Abstract
Background
This study aimed to describe the treatment strategies and outcomes for women with newly diagnosed advanced high‐grade serous or endometrioid ovarian cancer (OC).
Methods
This observational study collected real‐world medical record data from eight Western countries on the diagnostic workup, clinical outcomes, and treatment of adult women with newly diagnosed advanced (Stage III–IV) high‐grade serous or endometrioid OC. Patients were selected backward in time from April 1, 2018 (the index date), with a target of 120 patients set per country, followed for ≥20 months.
Results
Of the 1119 women included, 66.9% had Stage III disease, 11.7% had a deleterious BRCA mutation, and 26.6% received bevacizumab; 40.8% and 39.3% underwent primary debulking surgery (PDS) and interval debulking surgery (IDS), respectively. Of the patients who underwent PDS, 55.5% had no visible residual disease (VRD); 63.9% of the IDS patients had no VRD. According to physician‐assessed responses (at the first assessment after diagnosis and treatment), 53.2% of the total population had a complete response and 25.7% had a partial response to first‐line chemotherapy after surgery. After ≥20 months of follow‐up, 32.9% of the patients were disease‐free, 46.4% had progressive disease, and 20.6% had died. Bevacizumab use had a significant positive effect on overall survival (hazard ratio [HR], 0.62; 95% CI, 0.42–0.91; p = .01). A deleterious BRCA status had a significant positive effect on progression‐free survival (HR, 0.60; 95% CI, 0.41–0.84; p < .01).
Conclusions
Women with advanced high‐grade serous or endometrioid OC have a poor prognosis. Bevacizumab use and a deleterious BRCA status were found to improve survival in this real‐world population.
Lay summary
Patients with advanced (Stage III or IV) ovarian cancer (OC) have a poor prognosis.
The standard treatment options of surgery and chemotherapy extend life beyond diagnosis for 5 years or more in only approximately 45% of patients.
This study was aimed at describing the standard of care in eight Western countries and estimating how many patients who are diagnosed with high‐grade serous or endometrioid OC could potentially be eligible for first‐line poly(adenosine diphosphate ribose) polymerase inhibitor (PARPi) maintenance therapy.
The results highlight the poor prognosis for these patients and suggest that a significant proportion (79%) would potentially be eligible for first‐line PARPi maintenance treatment.
Keywords: bevacizumab, first‐line treatment, ovarian cancer, poly(adenosine diphosphate ribose) polymerase (PARP) inhibitor, real‐world data
Short abstract
In women diagnosed with high‐grade advanced serous or endometrioid ovarian cancer, bevacizumab use had a significant positive effect on overall survival (hazard ratio [HR], 0.62; 95% CI, 0.42–0.91; p = .01), and a deleterious BRCA status had a significant positive effect on progression‐free survival (HR, 0.60; 95% CI, 0.41–0.84; p < .01). According to clinical responses following surgery and first‐line chemotherapy, almost 8 in 10 women diagnosed with high‐grade advanced serous or endometrioid ovarian cancer would potentially be eligible for first‐line poly(adenosine diphosphate ribose) polymerase inhibitor maintenance treatment.
1. INTRODUCTION
Annually, there are an estimated 239,000 newly diagnosed cases of ovarian cancer (OC) and 152,000 deaths from OC worldwide. 1 In Europe, OC is the fifth most common cause of cancer death. The prognosis after treatment, which has remained largely unchanged over the last decade, is poor, with a 5‐year survival rate of approximately 45%, in part because of diagnoses occurring at an advanced stage (Stage III or IV) of disease. 2 , 3
Primary debulking surgery (PDS) followed by carboplatin plus paclitaxel is the standard of care (SoC) for OC. If upfront extensive surgery or complete tumor resection at PDS is not possible, then the standard treatment is neoadjuvant chemotherapy followed by interval debulking surgery (IDS) or palliative treatment. 4 Complete tumor resection at frontline surgery is the most important prognostic factor for patients with advanced ovarian cancer (AOC). 4 BRCA1 and/or BRCA2 mutations (BRCAm) are also a known predictive biomarker for a response to platinum‐based chemotherapy. 5
Oncologic treatment options for AOC, including cancers of the fallopian tubes and peritoneum (which behave and are treated similarly to AOC), include anti‐angiogenesis and molecularly targeted therapies. In the first‐line setting, bevacizumab is approved as maintenance therapy after carboplatin plus paclitaxel, and it has been shown in phase 3 randomized controlled trials (GOG‐0218 and ICON7) to improve progression‐free survival (PFS), but not overall survival (OS), in patients with high‐risk early‐stage disease, Stage III disease with visible residual disease (VRD) > 1 cm after surgery, or Stage IV disease. 4 , 6 , 7
Other approved molecularly targeted maintenance therapies for OC include the poly(adenosine diphosphate ribose) polymerase inhibitors (PARPi) niraparib, rucaparib, and olaparib; however, their use and reimbursement vary widely among European Union countries. 4 , 8 , 9 , 10 , 11 Data supporting PARPi use in the first‐line maintenance setting come from several randomized controlled trials showing significant improvements in PFS, although the trials differed considerably in terms of their control arms (placebo or active intervention), patient populations (platinum sensitivity and residual disease), timing of PARPi treatment (with chemotherapy or maintenance only), and planned duration of treatment. 8 , 9 , 10 , 11 , 12 , 13
The proportion of patients in representative clinical practice (i.e., in a real‐world setting) presenting with no evidence of disease (NED) after primary surgery or a clinical complete response (CR)/partial response (PR) after the completion of first‐line chemotherapy is largely unknown. Such patients would potentially be eligible for first‐line PARPi maintenance therapy with or without concomitant anti‐angiogenic treatment.
