Abstract
Objective
To investigate the association between hypoxic‐ischaemic insult timing and brain injury type in infants with severe cerebral palsy (CP).
Design
Longitudinal study.
Setting
Database of the Recurrence Prevention Committee, Japan Obstetric Compensation System for Cerebral Palsy.
Sample
Infants with severe CP born at ≥34 weeks of gestation.
Methods
The intrapartum fetal heart rate (FHR) strips were categorised as continuous bradycardia; persistently non‐reassuring (NR‐NR); reassuring‐prolonged deceleration (R‐PD); Hon's pattern (R‐Hon); persistently reassuring (R‐R); and unclassified. The brain magnetic resonance imaging (MRI) scans were categorised based on the predominant site involved: basal ganglia‐thalamus (BGT); white matter (WM); watershed (WS); stroke; normal; and unclassified.
Main outcome measures
Manifestations of the brain MRI types and the association between FHR evolution pattern and MRI type were analysed.
Results
Among 672 eligible infants, 76% had BGT‐dominant injury, 5.4% WM, 1.2% WS, 1.6% stroke, 1.9% normal, and 14% unclassified. Placental abruption and small‐for‐gestational age were associated with an increased (adjusted odds ratio [aOR] 8.02) and decreased (aOR 0.38) risk of BGT injury, respectively. The majority of infants had BGT injury in most FHR groups (bradycardia, 97%; NR‐NR, 75%; R‐PD, 90%; R‐Hon, 76%; and R‐R, 45%). The risk profiles in case of BGT in the NR‐NR group were similar to those in the R‐PD and R‐Hon groups.
Conclusion
BGT‐dominant brain damage accounted for three‐fourths of the cases of CP in term or near‐term infants, even in prenatal onset cases. Hypoxic‐ischaemic insult has a major impact on CP development during the antenatal period.
Tweetable abstract
Basal ganglia‐thalamus injury constitutes 76% of severe cerebral palsy cases, predominant even in antenatal‐onset cases.
Keywords: basal ganglia, brain injuries, cardiotocograph, cerebral palsy, hypoxia‐ischaemia, infant, perinatal, thalamus
Tweetable abstract
Basal ganglia‐thalamus injury constitutes 76% of severe cerebral palsy cases, predominant even in antenatal‐onset cases.
This article includes Author Insights, a video abstract available at https://vimeo.com/bjogabstracts/authorinsights17089.
1. INTRODUCTION
Various potential aetiologies for cerebral palsy (CP) other than hypoxia‐ischaemia (HI) have been confirmed, such as infection/inflammation, fetal growth restriction and genetic disorders. Although reports suggest that these aetiologies are common in antenatal‐onset cases, questions remain. 1 , 2 , 3 , 4 , 5 To understand the pathophysiology of CP and identify preventative measures, it is crucial to understand when and how the severe brain damage that leads to CP occurs. Fetal heart rate (FHR) evolution patterns during labour have been shown to be useful in evaluating the timing of perinatal brain injury. 6 , 7 , 8 , 9 , 10 FHR patterns can be classified into the following six patterns based on FHR patterns at hospital admission and those before delivery: bradycardia, non‐reassuring (NR)‐NR, reassuring (R)‐prolonged deceleration, R‐Hon's pattern (Hon), R‐R, and unclassified. 6 One study on 1069 infants with severe CP suggested that antepartum events are a substantial contributor to brain injury leading to CP. 11
Magnetic resonance imaging (MRI) is useful for predicting neurological outcomes prospectively, and for retrospectively evaluating the pathophysiological types and patterns that affect perinatal brain damage. Patterns are usually classified into deep nuclear grey matter injury, watershed injury (WS), white matter (WM) injury and vascular lesions. 12 Deep nuclear grey matter, which includes the basal ganglia and thalamus, is vulnerable to acute and severe HI stress, and selective neuronal necrosis is a characteristic of HI injury in both term and preterm infants. 13 WS injury principally relates to cerebral hypoperfusion. 12 , 13 As WS regions of the cerebral cortex and subcortical WM with a parasagittal distribution are the farthest away from the main arterial supplies, WS injury usually occurs as a result of mild‐to‐moderate arterial hypotension. 12 , 13 Damage to the WM is more common in preterm infants. 12 Ischaemia is thought to contribute to the development of WM injury. Specific cerebrovascular injuries such as focal arterial infarction, venous thrombosis or embolism and isolated intraparenchymal haemorrhage may also be associated with peripartum HI. 14
In addition to FHR evolution studies, MRI of infants could enable us to evaluate the pathophysiological mechanisms of CP in more detail; that is, insult severity and pattern, as well as insult timing. However, there is a lack of comprehensive studies on the correlations between FHR tracings and brain MRI findings. Herein, we aimed to investigate the association between MRI patterns and clinical factors. Further, we aimed to establish the association between FHR evolution and MRI pattern.
