Abstract
Objective
To compare the incidence of hypertension in people living with HIV receiving integrase strand transfer inhibitor (INSTI)‐based antiretroviral therapy (ART) versus non‐nucleoside reverse transcriptase inhibitors (NNRTIs) or boosted protease inhibitors (PIs) in the RESPOND consortium of HIV cohorts.
Methods
Eligible people with HIV were aged ≥18 years who initiated a new three‐drug ART regimen for the first time (baseline), did not have hypertension, and had at least two follow‐up blood pressure (BP) measurements. Hypertension was defined as two consecutive systolic BP measurements ≥140 mmHg and/or diastolic BP ≥90 mmHg or initiation of antihypertensives. Multivariable Poisson regression was used to determine adjusted incidence rate ratios (aIRRs) of hypertension, overall and in those who were ART naïve or experienced at baseline.
Results
Overall, 4606 people living with HIV were eligible (INSTIs 3164, NNRTIs 807, PIs 635). The median baseline systolic BP, diastolic BP, and age were 120 (interquartile range [IQR] 113–130) mmHg, 78 (70–82) mmHg, and 43 (34–50) years, respectively. Over 8380.4 person‐years (median follow‐up 1.5 [IQR 1.0–2.7] years), 1058 (23.0%) participants developed hypertension (incidence rate 126.2/1000 person‐years, 95% confidence interval [CI] 118.9–134.1). Participants receiving INSTIs had a higher incidence of hypertension than those receiving NNRTIs (aIRR 1.76; 95% CI 1.47–2.11), whereas the incidence was no different in those receiving PIs (aIRR 1.07; 95% CI 0.89–1.29). The results were similar when the analysis was stratified by ART status at baseline.
Conclusion
Although unmeasured confounding and channelling bias cannot be excluded, INSTIs were associated with a higher incidence of hypertension than were NNRTIs, but rates were similar to those of PIs overall, in ART‐naïve and ART‐experienced participants within RESPOND.
Keywords: antiretroviral agents, HIV, hypertension, integrase inhibitors
INTRODUCTION
Hypertension is a major cause of premature death worldwide and is a growing problem in people living with HIV [1]. Globally, 35% of all adult people living with HIV receiving antiretroviral therapy (ART) have hypertension [2]. The increasing burden of hypertension is partly due to the increasing prevalence of risk factors and ageing of people living with HIV [2, 3. However, the recent improvement in ART access and the associated control of viraemia, together with safer ART, may negate some of the increase in risk factors of hypertension due to ageing. Like individuals without HIV, hypertension is associated with a higher risk of cardiovascular disease (CVD) [4, 5 and mortality in people living with HIV [5, 6. The key risk factors for hypertension in the general population that are particularly important in people living with HIV include smoking, alcohol use, illicit drug use and dyslipidaemia [7, 8. In addition, uncontrolled HIV viraemia is an independent risk factor for hypertension [9] and CVD [10].
The potential mechanisms of hypertension in people living with HIV are diverse and include an interaction of adipogenesis and lipodystrophy activation of the adipocyte renin‐angiotensin system, immune reconstitution and metabolic changes, including dyslipidaemia, chronic systemic and vascular inflammation, and renal insufficiency [1, 7, 8. Furthermore, ART plays a role in the mechanism of hypertension [11, 12, 13, as has been reported with older protease inhibitors (PIs) [14, 15 and non‐nucleoside reverse transcriptase inhibitors (NNRTIs) [15, 16. However, the risk due to PIs and NNRTIs has been of less clinical relevance in some studies [15] and the risk due to contemporary regimens remains unclear.
Clear evidence associates integrase strand transfer inhibitors (INSTIs) with weight gain [17, 18, 19, 20, but the data linking these agents with hypertension are conflicting. Analyses from selected populations and small cohorts have reported a higher risk of hypertension or elevated blood pressure (BP) in people living with HIV using INSTIs [21, 22, 23 despite evidence linking INSTI use with favourable lipid profiles [24, 25, 26 and lower levels of vascular disease markers [27]. Randomized controlled trials have also not reported a higher risk of hypertension in people living with HIV receiving INSTIs [28, 29, although this has been investigated in few studies. In addition, the results from clinical trials may be inconclusive because of the highly selected participants and brief durations of follow‐up. The risk of hypertension in people living with HIV receiving INSTIs should be further investigated, especially in larger cohorts, since INSTIs are increasingly recommended as first‐line ART agents [30, 31. In this analysis, we compared the incidence of hypertension in people living with HIV receiving INSTI‐based regimens versus those receiving contemporary NNRTIs and PIs, within a large consortium of HIV cohorts.
METHODS
Study design
The analysis was within RESPOND, a consortium of 17 observational cohorts that follow 32 085 people living with HIV in Europe and Australia. The inclusion criteria and data management procedures in RESPOND have been previously described [32, 33. Briefly, individual cohorts use standard case report forms via the HIV Cohorts Data Exchange Protocol (https://hicdep.org/) to collect and transmit data on demographics, ART, BP, comorbidities (such as CVD, cancer, fractures, diabetes, etc.) and laboratory parameters to a central coordinating centre, annually. All cohorts and the coordination centre perform quality control checks to ensure data completeness and accuracy.
Study participants
Eligible participants were aged ≥18 years and initiated a three‐drug ART regimen or switched, for the first time, to a new regimen containing two nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) with either an INSTI (dolutegravir [DTG], raltegravir [RAL] or cobicistat‐boosted elvitegravir [EVG/c]), an NNRTI (rilpivirine [RPV] or efavirenz [EFV]) or a PI (cobicistat‐ or ritonavir‐boosted atazanavir [ATV/b] or cobicistat‐ or ritonavir‐boosted darunavir [DRV/b]) between 1 January 2012 and 1 January 2019. Therefore, all participants were naïve to the third antiretroviral drug (INSTI/NNRTI/PI) they initiated. Data on bictegravir, cabotegravir, or doravirine were limited, and these drugs were not included in the analysis. We excluded people living with HIV receiving non‐three drug ART regimens (booster not counted) and those receiving unboosted PIs. To determine the incidence, participants did not have hypertension before or at baseline (up to 7 days after the baseline date) and had at least two follow‐up BP results and 6 months of follow‐up. Individual cohorts with sparse data on BP measurements (≥20% of participants missing BP measurements) were excluded.
Study outcomes
The primary outcome for this analysis was the incidence of hypertension in people living with HIV receiving a three‐drug ART regimen. Hypertension was defined as occurring on the earliest date of the following events: two consecutive systolic BP (SBP) measurements ≥140 mmHg and/or diastolic BP (DBP) measurements ≥90 mmHg, performed on different days (with the date of the first raised measurement taken as the diagnosis date); one single SBP measurement ≥140 mmHg and/or DBP measurement ≥90 mmHg with the use of antihypertensives (including angiotensin‐converting enzyme inhibitors [ACEIs] and angiotensin II receptor blockers [ARBs]) within 6 months of this measurement (with the first date of either taken as the diagnosis date); or the initiation of antihypertensives without a recorded high BP (date of initiation taken as the diagnosis date). This definition has been used in other analyses in the Data Collection on Adverse Events of Anti‐HIV Drugs (D:A:D) cohort [15, 34.
Baseline covariates
The baseline date was the date of ART initiation (ART naïve) or the date of initiation of a new third antiretroviral drug (ART experienced). All covariates were time fixed, and the values closest to the baseline date, but within 1 year before and up to 7 days after the baseline date, were considered baseline. Diabetes mellitus was defined as the initiation of treatment and/or blood glucose level ≥11.1 mmol/L and/or glycated haemoglobin ≥6.5% and/or confirmed diagnosis. Participants were hepatitis C virus (HCV) positive if they had a positive antibody test and/or positive HCV‐RNA and/or HCV genotype tests or received HCV therapy. Hepatitis B virus (HBV) infection was defined as a positive HBV surface antigen and/or a prior positive HBV DNA. Chronic kidney disease (CKD) was defined as two successive estimated glomerular filtration rates (eGFR, Chronic Kidney Disease Epidemiology Collaboration formula) ≤60 ml/min/1.73 m2 at least 90 days apart. CVD was defined as stroke and/or acute myocardial infarction and/or invasive coronary procedure. Lipid‐lowering therapy was defined as the use of statins and/or fibrates.
