Abstract
Background
Studies suggest an association between osteoporosis and non‐alcoholic fatty liver disease (NAFLD), but whether patients with NAFLD are at increased risk of fractures is unknown.
Objectives
The aim was to determine the rate and risk of fractures and the mortality rate after fracture in patients with NAFLD compared to the general population.
Methods
This was a nationwide population‐based cohort study using data from the Swedish National Patient Registry on 10,678 patients with NAFLD from 1987 to 2016. Patients were matched for sex, age, and municipality with 99,176 controls from the Swedish Total Population Registry. Cox regression was used to estimate fracture rates. The risk of fractures was assessed while accounting for competing risks (death and liver transplantation).
Results
A total of 12,312 fractures occurred during 761,176 person‐years of follow‐up. Patients with NAFLD (17.5 per 1000 person‐years) had a slightly higher fracture rate than controls (16.1 per 1000 person‐years; adjusted hazard ratio 1.11, 95% confidence interval [CI] 1.05–1.19), although the 5‐year risk of fractures was similar (8.0%, 95% CI 7.4–8.6 versus 7.3%, 95% CI 7.2–7.5). Additionally, 1‐year mortality after fracture was similar in NAFLD and controls.
Conclusions
Patients with NAFLD have a slightly higher rate of fractures but long‐term risk of fractures comparable to the general population. This suggests that broad surveillance of risk factors for fractures in patients with NAFLD is not motivated.
Keywords: epidemiology, liver disease, metabolic dysfunction‐associated fatty liver disease, non‐alcoholic steatohepatitis, osteoporosis
Abbreviations
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- HR
hazard ratio
- ICD
International Classification of Disease
- IQR
interquartile range
- NPR
National Patient Registry
- PIN
personal identification number
Introduction
Non‐alcoholic fatty liver disease (NAFLD) is highly prevalent and associated with increased mortality [1, 2]. Additionally, NAFLD is associated with type 2 diabetes, insulin resistance, chronic inflammation, low physical activity, and vitamin D deficiency, which may result in a reduced bone mineral density [3]. Such reduction is supported by some studies [4, 5, 6, 7], although others show conflicting results [8, 9, 10, 11, 12, 13, 14]. However, it is currently unknown if the suggested demineralization in NAFLD translates to higher rates of fractures. Two cross‐sectional studies from China found that NAFLD was associated with a self‐reported history of osteoporotic fractures in men [15, 16], but no longitudinal studies have examined the rate of fractures in patients with NAFLD. Moreover, the absolute risk of fractures and the short‐term mortality rate after fracture in patients with NAFLD are both unknown. Therefore, we aimed to determine the rate and risk of fractures and the mortality rate after fracture in a national cohort of patients with NAFLD compared to controls from the general population.
Materials and methods
Data sources
The Swedish National Patient Registry (NPR) contains data on the International Classification of Disease (ICD) codes from inpatient care since 1964 and specialty outpatient care since 2001. The positive predictive value of the NPR has been estimated to be 85%–95% [17]. A unique personal identification number (PIN) is given to all Swedish residents. The PIN can be used to link the NPR to other Swedish registries, such as the Total Population Registry, which contains national data on date of birth, death, migration, and other demographic variables [18, 19]. Ethical approval for this study was provided by the regional ethics committee of Stockholm (protocol number 2017/1019‐31/1).
Study population
Data were obtained from the NPR on all patients with a diagnostic code for NAFLD between 1 January 1987 and 31 December 2016. NAFLD was defined according to the 9th or 10th revision of the ICD in agreement with a previous consensus statement (Table S1) [20]. Patients with NAFLD were matched for age, sex, and municipality with up to 10 controls from the general population using the Total Population Registry. The start of follow‐up was defined as the first date with a registered diagnostic code for NAFLD in the NPR. We excluded patients and controls meeting any of the following criteria before baseline: liver transplantation, a diagnostic code for any liver disease other than NAFLD, a code related to alcohol or drug abuse, or a re‐used PIN (Table S1, Fig. 1).
Fig. 1.
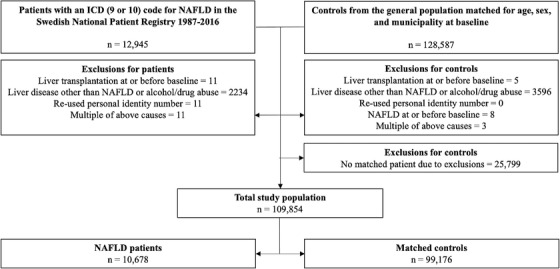
Flowchart of study inclusion.
