Abstract
Background and Aim
Total mesorectal excision (TME) remains the treatment of choice in T2N0 tumors. However, evidence suggest that one‐size‐fits‐all approach is not always beneficial for this group of patients. The aim of this study is to synthesize data on long‐term outcomes after neoadjuvant therapy (NAT) followed by local excision (LE) in T2N0 rectal cancer patients in the perspective of a rectal‐preserving strategy.
Methods
A systematic search of PubMed/MEDLINE, SCOPUS, and Web of Science databases was conducted until October 2021 to identify studies comparing LE after NAT and TME or reporting oncologic outcomes after conservative approach. A pooled analysis was conducted using a fixed‐effect model in the case of non‐significant heterogeneity (P > 0.1), and a random effect model (DerSimonian–Laird method) when significant heterogeneity was present (P < 0.1) CRD42022300344.
Results
Nine studies were included in the analysis. Three of them were comparative studies. The pooled 3‐year DFS, 5‐year DFS, 3‐year OS, 5‐year OS, local and distant recurrence rates were 92.8% (95% CI 81.6–99.5%), 91.3% (95% CI 88.3–94.3%), 96.1% (95% CI 90.5–100%), 72.6% (95% CI 57.5–87.7%), 4% (95% CI 18–63%), and 4.9% (95% CI 2–7.8%), respectively, in subjects treated with NAT followed by LE. No heterogeneity was found for all these analyses, except for the 5‐year OS sub‐analysis (I 2 95.5%, P < 0.001). Complete pathological response (ypT0) rate after NAT and LE ranges from 26.7% to 59%.
Conclusion
LE following neoadjuvant CRT may provide comparable survival benefit to radical surgery for patients with clinical stage T2N0 in selected patients although the evidence is still limited to provide solid recommendations. A personalized therapeutic approach taking into account tumor and patient‐related factors should be considered.
Keywords: local excision, neoadjuvant therapy, organ preservation, rectal cancer, T2 tumor
Introduction
Early rectal cancer is defined as a cancer with good prognostic features that might be safely removed by transanal local excision (LE) preserving the rectum and that will have a very limited risk of relapse. 1 According to the Association of Coloproctology of Great Britain & Ireland (ACPGBI), cT1‐2N0M0 tumors are included in the early stage of rectal cancer. 2 However, it is well established that LE has curative role only in T1 tumors with favorable pathologic characteristics (low‐risk pT1) while radical surgery remains the treatment of choice in T2 rectal cancer because of a not negligible risk of local recurrence (from 26% to 47%) and occult nodal disease. 3 , 4
Despite the benefits of the minimally invasive approaches for the surgical treatment of rectal cancer, 5 an high risk of perioperative complications, permanent stoma, and functional impairments are still associated with radical surgery. 6 , 7 , 8 In order to decrease morbidity related to major surgery, the use of organ‐preserving strategies based on a multidisciplinary approach is gaining support among surgeons. 9 , 10 Indeed, the possibility of avoiding a Total mesorectal excision (TME) with neoadjuvant chemoradiotherapy (CRT) followed by LE has been evaluated in the setting of clinical trials 11 , 12 with similar oncological outcomes, or for patients unfit for surgery or refusing permanent ostomy.
T2 rectal cancer patients are probably the best candidate for this approach because smaller and more superficial cancers are more likely to exhibit better response to CRT and they are less likely to develop a tumor regrowth than ≥ cT3 tumors once they have achieved a complete clinical response. 13 , 14 Downstaging and downsizing of the rectal lesion provide the opportunity to subsequently perform an LE to obtain a pathological evaluation of residual tumor with a low risk of nodes involvement.
Previous systematic reviews evaluated outcomes of LE after CRT 15 , 16 , 17 ; however, they included studies with any preoperative tumor stage and merged results. Therefore, we aim to conduct a systematic review of the literature in order to assess long‐term outcomes of LE following CRT in T2 rectal cancer patients exclusively. Evaluating the safety and effectiveness of the conservative management for cT2 rectal tumors could help shift the paradigm in favor of less invasive procedures without the postoperative inconveniences of major surgery.
Methods
Literature search and selection of primary studies
A systematic review of the existing literature was conducted in accordance with the preferred reporting items for systematic reviews and meta‐analyses (PRISMA) guidelines 18 to establish the evidence base regarding the use of neoadjuvant CRT followed by LE for the treatment of cT2 rectal tumors.
The systematic literature search was performed in PubMed/Medline, Scopus, and Web of Science databases to identify studies reporting oncologic outcomes from the beginning of indexing for each database till October 31, 2021. Bibliographic review of selected articles was assessed as secondary sources for full‐length articles of studies. A literature search was performed using the following index terms: “T2 rectal cancer,” “early rectal cancer,” “neoadjuvant therapy,” “preoperative chemoradiotherapy,” “local excision,” “transanal endoscopic microsurgery,” “transanal minimally invasive surgery,” and “transanal excision.”
