenia. Schizophrenia Research, 101(1–3), 76–83. 10.1016/j.schres.2008.02.002 [DOI] [PubMed] [Google Scholar]
- Madzarac, Z. , Tudor, L. , Sagud, M. , Erjavec, G. N. , Peles, A. M. , & Pivac, N. (2021). The associations between COMT and MAO‐B genetic variants with negative symptoms in patients with schizophrenia. Current Issues in Molecular Biology, 43(2), 618–636. 10.3390/cimb43020045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra, A. K. , Kestler, L. J. , Mazzanti, C. , Bates, J. A. , Goldberg, T. , & Goldman, D. (2002). A functional polymorphism in the COMT gene and performance on a test of prefrontal cognition. American Journal of Psychiatry, 159(4), 652–654. 10.1176/appi.ajp.159.4.652 [DOI] [PubMed] [Google Scholar]
- Mameli, M. , Bellone, C. , Brown, M. T. C. , & Lüscher, C. (2011). Cocaine inverts rules for synaptic plasticity of glutamate transmission in the ventral tegmental area. Nature Neuroscience, 14(4), 414–416. 10.1038/nn.2763 [DOI] [PubMed] [Google Scholar]
- Maurer, I. , Zierz, S. , & Möller, H. J. (2001). Evidence for a mitochondrial oxidative phosphorylation defect in brains from patients with schizophrenia. Schizophrenia Research, 48(1), 125–136. 10.1016/S0920-9964(00)00075-X [DOI] [PubMed] [Google Scholar]
- Maynard, T. M. , Meechan, D. W. , Dudevoir, M. L. , Gopalakrishna, D. , Peters, A. Z. , Heindel, C. C. , Sugimoto, T. J. , Wu, Y. , Lieberman, J. A. , & LaMantia, A. S. (2008). Mitochondrial localization and function of a subset of 22q11 deletion syndrome candidate genes. Molecular and Cellular Neuroscience, 39(3), 439–451. 10.1016/j.mcn.2008.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy, M. M. , Moore‐Kochlacs, C. , Gu, X. , Boyden, E. S. , Han, X. , & Kopell, N. (2011). Striatal origin of the pathologic beta oscillations in Parkinson's disease. Proceedings of the National Academy of Sciences of the United States of America, 108(28), 11620–11625. 10.1073/pnas.1107748108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meechan, D. W. , Rutz, H. L. H. , Fralish, M. S. , Maynard, T. M. , Rothblat, L. A. , & Lamantia, A. S. (2015). Cognitive ability is associated with altered medial frontal cortical circuits in the LgDel mouse model of 22q11.2DS. Cerebral Cortex, 25(5), 1143–1151. 10.1093/cercor/bht308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei, Y. Y. , Wu, D. C. , & Zhou, N. (2018). Astrocytic regulation of glutamate transmission in schizophrenia. Frontiers in Psychiatry, 9(November), 1–10. 10.3389/fpsyt.2018.00544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mena, A. , Ruiz‐Salas, J. C. , Puentes, A. , Dorado, I. , Ruiz‐Veguilla, M. , & De la Casa, L. G. (2016). Reduced prepulse inhibition as a biomarker of schizophrenia. Frontiers in Behavioral Neuroscience, 10(October), 1–9. 10.3389/fnbeh.2016.00202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer‐Lindenberg, A. S. , Olsen, R. K. , Kohn, P. D. , Brown, T. , Egan, M. F. , Weinberger, D. R. , & Berman, K. F. (2005). Regionally specific disturbance of dorsolateral prefrontal‐hippocampal functional connectivity in schizophrenia. Archives of General Psychiatry, 62(4), 379–386. 10.1001/archpsyc.62.4.379 [DOI] [PubMed] [Google Scholar]
- Middleton, F. A. , Mirnics, K. , Pierri, J. N. , Lewis, D. A. , & Levitt, P. (2002). Gene expression profiling reveals alterations of specific metabolic pathways in schizophrenia. Journal of Neuroscience, 22(7), 2718–2729. 10.1523/jneurosci.22-07-02718.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan, M. J. , Andrieux, A. , Bartzokis, G. , Cadenhead, K. , Dazzan, P. , Fusar‐Poli, P. , Gallinat, J. , Giedd, J. , Grayson, D. R. , Heinrichs, M. , Kahn, R. , Krebs, M. O. , Leboyer, M. , Lewis, D. , Marin, O. , Marin, P. , Meyer‐Lindenberg, A. , McGorry, P. , McGuire, P. , … Weinberger, D. (2016). Altering the course of schizophrenia: Progress and perspectives. Nature Reviews Drug Discovery, 15(7), 485–515. 10.1038/nrd.2016.28 [DOI] [PubMed] [Google Scholar]
- Moghaddam, B. , & Javitt, D. (2012). From revolution to evolution: The glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology, 37(1), 4–15. 10.1038/npp.2011.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel, L. , Higashimori, H. , Tolman, M. , & Yang, Y. (2014). VGluT1+ neuronal glutamatergic signaling regulates postnatal developmental maturation of cortical protoplasmic astroglia. Journal of Neuroscience, 34(33), 10950–10962. 10.1523/JNEUROSCI.1167-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita, Y. , Ujike, H. , Tanaka, Y. , Otani, K. , Kishimoto, M. , Morio, A. , Kotaka, T. , Okahisa, Y. , Matsushita, M. , Morikawa, A. , Hamase, K. , Zaitsu, K. , & Kuroda, S. (2007). A genetic variant of the serine racemase gene is associated with schizophrenia. Biological Psychiatry, 61(10), 1200–1203. 10.1016/j.biopsych.2006.07.025 [DOI] [PubMed] [Google Scholar]
