Abstract
Aim
The use of high‐concentration sodium hypochlorite (NaOCl) as an endodontic irrigant remains controversial because of its potential impact on the fracture strength of endodontically treated teeth. This study evaluated the effects of using different NaOCl concentrations, with 2‐min‐ethylenediaminetetraacetic acid (EDTA) as the final active irrigant, on the biomechanical and structural properties of root dentine.
Methodology
A new test method, which is more clinically relevant, was utilized to calculate the fracture strength of root dentine. Bovine incisors were used to obtain root dentine discs. The root canals were enlarged to mean diameter of 2.90 mm with a taper of 0.06. The resulting discs were divided into five groups (n = 20) and treated with different concentrations of NaOCl (5.25%, 2.5%, and 1.3%) for 30 min plus 17% EDTA for 2 min. The discs were then loaded to fracture by a steel rod with the same taper through the central hole. The fractured specimens were examined by scanning electron microscopy to evaluate changes in the dimensions of the remaining intertubular dentine and the tubular radius. Micro‐hardness was also measured with a Knoop diamond indenter along a radius to determine the depth of dentine eroded by the irrigation. Results were analysed by one‐way anova and the Tukey test. The level of significance was set at α = 0.05.
Results
The damage by NaOCl increased with its concentration. 5.25% NaOCl greatly reduced the fracture strength of root dentine from 172.10 ± 30.13 MPa to 114.58 ± 26.74 MPa. The corresponding reduction in micro‐hardness at the root canal wall was 34.1%. The damages reached a depth of up to 400 μm (p < .05). Structural changes involved the degradation of the intratubular wall leading to enlarged dentinal tubules and the loss of intertubular dentine. Changes in the microstructural parameters showed positive linear relationships with the fracture strength.
Conclusions
With the adjunctive use of EDTA, NaOCl caused destruction to the intratubular surface near the root canal and, consequently, reduced the root dentine's mechanical strength. The higher the concentration of NaOCl, the greater the effect. Therefore, endodontists should avoid using overly high concentration of NaOCl for irrigation to prevent potential root fracture in endodontically treated teeth.
Keywords: fracture strength, root canal irrigation, root dentine, root fracture, sodium hypochlorite
INTRODUCTION
Successful endodontic treatment requires debridement of the root canal system through mechanical instrumentation and chemical disinfection followed by sealing with appropriate materials. Root canal irrigation is essential since it can clean irregularly shaped canals and isthmuses that are inaccessible by instruments (Zehnder, 2006). The sequential use of 0.5% to 5.25% sodium hypochlorite (NaOCl) and 17% ethylenediaminetetraacetic acid (EDTA) has been recommended as a typical irrigation regimen (Dutner et al., 2012; Mostafa et al., 2020; Uzunoglu et al., 2012). NaOCl has excellent antimicrobial capacities and great efficacy in dissolving vital or necrotic tissues, while EDTA is used as an adjuvant to remove the smear layer (Mozayeni et al., 2009; Wright et al., 2020).
Currently, there is no consensus on the concentration of NaOCl that should be used for root canal irrigation, but there appears to be a tendency towards using higher concentrations for more effective disinfection and soft tissue removal (Dutner et al., 2012). A recent clinical survey found that 57% of endodontists used full‐strength NaOCl (>5.0%; Dutner et al., 2012). Even 8.25% NaOCl has become available (Cullen et al., 2015). However, high concentrations of NaOCl may irritate the periapical tissues and damage the mineralized root canal wall, which is in conflict with the contemporary medical principle of primum non nocere, which means ‘first, do no harm’ (Gershman & Boorjian, 2017; Gu et al., 2017; Sehgal et al., 2016). Furthermore, the degraded root dentine will make it more prone to fracture under subsequent load from treatment operation or mastication (Souza et al., 2014). Therefore, minimally invasive endodontics should limit the concentration of NaOCl used in root canal irrigation so as to minimize the risk of post‐treatment periapical tissue irritation and root fracture.
Dentine is a complex hard tissue composed of both organic and inorganic structures. The organic matrix, predominantly Type‐I collagen, is mineralized by apatite crystallites within both the intrafibrillar and extrafibrillar space (Bertassoni, Orgel, et al., 2012). While NaOCl is known as a non‐specific oxidizing and proteolytic agent that is capable of degrading organic components, the dentinal collagen fibrils are normally protected from denaturation by the encapsulating apatite (Armstrong et al., 2006). However, loss of surface integrity and erosion of subsurface intertubular dentine has been observed by transmission electron microscopy following the application of 5.25% NaOCl, with or without EDTA (Gu et al., 2017; Wagner et al., 2017; Zhang, Tay, et al., 2010), whereas polarized light microscopy revealed dentinal collagen alterations up to a depth of 300 μm from the root canal (Moreira et al., 2009). These results are attributed to the low molecular weight (74.4 Da) of NaOCl which allows it to penetrate into the internal environment of collagen fibrils to remove the organic phase. The collagen framework contributes considerably to the mechanical properties of dentin, such as viscoelasticity, toughness and fatigue resistance (Lu et al., 2018). NaOCl has been found to reduce dentine's micro‐hardness, modulus of elasticity and flexural/tensile/compressive strength (Pascon et al., 2009).
The fracture strength of NaOCl‐treated dentine has been extensively investigated, but the results are non‐conclusive. Most researches indicate a concentration‐dependent reduction in strength (Cecchin et al., 2015, 2017; Gu et al., 2017; Marending et al., 2007; Zhang, Kim, et al., 2010a), whereas others report no significant effect (Cullen et al., 2015; Machnick et al., 2003). There are many factors which could account for the inconsistent results, such as a lack of control of the samples' age, heterogeneity and anisotropy of tooth tissues and insensitive test methods. To date, most in vitro studies have been carried out by using 3/4‐point bending of dentine beams completely exposed to the irrigant. The limited volume of root dentin, which is anisotropic, makes it difficult to obtain an adequate number of rectangular beam specimens of consistent properties. Being concentrated on the surface, the degradation produced in these specimens is also not uniform. To improve the consistency and clinical relevance of the laboratory results—root fracture usually occurs longitudinally—hourglass‐shaped specimens from treated roots that are perpendicular to the long axis have been used to test the tensile strength of root dentine (Cecchin et al., 2015, 2017; Soares et al., 2007). But this specimen shape requires even more machining than the rectangular beams for flexural tests, resulting in much premature failure. Recently, a new method to test the fracture strength of treated dentine which considerably simplified specimen preparation was proposed (Xu et al., 2021). Thin root sections with enlarged canals were first treated with simulated intracanal irrigation and then fractured through the root canal with a metal rod of the same taper. This loading mode was similar to clinical operations that may cause root fracture, for example root canal preparation, obturation and post‐insertion. It could thus provide a more clinically relevant test for the fracture strength of treated root dentine (Fok & Chew, 2020; Munari et al., 2019).