Statistical analysis
We conducted a univariable analysis followed by multivariable Poisson regression to determine the adjusted incidence rate ratio (aIRR) of hypertension. We analysed all people living with HIV (regardless of ART exposure before baseline) and a priori performed separate analyses for ART‐naïve and ART‐experienced people living with HIV. Participants without hypertension were followed from the first time they started a new third drug and until they developed hypertension, they discontinued or switched any component of the initiated ART regimen, the last BP measurement date, or 1 January 2020, whichever occurred first. In the univariable analysis, the following baseline covariates were identified a priori and their significance assessed: age, race, sex, mode of transmission, calendar year, eGFR, smoking, body mass index (BMI), diabetes mellitus, prior AIDS, CVD, HBV and HCV status, HIV RNA, nadir, and baseline CD4 counts, time since HIV diagnosis, baseline BP and lipid levels, lipid‐lowering therapy, and baseline ART exposure (in the analysis for all people living with HIV). Covariates, including BMI, were fixed at baseline as they may be on the causal pathway to hypertension [21, 35. Covariates with a global p < 0.2 were considered for multivariable regression. The backward selection stepwise method was used to manually fit the final regression models while retaining confounders. The model with the smallest Akaike information criterion was considered the best fitting. We conducted several sensitivity analyses. First, we moved the baseline to 6 months after initiation of the third antiretroviral drug for ART‐experienced people living with HIV (i.e., washout period), as ART‐induced prohypertensive changes may not be immediately reversible after an ART regimen switch. Second, since CKD and CVD are associated with hypertension, we excluded participants with these comorbidities. Third, we determined the incidence of hypertension based on elevated BP alone (SBP <140 mmHg and/or DBP <90 mmHg), since antihypertensives can be used for non‐hypertensive indications. Conversely, since BP measurement may not be standardized, we also determined the incidence of hypertension based on the initiation of antihypertensive medication alone. Fourth, to assess the potential for channelling bias, we compared and adjusted for the baseline 5‐year D:A:D CVD risk score [36]. Furthermore, since BP monitoring may be less frequent for individuals with lower CVD risk, we limited the analysis to individuals with 5‐year predicted CVD risk scores <5%. Finally, we also investigated whether results were consistent when the baseline date was changed to the date, after 2014, of ART initiation or the initiation of the third antiretroviral drug for the first time since dolutegravir became widely available in Europe in 2014. All statistical tests were two‐sided, and statistical significance was set at p < 0.05. Data were prepared using SAS Enterprise Guide software version 9.4 (SAS Institute Inc.) and analysed with Stata version 16.0 (StataCorp).
RESULTS
Demographic and clinical characteristics of eligible participants
A total of 7030 participants had at least two prospective BP measures after baseline and initiated ART or switched to a new ART regimen. Of these, 2424 (34.5%) had hypertension before baseline and were excluded (Figure S1). Participants excluded for hypertension were more likely to be male, ART exposed, older and receiving lipid‐lowering therapy and to have diabetes mellitus, CKD, CVD and hypertriglyceridemia (Table S1). The characteristics and comparisons of participants with and without hypertension at baseline are presented in Table S2.
Overall, 4606 people living with HIV were included (2486 ART experienced, 2120 ART naïve), of whom 3164 (68.7%) initiated INSTIs (DTG = 1929, EVG/c = 777, RAL = 458), 807(17.5%) initiated NNRTIs (RPV = 487, EFV = 320) and 635 (13.8%) initiated PI‐based ART (ATV/b = 174, DRV/b = 461). In the 2486 ART‐experienced participants, 2090 (84.1%) switched to INSTIs (1289 switched from PIs), 305 switched to NNRTIs (276 from PIs) and 91 switched to PIs (81 from NNRTIs). The median baseline SBP (interquartile range [IQR]) and DBP were 120 (113–130) mmHg and 78 (70–82) mmHg, respectively, and did not differ by the initiated regimens. The median duration between consecutive BP tests was 6 (IQR 4–6) months in people living with HIV receiving INSTIs or PIs, but the duration between tests was 6 (IQR 4–12) months in those receiving NNRTIs (p < 0.001). The median age and CD4 count were 43 (IQR 34–50) years and 508 (IQR 329–720) cells/μl, respectively, but the participants who received INSTI were older and had higher CD4 counts (Table 1). Compared with ART‐naïve participants, ART‐experienced people were older, more likely to have prior AIDS and comorbidities (diabetes mellitus, HBV/HCV, CVD) and to be receiving lipid‐lowering therapy. However, baseline BP values were comparable between ART‐naïve and ART‐experienced participants (Table S3).
TABLE 1.
Baseline demographic and clinical characteristics of the study participants (n = 4606)
| INSTIs | NNRTIs | PIs | All | |||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| 3164 | 68.7 | 807 | 17.5 | 635 | 13.8 | 4606 | 100 | |
| Sex | ||||||||
| Male | 2284 | 72.2 | 610 | 75.6 | 485 | 76.4 | 3379 | 73.5 |
| Female | 880 | 27.8 | 197 | 24.4 | 150 | 23.6 | 1227 | 26.6 |
| Ethnicity | ||||||||
| Black | 233 | 7.4 | 40s | 5.0 | 52 | 8.2 | 325 | 7.1 |
| White | 2186 | 69.1 | 569 | 70.5 | 431 | 67.9 | 3186 | 69.2 |
| Other a | 745 | 23.6 | 198 | 24.5 | 152 | 23.9 | 1095 | 23.8 |
| Route of HIV acquisition | ||||||||
| MSM | 1545 | 48.8 | 438 | 54.3 | 333 | 52.4 | 2316 | 50.3 |
| IDU | 365 | 11.5 | 66 | 8.2 | 58 | 9.1 | 489 | 10.6 |
| Heterosexual | 1070 | 33.8 | 271 | 33.6 | 214 | 33.7 | 1555 | 33.8 |
| Other a | 184 | 5.8 | 32 | 4.0 | 30 | 4.7 | 246 | 5.3 |
| Treatment experience | ||||||||
| Naive | 1074 | 33.9 | 502 | 62.2 | 544 | 85.7 | 2120 | 46.0 |
| New class | 2090 | 66.1 | 305 | 37.8 | 91 | 14.3 | 2486 | 54.0 |
| Prior AIDS | ||||||||
| Yes | 580 | 18.3 | 92 | 11.4 | 84 | 13.2 | 756 | 16.4 |
| No | 2584 | 81.7 | 715 | 88.6 | 551 | 86.8 | 3850 | 83.6 |
| Hepatitis B | ||||||||
| Positive | 140 | 4.4 | 30 | 3.7 | 31 | 4.9 | 201 | 4.36 |
| Negative | 2768 | 87.5 | 647 | 80.2 | 470 | 74.0 | 3885 | 84.4 |
| Unknown | 256 | 8.1 | 130 | 16.1 | 134 | 21.1 | 520 | 11.3 |
| Hepatitis C | ||||||||
