Abstract
Background
SUCNR1 is a sensor of extracellular succinate, a Krebs cycle intermediate generated in excess during oxidative stress and has been linked to metabolic regulation and inflammation. While mast cells express SUCNR1, its role in mast cell reactivity and allergic conditions such as asthma remains to be elucidated.
Methods
Cord blood‐derived mast cells and human mast cell line LAD‐2 challenged by SUCNR1 ligands were analyzed for the activation and mediator release. Effects on mast cell‐dependent bronchoconstriction were assessed in guinea pig trachea and isolated human small bronchi challenged with antigen and anti‐IgE, respectively.
Results
SUCNR1 is abundantly expressed on human mast cells. Challenge with succinate, or the synthetic non‐metabolite agonist cis‐epoxysuccinate, renders mast cells hypersensitive to IgE‐dependent activation, resulting in augmented degranulation and histamine release, de novo biosynthesis of eicosanoids and cytokine secretion. The succinate‐potentiated mast cell reactivity was attenuated by SUCNR1 knockdown and selective SUCNR1 antagonists and could be tuned by pharmacologically targeting protein kinase C and extracellular signal‐regulated kinase. Both succinate and cis‐epoxysuccinate dose‐dependently potentiated antigen‐induced contraction in a mast cell‐dependent guinea pig airway model, associated with increased generation of cysteinyl‐leukotrienes and histamine in trachea. Similarly, cis‐epoxysuccinate aggravated IgE‐receptor‐induced contraction of human bronchi, which was blocked by SUCNR1 antagonism.
Conclusion
SUCNR1 amplifies IgE‐receptor‐induced mast cell activation and allergic bronchoconstriction, suggesting a role for this pathway in aggravation of allergic asthma, thus linking metabolic perturbations to mast cell‐dependent inflammation.
Keywords: allergic bronchoconstriction, eicosanoid, mast cell hyper‐reactivity, succinate, SUCNR1
This study analyses cord‐blood derived mast cells and human mast cell line LAD‐2 challenged by SUCNR1 ligands for activation and mediator release. Succinate challenge or cis‐epoxysuccinate challenge renders mast cells hypersensitive to IgE‐dependent activation, resulting in augmented degranulation and histamine release, de novo biosynthesis of eicosanoids and cytokine secretion. The succinate‐potentiated mast cell reactivity is attenuated by SUCNR1 knockdown and selective SUCNR1 antagonists and could be tuned by pharmacologically targeting protein kinase C and extracellular signal‐regulated kinase. Activation of SUCNR1 potentiates mast cell‐dependent bronchoconstriction.Abbreviations: AA, amino acid; cES, cis‐epoxysuccinate; cPLA2, cytosolic phospholipase A2; Cys‐LTs, cysteinyl leukotrienes; ERK, extracellular signal‐regulated kinase; GM‐CSF, granulocyte‐macrophage colony‐stimulating factor; Gq, heterotrimeric G protein; IL, interleukin; IgE, immunoglobulin E; LAD‐2, laboratory of allergic diseases 2; PGD2, prostaglandin D2; SUCNR1, succinate receptor 1; TNF, tumor necrosis factor; TXA2, thromboxane A2; XT1, selective SUCNR1 antagonist
1. INTRODUCTION
Mast cells are heterogeneous, tissue‐resident immune cells involved in the pathophysiology of acute allergic reactions 1 and chronic inflammatory diseases of the skin, gut, respiratory, and cardiovascular systems. 2 , 3 Typically, mast cells are activated by FcεRI crosslinking, which is induced by multivalent antigens; however, these cells can also be primed or functionally modulated by cytokines, growth factors, toll‐like receptor ligands and G protein‐coupled receptor (GPCR) ligands (e.g., β‐2‐adrenergic agonists, eicosanoids). 3 , 4 Degranulation and immediate release of pre‐formed mediators, such as histamine, are hallmarks of mast cell activation, which are usually followed by de novo biosynthesis of eicosanoids and subsequent release of inflammatory cytokines. These temporal actions of mast cells direct a series of secondary reactions in surrounding cells and tissues that will orchestrate and propagate the inflammatory response. 1
A range of endogenous metabolites including fatty acids, ketone bodies, and amino acids signal through GPCRs and recently, some of these receptors were found to be expressed on immune cells, thus offering distinct and potentially druggable molecular links between intermediary metabolism and the immune system. 5 , 6 SUCNR1 (GPR91) is a GPCR that senses extracellular succinate, a Krebs cycle intermediate, which accumulates intra‐ and extra‐cellularly under metabolic stress. 6 , 7 , 8 Expression of SUCNR1 has been detected in human tissues with the highest expression in kidney where activation of this receptor links high glucose levels with renin release. 9 , 10 To date, SUCNR1 has been strongly implicated in pathological conditions such as hypertension, ischemic tissue injury, diabetes, and rheumatoid arthritis. 5 , 11
Our work began with screening of human mast cells for expression of a range of metabolite receptors, which revealed very high levels of SUCNR1. The expression of this receptor in mast cells was reported, and mice deficient in SUCNR1 were subjected to mast cell related disease models with varying and unexpected outcomes. 12 While absence of the receptor reduced arthritis, markers of asthma were unaffected and, surprisingly, dermatitis aggravated. To gain further insights to the role of SUCNR1 in human mast cell reactivity and allergic asthma, we examined effects of specific receptor agonists and antagonists in isolated human mast cells and in guinea pig and human ex vivo models of mast cell‐dependent bronchoconstriction.
2. METHODS
2.1. Study approval
The study was approved by the Swedish Animal Experimentation Ethical Review Board (N143/14), the Regional Ethical Review Board in Stockholm (Ref no 2010/181‐31/2 and Ref no 2019–01729) and Swedish work environment authority (202100–2973 v127).
