Abstract
Iron deficiency is frequent in patients with chronic inflammatory conditions (e.g., chronic heart failure, chronic kidney disease, cancers, and bowel inflammatory diseases). Indeed, high concentrations of inflammatory cytokines increase hepcidin concentrations that lead to the sequestration of iron in cells of the reticuloendothelial system (functional iron deficiency). Iron parameters are often assessed only in the context of anemia, but iron deficiency, even without anemia, is present in about half of patients with inflammatory conditions. Iron deficiency worsens underlying chronic diseases and is an independent factor of morbidity and mortality. In daily practice, the most effective biomarkers of iron status are serum ferritin, which reflects iron storage, and transferrin saturation, which reflects the transport of iron. Serum ferritin is increased in an inflammatory context, and there is still no consensus on the threshold to be used in chronic inflammatory conditions. Nevertheless, recent recommendations of international guidelines agreed to define iron deficiency by serum ferritin <100 µg/L and/or transferrin saturation <20%. Iron parameters remain, however, insufficiently assessed in patients with chronic inflammatory conditions. Indeed, clinical symptoms of iron deficiency, such as fatigue, are not specific and often confused with those of the primary disease. Iron repletion, preferably by the intravenous route to bypass tissue sequestration, improves clinical signs and quality of life. Because of the negative impact of iron deficiency on chronic inflammatory diseases and the efficacy of intravenous iron repletion, screening of iron parameters should be part of the routine examination of all patients with chronic inflammatory diseases.
Keywords: bowel inflammatory disease, cancer, chronic heart failure, chronic inflammatory disease, chronic kidney disease, iron deficiency, serum ferritin, transferrin saturation
Abbreviations
- CHF
chronic heart failure
- CKD
chronic kidney disease
- CRP
C‐reactive protein
- ESA
erythropoiesis‐stimulating agents
- ESC
European Society of Cardiology
- ESMO
European Society for Medical Oncology
- HF
heart failure
- IBD
inflammatory bowel disease
- ID
iron deficiency
- LVEF
left ventricular ejection fraction
- NCCN
National Comprehensive Cancer Network
- NICE
National Institute for Health and Care Excellence
- TSAT
transferrin saturation
Iron deficiency is frequent in chronic conditions
Until very recently, iron deficiency (ID) was considered only in the context of iron‐deficiency anemia [1]. It is only in the last 10–15 years that ID has been considered as an autonomous pathological entity in which iron availability is insufficient to meet the body's needs [2]. Therefore, early ID detection and treatment became important goals, as iron is essential for the functioning of all organs. The fact that the biological definition of ID is debated and inconsistently defined by authors in different studies has probably contributed to preventing the recognition of ID as an independent condition [3]. An international multidisciplinary group of experts recently proposed a more comprehensive definition of ID: “Iron deficiency is a health‐related condition in which iron availability is insufficient to meet the body's needs and which can be present with or without anemia” [1]. This definition is important because it indicates that ID may have specific symptoms, diagnosis, and treatment, even in the absence of anemia. Furthermore, as detailed in the next section, ID is associated with poor prognosis in many diseases, thus highlighting the importance of its diagnosis.
Depending on the underlying pathophysiological mechanisms, two types of ID can be distinguished, with identical clinical symptoms: absolute ID, which is the consequence of a quantitative decrease in iron stores, and functional ID, which is due to the sequestration of iron in otherwise quantitatively normal or abundant stores. These two types of ID are not mutually exclusive and are often associated [4]. Understanding their underlying mechanisms is essential for the development of rational diagnostic biomarkers and therapeutic approaches.
In daily practice, the most effective biomarkers of iron status are serum ferritin, which reflects stored iron, and transferrin saturation (TSAT), which is indicative of transported iron. TSAT is always present; thus, the decrease of TSAT is an essential diagnostic criterion of absolute or functional ID. Since the reliability of serum ferritin measurement may be compromised in patients with chronic diseases or any inflammatory condition, there is a large consensus in guidelines to determine both serum ferritin and TSAT in the first line. There is still no consensus on the thresholds, but recent recommendations of international guidelines have shown agreement for defining ID by serum ferritin <100 µg/L and/or TSAT <20% [5, 6, 7, 8].
ID—associated with anemia or alone—is frequent, particularly in chronic diseases, usually associated with a biologically or clinically inflammatory state. Prevalence rates as high as 37%–61% were reported in chronic heart failure (CHF), 24%–85% in chronic kidney disease (CKD), 13%–90% in inflammatory bowel disease (IBD), 18%–82% in cancer, and an average of 23% in hospitalized patients [1, 4, 6, 9].
The French nationwide prospective CARENFER survey performed during 2019–2020 in hospitalized patients reported comparable high prevalence rates—57.9% (n = 1221) in patients with solid tumors, 49.6% (n = 1661) in CHF, 47.1% (n = 1211) in nondialyzed CKD, and 23.7% (n = 1090) in IBD [10, 11, 12, 13].
ID is a factor of poor prognosis
Chronic heart failure
Several studies have shown that ID significantly increased the relative risk (RR) of death in CHF patients by 40%–60% [14, 15, 16, 17]. A prospective study including nearly 550 CHF patients (mean age 55 years), mostly New York Heart Association (NYHA) class II–III (mean left ventricular ejection fraction [LVEF], 26%), showed in a multivariate analysis that ID increased by 58% at 3 years the adjusted RR of all‐cause death or heart transplantation (composite endpoint) while anemia had no independent effect [16]. In 150 patients with heart failure (HF) from the UK, there was a twofold greater risk for death in nonanemic ID patients compared to anemic iron‐replete patients [14].
In a pooled analysis of European cohort studies (1500 CHF patients followed for a mean of 2.5 years), ID—but not anemia—appeared as an independent risk factor for mortality (RR increased by 42% in multivariate analysis) [15].
Grote Beverborg et al. published in 2019 an analysis of data from two prospective observational studies and reported that lower TSAT (associated or not with lower serum ferritin) was associated with impaired 6‐min walking test [7]. However, only low iron storage (i.e., low serum ferritin) was independently associated with the composite end point of all‐cause mortality or HF hospitalizations (hazard ratio, 1.47; 95% confidence interval [CI], 1.26–1.71; p < 0.001), while lower TSAT was not (hazard ratio, 1.05; 95% CI, 0.87–1.26; p = 0.64). In another analysis by the same authors, a definition of ID based on bone marrow iron staining found that a TSAT ≤19.8% showed the best performance in selecting patients with ID and identifying HF patients at the highest risk of death [18]. In a recent Spanish retrospective study, 1701 patients hospitalized for decompensated HF were included. Lower TSAT (but not lower serum ferritin) was associated with a higher risk for the composite endpoint “30‐day readmission for HF or death” [19].
Analysis of 570 participants with self‐reported CHF included in the large US population‐based National Health and Nutrition Examination Survey III (NHANES III) showed that anemia was correlated with cardiovascular mortality in a multivariate analysis. In contrast, ID was not correlated with either cause of death [20]. However, the severity of CHF was not assessed in this study.
Except for the latter study, ID and, more specifically, low TSAT, is generally identified as a risk factor for poor prognosis in patients with CHF [21]; this prognostic factor appears to be independent of the other conventional factors, including anemia.
Chronic kidney disease
Patients undergoing maintenance hemodialysis usually have a negative iron balance due to reduced absorption and increased blood loss [22]. A US cohort study analyzed prospectively collected data over 2 years from 58,000 dialysis patients and observed that serum ferritin levels 200–1200 ng/ml and TSAT 30%–50% were associated with the lowest all‐cause and cardiovascular death risks [23].
The prospective Dutch PREVEND study analyzed the outcomes of 975 ambulatory CKD patients followed for an average of 8 years and observed that TSAT <10% was significantly correlated—independently of serum ferritin concentrations—with the adjusted RR of all‐cause mortality (×2.8), cardiovascular mortality (×4.1), and development of anemia (×3.0) [24].
A large retrospective cohort study conducted by the US Veterans Affairs analyzed the outcome of 80,000 patients with CKD, both diabetic and nondiabetic, with normal or abnormal iron status, indicative of absolute ID, functional ID, or even iron overload. After a mean follow‐up of 4 years, all three abnormalities were associated with a significant increase in the risk of all‐cause mortality, with the highest risk observed for functional ID [25].
