Abstract
Background
Hidradenitis suppurativa (HS) is a chronic, inflammatory, debilitating skin disease characterized by painful deep lesions and associated with substantial disease burden.
Objectives
The objective of this study was to describe physician‐ and patient‐reported clinical unmet needs from a real‐world perspective.
Methods
This study used data from the Adelphi HS Disease Specific Programme, a point‐in‐time survey of dermatologists and their patients with HS in Europe and the United States. Dermatologists completed patient record forms (PRFs) for 5–7 consecutively consulting patients with HS; patients or carers of patients also optionally completed a patient/carer self‐completion questionnaire (PSC/CSC). Data collection included demographics, symptomatology and impact on quality of life (QoL).
Results
Dermatologists (N = 312) completed PRFs for 1787 patients with HS; patient‐ and carer‐reported questionnaires (PSC/CSC) were completed for 33.1% (591/1787) of patients. The mean age was 34.4 ± 12.2 years and 57.6% of patients were female (1029/1787). Physician‐judged disease severity at sampling was categorized as mild in 66.0% (1179/1787), moderate in 29.3% (523/1787) and severe in 4.7% (85/1787) of patients. Deterioration or unstable condition over the previous 12 months was described by 17.1% [235/1372] and 12.6% [41/325] of physician‐ and patient/carer‐reported cases, respectively. Despite receiving treatment, high proportions of patients still experienced symptoms at sampling (general pain/discomfort [49.5%, 885/1787]; inflammation/redness of lesions/abscesses [46.1%, 823/1787] and itching [29.9%, 535/1787]); these symptoms were more frequent in patients with moderate or severe disease. Patients reported a mean Dermatology Life Quality Index score of 5.9 ± 5.4 (555/591; mild, 4.1 ± 4.3; moderate, 9.4 ± 5.4; severe, 13.3 ± 5.5) and a mean Hidradenitis Suppurativa Quality of Life score of 11.0 ± 10.6 (518/591; mild, 7.6 ± 8.3; moderate, 17.7 ± 10.0; severe, 31.0 ± 15.4) indicating a substantial impact on QoL.
Conclusions
Patients with HS experienced a high disease burden despite being actively treated by a dermatologist. This study demonstrates that the burden of HS disease is generally poorly managed with a considerable impact observed on patients' QoL.
Introduction
Hidradenitis Suppurativa (HS) is a chronic, inflammatory, debilitating skin disease characterized by painful deep lesions progressing to scarring and suppuration. 1 HS is difficult to manage and is associated with a high morbidity burden and disability, as well as a significant unmet medical and socioeconomic need. 2 , 3 , 4 Comorbid diseases are common with HS and contribute to reduced life expectancy. Many of the comorbid diseases are systemic in nature, including metabolic and cardiovascular comorbidities. 1 , 2 Further, patients with HS experience social stigma, isolation, anxiety and depression. Suicidal ideation and suicide completion rates are also elevated in patients with HS. 5 , 6 , 7
HS is a common skin disorder, with recent population‐based studies reporting the prevalence of up to 1.2%. 8 , 9 , 10 , 11 , 12 The diagnosis of HS is based solely on clinical presentation and medical history, with no specific serological markers available; correct diagnosis of HS is therefore reliant on the ability of the treating physician to accurately recognize HS symptomology. 13 , 14 Clinical presentation of HS is heterogeneous, and there are several distinct HS phenotypes, often leading to misdiagnosis. An average delay of 7–10 years has been reported between the onset of symptoms and HS diagnosis. 15 , 16 Potential reasons for this include a lack of disease awareness, misdiagnosis of HS and under‐reporting by patients due to shame and embarrassment.
The aim of this study was to describe the clinical unmet needs of a large, real‐world population of patients with HS across six countries, including disease characteristics and severity, as well as the physician‐ and patient‐reported impact of HS on patients' quality of life (QoL).
Methodology
Study design
The study was a retrospective analysis of a point‐in‐time market research survey to assess patient‐reported unmet clinical needs and burden of disease in the HS population, using data collected as part of the Adelphi HS Disease Specific Program (DSP™) across the United States (US) and five European countries (EU5; France, Germany, Italy, Spain, United Kingdom [UK]). This was a market research survey conducted by Adelphi Real World independently of Novartis. Data collection occurred between November 2020 and April 2021. The survey used a combination of physician‐reported survey data, physician‐completed medical record data extraction, and patient/carer‐reported survey data.
Inclusion and exclusion criteria
General dermatologists actively managing patients with HS were included in the survey; there were no exclusion criteria for dermatologists. Adult patients aged ≥18 years old and adolescents aged 10–17 years old with a diagnosis of HS and visiting a participating dermatologist for their HS at the time of sampling were included. Patients could have been receiving multiple treatment classes together (e.g., topical antibiotics/steroid, antiseptics, conventional systemic therapy, biologic therapy).