2.2. Cell culture and FcεRI crosslinking
Cord‐blood‐derived mast cells (CBMC) were cultured as previously described. 13 The human mast cell line LAD‐2 (kindly provided by Drs. A. Kirshenbaum and D. Metcalfe, NIH, Bethesda, MD) was maintained in StemPro‐34 SFM medium (Sigma‐Aldrich) supplemented with 100 ng/ml human stem cell factor (hSCF, kindly provided by Sobi, Stockholm, Sweden). 14 Monocyte‐derived macrophages and polymorphornuclear leukocytes (PMN) were isolated and cultured as previously described. 15
CBMC were incubated with 10 ng/ml IL‐4 prior to FcεRI crosslinking. For IgE sensitization, 1 µg/ml human IgE (Calbiochem, Minneapolis, MN) was added to CBMC or LAD‐2 at 24 h before FcεRI crosslinking, which was carried out with anti‐IgE antibody (0.4 µg/ml for CBMC and 100 µg/ml for LAD‐2 cells for 30 min, if not mentioned) (Sigma‐Aldrich). Calcium ionophore A23187 (2 µM, Sigma‐Aldrich) was used as a positive control for activation. The concentrations of both succinate and cES were 1 mM and both were added 30 min before anti‐IgE challenge unless otherwise specified.
2.3. Ex vivo setup
Male albino guinea pigs (Dunkin‐Hartley; 400–450 g; Envigo, Huntingdon, United Kingdom) were sensitized to ovalbumin (OVA) as previously described. 16 Macroscopically, healthy human lung tissue was collected after consent from patients undergoing lobectomy (n = 7, 4 female and 3 male; median 70 years, range: 60–75 years; (Table S1). After resection, the specimens were immediately put in ice‐cold Krebs‐Ringer PSS buffer solution supplemented with 2.5 mM calcium chloride and 2.1 g/L sodium bicarbonate. Within 1 h, isolated bronchial rings with an inner diameter between 0.5 and 2 mm were dissected under microscope and maintained as previously described. 17
2.4. Statistics
All results were presented as mean ± SEM. If not specified, differences among various groups were evaluated using paired Student's t‐test, one‐way or two‐way ANOVA, and a value of p < 0.05 was considered statistically significance.
Please refer to the supplementary materials for reagents and other methods used in the study.
3. RESULTS
3.1. SUCNR1 is highly expressed on human mast cells
Our study began with screening of human mast cells for a range of metabolite receptors, which revealed moderate mRNA expression of GPR35 and GPR41, while SUCNR1 was expressed at a very high level (Figure S1). Next, we examined SUCNR1 expression on human primary myeloid cells and cell lines by qPCR and Western blot. We found, as expected from the screening, that SUCNR1 is abundantly expressed in primary human CBMC and LAD‐2 cells, while minimal expression is observed in human PMN (Figure 1A), as previously reported. 18 The level of expression in mast cells is comparable with that in human monocyte‐derived macrophages, which are well known to express high levels of SUCNR1, 19 , 20 and was upregulated by IL‐4 (Figure 1B and Figure S2), a driver of Th2 responses in allergic inflammation.
FIGURE 1.
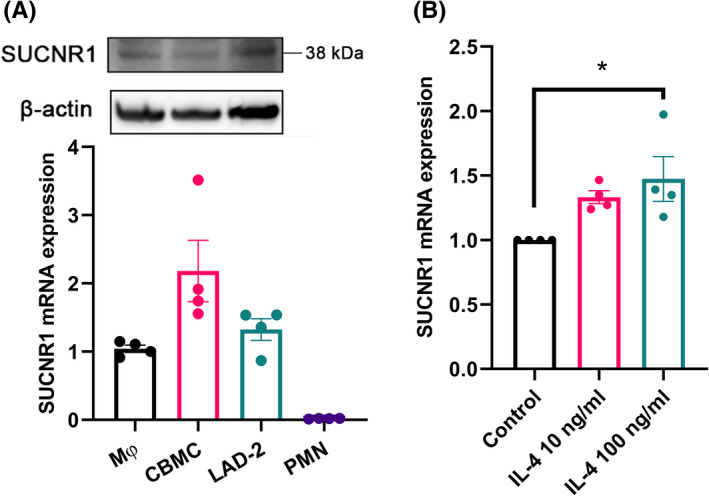
SUCNR1 is expressed in human CBMC and LAD‐2 cells. (A) WB and qPCR analysis of SUCNR1 expression on human CBMC, LAD‐2 cells, monocyte‐derived macrophages (MΦ) and PMN (n = 4). (B) qPCR analysis of SUCNR1 expression on CBMC after 24 h incubation with recombinant human IL‐4, normalized by expression of β‐actin (n = 4). One‐way ANOVA with Dunnett's multiple comparison test was applied. *p < 0.05
3.2. Activation of SUCNR1 enhances IgE‐receptor‐induced mast cell degranulation
We incubated IgE‐sensitized CBMC (referred to as resting mast cells) with succinate or cis‐epoxysuccinate (cES) and assessed CD63 expression—a marker of degranulation, and histamine release. Succinate (1 mM) induced CD63 expression (from 2% to 5% CD63 positive cells; Figure 2A). However, this was not accompanied by a significant induction of histamine release (Figure 2B). Similar effects were observed with cES on CD63 expression and histamine release (Figure 2C,2D), suggesting that SUCNR1 activation alone only causes mild degranulation in CBMC.
FIGURE 2.
