Abstract
Aim
To analyse patient factors and nurse staffing‐related issues involving hospital‐acquired pressure ulcers in patients at two types of hospital.
Background
Hospital‐acquired pressure ulcers are important for the safety of hospitalized patients. Hospital‐acquired pressure ulcers not only cause health problems, but also pose an economic burden to patients. In addition to patient factors such as mobility and skin integrity, hospital factors such as nurse staffing can also affect the management of such patients.
Methods
This study is a retrospective review of patient data and analysis of factors related to hospital‐acquired pressure ulcers using stratified Cox proportional hazards regression.
Results
A total of 53,923 patients were included. The incidence of hospital‐acquired pressure ulcers was 0.98 per 1,000 days. Hospital‐acquired pressure ulcers were affected by gender, age, previous falls, low oxygen levels, positioning and toilet use. When the levels of nurse staffing were determined as one of the hospital factors, the daily hours of patient care was increased thereby contributing to the reduced incidents of hospital‐acquired pressure ulcers.
Conclusion
Strategies for preventing hospital‐acquired pressure ulcers should be based on the analysis of risk factors.
Implications for Nursing Management
Most individual risk factors for hospital‐acquired pressure ulcers identified cannot be modified easily in a short time. Nurse staffing should be set at adequate levels to prevent hospital‐acquired pressure ulcers.
Keywords: hours per patient‐day, incidence, nurse staffing, pressure ulcer, risk factor
1. INTRODUCTION
Pressure ulcers (PUs) are localized skin injuries that can result in serious health problems (National Pressure Ulcer Advisory Panel, 2014). It is associated with extended hospital stays and substantial economic burden for patients (Bauer, Rock, Nazzal, Jones, & Qu, 2016). The incidence of hospital‐acquired pressure ulcers (HAPUs) is approximately 1.8%–14% (Bauer et al., 2016; Chaboyer et al., 2016; Fu Shaw, Chang, Lee, Kung, & Tung, 2014; Schneider & Geraedts, 2016). It varies depending on the detection methodology used. A HAPU is a preventable adverse event. While not all stages of HAPUs are direct causes of death, a deep HAPU is a strong risk factor for death (Brown, 2003; Khor et al., 2014). Thus, it is imperative to prevent the occurrence of HAPUs through various strategies.
Risk factors for HAPUs can be divided into patient factors and hospital factors. The most influential patient factor is restricted mobility (de Azevedo Macena et al., 2017; Li et al., 2019; Primiano et al., 2011). Increasing age, multiple comorbidities and poor nutritional status are other risk factors of HAPUs (Alhaug, Gay, Henriksen, & Lerdal, 2017; Cox & Rasmussen, 2014; Roberts, Chaboyer, & Desbrow, 2015). Hospital factors include health care types and nursing staff. Of different types of health care, nursing homes have higher incidents of PU, suggesting that patients admitted to nursing homes are more vulnerable to PUs (Bates‐Jensen, McCreath, & Pongquan, 2009; Cai, Mukamel, & Temkin‐Greener, 2010). However, structural issues such as staffing and facilities can also affect the risk of PU development. A number of studies have shown a direct relationship between nurse staffing and patient outcomes such as PUs, falls, infections and other safety indicators (Bae, Kelly, Brewer, & Spencer, 2014; Cho, Chin, Kim, & Hong, 2016; Kim & Bae, 2018; Kim, Kim, Park, & Lee, 2019; Schneider & Geraedts, 2016). The higher the level of nurse staffing, the lower the risk for PU development (Cho, Lee, June, Hong, & Kim, 2016; Choi & Staggs, 2014; Stalpers, de Brouwer, Kaljouw, & Schuurmans, 2015).
Even if nurse staffing has a direct influence on patient safety, hospitals in Korea cannot secure adequate numbers of nurses for inpatient care (OECD, 2018). Each Korean nurse cares for more patients than each nurse in some advanced countries (Aiken et al., 2012; Cho, Lee, et al., 2016). Hence, inpatients who are elderly or restricted in mobility would need a personal caregiver such as a family member or a paid informal caregiver during their hospital stay and treatment. However, economic burden involving the hiring of private caregivers and the low quality of care requires a resolution at a government level. To address this issue, the Korean government revised the nurse staffing policy in 2013 and designated an integrated nursing unit among existing nursing units. An integrated nursing unit, also known as the new inpatient care system in Korea, is established to provide all services by nursing staffs whose number is more than twice compared to that in a general nursing unit. Patients who do not have caregiver could be admitted to this nursing unit, irrespective of their medical department. The staffing standards in this unit differ according to hospital types and characteristics. The number of nursing staffs ranges from 5 to 12 patients cared for by a single registered nurse (RN) and from 25 to 40 patients cared for by a single nursing assistant (NA; Kim, Kim, Park, Jeong, & Lee, 2017).
It is difficult to completely heal HAPUs that occur during hospitalization in a short period of time. Even if HAPUs are completely healed, the recurrence of PU is high (Kuwahara et al., 2005). Therefore, risk factors affecting the development and recurrence of HAPUs during hospitalization must be evaluated. Known risk factors for HAPU development include immobility and impaired skin integrity (Clements, Moore, Tribble, & Blake, 2014; Coleman et al., 2013; Garcia‐Mayor et al., 2018; Li et al., 2019; Primiano et al., 2011). Several assessment tools such as the Braden Scale and the Waterlow scale have been developed to predict the risk of HAPUs (Pancorbo‐Hidalgo, Garcia‐Fernandez, Lopez‐Medina, & Alvarez‐Nieto, 2006). However, such risk assessment tools for HAPUs are limited to patient factors. In case of hospital factors, high nurse staffing has been reported to be a significant factor for the development of HAPUs (Schneider & Geraedts, 2016; Stalpers et al., 2015). However, previous studies did not include various patient factors. Hence, factors affecting HAPU development have not been analysed fully. Especially, no study has included both patient and hospital factors in the same model. To clearly predict and prevent HAPUs, it is necessary to consider not only patient factors, but also hospital factors. Therefore, the objective of this study was to investigate patient and nurse staffing factors related to HAPU development, focusing on the effectiveness of nurse staffing policy.