In another retrospective study on 78,500 veterans with nondialysis CKD, the same authors showed that iron status determined the risk of hospitalization for decompensated HF, independent of diabetic status and history of CHF. Compared with the reference group (TSAT 16%–28%, serum ferritin 55–205 µg/L), the adjusted mean risk of hospitalization for decompensated HF was increased by 29% in absolute ID (TSAT 0.4%–16%, serum ferritin 0.9–55 µg/L) and functional ID (TSAT 0.8%–16%, serum ferritin 109–2783 µg/L), whereas this risk was decreased by 18% with high iron stores (TSAT 28%–99%, serum ferritin 205–4941 µg/L) [26].
A recent international (Brazil, France, Germany, and United States) observational study included 1545 nondialyzed CKD patients with and without anemia [27]. Patients with TSAT ≤15% had the highest risks of all‐cause mortality and major adverse cardiovascular events, compared with patients with TSAT 26%–35%.
These studies show that ID is associated with increased mortality in nondialysis or dialysis CKD patients.
Cancers
In patients with common solid tumors, ID was associated with poor general health; advanced cancer, especially at diagnosis and undergoing chemotherapy [28, 29]; increased risk of incomplete response to therapy; and tumor progression [9]. Few studies directly evaluated the role of ID in cancer patients, but ID is the leading cause of anemia in these patients. Anemia was then considered as an additional risk factor for mortality in patients with cancer [30].
A nationwide study in the United States analyzed the outcomes of more than 23,000 scheduled colectomies (conventional or laparoscopic) and observed that the presence of preoperative anemia, including mild anemia, was associated with a significant and independent increase (approximately 50%–120%) in the RR of major postoperative complications (myocardial infarction, stroke, renal failure, or death) during the first postoperative month [31]. A Brazilian prospective observational study including 308 patients admitted for scheduled abdominal cancer surgery reported that preoperative anemia was associated with a doubling of the RR of major postoperative complications in the first month (all‐cause mortality or infectious, cardiovascular, respiratory, neurological, renal, or surgical complications) [32]. More generally, correction of preoperative anemia is important since it is now well demonstrated that any intraoperative blood transfusion is associated with a significant increase in mortality after surgery [33].
In a retrospective study, the outcomes of 141 nonanemic patients with colorectal cancer were compared according to their iron status before elective resection. Patients with ID compared to replete patients were significantly more frequently readmitted (25% vs. 4%) and had more postoperative infections (25% vs. 5%) [34].
In a recent study on 846 patients with colorectal cancer (including 64% with ID), there was an association between the depth of tumor invasion and ID with odds ratios of ID equal to 2.8, 4.22, and 4.63 for tumor stages 2, 3, and 4, respectively [35].
Altogether, these studies show that ID is associated with worsening disease progression in cancer patients undergoing chemotherapy and surgery.
Inflammatory bowel diseases
In IBD, research and recommendations have focused on the issue of ID anemia [36, 37, 38]. The impact of chronic fatigue related to ID anemia is considered intense, comparable to that of abdominal pain and diarrhea [39, 40], and with a huge impact on patient‐reported outcomes, such as quality of life [41], work capacity [42, 43, 44], cognitive performances [45], and emotional well‐being and fatigue [46, 47]. Despite the negative impact of ID anemia on patient life and disease course, ID anemia appears to remain untreated in half of the cases [48].
Given that targeting only abdominal pain and diarrhea in IBD does not prevent disease progression and that anemia is a late manifestation of ID, an innovative approach has recently been proposed [49]. The goal of this approach is to prevent anemia by early detection of ID, normalization of biomarkers related to ID with targeted therapy, and close monitoring to prevent anemia. This early management of ID anemia is expected to result in better outcomes in IBD patients.
From molecular mechanisms to clinical symptoms of ID
Besides its role in erythropoiesis and oxygen transport, iron has other functions as it is part of many proteins and enzymes. In hemoproteins, iron is associated with heme and allows oxygen fixation (hemoglobin, myoglobin) or oxidation reactions (cytochrome P450, cytochrome C oxidase, peroxidase, and catalase). In proteins with an iron–sulfur center, iron also participates in redox reactions in the mitochondrial respiratory chain. Therefore, iron plays a vital role in cellular energy production, energy metabolism of striated muscles and the heart, DNA replication and repair, cell growth and differentiation, and brain function.
Intestinal absorption of iron occurs by endocytosis at the apical pole of duodenal and early jejunal enterocytes (Fig. 1). Iron is then translocated to the basal pole of the enterocyte and released into plasma by ferroportin [50]. Iron exported into the circulation is bound to transferrin, which distributes it to target organs via a specific receptor present on the surface of most cells (RTf or CD71) [51, 52]. The transferrin/RTf complex is internalized by endocytosis, and iron is released into the cell. In the cytosol, iron is either incorporated in the cell metabolism (in the Fe‐S cluster mitochondrial synthesis or iron proteins synthesis) or stored as ferritin. Ferritin is mainly stored in macrophages of the liver (about 50%), bone marrow, spleen, and muscles [50].
Fig. 1.
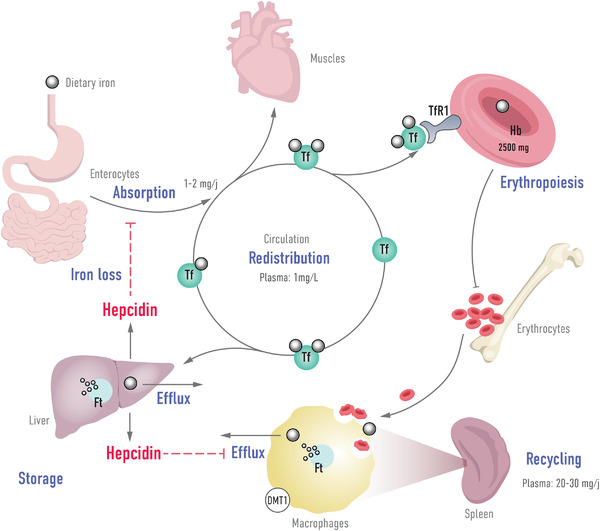
Iron homeostasis and role of hepcidin.
The primary regulator of iron homeostasis is hepcidin, a peptide hormone mainly synthesized by the liver. Hepcidin decreases circulating iron concentrations and is considered as an hyposideremic factor—its expression is, in physiological condition, inversely proportional to circulating iron concentrations [4]. Hepcidin binds to transmembrane ferroportin and induces its internalization and its degradation. This results in inhibition of iron export from iron‐containing cells (macrophages, hepatocytes, and intestinal cells) [51]. In a mouse model, hepcidin was also shown to decrease iron intestinal absorption and DMT1 protein expression [53].
Therefore, high hepcidin concentrations induce a decrease in iron availability to the functional compartment due to its sequestration in the storage compartment, accumulation in the reticuloendothelial recycling system (macrophages), and inhibition of intestinal absorption [54].
ID can be induced by an intake deficit (e.g., gastrointestinal diseases), blood loss (e.g., digestive bleeding), or a defect in mobilization to tissues in particular due to sequestration of iron in the stores [54]. Chronic diseases are accompanied by chronic inflammation that leads to increased hepcidin synthesis [55]. This synthesis is under the influence of IL‐6, which increases hepcidin transcription via the signal transducer and activator of transcription 3 [56]. In contrast, ID or any situation that stimulates erythropoiesis (e.g., hypoxia, bleeding) leads to decreased hepcidin synthesis. Inflammation in chronic diseases plays a crucial role in the establishment of hepcidin‐mediated functional ID.
Because of the role of iron outside of hematopoiesis (e.g., in ATP production), ID induces clinical symptoms even in the absence of anemia. The clinical manifestations of ID are varied and not very specific. Symptoms are usually of moderate intensity and therefore often overlooked because they are frequently confused with those of the underlying chronic disease [54, 57]. This is the case for fatigue, which is a significant symptom in ID [58]. The clinical signs are the same in absolute or functional ID [57]. ID has a significant negative impact on physical performance, quality of life, mood (depression), cognitive performance, and professional productivity [4, 54]. ID is a poor prognostic factor in chronic diseases, as described above. Fortunately, as detailed in the next section, the clinical consequences of ID are reversible by iron repletion, mainly via the intravenous route, which bypasses both the inhibition of intestinal absorption and the iron sequestration induced by chronic inflammation.
