Abstract
Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) is a rare but disabling disorder that often requires long‐term immunomodulatory treatment. Background incidence rates and prevalence and risk factors for developing CIDP are still poorly defined. In the current study, we used a longitudinal population‐based cohort study in The Netherlands to assess these rates and demographic factors and comorbidity associated with CIDP. We determined the incidence rate and prevalence of CIDP between 2008 and 2017 and the occurrence of potential risk factors in a retrospective Dutch cohort study using the Integrated Primary Care Information (IPCI) database. Cases were defined as CIDP if the diagnosis of CIDP was described in the electronic medical file. In a source population of 928 030 persons with a contributing follow‐up of 3 525 686 person‐years, we identified 65 patients diagnosed with CIDP. The overall incidence rate was 0.68 per 100 000 person‐years (95% CI 0.45‐0.99). The overall prevalence was 7.00 per 100 000 individuals (95% CI 5.41‐8.93). The overall incidence rate was higher in men compared to woman (IRR 3.00, 95% CI 1.27‐7.11), and higher in elderly of 50 years or older compared with people <50 years of age (IRR 17 95% CI 4‐73). Twenty percent of CIDP cases had DM and 9% a co‐existing other auto‐immune disease. These background rates are important to monitor changes in the frequency of CIDP following infectious disease outbreaks, identify potential risk factors, and to estimate the social and economic burden of CIDP.
Keywords: chronic inflammatory demyelinating polyradiculoneuropathy, CIDP, epidemiology, incidence, prevalence
1. INTRODUCTION
Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) is a treatable immune‐mediated disorder of the peripheral nervous system usually causing muscle weakness and sensory deficits in all extremities. Immunoglobulin, corticosteroids, and plasmapheresis are proven effective treatments for CIDP. 1 , 2 Most patients require maintenance treatment for years or even decades and suffer from residual disability despite of treatment. 1 , 3 CIDP is a rare disorder but the reported incidence and prevalence vary considerable between studies. In a recent systematic review of literature, the reported incidence rates ranged between 0.15 and 1.6 cases per 100 000 person‐years, and the prevalence ranged between 0.67 and 10.3 per 100 000. 4 Differences in study methodologies 4 and between regions or countries might explain this considerable variation. Importantly, previous studies were mainly limited to hospital‐based cohorts in which frequencies probably depend on references and policies of care. Risk factors for developing CIDP are still unknown but most studies indicated that CIDP is more frequent in males and elderly persons. 4 Infections preceding the onset of CIDP symptoms, diabetes mellitus (DM) and concomitant auto‐immune disorders have been reported in CIDP patients, 5 , 6 , 7 , 8 , 9 but frequencies vary considerable between studies and may differ per population, and the exact association with CIDP is unclear. Until now there is no epidemiological information on CIDP in The Netherlands.
We conducted a large population‐based study to determine the incidence and prevalence of CIDP in the general population in The Netherlands, and the occurrence of potential risk factors for developing CIDP. This information is needed to determine changes of CIDP incidence following exposure to infections or identify potential causes of CIDP and to estimate the social and economic burden of CIDP.
2. METHODS
2.1. Study design
We conducted a retrospective, population‐based cohort study using data from the Integrated Primary Care Information (IPCI) database. The IPCI database is a longitudinal observational database, containing medical information of patients from computer‐based records of general practitioners (GP) in the Netherlands. The IPCI patient population is representative of the general Dutch population regarding distribution of sex and age. 10 All residents of The Netherlands are registered with a GP, who acts as a gatekeeper to secondary and tertiary medical care in the Dutch healthcare system. The medical files from GPs contain medical history, including patient demographics, signs and symptoms, physical findings, laboratory results, drug prescriptions, and secondary care information such as hospital discharge letters and letters from medical specialists. The IPCI database complies with the European Union guidelines on the use of medical data for medical research and has been proven valid for (pharmaco)‐epidemiological studies. 10 The Governance Board of the IPCI database approved the current study.
2.2. Study population
The total study population comprised all patient files included in the IPCI database between January 1, 2008 and June 20, 2018. To have sufficient information on all cases, we only included GP systems in which neurological discharge letters were available, the GP practice had to be contributing data to the IPCI database for at least 1 year, and the patient had to be registered with the GP for at least 1 year. Follow‐up started on the first of January 2008 or on the date that valid history was available whichever came last. Follow‐up ended on the date that the patient left the GP practice, on the date of last data supply by the GP, death, or June 20, 2018.