2. METHODS
This was a retrospective cohort study. This study included patients who were admitted to integrated nursing units in Korea from April 2017 to June 2017 to identify the incidence and factors contributing to HAPU development. As these data were extracted from the National Health Insurance (NHI) in Korea, this research was certified as exempted by the IRB (No. E1710/002‐003).
2.1. Sample and data sources
We initially included all patients who were hospitalized and discharged from an integrated nursing unit for 3 months. Next, we investigated HAPU incidence and factors related to the newly acquired PUs, excluding patients who already had PUs on their first day of hospitalization. A total of 53,923 patients admitted to 175 hospitals were included in this study. We reviewed the hospitals and patients' data included in the NHI database. Hospital data consist of the type of hospital (tertiary or general) and nurse staffing in a nursing unit. Patient's data consist of gender, age, main disease, level of nursing needs and activities of daily living (ADL). We merged the hospital and patients' data collected daily during patients' hospitalization.
2.2. Variables
Hospital‐acquired pressure ulcer incidence was calculated as the number of PUs per 1,000 patient‐days. PUs were determined and categorized by nurses according to the guidelines stipulated by the National Pressure Ulcer Advisory Panel (National Pressure Ulcer Advisory Panel, 2014). Accordingly, PUs were divided into four types, ranging from the most superficial PU (stage 1) to the deepest PU (stage 4; National Pressure Ulcer Advisory Panel, 2014).
Hospital data used in this study include the hospital type and nursing staff including RNs and NAs. Tertiary and general hospitals differ according to organisation and patient. Therefore, the nurse staffing standards also vary according to the type of hospital. The RN‐to‐patient ratio ranged from 1:5 to 1:7 in a tertiary hospital and from 1:7 to 1:12 in a general hospital. The NA‐to‐patient ratio varied from 1:30 to 1:40 in a tertiary hospital and from 1:25 to 1:40 in a general hospital. The nurse‐to‐patient ratio was converted into hours per patient‐day (HPPD), dividing the total number of working hours in a day by the number of nurses in charge.
We extracted the patients' age, gender and the purpose of hospital visit, that is, the need for surgery or surgical procedures and the main diagnosis. Moreover, we used health status data such as the level of nursing needs and assistance for activities of daily living (ADL). These data were collected daily during hospitalization. The level of nursing needs was evaluated using 10 indicators and assessed according to the criteria. The score is high if patients meet several criteria. Among the indicators monitored, four indicators were based on performance frequency. These four indicators were: vital signs (7 times/day), intake and output (4 times/day), medication via intravenous route (IV; 6 times/day) and medication via other routes (subcutaneous, intramuscular or intradermal but not oral medication, 6 times/day). The other six indicators were assessed depending on whether or not the nurse provided services such as suction, monitoring, oxygen saturation analysis, drainage care, application of restraints and professional care such as transfusion, inotropic or anti‐cancer therapy. The ADL was assessed by the degree of functional ability required to move in bed (positioning), ambulate, use toilet facilities and eat. The score in each area ranged from 0 to 2, indicating three levels: independence (requires no assistance), partial dependence (some assistance is needed) and total dependence (complete assistance is needed).
2.3. Analysis
We conducted descriptive statistical analysis to report characteristics of hospitals and patients. The HAPU incidence was calculated as HAPUs per 1,000 patient‐days. HAPU stages were reported as frequencies and percentages. Nurse staffing was reported as means and SD of HPPD. It was calculated according to nurse staff‐to‐patient ratio.
Survival analysis was conducted to estimate the cumulative probability of HAPUs and investigate risk factors related to HAPU development. In survival analysis, HAPU development represented the occurrence of the event. The duration of hospitalization was indicated by the timing of the event. First, the Kaplan–Meier hazard curve was used to show differences in the probability of HAPU according to hospital type. Next, we conducted log‐rank tests to analyse the difference between two curves. The Cox proportional hazards regression was used to correlate the probability of HAPUs with covariates. Stata/SE version 14 was used for all statistical analyses. Before analysis, we computed and plotted the logarithm of the cumulative baseline hazards for predictors to assess the proportional hazard assumptions. In the case of hospital type (tertiary and general hospitals), the resulting curves were not parallel. The proportional hazard assumptions of other predictors were not violated (Mehrotra, Su, & Li, 2012). We also conducted stratified Cox proportional hazards regression analysis which treated hospital types as strata (Mehrotra et al., 2012; Oakes & Feng, 2010) to increase the accuracy of estimation and determine the effect of coefficient of patient factors and nurse staffing on HAPU. Univariate regression analysis with independent variables was conducted prior to multiple analysis. Variables with p < .15 in the univariate analysis were selected for the multivariate model. Results of the stratified Cox regression analysis are reported as hazard ratios and with 95% confidence intervals.