To address this question, we used real‐world observational data to describe the treatment strategies and outcomes for women with newly diagnosed advanced high‐grade serous or endometrioid OC. Secondary objectives included characterizing the effects of the BRCA status and bevacizumab use on clinical outcomes.
2. MATERIALS AND METHODS
2.1. Study design and patient selection
This observational, longitudinal, medical chart review study collected real‐world data from eight countries (Austria, Belgium, Denmark, Finland, Israel, the Netherlands, Norway, and Portugal). Women aged ≥18 years with histologically documented advanced (International Federation of Gynecology and Obstetrics [FIGO] Stage III–IV) high‐grade serous or endometrioid OC (including peritoneal or fallopian tube cancer) were eligible provided they were diagnosed (the index date was the date of diagnosis) no later than April 1, 2018. Patients were selected backward in time from this date until a target of 120 patients was identified in each participating country for the primary patient cohort, and they were followed up prospectively; this ensured a minimum of 20 months' follow‐up for all patients and no risk of immortal time bias. Patients who died or had disease progression during follow‐up were eligible.
Patients were ineligible if they had experienced other malignancies within the previous 5 years or were enrolled in clinical trials in which investigators were blinded to the treatments.
2.2. Data collection
All data were retrieved from national registries and/or medical records and sampled randomly according to the inclusion criteria until sufficient patient numbers were included. The following data were collected from the date of diagnosis: demographic and clinical characteristics; medical history and comorbidities; OC diagnosis and primary tumor; and BRCAm status. The data collected after the diagnosis until the end of each patient observation period (the last hospital visit 12 months after the completion of platinum‐based chemotherapy) included the following: therapeutic management, including the type (PDS, IDS, or no resection) and outcome of surgery (residual vs. no residual disease); pharmacological treatments (excluding supportive and palliative treatments); and treatment outcomes.
Physician‐assessed clinical responses to treatment were determined radiologically where possible. When imaging was not available and computed tomography scans were not assessed with the Response Evaluation Criteria in Solid Tumors by a radiologist, investigators applied the Response Evaluation Criteria in Solid Tumors retrospectively where possible before reverting to physician‐assessed responses of CR, PR, or NED.
There was no imputation of missing values, and data for subanalyses (e.g., the prognostic importance of the BRCA status) are presented for those with available data only.
2.3. Ethical considerations
The study was performed in accordance with the Declaration of Helsinki, the International Conference on Harmonisation's Good Clinical Practice guidelines, Good Publication Practice guidelines, and applicable legislation on noninterventional studies and observational studies. The collection of data adhered to all applicable data protection regulations and requirements regarding electronic records and database validation.
Informed consent was sought in accordance with local regulations. Consent was required for patients alive at the time that data collection started in Belgium, the Netherlands, Norway, and Portugal; patients who were deceased when data collection started were eligible for the study via a consent waiver. No consent was required for Austria, Denmark, Finland, or Israel. A total of 284 patients (25.4%) required consent (14 in Belgium, 18 in the Netherlands, 62 in Norway, and 190 in Portugal). Fourteen patients for whom some information had been entered into the electronic case report form were excluded for not fulfilling the inclusion criteria. Data from Finland were included in all descriptive analyses but were excluded from the survival analysis because of local data transfer regulations.
2.4. Study objectives
The primary objective was to estimate the proportion of patients potentially eligible for first‐line PARPi maintenance therapy by characterizing the percentage with a physician‐assessed CR/PR after surgery (PDS or IDS) and first‐line chemotherapy (at the first assessment after diagnosis and treatment).
Other objectives included determining the percentage of patients undergoing primary surgery and having no macroscopic residual disease after surgery or residual disease of ≤1 cm (i.e., traditionally considered ‘lower risk') as well as the percentage of patients who received angiogenesis inhibitor treatment.
Patient demographics and disease characteristics, including the FIGO stage distribution (only available as Stage III or Stage IV across all countries) and cancer antigen 125 (CA‐125) levels, were also assessed. A CA‐125 assessment after first‐line treatment was defined as the proportion of patients with a normal CA‐125 level or >90% decrease in the level from the baseline in accordance with the PRIMA study (NCT02655016). 10 The percentage of patients with VRD following surgery, OS, and PFS were also analyzed according to bevacizumab use and BRCAm status.
2.5. Statistical methods
All statistical evaluations were performed according to a predefined plan. An inclusion target of 120 patients per country was deemed adequate for providing precise estimates of local PARPi eligibility (4% error margin). Baseline parameters, clinical characteristics, and treatment patterns (and associated outcomes) were described with frequency counts and percentages for categorical variables and with means and interquartile ranges for continuous variables.
