Abstract
Background
Calcium plays an essential role in physiologic processes, including trauma's “Lethal Diamond.” Thus, inadequate serum calcium in trauma patients exacerbates the effects of hemorrhagic shock secondary to traumatic injury and subsequently poorer outcomes compared to those with adequate calcium levels. Evidence to date supports the consideration of calcium derangements when assessing the risk of mortality and the need for blood product transfusion in trauma patients. This review aims to further elucidate the predictive strength of this association for future treatment guidelines and clinical trials.
Methods
Publications were collected on the relationship between i‐Ca and the outcomes of traumatic injuries from PubMed, Web of Science, and CINAHL. Manuscripts were reviewed to select for English language studies. Hypocalcemia was defined as i‐Ca <1.2 mmol/L.
Results
Using PRISMA guidelines, we reviewed 300 studies, 7 of which met our inclusion criteria. Five papers showed an association between hypocalcemia and mortality.
Conclusions
In adult trauma patients, there has been an association seen between hypocalcemia, mortality, and the need for increased blood product transfusions. It is possible we are now seeing an association between low calcium levels prior to blood product administration and an increased risk for mortality and need for transfusion. Hypocalcemia may serve as a biomarker to show these needs. Therefore, hypocalcemia could potentially be used as an independent predictor for multiple transfusions such that ionized calcium measurements could be used predictively, allowing faster administration of blood products.
Keywords: hypocalcemia, military, mortality, transfusion, trauma
1. INTRODUCTION
Calcium plays an essential role in physiologic processes; calcium derangement is linked to complications of severe trauma, including hypothermia, coagulopathy, and acidosis. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 Inadequate serum calcium in trauma patients is associated with the exacerbation of hemorrhagic shock secondary to traumatic injury and subsequently poorer outcomes than those with adequate calcium levels. Hemorrhagic shock continues to be a significant cause of death after traumatic injury in both military and civilian settings. 9 , 10 Furthermore, studies have shown that seriously injured trauma patients commonly present with calcium derangements, specifically hypocalcemia. Calcium‐dependent pathways are key in vasomotor tone, platelet function, intrinsic and extrinsic pathway‐mediated coagulation, and therefore play a crucial role in hemorrhagic shock and resuscitation (Table 1). 5 , 11 , 12 Both trauma and transfusion procedures lead to worsening hypocalcemia. 3 The role of hypocalcemia in trauma patients has been a recent area of study, with goals to optimize resuscitation and understand the link between calcium derangements, risk of death, and need for transfusion. Increased focus on the measurement of ionized calcium in trauma patients has shown a strong association between hypocalcemia and negative outcomes, with Ditzel et al. arguing that hypocalcemia should be included in the Lethal Triangle of hypothermia, coagulopathy, and acidosis expanding it into a Lethal Diamond. 13 , 14 Calcium administration early on in resuscitation efforts are generally supported, there is disagreement about the best calcium‐replacement protocol. This is largely due to a lack of clinical research directly comparing the efficacy of calcium replacement strategies during hemostatic resuscitation.
TABLE 1.