- Moskvina, V. , Craddock, N. , Holmans, P. , Nikolov, I. , Pahwa, J. S. , Green, E. , Owen, M. J. , & O'Donovan, M. C. (2009). Gene‐wide analyses of genome‐wide association data sets: Evidence for multiple common risk alleles for schizophrenia and bipolar disorder and for overlap in genetic risk. Molecular Psychiatry, 14(3), 252–260. 10.1038/mp.2008.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothet, J. P. , Rouaud, E. , Sinet, P. M. , Potier, B. , Jouvenceau, A. , Dutar, P. , Videau, C. , Epelbaum, J. , & Billard, J. M. (2006). A critical role for the glial‐derived neuromodulator D‐serine in the age‐related deficits of cellular mechanisms of learning and memory. Aging Cell, 5(3), 267–274. 10.1111/j.1474-9726.2006.00216.x [DOI] [PubMed] [Google Scholar]
- Mukherjee, A. , Carvalho, F. , Eliez, S. , & Caroni, P. (2019). Long‐lasting Rescue of Network and Cognitive Dysfunction in a genetic schizophrenia model. Cell, 178(6), 1387–1402.e14. 10.1016/j.cell.2019.07.023 [DOI] [PubMed] [Google Scholar]
- Müller, D. J. , Zai, C. C. , Shinkai, T. , Strauss, J. , & Kennedy, J. L. (2011). Association between the DAOA/G72 gene and bipolar disorder and meta‐analyses in bipolar disorder and schizophrenia. Bipolar Disorders, 13(2), 198–207. 10.1111/j.1399-5618.2011.00905.x [DOI] [PubMed] [Google Scholar]
- Napoli, E. , Tassone, F. , Wong, S. , Angkustsiri, K. , Simon, T. J. , Song, G. , & Giulivi, C. (2015). Mitochondrial citrate transporter‐dependent metabolic signature in the 22q11.2 deletion syndrome. Journal of Biological Chemistry, 290(38), 23240–23253. 10.1074/jbc.M115.672360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni, P. , Noh, H. , Park, G. H. , Shao, Z. , Guan, Y. , Park, J. M. , Yu, S. , Park, J. S. , Coyle, J. T. , Weinberger, D. R. , Straub, R. E. , Cohen, B. M. , McPhie, D. L. , Yin, C. , Huang, W. , Kim, H. Y. , & Chung, S. (2020). iPSC‐derived homogeneous populations of developing schizophrenia cortical interneurons have compromised mitochondrial function. Molecular Psychiatry, 25(11), 2873–2888. 10.1038/s41380-019-0423-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberheim, N. A. , Wang, X. , Goldman, S. , & Nedergaard, M. (2006). Astrocytic complexity distinguishes the human brain. Trends in Neurosciences, 29(10), 547–553. 10.1016/j.tins.2006.08.004 [DOI] [PubMed] [Google Scholar]
- Okubo, Y. , Suharat, T. , Suzukit, K. , Kobayashit, K. , Lnouet, O. , Terasakl, O. , Someya, Y. , Sassa, T. , Sudot, Y. , Matsushima, E. , Lyot, M. , Tatenot, Y. , & Toru, M. (1997). Decreased prefrontal dopamine D1 receptors in schizophrenia revealed by PET. Nature, 385, 634–636. [DOI] [PubMed] [Google Scholar]
- Oliet, S. H. R. , & Mothet, J. P. (2009). Regulation of N‐methyl‐d‐aspartate receptors by astrocytic d‐serine. Neuroscience, 158(1), 275–283. 10.1016/j.neuroscience.2008.01.071 [DOI] [PubMed] [Google Scholar]
- Öngür, D. , Prescot, A. P. , Jensen, J. E. , Cohen, B. M. , & Renshaw, P. F. (2009). Creatine abnormalities in schizophrenia and bipolar disorder. Psychiatry Research ‐ Neuroimaging, 172(1), 44–48. 10.1016/j.pscychresns.2008.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osimo, E. F. , Beck, K. , Reis Marques, T. , & Howes, O. D. (2019). Synaptic loss in schizophrenia: A meta‐analysis and systematic review of synaptic protein and mRNA measures. Molecular Psychiatry, 24(4), 549–561. 10.1038/s41380-018-0041-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen, M. J. , O'Donovan, M. C. , Thapar, A. , & Craddock, N. (2011). Neurodevelopmental hypothesis of schizophrenia. British Journal of Psychiatry, 198(3), 173–175. 10.1192/bjp.bp.110.084384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard, M. , Mathew, D. , & Budnik, V. (2003). Wnts and TGFβ in synaptogenesis: old friends signalling at new places. Nature Reviews. Neuroscience, 23(1), 1–7. 10.1038/nrn1036.WNTS [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panatier, A. , Theodosis, D. T. , Mothet, J. P. , Touquet, B. , Pollegioni, L. , Poulain, D. A. , & Oliet, S. H. R. (2006). Glia‐derived d‐serine controls NMDA receptor activity and synaptic memory. Cell, 125(4), 775–784. 10.1016/j.cell.2006.02.051 [DOI] [PubMed] [Google Scholar]
- Pannasch, U. , Freche, D. , Dallérac, G. , Ghézali, G. , Escartin, C. , Ezan, P. , Cohen‐Salmon, M. , Benchenane, K. , Abudara, V. , Dufour, A. , Lübke, J. H. R. , Déglon, N. , Knott, G. , Holcman, D. , & Rouach, N. (2014). Connexin 30 sets synaptic strength by controlling astroglial synapse invasion. Nature Neuroscience, 17(4), 549–558. 10.1038/nn.3662 [DOI] [PubMed] [Google Scholar]
- Pannasch, U. , & Rouach, N. (2013). Emerging role for astroglial networks in information processing: From synapse to behavior. Trends in Neurosciences, 36(7), 405–417. 10.1016/j.tins.2013.04.004 [DOI] [PubMed] [Google Scholar]
