Summary
Background
Previous research indicates that the increased relative risk of colorectal cancer (CRC) in inflammatory bowel disease (IBD) is limited to young‐onset IBD.
Aim
To estimate risks of incident CRC and death from CRC in elderly‐onset IBD
Methods
Patients diagnosed with IBD at age ≥ 60 years between 1969 and 2017 were identified using Danish and Swedish National Patient Registers and histopathology data. We linked data to Cancer and Causes of Death Registers and used Cox regression to estimate hazard ratios (HRs) for CRC diagnosis and death compared to matched (by sex, age, and region) IBD‐free individuals.
Results
Among 7869 patients with Crohn's disease followed for 54,220 person‐years, and 21,224 patients with ulcerative colitis (UC) followed for 142,635 person‐years, 2.10% and 1.90% were diagnosed with CRC, compared to 2.26% and 2.34% of reference individuals (median follow‐up 6 and 7 years). The incidence of CRC was elevated during the first year after IBD diagnosis: 4.36 (95% CI = 3.33–5.71) in Crohn's disease and 2.48 (95% CI = 2.03–3.02) in UC, but decreased after the first year of follow‐up: 0.69 (95% CI = 0.56–0.86) and 0.78 (95% CI = 0.69–0.88). Once diagnosed with CRC, the risk of CRC death was similar for IBD patients and the general population.
Conclusion
The excess risk of CRC in elderly‐onset IBD was probably due to bias and not observed beyond the first year. From 2010, the HR for CRC diagnosis more than 1 year after initial IBD diagnosis was lower than in the largely unscreened reference population, supporting the benefit of endoscopic screening and surveillance in patients with IBD.
Colorectal cancer in elderly‐onset inflammatory bowel disease.

1. INTRODUCTION
Existing evidence has established an increased risk of colorectal cancer (CRC) in patients with inflammatory bowel disease (IBD) both overall, 1 and separately in patients with Crohn's disease (CD) 2 and ulcerative colitis (UC). 3 , 4 , 5 The risk of CRC is higher in IBD patients with extensive disease, 1 , 3 , 4 a family history of CRC, 6 primary sclerosing cholangitis (PSC), 7 and long disease duration. 1 , 3 Current endoscopic surveillance programmes thus recommend varied colonoscopy intervals according to the presence of risk factors. 8 , 9
When examining risks of CRC in relation to age at diagnosis, the highest relative risks of CRC has been reported for patients diagnosed with IBD at <30 years of age. 1 The relative risk is significantly lower for patients with adult‐onset IBD. 1 , 2 , 4 Approximately 4%–21% of patients with CD and 11%–25% of patients with UC are diagnosed at an elderly age (≥60–61 years). 10 , 11 , 12 , 13 , 14 For elderly patients, the underlying CRC risk is inherently higher than in younger patients, but the incidence of CRC in those with IBD has not been reported to be increased compared to age‐matched peers without IBD, based on population‐based studies conducted in the 2000s 15 , 16 , 17 , 18 (Table S1).
Although most studies found no excess incidence of CRC in patients with elderly‐onset IBD, cancer surveillance programmes do not take age of onset into account when establishing surveillance intervals. 9 , 19 The aim of the current study was to examine the risk of CRC diagnosis and death in a large cohort of individuals with elderly‐onset (60+ years) CD and UC, compared to matched reference individuals from the general population. This approach was taken to identify subgroups of elderly patients that might benefit from enhanced surveillance endoscopy.
2. METHODS
2.1. Setting and data sources
Denmark and Sweden are high‐income countries with populations of 5.7 20 and 10.1 million persons, 21 respectively, as of 2017. In both countries, healthcare is tax‐funded, access to care is universal, 21 and the personal identity number assigned to each resident allows for linkage of registers containing national data on demographics, morbidity, mortality and histopathology, with virtually no loss to follow‐up 22 (Table S2). Patients with IBD typically are followed by gastroenterologists in hospital‐based outpatient clinics. Guidelines for CRC surveillance in IBD patients were implemented in 2014 in Denmark 23 , 24 and 1995 in Sweden. 25
2.2. Participants
2.2.1. Patients with elderly‐onset IBD
We used International Classification of Diseases (ICD) codes (Table S3) in the National Patient Register to identify IBD cases (CD or UC) with onset at age 60 years or older from January 1979 to December 2011 in Denmark and January 1969–December 2017 in Sweden. We required either two records of IBD in the National Patient Registers (positive predictive value [PPV] = 93% in Sweden 26 , 27 , 28 and = 90% in Denmark 29 ) or one record of IBD in a National Patient Register combined with a biopsy suggestive of IBD from the Swedish ESPRESSO Biopsy Register (PPV = 95%) 30 , 31 or the Danish Pathology Register. 32 IBD could be identified only through inpatient data until 1995 in Denmark and until 2001 in Sweden. Thus individuals identified in 2001 and 2002 represent a mix of incident and prevalent cases. For this reason, we performed supplementary analyses restricted to incident cases during 2003–2017. Patients who had a CRC diagnosis or had undergone any type of colectomy before the start of follow‐up were excluded from the analyses.
2.2.2. Matched reference individuals
For each patient, we identified up to 10 reference individuals from the National Population Registers of the two countries 33 and matched them to the patients by sex, age and place of residence. The reference individuals had to be free of CRC and IBD and had not undergone colectomy at the start of follow‐up of their matched case. During the study period, the absolute majority of reference individuals had not been subject to CRC screening.
2.3. Covariates
We assessed patients by age (60–69, 70–79 and ≥80 years) and calendar period of IBD diagnosis (1969–1976, 1977–1989, 1990–2002 and 2003–2017). Extent and location of disease were categorised according to the Montreal classification (Table S4), 34 as were PSC and other extraintestinal manifestations (skin, eyes and joints) (Table S5). Colectomies were captured through surgery codes (Table S6) 35 and endoscopies through procedure codes (Table S7).
2.4. Outcomes
Our main outcomes were incident CRC diagnosis (from the National Cancer Registers in Sweden and Denmark) and CRC death (main or contributory cause of death in the National Cause of Death registers in Sweden and Denmark). Secondary outcomes were cancer location (Table S8), and stage (available from 2003).
2.5. Statistical analysis
Follow‐up of patients began on the date when inclusion criteria were met and on the same date for individually matched reference individuals. Follow‐up ended at death, emigration, proctocolectomy or end of follow‐up (31 December 2011 in Denmark and 31 December 2017 in Sweden), whichever came first. In analyses of incident CRC, follow‐up ended at the time of the first CRC diagnosis. A patient who had a colectomy with an intact rectum was considered at risk of rectal cancer until proctectomy.
We assessed incidence proportions and crude incidence rates (number of CRC diagnoses and deaths by person‐time at risk). Analyses were stratified by sex, age at IBD diagnosis, calendar period of diagnosis and follow‐up time. In stratum‐specific analyses of UC extent, CD location, PSC and other extraintestinal manifestations, follow‐up started on the date of the first corresponding register record for an IBD patient and on the same date for the individually matched reference individuals. We also performed separate analyses stratified by CRC location.
We used Cox regression, adjusted for age at IBD diagnosis, sex, birth year and country, to calculate hazard ratios (HRs) with 95% confidence intervals (CIs) for CRC diagnosis and death in patients compared with reference individuals. Log‐minus‐log curves and Schoenfeld residuals were used to test the proportional hazards assumption. To examine associations between CRC diagnosis and causes of death, we specified a multistate model via a series of Cox models. This allowed us to estimate cause‐specific HRs of a CRC diagnosis, CRC death and other causes of death, comparing patients with IBD to matched reference individuals and also adjusting for CRC tumour stage (I–IV).
We estimated calendar time trends of HRs for CRC diagnosis and CRC death. We also estimated HRs for CRC diagnoses and CRC deaths in patients potentially eligible for CRC surveillance according to current guidelines in the Nordic countries (i.e., IBD duration ≥8 years or concomitant PSC). 17 , 18
2.5.1. Ethics
The study was approved by the Regional Ethics Committee in Stockholm (Dnr 2007/785‐31/5, 2011/1509‐32, 2014/1287‐31/4, 2015/0004‐31 and 2016/192‐31/2) and the Danish Data Protection Agency. Individual informed consent was not required in this solely register‐based study. 36
3. RESULTS
3.1. Participants
We identified 29,093 patients with IBD onset at age 60 years or older in Denmark and Sweden during 1969–2017, and 280,185 matched reference individuals (Figure S1). Median age at IBD diagnosis was 69 years (IQR 64–76 years) in the 7869 patients with CD, and 70 years (65–76 years) in the 21,224 patients with UC. The majority were women: 57% of CD cases and 51% of UC cases. Median follow‐up time was 6 years for patients and 7 years for reference individuals, and 27% of patients were followed for 10 years or longer. By the end of follow‐up, 1.3% of patients with CD or UC had a diagnosis of PSC (Table 1).