The current study utilized this new test method to assess the impact of using different concentrations of NaOCl, with EDTA as an adjuvant, on the fracture strength of root dentin. Structural changes to the root canal wall and the dentinal tubules were characterized by scanning electron microscopy (SEM) and related to changes in the fracture strength of the root dentin. Micro‐hardness was also assessed to determine the depth of root dentine eroded by the irrigation. The null hypothesis was that there was no difference in the effect on the mechanical properties and microstructures of root dentine caused by the different concentrations of NaOCl.
MATERIALS AND METHODS
The manuscript of this laboratory study has been written according to the Preferred Reporting Items for Laboratory Studies in Endodontology (PRILE) 2021 Guidelines (Nagendrababu et al., 2021). The study design is illustrated by a PRILE flowchart in Figure 1. The sample size was determined based on a pilot study. The calculation was performed using a priori power calculation by software G*Power (Heinrich‐Heine‐Universitat) with a power of 0.8 and alpha error of 0.05. The F‐test of one‐way anova was used for the fracture test, and an effect size of 0.70 was estimated leading to a minimum sample size of six per group. For the Knoop micro‐indentation, the t‐test was conducted with a calculated effect size of 2.92, giving a sample size of four per group.
FIGURE 1.

PRILE 2021 flowchart explains the steps involved in conducting laboratory studies.
Specimen preparation and treatment
Bovine incisors with no visible damage were collected from 3‐year‐old cattle and stored in 0.5% chloramine‐T solution at 4°C prior to sample preparation. Twenty teeth were used for the fracture test. After embedding the teeth in a dental resin composite (Filtek™ Supreme Ultra Universal, 3 M OCSD) to increase their lateral dimensions for ease of mechanical testing, the tooth roots were horizontally cut into 2‐mm‐thick discs by an Isomet Low‐Speed Saw (Buehler; Figure 2a). The discs were divided into five groups according to the irrigant used: A: 5.25% NaOCl + 17% EDTA; B: 2.5% NaOCl + 17% EDTA; C: 1.3% NaOCl + 17% EDTA; D: Distilled water + 17% EDTA, and E: Distilled water (control). Five discs were cut from each of the 20 roots, and the discs were equally divided among the five groups such that each group had the same number of discs from a certain region of the root (n = 20). Four additional teeth were used for micro‐hardness testing, and four discs were obtained from each of the four roots. The discs were similarly assigned to groups A, B, C and D to make sure that each group had the same number of discs from a certain region of the root. Each disc would be split into two halves, one of which would be the control.
FIGURE 2.
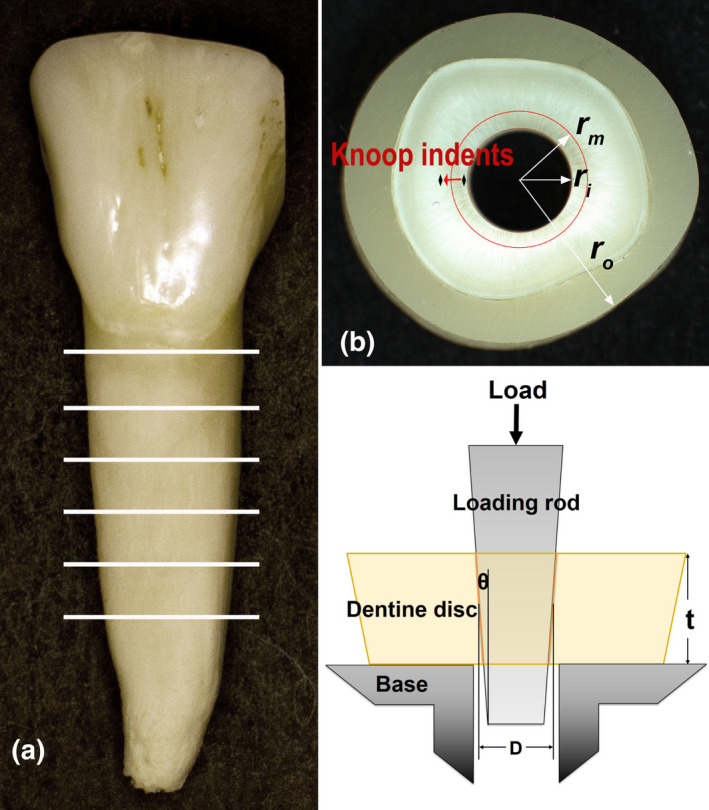
(a) Sectioning of bovine incisor roots to form dentine discs. (b) Specimen showing the radius of root canal (r i), surrounding resin composite (r o), and eroded dentine (r m), and micro‐indentation test locations. (c) Loading of root dentine disc. (b) and (c) are taken from our previous study (Xu et al., 2021).
The root canals were enlarged using a file with a constant taper of 0.06. All the discs were examined using a stereomicroscope (MVX10, Olympus) equipped with a camera (DP71, Olympus) to ascertain the absence of any crazes in them. The enlarged root canal diameters measured from the top and bottom surfaces of the discs were 2.96 ± 0.03 and 2.83 ± 0.03 mm, respectively. Afterwards, they were covered with a nail polish, exposing only the root canal wall. The resulting specimens were immersed in their corresponding irrigation solutions at room temperature, first in NaOCl for 30 min and then in EDTA as the final active irrigant for 2 min. After applying each agent, the specimens were washed three times with distilled water to remove the residual solution. The control specimens were simply immersed in distilled water for 32 min.