3. RESULTS
3.1. Patients' characteristics
A total of 53,923 patients admitted to tertiary and general hospitals in Korea were included in this study. Their characteristics are summarized in Table 1. The proportion of women was higher than that of men in general hospitals, while the proportion of men was higher in tertiary hospitals. The mean age of patients was 61.49 years. The age of patients in tertiary hospitals was higher than those admitted to general hospitals. Approximately 50% of patients in tertiary hospitals were admitted for treatment of neoplasms. Most (90.5%) patients were admitted because of a surgical procedure. The mean score of nursing needs of patients admitted to tertiary hospitals was 0.824, which was higher than that of patients in general hospitals (0.528). Among the 10 categories of nursing needs, professional care accounted for 27.1% was the highest in tertiary hospitals, followed by vital sign monitoring, IV medication, input and output assessments. For patients admitted to general hospitals, vital sign monitoring was the most frequently provided nursing care service, followed by IV medication and professional care. Dependence on assistance for ADL was also high in patients at tertiary hospitals. A higher number of patients showed partial dependence in four areas: ambulation (43.7%), eating (36.7%), toilet use (26.4%) and positioning in bed (24.3%). Patients were hospitalized for 7.58 days on average. The length of stay was shorter in tertiary hospitals (7.00 days) than in general hospitals (7.71 days).
TABLE 1.
Characteristics of inpatients at tertiary and general hospitals
| Total (n = 53,923) | Tertiary hospital (n = 10,370) | General hospital (n = 43,553) | |
|---|---|---|---|
| Gender | |||
| Women | 29,406 (54.5) | 4,583 (44.2) | 24,823 (57.0) |
| Men | 24,517 (45.5) | 5,787 (55.8) | 18,730 (43.0) |
| Age, mean ± SD | 61.49 ± 19.96 | 62.31 ± 16.73 | 61.29 ± 20.65 |
| Disease | |||
| Neoplasm | 10,074 (18.7) | 5,202 (50.2) | 4,872 (11.2) |
| Injury | 7,934 (14.7) | 475 (4.6) | 7,459 (17.1) |
| Musculoskeletal | 6,299 (11.7) | 474 (4.6) | 5,825 (13.4) |
| Digestive | 6,283 (11.7) | 1,269 (12.2) | 5,014 (11.5) |
| Respiratory | 4,722 (8.8) | 693 (6.7) | 4,029 (9.3) |
| Others | 18,611 (34.5) | 2,257 (21.8) | 16,354 (37.5) |
| Surgery/procedure | |||
| Yes | 48,779 (90.5) | 9,962 (96.1) | 38,817 (89.1) |
| No | 5,141 (9.5) | 407 (3.9) | 4,734 (10.9) |
| Nursing needs, mean ± SD | 0.585 ± 1.020 | 0.824 ± 1.190 | 0.528 ± 0.967 |
| Vital sign check | 6,860 (15.8) | 1,860 (17.9) | 5,000 (9.3) |
| I&O check | 2,662 (6.1) | 1,052 (10.1) | 1,610 (3.0) |
| Monitoring | 2,550 (5.9) | 443 (4.3) | 2,107 (3.9) |
| SpO2 monitoring | 2,523 (5.8) | 465 (4.5) | 2,058 (3.8) |
| Suction | 279 (0.6) | 54 (0.5) | 225 (0.4) |
| IV medication | 5,803 (13.3) | 1,305 (12.6) | 4,498 (8.3) |
| Other medication | 1,104 (2.5) | 69 (0.7) | 1,035 (1.9) |
| Drainage care | 1,793 (4.1) | 397 (3.8) | 1,396 (2.6) |
| Restraint apply | 684 (1.6) | 91 (0.9) | 593 (1.1) |
| Professional care | 7,300 (16.8) | 2,809 (27.1) | 4,491 (8.3) |
| ADL dependence, mean ± SD | 1.67 ± 2.25 | 1.84 ± 2.32 | 1.63 ± 2.23 |
| Position | |||
| Partial | 9,209 (17.1) | 2,436 (23.5) | 6,773 (15.6) |
| Total | 3,880 (7.2) | 795 (7.7) | 3,085 (7.1) |
| Ambulating | |||
| Partial | 17,324 (32.1) | 3,652 (35.2) | 13,672 (31.4) |
| Total | 6,253 (11.6) | 1,129 (10.9) | 5,124 (11.8) |
| Eating | |||
| Partial | 16,434 (30.5) | 3,168 (30.5) | 13,266 (30.5) |
| Total | 3,326 (6.2) | 739 (7.1) | 2,587 (5.9) |
| Toilet use | |||
| Partial | 8,144 (15.1) | 2,215 (21.4) | 5,929 (13.6) |
| Total | 6,113 (11.3) | 1,128 (10.9) | 4,985 (11.4) |
| Length of stay, mean ± SD | 7.58 ± 6.38 | 7.00 ± 5.82 | 7.71 ± 6.49 |
Partial dependent patients need some assistance, and total dependent patients need complete assistance.
Abbreviations: ADL, activities of daily living; I&O, input and output; IV, intravenous; SD, standard deviation; SpO2, saturation of oxygen.
3.2. HPPD by RN and NA, and pressure ulcer incidence
Hours per patient‐day data by RNs and NAs from 175 hospitals are shown in Table 2. The mean RN‐HPPD was 2.86 hr, which was higher in tertiary hospitals (4.09 hr) than in general hospitals (2.59 hr). In contrast to RN‐HPPD, the mean NA‐HPPD was 0.78 hr. This was higher in general hospitals. The HAPU incidence in this study was 0.98 cases per 1,000 patient‐days. Tertiary hospitals showed higher incidence (1.05/1,000 patient‐days) than general hospitals (0.97/1,000 patient‐days). Stage 1 HAPUs and stage 2 HAPUs accounted for 35.2% and 64.8% of all PU cases, respectively. The proportion of stage 2 HAPUs was higher in tertiary hospitals (73.7%) than in general hospitals (62.8%).
TABLE 2.