- Papouin, T. , Ladépêche, L. , Ruel, J. , Sacchi, S. , Labasque, M. , Hanini, M. , Groc, L. , Pollegioni, L. , Mothet, J. P. , & Oliet, S. H. R. (2012). Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell, 150(3), 633–646. 10.1016/j.cell.2012.06.029 [DOI] [PubMed] [Google Scholar]
- Pardiñas, A. F. , Holmans, P. , Pocklington, A. J. , Escott‐Price, V. , Ripke, S. , Carrera, N. , Legge, S. E. , Bishop, S. , Cameron, D. , Hamshere, M. L. , Han, J. , Hubbard, L. , Lynham, A. , Mantripragada, K. , Rees, E. , MacCabe, J. H. , McCarroll, S. A. , Baune, B. T. , Breen, G. , … Walters, J. T. R. (2018). Common schizophrenia alleles are enriched in mutation‐intolerant genes and in regions under strong background selection. Nature Genetics, 50(3), 381–389. 10.1038/s41588-018-0059-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterlini, M. , Zakharenko, S. S. , Lai, W. S. , Qin, J. , Zhang, H. , Mukai, J. , Westphal, K. G. C. , Olivier, B. , Sulzer, D. , Pavlidis, P. , Siegelbaum, S. A. , Karayiorgou, M. , & Gogos, J. A. (2005). Transcriptional and behavioral interaction between 22q11.2 orthologs modulates schizophrenia‐related phenotypes in mice. Nature Neuroscience, 8(11), 1586–1594. 10.1038/nn1562 [DOI] [PubMed] [Google Scholar]
- Pennington, K. , Beasley, C. L. , Dicker, P. , Fagan, A. , English, J. , Pariante, C. M. , Wait, R. , Dunn, M. J. , & Cotter, D. R. (2008). Prominent synaptic and metabolic abnormalities revealed by proteomic analysis of the dorsolateral prefrontal cortex in schizophrenia and bipolar disorder. Molecular Psychiatry, 13(12), 1102–1117. 10.1038/sj.mp.4002098 [DOI] [PubMed] [Google Scholar]
- Periyasamy, S. , John, S. , Padmavati, R. , Rajendren, P. , Thirunavukkarasu, P. , Gratten, J. , Vinkhuyzen, A. , McRae, A. , Holliday, E. G. , Nyholt, D. R. , Nancarrow, D. , Bakshi, A. , Hemani, G. , Nertney, D. , Smith, H. , Filippich, C. , Patel, K. , Fowdar, J. , McLean, D. , … Consortium, I. G. V . (2019). Association of Schizophrenia Risk with disordered niacin metabolism in an Indian genome‐wide association study. JAMA Psychiatry, 76(10), 1026–1034. 10.1001/jamapsychiatry.2019.1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkovic, N. , Sagud, M. , Zivkovic, M. , Uzun, S. , Nedic Erjavec, G. , Kozumplik, O. , Svob Strac, D. , Mimica, N. , Mihaljevic Peles, A. , & Pivac, N. (2020). Catechol‐O‐methyltransferase rs4680 and rs4818 haplotype association with treatment response to olanzapine in patients with schizophrenia. Scientific Reports, 10(1), 1–11. 10.1038/s41598-020-67351-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrelli, F. , & Bezzi, P. (2018). mGlu5‐mediated signalling in developing astrocyte and the pathogenesis of autism spectrum disorders. Current Opinion in Neurobiology, 48, 139–145. 10.1016/j.conb.2017.12.014 [DOI] [PubMed] [Google Scholar]
- Petrelli, F. , & Bezzi, P. (2016). Novel insights into gliotransmitters. Current Opinion in Pharmacology, 26(Table 1), 138–145. 10.1016/j.coph.2015.11.010 [DOI] [PubMed] [Google Scholar]
- Petrelli, F. , Dallérac, G. , Pucci, L. , Calì, C. , Zehnder, T. , Sultan, S. , Lecca, S. , Chicca, A. , Ivanov, A. , Asensio, C. S. , Gundersen, V. , Toni, N. , Knott, G. W. , Magara, F. , Gertsch, J. , Kirchhoff, F. , Déglon, N. , Giros, B. , Edwards, R. H. , … Bezzi, P. (2020). Dysfunction of homeostatic control of dopamine by astrocytes in the developing prefrontal cortex leads to cognitive impairments. Molecular Psychiatry, 25(4), 732–749. 10.1038/s41380-018-0226-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrelli, F. , Zehnder, T. , Pucci, L. , Cali, C. , Bondiolotti, B. M. , Perez, A. M. , Dallerac, G. , Déglon, N. , Giros, B. , Magara, F. , Magrassi, L. , Mothet, J.‐P. , Simmler, L. , & Bezzi, P. (2021). Astrocytic VMAT2 in the developing prefrontal cortex is required for normal grooming behavior in mice. BioRvix. https://hal.archives-ouvertes.fr/hal-03438361
- Petrie, K. A. , Schmidt, D. , Bubser, M. , Fadel, J. , Carraway, R. E. , & Deutch, A. Y. (2005). Neurotensin activates GABAergic interneurons in the prefrontal cortex. Journal of Neuroscience, 25(7), 1629–1636. 10.1523/JNEUROSCI.3579-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potkin, S. G. , Macciardi, F. , Guffanti, G. , Fallon, J. H. , Wang, Q. , Turner, J. A. , Lakatos, A. , Miles, M. F. , Lander, A. , Vawter, M. P. , & Xie, X. (2010). Identifying gene regulatory networks in schizophrenia. NeuroImage, 53(3), 839–847. 10.1016/j.neuroimage.2010.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabakaran, S. , Swatton, J. E. , Ryan, M. M. , Huffaker, S. J. , Huang, J. T. J. , Griffin, J. L. , Wayland, M. , Freeman, T. , Dudbridge, F. , Lilley, K. S. , Karp, N. A. , Hester, S. , Tkachev, D. , Mimmack, M. L. , Yolken, R. H. , Webster, M. J. , Torrey, E. F. , & Bahn, S. (2004). Mitochondrial dysfunction in schizophrenia: Evidence for compromised brain metabolism and oxidative stress. Molecular Psychiatry, 9(7), 684–697. 10.1038/sj.mp.4001511 [DOI] [PubMed] [Google Scholar]
