Abstract
Aim
The placebo effect and the specific effect are often thought to add up (additive model). Whether additivity holds can dramatically influence the external validity of a trial. This assumption of additivity was tested by Kleijnen et al in 1994 but the data produced since then have not been synthetized. In this review, we aimed to systematically review the literature to determine whether additivity held.
Methods
We searched Medline and PsychInfo up to 10 January 2019. Studies using the balanced placebo design (BPD), testing two different strengths of placebos, were included. The presence of interaction was evaluated by comparing each group in the BPD with analysis of variance or covariance.
Results
Thirty studies were included and the overall risk of bias was high: four found evidence of additivity and 16 studies found evidence of interaction (seven had evidence of positive additivity).
Conclusion
Evidence of additivity between placebo and specific features of treatments was rare in included studies. We suggest interventions for placebo‐sensitive ailments should be tested in trials designed to take interactions seriously once an exploratory RCTs has proven their efficacy with sufficient internal validity.
Keywords: clinical trials, drug effect, evidence‐based practice, placebo, therapeutic alliance, treatment outcome

1. INTRODUCTION
The total treatment effect is assumed to be the sum of its specific effect and of “nonspecific”, or “placebo” effects. 1 , 2 , 3 This is known as the additive model. 4 However, it has been noted since at least the 1960s that the placebo and treatment effects can interact. 5 , 6 , 7 , 8 If they interact, the specific treatment and placebo effects combine in ways that can be greater than the sum of the parts of (supra‐additive or synergistic), less than the sum of the parts of (subadditive or antagonistic) 9 or even reverse (qualitative interaction) the overall treatment effect. 10 , 11 , 12 , 13 , 14 The difference between these models is illustrated in Figure 1.
FIGURE 1.
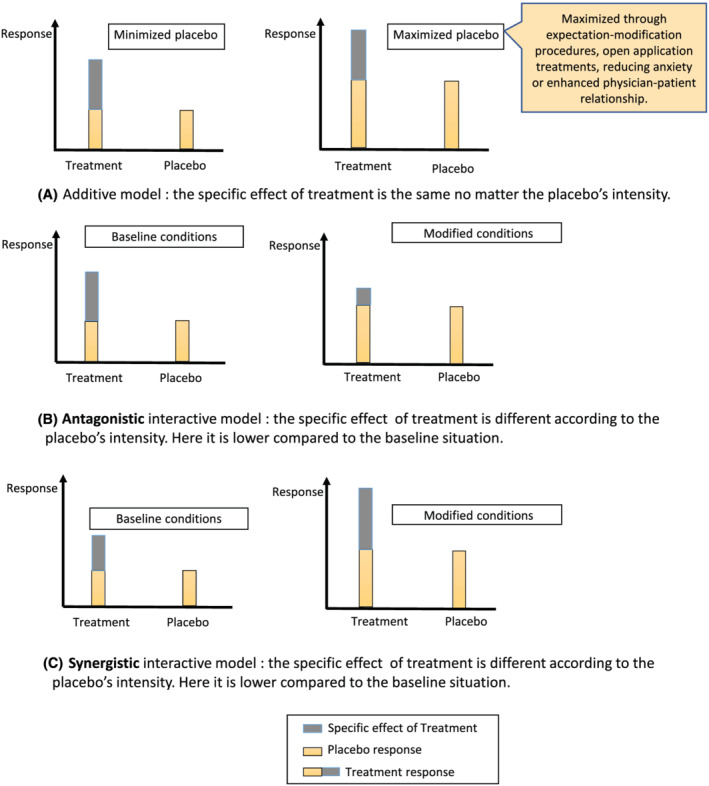
Additive versus interactive models
In 1994, Kleijnen et al 15 reviewed the potential evidence for interaction in 10 studies. They found that specific and nonspecific effects can at times be synergistic or antagonistic, thereby rendering overly reductive the presumed additive model of randomized clinical trials (RCTs). 4 , 15 For example, Bergmann et al 9 showed that the strength of the analgesic effect of naproxen depended on whether patients were correctly informed (and consent given) or not (P value interaction <0·10). Their results are illustrated in Figure 2. However, not all attempts to identify interactions found evidence of interaction. 16 In a two‐by‐two factorial, randomized, placebo‐controlled, double‐blind trial, chronic pain patients attending an outpatient clinic were randomized to receive a single oral dose of 50 mg of tramadol or placebo, and they were further randomized to receive positive or neutral information, verbally expressed by the physician, regarding the expected analgesic effect of the drug. However, the tramadol did not outperform the placebo, making it impossible to detect interactions. Overall, the clinical trials Kleijnen et al 15 identified had small populations and low quality. Also, a number of studies investigating additivity have been published since then which test the clinical pertinence of the placebo model, as discussed by Fava et al. 17 A recent review by Coleshill et al 18 tested how placebo analgesia interacted with active analgesic effects and identified seven studies suggesting that additivity did not hold in placebo analgesia. The review only included seven studies and concluded that data was unavailable to draw solid conclusions.
FIGURE 2.
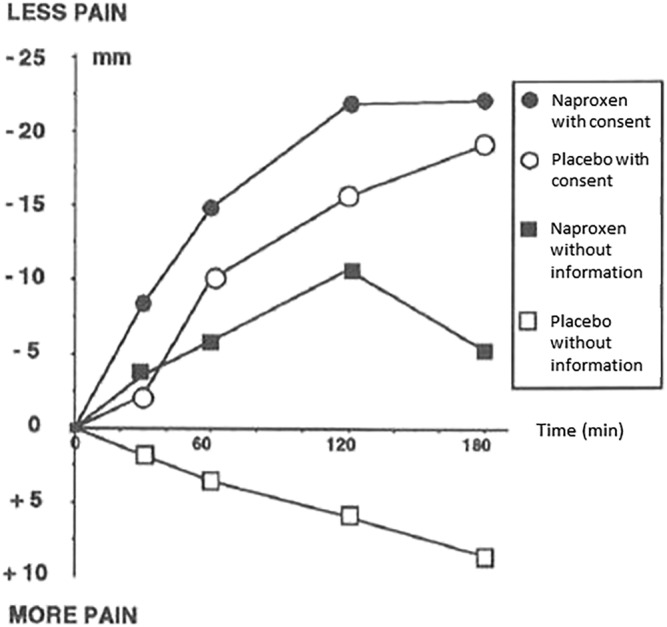
Bergmann et al results as published in 1994
In this study we aimed to update the findings from Kleijnen et al by systematically reviewing the available more recent literature to determine whether the additivity model of specific and nonspecific effects may be accepted as a general model.
2. METHODS
Our study protocol is available by contacting the study authors and is available in OSF Registries (https://osf.io/r5tzc). We followed PRISMA guidelines.
2.1. Eligibility criteria
This review included any randomised trials using the balanced placebo design (BPD), whereby there are at least two “intensities” of placebo effects (see Table 1). We included trials with any type of participants (clinical patients or healthy volunteers). To be comparable with Kleijnen et al’s earlier (1994) review, we excluded trials of alcohol, tobacco, acupuncture and homeopathy and we only included BPD trials (excluding pragmatic trials and other alternative designs). The BPD is a two‐by‐two factorial design and is described in Table 1. 19 , 20 , 21 , 22 It allows researchers to study the effect of the patient's expectation and the effect of the drug itself. In these trials, some patients in the treatment group are told they receive the treatment and others are told they receive placebo, which generates two different strengths of belief that the treatment will work. Likewise, some patients in the placebo group are told that they are receiving a placebo, while others are told they are receiving a treatment. If additivity holds, then the effect of the specific elements of the treatment should not change as a result of what patients are told, that is, (from Table 1) the difference C − A should be the same as D − B. Any statistically significant deviation from this means that additivity did not hold for that specific trial.
TABLE 1.
Balanced placebo design
| Told placebo | Told treatment | |
|---|---|---|
| Received placebo | A | B |
| Received treatment | C | D |
2.2. Information sources
We searched Medline and PsycInfo from 1964 (inception of Medline) to the 10 January 2019.
2.3. Search
The search equation was ([“Placebo Effect”[MeSH]] OR placebos[MeSH Terms])) OR “active placebo response”)) AND ((([“expectancies”] OR “expectancy”) OR “expectation”)))) NOT “alcohol”) NOT “smoking”) NOT “acupuncture”) AND Clinical Trial[ptyp])) OR (drug/placebo interaction AND Clinical Trial[ptyp])) OR (“balanced placebo design” AND Clinical Trial[ptyp]).
We also searched the bibliographies of each eligible study and searched for publications by the main authors of the trials included.
2.4. Study selection and data collection process
The searches were carried out independently by two researchers (R.Bo. and R.Ba.) and the results were pooled if possible. The two same researchers then read the full text of the selected studies and extracted the data into spreadsheets, which were then compared. In the event of doubt or disagreement a third researcher (F.G.) was intended to provide resolution, but this was not required.
2.5. Data items
The following data were extracted: study design, treatment and placebo used, analysis of risk of bias, number of study participants, endpoints, results about interaction between the specific effect and the placebo effect, and the authors' conclusion about the existence of interactions.