TABLE 1.
Characteristics at baseline and end of follow‐up for all patients with Crohn's disease and ulcerative colitis in Sweden (1969–2017) and Denmark (1977–2011) and reference individuals from the general population matched on sex, age and place of residence. Numbers are n (%) unless otherwise stated
| Crohn's disease | Ulcerative colitis | |||
|---|---|---|---|---|
| Patients | Reference individuals | Patients | Reference individuals | |
| Total | 7869 (100) | 75,654 (100) | 21,224 (100) | 204,531 (100) |
| Denmark | 2143 (27.2) | 20,854 (27.6) | 7956 (37.5) | 77,552 (37.9) |
| Sweden | 5726 (72.8) | 54,800 (72.4) | 13,268 (62.5) | 126,979 (62.1) |
| Sex | ||||
| Female | 4469 (56.8) | 43,119 (57.0) | 10,828 (51.0) | 104,530 (51.1) |
| Male | 3400 (43.2) | 32,535 (43.0) | 10,396 (49.0) | 100,001 (48.9) |
| Age at first diagnosis (years) | ||||
| Median (IQR) | 69 (64–76) | 69 (64–76) | 70 (65–76) | 70 (65–76) |
| 60–69 | 4171 (53.0) | 39,838 (52.7) | 10,625 (50.1) | 101,947 (49.8) |
| 70–79 | 2683 (34.1) | 25,782 (34.1) | 7620 (35.9) | 73,471 (35.9) |
| ≥80 | 1015 (12.9) | 9474 (12.5) | 2979 (14.0) | 27,977 (13.7) |
| Year of first IBD diagnosis | ||||
| 2003–2017 | 4084 (51.9) | 39,675 (52.4) | 10,783 (50.8) | 104,712 (51.2) |
| 1990–2002 | 2690 (34.2) | 25,825 (34.1) | 7787 (36.7) | 75,093 (36.7) |
| 1977–1989 | 919 (11.7) | 8547 (11.3) | 2267 (10.7) | 21,238 (10.4) |
| 1969–1976 | 176 (2.2) | 1607 (2.1) | 387 (1.8) | 3488 (1.7) |
| Age at end of follow‐up (years) | ||||
| Median (IQR) | 79 (73–85) | 80 (73–86) | 79 (74–85) | 80 (74–86) |
| 60–69 | 1230 (15.6) | 10,436 (13.8) | 2879 (13.6) | 25,509 (12.5) |
| 70–79 | 3128 (39.8) | 27,511 (36.4) | 8327 (39.2) | 74,928 (36.6) |
| ≥80 | 3511 (44.6) | 37,706 (49.8) | 10,018 (47.2) | 104,093 (50.9) |
| Length of follow‐up (years) | ||||
| Mean (SD) | 7 (6) | 8 (6) | 7 (6) | 8 (6) |
| Median (IQR) | 6 (2–10) | 7 (3–12) | 6 (2–11) | 7 (3–12) |
| Min‐Max | 0–33 | 0–48 | 0–42 | 0–47 |
| 0 to <1 | 1151 (14.6) | 6071 (8.0) | 2723 (12.8) | 16,272 (8.0) |
| 1 to <5 | 2415 (30.7) | 22,094 (29.2) | 6642 (31.3) | 61,695 (30.2) |
| 5 to <10 | 2198 (27.9) | 22,116 (29.2) | 5987 (28.2) | 59,987 (29.3) |
| 10 to <20 | 1834 (23.3) | 21,107 (27.9) | 5269 (24.8) | 57,447 (28.1) |
| 20 | 271 (3.4) | 4266 (5.6) | 603 (2.8) | 9130 (4.5) |
| Montreal classification of location at end of follow‐up | ||||
| N classified | 6812 | NA | NA | NA |
| L1/L3/LX (terminal ileum/ileocecal/not defined) | 4801 (61.0) | NA | NA | NA |
| L2 (colon) | 2011 (29.5) | NA | NA | NA |
| Perianal | 257 (3.8) | NA | NA | NA |
| Montreal Classification of extent at end of follow‐up | ||||
| N classified | NA | NA | 18,584 | NA |
| E1 (ulcerative proctitis) | NA | NA | 2245 (12.1) | NA |
| E2 (left sided UC) | NA | NA | 3009 (16.2) | NA |
| E3 (extensive UC) | NA | NA | 6482 (34.9) | NA |
| EX (extent not defined) | NA | NA | 6848 (36.8) | NA |
| Extraintestinal manifestations at end of follow‐up | ||||
| N classified | 7654 | NA | 20,649 | NA |
| Primary sclerosing cholangitis | 97 (1.3) | NA | 278 (1.3) | NA |
| Other extraintestinal manifestations | 888 (11.3) | NA | 1638 (7.7) | NA |
Abbreviations: IQR, interquartile range; N, number, NA, not applicable; SD, standard deviation.
3.2. Outcome data
Before the start of follow‐up, 734 patients with IBD and 4226 reference individuals were excluded because of a prior CRC diagnosis, and 72 patients with IBD and 23 reference individuals were excluded due to previous colectomy (Figure S1). During 54,220 person‐years of follow‐up, 165 patients with CD (2.10%) were diagnosed with CRC, while during 623,407 person‐years of follow‐up 1713 reference individuals (2.26%) were diagnosed with CRC. Patients with UC were followed for 142,635 person‐years, with 404 (1.90%) diagnosed with CRC. Among their reference subjects, 4776 individuals (2.34%) were diagnosed with CRC during 1,629,717 person‐years of follow‐up. CRC was the main or contributory cause of death in 1.45% of patients with CD versus 1.29% of their reference population. CRC was the main or contributory cause of death in 1.10% of patients with UC versus 1.25% of their reference population (Tables 2 and 3).
TABLE 2.
Absolute incidence rates of colorectal cancer diagnoses per 1000 person‐years (95% confidence intervals) in incident cases of inflammatory bowel disease cases and matched reference individuals from the general population
| Colorectal cancer diagnosis | ||||
| Crohn's disease | Ulcerative colitis | |||
| Patients | Reference | Patients | Reference | |
| N total | 7869 | 75,654 | 21,224 | 204,531 |
| N events | 165 | 1713 | 404 | 4776 |
| Incidence proportion (percent) | 2.10 | 2.26 | 1.90 | 2.34 |
| 1‐year cumulative incidence | 1.01 (0.78–1.24) | 0.24 (0.21–0.28) | 0.60 (0.49–0.70) | 0.25 (0.23–0.27) |
| 5‐year cumulative incidence | 1.79 (1.46–2.11) | 1.23 (1.14–1.32) | 1.29 (1.11–1.46) | 1.32 (1.26–1.38) |
| 10 year cumulative incidence | 2.46 (2.03–2.89) | 2.65 (2.49–2.80) | 2.50 (2.20–2.79) | 2.83 (2.73–2.92) |
| 20 year cumulative incidence | 5.25 (3.77–6.71) | 5.85 (5.47–6.23) | 5.81 (4.87–6.75) | 6.21 (5.96–6.46) |
| Person‐years | 54,220 | 623,407 | 142,635 | 1,629,717 |
| ev, IR (95% CI) a | ev, IR (95%CI) a | ev, IR (95%CI) a | ev, IR (95%CI) a | |
| Total, incidence rate (95% CI) | 165, 3.04 (2.61–3.54) | 1713, 2.75 (2.62–2.88) | 404, 2.83 (2.57–3.12) | 4776, 2.93 (2.85–3.01) |
| Denmark | 48, 3.79 (2.85–5.02) | 529, 3.66 (3.36–3.99) | 152, 3.30 (2.81–3.87) | 1895, 3.75 (3.58–3.92) |
| Sweden | 117, 2.82 (2.35–3.38) | 1184, 2.47 (2.34–2.62) | 252, 2.61 (2.31–2.95) | 2881, 2.56 (2.47–2.66) |
| Sex | ||||
| Female | 91, 2.85 (2.32–3.50) | 889, 2.40 (2.25–2.56) | 210, 2.83 (2.47–3.24) | 2132, 2.53 (2.42–2.64) |
| Male | 74, 3.32 (2.65–4.17) | 824, 3.25 (3.04–3.48) | 194, 2.84 (2.46–3.26) | 2644, 3.36 (3.23–3.49) |
| Age at first IBD diagnosis (years) | ||||
| 60–69 | 76, 2.18 (1.74–2.73) | 903, 2.37 (2.22–2.53) | 214, 2.52 (2.21–2.89) | 2440, 2.57 (2.47–2.67) |