Data sources and questionnaires employed
Each dermatologist completed a Patient Record Form (PRF) for the subsequent 5–7 patients with HS attending their practice. The PRF was comprised of 11 sections (A–K) covering the following topics: (A) Patient demographics, (B) Clinical profile, (C) Symptomatology, (D) Pattern of disease, (E) Concomitant conditions, (F) Current and historical treatment details, (G) Unmet needs with current treatment, (H) Consultation history, (I) Hospitalization/surgery, (J) Healthcare insurance, and (K) Survey feedback. Dermatologists also invited the same patients to complete a voluntary self‐completion questionnaire about their condition; these physician and patient data collection forms were linked at data processing. Patient self‐completion forms (PSC) were completed by all patients aged ≥12 years. The carer of the patient (i.e., patient/guardian) could complete a carer self‐completion form (CSC) on behalf of the patient if necessary (e.g., for younger patients or those with a disability); the age for completion of a CSC on behalf of a patients with HS was not pre‐specified in the survey. Only either a PSC or CSC were included per patient. The PSC/CSC was comprised of 10 sections (A–J) covering the following topics: (A) History of HS, (B) About the patient, (C) Severity and symptoms of HS, (D) Impact of HS on everyday life, (E) Dermatology Life Quality Index (DLQI, for patients ≥16 years) or Children's DLQI (CDLQI, validated for patients 4–16 years), (F) Hidradenitis Suppurativa Quality of Life scale (HiSQoL), (G) EuroQol EQ‐5D‐5L, (H) Work Productivity and Activity Impairment (WPAI), (I) HS treatment and (J) Feelings about HS.
Study objectives
The primary endpoint of this survey was to describe the clinical unmet need amongst the HS population (both physician‐ and patient/carer‐reported). Outcomes measured included:
Physician‐judged disease severity (categorized as mild, moderate or severe), recorded retrospectively at the time of first HS diagnosis and at the time of sampling (previously described in Seyger et al. 17 ).
Types and numbers of HS clinical signs (abscesses, inflammatory nodules, draining tunnels and scarring), also recorded both retrospectively at the time of first HS diagnosis and at the time of sampling.
Hurley staging (1–3) at the time of sampling.
Flare and physician‐judged remission status at the time of sampling.
Location and number of affected areas of the body, and type of symptoms at the time of sampling.
The key secondary objectives were to assess the levels of patient‐reported humanistic unmet needs of patients with HS by the assessment of QoL/patient‐reported outcome (PRO) measures and other activities of daily life amongst patients with HS and their caregivers.
Disease severity
Disease severity was physician‐judged, with no clinical definition applied. Dermatologists may have therefore considered multiple factors when subjectively defining a patient's disease severity (e.g., Hurley staging, lesion count, symptomatology, DLQI, HiSQoL). The retrospective rating of severity at first HS diagnosis was provided by a physician other than the dermatologist completing the survey in 47% of PRFs. In these cases, the retrospective rating of severity was extracted from the patient's case notes. Data are represented by severity at first HS diagnosis and at time of sampling, depending on the endpoint measured.
Data analysis
Patient‐reported data collected by the PSC and CSC were pooled; the exception to this was the DLQI and HiSQoL. Since the HiSQoL has only been validated in adults, this was not collected for patients <18 years. Further, instead of DLQI, the CDLQI was collected for patients aged 10–16 years. Basic descriptive statistics were derived using the software package StataCorp. 2019. Stata Statistical Software: Release 16.1, College Station, TX: StataCorp LLC. Data are represented as n/N (where n = number of patients per group, N = total number of patients with available data).
Regulatory and ethics considerations
The survey was performed in compliance with the European Pharmaceutical Market Research Association (EphMRA) and in full accordance with the US Health Insurance Portability and Accountability Act (HIPAA), 1996. Ethical approval was granted by the Western Copernicus Group Institutional Review Board (WCG‐IRB).
Results
Population
Data were collected from 312 dermatologists (US, n = 81 [26.0%]; EU5, n = 231 [74.0%]) representing a total of 1787 patients with HS (US, n = 482 [27.0%]; EU5, n = 1305 [73.0%]). In total, patient‐ and carer‐reported data were collected for 33.1% (591/1787) of patients: 31.8% (568/1787) of patients via the PSC and 1.3% (23/1787) of patients via the CSC. Breakdown of physician and patient sample collection by country are detailed in Table S1. Of particular note, no PSC/CSC were collected from the UK, and only 5.2% (31/591) and 8.3% (49/591) PSC/CSC were collected from Italy and France, respectively.
Of the overall HS population (N = 1787), the mean ± standard deviation (SD) age was 34.4 ± 12.2 years and 57.6% were female (1029/1787). The age range of patients completing a PSC was 14–75 years (mean 33.8 ± 12.0 years) and the age range of carers completing a CSC on behalf of the patient was 10–19 years (mean 13.0 ± 2.7 years; n = 9, <12 years; n = 14, 12–19 years). Additional demographics are detailed in Table 1 . Physician‐judged disease severity at the time of sampling was categorized as mild in 66.0% (1179/1787) patients, moderate in 29.3% (523/1787) patients and severe in 4.7% (85/1787) patients (Table 1). Of those patients completing a PSC/CSC (591/1787), the proportion of patients with mild, moderate and severe disease were similar to that of the full population (mild, 68.4% [404/591]; moderate, 28.9% [171/568]; severe, 2.7% [16/591]).
Table 1.
