Abstract
Objectives
Cenobamate is an antiseizure medication (ASM) approved in Europe as adjunctive therapy for adults with inadequately controlled focal seizures. This post hoc analysis reports onset of efficacy and characterizes time to onset, duration, and severity of the most common treatment‐emergent adverse events (TEAEs) during cenobamate titration.
Materials & Methods
Adult patients with uncontrolled focal seizures taking 1 to 3 concomitant ASMs were randomized to receive adjunctive cenobamate or placebo (double‐blind studies C013 and C017) or cenobamate (open‐label study C021). Outcome assessments included efficacy (median percentage change in seizure frequency and onset [studies C013 and C017]) and safety (onset, duration, and severity of TEAEs [all studies]).
Results
Onset of efficacy was observed by Weeks 1 to 4 of titration in studies C013 and C017 which used a faster titration schedule than study CO21. In study C013, the median percentage seizure frequency reduction was 36.7% in patients receiving cenobamate versus 16.3% in those taking placebo (p = .002); in study C017, significant differences in seizure frequency emerged in Week 1 and continued throughout titration between all cenobamate groups and placebo (p < .001). The most commonly reported TEAEs were somnolence, dizziness, fatigue, and headache, with first onset of each reported as early as Week 1; however, the majority resolved.
Conclusions
Reductions in seizure frequency occurred during titration with initial efficacy observed prior to reaching the target dose. These reductions were regarded as clinically meaningful because they may indicate early efficacy at lower doses than previously expected and had a considerable impact on patient quality of life. Long‐term treatment with adjunctive cenobamate was generally safe and well‐tolerated.
Keywords: anticonvulsants; cenobamate; clinical trial; double‐blind method; drug therapy, combination; drug‐related side effects and adverse reactions; drug‐resistant epilepsy; seizures
1. INTRODUCTION
A primary goal for patients in the treatment of epilepsy is to achieve seizure freedom while avoiding intolerable treatment‐related side effects. 1 , 2 Only about one‐half of patients become seizure‐free with their first antiseizure medication (ASM), and nearly one‐third continue to experience uncontrolled seizures despite appropriate treatment with additional ASMs. 2 , 3 , 4 Recently available adjunctive therapies should play an important role in improving the efficacy of epilepsy treatment and moving toward the goal of seizure freedom.
Cenobamate is an ASM approved in Europe as an adjunctive treatment for adults with inadequately controlled focal seizures who have previously failed at least 2 ASMs and is also approved in the United States as adjunctive therapy and monotherapy. Previously, two international, double‐blind, placebo‐controlled clinical trials (studies C013 and C017) demonstrated the efficacy and safety of cenobamate. 5 , 6 A large, international, open‐label safety study (study C021) demonstrated that cenobamate treatment was generally safe and well‐tolerated. 7 Pooled patient retention data across the cenobamate clinical development program reported cumulative cenobamate retention rates of 80% at 1 year and 72% at 2 years. 8
Data from studies C013 and C017 demonstrate that a significant reduction in seizure frequency from baseline may be observed impressively early and prior to reaching the recommended cenobamate maintenance dose of 200 mg/day. 5 , 6 Importantly, the phase 3 clinical trial (study C021) implemented a slower titration schedule than in the randomized controlled trials. The results of this trial demonstrated that initiating cenobamate at 12.5 mg and titrating up every two weeks reduces the number and severity of treatment‐emergent adverse events (TEAEs) compared to the randomized controlled studies. Additionally, a >50% responder rate was observed in 48.1% of patients in Weeks 1 to 4 (12.5–25 mg), even at the corresponding low doses, in the post hoc analysis of the study C021 open‐label trial. 9
The objective of this post hoc analysis was to determine onset of efficacy during titration of cenobamate in studies C013 and C017 and to characterize the onset, duration, and severity of the most common TEAEs in these trials as well as in study C021. Additional information about very early signs of efficacy and/or AEs may improve the therapeutic advice during cenobamate titration and may help to identify early and low‐dose responders so that the recommended first maintenance target dose might be reconsidered in some patients.
2. MATERIALS AND METHODS
2.1. Study design and patients
Study C013 (NCT01397968) was a multicenter, double‐blind, placebo‐controlled trial evaluating adults (18–65 years old) with focal seizures who were taking 1–3 ASMs. 5 Patients were randomized to either 200 mg/day cenobamate or placebo after an 8‐week baseline assessment. Details of the study have been previously published. 5 The dosing schedule is shown in Figure 1A. Changes to concomitant ASMs were not allowed during the double‐blind study. The primary outcome was the median percentage change in seizure frequency from baseline every 28 days. Safety outcomes assessed the incidence of TEAEs (treatment‐related and serious) and discontinuations.
FIGURE 1.