Roles of ionized calcium throughout the body and how hypocalcemia can affect resuscitation
| Organ system | Calcium's role | Likely effect on resuscitation |
|---|---|---|
| Musculoskeletal | Calcium triggers skeletal muscle contraction by reaction with regulatory proteins that in the absence of calcium prevent the interaction of Actin and myosin. https://doi.org/10.1016/S0006‐3495(75)85849‐8 | Decreased respiratory effort leading to hypoxia and hypercarbia |
| Calcium initiates smooth muscle contraction by binding to calmodulin and activating the enzyme myosin light chain kinase. Calcium may also enhance smooth muscle contractile activity by binding directly to myosin, the main component of the thick filament. https://doi.org/10.1016/0002‐9149(87)90076‐2 | Decreased vascular tone leading to hypotension, impaired tissue perfusion, and worsened shock. | |
| Calcium binds to troponin resulting in sliding of the thick and thin filaments, cell shortening, and thence the development of pressure within the ventricle and ejection of blood. https://doi.org/10.1161/CIRCRESAHA.117.310230 | Decreased cardiac output leading to impaired tissue perfusion, and worsened shock. | |
| Neurologic | Upon entering a presynaptic terminal, an action potential opens calcium channels and transiently increases the local calcium concentration at the presynaptic active zone. Calcium then triggers neurotransmitter release within a few hundred microseconds by activating synaptotagmins calcium. https://doi.org/10.1101/cshperspect.a011353 | Decreased release of catecholamines leading to decreased cardiac output and hypotension. |
| Calcium is involved in synaptic signaling processes, neuronal energy metabolism, and neurotransmission. https://doi.org/10.1007/s00018‐013‐1550‐7 | Increased chance of brain injury. | |
| Hematologic | Calcium ions play a major role in the tight regulation of the coagulation cascade which is paramount in the maintenance of hemostasis. Other than platelet activation, calcium ions are responsible for the complete activation of several coagulation factors, including coagulation Factor XIII (FXIII). https://doi.org/10.1038/s41598‐019‐47815‐z | Decreased ability to activate platelets and fibrin clots, leading to increased blood loss. |
| Clotting factor IV is a calcium ion that plays an important role in the intrinsic, extrinsic, and common pathways. www.ncbi.nlm.nih.gov/books/NBK507850 |
It is important to understand hypocalcemia and its potential role of confounding by indication in the context of trauma and hemostatic resuscitation. 15 It is unclear if hypocalcemia itself is a result of trauma and blood transfusions or an early indicator of poor outcomes and resuscitative needs.
The objective of this scoping review is to evaluate the existing data concerning hypocalcemia, mortality, and the need for transfusion in adult trauma patients to determine if sufficient evidence is available to conclude the demonstrated association between hypocalcemia, morbidity, and mortality as a predictive measure to inform researchers and clinicians for future use in clinical trials and treatment guidelines.
2. METHODS
This paper is a scoping review that followed the PRISMA‐ScR (Preferred Reporting Items for Systematic Review and Meta‐Analyses extension for Scoping Reviews) guidelines to display the number of papers used in this review (Figure 1). PubMed, Web of Science, and CINAHL databases were queried for all available full‐text articles that addressed hypocalcemia and its associations with mortality or blood transfusions in the adult trauma patient. Search terms included (hypocalcemia or low serum calcium) AND (trauma) AND (mortality OR death). Three hundred records were identified using the search criteria (Figure 1) and an additional two were known by SK, which were added to the screening process. Exclusion criteria included patients diagnosed with a chronic illness or significant comorbidities, associated mortality through multiple variable factors without isolating for ionized calcium, and a mean population age greater than 65. The search was conducted by NR and duplicated by SK. Initial articles were screened through title and abstract review for inclusion by authors SK and NR. Selected articles for full‐text review were screened by NR and SK independently. Authors SK, NR, and JE conducted further qualitative data extraction. JS was available as an independent decision‐maker had any disagreement occurred.
FIGURE 1.
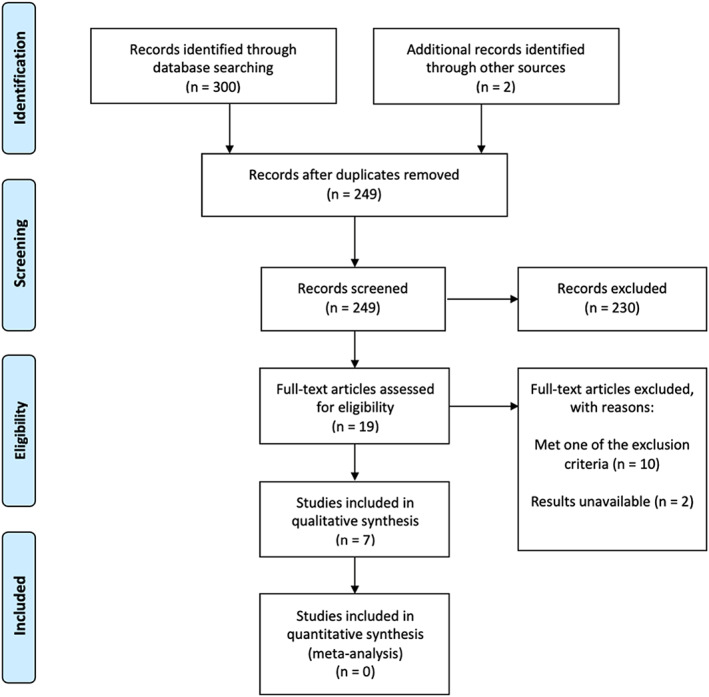
PRISMA flow diagram. Legend: The PRISMA diagram details the search and selection process applied during the scoping review
We assessed papers to show whether hypocalcemia was associated with increased mortality as well as the need for increased blood products. Additionally, it was assessed whether or not patients received blood transfusions or products prehospital to elicit citrate as a confounding variable (Table 2).