Hours per patient‐days and pressure ulcer incidence
| Total (n = 175) | Tertiary hospital (n = 38) | General hospital (n = 137) | |
|---|---|---|---|
| RN‐HPPD, mean ± SD | 2.86 ± 0.74 | 4.09 ± 0.35 | 2.59 ± 0.31 |
| NA‐HPPD, mean ± SD | 0.78 ± 0.11 | 0.75 ± 0.09 | 0.79 ± 0.10 |
| Pressure ulcer per 1,000 days, mean ± SD | 0.98 ± 1.55 | 1.05 ± 1.72 | 0.97 ± 1.50 |
| Case of pressure ulcer, n (%) | 401 (100.0) | 76 (100.0) | 325 (100.0) |
| Stage 1 | 141 (35.2) | 20 (26.3) | 121 (37.2) |
| Stage 2 | 260 (64.8) | 56 (73.7) | 204 (62.8) |
Stage 1 pressure ulcers only affect the upper layer of skin. Stage 2 pressure ulcers go deeper below the surface of the skin.
Abbreviations: HPPD, hours per patient‐days; NA, nursing assistant; RN, registered nurse; SD, standard deviation.
3.3. Cumulative probability of pressure ulcer and its influencing factors
The cumulative probability of a PU during a hospital stay is presented in Figure 1. When patients were hospitalized longer, they were more likely to have new PU development. According to hospital type, there was no significant difference in cumulative probability (χ 2 = 0.51, p = .475).
FIGURE 1.
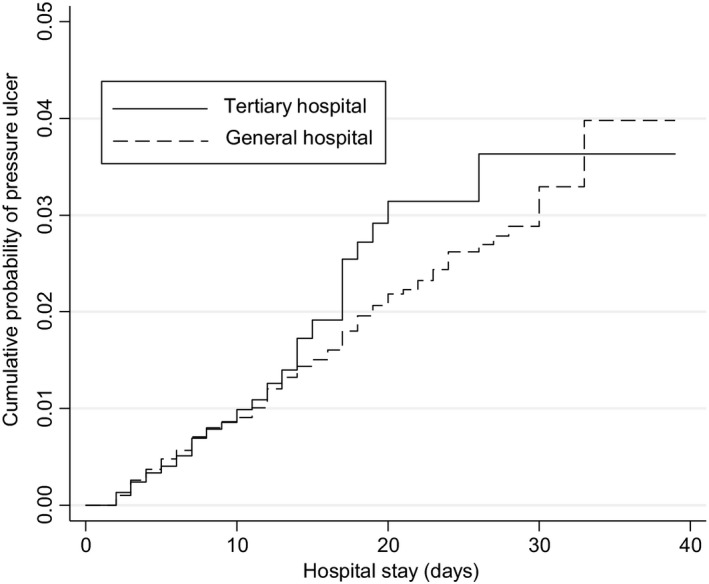
Cumulative probability of pressure ulcer. Note: Log‐rank test for equality of survivor functions χ 2 = 0.51 (p = .475)
Results of Cox proportional hazards regression survival analyses are shown in Table 3. Based on univariate analysis, a PU is more likely to develop in male and elderly patients. Moreover, there was a high probability of PU development after patient fell (HR = 19.55, p < .001). Most variables of nursing needs and ADL dependence were significantly associated with HAPU development. Professional care alone was not significantly associated with HAPU development. In staffing, only RN‐HPPD was significantly associated with HAPU in univariate analysis.
TABLE 3.
Cox proportional hazards regression analysis of development of pressure ulcers
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | p‐Value | Hazard ratio | 95% CI | p‐Value | |
| Gender (ref: female) | 1.36 | 1.12–1.65 | .002 | 1.54 | 1.26–1.88 | <.001 |
| Age | 1.01 | 1.00–1.01 | <.001 | 1.00 | 1.00–1.01 | .017 |
| Surgery/procedure | 0.95 | 0.67–1.36 | .787 | |||
| Fall | 19.55 | 10.08–37.93 | <.001 | 18.94 | 9.69–37.02 | <.001 |
| Nursing needs | ||||||
| Vital sign check | 2.45 | 1.95–3.07 | <.001 | 0.99 | 0.77–1.27 | .949 |
| I&O check | 2.57 | 1.92–3.43 | <.001 | 1.29 | 0.96–1.75 | .091 |
| Monitoring | 2.37 | 1.71–3.28 | <.001 | 0.84 | 0.59–1.21 | .351 |
| SpO2 monitoring | 4.82 | 3.82–6.06 | <.001 | 1.98 | 1.53–2.57 | <.001 |
| Suction | 4.80 | 3.01–7.64 | <.001 | 0.92 | 0.57–1.51 | .751 |
| IV medication | 2.12 | 1.67–2.68 | <.001 | 1.21 | 0.94–1.55 | .134 |
| Other medication | 2.00 | 1.36–2.95 | <.001 | 1.37 | 0.92–2.03 | .122 |
| Drainage care | 3.66 | 2.83–4.74 | <.001 | 1.22 | 0.93–1.61 | .158 |
| Use of restraints | 4.80 | 3.47–6.65 | <.001 | 0.94 | 0.66–1.33 | .722 |
| Professional care | 1.18 | 0.92–1.51 | .193 | |||
| ADL dependence (ref: independence) | ||||||
| Position | ||||||
| Partial | 5.55 | 4.19–7.35 | <.001 | 3.37 | 2.27–5.01 | <.001 |
| Total | 16.16 | 12.40–21.04 | <.001 | 5.84 | 3.63–9.40 | <.001 |
| Ambulating | ||||||
| Partial | 2.18 | 1.59–2.99 | <.001 | 0.66 | 0.44–1.00 | .049 |
| Total | 11.00 | 8.26–14.64 | <.001 | 0.68 | 0.42–1.09 | .110 |
| Eating | ||||||
| Partial | 3.24 | 2.48–4.22 | <.001 | 1.32 | 0.96–1.82 | .085 |
| Total | 11.67 | 8.90–15.30 | <.001 | 1.25 | 0.85–1.84 | .265 |
| Toilet use | ||||||
| Partial | 3.51 | 2.55–4.82 | <.001 | 1.73 | 1.13–2.65 | .012 |
| Total | 12.92 | 10.00–16.70 | <.001 | 3.25 | 2.05–5.14 | <.001 |
| Staffing | ||||||
| RN‐HPPD | 0.47 | 0.34–0.67 | <.001 | 0.20 | 0.13–0.30 | <.001 |
| NA‐HPPD | 0.45 | 0.19–1.08 | .073 | 0.12 | 0.04–0.34 | <.001 |
Partial dependent patients need some assistance. Total dependent patients need complete assistance.