2.3. Case identification
Potential cases of CIDP were first identified in the IPCI database using a computerized database search using disease specific terms (CIDP, DEMYEL + NEUROPAT, POLYRADICUL) (Step 1 Figure 1). The full electronic medical file of all patients, with one or more CIDP specific terms was manually reviewed by MB in order to eliminate non‐cases (Step 2, Figure 1). Cases were defined as CIDP for the current study if a patient had a diagnosis CIDP described in the electronic medical file. No exclusion criterion based on age was applied. The index date was defined as the date of the first diagnosis of CIDP. If the index date occurred after the start of follow‐up, this case was defined as incident. Patients with a CIDP diagnosis prior to start follow‐up were classified as prevalent.
FIGURE 1.
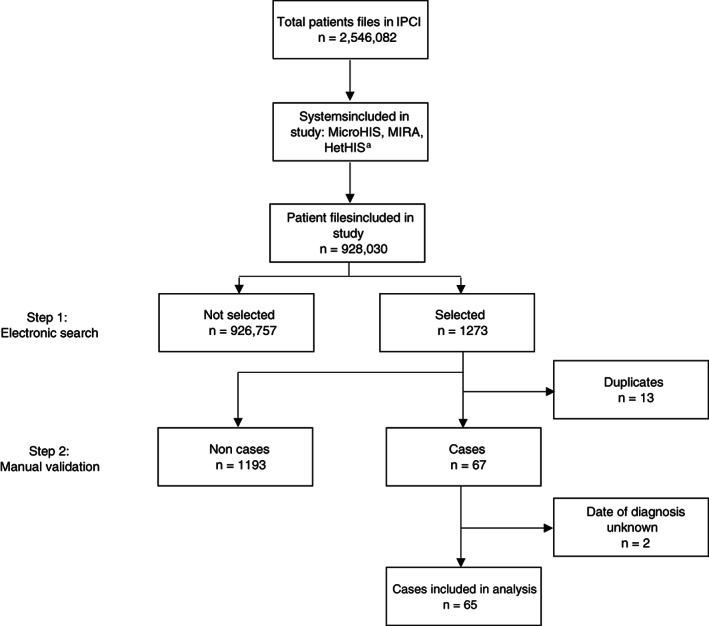
Identification of cases with CIDP from the total study population. CIDP, chronic inflammatory demyelinating polyradiculoneuropathy, IPCI, Integrated Primary Care Information database. aSelection of software systems containing sufficient discharge letters
2.4. Covariates
In both incident and prevalent cases, the full medical file was further explored to determine data regarding onset of symptoms, comorbidity, clinical features, electrophysiological findings, cerebrospinal fluid analysis, treatment, and diagnostic delay. Diagnostic delay was defined as the time between onset of symptoms and first CIDP diagnosis which was only evaluated in patients were this information was available. Improvement after immunotherapy was defined as any clinical improvement reported by the treating neurologist. We coded the appearance of concomitant auto‐immune disease and diabetes mellitus (DM) at study entry or during study follow‐up. In case information on auto‐immune disease was not present, we considered the patient not to have a co‐existing auto‐immune disease.
2.5. Statistical analyses
Continuous data are presented as medians with full ranges (minimum and maximum) and dichotomized or categorical data as numbers and proportions. Incidence rates of CIDP were calculated by dividing the total number of incident cases by the total number of person years at risk of the study population for each calendar year from 2008 to 2017. The prevalence of CIDP was calculated by dividing the number of patients with CIDP by the population on the first of January of each calendar year from 2008 to 2018 (defined as point prevalence). The 95% confidence intervals (CI) around the incidence and prevalence estimates were calculated using a Poisson distribution. Incidence rates and prevalence were calculated per calendar year, sex, and age (<50 years vs ≥50 years). The incidence rate of CIDP between sex‐ and age groups were compared with incidence rate ratio (IRR) estimated by Poisson regression. Prevalence between groups were compared using Chi‐squared or Fisher's exact tests. Two‐sided P values of <.05 were considered significant. We used Statistical Package for Social Sciences (SPSS) version 25 for data analysis.