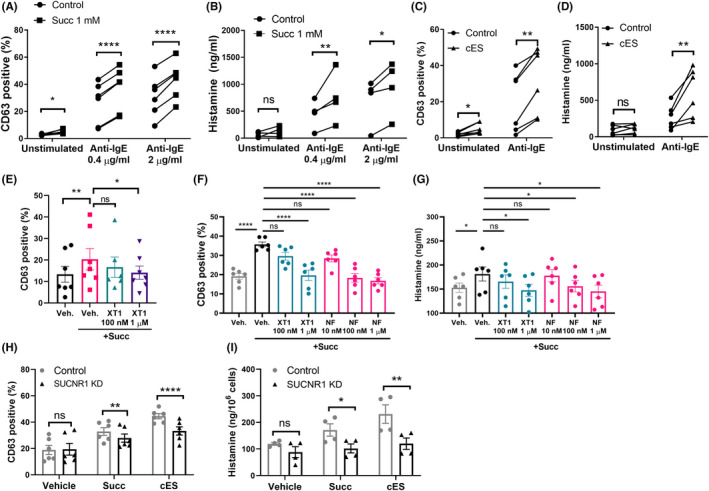
SUCNR1 activation enhances mast cell degranulation and histamine release. CD63 expression or histamine release was analyzed in CBMC treated with (A‐B) succinate or (C‐D) cES for 30 min followed by anti‐IgE challenge. Dotted lines link values from the same donors. (E) CBMC or (F‐G) LAD‐2 cells were treated with XT1 or NF‐56‐EJ40 at desired concentrations for 30 min, followed by succinate incubation and anti‐IgE challenge. (H‐I) Control and SUCNR1 KD LAD‐2 cells were incubated with succinate or cES, followed by anti‐IgE challenge. One‐way ANOVA (E‐G) with Dunnett's multiple comparison test, and RM Two‐way ANOVA with Sidak's multiple comparison test (A‐D, H‐I) were applied. *p < 0.05, **p < 0.01. ***p < 0.001. ****p < 0.0001
We further activated CBMC with 0.4 μg/ml or 2 μg/ml anti‐IgE after 30 min pre‐incubation with succinate, which led to significant induction of CD63 expression and histamine release. Pre‐incubation of CBMC with succinate significantly enhanced CD63 induction relative to cells activated with anti‐IgE alone, associated with increases of histamine release (Figure 2A,2B). Compared with succinate, pre‐incubation with the synthetic SUCNR1 agonist cES caused even greater potentiation of IgE‐receptor induced CD63 expression and histamine release (Figure 2C,2D). To further evaluate the role of SUCNR1 in mast cell degranulation, a potent and selective SUCNR1 antagonist, here denoted XT1, was synthesized according to the previous literature (Compound 4c in Bhuniya et al. 21 ). The binding of XT1 to SUCNR1 was assessed by molecular docking using the recently published crystal structure of humanized rat SUCNR1. 22 With this approach, we found that XT1 binds in the canonical ligand‐binding pocket with its trifluoromethyl moiety buried deep inside the receptor, and exhibits similar binding mode and binding pocket occupancy as observed for the recently reported human specific SUCNR1 antagonist NF‐56‐EJ40 22 (Figure S3). Antagonizing SUCNR1 by XT1 and NF‐56‐EJ40 effectively inhibited succinate potentiated mast cell degranulation (Figure 2E–F) and histamine release (Figure 2G). Moreover, the potentiating effects of succinate and cES on IgE/anti‐IgE‐induced mast cell degranulation and histamine release were reproduced in LAD‐2 cells (Figure S4) and here shRNA knockdown of SUCNR1 attenuated the effect (Figure 2H–I, Figure S5).
3.3. SUCNR1 activation increases de novo biosynthesis of eicosanoids in IgE‐receptor‐activated mast cells
Mast cell activation leads to de novo synthesis of eicosanoids, that is, prostaglandins (PG), thromboxanes (TX), and leukotrienes (LT), a family of lipid mediators derived from arachidonic acid (AA). 23 , 24 In activated cells, the biosynthesis of eicosanoids requires the liberation of AA by cytosolic phospholipase A2 (cPLA2) from membrane phospholipids for further metabolism via the cyclooxygenase (COX) or lipoxygenase (LOX) pathways (Figure 3A). To assess the effect of SUCNR1 activation on eicosanoid biosynthesis, we treated resting and IgE‐receptor‐activated mast cells with succinate and cES, and detected increased phosphorylation of cPLA2 (Figure 3B). Furthermore, in activated mast cells, succinate significantly and efficiently enhanced PGD2 production, associated with increased production of cys‐LTs, TXA2 (measured as its stable metabolite TXB2) and AA, as assessed by LC‐MS/MS (Figure 3C). Levels of the two major eicosanoid products, PGD2 and cys‐LTs, were further assessed by ELISA, which corroborated the potentiating effects of succinate and cES (Figure 3D,E). SUCNR1 antagonists XT1 or NF‐56‐EJ40 dose‐dependently blocked the potentiating effect of succinate on PGD2 production (Figure 3F–G). Knockdown of SUCNR1 in LAD‐2 cells attenuated succinate‐ and cES‐ enhanced cPLA2 phosphorylation (Figure 3H), PGD2 (Figure 3I) and cys‐LTs production (Figure 3J). Notably, 1 mM succinate was able to induce PGD2 release from resting LAD‐2 cells, an effect that was abolished by SUCNR1 knockdown (Figure S6).
FIGURE 3.
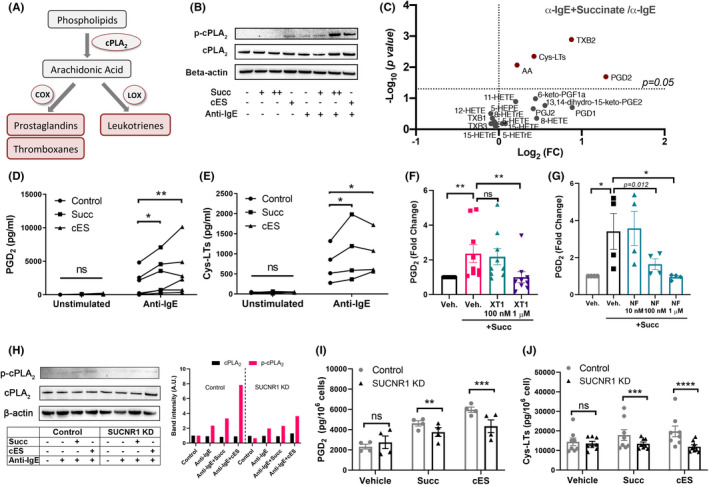
Activation of SUCNR1 promotes de novo biosynthesis of eicosanoids from human mast cells. (A) Biosynthesis of eicosanoids. (B) Western blot analysis of phosphorylated cPLA2 (p‐cPLA2) and cPLA2 in CBMC (+: 0.1 mM, ++: 1 mM). (C) Volcano Plot of eicosanoids in the supernatants of anti‐IgE activated CBMC challenged with succinate (n = 14), analyzed by LC‐MS/MS (cys‐LTs were analyzed by ELISA). (D‐E) PGD2 (n = 6) and cys‐LTs (n = 4) in the supernatants of CBMC challenged by succinate or cES in resting or activated mast cells. (F‐G) Effect of XT1 (n = 8) or NF‐56‐EJ40 (n = 4) on PGD2 released by CBMC. (H) p‐cPLA2 and cPLA2 protein levels in control and SUCNR1 KD LAD‐2 cells. Densitometry of the WB bands was analyzed. (I‐J) PGD2 (n = 4) and cys‐LTs (n = 8) in control and SUCNR1 KD LAD‐2 cells incubated with succinate or cES, followed by anti‐IgE challenge. Paired student's t‐test (C), RM one‐way ANOVA with Dunnett's multiple comparison test (F‐G), and RM Two‐way ANOVA with Sidak's multiple comparison test (D‐E, I‐J) were applied. *p < 0.05, **p < 0.01. ***p < 0.001. ****p < 0.0001
3.4. SUCNR1 activation promotes cytokine production from human mast cells
Activated mast cells release pre‐formed or newly synthesized cytokines, chemokines, and growth factors, contributing to late phase inflammation and tissue remodeling. 25
IL‐8, the most predominant cytokine secreted by mast cells, was found to be significantly upregulated by succinate and cES with or without anti‐IgE activation (Figure 4A,B). Furthermore, succinate or cES alone induced a panel of cytokines from resting mast cells, best represented by GM‐CSF and IL‐13, as well as TNF‐α, IL‐6, IFN‐γ, IL‐5, IL‐1β, IL‐17A, and MIP1β. Moreover, activation of SUCNR1 and FcεRI signaling showed synergy in producing GM‐CSF, TNF‐α, IL‐5, IL‐13, IL‐4, IL‐2, IL‐1β, IL‐17A, and G‐CSF from human CBMC, whereas the levels of IFN‐γ, IL‐7, MCP‐1, MIP1β, or IL‐10 were not influenced by succinate or cES in activated mast cells (Figure 4C, Table S2). In addition, the relative amounts of cytokines were analyzed, and a marked change in cytokine signature was observed under SUCNR1 activating conditions (Figure 4D,E).
