Abstract
Background
It is unknown how use of newer glucose‐lowering drugs (GLDs) has changed in Australia following the publication of clinical trials demonstrating definitive clinical advantages for glucagon‐like peptide‐1 receptor agonists (GLP‐1 RAs) and sodium‐glucose co‐transporter 2 inhibitors (SGLT2is), and whether this varies by socio‐economic disadvantage.
Methods
We included 1,064,645 people with type 2 diabetes registered on the National Diabetes Services Scheme. This cohort was linked to the Pharmaceutical Benefits Scheme database to evaluate trends in diabetes medication receipt and variation by socio‐economic disadvantage between 2013 and 2019.
Results
The proportion of people with type 2 diabetes receiving ≥3 GLDs concurrently increased from 12% in 2013 to 25% in 2019. By 2019, 6% of people with diabetes were receiving a GLP‐1 RA and 21% an SGLT2i. Disparities in receipt of GLP‐1 RAs and SGLT2is by socio‐economic disadvantage decreased over time (ORs for most vs. least disadvantaged quintile were 0.80 [0.77–0.85] and 0.87 [0.82–0.94] in 2014 and 0.95 [0.92–0.98] and 1.07 [1.05–1.09] in 2019 for GLP‐1 RAs and SGLT2is, respectively). However, people in more disadvantaged areas were more likely to receive multiple GLDs. After stratifying by number of concurrent GLDs received, people in more disadvantaged areas were less likely to receive GLP‐1 RAs and SGLT2is in 2019 (ORs for most vs. least disadvantaged: 0.81 [0.78–0.84] and 0.90 [0.87–0.93] for people receiving ≥3 GLDs, respectively).
Conclusions
After controlling for intensity of glucose‐lowering therapy, people in more disadvantaged areas were less likely to receive cardioprotective GLDs, although disparities decreased over time.
What's new?
The known—Recent clinical trials have shown definitive cardiovascular benefits of GLP‐1 RAs and SGLT2is over other diabetes medications. However, it is unknown how prescribing habits have changed in response or whether previous socio‐economic disparities observed in receipt of these medications persist.
The new—DPP4is were still the most common second‐line diabetes medication in 2019, despite clear benefits of GLP‐1 RAs and SGLT2is. We observed persistent disparities in receipt, persistence and adherence to GLP‐1 RAs and SGLT2is by socio‐economic disadvantage.
The implications—Expanding the Pharmaceutical Benefits Scheme criteria for GLP‐1 RAs and SGLT2is to recognise their effects beyond glycaemia may be warranted.
1. INTRODUCTION
Cardiovascular and kidney disease are common complications of type 2 diabetes and are both burdensome and costly. 1 Recently, two new classes of glucose‐lowering drug (GLD), glucagon‐like peptide‐1 receptor agonists (GLP‐1 RAs) and sodium‐glucose co‐transporter 2 inhibitors (SGLT2is), have been shown to be effective at preventing cardiovascular disease and kidney disease in people with diabetes. 2 , 3 , 4 Our group has recently shown that when they first became widely available in Australia, there was decreased receipt of these GLDs with increasing socio‐economic disadvantage, 5 which did not appear to be due to affordability or access to specialists. However, the cardiovascular and kidney benefits of GLP‐1 RAs and SGLT2is were unknown at the time of their listing on the Pharmaceutical Benefits Scheme (PBS; in August 2010 and December 2013, respectively), thus our study could not capture the effect of socio‐economic status on receipt of GLDs at a time when there were clear clinical advantages for these GLDs.
It is therefore of interest to examine trends in the receipt of these GLDs by socio‐economic disadvantage following the publication of clinical trials showing their benefits for preventing cardiovascular events for the first time (in November 2015 for SGLT2is and July 2016 for GLP‐1 RAs 6 , 7 ), as this may highlight a disparity in communicating novel benefits of existing medications by socio‐economic status. Therefore, we linked the National Diabetes Services Scheme (NDSS), the Australian diabetes registry, to the PBS to estimate trends in the receipt of each GLD class and how this varied by socio‐economic disadvantage among people with type 2 diabetes in Australia from 2013 to 2019.
2. METHODS
2.1. Data sources
The study population was derived from the NDSS. The NDSS is the Australian diabetes registry, and is estimated to cover 80%–90% of people with diagnosed diabetes in Australia. 8 We included members of the NDSS with type 2 diabetes in Victoria, New South Wales, the Australian Capital Territory and Queensland who were registered on the NDSS before 25 December 2019. Assignment of diabetes type in this study was as previously described. 9 This cohort was linked to the PBS and National Death Index (NDI) by the Australian Institute of Health and Welfare. The PBS is administered by the Australian Government and subsidises medications and collects information on all prescriptions covered by the scheme. The PBS covers the vast majority of diabetes medications dispensed in Australia; timelines of major cardiovascular outcomes trials, the date each SGLT2i and GLP‐1 RA was listed on the PBS and the date each received approval for cardiovascular disease prevention by the Therapeutic Goods Administration (TGA) of Australia are shown in Figures S1, S2. Our analysis was restricted to Australians who do not identify as Aboriginal or Torres Strait Islander because Aboriginal and Torres Strait Islander Australians are able to access PBS‐listed medications via the Remote Area Aboriginal Health Services Program, and thus may not have medication dispensations recorded in the PBS.