Knoop micro‐indentation
As mentioned above, the discs for micro‐indentation were split into two halves before irrigation. One half was treated while the other half was used as control. After removing the nail polish, the flat surfaces were polished using a series of SiC papers (up to 1200 grit) and a diamond paste. The specimens were then cleaned ultrasonically and tested immediately. Knoop hardness (KHN) was measured on a micro‐hardness testing machine (Micromet 5104) with a Knoop diamond indenter. The long diagonal of the indenter was perpendicular to the radial direction of the disc. A 50‐g load was applied with a dwell time of 20 s. Measurement started at a point 50 μm from the root canal wall and continued radially outward at 50.8‐μm intervals. Two lines of measurement were made for each half specimen, and each line had 12 indentations, reaching a depth of 608.8 μm (Figure 2b).
Micro‐hardness of the treated and control halves were compared at the same depths from the root canal wall. The maximum depth at which the micro‐hardness values still showed a statistically significant difference was taken as the depth () of dentine eroded by the irrigation regimen.
Fracture test
The roots for fracture testing were first built up with an outer layer of resin composite before sectioning to make the ratio between the root canal radius (r i) and the outer section radius (r o) smaller than 0.33 (Figure 2b). This was required for the formulae used to calculate the stresses to be applicable (Xu et al., 2021). r i and r o were measured by using the stereomicroscope.
The treated disc sections were then loaded through the enlarged root canals by a stainless‐steel rod of the same taper at a speed of 0.5 mm/min until fracture (Figure 2c). The loading rod was mounted on the crosshead of a universal test system (MTS 858S). The fracture load was determined from the peak load on the load–displacement curve.
Fracture strength calculation
The specimens in the control group, E, were assumed to be homogeneous in material properties. Results from the micro‐hardness and fracture tests showed that a 2‐min application of EDTA made no significant difference to these parameters when compared with treatment using distilled water. Therefore, the fracture strength () of Groups D and E was determined by the following equations for homogeneous discs based on the thick‐walled cylinder theory (Fok & Chew, 2020; Munari et al., 2019), that is
| (1) |
where , the internal pressure exerted by the loading rod on the root canal wall, has the form
| (2) |
is the geometry‐dependent stress concentration factor; is the fracture load; is the frictional coefficient between the steel rod and root canal wall, taken as 0.3 in this study; , and are the diameter of the root canal, thickness of the disc section and taper angle (radians) of the root canal or steel rod, respectively (Figure 2c).
For Groups A, B and C, more complicated equations were needed to account for the heterogeneity induced by dentine erosion near the root canal wall (Xu et al., 2021). Thus,
| (3) |
with
| (4) |
where , and are the radius of the root canal, outer section and eroded dentine cylinder, respectively, with ; and are the pressure on the root canal wall and the radial stress at the boundary between eroded and sound dentin, respectively; and are the elastic modulus of eroded and sound dentin, respectively; and is Poisson's ratio of dentin, taken as 0.31. The ratio for the different NaOCl concentrations was referenced from the literature (Marending et al., 2007; Xu et al., 2021), with appropriate scaling between the values reported.
SEM
The fractured specimens were mounted on an aluminium stub using conductive carbon tapes. The root canal surface was examined with a tabletop SEM (TM‐3000, Hitachi, High‐Technologies Corporation) using an acceleration voltage of 15 kV. Images (×1500) were imported to ImageJ 1.52a (Java 1.8.0_112 version, NIH) to measure the area fraction and width (μm) of the intertubular dentin, as well as the ratio between the intertubular dentine width and dentine tubular radius. The dentinal tubules in Group E were not measured as they were covered by a smear layer.
To further investigate the microstructural changes caused by the different combinations of irrigants, more specimens were prepared, sputter‐coated with iridium and observed under a more powerful SEM (Hitachi SU8230). Images with higher magnifications (×50 000 and ×10 000) were obtained, from the fracture surfaces, of dentinal tubules at intervals of 200 μm, covering a total distance of ~600 μm from the root canal wall.
Statistical analysis
The Kolmogorov–Smirnov and Shapiro–Wilk tests were used to verify the normal distribution of the data before statistical comparison. The results were then analysed by using one‐way anova with the level of significance set at α = 0.05 (SPSS 17.0, SPSS Inc.). Multiple comparisons among the different groups were performed by using the Tukey HSD test. The fracture strength was plotted against the microstructural parameters of the treated root dentine, and regression analysis was performed.
RESULTS
Knoop micro‐hardness
Figure 3 compares the micro‐hardness between the treated and untreated halves of the root dentine discs for the different irrigating regimens. Using 5.25%, 2.5% and 1.3% NaOCl plus 2 min of EDTA reduced the micro‐hardness of dentine at the root canal wall by 34.1%, 20.8% and 16.7%, respectively. The reduction was statistically significant up to a depth (δ) of ~400 μm for 5.25% and 2.5% NaOCl, and ~350 μm for 1.3% NaOCl (p < .05). Figure 3d shows that 17% EDTA did not make a statistically significant difference to the micro‐hardness when compared to distilled water (p > .05).
FIGURE 3.
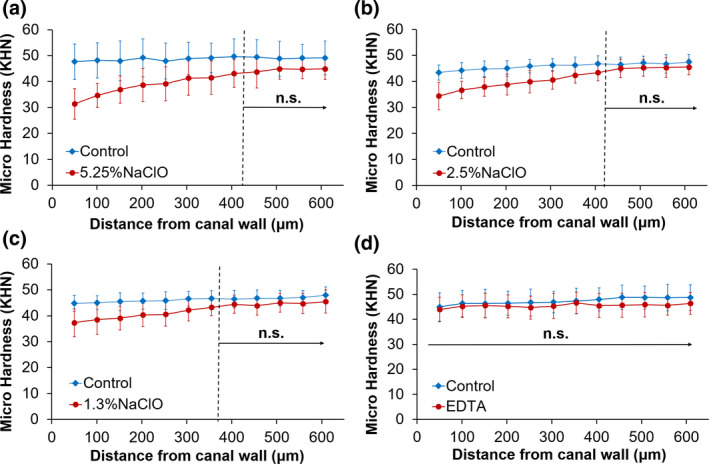
Knoop micro‐hardness of treated and untreated dentine from opposing halves of the same specimen. ‘N.s.’ indicates no statistically significant difference between treated and untreated dentine (p > .05).