FIGURE 4.
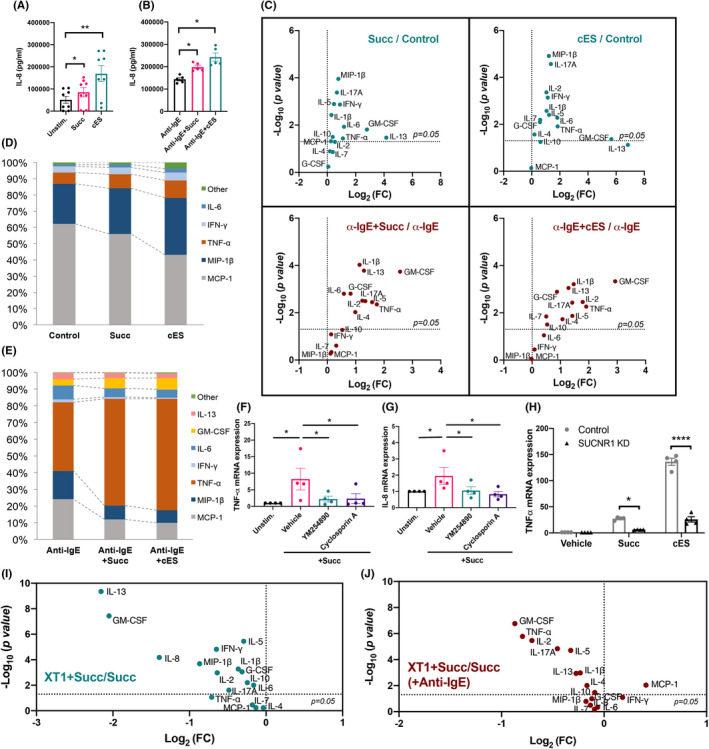
SUCNR1 ligation promotes cytokine release from resting and IgE‐receptor activated mast cells. (A‐E) CBMC were pre‐incubated with succinate or cES, followed by 24 h incubation with 0.4 μg/ml anti‐IgE. Afterward, the supernatants were collected and analyzed by Bio‐plex assays (n = 8 and n = 4) and 17‐plex cytokine assay (n = 10). (F‐G) qPCR analysis of TNF and IL‐8 mRNA expression in IgE‐sensitized CBMC, pre‐incubated 30 min with or without 100 nM YM254890 or 1 μM cyclosporin A, followed by incubation of succinate (n = 4). (H) qPCR analysis of TNF mRNA in control and SUCNR1 KD LAD‐2 cells pre‐incubated with vehicle succinate or cES, followed by 24 h incubation of 100 μg/ml anti‐IgE (n = 4, normalized to β‐actin expression). (I‐J) Multiplex cytokine analysis of CBMC incubated with 1 µM XT1, followed by succinate and anti‐IgE (0.4 μg/ml, 24 h) (n = 8). Paired student's t‐test (C, I‐J), RM one‐way ANOVA with Dunnett's multiple comparison test (F‐G), and RM two‐way ANOVA with Sidak's multiple comparison test (H) were applied. *p < 0.05, **p < 0.01. ***p < 0.001. ****p < 0.0001
In line with the cytokine secretion, mRNA expression of TNF‐α and IL‐8, two of the most abundantly expressed cytokines in CBMC, were upregulated by succinate and cES, suggesting that SUCNR1 influences the transcriptional regulation of cytokine synthesis. Furthermore, the upregulation of TNF‐α and IL‐8 transcripts was blocked by YM254890, a Gq inhibitor, (Figure 4F) and cyclosporin A, an inhibitor of nuclear factor of activated T cells (NFAT), a key family of transcription factors involved in cytokine expression in mast cells (Figure 4G). Compared with control cells, SUCNR1 deficiency dramatically reduced TNF‐α expression in succinate‐ or cES‐ treated cells (Figure 4H). Moreover, 1 μM XT1 counteracted the effects of succinate or cES on specific cytokine production in resting and activated mast cells (Figure 4I,J and Table S3).
3.5. Succinate signals through SUCNR1—protein kinase C (PKC)—extracellular signal‐regulated kinase (ERK) pathway to enhance mast cell activation
Using CBMC or LAD‐2 cells, we found that CD63 expression was blocked by the Gq inhibitor YM254890 (Figure 5A) but not the Gi inhibitor pertussis toxin (PTX) (Figure S7), suggesting SUCNR1 signals through Gq to potentiate mast cell degranulation. Succinate‐ or cES‐induced a rapid and significant increase in [Ca2+]i (Figure 5B). SUCNR1 activation also enhanced ERK phosphorylation in IgE‐receptor‐activated cells (Figure 5C), an effect that could be reduced by SUCNR1 antagonist and genetic knockdown (Figure 5D). Both ERK phosphorylation and accompanying PGD2 production in LAD‐2 cells could also be blocked by PKC inhibitors Bisindolylmaleimide and Gö 6976 (Figure 5E,F), suggesting an involvement of PKC in mediating the SUCNR1 signaling in mast cells. SUCNR1 antagonists XT1 and NF‐56‐EJ40, the Gq inhibitor YM254890 and the ERK inhibitor U0126 were all able to attenuate succinate‐induced cPLA2 phosphorylation, whereas the p38 inhibitor SB203580 was without effect, demonstrating the activation of SUCNR1(Gq)‐PKC‐ERK pathway in succinate‐challenged mast cells (Figure 5G). Moreover, targeting the ERK signaling by U0126 also decreased CD63 expression potentiated by succinate (Figure S8).