Managing ID in CHF
Characteristics of ID in CHF
ID impairs the functional status in CHF patients independent of hemoglobin levels [59]. Cardiac muscle is particularly susceptible to the effects of ID [60, 61]. Cardiomyocytes contain numerous mitochondria that produce the energy necessary to maintain continuous contractions. ID is associated with significant impairment of the contraction force and relaxation phase of cardiac muscle [62]. ID in CHF is generally associated with absolute and functional ID [60]. Absolute deficiency may result from anorexia, decreased absorption due to intestinal edema, digestive bleeding due to the administration of antiplatelet agents, non steroid anti iflammatory drugs, or anticoagulants. CHF is also characterized by a systemic inflammatory state leading to functional ID.
Iron biomarker thresholds
According to the European Society of Cardiology (ESC) criteria, serum ferritin <100 µg/L defines absolute ID, and a serum ferritin level between 100 and 299 µg/L with a TSAT <20% define functional ID [63].
Clinical evidence for iron therapy
In line with the impact of ID on the functional capacity of the cardiac muscle, treatment with intravenous iron improves exercise capacity and symptoms in HF patients with ID, whether they are anemic or not [64, 65]. The pioneering work of Silverberg et al. showed the importance of intravenous iron treatment for cardiac function in anemic patients with CHF, whether treated or not by erythropoiesis‐stimulating agents (ESA). [66, 67, 68]. More recent data from two small clinical studies in patients with HF and renal failure showed that intravenous iron improved LVEF, as also shown in an experimental model [61, 69, 70].
The meta‐analysis of Jankowska et al. [71] included five randomized clinical (851 patients) with systolic CHF and ID [72, 73, 74, 75, 76]. Patients were treated with an intravenous iron preparation and various comparators (oral or intravenous placebo, oral iron). Results in all included patients (anemic and nonanemic) showed a mean 56% RR reduction in the composite endpoint “all‐cause death or cardiovascular hospitalization” and a 61% RR reduction in the composite endpoint “cardiovascular death or hospitalization for progression of CHF”. The results were comparable in the subgroup of nonanemic patients with a significant decrease in the composite criterion “all‐cause death or cardiovascular hospitalization”. When analyzed separately, the mortality endpoints (all‐cause or cardiovascular) were not significantly improved, probably because of a lack of power due to the small number of deaths in the included studies.
A second meta‐analysis by Anker et al. [77] included data from 839 patients with systolic HF and ID from four double‐blind, randomized clinical trials (FER‐CARS‐01, FAIR‐HF, EFFICACY‐HF, and CONFIRM‐HF) [72, 75, 78, 79] that compared intravenous ferric carboxymaltose (n = 504) with placebo (n = 335). The analysis showed that ferric carboxymaltose treatment was associated with a 41% decrease in the RR of cardiovascular hospitalization or cardiovascular death (primary combined endpoint).
A recent study suggested that administration of iron intravenously in a patient with stable HF and ID lead to myocardial iron repletion, as shown by using a noninvasive cardiac magnetic resonance method [80]. Martens et al. showed that intravenous iron supplementation significantly improved cardiac remodeling (increased LVEF) in patients who benefited from cardiac resynchronization therapy [81].
The multicenter, double‐blind phase II IRONOUT‐HF trial with 225 patients with stable symptomatic systolic HF (NYHA classes II–IV) and ID (FAIR HF/ESC 2016 criteria) compared the effects of oral iron (150 mg × 2/day) administered for 16 weeks with placebo [82]. Results showed no difference between oral iron and placebo groups in the change in functional capacity assessed by the peak oxygen uptake (primary endpoint) and 6‐min walk test. This resistance to oral iron despite a high total dose contrasts with the efficacy of intravenous iron given at 15‐fold lower doses (about 2 g vs. 33 g) to comparable patients in the FAIR‐HF, CONFIRM‐HF, FERRIC‐HF, and EFFECT‐HF trials [72, 74, 75, 83]. The difference in efficacy between oral and intravenous iron is mainly explained by the action of high hepcidin concentrations in CHF patients [82] leading to a low absorption rate of oral iron [84].
The effect of iron supplementation on mortality has been explored in the placebo‐controlled AFFIRM‐HF trial, which included patients with HF (LVEF <50%) and ID (defined by the FAIR‐HF/ESC 2016 criteria) hospitalized for acute HF [85]. After stabilization, participants were randomized at discharge to receive either intravenous ferric carboxymaltose or placebo, and followed for 52 weeks (1108 evaluable participants). Results showed that, compared with placebo, intravenous ferric carboxymaltose reduced the RR of hospitalization for new decompensation by 26%, with no apparent effect on cardiovascular mortality.
Overall, these trials demonstrate that intravenous iron repletion is associated with clinically significant benefits in HF patients with ID, whether or not they are anemic. Therefore, ID should be considered as an independent therapeutic objective in this population, that is, as a comorbidity that should be routinely detected and treated [1].
Current recommendations for treatment
Based on the results of epidemiological studies and therapeutic trials, the recent 2021 ESC guidelines for HF recommend that all patients with HF should be screened periodically for anemia and ID (blood count, serum ferritin, and TSAT) [86]. In addition, intravenous iron repletion with ferric carboxymaltose should be considered in symptomatic patients with LVEF ≤45% and ID (defined as serum ferritin <100 µg/L or serum ferritin between 100 and 299 µg/L and TSAT <20%) to alleviate HF symptoms and improve exercise capacity and quality of life. In hospitalized symptomatic HF patients with LVEF <50% and ID, supplementation with ferric carboxymaltose should be considered to reduce the risk of HF hospitalization.
Knowledge gaps
Due to lack of data, the ESC recommendation for supplementation with intravenous iron does not apply to CHF with preserved LVEF. In addition, no trial was powered to evaluate the efficacy of intravenous iron on significant outcomes or to assess its effectiveness separately in patients with or without anemia. There is also a lack of data on the long‐term safety of intravenous iron in this patient population. Therefore, future studies are expected to address these points.
Managing ID in CKD
Characteristics of ID in CKD
CKD is associated with a decrease in the amount of erythropoietin produced by the kidney, frequently leading to anemia. The treatment of anemia has been revolutionized by the availability of ESA. The association of ID with CKD is complex because erythropoiesis requires large amounts of iron, and ESA by stimulating erythropoiesis may worsen ID, which induces resistance to the effects of ESA. For this reason, the National Institute for Health and Care Excellence (NICE) in the UK has recommended that ESA therapy should not be started until the ID problem has been addressed [87]. Absolute ID can be caused during CKD by malnutrition, decreased intestinal absorption, blood spoliation related to iterative blood testing, and hemodialysis. In addition, CKD is associated with an inflammatory state leading to excessive hepcidin secretion and functional ID.
Iron biomarker thresholds
Iron biomarker thresholds for the diagnosis of ID in CKD currently tend to approach those used in HF. An expert consensus from the international IRON CORE Group proposed defining ID by serum ferritin <100 µg/L or TSAT <20% in both dialysis and nondialysis patients [1]. In the UK, the NICE guidelines recommended the same thresholds to screen for ID every 3 months in patients with nondialysis CKD and every 1–3 months in dialysis patients [87].
Clinical evidence for iron therapy
The FIND‐CKD multicenter randomized trial included 626 patients with nondialysis CKD, anemia, and ID not treated with ESA [88]. Participants received ferric carboxymaltose at dosages adjusted to achieve low (100–200 µg/L) or high (400–600 µg/L) serum ferritin or oral supplementation (ferrous sulfate, 100 mg × 2/day × 52 weeks) and were followed for 1 year. The primary endpoint was the time to the need for additional antianemic therapy (e.g., ESA) or achievement of hemoglobinemia <10 g/dl. Results showed that the incidence of the primary endpoint was significantly lower in the high‐ferritin ferric carboxymaltose group (23.5%) than in the oral supplementation group (31.8%), resulting in an RR reduction of 35%. The results in the low‐ferritin ferric carboxymaltose arm were comparable to those in the oral iron group. No safety alert was reported in this study in patients receiving a high dose of intravenous iron.
Comparable efficacy was shown in a multicentric noninferiority trial, which included 350 nondialysis CKD patients with anemia. The effects of parenteral supplementation (isomaltose iron 1000 mg/injection) versus oral ferrous sulfate (100 mg × 2/day × 8 weeks) were compared. Parenteral supplementation was noninferior to oral supplementation in terms of increase in hemoglobin level at week 4 (primary endpoint). Correction of the serum ferritin and TSAT was also significantly better with intravenous supplementation [89].