Fracture load and fracture strength
As shown in Table 1, concentration‐dependent reductions were found in both the fracture load and fracture strength. Compared with Group E (control), the difference in Group C (1.3% NaOCl) was not statistically significant (p > .05), whereas that in Group A (5.25% NaOCl) was for both fracture parameters (p < .05). Group B (2.5% NaOCl) showed a significant reduction in the fracture strength (p < .05) despite showing no significant difference in the fracture load (p > .05). EDTA alone did not make a significant difference to either the fracture load or fracture strength when compared to distilled water (p > .05).
TABLE 1.
Fracture load (N), fracture strength (MPa), intertubular dentine area fraction, intertubular dentine width (μm) and ratio between intertubular dentine width and tubular radius for the different treatment groups
| Group | Fracture load | Fracture strength | Intertubular dentine area fraction | Intertubular dentine width | Intertubular dentine width/tubular radius |
|---|---|---|---|---|---|
| A | 696.71 ± 162.63* | 114.58 ± 26.74α | 0.62 ± 0.07a | 1.52 ± 0.26e | 0.91 ± 0.28h |
| B | 788.78 ± 165.34*# | 145.38 ± 30.33β | 0.71 ± 0.02b | 2.08 ± 0.31f | 1.32 ± 0.11i |
| C | 821.66 ± 142.09*# | 157.63 ± 28.21βγ | 0.77 ± 0.03c | 2.51 ± 0.36g | 1.73 ± 0.24j |
| D | 854.88 ± 149.30# | 172.10 ± 30.13γ | 0.84 ± 0.02d | 2.78 ± 0.25g | 2.52 ± 0.32k |
| E | 904.40 ± 176.15# | 181.47 ± 35.61γ | – | – | – |
Note: Different superscripts in each column indicate statistically significant difference (p < .05).
SEM of microstructures
SEM images of the root canal surface after the different treatments are compared in Figure 4. In the NaOCl‐treated groups (a–c), the loss of intertubular dentine and the associated enlargement of the dentinal tubules were obvious. The extent of erosion seemed to be positively related to the NaOCl concentration, and joining of adjacent dentinal tubules as a result of complete loss of intertubular dentine was observed in the 5.25% NaOCl group. Figure 4d,e indicate that EDTA removed the smear layer, which prevented measurement of the tubules and intertubular dentine in Group E, created by the filing. The microstructural changes in Groups A–D are quantified and presented in Table 1. Similar to the mechanical properties, the area fraction and width of intertubular dentin, and the ratio between intertubular dentine width and tubular radius showed reduction with increasing NaOCl concentration. These microstructural parameters all showed a positive linear relationship with the fracture strength, as shown in Figure 5. However, when the origin was added as a data point, as zero intertubular dentine width necessarily gives zero strength, it was the area fraction of intertubular dentine that gave the best linear relation (Figure 5d).
FIGURE 4.
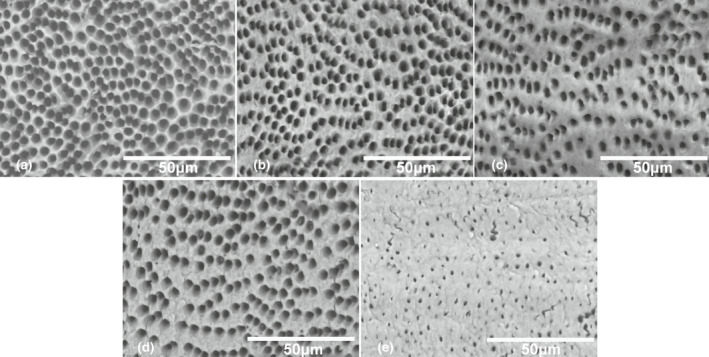
SEM images (×1500) of root canal surface treated with (a) 5.25% NaOCl + EDTA, (b) 2.5% NaOCl + EDTA, (c) 1.3% NaOCl + EDTA, (d) distilled water + EDTA and (e) distilled water only. (a–c) Show NaOCl concentration‐related loss of intertubular dentine with associated enlargement of dentinal tubules. (d) Shows that EDTA removed the smear layer (evident in e) which covered the dentine surface and partially blocked the tubules.
FIGURE 5.
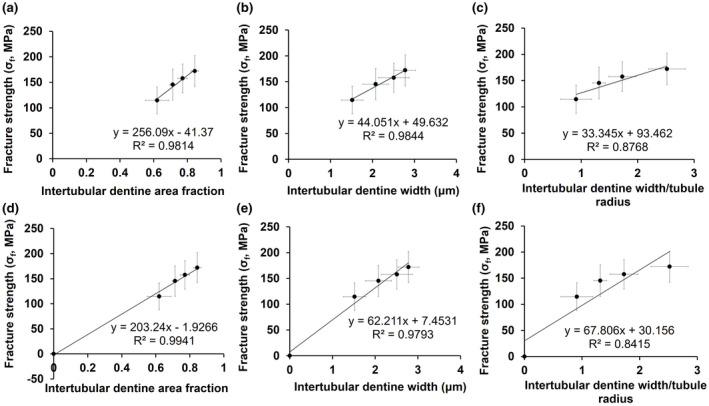
Fracture strength as a function of (a) intertubular dentine area fraction. (b) Intertubular dentine width and (c) intertubular dentine width/tubular radius. (d), (e) and (f) are corresponding figures with the origin added as a data point.