This study was approved by the Alfred Hospital Ethics Committee (Project No: 463/18) and the Australian Institute of Health and Welfare Ethics Committee (EO2018/5/501).
2.2. Measure of socio‐economic disadvantage
Socio‐economic disadvantage was assigned based on an individual's residential postcode; we only included NDSS registrants with available postcode information (>99% of those who otherwise met our inclusion criteria). Socio‐economic disadvantage was measured using the Socio‐Economic Index for Areas: Index of Relative Socio‐economic Disadvantage (IRSD). The IRSD is produced by the Australian Bureau of Statistics after each census and ranks areas in Australia according to various measures of socio‐economic disadvantage (such as income, education and employment). 10 IRSD quintiles were used in this study, where the highest quintile represents individuals living in areas of least socio‐economic disadvantage.
2.3. Data analysis
2.3.1. Receipt of GLDs
First, we estimated the proportion of people with type 2 diabetes receiving 0, 1, 2 or 3 or more GLDs concurrently, by calendar year. Then, we estimated the proportion of people with type 2 diabetes receiving each class of GLD (metformin, sulfonylureas, dipeptidyl‐peptidase 4 inhibitors [DPP4is], SGLT2is, thiazolidinediones, GLP‐1 RAs, alpha glucosidase inhibitors and insulin) within each calendar year from 2013 to 2019. These proportions were calculated as the total number of people dispensed ≥1 prescription for the respective GLD class, divided by the total number of people with type 2 diabetes who were registered on the NDSS before the end of that calendar year who survived the full year. Current guidelines recommend metformin monotherapy as the initial GLD for people with type 2 diabetes 11 , 12 ; GLP‐1 RAs and SGLT2is are only approved as second‐ and third‐line GLDs in Australia. Therefore, we subsequently calculated the proportional receipt of each class of GLD as an add‐on medication, as previously described. 5 These proportions were calculated as the total number of people adding‐on the specific GLD, divided by the total number of add‐on events in a given calendar year. To ensure add‐on events were correctly defined in 2013, we included data from 1 July 2011 to define add‐on events (i.e. an 18‐month lookback period). Definitions of concurrent and add‐on GLD receipt are available in the Appendix S1.
2.3.2. Adherence and persistence
We also estimated adherence and persistence for the first year of therapy with GLP‐1 RAs and SGLT2is. Therefore, we only included people who initiated a GLP‐1 RA or SGLT2i from 2013 to 2018 in these analyses. Persistence was defined as no >90 day gap in coverage for the respective GLD class during the first year of therapy, where coverage was defined as the product of quantity supplied and defined daily dose. Adherence was calculated via the proportion of days covered (PDCs) over the first year of therapy, and analysed as a binary variable (adherent vs. non‐adherent), where individuals were considered adherent if they had >80% of days covered throughout the year. 13 PDCs was calculated as the number of days the GLD class was available (from quantity supplied and defined daily dose), divided by 365. When dispensations occurred before coverage expired, it was assumed individuals would finish the current supply before commencing the next.
In the primary analysis, we only included individuals who persisted for at least two dispensings of the GLD; we included results from all initiators in a sensitivity analysis. Moreover, because medications are not always prescribed at the defined daily dose, we conducted another sensitivity analysis where coverage was defined as the time period in which 75% of the population refilled a prescription for that item. Finally, to determine the effect of medication cost on persistence and adherence, we conducted a sensitivity analysis estimating persistence and adherence including only people who received the majority of their prescriptions (>80%) under a concession, a group in which medication costs are substantially reduced.
2.3.3. Differences by socio‐economic disadvantage
Differences in number of GLDs concurrently received (0, 1, 2 or 3 or more GLDs received concurrently) by the IRSD were assessed using linear regression. We conducted logistic regression to assess differences in receipt of each GLD class by the IRSD (where the outcome was a dispensation of the GLD within that calendar year), as previously described. 5 To account for differences in intensity of diabetes treatment across the IRSD, 5 logistic regression analyses were stratified by number of concurrent GLDs (two or three or more). We then performed logistic regression with the outcome as receiving a given GLD class as an add‐on among only people who received an add‐on GLD within a given calendar year, by the IRSD. Finally, we conducted logistic regression analyses to assess differences in persistence and adherence, with the outcome being 1‐year persistence or adherence, among people who initiated GLP‐1 RAs and SGLT2is.