Outcomes
The primary outcome was time until the first fracture during the follow‐up. Secondary outcomes were time until subcategories of fractures based on the presumed mechanism of the fracture or the fracture site. Presumed fracture mechanisms were osteoporotic fracture or non‐osteoporotic fracture. An osteoporotic fracture was defined as a fracture of the vertebrae, proximal humerus, distal forearm, pelvis, or hip, without any coding for high‐energy trauma (a fall from one level to another, or a traffic accident involving motor vehicles). Fractures were further classified based on their location, that is, a fracture of the skull, vertebrae, shoulder, humerus, proximal forearm, distal forearm, hand, ribs or sternum, pelvis, hip, femur shaft, lower leg, or foot. All fracture definitions according to their respective ICD codes are specified in Table S2. Secondary outcomes also included time until all‐cause post‐fracture mortality. This analysis only included patients and controls with a fracture during follow‐up. Mortality was analysed at 1 year after any fracture, osteoporotic fracture, and non‐osteoporotic fracture.
Subgroups
The fracture rate was further analysed for subgroups according to sex, age (18‐50, 51–65, or >65 years), the presence of cirrhosis, diabetes, and inclusion year (1987–1999, 2000–2009, or 2010–2016). Moreover, the rate of fractures was analysed for the subgroup of patients and controls with osteoporosis at baseline. Osteoporosis was defined according to the corresponding ICD codes (Table S3) or by an ICD code for a previous fracture of the vertebrae, proximal humerus, distal forearm, pelvis, or hip not related to high‐energy trauma.
Sensitivity analyses
As a sensitivity analysis, osteoporotic fracture was re‐defined as a fracture of the vertebrae, proximal humerus, distal forearm, pelvis, or hip, in mandatory combination with ICD coding for osteoporosis as defined in Table S3 before, at, or within 3 months after the fracture. The time lag of 3 months after the fracture event was chosen because osteoporosis is often asymptomatic and may not have been discovered until after a fracture. The analysis of osteoporotic fractures was then repeated using this stricter definition.
Statistics
The rates of fractures were analysed in Cox regression models for patients with NAFLD compared to their matched controls to estimate hazard ratios (HRs) and their 95% confidence intervals (CIs). Adjustments were made in three different models. In the first model, analyses were done within matched patient‐control strata with no further adjustments, and in the second model, adjustments were made for covariates possibly associated with increased rates of fractures: country of birth, rheumatic disease, chronic obstructive pulmonary disease (COPD), dementia, and cancer. The third model included all covariates in the second model in addition to variables associated with the metabolic syndrome (chronic kidney disease, cardiovascular disease, diabetes, and obesity). Comorbidities were defined by their respective ICD codes before or at baseline (Table S3). We used different adjustment models because of the unclarity of the pathophysiological relationship between NAFLD and the metabolic syndrome, that is, if NAFLD occurs upstream or downstream of other components in the metabolic syndrome. The cumulative incidence of any first fracture was estimated while accounting for the competing risks of death and liver transplantation [21]. Study participants were followed until the occurrence of a fracture, death, liver transplantation, coding for another chronic liver disease than NAFLD, coding for alcohol or drug abuse, emigration from Sweden, or the end of the study period (31 December 2016), whichever occurred first. All‐cause mortality after a fracture was also analysed in three separate Cox regression models, using the date of the first fracture as the start of follow‐up. Here, the first model included adjustments for age, sex, municipality, and inclusion year, and the second and third models were the same as those presented above. Mortality was presented in Kaplan–Meier failure functions. A two‐tailed p‐value less than 0.05 was considered statistically significant. All analyses were done in Stata 17.0 (StataCorp, College Station, TX).
Results
Background characteristics
During the study period, 10,678 patients with NAFLD and 99,176 matched controls were included and followed for 761,176 person‐years (63,531 in patients and 697,645 in controls). For patients and controls, 52% were male, and the median age was 55 years (Table 1). A lower proportion of patients were born in a Nordic country (84%) than controls (88%). Patients had higher frequencies of most comorbidities, including cardiovascular disease and diabetes. Osteoporosis was found equally often in patients and controls, and the proportion of individuals with a previous fracture of any kind was similar in both groups (Table 1). Liver transplantation was performed in 53 (0.5%) patients with NAFLD and three (0.0%) controls.