OS and PFS were estimated with Kaplan–Meier methodology, and p values were calculated with the log‐rank test. A multivariate Cox proportional hazards regression analysis was performed (for subanalyses: outcomes by BRCAm status and effect of bevacizumab [yes/no]) to assess for confounding by age (continuous and linear), country, stage, BRCAm status, and indication of good prognosis (defined as primary surgery and no VRD).
3. RESULTS
Across eight countries, 1119 women diagnosed with advanced high‐grade serous or endometrioid OC between 2002 and 2018 were included (>98.5% were enrolled from 2010 onward; Figure S1).
Most patients had tumors with serous histology (97.5%; 1091 of 1119); tumors were most often located in the ovaries (66.7%; 746 of 1119), with 66.9% of all patients (749 of 1119) diagnosed at FIGO Stage III (Table 1). A total of 66.0% of the patients (739 of 1119) had available BRCA testing; 11.6% had a deleterious germline BRCAm, 5.8% had tumors with a deleterious somatic BRCAm (out of 37.2% [275 of 739] tested for somatic BRCAm), and 83.9% (620 of 739) had no BRCAm. Forty‐one percent of the patients (456 of 1119) underwent PDS, and 39.3% (440 of 1119) underwent IDS. Approximately one quarter of the patients (26.6%; 298 of 1119) received bevacizumab concomitantly with first‐line chemotherapy and as maintenance.
TABLE 1.
Patient characteristics
| Variable | Total (N = 1119) |
|---|---|
| Age, median (IQR), years | 66 (57–73) |
| Histology, No. (%) | |
| High‐grade serous | 1091 (97.5) |
| High‐grade endometrioid | 23 (2.1) |
| Mixed | 5 (0.4) |
| Tumor location, No. (%) | |
| Ovarian | 746 (66.7) |
| Fallopian tube | 200 (17.9) |
| Peritoneal | 97 (8.7) |
| Unknown | 76 (6.8) |
| FIGO stage, No. (%) | |
| III | 749 (66.9) |
| IV | 370 (33.1) |
| ECOG performance status, No. (%) | |
| 0 | 459 (41.0) |
| 1 | 318 (28.4) |
| 2 | 70 (6.3) |
| 3 | 15 (1.3) |
| Unknown | 257 (23.0) |
| BRCAm, No. (%) | |
| Test result available | 739 (66.0) |
| Deleterious germline | 86/739 (11.6) |
| Deleterious somatic | 43/739 (5.8) |
| No mutation | 620/739 (83.9) |
| Surgery, No. (%) | |
| No surgerya | 223 (19.9) |
| Primary debulking surgery | 456 (40.8) |
| Interval debulking surgery | 440 (39.3) |
| Bevacizumab, No. (%) | |
| Yes | 298 (26.6) |
| No | 806 (72.0) |
| Unknown | 15 (1.3) |
| Chemotherapy setting, No. (%) | |
| Any | 1040 (92.9) |
| Neoadjuvant | 515 (46.0) |
| Type of chemotherapy, No. (%)b | |
| Carboplatin | 1036 (92.6) |
| Paclitaxel | 876 (78.3) |
| Docetaxel | 154 (13.8) |
| Gemcitabine | 66 (5.9) |
| Other | 226 (20.2) |
| Country, No. (%) | |
| Austria | 121 (10.8) |
| Belgium | 120 (10.7) |
| Denmark | 246 (22.0) |
| Finland | 120 (10.7) |
| Israel | 120 (10.7) |
| Netherlands | 82 (7.3) |
| Norway | 120 (10.7) |
| Portugal | 190 (17.0) |
Abbreviations: BRCAm, BRCA1 and/or BRCA2 mutation; ECOG, Eastern Cooperative Oncology Group; FIGO, International Federation of Gynecology and Obstetrics; IQR, interquartile range.
No surgery corresponds to no attempt to reduce a tumor (i.e., diagnostic laparoscopy and palliative operations were not considered reductive surgery).
Chemotherapy is presented by agent and could be administered in combination.
Of the patients who underwent PDS, 55.5% (253 of 456) had no VRD, and 15.1% (69 of 456) had residual disease ≤1 cm. Of the patients who underwent IDS, 63.9% (281 of 440) had no VRD, and 20.9% (92 of 440) had residual disease ≤1 cm. When we considered the outcomes of surgery in the overall population (including the 19.9% [223 of 1119] who did not undergo surgery), 22.6% (253 of 1119) had no VRD and 6.2% (69 of 1119) had residual disease ≤1 cm after PDS, whereas 25.1% (281 of 1119) had no VRD and 8.2% (92 of 1119) had residual disease ≤1 cm after IDS (Table 2). Among all patients, 18.0% (201 of 1119) had residual disease >1 cm or an unknown status.
TABLE 2.