Survey population demographics overall and based on physician‐judged severity at the time of sampling
| Characteristic | Physician‐judged severity at the time of sampling | |||
|---|---|---|---|---|
| Overall (N = 1787) | Mild (N = 1179) | Moderate (N = 523) | Severe (N = 85) | |
| Patient age, mean ± SD | 34.4 ± 12.2 | 33.8 ± 12.1 | 35.4 ± 12.4 | 37.8 ± 12.5 |
| Sex, Female, n (%) | 1029 (57.6) | 686 (58.2) | 303 (57.9) | 40 (47.1) |
| Ethnicity, n (%) | ||||
| White | 1388 (77.7) | 938 (79.6) | 389 (74.4) | 61 (71.8) |
| African American | 102 (5.7) | 61 (5.2) | 35 (6.7) | 6 (7.1) |
| Asian | 67 (3.7) | 45 (3.8) | 16 (3.1) | 6 (7.1) |
| Hispanic/Latino | 96 (5.4) | 55 (4.7) | 38 (7.3) | 3 (3.5) |
| Weight, kg, mean ± SD | 80.2 ± 16.5 | 79.2 ± 16.2 | 81.5 ± 16.0 | 86.4 ± 20.8 |
| BMI, mean ± SD | 27.8 ± 5.3 | 27.5 ± 5.1 | 28.3 ± 5.2 | 29.6 ± 7.1 |
| Smoking status, n (%) | (N = 1570) | (N = 1032) | (N = 460) | (N = 78) |
| Current smoker | 550 (35.0) | 364 (35.3) | 151 (32.8) | 35 (44.9) |
| Ex‐smoker | 380 (24.2) | 249 (24.1) | 117 (25.4) | 14 (17.9) |
| Currently flaring, yes, n (%) | 216 (21.3) (N = 1014) | 57 (9.9) (N = 575) | 120 (32.4) (N = 370) | 39 (56.5) (N = 69) |
| Currently in remission, yes, n (%) | 805 (45.9) (N = 1755) | 741 (63.7) (N = 1163) | 60 (11.8) (N = 510) | 4 (4.9) (N = 82) |
| Hurley staging | ||||
| Hurley 1 | 978 (54.7) | 854 (72.4) | 122 (23.3) | 2 (2.4) |
| Hurley 2 | 661 (37.0) | 291 (24.7) | 355 (67.9) | 15 (17.6) |
| Hurley 3 | 148 (8.3) | 34 (2.9) | 46 (8.8) | 68 (80.0) |
| Time since 1st physician consultation, months, mean ± SD | 54.7 ± 68.4 (N = 991) | 52.9 ± 68.3 (N = 658) | 53.6 ± 65.1 (N = 290) | 90.2 ± 83.5 (N = 43) |
| Time since 1st HS diagnosis, months, mean ± SD | 41.6 ± 54.2 (N = 1247) | 38.9 ± 47.7 (N = 823) | 40.4 ± 52.7 (N = 367) | 88.7 ± 107.0 (N = 57) |
BMI, Body Mass Index; HS, hidradenitis suppurativa; kg, kilogram; SD, standard deviation; n, number of patients with characteristic; N, number of patients per group.
Physician‐judged severity at time of sampling was generally aligned with current Hurley staging of the worst affected skin region: the majority of mild patients were Hurley 1, and the majority of severe patients were Hurley 2/3 (Fig. S1). Overall, the most commonly reported comorbidities, as reported by physicians, were acne (24.2%, 433/1787), obesity (20.5%, 367/1787), anxiety (13.4%, 240/1787), depression (12.0%, 215/1787) and dyslipidaemia (8.4%, 151/1787) (Table S1). In particular, anxiety, depression and obesity were more frequent with increasing disease severity.
HS disease severity over time
Using severity classifications both retrospectively at the time of first HS diagnosis and at the time of sampling, the unmet need in patients with HS could be ascertained and is illustrated in Fig. 1 . Of those patients with mild HS at diagnosis, 17.0% (80/472) and 2.3% (11/472) reported moderate or severe disease at sampling, respectively (mean duration between diagnosis and sampling was 38.9 months) (Fig. 1). Of those patients with moderate disease at diagnosis, 31.4% (301/959) remained with a moderate disease and 2.8% (27/959) progressed to severe disease at sampling (mean duration between diagnosis and sampling was 40.4 months). Similarly, of those patients with severe disease at diagnosis, 39.9% (142/356) and 13.2% (47/356) had a moderate or severe disease at sampling, respectively (mean duration between diagnosis and sampling was 88.7 months).
Figure 1.
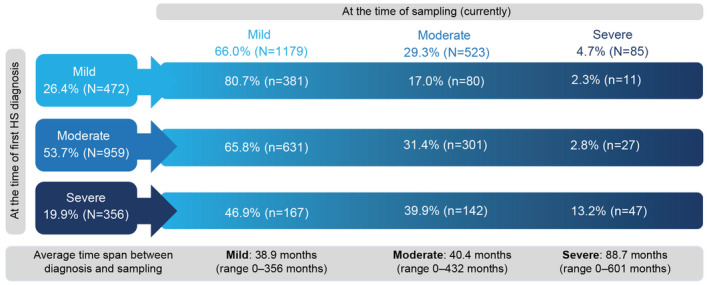
Physician‐judged disease severity retrospectively at the time of diagnosis vs currently at the time of sampling. Disease severity (mild, moderate, severe) at the time of diagnosis and the time of sampling. HS, hidradenitis suppurativa; N, total number of patients per group. [Colour figure can be viewed at wileyonlinelibrary.com]
Further, when dermatologists and patients were asked about disease progression over the past 12 months, only approximately half (physician‐reported, 48.5% [666/1372]; patient/carer‐reported, 62.2% [202/325]) reported an improvement in their condition (Fig. S1). Additionally, large proportions of moderate and severe patients reported a disease which was deteriorating or unstable in the previous 12 months (as reported by physicians, Fig. S1A; as reported by patient/carers, Fig. S1B).