Table 1.
Background characteristics of patients with non‐alcoholic fatty liver disease (NAFLD) and matched controls
| NAFLD | Controls | |
|---|---|---|
| Included persons, n | 10,678 | 99,176 |
| Follow‐up (years) median (IQR) | 4.1 (1.5–8.8) | 5.4 (2.2–10.4) |
| Sex, male n (%) | 5553 (52.0) | 51,152 (51.6) |
| Age at baseline (years) median (IQR) | 55 (41–65) | 55 (40–65) |
| Period of inclusion n (%) | ||
| 1987–1999 | 1220 (11.4) | 11,724 (11.8) |
| 2000–2009 | 3972 (37.2) | 37,101 (37.4) |
| 2010–2016 | 5486 (51.4) | 50,351 (50.8) |
| Country of birth n (%) | ||
| Nordic | 8913 (83.5) | 87,574 (88.3) |
| Other | 1765 (16.5) | 11,602 (11.7) |
| Comorbidity at or before baseline n (%) | ||
| Cardiovascular disease | 3342 (31.3) | 13,036 (13.1) |
| Diabetes | 1900 (17.8) | 3647 (3.7) |
| Obesity | 1264 (11.8) | 1475 (1.5) |
| Cirrhosis | 246 (2.3) | 0 (0.0) |
| Dementia | 39 (0.4) | 432 (0.4) |
| Chronic obstructive pulmonary disease | 310 (2.9) | 971 (1.0) |
| Cancer | ||
| Hepatocellular carcinoma | 21 (0.2) | 5 (0.01) |
| Other cancers | 886 (8.3) | 6298 (6.4) |
| Chronic kidney disease | 138 (1.3) | 406 (0.4) |
| Rheumatic disease | 274 (2.6) | 1026 (1.0) |
| Osteoporosis | 463 (4.3) | 4296 (4.3) |
| Any previous fracture | 1242 (11.6) | 12,202 (12.3) |
Abbreviations: IQR, interquartile range; NAFLD, non‐alcoholic fatty liver disease.
Fractures
A total of 12,312 first fractures occurred during follow‐up: 1113 (10.4%) in patients and 11,199 (11.3%) in controls. The presumed underlying cause was osteoporosis in 433 (38.9%) fractures for patients and in 4758 (42.5%) fractures for controls. The incidence of fractures was marginally higher in patients with NAFLD (17.5 per 1000 person‐years) compared to controls (16.1 per 1000 person‐years; unadjusted HR 1.13, 95% CI 1.06‐1.21, p < 0.001) (Table 2). We found similar results when adjusting for comorbidities associated with an increased rate of fractures (HR 1.11, 95% CI 1.05–1.19, p = 0.001), but no difference was found between patients and controls when further adjusting for comorbidities associated with the metabolic syndrome (HR 1.05, 95% CI 0.98–1.12, p = 0.139). The increased fracture rate was only found for non‐osteoporotic as opposed to osteoporotic fractures (Table 2). The osteoporotic fracture rate was similar when using a stricter osteoporotic fracture definition as described above. Patients with NAFLD had an increased rate of fractures of the vertebrae, ribs or sternum, and lower leg in all regression models compared to controls. However, the rate of forearm fractures was lower for patients with NAFLD than for controls (Table 2). The rate of any fracture was similar when analyses were stratified on sex, age groups, or inclusion year (Table S4). The small increase in the fracture rate for patients with NAFLD compared to controls was more pronounced for patients with baseline cirrhosis or diabetes, although still small (Table S4). For patients and controls who had osteoporosis at baseline, there was a slightly increased rate of fractures associated with NAFLD (unadjusted HR 1.25, 95% CI 1.02–1.54, p = 0.028), with similar results after adjustments for confounders associated with a higher fracture rate (HR 1.21, 95% CI 0.99–1.48, p = 0.068) and confounders associated with the metabolic syndrome (HR 1.19, 95% CI 0.97–1.47, p = 0.100).
Table 2.