Figure 6 illustrates the microstructural changes along the dentinal tubules for the different groups. Specimens treated with distilled water (Figure 6a) or EDTA (Figure 6b) showed a similar micromorphology expected of intact dentin, with a sheet‐like membrane (lamina limitans) covering the entire length of each dentinal tubule (Bertassoni, Stankoska, & Swain, 2012; Thomas, 1984; Yoshiba et al., 2002). However, NaOCl alone could dissolve this intratubular membrane, exposing the underlying collagen fibres and leaving behind some dense particles (Figure 6c). The dual irrigation regime resulted in complete dissolution of the intratubular membrane near the root canal wall, as well as demineralization of the exposed collagen fibres (Figure 6d). The depth of dentine eroded determined from these images was again ~400 μm, similar to that given by micro‐hardness testing.
FIGURE 6.
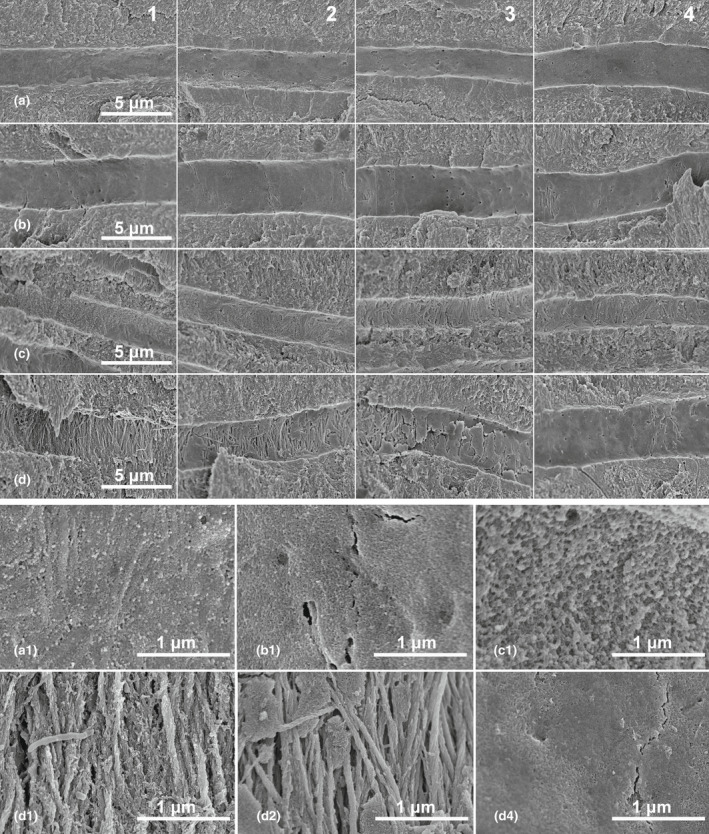
High‐magnification SEM images of fracture surfaces from specimens treated with (a) distilled water, (b) 17% EDTA, (c) 5.25% NaOCl, and (d) 5.25% NaOCl + 17% EDTA. Images were taken from regions (1) near canal wall, (2) 200 μm, (3) 400 μm and (4) 600 μm from canal wall. The bottom two rows are higher magnification (×50 000) images of the tubular wall from representative regions in the top 4 rows (×10 000). In (a) and (b), a sheet‐like membrane (lamina limitans) covers the entire length of the tubule. In (c), the lamina limitans has been damaged by NaOCl, exposing the mineralized collagen fibres and leaving behind some dense particles (c1). In (d), the lamina limitans near the canal wall is completely dissolved (d1) and the underlying collagen fibres are demineralized up to a depth of 400 μm.
DISCUSSION
This study attempted to relate the reduction in mechanical properties of root dentine caused by the use of NaOCl as an irrigant to changes in the dentinal microstructures. The mechanical testing of the disc specimens from Groups A and E to validate the method and equations used for measuring the fracture strength of root dentine has already been published (Xu et al., 2021). The other groups considered in the current study were tested under the same experimental condition; hence, all the results could be compared directly.
When using the spatial variation of dentine's micro‐hardness to determine the depth of dentine eroded by the irrigation (Slutzky‐Goldberg et al., 2004; Xu et al., 2014), both the control and experimental segments came from the same specimen. This helped to minimize differences due to variations among specimens. In line with results reported by others, a concentration‐related reduction in dentine's micro‐hardness was found (Ghisi et al., 2014; Slutzky‐Goldberg et al., 2004). However, the depth of dentine eroded (δ), which was about 0.35–0.40 mm, showed less dependence on the concentration of NaOCl. This also agreed with previous mechanical and structural analyses performed by others (Moreira et al., 2009; Oliveira et al., 2007; Slutzky‐Goldberg et al., 2002, 2004). The SEM images of the dentinal tubules indicated that structural damages from the application of 5.25% NaOCl plus EDTA also reached a depth of up to 400 μm. This value of δ was thus used in Equations (3) and (4) to evaluate the fracture strength of the eroded dentin.
The fracture strength values obtained from the present study confirmed the adverse effect of NaOCl, especially high‐concentration NaOCl, on root dentine (Marending et al., 2007; Pascon et al., 2009; Zhang, Kim, et al., 2010). However, the current results are perhaps more clinically relevant because the disc specimens used fractured in a way similar to that of endodontically treated teeth, that is along the dentinal tubule direction. The reduction in the fracture strength of eroded dentine was not so obvious from the fracture load of the discs. This was because the layer of eroded dentine was thin compared with the radius of the disc. But when the softer eroded dentine layer was taken into account by using equations for a compound cylinder with different elastic moduli, the reduction in the dentine's fracture strength became obvious. Therefore, when measuring the fracture strength of eroded dentine using the disc specimen presented in this study, changes in the elastic modulus of the eroded dentine must be taken into account. As shown in this study, this could be done by measuring changes in the micro‐hardness. However, it should be pointed out that dentine is viscoelastic (Herkströter et al., 1989), and may become more so following erosion. This may have introduced errors to the measured micro‐hardness and, hence, elastic modulus for the eroded dentin.