| 70–79 | 71, 4.57 (3.62–5.77) | 663, 3.49 (3.23–3.76) | 139, 3.00 (2.54–3.54) | 1853, 3.46 (3.31–3.62) |
| ≥80 | 18, 4.72 (2.97–7.49) | 137, 2.94 (2.48–3.47) | 51, 4.46 (3.39–5.87) | 463, 3.52 (3.22–3.86) |
| Year of first diagnosis | ||||
| 2003–2017 | 67, 3.47 (2.73–4.40) | 474, 2.27 (2.08–2.49) | 125, 2.45 (2.06–2.92) | 1390, 2.58 (2.45–2.72) |
| 1990–2002 | 70, 2.95 (2.33–3.72) | 885, 3.18 (2.97–3.39) | 186, 2.73 (2.37–3.16) | 2486, 3.19 (3.07–3.32) |
| 1977–1989 | 18, 1.90 (1.20–3.02) | 305, 2.64 (2.36–2.96) | 70, 3.39 (2.68–4.29) | 783, 2.91 (2.71–3.12) |
| 1969–1976 | 10, 6.01 (3.23–11.2) | 49, 2.36 (1.78–3.12) | 23, 7.58 (5.04–11.4) | 117, 2.64 (2.21–3.17) |
| Years of follow‐up | ||||
| 0 to <1 | 76, 10.7 (8.56–13.4) | 177, 2.44 (2.11–2.83) | 121, 6.26 (5.24–7.48) | 495, 2.52 (2.31–2.75) |
| 1 to <5 | 42, 1.96 (1.45–2.66) | 571, 2.46 (2.27–2.68) | 102, 1.75 (1.44–2.13) | 1671, 2.68 (2.56–2.81) |
| 5 to <10 | 23, 1.50 (1.00–2.26) | 503, 2.84 (2.61–3.10) | 98, 2.42 (1.99–2.95) | 1426, 3.05 (2.89–3.21) |
| 10 to <20 | 21, 2.21 (1.44–3.39) | 418, 3.35 (3.04–3.69) | 76, 3.29 (2.63–4.13) | 1079, 3.50 (3.30–3.72) |
| ≥20 | 3, 3.18 (1.03–9.88) | 44, 2.50 (1.86–3.36) | 7, 4.38 (2.09–9.19) | 105, 3.08 (2.55–3.73) |
| Excluding the first year | 89, 1.89 (1.53–2.32) | 1536, 2.79 (2.65–2.93) | 283, 2.30 (2.04–2.58) | 4281, 2.99 (2.90–3.08) |
| Location or extent | ||||
| N classified since 1997 | 6812 | NA | 18,584 | NA |
| L1/L3/LX | 93, 3.27 (2.67–4.00) | NA | NA | NA |
| L2 | 34, 2.71 (1.94–3.79) | NA | NA | NA |
| E1 (proctitis) | NA | NA | 35, 2.52 (1.81–3.51) | NA |
| E2 (left sided colitis) | NA | NA | 43, 2.22 (1.65–3.00) | NA |
| E3 (extensive colitis) | NA | NA | 117, 3.01 (2.51–3.60) | NA |
| EX (undefined) | NA | NA | 97, 2.50 (2.05–3.05) | NA |
| Extraintestinal manifestations | ||||
| Primary sclerosing cholangitis | 1, 1.69 (0.24–12.0) | NA | 8, 4.23 (2.11–8.45) | NA |
| Other extraintestinal manifestations | 6, 0.88 (0.39–1.95) | NA | 11, 0.95 (0.53–1.71) | NA |
Events (ev) in IBD cases, incidence rate per 1000 person‐years (95% confidence intervals).
TABLE 3.
Absolute incidence rates of colorectal cancer deaths per 1000 person‐years (95% confidence intervals) in incident cases of inflammatory bowel disease cases and matched reference individuals from the general population
| Colorectal cancer death | ||||
|---|---|---|---|---|
| Crohn's disease | Ulcerative colitis | |||
| Patients | Reference | Patients | Reference | |
| N total | 7869 | 75,654 | 21,224 | 204,531 |
| N events | 114 | 974 | 233 | 2553 |
| Incidence proportion (%) | 1.45 | 1.29 | 1.10 | 1.25 |
| 1‐year cumulative incidence | 0.30 (0.18–0.43) | 0.06 (0.05–0.08) | 0.17 (0.11–0.22) | 0.05 (0.04–0.06) |
| 5‐year cumulative incidence | 1.09 (0.82–1.36) | 0.53 (0.47–0.59) | 0.55 (0.44–0.67) | 0.53 (0.49–0.56) |
| 10‐year cumulative incidence | 2.01 (1.58–2.44) | 1.31 (1.20–1.42) | 1.33 (1.10–1.55) | 1.39 (1.32–1.46) |
| 20‐year cumulative incidence | 3.52 (2.63–4.39) | 4.07 (3.71–4.42) | 4.53 (3.58–5.46) | 4.01 (3.78–4.24) |
| Person‐years | 55,378 | 630,030 | 151,134 | 1,646,320 |
| ev, IR (95% CI) a | ev, IR (95% CI) a | ev, IR (95% CI) a | ev, IR (95% CI) a | |
| Total, incidence rate (95% CI) | 114, 2.06 (1.71–2.47) | 974, 1.55 (1.45–1.65) | 233, 1.54 (1.36–1.75) | 2553, 1.55 (1.49–1.61) |
| Denmark | 23, 1.78 (1.18–2.68) | 261, 1.79 (1.58–2.02) | 66, 1.37 (1.08–1.75) | 832, 1.63 (1.52–1.75) |
| Sweden | 91, 2.14 (1.75–2.63) | 713, 1.47 (1.37–1.59) | 167, 1.62 (1.39–1.88) | 1721, 1.51 (1.44–1.59) |
| Sex | ||||
| Female | 67, 2.06 (1.62–2.61) | 506, 1.35 (1.24–1.48) | 128, 1.63 (1.37–1.94) | 1142, 1.34 (1.27–1.42) |
| Male | 47, 2.06 (1.55–2.74) | 468, 1.83 (1.67–2.00) | 105, 1.44 (1.19–1.75) | 1411, 1.77 (1.68–1.87) |
| Age at first IBD diagnosis (years) | ||||
| 60–69 | 45, 1.26 (0.94–1.69) | 448, 1.17 (1.06–1.28) | 114, 1.25 (1.04–1.50) | 1184, 1.23 (1.17–1.31) |
| 70–79 | 52, 3.26 (2.49–4.28) | 398, 2.07 (1.87–2.28) | 87, 1.80 (1.46–2.22) | 1032, 1.91 (1.79–2.03) |
| ≥80 | 17, 4.41 (2.74–7.09) | 122, 2.60 (2.18–3.10) | 32, 2.75 (1.95–3.89) | 324, 2.45 (2.19–2.73) |
| Year of first diagnosis | ||||
| 2003–2017 | 43, 2.20 (1.63–2.96) | 226, 1.08 (0.95–1.23) | 45, 0.86 (0.64–1.16) | 539, 1.00 (0.91–1.08) |
| 1990–2002 | 51, 2.10 (1.60–2.76) | 494, 1.75 (1.60–1.91) | 111, 1.54 (1.28–1.85) | 1373, 1.74 (1.65–1.84) |
| 1977–1989 | 12, 1.23 (0.70–2.16) | 214, 1.83 (1.60–2.09) | 58, 2.49 (1.93–3.23) | 545, 2.00 (1.84–2.18) |
| 1969–1976 | 8, 4.60 (2.30–9.19) | 40, 1.91 (1.40–2.61) | 19, 5.35 (3.41–8.39) | 96, 2.15 (1.76–2.63) |
| Years of follow‐up | ||||
| 0 to <1 | 22, 3.07 (2.02–4.67) | 46, 0.63 (0.48–0.85) | 33, 1.68 (1.20–2.37) | 106, 0.54 (0.45–0.65) |
| 1 to <5 | 43, 1.98 (1.47–2.67) | 266, 1.14 (1.01–1.29) | 58, 0.96 (0.75–1.25) | 726, 1.16 (1.08–1.25) |
| 5 to <10 | 29, 1.85 (1.28–2.66) | 281, 1.57 (1.40–1.76) | 64, 1.48 (1.16–1.89) | 819, 1.73 (1.61–1.85) |
| 10 to <20 | 18, 1.83 (1.16–2.91) | 325, 2.55 (2.28–2.84) | 69, 2.65 (2.09–3.36) | 757, 2.41 (2.24–2.58) |
| ≥20 | 2, 1.99 (0.50–7.95) | 56, 3.09 (2.38–4.01) | 9, 4.20 (2.18–8.06) | 145, 4.13 (3.51–4.86) |
| Excluding the first year | 92, 1.91 (1.56–2.34) | 928, 1.66 (1.56–1.77) | 200, 1.52 (1.32–1.75) | 2447, 1.69 (1.62–1.76) |
| Location or extent | ||||
| N classified since 1997 | 6812 | NA | 18,584 | NA |
| L1/L3/LX | 74, 2.56 (2.04–3.21) | NA | NA | NA |
| L2 | 19, 1.48 (0.94–2.32) | NA | NA | NA |
| E1 (proctitis) | NA | NA | 16, 1.13 (0.69–1.85) | NA |
| E2 (left sided colitis) | NA | NA | 28, 1.42 (0.98–2.05) | NA |
| E3 (extensive colitis) | NA | NA | 63, 1.50 (1.17–1.92) | NA |
| EX (undefined) | NA | NA | 62, 1.55 (1.21–1.99) | NA |
| Extraintestinal manifestations | ||||
| Primary sclerosing cholangitis | 2, 3.37 (0.84–13.5) | NA | 9, 4.58 (2.38–8.80) | NA |
| Other extraintestinal manifestations | 4, 0.58 (0.22–1.54) | NA | 8, 0.67 (0.34–1.34) | NA |
Events (ev) in IBD cases, incidence rate per 1000 person‐years (95% confidence intervals).