Fracture outcomes for patients with non‐alcoholic fatty liver disease (NAFLD) (n = 10,678) and matched controls (n = 99,176)
| NAFLD | Controls | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Events, n (%) | Incidence per 1000 person‐years (95% CI) | Events, n (%) | Incidence per 1000 person‐years (95% CI) | HRmodel 1 (95% CI) | p | HRmodel 2 (95% CI) | p | HRmodel 3 (95% CI) | p | |
| Any fracture | 1113 (10.4) | 17.5 (16.5–18.6) | 11,199(11.3) | 16.1 (15.8–16.4) | 1.13 (1.06–1.21) | <0.001 | 1.11 (1.05–1.19) | 0.001 | 1.05 (0.98–1.12) | 0.139 |
| Fracture by presumed mechanism | ||||||||||
| Osteoporotic | 499 (4.7) | 7.5 (6.9–8.2) | 5580 (5.6) | 7.7 (7.5–7.9) | 1.07 (0.98–1.18) | 0.138 | 1.04 (0.95–1.15) | 0.391 | 0.99 (0.89–1.09) | 0.771 |
| Non‐osteoporotic | 734 (6.9) | 11.3 (10.5–12.1) | 7069 (7.1) | 9.9 (9.6–10.1) | 1.15 (1.07–1.24) | <0.001 | 1.14 (1.06–1.23) | 0.001 | 1.07 (0.98–1.16) | 0.112 |
| Fracture by site | ||||||||||
| Skull | 53 (0.5) | 0.8 (0.6–1.0) | 584 (0.6) | 0.8 (0.7–0.8) | 0.97 (0.73–1.29) | 0.842 | 0.95 (0.71–1.26) | 0.703 | 0.82 (0.61–1.1) | 0.191 |
| Vertebrae | 154 (1.4) | 2.3 (1.9–2.7) | 1179 (1.2) | 1.6 (1.5–1.7) | 1.58 (1.32–1.88) | <0.001 | 1.46 (1.22–1.75) | <0.001 | 1.37 (1.14–1.65) | 0.001 |
| Shoulder | 43 (0.4) | 0.6 (0.5–0.9) | 529 (0.5) | 0.7 (0.6–0.8) | 0.89 (0.65–1.23) | 0.485 | 0.88 (0.64–1.21) | 0.425 | 0.81 (0.59–1.13) | 0.223 |
| Humerus | 129 (1.2) | 1.9 (1.6–2.3) | 1257 (1.3) | 1.7 (1.6–1.8) | 1.17 (0.97–1.41) | 0.096 | 1.18 (0.98–1.42) | 0.086 | 1.05 (0.86–1.27) | 0.643 |
| Proximal forearm | 46 (0.4) | 0.7 (0.5–0.9) | 676 (0.7) | 0.9 (0.8–1.0) | 0.75 (0.56–1.02) | 0.068 | 0.72 (0.53–0.98) | 0.038 | 0.72 (0.52–0.98) | 0.038 |
| Distal forearm | 137 (1.3) | 2.0 (1.7–2.4) | 2178 (2.2) | 2.9 (2.8–3.1) | 0.70 (0.59–0.83) | <0.001 | 0.70 (0.59–0.84) | <0.001 | 0.70 (0.58–0.84) | <0.001 |
| Hand | 126 (1.2) | 1.9 (1.6–2.2) | 1556 (1.6) | 2.1 (2.0–2.2) | 0.86 (0.72–1.04) | 0.120 | 0.87 (0.72–1.05) | 0.142 | 0.82 (0.68–1.00) | 0.047 |
| Ribs/sternum | 113 (1.1) | 1.7 (1.4–2.0) | 954 (1.0) | 1.3 (1.2–1.4) | 1.39 (1.14–1.70) | 0.001 | 1.36 (1.11–1.67) | 0.003 | 1.28 (1.04–1.57) | 0.021 |
| Pelvis | 35 (0.3) | 0.5 (0.4–0.7) | 404 (0.4) | 0.5 (0.5–0.6) | 1.17 (0.82–1.68) | 0.378 | 1.13 (0.79–1.62) | 0.509 | 1.11 (0.77–1.60) | 0.576 |
| Hip | 161 (1.5) | 2.4 (2.0–2.8) | 1882 (1.9) | 2.5 (2.4–2.6) | 1.15 (0.97–1.36) | 0.097 | 1.13 (0.95–1.34) | 0.159 | 0.98 (0.82–1.17) | 0.847 |
| Femur shaft | 30 (0.3) | 0.4 (0.3–0.6) | 228 (0.2) | 0.3 (0.3–0.3) | 1.69 (1.14–2.50) | 0.009 | 1.56 (1.04–2.3) | 0.031 | 1.28 (0.83–1.97) | 0.264 |
| Lower leg | 240 (2.3) | 3.6 (3.2–4.1) | 1815 (1.8) | 2.4 (2.3–2.6) | 1.50 (1.31–1.72) | <0.001 | 1.51 (1.32–1.74) | <0.001 | 1.45 (1.25–1.68) | <0.001 |
| Foot | 102 (1.0) | 1.5 (1.2–1.8) | 851 (0.9) | 1.1 (1.1–1.2) | 1.29 (1.05–1.59) | 0.015 | 1.26 (1.02–1.56) | 0.029 | 1.19 (0.95–1.48) | 0.126 |
Note: Model 1: Analysed within patient‐control strata with no further adjustments. Model 2: Model 1 + country of birth, rheumatic disease, chronic obstructive pulmonary disease, dementia, and cancer (other than hepatocellular carcinoma). Model 3: Model 2 + chronic kidney disease, cardiovascular disease, diabetes, and obesity.