| Positive | 659 | 20.8 | 132 | 16.4 | 90 | 14.2 | 881 | 19.1 |
| Negative | 2380 | 75.2 | 581 | 72.0 | 456 | 71.8 | 3417 | 74.2 |
| Unknown | 125 | 4.0 | 94 | 11.7 | 89 | 14.0 | 308 | 6.69 |
| Smoking status | ||||||||
| Current | 1246 | 39.4 | 297 | 36.8 | 217 | 34.2 | 1760 | 38.2 |
| Prior | 365 | 11.5 | 76 | 9.4 | 32 | 5.0 | 473 | 10.3 |
| Never | 909 | 28.7 | 261 | 32.3 | 168 | 26.5 | 1338 | 29.1 |
| Unknown | 644 | 20.4 | 173 | 21.4 | 218 | 34.3 | 1035 | 22.5 |
| Diabetes mellitus | ||||||||
| Yes | 89 | 2.8 | 22 | 2.7 | 6 | 0.9 | 117 | 2.5 |
| No | 2758 | 87.2 | 651 | 80.7 | 507 | 79.8 | 3916 | 85.0 |
| Unknown | 317 | 10.0 | 134 | 16.6 | 122 | 19.2 | 573 | 12.4 |
| CVD | ||||||||
| Yes | 14 | 0.4 | 1 | 0.1 | 1 | 0.2 | 16 | 0.4 |
| No | 2627 | 83.0 | 673 | 83.4 | 479 | 75.4 | 3779 | 82.1 |
| Unknown | 523 | 16.5 | 133 | 16.5 | 155 | 24.4 | 811 | 17.6 |
| Lipid‐lowering therapy | ||||||||
| Yes | 167 | 5.3 | 26 | 3.2 | 6 | 0.9 | 199 | 4.3 |
| No | 2997 | 94.7 | 781 | 96.8 | 629 | 99.1 | 4407 | 95.7 |
| NRTI | ||||||||
| TAF/FTC | 614 | 19.4 | 43 | 5.3 | 17 | 2.7 | 674 | 14.6 |
| TDF/FTC | 1348 | 42.6 | 691 | 85.6 | 501 | 78.9 | 2540 | 55.2 |
| TDF/3TC | 26 | 0.8 | 14 | 1.7 | 7 | 1.1 | 47 | 1.0 |
| ABC/3TC | 1153 | 36.4 | 31 | 3.8 | 92 | 14.5 | 1276 | 27.7 |
| Other | 23 | 0.7 | 28 | 3.5 | 18 | 2.8 | 69 | 1.5 |
| Median (IQR) | Number (%) missing | Median (IQR) | Number (%) missing | Median (IQR) | Number (%) missing | Median (IQR) | Number (%) missing | |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 45 (36–51) | 0 (0) | 39 (33–47) | 0 (0) | 36 (30–45) | 0 (0) | 43 (34–50) | 0 (0) |
| Baseline CD4 (cells/µl) | 560 (370–767) | 0 (0) | 474 (330–652) | 0 (0) | 344 (181–529) | 0 (0) | 508 (329–720) | 0 (0) |
| Nadir CD4 (cells/µl) | 243 (124–383) | 0 (0) | 307 (197–420) | 0 (0) | 280 (143–418) | 0 (0) | 260 (137–395) | 0 (0) |
| HIV RNA (copies/ml) | 39 (19–9492) | 0 (0) | 525 (33–2500) | 0 (0) | 2290 (861–165 546) | 0 (0) | 49 (19–26 500) | 0 (0) |
| BMI (kg/m2) | 23.4 (21.1–25.7) | 391 (12.4) | 23.4 (21.3–25.7) | 123 (15.2) | 22.6 (20.8–24.9) | 106 (16.7) | 23.2 (21.1–25.6) | 620 (13.5) |
| HDL (mg/dl) | 46.4 (38.7–58.0) | 570 (18.0) | 42.5 (34.8–54.1) | 198 (24.5) | 38.7 (30.9–50.3) | 206 (32.4) | 46.4 (34.8–58.0) | 974 (21.1) |
| CHOL (mg/dl) | 181.8 (154.7–212.7) | 402 (12.7) | 174.0 (150.8–201.1) | 132 (16.4) | 158.6 (135.4–185.6) | 151 (23.8) | 177.9 (150.8–208.8) | 685 (14.9) |
| TRIG (mg/dl) | 115.1 (79.7–168.3) | 467 (14.8) | 115.1 (79.7–159.4) | 163 (20.2) | 106.3 (79.7–141.7) | 164 (25.8) | 115.1 (79.7–168.3) | 794 (17.2) |
| LDL (mg/dl) | 108.3 (85.1–131.5) | 1757 (55.5) | 100.5 (85.1–127.6) | 468 (58.0) | 96.7 (77.3–119.9) | 387 (60.9) | 104.4 (85.1–131.5) | 2612 (56.7) |
| eGFR (ml/min/1.73 m2) | 99.0 (84.4–110.3) | 261 (8.2) | 104.0 (90.9–115.3) | 53 (6.6) | 109.0 (95.7–117.9) | 93 (14.6) | 101.1 (86.8–112.4) | 407 (8.8) |
| SBP (mmHg) | 121.0 (113–130) | 0 (0) | 120 (113–130) | 0 (0) | 120 (112–130) | 0 (0) | 120 (113–130) | 0 (0) |
| DBP (mmHg) | 78.0 (70.0–82.0) | 0 (0) | 77.0 (70.0–81.0) | 0 (0) | 78.0 (70.0–83.0) | 0 (0) | 78 (70–82) | 0 (0) |
| ART duration (years) | 7.3 (0.0–14.5) | 0 (0) | 0.0 (0.0–4.6) | 0 (0) | 0.0 (0.0–0.0) | 0 (0) | 3.5 (0.0–11.3) | 0 (0) |
| 5‐year predicted CVD | 2.2 (1.0–4.0) | 576 (18.2) | 1.5 (0.7–3.0) | 200 (24.8) | 1.2 (0.5–2.7) | 210 (33.1) | 1.9 (0.9–3.7) | 986 (21.4) |
| BP measure rate (per year) | 2 (2–3) | 0 (0) | 2 (1–3) | 0 (0) | 2 (2–3) | 0 (0) | 2 (2–3) | 0 (0) |
| Baseline date (mm/yy) | 10/15 (11/14–01/17) | 0 (0) | 10/13 (11/12–01/15) | 06/13 (08/12–09/14) | 0 (0) | 05/15 (12/13–08/16) | 0 (0) |
| Duration of prior exposure (years) to antiretrovirals before baseline b | Median (IQR) | Number (%) exposed | Median (IQR) | Number (%) exposed | Median (IQR) | Number (%) exposed | Median (IQR) | Number (%) exposed |
|---|---|---|---|---|---|---|---|---|
| Prior exposure to INSTIs | – | 0 (0) | 1.9 (1.9–1.9) | 1 (0.1) | 0.5 (0.5–0.5) | 1 (0.2) | 1.2 (0.5–1.9) | 2 (0.0) |
| Prior exposure to NNRTIs | 5.6 (1.8–9.7) | 1218 (38.6) | – | 0 (0) | 3.4 (1.7–7.3) | 84 (13.2) | 5.4 (1.8–9.6) | 1302 (28.3) |
| Prior exposure to PIs | 7.7 (4.2–11.5) | 1692 (53.6) | 5.1 (2.5–8.1) | 290 (35.9) | – | 0 (0) | 7.2 (3.9–11.0) | 1982 (43.0) |
| Prior exposure to NRTIs | 10.2 (6.7–15.2) | 2085 (66.1) | 5.8 (2.6–8.9) | 302 (37.4) | 3.5 (2.0–8.4) | 88 (13.9) | 9.5 (5.9–14.5) | 2475 (53.7) |
| Prior exposure to ABC | 5.8 (2.5–9.5) | 917 (29.1) | 4.8 (2.3–8.7) | 93 (11.5) | 3.2 (1.3–6.5) | 21 (3.3) | 5.6 (2.4–9.4) | 1031 (22.4) |
| Prior exposure to TDF | 6.5 (4.2–9.0) | 1647 (52.2) | 4.1 (2.0–6.7) | 201 (24.9) | 2.9 (1.6–4.2) | 60 (9.4) | 6.2 (3.7–8.7) | 1908 (41.4) |
| Prior exposure to TAF | 0.7 (0.3–1.1) | 55 (1.7) | 1.2 (1.0–1.3) | 8 (1.0) | – | 0 (0) | 0.7 (0.3–1.2) | 63 (1.4) |
| Prior exposure to AZT | 3.5 (1.0–7.7) | 1159 (36.7) | 3.0 (0.5–7.8) | 136 (16.9) | 4.4 (2.9–9.9) | 31 (4.93) | 3.5 (1.0–7.7) | 1326 (28.8) |
| Prior exposure to d4T | 3.0 (1.3–5.1) | 551 (17.5) | 2.9 (1.1–5.2) | 35 (4.39) | 1.8 (0.5–2.9) | 3 (0.5) | 2.9 (1.2–5.1) | 589 (12.8) |
| Prior exposure to ddI | 2.4 (0.9–5.2) | 455 (14.4) | 3.3 (1.4–5.9) | 19 (2.4) | 2.7 (0.8–7.7) | 6 (0.9) | 2.5 (0.9–5.2) | 480 (10.48) |
| Prior exposure to ddC | 1.1 (0.5–2.2) | 121 (3.82) | 1.6 (0.9–1.9) | 5 (0.6) | 5.0 (5.0–5.0) | 1 (0.2) | 1.1 (0.5–2.4) | 127 (2.8) |
All lipids are in mg/dl. To convert triglycerides from mg/dl to mmol/L, divide by 88.57. For HDL, LDL and total cholesterol, divide by 38.67.
Abbreviations: ABC, abacavir; ART, antiretroviral therapy; AZT, azidothymidine; BMI, body mass index; BP, blood pressure; CHOL, total cholesterol; CVD, cardiovascular disease; DBP, diastolic blood pressure; ddC, zalcitabine; ddI, didanosine; d4T, stavudine; eGFR, estimated glomerular filtration rate using the Chronic Kidney Disease Epidemiology Collaboration equation; FTC, emtricitabine; HDL, high‐density lipoprotein; IDU, intravenous drug use; INSTI, integrase strand transfer inhibitors; IQR, interquartile range; LDL, low‐density lipoprotein; MSM, men who have sex with men; NNRTI, non‐nucleoside reverse transcriptase inhibitors; NRTI, nucleoside/nucleotide reverse transcriptase inhibitor; PI, protease inhibitors; SBP, systolic blood pressure; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate; TRIG, triglycerides; 3TC, lamivudine.
Other categories (other than those stated), including unknown status.
Eligible participants must have been naïve to the ART regimen they initiated.