The cumulative incidence proportion of CRC diagnosis during the first year of follow‐up was higher in patients with CD (95%CI 1.01% [0.78–1.24])and UC (0.60% [0.49–0.70]) than in the reference population (0.24% [0.21–0.28] and 0.25% [0.23–0.27]). Ten‐ and 20‐year cumulative incidences were similar for patients and their reference population (overlapping CIs), but with lower point estimates for the patients. The cumulative incidence of CRC deaths in both CD and UC patients was lower than the cumulative incidence of CRC diagnoses but followed the same pattern of decreasing excess risk with longer follow‐up (Tables 2 and 3).
Colectomy with or without proctectomy in the absence of a CRC diagnosis was more common in Denmark than in Sweden (cumulative 20‐year incidence in CD patients was 5% in Denmark vs 3% in Sweden and in UC patients 12% in Denmark vs 10% in Sweden) (Figure S2).
3.3. CRC by location, including the first year of follow‐up
Compared to the reference population, the HR of a CRC diagnosis at any location was not elevated in patients with elderly‐onset CD (1.12 [0–96‐1.32]) or UC (0.98 [0.88–1.08]). However, the HR for colon cancer was increased in patients with CD (1.48 [95% CI: 1.25–1.75]), while the HR for rectal cancer was decreased (0.33 [95% CI: 0.20–0.55]) (Figure 1, Table S9). The overall HR for CRC death was increased only in CD patients (1.43 [95% CI: 1.18–1.74]), driven by colon cancer (1.70 [95% CI: 1.38–2.10]) but not rectal cancer (0.73 [95% CI: 0.44–1.20]). In CD patients, the HR of a CRC diagnosis and death by calendar year of IBD onset was U‐shaped, with increased HRs both during earlier calendar periods (1969–1979) and during later calendar periods (2003–2017). In contrast, the HR in UC patients was increased only during earlier calendar years of disease diagnosis (1969–1976) (Figure 1). After stratifying for tumour location within the large bowel, HRs for CRC diagnosis and death in both CD and UC were increased mainly for proximal tumours and tumours with an undefined location (Table S10).
FIGURE 1.
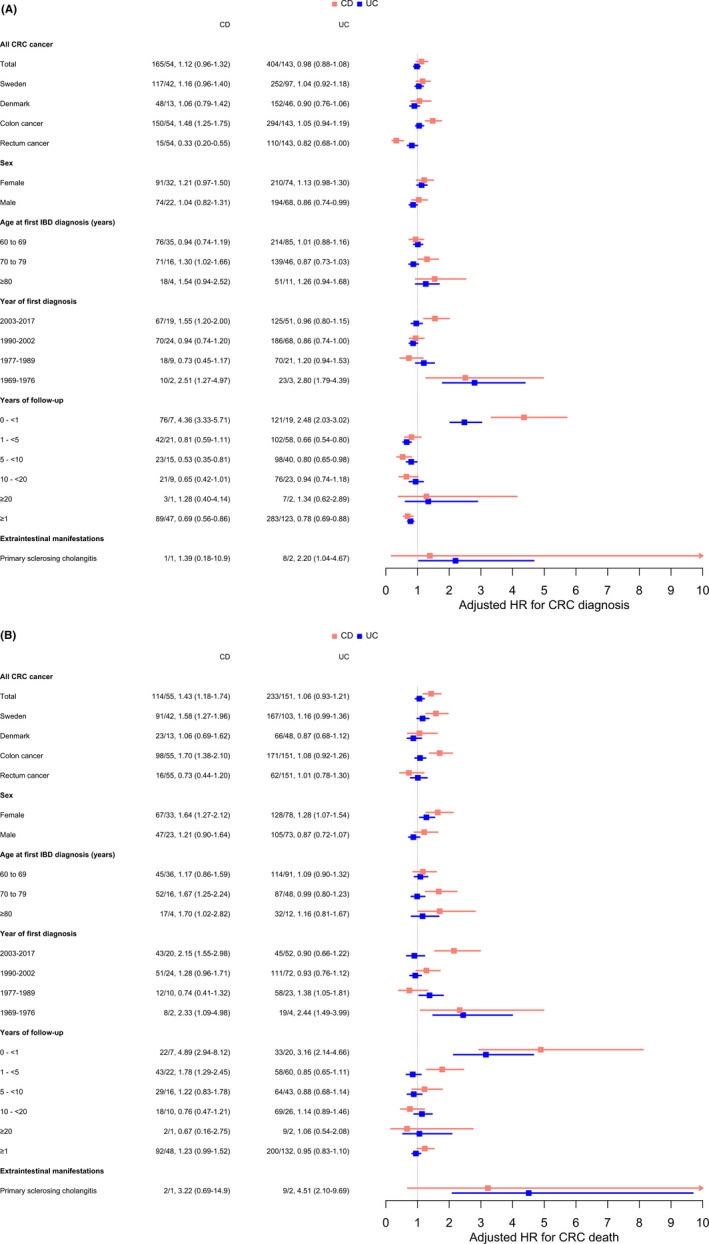
Hazard ratios for (A) incident colorectal cancer diagnosis and (B) colorectal cancer death during all available follow‐up time (1969–2017 in Sweden and 1977–2011 in Denmark) in patients with Crohn's disease and ulcerative colitis and matched general population comparators. Numbers represent: Number of events in IBD patients/1000 person‐years of follow‐up, hazard ratio (95% confidence interval).
3.4. Competing risks and adjustment for tumour stage
We used a multi‐state model to compute HRs for transitions from the start of follow‐up until CRC diagnosis, CRC death and other death. Both in patients with CD and UC, the cause‐specific HRs were increased for the transitions from the start of follow‐up to CRC death and from the start of follow‐up to other death, but not from the start of follow‐up to CRC diagnosis compared to the matched reference population (Figure 2, Table S12). In a comparison of IBD patients diagnosed with CRC with reference individuals also diagnosed with CRC, the crude HR of CRC death since 2003 was not increased (0.89 [95% CI: 0.73–1.09]). The distribution of cancer stage also was similar between patients with CD and their reference individuals (Table S11). Patients with UC were more often diagnosed with stage I CRC than the reference population (20% vs 11%) and less frequently with stage IV CRC (8.5% vs 17%). After adjustment for cancer stage, the HR for CRC death was increased for patients with UC (1.30 [95% CI: 1.01–1.68]), but not for patients with CD (0.90 [95% CI: 0.63–1.28]).
FIGURE 2.