- Pulver, A. E. , Mulle, J. , Nestadt, G. , Swartz, K. L. , Blouin, J. L. , Dombroski, B. , Liang, K. Y. , Housman, D. E. , Kazazian, H. H. , Antonarakis, S. E. , Lasseter, V. K. , Wolyniec, P. S. , Thornquist, M. H. , & McGrath, J. A. (2000). Genetic heterogeneity in schizophrenia: Stratification of genome scan data using co‐segregating related phenotypes. Molecular Psychiatry, 5(6), 650–653. 10.1038/sj.mp.4000814 [DOI] [PubMed] [Google Scholar]
- Rajkowska, G. , Selemon, L. D. , & Goldman‐Rakic, P. S. (1998). Neuronal and glial somal size in the prefrontal cortex. Archives of General Psychiatry, 55(3), 215–224. 10.1001/archpsyc.55.3.215 [DOI] [PubMed] [Google Scholar]
- Rangaraju, V. , Lewis, T. L. , Hirabayashi, Y. , Bergami, M. , Motori, E. , Cartoni, R. , Kwon, S. K. , & Courchet, J. (2019). Pleiotropic mitochondria: The influence of mitochondria on neuronal development and disease. Journal of Neuroscience, 39(42), 8200–8208. 10.1523/JNEUROSCI.1157-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport, J. L. , Giedd, J. N. , & Gogtay, N. (2012). Neurodevelopmental model of schizophrenia: Update 2012. Molecular Psychiatry, 17(12), 1228–1238. 10.1038/mp.2012.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath, M. , Korenke, G. C. , Najm, J. , Hoffmann, G. F. , Hagendorff, A. , Strom, T. M. , & Felbor, U. (2017). Exome sequencing results in identification and treatment of brain dopamine‐serotonin vesicular transport disease. Journal of the Neurological Sciences, 379, 296–297. 10.1016/j.jns.2017.06.034 [DOI] [PubMed] [Google Scholar]
- Regenold, W. T. , Pratt, M. , Nekkalapu, S. , Shapiro, P. S. , Kristian, T. , & Fiskum, G. (2012). Mitochondrial detachment of hexokinase 1 in mood and psychotic disorders: Implications for brain energy metabolism and neurotrophic signaling. Journal of Psychiatric Research, 46(1), 95–104. 10.1016/j.jpsychires.2011.09.018 [DOI] [PubMed] [Google Scholar]
- Regenold, W. T. , Phatak, P. , Marano, C. M. , Sassan, A. , Conley, R. R. , & Kling, M. A. (2009). Elevated cerebrospinal fluid lactate concentrations in patients with bipolar disorder and schizophrenia: Implications for the mitochondrial dysfunction hypothesis. Biological Psychiatry, 65(6), 489–494. 10.1016/j.biopsych.2008.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenbach, A. , Derouiche, A. , & Kirchhoff, F. (2010). Morphology and dynamics of perisynaptic glia. Brain Research Reviews, 63(1–2), 11–25. 10.1016/j.brainresrev.2010.02.003 [DOI] [PubMed] [Google Scholar]
- Remington, G. , Addington, D. , Honer, W. , Ismail, Z. , Raedler, T. , & Teehan, M. (2017). Guidelines for the pharmacotherapy of schizophrenia in adults. Canadian Journal of Psychiatry, 62(9), 604–616. 10.1177/0706743717720448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilstone, J. J. , Alkhater, R. A. , & Minassian, B. A. (2013). Brain dopamine–serotonin vesicular transport disease and its treatment. New England Journal of Medicine, 368(6), 543–550. 10.1056/nejmoa1207281 [DOI] [PubMed] [Google Scholar]
- Ripke, S. , O'Dushlaine, C. , Chambert, K. , Moran, J. L. , Kähler, A. K. , Akterin, S. , Bergen, S. E. , Collins, A. L. , Crowley, J. J. , Fromer, M. , Kim, Y. , Lee, S. H. , Magnusson, P. K. E. , Sanchez, N. , Stahl, E. A. , Williams, S. , Wray, N. R. , Xia, K. , Bettella, F. , … Sullivan, P. F. (2013). Genome‐wide association analysis identifies 13 new risk loci for schizophrenia. Nature Genetics, 45(10), 1150–1159. 10.1038/ng.2742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto, R. , De Stefani, D. , Raffaello, A. , & Mammucari, C. (2012). Mitochondria as sensors and regulators of calcium signalling. Nature Reviews Molecular Cell Biology, 13(9), 566–578. 10.1038/nrm3412 [DOI] [PubMed] [Google Scholar]
- Rodriguez‐Murillo, L. , Gogos, J. A. , & Karayiorgou, M. (2012). The genetic architecture of schizophrenia: New mutations and emerging paradigms. Annual Review of Medicine, 63, 63–80. 10.1146/annurev-med-072010-091100 [DOI] [PubMed] [Google Scholar]
- Roeper, J. (2013). Dissecting the diversity of midbrain dopamine neurons. Trends in Neurosciences, 36(6), 336–342. 10.1016/j.tins.2013.03.003 [DOI] [PubMed] [Google Scholar]
- Romero‐Calderón, R. , Uhlenbrock, G. , Borycz, J. , Simon, A. F. , Grygoruk, A. , Yee, S. K. , Shyer, A. , Ackerson, L. C. , Maidment, N. T. , Meinertzhagen, I. A. , Hovemann, B. T. , & Krantz, D. E. (2008). A glial variant of the vesicular monoamine transporter is required to store histamine in the drosophila visual system. PLoS Genetics, 4(11), e1000245. 10.1371/journal.pgen.1000245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi, D. , Brambilla, L. , Valori, C. F. , Crugnola, A. , Giaccone, G. , Capobianco, R. , Mangieri, M. , Kingston, A. E. , Bloc, A. , Bezzi, P. , & Volterra, A. (2005). Defective tumor necrosis factor‐α‐dependent control of astrocyte glutamate release in a transgenic mouse model of Alzheimer disease. Journal of Biological Chemistry, 280(51), 42088–42096. 10.1074/jbc.M504124200 [DOI] [PubMed] [Google Scholar]