FIGURE 5.
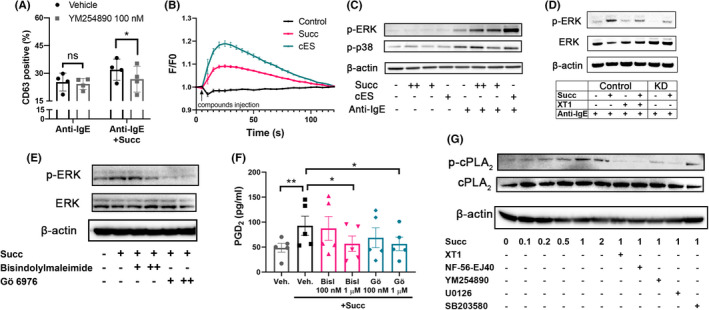
Succinate signals through SUCNR1‐PKC‐ERK axis in human mast cells. (A) CD63 expression of CBMC with vehicle or 100 nM YM254890, followed by succinate and anti‐IgE incubation (n = 8). (B) Intracellular calcium level was measured continuously for 120 s in LAD‐2 cells challenged with or without succinate or cES. (C) Phosphorylated ERK and p38 (p‐ERK, p‐p38) in CBMC challenged with succinate or cES followed by anti‐IgE (+: 0.1 mM, ++: 1 mM). (D) p‐ERK and ERK protein levels in control and SUCNR1 KD LAD‐2 cells with XT1, succinate and anti‐IgE. (E‐F) p‐ERK and ERK protein levels and PGD2 production in LAD‐2 cells pre‐incubated with bisindolylmaleimide or Gö 6976 (+: 0.1 μM, ++: 1 μM), followed by challenge with succinate (n = 5). (G) p‐cPLA2 and cPLA2 protein levels in LAD‐2 cells pre‐incubated with 1 uM different inhibitors or antagonists as indicated, followed by succinate challenge. RM two‐way ANOVA with Sidak's multiple comparison test (A) and RM one‐way ANOVA with Dunnett's multiple comparison test (F) were applied. *p < 0.05, **p < 0.01
3.6. SUCNR1‐dependent mast cell hyper‐reactivity enhances bronchoconstriction
Mast cells are key players in the early phase of allergic airway inflammation, which is characterized by rapid airway smooth muscle contraction, vasodilation, and mucosal plasma exudation in response to mast cell‐derived mediators. 26 To explore whether SUCNR1 activation influences allergen‐induced smooth muscle contraction in complex tissues, we performed guinea pig organ bath experiments with isolated tracheal rings from sensitized animals. Addition of OVA induced a concentration‐dependent contraction, which was shifted leftward by pretreatment with either succinate or cES (Figure 6A,B). At the highest concentration, both succinate (1 mM) and cES (1 mM) caused a significant leftward shift of the OVA‐induced contraction curve, with a significantly lower EC50 value. As observed in CBMC, cES was more potent than succinate in promoting tracheal contraction (Figure 6C,D). Increased levels of histamine and cys‐LTs were detected in the organ bath fluid of cES‐treated tissues (Figure 6E,F), which indicates that guinea pig airway mast cells become hyper‐reactive when SUCNR1 is activated. Antagonizing SUCNR1 by XT1 significantly attenuated the effects of both succinate and cES on OVA‐induced trachea contraction (Figure 6G,H) but had no significant effect on OVA‐induced contraction in the absence of SUCNR1 agonists (Figure S9), demonstrating the specificity of XT1 in targeting the receptor.
FIGURE 6.
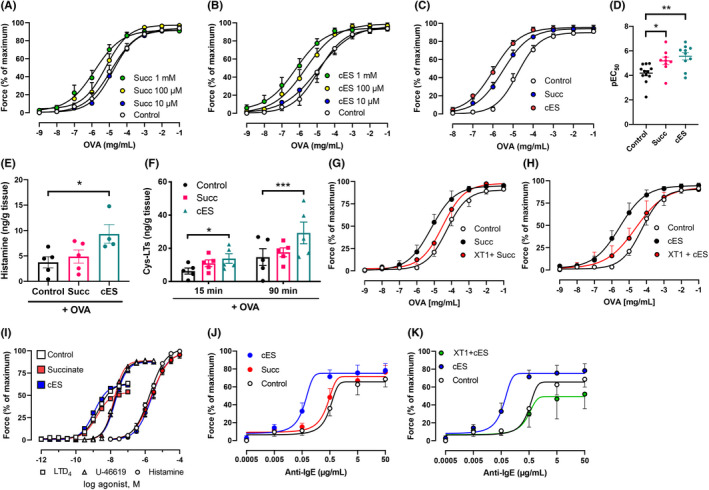
SUCNR1 activation potentiates mast cell‐dependent bronchoconstriction. (A‐H) Contractile force and mediator release of guinea pig trachea segments stimulated by increase doses of OVA. (A‐B) Effects of succinate or cES pre‐incubation on tracheal contraction (n:7–10). (C‐D) The comparison of contractile force of tracheal rings pre‐incubated with 1 mM succinate or cES (n:7–10). (E‐F) Levels of histamine and cys‐LTs in the organ bath fluids were assessed after pre‐incubation of succinate or cES and 15 min (histamine and cys‐LTs) or 90 min (cys‐LTs) challenge with 1 μM OVA (n = 5). (G‐H) Effect of 1 μM XT1 on contractile force (succinate or cES: 1 mM, 30 min) (n = 6). (I) Effects of succinate or cES on LTD4‐, U‐46619‐, or histamine‐induced contraction (n = 6). (J) Effects of succinate or cES on IgE‐/anti‐IgE‐induced human bronchus contraction (n = 6). (K) Effect of 1 µM XT1 on cES potentiated human bronchi contraction (n = 6). One‐way (D‐E) or two‐way (F) ANOVA with Dunnett's multiple comparison test was applied. *p < 0.05, **p < 0.01, ***p < 0.001
Furthermore, when tracheal contraction was triggered by exogenous histamine, LTD4, or U‐46619, which all directly activate smooth muscle cells in a mast cell‐independent manner, there was no significant effect of either 1 mM succinate or cES (Figure 6I). This indicates that SUCNR1 ligands do not directly regulate smooth muscle cells. This is in line with the receptor expression analysis in isolated airway smooth muscle cells, where no SUCNR1 transcripts were detected (data not shown).