3. RESULTS
3.1. Study population
The source population for the study comprised 928 030 patients, contributing a total follow‐up time of 3 525 686 person‐years (median 3, range 0‐10 person‐years). Within this population, 1273 records of potential CIDP cases were identified based on the automated search. After the manual review, we retained 67 cases. We subsequently excluded two cases because the date of diagnosis could not be assessed, leaving 65 cases for the analysis (Figure 1), of which 27 were incident cases. Patient characteristics are shown in Table 1. The median age at time of diagnosis was 58 years (range 12‐81 years) and 66% were male. Median time from onset of symptoms to GP visit, neurologist visit and diagnosis of CIDP, was 0.2 months (range 0.1‐4.1), 1.1 months (range 0.1‐28.3) and 4.0 months (range 0.3‐40.7) respectively. Twenty percent of CIDP cases had DM and 9% a co‐existing auto‐immune disease. Fifty‐four of 57 cases received CIDP treatment, in which in 90% (38/42) of patients improvement was reported. In the remaining 8 cases, no information on treatment was given.
TABLE 1.
Characteristics of cases with CIDP (n = 65)
| Demographics | |
| Age at diagnosis, years, median (range) | 58 (12‐81) |
| Male (n) | 66% (43) |
| Time onset till first visit GP, months, median (range) | 0.2 (0.1–4.1) a |
| Time onset till hospital visit, months, median (range) | 1.1 (0.1–28.3) b |
| Time onset till diagnosis, months, median (range) | 4.0 (0.3–40.7) c |
| Comorbidity | |
| Diabetes mellitus | 20% (11/55) d |
| Co‐existing auto‐immune disease | 9% (6) |
| Diagnostic features | |
| Limb weakness (n/N) | 100% (51/51) |
| Proximal limb weakness (n/N) | 81% (35/43) |
| Sensory deficits (n/N) | 100% (56/56) |
| Absent or low reflexes (n/N) | 100% (49/49) |
| Elevated protein level CSF (n/N) | 76% (22/29) |
| Demyelinating features on NCS e (n/N) | 96% (43/45) |
| M‐protein | |
| Excluded (n/N) | 60% (12/20) |
| Demonstrated (n/N) | 40% (8/20) |
| Treatment | |
| Treated with immunotherapy (n/N) | 95% (54/57) |
| Improvement after immunotherapy (n/N) | 90% (38/42) |
Abbreviations: CIDP, chronic inflammatory demyelinating polyradiculoneuropathy.
Missing n = 46.
Missing n = 47.
Missing n = 39.
Type 1 n = 2, type 2 n = 8, unknown n = 1.
Electronic medical record stated that demyelinating features on NCS were confirmed by a neurologist, no raw data of NCS were available.
3.2. Incidence rates
The overall incidence rate was 0.68 per 100 000 person‐years (95% CI 0.45‐0.99) (Table 2). No trend was found in IRs over the different calendar years. The overall incidence rate was higher in men compared to woman (IRR 3.00, 95% CI 1.27‐7.11), and higher in elderly of 50 years or older compared with people <50 years of age (IRR 17 95% CI 4‐73).
TABLE 2.
Incidence rates of CIDP per 100.000 person years by calendar year, gender, and age
| N a | Person‐years | Incidence rate (95% CI) | |
|---|---|---|---|
| Calendar year | |||
| 2008 | 0 | 209 536 | 0.00 |
| 2009 | 0 | 242 189 | 0.00 |
| 2010 | 1 | 271 071 | 0.37 (0.01‐2.06) |
| 2011 | 5 | 407 158 | 1.23 (0.40‐2.87) |
| 2012 | 3 | 460 883 | 0.65 (0.13‐1.90) |
| 2013 | 4 | 493 328 | 0.81 (0.22‐2.08) |
| 2014 | 1 | 461 058 | 0.22 (0.01‐1.21) |
| 2015 | 6 | 495 716 | 1.21 (0.44‐2.63) |
| 2016 | 4 | 464 488 | 0.86 (0.23‐2.20) |
| 2017 | 3 | 472 809 | 0.63 (0.13‐1.85) |
| Overall IR (2008‐2017) | 27 | 3 978 236 | 0.68 (0.45‐0.99) |
| Overall IR (2008‐2017) | |||
| Men | 20 | 1 938 708 | 1.03 (0.63‐1.59) |
| Woman | 7 | 2 039 528 | 0.34 (0.14‐0.71) |
| Overall IR (2008‐2017) | |||
| <50 years | 2 | 2 307 910 | 0.09 (0.01‐0.31) |
| ≥ 50 years | 25 | 1 670 326 | 1.50 (0.97‐2.21) |
Abbreviations: CI, confidence interval; CIDP, chronic inflammatory demyelinating polyradiculoneuropathy; and IR, incidence rate.