All regression analyses were adjusted for age, sex, duration of diabetes and the Accessibility and Remoteness Index of Australia (ARIA), which have all been shown to be associated with prescribing of GLDs, 5 as well as the RxRisk comorbidity index. The ARIA is a measure of remoteness, based on relative access to services within an area, 14 classified in this study as major city, inner regional, outer regional or remote. The RxRisk comorbidity index is a weighted comorbidity score calculated via all prescriptions dispensed in the preceding year. 15 Persistence and adherence analyses were additionally adjusted for date of initiation and initiating medication (exenatide or dulaglutide for GLP‐1 RAs; dapagliflozin or empagliflozin for SGLT2is). Finally, to assess the contribution of access to specialist care on receipt of GLDs, we repeated the analyses described with further adjustment for the type of doctor providing the prescription (measured as a binary variable, with a value of 1 for receipt of any prescription for a GLD from a specialist in a given year and a value of 0 for no prescriptions from a specialist).
Analyses were performed in the Stata statistical software, version 15.
3. RESULTS
3.1. Trends in GLD dispensing
Characteristics of the study population are shown in Table 1. The proportion of people receiving 0, 1 or 2 GLDs concurrently decreased over time, while the proportion of people receiving 3 or more increased from 12% in 2013 to 25% in 2019 (Figure 1). The proportion of people with type 2 diabetes receiving each GLD class from 2013 to 2019 is shown in Figure 2, and the receipt of each GLD class as an add‐on GLD is shown in Figure 3. DPP4is remained the most common add‐on GLD throughout the study period, with SGLT2is becoming the second most common by 2016. By 2019, 32% of add‐on prescriptions were for DPP4is versus 30% for SGLT2is. When stratified by second‐ and third‐line add‐on, SGLT2is were the most common third‐line add‐on by 2015 and remained so through 2019, when SGLT2is accounted for 35% of all third‐line add‐ons (vs. 24% for DPP4is and 8% for GLP‐1 RAs; data not shown).
TABLE 1.
Population characteristics, by socio‐economic disadvantage (IRSD)
| IRSD | Total | |||||
|---|---|---|---|---|---|---|
| 5 (least disadvantaged) | 4 | 3 | 2 | 1 (most disadvantaged) | ||
| Number of people | 159,961 (15.0%) | 184,940 (17.4%) | 207,854 (19.5%) | 232,832 (21.9%) | 279,135 (26.2%) | 1,064,722 (100.0%) |
| Number of men | 90,508 (56.6%) | 102,855 (55.6%) | 114,433 (55.1%) | 126,485 (54.3%) | 149,404 (53.5%) | 583,685 (54.8%) |
| Age at diagnosis of diabetes | 58.8 (49.3, 67.8) | 57.9 (48.6, 66.9) | 57.3 (47.9, 66.3) | 57.7 (48.5, 66.6) | 57.3 (48.0, 66.2) | 57.7 (48.4, 66.7) |
| Age at end of follow‐up | 71.2 (61.2, 80.2) | 70.0 (60.0, 79.0) | 69.4 (59.2, 78.4) | 70.2 (60.1, 79.1) | 69.6 (59.6, 78.4) | 70.0 (59.9, 78.9) |
| Number dispensed a GLP‐1 RA a | 11,551 (7.2%) | 14,421 (7.8%) | 17,017 (8.2%) | 19,377 (8.3%) | 22,721 (8.1%) | 85,087 (8.0%) |
| Number dispensed an SGLT2i a | 34,440 (21.5%) | 40,348 (21.8%) | 47,388 (22.8%) | 52,336 (22.5%) | 63,634 (22.8%) | 238,146 (22.4%) |
Note: Ages presented as median (25th, 75th percentile).Abbreviations: GLP‐1 RA, Glucagon‐like peptide‐1 receptor agonist; IRSD: Index of Relative Socio‐economic Disadvantage; SGLT2i, Sodium‐glucose co‐transporter 2 inhibitor.
At any point during 2013–2019.
FIGURE 1.
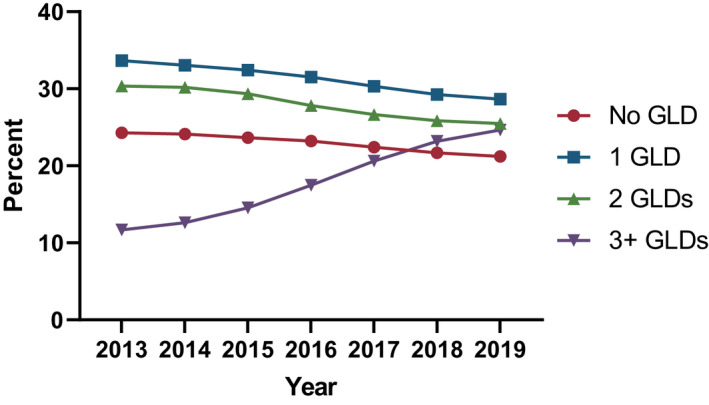
Proportion of people with type 2 diabetes receiving 0, 1, 2 or 3 or more glucose‐lowering drugs (GLDs) concurrently by calendar year
FIGURE 2.