Patient outcomes at the first assessment after diagnosis and treatment
| Variable | FIGO Stages III and IV (N = 1119) | FIGO Stage III (N = 749) | FIGO Stage IV (N = 370) |
|---|---|---|---|
| Outcome after surgery, No. (%) | |||
| Primary debulking surgery | |||
| No visible residual disease | 253 (22.6) | 215 (28.7) | 38 (10.3) |
| Residual disease ≤1 cm | 69 (6.2) | 59 (7.9) | 10 (2.7) |
| Residual disease >1 cm | 91 (8.1) | 66 (8.8) | 25 (6.8) |
| Unknown | 43 (3.8) | 36 (4.8) | 7 (1.9) |
| Total | 456 (40.8) | 376 (50.2) | 80 (21.6) |
| Interval debulking surgery | |||
| No visible residual disease | 281 (25.1) | 153 (20.4) | 128 (34.6) |
| Residual disease ≤1 cm | 92 (8.2) | 58 (7.7) | 34 (9.2) |
| Residual disease >1 cm | 52 (4.6) | 35 (4.7) | 17 (4.6) |
| Unknown | 15 (1.3) | 10 (1.3) | 5 (1.4) |
| Total | 440 (39.3) | 256 (34.2) | 184 (49.7) |
| No surgery, No. (%)a | 223 (19.9) | 117 (15.6) | 106 (28.6) |
| Response to treatment | FIGO Stages III and IV (N = 1119) | ||
| CA‐125 normal at baseline, No. (%)b , c | |||
| Yes | 158/706 (22.4) | ||
| No | 548/706 (77.6) | ||
| CA‐125 normal at assessment, No. (%)b , c | |||
| Yes | 310/548 (56.6) | ||
| No | 238/548 (43.4) | ||
| CA‐125 90% reduction, No. (%)b | |||
| Yes | 302/548 (55.1) | ||
| No | 246/548 (44.9) | ||
| CA‐125 response, No. (%)b | |||
| Yes | 363/548 (66.2) | ||
| No | 185/548 (33.8) | ||
| Physician‐assessed response, No. (%)d | |||
| Complete response/no macroscopic residual diseasee | 595 (53.2) | ||
| Partial response | 288 (25.7) | ||
| Otherf | 119 (10.6) | ||
| No assessment | 117 (10.5) | ||
| Potentially eligible for PARPi, No. (%) | |||
| Complete response/no macroscopic residual disease/partial response | 883 (78.9) | ||
Abbreviations: CA‐125, cancer antigen 125; FIGO, International Federation of Gynecology and Obstetrics; PARPi, poly(adenosine diphosphate ribose) polymerase inhibitor.
No surgery corresponds to no attempt to reduce a tumor (i.e., diagnostic laparoscopy and palliative operations were not considered reductive surgery).
The response according to CA‐125 is based only on data for patients with measurements at diagnosis and at assessment and with a CA‐125 value above normal at diagnosis, and it is defined as the proportion of patients with a normal CA‐125 level or >90% decrease in the level from the baseline.
A normal CA‐125 level is <35 U/mL.
Clinical responses were assessed and reported by a physician.
No macroscopic residual disease is defined as undergoing surgery and having no macroscopic residual disease after surgery.
Patients with stable disease, patients with progressive disease, and deceased patients.
According to CA‐125 levels for the patients with data available at diagnosis and the first response assessment, 66.2% of the patients with available measurements (363 of 548) had a response to treatment (defined as the proportion of patients with a normal CA‐125 level or >90% decrease in the level from the baseline; Table 2); according to physician‐assessed responses, 53.2% of the patients (595 of 1119) had a CR (or NED as the tumor was not identified), and 25.7% (288 of 1119) had a PR. The percentage of patients who would potentially have been eligible for PARPi maintenance therapy (the CR + PR rate; Table 2) was 78.9% (883 of 1119). The response according to CA‐125 levels and the physician‐assessed response (CR or PR) were concordant in 75.0% of the patients with both measures (baseline and response assessments) available.
The disease status of all patients was followed up for a minimum of 20 months (median follow‐up time since diagnosis, 24.0 months for OS and 23.1 months for PFS; data for Finland were not included in this analysis). Six months after the completion of chemotherapy, 71.9% (718 of 999), 26.2% (262 of 999), and 1.9% (19 of 999) of the patients were disease‐free, had progressive disease, and had died, respectively; after at least 20 months of follow‐up, 32.9% (329 of 999), 46.4% (464 of 999), and 20.6% (206 of 999) of the patients were disease‐free, had progressive disease, and had died, respectively (Figure 1).
FIGURE 1.
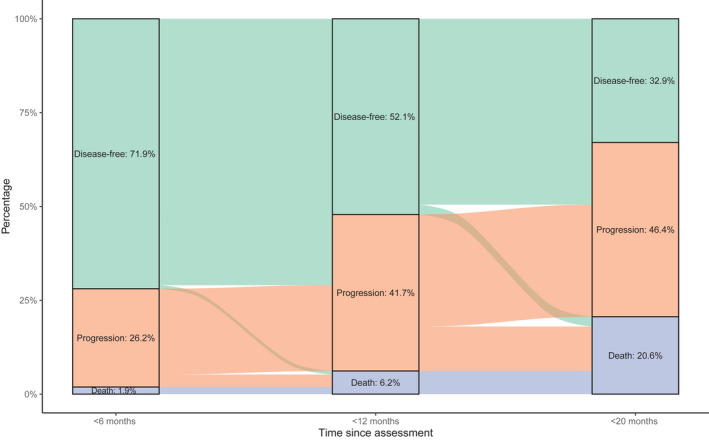
Disease status since the response assessment (N = 999). Data for Finland were not included in this analysis.