2. METHODS
2.1. Study population and data collection
A longitudinal, nationwide cohort study was conducted. All data in this study were obtained from the nationwide database of the Recurrence Prevention Committee, Japan Obstetric Compensation System for Cerebral Palsy [JOCSC] for infants enrolled between 28 September 2009 and 30 September 2016). 15 Infants were enrolled in the database upon being diagnosed with severe CP (equivalent to levels 3–5 of the Gross Motor Function Classification System‐Expanded and Revised) 16 and were approved by the JOCSC for disability support before the age of 5 years. All clinical data, including cardiotocograph (CTG) strips and MRIs, were obtained retrospectively after enrolment from the birth and neonatal care hospitals. Cases eligible for this study were infants with severe CP born at or beyond 34 weeks of gestation between 2009 and 2014. Infants whose cardiotocograph (CTG) tracings on admission or at delivery were missing or uninterpretable, or whose brain MRI data within 6 weeks after birth were not available, were excluded. MRI was performed on a 1.5–3‐Tesla scanner (slice width 1.5–6 mm) depending on the facility. Neonatal asphyxia was defined as impaired neonatal condition due to lack of oxygen at delivery with metabolic acidosis (umbilical artery blood pH <7.0 and base deficit >12 mEq/L) and low Apgar scores (<7). Umbilical cord abnormality was diagnosed at birth and included anatomical abnormalities such as cord entanglement, marginal or velamentous insertion, abnormal twisting or coiling, deficiency of Wharton's jelly, long (≥70 cm) or short (≤25 cm) cords, single umbilical artery or true knot. Intrauterine infection was defined as maternal pyrexia associated with evidence of clinical or pathological chorioamnionitis, or neonatal sepsis. Small‐for‐gestational‐age (SGA) was defined as birthweight <10th percentile. The diagnoses of remaining factors such as placental abruption, placenta praevia or low‐lying placenta, uterine rupture, umbilical cord prolapse, feto‐maternal transfusion, rhesus factor alloimmunisation, shoulder dystocia and twin‐to‐twin transfusion syndrome (TTTS), and postnatal complications such as neonatal sepsis, intracranial haemorrhage, and hypoglycaemia were collected from the medical records of the perinatal care facilities.
2.2. FHR pattern analysis
Three obstetricians (MN, AO and JH), blinded to the infants' clinical information, independently analysed the individual CTG strips throughout labour. FHR patterns were categorised into the six groups listed below according to the national guidelines 17 after agreement by at least two of the three reviewers, based on the FHR pattern on the earliest recorded CTG (on admission or during labour), compared with the pattern just before delivery:
bradycardia: bradycardia (<110 bpm) on admission persisting until delivery;
persistently non‐reassuring (NR‐NR) pattern: a persistently decreased or absent variability (≤5 bpm) without bradycardia on admission and persisting until delivery;
reassuring‐prolonged deceleration (R‐PD): abruptly (<1 h) evolving pattern during labour from the initial reassuring pattern (baseline at 110–160 bpm with variability of 6–25 bpm) on admission to severe PD (bottom FHR <80 bpm) or bradycardia just before delivery;
Hon's pattern (R‐Hon): gradually (≥1 h) evolving pattern during labour from reassuring on admission to recurrent decelerations that become wider and deeper, combined with tachycardia and a decrease or loss of variability, followed by terminal bradycardia;
persistent reassuring (R‐R) pattern: reassuring admission test that persists within normal range (moderate variability) throughout delivery;
unclassified: patterns without agreement among the three readers, or indeterminable due to intermittent monitoring, tachycardia with normal variability on admission, a normal admission test followed by an NR pattern without terminal bradycardia, and a sinusoidal or checkmark pattern.
The timing of brain injury was estimated to be prenatal in the bradycardia and NR‐NR pattern groups, during delivery in the R‐PD and R‐Hon pattern groups, and prenatal or postnatal in the R‐R pattern group (Table S1).
2.3. Brain MRI analysis
Transverse T1‐ and T2‐weighted images and, when appropriate, diffusion‐weighted images were reviewed by a specialised paediatric neurologist (YN) who was blinded to the clinical background of the infant. Cases in which the basal ganglia‐thalamus (BGT) region was injured, were classified as BGT injury. Cases where the BGT region was preserved, divided into five categories. Among these, cases with WM affected predominantly were classified as WM injury or WS injury if distributed in the parasagittal subcortical WM in particular. The remainder categories comprised: stroke (focal disruption of cerebral blood flow due to arterial or venous thrombosis or embolism), normal finding (no significantly aberrant signals in the whole brain), and unclassified (did not fit one of the other five categories).
According to previously described criteria, 18 , 19 we graded BGT injuries as mild (abnormal signal intensities in the focal area, usually in the ventrolateral nuclei of the thalami); moderate (multifocal or more diffuse abnormalities extending to multiple areas of the thalamus); and severe (widespread abnormalities including the whole BGT area). Furthermore, cortical and brainstem involvement, commonly associated with severe forms of BGT injury, were classified. Cortical injuries, usually observed as a loss of grey–WM differentiation or cortical highlighting, were classified as mild (1–2 sites of signal changes), moderate (3 sites), or severe (>3 sites). Damage to the brainstem was defined as moderate when loss of anatomical details, over‐differentiation of the anterior and posterior pons, focal signal abnormality or mild asymmetries were observed, and as severe if widespread abnormalities, abnormal myelination, marked asymmetries or atrophy was detected.
The outcomes assessed included the manifestations of brain damage and the association between FHR evolution pattern and MRI type.