Abbreviations: CI, confidence interval; HR, hazard ratio; NAFLD, non‐alcoholic fatty liver disease.
The absolute risk of fractures was similar for patients with NAFLD and controls at 1 year (2.1%, 95% CI 1.9–2.4 vs. 1.8%, 95% CI 1.7–1.8) and 5 years (8.0%, 95% CI 7.4–8.6 vs. 7.3%, 95% CI 7.2–7.5) when taking competing risks into account. Patients with NAFLD had a marginally higher risk of fractures after 10 years of follow‐up (14.8%, 95% CI 13.9–15.8 vs. 13.7%, 95% CI 13.4–14.0), but after the full follow‐up of 30 years, the risk was lower for patients than for controls (30.0%, 95% CI 26.5–33.6 vs. 34.7%, 95% CI 33.5–35.9) (Fig. 2A). Comparable observations were made when analyzing osteoporotic and non‐osteoporotic fractures separately (Fig. 2B,C).
Fig. 2.
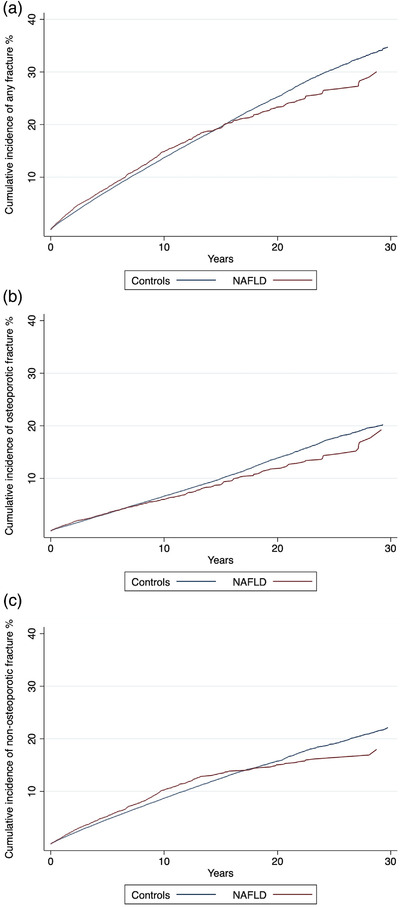
Cumulative incidence of any first fracture (a), osteoporotic fracture (b), and non‐osteoporotic fracture (c) in patients with non‐alcoholic fatty liver disease (NAFLD) (n = 10,678) and controls from the general population (n = 99,176) accounting for competing risks (death and liver transplantation).
Post‐fracture mortality
The median time to death in patients with NAFLD who died after having had a fracture during follow‐up was 2.4 years (interquartile range [IQR] 0.7–5.1) compared to 2.7 years (IQR 0.8–5.4) in controls. Of the 1113 patients with NAFLD who had a fracture, 78 died from any cause within 1 year. The post‐fracture 1‐year mortality rate was higher for patients with NAFLD (80.4 per 1000 person‐years) than for controls (67.2 per 1000 person‐years; unadjusted HR 1.48, 95% CI 1.17–1.87, p = 0.001) (Table S5). Similar results were noted when adjusting for comorbidities associated with increased fracture rate (HR 1.39, 95% CI 1.10–1.77, p = 0.006), but not in the fully adjusted model that also included comorbidities associated with the metabolic syndrome (HR 1.20, 95% CI 0.94–1.55, p = 0.143). Comparable results were found when looking at osteoporotic and non‐osteoporotic fractures separately (Table S5). As seen in Fig. 3, the small increase in post‐fracture mortality related to NAFLD was restricted to later time points beyond the first 3–6 months. Early after fracture, mortality rates did not differ between patients with NAFLD and controls (Fig. 3).