TABLE 2.
Prehospital blood transfusion status
| Authors | Prehospital blood transfusion |
|---|---|
| Cherry et al. | No |
| Choi and Hwang | No |
| Magnotti et al. | No |
| Giancarelli et al. | No |
| Vasudeva et al. | No |
| Connor et al. | 37.60% |
| Chanthima et al. | Yes |
3. RESULTS
Of the seven articles compiled, five were retrospective, one was prospective, and one was a combined prospective and retrospective study (Table 3). Of the five retrospective studies, one derived data from the Penn State Shock Trauma Center and the Departments of Surgery, Medicine, and Health Evaluation Sciences, Penn State Milton S. Hershey Medical Center, one from the Orlando Regional Medical Center, one from the Harborview Injury Prevention and Research Center, Seattle, Washington, one from the Alfred Trauma Registry and the Alfred Applications and Knowledge Management Department, Melbourne, Australia, and one from the Association of Military Surgeons. The one prospective study derived data from all trauma activations during 9 months in the Department of Surgery, University of Tennessee Health Science Center. The combined prospective and retrospective article derived data from the Departments of Surgery and Emergency Medicine, Masan Samsung Hospital, Sungkyunkwan University School of Medicine, Masan, Korea. Therefore, there are likely zero overlaps of patient data between studies used in this scoping review.
TABLE 3.
Data collection type
| Authors | Year published | Sample size (n) | Data collection | Level of evidence |
|---|---|---|---|---|
| Cherry et al. | 2006 | 396 | Retrospective observational | Level 2b |
| Choi and Hwang | 2008 | 255 | Retrospective and prospective observational | Level 2b |
| Magnotti et al. | 2011 | 591 | Prospective observational | Level 1b |
| Giancarelli et al. | 2016 | 156 | Retrospective observational | Level 2b |
| Vasudeva et al. | 2020 | 226 | Retrospective observational | Level 2b |
| Connor et al. | 2021 | 101 | Retrospective observational | Level 2b |
| Chanthima et al. | 2021 | 346 | Retrospective observational | Level 2b |
In 2006, the Penn State Shock Trauma Center and the Departments of Surgery, Medicine, and Health Evaluation Sciences at Penn State Milton S. Hershey Medical Center conducted a retrospective cohort study on the relationship between ionized calcium (iCa) levels drawn upon arrival to the emergency department, with injury severity, acidosis, hypotension, and mortality. No patients received blood products prior to arrival at the Emergency Department and there was no change in the timing of the administration of blood products based upon iCa levels. Three hundred ninety‐six Level I trauma patients were analyzed, and it was noted in the study that mortality significantly increased in the iCa ≤1 mM/L compared to the iCa >1 mM/L group (26.4% vs. 16.7%, p < .05; OR 1.92). Of the patients who died, those with iCa levels >1 lived longer than those with iCa levels ≤1. The examiners predicted mortality using iCa ≤1 alone (p < .02, OR 3.28), iCa ≤1 + base deficit (p < .02, OR 2.00), and base deficit alone (p = .06, OR 1.5). Low iCa levels were found to be associated with prehospital hypotension and shown to be a better predictor of mortality than base deficit. Lastly, the study noted that additional research is recommended to highlight further the effect of acidosis on the relationship between base deficit and iCa. 16
The Departments of Surgery and Emergency Medicine, Masan Samsung Hospital, Sungkyunkwan University School of Medicine, Masan, Korea, published a combined prospective and retrospective study examining the correlation between iCa levels in trauma patients and mortality risk. The study also evaluated mortality risk against three other triage tools ‐ base deficit, systemic inflammatory response syndrome (SIRS) score, and triage‐revised trauma score (t‐RTS). Two hundred fifty‐five trauma patients who underwent arterial blood gas analysis (ABGA) of iCa levels within 10 min of arrival were examined to determine the best predictor of mortality. Serum iCa, pH, PaO2, PaCO2, bicarbonate, base deficit, and SaO2 were all measured automatically by the ABGA. The mean age of the cohort was 47.2 ± 16 years with a range of 16–90 years old ‐ males made up most of the study at 76.5% (195 