2.4. Statistical analysis
Continuous or integer variables are presented as medians with interquartile ranges and categorical variables as numbers and percentages. Interobserver agreement for FHR classification was analysed using Cohen's kappa coefficient (two observers) and Fleiss's kappa coefficient (three observers). Univariate analyses among the BGT, WS‐WM, and remainder categories groups were performed using the Kruskal–Wallis test for continuous variables and the chi‐square test or Fisher's exact test for categorical variables. Comparisons of categorical variables between FHR evolution classes were performed using multinomial logistic regression. Odds ratios (ORs) and 95% confidence intervals (CIs) were obtained using multinomial logistic regression models, with BGT injury, severity of BGT injury and cortical/brainstem involvement as dependent variables. Multivariate analyses were performed using multinomial logistic regression to verify the FHR pattern and risk factors associated with BGT injury compared with WS‐WM injury, BGT severity, or damage to the cortex or brainstem as independent variables, with covariates selected based on the obstetrical background, including maternal age, pre‐pregnancy body mass index, parity, history of miscarriage, history of fertility treatment, smoking, alcohol consumption, preterm birth, infantile sex and therapeutic hypothermia. p‐values <0.05 were considered statistically significant. Pairwise deletion was used in all statistical analyses to address the missing data. All analyses were performed with STATA version 16.0 (Stata Corporation).
3. RESULTS
3.1. FHR evolution groups
Among 1593 infants, 672 met the eligibility criteria (Figure S1). Infants were stratified according to the FHR evolution pattern as follows: bradycardia, 9% (n = 59); NR‐NR, 25% (n = 169); R‐PD, 17% (n = 115); R‐Hon, 17% (n = 111); R‐R, 13% (n = 86); and unclassified, 20% (n = 132) (Figure 1A). A total of 20% cases (n = 132) were unclassified due to disagreement (n = 47), intermittent monitoring (n = 45), no terminal bradycardia (n = 19), sinusoidal or checkmark pattern (n = 11) and tachycardia with moderate variability on admission (n = 10). The overall interobserver agreement was moderate (kappa, 0.60), between 0.59 and 0.62 across each pair of reviewers. The agreement for each FHR pattern was as follows: bradycardia, kappa 0.82; NR‐NR, kappa 0.68; R‐PD, kappa 0.60; R‐Hon, kappa 0.47; R‐R, kappa 0.55; unclassified, kappa 0.54. As in our previous report, 11 brain damage in at least 34% of infants was presumed to have occurred during the prenatal period (bradycardia and NR‐NR) and brain injuries associated with a hypoxic event during labour (R‐PD and R‐Hon) also accounted for 34% of cases.
FIGURE 1.
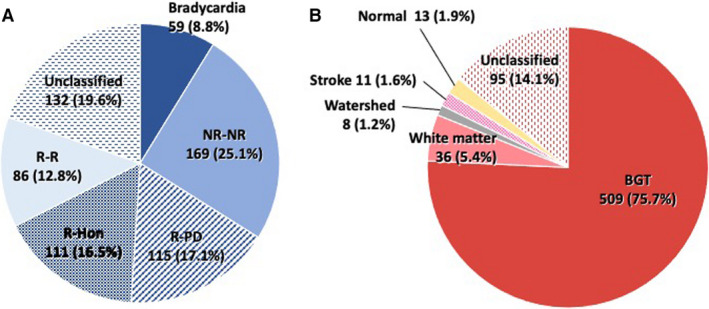
Analysis of fetal heart rate evolution pattern and infant brain MRI. (A) Analysis of fetal heart rate evolution pattern. NR‐NR, decreased variability on admission; R‐Hon, gradual deterioration followed by a decline in base rate; R‐PD, reassuring on admission and abruptly changed immediately before delivery; R‐R, fetal heart rate variability maintained throughout delivery. (B) Analysis of magnetic resonance imaging. BGT, basal ganglia‐thalamus
3.2. Brain MRI types
The details of brain damage types are shown in Figure 1B. The median age at MRI scan was 15 days (interquartile range 10–23). Overall, 76% (n = 509) of infants developed BGT‐dominant lesions, indicating acute, profound HI insults as the cause. The BGT region was spared in the remaining 24% of infants (n = 163), which included 5.4% (n = 36) WM injury, 1.2% (n = 8) WS injury, 1.6% (n = 11) stroke, 1.9% (n = 13) normal findings, and 14% (n = 95) unclassified types of injury. The unclassified findings comprised: haematogenous or meningeal injury (n = 26), multicystic encephalomalacia with preserved BGT region (n = 22), mixed lesions where the primary locus was unclear (n = 20), extrinsic brain haemorrhage (n = 11), brain herniation of unknown origin (n = 3) and nonspecific injuries (n = 9).
Maternal and neonatal characteristics are outlined in the Table S2 and S3. In comparison between the three groups, the mothers' median age was 31 years, and ~60% were nulliparous. The number of infants born by operative vaginal or caesarean section delivery was higher in the BGT group than in the WS‐WM and remainder categories group (71% vs. 55% vs. 53%, p < 0.01). Preterm birth rates were higher in the WS‐WM group compared with the other groups (15% vs. 34% vs. 18%, p < 0.01). The BGT group showed higher rates of neonatal asphyxia (umbilical artery pH 6.96 vs. 7.16 vs. 7.20; base deficit 15.8 vs. 7.4 vs. 8.2 mEq/L; Apgar score at 1/5 min 1/3 vs. 5/7 vs. 6/8; all p < 0.01). Therapeutic hypothermia was performed in 51% of infants in the BGT group, compared with only 14% and 15% of those in the WS‐WM and remainder categories groups (p < 0.01).