Abbreviations: ADL, activities of daily living; CI, confidence interval; HPPD, hours per patient‐day; NA, nursing assistant; RN, registered nurse.
Multivariate analysis also showed that new HAPUs are more likely to develop in male and elderly patients. Patients sustaining falls are 18.94 times more likely to develop HAPUs compared with patients without falls. In cases requiring several types of nursing care, most of them were not associated with HAPUs in multivariate analysis. Only oxygen saturation (SpO2) monitoring was a significant factor in PU development. Hence, patients with low oxygen levels are 1.98 times more likely to develop HAPUs (p < .001). Among different ADLs, dependence on bed positioning and toilet use was significantly associated with HAPUs. According to the level of dependence, fully dependent patients are more likely to develop PUs than partially dependent patients. The risk for HAPU development was 5.84‐fold higher for patients fully dependent on assistance for bed positioning compared to patients without such need. In multivariate analysis, HPPD by RNs and NAs was a significant factor in developing HAPUs. HAPUs are less likely to develop during higher levels of RN‐HPPD (HR = 0.20, p < .001) and NA‐HPPD care (HR = 0.12, p < .001).
4. DISCUSSION
This study investigated the patient characteristics and nursing needs associated with HAPU development. The HAPU incidence was 0.98 per 1,000 patient‐days, which was lower than the results of previous studies (Bauer et al., 2016; Fu Shaw et al., 2014; Schneider & Geraedts, 2016). The HAPU incidence, which might be under‐reported, varies with the detection methodology used. Regardless of the used criteria, the incidence of HAPUs was extremely low which can be attributed to the focus on the outcome of integrated nursing units, where nurses monitor patients' skin and document the stage of HAPUs daily. Moreover, nurses are required to regularly reposition patients with impaired mobility. Periodic skin assessment and repositioning are components of an effective PU prevention strategy for HAPU (Chaboyer et al., 2016; Tayyib, Coyer, & Lewis, 2016). Partial application of the prevention strategy might have contributed to the low incidence of HAPUs in this study. Since most patients in integrated nursing units are hospitalized for a short time, the average length of stay in our study was short (7.58 days). Such short period of hospitalization was associated with a low HAPU incidence. Since not all hospital units are integrated nursing units, the HAPU incidence in this study cannot be applied to the whole of South Korea.
Regarding the initial stage of HAPU, 64.8% were at stage 2 and the remainder were at stage 1. The proportion of stage 2 HAPUs was higher in tertiary hospitals than in general hospitals (62.8%). No deep ulcer such as stage 3 or stage 4 was detected. Previous studies have reported that the proportion of stage 1 is higher than stage 2 or higher (Alhaug et al., 2017; Chaboyer et al., 2016; Gray & Giuliano, 2018). In the present study, the proportion of stage 2 was relatively high even in the absence of deep ulcers. Further studies are needed because of several factors such as accuracy of assessment, and late detection might have resulted in a high proportion of stage 2 HAPUs.
Since patients admitted to tertiary hospitals had high nursing needs and ADL dependence, the cumulative probability of HAPUs in tertiary hospitals was not significantly different from the general hospitals. Despite the risk of PUs, similar findings in tertiary and general hospitals analysed in this study might be attributed to differences in nurse staffing. The average standard of nurse staffing was 1:6 in a tertiary hospital while 1:10 in a general hospital (Kim et al., 2017). The gap between these two hospital types was quite different, suggesting that the RN‐HPPD in tertiary hospitals (4.0 hr) was 1.6 hr more than in general hospitals (2.4 hr). Despite the poor condition of patients and the severity of their nursing needs and assistance with ADL in tertiary hospitals, the risk of HAPU development was low because of adequate nursing staff. Therefore, appropriate and adequate levels of nursing staff are critical for the prevention of HAPU.
The effectiveness of nurse staffing on HAPU development was also analysed via Cox regression. In multivariate Cox regression analysis, HPPDs provided by RNs and NAs were significant factors influencing the development of HAPUs. The increase in the RN‐HPPD or NA‐HPPD hours lowered the likelihood of HAPU development. Several studies have reported the association between high levels of nursing staff and better outcomes (Schneider & Geraedts, 2016; Stalpers et al., 2015) not only in preventing HAPUs and patient falls (Cho, Lee, et al., 2016; Kim & Bae, 2018), but also in reducing mortality rates (Kim & Bae, 2018). The high incidence of HAPUs in patients has been associated with low levels of nurse staffing (Schneider & Geraedts, 2016; Stalpers et al., 2015). In the present study, an extra hour of nursing service provided by RN reduced the risk for HAPU development by 80% (HR = 0.20, 95% CI: 0.13–0.30). Hence, adequate nurse staffing might contribute to the reduction of HAPU incidence in hospitals, despite the patient‐related risk factors. Besides RNs, levels of NA staffing also affected the risk of HAPU development. Since supportive care such as patient repositioning is a significant factor in the prevention of HAPUs (Chaboyer et al., 2016; Joyce, Moore, & Christie, 2018), NA staffing issues might be associated with HAPU development in this study, similar to the results of previous studies. Total staffing as well as the RN skill mix can affect HAPU development (Choi & Staggs, 2014; Schneider & Geraedts, 2016). These findings suggest that all nursing staff personnel can contribute to the prevention of HAPUs: RNs can evaluate patients' skin and other risk factors, while NAs provide ADL support. It is crucial to protect patients' safety. Thus, hospital managers should identify risk factors in patients and ensure appropriate RN staffing levels. The entire nursing workforce is needed to reduce these risks.