Number of cases.
3.3. Prevalence
The overall prevalence was 7.00 per 100 000 individuals (95% CI 5.41‐8.93) (Table 3). The point prevalence on January 1, 2018 was 9.99 per 100 000 individuals (7.26‐13.4). The point prevalence on January 1, 2018 seemed to be higher in men compared to woman (11.62 95% CI 7.52‐17.16 vs 8.43 95% CI 5.07‐13.16)(P = .29). The point prevalence on January 1, 2018 was higher for elderly compared with people <50 years of age (20.51 95% CI 14.28‐28.52 vs 3.33 95% CI 1.52‐6.33) (P < .01).
TABLE 3.
Prevalence of CIDP per 100.000 persons by calendar year, gender, and age
| N a | Population | Prevalence (95% CI) | |
|---|---|---|---|
| Calendar year b | |||
| 2008 | 7 | 138 011 | 5.07 (2.04‐10.45) |
| 2009 | 10 | 202 866 | 4.93 (2.36‐9.07) |
| 2010 | 11 | 232 440 | 4.73 (2.36‐8.47) |
| 2011 | 14 | 251 610 | 5.56 (3.04‐9.34) |
| 2012 | 21 | 325 844 | 6.44 (3.99‐9.85) |
| 2013 | 28 | 419 786 | 6.67 (4.43‐9.64) |
| 2014 | 31 | 384 059 | 8.07 (5.48‐11.46) |
| 2015 | 28 | 396 470 | 7.06 (4.69‐10.21) |
| 2016 | 40 | 425 324 | 9.40 (6.72‐12.81) |
| 2017 | 42 | 440 270 | 9.54 (6.87‐12.90) |
| 2018 | 44 | 440 552 | 9.99 (7.26‐13.41) |
| Overall prevalence at the end of follow‐up | 65 | 928 030 | 7.00 (5.41–8.93) |
| Point prevalence on 1 January 2018 c | |||
| Male | 25 | 215 090 | 11.62 (7.52‐17.16) |
| Female | 19 | 225 462 | 8.43 (5.07–13.16) |
| Point prevalence on 1 January 2018 c , d | |||
| <50 years | 9 | 269 893 | 3.33 (1.52‐6.33) |
| ≥50 years | 35 | 170 659 | 20.51 (14.28‐28.52) |
Abbreviations: CI, confidence interval; CIDP, chronic inflammatory demyelinating polyradiculoneuropathy.
Number of cases.
On the first of January.
P = .29.
P < .01.
4. DISCUSSION
In this study, we found an overall incidence rate of CIDP of 0.68 per 100 000 person‐years (95% CI 0.45‐0.99) per 100 000 person‐years and an overall prevalence of 7.00 (95% CI 5.41‐8.93) per 100 000 persons in The Netherlands. The incidence rate and prevalence of CIDP was higher in males and in persons of 50 years or older. We observed no evident change in the incidence rate during the follow‐up of the study (2008‐2017).
Our findings are in line with previous studies on the incidence and prevalence of CIDP, 4 , 11 in which incidence rates ranged from 0.15‐1.6 cases per 100 000 person‐years and the prevalence ranged from 0.67‐10.3 per 100 000 persons, but are higher than the previously found pooled incidence (0.33 cases per 100 000 person‐years) and prevalence (2.81 per 100 000 persons) of CIDP. A high awareness of this disease could be an explanation, as The Netherlands is a relatively densely populated, urbanized country with the proximity of neuromuscular specialists. As the IPCI population is a good reflection of the general Dutch population regarding the distribution of sex and age, we estimate that for The Netherlands with 17470459 12 inhabitants there are between 79 and 173 new cases of CIDP yearly, and that between 945 and 1560 cases currently have CIDP.