2.6. Summary measures and synthesis of results
We predicted there would be a high risk of bias on average because Kleijnen et al included 10 studies at high risk of bias in 1994. We took this into consideration when planning to pool our results: statistical analysis of interaction was planned with only low and/or intermediate risk of bias studies. The initial strategy was to calculate the effect sizes of treatments and placebos for each intensity of placebo administration (in accordance with Cochrane methods). 23 We initially planned to pool our results, but this was not possible due to lack of sufficient data in the included studies.
After the initial database search, and when analysing the data, we decided to present the results according to context (clinical context or healthy volunteers).
2.7. Risk of bias in individual studies
Analysis of bias risk was planned for each study. We used the Revised Cochrane risk‐of‐bias tool for randomized trials, RoB 2.0. 24 We took into account randomization, effects of the intervention on unblinding, missing data, primary endpoint measurement and transcription of study results. See Appendix 1 for detailed risk analysis.
2.8. Interactive model
There are three possible types of interactions: synergistic, antagonistic and reversal (qualitative interaction). In the case of antagonistic interaction, the total effect of a treatment is inferior to the sum total of the placebo effect and the specific effect of the treatment, whereas in the synergistic model the total effect of a treatment is superior to the sum total of the placebo effect and the specific effect. 6 , 12 , 18 In the case of reversal of effect (or qualitative interaction), the placebo effect will reverse the specific effect (like when pain is experienced when a topical analgesic is applied with nocebo information in the trial by Aslaksen et al). 10
3. RESULTS
3.1. Study selection
Figure 3 is a flowchart illustrating the selection process of studies for this review. Our search identified 1744 articles; only 30 studies were eligible for inclusion. 7 , 9 , 10 , 16 , 20 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 A considerable number of these studies (40%) were pain studies.
FIGURE 3.
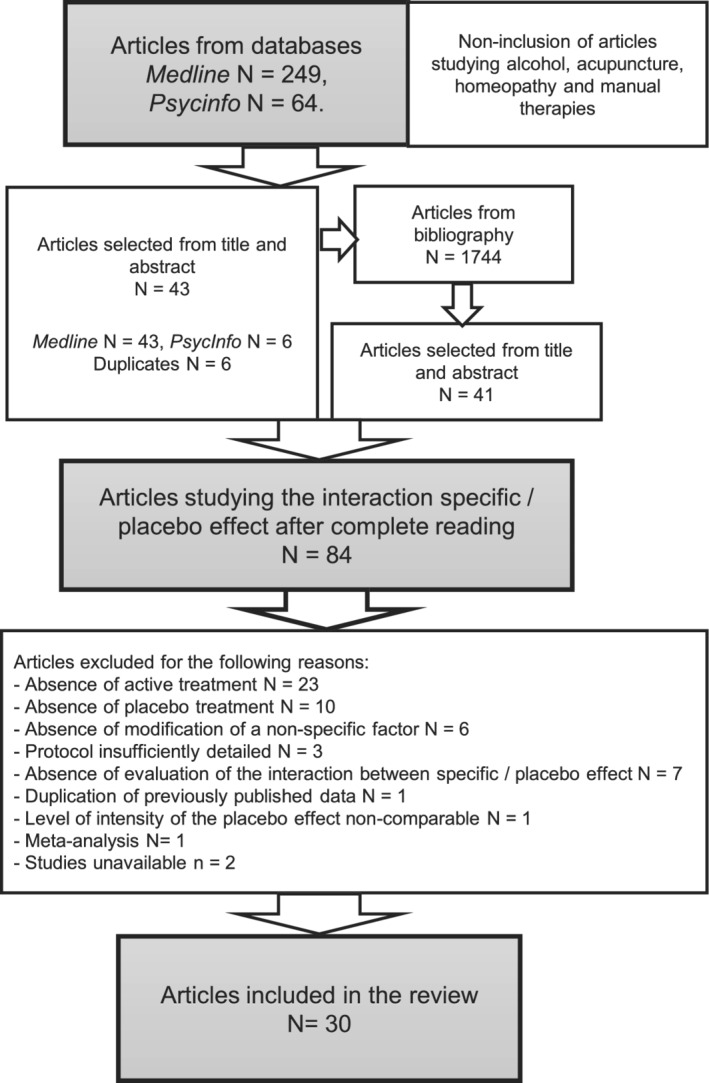
Flowchart illustrating the selection process
3.2. Study characteristics and risk of bias within studies
Study characteristics are presented in Table 2 and a more detailed version is available Appendix 2.
TABLE 2.
Study characteristics and results
| (a) Trials including patients | ||||||
|---|---|---|---|---|---|---|
| Trials | Population | Information modifying the power of the placebo effect | Treatment group | Endpoints | Interaction found? (which model?) | Risk of bias |
| Faasse et al (2016) 41 | 87 patients with chronic headaches | Oral information provided on the treatment brand administered: minimized or maximized situations | Ibuprofen | Pain | Yes (antagonistic) | Unclear |
| Bergmann et al (1994) 9 | 49 cancer patients | Oral information provided or not on the study procedure: neutral or maximized situations | Naproxen | Pain | Yes (antagonistic) | High |
| Wise et al (2009) 42 | 601 poorly controlled asthmatics | Oral information provided on the treatment administered, its brand and its colour: neutral or maximized situations | Montelukast | Peak expiratory flow, spirometry and four self‐assessment asthma scales | Yes (antagonistic) | High |
| Levine et al (1984) 45 | 96 patients having undergone dental extraction | Hidden administration of treatments, manually or by a machine: minimized, neutral or maximized situations | Naloxone | Pain | Yes (antagonistic) | High |
| Uhlenhuth et al (1959) 7 | 52 psychiatric patients suffering from anxiety | Neutral or positive attitude concerning the treatments administered: neutral or maximized situations | Meprobamate or phenobarbital | Improvement perceived by patients, assessment by a psychiatrist and a scale grouping together 45 symptoms | Yes (synergistic) | High |
| Uhlenhuth et al (1966) 46 | 138 patients referred to psychiatric clinic | Neutral or positive attitude concerning the treatments administered: neutral or maximized situations | Meprobamate in neutral or maximized situation | Modifications on different scales | Yes (antagonistic) | High |
| Kam‐Hansen et al (2014) 25 | 66 chronic migraine patients | Oral information on the treatment administered: minimized, neutral or maximized situations | Razatriptan | Pain | No (additive) | Low |
| Kemeny et al (2007) 43 | 55 poorly controlled asthmatics | Oral information provided on the treatment administered: neutral or maximized situations | Salmeterol | Concentration of methacholine needed to induce a 20% FEV1 decrease | No (no effect) | Low |
| Mathews et al (1983) 47 | 48 couples presenting with sexual disorders | Frequency of administration and number of therapists: weekly, monthly and at least one therapist | Testosterone | Improvement of symptoms evaluated by an outside investigator and the couples themselves | No (no effect) | High |
| De Craen et al (2001) 16 | 112 chronic pain patients | Written information on the treatment administered: neutral or maximized situations | Tramadol | Pain | No (no effect) | High |
| Brandwhaite et al (1981) 36 | 835 women with chronic headaches | Oral information provided on the “brand” of treatment administered: minimized or maximized situations | Aspirin | Pain | No (additive) | High |
| (b) Trials including healthy volunteers | ||||||
|---|---|---|---|---|---|---|
| Trials | Population | Information modifying the power of the placebo effect | Treatment group | Endpoints | Interaction found? (which model?) | Risk ok bias |
| Schenk et al (2013) 40 | 34 healthy volunteers | Oral information provided on the treatment administered: minimized or maximized situation | Lidocaine | Pain after painful thermal stimulus | Yes (synergistic) | Low |
| Hammami et al (2016) 27 | 480 healthy volunteers | Oral information on the treatment administered: minimized, neutral or maximized situations | Hydroxyzine | Drowsiness and dry mouth | Yes (synergistic) | Low |
| Berna et al (2017) 33 | 100 healthy volunteers | Oral information that an analgesic yielding a dry mouth would be administered (in fact, it was atropine): minimized or maximized situations | Diclofenac | Pain after painful thermal stimulus | Yes (synergistic) | Low |
| Lund et al (2014) 31 | 46 healthy volunteers | Oral information on the treatment administered: minimized or maximized situations | Lidocaine | Self‐assessed pain duration and its maximal intensity after painful stimulus by IM injection | Yes (antagonistic) | Unclear |
| Kirsch et al (1993) 38 | 100 healthy volunteers | Oral information on the treatment administered: minimized or maximized situation | Caffeine | Level of alertness and stress, systolic and diastolic tension and cardiac rhythm | Yes (synergistic) | High |
| Penick et al (1965) 39 | 14 healthy volunteers | Oral information on the treatment administered: minimized or maximized situations | Epinephrine | Level of perceived stress, glucose and free fatty acid concentration and cardiac rhythm | Yes (synergistic) | High |
| Van Der Molen et al (1988) 48 | 13 healthy volunteers | Oral information provided on the treatment administered: minimized (relaxing information) and maximized (stressful information) situations | Lactate | Anxiety, pCO2 and respiratory rate | Yes (synergistic) | High |
| Rose et al (2001) 44 | 53 healthy volunteers | Oral and written information on the treatment administered: minimized or maximized situations | Melatonin | 12‐question assessment sleeping scale | Yes (antagonistic) | High |
| Mitchell et al (1996) 30 | 40 healthy volunteers | Oral information on the treatment administered: minimized or maximized situations | d‐amphetamine | Different scales of drug response (ARCI, DEQ, POMS) | Yes (antagonistic) | High |
| Hammami et al (2010) 28 | 180 healthy volunteers | Oral information on the treatment administered: maximized or minimized situations | Caffeine | Subjective self‐assessed (energy, fatigue, nausea) and objective parameters (systolic blood pressure) | Yes (antagonistic) | High |
| Butcher et al (2012) 32 | 20 healthy volunteers | Oral information on the treatment administered: minimized or maximized situations | Ibuprofen | Pain after painful electric stimulus | No (no effect) | Low |