patients). Univariate analysis with a Student's t‐test or chi‐square test showed a statistical significance in mortality risk that was correlated with all of the following metrics ‐ iCa, Glasgow coma scale score, serum bicarbonate, base deficit, SaO2, initial systolic blood pressure, pulse rate, body temperature, SIRS, t‐RTS, emergency operation, transfusion amount, injury severity score, and revised trauma score. The receiver operating characteristic (ROC) curve analysis and the recognized cut‐off point for normal conditions were used to categorize iCa levels as follows – normal iCa concentration (≥ 1.15 mM/L), mild ionized hypocalcemia (0.89–1.14 mM/L), and severe ionized hypocalcemia (≤ 0.88 mM/L). Multivariate logistic regression analysis confirmed that patients with iCa ≤0.88 had a significant increase in risk for mortality (30.2% vs. 14.3%; p = .003). Although iCa ROC curve analysis levels correlated with mortality risk, displaying a high sensitivity and accuracy, the t‐RTS proved to be a better predictor of specific patient mortality. Still, the study highlights the strong correlation of iCa to mortality risk. It suggests that this metric could be used in the future, with additional factors, to predict mortality better – even against the t‐RTS test metric. 17
In 2015 the Departments of Pharmacy and Surgical Education, Orlando Regional Medical Center published a retrospective study that analyzed the incidence of hypocalcemia and severe hypocalcemia in trauma patients who received a massive transfusion protocol (MTP) and compared characteristics of patients with severe versus non‐severe hypocalcemia. MTP was activated by the presence of a systolic blood pressure ≤90 mm Hg, heart rate ≥120 bpm, positively focused sonography for trauma examination, or pH ≤7.24, and if four or more units of PRBCs were transfused over 1 h or if over 10 units were expected to be transfused within 24 h; there was no mention of timing to transfusion. Measurements of iCa were collected within 24 h after MTP discontinuation. One hundred fifty‐six patients were examined in the study and split into two groups – iCa <0.90 mmol/L (n = 111) and iCa ≥0.90 mmol/L (n = 45). Mortality was higher in the iCa <0.90 mmol/L group ‐ 49% death rate versus 24% in the iCa ≥0.90 group (p = .007). In addition, patients in the iCa <0.90 mmol/L group received more blood products (34 [23–58] vs. 22 [18–30] units, p < .001) and calcium chloride (4 [2–7] vs. 3 [1–4] g, p = .002), possibly showing a bias by indication in this study. There was no difference in the duration (hours) of the MTP or final iCa levels. Based on a ROC analysis, the study concluded that the higher the number of blood products used during an MTP correlated with an increase in severe hypocalcemia, likely secondary to the massive transfusion. Overall, this study found that hypocalcemia was common in patients who received a massive transfusion and iCa levels are important in determining the mortality risk. Lastly, it concluded that more research is required to manage hypocalcemia throughout massive transfusions effectively. 18
The Department of Surgery from the University of Tennessee conducted a prospective study on 591 trauma patients over 9 months to determine if iCa levels upon admission impacted the number of transfusions required during treatment. Patients were not eligible to be included in the study if they received any blood product transfusion prehospital. The patients were split into two groups ‐ iCa <1.00 (lo‐Cal group ‐ 332 patients) and iCa ≥1.00 (hi‐Cal group ‐ 259 patients). Mortality (15.5% vs. 8.7%, p = .036) and the need for multiple transfusions (defined as transfusion of 5 units of PRBCs in 24 h; 17.1% vs. 7.1%, p = .005) increased in the lo‐Cal group, along with a fourfold increase in the need for massive transfusion (defined as transfusion of 10 units PRBCs in 24 h; 8.2% vs. 2.2%, p = .017). Additionally, in a multivariable logistic regression analysis, this study identified iCa <1.00 as an independent predictor of the need for multiple transfusions after adjusting for age and injury severity (odds ratio = 2.294, 95% confidence interval = 1.053–4.996). The admission iCa levels proved to be a key marker when determining patient mortality risk and blood transfusion requirements, and in conclusion, should be a leading indicator when preparing to treat trauma patients. 19