Among individual factors, gender, age and previous falls were significant risk factors for HAPU development. The risk of HAPU development was higher in men and the elderly, consistent with results of previous studies (Bauer et al., 2016; Primiano et al., 2011). Patients who experienced falls during hospitalization had an extremely high risk of subsequent HAPU development (HR = 18.94, p < .001). It might be attributed to the change in mobility after a fall. If a patient fell, the risk for HAPUs is significantly increased because the patient is usually restricted from moving due to fall‐related injuries (Doran et al., 2014). The patient's mobility is more likely to be fully restricted in the case of a fall‐related injury. The risk of HAPUs after falls is the most influencing patient factor in this study; adequate nursing staff is needed to provide preventative care to such patients.
Among nursing needs, only oxygen saturation monitoring was significantly associated with HAPUs. Patients who are measured for oxygen saturation have the potential for low oxygen levels. There is a lack of research for investigating the direct relationship between oxygen levels and HAPUs. However, low oxygen level might be related to peripheral perfusion and tissue oxygenation, and it may lead to the breakdown of skin integrity (Clements et al., 2014; Garcia‐Mayor et al., 2018). Low oxygen levels are not included among the assessment scales such as the Braden scale or the Waterlow scale (Pancorbo‐Hidalgo et al., 2006). However, since low oxygen level is likely to affect skin integrity, further studies investigating the relationship between oxygen level and HAPUs are needed.
Mobility impairment is a significant factor in HAPU development (Coleman et al., 2013; Li et al., 2019; Primiano et al., 2011). Patients who need repositioning in bed and toilet use have high risks for HAPU development. Patients with full dependence on positioning were at a 5.84‐fold higher risk of developing HAPUs than patients without any restrictions. Mobility was included as a factor in all assessment tools because previous studies showed its role as a risk factor for HAPU hence requiring nurses' attention. Patients with dependence on toilet use were at high risk for HAPUs as well. Since it is difficult for these patients to walk to the toilet and clean themselves, skin integrity around the buttocks might be disrupted due to incontinence and poor hygiene (Garcia‐Mayor et al., 2018). Excessively moist skin compromises tissue strength and increases the risk of skin damage under pressure and shear forces (Bates‐Jensen et al., 2009; Coleman et al., 2013). In addition to restricted mobility contributing to dependence of toilet use, patients might have two coexisting ADL problems. Patients with limited position in bed and toilet use were more likely to develop HAPUs compared to patients who had a single limitation. It is difficult to resolve these limitations in a short span of time. Thus, active preventive strategies are needed for patients with compound risk factors for HAPUs. Adequate nursing staff personnel are needed to assess risk factors and implement prevention strategies.
The study has few limitations. First, results were analysed only in the case of integrated nursing units because we could not use data from other nursing units. Therefore, the incidence of HAPU cannot be generalized to the entire Korea. Second, this study did not verify whether all hospitals adopted similar reporting criteria for HAPU. It did not include all factors related to HAPU because only secondary data were analysed.
5. CONCLUSION
This study analysed patient factors and nursing staff‐related issues influencing the development of HAPU. Gender, age, previous falls, low oxygen levels and dependence of position in bed and toilet use were significant patient factors influencing HAPUs. To prevent HAPUs, risk factors should be analysed first so that prevention strategies could be implemented accordingly. In addition, adequate and appropriate nurse staffing could contribute to the prevention of HAPUs.
6. IMPLICATIONS TO NURSING MANAGEMENT
This study showed that several individual risk factors and factors related to nurse staffing were associated with HAPUs. Appropriate inpatient care via preventative nursing services tailored to individual patients' risk factors can reduce the incidence of HAPUs. As most risk factors are not easy to modify in a short time, preventive strategies in clinical practice should be developed and applied according to risk factors identified in this study. Moreover, nurse staffing should be equipped adequately. Given the current state of nursing shortage worldwide, further studies are needed to maintain meaningful levels of RNs and NAs at hospitals with similar patient severity and characteristics.
ETHICAL APPROVAL
Seoul National University (E1710/002‐003).
ACKNOWLEDGEMENTS
This work was supported by Mid‐career Researcher Program through the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP; No. 2016R1A2B4015298).
Kim J, Lee J‐Y, Lee E. Risk factors for newly acquired pressure ulcer and the impact of nurse staffing on pressure ulcer incidence. J Nurs Manag. 2022;30:O1–O9. 10.1111/jonm.12928
Contributor Information
Jinhyun Kim, Email: jinhyun@snu.ac.kr.
Eunhee Lee, Email: ehlee@hallym.ac.kr.