- Rouach, N. , Koulakoff, A. , Abudara, V. , Willecke, K. , & Giaume, C. (2008). Astroglial metabolic networks sustain hippocampal synaptic transmission. Science, 322(December), 1551–1555. http://science.sciencemag.org/content/322/5907/1551.abstract [DOI] [PubMed] [Google Scholar]
- Roussos, P. , Giakoumaki, S. G. , Pavlakis, S. , & Bitsios, P. (2008). Planning, decision‐making and the COMT rs4818 polymorphism in healthy males. Neuropsychologia, 46(2), 757–763. 10.1016/j.neuropsychologia.2007.10.009 [DOI] [PubMed] [Google Scholar]
- Russo, M. , Levine, S. Z. , Demjaha, A. , Di Forti, M. , Bonaccorso, S. , Fearon, P. , Dazzan, P. , Pariante, C. M. , David, A. S. , Morgan, C. , Murray, R. M. , & Reichenberg, A. (2014). Association between symptom dimensions and categorical diagnoses of psychosis: A cross‐sectional and longitudinal investigation. Schizophrenia Bulletin, 40(1), 111–119. 10.1093/schbul/sbt055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santello, M., & Volterra, A. (2011). TNFα in synaptic function: switching gears. Trends in neurosciences, 35(10), 638–647. doi: 10.1016/j.tins.2012.06.001. [DOI] [PubMed]
- Santello, M., Calì, C., & Bezzi, P. (2012). Gliotransmission and the tripartite synapse. Advances in experimental medicine and biology, 970, 307–331. 10.1007/978-3-7091-0932-8_14 [DOI] [PubMed]
- Santello, M. , Toni, N. , & Volterra, A. (2019). Astrocyte function from information processing to cognition and cognitive impairment. Nature Neuroscience, 22(2), 154–166. 10.1038/s41593-018-0325-8 [DOI] [PubMed] [Google Scholar]
- Schiweck, J., Eickholt, B. J., & Murk, K. (2018). Important shapeshifter: mechanisms allowing astrocytes to respond to the changing nervous system during development, injury and disease. Frontiers in cellular neuroscience, 12, 261. 10.3389/fncel.2018.00261 [DOI] [PMC free article] [PubMed]
- Schork, A. J. , Won, H. , Appadurai, V. , Nudel, R. , Gandal, M. , Delaneau, O. , Revsbech Christiansen, M. , Hougaard, D. M. , Bækved‐Hansen, M. , Bybjerg‐Grauholm, J. , Giørtz Pedersen, M. , Agerbo, E. , Bøcker Pedersen, C. , Neale, B. M. , Daly, M. J. , Wray, N. R. , Nordentoft, M. , Mors, O. , Børglum, A. D. , … Werge, T. (2019). A genome‐wide association study of shared risk across psychiatric disorders implicates gene regulation during fetal neurodevelopment. Nature Neuroscience, 22(3), 353–361. 10.1038/s41593-018-0320-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulmann, A. , Ryu, E. , Goncalves, V. , Rollins, B. , Christiansen, M. , Frye, M. A. , Biernacka, J. , & Vawter, M. P. (2019). Novel complex interactions between mitochondrial and nuclear DNA in schizophrenia and bipolar disorder. Molecular Neuropsychiatry, 5(1), 13–27. 10.1159/000495658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi, E. , Saito, K. , Sano, F. , & Koizumi, S. C. (2019). Aberrant calcium signals in reactive astrocytes: A key process in neurological disorders. International Journal of Molecular Sciences, 20(4), 996. 10.3390/ijms20040996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbereis, J. C. , Pochareddy, S. , Zhu, Y. , Li, M. , & Sestan, N. (2016). The cellular and molecular landscapes of the developing human central nervous system. Neuron, 176(3), 139–148. 10.1016/j.neuron.2015.12.008.The [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson, E. H. , Kellendonk, C. , & Kandel, E. (2010). A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron, 65(5), 585–596. 10.1016/j.neuron.2010.02.014.A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, T. , Poterba, T. , Curtis, D. , Akil, H. , Al Eissa, M. , Barchas, J. D. , Bass, N. , Bigdeli, T. B. , Breen, G. , Bromet, E. J. , Buckley, P. F. , Bunney, W. E. , Bybjerg‐Grauholm, J. , Byerley, W. F. , Chapman, S. B. , Chen, W. J. , Churchhouse, C. , Craddock, N. , Cusick, C. M. , … Daly, M. J. (2022). Rare coding variants in ten genes confer substantial risk for schizophrenia. Nature, 604(7906), 509–516. 10.1038/s41586-022-04556-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew, M. V. (2015). Astrocyte barriers to neurotoxic inflammation. Nature Reviews Neuroscience, 16(5), 249–263. 10.1038/nrn3898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew, M. V. (2020). Astrocyte reactivity: Subtypes, states and functions in CNS innate immunity. Trends Immunology, 41(9), 758–770. 10.1016/j.it.2020.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew, M. V. (2014). Astrogliosis. Cold Spring Harbor Perspectives in Biology, 7(2), a020420. 10.1101/cshperspect.a020420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangaro, M. , Bosia, M. , Zanoletti, A. , Bechi, M. , Cocchi, F. , Pirovano, A. , Lorenzi, C. , Bramanti, P. , Benedetti, F. , Smeraldi, E. , & Cavallaro, R. (2012). Cognitive dysfunction and glutamate reuptake: Effect of EAAT2 polymorphism in schizophrenia. Neuroscience Letters, 522(2), 151–155. 10.1016/j.neulet.2012.06.030 [DOI] [PubMed] [Google Scholar]