Eligibility criteria
Two reviewers (R. P. and M. M. D. N.) independently evaluated all the studies retrieved according to the eligibility criteria and any differences between the datasets were resolved by discussion. Studies were included if they met the following criteria: evaluation of oncologic outcomes in terms of recurrence rate or survival after neoadjuvant CRT followed by LE in not comparative or in comparative studies with conventional treatment for cT2 rectal cancer. We excluded the articles if there was no sufficient documentation, if data were combined with those of other tumor stages, if sample size was ≤ 10 patients and if they were in languages other than English. Narrative reviews, duplicate publications, editorials, and abstracts were also excluded.
Data extraction and management
Data were extracted independently and entered into standardized Excel spreadsheets (Microsoft Inc., Redmond, Washington, USA). Data were presented as frequencies and percentages. Any disagreements were resolved through discussion. The following data were extracted from each study: first author, study period, study design, inclusion and exclusion criteria, number of participants, mean age, type of surgery ad platforms for LE, surgery indication, neoadjuvant regimen, ypT0 rate, survival, recurrence, and mortality rates. Study outcomes included disease‐free survival or overall survival and local o distant recurrence rate.
Assessment of the methodological quality of studies
All studies were assessed for methodological quality. For randomized studies, the validated score described by Jadad et al. 19 was used. The scale consists of three items pertaining to descriptions of randomization, masking, and dropouts and withdrawals in the report of an RCT. The scale ranges from 0 to 5, with higher scores indicating better reporting. High‐quality trials scored more than 2 out of a maximum possible score of 5. Low‐quality trials scored 2 or less out of a maximum possible score of 5. The Methodologic Index for Nonrandomized Studies (MINORS) 20 tool was also used to assess quality of the studies included in the review. For noncomparative studies, a maximum score of 16 could be achieved using the MINORS tool, with a maximum score of 24 available for comparative studies.
Statistical analysis
Statistical analyses were performed by using Comprehensive Meta‐analysis Software version 3.0 (Biostat, Englewood, New Jersey, USA). Heterogeneity was assessed by using chi‐squared statistics and I 2 measure of inconsistency.
A pooled analysis was conducted using a fixed‐effect model in the case of non‐significant heterogeneity (P > 0.1), and a random effect model (DerSimonian–Laird method) when significant heterogeneity was present (P < 0.1). Corresponding forest plots were constructed for the pooled estimates of the abovementioned outcomes and weight of individual studies are represented by the size of individual squares.
A P value < 0.05 was considered statistically significant for all outcomes.
Results
Figure 1 shows the PRISMA flow diagram of the literature selection process. The search strategy identified a total of 900 publications in the initial search. After the screening of title and abstract and removal of duplicates, 47 articles were selected for further review. After exclusion of 36 articles based on aforementioned criteria, 10 studies were initially included. 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 However, two of them evaluated the results of the same national database during the same period. 22 , 23 Therefore, we decided to exclude the study with fewer patients 23 from quantitative analysis.
Figure 1.
PRISMA flowchart outlining search strategy and selection of included studies.
Only three retrospective studies 22 , 23 , 24 and one RCT 21 compared neoadjuvant therapy and LE with radical surgery. The remaining six studies were prospective 25 , 26 and retrospectives 27 , 28 , 29 , 30 articles which investigated oncological outcomes of LE after neoadjuvant CRT. Two of them were multicenter studies. 26 , 29 One study 25 which considered T2 and T3s tumors was also included as T3s have the same conventional treatment as T2 cancers (TME).
Six 24 , 25 , 26 , 27 , 28 , 30 of 10 studies have as indication for LE of T2 rectal tumor patients who refuse major surgery, or patients with poor performance status or complete response after CRT. Details and quality assessment of the studies are showed in Table 1.
Table 1.
Characteristics of the studies included in the review
| Authors | Study type | Study period | Inclusion criteria | Neoadjuvant therapy | Surgical procedure | Quality assessment |
|---|---|---|---|---|---|---|
| Lezoche et al. 21 | RCT | 1997–2004 | cT2N0 < 3 cm, within 6 cm of a.v., G1–2, ASA I‐II | 50.4 Gy + 5‐FU | TEM vs TME | 3/5 |
| Jawitz et al. 22 | Retro | 2004–2015 | cT2N0M0 rectal cancer, > 18 yo | TEM vs TME | 16/24 | |
| Lee et al. 23 | Retro | 2004–2014 | cT2N0M0 | TEM vs TME | 16/24 | |
| Lynn et al. 24 | Retro |
2006–2009 1996–1999 |
cT2N0 < 4 cm, involving < 40% rectal circum, within 8 cm of a.v., ECOG Performance Status ≤ 2; pT2N0M0 within 8 cm of a.v. | 54 Gy, 50.4 Gy + CAPOX; Short‐course radiotherapy | TEM, TAE vs TME | 18/24 |
| Pericay et al. 25 | Pro | 2007–2013 | cT2‐T3sN0 rectal cancer, G1 or G2 who refused radical surgery | 45 Gy + 5FU or Capecitabine | TEM,TEO | 12/16 |
| Garcia‐Aguilar et al. 26 | Pro | 2006–2009 | cT2N0 < 4 cm, involving < 40% rectal circum, within 8 cm of a.v., ECOG Performance Status ≤ 2 | 54 Gy, 50.4 Gy + CAPOX | TEM, TAE | 12/16 |
| Noh et al. 27 | Retro | 2002–2009 | Patients refusal of radical surgery and poor performance status | 45 Gy, 50.4 Gy, 44 Gy + 5‐FU, Capecitabine, S1 + IRINOTECAN | NS | 10/16 |
| Shin et al. 28 | Retro | 2006–2014 | cT2N0M0 rectal cancer within 7 cm from the a.v., cCR or near cCR | 50.0 Gy + 5‐FU or Capecitabine | TAE, TAMIS | 10/16 |
| Yu et al. 29 | Retro | 2000–2009 | cT2N0M0 rectal cancer below 5 cm from a.v. | 50.4 Gy + 5‐FU or Capecitabine | NS | 10/16 |
| Guerrieri et al. 30 | Retro | 1992–2013 | cT2N0M0 rectal cancer < 3 cm, high‐risk patients (ASA III‐IV) or who refused conventional resection | 50.4 Gy + 5‐FU or Capecitabine | TEM | 12/16 |
Quality assessment was carried out using the MINORS score for non‐randomized studies and using a Jadad score for RCT.