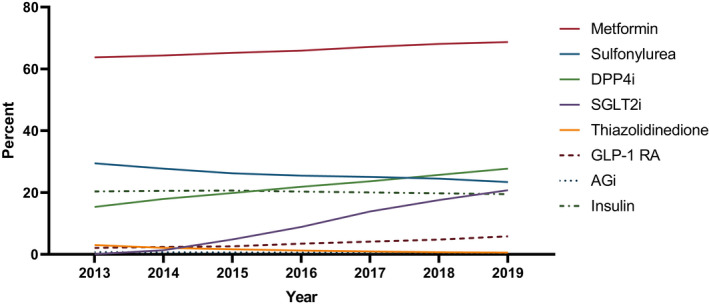
Proportion of people with type 2 diabetes dispensed a prescription for each glucose‐lowering drug class by calendar year. AGi, Alpha Glucosidase inhibitor; DPP4i, Dipeptidyl peptidase‐4 inhibitor; GLP‐1 RA, Glucagon‐like peptide‐1 receptor agonist; SGLT2i, Sodium‐glucose co‐transporter 2 inhibitor.
FIGURE 3.
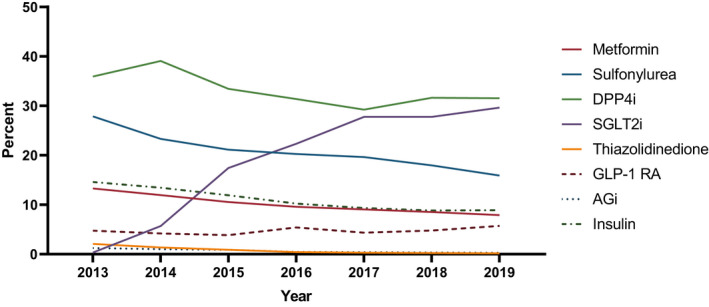
Proportional receipt of each glucose‐lowering drug as an add‐on glucose‐lowering drug class by calendar year. AGi, Alpha Glucosidase inhibitor; DPP4i, Dipeptidyl peptidase‐4 inhibitor; GLP‐1 RA, Glucagon‐like peptide‐1 receptor agonist; SGLT2i, Sodium‐glucose co‐transporter 2 inhibitor.
3.2. Socio‐economic disadvantage and GLD dispensing
In 2013, the odds ratio for receipt of a GLP‐1 RA for people living in the most disadvantaged quintile versus least disadvantaged quintile was 0.72 (95% CI: 0.68–0.76), with this disparity decreasing by 2019, as shown by the odds ratio of 0.95 (0.92, 0.98) by 2019 (Figure 4 and Table S1). In 2014, the odds ratio for receipt of an SGLT2i was 0.87 (0.82, 0.94) for people in the most versus least disadvantaged quintile; from 2016 onwards, overall receipt of SGLT2is was more common with increasing socio‐economic disadvantage (Figure 4 and Table S1). Receipt of metformin, sulfonylureas, thiazolidinediones, DPP4is and insulin increased with increasing socio‐economic disadvantage (Table S1).
FIGURE 4.
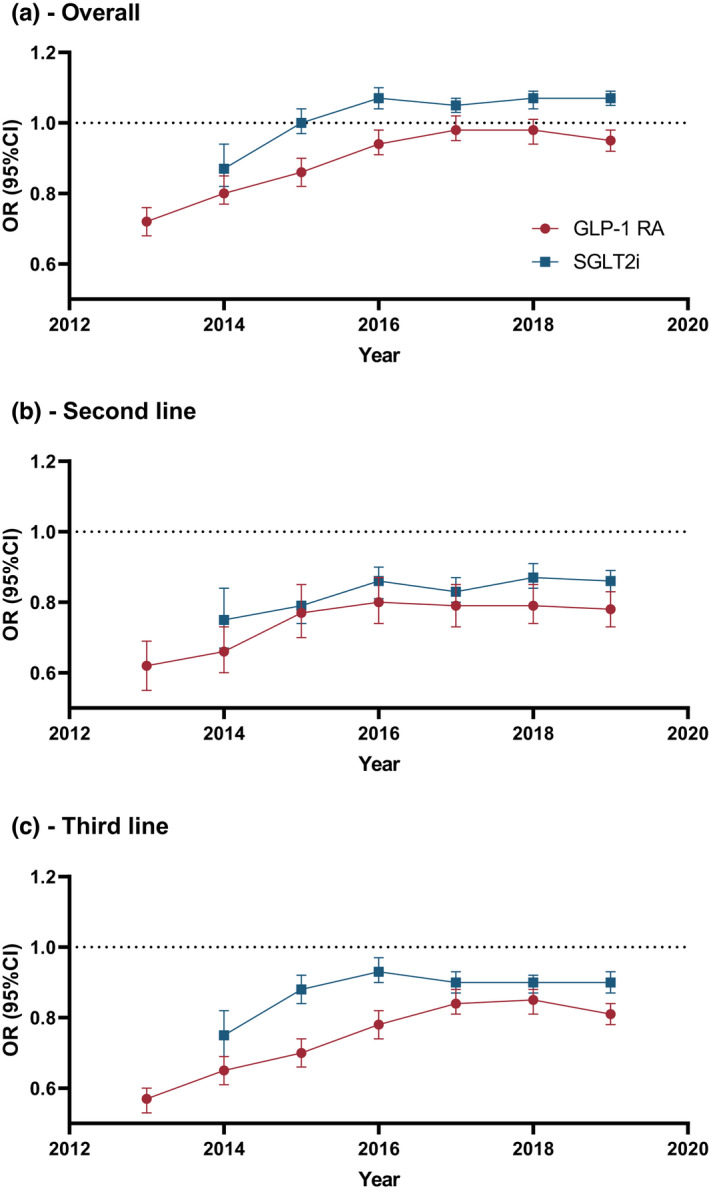
Odds ratio for receipt of a glucagon‐like peptide‐1 receptor agonist (GLP‐1 RA) and sodium‐glucose co‐transporter 2 inhibitor (SGLT2i) for people in the most vs. least disadvantaged quintile of socio‐economic disadvantage by calendar year. A – In the whole population, B – Among people receiving two glucose‐lowering drugs (GLDs), C – Among people receiving three or more GLDs. Odds ratios from models adjusted for age, sex, duration of diabetes, ARIA and the RxRisk comorbidity index.
There was a significant association between increasing socio‐economic disadvantage and increased number of concurrent GLDs received (p < 0.001 in all years; data not shown). Therefore, we subsequently stratified our analyses by number of concurrent GLDs (two or three or more; Figure 4 and Table S1). In this analysis, people living in more disadvantaged areas were less likely to receive GLP‐1 RAs and SGLT2is, with the differences between the least and most disadvantaged quintile decreasing over time (from ORs of 0.65 [0.61, 0.69] and 0.75 [0.69, 0.82] in 2014 to 0.81 [0.78, 0.84] and 0.90 [0.87, 0.93] in 2019 among people receiving three or more GLDs for GLP‐1 RAs and SGLT2is respectively). However, absolute differences in receipt of GLDs were small (results not