- Spencer, J. P. E. , Jenner, P. , Daniel, S. E. , Lees, A. J. , Marsden, D. C. , & Halliwell, B. (1998). Conjugates of catecholamines with cysteine and GSH in Parkinson's disease: Possible mechanisms of formation involving reactive oxygen species. Journal of Neurochemistry, 71(5), 2112–2122. 10.1046/j.1471-4159.1998.71052112.x [DOI] [PubMed] [Google Scholar]
- Stanwood, G. D. , & Levitt, P. (2007). Prenatal exposure to cocaine produces unique developmental and long‐term adaptive changes in dopamine D1 receptor activity and subcellular distribution. Journal of Neuroscience, 27(1), 152–157. 10.1523/JNEUROSCI.4591-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanwood, G. D. , Parlaman, J. P. , & Levitt, P. (2005). Anatomical abnormalities in dopaminoceptive regions of the cerebral cortex of dopamine D 1 receptor mutant mice. Journal of Comparative Neurology, 487(3), 270–282. 10.1002/cne.20548 [DOI] [PubMed] [Google Scholar]
- Stanwood, G. D. , Washington, R. A. , & Levitt, P. (2001). Identification of a sensitive period of prenatal cocaine exposure that alters the development of the anterior cingulate cortex. Cerebral Cortex, 11(5), 430–440. 10.1093/cercor/11.5.430 [DOI] [PubMed] [Google Scholar]
- Stark, K. L. , Xu, B. , Bagchi, A. , Lai, W. S. , Liu, H. , Hsu, R. , Wan, X. , Pavlidis, P. , Mills, A. A. , Karayiorgou, M. , & Gogos, J. A. (2008). Altered brain microRNA biogenesis contributes to phenotypic deficits in a 22q11‐deletion mouse model. Nature Genetics, 40(6), 751–760. 10.1038/ng.138 [DOI] [PubMed] [Google Scholar]
- Steeds, H. , Carhart‐Harris, R. L. , & Stone, J. M. (2015). Drug models of schizophrenia. Therapeutic Advances in Psychopharmacology, 5(1), 43–58. 10.1177/2045125314557797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffek, A. E. , Haroutunian, V. , & Meador‐Woodruff, J. H. (2006). Serine racemase protein expression in cortex and hippocampus in schizophrenia. Neuroreport, 17(11), 1181–1185. 10.1097/01.wnr.0000230512.01339.72 [DOI] [PubMed] [Google Scholar]
- Stogsdill, J. A. , & Eroglu, C. (2017). The interplay between neurons and glia in synapse development and plasticity. Current Opinion in Neurobiology, 42, 1–8. 10.1016/j.conb.2016.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stogsdill, J. A. , Ramirez, J. , Liu, D. , Kim, Y. H. , Baldwin, K. T. , Enustun, E. , Ejikeme, T. , Ji, R. R. , & Eroglu, C. (2017). Astrocytic neuroligins control astrocyte morphogenesis and synaptogenesis. Nature, 551(7679), 192–197. 10.1038/nature24638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, D. , Ching, C. R. K. , Lin, A. , Forsyth, J. K. , Kushan, L. , Vajdi, A. , Jalbrzikowski, M. , Hansen, L. , Villalon‐Reina, J. E. , Qu, X. , Jonas, R. K. , van Amelsvoort, T. , Bakker, G. , Kates, W. R. , Antshel, K. M. , Fremont, W. , Campbell, L. E. , McCabe, K. L. , Daly, E. , … Bearden, C. E. (2020). Large‐scale mapping of cortical alterations in 22q11.2 deletion syndrome: Convergence with idiopathic psychosis and effects of deletion size. Molecular Psychiatry, 25(8), 1822–1834. 10.1038/s41380-018-0078-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, J. , Jia, P. , Fanous, A. H. , van Den Oord, E. , Chen, X. , Riley, B. P. , Amdur, R. L. , Kendler, K. S. , & Zhao, Z. (2010). Schizophrenia gene networks and pathways and their applications for novel candidate gene selection. PLoS One, 5(6), e11351. 10.1371/journal.pone.0011351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo, A. , Akkouh, I. A. , Vandenberghe, M. , Osete, J. R. , Hughes, T. , Heine, V. , Smeland, O. B. , Glover, J. C. , Andreassen, O. A. , & Djurovic, S. (2021). A human iPSC‐astroglia neurodevelopmental model reveals divergent transcriptomic patterns in schizophrenia. Translational Psychiatry, 11(1), 1–13. 10.1038/s41398-021-01681-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura, M. , Mukai, J. , Gordon, J. A. , & Gogos, J. A. (2016). Developmental inhibition of Gsk3 rescues behavioral and neurophysiological deficits in a mouse model of schizophrenia predisposition. Neuron, 89(5), 1100–1109. 10.1016/j.neuron.2016.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon, R. , Keshavan, M. S. , & Nasrallah, H. A. (2008). Schizophrenia, “just the facts” what we know in 2008. 2. Epidemiology and etiology. Schizophrenia Research, 102(1–3), 1–18. 10.1016/j.schres.2008.04.011 [DOI] [PubMed] [Google Scholar]