RCT, randomized controlled trial; Retro, retrospective study; Pro, prospective study; ASA, American Society of Anesthesiology Score; a.v., anal verge; ECOG, Eastern Cooperative Oncology Group; cCR, complete clinical response; TEM, transanal endoscopic microsurgery; TAMIS, transanal minimally invasive surgery; TAE, transanal excision; NS, not specified; TME, total mesorectal excision.
Oncologic outcomes and pooled analysis
All studies which compared neoadjuvant therapy followed by transanal LE with transabdominal TME showed comparable survival outcomes for patients with cT2N0 rectal cancer (Table 2). In the only RCT, 21 5‐year DFS and OS was 89% and 72% for organ‐preserving treatment and 94% and 80% for radical surgery respectively, with no statistically difference. Likewise, local and distant recurrence rate did not differ between two groups (8% vs 6% and 4% vs 4%, respectively).
Table 2.
Summary of oncological outcomes
| Authors | Surgical procedure | No. of patients | Median age | ypT0 (%) | 5y‐DFS (%) | 5y‐OS (%) | 3y‐DFS (%) | 3y‐OS (%) | LR (%) | DR (%) | 30‐day mortality | 90‐day mortality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lezoche et al. 21 | NAT + LE | 50 | 66 (58–70) | 14 (28) | 89 | 72 | 4 (8) | 2 (4) | 0 | |||
| TME | 50 | 66 (60–69) | 13 (26) | 94 | 80 | 3 (6) | 2 (4) | 0 | ||||
| Jawitz et al. 22 | NAT + LE | 695 | 63 (16) | 77.7 | 5 (0.7) | 10 (1.4) | ||||||
| TME | 6629 | 66 (20) | 75.1 | 86 (1.3) | 176 (2.7) | |||||||
| Lynn et al. 24 | NAT + LE | 79 | 62.7 (11.24) | 38 (50) | 88.2 | 90.3 | 3 (4) | |||||
| TME | 79 | 64.4 (11.25) | 88.3 | 88.4 | 1 (1.3) | |||||||
| Pericay et al. 25 | NAT + LE | 15 | 76 (57–87) | 4 (26.7) | 91 | 73 | 0 | 1 (6.7) | ||||
| Garcia‐Aguilar et al. 26 | NAT + LE | 79 | 62 (30–83) | 38 (49) | 86.9 | 95.7 | 3 (4) | 5 (6) | ||||
| Noh et al. 27 | NAT + LE | 17 | 63 (38–79) | 10 (59) | 82 | 2 (11.7) | 2 (11.7) | |||||
| Shin et al. 28 | NAT + LE | 34 | 63.6 (36–83) | 19 (55.9) | 97.1 | 100 | 1 (2.9) | 1 (2.9) | ||||
| Yu et al. 29 | NAT + LE | 18 | 9 (50) | 0 | 1 (5.5) | |||||||
| Guerrieri et al. 30 | NAT + LE | 185 | 68 (60–74) | 63 (34.1) | 93 | 50 | 24 (13) a |
Local and distant recurrences.
DFS, disease‐free survival; DR, distant recurrence; LR, local recurrence; OS, overall survival.
One study retrospectively reviewed American National Cancer Database (NCDB) to determine the effect of LE with preoperative CRT and major surgery. There were no difference in OS (77.7% vs 75.1% 22 ) with similar rate in terms of 30‐ and 90‐day postoperative mortality.
Finally, Lynn et al. 24 compared 79 patients with cT2N0 rectal cancer treated with CRT and LE in ACOSOG Z6041 trial with a similar group of patients with pT2N0 tumors who underwent upfront TME in the Dutch TME trial. Even in this comparative study, no difference regarding 5‐year DFS (88.2% vs 88.3%), 5‐year OS (90.3% vs 88.4%) and LR rate (4% vs 1.3%) emerged between groups.