shown). Stratification generally attenuated disparities in receipt of other GLDs relative to the overall analysis (Table S1). GLP‐1 RAs and SGLT2is were less likely to be the add‐on GLD for people living in increasingly disadvantaged areas (Table S1); this was generally consistent when received as a second or third‐line GLD (data not shown). The associations between socio‐economic disadvantage and receipt of most GLDs were generally unaffected by adjustment for specialist prescription (Table S2). However, adjusting for specialist prescribing attenuated the association between socio‐economic disadvantage and receipt of SGLT2is and GLP‐1 RAs, with a more marked effect on GLP‐1 RAs.
3.3. Socio‐economic disadvantage, adherence and persistence
In the primary analyses, where only people who received at least two prescriptions for a GLP‐1 RA or SGTL2i were considered, 60.3% and 73.7% of people were persistent at 1 year for GLP‐1 RAs and SGLT2is respectively (Table S3). The median (IQR) PDCs was 0.82 (0.46, 0.96) and 0.91 (0.61, 0.99), with 51.7% and 64.1% of people considered adherent (>80% PDCs) at 1 year for GLP‐1 RAs and SGLT2is respectively. Including all initiators reduced measured persistence (52.0% and 65.7% for GLP‐1 RAs and SGLT2is respectively) and adherence (44.6% and 57.2%). Conversely, when coverage was defined as 75% of the refill time, persistence (64.5% and 76.0%) and adherence (63.0% and 71.4%) increased. Persistence and adherence were slightly higher in the concession population for GLP‐1 RAs (61.5% and 54.6% respectively), while only adherence was higher in the concession population for SGLT2is (persistence an adherence were 72.8% and 66.0% respectively). Persistence and adherence were higher for individuals who initiated with dulaglutide (vs. exenatide) and empagliflozin (vs. dapagliflozin) (Table S3).
For GLP‐1 RAs, there was reduced persistence with increasing socio‐economic disadvantage (OR for most vs. least disadvantaged quintile: 0.92 [0.86, 0.97]; Table S3) and this was broadly consistent in sensitivity analyses and the concession population (data not shown). However, there was no consistent association between socio‐economic disadvantage and adherence to GLP‐1 RAs. Conversely, there was a significant association between socio‐economic disadvantage and both persistence (OR for most vs. least disadvantaged quintile: 0.92 [0.89, 0.96]) and adherence (0.90 [0.87, 0.93]) for SGLT2is (Table S3), both of which were consistent in sensitivity analyses including all initiators and altering the refill period (data not shown). However, the association of socio‐economic disadvantage with both persistence and adherence to SGLT2is was attenuated in the concession population (data not shown).