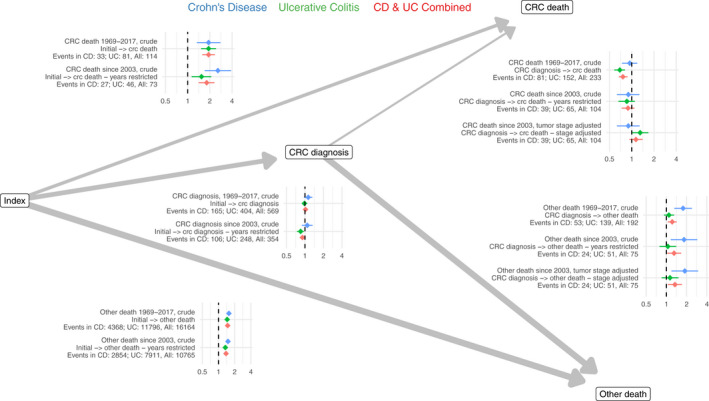
Cause‐specific hazard ratios for transitions from the start of follow‐up to colorectal cancer diagnosis, colorectal cancer death and other death, in patients with inflammatory bowel disease (Crohn's disease [CD] and ulcerative colitis [UC]) and the matched reference population.
3.5. CRC over follow‐up time
When plotting the incidence rate of CRC in relation to the start of follow‐up, we observed an extreme increase in CRC cases during the months just before and after IBD diagnosis among IBD patients, but a constant CRC incidence in the reference population (Figure 3). The HR for a CRC diagnosis was 4.36 (95% CI: 3.33–5.71) in CD patients and 2.48 (95% CI: 2.03–3.02) in UC patients during the first year of follow‐up (Figure 1). When the first year of follow‐up was excluded, the HR for CRC diagnosis was decreased in IBD patients compared to the reference population: 0.69 (95% CI: 0.56–0.86) for CD patients and 0.78 (95% CI: 0.69–0.88) for UC patients. The HR for CRC death was 4.89 (95% CI: 2.94–8.12) for CD patients and 3.16 (95% CI: 2.14–4.66) for UC patients during the first year after their IBD diagnosis (Figure 1). It was not significantly increased when the first year of follow‐up was excluded.
FIGURE 3.
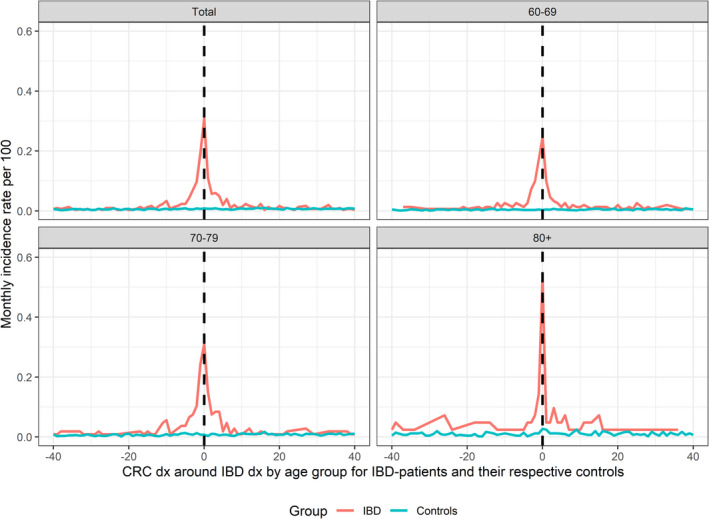
Incidence rate of colorectal cancer (CRC) per month in relation to the date of inflammatory bowel disease (IBD) diagnosis in patients with elderly‐onset IBD (60–69 years, 70–79 years, 80+ years and overall, at diagnosis).
3.6. CRC beyond the first year of follow‐up and CRC in IBD patients eligible for CRC surveillance
In additional analyses, we excluded the first year of follow‐up. Over succeeding calendar periods up to the end of follow‐up, the HR for CRC diagnosis and death decreased for patients with UC from the 1990s to 2005 but remained unchanged for patients with CD (Figure 4). Starting in 2010, the HR for CRC diagnosis (but not the HR for CRC death) remained below 1 in both CD and UC patients, compared to the reference population.
FIGURE 4.
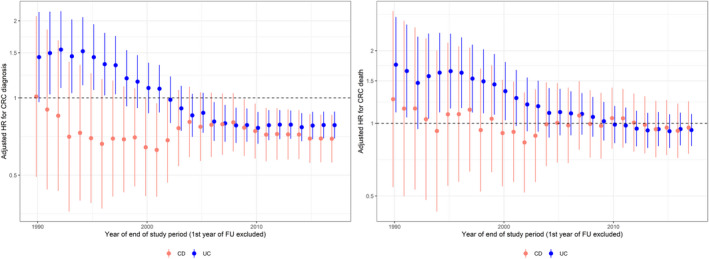
Adjusted hazard ratio (HR) for colorectal cancer (CRC) diagnosis (left panel) and death (right panel) in patients with Crohn's disease (CD) and ulcerative colitis (UC), excluding the first year of follow‐up (FU). Data are presented by calendar year in relation to a matched reference population.
In patients with CD who were eligible for CRC surveillance (i.e., patients followed ≥8 years or diagnosed with PSC), the HR for CRC diagnosis was lower than in the reference population (HR 0.61 [95% CI: 0.42–0.88]). In patients with UC who were eligible for CRC surveillance, the HR for CRC diagnosis was similar to that in the reference population (HR 0.93 [95% CI: 0.77–1.12]). The HR of CRC death did not significantly differ from that in the reference population for both patients with CD (HR 0.96 [95% CI: 0.64–1.42]) and UC (1.23 [95% CI: 0.99–1.53]). The HR was increased for patients with PSC but based on a few events and with wide CIs (Figure 5, Table S15). Country‐specific estimates show that the HRs for CRC diagnosis decreased in both countries over time, also before the date of the start of surveillance programmes for IBD patients (Figure S3).
FIGURE 5.
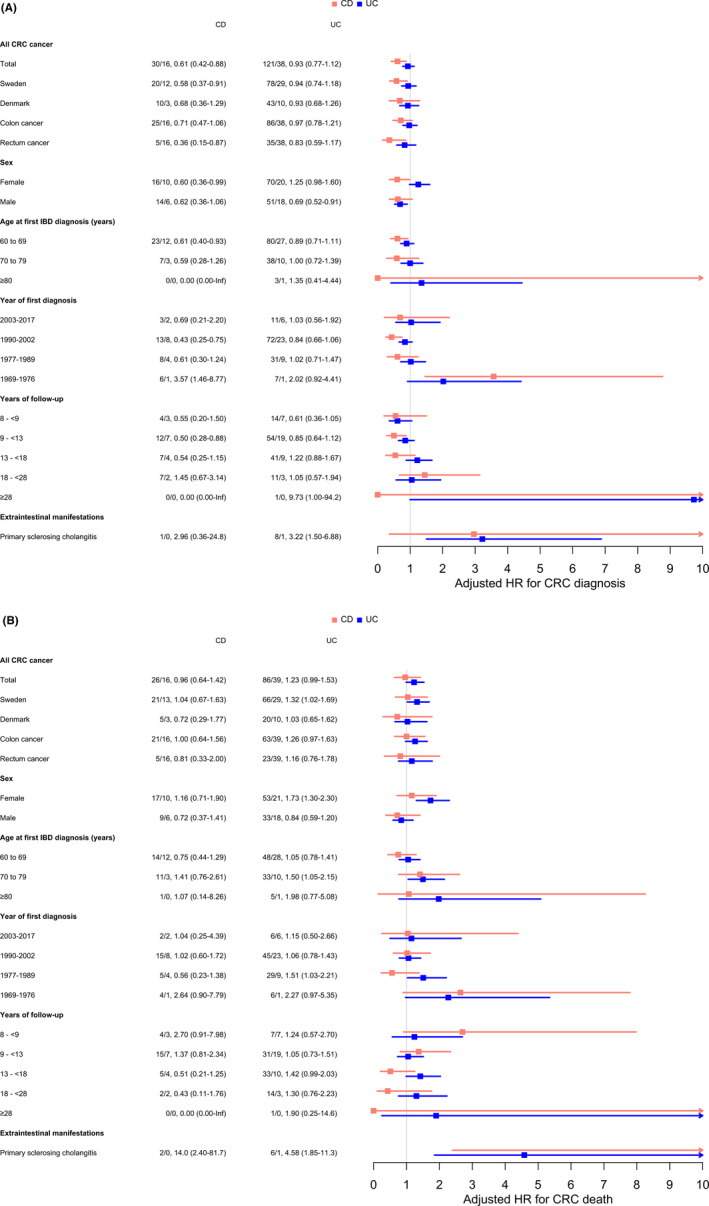
Colorectal cancer in elderly‐onset inflammatory bowel disease patients eligible for cancer surveillance according to international guidelines: Hazard ratios for (A) incident colorectal cancer diagnosis and (B) colorectal cancer death during all available follow‐up time (1969–2017 in Sweden and 1977–2011 in Denmark) in patients with Crohn's disease and ulcerative colitis, at risk after 8 years of follow‐up or from the date of a primary sclerosing cholangitis diagnosis, and matched general population comparators. Numbers represent number of events in IBD patients/1000 person‐years of follow‐up, hazard ratio (95% confidence interval).