Among noncomparative studies, 3‐year DFS and OS rates were reported in three articles 25 , 26 , 28 ranging from 86.9% to 97.1% and from 73% to 100%, respectively. In the Korean Radiation Oncology Group (KROG) 12‐06 study, 27 5‐year DFS was 82% for cT2 rectal cancer patients who refused radical surgery. Similarly, Guerrieri et al. 30 had 93% 5‐year DFS and 50% 5‐year OS for stage T2 patients who underwent preoperative CRT and LE. Recurrence rate was reported in all noncomparative studies up to 11.7% of cases.
Complete pathological response (ypT0) rate after CRT and LE was reported in eight studies, 21 , 24 , 25 , 26 , 27 , 28 , 29 , 30 and it ranges from 26.7% to 59%.
The pooled 3‐year DFS, 5‐year DFS, 3‐year OS, 5‐year OS, local and distant recurrence rates were 92.8% (95% CI 81.6–99.5%), 91.3% (95% CI 88.3–94.3%), 96.1% (95% CI 90.5–100%), 72.6% (95% CI 57.5–87.7%), 4% (95%CI 18–63%) and 4.9% (95%CI 2–7.8%), respectively, in subjects treated with neoadjuvant therapy followed by transanal LE (Fig. 2). No heterogeneity was found for all these analyses, except for the 5‐year OS sub‐analysis (I 2 94%, P < 0.001).
Figure 2.
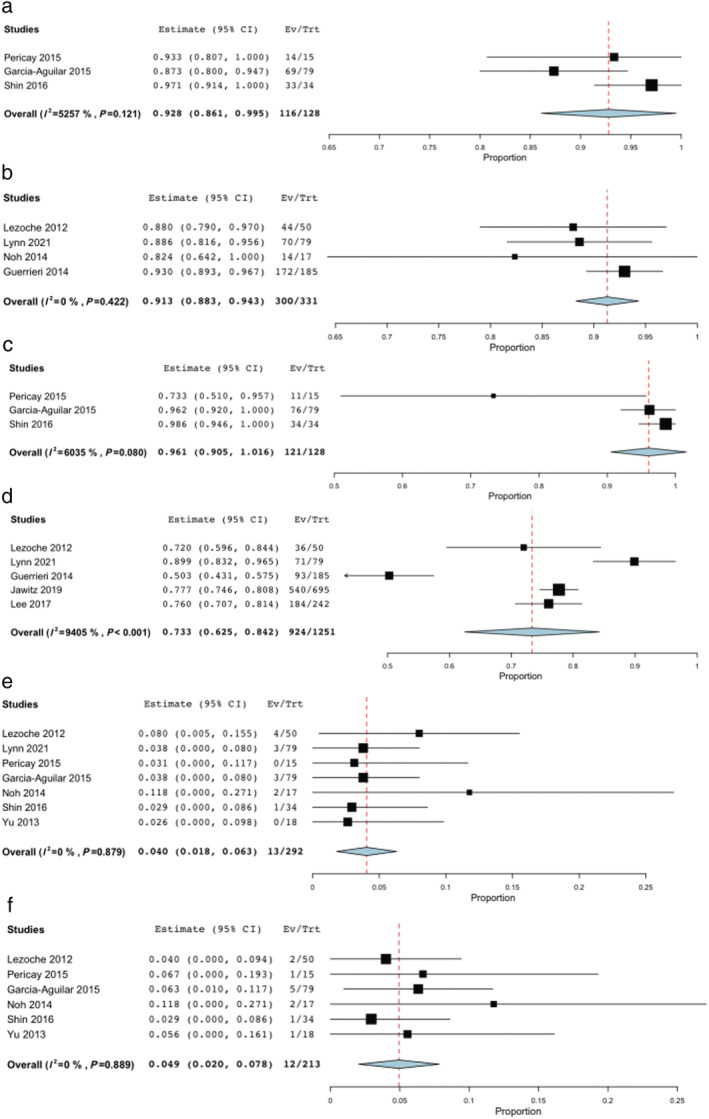
Pooled analysis for Local Excision. (a) 3‐year DFS. (b) 5‐year DFS. (c) 3‐year OS. (d) 5‐year OS. (e) Local Recurrence. (f) Distant Recurrence.
Discussion
The results of this review indicate that LE after CRT confers equivalent survival advantages as radical surgery in cT2 rectal cancer patients. Pooled 5‐year DFS rate was 91.3%, 5‐year OS was 73.3% and LR rate was 4% after treatment. Therefore, organ preservation seems a feasible alternative to TME in this setting.
Although local recurrences were less after TME in Dutch trial (1.3%), 5‐year DFS rate is similar (88.3%) while 5‐year OS is 88.4%. 24 , 31 This slight difference in OS is probably due to the inclusion of patients with more severe comorbidities (unfit for major surgery) in the conservative treatment group of the selected studies.