Presence of HS clinical signs over time
Using the reported occurrence of HS clinical signs retrospectively and currently at the time of sampling, the effect of receiving a diagnosis of HS later in the disease course could be hypothesized (Fig. 2). For those patients receiving a diagnosis of HS when disease severity was mild, the frequency of HS clinical signs at the time of sampling was greatly reduced. In particular, 48.2% (204/423) of mild patients reported the presence of 2 or more inflammatory nodules at first HS diagnosis, compared to 10.7% (126/1174) at sampling (Fig. 2b).
Figure 2.
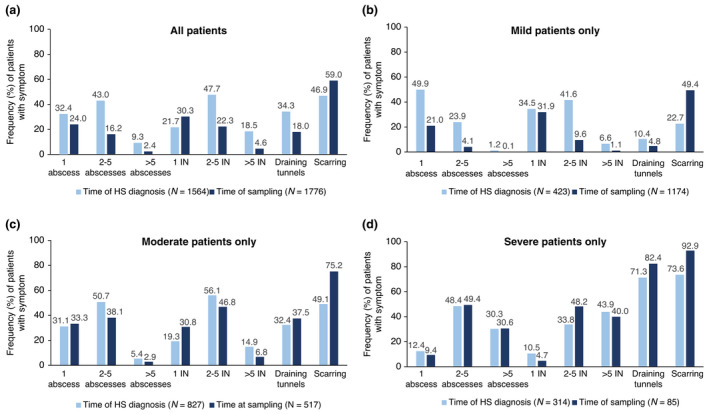
Presence and number of HS clinical signs based on physician‐judged severity between time of diagnosis vs currently at the time of sampling. Proportion of patients (%) experiencing clinical signs both at time of diagnosis and sampling (a) overall, (b) mild patients only, (c) moderate patients only and (d) severe patients only. HS, hidradenitis suppurativa; IN, inflammatory nodule; N, number of patients per group. [Colour figure can be viewed at wileyonlinelibrary.com]
For those patients receiving a diagnosis of HS with a more advanced disease severity (moderate or severe), the frequency of severe HS clinical signs remained relatively unchanged or had worsened at the time of sampling. This was particularly evident in patients with physician‐judged moderate or severe disease (Fig. 2c,d). Overall, almost half (46.9% [733/1564]) of patients with HS reported scarring at diagnosis. At the time of sampling, nearly all moderate and severe patients reported the presence of scarring (moderate, 75.2% [389/517]; severe, 92.9% [79/85]) (Fig. 2c,d).
Uncontrolled current HS symptoms and areas affected
Despite receiving treatment for their HS, large proportions of patients still experienced HS symptoms at the time of sampling (Table 2). In particular, troublesome problems such as drainage from lesions, malodorous drainage and low mood/depression were substantially more frequent in patients with moderate or severe disease. Overall, an average of 2.4 ± 1.6 body areas were affected by HS. This was higher in patients with greater disease severity at the time of sampling (mild, 2.1 ± 1.4 areas; moderate, 2.7 ± 1.7 areas; severe, 4.2 ± 1.8 areas) (Table 2). The most common areas affected overall were the armpits (60.4%, 1080/1787), groin (45.7%, 817/1787) and buttocks (26.5%, 474/1787).
Table 2.