The morphological changes in the eroded dentine were responsible for the reduction in its mechanical strength, as can be seen from their correlation (Figure 5). Erosion of the root canal surface was characterized by dissolution of the intertubular dentin, which led to the enlargement and eventually joining of dentinal tubules with increasing NaOCl concentration. The compromised structural integrity of root dentine would reduce its resistance to crack initiation and propagation. In addition, since collagen plays an important role in the toughness and strength of mineralized tissues (Wang et al., 2001), removal of the collagen matrix would create dentine that is more brittle and more vulnerable to crack propagation. Furthermore, collagen‐sparse dentine could be more permeable to EDTA (Surapipongpuntr et al., 2008), which, in turn, helped dissolve the apatite phase and further weakened the dentin. The irrigants could easily travel along the dentinal tubules to a considerable depth. NaOCl alone could penetrate the smear layer and dissolve the organic surface layer on the tubular wall, leaving behind some dense particles (Figure 6c1). These particles, probably unbound apatite crystallites, would subsequently be dissolved by EDTA, bringing about the complete destruction of the intratubular surface structure and exposing the underlying dentine for demineralization by EDTA (Figure 6d).
It is interesting to see that EDTA alone could not enlarge the dentinal tubules even though it was capable of removing the smear layer (Figure 6b). This showed that EDTA cannot dissolve the lamina limitans, a sheet‐like structure which coats the tubular wall throughout the length of the tubules. This is an organic membrane, primarily proteoglycan protein cores, which could hinder the demineralization by EDTA (Bertassoni, Stankoska, & Swain, 2012). Therefore, tubular dentine demineralization by EDTA appeared to require NaOCl to be used beforehand to remove the lamina limitans. This was evident from the mechanical properties of EDTA‐treated specimens from Group D which showed no significant differences from those treated with distilled water (Group E). This was also consistent with results reported in the literature that, when used alone for 2 min, 17% EDTA did not cause statistically significant changes to dentine's structure or flexural strength, despite a reduction in the apatite/collagen ratio (Moreira et al., 2009; Zhang, Kim, et al., 2010). It is possible, though, that prolonged treatment with EDTA could result in more significant demineralization and strength reduction. However, as far as the common irrigation regimen is concerned, these observations indicate that it is the concentration of NaOCl rather than that of EDTA that we should pay attention to in order to preserve the structure and mechanical strength of root dentin. With this regimen, dentine erosion is initiated by the use of NaOCl which dissolves the organic matters that cover the root canal wall and intratubular surfaces. The use of EDTA as a final active irrigant then completes the removal of the smear layer and the collagen‐depleted apatite, and demineralize the exposed underlying dentin.
1%–5.25% NaOCl has been reported to have similar antimicrobial efficacy (Siqueira et al., 2000). Therefore, from a clinical perspective, to reduce the susceptibility to root fracture, overly high concentration of NaOCl should be avoided for root canal irrigation. In this study, 1.3% NaOCl was found not to cause significant changes to the fracture strength of root dentine despite the microstructural changes observed. While using a low concentration of NaOCl may reduce the soft tissue‐dissolving effectiveness, this could be compensated for by other safer means, for example using a higher temperature, continuous agitation, surfactants or simply more frequent replenishing of the irrigants (Stojicic et al., 2010). Furthermore, we should look for an alternative substance with suitable antimicrobial and soft‐tissue‐dissolving properties but less deleterious to dentine structurally and mechanically.
The time of contact between the irrigant and the root dentine used was based on previous studies on NaOCl/EDTA irrigation regimens (Gu et al., 2017; Moreira et al., 2009; Zhang, Kim, et al., 2010; Zhang, Tay, et al., 2010). This length of time was approximately that for the whole instrumentation procedure. However, clinically, a thin layer of the dentinal wall is removed during mechanical instrumentation every time a new file is used. This was not simulated in the present study. Nevertheless, this omission does not alter the role the irrigation regimen played in affecting the root dentine structurally and mechanically. Furthermore, considering the larger size of the bovine root, the extended contact time for the irrigant was justified.
CONCLUSION
Within the limitations of this study, the null hypothesis that there was no difference in the effect on the mechanical properties and microstructures of root dentine caused by the different concentrations of NaOCl used in the irrigation regimen was rejected. NaOCl caused destruction to the root canal wall and intratubular surface, allowing EDTA to demineralize the exposed underlying dentine and thus reducing the root's mechanical strength. The higher the NaOCl concentration, the greater the effects. Endodontists should follow the modern concept of minimally invasive dentistry and avoid using overly high concentration of NaOCl for the sole purpose of more effective disinfection. This will help prevent root fracture after endodontic treatment.
AUTHOR CONTRIBUTIONS
H. Xu conceived the research idea, performed the experiments, analysed the data and prepared the manuscript. Z. Ye and A. Zhang performed the SEM and image analysis. F. Lin and J. Fu helped prepare the specimens and contributed significantly to the data analysis and discussion of the results. A. Fok designed the experiments, guided the data analysis and interpretation of the results and edited the manuscript.
FUNDING INFORMATION
This work was supported by the National Natural Science Foundation of China (No. 81600903).
CONFLICT OF INTEREST
The authors declare no potential conflicts of interest in connection with this article.
ETHICAL APPROVAL
The protocol (no. STUDY00010231) was approved by the Institutional Review Board of the University of Minnesota.
ACKNOWLEDGEMENTS
Haiping Xu would like to thank the Minnesota Dental Research Center for Biomaterials and Biomechanics for hosting her visit, during which this study was performed. This work was supported by the National Natural Science Foundation of China (No. 81600903).
Xu, H. , Ye, Z. , Zhang, A. , Lin, F. , Fu, J. & Fok, A.S.L. (2022) Effects of concentration of sodium hypochlorite as an endodontic irrigant on the mechanical and structural properties of root dentine: A laboratory study. International Endodontic Journal, 55, 1091–1102. Available from: 10.1111/iej.13800
DATA AVAILABILITY STATEMENT
Data available on request from the authors.