4. DISCUSSION
4.1. Main results
We found a dichotomous pattern of cancer risk with time. Patients with elderly‐onset IBD had an increased risk of a CRC diagnosis and CRC death during the first year after an IBD diagnosis. The excess risk of CRC was not present in UC patients after the first year, but CD patients experienced increased CRC mortality during the first 5 years of follow‐up. Our follow‐up data show that the relative risk for a CRC diagnosis has decreased over the past 20 years, regardless of whether CRC surveillance programmes in IBD had been introduced or not. From 2010 onwards, both patients with CD and UC had a lower risk of incident CRC than the general population (except during the year following IBD diagnosis).
4.2. Comparison to other studies
The risk of CRC has been investigated extensively in IBD patients, but few studies have evaluated this risk in patients with elderly‐onset IBD. 15 , 16 , 17 , 18 , 37 Ekbom et al. found an increased risk in patients with elderly‐onset UC in a 1990 study, but later publications reported non‐significantly lower standardised incidence ratios (SIRs) for UC patients, and non‐significantly higher SIRs for patients with CD. 15 , 16 Meta‐analyses, which included IBD patients of all ages, have reported that age at diagnosis of UC or CD in adults does not influence the relative risk of CRC, 2 , 4 and that the incidence ratio was not significantly increased for patients with IBD onset >30 years of age. 1 A less aggressive phenotype has been described in elderly‐onset UC, with predominantly left‐sided colitis and less extensive colitis. 38 , 39 However, more colonic involvement is reported in the elderly population with CD. 38 , 39
In the present study, the HR for a CRC diagnosis in IBD patients after the first year of follow‐up was lower than in the reference population: 0.69 (95% CI: 0.56–0.86) in CD patients and 0.78 (95% CI: 0.69–0.88) in UC patients (Figure 1). Further, the incidence did not differ significantly between phenotypes of CD and UC (Table 2). Patients with both UC and PSC were the only subgroup of elderly patients that could be identified as high‐risk in this study (Table 2, Figure 1, Table S10, Figure 5, Table S14). However, this was based on only 8 CRC events in patients with both diagnoses. In contrast, in another very large cohort of IBD patients with and without PSC (but no non‐IBD controls), no difference in CRC risk could be found between patients with IBD‐PSC and IBD alone among those diagnosed after 60 years of age (based on 44 events in patients with IBD‐PSC and IBD). 40
Beyond the first year following an IBD diagnosis, the HRs of both a CRC diagnosis and death from CRC decreased in UC patients over calendar time (Figure 4). This finding, which has been reported previously, 3 , 17 may reflect the increased availability and quality of colonoscopy, 41 implementation of surveillance strategies that detect precancerous lesions, 23 , 25 and introduction of drugs that control inflammation effectively. The reason also may be partly administrative, as outpatient data were added to Danish registers in 1995 and to Swedish registers in 2001, potentially contributing some less severe IBD cases to the cohort. However, we observed no large differences in numbers of CD patients as an effect of calendar period. It should be noted that the decreasing HR for CRC over calendar periods was seen in both Sweden and Denmark, although surveillance was recommended much later in Denmark and could not have affected the Danish estimates in this study (Figure S3).
4.3. Strengths and limitations
The main strengths of this study are its large population‐based study design, data prospectively recorded during a long follow‐up period, and availability of registers with validated exposure definitions and almost complete coverage of outcome measures. As well, access to histopathology data helped to define IBD onset more accurately than in previous reports and also increased sensitivity, especially during years when the Danish and Swedish patient registers were restricted to inpatient data. The use of a matched reference cohort allowed for the calculation of incidence rates and HRs of truly incident cancers.
Study limitations include absence of data on confounding from lifestyle risk factors, including intake of red and processed meats, obesity, tobacco use and alcohol use. However, such unmeasured confounding is extremely unlikely to explain our findings. 17 , 18 Another limitation is the absence of data on IBD medication and disease activity, for example laboratory markers, endoscopic activity and histological findings. As well, phenotype was determined from ICD codes, which have limited precision. 28 , 34 Nevertheless, in our cohort, the proportion of CD patients with only colonic involvement at end of follow‐up was 30%, compared to 28% 39 and 62% at diagnosis 10 in other well‐characterised cohorts. The proportion of UC patients with isolated proctitis was 12% in the current study, compared to 20% 39 and 21% 10 found at diagnosis in other studies. The proportion of UC patients with extensive colitis at end of follow‐up was 35% in our study, compared to 33% and 18% at diagnosis in other studies. 10 , 38
Some informative censoring can be assumed, i.e. patients who were censored due to proctocolectomy may have had a higher risk of developing CRC than other patients, thus possibly leading to underestimation of overall CRC risk. However, the cumulative incidence of colectomy has decreased over time in both CD and UC (Figure S2), and the relative risk of CRC has also decreased (Figure S3).
4.4. Clinical implications
The increased risk of CRC in patients with IBD is thought to result from the pro‐neoplastic effect of chronic intestinal inflammation. 42 Data from the current study suggest that this either is not the case in elderly‐onset IBD, or that such effect is effectively counterbalanced by planned or opportunistic screening and colectomies. The pronounced increase in CRC incidence around the time of IBD diagnosis suggests that a large proportion of such IBD‐CRC cases is due either to detection bias or misclassification. CRC cases may have been discovered accidentally due to IBD symptoms and work‐up, or IBD could have been diagnosed in conjunction with a CRC diagnosis. As cancer development requires a reasonable induction time, the only way in which excess CRC cases during the first year following IBD diagnosis can be attributed to IBD is if they result from longstanding undiagnosed IBD, which was finally discovered and diagnosed due to symptoms from CRC. Diagnostic delay is more common in CD 43 , 44 than UC, which could explain why the absolute and relative risk of CRC was higher in CD than in UC patients during the first year. It is also well‐known that the use of immunosuppressants is much lower in the elderly population than in children and adults with IBD 14 likely because of comorbidity and polypharmacy. 45 Insufficiently treated inflammation may contribute to the development of CRC in the elderly.
Our study documented differences in cancer incidence between a screened population (all patients diagnosed with IBD who undergo colonoscopy during the year of IBD diagnosis and are thereby screened for CRC and premalignant polyps at that timepoint) and an unscreened population (most reference individuals). Most reference individuals did not participate in CRC screening programmes, since only two regions in Sweden (Stockholm and Gotland) offer screening programmes for individuals aged 60–69 years since 2008 and the national programme Screening of Swedish colons (SCREESCO), targeting individuals aged 59–62 years, was initiated in 2014. Also in Denmark, CRC screening with FIT‐test (faecal immunochemical test) was not initiated for ages 50–74 until 2014.
The HRs of 0.69 in CD patients and 0.78 in UC patients for a CRC diagnosis after the first year of follow‐up can be compared with 0.83 (95% CI 0.71–0.96) after 10 years of follow‐up in a randomised controlled trial of sigmoidoscopy screening in person aged between 55 and 64 years (intention‐to‐treat analysis with 63% adherence). 46 Similarly, the implementation of organised CRC screening with FIT and follow‐up colonoscopy in persons aged 50–75 was reported to have led to an initial rise in CRC incidence, followed by a 25.5% decline in incidence over 12–14 years. 47 The tumours in patients with UC in our study were detected at an earlier stage than in the reference population (Table S12), also likely an effect of intensified screening particularly during the year of IBD diagnosis. Consequently, patients with UC had a better prognosis than the reference population (HR 0.69 [95% CI: 0.58–0.81] from CRC diagnosis to CRC death). However, after adjusting for tumour stage, the HR increased to 1.30 (95% CI: 1.01–1.68), implying that CRC prognosis in UC patients is worse than in sporadic cases.
Based on data from this study and others, we conclude that the relative risk of CRC diagnosis is not increased in patients with elderly‐onset IBD beyond the period around IBD diagnosis, with current endoscopy and colectomy routines. However, the absolute risk is high due to age alone, which warrants implementing screening colonoscopy according to guidelines for the general population. 48 The risk of CRC death without a previous CRC diagnosis was noticeably high in IBD patients. The increased risk of CRC death following a CRC diagnosis in patients with UC also warrants further investigation.
5. CONCLUSION
We found that patients with elderly‐onset IBD had an increased risk of CRC diagnosis only during the year following IBD diagnosis. Beyond the first year there was no excess risk, and from 2010 and onward the HR for CRC diagnosis more than 1 year after initial IBD diagnosis was significantly lower than that in the largely unscreened reference population. Our findings thus support the benefit of endoscopic screening and surveillance in patients with IBD.