Although a recent meta‐analysis showed no impact of ID correction on mortality in CKD patients, intravenous iron compared with oral iron increased hemoglobin, serum ferritin, and TSAT and reduced ESA requirements [90].
High‐dose intravenous iron was suspected of increasing the risk of infections, arterial calcifications, atherothrombosis, and oxidative stress. The PIVOTAL study included 2141 dialysis patients with serum ferritin <400 µg/L and TSAT <30% [91]. Patients were randomized to receive high‐dose intravenous iron (400 mg per month as long as serum ferritin was <700 ng/ml and TSAT <40%) or low‐dose intravenous iron (0–400 mg only if serum ferritin was <200 ng/ml or TSAT <20%). The primary endpoint was a composite of nonfatal myocardial infarction, nonfatal stroke, hospitalization for HF, or death. The authors concluded that in dialysis patients, the use of a proactively administered high‐dose intravenous iron regimen resulted in a significantly lower risk of death or major nonfatal cardiovascular events as compared with that observed with a reactive, low‐dose regimen. A significantly lower dose of ESA and a lower incidence of blood transfusion were also observed. The incidence of infection and hospitalization for any cause was comparable in the two treatment groups.
Current recommendations for treatment
The 2012 KDIGO guidelines recommend intravenous iron in adult patients with TSAT <30% and serum ferritin <500 mg/L if an increase in hemoglobin without starting ESA treatment is preferred or if a decrease in the current ESA dose is wanted. In patients with nondialysis dependent CKD, a 1–3 month trial of oral administration is suggested. In patients with end‐stage CKD on regular dialysis, the oral route is not recommended [92].
The 2013 European recommendations are similar but with a different threshold for iron supplementation, that is, TSAT <20% and serum ferritin <100 ng/ml (absolute ID) or—if an increase in Hb concentration without starting ESA treatment is desired—TSAT <25% and serum ferritin <200 ng/ml in nondialysis CKD patients and TSAT <25% and serum ferritin <300 ng/ml in dialysis patients [93]. The limit of TSAT of 30% and serum ferritin of 500 ng/ml should not be exceeded in both nondialysis and dialysis CKD patients.
Knowledge gaps
ID in CKD patients is most often, if not exclusively, considered in the setting of anemia. Future studies should evaluate the efficacy of iron therapy in nondialysis CKD patients with ID but without anemia. Long‐term studies with mortality are also needed. There is a relationship between fatigue in CKD patients and the role of iron in ATP production in muscle cells. However, the impact of ID, independent of anemia, on neurological and cognitive disorders in CKD patients is also an insufficiently explored field.
Managing ID in cancer
Characteristics of ID in cancer
Many factors possibly lead to ID in cancer—the cancer disease itself, chemotherapy treatment, malnutrition, blood loss, inflammatory state, use of ESA, and so on. ID in these patients is frequently both absolute and functional, but with a predominant role of functional ID due to chronic inflammation [94].
Iron biomarker thresholds
The European Society for Medical Oncology (ESMO) and the US National Comprehensive Cancer Network (NCCN) have proposed criteria for the diagnosis of ID in cancer patients [95, 96]. In the absence of consensus on threshold values, these recommendations agree on the importance of joint determination of serum ferritin and TSAT. In the 2018 ESMO guidelines, ID is defined by serum ferritin <100 µg/L (absolute ID) or serum ferritin ≥100 µg/L and TSAT <20% (functional ID) [95].
In the recent CARENFER study, among cancer patients with ID as defined by the ESMO criteria, TSAT was <20% in 85.8% of patients with neoadjuvant treatment, 90.1% with adjuvant therapy, and 88.6% with metastatic treatment [10, 97]. This indicates that TSAT <20% as the sole criterion for defining ID in cancer patients had high sensitivity regardless of the disease stage.
Clinical evidence for iron therapy
A meta‐analysis of eight randomized clinical trials (including 1600 patients) showed that the combination of intravenous iron with ESA decreased the frequency of transfusions by 23% and increased the hematopoietic response rate by 29%, compared with ESA alone, while both efficacy endpoints were unchanged by oral iron [98].
A systematic review identified 11 randomized clinical trials (1680 participants) that compared intravenous iron with oral iron or no iron supplementation in treating patients with anemia associated with cancer chemotherapy. The addition of intravenous iron to ESA increased the hematopoietic response rate by 28%. It significantly decreased the frequency of transfusions by 24% when combined with ESA and by 48% when no ESA was given. There was no difference between intravenous iron and comparator treatments in mortality or adverse events [99].
Current recommendations for treatment
Recommendations for iron supplementation vary according to the guidelines. The NCCN guidelines are mainly for anemic patients receiving ESA, not with ID alone [96]. Intravenous or oral iron is recommended for absolute ID (serum ferritin <30 ng/ml and TSAT <20%); failure to correct anemia within 4 weeks should lead to consideration of functional ID. Intravenous iron is recommended in functional ID (serum ferritin 30–500 µg/L and TSAT <20%) in patients on ESA therapy.
According to ESMO guidelines, ID should be corrected as soon as serum ferritin levels are <100 µg/L and TSAT is <20% regardless of anemia [95]. Oral iron treatment can only be considered in patients with absolute ID with ferritin <30 µg/L and in the absence of an inflammatory state (C‐reactive protein [CRP] <5 mg/L) [95].
Knowledge gaps
ID remains insufficiently diagnosed in cancer patients and guidelines recommendations are insufficiently applied. Iron therapy is generally considered only a supportive care treatment, combined with ESA in advanced cancer disease. There is a lack of data on ID with or without anemia in the early phases of cancer in patients with adjuvant or neoadjuvant treatment. The insufficient use of iron therapy by oncologists may be related to previous concerns and uncertainties regarding iron administration in this setting. Although iron therapy is recommended in patients controlled with antitumor treatment, iron is also considered as a promising target in cancer progression because it facilitates cell proliferation and growth [100]. In addition, blood transfusion has been associated with an increased risk of cancer development [101]. Long‐term studies on the efficacy and safety of iron therapy could help convince oncologists to systematically diagnose and treat ID, especially during the early phase of the cancer disease, thus avoiding the need for ESA or transfusions when anemia is established and more problematic to treat.
Managing ID in IBDs
Characteristics of ID in IBDs
In the majority of cases, both absolute and functional ID are present in patients with IBD, resulting from the combination of different mechanisms—digestive blood loss (mainly chronic), malnutrition, impaired intestinal iron absorption, intestinal resection, inflammatory syndrome, and so on. [39, 102, 103].
Iron biomarker thresholds
The criteria for the biological diagnosis of ID are controversial. The choice and definition of threshold values are complicated by the variable intensity of the inflammatory state that accompanies the relapses and remissions of chronic IBD. The European Crohn's and Colitis Organisation consensus proposes a definition that considers an associated inflammatory syndrome [104]. ID is defined as serum ferritin ≤100 µg/L in the presence of concomitant inflammation (CRP ≥5 mg/L) or serum ferritin <30 µg/L in the absence of inflammation (CRP <5 mg/L); ID is absolute if serum ferritin is <30 µg/L or TSAT is <16% whereas ID is functional if serum ferritin is <100 µg/L and TSAT is <16%. According to the British Society of Gastroenterology, serum ferritin is the most specific assay for ID in the absence of inflammation—serum ferritin <30 µg/L is generally indicative of low iron stores, and serum ferritin <15 µg/L is indicative of absent iron stores [105]. Therefore, serum ferritin <15 µg/L is considered highly specific for ID; TSAT <20% indicates iron restriction. In the presence of inflammation (for example, elevated CRP), the threshold for ID is serum ferritin <100 µg/L [105].
Clinical evidence for iron therapy
A systematic review of randomized clinical trials comparing oral versus parenteral supplementation in adult patients with IBD included five trials with nearly 700 participants. Meta‐analysis of the data showed that the likelihood of increasing hemoglobin ≥2 g/dl was significantly higher with intravenous iron than with oral iron. In contrast, the latter was associated with a substantially higher incidence of treatment discontinuations for adverse events [106].
Treatment of ID is associated with a significant improvement in physical and mental quality of life, including in patients with ID without anemia [41, 107].
Current recommendations for iron treatment
A recent international expert consensus proposed an algorithm for managing ID associated with chronic IBDs. ID was defined as serum ferritin <100 µg/L or TSAT <20% [1]. Recommendations propose that all patients should be treated to correct anemia and iron stores [36, 37].