REFERENCES
- Aiken, L. H. , Sermeus, W. , Van den Heede, K. , Sloane, D. M. , Busse, R. , McKee, M. , … Kutney‐Lee, A. (2012). Patient safety, satisfaction, and quality of hospital care: Cross sectional surveys of nurses and patients in 12 countries in Europe and the United States. British Medical Journal, 344, e1717. 10.1136/bmj.e1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhaug, J. , Gay, C. L. , Henriksen, C. , & Lerdal, A. (2017). Pressure ulcer is associated with malnutrition as assessed by Nutritional Risk Screening (NRS 2002) in a mixed hospital population. Food & Nutrition Research, 61(1), 1324230. 10.1080/16546628.2017.1324230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae, S. H. , Kelly, M. , Brewer, C. S. , & Spencer, A. (2014). Analysis of nurse staffing and patient outcomes using comprehensive nurse staffing characteristics in acute care nursing units. Journal of Nursing Care Quality, 29(4), 318–326. 10.1097/NCQ.0000000000000057 [DOI] [PubMed] [Google Scholar]
- Bates‐Jensen, B. M. , McCreath, H. E. , & Pongquan, V. (2009). Sub‐epidermal moisture is associated with early pressure ulcer damage in nursing home residents with dark skin tones: Pilot findings. Journal of Wound, Ostomy, and Continence Nursing, 36(3), 277–284. 10.1097/WON.0b013e3181a19e53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer, K. , Rock, K. , Nazzal, M. , Jones, O. , & Qu, W. (2016). Pressure ulcers in the United States' inpatient population from 2008 to 2012: Results of a retrospective nationwide study. Ostomy Wound Management, 62(11), 30–38. [PubMed] [Google Scholar]
- Brown, G. (2003). Long‐term outcomes of full‐thickness pressure ulcers: Healing and mortality. Ostomy Wound Management, 49, 42–50. [PubMed] [Google Scholar]
- Cai, S. , Mukamel, D. B. , & Temkin‐Greener, H. (2010). Pressure ulcer prevalence among black and white nursing home residents in New York state: Evidence of racial disparity? Medical Care, 48(3), 233. 10.1097/MLR.0b013e3181ca2810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaboyer, W. , Bucknall, T. , Webster, J. , McInnes, E. , Gillespie, B. M. , Banks, M. , … Wallis, M. (2016). The effect of a patient centred care bundle intervention on pressure ulcer incidence (INTACT): A cluster randomised trial. International Journal of Nursing Studies, 64, 63–71. 10.1016/j.ijnurstu.2016.09.015 [DOI] [PubMed] [Google Scholar]
- Cho, E. , Chin, D. L. , Kim, S. , & Hong, O. (2016). The relationships of nurse staffing level and work environment with patient adverse events. Journal of Nursing Scholarship, 48(1), 74–82. 10.1111/jnu.12183 [DOI] [PubMed] [Google Scholar]
- Cho, S.‐H. , Lee, J.‐Y. , June, K.‐J. , Hong, K. J. , & Kim, Y. (2016). Nurse staffing levels and proportion of hospitals and clinics meeting the legal standard for nurse staffing for 1996–2013. Journal of Korean Academy of Nursing Administration, 22(3), 209–219. 10.11111/jkana.2016.22.3.209 [DOI] [Google Scholar]
- Choi, J. , & Staggs, V. S. (2014). Comparability of nurse staffing measures in examining the relationship between RN staffing and unit‐acquired pressure ulcers: A unit‐level descriptive, correlational study. International Journal of Nursing Studies, 51(10), 1344–1352. 10.1016/j.ijnurstu.2014.02.011 [DOI] [PubMed] [Google Scholar]
- Clements, L. , Moore, M. , Tribble, T. , & Blake, J. (2014). Reducing skin breakdown in patients receiving extracorporeal membranous oxygenation. Nursing Clinics of North America, 49(1), 61–68. 10.1016/j.cnur.2013.11.003 [DOI] [PubMed] [Google Scholar]
- Coleman, S. , Gorecki, C. , Nelson, E. A. , Closs, S. J. , Defloor, T. , Halfens, R. , … Nixon, J. (2013). Patient risk factors for pressure ulcer development: Systematic review. International Journal of Nursing Studies, 50(7), 974–1003. 10.1016/j.ijnurstu.2012.11.019 [DOI] [PubMed] [Google Scholar]
- Cox, J. , & Rasmussen, L. (2014). Enteral nutrition in the prevention and treatment of pressure ulcers in adult critical care patients. Critical Care Nurse, 34(6), 15–27. 10.4037/ccn2014950 [DOI] [PubMed] [Google Scholar]
- de Azevedo Macena, M. S. , da Costa Silva, R. S. , Dias Fernandes, M. , de Almeida Medeiros, A. B. , Batista Lucio, K. D. , & de Carvalho Lira, A. L. B. (2017). Pressure ulcer risk evaluation in critical patients: Clinical and social characteristics. The Open Nursing Journal, 11, 91–97. 10.2174/1874434601711010091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran, D. , Lefebre, N. , O'Brien‐Pallas, L. , Estabrook, C. A. , White, P. , Carryer, J. , … Li, M. (2014). The relationship among evidence‐based practice and client dyspnea, pain, falls, and pressure ulcer outcomes in the community setting. Worldviews on Evidence‐Based Nursing, 11(5), 274–283. 10.1111/wvn.12051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García‐Mayor, S. , Morilla‐Herrera, J. C. , Lupiáñez‐Pérez, I. , Kaknani Uttumchandani, S. , León Campos, Á. , Aranda‐Gallardo, M. , … Morales‐Asencio, J. M. (2018). Peripheral perfusion and oxygenation in areas of risk of skin integrity impairment exposed to pressure patterns. A phase I trial (POTER Study). Journal of Advanced Nursing, 74(2), 465–471. 10.1111/jan.13414 [DOI] [PubMed] [Google Scholar]