A 2021 retrospective cohort study completed by the Harborview Injury Prevention and Research Center in Seattle, Washington, examined the effects of transfused citrated blood products on resuscitation‐induced hypocalcemia and trauma outcomes. Three hundred forty‐six patients were analyzed – 288 (83.2%) had hypocalcemia at first iCa determination, 296 (85.6%) had hypocalcemia in the last determination in the first 3 h of hospital care, and 177 (51.2%) received at least one calcium replacement dose during that time. The study found that neither first iCa determination nor the administered replacement calcium dose corrected for citrate load and was not effective at predicting mortality. 20
A 2019 study by the National Trauma Research Institute in Melbourne, Australia, utilized the Alfred Trauma Registry and the Alfred Applications and Knowledge Management Department to evaluate the correlation between admission hypocalcemia and adverse outcomes. Patients were excluded from the study if they received a blood transfusion prehospital or had a shock index <1 (which assumes there were no occult derangements), excluding 3045 patients from the study. Ultimately, 226 patients were analyzed in the study ‐ 113 (50%; 95% CI: 43.54–56.46%) had an admission iCa of whom six (2.6%; 95% CI: 1.22–5.66%) patients had severe hypocalcemia. Hypocalcemia was significantly associated with an increased need for blood transfusions (p < .001), coagulopathy upon arrival (OR 5.5, 95% CI 2.8–10.8, p < .001), and death upon hospital discharge (p = .047). Although hypocalcemia was an accurate indicator for patient outcomes (25.6% vs. 15.0%; p = .047), the study noted that further research is warranted on calcium administration for trauma patients in hemorrhagic shock. 21
In 2021 the Association of Military Surgeons published a retrospective study on military casualties at different locations in Afghanistan from August 2018 to February 2019, examining both initial admission calcium levels in trauma patients, and separately the effects of prehospital treatment with blood product administration and its relationship with admission calcium levels. One hundred one patients were analyzed, and of this cohort, 55 (54.5%) had hypocalcemia (iCa <1.20 mmol/L) on arrival to the Forward Surgical Team (FST) with a mean iCa of 1.16 mmol/L (95% CI 1.14 to 1.18). In addition, 33/38 (86.8%) of the patients requiring blood product transfusion after arrival to the FST were hypocalcemic on admission. The mean iCa levels of patients requiring blood transfusion were significantly lower than those who did not require transfusion (1.13 mmol/L [95% CI 1.08–1.18] vs. 1.19 mmol/L [95% CI 1.17–1.20]; p = .01). Patients with hypocalcemia (iCa <1.2) who required a blood transfusion made up 65.7% (25/38) of the cohort, while patients who had an iCa >1.2 made up 34.2% (13/38) of the patients requiring a transfusion. 71% (27/38) of patients received at least four units of blood products containing citrate, and 18.4% (7/38) required an MTP. The study found that hypocalcemia was prevalent in military casualties, and early administration of calcium during resuscitation may benefit patient outcomes, although further research was recommended to confirm the results. 22
4. DISCUSSION
Hypocalcemia in trauma remains a relevant clinical and research topic of discussion today. With the availability of pre‐hospital and in‐hospital laboratory technologies, iCa levels are a simple biomarker to obtain for the military and civilian population that could potentially serve as a substantial predictive value for mortality. The seven papers included in this study found that iCa was an independent predictor for mortality (Table 4); while iCa alone was an independent predictor for mortality, secondary findings showed iCa with another biomarker such as hypotension or iCa with the base deficit was more predictive of mortality than base deficit alone. Furthermore, it was found in the Choi study that transfusion was also a significant predictor of mortality. It is possible that the associations described here between mortality and transfusion could be a result of indication and spectrum bias.
TABLE 4.