The criteria for choosing between oral and parenteral supplementation remain debated [36, 108, 109]. Factors such as etiology, severity of anemia, and quiescent or active nature of the IBD must be considered [1, 37]. In the active forms, the intravenous route avoids the risk of oxidative damage to the intestinal mucosa and preserves the intestinal microbiome, unlike oral supplementation [38, 108]. The indication for oral supplementation in the first line is preferably in quiescent forms, with mild anemia (Hb ≥10 g/dl) [1, 37, 110]. The parenteral route is indicated in case of intolerance or inefficacy of the oral route, or at the first line in active disease and/or more profound anemia (Hb <10 g/dl) [1, 108, 110]. The British Society of Gastroenterology guidelines recommend that parenteral iron be considered when oral iron is contraindicated, ineffective, or not tolerated [105]. Parenteral iron can be administered early if oral iron replacement therapy is deemed unlikely to be effective or if the correction of ID anemia is urgent.
Knowledge gaps
There is a lack of data on the benefits of ID therapy (relief of symptoms, quality of life) in these generally young patients and no long‐term data on morbidity and mortality.
Conclusion
It is now well established that anemia and ID can exist independently. ID is a proved independent predictor of poor prognosis for chronic inflammatory conditions such as CHF and CKD. Because ID is frequent in patients with chronic inflammatory diseases, iron parameters (both serum ferritin and TSAT) should be part of the routine examination of all these patients (Fig. 2). Iron repletion, preferably via the intravenous route to bypass tissue sequestration and poor intestinal absorption, improves clinical signs and quality of life. There are still knowledge gaps, unmet medical needs, and a need for harmonization of ID definition. ID is an independent factor of mortality, but a decrease of mortality related to iron supplementation remains to be demonstrated in patients with chronic inflammatory conditions.
Fig. 2.
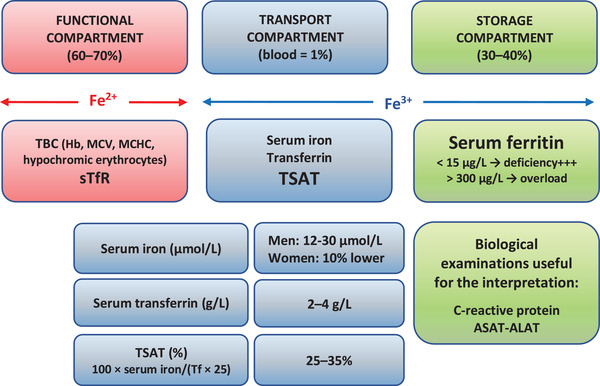
Biological exploration of iron metabolism in routine practice. Hb, hemogobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; sTfR, soluble transferrin receptor; TBC, total blood count; Tf, transferrin; TSAT, transferrin saturation.
Conflict of interest
Patrice Cacoub received consultancies, honoraria, advisory board, and speakers’ fees from Alnylam, Innotech, Mylan, Pfizer, Servier, and Vifor. Sigismond Lasocki is a lecturer personally funded by Pfizer, Vifor Pharma, and Masimo; is consulting for Pfizer and Vifor Pharma; and has received research support from Vifor Pharma. Gabriel Choukroun received honorarium for advisory board and scientific communication from Vifor Pharma, Amgen, Astellas, AstraZeneca, Sanofi, and Takeda. Alain Cohen‐Solal received fees from Vifor for boards, meeting, and scientific presentations and has received fees outside the submitted work from Sanofi, Astra Zeneca, Bayer, Merck, Novartis, Wii Health, Boehringer Ingelheim, Leo, and Cytokinetics. Jean‐Noël Trochu received personal fees from ViforPharma for the work under consideration (advisory boards) and outside the submitted work has received research and institutional grants from Akcea, Carmat, Novartis, and Boston Scientific and personal fees from Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Carmat, Novartis, and Resmed, and is an unpaid member of the Corvia Medical Scientific Advisory Group. Elisabeth Luporsi, Laurent Peyrin‐Biroulet, Katell Peoc'h, Valérie Andrieu, and Hervé Puy received personal fees from Vifor Pharma for the work under consideration (advisory boards).
Author contributions
All authors participated in the literature search in their respective areas of expertise and contributed to the initial drafting of specific sections of the article. Patrice Cacoub supervised the writing of the article. All authors read and edited the manuscript to the final version.
Cacoub P, Choukroun G, Cohen‐Solal A, Luporsi E, Peyrin‐Biroulet L, Peoc'h K, et al. Iron deficiency screening is a key issue in chronic inflammatory diseases: A call to action. J Intern Med. 2022;292:542–556.
References
- 1. Cappellini MD, Comin‐Colet J, De Francisco A, Dignass A, Doehner W, Lam CS, et al. Iron deficiency across chronic inflammatory conditions: international expert opinion on definition, diagnosis, and management. Am J Hematol. 2017;92:1068–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pratt JJ, Khan KS. Non‐anaemic iron deficiency—a disease looking for recognition of diagnosis: a systematic review. Eur J Haematol. 2016;96:618–28. [DOI] [PubMed] [Google Scholar]
- 3. Cacoub P. [Iron deficiency: recent pathophysiological approach and treatment consequences]. Rev Med Interne. 2018;39:381–5. [DOI] [PubMed] [Google Scholar]
- 4. Lopez A, Cacoub P, Macdougall IC, Peyrin‐Biroulet L. Iron deficiency anaemia. Lancet. 2016;387:907–16. [DOI] [PubMed] [Google Scholar]
- 5. Cacoub P, Vandewalle C, Peoc'h K. Using transferrin saturation as a diagnostic criterion for iron deficiency: a systematic review. Crit Rev Clin Lab Sci. 2019;56:526–32. [DOI] [PubMed] [Google Scholar]
- 6. Peyrin‐Biroulet L, Williet N, Cacoub P. Guidelines on the diagnosis and treatment of iron deficiency across indications: a systematic review. Am J Clin Nutr. 2015;102:1585–94. [DOI] [PubMed] [Google Scholar]
- 7. Grote Beverborg N, Van Der Wal HH, Klip IT, Anker SD, Cleland J, Dickstein K, et al. Differences in clinical profile and outcomes of low iron storage vs defective iron utilization in patients with heart failure: results from the DEFINE‐HF and BIOSTAT‐CHF studies. JAMA Cardiol. 2019;4:696–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cacoub P, Choukroun G, Cohen‐Solal A, Luporsi E, Peyrin‐Biroulet L, Peoc'h K, et al. Towards a common definition for the diagnosis of iron deficiency in chronic inflammatory diseases. Nutrients. 2022;14:1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ludwig H, Müldür E, Endler G, Hübl W. Prevalence of iron deficiency across different tumors and its association with poor performance status, disease status and anemia. Ann Oncol. 2013;24:1886–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Luporsi E, Turpin A, Massard V, Morin S, Chauffert B, Carnot A, et al. Iron deficiency in patients with cancer: a prospective cross‐sectional study. BMJ Support Palliat Care. 2021. 10.1136/bmjspcare-2021-002913 [DOI] [PubMed] [Google Scholar]
- 11. Cohen‐Solal A, Philip J‐L, Picard F, Delarche N, Taldir G, Gzara H, et al. Iron deficiency in heart failure patients: the French CARENFER prospective study. ESC Heart Fail. 2022;9:874–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Choukroun G, Cacoub P, Trochu J. Prevalence of iron deficiency in patients with non‐dialysis chronic kidney disease: a national, multicenter, observational study. European Renal Association—European Dialysis and Transplant Association, editor. 58th ERA‐ADTA Congress, June 5–8, 2021.
- 13. Peyrin‐Biroulet L, Bouguen G, Laharie D. Objectifs et rationnel de l’étude de prévalence de la carence martiale dans une population de patients présentant une maladie inflammatoire chronique. Journées Francophones d'Hépato‐gastroentérologie et d'Oncologie Digestive, 18–21 Mars 2021.