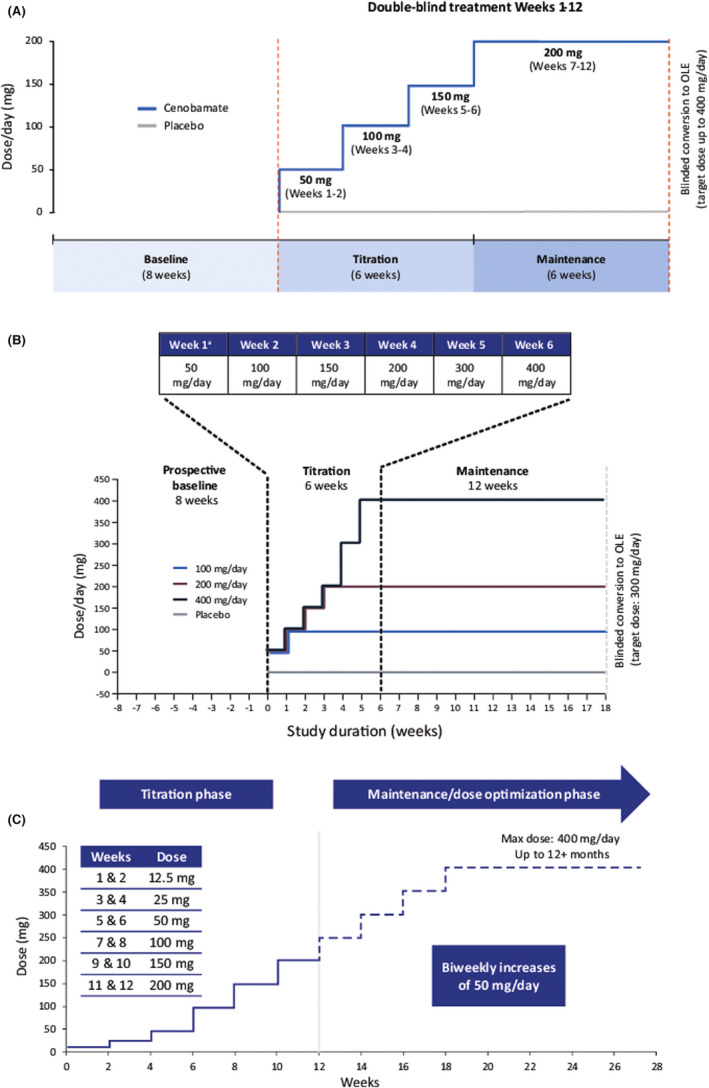
Cenobamate titration schedule during the double‐blind treatment period of (A) study C013, (B) study C017, and (C) open‐label study C021. *The initial starting dose of cenobamate in the original faster titration schedule was 100 mg/day, with weekly increments of 100 mg/day to the target dose. The amended titration schedule reduced the initial starting dose to 50 mg/day and slowed the titration rate to the target dose to improve tolerability
Study C017 (NCT01866111) was the second multicenter, double‐blind, randomized, placebo‐controlled, dose–response trial. 6 Details were previously published, and the dosing schedule is shown in Figure 1B. 6 Adult patients (18–70 years old) with focal seizures who had taken 1–3 ASMs were randomly assigned to adjunctive cenobamate at 100, 200, 400 mg, or placebo following the 8‐week baseline period. No changes to concomitant ASMs were allowed during the double‐blind study. Primary efficacy outcomes included the median percentage change in focal seizure frequency from baseline per 28 days and responder rates (≥50% reduction). Safety and tolerability across treatment groups were compared descriptively.
In the open‐label phase 3 safety and pharmacokinetic study C021 (NCT02535091), patients 18–70 years old with uncontrolled focal seizures taking 1–3 ASMs were enrolled; details were published previously. 7 During the 12‐week titration phase, concomitant ASMs and cenobamate doses could be adjusted as clinically needed. However, patients taking concomitant phenytoin or phenobarbital were not allowed to adjust the cenobamate titration rate or other concomitant ASMs, but their current doses of phenytoin/phenobarbital could be decreased by 25%–33% at any visit with a maximum reduction up to two‐thirds of the baseline dose due to dose‐related toxicity. During the open‐label maintenance phase, cenobamate and other ASMs could be adjusted, removed, or added (with the exception of the addition of phenytoin or phenobarbital).
2.2. Outcome assessments
The median percentage change in seizure frequency and onset of efficacy in patients receiving cenobamate versus placebo were evaluated during the 6‐week titration phase in studies C013 and C017. Onset of efficacy examined the reduction in seizure frequency at each week compared to baseline. Time of first onset of TEAEs, duration of TEAE occurrences, and severity of somnolence, dizziness, and fatigue were examined in all 3 studies (C013, C017, and C021). Severity of TEAEs was defined as follows: mild, the AE was easily tolerated and did not interfere with daily activity; moderate, the AE interfered with daily activity, but the subject was still able to function; and severe, the AE was incapacitating and required medical intervention.
2.3. Statistical analysis
Post hoc analysis of efficacy examined the percentage reduction in seizure frequency from baseline to each week during titration using a Wilcoxon rank‐sum test (study C013) or a non‐parametric ANCOVA model (study C017) using ranked data with treatment group as the fixed factor and ranked values of baseline seizure rate as the covariate. The safety and tolerability analysis evaluated the duration, occurrence, and severity of TEAEs using descriptive statistics.
3. RESULTS
3.1. Patients
3.1.1. Demographics and disposition
Patient demographics and baseline characteristics have been previously described and are summarized in Table S1. In study C013, a total of 222 patients were enrolled, all patients received at least 1 dose of cenobamate and were included in the safety population, and rates of study discontinuation were similar in both cenobamate and placebo groups (primary reason for withdrawal was TEAEs). In study C017, the modified intention‐to‐treat safety population included 437 patients, 82% of whom completed the study. TEAEs were the primary reason for discontinuation. In study C021 (median treatment duration 33.4 months; data cutoff June 2020), 1340 patients were enrolled, of whom 424 (31.6%) discontinued, most commonly due to TEAEs and withdrawn consent for reasons other than a TEAE; 1340 patients received ≥1 treatment dose (median modal dose = 200 mg), and 904 patients were ongoing at data cutoff (June 2020).
3.2. Efficacy during titration
3.2.1. Study C013
At Week 2 of titration at the initial dose of 50 mg/day, patients receiving cenobamate had significant reductions in median percentage seizure frequency versus placebo (−26.7% vs. −15.1%; p = .013) (Figure 2A). At Week 4, patients randomized to cenobamate were receiving 100 mg/day and the median percentage reduction in seizure frequency was −36.7% compared with −16.3% in the placebo group (p = .002).