AUTHOR CONTRIBUTIONS
Åsa Everhov: Conceptualization (supporting); methodology (equal); writing – original draft (lead); writing – review and editing (lead). Rune Erichsen: Methodology (equal); writing – review and editing (equal). Jacob J är ås: Formal analysis (lead); methodology (equal); writing – review and editing (equal). Lars Pedersen: Methodology (equal); writing – review and editing (equal). Jonas Halfvarson: Methodology (equal); writing – review and editing (equal). Johan Askling: Methodology (equal); writing – review and editing (equal). Anders Ekbom: Methodology (equal); writing – review and editing (equal). Jonas F Ludvigsson: Methodology (equal); writing – review and editing (equal). Henrik Toft S ørensen: Data curation (lead); methodology (equal); writing – review and editing (equal). Ola Ol én: Conceptualization (lead); data curation (lead); methodology (lead); writing – review and editing (equal). All authors approved the final version of the article.
FUNDING INFORMATION
This project was supported by grants from Karolinska Institutet (KI SÖS), Bengt Ihre Research Foundation, Bengt Ihre Research Fellowship, the Swedish Medical Society, the Swedish Research Council, the Young Scholar Award from the Strategic Research Area Epidemiology Program at Karolinska Institutet, the FORTE Foundation, the Swedish Cancer Foundation, The Swedish Heart Lung Foundation, the Independent Research Fund Denmark, the Danish Cancer Association, the Novo Nordisk Foundation and the Regional Agreement on Medical Training and Clinical Research between Stockholm County Council and Karolinska Institutet (ALF). None of the funding organisations had any role in the design and conduct of the study; in the collection, management and analysis of the data; or in the preparation, review and approval of the manuscript.
AUTHORSHIP
Guarantor of the article: Ola Olén.
Supporting information
Appendix S1
ACKNOWLEDGEMENT
Declaration of personal interests: Å H Everhov has worked on projects at Karolinska Institutet and SWIBREG partly financed by grants from Ferring and Jansen. J F Ludvigsson coordinates a study on behalf of the Swedish IBD quality register (SWIBREG), which has received funding from the Jansen Corporation. O Olén has been PI on projects at Karolinska Institutet, partly financed by investigator‐initiated grants from Janssen and Ferring. Karolinska Institutet has received fees for lectures and participation on advisory boards from Janssen, Ferring, Takeda and Pfizer. O Olén also reports a grant from Pfizer addressing a national safety monitoring programme. J Askling acts or has acted as PI in agreements between Karolinska Institutet and the following entities, mainly regarding safety monitoring of rheumatology immunomodulators: Abbvie, AstraZeneca, BMS, Eli Lilly, Janssen, MSD, Pfizer, Roche, Samsung Bioepis, Sanofi and UCB. J Halfvarson served as speaker and/or advisory board member for AbbVie, Celgene, Celltrion, Dr Falk Pharma and the Falk Foundation, Ferring, Hospira, Janssen, MEDA, Medivir, MSD, Novartis, Olink Proteomics, Pfizer, Prometheus Laboratories, Sandoz, Shire, Takeda, Thermo Fisher Scientific, Tillotts Pharma and Vifor Pharma (UCB), and received grant support from Janssen, MSD and Takeda. H T Sørensen, R Erichsen and L Pedersen report that the Department of Clinical Epidemiology, Aarhus University, receives funding for other studies from companies in the form of institutional research grants to (and administered by) Aarhus University. None of these studies have any relation to the present study.
Everhov ÅH, Erichsen R, Järås J, Pedersen L, Halfvarson J, Askling J et al. Colorectal cancer in elderly‐onset inflammatory bowel disease: a 1969–2017 Scandinavian register‐based cohort study. Aliment Pharmacol Ther. 2022;56:1168–1182. 10.1111/apt.17175
Henrik Toft Sørensen and Ola Olén contributed equally.
The Handling Editor for this article was Professor Peter Gibson, and this was accepted for publication after full peer‐review.
REFERENCES
- 1. Lutgens MW, van Oijen MG, van der Heijden GJ, Vleggaar FP, Siersema PD, Oldenburg B. Declining risk of colorectal cancer in inflammatory bowel disease: an updated meta‐analysis of population‐based cohort studies. Inflamm Bowel Dis. 2013;19(4):789–99. [DOI] [PubMed] [Google Scholar]
- 2. Laukoetter MG, Mennigen R, Hannig CM, Osada N, Rijcken E, Vowinkel T, et al. Intestinal cancer risk in Crohn's disease: a meta‐analysis. J Gastrointest Surgery. 2011;15(4):576–83. [DOI] [PubMed] [Google Scholar]
- 3. Castano‐Milla C, Chaparro M, Gisbert JP. Systematic review with meta‐analysis: the declining risk of colorectal cancer in ulcerative colitis. Aliment Pharmacol Ther. 2014;39(7):645–59. [DOI] [PubMed] [Google Scholar]
- 4. Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta‐analysis. Gut. 2001;48(4):526–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jess T, Rungoe C, Peyrin‐Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta‐analysis of population‐based cohort studies. Clin Gastroenterol Hepatol. 2012;10(6):639–45. [DOI] [PubMed] [Google Scholar]
- 6. Askling J, Dickman PW, Karlen P, Brostrom O, Lapidus A, Lofberg R, et al. Family history as a risk factor for colorectal cancer in inflammatory bowel disease. Gastroenterology. 2001;120(6):1356–62. [DOI] [PubMed] [Google Scholar]
- 7. Zheng HH, Jiang XL. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and inflammatory bowel disease: a meta‐analysis of 16 observational studies. Eur J Gastroenterol Hepatol. 2016;28(4):383–90. [DOI] [PubMed] [Google Scholar]
- 8. Magro F, Gionchetti P, Eliakim R, Ardizzone S, Armuzzi A, Barreiro‐de Acosta M, et al. Third European evidence‐based consensus on diagnosis and Management of Ulcerative Colitis. Part 1: definitions, diagnosis, extra‐intestinal manifestations, pregnancy, cancer surveillance, surgery, and Ileo‐anal pouch disorders. J Crohns Colitis. 2017;11(6):649–70. [DOI] [PubMed] [Google Scholar]
- 9. Farraye FA, Odze RD, Eaden J, Itzkowitz SH. AGA technical review on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138(2):746–74, 74 e1‐4; quiz e12‐3. [DOI] [PubMed] [Google Scholar]
- 10. Lakatos PL, David G, Pandur T, Erdelyi Z, Mester G, Balogh M, et al. IBD in the elderly population: results from a population‐based study in Western Hungary, 1977‐2008. J Crohns Colitis. 2011;5(1):5–13. [DOI] [PubMed] [Google Scholar]
- 11. Charpentier C, Salleron J, Savoye G, Fumery M, Merle V, Laberenne JE, et al. Natural history of elderly‐onset inflammatory bowel disease: a population‐based cohort study. Gut. 2014;63(3):423–32. [DOI] [PubMed] [Google Scholar]
- 12. Jeuring SF, van den Heuvel TR, Zeegers MP, Hameeteman WH, Romberg‐Camps MJ, Oostenbrug LE, et al. Epidemiology and long‐term outcome of inflammatory bowel disease diagnosed at elderly age‐an increasing distinct entity? Inflamm Bowel Dis. 2016;22(6):1425–34. [DOI] [PubMed] [Google Scholar]
- 13. Norgard BM, Nielsen J, Fonager K, Kjeldsen J, Jacobsen BA, Qvist N. The incidence of ulcerative colitis (1995‐2011) and Crohn's disease (1995‐2012) – based on nationwide Danish registry data. J Crohns Colitis. 2014;8(10):1274–80. [DOI] [PubMed] [Google Scholar]
- 14. Everhov AH, Halfvarson J, Myrelid P, Sachs MC, Nordenvall C, Soderling J, et al. Incidence and treatment of patients diagnosed with inflammatory bowel diseases at 60 years or older in Sweden. Gastroenterology. 2018;154(3):518–28 e15. [DOI] [PubMed] [Google Scholar]
- 15. Jess T, Horvath‐Puho E, Fallingborg J, Rasmussen HH, Jacobsen BA. Cancer risk in inflammatory bowel disease according to patient phenotype and treatment: a Danish population‐based cohort study. Am J Gastroenterol. 2013;108(12):1869–76. [DOI] [PubMed] [Google Scholar]
- 16. Cheddani H, Dauchet L, Fumery M, Charpentier C, Marie Bouvier A, Dupas JL, et al. Cancer in elderly onset inflammatory bowel disease: a population‐based study. Am J Gastroenterol. 2016;111(10):1428–36. [DOI] [PubMed] [Google Scholar]
- 17. Olen O, Erichsen R, Sachs MC, Pedersen L, Halfvarson J, Askling J, et al. Colorectal cancer in ulcerative colitis: a Scandinavian population‐based cohort study. Lancet. 2020;395(10218):123–31. [DOI] [PubMed] [Google Scholar]
- 18. Olen O, Erichsen R, Sachs MC, Pedersen L, Halfvarson J, Askling J, et al. Colorectal cancer in Crohn's disease: a Scandinavian population‐based cohort study. Lancet Gastroenterol Hepatol. 2020;5(5):475–84. [DOI] [PubMed] [Google Scholar]
- 19. ECCO . Colorectal carcinoma surveillance. http://www.e‐guide.ecco‐ibd.eu/interventions‐investigational/colorectal‐carcinoma‐surveillance.