REFERENCES
- Armstrong, S.R. , Jessop, J.L.P. , Winn, E. , Tay, F.R. & Pashley, D.H. (2006) Denaturation temperatures of dentin matrices. I. Effect of demineralization and dehydration. Journal of Endodontics, 32, 638–641. [DOI] [PubMed] [Google Scholar]
- Bertassoni, L.E. , Orgel, J.P. , Antipova, O. & Swain, M.V. (2012) The dentin organic matrix – limitations of restorative dentistry hidden on the nanometer scale. Acta Biomaterialia, 8, 2419–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertassoni, L.E. , Stankoska, K. & Swain, M.V. (2012) Insights into the structure and composition of the peritubular dentin organic matrix and the lamina limitans. Micron, 43, 229–236. [DOI] [PubMed] [Google Scholar]
- Cecchin, D. , Farina, A.P. , Souza, M.A. , Albarello, L.L. , Schneider, A.P. , Vidal, C.M.P. et al. (2015) Evaluation of antimicrobial effectiveness and dentine mechanical properties after use of chemical and natural auxiliary irrigants. Journal of Dentistry, 43, 695–702. [DOI] [PubMed] [Google Scholar]
- Cecchin, D. , Soares Giaretta, V. , Granella Cadorin, B. , Albino Souza, M. , Vidal, C.M.P. & Paula Farina, A. (2017) Effect of synthetic and natural‐derived novel endodontic irrigant solutions on mechanical properties of human dentin. Journal of Materials Science: Materials in Medicine, 28, 141. [DOI] [PubMed] [Google Scholar]
- Cullen, J.K. , Wealleans, J.A. , Kirkpatrick, T.C. & Yaccino, J.M. (2015) The effect of 8.25% sodium hypochlorite on dental pulp dissolution and dentin flexural strength and modulus. Journal of Endodontics, 41, 920–924. [DOI] [PubMed] [Google Scholar]
- Dutner, J. , Mines, P. & Anderson, A. (2012) Irrigation trends among American Association of Endodontists members: a web‐based survey. Journal of Endodontics, 38, 37–40. [DOI] [PubMed] [Google Scholar]
- Fok, A. & Chew, H.P. (2020) Endodontic treatment and vertical root fracture. In: Fok, A. & Chew, H.P. (Eds.) Mathematical models for dental materials research. Cham: Springer, pp. 9–15. [Google Scholar]
- Gershman, B. & Boorjian, S.A. (2017) Primum non nocere: critically assessing the morbidity of prostate biopsy. European Urology, 71, 366–367. [DOI] [PubMed] [Google Scholar]
- Ghisi, A.C. , Kopper, P.M. , Baldasso, F.E. , Stürmer, C.P. , Rossi‐Fedele, G. , Steier, L. et al. (2014) Effect of super‐oxidized water, sodium hypochlorite and EDTA on dentin microhardness. Brazilian Dental Journal, 25, 420–424. [DOI] [PubMed] [Google Scholar]
- Gu, L.S. , Huang, X.Q. , Griffin, B. , Bergeron, B.R. , Pashley, D.H. , Niu, L.N. et al. (2017) Primum non nocere – the effects of sodium hypochlorite on dentin as used in endodontics. Acta Biomaterialia, 61, 144–156. [DOI] [PubMed] [Google Scholar]
- Herkströter, F.M. , Witjes, M. , Ruben, J. & Arends, J. (1989) Time dependency of microhardness indentations in human and bovine dentine compared with human enamel. Caries Research, 23, 342–344. [DOI] [PubMed] [Google Scholar]
- Lu, X. , Rawson, S.D. & Withers, P.J. (2018) Effect of hydration and crack orientation on crack‐tip strain, crack opening displacement and crack‐tip shielding in elephant dentin. Dental Materials, 34, 1041–1053. [DOI] [PubMed] [Google Scholar]
- Machnick, T.K. , Torabinejad, M. , Munoz, C.A. & Shabahang, S. (2003) Effect of MTAD on flexural strength and modulus of elasticity of dentin. Journal of Endodontics, 29, 747–750. [DOI] [PubMed] [Google Scholar]
- Marending, M. , Luder, H.U. , Brunner, T.J. , Knecht, S. , Stark, W.J. & Zehnder, M. (2007) Effect of sodium hypochlorite on human root dentine – mechanical, chemical and structural evaluation. International Endodontic Journal, 40, 786–793. [DOI] [PubMed] [Google Scholar]
- Moreira, D.M. , Almeida, J.F.A. , Ferraz, C.C.R. , Gomes, B.P.F.D.A. & Zaia, A.A. (2009) Structural analysis of bovine root dentin after use of different endodontics auxiliary chemical substances. Journal of Endodontics, 35, 1023–1027. [DOI] [PubMed] [Google Scholar]
- Mostafa, M. , El‐Shrief, Y.A.I. , Anous, W.I.O. , Hassan, M.W. , Salamah, F.T.A. , El Boghdadi, R.M. et al. (2020) Postoperative pain following endodontic irrigation using 1.3% versus 5.25% sodium hypochlorite in mandibular molars with necrotic pulps: a randomized double‐blind clinical trial. International Endodontic Journal, 53, 154–166. [DOI] [PubMed] [Google Scholar]
- Mozayeni, M.A. , Javaheri, G.H. , Poorroosta, P. , Ashari, M.A. & Javaheri, H.H. (2009) Effect of 17% EDTA and MTAD on intracanal smear layer removal: a scanning electron microscopic study. Australian Endodontic Journal, 35, 13–17. [DOI] [PubMed] [Google Scholar]
- Munari, L.S. , Bowles, W.R. & Fok, A.S.L. (2019) Relationship between canal enlargement and fracture load of root dentin sections. Dental Materials, 35, 818–824. [DOI] [PubMed] [Google Scholar]
- Nagendrababu, V. , Murray, P.E. , Ordinola‐Zapata, R. , Peters, O.A. , Rôças, I.N. , Siqueira, J.F., Jr. et al. (2021) PRILE 2021 guidelines for reporting laboratory studies in endodontology: a consensus‐based development. International Endodontic Journal, 54, 1482–1490. [DOI] [PubMed] [Google Scholar]