Table 1 summarises the perinatal risk factors underlying CP. Umbilical cord abnormality was the most common aetiology in all three groups (>40%), followed by placental abruption (23%), intrauterine infection (18%) and SGA (13%) (including duplicates) in the BGT group. Postnatal complications were the second most common aetiology only in the remainder categories groups (40%). Furthermore, 20% of infants in the BGT group, 30% in the WS‐WM group and 21% in the remainder categories group developed CP without apparent aetiologies. Multivariate analysis showed that placental abruption (adjusted OR [aOR] 8.02, 95% CI 1.53–41.95) was significantly associated with an increased risk of BGT injury, whereas SGA (aOR 0.38, 95% CI 0.17–0.86) was significantly associated with a low risk of BGT injury, indicating an association with WS‐WM injuries (Table 2).
TABLE 1.
Perinatal risk factor and the fetal heart rate class: a comparison of basal‐ganglia thalamus, watershed‐white matter and the remainder categories groups
| BGT (n = 509) | WS‐WM (n = 44) | Remainder (n = 119) | p‐Value | |
|---|---|---|---|---|
| Perinatal risk factor underlying CP (data includes duplicates), n (%) | ||||
| Umbilical abnormalities (n = 289) | 217 (42.6) | 18 (40.9) | 54 (45.4) | 0.83 |
| Placental abruption (n = 127) | 119 (23.4) | 2 (4.5) | 6 (5.0) | <0.01 |
| Intrauterine infection (n = 121) | 91 (17.9) | 6 (13.6) | 24 (20.2) | 0.62 |
| SGA (n = 94) | 65 (12.8) | 11 (25.0) | 18 (15.1) | 0.08 |
| Feto‐maternal transfusion (n = 21) | 10 (2.0) | 4 (9.1) | 7 (5.9) | <0.01 |
| Umbilical cord prolapse (n = 21) | 20 (3.9) | 0 (0) | 1 (0.8) | 0.13 |
| Inappropriate operative delivery (n = 18) | 16 (3.1) | 0 (0) | 2 (1.7) | 0.56 |
| Uterine rupture (n = 18) | 18 (3.5) | 0 (0) | 0 (0) | 0.05 |
| Maternal cardiopulmonary collapse (n = 13) | 12 (2.4) | 0 (0) | 1 (0.8) | 0.50 |
| Uterine hypertonus or tachysystole (n = 12) | 10 (2.0) | 1 (2.3) | 1 (0.8) | 0.56 |
| Shoulder dystocia (n = 9) | 9 (1.8) | 0 (0) | 0 (0) | 0.36 |
| TTTS (n = 3) | 1 (0.2) | 1 (2.3) | 1 (0.8) | 0.08 |
| Abruption of placenta praevia or low‐lying placenta (n = 2) | 2 (0.4) | 0 (0) | 0 (0) | 1.00 |
| Rh(D) alloimmunisation (n = 1) | 0 (0) | 0 (0) | 1 (0.8) | 0.24 |
| Postnatal complications (n = 66) | 15 (2.9) | 3 (6.8) | 48 (40.3) | <0.01 |
| No risk factor (n = 141) | 103 (20.2) | 13 (29.5) | 25 (21.0) | 0.35 |
| FHR evolution class (n = 672), n (%) | ||||
| Bradycardia (n = 59) | 57 (11.2) | 2 (4.5) | 0 (0) | <0.01 |
| NR‐NR (n = 169) | 127 (25.0) | 17 (38.6) | 25 (21.0) | |
| R‐PD (n = 115) | 104 (20.4) | 3 (6.8) | 8 (6.7) | |
| R‐Hon (n = 111) | 84 (16.5) | 6 (13.6) | 21 (17.6) | |
| R‐R (n = 86) | 39 (7.7) | 7 (15.9) | 40 (33.6) | |
| Unclassified (n = 132) | 98 (18.5) | 9 (20.5) | 25 (21.0) | |
Abbreviations: BGT, basal ganglia thalamus; BMI, body mass index; CP, cerebral palsy; FHR, fetal heart rate; NR‐NR, decreased variability on admission; Rh(D), rhesus D factor; R‐Hon, gradual deterioration followed by decline in base rate; R‐PD, reassuring on admission and abruptly changed immediately before delivery; R‐R, fetal heart rate variability maintained throughout delivery; SGA, small‐for‐gestational age (birthweight <10th percentile); TTTS, twin‐twin transfusion syndrome; WM, white matter; WS, watershed.
TABLE 2.
Multinomial logistic regression analysis of perinatal risk factor and fetal heart rate evolution class for basal ganglia‐thalamus injury in cerebral palsy: comparison of watershed–white matter injury
| Variables | Adjusted OR a | 95% CI |
|---|---|---|
| Perinatal risk factor underlying CP (n = 672) (data include duplicates) | ||
| Umbilical abnormalities (n = 289) | 1.36 | 0.68–2.73 |
| Placental abruption (n = 127) | 8.02 | 1.53–41.95* |
| Intrauterine infection (n = 121) | 1.28 | 0.47–3.43 |
| SGA (n = 94) | 0.38 | 0.17–0.86* |
| Feto‐maternal transfusion (n = 21) | 0.25 | 0.06–1.01 |
| Uterine hypertonus or tachysystole (n = 12) | 0.42 | 0.04–4.11 |
| TTTS (n = 3) | 0.14 | <0.01–4.47 |
| Postnatal complications (n = 66) | 0.68 | 0.17–2.69 |
| FHR class (n = 672) | ||
| Bradycardia (n = 59) | 1.25 | 0.19–8.20 |
| NR‐NR (n = 169) | 1.24 | 0.43–3.58 |
| R‐PD (n = 115) | 3.46 | 0.78–15.37 |
| R‐Hon (n = 111) | 1.09 | 0.31–3.88 |
| R‐R (n = 86) | (reference) | (reference) |
| Unclassified (n = 132) | 1.19 | 0.37–3.79 |
Abbreviations: BGT, basal ganglia‐thalamus; CI, confidence interval; CP, cerebral palsy; FHR, fetal heart rate; n, number; NR‐NR, decreased variability on admission; OR, odds ratio; R‐Hon, gradual deterioration followed by decline in base rate; R‐PD, reassuring on admission and abruptly change immediately before delivery; R‐R, fetal heart rate variability maintained throughout delivery; SGA, small‐for gestational age (birth weight <10th percentile); TTTS, twin‐twin transfusion syndrome.