Hypocalcemia and associated mortality versus non‐hypocalcemia groups
| Authors | Outcome (%) | Odds ratio (confidence intervals) | p value |
|---|---|---|---|
| Cherry et al. | 26.4 versus 16.7 (iCa < 1.0 mmol/L) | 1.92 (1.1–3.5) | <.05 |
| Choi and Hwang | 30.2 versus 14.3 (iCa <0.88 mmol/L) | 3.10 (1.11–8.62) | .003 |
| Magnotti et al. | 15.5 versus 8.7 (iCa <1.0 mmol/L) | [not provided] | .036 |
| Giancarelli et al. | 49 versus 24 (iCa <0.09 mmol/L) | 2.93 (1.35–6.36) | .007 |
| Vasudeva et al. | 25.6 versus 15.0 (iCa <1.11 mmol/L) | 1.95 (1–1.38) | .047 |
Multiple papers showed an association between iCa and blood product requirements (Table 5). Since blood transfusions are the standard treatment for trauma patients, it is crucial to identify associations between hypocalcemia and transfusion. 17 A recent study conducted by Conner et al. showed that 54.5% of military casualties with hemorrhagic shock (n = 110) in Iraq who arrived at their FST from August 2018 to February 2019 were hypocalcemic (n = 55). Additionally, they found that 87% of the patients who required blood transfusion were hypocalcemic upon arrival at the FST. Of the group that received blood products prior to arrival to the FST or during aeromedical evacuation to the FST, 75% of them were hypocalcemic upon arrival (8/101 received products and 6/8 were hypocalcemic). Conversely, of those who did not receive blood products until they arrived at the FST (38/101), 71.1% of these patients required at least four units of citrated blood, while only 18.4% (7/38) required MTP. Out of this in‐FST treatment group, 33 of the 338 casualties were hypocalcemic on arrival or became hypocalcemic and 24/38 received calcium supplementation. 22 This also likely represents indication, selection, and spectrum bias, as all the patients requiring MTP and those requiring pre‐arrival blood had hypocalcemia. Even so, these findings are significant because it has been often thought that hypocalcemia in this patient population is solely due to calcium chelation from the addition of the anticoagulant citrate into blood and blood products (3 g citrate per CPD and 1.66 g per CPDA‐1 bag). However, this does not fully define whether there is a dose‐dependent relationship between total blood loss and calcium levels, but it does provide insight that hypocalcemia in hemorrhagic shock could be caused by a variable other than massive transfusion. Since massive transfusion is a typical predictive value for morbidity and mortality, hypocalcemia may be a confounding variable that has yet to be effectively studied. Correcting hypocalcemia may decrease the overall amount of blood and blood products needed during transfusion, albeit resuscitation needs still need to be corrected per immediate versus ongoing requirements according to Starling curve principles; perhaps calcium may provide at least an effective and synergistic resuscitation effect when using proper ratios, and assisting with vasomotor tone. This theory is possible due to calcium's role throughout the clotting cascade and its effects on cardiac output and smooth muscle mechanics. 22
TABLE 5.
Hypocalcemia and transfusion‐related data versus non‐hypocalcemia groups
| Authors | Variable | Difference (%) | Odds ratio (confidence intervals) | p value |
|---|---|---|---|---|
| Magnotti et al. | Need for multiple transfusions | 17.1 versus 7.1 | 2.294(1.053–4.996) | .005 |
| Giancarelli et al. | Units of blood received during massive transfusion protocol | 34 versus 22 | 2.93(1.35–6.36) | <.001 |
| Vasudeva et al. | Patients requring transfusion in first 24 h post admission | 62.5 versus 37.5 | 1.95(1–1.38) | <.001 |
| Conner et al. | Patients requiring transfusion | 86.8 versus 13.2 | [not provided] | [not provided] |
Vasudeva and colleagues supported the hypocalcemia and transfusion hypothesis in 2020 when they published their results from a retrospective review they conducted from 1 July 2014 to 30 June 2018. The authors found that admission ionized hypocalcemia was associated with both the need for blood transfusion within the first 24 h (62.5%, p < .001) and death at hospital discharge (25.6%, p < .047). This is further supported by Magnotti et al., who found that patients with iCa <1.0 was an independent predictor for multiple transfusions (OR = 2.294, 95% CI = 1.053–4.996). However, while the results of this review and the findings of these two studies show that hypocalcemia is associated with mortality prediction (either directly or through the need for more transfusions), they do not determine if the correction of the derangement will decrease the mortality in these populations. 19 , 21
Moreover, the data does not suggest an evidence‐based protocol to correct hypocalcemia, likely due to the current gap in the literature, which warrants more prospective and controlled research in this area. Confounding by indication 15 could also be a factor insofar as hypocalcemia could be masking the actual factor/s that lead to poor outcomes in trauma. The current military Joint Trauma Systems (JTS) Damage Control Clinical Practice guideline (CPG) indicates that hemorrhagic shock patients can receive 1 g of calcium chloride or 3 g of calcium gluconate before or concurrently with the first unit of blood and then repeated after every fourth unit is administered.24 The protocol also includes that calcium should be given if the iCa is <1.2 mmol/L, but does not suggest a stopping point. This is concerning for this population because the data on hypercalcemia in trauma as a predictive value for mortality is even less available than the data for hypocalcemia. These findings suggest that while hypocalcemia is a crucial value to monitor and study, the limitations in the data propose a high level of risk, and further investigation should be conducted.