Primary outcome: Incidence of hypertension
Of the 4606 people living with HIV without hypertension at baseline, 1058 (23.0%) developed hypertension during 8380.4 person‐years of follow‐up (incidence rate [IR] 126.2 per 1000 person‐years; 95% CI 118.9–134.1). The median follow‐up period was 1.5 (IQR 1.0–2.7) years. Of the 1058 hypertension events, hypertension was diagnosed based on elevated BP alone in 776 (73.3%) participants, 134 of whom developed SBP ≥160 mmHg and/or DBP ≥100 mmHg. In 282 (26.7%) participants, the diagnosis was made by the initiation of antihypertensives, 191 (67.7%) of whom initiated either ARBs or ACEIs. Overall, participants receiving INSTIs had a 76% higher incidence of hypertension than those receiving NNRTIs (aIRR 1.76; 95% CI 1.47–2.11). In the univariable analysis, the incidence of hypertension was 31% lower with INSTIs than with PIs (aIRR 0.69; 95% CI 0.58–0.81), but the incidence was not significantly different after adjusting for prior exposure to ART (aIRR 1.07; 95% CI 0.75–1.06). In the final adjusted multivariable regression model, adjusted for other potential confounders, the incidence of hypertension was also not significantly different in those receiving INSTIs or PIs (aIRR 1.07; 95% CI 0.89–1.29) (Figure 1). The association between ART class and hypertension did not differ by sex (interaction p = 0.737), age (interaction p = 0.732), HCV status (interaction p = 0.253) or baseline 5‐year predicted CVD risk scores (interaction p = 0.239).
FIGURE 1.
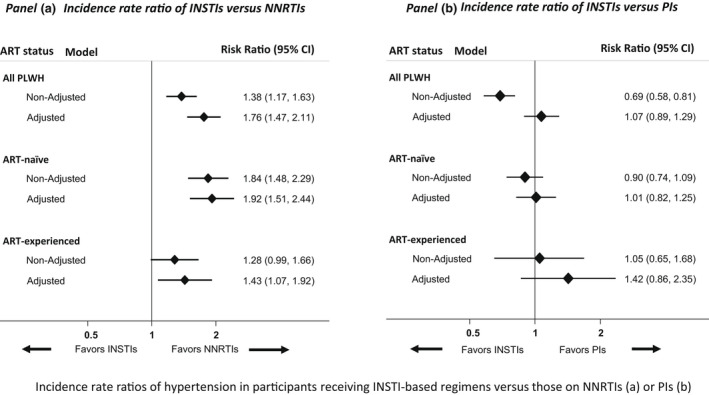
Incidence rate ratios of hypertension in participants receiving INSTI‐based regimens versus those on NNRTIs (a) or PIs (b)
Note: The final multivariable model was adjusted for the nucleoside/nucleotide reverse transcriptase inhibitor (NRTI) backbone, age, ethnicity, sex, mode of transmission, calendar year, estimated glomerular filtration rate, smoking, body mass index, diabetes mellitus, prior AIDS, cardiovascular disease, hepatitis B and hepatitis C virus status, HIV RNA, nadir, and baseline CD4 counts, time since HIV diagnosis, baseline blood pressure and lipid levels, lipid‐lowering therapy, and prior ART (in the analysis for all people living with HIV). Abbreviations: ART, antiretroviral therapy; CI, confidence interval; INSTI, integrase strand transfer inhibitor; NNRTI, non‐nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; PLWH, people living with HIV
Predictors of hypertension in people living with HIV
In the final multivariable regression model adjusted for baseline BP levels and other confounders, the predictors of hypertension included INSTI‐ and PI‐based ART regimens, compared with NNRTIs (INSTIs: aIRR 1.76; 95% CI 1.47–2.11; PIs: aIRR 1.65; 95% CI 1.33–2.03), prior AIDS (aIRR 1.31; 95% CI 1.11–1.54), baseline CD4 count <350 cells/µl (aIRR 1.48; 95% CI 1.27–1.74), being ART naïve (aIRR 1.44; 95% CI 1.14–1.81) and having BMI ≥30 kg/m2 (aIRR 1.32; 95% CI 1.05–1.65). Furthermore, baseline BP higher than the optimal level was associated with a higher incidence of hypertension (Table 2). The incidence of hypertension also increased linearly for every 5‐year increase in age (aIRR 1.16; 95% CI 1.12–1.19). Compared with people living with HIV aged <35 years, those aged >55 years were twice as likely to develop hypertension (aIRR 2.42; 95% CI 1.97–2.98). Diabetes mellitus, hypertriglyceridemia (≥200 mg/dl), low eGFR and male sex were associated with a higher risk of hypertension in univariable but not multivariable analysis.
TABLE 2.
Incidence of hypertension in all people living with HIV (ART naïve and ART experienced) (n = 4606)
| Variable | Variable categories | Events/PY | IR/1000 PY (95% CI) | Crude IRR (95% CI) | p‐Value | Global p | Adjusted IRR (95% CI) | p‐Value | Global p |
|---|---|---|---|---|---|---|---|---|---|
| ART class | INSTIs | 694/5441.7 | 127.5 (118.4–137.4) | INSTI vs NNRTI a : 1.38 (1.17–1.63) | <0.001 | <0.001 | INSTI vs. NNRTI a : 1.76 (1.47–2.11) | <0.001 | <0.001 |
| INSTI vs PIs b : 0.69 (0.58–0.81) | <0.001 | INSTI vs PIs b : 1.07 (0.89–1.29) | 0.460 | ||||||
| NNRTIs | 180/1948.2 | 92.4 (79.8–106.9) | |||||||
| PIs | 184/990.4 | 185.8 (160.8–214.7) | |||||||
| Sex | Male | 841/6119.0 | 137.4 (128.5–147.1) | 1.43 (1.23–1.66) | <0.001 | <0.001 | 1.11 (0.94–1.30) | 0.217 | 0.217 |
| Female | 217/2261.4 | 96.0 (84.0–109.6) | Ref | Ref | |||||
| Race | White | 72/601.4 | 119.7 (95.0–150.8) | Ref | <0.001 | Ref | <0.001 | ||
| Black | 807/5771.8 | 139.8 (130.5–149.8) | 0.86 (0.67–1.09) | 0.207 | 0.98 (0.76–1.25) | 0.858 | |||
| Other c | 179/2007.2 | 89.2 (77.0–103.2) | 0.64 (0.54–0.75) | <0.001 | 0.63 (0.53–0.74) | <0.001 | |||
| Treatment experience | Prior ART | 497/4894.5 | 101.5 (93.0–110.9) | Ref | <0.001 | Ref | 0.001 | ||
| ART naïve | 561/3485.8 | 160.9 (148.2–174.8) | 1.58 (1.j–1.79) | <0.001 | 1.44 (1.14–1.81) | 0.002 | |||
| Prior AIDS | Yes | 195/1248.5 | 156.2 (135.7–179.7) | 1.29 (1.10–1.51) | 0.001 | <0.001 | 1.31 (1.11–1.54) | 0.001 | <0.001 |
| No | 863/7131.9 | 121.0 (113.2–129.4) | Ref | Ref | |||||
| Smoking history | Never | 280/2538.1 | 110.3 (98.1–124.0) | Ref | 0.045 | Ref | 0.003 | ||
| Current | 384/3223.6 | 119.1 (107.8–131.7) | 1.08 (0.93–1.26) | 0.328 | 1.14 (0.97–1.33) | 0.116 | |||
| Prior | 110/927.2 | 118.6 (98.4–143.0) | 1.08 (0.86–1.34) | 0.518 | 1.13 (0.90–1.41) | 0.298 | |||
| Unknown | 284/1691.4 | 167.9 (149.5–188.6) | 1.52 (1.29–1.80) | <0.001 | 1.39 (1.17–1.66) | <0.001 | |||
| Diabetes mellitus | Yes | 38/207.3 | 183.3 (133.4–252.0) | 1.53 (1.10–2.12) | 0.010 | 0.010 | 1.12 (0.80–1.56) | 0.510 | 0.003 |
| No | 871/7261.3 | 120.0 (112.2–128.2) | Ref | Ref | |||||
| Unknown | 149/911.8 | 163.4 (139.2–191.9) | 1.36 (1.15–1.62) | <0.001 | 1.37 (1.14–1.64) | 0.001 | |||
| Age | Per 5 years | 1058/8380.4 | 126.2 (118.9–134.1) | 1.11 (1.07–1.14) | <0.001 | <0.001 | 1.16 (1.12–1.19) | <0.001 | <0.001 |
| Baseline HDL (mg/dl) | <40 | 324/2362.4 | 137.1 (123.0–152.9) | 1.31 (1.14–1.51) | <0.001 | <0.001 | 1.01 (0.87–1.17) | 0.938 | 0.684 |
| ≥40 | 474/4524.7 | 104.8 (95.7–114.6) | Ref | Ref | |||||
| Unknown | 260/1493.2 | 174.1 (154.2–196.6) | 1.66 (1.43–1.93) | <0.001 | 1.32 (1.12–1.55) | 0.001 | |||
| Baseline TRIG (mg/dl) | <200 | 684/6000.9 | 114.0 (105.8–122.9) | Ref | 0.018 | Ref | 0.220 | ||
| ≥200 | 157/1117.0 | 140.6 (120.2–164.4) | 1.23 (1.04–1.47) | 0.018 | 1.14 (0.96–1.37) | 0.145 | |||
| Unknown | 217/1262.4 | 171.9 (150.5–196.4) | 1.51 (1.29–1.76) | <0.001 | 1.26 (1.08–1.48) | 0.004 | |||
| HIV RNA (copies/ml) | <200 | 525/5128.9 | 102.4 (94.0–111.5) | Ref | <0.001 | Ref | 0.314 | ||
| ≥200 | 533/3251.4 | 163.9 (150.6–178.5) | 1.60 (1.42–1.81) | <0.001 | 1.11 (0.90–1.36) | 0.314 | |||
| Baseline CD4 (cells/ml) | <350 | 365/1885.3 | 193.6 (174.7–214.5) | 1.90 (1.65–2.17) | <0.001 | <0.001 | 1.48 (1.27–1.74) | <0.001 | <0.001 |