A male predominance and increasing occurrence of CIDP by age is extensively reported in the literature. 4 The male predominance in CIDP is unexplained and deviates from a female predominance that is seen in most classic auto‐immune disorders. 13 An increasing incidence with age has also been described in polyneuropathies in general 14 , 15 implying that elderly are more at risk to develop a polyneuropathy. We found that 20% of the CIDP patients also had DM, which is considerably higher than that of the general Dutch population (6.47%). 16 The possible association between DM and CIDP has been studied for several decades, but frequencies and study designs vary considerably. 11 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 Several studies reported high frequencies of DM in patients with CIDP, 18 , 19 , 21 , 22 , 23 , 24 , 25 while other studies suggest no association of CIDP with DM. 11 , 17 , 20 Whether CIDP is associated with DM type 1 as concomitant auto‐immune disorder is unknown, but one study showed that de frequency of CIDP is the same in type 1 and type 2 diabetes. 23 A CIDP diagnosis can be difficult in patients with DM. (Demyelinating) features on NCS based on a diabetic polyneuropathy can be misdiagnosed as CIDP. 27 , 28 In patients with diabetes, demyelinating features can also be misclassified as diabetic polyneuropathy leading to withhold of treatment for CIDP. 29 Whether DM is a true risk factor for CIDP remain uncertain. In our study, the high incidence of DM in CIDP patients could be in part explained by the higher age of CIDP patients compared with the general Dutch population, as a higher age is risk factor for developing DM, but we cannot exclude the possibility that some patient had diabetic polyneuropathy instead of CIDP. In our study, 9% of CIDP patients had a co‐existing auto‐immune disease. Data on the prevalence of auto‐immune diseases for the general Dutch population are not available. A recent cohort study in Italy showed a higher frequency of auto‐immune disorders than expected in the general Italian population (16% vs 8.6%), while in a Dutch survey study only 5% of CIDP patients reported a common auto‐immune disorder. 30
In our study, the median time from onset of symptoms to the first hospital visit was 1.1 months and the median time to CIDP diagnosis 4 months. This finding is in line with a previous Dutch study, in which median time from onset to the first hospital visit was 2 months, and the median time from onset to CIDP diagnosis was 5 months. 3 However, these time windows are considerable shorter than found in study performed in the United States in which the median symptom duration before presentation was 10 months. Differences in healthcare systems, including the accessibility of a neuromuscular specialist, might explain this. This is the first study that describes the time between onset of symptoms and GP visit in a population‐based study. A rapid diagnosis of CIDP is essential to initiate treatment at an early stage of disease and thereby may prevent or reduce secondary and potentially irreversible axonal nerve damage and related disability. 31
The strengths of IPCI are its population‐based design, the large sample size, limited selection bias as all Dutch citizens need to be registered with a GP practice, and the representativeness of the overall Dutch population. 10 The observed incidence rate en prevalence falls within the range of previous reported estimates, 4 but we cannot rule out an underestimation in case CIDP was not well documented by the GP. On the other hand, in clinic there is an overdiagnosis of CIDP and we cannot exclude the possibility of overdiagnosis in this study. 27 Although data regarding clinical features were present in most patients in our study and the diagnosis was re‐evaluated by the authors in all CIDP patients as much as possible based on this information provided in the IPCI database. Raw NCS data were not available. Therefore, fulfilment of the diagnostic criteria according to the European Academy of Neurology/Peripheral Nerve Society (EAN/PNS) guideline on diagnosis and treatment of CIDP could not be assessed, which is a limitation of our study. The IPCI database does not contain all data regarding infections, and as CIDP has a relatively slowly progressive disease course, the exact onset data are often missing. Therefore, a study on the association of infections preceding the onset of CIDP symptoms and CIDP was not possible. Because data regarding the onset of symptoms, first GP visit and first hospital visit were often missing, time from CIDP onset till first GP visit, hospital visit, and diagnosis, should be read cautiously.
In conclusion, we found an overall incidence rate of 0.68 (95% CI 0.45‐0.99) per 100 000 person‐years and an overall prevalence of 7.00 per 100 000 individuals (95% CI 5.41‐8.93). Our findings are important to address an increase in CIDP incidence following an infectious disease outbreak, identify potential risk factors, and to estimate the true social and economic burden of CIDP.
CONFLICT OF INTEREST
M.C. Broers and H.F. Lingsma report grants from Dutch Prinses Beatrix Spierfonds, during the conduct of the study. M. de Wilde, J. van der Lei and K. Verhamme work for a research institute who receives/received unconditional research grants from Yamanouchi, Pfizer‐Boehringer Ingelheim, GSK, Amgen, UCB, Novartis, Astra‐Zeneca, Chiesi, Janssen Research and Development, none of which relate to the content of this work. B.C. Jacobs reports grants from Dutch Prinses Beatrix Spierfonds during the conduct of the study; grants from Dutch Prinses Beatrix Spierfonds, Horizon 2020, GBS‐CIDP Foundation International, Baxalta, Grifols, CSL Behring, Annexon and Hansa Biopharma, outside the submitted work.