Fig. 3.
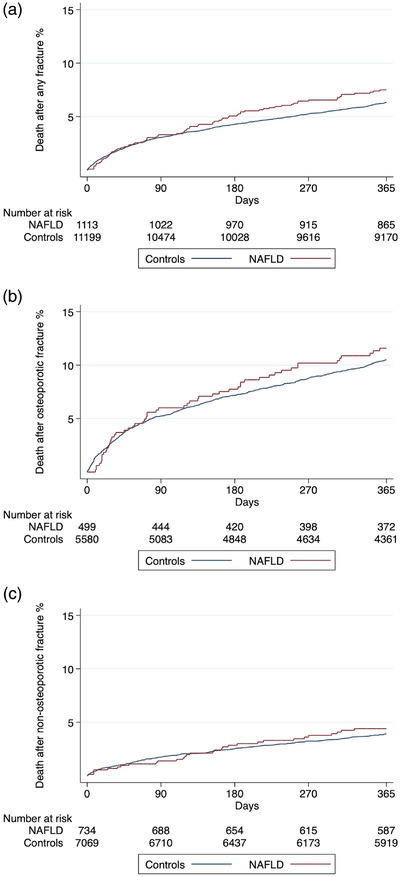
Kaplan–Meier failure functions for all‐cause mortality after any first fracture (a), osteoporotic fracture (b), and non‐osteoporotic fracture (c) in patients with non‐alcoholic fatty liver disease (NAFLD) (n = 1113) and controls from the general population (n = 11,199) who had a fracture during the study period.
Discussion
The key findings of this nationwide population‐based cohort study of patients with NAFLD and matched controls were as follows: (1) NAFLD was associated with a marginally increased fracture rate; (2) the absolute risk of fractures was comparable for patients with NAFLD and controls; (3) 1‐year mortality after fracture was low and similar between patients and controls, especially early after the fracture. The fracture rate remained similar or slightly increased for patients with NAFLD compared to controls for osteoporotic and non‐osteoporotic fractures, different fracture sites, and throughout a range of pre‐specified subgroup and sensitivity analyses. Taken together, this suggests that patients with NAFLD do not need specific investigation related to fracture risk, such as measurement of vitamin D levels or bone densitometry.
Fractures
Previous studies on the relationship between NAFLD and fractures are rare. Li and colleagues did a cross‐sectional study of 7797 Chinese adults older than 40 years, of whom 2352 patients were considered to have NAFLD based on examination with ultrasound [15]. They found NAFLD to be associated with more than twofold higher odds of previous osteoporotic fractures in men, but not in women. A similar cross‐sectional study from China of 614 patients with NAFLD supported these findings [16]. In contrast, the current study found only a marginally increased fracture rate in a cohort of more than 10,000 patients with NAFLD compared to nearly 100,000 matched controls. This increased rate was primarily driven by non‐osteoporotic fractures and varied according to fracture site. However, our competing risk analysis showed that despite this increase in fracture rate, the absolute risk of fractures was comparable between patients with NAFLD and controls for up to 30 years of follow‐up.
Some studies have indicated an association between NAFLD and higher risk of osteoporosis [4, 5, 6, 7], while others did not support such connection [8, 9, 10, 11, 12, 13, 14]. In our study, a diagnosis of osteoporosis was equally frequent among patients with NAFLD and in matched controls. Moreover, we found the relationship between NAFLD and fracture rate to be similar in the total cohort and in the subgroup of patients and controls with baseline osteoporosis, indicating that a potential association between NAFLD and fractures might not be explained by higher risk of osteoporosis.
Post‐fracture death
Patients with NAFLD had a higher mortality rate than controls the first year after fracture. However, mortality was similar the first months, indicating that the small difference in mortality may be due to other factors than the fracture, for example, cancer or cardiovascular disease [2].
Clinical implications
Chronic liver disease, especially complicated by cirrhosis, is related to osteoporosis and fracture rates higher than in the general population, including alcohol‐related liver disease and primary biliary cholangitis [22, 23], which might motivate preventive interventions in these populations. However, despite previous studies implying higher risk of fractures in NAFLD [15, 16], our study found this risk to be comparable to the general population, suggesting that broad surveillance of fracture risk factors in NAFLD might not be indicated.