- 14. Okonko DO, Mandal AKJ, Missouris CG, Poole‐Wilson PA. Disordered iron homeostasis in chronic heart failure: prevalence, predictors, and relation to anemia, exercise capacity, and survival. J Am Coll Cardiol. 2011;58:1241–51. [DOI] [PubMed] [Google Scholar]
- 15. Klip IT, Comin‐Colet J, Voors AA, Ponikowski P, Enjuanes C, Banasiak W, et al. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J. 2013;165:575–82.e3. [DOI] [PubMed] [Google Scholar]
- 16. Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, et al. Iron deficiency: an ominous sign in patients with systolic chronic heart failure. Eur Heart J. 2010;31:1872–80. [DOI] [PubMed] [Google Scholar]
- 17. Martens P, Nijst P, Verbrugge FH, Smeets K, Dupont M, Mullens W. Impact of iron deficiency on exercise capacity and outcome in heart failure with reduced, mid‐range and preserved ejection fraction. Acta Cardiol. 2018;73:115–23. [DOI] [PubMed] [Google Scholar]
- 18. Grote Beverborg N, Klip IT, Meijers WC, Voors AA, Vegter EL, Van Der Wal HH, et al. Definition of iron deficiency based on the gold standard of bone marrow iron staining in heart failure patients. Circ Heart Fail. 2018;11:e004519. [DOI] [PubMed] [Google Scholar]
- 19. Palau P, Llàcer P, Domínguez E, Tormo JP, Zakarne R, Mollar A, et al. Iron deficiency and short‐term adverse events in patients with decompensated heart failure. Clin Res Cardiol. 2021;110:1292–8. [DOI] [PubMed] [Google Scholar]
- 20. Parikh A, Natarajan S, Lipsitz SR, Katz SD. Iron deficiency in community‐dwelling US adults with self‐reported heart failure in the National Health and Nutrition Examination Survey III: prevalence and associations with anemia and inflammation. Circ Heart Fail. 2011;4:599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moliner P, Jankowska EA, Van Veldhuisen DJ, Farre N, Rozentryt P, Enjuanes C, et al. Clinical correlates and prognostic impact of impaired iron storage versus impaired iron transport in an international cohort of 1821 patients with chronic heart failure. Int J Cardiol. 2017;243:360–6. [DOI] [PubMed] [Google Scholar]
- 22. Wish JB, Aronoff GR, Bacon BR, Brugnara C, Eckardt K‐U, Ganz T, et al. Positive iron balance in chronic kidney disease: how much is too much and how to tell? Am J Nephrol. 2018;47:72–83. [DOI] [PubMed] [Google Scholar]
- 23. Kalantar‐Zadeh K, Regidor DL, Mcallister CJ, Michael B, Warnock DG. Time‐dependent associations between iron and mortality in hemodialysis patients. J Am Soc Nephrol. 2005;16:3070–80. [DOI] [PubMed] [Google Scholar]
- 24. Eisenga MF, Nolte IM, Van Der Meer P, Bakker SJL, Gaillard CAJM. Association of different iron deficiency cutoffs with adverse outcomes in chronic kidney disease. BMC Nephrol. 2018;19:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cho ME, Hansen JL, Peters CB, Cheung AK, Greene T, Sauer BC. An increased mortality risk is associated with abnormal iron status in diabetic and non‐diabetic veterans with predialysis chronic kidney disease. Kidney Int. 2019;96:750–60. [DOI] [PubMed] [Google Scholar]
- 26. Cho ME, Hansen JL, Sauer BC, Cheung AK, Agarwal A, Greene T. Heart failure hospitalization risk associated with iron status in veterans with CKD. Clin J Am Soc Nephrol. 2021;16:522–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guedes M, Muenz DG, Zee J, Bieber B, Stengel B, Massy ZA, et al. Serum biomarkers of iron stores are associated with increased risk of all‐cause mortality and cardiovascular events in nondialysis CKD patients, with or without anemia. J Am Soc Nephrol. 2021;32:2020–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abiri B, Vafa M. Iron deficiency and anemia in cancer patients: the role of iron treatment in anemic cancer patients. Nutr Cancer. 2020;72:864–72. [DOI] [PubMed] [Google Scholar]
- 29. Ludwig H, Van Belle S, Barrett‐Lee P, Birgegård G, Bokemeyer C, Gascón P, et al. The European Cancer Anaemia Survey (ECAS): a large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur J Cancer. 2004;40:2293–306. [DOI] [PubMed] [Google Scholar]
- 30. Caro JJ, Salas M, Ward A, Goss G. Anemia as an independent prognostic factor for survival in patients with cancer: a systemic, quantitative review. Cancer. 2001;91:2214–21. [PubMed] [Google Scholar]
- 31. Leichtle SW, Mouawad NJ, Lampman R, Singal B, Cleary RK. Does preoperative anemia adversely affect colon and rectal surgery outcomes? J Am Coll Surg. 2011;212:187–94. [DOI] [PubMed] [Google Scholar]
- 32. Simões CM, Carmona MJC, Hajjar LA, Vincent J‐L, Landoni G, Belletti A, et al. Predictors of major complications after elective abdominal surgery in cancer patients. BMC Anesthesiol. 2018;18:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fowler AJ, Ahmad T, Phull MK, Allard S, Gillies MA, Pearse RM. Meta‐analysis of the association between preoperative anaemia and mortality after surgery. Br J Surg. 2015;102:1314–24. [DOI] [PubMed] [Google Scholar]
- 34. Miles LF, Sandhu RNs, Grobler AC, Heritier S, Burgess A, Burbury KL, et al. Associations between non‐anaemic iron deficiency and outcomes following surgery for colorectal cancer: an exploratory study of outcomes relevant to prospective observational studies. Anaesth Intensive Care. 2019;47:152–9. [DOI] [PubMed] [Google Scholar]
- 35. Ploug M, Kroijer R, Qvist N, Lindahl CH, Knudsen T. Iron deficiency in colorectal cancer patients: a cohort study on prevalence and associations. Colorectal Dis. 2021;23:853–9. [DOI] [PubMed] [Google Scholar]
- 36. Goddard AF, James MW, Mcintyre AS, Scott BB, British Society of Gastroenterology . Guidelines for the management of iron deficiency anaemia. Gut. 2011;60:1309–16. [DOI] [PubMed] [Google Scholar]
- 37. Van Assche G, Dignass A, Bokemeyer B, Danese S, Gionchetti P, Moser G, et al. Second European evidence‐based consensus on the diagnosis and management of ulcerative colitis part 3: special situations. J Crohns Colitis. 2013;7:1–33. [DOI] [PubMed] [Google Scholar]
- 38. Lee T, Clavel T, Smirnov K, Schmidt A, Lagkouvardos I, Walker A, et al. Oral versus intravenous iron replacement therapy distinctly alters the gut microbiota and metabolome in patients with IBD. Gut. 2017;66:863–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gasche C. Iron, anaemia, and inflammatory bowel diseases. Gut. 2004;53:1190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Danese S, Hoffman C, Vel S, Greco M, Szabo H, Wilson B, et al. Anaemia from a patient perspective in inflammatory bowel disease: results from the European Federation of Crohn's and Ulcerative Colitis Association's online survey. Eur J Gastroenterol Hepatol. 2014;26:1385–91. [DOI] [PubMed] [Google Scholar]
- 41. Wells CW, Lewis S, Barton RJ, Corbett S. Effects of changes in hemoglobin level on quality of life and cognitive function in inflammatory bowel disease patients. Inflamm Bowel Dis. 2006;12:123–30. [DOI] [PubMed] [Google Scholar]
- 42. Haas JD, Brownlie T. Iron deficiency and reduced work capacity: a critical review of the research to determine a causal relationship. J Nutr. 2001;131:676S–90S. [DOI] [PubMed] [Google Scholar]
- 43. Van Der Valk ME, Mangen M‐JJ, Leenders M, Dijkstra G, Van Bodegraven AA, Fidder HH, et al. Healthcare costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti‐TNFalpha therapy: results from the COIN study. Gut. 2014;63:72–9. [DOI] [PubMed] [Google Scholar]
- 44. Høivik ML, Moum B, Solberg IC, Henriksen M, Cvancarova M, Bernklev T. Work disability in inflammatory bowel disease patients 10 years after disease onset: results from the IBSEN Study. Gut. 2013;62:368–75. [DOI] [PubMed] [Google Scholar]
- 45. Andro M, Le Squere P, Estivin S, Gentric A. Anaemia and cognitive performances in the elderly: a systematic review. Eur J Neurol. 2013;20:1234–40. [DOI] [PubMed] [Google Scholar]
- 46. Sawada T, Konomi A, Yokoi K. Iron deficiency without anemia is associated with anger and fatigue in young Japanese women. Biol Trace Elem Res. 2014;159:22–31. [DOI] [PubMed] [Google Scholar]