Bevacizumab had a significant positive effect on OS (univariate log‐rank analysis p < .01; adjusted Cox regression analysis hazard ratio [HR], 0.62; 95% CI, 0.42–0.91; p = .01); the effect of bevacizumab on PFS was not significant (Figure 2 and Table S1).
FIGURE 2.
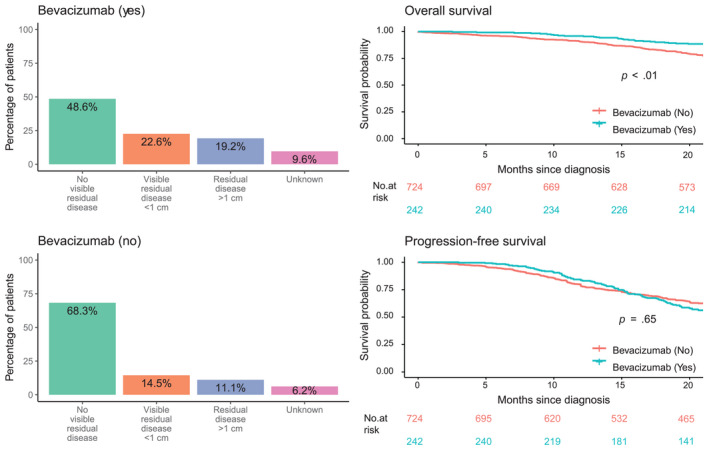
Residual disease status after surgery and effect of bevacizumab on overall survival and progression‐free survival. In the adjusted analysis, bevacizumab remained a significant factor for overall survival but not for progression‐free survival.
The presence of a BRCAm had a significant positive effect on PFS (univariate log‐rank analysis p < .01; HR, 0.60; 95% CI, 0.41–0.84; p < .01). Although the presence of a BRCAm had a significant effect on OS in the univariate log‐rank analysis (p = .015; Figure 3), it did not have a significant effect in the adjusted Cox regression analysis (Table S1).
FIGURE 3.
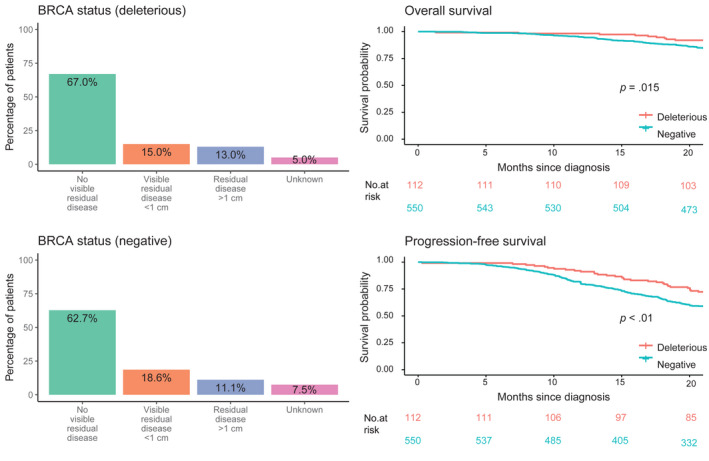
Prognostic importance of the BRCA status for visible residual disease, overall survival, and progression‐free survival in the subgroup of patients who underwent BRCA testing. In the adjusted analysis, the BRCA status (deleterious) remained a significant factor for progression‐free survival but not for overall survival.
4. DISCUSSION
This large observational study, conducted across eight countries, characterized the real‐world treatment patterns and outcomes of patients with newly diagnosed advanced high‐grade serous or endometrioid OC. The study included patients diagnosed no later than April 1, 2018 (a time when PARPi were not commercially available), in order to estimate the number of high‐risk patients who would be eligible for PARPi therapy.
The percentage of patients with a physician‐assessed clinical CR/PR following surgery (PDS or IDS) and first‐line chemotherapy was calculated as a surrogate for PARPi eligibility. As such, 79% of the patients in the current study would potentially have been eligible for PARPi maintenance therapy (with or without anti‐angiogenic therapy). However, it should be noted that this does not take into account biomarker status; access to PARPi can vary by country according to biomarker status because of national reimbursement policies despite approval by the European Medicines Agency. 8 Nevertheless, 2 years after their diagnosis and after receiving the SoC, only one third of the women (33%) were disease‐free, whereas 47% had progression, and 21% had died; this underlines the unmet need for better treatments in this setting.