Strengths and limitations
The main strength of this study was its population‐based and cohort design, including all patients with a formal diagnosis of NAFLD across three decades on a national level in Sweden. The study further benefited from the high validity of the NPR, minimizing selection bias, and providing high accuracy [17]. Additionally, the internal validity of our study was strengthened by the inclusion of matched controls and extensive data on comorbidities to allow for several subgroup analyses. Moreover, fractures likely lead to patients seeking specialty care, allowing us to presumably capture all fracture events.
Our study also had some limitations. First, we missed data on possible confounders, such as body mass index, physical activity, and smoking. This was partly accounted for by including COPD in our adjustment model as a proxy for smoking. Second, most patients with NAFLD are cared for in primary care, which is not covered by the NPR. It is therefore likely that our design predominantly captured a sicker proportion of patients with NAFLD, who may have a higher risk of fractures and risk of death after fracture than completely unselected patients with NAFLD. However, inclusion of more unselected patients would probably reduce the already small difference in fracture risk that we found and not change our conclusion that this risk is comparable between patients with NAFLD and matched controls. Third, NAFLD is believed to be highly underdiagnosed, and it is therefore plausible that some controls in our study also had NAFLD, although undiagnosed, which may have diluted the difference between patients and controls.
Conclusion
Patients with NAFLD have a slightly higher fracture rate but an absolute risk of fractures comparable to the general population. Osteoporosis was not more common in patients with NAFLD, and the short‐term mortality after fracture was equal in NAFLD and controls. Targeted preventive interventions or general surveillance of risk factors for fractures are not motivated in NAFLD.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
Study conception and design, analysis and interpretation of data, critical revision, and guarantor of article: Axel Wester and Hannes Hagström. Acquisition of data: Hannes Hagström. Statistical analysis and drafting of manuscript: Axel Wester. All authors approved the final version of the article, including the authorship list.
Supporting information
Supplemental Table 1. International Classification of Disease (ICD) codes from the 9th and 10th revision for NAFLD, its differential diagnoses, alcohol or drug abuse, and liver transplantation.
Supplemental Table 2. International Classification of Disease (ICD) codes from the 9th and 10th revision for fractures.
Supplemental Table 3. International Classification of Disease (ICD) codes from the 9th and 10th revision for comorbidities.
Supplemental Table 4. Fracture outcomes for patients with NAFLD (n=10,678) and matched controls (n=99,176) stratified for sex, age group, disease severity, the presence of diabetes, and inclusion year.
Supplemental Table 5. Post‐fracture mortality at 1 year for patients with NAFLD (n=1113) and controls (n=11,199) who had a fracture during the study period.
Acknowledgements
AW was supported by the Syskonen Svensson Foundation for medical research. HH was supported by grants from Region Stockholm. The funders had no role in the conduct of this study.
Wester A, Hagström H. Risk of fractures and subsequent mortality in non‐alcoholic fatty liver disease: A nationwide population‐based cohort study. J Intern Med. 2022;292:492–500.
References
- 1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of non‐alcoholic fatty liver disease—meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- 2. Simon TG, Roelstraete B, Khalili H, Hagstrom H, Ludvigsson JF. Mortality in biopsy‐confirmed non‐alcoholic fatty liver disease: results from a nationwide cohort. Gut 2021;70:1375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Targher G, Lonardo A, Rossini M. Nonalcoholic fatty liver disease and decreased bone mineral density: is there a link? J Endocrinol Invest. 2015;38:817–25. [DOI] [PubMed] [Google Scholar]
- 4. Cui R, Sheng H, Rui XF, Cheng X‐Y, Sheng C‐J, Wang J‐Y, et al. Low bone mineral density in Chinese adults with non‐alcoholic Fatty liver disease. Int J Endocrinol. 2013;2013:396545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee DY, Park JK, Hur KY, Um SH. Association between non‐alcoholic fatty liver disease and bone mineral density in postmenopausal women. Climacteric 2018;21:498–501. [DOI] [PubMed] [Google Scholar]
- 6. Moon SS, Lee YS, Kim SW. Association of non‐alcoholic fatty liver disease with low bone mass in postmenopausal women. Endocrine 2012;42:423–9. [DOI] [PubMed] [Google Scholar]