- 47. Guthrie E, Jackson J, Shaffer J, Thompson D, Tomenson B, Creed F. Psychological disorder and severity of inflammatory bowel disease predict health‐related quality of life in ulcerative colitis and Crohn's disease. Am J Gastroenterol. 2002;97:1994–9. [DOI] [PubMed] [Google Scholar]
- 48. Ott C, Liebold A, Takses A, Strauch UG, Obermeier F. High prevalence but insufficient treatment of iron‐deficiency anemia in patients with inflammatory bowel disease: results of a population‐based cohort. Gastroenterol Res Pract. 2012;2012:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Peyrin‐Biroulet L, Lopez A, Cummings JRF, Dignass A, Detlie TE, Danese S. Review article: treating‐to‐target for inflammatory bowel disease‐associated anaemia. Aliment Pharmacol Ther. 2018;48:610–7. [DOI] [PubMed] [Google Scholar]
- 50. Zhang A‐S, Enns CA. Molecular mechanisms of normal iron homeostasis. Hematology Am Soc Hematol Educ Program. 2009;2009:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hentze MW, Muckenthaler MU, Galy B, Camaschella C. Two to tango: regulation of mammalian iron metabolism. Cell. 2010;142:24–38. [DOI] [PubMed] [Google Scholar]
- 52. Koulaouzidis A, Said E, Cottier R, Saeed AA. Soluble transferrin receptors and iron deficiency, a step beyond ferritin. A systematic review. J Gastrointestin Liver Dis. 2009;18:345–52. [PubMed] [Google Scholar]
- 53. Brasse–Lagnel C, Karim Z, Letteron P, Bekri S, Bado A, Beaumont C. Intestinal DMT1 cotransporter is down‐regulated by hepcidin via proteasome internalization and degradation. Gastroenterology. 2011;140:1261–71.e1. [DOI] [PubMed] [Google Scholar]
- 54. Camaschella C. Iron deficiency. Blood. 2019;133:30–9. [DOI] [PubMed] [Google Scholar]
- 55. Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, et al. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110:1037–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wrighting DM, Andrews NC. Interleukin‐6 induces hepcidin expression through STAT3. Blood. 2006;108:3204–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Weiss G, Ganz T, Goodnough LT. Anemia of inflammation. Blood. 2019;133:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Patterson AJ, Brown WJ, Powers JR, Roberts DCK. Iron deficiency, general health and fatigue: results from the Australian Longitudinal Study on Women's Health. Qual Life Res. 2000;9:491–7. [DOI] [PubMed] [Google Scholar]
- 59. Anand IS, Gupta P. Anemia and iron deficiency in heart failure: current concepts and emerging therapies. Circulation. 2018;138:80–98. [DOI] [PubMed] [Google Scholar]
- 60. Van Veldhuisen DJ, Anker SD, Ponikowski P, Macdougall IC. Anemia and iron deficiency in heart failure: mechanisms and therapeutic approaches. Nat Rev Cardiol. 2011;8:485–93. [DOI] [PubMed] [Google Scholar]
- 61. Rineau E, Gaillard T, Gueguen N, Procaccio V, Henrion D, Prunier F, et al. Iron deficiency without anemia is responsible for decreased left ventricular function and reduced mitochondrial complex I activity in a mouse model. Int J Cardiol. 2018;266:206–12. [DOI] [PubMed] [Google Scholar]
- 62. Hoes MF, Grote Beverborg N, Kijlstra JD, Kuipers J, Swinkels DW, Giepmans BNG, et al. Iron deficiency impairs contractility of human cardiomyocytes through decreased mitochondrial function. Eur J Heart Fail. 2018;20:910–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–200. [DOI] [PubMed] [Google Scholar]
- 64. Davies KJ, Maguire JJ, Brooks GA, Dallman PR, Packer L. Muscle mitochondrial bioenergetics, oxygen supply, and work capacity during dietary iron deficiency and repletion. Am J Physiol. 1982;242:E418–27. [DOI] [PubMed] [Google Scholar]
- 65. Brunner‐La Rocca H‐P, Eurlings L, Richards AM, Januzzi JL, Pfisterer ME, Dahlström U, et al. Which heart failure patients profit from natriuretic peptide guided therapy? A meta‐analysis from individual patient data of randomized trials. Eur J Heart Fail. 2015;17:1252–61. [DOI] [PubMed] [Google Scholar]
- 66. Silverberg DS, Wexler D, Sheps D, Blum M, Keren G, Baruch R, et al. The effect of correction of mild anemia in severe, resistant congestive heart failure using subcutaneous erythropoietin and intravenous iron: a randomized controlled study. J Am Coll Cardiol. 2001;37:1775–80. [DOI] [PubMed] [Google Scholar]
- 67. Silverberg DS, Wexler D, Blum M, Keren G, Sheps D, Leibovitch E, et al. The use of subcutaneous erythropoietin and intravenous iron for the treatment of the anemia of severe, resistant congestive heart failure improves cardiac and renal function and functional cardiac class, and markedly reduces hospitalizations. J Am Coll Cardiol. 2000;35:1737–44. [DOI] [PubMed] [Google Scholar]
- 68. Usmanov RI, Zueva EB, Silverberg DS, Shaked M. Intravenous iron without erythropoietin for the treatment of iron deficiency anemia in patients with moderate to severe congestive heart failure and chronic kidney insufficiency. J Nephrol. 2008;21:236–42. [PubMed] [Google Scholar]
- 69. Núñez J, Monmeneu JV, Mollar A, Núñez E, Bodí V, Miñana G, et al. Left ventricular ejection fraction recovery in patients with heart failure treated with intravenous iron: a pilot study. ESC Heart Fail. 2016;3:293–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Toblli JE, Di Gennaro F, Rivas C. Changes in echocardiographic parameters in iron deficiency patients with heart failure and chronic kidney disease treated with intravenous iron. Heart Lung Circ. 2015;24:686–95. [DOI] [PubMed] [Google Scholar]
- 71. Jankowska EA, Tkaczyszyn M, Suchocki T, Drozd M, Von Haehling S, Doehner W, et al. Effects of intravenous iron therapy in iron‐deficient patients with systolic heart failure: a meta‐analysis of randomized controlled trials. Eur J Heart Fail. 2016;18:786–95. [DOI] [PubMed] [Google Scholar]
- 72. Ponikowski P, Van Veldhuisen DJ, Comin‐Colet J, Ertl G, Komajda M, Mareev V, et al. Beneficial effects of long‐term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiencydagger. Eur Heart J. 2015;36:657–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Toblli JE, Lombraña A, Duarte P, Di Gennaro F. Intravenous iron reduces NT‐pro‐brain natriuretic peptide in anemic patients with chronic heart failure and renal insufficiency. J Am Coll Cardiol. 2007;50:1657–65. [DOI] [PubMed] [Google Scholar]
- 74. Okonko DO, Grzeslo A, Witkowski T, Mandal AKJ, Slater RM, Roughton M, et al. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency FERRIC‐HF: a randomized, controlled, observer‐blinded trial. J Am Coll Cardiol. 2008;51:103–12. [DOI] [PubMed] [Google Scholar]
- 75. Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361:2436–48. [DOI] [PubMed] [Google Scholar]
- 76. Beck‐Da‐Silva L, Piardi D, Soder S, Rohde LE, Pereira‐Barretto AC, De Albuquerque D, et al. IRON‐HF study: a randomized trial to assess the effects of iron in heart failure patients with anemia. Int J Cardiol. 2013;168:3439–42. [DOI] [PubMed] [Google Scholar]
- 77. Anker SD, Kirwan B‐A, Van Veldhuisen DJ, Filippatos G, Comin‐Colet J, Ruschitzka F, et al. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron‐deficient heart failure patients: an individual patient data meta‐analysis. Eur J Heart Fail. 2018;20:125–33. [DOI] [PubMed] [Google Scholar]
- 78. Anker SD, Colet JC, Filippatos G, Willenheimer R, Dickstein K, Drexler H, et al. Rationale and design of Ferinject assessment in patients with IRon deficiency and chronic Heart Failure (FAIR‐HF) study: a randomized, placebo‐controlled study of intravenous iron supplementation in patients with and without anaemia. Eur J Heart Fail. 2009;11:1084–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Arutyunov G, Bylova N, Ivleva A, Kobalava Z. The safety of intravenous (IV) ferric carboxymaltose versus IV iron sucrose on patients with chronic heart failure (CHF) and chronic kidney disease (CKD) with iron deficincy (ID). Eur J Heart Fail. 2009;8:ii71. [Google Scholar]