In last few years, attention on organ preservation strategies with multimodal approach (CRT and LE) has increased. GRECCAR 2 is a prospective randomized multicenter trial which compared TME with LE in both after NAT. A three‐step strategy was adopted to identify patients who can benefit from an organ‐preserving treatment: selection occurs first at the moment of the initial clinical staging, then at the restaging 8 weeks after CRT by pelvic MRI and finally at the evaluation of the pathological response. 32 The authors found no significant difference in terms of survival and recurrence rates between patients who had a good clinical response after chemoradiotherapy for small T2T3 low rectal cancer at 5‐year follow‐up. 12 Likewise, in CARTS study, including 55 patients with cT1‐3N0 tumor, organ preservation was achieved in 35 patients (64%) with acceptable long‐term oncological outcomes and health‐related quality of life. 11 In TREC trial, 55 patients with cT1T2N0 rectal cancer were randomly assigned to radical surgery group or short‐course radiotherapy and LE group. 33 Although primary endpoint was the feasibility of recruiting to a RCT comparing rectal‐sparing strategy with TME, no difference in DFS and OS between groups emerged assuring high level of organ preservation with relatively low morbidity.
Despite the encouraging results of the aforementioned studies, patient selection for LE after CRT is still challenging due to balance between the risk of undertreatment and surgical morbidity. Pelvic MRI is the preferred modality when a T2 or larger tumor is suspected because it has higher accuracy than endorectal ultra‐sound (ERUS) for detection of mesorectal infiltration. High‐resolution MRI staging allows to assess tumors infiltrating the muscolaris propria (T2), to measure the depth of extramural spread (T3a‐d) and to evaluate lymph node involvement. Recent technological advancements, e.g. diffusion‐weighted imaging (DWI) 34 and artificial intelligence‐based reconstructions, 35 may provide additional information to MRI and improve the accuracy of pre‐treatment staging. ERUS should typically be considered complementary to MRI for purposes of clinical staging, 34 and is most useful in differentiating between early T stages (i.e., T1 versus T2 tumors) or when MRI is contraindicated.
Upfront TME is standard for rectal tumors limited to the muscolaris propria (T2) with negative nodes 3 , 36 because the risk of recurrence after LE and of harboring occult nodal disease is not negligible. Indeed, compared with LE alone, radical surgery offers a significant decreased LR rate: 7% versus 13% in T2 rectal cancer. 37 However, 30‐day mortality rate after radical rectal excision is 2%, 6 and it increases in elderly patients up to 6% after 75 years and 12% above 85 years. 38 Furthermore, postoperative morbidity is present in 30–50% of cases 39 , 40 , 41 , 42 wih a significant adverse impact on quality of life, bowel, urinary, and sexual dysfunction, often including the need for a definitive or permanent stoma. 43 , 44 , 45 , 46 Thus, it is not surprising that LE may seem a desirable alternative in selected patients such as the elderly and frail patients if oncologic outcomes are not compromised. The main characteristics that patients should fulfill in order to benefit from neoadjuvant CRT and LE include tumors < 10 cm from the anal verge, ≤ 4 cm in diameter, with low risk of positive lymph nodes on MRI imaging, and with a good clinical response after nCRT. 47
Although both immunological and individual factors can contribute to bowel diseases, 48 , 49 preoperative CRT has certainly demonstrated its efficacy to decrease the local recurrence rate after rectal excision for locally advanced rectal cancer. 50 , 51 Usually, patients who have a good response after CRT have a better prognosis than those having a bad response. As found in the present study, complete pathological response (ypT0) rate in T2N0 is high: ≥ 50% in five studies 24 , 26 , 27 , 28 , 29 and between 27% and 34% in three studies. 21 , 25 , 30 Thus, in addiction to LE, this rectal‐preserving approach may avoid morbidity of major surgery without jeopardizing survival.
Furthermore, we assume that LE after neoadjuvant CRT in T2 cancers improves local control of the disease if compared with LE and adjuvant CRT. In fact, we found a pooled LR rate of 4% that is significantly lower than 12% 52 and 14% 53 reported by the Cancer and Leukemia Group B studies.
There are some limitations to our study. Few studies reported long‐term oncological outcomes of LE after neoadjuvant CRT for T2 rectal cancer. Three of them were comparative retrospective studies and only one RCT. Thus, it is difficult to interpret the results with accuracy. However, all studies show similar outcomes compared with conventional surgical treatment. The selection criteria for conservative strategy were not.
standardized among studies as well as neoadjuvant therapy regimen and technical aspect to perform LE. As the comparison of different cancer treatments should be made for similar staging, we focused on cT2N0 rectal lesions avoiding merging both early and advanced tumors results as reported elsewhere. 15 , 16 , 17 We are aware that further studies are warranted to confirm our findings. In this setting, preliminary results of a phase III multicenter RCT (NCT01308190) demonstrated no difference in terms of LR and DR between T2‐T3s cancers treated with CRT and LE compared with TME at 2‐year follow up. 54
Conclusion
LE following neoadjuvant CRT may provide comparable survival benefit to radical surgery for patients with clinical stage T2N0. This strategy may be considered as an alternative approach for patients unfit for major abdominal resection or who refuse surgery and it may also represent a viable treatment modality for all subjects. However, further studies are needed to draw firm conclusions.