Physician‐reported frequency of symptoms present, and areas affected overall and based on physician‐judged severity at the time of sampling
| Physician‐judged severity at the time of sampling | ||||
|---|---|---|---|---|
| Overall (N = 1787) | Mild (N = 1179) | Moderate (N = 523) | Severe (N = 85) | |
| Symptom, n (%) | ||||
| General pain/discomfort | 885 (49.5) | 452 (38.3) | 364 (69.6) | 69 (81.2) |
| Inflammation/redness of HS lesions/abscesses | 823 (46.1) | 411 (34.9) | 342 (65.4) | 70 (82.4) |
| Itching | 535 (29.9) | 333 (28.2) | 183 (35.0) | 19 (22.4) |
| Drainage from HS lesions/abscesses | 474 (26.5) | 175 (14.8) | 233 (44.6) | 66 (77.6) |
| Pain on sitting | 428 (24.0) | 179 (15.2) | 191 (36.5) | 58 (68.2) |
| Restricted/painful movement of arms/legs | 421 (23.6) | 184 (15.6) | 194 (37.1) | 43 (50.6) |
| Infection of HS lesions/abscesses | 295 (16.5) | 121 (10.3) | 141 (27.0) | 33 (38.8) |
| Malodorous drainage | 278 (15.6) | 98 (8.3) | 129 (24.7) | 51 (60.0) |
| Low mood/depression | 260 (14.5) | 116 (9.8) | 104 (19.9) | 40 (47.1) |
| Fatigue | 228 (12.8) | 92 (7.8) | 110 (21.0) | 26 (30.6) |
| Number of current body areas affected, mean ± SD | 2.4 ± 1.6 | 2.1 ± 1.4 | 2.7 ± 1.7 | 4.2 ± 1.8 |
| Armpits | 1080 (60.4) | 690 (58.5) | 330 (63.1) | 60 (70.6) |
| Groin | 817 (45.7) | 493 (41.8) | 272 (52.0) | 52 (61.2) |
| Buttocks | 474 (26.5) | 255 (21.6) | 162 (31.0) | 57 (67.1) |
| Genitals or pubic region | 398 (22.3) | 202 (17.1) | 152 (29.1) | 44 (51.8) |
| Breast and chest | 345 (19.3) | 190 (16.1) | 128 (24.5) | 27 (31.8) |
| Inner thighs | 288 (16.1) | 164 (13.9) | 92 (17.6) | 32 (37.6) |
| Anus and perianal skin | 233 (13.0) | 122 (10.3) | 83 (15.9) | 28 (32.9) |
| Nape of neck | 150 (8.4) | 88 (7.5) | 50 (9.6) | 12 (14.1) |
| Back | 137 (7.7) | 82 (7.0) | 47 (9.0) | 8 (9.4) |
| Abdomen | 123 (6.9) | 67 (5.7) | 44 (8.4) | 12 (14.1) |
Only the 10 most frequently experienced symptoms or areas affected are listed.
HS, hidradenitis suppurativa; n, number of patients with symptom; N, number of patients per group; SD, standard deviation.
Effect of HS on quality of life
Patients reported a substantial impact of HS on their QoL at sampling (Fig. 3); patients reported an average DLQI score of 5.9 ± 5.4 (mild, 4.1 ± 4.3; moderate, 9.4 ± 5.4; severe, 13.3 ± 5.5; Fig. 3a) and an average HiSQoL score of 11.0 ± 10.6 (mild, 7.6 ± 8.4; moderate, 17.7 ± 10.0; severe, 31.0 ± 15.4; Fig. 3c). Despite having a physician‐judged mild disease, only 36.1% (137/379) of patients were experiencing no impact of disease on QoL (DLQI 0–1); Further, 37.3% (60/161) and 66.7% (10/15) of moderate and severe patients were experiencing a very‐to‐extremely large effect on their QoL (DLQI 11–30; Fig. 3b). CDLQI was included for patients 10–15 years (since CDLQI is validated for paediatric patients aged ≤15 years; collected by CSC, 18/591). The mean CDLQI was 7.2 ± 5.2 (mild, 6.9 ± 5.4 [16/18]; moderate, 10.2 ± 2.8 [2/18]).
Figure 3.
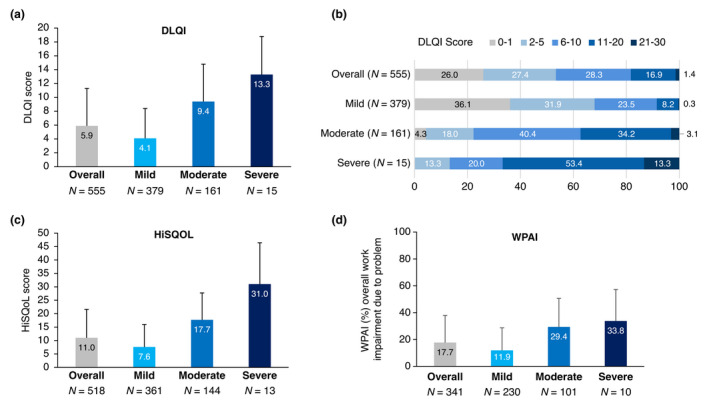
Effect of HS on patient quality of life overall, and by physician‐judged severity at sampling. Bar graphs demonstrating (a) average DLQI scores, (b) proportion of patients (%) with DLQI ranges (PSC, N = 555 for both A and B), (c) average HiSQoL scores (PSC, N = 518) and (d) overall work impairment due to HS (PSC, N = 341). Patient‐reported data includes PSC and CSC responses only. CSC, carer self‐completion questionnaire; DLQI, Dermatology Life Quality Index; HiSQoL, Hidradenitis Suppurativa Quality of Life; n, number of patients per group; PSC, patient self‐completion questionnaire; WPAI, Work Productivity and Activity Impairment. [Colour figure can be viewed at wileyonlinelibrary.com]
WPAI assessment uncovered a mean overall work impairment of 17.7%, which was substantially more pronounced with increasing disease severity (mild, 11.9%; moderate, 29.4%; severe, 33.8%; Fig. 3d).
Patients were asked about the impact HS had on everyday activities (scale: no effect, rarely affected, sometimes affected and greatly affected; Fig. 4). Overall, the most affected areas were personal appearance and self‐confidence (15.1%, 87/577), mood (12.7%, 73/576), close personal relationships (10.2%, 59/578), feelings about the future (6.8%, 39/573), leisure activities (6.8%, 39/572) and motivation (6.5%, 37/567). The biggest impact was observed in moderate and severe patients, although many patients with mild disease were also sometimes affected (Fig. 4a–f).