- Tarasov, V. V. , Svistunov, A. A. , Chubarev, V. N. , Sologova, S. S. , Mukhortova, P. , Levushkin, D. , Somasundaram, S. G. , Kirkland, C. E. , Bachurin, S. O. , & Aliev, G. (2020). Alterations of astrocytes in the context of schizophrenic dementia. Frontiers in Pharmacology, 10(February), 1–13. 10.3389/fphar.2019.01612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, J. L. , Pogue‐Geile, M. F. , & Grace, A. A. (2004). Developmental pathology, dopamine, and stress: A model for the age of onset of schizophrenia symptoms. Schizophrenia Bulletin, 30(4), 875–900. 10.1093/oxfordjournals.schbul.a007139 [DOI] [PubMed] [Google Scholar]
- Tomasella, E. , Bechelli, L. , Ogando, M. B. , Mininni, C. , Di Guilmi, M. N. , De Fino, F. , Zanutto, S. , Elgoyhen, A. B. , Marin‐Burgin, A. , & Gelman, D. M. (2018). Deletion of dopamine D2 receptors from parvalbumin interneurons in mouse causes schizophrenialike phenotypes. Proceedings of the National Academy of Sciences of the United States of America, 115(13), 3476–3481. 10.1073/pnas.1719897115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trépanier, M. O. , Hopperton, K. E. , Mizrahi, R. , Mechawar, N. , & Bazinet, R. P. (2016). Postmortem evidence of cerebral inflammation in schizophrenia: A systematic review. Molecular Psychiatry, 21(8), 1009–1026. 10.1038/mp.2016.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch, N. X. , & Sabatini, B. L. (2012). Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron, 76(1), 33–50. 10.1016/j.neuron.2012.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trubetskoy, V. , Pardiñas, A. F. , Qi, T. , Panagiotaropoulou, G. , Awasthi, S. , Bigdeli, T. B. , Bryois, J. , Chen, C. Y. , Dennison, C. A. , Hall, L. S. , Lam, M. , Watanabe, K. , Frei, O. , Ge, T. , Harwood, J. C. , Koopmans, F. , Magnusson, S. , Richards, A. L. , Sidorenko, J. , … Van Os, J. (2022). Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature, 604, 502–508. 10.1038/s41586-022-04434-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng, K. Y. , Lewis, B. L. , Lipska, B. K. , & O'Donnell, P. (2007). Post‐pubertal disruption of medial prefrontal cortical dopamine‐glutamate interactions in a developmental animal model of schizophrenia. Biological Psychiatry, 62(7), 730–738. 10.1016/j.biopsych.2006.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng, K. Y. , & O'Donnell, P. (2007). Dopamine modulation of prefrontal cortical interneurons changes during adolescence. Cerebral Cortex, 17(5), 1235–1240. 10.1093/cercor/bhl034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunbridge, E. M. , Narajos, M. , Harrison, C. H. , Beresford, C. , Cipriani, A. , & Harrison, P. J. (2019). Which dopamine polymorphisms are functional? Systematic review and meta‐analysis of COMT, DAT, DBH, DDC, DRD1–5, MAOA, MAOB, TH, VMAT1, and VMAT2. Biological Psychiatry, 86(8), 608–620. 10.1016/j.biopsych.2019.05.014 [DOI] [PubMed] [Google Scholar]
- Uhlhaas, P. J. , & Singer, W. (2011). The development of neural synchrony and large‐scale cortical networks during adolescence: Relevance for the pathophysiology of schizophrenia and neurodevelopmental hypothesis. Schizophrenia Bulletin, 37(3), 514–523. 10.1093/schbul/sbr034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas, P. J. , & Singer, W. (2015). Oscillations and neuronal dynamics in schizophrenia: The search for basic symptoms and translational opportunities. Biological Psychiatry, 77(12), 1001–1009. 10.1016/j.biopsych.2014.11.019 [DOI] [PubMed] [Google Scholar]
- Van Kesteren, C. F. M. G. , Gremmels, H. , De Witte, L. D. , Hol, E. M. , Van Gool, A. R. , Falkai, P. G. , Kahn, R. S. , & Sommer, I. E. C. (2017). Immune involvement in the pathogenesis of schizophrenia: A meta‐analysis on postmortem brain studies. Translational Psychiatry, 7(3), e1075–e1011. 10.1038/tp.2017.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zundert, B. , Yoshii, A. , & Constantine‐Paton, M. (2004). Receptor compartmentalization and trafficking at glutamate synapses: A developmental proposal. Trends in Neurosciences, 27(7), 428–437. 10.1016/j.tins.2004.05.010 [DOI] [PubMed] [Google Scholar]
- Vawter, M. , Morgan, L. , Akil, H. , Watson, S. , Myers, R. , Lee, F. , Schatzberg, A. , Bunney, W. , Hjelm, B. , Manojlovic, Z. , Jin, Y. , & Rollins, B. (2021). Mitochondria genetics of schizophrenia and bipolar disorder. Biological Psychiatry, 89(9), S5. 10.1016/j.biopsych.2021.02.033 [DOI] [Google Scholar]
- Ventura, R. , & Harris, K. M. (1999). Three‐dimensional relationships between hippocampal synapses and astrocytes. Journal of Neuroscience, 19(16), 6897–6906. 10.1523/jneurosci.19-16-06897.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky, A. , & Nedergaard, M. (2018). Physiology of astroglia. Physiological Reviews, 98(1), 239–389. 10.1152/physrev.00042.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz, H. P. , Riehemann, S. , Maurer, I. , Smesny, S. , Sommer, M. , Rzanny, R. , Holstein, W. , Czekalla, J. , & Sauer, H. (2000). Reduced phosphodiesters and high‐energy phosphates in the frontal lobe of schizophrenic patients: A 31P chemical shift spectroscopic‐imaging study. Biological Psychiatry, 47(11), 954–961. 10.1016/S0006-3223(00)00235-3 [DOI] [PubMed] [Google Scholar]