| Flaten et al (2004) 35 | 94 healthy volunteers | Oral information on the treatment administered: minimized, neutral or maximized situation | Carisoprodol or caffeine | Eyeblink reflex, self‐assessment of level of wakefulness and calm, skin conductance, cardiac rhythm, arterial tension | No (no effect) | Low |
| Alasken et al (2015) 10 | 142 healthy volunteers | Oral information that analgesic or hyperalgesic cream was going to be administered: minimized or maximized situations | EMLA cream | Endpoints evaluated after painful stimulus, including pain, stress and blood pressure | No (additive) | Unclear |
| Atlas et al (2012) 37 | 14 healthy volunteers | Oral information on the treatment administered: minimized or maximized situation | Remifentanil | Pain after painful thermal stimulus | No (additive) | Unclear |
| Ross et al (1962) 19 | 80 healthy volunteers | Hidden administration of treatments to minimize their effect: minimized or neutral situations | d‐amphetamine | Mood swings (Clyde mood scale) and level of performance (tapping task and H‐bar test) | No (no effect) | High |
| Walach et al (2009) | 75 healthy volunteers | Oral information on the treatment administered | Caffeine | Objective parameters (SAT, DAT, CF, reaction time) and subjective parameters | No (no effect) | High |
| Bjorkedal et al (2011) 29 | 20 healthy volunteers | Oral information that a powerful painkiller was administered (in fact, caffeine): minimized or maximized situations | Caffeine | Wakefulness, stress, pain, expectations and laser‐evoked potentials | No (no effect) | High |
| Flaten et al (1999) 34 | 66 healthy volunteers | Oral information on the treatment administered: minimized, neutral or maximized situations | Carisoprodol | Eyeblink reflex, skin conductance, self‐assessment of level of stress and drowsiness | No (no effect) | High |
| Lyerly et al (1964) 49 | 90 veterans and 90 young employees | Oral information provided on the treatment administered: minimized, neutral or maximized situations | Amphetamine and chloral hydrate | Mood swings (Clyde mood scale) and level of performance (tapping task and H‐bar test) | No (no effect) | High |
Abbreviations: ARCI, addiction research center inventory; CF, cognitive function; DAT, divided attention task; DEQ, drug effect questionnaire; EMLA, eutectic mixture of local anaesthetics; FEV1, forced expiratory volume in 1 second; IM, intramuscular; pCO2, partial pressure of carbon dioxide; POMS, profile of mood states; SAT, spontaneous awakening trials; VAS, visual analogue scale.
3.3. Results of individual studies
The 30 included studies were published between 1959 and 2017. Of these, 19 (63%) involved healthy volunteers, 10 , 20 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 37 , 38 , 39 , 40 , 44 , 48 , 49 with six of these using painful stimulus. 10 , 31 , 32 , 33 , 37 , 40 Eleven other studies tested symptomatic patients 7 , 9 , 16 , 25 , 36 , 41 , 43 , 45 , 46 , 47 : six for pain management, 9 , 16 , 25 , 36 , 41 , 45 two for psychological disorders, 7 , 46 two for asthma symptoms, 42 , 43 and one for sexual disorders. 47 Pain, whether provoked for the study or not, was the outcome in 40% of studies included (12 out of 30).
The number of patients included varied from 13 to 835 (median 70.5).
The presence or not of interaction was evaluated in most included studies by comparing the variables in each group in BPD with analysis of variance (ANOVA) or covariance (ANCOVA). 7 , 10 , 18 , 20 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 36 , 38 , 42 , 43 , 44 , 45 , 46 , 48 When it was detailed, the level of significance chosen was 0.05 10 , 20 , 25 , 26 , 27 , 28 , 30 , 32 , 33 , 38 , 39 (except for Bergmann et al at 0.10). 9 Linear regression models were also used to demonstrate interaction. 10 , 23 , 35 , 38 , 39 , 40 , 41
Three studies did not sufficiently detail the statistical analysis. 16 , 37 , 47
3.4. Synthesis of results
Our review allowed us to include 22 new studies that were not included in Kleijnen et al’s review. However, only eight 7 , 9 , 20 , 45 , 46 , 47 , 48 , 49 of the 10 studies included by Kleijnen et al could be reanalysed (the two others were unavailable 50 , 51 ).
As illustrated in Tables 2, 16 studies found interaction between treatment effect and placebo effect. 7 , 9 , 27 , 28 , 30 , 31 , 33 , 38 , 39 , 40 , 41 , 42 , 44 , 45 , 46 , 48 Among these 16, seven described a synergistic model 7 , 27 , 33 , 38 , 39 , 40 , 48 and six an antagonistic model six with an antagonistic model. 9 , 28 , 30 , 31 , 41 , 42 , 44 , 45 , 46 Four studies provided evidence of additivity. 10 , 25 , 36 , 37
There was evidence of effect reversal in two studies. In the study by Alasken et al, 10 informed participants were told that the eutectic mixture of local anaesthetics (EMLA) cream would exacerbate pain, and so it did. Similarly, in the study by Flaten et al, 35 the calming effect of a beta‐blocker was reversed once participants were informed that a stimulant treatment would be applied.
The 14 other studies found no significant interaction. 10 , 16 , 20 , 25 , 26 , 29 , 32 , 34 , 35 , 36 , 37 , 43 , 47 , 49 The lack of evidence for interactions in some studies was due to the fact that there was no effect at all. 16 , 20 , 26 , 29 , 32 , 34 , 35 , 43 , 47 In the other studies, the hypothesis was not tested. 10 , 25 , 36 , 37 , 47
Statistical meta‐analysis of the interaction was not carried out due to lack of available data (means and standard deviation were missing).
3.5. Risk of bias across studies
Risk of bias was evaluated as low for seven studies, 25 , 27 , 32 , 33 , 35 , 40 , 43 intermediate for four 10 , 31 , 37 , 41 and high for the rest. 7 , 16 , 20 , 26 , 28 , 29 , 30 , 34 , 36 , 38 , 39 , 42 , 44 , 45 , 46 , 47 , 48 , 49
3.6. Amendment to the initial protocol: Analysis of results according to context
This was not planned a priori but the studies were set either in clinical context (symptomatic patients) or in laboratories (healthy volunteers). Among the 19 studies carried out in laboratories, 10 , 20 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 37 , 38 , 39 , 40 , 44 , 48 , 49 10 (52%) showed an interaction. Among the 11 studies carried out in a clinical context, 7 , 9 , 16 , 25 , 36 , 41 , 42 , 43 , 45 , 46 , 47 six (54%) showed an interaction.
4. DISCUSSION
4.1. Summary of results
There was evidence of interaction in over half of our sample. The trials included were of poor quality overall and 18 out of 30 included healthy volunteers, making it difficult to judge the clinical pertinence of our results. Indeed, interaction can only exist if there is a specific and a placebo effect. On the one hand, some situations are “sensitive” to the placebo effect, 52 especially in cases of perceived disorders such as pain, 52 nausea, 52 anxiety, 52 coughing 53 and shortness of breath. 54 On the other hand, in situations where neither the drug nor the placebo is effective, no interaction (or additivity for that matter) can take place. To our knowledge, there is no direct proof showing that the placebo effect exists for hard outcomes, such as morbidity/mortality. 52 The high prevalence of studies on healthy volunteers may be the result of ethical limitations.
4.2. Comparison with other studies
Our work confirms and adds to the earlier systematic review by Kleijnen et al 15 and a more recent literature review carried out in 2018 by Coleshill et al 18 (which only included studies pertaining to pain). The evidence of effect reversal corroborates the initial experiments of Stewart Wolf in the 1950s 55 as well as those of more recent studies. 56
4.3. Limitations
The study had limitations. The included studies were small (only nine had over 100 participants) and of poor quality. Publication bias was also possible. Although the placebo effect is a subject that has received considerable attention, this is not the case for interaction between the placebo effect and the specific effect. However, this difficulty had been anticipated in our research protocol. We had decided to restrict our initial research to facilitate systematic analysis of the bibliographies of the included studies and of the authors of several relevant publications. For example, we identified two studies which supported the interactive model, but they were not included in our analysis because no test and no interpretation of the interaction were included in the publication. 57 , 58 However, negative studies may not have been published. Selective reporting was another potential problem. While several endpoints were often measured in the studies, interaction was analysed for only one endpoint.