Adjusted for maternal age, pre‐pregnancy BMI, parity, history of miscarriage, history of fertility treatment, smoking, alcohol consumption, preterm birth, infantile sex and therapeutic hypothermia. Reference for the outcome variables was watershed–white matter injury. Adjusted ORs for the remainder categories group are not shown.
p < 0.05.
3.3. FHR evolution groups and brain MRI types
The brain MRI types in each FHR evolution group are shown in Figure S2. Brain damage to the BGT accounted for a substantial proportion of infants in all FHR pattern groups (bradycardia, 97%; NR‐NR, 75%; R‐PD, 90%; R‐Hon, 76%; R‐R, 45%; unclassified, 45%). Even in the NR‐NR group (n = 169), 75% of infants (n = 127) showed a BGT pattern, followed by WM injury (n = 13; 8%), WS injury (n = 4; 2%), stroke (n = 4; 2%), normal findings (n = 1; 0.6%) and unclassified (n = 20; 12%). Compared with the R‐R group, the incidence of BGT injury was significantly higher in all the FHR evolution groups, especially in the bradycardia and R‐PD groups. However, after adjustment for clinical background, there was no significant difference between the incidence of BGT injury and WS‐WM injury in each FHR group (Table 2).
Most infants with BGT injury in all FHR evolution groups showed severe damage (Figure S3). After adjusting for clinical background, there was a significant difference in severe injury in the bradycardia (aOR 6.71, 95% CI 1.26–35.75), NR‐NR (aOR 2.51, 95% CI 1.10–5.73) and R‐PD (aOR 7.04, 95% CI 2.24–22.10) groups when compared with that in the R‐R group.
Figure S4 demonstrates the severity analysis of cortical brain damage for BGT injury in each FHR evolution group. Cortical damage accompanied most cases in all groups. Bradycardia (aOR 5.61, 95% CI 1.20–26.33), NR‐NR (aOR 4.57, 95% CI 1.33–15.68) and R‐Hon (aOR 4.69, 95% CI 1.22–18.00) patterns were significantly associated with more severe involvement than the R‐R pattern was after adjusting for clinical background. Figure S5 shows the severity analysis of brainstem damage for BGT injury in each FHR evolution group. Brainstem injuries were less frequent, ranging from 23% to 32%, and there was no difference in prevalence between the groups.
3.4. Risk profile of each FHR evolution group
The profiles of the underlying aetiology in each FHR evolution group for BGT injury, WS‐WM injury and the remainder categories are shown in Figure 2. Most cases in the bradycardia group progressed to BGT injury caused by placental abruption (45/59; 76%). It is intriguing to note that in the NR‐NR group (antenatal cause suspected), the causal pattern of BGT injury was similar to those in the R‐PD and R‐Hon groups (intrapartum cause suspected); that is, umbilical abnormalities, intrauterine infection, placental abruption and SGA, in that order.
FIGURE 2.
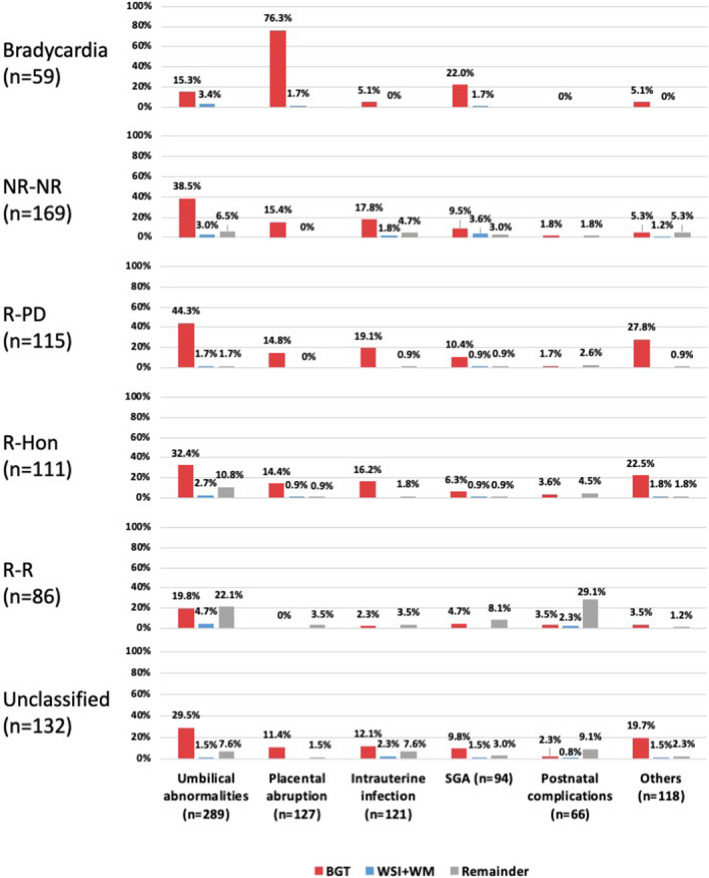
Profile of risk factors in each FHR class stratified by BGT injury, WS‐WM injury and remainder categories. Risk profiles of BGT injury in the NR‐NR (antenatal cause suspected) group were similar to those in the intrapartum causal groups (R‐PD and the R‐Hon groups), that is, umbilical abnormalities, intrauterine infection, placental abruption and SGA, in that order. BGT, basal ganglia‐thalamus; FHR, fetal heart rate; NR‐NR, decreased variability on admission; R‐Hon, gradual deterioration followed by decline in base rate; R‐PD, reassuring on admission and abruptly changed immediately before delivery; R‐R, fetal heart rate variability maintained throughout delivery; SGA, small‐for‐gestational age; WS‐WM, watershed‐white matter
4. DISCUSSION
4.1. Main findings
This longitudinal, nationwide cohort study demonstrated the association between the timing of insult based on FHR evolution and the pattern and severity of brain damage based on MRI classification in term or near‐term infants with severe CP. First, the brain MRI analysis demonstrated that three‐fourths of term or near‐term infants with severe CP developed a BGT injury. Secondly, BGT injuries were more frequently characterised by severe neonatal asphyxia compared with non‐BGT injuries. This is supported by the fact that placental abruption was associated with an increased risk of BGT injury, whereas SGA was less likely to contribute to BGT injury. Thirdly, BGT injury accounted for most cases, even in the NR‐NR group where antenatal causes were suspected. These findings suggest that cases of CP with an antenatal onset would be compromised by HI insult. Moreover, the risk profiles of cases of BGT injury in the NR‐NR group were similar to those in the R‐PD and R‐Hon groups.