Figure 4.
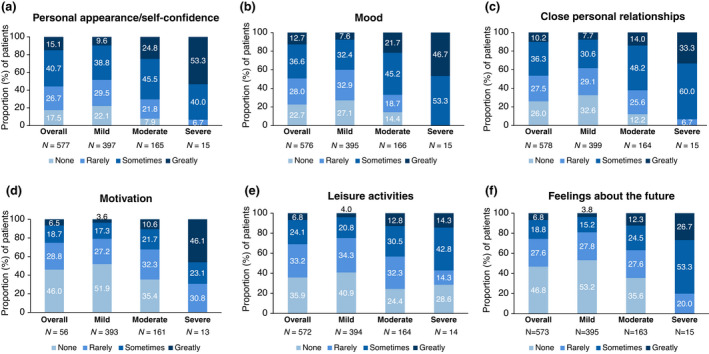
Patient‐reported impact of HS on everyday life by physician‐reported severity at sampling. Bar graphs demonstrating the effect of HS on (a) daily activities including personal appearance/self‐confident, (b) mood, (c) close personal relationships, (d) motivation, (e) leisure activities and (f) feelings about the future (F). Data is represented as proportion of patients (%). Patient‐reported data includes responses from both the PSC (N = 556) and CSC (N = 23). CSC, carer self‐completion questionnaire; PSC, patient self‐completion questionnaire; N, number of patients per group. [Colour figure can be viewed at wileyonlinelibrary.com]
Discussion
HS is a chronic debilitating skin disease, associated with a substantial burden both on the patient, due to the substantial impact on many aspects of QoL and on the physician, due to lack of recognition of the condition and its challenging management. The primary aim of this study was to assess physician‐ and patient/carer‐reported clinical unmet needs amongst a large HS population across six countries in two continents. This survey was designed to facilitate understanding of real‐world clinical practice; the minimal inclusion and exclusion criteria ensured a broad cross‐section of physicians and patients, allowing the data to be representative of patients with HS consulting a dermatologist. Further, the geographic distribution of participating countries allows extrapolation of these findings to diverse clinical practices.
These data demonstrate that over 70% of patients were diagnosed with a disease that was already characterized as moderate to severe in nature. Since the clinical presentation and the progression of HS is heterogenous, delays in diagnosis and misdiagnosis of HS are a constant feature of this disease. 18 , 19 , 20 This may account for the high proportion of patients presenting with more advanced disease. Kokolakis et al. recently described the association of delay in diagnosis with greater disease severity and increased number of surgically treated sites and comorbidities, indicating that a delay in diagnosis results in poorer outcomes for patients. 21 Additionally, despite being currently treated by a dermatologist for their HS, approximately 34% of patients continued to have moderate to severe disease. Within the previous year from start of the survey, approximately 17% of patients had unstable or deteriorating HS.
At diagnosis, patients were already presenting with many clinical signs of HS, including multiple abscesses and inflammatory nodules, and draining tunnels. Furthermore, almost half of patients already reported scarring at the time of diagnosis. Patients were relatively successfully managed when their disease was diagnosed at a mild stage, compared to diagnosis with an already‐substantial inflammatory burden (i.e., moderate or severe). In particular, the presence of draining tunnels was unchanged or more prevalent between diagnosis and sampling timepoints in moderate and severe patients. These data highlight the importance of early recognition and diagnosis of HS, since the inflammatory burden associated with a more severe disease is more challenging to treat. 21 , 22
The impact of HS on QoL as reported by patients was substantial and increased with greater HS disease severity. More severe HS was also generally associated with an increased burden of comorbidities. Anxiety and depression were amongst the most frequently experienced comorbidities and the DLQI and HiSQoL results indicated that patients with HS were highly impacted by their disease. Compared to the global VOICE study, the proportion of patients reporting anxiety and depression was lower in the present study, 16 however the severity of patients included in VOICE was not reported and it could represent a more advanced population. The areas most affected by patients with HS were personal appearance and self‐confidence, mood and close personal relationships. These results support previous studies which described the negative social and emotional consequences associated with HS, in which patients frequently experience shame and stigma due to their HS. 2 , 23 , 24
Potential limitations of this survey include the use of subjective, physician‐judged severity assessment. Additionally, there were no patient‐reported data included for the UK and few responses from Italy and France. Therefore, patient‐reported data are skewed to the German, US and Spanish populations. Recall bias may have also affected the responses of physicians to the questionnaires. This survey is cross‐sectional in nature and cannot be used to demonstrate cause and effect. Finally, this survey was conducted during the Coronavirus (COVID‐19) pandemic and a large proportion of consultations occurred virtually (16.9%, 302/1787), which may have biased clinical and severity assessments. Furthermore, virtual consultations may account for the low number or absence of PSC completed in the UK and Italy. However, demographics between those who consulted virtually and face to face were similar, suggesting virtual consultations did not affect data collection.