- 80. Núñez J, Miñana G, Cardells I, Palau P, Llàcer P, Fácila L, et al. Noninvasive imaging estimation of myocardial iron repletion following administration of intravenous iron: the myocardial‐IRON trial. J Am Heart Assoc. 2020;9:e014254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Martens P, Dupont M, Dauw J, Nijst P, Herbots L, Dendale P, et al. The effect of intravenous ferric carboxymaltose on cardiac reverse remodelling following cardiac resynchronization therapy‐the IRON‐CRT trial. Eur Heart J. 2021;42:4905–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lewis GD, Malhotra R, Hernandez AF, Mcnulty SE, Smith A, Felker GM, et al. Effect of oral iron repletion on exercise capacity in patients with heart failure with reduced ejection fraction and iron deficiency: the IRONOUT HF randomized clinical trial. JAMA. 2017;317:1958–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. van Veldhuisen DJ, Ponikowski P, van der Meer P, Metra M, Böhm M, Doletsky A, et al. Effect of ferric carboxymaltose on exercise capacity in patients with chronic heart failure and iron deficiency. Circulation. 2017;136:1374–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Stoffel NU, Cercamondi CI, Brittenham G, Zeder C, Geurts‐Moespot AJ, Swinkels DW, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice‐daily split dosing in iron‐depleted women: two open‐label, randomised controlled trials. Lancet Haematol. 2017;4:e524–33. [DOI] [PubMed] [Google Scholar]
- 85. Ponikowski P, Kirwan B‐A, Anker SD, Mcdonagh T, Dorobantu M, Drozdz J, et al. Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: a multicentre, double‐blind, randomised, controlled trial. Lancet. 2020;396:1895–904. [DOI] [PubMed] [Google Scholar]
- 86. Mcdonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–726. [DOI] [PubMed] [Google Scholar]
- 87. National Institute for Health and Care Excellence . Chronic kidney disease: managing anaemia. NICE guideline [NG8]. https://www.nice.org.uk/guidance/ng8 (2015). Accessed April 27, 2022. [PubMed]
- 88. Macdougall IC, Bock AH, Carrera F, Eckardt K‐U, Gaillard C, Van Wyck D, et al. FIND‐CKD: a randomized trial of intravenous ferric carboxymaltose versus oral iron in patients with chronic kidney disease and iron deficiency anaemia. Nephrol Dial Transplant. 2014;29:2075–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kalra PA, Bhandari S, Saxena S, Agarwal D, Wirtz G, Kletzmayr J, et al. A randomized trial of iron isomaltoside 1000 versus oral iron in non‐dialysis‐dependent chronic kidney disease patients with anaemia. Nephrol Dial Transplant. 2016;31:646–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. O'Lone EL, Hodson EM, Nistor I, Bolignano D, Webster AC, Craig JC. Parenteral versus oral iron therapy for adults and children with chronic kidney disease. Cochrane Database Syst Rev. 2019;2:CD007857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Macdougall IC, White C, Anker SD, Bhandari S, Farrington K, Kalra PA, et al. Intravenous iron in patients undergoing maintenance hemodialysis. N Engl J Med. 2019;380:447–58. [DOI] [PubMed] [Google Scholar]
- 92. McMurray JJV, Parfrey PS, Adamson JW, Aljama P, Berns JS, Bohlius J, et al. Kidney Disease: Improving Global Outcomes (KDIGO) anemia work group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl. 2012;2:279–335. [Google Scholar]
- 93. Locatelli F, Bárány P, Covic A, De Francisco A, Del Vecchio L, Goldsmith D, et al. Kidney Disease: Improving Global Outcomes guidelines on anaemia management in chronic kidney disease: a European Renal Best Practice position statement. Nephrol Dial Transplant. 2013;28:1346–59. [DOI] [PubMed] [Google Scholar]
- 94. Wilson MJ, Dekker JWT, Harlaar JJ, Jeekel J, Schipperus M, Zwaginga JJ. The role of preoperative iron deficiency in colorectal cancer patients: prevalence and treatment. Int J Colorectal Dis. 2017;32:1617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Aapro M, Beguin Y, Bokemeyer C, Dicato M, Gascón P, Glaspy J, et al. Management of anaemia and iron deficiency in patients with cancer: ESMO clinical practice guidelines. Ann Oncol. 2018;29:iv96–110. [DOI] [PubMed] [Google Scholar]
- 96. National Comprehensive Cancer Network . NCCN guidelines. Hematopoïetic growth factors. Management of cancer‐ and chemotherapy‐induced anemia. https://www.nccn.org/guidelines/guidelines‐detail?category=3&id=1493 (2021). Accessed April 27, 2022.
- 97. Luporsi‐Gely E, Turpin A, Massard V, Morin S, Simon H, Ruppert A‐M, et al. Transferrin saturation shows high prevalence of iron deficiency in cancer patients under adjuvant and neo‐adjuvant treatment. Ann Oncol. 2021;32:S385–6. [Google Scholar]
- 98. Petrelli F, Borgonovo K, Cabiddu M, Lonati V, Barni S. Addition of iron to erythropoiesis‐stimulating agents in cancer patients: a meta‐analysis of randomized trials. J Cancer Res Clin Oncol. 2012;138:179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Gafter‐Gvili A, Rozen‐Zvi B, Vidal L, Leibovici L, Vansteenkiste J, Gafter U, et al. Intravenous iron supplementation for the treatment of chemotherapy‐induced anaemia—systematic review and meta‐analysis of randomised controlled trials. Acta Oncol. 2013;52:18–29. [DOI] [PubMed] [Google Scholar]
- 100. Jung M, Mertens C, Tomat E, Brüne B. Iron as a central player and promising target in cancer progression. Int J Mol Sci. 2019;20:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Hjalgrim H, Edgren G, Rostgaard K, Reilly M, Tran TN, Titlestad KE, et al. Cancer incidence in blood transfusion recipients. J Natl Cancer Inst. 2007;99:1864–74. [DOI] [PubMed] [Google Scholar]
- 102. Nielsen O, Soendergaard C, Vikner M, Weiss G. Rational management of iron‐deficiency anaemia in inflammatory bowel disease. Nutrients. 2018;10:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Bager P, Befrits R, Wikman O, Lindgren S, Moum B, Hjortswang H, et al. The prevalence of anemia and iron deficiency in IBD outpatients in Scandinavia. Scand J Gastroenterol. 2011;46:304–9. [DOI] [PubMed] [Google Scholar]
- 104. Dignass AU, Gasche C, Bettenworth D, Birgegård G, Danese S, Gisbert JP, et al. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J Crohns Colitis. 2015;9:211–22. [DOI] [PubMed] [Google Scholar]
- 105. Snook J, Bhala N, Beales ILP, Cannings D, Kightley C, Logan RPH, et al. British Society of Gastroenterology guidelines for the management of iron deficiency anaemia in adults. Gut. 2021;70:2030–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Bonovas S, Fiorino G, Allocca M, Lytras T, Tsantes A, Peyrin‐Biroulet L, et al. Intravenous versus oral iron for the treatment of anemia in inflammatory bowel disease: a systematic review and meta‐analysis of randomized controlled trials. Medicine (Baltimore). 2016;95:e2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Çekiç C, İpek S, Aslan F, Akpınar Z, Arabul M, Topal F, et al. The effect of intravenous iron treatment on quality of life in inflammatory bowel disease patients with nonanemic iron deficiency. Gastroenterol Res Pract. 2015;2015:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Nielsen OH, Ainsworth M, Coskun M, Weiss G. Management of iron‐deficiency anemia in inflammatory bowel disease: a systematic review. Medicine (Baltimore). 2015;94:e963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Reinisch W, Staun M, Tandon RK, Altorjay I, Thillainayagam AV, Gratzer C, et al. A randomized, open‐label, non‐inferiority study of intravenous iron isomaltoside 1,000 (Monofer) compared with oral iron for treatment of anemia in IBD (PROCEED). Am J Gastroenterol. 2013;108:1877–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. van Rheenen PF, Aloi M, Biron IA, Carlsen K, Cooney R, Cucchiara S, et al. European Crohn's and Colitis Organisation topical review on transitional care in inflammatory bowel disease. J Crohns Colitis. 2017;11:1032–8. [DOI] [PubMed] [Google Scholar]