FIGURE 2.
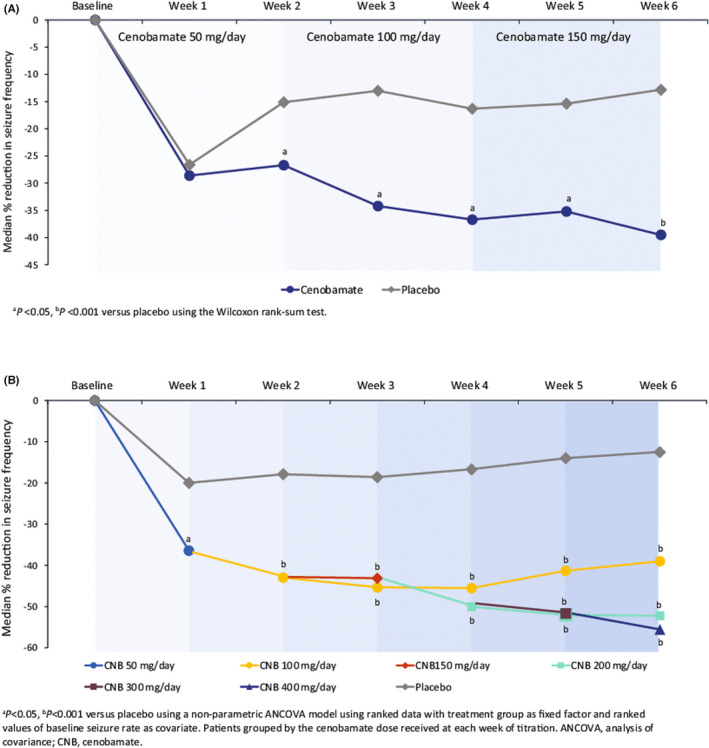
Median percentage reduction in seizure frequency during the titration phase in (A) study C013 and (B) study C017
3.2.2. Study C017
During the titration phase in study C017, already at the end of Week 1, a significant difference was observed in the median percentage reduction in seizure frequency between the cenobamate treatment group (50 mg/day) and the placebo group (−36.4% vs. −20.0%; p = .041) (Figure 2B). For the remaining 5 weeks of the titration phase, significant differences were reported between the groups of patients receiving cenobamate at any dose versus the placebo group. By Week 2, a 42.9% median percentage reduction in seizure frequency was observed in those patients taking 100 mg/day cenobamate compared to 17.9% in those receiving placebo (p < .001). At Week 3, patients in the 100 and 150 mg/day cenobamate groups had a 45.3% and 43.1% median percentage reduction in seizure frequency, respectively, versus 18.6% in the placebo group (p < .001). By Week 4, patients in the 100 mg/day and the 200 mg/day cenobamate groups were observed to have a significant reduction in median percentage seizure frequency versus the placebo group (100 mg, −45.5%; 200 mg, −50.0%; placebo, −16.7%; p < .001). At Week 5, all patients in the cenobamate groups (100 mg, 200 mg, and 300 mg/day) had a significant median percentage reduction in seizure frequency compared to patients receiving placebo (−41.3%, −52.0%, and −51.6%, respectively, vs. placebo −14.0%; p < .001). Finally, by Week 6 there was a significant median percentage reduction in seizure frequency between all cenobamate treatment groups and the placebo group (100 mg, −39.0%; 200 mg, −52.2%; 400 mg, −55.5%; placebo, −12.5%; p < .001).
3.3. Safety
3.3.1. Studies C013 and C017: Pooled double‐blind population
In the pooled double‐blind population, TEAEs occurred in 67% of patients (n = 296) taking cenobamate and in 54.6% (n = 118) receiving placebo during the titration period (Table 1). Nervous system disorders were cited as the most commonly occurring TEAEs in both the pooled cenobamate groups (46.2%; n = 204) and in the placebo group (28.2%; n = 61). TEAEs occurring in ≥5% of all patients taking cenobamate included somnolence (21.3% vs. 6.5% placebo), dizziness (19.7% vs. 11.6% placebo), and headache (6.3% vs. 7.9% placebo). Additionally, nystagmus, balance disorder, ataxia, and dysarthria were reported in 6.3%–8.1% of patients in the 400 mg/day group (Table S2). The incidence of TEAEs increased in a dose‐dependent manner.
TABLE 1.