Conclusion
In a large real‐world population of patients with HS across Europe and the US, there was a high burden of disease despite active treatment by a dermatologist; over 70% of patients were already exhibiting moderate or severe disease when first diagnosed with HS. This survey suggests that HS is poorly controlled in the general population which leads to flares and disease progression over time. Further, a considerable impact was observed on patients' QoL. The high inflammatory burden, the significant impact on patients' lives and the progressive nature of the disease suggests that greater disease awareness, shortening of time to first diagnosis and availability of better therapeutic options to control symptoms are highly desirable goals.
Supporting information
Table S1 Physician and patient sample by country.
Table S2 Common comorbidities suffered by patients with HS by physician‐judged severity at the time of sampling.
Figure S1 Hurley staging in physician‐judged mild, moderate and severe patients at the time of sampling.
Figure S2 Physician‐ and patient‐/carer‐reported perception of disease progression in the previous 12 months by physician‐judged severity at sampling.
Acknowledgements
The authors thank Trudy McGarry, PhD for providing medical writing support, which was funded by Novartis Pharma AG, Basel, Switzerland in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Funding sources
This study was funded by Novartis Pharma AG, Basel, Switzerland.
Conflicts of interest
JR Ingram has acted as a consultant and/or advisory board member for Novartis, UCB, ChemoCentryx, Boehringer Ingelheim, Insmed, Viela Bio and Kymera Therapeutics. JR Ingram also receives an editorial stipend from the British Journal of Dermatology as Editor‐in‐Chief and an author honorarium from UpToDate. JR Ingram is co‐copyright holder of HiSQOL and Investigator and Patient Global Assessment instruments for HS. V Bettoli has acted as a consultant, advisory board member, and investigator and received honoraria from AbbVie, Beiersdorf, Bioderma, Biogena, Difa‐Cooper, Galderma, GSK, ICF, LEO Pharma, L'Oreal, Meda, Menarini‐Relife, Mylan, Novartis, Pharcos‐Biodue, UCB and has received research support from AbbVie. JI Espy has nothing to disclose. G Kokolakis has received honoraria and travel grants and acted as an advisory board member and investigator for AbbVie, Abbott, Actelion Pharmaceuticals, Amgen, Basilea Pharmaceutica, Bayer, Biogen IDEC, Boehringer Ingelheim, Bristol‐Myers Squibb, Celgene, Hexal, Janssen‐Cilag, LEO Pharma, Lilly, MSD Sharp and Dohme, Mylan, Novartis, Parexel, Pfizer and UCB. A Martorell has acted as a consultant, advisory board member, and investigator and received honoraria from Novartis, AbbVie, Janssen Cilag, UCB, Lilly, LEO Pharma, L'Oreal, Sanofi, Sandoz and Amgen. AP Villani has acted as a consultant for AbbVie, Almirall, Janssen, LEO Pharma, Lilly, MSD, Novartis and UCB. C Richardson, T Kasparek and E Mucsianisi are employed by Novartis Pharma AG, Basel, Switzerland. H Wallinger and E Coak are employed by Adelphi Real World, Bollington, United Kingdom. AB Kimball is a consultant and investigator for Abbvie, Janssen, Eli Lilly, Novartis, Pfizer, and UCB; investigator for Incyte, Bristol Meyers Squibb, and Anapyts Bio; consultant for Bayer, Ventyx, Moonlake, Concert, EvoImmune, receives fellowship funding from Janssen and Abbvie; and served as previous Board of Directors and Past President of the International Psoriasis Council, Board of Directors of the HS Foundation, and Board of Directors for Almirall.
Data availability statement
Data collection was undertaken by Adelphi Real World as part of an independent survey, entitled the Hidradenitis Suppurativa Disease Specific ProgrammeTM (DSP), subscribed to by multiple pharmaceutical companies, of which, one was Novartis Pharma AG. Novartis Pharma AG did not influence the original survey through either contribution to the design of questionnaires or data collection. All data that support the findings of this study are the intellectual property of Adelphi Real World. All requests for access should be addressed directly to Hayley Wallinger at hayley.wallinger@adelphigroup.com.