| 350–499 | 225/1910.3 | 117.8 (103.4–134.2) | 1.15 (0.98–1.35) | 0.078 | 1.06 (0.90–1.25) | 0.514 | |||
| >499 | 468/4584.8 | 102.1 (93.2–111.8) | Ref | Ref | |||||
| Nadir CD4 (cells/ml) | <200 | 416/2888.2 | 144.0 (130.8–158.6) | 1.25 (1.08–1.45) | 0.003 | 0.002 | 1.05 (0.85–1.29) | 0.668 | 0.563 |
| 200–349 | 326/2744 | 118.8 (106.6–132.4) | 1.03 (0.89–1.21) | 0.679 | 0.97 (0.81–1.18) | 0.776 | |||
| >350 | 316/2748.2 | 115.0 (103.0–128.4) | Ref | Ref | |||||
| BMI (kg/m2) | <25 | 570/5122.4 | 111.3 (102.5–120.8) | Ref | <0.001 | Ref | 0.039 | ||
| 25–29 | 251/1725.5 | 145.5 (128.5–164.6) | 1.31 (1.13–1.52) | <0.001 | 1.06 (0.91–1.24) | 0.452 | |||
| >30 | 93/501.6 | 185.4 (151.3–227.2) | 1.67 (1.34–2.07) | <0.001 | 1.32 (1.05–1.65) | 0.015 | |||
| Unknown | 144/1030.8 | 139.7 (118.6–164.5) | 1.26 (1.05–1.51) | 0.015 | 1.11 (0.92–1.34) | 0.284 | |||
| HIV Duration (years) | <5 | 180/1705.2 | 105.6 (91.2–122.2) | 1.33 (1.08–1.64) | 0.007 | 0.007 | 0.86 (0.68–1.09) | 0.219 | 0.456 |
| 5+ | 173/2184.2 | 79.2 (68.2–91.9) | Ref | Ref | |||||
| Unknown | 705/4490.9 | 157.0 (145.8–169.0) | 1.98 (1.68–2.34) | <0.001 | 1.65 (1.36–1.99) | <0.001 | |||
| GFR (ml/min/1.73 m2) | <90 | 312/2251.5 | 138.6 (124.0–154.8) | 1.23 (1.08–1.41) | 0.002 | 0.002 | 1.12 (0.96–1.30) | 0.151 | 0.120 |
| >90 | 626/5572 | 112.3 (103.9–121.5) | Ref | Ref | |||||
| Unknown | 120/556.9 | 215.5 (180.2–257.7) | 1.92 (1.58–2.33) | <0.001 | 1.37 (1.11–1.68) | 0.004 | |||
| Lipid‐lowering therapy | Yes | 65/373.7 | 173.9 (136.4–221.8) | 1.40 (1.09–1.80) | 0.008 | 0.008 | 1.28 (0.98–1.66) | 0.068 | 0.068 |
| No | 993/8006.6 | 124.0 (116.5–132.0) | Ref | Ref | |||||
| SBP (mmHg) | <120 | 206/3336.1 | 61.7 (53.9–70.8) | Ref | <0.001 | Ref | <0.001 | ||
| 121–129 | 334/2729.4 | 122.4 (109.9–136.2) | 1.98 (1.67–2.36) | <0.001 | 1.58 (1.32–1.90) | <0.001 | |||
| ≥130 | 518 /2314.8 | 223.8 (205.3–243.9) | 3.62 (3.08–4.26) | <0.001 | 2.24 (1.86–2.69) | <0.001 | |||
| DBP Categories (mmHg) | <80 | 370 /4867.6 | 76.0 (68.6–84.2) | Ref | <0.001 | Ref | <0.001 | ||
| 80–84 | 320/2088.4 | 153.2 (137.3–171.0) | 2.02 (1.74–2.34) | <0.001 | 1.55 (1.32–1.82) | <0.001 | |||
| ≥85 | 368/1424.3 | 258.4 (233.3–286.2) | 3.40 (2.94–3.93) | <0.001 | 2.14 (1.82–2.52) | <0.001 | |||
| ART duration | <5 years | 654/4373.8 | 149.5 (138.5–161.4) | Ref | <0.001 | Ref | <0.001 | ||
| 5–9 years | 160/1419.7 | 112.7 (96.5–131.6) | 0.75 (0.63–0.90) | 0.001 | 1.09 (0.84–1.41) | 0.529 | |||
| ≥10 years | 244/2586.9 | 94.3 (83.2–106.9) | 0.63 (0.54–0.73) | <0.001 | 0.72 (0.56–0.92) | 0.010 |
At the bivariable analysis stage, the mode of HIV transmission, baseline year, hepatitis B virus, hepatitis C virus, total cholesterol and low‐density lipoprotein cholesterol levels, and cardiovascular disease were not significant (global p > 0.200). These variables were not considered in the multivariable regression, and the parameter estimates for these excluded covariates are not presented. The final multivariable model was also adjusted for the nucleoside/nucleotide reverse transcriptase inhibitor backbone. The variables that were considered but not significant in the final multivariable regression model are in italics. The covariates that were significant in the final model are in bold. All lipids are in mg/dl; to convert triglycerides from mg/dl to mmol/L, divide by 88.57. For HDL, divide by 38.67.
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; CI, confidence interval; DBP, diastolic blood pressure; GFR, glomerular filtration rate determined using the Chronic Kidney Disease Epidemiology Collaboration equation; HDL, high‐density lipoprotein; INSTI, integrase strand transfer inhibitors; IR, incidence rate; IRR, incidence rate ratio; NNRTI, non‐nucleoside reverse transcriptase inhibitors; PI, protease inhibitors; PY, person‐years; SBP, systolic blood pressure; TRIG, triglycerides.
NNRTI is the reference group.
PI is the reference group.
Other categories (other than those stated), including unknown status.
Incidence of hypertension in ART‐naïve and ART‐experienced people living with HIV
Of the 2436 people living with HIV on ART before baseline, 497 (20.4%) developed hypertension over 4894.5 person‐years (IR 101.5/1000 person‐years, 95% CI 93.0–110.95). Hypertension was 43% more common in those receiving INSTIs than in those receiving NNRTIs (aIRR 1.43; 95% CI 1.07–1.92). In contrast, the incidence of hypertension in people living with HIV receiving INSTIs was not different from in those receiving PIs (aIRR 1.42; 95% CI 0.86–2.35) (Figure 1). Table S5 shows the predictors of hypertension in people living with HIV with prior ART exposure at baseline.
Of the 2120 people living with HIV who were ART naïve, 561 (26.5%) developed hypertension over 3485.8 person‐years (IR 160.9/1000 person‐years, 95% CI 148.2–174.8). The incidence of hypertension was 92% higher in participants receiving INSTIs than in those receiving NNRTIs (aIRR 1.92; 95% CI 1.51–2.44). Also, the higher incidence of hypertension in people living with HIV receiving INSTIs compared with those on NNRTIs was greater in ART‐naïve than in ART‐experienced people living with HIV. In contrast, the incidence of hypertension in people living with HIV receiving INSTIs was not different from that in participants receiving PIs (aIRR 1.01; 95% CI 0.82–1.25) (Figure 1). Table S6 shows the predictors of hypertension in people living with HIV who were ART naïve at baseline.
Sensitivity analysis
In all sensitivity analyses, the incidence of hypertension was consistently higher in people living with HIV receiving INSTIs than in those receiving NNRTIs but not different than in participants receiving PIs (Table 3). First, we investigated potential channelling bias. Participants receiving INSTIs had relatively higher 5‐year predicted estimated CVD risk scores based on the D:A:D risk equation (2.2%; IQR 1.0%–4.0%) than those receiving NNRTIs (1.5%; IQR 0.7%–3.0%) or PIs (1.2%; IQR 0.5%–2.7%). However, even after adjusting for CVD risk scores, participants on INSTIs remained at a higher risk of hypertension than those receiving NNRTIs (aIRR 1.62; 95% CI 1.35–1.94) but the risks were comparable to those receiving PIs (aIRR 0.94; 95% CI 0.78–1.13). Second, after a 6‐month washout period for ART‐experienced people living with HIV, the incidence of hypertension remained higher in people living with HIV receiving INSTIs than in those on NNRTIs but was comparable to the incidence in those receiving PIs. Third, the results were also consistent after excluding participants with CKD or CVD and when hypertension was defined solely based on high BP or initiation of antihypertensives (Table 3). Finally, the results also remained consistent when the analysis was limited to individuals with 5‐year predicted CVD risk scores <5% and when the baseline date was changed to the date after 2014 of ART initiation or the initiation of the third antiretroviral drug for the first time.