As a final translational test, we investigated whether SUCNR1 activation could affect IgE‐induced smooth muscle cell contraction in human tissue. For this, small airways with an inner diameter of 1 mm or less were collected from patients undergoing lobectomies. In this model, 1 mM cES significantly enhanced the efficacy and the maximal response of airway contraction to anti‐IgE (Figure 6J). In addition, pretreatment with XT1 totally blocked the effect of cES (Figure 6K and Table S4), suggesting a potentiating effect of SUCNR1 signaling in human airways as observed in isolated cells and guinea pig trachea.
4. DISCUSSION
In this study, we find that SUCNR1 activation significantly enhances the early phase of mast cell activation with degranulation, histamine, and eicosanoid release that is induced by FcεRI crosslinking. Degranulation and histamine release are the primary outcomes of FcεRI‐induced mast cell activation, key events in allergic inflammation. PGD2 and cys‐LTs are major eicosanoid products of mast cells, whose synthesis requires cPLA2‐dependent release of free AA from phospholipids at the nuclear and ER membranes. 27 We find that SUCNR1 activation increases cPLA2 phosphorylation and subsequent AA release in succinate‐treated cells, demonstrating hyperactivation of an early and critical step in the eicosanoid biosynthetic pathway (Figure 3B). The increases in cys‐LTs, PGD2, and TXA2 predict a pro‐contractile effect of SUCNR1 activation in smooth muscles (Figure 6), and could contribute to other events in local inflammation, such as vasodilation and mucus secretion. Moreover, the increases in cys‐LTs and PGD2 levels may indicate a contribution of SUCNR1 activation in Type 2 inflammation in severe asthma. 28
Succinate promotes the release of certain cytokines from CBMC, indicating a significant impact of SUCNR1 activation on the late phase response of mast cells, and a potential to promote chronic inflammation and tissue remodeling. The effect of succinate in resting mast cells suggests a link between succinate accumulation and low‐grade inflammation. Comparing effects of SUCNR1 signaling with or without FcεRI crosslinking, reveals some qualitative differences. Thus, in resting cells, the production of the Th1 cytokine IFN‐γ was enhanced, while generation of IL‐4 was only promoted in FcεRI‐activated cells (Figure 4C–E). These results suggest that FcεRI crosslinking may shift the impact of SUCNR1 activation from a general pro‐inflammatory effect toward a more Type 2 inflammation‐oriented effect.
Previous studies have identified at least two SUCNR1‐coupled G proteins ‐ Gi and Gq. 6 , 8 , 29 We report that the potentiating effect of SUCNR1 on mast cell activation is via the PTX‐insensitive Gq pathway and further downstream, the receptor connects with PKC and ERK pathways (Figure 5F,G, Figure S8). Though SUCNR1 and FcεRI activation have synergistic effects on mast cell reactivity, a link in signal transduction was mainly observed at a distal level, that is, ERK MAPK, which in turn suggests that SUCNR1 activation may affect additional functional mast cell responses involving the ERK MAPK hub, such as differentiation and cell fate determination.
In the current study, we used isolated guinea pig trachea to study effects of SUCNR1 activation in mast cell‐mediated allergic reactions of the airways and found a robust enhancement of the contractile response, which was associated with the release of histamine and cys‐LTs (Figure 6). Similar results were also observed with isolated human bronchi pointing to a role for succinate/SUCNR1 in allergic inflammation of the human airways. In mice, it has been shown that SUCNR1 is enriched in mucosal mast cells which express more β7 integrin, 30 which—unlike constitutive mast cells—would mainly add to airway inflammation after immunological challenge and contribute to tissue hyper‐reactivity and remodeling. 31 , 32 It is interesting to note that, a potentiating effect of succinate on cys‐LTs and histamine release from lung tissues was observed by Austen and Brocklehurst already in 1961, without knowing the existence of specific metabolite receptors. 33 Now, 60 years later, we demonstrate that SUCNR1 is key to succinate‐potentiated release of proinflammatory and contractile agents resulting in allergic bronchoconstriction (Figure 6).
In a previous study, the role of SUCNR1 in mast cell‐related pathologies was examined in Sucnr1 −/− mice. 12 Receptor deficiency attenuated disease in models of arthritis, left asthmatic scores unaltered and lead to a paradoxical aggravation of allergic contact dermatitis. 12 It was suggested that these surprising results were caused by a defect in mouse mast cell maturation induced by global SUCNR1 deficiency. In the present study, we focused on human cells and used other experimental approaches to complement and extend the observations with knockout mice. Though mice have been widely used to study the pathogenesis of respiratory diseases, they have limited predictive value for human mast cells and allergic asthma. Thus, we focused on models of allergic bronchoconstriction believed to better reflect human asthma and observed a significant effect of SUCNR1 activation on promoting airway smooth muscle contraction, a critical component of human asthma. We identify cys‐LTs and histamine as messengers downstream of the SUCNR1 receptor (Figure 6E,F), neither of which induces airway smooth muscle contraction in mice. 34
In line with the notion that SUCNR1 signaling is involved in allergic airway inflammation, increased level of succinate was indeed observed in certain asthmatic population in comparison to healthy individuals. 35 , 36 , 37 Generally, host tissues under hypoxic conditions and residential microbiota in dysbiosis are two major sources of excessive succinate. In such conditions, millimolar range of succinate could be easily detected. 38 , 39 , 40 , 41 Thus, SUCNR1 is a candidate molecular link between local hypoxia and severe asthma, which in many cases happen in parallel and influence each other. 42 Moreover, it provides a possible explanation for the association between residential microbiota and asthma. 43 , 44 , 45 , 46
In summary, the current study demonstrates a novel role of succinate receptor SUCNR1 in promoting human mast cell reactivity and mast cell‐mediated bronchoconstriction. Our findings may provide new therapeutic insights for allergic asthma.