References
- 1. Sabat R, Jemec GBE, Matusiak L, Kimball AB, Prens E, Wolk K. Hidradenitis suppurativa. Nat Rev Dis Primers 2020; 6: 18. [DOI] [PubMed] [Google Scholar]
- 2. Dufour DN, Emtestam L, Jemec GB. Hidradenitis suppurativa: a common and burdensome, yet under‐recognised, inflammatory skin disease. Postgrad Med J 2014; 90: 216–221 quiz 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kirby JS, Miller JJ, Adams DR, Leslie D. Health care utilization patterns and costs for patients with hidradenitis suppurativa. JAMA Dermatol 2014; 150: 937–944. [DOI] [PubMed] [Google Scholar]
- 4. Jemec GB, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol 2015; 73: S4–S7. [DOI] [PubMed] [Google Scholar]
- 5. Onderdijk AJ, van der Zee HH, Esmann S et al. Depression in patients with hidradenitis suppurativa. J Eur Acad Dermatol Venereol 2013; 27: 473–478. [DOI] [PubMed] [Google Scholar]
- 6. Machado MO, Stergiopoulos V, Maes M et al. Depression and anxiety in adults with hidradenitis suppurativa: a systematic review and meta‐analysis. JAMA Dermatol 2019; 155: 939–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thorlacius L, Cohen AD, Gislason GH, Jemec GBE, Egeberg A. Increased suicide risk in patients with hidradenitis suppurativa. J Invest Dermatol 2018; 138: 52–57. [DOI] [PubMed] [Google Scholar]
- 8. Delany E, Gormley G, Hughes R et al. A cross‐sectional epidemiological study of hidradenitis suppurativa in an Irish population (SHIP). J Eur Acad Dermatol Venereol 2018; 32: 467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ingram JR, Jenkins‐Jones S, Knipe DW, Morgan CLI, Cannings‐John R, Piguet V. Population‐based clinical practice research datalink study using algorithm modelling to identify the true burden of hidradenitis suppurativa. Br J Dermatol 2018; 178: 917–924. [DOI] [PubMed] [Google Scholar]
- 10. Theut Riis P, Pedersen OB, Sigsgaard V et al. Prevalence of patients with self‐reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross‐sectional study. Br J Dermatol 2019; 180: 774–781. [DOI] [PubMed] [Google Scholar]
- 11. Garg A, Kirby JS, Lavian J, Lin G, Strunk A. Sex‐ and age‐adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol 2017; 153: 760–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jemec GB, Heidenheim M, Nielsen NH. The prevalence of hidradenitis suppurativa and its potential precursor lesions. J Am Acad Dermatol 1996; 35: 191–194. [DOI] [PubMed] [Google Scholar]
- 13. Zouboulis CC, Desai N, Emtestam L et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol 2015; 29: 619–644. [DOI] [PubMed] [Google Scholar]
- 14. Wang SC, Wang SC, Sibbald RG, Alhusayen R, Bashash M, Alavi A. Hidradenitis suppurativa: a frequently missed diagnosis, part 1: a review of pathogenesis, associations, and clinical features. Adv Skin Wound Care 2015; 28: 325–332 quiz 333‐324. [DOI] [PubMed] [Google Scholar]
- 15. Saunte DM, Boer J, Stratigos A et al. Diagnostic delay in hidradenitis suppurativa is a global problem. Br J Dermatol 2015; 173: 1546–1549. [DOI] [PubMed] [Google Scholar]
- 16. Garg A, Neuren E, Cha D et al. Evaluating patients' unmet needs in hidradenitis suppurativa: results from the global survey of impact and healthcare needs (VOICE) project. J Am Acad Dermatol 2020; 82: 366–376. [DOI] [PubMed] [Google Scholar]
- 17. Seyger M, Bachhuber T, Fang J, Scott A, Lucas J, Meakin S, et al. Unmet clinical needs of pediatric psoriasis patients based on real‐world data from the EU5 and US. European Society for Pediatric Dermatology (ESPD) 20th Annual Meeting. Virtual Meeting. P0351, 2021.
- 18. Andersen RK, Jemec GB. Treatments for hidradenitis suppurativa. Clin Dermatol 2017; 35: 218–224. [DOI] [PubMed] [Google Scholar]
- 19. Gulliver W, Zouboulis CC, Prens E, Jemec GB, Tzellos T. Evidence‐based approach to the treatment of hidradenitis suppurativa/acne inversa, based on the European guidelines for hidradenitis suppurativa. Rev Endocr Metab Disord 2016; 17: 343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bettoli V, Zauli S, Borghi A et al. Oral clindamycin and rifampicin in the treatment of hidradenitis suppurativa‐acne inversa: a prospective study on 23 patients. J Eur Acad Dermatol Venereol 2014; 28: 125–126. [DOI] [PubMed] [Google Scholar]
- 21. Kokolakis G, Wolk K, Schneider‐Burrus S et al. Delayed diagnosis of hidradenitis suppurativa and its effect on patients and healthcare system. Dermatology 2020; 236: 421–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Duran C, Baumeister A. Recognition, diagnosis, and treatment of hidradenitis suppurativa. JAAPA 2019; 32: 36–42. [DOI] [PubMed] [Google Scholar]
- 23. Keary E, Hevey D, Tobin AM. A qualitative analysis of psychological distress in hidradenitis suppurativa. Br J Dermatol 2020; 182: 342–347. [DOI] [PubMed] [Google Scholar]
- 24. Koumaki D, Efthymiou O, Bozi E, Katoulis AC. Perspectives on perceived stigma and self‐stigma in patients with hidradenitis suppurativa. Clin Cosmet Investig Dermatol 2019; 12: 785–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Physician and patient sample by country.
Table S2 Common comorbidities suffered by patients with HS by physician‐judged severity at the time of sampling.
Figure S1 Hurley staging in physician‐judged mild, moderate and severe patients at the time of sampling.
Figure S2 Physician‐ and patient‐/carer‐reported perception of disease progression in the previous 12 months by physician‐judged severity at sampling.
Data Availability Statement
Data collection was undertaken by Adelphi Real World as part of an independent survey, entitled the Hidradenitis Suppurativa Disease Specific ProgrammeTM (DSP), subscribed to by multiple pharmaceutical companies, of which, one was Novartis Pharma AG. Novartis Pharma AG did not influence the original survey through either contribution to the design of questionnaires or data collection. All data that support the findings of this study are the intellectual property of Adelphi Real World. All requests for access should be addressed directly to Hayley Wallinger at hayley.wallinger@adelphigroup.com.