Incidence of treatment‐emergent adverse events during the titration phase in the pooled DB period and study C021
| C013 | C017 | Pooled DB | Study C021 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CNB 200 mg/day | PBO | CNB 100 mg/day | CNB 200 mg/day | CNB 400 mg/day | PBO | Cenobamate | Placebo | Cenobamate | |
| n, % | n = 113 | n = 109 | n = 108 | n = 110 | n = 111 | n = 107 | n = 442 | n = 216 | n = 1340 |
| Subjects with ≥1 TEAE | 74 (65.5) | 57 (52.3) | 57 (52.8) | 69 (62.7) | 96 (86.5) | 61 (57.0) | 296 (67.0) | 118 (54.6) | 960 (71.6) |
| Mild | 36 (31.9) | 32 (29.4) | 31 (28.7) | 37 (33.6) | 35 (31.5) | 41 (38.3) | 139 (31.4) | 73 (33.8) | 589 (44.0) |
| Moderate | 37 (32.7) | 21 (19.3) | 21 (19.4) | 24 (21.8) | 46 (41.4) | 16 (15.0) | 128 (29.0) | 37 (17.1) | 324 (24.2) |
| Severe | 1 (0.9) | 4 (3.7) | 5 (4.6) | 8 (7.3) | 15 (13.5) | 4 (3.7) | 29 (6.6) | 8 (3.7) | 47 (3.5) |
| TEAEs ≥5% | |||||||||
| Nervous system disorders | 44 (38.9) | 34 (31.2) | 41 (38.0) | 46 (41.8) | 73 (65.8) | 27 (25.2) | 204 (46.2) | 61 (28.2) | 612 (45.7) |
| Somnolence | 20 (17.7) | 7 (6.4) | 15 (13.9) | 19 (17.3) | 40 (36.0) | 7 (6.5) | 94 (21.3) | 14 (6.5) | 295 (22.0) |
| Dizziness | 22 (19.5) | 14 (12.8) | 15 (13.9) | 18 (16.4) | 32 (28.8) | 11 (10.3) | 87 (19.7) | 25 (11.6) | 222 (16.6) |
| Headache | 8 (7.1) | 11 (10.1) | 5 (4.6) | 7 (6.4) | 8 (7.2) | 6 (5.6) | 28 (6.3) | 17 (7.9) | 101 (7.5) |
| Discontinuation during titration due to TEAEs, n (%) | |||||||||
| 4 (3.5) | 4 (3.7) | 5 (4.6) | 11 (10.0) | 20 (18.0) | 1 (0.9) | 40 (9.0) | 5 (2.3) | 102 (7.6) | |
| TEAEs leading to study discontinuation, ≥2% | |||||||||
| Ataxia | 0 | 1 (0.9) | 0 | 3 (2.7) | 4 (3.6) | 0 | 7 (1.6) | 1 (0.5) | 3 (0.2) |
| Somnolence | 0 | 1 (0.9) | 0 | 2 (1.8) | 3 (2.7) | 0 | 5 (1.1) | 1 (0.5) | 6 (0.4) |
| Dizziness | 0 | 0 | 0 | 0 | 4 (3.6) | 0 | 4 (0.9) | 0 | 10 (0.7) |
| Nystagmus | 0 | 0 | 0 | 1 (0.9) | 3 (2.7) | 0 | 4 (0.9) | 0 | 0 |
3.3.2. Study C021
During the titration period in study C021, 960 patients (71.6%) reported experiencing at least 1 TEAE (Table 2). Nervous system disorders were the most commonly observed TEAEs and occurred in 45.7% (n = 612) of patients taking cenobamate. In those patients taking concomitant phenytoin or phenobarbital, central nervous system disorders were reported in 47.0% and 48.6% of patients, respectively, and in 45.5% of patients taking other concomitant ASMs (Table S3). Somnolence was the most often reported TEAE and occurred in 22% (n = 295) of all patients, in 16.9% of those taking cenobamate with phenytoin, in 29.7% of those taking phenobarbital, and in 22.1% of patients on other concomitant ASMs.
TABLE 2.
Resolution of treatment‐emergent adverse events during the titration phase in the pooled DB period and study C021
| Pooled DB | Study C021 | |||||
|---|---|---|---|---|---|---|
| Occurred, n | Resolved, n (%) | Median duration, days | Occurred, n | Resolved, n (%) | Median duration, days | |
| Total | 955 | 813 (85.0) | 11.0 | 2797 | 2367 (84.6) | 15.0 |
| Dizziness | 127 | 115 (90.6) | 9 | 241 | 216 (89.6) | 15.0 |
| Somnolence | 116 | 98 (84.5) | 26.5 | 323 | 256 (79.3) | 29.0 |
| Fatigue | 71 | 51 (71.8) | 23.0 | 186 | 123 (66.1) | 41.0 |
Abbreviations: DB, double blind; TEAE, treatment‐emergent adverse event.
3.4. Central nervous system treatment‐emergent adverse events
3.4.1. Somnolence
During the double‐blind titration period in studies C013 and C017, a greater percentage of study participants taking cenobamate reported first onset of somnolence beginning in Week 1 versus study participants taking placebo (Figure 3A). By the end of the titration periods, the incidences of first onset of somnolence reported by the individuals randomized to receive cenobamate across both studies in all cenobamate treatment groups had decreased to ≤1.8%.
FIGURE 3.
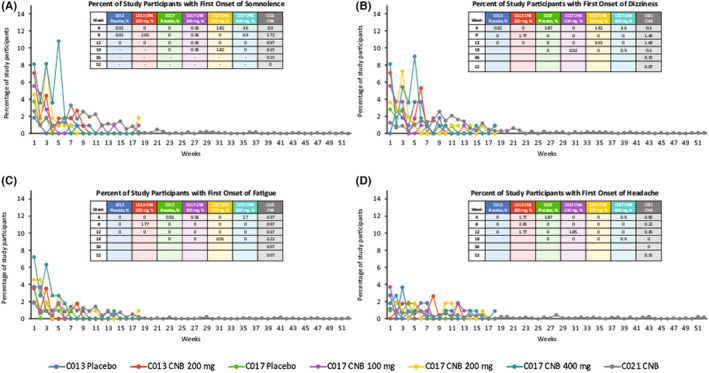
Time to onset of treatment‐emergent adverse events: somnolence (A), dizziness (B), fatigue (C), and headache (D). CNB, cenobamate. The 52 weeks presented along the x‐axis include the pooled double‐blind titration and maintenance phases as well as the open‐label extension period
In study C021, first onset of somnolence was reported during the titration period as early as Week 1. By the end of the 12‐week titration period, the incidence of first onset of somnolence was reported by fewer than 1.4% of patients and remained beneath this threshold for the duration of the study.