CONFLICT OF INTEREST
The authors have declared that there is no conflict of interest.
AUTHOR CONTRIBUTIONS
XT designed the project, performed the experiments, analyzed data, and wrote the manuscript. ER and GN provided mast cell‐related expertise, cells and reagents, set up the in vitro experiments, and contributed to the manuscript writing. JS performed ex vivo experiments with guinea pig trachea and human tissues, analyzed data, and contributed to the manuscript writing. M. Thulasingam did computational modeling of ligand‐receptor interaction. M. Trauelsen and TWS provided expertise, insights, and reagents of SUCNR1‐related pharmacology. CEW provided protocols and expertise of LC‐MS/MS. SEW provided expertise and pharmacological insights of allergic inflammation. JZH initiated, designed, and supervised the project and wrote the manuscript.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
The authors are grateful to Prof. Per Uhlén for valuable discussion on calcium signaling, Dr. Anna‐Karin Johnsson for kindly providing human airway smooth muscle cells, and Dr. Xiaofei Li for the suggestions on omics data analysis. This study was supported by the Swedish Research Council (Grants 2018‐02818, 2016‐02798, 2016‐01157), the Swedish Heart and Lung Foundation and an immunometabolism grant (NNF15CC0018346 and 0064142) from the Novo Nordisk Foundation to University of Copenhagen (TWS), Karolinska Institutet (JZH), and Oxford University. The NNF Center for Basic Metabolic Research is supported by an unconditional grant (NNF10CC1016515) from the Novo Nordisk Foundation to University of Copenhagen (TWS). XT was supported by the Konsul Th C Berghs Foundation. ER was supported by the Swedish Society for Medical Research, the Lars Hiertas Memorial Fund, the Konsul Th C Berghs Foundation, and the O. E. and Edla Johanssons Foundation.
Tang X, Rönnberg E, Säfholm J, et al. Activation of succinate receptor 1 boosts human mast cell reactivity and allergic bronchoconstriction. Allergy. 2022;77:2677–2687. doi: 10.1111/all.15245
Elin Rönnberg, Jesper Säfholm contributed equally.
REFERENCES
- 1. Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18(5):693‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shi GP, Bot I, Kovanen PT. Mast cells in human and experimental cardiometabolic diseases. Nat Rev Cardiol. 2015;12(11):643‐658. [DOI] [PubMed] [Google Scholar]
- 3. Siebenhaar F, Redegeld FA, Bischoff SC, Gibbs BF, Maurer M. Mast cells as drivers of disease and therapeutic targets. Trends Immunol. 2018;39(2):151‐162. [DOI] [PubMed] [Google Scholar]
- 4. Halova I, Ronnberg E, Draberova L, Vliagoftis H, Nilsson GP, Draber P. Changing the threshold‐Signals and mechanisms of mast cell priming. Immunol Rev. 2018;282(1):73‐86. [DOI] [PubMed] [Google Scholar]
- 5. Husted AS, Trauelsen M, Rudenko O, Hjorth SA, Schwartz TW. GPCR‐mediated signaling of metabolites. Cell Metab. 2017;25(4):777‐796. [DOI] [PubMed] [Google Scholar]
- 6. He W, Miao FJ, Lin DC, et al. Citric acid cycle intermediates as ligands for orphan G‐protein‐coupled receptors. Nature. 2004;429(6988):188‐193. [DOI] [PubMed] [Google Scholar]
- 7. Brosnan JT, Krebs HA, Williamson DH. Effects of ischaemia on metabolite concentrations in rat liver. Biochem J. 1970;117(1):91‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Castro FM, Aguiar CJ, da Rocha Franco JA, Gingold RN, Leite MF. GPR91: expanding the frontiers of Krebs cycle intermediates. Cell Commun Signal. 2016;14:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fagerberg L, Hallstrom BM, Oksvold P, et al. Analysis of the human tissue‐specific expression by genome‐wide integration of transcriptomics and antibody‐based proteomics. Mol Cell Proteomics. 2014;13(2):397‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Toma I, Kang JJ, Sipos A, et al. Succinate receptor GPR91 provides a direct link between high glucose levels and renin release in murine and rabbit kidney. J Clin Invest. 2008;118(7):2526‐2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tan JK, McKenzie C, Marino E, Macia L, Mackay CR. Metabolite‐sensing G protein‐coupled receptors‐facilitators of diet‐related immune regulation. Annu Rev Immunol. 2017;35:371‐402. [DOI] [PubMed] [Google Scholar]
- 12. Rubic‐Schneider T, Carballido‐Perrig N, Regairaz C, et al. GPR91 deficiency exacerbates allergic contact dermatitis while reducing arthritic disease in mice. Allergy. 2017;72(3):444‐452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Enoksson M, Ejendal KF, McAlpine S, Nilsson G, Lunderius‐Andersson C. Human cord blood‐derived mast cells are activated by the Nod1 agonist M‐TriDAP to release pro‐inflammatory cytokines and chemokines. J Innate Immun. 2011;3(2):142‐149. [DOI] [PubMed] [Google Scholar]
- 14. Kirshenbaum AS, Akin C, Wu Y, et al. Characterization of novel stem cell factor responsive human mast cell lines LAD 1 and 2 established from a patient with mast cell sarcoma/leukemia; activation following aggregation of FcepsilonRI or FcgammaRI. Leuk Res. 2003;27(8):677‐682. [DOI] [PubMed] [Google Scholar]
- 15. Tang X, Basavarajappa D, Haeggstrom JZ, Wan M. P2X7 receptor regulates internalization of antimicrobial peptide LL‐37 by human macrophages that promotes intracellular pathogen clearance. J Immunol. 2015;195(3):1191‐1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Safholm J, Dahlen SE, Adner M. Antagonising EP1 and EP2 receptors reveal that the TP receptor mediates a component of antigen‐induced contraction of the guinea pig trachea. Eur J Pharmacol. 2013;718(1–3):277‐282. [DOI] [PubMed] [Google Scholar]
- 17. Safholm J, Manson ML, Bood J, et al. Prostaglandin E2 inhibits mast cell‐dependent bronchoconstriction in human small airways through the E prostanoid subtype 2 receptor. J Allergy Clin Immunol. 2015;136(5):1232‐1239. [DOI] [PubMed] [Google Scholar]