Moreover, most (19/30, 63%) of the experimentations took place in a laboratory setting and involved healthy volunteers, a factor possibly limiting extrapolation of the results to routine clinical practice. Intensity of the placebo effect was probably higher in patients presenting with clinical symptoms such as pain. 59 However, an analysis of the data in two subgroups found that interaction presented similarly in both contexts. In the end, we were unable to undertake our planned meta‐analysis, the data being too fragmented to pool. Indeed, for three‐quarters of the studies, we were unable to obtain the necessary data to carry out a meta‐analysis according to Cochrane standards.
4.4. Consequences and implications
In spite of its limitations, our study shows that additivity cannot be the default assumption, at least in trials where placebo effects exist. The existence of interaction between pharmacological effect and placebo effect has consequences for medical practice, and clinical trials in particular; the effect of a treatment can no longer be considered independently of supposedly nonspecific factors. BPD trials should be carried out to better evaluate those factors that could modify the interaction, and statistical simulations could also be used to optimise the study design. Indeed, a recent expert consensus recommended a number of attitudes to be adopted in clinical practice to maximize treatment effects and minimize adverse effects. 60 In clinical practice, this reinforces the need for a biopsychosocial therapeutic model. 60 , 61 It becomes of utmost importance to understand how psychobiological factors affect therapeutic outcome to maintain or regain the trust of patients in medical science, as argued by Benedetti et al. 62 As suggested by Berna et al 33 and also Schenck et al, 40 a minimal placebo intensity may be necessary to get a treatment response. On the contrary, minimizing the placebo effect with an unempathetic approach 60 may decrease treatment response. At most, there may be an inverted effect of the drug depending on the information given. 10 , 34
The presence of interactions also implies that the external validity of trial results (in areas that are placebo‐sensitive) cannot be assumed. 63 , 64 , 65 This extends beyond traditional worries about external validity. Indeed, instead of focusing on the potentially different responses to interventions in trial and target populations, our analysis revealed that the very difference between intervention and placebo cannot be assumed to be stable across trial and target populations. Our sample showed that, albeit in rare cases, the effect direction of the intervention‐placebo difference can be reversed. 66 , 67 , 68 Pragmatic trials would overcome the worries related to external validity that our analysis illustrates. In France, for example, the Haute Autorité de Santé (French National Authority for Health) has mandated post‐registration meta‐analyse to test glifozins (SGLT2 inhibitors, antidiabetic drugs) against older antidiabetic drugs. 69 The former have been submitted to solid placebo‐controlled trials, but the latter have not. Comparing different treatment strategies, post‐registration, as prescribed in clinical practice, would allow a better analysis of the treatment effect with an evaluation of the nocebo and placebo effects specific to each treatment strategy.
Moreover, interactions may explain the variability observed between RCTs and “real world evidence”. 64 , 65 , 70 For example, a recent systematic review involving 347 trials (89 183 patients) compared trials that used placebo run‐in periods with trials that did not. 71 In these trials, patients who respond to placebo in the run‐in period are excluded from the eventual trial. The authors found that the drug‐placebo difference was smaller in trials that used placebo run‐in periods. Whereas an additive model would predict that the drug‐placebo difference was constant, our results offer a plausible explanation for the findings of this systematic review. Since some patients in routine practice will be placebo responders, trials that use placebo run‐in periods are not representative and, to estimate an intervention's real world effects, alternatives such as pragmatic trials or enabling technologies should be considered.
Finally, interaction between the treatment effect and the placebo effect challenges the concept of a “specific” effect of a treatment and of its “intrinsic” effect. 72 , 73 , 74 , 75 , 76 , 77 Any and every therapeutic intervention can be considered as “complex”. 76 , 77
4.5. Conclusion
The therapeutic effect of a treatment can be increased, decreased or even reversed depending on the intensity of the placebo effect. Because placebo effects are likely to differ in trial and “real‐world” contexts, 70 interventions for placebo‐sensitive ailments may have very different specific effects in trials than they do in actual practice. To overcome this problem, interventions for placebo‐sensitive ailments should be tested in pragmatic trials once an exploratory RCT has proven their efficacy with sufficient internal validity.
CONFLICT OF INTEREST
The authors declare that they have no competing interests. No funding was received.
CONTRIBUTORS
R.Bo. conceived the study, organised the research, verified the methodology, interpreted the results and wrote the initial draft. J.H. provided methodological guidance, helped interpret the results and critically reviewed the final draft of the manuscript. R.Ba. organised the database search, and helped with the methodology and the critical review of the first draft of the manuscript. F.N. participated in the critical review of the manuscript and reread the final draft, helped interpret the results and critically reviewed the final draft of the manuscript. B.F. participated in the critical review of the manuscript and reread the final draft, helped interpret the results and critically reviewed the final draft of the manuscript. G.H.‐G. participated in the critical review of the manuscript and reread the final draft. W.I. participated in the critical review of the manuscript and reread the final draft. F.G. provided methodological guidance, participated in the critical review of the manuscript and reread the final draft, verified the methodology and interpreted the results. N.J. participated in the critical review of the manuscript and reread the final draft. C.B. interpreted the results, translated the initial draft into English, responded to the reviewers’ critiques, managed the project and is the corresponding author. All authors read and approved the final manuscript.
ACKNOWLEDGEMENT
No funding was received for this study by any of the co‐authors.
APPENDIX 1. RISK OF BIAS ANALYSIS
The following table is an example of how risk of bias was evaluated in this study using the RoB2 tool for the analysis here of Hammami et al (2010). Please see below for the detailed analysis of all the included trials.
Domain 1: Risk of bias arising from the randomization process
| Signalling questions | Description | Response options |
|---|---|---|
| 1.1 Was the allocation sequence random? |
The randomization schedule was generated by one of the authors (M.M.H.) using a program available online (http://www.randomization.com) Group assignment was concealed before randomization from participants and the study coordinators who enrolled them |
Y |
| 1.2 Was the allocation sequence concealed until participants were enrolled and assigned to interventions? | Y | |
| 1.3 Did baseline differences between intervention groups suggest a problem with the randomization process? | Baseline characteristics of study groups are shown in Table 1 | N |
| Risk‐of‐bias judgement | Low |
Note: N, No; Y, Yes.
Domain 2: Risk of bias due to deviations from the intended interventions (effect of assignment to intervention)
| Signalling questions | Description | Response options |
|---|---|---|
| 2.1. Were participants aware of their assigned intervention during the trial? |
None (participants) indicated that they guessed the actual study aims Study coordinators guessed that 52%, 51%, 41% and 44% of participants who received caffeine described as caffeine, caffeine described as placebo, placebo described as placebo and placebo described as caffeine, respectively, received caffeine, indicating the success of blinding |
PN |
| 2.2. Were carers and people delivering the interventions aware of participants' assigned intervention during the trial? | PN | |
| 2.3. If Y/PY/NI to 2.1 or 2.2: Were there deviations from the intended intervention that arose because of the experimental context? | NA | |
| 2.4. If Y/PY to 2.3: Were these deviations from intended intervention balanced between groups? | NA | |
| 2.5 If N/PN/NI to 2.4: Were these deviations likely to have affected the outcome? | NA | |
| 2.6 Was an appropriate analysis used to estimate the effect of assignment to intervention? | PY | |
| 2.7 If N/PN/NI to 2.6: Was there potential for a substantial impact (on the result) of the failure to analyse participants in the group to which they were randomized? | NA | |
| Risk‐of‐bias judgement | Low |
Note: N, No; NI, No Information; PN, Probably No; PY, Probably Yes; Y, Yes.
Domain 3: Missing outcome data
| Signalling questions | Description | Response options |
|---|---|---|
| 3.1 Were data for this outcome available for all, or nearly all, participants randomized? |
180 were equally randomized to caffeine or placebo cross‐over arms We excluded from analysis participants who later withdrew from the study (three randomized to placebo, two to caffeine) or did not adequately abstain from caffeine (baseline caffeine levels in the study periods differed by ≥1 μg/mL (two randomized to placebo and five to caffeine). A flowchart is presented in Figure 2 |
Y |
| 3.2 If N/PN/NI to 3.1: Is there evidence that the result was not biased by missing outcome data? | NA | |
| 3.3 If N/PN to 3.2: Could missingness in the outcome depend on its true value? | NA | |
| 3.4 If Y/PY/NI to 3.3: Do the proportions of missing outcome data differ between intervention groups? | NA | |
| 3.5 If Y/PY/NI to 3.3: Is it likely that missingness in the outcome depended on its true value? | NA | |
| Risk‐of‐bias judgement | Low |
Note: N, No; NI, No Information; PN, Probably No; PY, Probably Yes; Y, Yes.
Domain 4: Risk of bias in measurement of the outcome
| Signalling questions | Description | Response options |
|---|---|---|
| 4.1 Was the method of measuring the outcome inappropriate? | PN | |
| 4.2 Could measurement or ascertainment of the outcome have differed between intervention groups? | N | |
| 4.3 If N/PN/NI to 4.1 and 4.2: Were outcome assessors aware of the intervention received by study participants? | N | |
| 4.4 If Y/PY/NI to 4.3: Could assessment of the outcome have been influenced by knowledge of intervention received? | NA | |
| 4.5 If Y/PY/NI to 4.4: Is it likely that assessment of the outcome was influenced by knowledge of the intervention received? | NA | |
| Risk‐of‐bias judgement | Low |
Note: N, No; NI, No Information; PN, Probably No; PY, Probably Yes; Y, Yes.