3.4.2. Dizziness
During the double‐blind titration period in studies C013 and C017, a greater percentage of study participants taking cenobamate reported first onset of dizziness beginning in Week 1 (cenobamate dose 50 mg/day) versus study participants taking placebo (Figure 3B). During the maintenance phase of the double‐blind studies, the percentage of patients reporting first onset of dizziness in all cenobamate groups after Week 8 was <1.0%.
During the titration period of study C021, first onset of dizziness peaked at Week 9 in patients receiving cenobamate. After titration, the incidence of first onset of dizziness remained low, with fewer than 1.4% of patients reporting first onset of dizziness after Week 12.
3.4.3. Fatigue
During the titration period of study C013, first onset of fatigue was highest among patients randomized to cenobamate during Week 1 and Week 3 compared to study participants randomized to receive placebo for whom first onset of fatigue peaked during Week 1 (Figure 3C).
During the titration period of study C017, first onset of fatigue was reported during Week 1 by patients in the cenobamate 100, 200, and 400 mg/day groups (actual cenobamate dose 50 mg/day). During the maintenance phase of both double‐blind studies, the percentage of patients reporting first onset of fatigue across all treatment arms was low.
During the titration period of study C021, first onset of fatigue was highest in Week 1 for patients taking cenobamate (cenobamate dose 12.5 mg/day). By the end of the 12‐week titration period, the incidence of first onset of fatigue was reported by <1.0% of patients and remained beneath this threshold for the rest of the study.
3.4.4. Headache
During the double‐blind titration period in study C013, first onset of headache was higher at Week 1 for patients randomized to placebo (2.8%) versus patients randomized to cenobamate treatment (0%; cenobamate dose 50 mg/day) (Figure 3D). During the double‐blind titration period in study C017, a greater percentage of study participants taking cenobamate reported first onset of headache beginning in Week 1 versus those taking placebo (cenobamate dose 50 mg/day).
During the maintenance phase of both double‐blind studies, the percentage of patients randomized to cenobamate treatment who reported first onset of headache across all treatment arms was low compared with patients reporting first onset of headache in the placebo groups.
In study C021, first onset of headache was reported during Week 1 of the titration period (1.2%; cenobamate dose 12.5 mg/day). The incidence of first onset of headache continued to fall after Week 1 — by the end of the 12‐week titration period, the incidence of first onset of headache was reported by fewer than 0.5% of patients and remained beneath this threshold for the rest of the study.
3.5. Categorization of treatment‐emergent adverse event severity
Of the TEAEs reported during the studies, 31.4% of these were mild, 29.0% were moderate, and 6.6% were categorized as severe (placebo, 33.8%, 17.1%, and 3.7%, respectively) (Figure 4). Nervous system disorders were the most common TEAEs reported during the titration phase, and somnolence (Figure 4A), dizziness (Figure 4B), and fatigue (Figure 4C) were the most frequently observed events.
FIGURE 4.
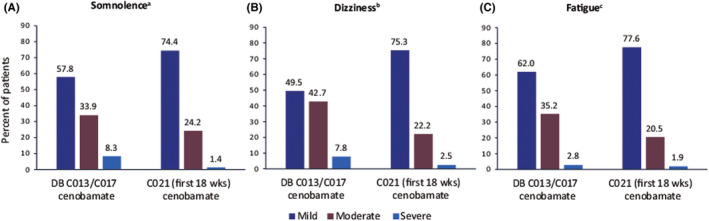
Severity of most common serious treatment‐emergent adverse events in studies C013 and C017 (pooled double‐blind Period) and study C021: (A) somnolence, (B) dizziness, and (C) fatigue. aTotal patients reporting somnolence in DB: 109/442 (24.7%); in C021: 356/1340 (26.6%). bTotal patients reporting dizziness in DB: 103/442 (23.3%); in C021: 275/1340 (20.5%). cTotal patients reporting fatigue in DB: 71/442 (16.1%); in C021: 210/1340 (15.7%). AEs, adverse events; DB, double‐blind period
3.6. Treatment‐emergent adverse events leading to study discontinuation and resolution of adverse events
3.6.1. Discontinuation during titration
Overall, 40 patients taking cenobamate (9.0%) in the pooled double‐blind population had at least 1 TEAE that started in the titration period and led to study drug discontinuation (Table 1). A clear dose–response pattern was observed: 4.6% of patients in the 100 mg/day group, 10.0% in the 200 mg/day group, and 18.0% in the 400 mg/day group discontinued cenobamate treatment versus 0.9% in the placebo group. In the pooled double‐blind period, nervous system disorders were the most common TEAEs leading to discontinuation during titration and occurred in 5.4% (n = 24) of patients. The top 4 TEAEs leading to study discontinuation observed in all patients taking cenobamate in the pooled double‐blind period were ataxia (1.6%; n = 7), somnolence (1.1%; n = 5), dizziness (0.9%; n = 4), and nystagmus (0.9%; n = 4).
In study C021, 102 patients (7.6%) discontinued the study drug due to a TEAE that started during the titration period (Table 1). The most commonly reported TEAEs leading to discontinuation during the titration period were skin and subcutaneous tissue disorders (2.6% of patients; n = 35), including rash (0.6%; n = 8), pruritus (0.2%; n = 3), rash erythematous (0.2%; n = 3), and urticaria (0.2%; n = 3). These TEAEs leading to study discontinuation during the titration period were not observed in any patients taking concomitant phenytoin or phenobarbital.
3.6.2. Resolution of adverse events
The majority of TEAEs resolved on their own without need for treatment. Of the 955 TEAEs that occurred during the titration period in the pooled double‐blind period, 813 (85%) resolved (Table 2). Dizziness in the titration phase of the double‐blind period was resolved in 90.6% (115/127) of instances. The median duration of dizziness was 9.0 days among patients receiving cenobamate. Similarly, 84.5% (98/116) of somnolence reported during the titration phase was resolved. The median duration of somnolence was 26.5 days. Among patients taking cenobamate who reported events of fatigue during titration in the pooled double‐blind period, 71.8% (51/71) of these events resolved, and the median duration of resolved events was 23.0 days.