5. LIMITATIONS
The available data on hypocalcemia as a predictive factor for mortality is low (n = 7). During this study's review process, only one paper was prospective, while the other six were retrospective. The lack of prospective data is a significant concern for the authors of this paper as the data cannot show causation. Because we did not intend to perform a meta‐analysis, we did not systematically assess for bias, level of evidence, or perform a pooled analysis.
6. CONCLUSION
This review found that the current data concerning hypocalcemia, mortality, and transfusion need is limited (n = 7). While the data shows the correlation between hypocalcemia in mortality, only one prospective study was available, which does not provide enough evidence to support causation. The research showed that hypocalcemia was an independent predictor for mortality, albeit due to the limited nature of this data, the researchers suggest that a multi‐center prospective study should be conducted to investigate if there is any causal relationship between hypocalcemia and mortality. The relationship between massive (or any transfusions) and hypocalcemia, as well as the hemodynamic endpoints for blood product requirements and hypocalcemia, need to be clinically investigated.
CONFLICT OF INTEREST
The authors have no conflicts to declare.
ACKNOWLEDGMENT
The authors have no financial conflicts of interest, and no funding or grants were received.
Kronstedt S, Roberts N, Ditzel R, Elder J, Steen A, Thompson K, et al. Hypocalcemia as a predictor of mortality and transfusion. A scoping review of hypocalcemia in trauma and hemostatic resuscitation. Transfusion. 2022;62(S1):S158–S166. 10.1111/trf.16965
REFERENCES
- 1. De Robertis E, Kozek‐Langenecker S, Tufano R, Romano G, Piazza O, Zito MG. Coagulopathy induced by acidosis, hypothermia and hypocalcaemia in severe bleeding. Minerva Anestesiol. 2015;81(1):65–75. [PubMed] [Google Scholar]
- 2. Ho K, Leonard A. Concentration‐dependent effect of hypocalcaemia on mortality of patients with critical bleeding requiring massive transfusion: a cohort study. Anaesth Intensive Care. 2011;39(1):46–54. [DOI] [PubMed] [Google Scholar]
- 3. Ho K, Yip C. Concentration‐dependent effect of hypocalcaemia on in vitro clot strength in patients at risk of bleeding: a retrospective cohort study. Transfus Med. 2016;26(1):57–62. [DOI] [PubMed] [Google Scholar]
- 4. James MF, Roche AM. Dose‐response relationship between plasma ionized calcium concentration and thrombelastography. J Cardiothorac Vasc Anesth. 2004;18(5):581–6. 10.1053/j.jvca.2004.07.016 [DOI] [PubMed] [Google Scholar]
- 5. Lier H, Krep H, Schroeder S, Stuber F. Preconditions of hemostasis in trauma: a review. The influence of acidosis, hypocalcemia, anemia, and hypothermia on functional hemostasis in trauma. J Trauma. 2008;65(4):951–60. 10.1097/TA.0b013e318187e15b [DOI] [PubMed] [Google Scholar]
- 6. Maxwell MJ, Wilson MJ. Complications of blood transfusion. Contin Educ Anaes Critic Care Pain. 2006;6(6):225–9. [Google Scholar]
- 7. Vivien B, Langeron O, Morell E, Devilliers C, Carli PA, Coriat P, et al. Early hypocalcemia in severe trauma. Crit Care Med. 2005;33(9):1946–52. [DOI] [PubMed] [Google Scholar]
- 8. Wray JP, Bridwell RE, Schauer SG, Shackelford SA, Bebarta VS, Wright FL, et al. The diamond of death: hypocalcemia in trauma and resuscitation. Am J Emerg Med. 2021;41:104–9. 10.1016/j.ajem.2020.12.065 [DOI] [PubMed] [Google Scholar]