Acknowledgment
Open Access Funding provided by Universita degli Studi della Campania Luigi Vanvitelli within the CRUI‐CARE Agreement.
Peltrini, R. , Imperatore, N. , Di Nuzzo, M. M. , and Pellino, G. (2022) Towards personalized treatment of T2N0 rectal cancer: A systematic review of long‐term oncological outcomes of neoadjuvant therapy followed by local excision. Journal of Gastroenterology and Hepatology, 37: 1426–1433. 10.1111/jgh.15898.
Declaration of conflict of interest: The authors have no conflicts of interest to disclose.
Financial support: No funding was received for the preparation of this manuscript.
References
- 1. Morino M, Risio M, Bach S et al. Early rectal cancer: the European Association for Endoscopic Surgery (EAES) clinical consensus conference. Surg. Endosc. 2015; 29: 755–773. [DOI] [PubMed] [Google Scholar]
- 2. Gollins S, Moran B, Adams R et al. Association of Coloproctology of Great Britain & Ireland (ACPGBI): Guidelines for the Management of Cancer of the Colon, Rectum and Anus (2017) ‐ Multidisciplinary Management. Colorectal Dis. 2017; 19: 37–66. [DOI] [PubMed] [Google Scholar]
- 3. You YN, Hardiman KM, Bafford A et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Rectal Cancer. Dis. Colon Rectum 2020; 63: 1191–1222. [DOI] [PubMed] [Google Scholar]
- 4. Halverson AL, Morris AM, Cleary RK, Chang GJ. For Patients with Early Rectal Cancer, Does Local Excision Have an Impact on Recurrence, Survival, and Quality of Life Relative to Radical Resection? Ann. Surg. Oncol. 2019; 26: 2497–2506. [DOI] [PubMed] [Google Scholar]
- 5. Peltrini R, Luglio G, Cassese G et al. Oncological Outcomes and Quality of Life After Rectal Cancer Surgery. Open Med (Wars) 2019; 14: 653–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Paun BC, Cassie S, MacLean AR, Dixon E, Buie WD. Postoperative complications following surgery for rectal cancer. Ann. Surg. 2010; 251: 807–818. [DOI] [PubMed] [Google Scholar]
- 7. Lange MM, van de Velde CJ. Urinary and sexual dysfunction after rectal cancer treatment. Nat. Rev. Urol. 2011; 8: 51–57. [DOI] [PubMed] [Google Scholar]
- 8. Floodeen H, Lindgren R, Hallböök O, Matthiessen P. Evaluation of long‐term anorectal function after low anterior resection: a 5‐year follow‐up of a randomized multicenter trial. Dis. Colon Rectum 2014; 57: 1162–1168. [DOI] [PubMed] [Google Scholar]
- 9. Beets GL, Figueiredo NL, Habr‐Gama A, van de Velde CJ. A new paradigm for rectal cancer: Organ preservation: Introducing the International Watch & Wait Database (IWWD). Eur. J. Surg. Oncol. 2015; 41: 1562–1564. [DOI] [PubMed] [Google Scholar]
- 10. Peltrini R, Sacco M, Luglio G, Bucci L. Local excision following chemoradiotherapy in T2‐T3 rectal cancer: current status and critical appraisal. Updates Surg. 2020; 72: 29–37. [DOI] [PubMed] [Google Scholar]
- 11. Stijns RCH, de Graaf EJR, Punt CJA et al. Long‐term Oncological and Functional Outcomes of Chemoradiotherapy Followed by Organ‐Sparing Transanal Endoscopic Microsurgery for Distal Rectal Cancer: The CARTS Study. JAMA Surg. 2019; 154: 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rullier E, Vendrely V, Asselineau J et al. Organ preservation with chemoradiotherapy plus local excision for rectal cancer: 5‐year results of the GRECCAR 2 randomised trial. Lancet Gastroenterol. Hepatol. 2020; 5: 465–474. [DOI] [PubMed] [Google Scholar]
- 13. Habr‐Gama A, São Julião GP, Gama‐Rodrigues J et al. Baseline T Classification Predicts Early Tumor Regrowth After Nonoperative Management in Distal Rectal Cancer After Extended Neoadjuvant Chemoradiation and Initial Complete Clinical Response. Dis. Colon Rectum 2017; 60: 586–594. [DOI] [PubMed] [Google Scholar]
- 14. Chadi SA, Malcomson L, Ensor J et al. Factors affecting local regrowth after watch and wait for patients with a clinical complete response following chemoradiotherapy in rectal cancer (InterCoRe consortium): an individual participant data meta‐analysis. Lancet Gastroenterol. Hepatol. 2018; 3: 825–836. [DOI] [PubMed] [Google Scholar]
- 15. Shaikh I, Askari A, Ourû S, Warusavitarne J, Athanasiou T, Faiz O. Oncological outcomes of local excision compared with radical surgery after neoadjuvant chemoradiotherapy for rectal cancer: a systematic review and meta‐analysis. Int. J. Colorectal Dis. 2015; 30: 19–29. [DOI] [PubMed] [Google Scholar]
- 16. Hallam S, Messenger DE, Thomas MG. A Systematic Review of Local Excision After Neoadjuvant Therapy for Rectal Cancer: Are ypT0 Tumors the Limit? Dis. Colon Rectum 2016; 59: 984–997. [DOI] [PubMed] [Google Scholar]