ACKNOWLEDGEMENTS
This study is funded by the Dutch Prinses Beatrix Spierfonds (grant application number: W.OR16‐18).
Broers MC, de Wilde M, Lingsma HF, van der Lei J, Verhamme KMC, Jacobs BC. Epidemiology of chronic inflammatory demyelinating polyradiculoneuropathy in The Netherlands. J Peripher Nerv Syst. 2022;27(3):182‐188. doi: 10.1111/jns.12502
Funding information Prinses Beatrix Spierfonds, Grant/Award Number: W.OR16‐18
DATA AVAILABILITY STATEMENT
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data is not available.
REFERENCES
- 1. Oaklander AL, Lunn MP, Hughes RA, van Schaik IN, Frost C, Chalk CH. Treatments for chronic inflammatory demyelinating polyradiculoneuropathy (CIDP): an overview of systematic reviews. Cochrane Database Syst Rev. 2017;2017(1):CD010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van Schaik IN, Bril V, van Geloven N, et al. Subcutaneous immunoglobulin for maintenance treatment in chronic inflammatory demyelinating polyneuropathy (PATH): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Neurol. 2018;17(1):35‐46. [DOI] [PubMed] [Google Scholar]
- 3. Bunschoten C, Blomkwist‐Markens PH, Horemans A, van Doorn PA, Jacobs BC. Clinical factors, diagnostic delay, and residual deficits in chronic inflammatory demyelinating polyradiculoneuropathy. J Peripher Nerv Syst. 2019;24(3):253‐259. [DOI] [PubMed] [Google Scholar]
- 4. Broers MC, Bunschoten C, Nieboer D, Lingsma HF, Jacobs BC. Incidence and prevalence of chronic inflammatory demyelinating polyradiculoneuropathy: a systematic review and meta‐analysis. Neuroepidemiology. 2019;52(3–4):161‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Doneddu PE, Bianchi E, Cocito D, et al. Risk factors for chronic inflammatory demyelinating polyradiculoneuropathy (CIDP): Antecedent events, lifestyle and dietary habits. Data from the Italian CIDP satabase. Eur J Neurol. 2020;27(1):136‐143. [DOI] [PubMed] [Google Scholar]
- 6. Rajabally YA, Stettner M, Kieseier BC, Hartung HP, Malik RA. CIDP and other inflammatory neuropathies in diabetes ‐ diagnosis and management. Nat Rev Neurol. 2017;13(10):599‐611. [DOI] [PubMed] [Google Scholar]
- 7. Ferrari SM, Fallahi P, Ruffilli I, et al. The association of other autoimmune diseases in patients with Graves' disease (with or without ophthalmopathy): review of the literature and report of a large series. Autoimmun Rev. 2019;18(3):287‐292. [DOI] [PubMed] [Google Scholar]
- 8. Bibbo S, Pes GM, Usai‐Satta P, et al. Chronic autoimmune disorders are increased in coeliac disease: a case‐control study. Medicine. 2017;96(47):e8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bar Yehuda S, Axlerod R, Toker O, et al. The association of inflammatory bowel diseases with autoimmune disorders: a report from the epi‐IIRN. J Crohns Colitis. 2019;13(3):324‐329. [DOI] [PubMed] [Google Scholar]
- 10. Vlug AE, van der Lei J, Mosseveld BM, et al. Postmarketing surveillance based on electronic patient records: the IPCI project. Methods Inf Med. 1999;38(4–5):339‐344. [PubMed] [Google Scholar]
- 11. Cea G, Idiaquez JF, Salinas R, Matamala JM, Villagra R, Stuardo A. Epidemiology of chronic inflammatory demyelinating polyneuropathy in the south‐eastern area of Santiago, Chile. J Clin Neurosci. 2020;74:271‐273. [DOI] [PubMed] [Google Scholar]
- 12. Bevolkingsteller: CBS. 2020. [updated November 3, 2020. Available from: https://www.cbs.nl/nl-nl/visualisaties/bevolkingsteller.