In the European Union, PARPi approved for first‐line maintenance include olaparib (for patients with BRCAm and in combination with bevacizumab for homologous recombination deficiency [HRD]–positive AOC) and niraparib (regardless of the biomarker status or histological subtype). 14 , 15 , 16 Olaparib, niraparib, and rucaparib are also indicated as maintenance therapy in the platinum‐sensitive relapsed disease setting. 14 , 15 , 16 PARPi maintenance therapy has been shown to lower the disease progression risk after surgical resection and platinum‐based chemotherapy for women with newly diagnosed AOC, with the largest benefit observed in patients positive for HRD or a BRCAm, although significant benefits were observed regardless of the biomarker status. 10 , 11 , 12 , 13 An exploratory analysis of SOLO1 and PAOLA‐1 indicated that both ‘lower‐risk' patients (Stage III without residual disease after PDS) and ‘higher‐risk' patients (Stage IV, Stage III with residual disease after PDS, inoperable Stage III, or Stage III with IDS) benefited from first‐line PARPi (olaparib) maintenance. 17 , 18
In our study, complete cytoreduction was achieved in 56% of the patients who underwent PDS and in 64% who underwent IDS. These proportions are higher than those observed in a real‐world Danish study of 2092 women diagnosed with advanced epithelial OC (2005–2016) undergoing PDS, which showed complete cytoreduction in 47% and 38% of patients with Stage IIIB–IIIC and IV epithelial OC, respectively. 19 Our study also included nonoperated patients in the denominator for calculations of surgical outcomes, although the reasons for patients not receiving surgery were not recorded. Of our entire study population, only a small proportion (23%) could be considered to have a good prognosis with no VRD and PDS. The remaining patients (77%) had a poor prognosis (i.e., those with VRD, IDS, or no surgery) under the SoC.
The high rates of BRCA status testing observed were encouraging, although somatic testing rates were lower. The percentages of tested patients with a germline BRCAm and a somatic BRCAm were somewhat lower than those reported previously in real‐world and clinical trial settings (20%–25% for germline and somatic mutations combined). 20 , 21 , 22 Most of the patients with germline or somatic BRCA testing available were BRCA wild type, and for these patients, HRD testing is important for identifying those who would potentially have the largest benefit from PARPi maintenance.
The Kaplan–Meier survival analysis, controlled by BRCA status, indicated that patients with a BRCAm had longer PFS. 5 Differences in surgical outcomes were nonsignificant between patients with and without a BRCAm; this contradicts previous reports of the presence of a BRCAm being predictive of NED after PDS in patients with high‐grade serous OCs. 23
Bevacizumab had a significant positive effect on OS in our study. In the ICON7 trial, ‘high‐risk' patients with OC treated with bevacizumab maintenance therapy showed an OS treatment effect (HR, 0.78; 95% CI, 0.63–0.97) in comparison with ‘non–high‐risk' patients (HR, 1.14; 95% CI, 0.93–1.40). 24 The profile of the real‐world patients in the current study indicates that those with no VRD after surgery were less likely to receive bevacizumab, whereas ‘higher‐risk' patients were receiving and benefiting from bevacizumab maintenance therapy (Figure 2). The observed survival benefit induced by bevacizumab is even more important when we consider the lower risk profile of the patients. This is consistent with the survival benefit reported in the ICON7 trial, in which bevacizumab was used as the first line. 24 A multivariate Cox model that was adjusted for age, country, stage, assessment result, and BRCAm status was used in our analysis to minimize the selection bias.
4.1. Strengths and limitations
This study fills an important gap in the literature on clinical practice for patients newly diagnosed with advanced high‐grade serous or endometrioid OC. The main strength is the multinational setting: We used robust data from clinical records or registries to capture baseline characteristics, treatments, and clinical outcomes, and this reduced the risk of residual confounding and differential misclassification. A large sample of patients (N = 1119) was enrolled from a broad range of centers across eight countries, and this improved external validity. Adjustments for well‐known confounders limited the risk of a selection bias.
Although not necessarily generalizable to other regions, this study provides an important picture of the treatment landscape in Europe. An analysis by participating country is planned in the future. Differences in the diagnostic workup, definition of stage, and treatment protocols between the participating countries inevitably led to variations in treatments and outcomes. The target population was not reached in the Netherlands (not all the necessary approvals could be obtained within the available study time frame to reach the target population), and the Portuguese and Danish populations were larger than the target. Furthermore, the number of (excluded) patients who did not provide consent was not collected.
The retrospective study design is associated with several inherent limitations, and the results should be interpreted in the context of possible channeling bias and unmeasured confounding. Because of required informed consent in some countries, there was a risk of ascertainment bias. Patients who previously participated in blinded clinical trials were ineligible, and enrollment was not consecutive in some countries because of consent requirements. Potential unmeasured confounding included frailty, which may have been correlated with treatment and outcomes. The high proportion of patients with serous histology (97.5%) may also indicate a selection bias, but this was expected because of the rarity of high‐grade endometrioid OC. All data were retrieved from registries and/or medical records; therefore, not all parameters, including the BRCA and HRD status, were collected or available for all patients. Furthermore, imaging was not available for all patients, so in some cases, the response was assessed by investigators. Data were collected for the predefined patient observation period (from diagnosis to the last hospital visit 12 months after the completion of platinum‐based chemotherapy), and longer term follow‐up was not planned.
In conclusion, this large retrospective, observational study illustrates real‐world SoC treatment of newly diagnosed advanced high‐grade serous or endometrioid OC in eight countries representative of Western Europe. A significant proportion of the patients were potentially eligible for first‐line PARPi maintenance treatment, with 79% showing a clinical CR/PR after surgery (PDS or IDS) and first‐line chemotherapy. Although bevacizumab had a significant positive effect on OS and BRCAm had a significant positive effect on PFS, the overall outcomes after surgery and the disease status at the most recent assessment highlight the poor prognosis of this population and the urgent need for better treatments.