Domain 5: Risk of bias in selection of the reported result
| Signalling questions | Description | Response options |
|---|---|---|
| 5.1 Was the trial analysed in accordance with a pre‐specified plan that was finalized before unblinded outcome data were available for analysis? |
Trial registration: ClinicalTrials.gov identification number NCT00426010 There were no changes to study outcomes after study commencement |
Y |
| Is the numerical result being assessed likely to have been selected, on the basis of the results, from… | ||
| 5.2. … Multiple outcome measurements (e.g. scales, definitions, time points) within the outcome domain? | Measure of fatigue, energy, nausea, systolic blood pressure and conclusion according to results of energy and fatigue only, while no tests found significant difference | Y |
| 5.3 … Multiple analyses of the data? | PN | |
| Risk‐of‐bias judgement | High |
Note: PN, Probably No; Y, Yes.
Overall risk of bias
| Risk‐of‐bias judgement | High |
Table of risk of bias evaluation for included trials according to RoB2
| Included trials (year of publication) | Domains | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Overall risk of bias | |
| Kam‐Hansen et al (2014) 25 | Low | Low | Low | Low | Low | Low |
| Walach et al (2009) 26 | Low | Low | Low | Low | High | High |
| Hammami et al (2016) 27 | Low | Low | Low | Low | Low | Low |
| De Craen et al (2001) 16 | Some concerns | High | Low | Some concerns | High | High |
| Hammami et al (2010) 28 | Low | Low | Low | Low | High | High |
| Bjorkedal et al (2011) 29 | Low | Low | Low | Low | High | High |
| Mitchell et al (1996) 30 | Low | High | Low | Low | Some concerns | High |
| Aslasken et al (2015) 10 | Some concerns | Some concerns | Low | Low | Low | Some concerns |
| Lund et al (2014) 31 | Low | Low | Low | Low | Some concerns | Some concerns |
| Butcher et al (2012) 32 | Low | Low | Low | Low | Low | Low |
| Berna et al (2017) 33 | Low | Low | Low | Low | Low | Low |
| Flaten et al (1999) 34 | Low | Low | Low | Low | High | High |
| Flaten et al (2004) 35 | Low | Low | Low | Low | Low | Low |
| Brandwhaite et al (1981) 36 | Low | Low | Low | Low | Some concerns | Low |
| Atlas et al (2012) 37 | Some concerns | Some concerns | Low | Some concerns | Some concerns | Some concerns |
| Kirsch et al (1993) 38 | Low | Some concerns | Low | Low | High | High |
| Penick et al (1965) 39 | High | High | Low | High | Low | High |
| Schenk et al (2014) 40 | Low | Low | Low | Low | Low | Low |
| Faasse et al (2016) 41 | Low | Some concerns | Low | Some concerns | Some concerns | Some concerns |
| Wise et al (2009) 42 | Low | Low | Low | Low | High | High |
| Kemeny et al (2007) 43 | Low | Low | Low | Low | Low | Low |
| Rose et al (2001) 44 | Low | Low | Some concerns | Some concerns | High | High |
| Ross et al (1962) 19 | Some concerns | Some concerns | Some concerns | Some concerns | Some concerns | High |
| Levine et al (1984) 45 | Low | Some concerns | Some concerns | Some concerns | Low | High |
| Uhlenhuth et al (1959) 7 | Low | Low | Some concerns | Some concerns | Low | High |
| Uhlenhuth et al (1966) 46 | Low | Some concerns | Some concerns | Low | High | High |
| Mathews et al (1983) 47 | Some concerns | High | Some concerns | Some concerns | Low | High |
| Van Der Molen et al (1988) 48 | Some concerns | Some concerns | Low | Some concerns | High | High |
| Lyerly et al (1964) 49 | Some concerns | Some concerns | Low | Some concerns | High | High |
| Bergmann et al (1994) 9 | Low | Low | Some concerns | Low | Low | Some concerns |
APPENDIX 2.
| Trials | Hypothesis tested | Population | Modification of the power of the placebo effect | Treatments | Endpoints |
|---|---|---|---|---|---|
| Kam‐Hansen et al (2014) 25 | Additive model and interactive model | 66 chronic migraine patients | Oral information on the treatment administered | Treatment group (razatriptan) and placebo in minimized, neutral or maximized situation | Relief 2 h after onset of migraine symptoms and number of subjects without pain at 2.5 h |
| Walach et al (2009) 26 | Placebo effect depending on a nonlocal correlation with response to treatment | 75 healthy volunteers | Oral information on the treatment administered | Treatment group (caffeine) and placebo in maximized or neutral situation | Objective parameters (SAT, DAT, CF, reaction time) and subjective parameters (calm, mood and alertness) |
| Hammami et al (2016) 27 | Interactive model | 480 healthy volunteers | Oral information on the treatment administered | Treatment group (hydroxyzine) and placebo in minimized, neutral or maximized situation | Drowsiness and dry mouth, self‐assessed by the participants during the 7 h following treatment |
| De Craen et al (2001) 16 | Interactive model | 112 chronic pain patients | Written information on the treatment administered | Treatment group (tramadol) and placebo in maximized or neutral situation | Primary endpoint: pain reduction on self‐assessing VAS |
| Hammami et al (2010) 28 | Interactive model and pharmacokinetic modification of the placebo effect | 180 healthy volunteers | Oral information on the treatment administered | Treatment group (caffeine) and placebo in maximized or minimized situation | Subjective self‐assessed (energy, fatigue, nausea) and objective parameters (systolic blood pressure) |
| Bjorkedal et al (2011) 29 | Interactive model: variation of treatment activity according to adverse effects | 20 healthy volunteers | Oral information that a powerful painkiller was administered (in fact, caffeine) | Treatment (caffeine) and placebo groups in maximized or minimized situations | Wakefulness, stress, pain, expectations and laser‐evoked potentials |
| Mitchell et al (1996) 30 | Interactive model | 40 healthy volunteers | Oral information on the treatment administered | Treatment group (d‐amphetamine) and placebo in maximized or minimized situation | Different scales of drug response (ARCI, DEQ, POMS) |
| Alasken et al (2015) 10 | Interactive model: inversion of treatment effects by means of information | 142 healthy volunteers | Oral information that analgesic or hyperalgesic cream was going to be administered | Treatment group (EMLA cream) and placebo in minimized or maximized situation | Endpoints evaluated after painful stimulus, including pain, stress and blood pressure |
| Lund et al (2014) 31 | Interactive model, being of more import with powerful placebo effect | 46 healthy volunteers | Oral information on the treatment administered | Treatment group (lidocaine) and placebo in minimized or maximized situation | Self‐assessed pain duration and its maximal intensity after painful stimulus by IM injection |
| Butcher et al (2012) 32 | Variation of the placebo effect according to gender | 20 healthy volunteers | Oral information on the treatment administered | Treatment group (ibuprofen) and placebo in minimized or maximized situation | Self‐assessed pain after painful electric stimulus |
| Berna et al (2017) 33 | Interactive model: activity variation according to adverse effects | 100 healthy volunteers | Oral information that an analgesic yielding a dry mouth would be administered (in fact, it was atropine) | Treatment group (diclofenac) and placebo in minimized or maximized situation | Analgesia evaluated by VAS after painful thermal stimulus |
| Flaten et al (1999) 34 | Interactive model | 66 healthy volunteers | Oral information on the treatment administered | Treatment group (carisoprodol) and placebo in minimized, neutral or maximized situation | Eyeblink reflex, skin conductance, self‐assessment of level of stress and drowsiness |
| Flaten et al (2004) 35 | Interactive model | 94 healthy volunteers | Oral information on the treatment administered | Treatment group (carisoprodol), caffeine and placebo in minimized, neutral or maximized situation | Eyeblink reflex, self‐assessment of level of wakefulness and calm, skin conductance, cardiac rhythm, arterial tension |
| Brandwhaite et al (1981) 36 | Interactive model | 835 women presenting with chronic headaches | Oral information provided on the “brand” of treatment administered | Treatment group (aspirin) and placebo in minimized or maximized situation | Pain self‐assessment 30 min and 1 h after headaches |