- 20. Danmarks Statistik. https://www.dst.dk/da.
- 21. Schmidt M, Schmidt SAJ, Adelborg K, Sundboll J, Laugesen K, Ehrenstein V, et al. The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol. 2019;11:563–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Laugesen K, Ludvigsson JF, Schmidt M, Gissler M, Valdimarsdottir UA, Lunde A, et al. Nordic health registry‐based research: a review of health care systems and key registries. Clin Epidemiol 2021;13(1179–1349 [Print]):533–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. C A. Koloskopiovervågning af patienter med kronisk inflammatorisk tarmsygdom (IBD) med henblik på udvikling af dysplasi og kolorektal cancer. https://www.dsgh.dk/images/Guidelines/pdf/ibdkrc_dsgh_guideline.pdf.
- 24. Ward DA‐O, Neumann AA‐O, Hendel JA‐O, Riis LA‐O, Tøttrup A, Jess TA‐O, et al. Danish Society for Gastroenterology and Hepatology's clinical recommendations for colonoscopic surveillance for colorectal dysplasia and cancer in patients with inflammatory bowel disease. (1502–7708 [Electronic]). [DOI] [PubMed]
- 25. Hertervig E. Koloskopisk övervakning av IBD. https://svenskgastroenterologi.se/wp‐content/uploads/2018/01/2017‐Koloskopisk‐övervakning‐av‐IBD.pdf.
- 26. Everhov AH, Sachs MC, Malmborg P, Nordenvall C, Myrelid P, Khalili H, et al. Changes in inflammatory bowel disease subtype during follow‐up and over time in 44,302 patients. Scand J Gastroenterol. 2019;54(1):55–63. [DOI] [PubMed] [Google Scholar]
- 27. Jakobsson GL, Sternegard E, Olen O, Myrelid P, Ljung R, Strid H, et al. Validating inflammatory bowel disease (IBD) in the Swedish National Patient Register and the Swedish quality register for IBD (SWIBREG). Scand J Gastroenterol. 2017;52(2):216–21. [DOI] [PubMed] [Google Scholar]
- 28. Lo B, Vind I, Vester‐Andersen MK, Burisch JA‐O. Validation of ulcerative colitis and Crohn's disease and their phenotypes in the Danish National Patient Registry using a population‐based cohort (1502–7708 [Electronic]). [DOI] [PubMed]
- 29. Rye CA‐O, Rubin KA‐OX, Moller FA‐O, Julsgaard MA‐O, Jess TA‐O, Andersen VA‐O. Positive Predictive Value of Diagnostic Codes for Inflammatory Bowel Disease in the Danish National Patient Registry Among Individuals 50+ Years, Using Patient Records as Reference Standard (1179–1349 [Print]). [DOI] [PMC free article] [PubMed]
- 30. Ludvigsson JF, Lashkariani M. Cohort profile: ESPRESSO (epidemiology strengthened by histoPathology reports in Sweden). Clin Epidemiol. 2019;11:101–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nguyen LH, Ortqvist AK, Cao Y, Simon TG, Roelstraete B, Song M, et al. Antibiotic use and the development of inflammatory bowel disease: a national case‐control study in Sweden. Lancet Gastroenterol Hepatol. 2020;5(11):986–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bjerregaard B, Larsen OB. The Danish pathology register. Scand J Public Health. 2011;39(7 Suppl):72–4. [DOI] [PubMed] [Google Scholar]
- 33. Ludvigsson JF, Almqvist C, Bonamy AE, Ljung R, Michaelsson K, Neovius M, et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol. 2016;31(2):125–36. [DOI] [PubMed] [Google Scholar]
- 34. Shrestha S, Olen O, Eriksson C, Everhov AH, Myrelid P, Visuri I, et al. The use of ICD codes to identify IBD subtypes and phenotypes of the Montreal classification in the Swedish National Patient Register. Scand J Gastroenterol. 2020;55(4):430–5. [DOI] [PubMed] [Google Scholar]
- 35. Forss A, Myrelid P, Olen O, Everhov AH, Nordenvall C, Halfvarson J, et al. Validating surgical procedure codes for inflammatory bowel disease in the Swedish National Patient Register. BMC Med Inform Decis Mak. 2019;19(1):217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ludvigsson JF, Haberg SE, Knudsen GP, Lafolie P, Zoega H, Sarkkola C, et al. Ethical aspects of registry‐based research in the Nordic countries. Clin Epidemiol. 2015;7:491–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population‐based study. N Engl J Med. 1990;323(18):1228–33. [DOI] [PubMed] [Google Scholar]
- 38. Ananthakrishnan AN, Shi HY, Tang W, Law CC, Sung JJ, Chan FK, et al. Systematic review and meta‐analysis: phenotype and clinical outcomes of older‐onset inflammatory bowel disease. J Crohns Colitis. 2016;10(10):1224–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Manosa M, Calafat M, de Francisco R, Garcia C, Casanova MJ, Huelin P, et al. Phenotype and natural history of elderly onset inflammatory bowel disease: A multicentre, case‐control study. Aliment Pharmacol Ther. 2018;47(5):605–14. [DOI] [PubMed] [Google Scholar]
- 40. Trivedi PJ, Crothers H, Mytton J, Bosch S, Iqbal T, Ferguson J, et al. Effects of primary sclerosing cholangitis on risks of cancer and death in people with inflammatory bowel disease, based on sex, race, and age. Gastroenterology. 2020;159(3):915–28. [DOI] [PubMed] [Google Scholar]
- 41. Forsberg A, Widman L, Bottai M, Ekbom A, Hultcrantz R. Postcolonoscopy colorectal cancer in Sweden from 2003 to 2012: survival, tumor characteristics, and risk factors. Clin Gastroenterol Hepatol. 2020;18(12):2724–33. e3. [DOI] [PubMed] [Google Scholar]
- 42. Kimmel JA‐O, Axelrad JA‐O. The complex interplay between inflammatory bowel disease and malignancy (1534‐312X [Electronic]). [DOI] [PubMed]
- 43. Schoepfer AM, Dehlavi MA, Fournier N, Safroneeva E, Straumann A, Pittet V, et al. Diagnostic delay in Crohn's disease is associated with a complicated disease course and increased operation rate. Am J Gastroenterol. 2013;108(11):1744–53. quiz 54. [DOI] [PubMed] [Google Scholar]
- 44. Pimentel M, Chang M, Chow EJ, Tabibzadeh S, Kirit‐Kiriak V, Targan SR, et al. Identification of a prodromal period in Crohn's disease but not ulcerative colitis. Am J Gastroenterol. 2000;95(12):3458–62. [DOI] [PubMed] [Google Scholar]
- 45. Kochar B, Jylhävä J, Söderling J, Ritchie CS, Ludvigsson JF, Khalili H, et al. Prevalence and implications of frailty in older adults with incident inflammatory bowel diseases: a nationwide cohort study. LID ‐ S1542‐3565(22)00007–6 [pii] LID. 10.1016/j.cgh.2022.01.001 [doi]. (1542–7714 [Electronic]). [DOI] [PMC free article] [PubMed]
- 46. Holme O, Loberg M, Kalager M, Bretthauer M, Hernan MA, Aas E, et al. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: a randomized clinical trial. JAMA. 2014;312(6):606–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Levin TR, Corley DA, Jensen CD, Schottinger JE, Quinn VP, Zauber AG, et al. Effects of organized colorectal cancer screening on cancer incidence and mortality in a large community‐based population. Gastroenterology. 2018;155(5):1383–91 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ladabaum U, Dominitz JA, Kahi C, Schoen RE. Strategies for colorectal cancer screening. (1528–0012 [Electronic]). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