- Oliveira, L.D. , Carvalho, C.A. , Nunes, W. , Valera, M.C. , Camargo, C.H. & Jorge, A.O. (2007) Effects of chlorhexidine and sodium hypochlorite on the microhardness of root canal dentin. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 104, e125–e128. [DOI] [PubMed] [Google Scholar]
- Pascon, F.M. , Kantovitz, K.R. , Sacramento, P.A. , Nobre‐dos‐Santos, M. & Puppin‐Rontani, R.M. (2009) Effect of sodium hypochlorite on dentine mechanical properties. A review. Journal of Dentistry, 37, 903–908. [DOI] [PubMed] [Google Scholar]
- Sehgal, I.S. , Dhooria, S. , Aggarwal, A.N. , Chaudhry, D. & Agarwal, R. (2016) Noninvasive ventilation in acute respiratory distress syndrome: primum non nocere. Journal of Critical Care, 32, 226. [DOI] [PubMed] [Google Scholar]
- Siqueira, J.F., Jr. , Rôças, I.N. , Favieri, A. & Lima, K.C. (2000) Chemomechanical reduction of the bacterial population in the root canal after instrumentation and irrigation with 1%, 2.5%, and 5.25% sodium hypochlorite. Journal of Endodontics, 26, 331–334. [DOI] [PubMed] [Google Scholar]
- Slutzky‐Goldberg, I. , Liberman, R. & Heling, I. (2002) The effect of instrumentation with two different file types, each with 2.5% NaOCl irrigation on the microhardness of root dentin. Journal of Endodontics, 28, 311–312. [DOI] [PubMed] [Google Scholar]
- Slutzky‐Goldberg, I. , Maree, M. , Liberman, R. & Heling, I. (2004) Effect of sodium hypochlorite on dentin microhardness. Journal of Endodontics, 30, 880–882. [DOI] [PubMed] [Google Scholar]
- Soares, C.J. , Santana, F.R. , Silva, N.R. , Preira, J.C. & Pereira, C.A. (2007) Influence of the endodontic treatment on mechanical properties of root dentin. Journal of Endodontics, 33, 603–606. [DOI] [PubMed] [Google Scholar]
- Souza, E.M. , Calixto, A.M. , Lima, C.N. , Pappen, F.G. & De‐Deus, G. (2014) Similar influence of stabilized alkaline and neutral sodium hypochlorite solutions on the fracture resistance of root canal‐treated bovine teeth. Journal of Endodontics, 40, 1600–1603. [DOI] [PubMed] [Google Scholar]
- Stojicic, S. , Zivkovic, S. , Qian, W. , Zhang, H. & Haapasalo, M. (2010) Tissue dissolution by sodium hypochlorite: effect of concentration, temperature, agitation, and surfactant. Journal of Endodontics, 36, 1558–1562. [DOI] [PubMed] [Google Scholar]
- Surapipongpuntr, P. , Duangcharee, W. , Kwangsamai, S. & Ekka, A. (2008) Effect of root canal irrigants on cervical dentine permeability to hydrogen peroxide. International Endodontic Journal, 41, 821–827. [DOI] [PubMed] [Google Scholar]
- Thomas, H.F. (1984) The lamina limitans of human dentinal tubules. Journal of Dental Research, 63, 1064–1066. [DOI] [PubMed] [Google Scholar]
- Uzunoglu, E. , Aktemur, S. , Uyanik, M.O. , Durmaz, V. & Nagas, E. (2012) Effect of ethylenediaminetetraacetic acid on root fracture with respect to concentration at different time exposures. Journal of Endodontics, 38, 1110–1113. [DOI] [PubMed] [Google Scholar]
- Wagner, M.H. , da Rosa, R.A. , de Figueiredo, J.A.P. , Duarte, M.A.H. , Pereira, J.R. & Só, M.V.R. (2017) Final irrigation protocols may affect intraradicular dentin ultrastructure. Clinical Oral Investigations, 21, 2173–2182. [DOI] [PubMed] [Google Scholar]
- Wang, X. , Bank, R.A. , TeKoppele, J.M. & Agrawal, C.M. (2001) The role of collagen in determining bone mechanical properties. Journal of Orthopaedic Research, 19, 1021–1026. [DOI] [PubMed] [Google Scholar]
- Wright, P.P. , Scott, S. , Kahler, B. & Walsh, L.J. (2020) Organic tissue dissolution in clodronate and etidronate mixtures with sodium hypochlorite. Journal of Endodontics, 46, 289–294. [DOI] [PubMed] [Google Scholar]
- Xu, H. , Ye, N. , Lin, F. , Heo, Y.C. & Fok, A.S.L. (2021) A new method to test the fracture strength of endodontically‐treated root dentin. Dental Materials, 37, 796–804. [DOI] [PubMed] [Google Scholar]
- Xu, H. , Zheng, Q. , Shao, Y. , Song, F. , Zhang, L. , Wang, Q. et al. (2014) The effects of ageing on the biomechanical properties of root dentine and fracture. Journal of Dentistry, 42, 305–311. [DOI] [PubMed] [Google Scholar]
- Yoshiba, K. , Yoshiba, N. , Ejiri, S. , Iwaku, M. & Ozawa, H. (2002) Odontoblast processes in human dentin revealed by fluorescence labeling and transmission electron microscopy. Histochemistry and Cell Biology, 118, 205–212. [DOI] [PubMed] [Google Scholar]
- Zehnder, M. (2006) Root canal irrigants. Journal of Endodontics, 32, 389–398. [DOI] [PubMed] [Google Scholar]
- Zhang, K. , Kim, Y.K. , Cadenaro, M. , Bryan, T.E. , Sidow, S.J. , Loushine, R.J. et al. (2010) Effects of different exposure times and concentrations of sodium hypochlorite/ethylenediaminetetraacetic acid on the structural integrity of mineralized dentin. Journal of Endodontics, 36, 105–109. [DOI] [PubMed] [Google Scholar]
- Zhang, K. , Tay, F.R. , Kim, Y.K. , Mitchell, J.K. , Kim, J.R. , Carrilho, M. et al. (2010) The effect of initial irrigation with two different sodium hypochlorite concentrations on the erosion of instrumented radicular dentin. Dental Materials, 26, 514–523. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request from the authors.