- 17. Smith FM, Ahad A, Perez RO, Marks J, Bujko K, Heald RJ. Local Excision Techniques for Rectal Cancer After Neoadjuvant Chemoradiotherapy: What Are We Doing? Dis. Colon Rectum 2017; 60: 228–239. [DOI] [PubMed] [Google Scholar]
- 18. PRISMA‐P Group , Moher D, Shamseer L et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst. Rev. 2015; 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jadad AR, Moore RA, Carroll D et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control. Clin. Trials 1996; 17: 1–12. [DOI] [PubMed] [Google Scholar]
- 20. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non‐randomized studies (minors): development and validation of a new instrument. ANZ J. Surg. 2003; 73: 712–716. [DOI] [PubMed] [Google Scholar]
- 21. Lezoche E, Baldarelli M, Lezoche G, Paganini AM, Gesuita R, Guerrieri M. Randomized clinical trial of endoluminal locoregional resection versus laparoscopic total mesorectal excision for T2 rectal cancer after neoadjuvant therapy. Br. J. Surg. 2012; 99: 1211–1218. [DOI] [PubMed] [Google Scholar]
- 22. Jawitz OK, Adam MA, Turner MC, Gilmore BF, Migaly J. Neoadjuvant chemoradiation followed by transanal local excision for T2 rectal cancer confers equivalent survival benefit as traditional transabdominal resection. Surgery 2019; 165: 1193–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee L, Kelly J, Nassif GJ et al. Chemoradiation and Local Excision for T2N0 Rectal Cancer Offers Equivalent Overall Survival Compared to Standard Resection: a National Cancer Database Analysis. J. Gastrointest. Surg. 2017; 21: 1666–1674. [DOI] [PubMed] [Google Scholar]
- 24. Lynn PB, van der Valk M, Claassen YHM et al. Chemoradiation and Local Excision versus Total Mesorectal Excision for T2N0 Rectal Cancer: Comparison of Short‐ and Long‐Term Outcomes from Two Prospective Studies. Ann. Surg. 2021; 2. 10.1097/SLA.0000000000005052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pericay C, Serra‐Aracil X, Ocaña‐Rojas J et al. Further evidence for preoperative chemoradiotherapy and transanal endoscopic surgery (TEM) in T2–3s,N0,M0 rectal cancer. Clin. Transl. Oncol. 2016; 18: 666–671. [DOI] [PubMed] [Google Scholar]
- 26. Garcia‐Aguilar J, Renfro LA, Chow OS et al. Organ preservation for clinical T2N0 distal rectal cancer using neoadjuvant chemoradiotherapy and local excision (ACOSOG Z6041): results of an open‐label, single‐arm, multi‐institutional, phase 2 trial. Lancet Oncol. 2015; 16: 1537–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Noh JM, Park W, Kim JS et al. Outcome of Local Excision Following Preoperative Chemoradiotherapy for Clinically T2 Distal Rectal Cancer: A Multicenter Retrospective Study (KROG 12‐06). Cancer Res Treat 2014; 46: 243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shin YS, Yoon YS, Lim SB et al. Preoperative chemoradiotherapy followed by local excision in clinical T2N0 rectal cancer. Radiat. Oncol. J. 2016; 34: 177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yu CS, Yun HR, Shin EJ et al. Local excision after neoadjuvant chemoradiation therapy in advanced rectal cancer: a national multicenter analysis. Am. J. Surg. 2013; 206: 482–487. [DOI] [PubMed] [Google Scholar]
- 30. Guerrieri M, Gesuita R, Ghiselli R, Lezoche G, Budassi A, Baldarelli M. Treatment of rectal cancer by transanal endoscopic microsurgery: experience with 425 patients. World J. Gastroenterol. 2014; 20: 9556–9563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kapiteijn E, Marijnen CA, Nagtegaal ID et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N. Engl. J. Med. 2001; 345: 638–646. [DOI] [PubMed] [Google Scholar]
- 32. Rullier E, Rouanet P, Tuech JJ et al. Organ preservation for rectal cancer (GRECCAR 2): a prospective, randomised, open‐label, multicentre, phase 3 trial. Lancet 2017; 390: 469–479. [DOI] [PubMed] [Google Scholar]
- 33. Bach SP, Gilbert A, Brock K et al. Radical surgery versus organ preservation via short‐course radiotherapy followed by transanal endoscopic microsurgery for early‐stage rectal cancer (TREC): a randomised, open‐label feasibility study. Lancet Gastroenterol. Hepatol. 2021; 6: 92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Reginelli A, Clemente A, Sangiovanni A et al. Endorectal Ultrasound and Magnetic Resonance Imaging for Rectal Cancer Staging: A Modern Multimodality Approach. J. Clin. Med. 2021; 10: 641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Garcia‐Granero A, Pellino G, Giner F et al. A mathematical 3D‐method applied to MRI to evaluate prostatic infiltration in advanced rectal cancer. Tech. Coloproctol. 2020; 24: 605–607. [DOI] [PubMed] [Google Scholar]