AUTHOR CONTRIBUTIONS
Christian Marth: Conceptualization, investigation, methodology, project administration, supervision, writing–original draft, and writing–review and editing. Miguel Henriques Abreu: Investigation, project administration, supervision, writing–original draft, and writing–review and editing. Klaus Kaae Andersen: Conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, validation, visualization, writing–original draft, and writing–review and editing. Karoliina M. Aro: Investigation, project administration, supervision, writing–original draft, and writing–review and editing. Maria de Lurdes Batarda: Investigation, project administration, supervision, writing–original draft, and writing–review and editing. Dorry Boll: Investigation, project administration, supervision, writing–original draft, and writing–review and editing. Anne Weng Ekmann‐Gade: Investigation, project administration, supervision, writing–original draft, and writing–review and editing. Ulla‐Maija Haltia: Investigation, project administration, supervision, writing–original draft, and writing–review and editing. Jesper Hansen: Conceptualization, investigation, methodology, project administration, resources, writing–original draft, and writing–review and editing. Ala Jabri Haug: Investigation, project administration, supervision, writing–original draft, and writing–review and editing. Claus Høgdall: Investigation, project administration, supervision, writing–original draft, and writing–review and editing. Jacob Korach: Investigation, project administration, supervision, writing–original draft, and writing–review and editing. Heini Lassus: Investigation, project administration, supervision, writing–original draft, and writing–review and editing. Kristina Lindemann: Investigation, project administration, supervision, writing–original draft, and writing–review and editing. Els Van Nieuwenhuysen: Investigation, project administration, supervision, writing–original draft, and writing–review and editing. Petronella B. Ottevanger: Investigation, project administration, supervision, writing–original draft, and writing–review and editing. Stephan Polterauer: Investigation, project administration, supervision, writing–original draft, and writing–review and editing. Tine Henrichsen Schnack: Investigation, project administration, supervision, writing–original draft, and writing–review and editing.
FUNDING INFORMATION
This study was funded by AstraZeneca and is part of an alliance between AstraZeneca and Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc (Kenilworth, New Jersey).
CONFLICT OF INTEREST
Christian Marth reports consulting fees from Roche, Novartis, Amgen, MSD, AstraZeneca, Pfizer, PharmaMar, Cerulean, Vertex, Tesaro, GSK, and Seagen; honoraria from Roche, Novartis, Amgen, MSD, AstraZeneca, PharmaMar, Tesaro, GSK, and Seagen; support for attending meetings from Roche and AstraZeneca; and participation on data safety monitoring or advisory boards for Roche, Novartis, Amgen, MSD, AstraZeneca, Pfizer, PharmaMar, Cerulean, Vertex, Tesaro, GSK, and Seagen. Klaus Kaae Andersen is an employee of and holds stock in AstraZeneca. Karoliina M. Aro reports payments or honoraria from Gedeon Richter. Maria de Lurdes Batarda reports support for attending meetings and/or travel from AstraZeneca. Anne Weng Ekmann‐Gade has received honoraria for consultation from AstraZeneca (this study). Ulla‐Maija Haltia has received honoraria for consultation from AstraZeneca (this study). Christian Marth has received honoraria for consultation from AstraZeneca (this study). Maria de Lurdes Batarda has received honoraria for consultation from AstraZeneca (this study). Jesper Hansen is an employee of AstraZeneca. Heini Lassus has received honoraria for consultation or lectures from AstraZeneca (this study), GSK, Eisai, and Roche. Kristina Lindemann reports consulting fees from AstraZeneca (paid to her institution); participation on boards for GSK, MSD, and Eisai; and leadership or fiduciary roles with the Nordic Society of Gynaecological Oncology and the Cancer Registry of Norway. Stephan Polterauer reports honoraria for consultation from AstraZeneca, Celgene, GSK, Eisai, MSD, PharmaMar, Roche, Roche Diagnostics, Meda Pharma, Tesaro, and Vifor Pharma; honoraria for lectures from AstraZeneca, Eisai, MSD, GSK, Tesaro, MedAhead, and KLI; and participation on advisory boards for AstraZeneca, Eisai, MSD, GSK, Tesaro, and PharmaMar. The other authors made no disclosures.
Supporting information
Supplementary Table and Figure
ACKNOWLEDGMENTS
We thank the patients who participated in this trial and their families. This study was funded by AstraZeneca and is part of an alliance between AstraZeneca and Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc (Kenilworth, New Jersey). Trude Ågesen (AstraZeneca) provided support for the study. Medical writing assistance, in the form of the preparation and revision of this article, was provided by C. L. Attwell, PhD, and B. Drever, PhD (AMICULUM Limited), and was funded by AstraZeneca, and is part of an alliance between AstraZeneca and Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc (Kenilworth, New Jersey).
DATA AVAILABILITY STATEMENT
The data underlying the findings described in this article may be obtained in accordance with AstraZeneca's data sharing policy, which is described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
REFERENCES
- 1. Reid BM, Permuth JB, Sellers TA. Epidemiology of ovarian cancer: a review. Cancer Biol Med. 2017;14:9–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Estimates of cancer incidence and mortality in 2020, for all cancer sites . European Cancer Information System. https://ecis.jrc.ec.europa.eu/index.php. Accessed July 6, 2021.