- 9. Gurney JM, Spinella PC. Blood transfusion management in the severely bleeding military patient. Curr Opin Anaesthesiol. 2018;31(2):207–14. [DOI] [PubMed] [Google Scholar]
- 10. Centers for Disease Control and Prevention , National Center for Injury Prevention and Control . Web‐based Injury Statistics Query and Reporting System (WISQARS) [online]. (2005) [cited Year Month (abbreviated) Day]. Available from URL: www.cdc.gov/injury/wisqars
- 11. Desai TK, Carlson RW, Thill‐Baharozian M, Geheb MA. A direct relationship between ionized calcium and arterial pressure among patients in an intensive care unit. Crit Care Med. 1988;16(6):578–82. [DOI] [PubMed] [Google Scholar]
- 12. Rutmann T. Coagulation for the clinician. S Afr J Surg. 2006;44(1):22–37. [PubMed] [Google Scholar]
- 13. Ditzel RM Jr, Anderson JL, Eisenhart WJ, Rankin CJ, DeFeo DR, Oak S, et al. A review of transfusion‐and trauma‐induced hypocalcemia: is it time to change the lethal triad to the lethal diamond? J Trauma Acute Care Surg. 2020;88(3):434–9. [DOI] [PubMed] [Google Scholar]
- 14. MacKay EJ, Stubna MD, Holena DN, Reily PM, Seamon MJ, Smith BP, et al. Abnormal calcium levels during trauma resuscitation are associated with increased mortality, increased blood product use, and greater hospital resource consumption: a pilot investigation. Anesth Analgesia. 2017;125(3):895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Middelburg RA, van de Watering LM, van der Bom JG. Blood transfusions: good or bad? Confounding by indication, an underestimated problem in clinical transfusion research. Transfusion. 2010;50(6):1181–3. 10.1111/j.1537-2995.2010.02675.x [DOI] [PubMed] [Google Scholar]
- 16. Cherry RA, Bradburn E, Carney DE, Shaffer ML, Gabbay RA, Cooney RN. Do early ionized calcium levels really matter in trauma patients? J Trauma Acute Care Surg. 2006;61(4):774–9. [DOI] [PubMed] [Google Scholar]
- 17. Choi YC, Hwang SY. The value of initial ionized calcium as a predictor of mortality and triage tool in adult trauma patients. J Korean Med Sci. 2008;23(4):700–5. 10.3346/jkms.2008.23.4.700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Giancarelli A, Birrer KL, Alban RF, Hobbs BP, Liu‐DeRyke X. Hypocalcemia in trauma patients receiving massive transfusion. J Surg Res. 2016;202(1):182–7. 10.1016/j.jss.2015.12.036 [DOI] [PubMed] [Google Scholar]
- 19. Magnotti LJ, Bradburn EH, Webb DL, Berry SD, Fischer PE, Zarzaur BL, et al. Admission ionized calcium levels predict the need for multiple transfusions: a prospective study of 591 critically ill trauma patients. J Trauma. 2011;70(2):391–5; discussion 395‐7. 10.1097/TA.0b013e31820b5d98 [DOI] [PubMed] [Google Scholar]
- 20. Chanthima P, Yuwapattanawong K, Thamjamrassri T, Nathwani R, Stansbury LG, Vavilala MS, et al. Association between ionized calcium concentrations during hemostatic transfusion and calcium treatment with mortality in major trauma. Anesth Analg. 2021;132(6):1684–91. 10.1213/ANE.0000000000005431 [DOI] [PubMed] [Google Scholar]
- 21. Vasudeva M, Mathew JK, Fitzgerald MC, Cheung Z, Mitra B. Hypocalcaemia and traumatic coagulopathy: an observational analysis. Vox Sang. 2020;115(2):189–95. 10.1111/vox.12875 [DOI] [PubMed] [Google Scholar]
- 22. Conner JR, Benavides LC, Shackelford SA, Gurney JM, Burke EF, Remley MA, et al. Hypocalcemia in military casualties from point of injury to surgical teams in Afghanistan. Mil Med. 2021;186(Suppl 1):300–4. 10.1093/milmed/usaa267 [DOI] [PubMed] [Google Scholar]