TABLE 3.
Sensitivity analyses of Incidence of hypertension by ART regimen
| Sensitivity analysis description | Number included | Number of hypertension events | Variable categories | Adjusted IRR (95% CI) | p‐Value |
|---|---|---|---|---|---|
| Primary analysis | 4606 | 1058 | INSTIs vs. NNRTIs | 1.76 (1.47–2.11) | <0.001 |
| INSTIs vs. PIs | 1.07 (0.89–1.29) | 0.460 | |||
| Only PLWH with normal BP (SBP <130 and DBP <85) included in the analysis | 2914 | 371 | INSTIs vs. NNRTIs | 2.19 (1.63–2.95) | <0.001 |
| INSTIs vs. PIs | 1.01 (0.76–1.34) | 0.937 | |||
| PLWH with CVD or CKD excluded from analysis | 4503 | 1038 | INSTIs vs. NNRTIs | 1.76 (1.47–2.11) | <0.001 |
| INSTIs vs. PIs | 1.07 (0.89–1.29) | 0.471 | |||
| Primary analysis but with adjustment made for D:A:D cardiovascular risk scores at baseline | 4606 | 1058 | INSTIs vs. NNRTIs | 1.62 (1.35–1.94) | <0.001 |
| INSTIs vs. PIs | 0.94 (0.78–1.13) | 0.481 | |||
| Primary analysis but with adjustment made for cohort | 4606 | 1058 | INSTIs vs. NNRTIs | 1.75 (1.46–2.10) | <0.001 |
| INSTIs vs. PIs | 1.08 (0.90–1.30) | 0.426 | |||
| Primary analysis but with primary endpoint based on BP only (initiation of antihypertensives ignored) | 4606 | 933 | INSTIs vs. NNRTIs | 1.75 (1.45–2.12) | <0.001 |
| INSTIs vs. PIs | 1.08 (0.89–1.31) | 0.461 | |||
| Primary analysis with the definition of hypertension just based on the initiation of anti‐hypertensives (high BP ignored) | 4606 | 282 | INSTIs vs. NNRTIs | 1.74 (1.18–2.57) | 0.005 |
| INSTIs vs. PIs | 0.83 (0.56–1.22) | 0.345 | |||
| 6 months washout | 4150 | 911 | INSTIs vs. NNRTIs | 1.63 (1.35–1.98) | <0.001 |
| INSTIs vs. PIs | 0.99 (0.82–1.20) | 0.918 | |||
| Baseline period pushed to 2014 a or after | 4617 | 1060 | INSTIs vs. NNRTIs | 1.77 (1.48–2.12) | <0.001 |
| INSTIs vs. PIs | 1.07 (0.89–1.29) | 0.454 | |||
| Participants with missing data excluded b | 3027 | 763 | INSTIs vs. NNRTIs | 1.83 (1.48–2.27) | <0.001 |
| INSTIs vs. PIs | 1.15 (0.92–1.43) | 0.225 | |||
| Participants with predicted 5‐year risk scores <5% | 4042 | 877 | INSTIs vs. NNRTIs | 1.75 (1.44–2.13) | <0.001 |
| INSTIs vs. PIs | 1.05 (0.86–1.28) | 0.610 |
In each of the presented analyses, we adjusted for potential confounders including the nucleoside/nucleotide reverse transcriptase inhibitor backbone, baseline BP levels and other covariates adjusted for in the primary analysis (Table 2). INSTIs vs. NNRTIs means that NNRTIs is the reference group; similarly, INSTIs vs. PIs means that PIs is the reference group.
Abbreviations: ART, antiretroviral therapy; BP, blood pressure; CI, confidence interval; CKD, chronic kidney disease; CVD, cardiovascular disease; D:A:D, Data Collection on Adverse Events of Anti‐HIV Drugs; DBP, diastolic blood pressure; INSTI, integrase strand transfer inhibitor; IRR, incidence rate ratio; NNRTI, non‐nucleoside reverse transcriptase inhibitor; PI, protease inhibitors; PLWH, people living with HIV; SBP, systolic blood pressure.
The date of initiation of ART initiation (ART‐naïve) or commencement of a new third antiretroviral regimen (for ART‐experienced) after 2014 (i.e., the date when dolutegravir was approved for use in Europe).
Model with participants with any missing data on the variables in the final models excluded.
DISCUSSION
In this study, we found an incidence of hypertension of 126 per 1000 person‐years in people living with HIV receiving contemporary ART within a large consortium of heterogeneous HIV cohorts. The incidence was higher in people living with HIV receiving INSTIs than in those receiving NNRTIs overall, but not different from that of participants receiving PIs. In the ART‐naïve participants, hypertension was more common in those receiving INSTIs than in those receiving NNRTIs but was comparable to those receiving PIs. In contrast, the incidence of hypertension was higher in those who switched to INSTIs than in those who switched to NNRTIs or PIs in the ART‐experienced group. The results were consistent in several sensitivity analyses and separate analyses for ART‐naïve and ART‐experienced people living with HIV. Furthermore, we demonstrated that prior AIDS, CD4 count <350 cells/µl, older age and obesity were predictors of hypertension.
Consistent with some cohorts that have reported greater elevation of BP with INSTIs [21, 22, 23, we found a higher incidence of hypertension with INSTIs than with NNRTIs. Although the mechanism for hypertension in people living with HIV receiving INSTIs is still unclear, there is an association between the use of INSTIs and weight gain [17, 18, 19, 20, a key risk factor for hypertension. The association between INSTI‐associated weight gain and hypertension is being investigated in a different analysis in RESPOND. The use of INSTIs has also been associated with adipogenesis, oxidative stress and insulin resistance [22, 37, 38 and these metabolic derangements have been associated with hypertension [39, 40. Additional studies have linked INSTIs with other predictors of hypertension such as dyslipidaemia and diabetes mellitus [26, 38, 41. The lower incidence of hypertension in individuals receiving NNRTIs than in those receiving INSTIs may be related to a favourable lipid profile associated with newer NNRTIs [26], especially RPV [42]. However, several studies have not found a higher risk of hypertension associated with the use of EFV [15, 34, 35 despite it being associated with dyslipidaemia [42]. Therefore, the lower risk of hypertension associated with NNRTIs may not be mediated by a favourable lipid profile alone, and other underlying mechanisms need further investigation. In this analysis, the use of PIs or INSTIs was associated with a higher risk of hypertension compared with the use of NNRTIs. Although the use of older PIs has been associated with hypertension [14, 15, the risk due to contemporary PIs is unclear. However, a recent analysis within the D:A:D cohort reported a lower risk of hypertension with cumulative exposure to ATV and DRV [15]. This possibly explains the lower risk with PIs in this study compared with prior studies that reported on older drugs. The relationship between ART regimens and hypertension incidence differed by ART status at baseline, which may be due to the confounding effect of ART exposure or differences in the characteristics of ART‐naïve versus ART‐experienced people living with HIV.
The predictors of hypertension in the present study are generally consistent with findings from other cohorts [15, 43. The results suggest that individuals with prior AIDS or traditional hypertension risk factors should have their BP measured during clinic visits. The incidence of hypertension was generally higher in participants with baseline BP higher than the optimal level (SBP >120 mmHg and DBP >80 mmHg). Therefore, while these BP levels do not require treatment, such individuals should be considered for lifestyle modification and closer BP monitoring. In this analysis, ART‐naïve people living with HIV paradoxically had a higher incidence of hypertension, contrary to findings in other studies [11, 12. This finding is possibly due to confounding by indication, since ART‐naïve participants are more likely to have more review visits following ART initiation and therefore more frequent BP monitoring. Furthermore, participants with prior ART exposure and longer durations on ART had higher odds of prevalent hypertension at baseline and were likely differentially excluded. We did not find a significant association between incident hypertension and other previously reported risk factors such as diabetes and abnormal lipid levels [15, 34. These risk factors may require longer exposure to have an impact on BP. The lack of association between Black race and hypertension is possibly due to restrictions on reporting race in certain cohorts and the underrepresentation of Black people in RESPOND, which is primarily based in Europe and Australia. The missingness of data on other analyzed variables could have resulted in the loss of statistical power, although the proportion of participants with missing data was generally small.