- 18. Tang X, Fuchs D, Tan S, et al. Activation of metabolite receptor GPR91 promotes platelet aggregation and transcellular biosynthesis of leukotriene C4. J Thromb Haemost. 2020;18(4):976‐984. [DOI] [PubMed] [Google Scholar]
- 19. Littlewood‐Evans A, Sarret S, Apfel V, et al. GPR91 senses extracellular succinate released from inflammatory macrophages and exacerbates rheumatoid arthritis. J Exp Med. 2016;213(9):1655‐1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Trauelsen M, Rexen Ulven E, Hjorth SA, et al. Receptor structure‐based discovery of non‐metabolite agonists for the succinate receptor GPR91. Mol Metab. 2017;6(12):1585‐1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bhuniya D, Umrani D, Dave B, et al. Discovery of a potent and selective small molecule hGPR91 antagonist. Bioorg Med Chem Lett. 2011;21(12):3596‐3602. [DOI] [PubMed] [Google Scholar]
- 22. Haffke M, Fehlmann D, Rummel G, et al. Structural basis of species‐selective antagonist binding to the succinate receptor. Nature. 2019;574(7779):581‐585. [DOI] [PubMed] [Google Scholar]
- 23. Boyce JA. Mast cells and eicosanoid mediators: a system of reciprocal paracrine and autocrine regulation. Immunol Rev. 2007;217:168‐185. [DOI] [PubMed] [Google Scholar]
- 24. Haeggstrom JZ. Leukotriene biosynthetic enzymes as therapeutic targets. J Clin Invest. 2018;128(7):2680‐2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mukai K, Tsai M, Saito H, Galli SJ. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol Rev. 2018;282(1):121‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454(7203):445‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haeggstrom JZ, Funk CD. Lipoxygenase and leukotriene pathways: biochemistry, biology, and roles in disease. Chem Rev. 2011;111(10):5866‐5898. [DOI] [PubMed] [Google Scholar]
- 28. Kolmert J, Gomez C, Balgoma D, et al. Urinary leukotriene E4 and prostaglandin D2 metabolites increase in adult and childhood severe asthma characterized by type 2 inflammation. a clinical observational study. Am J Respir Crit Care Med. 2021;203(1):37‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mills E, O'Neill LA. Succinate: a metabolic signal in inflammation. Trends Cell Biol. 2014;24(5):313‐320. [DOI] [PubMed] [Google Scholar]
- 30. Derakhshan T, Samuchiwal SK, Hallen N, et al. Lineage‐specific regulation of inducible and constitutive mast cells in allergic airway inflammation. J Exp Med. 2021;218(1):e20200321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bankova LG, Dwyer DF, Liu AY, Austen KF, Gurish MF. Maturation of mast cell progenitors to mucosal mast cells during allergic pulmonary inflammation in mice. Mucosal Immunol. 2015;8(3):596‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Olivera A, Beaven MA, Metcalfe DD. Mast cells signal their importance in health and disease. J Allergy Clin Immunol. 2018;142(2):381‐393. [DOI] [PubMed] [Google Scholar]
- 33. Austen KF, Brocklehurst WE. Anaphylaxis in chopped guinea pig lung. II. Enhancement of the anaphylactic release of histamine and slow reacting substance by certain dibasic aliphatic acids and inhibition by monobasic fatty acids. J Exp Med. 1961;113:541‐557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Adner M, Canning BJ, Meurs H, et al. Back to the future: re‐establishing guinea pig in vivo asthma models. Clin Sci (Lond). 2020;134(11):1219‐1242. [DOI] [PubMed] [Google Scholar]
- 35. Saude EJ, Skappak CD, Regush S, et al. Metabolomic profiling of asthma: diagnostic utility of urine nuclear magnetic resonance spectroscopy. J Allergy Clin Immunol. 2011;127(3):757‐764. [DOI] [PubMed] [Google Scholar]
- 36. Jung J, Kim SH, Lee HS, et al. Serum metabolomics reveals pathways and biomarkers associated with asthma pathogenesis. Clin Exp Allergy. 2013;43(4):425‐433. [DOI] [PubMed] [Google Scholar]
- 37. Chang C, Guo ZG, He B, Yao WZ. Metabolic alterations in the sera of Chinese patients with mild persistent asthma: a GC‐MS‐based metabolomics analysis. Acta Pharmacol Sin. 2015;36(11):1356‐1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chouchani ET, Pell VR, Gaude E, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515(7527):431‐435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tannahill GM, Curtis AM, Adamik J, et al. Succinate is an inflammatory signal that induces IL‐1beta through HIF‐1alpha. Nature. 2013;496(7444):238‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fernandez‐Veledo S, Vendrell J. Gut microbiota‐derived succinate: Friend or foe in human metabolic diseases? Rev Endocr Metab Disord. 2019;20(4):439‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Correa PR, Kruglov EA, Thompson M, Leite MF, Dranoff JA, Nathanson MH. Succinate is a paracrine signal for liver damage. J Hepatol. 2007;47(2):262‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Baek KJ, Cho JY, Rosenthal P, Alexander LEC, Nizet V, Broide DH. Hypoxia potentiates allergen induction of HIF‐1alpha, chemokines, airway inflammation, TGF‐beta1, and airway remodeling in a mouse model. Clin Immunol. 2013;147(1):27‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chung KF. Airway microbial dysbiosis in asthmatic patients: A target for prevention and treatment? J Allergy Clin Immunol. 2017;139(4):1071‐1081. [DOI] [PubMed] [Google Scholar]
- 44. Sharma A, Laxman B, Naureckas ET, et al. Associations between fungal and bacterial microbiota of airways and asthma endotypes. J Allergy Clin Immunol. 2019;144(5):1214‐1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wypych TP, Marsland BJ. Antibiotics as instigators of microbial dysbiosis: implications for asthma and Allergy. Trends Immunol. 2018;39(9):697‐711. [DOI] [PubMed] [Google Scholar]
- 46. Trivedi R, Barve K. Gut microbiome a promising target for management of respiratory diseases. Biochem J. 2020;477(14):2679‐2696. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