| Atlas et al (2012) 37 | Interactive model | 14 healthy volunteers | Oral information on the treatment administered | Treatment group (remifentanil) and placebo in minimized or maximized situation | Self‐assessed pain after painful thermal stimulus |
| Kirsch et al (1993) 38 | Interactive model | 100 healthy volunteers | Oral information on the treatment administered | Treatment group (caffeine) and placebo in minimized or maximized situation | Level of alertness and stress, systolic and diastolic tension and cardiac rhythm at 15, 30 and then 45 min after ingestion |
| Penick et al (1965) 39 | Interactive model | 14 healthy volunteers | Oral information on the treatment administered |
Treatment group (epinephrine) and placebo in minimized or maximized situation Endpoints: l |
Level of perceived stress, glucose and free fatty acid concentration and cardiac rhythm |
| Schenk et al (2013) 40 | Interactive model | 34 healthy volunteers | Oral information provided on the treatment administered | Treatment group (lidocaine) and placebo in minimized or maximized situation | Self‐assessment of pain on VAS after painful thermal stimulus |
| Faasse et al (2016) 41 | Additive model | 87 patients presenting with chronic headaches | Oral information provided on the treatment brand administered | Treatment group (ibuprofen) and placebo in minimized or maximized situation | Home self‐assessment of pain following headache episodes and reported adverse effects |
| Wise et al (2009) 42 | Interactive model | 601 poorly controlled asthmatics | Oral information provided on the treatment administered, its brand and its colour | Treatment group (montelukast) and placebo in neutral or maximized situation | Improvement at 4 wk of peak expiratory flow, improvement of pulmonary functions evaluated by spirometry and asthma control evaluated by four self‐assessment scales |
| Kemeny et al (2007) 43 | Variation of the placebo effect and its determinants | 55 poorly controlled asthmatics | Oral information provided on the treatment administered | Treatment group (salmeterol) and placebo in maximized or neutral situation | Concentration of methacholine needed to induce a 20% FEV1 decrease |
| Rose et al (2001) 44 | Interactive model | 53 healthy volunteers | Oral and written information on the treatment administered | Treatment group (melatonin) and placebo in minimized or maximized situation | Subjective sleep evaluated by a 12‐question assessment scale |
| Ross et al (1962) 19 | Interactive model | 80 healthy volunteers | Hidden administration of treatments to minimize their effect | Treatment group (d‐amphetamine) and placebo in the same neutral or minimized situations | Mood swings (Clyde mood scale) and level of performance (tapping task and H‐bar test) |
| Levine et al (1984) 45 | Placebo effect independent of the means of administration | 96 patients having undergone dental extraction | Hidden administration of treatments, manually or by a machine | Treatment group (naloxone) and placebo in minimized, neutral or maximized situation | Self‐assessment of pain 50 min after treatment administration |
| Uhlenhuth et al (1959) 7 | Interactive model | 52 psychiatric patients suffering from anxieties | Neutral or positive attitude concerning the treatments administered | Treatment group (meprobamate or phenobarbital) and placebo in neutral or maximized situation | Improvement perceived by patients, assessment by a psychiatrist and a scale grouping together 45 symptoms |
| Uhlenhuth et al (1966) 46 | Interactive model | 138 patients referred to psychiatric clinic | Neutral or positive attitude concerning the treatments administered | Treatment group (meprobamate) in neutral or maximized situations and placebo in the same situations | Modifications on different scales |
| Mathews et al (1983) 47 | Interactive model | 48 couples presenting with sexual disorders | Frequency of administration and number of therapists | Treatment group (testosterone) and placebo with weekly or monthly administration and at least one therapist | Improvement of symptoms evaluated by an outside investigator and the couples themselves |
| Van Der Molen et al (1988) 48 | Hyperventilation in the event of lactate injection or stressful information | 13 healthy volunteers | Oral information provided on the treatment administered | Treatment group (lactate) and placebo in minimized (relaxing information) and maximized (stressful information) situations | Anxiety, pCO2 and respiratory rate |
| Lyerly et al (1964) 49 | Interactive model | 90 veterans and 90 young employees | Oral information provided on the treatment administered | Treatment group (amphetamine and chloral hydrate) versus placebo in minimized, neutral or maximized situation | Mood swings (Clyde mood scale) and level of performance (tapping task and H‐bar test). |
| Bergmann et al (1994) 9 | Interactive model | 49 cancer patients | Oral information provided or not on the study procedure | Treatment group (500 mg of naproxen) and placebo in neutral or maximized situation | Self‐assessment of pain on VAS up to 3 h after administration |
Detailed study characteristics. A minimized situation corresponds to less placebo effect power compared to a neutral or maximized situation.
Abbreviations: ARCI, addiction research center inventory; CF, cognitive function; DAT, direct antiglobulin test; DEQ, drug effect questionnaire; EMLA, eutectic mixture of local anaesthetics; FEV1, forced expiratory volume in 1 second; IM, intramuscular; pCO2, partial pressure of carbon dioxide; POMS, profile of mood states; SAT, spontaneous awakening trials; VAS, visual analogue scale.
Interaction and risk of bias (⋯: missing data)
| Studies | Effect of the treatment | Effect of the information | Interaction | Model | Risk of bias |
|---|---|---|---|---|---|
| Hammami et al (2016) 27 | Yes | Yes | Yes | Synergistic | Low |
| Berna et al (2017) 33 | No | No | Yes | Synergistic | Low |
| Schenk et al (2013) 40 | Yes | No | Yes | Synergistic | Low |
| Lund et al (2014) 31 | Yes | Yes | Yes | Antagonistic | Unclear |
| Faasse et al (2016) 41 | Yes | Yes | Yes | Antagonistic | Unclear |
| Hammami et al (2010) 28 | Yes | Yes | Yes | Antagonistic | High |
| Wise et al (2009) 42 | Yes | Yes | Yes | Antagonistic | High |
| Rose et al (2001) 44 | No | Yes | Yes | Antagonistic | High |
| Bergmann et al (1994) 9 | Yes | Yes | Yes | Antagonistic | High |
| Van Der Molen et al 1988) 48 | Yes | Yes | Yes | Synergistic | High |
| Kirsch et al (1993) 38 | Yes | Yes | Yes | Synergistic | High |
| Penick et al (1965) 39 | … | No | Yes | Synergistic | High |
| Uhlenhuth et al (1959) 7 | … | Yes | Yes | Synergistic | High |
| Uhlenhuth et al (1966) 46 | … | Yes | Yes | Antagonistic | High |
| Levine et al (1984) 45 | No | Yes | Yes | Antagonistic | High |
| Mitchell et al (1996) 30 | Yes | Yes | Yes | Antagonistic | High |
| Kam‐Hansen et al (2014) 25 | Yes | Yes | No | Additive | Low |
| Butcher et al (2012) 32 | No | No | No | No effect | Low |
| Flaten et al (2004) 35 | Yes | No | No | No effect | Low |
| Kemeny et al (2007) 43 | Yes | No | No | No effect | Low |
| Alasken et al (2015) 10 | Yes | Yes | No | Additive | Unclear |
| Atlas et al (2012) 37 | Yes | Yes | No | Additive | Unclear |
| Brandwhaite et al (1981) 36 | Yes | Yes | No | Additive | High |
| De craen et al (2001) 16 | No | No | No | No effect | High |
| Flaten et al (1999) 34 | No | Yes | No | No effect | High |
| Bjorkedal et al (2011) 29 | No | No | No | No effect | High |
| Ross et al (1962) 19 | No | No | No | No effect | High |
| Lyerly et al (1964) 49 | No | No | No | No effect | High |
| Walach et al (2009) 26 | No | No | No | No effect | High |
| Mathews et al (1983) 47 | No | No | No | No effect | High |
Boussageon R, Howick J, Baron R, et al. How do they add up? The interaction between the placebo and treatment effect: A systematic review. Br J Clin Pharmacol. 2022;88(8):3638‐3656. doi: 10.1111/bcp.15345
[Correction added on 4 August 2022, after first online publication: The copyright line was changed.]