- 3.European Institute of Women's Health. Ovarian cancer: a silent killer. Eurohealth. https://eurohealth.ie/policy‐brief‐women‐and‐ovarian‐cancer‐in‐the‐eu‐2018/. Accessed July 6, 2021.
- 4. Colombo N, Sessa C, du Bois A, et al. ESMO‐ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann Oncol. 2019;30:672–705. [DOI] [PubMed] [Google Scholar]
- 5. Madariaga A, Lheureux S, Oza AM. Tailoring ovarian cancer treatment: implications of BRCA1/2 mutations. Cancers (Basel). 2019;11:416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tewari KS, Burger RA, Enserro D, et al. Final overall survival of a randomized trial of bevacizumab for primary treatment of ovarian cancer. J Clin Oncol. 2019;37:2317–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Perren TJ, Swart AM, Pfisterer J, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484–2496. [DOI] [PubMed] [Google Scholar]
- 8. Mirza MR, Coleman RL, González‐Martín A, et al. The forefront of ovarian cancer therapy: update on PARP inhibitors. Ann Oncol. 2020;31:1148–1159. [DOI] [PubMed] [Google Scholar]
- 9. Banerjee S, Moore KN, Colombo N, et al. Maintenance olaparib for patients (pts) with newly diagnosed, advanced ovarian cancer (OC) and a BRCA mutation (BRCAm): 5‐year (y) follow‐up (f/u) from SOLO1. Ann Oncol. 2020;31(suppl 4):S613. [Google Scholar]
- 10. Gonzalez‐Martin A, Pothuri B, Vergote I, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381:2391–2402. [DOI] [PubMed] [Google Scholar]
- 11. Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495–2505. [DOI] [PubMed] [Google Scholar]
- 12. Coleman RL, Fleming GF, Brady MF, et al. Veliparib with first‐line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019;381:2403–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ray‐Coquard I, Pautier P, Pignata S, et al. Olaparib plus bevacizumab as first‐line maintenance in ovarian cancer. N Engl J Med. 2019;381:2416–2428. [DOI] [PubMed] [Google Scholar]
- 14.Lynparza: summary of product characteristics. European Medicines Agency. https://www.ema.europa.eu/en/documents/product‐information/lynparza‐epar‐product‐information_en.pdf. Accessed July 6, 2021.
- 15.Zejula: summary of product characteristics. European Medicines Agency. https://www.ema.europa.eu/en/documents/product‐information/zejula‐epar‐product‐information_en.pdf. Accessed July 6, 2021.
- 16.Rubraca: summary of product characteristics. European Medicines Agency. https://www.ema.europa.eu/en/documents/product‐information/rubraca‐epar‐product‐information_en.pdf. Accessed July 6, 2021.
- 17. Colombo N, Bradley W, Moore K, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer and a BRCA mutation: subgroup analysis by risk in the phase III SOLO1 study. Int J Gynecol Cancer. 2020;30(suppl 4):A76–A77. [Google Scholar]
- 18. Harter P, Petran D, Scambia G, et al. Efficacy of maintenance olaparib plus bevacizumab by biomarker status in clinical higher‐ and lower‐risk patients with newly diagnosed, advanced ovarian cancer in the PAOLA‐1 trial. Int J Gynecol Cancer. 2020;30(suppl 3):A13–A14. [Google Scholar]
- 19. Sørensen SM, Schnack TH, Høgdall C. Impact of residual disease on overall survival in women with Federation of Gynecology and Obstetrics Stage IIIB‐IIIC vs Stage IV epithelial ovarian cancer after primary surgery. Acta Obstet Gynecol Scand. 2019;98:34–43. [DOI] [PubMed] [Google Scholar]
- 20. Hennessy BT, Timms KM, Carey MS, et al. Somatic mutations in BRCA1 and BRCA2 could expand the number of patients that benefit from poly (ADP ribose) polymerase inhibitors in ovarian cancer. J Clin Oncol. 2010;28:3570–3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Konstantinopoulos PA, Ceccaldi R, Shapiro GI, D'Andrea AD. Homologous recombination deficiency: exploiting the fundamental vulnerability of ovarian cancer. Cancer Discov. 2015;5:1137–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ledermann JA, Drew Y, Kristeleit RS. Homologous recombination deficiency and ovarian cancer. Eur J Cancer. 2016;60:49–58. [DOI] [PubMed] [Google Scholar]
- 23. Kim RS, Malcolmson J, Li X, Bernardini M, Hogen LF, May T. The correlation between BRCA status and surgical cytoreduction in high‐grade serous ovarian carcinoma. J Clin Oncol. 2021;39(15)(suppl):5543. [DOI] [PubMed] [Google Scholar]
- 24. Oza AM, Cook AD, Pfisterer J, et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a phase 3 randomised trial. Lancet Oncol. 2015;16:928–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table and Figure
Data Availability Statement
The data underlying the findings described in this article may be obtained in accordance with AstraZeneca's data sharing policy, which is described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.