- Gray, M. , & Giuliano, K. K. (2018). Incontinence‐associated dermatitis, characteristics and relationship to pressure injury: a multisite epidemiologic analysis. Journal of Wound Ostomy & Continence Nursing, 45(1), 63–67. 10.1097/WON.0000000000000390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce, P. , Moore, Z. E. , & Christie, J. (2018). Organisation of health services for preventing and treating pressure ulcers. Cochrane Database of Systematic Reviews, 12, CD012132. 10.1002/14651858.CD012132.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khor, H. M. , Tan, J. , Saedon, N. I. , Kamaruzzaman, S. B. , Chin, A. V. , Poi, P. J. , … Tan, M. P. (2014). Determinants of mortality among older adults with pressure ulcers. Archives of Gerontology and Geriatrics, 59, 536–541. 10.1016/j.archger.2014.07.011 [DOI] [PubMed] [Google Scholar]
- Kim, C. G. , & Bae, K. S. (2018). Relationship between nurse staffing level and adult nursing‐sensitive outcomes in tertiary hospitals of Korea: Retrospective observational study. International Journal of Nursing Studies, 80, 155–164. 10.1016/j.ijnurstu.2018.01.001 [DOI] [PubMed] [Google Scholar]
- Kim, J. , Kim, S. , Park, E. , Jeong, S. , & Lee, E. (2017). Policy issues and new direction for comprehensive nursing service in the national health insurance. Journal of Korean Academy of Nursing Administration, 23(3), 312–322. 10.11111/jkana.2017.23.3.312 [DOI] [Google Scholar]
- Kim, J. , Kim, S. , Park, J. , & Lee, E. (2019). Multilevel factors influencing falls of patients in hospital: The impact of nurse staffing. Journal of Nursing Management, 27(5), 1011–1019. 10.1111/jonm.12765 [DOI] [PubMed] [Google Scholar]
- Kuwahara, M. , Tada, H. , Mashiba, K. , Yurugi, S. , Iioka, H. , Niitsuma, K. , & Yasuda, Y. (2005). Mortality and recurrence rate after pressure ulcer operation for elderly long‐term bedridden patients. Annals of Plastic Surgery, 54(6), 629–632. 10.1097/01.sap.0000164465.40841.0b [DOI] [PubMed] [Google Scholar]
- Li, J. , Wu, X. , Li, Z. , Zhou, X. , Cao, J. , Jia, Z. , … Cheng, S. (2019). Nursing resources and major immobility complications among bedridden patients: A multicenter descriptive study in China. Journal of Nursing Management, 27(5), 930–938. 10.1111/jonm.12731 [DOI] [PubMed] [Google Scholar]
- Mehrotra, D. V. , Su, S. C. , & Li, X. (2012). An efficient alternative to the stratified Cox model analysis. Statistics in Medicine, 31(17), 1849–1856. 10.1002/sim.5327 [DOI] [PubMed] [Google Scholar]
- National Pressure Ulcer Advisory Panel (2014). New 2014 prevention and treatment of pressure ulcers: Clinical practice guideline. Retrieved from http://www.npuap.org/wp-content/uploads/2014/08/Quick-Reference-Guide-DIGITAL-NPUAP-EPUAP-PPPIA-Jan2016.pdf [Google Scholar]
- Oakes, D. , & Feng, C. (2010). Combining stratified and unstratified log‐rank tests in paired survival data. Statistics in Medicine, 29(16), 1735–1745. 10.1002/sim.3921 [DOI] [PubMed] [Google Scholar]
- OECD (2018). OECD health statistics 2018. Retrieved from http://www.oecd.org/els/health-systems/health-data.htm [Google Scholar]
- Pancorbo‐Hidalgo, P. L. , Garcia‐Fernandez, F. P. , Lopez‐Medina, I. M. , & Alvarez‐Nieto, C. (2006). Risk assessment scales for pressure ulcer prevention: A systematic review. Journal of Advanced Nursing, 54(1), 94–110. 10.1111/j.1365-2648.2006.03794.x [DOI] [PubMed] [Google Scholar]
- Primiano, M. , Friend, M. , McClure, C. , Nardi, S. , Fix, L. , Schafer, M. , … McNett, M. (2011). Pressure ulcer prevalence and risk factors during prolonged surgical procedures. AORN Journal, 94(6), 555–566. 10.1016/j.aorn.2011.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, S. , Chaboyer, W. , & Desbrow, B. (2015). Nutrition care‐related practices and factors affecting nutritional intakes in hospital patients at risk of pressure ulcers. Journal of Human Nutrition and Dietetics, 28(4), 357–365. 10.1111/jhn.12258 [DOI] [PubMed] [Google Scholar]
- Schneider, P. P. , & Geraedts, M. (2016). Staffing and the incidence of pressure ulcers in German hospitals: A multicenter cross‐sectional study. Nursing and Health Sciences, 18(4), 457–464. 10.1111/nhs.12292 [DOI] [PubMed] [Google Scholar]
- Shaw, L. F. , Chang, P.‐C. , Lee, J.‐F. , Kung, H.‐Y. , & Tung, T.‐H. (2014). Incidence and predicted risk factors of pressure ulcers in surgical patients: Experience at a medical center in Taipei, Taiwan. BioMed Research International, 2014(2), 416896. 10.1155/2014/416896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalpers, D. , de Brouwer, B. J. , Kaljouw, M. J. , & Schuurmans, M. J. (2015). Associations between characteristics of the nurse work environment and five nurse‐sensitive patient outcomes in hospitals: A systematic review of literature. International Journal of Nursing Studies, 52(4), 817–835. 10.1016/j.ijnurstu.2015.01.005 [DOI] [PubMed] [Google Scholar]
- Tayyib, N. , Coyer, F. , & Lewis, P. A. (2016). Implementing a pressure ulcer prevention bundle in an adult intensive care. Intensive and Critical Care Nursing, 37, 27–36. 10.1016/j.iccn.2016.04.005 [DOI] [PubMed] [Google Scholar]