REFERENCES
- 1. Beecher HK. The powerful placebo. Jama. 1955;159(17):1602‐1606. doi: 10.1001/jama.1955.02960340022006 [DOI] [PubMed] [Google Scholar]
- 2. De Craen AJ, Kaptchuk TJ, Tijssen JG, Kleijnen J. Placebos and placebo effects in medicine: historical overview. J R Soc Med. 1999;92(10):511‐515. doi: 10.1177/014107689909201005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Finniss DG, Kaptchuk TJ, Miller F, Benedetti F. Placebo effects: Biological, clinical and ethical advances. Lancet. 2010;375(9715):686‐695. doi: 10.1016/S0140-6736(09)61706-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boussageon R, Gueyffier F, Bejan‐Angoulvant T, Felden‐Dominiak G. Critical of the additive model of the randomized controlled trial. Therapie. 2008;63(1):29‐35. doi: 10.2515/therapie:2008015 [DOI] [PubMed] [Google Scholar]
- 5. Modell W, Garrett M. Interactions between pharmacodynamic and placebo effects in drug evaluations in man. Nature. 1960;185(4712):538‐539. doi: 10.1038/185538a0 [DOI] [PubMed] [Google Scholar]
- 6. Enck P, Bingel U, Schedlowski M, Rief W. The placebo response in medicine: minimize, maximize or personalize? Nat Rev Drug Discov. 2013;12(3):191‐204. doi: 10.1038/nrd3923 [DOI] [PubMed] [Google Scholar]
- 7. Uhlenhuth EH, Canter A, Neustadt JO, Payson HE. The symptomatic relief of anxiety with meprobamate, phenobarbital and placebo. Am J Psychiatry. 1959;115(10):905‐910. doi: 10.1176/ajp.115.10.905 [DOI] [PubMed] [Google Scholar]
- 8. Puech J, Ortlieb JJ. Evaluation de la valeur thérapeutique d'un médicament. Méthode des placebos. Critique méthodologique. Presse Med. 1963;71:2168‐2170. [PubMed] [Google Scholar]
- 9. Bergmann JF, Chassany O, Gandiol J, et al. A randomised clinical trial of the effect of informed consent on the analgesic activity of placebo and naproxen in cancer pain. Clin Trials Metaanal. 1994;29(1):41‐47. [PubMed] [Google Scholar]
- 10. Aslaksen PM, Zwarg ML, Eilertsen H‐IH, Gorecka MM, Bjørkedal E. Opposite effects of the same drug: reversal of topical analgesia by nocebo information. Pain. 2015;156(1):39‐46. doi: 10.1016/j.pain.0000000000000004 [DOI] [PubMed] [Google Scholar]
- 11. Boehm K, Berger B, Weger U, Heusser P. Does the model of additive effect in placebo research still hold true? A narrative review. JRSM Open. 2017;8(3):20. doi: 10.1177/2054270416681434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kube T, Rief W. Are placebo and drug‐specific effects additive? Questioning basic assumptions of double‐blinded randomized clinical trials and presenting novel study designs. Drug Discov Today. 2017;22(4):729‐735. doi: 10.1016/j.drudis.2016.11.022 [DOI] [PubMed] [Google Scholar]
- 13. Colagiuri B. Participant expectancies in double‐blind randomized placebo‐controlled trials: potential limitations to trial validity. Clin Trials. 2010;7(3):246‐255. doi: 10.1177/1740774510367916 [DOI] [PubMed] [Google Scholar]
- 14. Kirsch I. Are drug and placebo effects in depression additive? Biol Psychiatry. 2000;47(8):733‐735. doi: 10.1016/S0006-3223(00)00832-5 [DOI] [PubMed] [Google Scholar]
- 15. Kleijnen J, de Craen AJ, van Everdingen J, Krol L. Placebo effect in double‐blind clinical trials: A review of interactions with medications. Lancet. 1994;344(8933):1347‐1349. doi: 10.1016/S0140-6736(94)90699-8 [DOI] [PubMed] [Google Scholar]
- 16. De Craen AJ, Lampe‐Schoenmaeckers AJ, Kraal JW, Tijssen JG, Kleijnen J. Impact of experimentally‐induced expectancy on the analgesic efficacy of tramadol in chronic pain patients: a 2 × 2 factorial, randomized, placebo‐controlled, double‐blind trial. J Pain Symptom Manage. 2001;21(3):210‐217. doi: 10.1016/S0885-3924(01)00265-2 [DOI] [PubMed] [Google Scholar]
- 17. Fava GA, Guidi J, Rafanelli C, Rickels K. The clinical inadequacy of the placebo model and the development of an alternative conceptual framework. Psychother Psychosom. 2017;86(6):332‐340. doi: 10.1159/000480038 [DOI] [PubMed] [Google Scholar]
- 18. Coleshill MJ, Sharpe L, Colloca L, Zachariae R, Colagiuri B. Placebo and active treatment additivity in placebo analgesia: Research to date and future directions. Int Rev Neurobiol. 2018;139:407‐441. doi: 10.1016/bs.irn.2018.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Enck P, Klosterhalfen S, Zipfel S. Novel study designs to investigate the placebo response. BMC Med Res Methodol. 2011;11(1):90. doi: 10.1186/1471-2288-11-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ross S, Krugman AD, Lyerly SB, Clyde DJ. Drugs and placebos: a model design? Psychol Rep. 1962;10(2):382‐392. doi: 10.2466/pr0.1962.10.2.383 [DOI] [Google Scholar]
- 21. Rohsenow DJ, Marlatt GA. The balanced placebo design: methodological considerations. Addict Behav. 1981;6(2):107‐122. doi: 10.1016/0306-4603(81)90003-4 [DOI] [PubMed] [Google Scholar]
- 22. Colloca L, Lopiano L, Lanotte M, Benedetti F. Overt versus covert treatment for pain, anxiety, and Parkinson's disease. Lancet Neurol. 2004;3(11):679‐684. doi: 10.1016/S1474-4422(04)00908-1 [DOI] [PubMed] [Google Scholar]
- 23. Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. 2011.
- 24. Sterne JAC, Savović J, Page MJ, et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 25. Kam‐Hansen S, Jakubowski M, Kelley JM, et al. Altered placebo and drug labeling changes the outcome of episodic migraine attacks. Sci Transl Med. 2014;6(218):218‐215. doi: 10.1126/scitranslmed.3006175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Walach H, Schneider R. Does the presence of a pharmacological substance alter the placebo effect? Results of two experimental studies using the placebo‐caffeine paradigm. Hum Psychopharmacol. 2009;24(7):549‐558. doi: 10.1002/hup.1054 [DOI] [PubMed] [Google Scholar]
- 27. Hammami MM, Hammami S, Al‐Swayeh R, Al‐Gaai E, Farah FA, De Padua SJS. Drug*placebo interaction effect may bias clinical trials interpretation: hybrid balanced placebo and randomized placebo‐controlled design. BMC Med Res Methodol. 2016;16(1):166. doi: 10.1186/s12874-016-0269-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hammami MM, Al‐Gaai EA, Alvi S, Hammami MB. Interaction between drug and placebo effects: a cross‐over balanced placebo design trial. Trials. 2010;11(1):110. doi: 10.1186/1745-6215-11-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bjørkedal E, Flaten MA. Interaction between expectancies and drug effects: an experimental investigation of placebo analgesia with caffeine as an active placebo. Psychopharmacology (Berl). 2011;215(3):537‐548. doi: 10.1007/s00213-011-2233-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mitchell SH, Laurent CL, de Wit H. Interaction of expectancy and the pharmacological effects of d‐amphetamine: subjective effects and self‐administration. Psychopharmacology (Berl). 1996;125(4):371‐378. doi: 10.1007/BF02246020 [DOI] [PubMed] [Google Scholar]
- 31. Lund K, Vase L, Petersen GL, Jensen TS, Finnerup NB. Randomised controlled trials may underestimate drug effects: balanced placebo trial design. PLoS One. 2014;9(1):e84104. doi: 10.1371/journal.pone.0084104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Butcher BE, Carmody JJ. Sex differences in analgesic response to ibuprofen are influenced by expectancy: a randomized, crossover, balanced placebo‐designed study. Eur J Pain. 2012;16(7):1005‐1013. doi: 10.1002/j.1532-2149.2011.00104.x [DOI] [PubMed] [Google Scholar]
- 33. Berna C, Kirsch I, Zion SR, et al. Side effects can enhance treatment response through expectancy effects: an experimental analgesic randomized controlled trial. Pain. 2017;158(6):1014‐1020. doi: 10.1097/j.pain.0000000000000870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Flaten MA, Simonsen T, Olsen H. Drug‐related information generates placebo and nocebo responses that modify the drug response. Psychosom Med. 1999;61(2):250‐255. doi: 10.1097/00006842-199903000-00018 [DOI] [PubMed] [Google Scholar]
- 35. Flaten MA, Simonsen T, Zahlsen K, Aamo T, Sager G, Olsen H. Stimulant and relaxant drugs combined with stimulant and relaxant information: a study of active placebo. Psychopharmacology (Berl). 2004;176(3‐4):426‐434. doi: 10.1007/s00213-004-1886-7 [DOI] [PubMed] [Google Scholar]
- 36. Branthwaite A, Cooper P. Analgesic effects of branding in treatment of headaches. Br Med J. 1981;282(6276):1576‐1578. doi: 10.1136/bmj.282.6276.1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Atlas LY, Whittington RA, Lindquist MA, Wielgosz J, Sonty N, Wager TD. Dissociable influences of opiates and expectations on pain. J Neurosci. 2012;32(23):8053‐8064. doi: 10.1523/JNEUROSCI.0383-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kirsch I, Rosadino MJ. Do double‐blind studies with informed consent yield externally valid results? An Empirical Test Psychopharmacology (Berl). 1993;110(4):437‐442. doi: 10.1007/BF02244650 [DOI] [PubMed] [Google Scholar]