In study C021, 84.6% (2367/2797) of the TEAEs that occurred during the titration period resolved (Table 2). In cenobamate‐treated patients, somnolence reported during the titration phase was resolved in 79.3% (256/323) of these events. The median duration of resolved events was 29.0 days. Events of dizziness reported during cenobamate titration were resolved in 89.6% (216/241) of instances, and the median duration of resolved events was 15.0 days. During the titration period, 66.1% (123/186) of reported fatigue events resolved, and the median duration of resolved events was 41.0 days.
4. DISCUSSION
A substantial proportion of people with epilepsy (estimated at up to ~40%) do not achieve seizure freedom despite treatment with more than 1 ASM, and many refractory patients may be administered treatment regimens that include 2 to 4 concomitant ASMs. 10 , 11 Clinical trial results have demonstrated that adjunctive cenobamate significantly decreases seizure frequency in adults with uncontrolled focal onset seizures while providing an acceptable and well‐characterized safety profile. Post hoc analyses of studies C013 and C017 indicate that initial signs of efficacy begin to occur early in the titration period prior to reaching the recommended target dose. Sustained significant decreases in seizure frequency with cenobamate versus placebo were observed throughout the 6‐week titration period, with patients reporting clinically meaningful improvement early in the first 4 weeks of treatment, defined as a significant reduction in observed seizure frequency leading to a substantial impact on patient quality of life. Of note, the data suggest that in some instances, doses lower than the previously recommended maintenance dose may be sufficient in some patients and may offer potentially improved tolerability (see following section). The slow titration recommended in the current labeling may help to identify early and possibly sustained low‐dose responders.
In the 3 studies, treatment‐emergent nervous system disorders manifested early in the titration phase (Week 1), but generally decreased over time such that ≤1% of patients in the pooled double‐blind period were reporting somnolence, dizziness, or fatigue by the start of the maintenance phase. The most commonly reported TEAEs that occurred in ≥5% of all patients receiving cenobamate were nervous system disorders, including somnolence, dizziness, and headache. The occurrence of TEAEs increased in a dose‐dependent manner; however, the majority of TEAEs were self‐limited and resolved without treatment.
Clinical data suggest that a slower titration schedule reduces the occurrence and severity of adverse effects for many commonly used ASMs. 12 , 13 , 14 , 15 , 16 A slower titration schedule was used in the long‐term safety study C021. Somnolence, dizziness, and fatigue were reported in a smaller percentage of patients receiving cenobamate during the titration phase of study C021 using the longer titration schedule versus those in the pooled double‐blind studies with faster titration schedules. This finding supports the “start low, go slow” titration protocol utilized in study C021 as an effective strategy to improve tolerability.
A protocol amendment to study C021 allowed for the post hoc collection of seizure data from patient diaries and clinic notes to assess seizure outcomes. Analysis of high‐quality seizure data from a subpopulation of 240 patients at 10 US study sites demonstrated that patients began to respond to cenobamate relatively early in the titration phase. 9 During the first 4 weeks of titration, 48.1% of patients achieved responder rates of ≥50% corresponding to cenobamate doses of 12.5–25 mg/day. Over 60% of patients achieved a ≥ 50% seizure reduction during Weeks 5 to 8 of the titration period with doses of 50–100 mg/day.
Regarding the comparison of initial efficacy observed between studies C013 and C017, a starting dose of 50 mg/day in study C017 demonstrated significantly higher efficacy over placebo after one week and a significant effect was shown at Week 2 in both studies. We can only speculate about this difference, which might be partly explained by population size (there were fewer patients in study C013), and which may have resulted in a reduced chance of revealing statistically significant differences. In addition, baseline seizure frequency was higher in study C017 compared to study C013 (Table S1), which may have also impacted these results.
Indirect treatment comparisons have been made in the scientific literature evaluating commonly used ASMs. The intention of this post hoc analysis was to analyze the efficacy and tolerability of cenobamate during the titration phase, and therefore, discussion of indirect treatment comparisons beyond the titration period based on this post hoc analysis may be open to discussion. However, in a recently published meta‐analysis, it was concluded that cenobamate was associated with a higher responder rate and a better likelihood of providing seizure freedom compared to brivaracetam, eslicarbazepine acetate, lacosamide, and perampanel. 17 The superiority of cenobamate for seizure reduction was reported in a recent review that showed that FDA‐recommended maintenance doses nearly doubled the chances of ≥50% seizure reduction and found that the tolerability profile of cenobamate was consistent with other ASMs in its class. 18
Interactions occurring between concomitant ASMs with varying mechanisms of action may lead to tolerability issues that affect patient compliance. Cenobamate acts as a CYP2C19 inhibitor, significantly increasing exposure to concomitant phenytoin or phenobarbital therapies when used as adjunctive treatment. 19 , 20 Additionally, interactions may occur with use of concomitant clobazam, as its active metabolite (N‐desmethylclobazam) is also metabolized by CYP2C19. 21 , 22 When clobazam is co‐administered with another CYP2C19 inhibitor (like cenobamate), levels of this metabolite may increase twofold to sixfold (based on the interaction between cannabidiol and clobazam and similar interactions), resulting in a greater number of TEAEs. 23 Data from a subpopulation of 240 patients at 10 US study sites from study C021 showed that dizziness and somnolence were reported in some patients receiving lacosamide and cenobamate. These side effects could be due to a pharmacodynamic interaction at lower cenobamate dosages and a possible minimal pharmacokinetic CYP2C19 interaction at 400 mg/day cenobamate. Dose levels of these concomitant ASMs may need to be reduced early in treatment. 24
Limitations in the double‐blind studies C013 and C017 include relatively short treatment duration, potential effects of concomitant ASMs, the use of self‐reported seizure type and frequency, stringent eligibility criteria, and the allowance for changes to concomitant ASMs only during the open‐label extension period. 5 , 6 , 7 Limitations of the safety and pharmacokinetics study C021 included its open‐label design, less stringent eligibility criteria, use of concomitant ASMs, and allowance of changes to concomitant ASMs and/or cenobamate doses.