4.2. Interpretation
Consistent with previous studies, 12 BGT damage was the most common manifestation in our study. Furthermore, severe BGT lesions were more prevalent in acute and total insult, and cortical involvement accompanying BGT damage was characterised by a gradual and partial insult.
It is important to note that infants in this study were serially registered in the JOCSC with several mandatory requirements. First, the infants must have severe CP equivalent to levels 3–5 of the Gross Motor Function Classification System – Expanded and Revised. 16 If less severe CP cases were included, the incidence of non‐BGT would have increased. WS injury is associated with mild hypoxic insult or prolonged hypoperfusion. 12 Stroke cases may not become apparent until a later period when higher brain functions are developing. 12 The WM injury rate would have increased had we included infants born before 34 gestational weeks. 20
Secondly, infants who died before 6 months of age are not included in the JOCSC. If those cases had been included, the incidence of brainstem lesion in the BGT would have increased, as brainstem integrity is vital to survival. That might be the reason why we did not identify a significant difference between the FHR evolution groups.
BGT was characterised by a more severe neonatal asphyxia compared with WS‐WM injury. Placental abruption usually leads to abrupt fetal hypoxia, which is likely to damage the BGT region. In contrast, SGA is potentially associated with chronic (less severe) hypoxia, and CP associated with feto‐maternal transfusion is characterised by prolonged ischaemia (followed by hypoxia) during the antenatal period, resulting in WM or WS subcortical injury, or stroke, rather than BGT. As for postnatal issues, neonatal bacterial or viral infections of the central nervous system cause diffuse structural or signal changes such as multicystic encephalomalacia, leptomeningeal enhancement or infarctions. 21 , 22 Although neonatal hypoglycaemia (n = 6) has been shown to cause WM abnormalities with a particular predilection for the posterior cerebrum, 23 all cases in this study were multifactorial, with five of six cases in our database being unclassifiable due to mixed lesions. This could explain the high incidence of postnatal complications only in the remainder categories group.
In this study, BGT injury was the most common manifestation, even in the NR‐NR group. We have previously reported that most infants in the NR‐NR pattern group developed CP without severe acidosis at birth, representing antenatal causation. 11 Cowan et al. concluded that perinatal hypoxic events were the most important factors in neonatal encephalopathy. 24 Conversely, most studies have suggested that genetic factors, infection/inflammation, fetal growth restriction and placental abnormalities are more important causes of CP than hypoxia, especially in antenatal causal cases. 2 , 3 , 25 , 26 The result of our study indicated that HI stress is important when a fetus develops severe brain damage leading to CP before labour.
Interestingly, risk profiles of BGT injury in the NR‐NR group were similar to those in the intrapartum causal groups (R‐PD and the R‐Hon groups), that is, umbilical abnormalities, intrauterine infection, placental abruption and SGA, in that order. One possibility is that causes of antenatal HI injury such as umbilical abnormalities and intrauterine infection may be reversible or not severe enough to have a negative influence on fetal survival, the alternative of which would be a stillbirth. As such, causes of antenatal HI injury are generally similar to those of intrapartum HI injury, in which the stress may be acute (R‐PD) or subacute (R‐Hon), and only the timing is different.
4.3. Strengths and limitations
The strength of this study was that it represents a more extensive survey than a previous report. 27 We have demonstrated the association between FHR evolution patterns and neonatal brain MRI findings in cases of severe CP, highlighting the impact of prenatal HI stress on CP development.