4. DISCUSSION
There are several important findings of this work. First, we have found that the proportion of people with type 2 diabetes receiving three or more GLDs concurrently doubled in a relatively short period of time, from 12% in 2013 to 25% in 2019. Second, we have shown a marked increase in the use of SGLT2is, while use of GLP‐1 RAs increased more slowly over time. Nevertheless, DPP4is were still the most common add‐on GLD in 2019, but only as a second‐line GLD. Third, we have shown that by 2019, overall receipt of SGLT2is was more likely with increasing disadvantage and that the relative disparity in receipt of GLP‐1 RAs had decreased to only 4% between the highest and lowest quintiles of socio‐economic disadvantage. However, people living in more disadvantaged areas were consistently less likely to receive these medications at a given intensity of therapy. Finally, we found persistence on GLP‐1 RAs and SGLT2is, and adherence to SGLT2is was lower for people in more disadvantaged areas.
An increase in the proportion of people receiving three or more concurrent GLDs may reflect an increase in the ability to intensify glycaemic control without substantially adding to the risk of hypoglycaemia owing to the availability of newer GLDs. It is interesting that this finding is in contrast to results from the United States, where the proportion of people with diabetes on ≥3 GLDs remained relatively stable through the same time period. 16 Moreover, despite a similar number of people receiving any GLD, receipt of DPP4is, SGLT2is and GLP‐1 RAs was higher in Australia than the United States, 16 a finding likely due to the high costs of these GLDs in the United States. 17 Nevertheless, the fact that DPP4is, and not SGLT2is or GLP‐1 RAs, still remained the preferential add‐on therapy in 2019 represents an opportunity to further improve pharmacological management of diabetes in Australia with respect to reducing the risk of cardiovascular and kidney disease.
Our observed patterns of GLD receipt by socio‐economic disadvantage accord with studies from other countries. The most recent of these was in Denmark, from 2012 to 2020, which found significantly lower probabilities of initiating a GLP‐1 RA or SGLT2i for people with low socio‐economic position, 18 and this was independent of age sex, ethnicity, access to specialist care and prior cardiovascular disease. Similarly, a study in the United Kingdom from 2014 to 2017 found people with lower socio‐economic status were less likely to initiate an SGLT2i as second‐line therapy. 19 Findings of a socio‐economic gradient for receipt of GLDs in Denmark, the United Kingdom and Australia is especially concerning considering these countries all have ‘universal’ healthcare, and it is thus likely disparities are worse in countries with more inequitable healthcare systems.
Importantly, we found evidence that part of this disparity in Australia is attributable to access to specialist care, especially for GLP‐1 RAs. However, this analysis should be interpreted with caution, as it is not uncommon in Australia for specialists to recommend medications for general practitioners to prescribe, which would have resulted in misclassification in our analysis. Nevertheless, this suggests that increasing specialist access for people in more disadvantaged areas of Australia could improve access to newer, more effective medications for the treatment of chronic disease. The reason for the greater effects of socio‐economic disadvantage and specialist prescribing on the receipt of GLP‐1 RAs than SGLT2is is unclear, but is unlikely related to the fact that it is an injectable, as the effect of socio‐economic disadvantage on receipt of insulin was in the opposite direction. Indeed, other factors underlying the disparities we observed in this study are unclear and will require further study.
The disparities by socio‐economic disadvantage also have implications for cardiovascular and kidney disease. First, disparities in receipt of SGLT2is and GLP‐1 RAs by disadvantage did not worsen following the publication of clinical trials showing their cardiovascular benefits, although there was little evidence of any impact of these publications on trends in the receipt of these GLDs overall. The latter finding may reflect the fact that the PBS reimbursement criteria for prescribing these medications have not changed in response to these trials (Figures S1, S2), and in fact still considers these GLDs as anti‐hyperglycaemics only (with no subsidised indications for CVD, kidney disease or obesity). This is in contrast to international guidelines, which recommend these agents on the basis of their cardiovascular and kidney benefits, at least for people with or at high risk for cardiovascular and kidney disease. 11 , 12 Limiting access to these medications for people who are likely to benefit from them is likely to have consequences on the incidence of diabetes complications at the population level, 20 and therefore the current reimbursement criteria for these GLDs may need to be reconsidered.
Second, there was minimal overall disparity in receipt of SGLT2is and GLP‐1 RAs by socio‐economic disadvantage. However, this was achieved only because of the use of a greater number of GLDs in more disadvantaged areas. After stratifying by number of concurrent GLDs, people in more disadvantaged areas were receiving these medications less frequently at any given level of therapy. This is unlikely to be due to higher costs, because this association occurs among people on concessions, 5 and, given that cardiovascular and kidney disease risk is higher for people in more disadvantaged areas, 21 , 22 is unlikely to be because of a lesser need for cardiovascular disease prevention.
Third, we also observed small, but potentially important difference in persistence and adherence by socio‐economic disadvantage. Importantly, some of these results were attenuated in the concession population, suggesting that cost may play a role in persistence and adherence, with this disproportionately affecting more disadvantaged individuals.