- 7. Xia MF, Lin HD, Yan HM, Bian H, Chang X‐X, Zhang L‐S, et al. The association of liver fat content and serum alanine aminotransferase with bone mineral density in middle‐aged and elderly Chinese men and postmenopausal women. J Transl Med. 2016;14:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bhatt SP, Nigam P, Misra A, Guleria R, Qadar Pasha MA. Independent associations of low 25 hydroxy vitamin D and high parathyroid hormonal levels with non‐alcoholic fatty liver disease in Asian Indians residing in north India. Atherosclerosis 2013;230:157–63. [DOI] [PubMed] [Google Scholar]
- 9. Kaya M, Isik D, Bestas R, Evliyaoğlu O, Akpolat V, Büyükbayram H, et al. Increased bone mineral density in patients with non‐alcoholic steatohepatitis. World J Hepatol. 2013;5:627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim G, Kim KJ, Rhee Y, Lim SK. Significant liver fibrosis assessed using liver transient elastography is independently associated with low bone mineral density in patients with non‐alcoholic fatty liver disease. PLoS One. 2017;12:e0182202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee SH, Yun JM, Kim SH, Seo YG, Min H, Chung E, et al. Association between bone mineral density and non‐alcoholic fatty liver disease in Korean adults. J Endocrinol Invest. 2016;39:1329–36. [DOI] [PubMed] [Google Scholar]
- 12. Mantovani A, Dauriz M, Gatti D, Viapiana O, Zoppini G, Lippi G, et al. Systematic review with meta‐analysis: non‐alcoholic fatty liver disease is associated with a history of osteoporotic fractures but not with low bone mineral density. Aliment Pharmacol Ther. 2019;49:375–88. [DOI] [PubMed] [Google Scholar]
- 13. Mantovani A, Sani E, Fassio A, Colecchia A, Viapiana O, Gatti D, et al. Association between non‐alcoholic fatty liver disease and bone turnover biomarkers in post‐menopausal women with type 2 diabetes. Diabetes Metab. 2019;45:347–55. [DOI] [PubMed] [Google Scholar]
- 14. Umehara T. Nonalcoholic fatty liver disease with elevated alanine aminotransferase levels is negatively associated with bone mineral density: cross‐sectional study in U.S. adults. PLoS One. 2018;13:e0197900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li M, Xu Y, Xu M, Zhang R, Gao L, Han X, et al. Association between non‐alcoholic fatty liver disease (NAFLD) and osteoporotic fracture in middle‐aged and elderly Chinese. J Clin Endocrinol Metab. 2012;97:2033–8. [DOI] [PubMed] [Google Scholar]
- 16. Wang Y, Wen G, Zhou R, Zhong W, Lu S, Hu C, et al. Association of nonalcoholic fatty liver disease with osteoporotic fractures: a cross‐sectional retrospective study of Chinese individuals. Front Endocrinol (Lausanne). 2018;9:408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim J‐L, Reuterwall C, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ludvigsson JF, Almqvist C, Bonamy AK, Ljung R, Michaëlsson K, Neovius M, et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol. 2016;31:125–36. [DOI] [PubMed] [Google Scholar]
- 19. Ludvigsson JF, Otterblad‐Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24:659–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hagstrom H, Adams LA, Allen AM, Byrne CD, Chang Y, Grønbaek H, et al. Administrative coding in electronic health care record‐based research of NAFLD: an expert panel consensus statement. Hepatology 2021;74:474–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation 2016;133:601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Otete H, Deleuran T, Fleming KM, Card T, Aithal GP, Jepsen P, et al. Hip fracture risk in patients with alcoholic cirrhosis: a population‐based study using English and Danish data. J Hepatol. 2018;69:697–704. [DOI] [PubMed] [Google Scholar]
- 23. Solaymani‐Dodaran M, Card TR, Aithal GP, West J. Fracture risk in people with primary biliary cirrhosis: a population‐based cohort study. Gastroenterology 2006;131:1752–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. International Classification of Disease (ICD) codes from the 9th and 10th revision for NAFLD, its differential diagnoses, alcohol or drug abuse, and liver transplantation.
Supplemental Table 2. International Classification of Disease (ICD) codes from the 9th and 10th revision for fractures.
Supplemental Table 3. International Classification of Disease (ICD) codes from the 9th and 10th revision for comorbidities.
Supplemental Table 4. Fracture outcomes for patients with NAFLD (n=10,678) and matched controls (n=99,176) stratified for sex, age group, disease severity, the presence of diabetes, and inclusion year.
Supplemental Table 5. Post‐fracture mortality at 1 year for patients with NAFLD (n=1113) and controls (n=11,199) who had a fracture during the study period.