This study also had several limitations. First, it was a retrospective, observational study that only included cases of severe CP, and the data were not compared with data on a control group. Thus, there might have been an inherent selection bias, and our results may not be extrapolated to milder forms of CP or milder injuries not leading to CP. Secondly, only MRIs performed within 6 weeks after birth were included. Given that cerebral abnormalities would become most evident between 1 and 2 weeks of life and that the optimal timing might differ according to the nature and timing of the cerebral injury, 12 , 28 the accuracy of MRI at 6 weeks in terms of classifying the brain injury is debatable. Thirdly, sampling bias might have occurred due to the small number of cases for some of the risk factors. Therefore, further studies with larger sample sizes are needed for generalisability.
5. CONCLUSIONS
In this study, we demonstrated that BGT‐dominant brain damage accounted for three‐fourths of severe CP cases in term or near‐term infants. Placental abruption was associated with an increased risk of BGT injury, and SGA was associated with a decreased risk. HI injury may have a major impact on the development of CP, even during the antepartum period. However, our results were only applicable to a subset of patients who underwent MRIs during the neonatal period, and could not be generalised to milder forms of CP or milder injuries not leading to CP. Therefore, further investigation with a larger sample size, including control and mild cases, is needed to comprehend the entire pathophysiology of CP and infer a strategy for preventing brain injuries.
CONFLICT OF INTERESTS
None declared. Completed disclosure of interest forms are available to view online as supporting information.
AUTHOR CONTRIBUTIONS
MN, YN, and TI designed the study. MN, AO, and JH performed the analysis of FHR strips, and YN analysed MRI. MN and ST performed the statistical analysis, and all authors made significant contributions in interpreting data. MN drafted, and TI critically reviewed the manuscript. All authors read and approved the final manuscript.
ETHICS APPROVAL
The ethics committee of JOCSC approved the protocol (No. 30‐01, July 20, 2018). Informed consent was waived by the review board, as this was a retrospective analysis of anonymised data.
Supporting information
Figures S1–S5
Tables S1–S3
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to acknowledge the following persons for their excellent support in data collection: Hitomi Yuasa, Natsumi Tsuchiya, Sana Ohno, Nozomi Kobayashi, Mio Sano, Asami Nagatani, Miyuki Takeuchi, Saori Ikeda, Miki Osa, and Yuka Yamazaki, who are members of the Recurrence Prevention Committee, Japan Obstetric Compensation System for Cerebral Palsy, Public Interest Incorporated Foundation, Japan Council for Quality Health Care.
Nakao M, Nanba Y, Okumura A, Hasegawa J, Toyokawa S, Ichizuka K, Kanayama N, Satoh S, Tamiya N, Nakai A, Fujimori K, Maeda T, Suzuki H, Iwashita M, Oka A, Ikeda T. (2022). Correlation between fetal heart rate evolution patterns and magnetic resonance imaging findings in severe cerebral palsy: A longitudinal study. BJOG: Int J Obstet Gy. 2022;129:1574–1582. 10.1111/1471-0528.17089
This article includes Author Insights, a video abstract available at https://vimeo.com/bjogabstracts/authorinsights17089.
Funding information
None.
DATA AVAILABILITY STATEMENT
The author have elected not to share data.
REFERENCES
- 1. Kodama Y, Sameshima H, Ikeda T, Ikenoue T. Intrapartum fetal heart rate patterns in infants (> or =34 weeks) with poor neurological outcome. Early Hum Dev. 2009;85(4):235–8. [DOI] [PubMed] [Google Scholar]
- 2. McIntyre S, Blair E, Badawi N, Keogh J, Nelson KB. Antecedents of cerebral palsy and perinatal death in term and late preterm singletons. Obstet Gynecol. 2013;122(4):869–77. [DOI] [PubMed] [Google Scholar]
- 3. MacLennan AH, Thompson SC, Gecz J. Cerebral palsy: causes, pathways, and the role of genetic variants. Am J Obstet Gynecol. 2015;213(6):779–88. [DOI] [PubMed] [Google Scholar]
- 4. Badawi N, Kurinczuk JJ, Keogh JM, Alessandri LM, O'Sullivan F, Burton PR, et al. Antepartum risk factors for newborn encephalopathy: the Western Australian case‐control study. BMJ. 1998;317(7172):1549–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Korzeniewski SJ, Slaughter J, Lenski M, Haak P, Paneth N. The complex aetiology of cerebral palsy. Nat Rev Neurol. 2018;14(9):528–43. [DOI] [PubMed] [Google Scholar]
- 6. Phelan JP, Ahn MO. Fetal heart rate observations in 300 term brain‐damaged infants. J Matern Fetal Investig. 1998;8(1):1–5. [PubMed] [Google Scholar]
- 7. Phelan JP, Ahn MO. Perinatal observations in forty‐eight neurologically impaired term infants. Am J Obstet Gynecol. 1994;171(2):424–31. [DOI] [PubMed] [Google Scholar]