Strengths of this study include: a large sample size; access to actual dispensing data (and not just clinical prescribing records); ability to control for differences in diabetes prevalence by socio‐economic disadvantage through use of the NDSS; and ability to control for duration of diabetes, an important determinant of GLD receipt. 5 Nevertheless, there are some important limitations that deserve mention. First, receipt of medications does not necessarily mean these GLDs are being used. Second, we did not have access to clinical information, such as HbA1c, BMI, history of cardiovascular disease or eGFR, and therefore cannot comment on the appropriateness of prescribing observed in this study. Indeed, the disparities observed in this study do not necessarily imply inadequate care—clinical indication and patient preference could not be accounted for. However, because CVD and CKD prevalence are both higher in more disadvantaged areas of Australia, 23 , 24 it is likely that people in these areas have a greater requirement for SGLT2is and GLP‐1 RAs. Nevertheless, for most of our observation period, an eGFR <45 ml/min/1.73 m2 was a contra‐indication for SGLT2is, which may have affected our results. Third, not all GLP‐1 RAs approved for use in Australia by the TGA are available on the PBS (Figures S1, S2). Importantly, liraglutide is approved in Australia, but not listed on the PBS, and was the first of the GLP‐1 RAs to show cardiovascular benefits, which likely influenced its use in ways we could not observe among people who were willing and able to pay the full price out‐of‐pocket. However, the number of people who do this is likely very small, and would predominately include people in the least disadvantaged areas of Australia, suggesting that the effect of including these prescriptions would have been to enhance the socio‐economic gradient observed in this study. Fourth, duration of diabetes is frequently self‐reported in the NDSS and may have limited accuracy. Fifth, we excluded Aboriginal and Torres Strait Islander Australians with diabetes from this analysis, and were unable to stratify by ethnicity. 25 Whether our findings generalise to these disadvantaged groups requires further study. Finally, the measure of socio‐economic disadvantage in this study was an area‐level measures, and is therefore only a proxy for individual socio‐economic disadvantage, which may have biased our estimates towards the null via non‐differential misclassification.
In conclusion, the availability of newer GLDs has been associated with an increase in the use of multiple GLDs. However, despite clear advantages of SGLT2is and GLP‐1 RAs over other second‐line GLDs, DPP4is were still the most common second‐line GLD in 2019. Indeed, expanding the PBS criteria for GLP‐1 RAs and SGLT2is in a manner that recognises their effects beyond glycaemia may be warranted to increase use among individuals who stand to benefit from their cardio‐ and reno‐protective effects. Finally, there are persistent disparities in receipt of GLP‐1 RAs, and receipt, persistence and adherence to SGLT2is, by socio‐economic disadvantage, which may represent a point of intervention to reduce socio‐economic disparity in diabetes outcomes.
AUTHOR CONTRIBUTIONS
J.I.M. contributed to the design of the study and acquisition and interpretation of data, performed the statistical analysis and literature search and wrote and revised the manuscript. J.I. contributed to the design of the study, interpretation of data and revision of the manuscript. D.J.M. and J.E.S. made contributions to the design of the study, acquisition and interpretation of the data and revision of the manuscript. J.I.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors have read and approved the final version of this manuscript.
FUNDING INFORMATION
J.I.M is supported by an Australian Government Research Training Program (RTP) Scholarship and Monash Graduate Excellence Scholarship. DJM is supported by a National Health and Medical Research Council Senior Research Fellowship. JES is supported by a National Health and Medical Research Council Investigator Grant. This work is partially supported by the Victorian Government's Operational Infrastructure Support Program.
CONFLICT OF INTEREST
JI has consulted for Astra Zeneca and received grant funding from Amgen. JES has received honoraria from Astra Zeneca, Sanofi, Novo Nordisk, MSD, Pfizer, Eli Lilly, Abbott, Mylan and Boehringer Ingelheim.
Supporting information
Appendix S1
ACKNOWLEDGEMENT
Open access publishing facilitated by Monash University, as part of the Wiley ‐ Monash University agreement via the Council of Australian University Librarians.
Morton JI, Ilomӓki J, Magliano DJ, Shaw JE. Persistent disparities in diabetes medication receipt by socio‐economic disadvantage in Australia. Diabet Med. 2022;39:e14898. doi: 10.1111/dme.14898
Dianna J. Magliano and Jonathan E. Shaw are joint senior authors.
DATA AVAILABILITY STATEMENT
The datasets analysed during the current study are not publicly available due to privacy concerns.