5. CONCLUSIONS
Significant reductions in seizure frequency occurred during cenobamate titration in studies C013 and C017, with initial efficacy improvements observed as early as the first week of the titration phase, prior to reaching the target dose. First onset of the most commonly occurring TEAEs (somnolence, dizziness, headache, and fatigue) was reported as early as Week 1 of cenobamate titration in all 3 studies, with the exception of headache in study C013; however, TEAEs were usually self‐limited in duration and mostly mild to moderate in severity. Adhering to the C021 titration strategy of initiating cenobamate at 12.5 mg and titrating up every two weeks and the option of lowering concomitant ASMs lead to improved tolerability as evidenced by fewer severe TEAEs reported during the titration period. This approach is a clinically meaningful strategy to reduce emergence of adverse events during cenobamate titration. Furthermore, this strategy may facilitate identification of low‐dose responders who might not need high individual doses to achieve both satisfying efficacy and tolerability.
CONFLICT OF INTEREST
Bernhard J. Steinhoff: Consultant/advisor, speaker honoraria: Al‐Jazeera, Angelini Pharma S.p.a., B. Braun Melsungen, Desitin, Eisai, GW Pharmaceuticals, UCB Pharma, Precisis, Zogenix; Research support: Eisai, GW Pharmaceuticals, SK Life Science, Inc., UCB Pharma. Zogenix. Elinor Ben‐Menachem: Consultant/advisor: Arvelle Therapeutics, GW Pharmaceuticals, UCB Pharma, Sandoz; Research support: Eisai, SK Life Science, Inc., Zogenix; Editor, Acta Neurologica Scandinavica. Christian Brandt: Consultant/advisor: Arvelle Therapeutics, Desitin, Eisai, GW Pharmaceuticals, Idorsia, Novartis, UCB Pharma; Speaker: Eisai, SK Life Science, Inc., UCB Pharma, Upsher‐Smith, Zogenix. Irene García Morales: Nothing to disclose. William E. Rosenfeld: Consultant/advisor: SK Life Science, Inc.; Speaker: Eisai, Greenwich Biosciences (GW Pharmaceuticals), SK Life Science, Inc., Sunovion, UCB Pharma; Research support: Greenwich Biosciences, Marinus, Medtronic, Neurelis, Ovid, SK Life Science, Inc., Takeda, UCB Pharma, Upsher‐Smith. Estevo Santamarina: Consultant/advisor: Eisai, Ferrer, UCB Pharma; Speaker: Eisai, Esteve, Exeltis, UCB Pharma. José M. Serratosa: Consultant/advisor: Arvelle Therapeutics, BIAL, Eisai, GW Pharmaceuticals, Sanofi, UCB Pharma; Speaker: Arvelle Therapeutics, BIAL, Eisai, Sanofi, UCB Pharma.
Supporting information
Table S1–S3
ACKNOWLEDGMENT
The double‐blind studies and open‐label study were funded by SK Life Science, Inc. (Paramus, NJ, USA). Study data were pooled and analyzed by Angelini S.p.a. Nicole Day, PhD, of MedVal Scientific Information Services, LLC (Princeton, NJ, USA) provided medical writing assistance, funded by Angelini S.p.a. The manuscript was prepared according to the International Society for Medical Publication Professionals' “Good Publication Practice for Communicating Company‐Sponsored Medical Research: GPP3.”
Steinhoff, B. J. , Ben‐Menachem, E. , Brandt, C. , García Morales, I. , Rosenfeld, W. E. , Santamarina, E. & Serratosa, J. M. (2022). Onset of efficacy and adverse events during Cenobamate titration period. Acta Neurologica Scandinavica, 146, 265–275. 10.1111/ane.13659
DATA AVAILABILITY STATEMENT
Anonymized data from this study, the study protocol, and statistical analysis plan are available by request from the corresponding author, investigators, or SK Life Science, Inc. (Paramus, NJ, USA), the company that sponsored the clinical development of cenobamate for the treatment of focal epilepsy. The post hoc analysis plan is available by request from the corresponding author or Angelini Pharma, S.p.a (Rome, Italy). At the time of the request, the format and scope of the data to be disseminated will be determined by the authors and Angelini Pharma. A data sharing agreement will need to be signed.