- 8. Clark SL, Hamilton EF, Garite TJ, Timmins A, Warrick PA, Smith S. The limits of electronic fetal heart rate monitoring in the prevention of neonatal metabolic acidemia. Am J Obstet Gynecol. 2017;216(2):163.e1–6. [DOI] [PubMed] [Google Scholar]
- 9. Vintzileos AM, Smulian JC. Timing intrapartum management based on the evolution and duration of fetal heart rate patterns. J Matern Fetal Neonatal Med. 2021;13:1–6. [DOI] [PubMed] [Google Scholar]
- 10. Gaffney G, Sellers S, Flavell V, Squier M, Johnson A. Case‐control study of intrapartum care, cerebral palsy, and perinatal death. BMJ. 1994;308(6931):743–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nakao M, Okumura A, Hasegawa J, Toyokawa S, Ichizuka K, Kanayama N, et al. Fetal heart rate pattern in term or near‐term cerebral palsy: a nationwide cohort study. Am J Obstet Gynecol. 2020;223(6):907.e1–13. [DOI] [PubMed] [Google Scholar]
- 12. American College of Obstetricians and Gynecoogists . Focal ischemic stroke, and role of neuroimaging. In: American College of Obstetricians and Gynecologists , editor. Neonatal encephalopathy and neurologic outcome. 2nd ed. (reaffirmed 2019). Washington, DC: American College of Obstetricians and Gynecologists; 2014. pp. 125–38, and 149–72. [cited 2021 July 15]. Available from: https://www.acog.org/store/products/clinical‐resources/neonatal‐encephelopathy‐and‐neurologic‐outcome [Google Scholar]
- 13. Volpe JJ, editor. Hypoxic‐ischemic injury in the term infant: neuropathology, and pathophysiology. In: Volpe's neurology of the newborn. 6th ed. Philadelphia, PA: Elsevier; 2018. pp. 484–99, 500–83. [Google Scholar]
- 14. Michoulas A, Basheer SN, Roland EH, Poskitt K, Miller S, Hill A. The role of hypoxia‐ischemia in term newborns with arterial stroke. Pediatr Neurol. 2011;44(4):254–8. [DOI] [PubMed] [Google Scholar]
- 15. Hasegawa J, Toyokawa S, Ikenoue T, Asano Y, Satoh S, Ikeda T, et al. Relevant obstetric factors for cerebral palsy: from the nationwide obstetric compensation system in Japan. PLoS One. 2016;11(1):e0148122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39(4):214–23. [DOI] [PubMed] [Google Scholar]
- 17. Okai T, Ikeda T, Kawarabayashi T, Kozuma S, Sugawara J, Chisaka H, et al. Intrapartum management guidelines based on fetal heart rate pattern classification. J Obstet Gynaecol Res. 2010;36(5):925–8. [DOI] [PubMed] [Google Scholar]
- 18. Okereafor A, Allsop J, Counsell SJ, Fitzpatrick J, Azzopardi D, Rutherford MA, et al. Patterns of brain injury in neonates exposed to perinatal sentinel events. Pediatrics. 2008;121(5):906–14. [DOI] [PubMed] [Google Scholar]
- 19. Martinez‐Biarge M, Diez‐Sebastian J, Rutherford MA, Cowan FM. Outcomes after central grey matter injury in term perinatal hypoxic‐ischaemic encephalopathy. Early Hum Dev. 2010;86(11):675–82. [DOI] [PubMed] [Google Scholar]
- 20. Korzeniewski SJ, Birbeck G, DeLano MC, Potchen MJ, Paneth N. A systematic review of neuroimaging for cerebral palsy. J Child Neurol. 2008;23(2):216–27. [DOI] [PubMed] [Google Scholar]
- 21. Bajaj M, Mody S, Natarajan G. Clinical and neuroimaging findings in neonatal herpes simplex virus infection. J Pediatr. 2014;165(2):404–7. [DOI] [PubMed] [Google Scholar]
- 22. Oliveira CR, Morriss MC, Mistrot JG, Cantey JB, Doern CD, Sánchez PJ. Brain magnetic resonance imaging of infants with bacterial meningitis. J Pediatr. 2014;165(1):134–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Burns CM, Rutherford MA, Boardman JP, Cowan FM. Patterns of cerebral injury and neurodevelopmental outcomes after symptomatic neonatal hypoglycemia. Pediatrics. 2008;122(1):65–74. [DOI] [PubMed] [Google Scholar]
- 24. Cowan F, Rutherford M, Groenendaal F, Eken P, Mercuri E, Bydder GM, et al. Origin and timing of brain lesions in term infants with neonatal encephalopathy. Lancet. 2003;361(9359):736–42. [DOI] [PubMed] [Google Scholar]
- 25. Moreno‐De‐Luca A, Ledbetter DH, Martin CL. Genetic [corrected] insights into the causes and classification of [corrected] cerebral palsies. Lancet Neurol. 2012;11(3):283–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kaneko M, Sameshima H, Ikeda T, Ikenoue T, Minematsu T. Intrapartum fetal heart rate monitoring in cases of cytomegalovirus infection. Am J Obstet Gynecol. 2004;191(4):1257–62. [DOI] [PubMed] [Google Scholar]
- 27. Yatham SS, Whelehan V, Archer A, Chandraharan E. Types of intrapartum hypoxia on the cardiotocograph (CTG): do they have any relationship with the type of brain injury in the MRI scan in term babies? J Obstet Gynaecol. 2020;40(5):688–93. [DOI] [PubMed] [Google Scholar]
- 28. Rutherford M, Srinivasan L, Dyet L, Ward P, Allsop J, Counsell S, et al. Magnetic resonance imaging in perinatal brain injury: clinical presentation, lesions and outcome. Pediatr Radiol. 2006;36(7):582–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1–S5
Tables S1–S3
Supplementary Material
Data Availability Statement
The author have elected not to share data.