REFERENCES
- 1. Li R, Bilik D, Brown MB, et al. Medical costs associated with type 2 diabetes complications and comorbidities. Am J Manag Care. 2013;19(5):421‐430. [PMC free article] [PubMed] [Google Scholar]
- 2. Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta‐analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31‐39. [DOI] [PubMed] [Google Scholar]
- 3. Kristensen SL, Rørth R, Jhund PS, et al. Cardiovascular, mortality, and kidney outcomes with GLP‐1 receptor agonists in patients with type 2 diabetes: a systematic review and meta‐analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7(10):776‐785. [DOI] [PubMed] [Google Scholar]
- 4. Neuen BL, Young T, Heerspink HJL, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta‐analysis. Lancet Diabetes Endocrinol. 2019;7(11):845‐854. [DOI] [PubMed] [Google Scholar]
- 5. Morton JI, Ilomӓki J, Magliano DJ, Shaw JE. The association of socioeconomic disadvantage and remoteness with receipt of type 2 diabetes medications in Australia: a nationwide registry study. Diabetologia. 2021;64(2):349‐360. [DOI] [PubMed] [Google Scholar]
- 6. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117‐2128. [DOI] [PubMed] [Google Scholar]
- 7. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Australian Institute of Health and Welfare . Diabetes prevalence in Australia: an assessment of national data sources. Diabetes series no. 12. Cat. no. CVD 46. AIHW; 2009. [Google Scholar]
- 9. Morton JI, Liew D, McDonald SP, Shaw JE, Magliano DJ. The association between age of onset of type 2 diabetes and the long‐term risk of end‐stage kidney disease: a National Registry Study. Diabetes Care. 2020;43(8):1788‐1795. [DOI] [PubMed] [Google Scholar]
- 10. Australian Bureau of Statistics Socio‐Economic Index for Areas (SEIFA). Technical Paper. 2016. Available at: https://www.abs.gov.au/.
- 11. 9. harmacologic Approaches to Glycemic Treatment: Standards of medical Care in Diabetes ‐ 2020. Diabetes Care. 2020;43(Supplement 1):S98‐S110. [DOI] [PubMed] [Google Scholar]
- 12. Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC guidelines on diabetes, pre‐diabetes, and cardiovascular diseases developed in collaboration with the EASD: the task force for diabetes, pre‐diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Association for the Study of diabetes (EASD). Eur Heart J. 2020;41(2):255‐323. [DOI] [PubMed] [Google Scholar]
- 13. Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11(1):44‐47. [DOI] [PubMed] [Google Scholar]
- 14. Australian Bureau of Statistics . ABS Views on Remoteness. Information Paper; 2001. Available at: https://www.abs.gov.au/ [Google Scholar]
- 15. Pratt NL, Kerr M, Barratt JD, et al. The validity of the Rx‐risk comorbidity index using medicines mapped to the anatomical therapeutic chemical (ATC) classification system. BMJ Open. 2018;8(4):e021122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fang M, Wang D, Coresh J, Selvin E. Trends in diabetes treatment and control in U.S. adults, 1999–2018. N Engl J Med. 2021;384(23):2219‐2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Taylor SI. The high cost of diabetes drugs: disparate impact on the Most vulnerable patients. Diabetes Care. 2020;43(10):2330‐2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Falkentoft AC, Andersen J, Malik ME, et al. Impact of socioeconomic position on initiation of SGLT‐2 inhibitors or GLP‐1 receptor agonists in patients with type 2 diabetes – a Danish nationwide observational study. Lancet Regional Health ‐ Europe. 2022;14:100308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wilkinson S, Douglas IJ, Williamson E, et al. Factors associated with choice of intensification treatment for type 2 diabetes after metformin monotherapy: a cohort study in UKprimary care. Clin Epidemiol. 2018;10:1639‐1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Morton JI, McDonald SP, Salim A, Liew D, Shaw JE, Magliano DJ. Projecting the incidence of type 2 diabetes–related end‐stage kidney disease until 2040: A comparison between the effects of diabetes prevention and the effects of diabetes treatment. Diabetes Care. 2021;44(7):1515‐1523. [DOI] [PubMed] [Google Scholar]
- 21. Koye DN, Magliano DJ, Reid CM, et al. Trends in incidence of ESKD in people with type 1 and type 2 diabetes in Australia, 2002–2013. Am J Kidney Dis. 2019;73(3):300‐308. [DOI] [PubMed] [Google Scholar]
- 22. Korda RJ, Soga K, Joshy G, et al. Socioeconomic variation in incidence of primary and secondary major cardiovascular disease events: an Australian population‐based prospective cohort study. Int J Equity Health. 2016;15(1):189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Australian Institute of Health and Welfare . Chronic Kidney Disease. Cat. No: CDK 16. AIHW; 2020. [Google Scholar]
- 24. Australian Institute of Health and Welfare 2021. Heart, Stroke and Vascular Disease—Australian Facts. Cat. No: CVD 92. AIHW. [Google Scholar]
- 25. Whyte MB, Hinton W, McGovern A, et al. Disparities in glycaemic control, monitoring, and treatment of type 2 diabetes in England: a retrospective cohort analysis. PLoS Med. 2019;16(10):e1002942. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
The datasets analysed during the current study are not publicly available due to privacy concerns.