The present analysis has several limitations. First, we cannot rule out residual confounding, as this was an observational study. There is also potential channeling bias, since 66.7% of participants receiving INSTIs had been on PI‐based regimens, and participants on INSTIs generally had higher estimated 5‐year predicted CVD risk scores. However, the use of INSTIs was still associated with a higher incidence, even when we considered a 6‐month washout period and adjusted for 5‐year predicted CVD risk scores at baseline. However, the hypertensive effects of prior ART regimens, such as atherosclerosis, may not be reversed within 6 months. Second, we did not evaluate other hypertension risk factors such as non‐ART pro‐hypertensive medications, anxiety, diet, physical inactivity and family history of hypertension. In addition, the analysis mainly focused on ART‐class comparisons and was not powered for individual antiretroviral drug comparisons, although the risk of hypertension may differ among individual INSTIs, as has been demonstrated with PIs [15]. Furthermore, few participants were receiving bictegravir, cabotegravir, or doravirine, which were excluded from the analysis despite the increasing use of these agents. Individual drug comparisons, including NRTIs, should be considered in larger cohorts with greater exposure to these agents. Finally, BP measurements are not standardized across cohorts, which may limit the generalizability of results. However, we excluded cohorts with sparse BP monitoring.
Furthermore, participants may also have had BP measured outside HIV settings. The strength of this analysis is that it used routinely collected clinical data from heterogeneous cohorts with older populations at significant CVD risk. Additionally, in all analyses, adjustment was made for the baseline calendar year, since BP monitoring frequency may increase with the increasing concern about CVD.
Our results have several implications. First, they suggest that hypertension is common in people living with HIV, even in the era of contemporary ART, and indicates that people living with HIV, especially those receiving INSTIs or PIs, should have regular BP monitoring. This is important given that INSTIs are increasingly the preferred ART option [44, 45, 46. The incidence of hypertension in this study was higher than in other predominantly Caucasian cohorts [15, 35 but comparable to that in cohorts with significant CVD risk [47, 48. The high incidence in the present study possibly reflects an ageing and predominantly ART‐experienced population. Furthermore, participants with prior AIDS or traditional risk factors should be routinely monitored for hypertension.
In conclusion, the results of this analysis show a high incidence of hypertension in people living with HIV and suggest that contemporary ART regimens are differentially associated with hypertension. Hypertension was more common with INSTIs than with NNRTIs, but the incidence was not different with PIs, although we cannot entirely exclude potential channelling bias and residual confounding. Whether the higher risk of hypertension with INSTIs is associated with weight gain will be explored in a planned analysis within RESPOND. Our results suggest that participants with prior AIDS or traditional risk factors should be monitored for hypertension at each visit, especially after initiating INSTIs and PIs. Future studies should investigate the risk of hypertension due to individual INSTIs and the relationship between INSTI‐associated weight gain and hypertension.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHORS’ CONTRIBUTIONS
DMB, supervised by KP, MNP, and ML, conceived the idea and developed the project proposal and a statistical analysis plan. LR and AM also provided additional input into the proposal and the analysis plan. All authors reviewed the proposal and contributed to the revised proposal and analysis plan. DMB, with the supervision of KP and ML, performed the statistical analysis and wrote the analysis report, which was reviewed and commented on by all authors. DMB developed the first draft of the manuscript and revised the subsequent drafts. DMB, KP, MNP, and ML reviewed all versions of the manuscript and interpreted the data. MS, RM, DLB, AC, CH, SdW, FW, EF, JJV, BN, LG, HG, JG, AVA, AÖ, IM‐L, BS, VS, CN, and MvdV contributed to the interpretation of data and reviewed and provided input into the final draft of the manuscript.
Supporting information
Fig S1
Table S1
Table S2
Table S3
Table S4
Table S5
Table S6
ACKNOWLEDGEMENTS
The International Cohort Consortium of Infectious Disease (RESPOND) has received funding from ViiV Healthcare LLC and Gilead Sciences and Merck Sharp & Dohme. Additional support has been provided by participating cohorts contributing data in‐kind and/or statistical support: Austrian HIV Cohort Study (AHIVCOS); The Australian HIV Observational Database (AHOD); CHU Saint‐Pierre, University Hospital Cologne; EuroSIDA; Frankfurt HIV Cohort Study; Georgian National AIDS Health Information System (AIDS HIS); Modena HIV Cohort; San Raffaele Scientific Institute; Swiss HIV Cohort Study (SHCS); AIDS Therapy Evaluation in the Netherlands Cohort (ATHENA); Royal Free HIV Cohort Study. AHOD is further supported by grant no. U01‐AI069907 from the U.S. National Institutes of Health and GNT1050874 of the National Health and Medical Research Council, Australia. Funding companies had no direct involvement in the conduct of scientific projects. Funders had no direct role in study design, data collection, data analysis and/or data interpretation.
The International Multicohort Consortium of Infectious Disease study group: AIDS Therapy Evaluation in the Netherlands Cohort (ATHENA): F Wit, M vd Valk, M Hillebregt, Stichting HIV Monitoring (SHM), Amsterdam, Netherlands. The Australian HIV Observational Database (AHOD): M Law, K Petoumenos, N Rose, J Hutchinson, UNSW Sydney, Sydney, Australia. Austrian HIV Cohort Study (AHIVCOS): R Zangerle, H Appoyer, Medizinische Universität Innsbruck, Innsbruch, Austria. CHU Saint‐Pierre: S de Wit, M Delforge, Centre de Recherche en Maladies Infectieuses a.s.b.l., Brussels, Belgium. EuroSIDA Cohort: Gilles Wandeler, CHIP, Rigshospitalet, RegionH, Copenhagen, Denmark. Frankfurt HIV Cohort Study: C Stephan, M Bucht, Johann Wolfgang Goethe‐University Hospital, Frankfurt, Germany. Georgian National AIDS Health Information System (AIDS HIS): National AIDS Health Information System (AIDS HIS): N Chkhartishvili, O Chokoshvili, Infectious Diseases, AIDS and Clinical Immunology Research Center, Tbilisi, Georgia. Italian Cohort Naive Antiretrovirals (ICONA): A d’Arminio Monforte, A Rodano, A Tavelli, ASST Santi Paolo e Carlo, Milan, Italy; I Fanti, Icona Foundation, Milan, Italy. Modena HIV Cohort: C Mussini, V Borghi, Università degli Studi di Modena, Modena, Italy. Nice HIV Cohort: C Pradier, E Fontas, K Dollet, C Caissotti, Université Côte d’Azur et Centre Hospitalier Universitaire, Nice, France. PISCIS Cohort Study: J Casabona, JM Miro, Centre Estudis Epidemiologics de ITS i VIH de Catalunya (CEEISCAT), Badalona, Spain. Royal Free Hospital Cohort: C Smith, F Lampe, M Johnson, F Burns, C Chaloner, Royal Free Hospital, University College London, London, United Kingdom. San Raffaele Scientific Institute: A Castagna, A Lazzarin, A Poli, Università Vita‐Salute San Raffaele, Milano, Italy. Swedish InfCare HIV Cohort: A Sönnerborg, K Falconer, V Svedhem, Karolinska University Hospital, Stockholm, Sweden. Swiss HIV Cohort Study (SHCS): H Günthard, B Ledergerber, H Bucher, A Scherrer, University of Zurich, Zurich, Switzerland. University Hospital Bonn: JC Wasmuth, J Rockstroh, Bonn, Germany. University Hospital Cologne: JJ Vehreschild, G Fätkenheuer, M Stecher, N Schulze, B Franke, Cologne, Germany.
RESPOND executive committee: Lene Ryom (Chair), J Rooney, I McNicholl, V Vannappagari, H Garges, G Wandeler, M Law, R Zangerle, C Smith, S de Wit, J Lundgren, H Günthard.
RESPOND scientific steering committee: J Lundgren (Chair), H Günthard (Chair), J Kowalska, D Raben, L Ryom, A Mocroft, J Rockstroh, L Peters, A Volny Anne, N Dedes, ED Williams, N Chkhartishvili, R Zangerle, M Law, F Wit, C Necsoi, G Wandeler, C Stephan, C Pradier, A D’Arminio Monforte, C Mussini, A Bruguera, H Bucher, A Sönnerborg, JJ Vehreschild, JC Wasmuth, C Smith, A Castagna, J Rooney, I McNicholl, V Vannappagari, H Garges.
Community representatives: Alain Volny‐Anne, Nikos Dedes, Luis Mendao (European AIDS Treatment Group), Esther Dixon Williams (UK).