- 39. Penick SB, Fisher S. Drug‐set interaction: psychological and physiological effects of epinephrine under differential expectations. Psychosom Med. 1965;27(2):177‐182. doi: 10.1097/00006842-196503000-00010 [DOI] [PubMed] [Google Scholar]
- 40. Schenk LA, Sprenger C, Geuter S, Büchel C. Expectation requires treatment to boost pain relief: an fMRI study. Pain. 2014;155(1):150‐157. doi: 10.1016/j.pain.2013.09.024 [DOI] [PubMed] [Google Scholar]
- 41. Faasse K, Martin LR, Grey A, Gamble G, Petrie KJ. Impact of brand or generic labeling on medication effectiveness and side effects. Health Psychol. 2016;35(2):187‐190. doi: 10.1037/hea0000282 [DOI] [PubMed] [Google Scholar]
- 42. Wise RA, Bartlett SJ, Brown ED, et al. Randomized trial of the effect of drug presentation on asthma outcomes: the American Lung Association Asthma Clinical Research Centers. J Allergy Clin Immunol. 2009;124(3):436‐444.e8. doi: 10.1016/j.jaci.2009.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kemeny ME, Rosenwasser LJ, Panettieri RA, Rose RM, Berg‐Smith SM, Kline JN. Placebo response in asthma: a robust and objective phenomenon. J Allergy Clin Immunol. 2007;119(6):1375‐1381. doi: 10.1016/j.jaci.2007.03.016 [DOI] [PubMed] [Google Scholar]
- 44. Rose DA, Kahan TL. Melatonin and sleep qualities in healthy adults: pharmacological and expectancy effects. J Gen Psychol. 2001;128(4):401‐421. doi: 10.1080/00221300109598918 [DOI] [PubMed] [Google Scholar]
- 45. Levine JD, Gordon NC. Influence of the method of drug administration on analgesic response. Nature. 1984;312(5996):755‐756. doi: 10.1038/312755a0 [DOI] [PubMed] [Google Scholar]
- 46. Uhlenhuth EH, Rickels K, Fisher S, Park LC, Lipman RS, Mock J. Drug, Doctor's verbal attitude and clinic settings in the symptomatic response to pharmacotherapy. Psychopharmacologia (Berl). 1966;9(5):392‐418. doi: 10.1007/BF00406450 [DOI] [PubMed] [Google Scholar]
- 47. Mathews A, Whitehead A, Kellett J. Psychological and hormonal factors in the treatment of female sexual dysfunction. Psychol Med. 1983;13(1):83‐92. doi: 10.1017/S0033291700050091 [DOI] [PubMed] [Google Scholar]
- 48. van der Molen GM, van den Hout MA. Expectancy effects on respiration during lactate infusion. Psychosom Med. 1988;50(4):439‐443. doi: 10.1097/00006842-198807000-00011 [DOI] [PubMed] [Google Scholar]
- 49. Lyerly SB, Ross S, Krugman AD, Clyde DJ. Drugs and placebos: the effect of instructions upon performance and mood under amphetamine sulphate and chloral hydrate. J Abnorm Psychol. 1964;68(3):321‐327. doi: 10.1037/h0044351 [DOI] [PubMed] [Google Scholar]
- 50. Wied GI. Über die Bedeutung der Suggestion in der Therapie klimakterischer Ausfallerscheinungen. Arztl Wochensch. 1953;8(26):623‐625. [PubMed] [Google Scholar]
- 51. Affleck C, Eaton M, Mansfield E. The action of a medication and the physician's expectation. Nebr State Med J. 1966;331‐334. [PubMed] [Google Scholar]
- 52. Hróbjartsson A, Gøtzsche PC. Placebo interventions for all clinical conditions. Cochrane Database Syst Rev. 2010;(1):CD003974. doi: 10.1002/14651858.CD003974.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Paul IM, Beiler JS, Vallati JR, Duda LM, King TS. Placebo effect in the treatment of acute cough in infants and toddlers: a randomized clinical trial. JAMA Pediatr. 2014;168(12):1107‐1113. doi: 10.1001/jamapediatrics.2014.1609 [DOI] [PubMed] [Google Scholar]
- 54. Wechsler ME, Kelley JM, Boyd IO, et al. Active albuterol or placebo, sham acupuncture, or no intervention in asthma. N Engl J Med. 2011;365(2):119‐126. doi: 10.1056/NEJMoa1103319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wolf S. Effects of suggestion and conditioning on the action of chemical agents in human subjects. The pharmacology of placebos. J Clin Investig. 1950;29(1):100‐109. doi: 10.1172/JCI102225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bingel U, Wanigasekera V, Wiech K, et al. The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Sci Transl Med. 2011;3(70):70ra14. doi: 10.1126/scitranslmed.3001244 [DOI] [PubMed] [Google Scholar]
- 57. Benedetti F, Amanzio M, Maggi G. Potentiation of placebo analgesia by proglumide. Lancet. 1995;346(8984):1231. doi: 10.1016/S0140-6736(95)92938-X [DOI] [PubMed] [Google Scholar]
- 58. Amanzio M, Benedetti F. Neuropharmacological dissection of placebo analgesia: Expectation‐activated opioid systems versus conditioning activated specific subsystems. J Neurosci. 1999;19(1):484‐494. doi: 10.1523/JNEUROSCI.19-01-00484.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Amanzio M, Pollo A, Maggi G, Benedetti F. Response variability to analgesics: a role for non‐specific activation of endogenous opioids. Pain. 2001;90(3):205‐215. doi: 10.1016/S0304-3959(00)00486-3 [DOI] [PubMed] [Google Scholar]
- 60. Evers AWM, Colloca L, Blease C, et al. Implications of placebo and nocebo effects for clinical practice: Expert consensus. Psychother Psychosom. 2018;87(4):204‐210. doi: 10.1159/000490354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. White L, Tursky B, Schwartz GE. Proposed synthesis of placebo models. In: White L, Tursky B, Schwartz GE, eds. Placebo: Theory, Research, and Mechanisms. New York: Guilford Press; 1985:431‐448. [Google Scholar]
- 62. Benedetti F, Frisaldi E, Shaibani A. Thirty years of neuroscientific investigation of placebo and nocebo: The interesting, the good, and the bad. Annu Rev Pharmacol Toxicol. 2021;62(1):323‐340. doi: 10.1146/annurev-pharmtox-052120-104536. Epub ahead of print PMID: 34460317 [DOI] [PubMed] [Google Scholar]
- 63. Rothwell PM. External validity of randomised controlled trials: "to whom do the results of this trial apply?". Lancet. 2005;365(9453):82‐93. doi: 10.1016/S0140-6736(04)17670-8 [DOI] [PubMed] [Google Scholar]
- 64. Kaptchuk TJ. The double‐blind, randomised, placebo‐controlled trial: Gold standard or golden calf? J Clin Epidemiol. 2001;54(6):541‐549. doi: 10.1016/S0895-4356(00)00347-4 [DOI] [PubMed] [Google Scholar]
- 65. Howick J. Questioning the methodologic superiority of 'placebo' over 'active' controlled trials. Am J Bioeth. 2009;9(9):34‐48. doi: 10.1080/15265160903090041 [DOI] [PubMed] [Google Scholar]
- 66. Rief W, Glombiewski JA. The hidden effects of blinded, placebo‐controlled randomized trials: an experimental investigation. Pain. 2012;153(12):2473‐2477. doi: 10.1016/j.pain.2012.09.007 [DOI] [PubMed] [Google Scholar]
- 67. Boussageon R, Gueyffier F, Moreau A, Boussageon V. The difficulty of measurement of placebo effect. Therapie. 2006;61(3):185‐190. doi: 10.2515/therapie:2006049 [DOI] [PubMed] [Google Scholar]
- 68. Hróbjartsson A. The uncontrollable placebo effect. Eur J Clin Pharmacol. 1996;50(5):345‐348. doi: 10.1007/s002280050120 [DOI] [PubMed] [Google Scholar]
- 69. French National Authority for Health . “Commission de Transparence. Rapport d'évaluation des antidiabétiques de type 2 de la classe des gliflozines ou inhibiteurs du SGLT2. Direction de l'Evaluation Médicale, Economique et de Santé Publique”. Definitive version modified on the 3rd of December 2020.
- 70. Dal‐Re R, Janiaud P, Ioannidis J. Real‐world evidence: How pragmatic are randomized controlled trials labeled as pragmatic? BMC Med. 2018;16(1):49. doi: 10.1186/s12916-018-1038-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Scott AJ, Sharpe L, Quinn V, Colagiuri B. Association of single‐blind placebo run‐in periods with the placebo response in randomized clinical trials of antidepressants: A systematic review and meta‐analysis. JAMA Psychiat. 2022;79(1):42‐49. doi: 10.1001/jamapsychiatry.2021.3204. PMID: 34757405; PMCID: PMC8581773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Temkin O. The scientific approach to disease: specific entity and individual sickness. In: Crombie AC, ed. Scientific Change. Historical studies in the intellectual, social and technical conditions for scientific discovery and technical invention, from antiquity to the present. Glasgow: The University Press; 1963:629‐647. [Google Scholar]
- 73. Shepherd M. The placebo: from specificity to the non‐specific and back. Psychol Med. 1993. Aug;23(3):569‐578. doi: 10.1017/S0033291700025356 [DOI] [PubMed] [Google Scholar]
- 74. Gadow KD, White L, Fergusson DG. Placebo controls and double‐blind conditions: placebo theory in experimental design. In: Gadow KD, Poling A, eds. Methodological issues in human psychopharmacology. Advances in Learning and Behavioral Disabilities, S1. London: Jai Press; 1986:57. [Google Scholar]
- 75. Dagognet F. La Raison et les Remèdes. Paris cedex 14. France: Presses Universitaires de France; 1984. doi: 10.3917/puf.dago.1984.01 [DOI] [Google Scholar]
- 76. Boussageon R. L'efficacité thérapeutique. Objectivité curative et effet placebo (Thesis), University of Lyon, France; 2010.
- 77. Paterson C, Dieppe P. Characteristic and incidental (placebo) effects in complex interventions such as acupuncture. BMJ. 2005;330(7501):1202‐1205. doi: 10.1136/bmj.330.7501.1202 [DOI] [PMC free article] [PubMed] [Google Scholar]