- 36. Glynne‐Jones R, Wyrwicz L, Tiret E et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann. Oncol. 2017; 28: iv22‐iv40. [DOI] [PubMed] [Google Scholar]
- 37. You YN, Baxter NN, Stewart A, Nelson H. Is the increasing rate of local excision for stage I rectal cancer in the United States justified?: a nationwide cohort study from the National Cancer Database. Ann. Surg. 2007; 245: 726–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Simmonds PD et al. Surgery for colorectal cancer in elderly patients: a systematic review. Lancet 2000; 356: 968–974. [PubMed] [Google Scholar]
- 39. Penninckx F, Kartheuser A, Van de Stadt J et al. Outcome following laparoscopic and open total mesorectal excision for rectal cancer. Br. J. Surg. 2013; 100: 1368–1375. [DOI] [PubMed] [Google Scholar]
- 40. Påhlman L, Bohe M, Cedermark B et al. The Swedish rectal cancer registry. Br. J. Surg. 2007; 94: 1285–1292. [DOI] [PubMed] [Google Scholar]
- 41. Peltrini R, Imperatore N, Carannante F et al. Age and comorbidities do not affect short‐term outcomes after laparoscopic rectal cancer resection in elderly patients. A multi‐institutional cohort study in 287 patients. Updates Surg. 2021; 73: 527–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. EuroSurg Collaborative . Body mass index and complications following major gastrointestinal surgery: a prospective, international cohort study and meta‐analysis. Colorectal Dis. 2018; 20: O215–O225. [DOI] [PubMed] [Google Scholar]
- 43. Hendren SK, O'Connor BI, Liu M, Asano T, Cohen Z, Swallow CJ et al. Prevalence of male and female sexual dysfunction is high following surgery for rectal cancer. Ann. Surg. 2005; 242: 212–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Engel J, Kerr J, Schlesinger‐Raab A, Eckel R, Sauer H, Hölzel D. Quality of life in rectal cancer patients: a four‐year prospective study. Ann. Surg. 2003; 238: 203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wallner C, Lange MM, Bonsing BA et al. Causes of fecal and urinary incontinence after total mesorectal excision for rectal cancer based on cadaveric surgery: a study from the Cooperative Clinical Investigators of the Dutch total mesorectal excision trial. J. Clin. Oncol. 2008; 26: 4466–4472. [DOI] [PubMed] [Google Scholar]
- 46. Celerier B, Denost Q, Van Geluwe B, Pontallier A, Rullier E. The risk of definitive stoma formation at 10 years after low and ultralow anterior resection for rectal cancer. Colorectal Dis. 2016; 18: 59–66. [DOI] [PubMed] [Google Scholar]
- 47. Rullier E, Denost Q. Transanal surgery for cT2T3 rectal cancer: Patient selection, adjuvant therapy, and outcomes. Semin. Colon Rectal Surgery 2015; 26: 26–31. [Google Scholar]
- 48. Gausman V, Dornblaser D, Anand S, Hayes RB, O'Connell K, Du M, Liang PS. Risk Factors Associated With Early‐Onset Colorectal Cancer. Clin. Gastroenterol. Hepatol. 2020; 18: 2752–2759.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fina D, Franzè E, Rovedatti L et al. Interleukin‐25 production is differently regulated by TNF‐α and TGF‐β1 in the human gut. Mucosal Immunol. 2011; 4: 239–244. [DOI] [PubMed] [Google Scholar]
- 50. Sauer R, Becker H, Hohenberger W et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N. Engl. J. Med. 2004; 351: 1731–1740. [DOI] [PubMed] [Google Scholar]
- 51. Bosset JF, Collette L, Calais G et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N. Engl. J. Med. 2006; 355: 1114–1123. [DOI] [PubMed] [Google Scholar]
- 52. Greenberg JA, Shibata D, Herndon JE 2nd, Steele GD Jr, Mayer R, Bleday R. Local excision of distal rectal cancer: an update of cancer and leukemia group B 8984. Dis. Colon Rectum 2008; 51: 1185–1191 discussion 1191‐4. [DOI] [PubMed] [Google Scholar]
- 53. Steele GD Jr, Herndon JE, Bleday R et al. Sphincter‐sparing treatment for distal rectal adenocarcinoma. Ann. Surg. Oncol. 1999; 6: 433–441. [DOI] [PubMed] [Google Scholar]
- 54. Serra‐Aracil X, Mora L, Pericay C, Delgado S. Phase III multicenter, prospective, controlled, randomized trial to evaluate the safety and efficacy of treatment of rectal cancer T2–T3S (superficial) N0, M0 with preoperative chemoradiotherapy and transanal endoscopic microsurgery versus total mesorecta. Dis. Colon Rectum 2015; 58: e323–e324. [Google Scholar]