- 13. Beeson PB. Age and sex associations of 40 autoimmune diseases. Am J Med. 1994;96(5):457‐462. [DOI] [PubMed] [Google Scholar]
- 14. Sejvar JJ, Baughman AL, Wise M, Morgan OW. Population incidence of Guillain‐Barre syndrome: a systematic review and meta‐analysis. Neuroepidemiology. 2011;36(2):123‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hanewinckel R, van Oijen M, Ikram MA, van Doorn PA. The epidemiology and risk factors of chronic polyneuropathy. Eur J Epidemiol. 2016;31(1):5‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peters ML, Huisman EL, Schoonen M, Wolffenbuttel BHR. The current total economic burden of diabetes mellitus in The Netherlands. Neth J Med. 2017;75(7):281‐297. [PubMed] [Google Scholar]
- 17. Chio A, Plano F, Calvo A, et al. Comorbidity between CIDP and diabetes mellitus: only a matter of chance? Eur J Neurol. 2009;16(6):752‐754. [DOI] [PubMed] [Google Scholar]
- 18. Rotta FT, Sussman AT, Bradley WG, Ram Ayyar D, Sharma KR, Shebert RT. The spectrum of chronic inflammatory demyelinating polyneuropathy. J Neurol Sci. 2000;173(2):129‐139. [DOI] [PubMed] [Google Scholar]
- 19. Mahdi‐Rogers M, Hughes RA. Epidemiology of chronic inflammatory neuropathies in Southeast England. Eur J Neurol. 2014;21(1):28‐33. [DOI] [PubMed] [Google Scholar]
- 20. Laughlin RS, Dyck PJ, Melton LJ 3rd, Leibson C, Ransom J, Dyck PJ. Incidence and prevalence of CIDP and the association of diabetes mellitus. Neurology. 2009;73(1):39‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rajabally YA, Simpson BS, Beri S, Bankart J, Gosalakkal JA. Epidemiologic variability of chronic inflammatory demyelinating polyneuropathy with different diagnostic criteria: study of a UKpopulation. Muscle Nerve. 2009;39(4):432‐438. [DOI] [PubMed] [Google Scholar]
- 22. Bril V, Blanchette CM, Noone JM, Runken MC, Gelinas D, Russell JW. The dilemma of diabetes in chronic inflammatory demyelinating polyneuropathy. J Diabetes Complications. 2016;30(7):1401‐1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sharma KR, Cross J, Farronay O, Ayyar DR, Shebert RT, Bradley WG. Demyelinating neuropathy in diabetes mellitus. Arch Neurol. 2002;59(5):758‐765. [DOI] [PubMed] [Google Scholar]
- 24. Doneddu PE, Cocito D, Manganelli F, et al. Frequency of diabetes and other comorbidities in chronic inflammatory demyelinating polyradiculoneuropathy and their impact on clinical presentation and response to therapy. J Neurol Neurosurg Psychiatry. 2020;91:1092‐1099. [DOI] [PubMed] [Google Scholar]
- 25. Rajabally YA, Peric S, Cobeljic M, et al. Chronic inflammatory demyelinating polyneuropathy associated with diabetes: a European multicentre comparative reappraisal. J Neurol Neurosurg Psychiatry. 2020;91(10):1100‐1104. [DOI] [PubMed] [Google Scholar]
- 26. Chio A, Cocito D, Bottacchi E, et al. Idiopathic chronic inflammatory demyelinating polyneuropathy: an epidemiological study in Italy. J Neurol Neurosurg Psychiatry. 2007;78(12):1349‐1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Allen JA, Lewis RA. CIDP diagnostic pitfalls and perception of treatment benefit. Neurology. 2015;85(6):498‐504. [DOI] [PubMed] [Google Scholar]
- 28. Allen JA, Ney J, Lewis RA. Electrodiagnostic errors contribute to chronic inflammatory demyelinating polyneuropathy misdiagnosis. Muscle Nerve. 2018;57(4):542‐549. [DOI] [PubMed] [Google Scholar]
- 29. Dunnigan SK, Ebadi H, Breiner A, et al. Conduction slowing in diabetic sensorimotor polyneuropathy. Diabetes Care. 2013;36(11):3684‐3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kuitwaard K, Bos‐Eyssen ME, Blomkwist‐Markens PH, van Doorn PA. Recurrences, vaccinations and long‐term symptoms in GBS and CIDP. J Peripher Nerv Syst. 2009;14(4):310‐315. [DOI] [PubMed] [Google Scholar]
- 31. Bouchard C, Lacroix C, Plante V, et al. Clinicopathologic findings and prognosis of chronic inflammatory demyelinating polyneuropathy. Neurology. 1999;52(3):498‐503. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data is not available.