- Wang, C. , Aleksic, B. , & Ozaki, N. (2015). Glia‐related genes and their contribution to schizophrenia. Psychiatry and Clinical Neurosciences, 69(8), 448–461. 10.1111/pcn.12290 [DOI] [PubMed] [Google Scholar]
- Washizuka, S. , Iwamoto, K. , Kakiuchi, C. , Bundo, M. , & Kato, T. (2009). Expression of mitochondrial complex I subunit gene NDUFV2 in the lymphoblastoid cells derived from patients with bipolar disorder and schizophrenia. Neuroscience Research, 63(3), 199–204. 10.1016/j.neures.2008.12.004 [DOI] [PubMed] [Google Scholar]
- Wei, Y. L. , Li, C. X. , Li, S. B. , Liu, Y. , & Hu, L. (2011). Association study of monoamine oxidase a/B genes and schizophrenia in Han Chinese. Behavioral and Brain Functions, 7(1), 42. 10.1186/1744-9081-7-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger, D. R. , Egan, M. F. , Bertolino, A. , Callicott, J. H. , Mattay, V. S. , Lipska, B. K. , Berman, K. F. , & Goldberg, T. E. (2001). Prefrontal neurons and the genetics of schizophrenia. Biological Psychiatry, 50(11), 825–844. 10.1016/S0006-3223(01)01252-5 [DOI] [PubMed] [Google Scholar]
- Wesseling, H. , Xu, B. , Want, E. J. , Holmes, E. , Guest, P. C. , Karayiorgou, M. , Gogos, J. A. , & Bahn, S. (2017). System‐based proteomic and metabonomic analysis of the Df(16)a+/− mouse identifies potential miR‐185 targets and molecular pathway alterations. Molecular Psychiatry, 22(3), 384–395. 10.1038/mp.2016.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windrem, M. S. , Osipovitch, M. , Liu, Z. , Bates, J. , Chandler‐Militello, D. , Zou, L. , Munir, J. , Schanz, S. , McCoy, K. , Miller, R. H. , Wang, S. , Nedergaard, M. , Findling, R. L. , Tesar, P. J. , & Goldman, S. A. (2017). Human iPSC glial mouse chimeras reveal glial contributions to schizophrenia. Cell Stem Cell, 21(2), 195–208.e6. 10.1016/j.stem.2017.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, A. H. C. , & Van Tol, H. H. M. (2003). Schizophrenia: From phenomenology to neurobiology. Neuroscience and Biobehavioral Reviews, 27(3), 269–306. 10.1016/S0149-7634(03)00035-6 [DOI] [PubMed] [Google Scholar]
- Wu, Z. , Puigserver, P. , Andersson, U. , Zhang, C. , Adelmant, G. , Mootha, V. , Troy, A. , Cinti, S. , Lowell, B. , Scarpulla, R. C. , & Spiegelman, B. M. (1999). Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC‐1. Cell, 98(1), 115–124. 10.1016/S0092-8674(00)80611-X [DOI] [PubMed] [Google Scholar]
- Yashiro, K. , & Philpot, B. D. (2008). Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology, 55(7), 1081–1094. 10.1016/j.neuropharm.2008.07.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa, T. , Naganuma, F. , Iida, T. , Nakamura, T. , Harada, R. , Mohsen, A. S. , Kasajima, A. , Sasano, H. , & Yanai, K. (2013). Molecular mechanism of histamine clearance by primary human astrocytes. Glia, 61(6), 905–916. 10.1002/glia.22484 [DOI] [PubMed] [Google Scholar]
- Youdim, M. B. H. , Edmondson, D. , & Tipton, K. F. (2006). The therapeutic potential of monoamine oxidase inhibitors. Nature Reviews Neuroscience, 7(4), 295–309. 10.1038/nrn1883 [DOI] [PubMed] [Google Scholar]
- Yu, X. , Taylor, A. M. W. , Nagai, J. , Golshani, P. , Evans, C. J. , Coppola, G. , & Khakh, B. S. (2018). Reducing astrocyte calcium signaling in vivo alters striatal microcircuits and causes repetitive behavior. Neuron, 99(6), 1170–1187.e9. 10.1016/j.neuron.2018.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehnder, T. , Petrelli, F. , Romanos, J. , De Oliveira Figueiredo, E. C. , Lewis, T. L. , Déglon, N. , Polleux, F. , Santello, M. , & Bezzi, P. (2021). Mitochondrial biogenesis in developing astrocytes regulates astrocyte maturation and synapse formation. Cell Reports, 35(2), 108952. 10.1016/j.celrep.2021.108952 [DOI] [PubMed] [Google Scholar]
- Zhang, F. , Gradinaru, V. , Adamantidis, A. R. , Durand, R. , Airan, R. D. , De Lecea, L. , & Deisseroth, K. (2010). Optogenetic interrogation of neural circuits: Technology for probing mammalian brain structures. Nature Protocols, 5(3), 439–456. 10.1038/nprot.2009.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Sloan, S. A. , Clarke, L. E. , Caneda, C. , Plaza, C. A. , Blumenthal, P. D. , Vogel, H. , Steinberg, G. K. , Edwards, M. S. B. , Li, G. , Duncan, J. A. , Cheshier, S. H. , Shuer, L. M. , Chang, E. F. , Grant, G. A. , Gephart, M. G. H. , & Barres, B. A. (2016). Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron, 89(1), 37–53. 10.1016/j.neuron.2015.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study