REFERENCES
- 1. Halford JJ, Edwards JC. Seizure freedom as an outcome in epilepsy treatment clinical trials. Acta Neurol Scand. 2020;142:91‐107. [DOI] [PubMed] [Google Scholar]
- 2. Leppik I, De Rue K, Edrich P, Perucca E. Measurement of seizure freedom in adjunctive therapy studies in refractory partial epilepsy: the levetiracetam experience. Epileptic Disord. 2006;8:118‐130. [PubMed] [Google Scholar]
- 3. Tian N, Boring M, Kobau R, Zack MM, Croft JB. Active epilepsy and seizure control in adults ‐ United States, 2013 and 2015. MMWR Morb Mortal Wkly Rep. 2018;67:437‐442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen Z, Brodie MJ, Liew D, Kwan P. Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs: a 30‐year longitudinal cohort study. JAMA Neurol. 2018;75:279‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chung SS, French JA, Kowalski J, et al. Randomized phase 2 study of adjunctive cenobamate in patients with uncontrolled focal seizures. Neurology. 2020;94:e2311‐e2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Krauss GL, Klein P, Brandt C, et al. Safety and efficacy of adjunctive cenobamate (YKP3089) in patients with uncontrolled focal seizures: a multicentre, double‐blind, randomised, placebo‐controlled, dose‐response trial. Lancet Neurol. 2020;19:38‐48. [DOI] [PubMed] [Google Scholar]
- 7. Sperling MR, Klein P, Aboumatar S, et al. Cenobamate (YKP3089) as adjunctive treatment for uncontrolled focal seizures in a large, phase 3, multicenter, open‐label safety study. Epilepsia. 2020;61:1099‐1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sander JW, Rosenfeld WE, Halford JJ, Steinhoff BJ, Biton V, Toledo M. Long‐term individual retention with cenobamate in adults with focal seizures: pooled data from the clinical development program. Epilepsia. 2021;63:139‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sperling MR, Abou‐Khalil B, Aboumatar S, et al. Efficacy of cenobamate for uncontrolled focal seizures: post‐hoc analysis of a phase 3, multicenter, open‐label study. Epilepsia. 2021;62:3005‐3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sirven JI. Evaluation and management of drug‐resistant epilepsy. In: Garcia P, Dashe JF, eds. UpToDate. UpToDate, Inc; 2017. https://www.uptodate.com/contents/evaluation‐and‐management‐of‐drug‐resistant‐epilepsy. Accessed February 24, 2022. [Google Scholar]
- 11. Brodie MJ. Sodium channel blockers in the treatment of epilepsy. CNS Drugs. 2017;31:527‐534. [DOI] [PubMed] [Google Scholar]
- 12. Basheikh M, Sadler RM. Retention rate and efficacy of perampanel with a slow titration schedule in adults. Can J Neurol Sci. 2021;48:105‐111. [DOI] [PubMed] [Google Scholar]
- 13. Biton V, Edwards KR, Montouris GD, Sackellares JC, Harden CL, Kamin M. Topiramate titration and tolerability. Ann Pharmacother. 2001;35:173‐179. [DOI] [PubMed] [Google Scholar]
- 14. Krauss G, Biton V, Harvey JH, et al. Influence of titration schedule and maintenance dose on the tolerability of adjunctive eslicarbazepine acetate: an integrated analysis of three randomized placebo‐controlled trials. Epilepsy Res. 2018;139:1‐8. [DOI] [PubMed] [Google Scholar]
- 15. D'Onofrio G, Kuchenbuch M, Hachon‐Le Camus C, et al. Slow titration of cannabidiol add‐on in drug‐resistant epilepsies can improve safety with maintained efficacy in an open‐label study. Front Neurol. 2020;11:829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schmitz B, Montouris G, Schäuble B, Caleo S. Assessing the unmet treatment need in partial‐onset epilepsy: looking beyond seizure control. Epilepsia. 2010;51:2231‐2240. [DOI] [PubMed] [Google Scholar]
- 17. Lattanzi S, Trinka E, Zaccara G, et al. Third‐generation antiseizure medications for adjunctive treatment of focal‐onset seizures in adults: a systematic review and network meta‐analysis. Drugs. 2022;82:199‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Privitera M, Richy FF, Schabert VF. Indirect treatment comparison of cenobamate to other ASMs for the treatment of uncontrolled focal seizures. Epilepsy Behav. 2022;126:108429. [DOI] [PubMed] [Google Scholar]
- 19. XCOPRI® (Cenobamate Tablets), for Oral Use, CV [Prescribing Information]. SK Life Science, Inc.; 2021. [Google Scholar]
- 20. Vernillet L, Greene SA, Kamin M. Pharmacokinetics of cenobamate: results from single and multiple oral ascending‐dose studies in healthy subjects. Clin Pharmacol Drug Dev. 2020;9:428‐443. [DOI] [PubMed] [Google Scholar]
- 21. Giraud C, Tran A, Rey E, Vincent J, Tréluyer JM, Pons G. In vitro characterization of clobazam metabolism by recombinant cytochrome P450 enzymes: importance of CYP2C19. Drug Metab Dispos. 2004;32:1279‐1286. [PubMed] [Google Scholar]
- 22. Greene S, Orlinsky L, Streicher C, Vermillet L. The pharmacokinetics of cenobamate in special populations [abstract]. Neurology. 2019;92(suppl 15):P1.5‐P1.134. [Google Scholar]
- 23. Geffrey AL, Pollack SF, Bruno PL, Thiele EA. Drug‐drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia. 2015;56:1246‐1251. [DOI] [PubMed] [Google Scholar]
- 24. Rosenfeld WE, Abou‐Khalil B, Aboumatar S, et al. Post‐hoc analysis of a phase 3, multicenter, open‐label study of cenobamate for treatment of uncontrolled focal seizures: effects of dose adjustments of concomitant antiseizure medications. Epilepsia. 2021;62:3016‐3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1–S3
Data Availability Statement
Anonymized data from this study, the study protocol, and statistical analysis plan are available by request from the corresponding author, investigators, or SK Life Science, Inc. (Paramus, NJ, USA), the company that sponsored the clinical development of cenobamate for the treatment of focal epilepsy. The post hoc analysis plan is available by request from the corresponding author or Angelini Pharma, S.p.a (Rome, Italy). At the time of the request, the format and scope of the data to be disseminated will be determined by the authors and Angelini Pharma. A data sharing agreement will need to be signed.