RESPOND Staff: Coordinating centre staff: JF Larsen, B Neesgaard, N Jaschinski, D Raben, L Ryom, L Peters, ML Jakobsen, T Bruun, A Bojesen. Data management staff: EV Hansen, AK Traytel, TW Elsing, D Kristensen, T Weide. Statistical staff: A Mocroft, L Greenberg, L Bansi‐Matharu, A Pelchen‐Matthews, K Petoumenos, N Rose, D. Byonanebye. Open access publishing facilitated by University of New South Wales, as part of the Wiley ‐ University of New South Wales agreement via the Council of Australian University Librarians. [Correction added on 17 May 2022, after first online publication: CAUL funding statement has been added.]
Byonanebye DM, Polizzotto MN, Neesgaard B, et al; the RESPOND study group . Incidence of hypertension in people with HIV who are treated with integrase inhibitors versus other antiretroviral regimens in the RESPOND cohort consortium. HIV Med. 2022;23:895–910. doi: 10.1111/hiv.13273
Contributor Information
Dathan M. Byonanebye, Email: dbyonanebye@kirby.unsw.edu.au.
the RESPOND study group:
M Hillebregt, N Rose, J Hutchinson, R Zangerle, H Appoyer, M Delforge, C Stephan, M Bucht, N Chkhartishvili, O Chokoshvili, C Mussini, V Borghi, C Pradier, K Dollet, C Caissotti, J Casabona, JM Miro, C Smith, F Lampe, M Johnson, F Burns, C Chaloner, A Lazzarin, A Poli, A Sönnerborg, K Falconer, V Svedhem, H Günthard, B Ledergerber, H Bucher, A Scherrer, JC Wasmuth, J Rockstroh, G Fätkenheuer, M Stecher, N Schulze, B Franke, Lene Ryom, J Rooney, I McNicholl, V Vannappagari, G Wandeler, J Lundgren, J Kowalska, D Raben, A Mocroft, L Peters, ED Williams, C Necsoi, A D’Arminio Monforte, A Bruguera, Nikos Dedes, Luis Mendao, JF Larsen, N Jaschinski, ML Jakobsen, T Bruun, A Bojesen, EV Hansen, AK Traytel, TW Elsing, D Kristensen, T Weide, L Bansi‐Matharu, and A Pelchen‐Matthews
REFERENCES
- 1. Fahme SA, Bloomfield GS, Peck R. Hypertension in HIV‐infected adults. Hypertension. 2018;72(1):44‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xu Y, Chen X, Wang K. Global prevalence of hypertension among people living with HIV: a systematic review and meta‐analysis. J Am Soc Hypertens. 2017;11(8):530‐540. [DOI] [PubMed] [Google Scholar]
- 3. Smit M, Brinkman K, Geerlings S, et al. Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis. 2015;15(7):810‐818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Socio GV, Ricci E, Maggi P, et al. Time trend in hypertension prevalence, awareness, treatment, and control in a contemporary cohort of HIV‐infected patients: the HIV and Hypertension Study. J Hypertens. 2017;35(2):409‐416. [DOI] [PubMed] [Google Scholar]
- 5. Nüesch R, Wang Q, Elzi L, et al. Risk of cardiovascular events and blood pressure control in hypertensive HIV‐infected patients: Swiss HIV cohort study (SHCS). J Acquir Immune Defic Syndr. 2013;62(4):396‐404. [DOI] [PubMed] [Google Scholar]
- 6. Bloomfield GS, Hogan JW, Keter A, et al. Blood pressure level impacts risk of death among HIV seropositive adults in Kenya: a retrospective analysis of electronic health records. BMC Infect Dis. 2014;14:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Touloumi G, Kalpourtzi N, Papastamopoulos V, et al. Cardiovascular risk factors in HIV infected individuals: comparison with general adult control population in Greece. PLoS One. 2020;15(3):e0230730. doi: 10.1371/journal.pone.0230730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Zoest RA, van den Born B‐JH, Reiss P. Hypertension in people living with HIV. Curr Opin HIV AIDS. 2017;12(6):513‐522. [DOI] [PubMed] [Google Scholar]
- 9. Xu Y, Chen X, Wijayabahu A, et al. Cumulative HIV viremia copy‐years and hypertension in people living with HIV. Curr HIV Res. 2020;18(3):143‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hsue PY, Waters DD. Time to recognize HIV infection as a major cardiovascular risk factor. Circulation. 2018;138(11):1113‐1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nduka CU, Stranges S, Sarki AM, Kimani PK, Uthman OA. Evidence of increased blood pressure and hypertension risk among people living with HIV on antiretroviral therapy: a systematic review with meta‐analysis. J Hum Hypertens. 2016;30(6):355‐362. [DOI] [PubMed] [Google Scholar]
- 12. Nduka CU, Stranges S, Bloomfield GS, et al. A plausible causal link between antiretroviral therapy and increased blood pressure in a sub‐Saharan African setting: a propensity score‐matched analysis. Int J Cardiol. 2016;220:400‐407. [DOI] [PubMed] [Google Scholar]
- 13. Seaberg EC, Muñoz A, Lu M, et al. Association between highly active antiretroviral therapy and hypertension in a large cohort of men followed from 1984 to 2003. AIDS. 2005;19(9):953‐960. [DOI] [PubMed] [Google Scholar]
- 14. Crane HM, van Rompaey SE, Kitahata MM. Antiretroviral medications associated with elevated blood pressure among patients receiving highly active antiretroviral therapy. AIDS. 2006;20(7):1019‐1026. [DOI] [PubMed] [Google Scholar]
- 15. Hatleberg CI, Ryom L, d'Arminio Monforte A, et al. Association between exposure to antiretroviral drugs and the incidence of hypertension in HIV‐positive persons: the Data Collection on Adverse Events of Anti‐HIV Drugs (D:A:D) study. HIV Med. 2018;19(9):605‐618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brennan AT, Jamieson L, Crowther NJ, et al. Prevalence, incidence, predictors, treatment, and control of hypertension among HIV‐positive adults on antiretroviral treatment in public sector treatment programs in South Africa. PLoS One. 2018;13(10):e0204020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pérez SE, Chow SP, Kania A, Goldberg R, Badowski ME. Weighing in on the role of Integrase Strand Transfer Inhibitors (INSTIs) on weight gain: fact or fiction? Curr Infect Dis Rep. 2020;22(7):1‐11.31933158 [Google Scholar]
- 18. Bourgi K, Jenkins CA, Rebeiro PF, et al. Weight gain among treatment‐naïve persons with HIV starting integrase inhibitors compared to non‐nucleoside reverse transcriptase inhibitors or protease inhibitors in a large observational cohort in the United States and Canada. J Int AIDS Soc. 2020;23(4):e25484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kerchberger AM, Sheth AN, Angert CD, et al. Weight gain associated with integrase stand transfer inhibitor use in women. Clin Infect Dis. 2020;71(3):593‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sax PE, Erlandson KM, Lake JE, et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis. 2020;71(6):1379‐1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Galdamez R, García JA, Fernández M, et al. Short‐term increase in risk of overweight and concomitant systolic blood pressure elevation in treatment naïve persons starting INSTI‐based antiretroviral therapy. Open Forum Infect Dis. 2019;6(12):ofz491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Summers NA, Lahiri CD, Angert CD, et al. Metabolic changes associated with the use of integrase strand transfer inhibitors among virally controlled women. J Acquir Immune Defic Syndr. 2020;85(3):355‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Saums MK, King CC, Adams JC, et al. Combination antiretroviral therapy and hypertensive disorders of pregnancy. Obstet Gynecol. 2019;134(6):1205‐1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Snedecor SJ, Radford M, Kratochvil D, Grove R, Punekar YS. Comparative efficacy and safety of dolutegravir relative to common core agents in treatment‐naïve patients infected with HIV‐1: a systematic review and network meta‐analysis. BMC Infect Dis. 2019; 19(1):484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gatell JM, Assoumou L, Moyle G, et al. Switching from a ritonavir‐boosted protease inhibitor to a dolutegravir‐based regimen for maintenance of HIV viral suppression in patients with high cardiovascular risk. AIDS. 2017;31(18):2503‐2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. The RESPOND Study Group . Incidence of dyslipidemia in people with HIV who are treated with integrase inhibitors versus other antiretroviral agents. AIDS. 2021;35(6):869‐882. [DOI] [PubMed] [Google Scholar]
- 27. Castley A, Williams L, James I, Guelfi G, Berry C, Nolan D. Plasma CXCL10, sCD163 and sCD14 levels have distinct associations with antiretroviral treatment and cardiovascular disease risk factors. PLoS One. 2016;11(6):e0158169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baker JV, Sharma S, Achhra AC, et al. Changes in cardiovascular disease risk factors with immediate versus deferred antiretroviral therapy initiation among HIV‐positive participants in the START (Strategic Timing of Antiretroviral Treatment) Trial. J Am Heart Assoc. 2017; 6(5):e004984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Venter WDF, Moorhouse M, Sokhela S, et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med. 2019;381(9):803‐815. [DOI] [PubMed] [Google Scholar]
- 30. World Health Organization . Updated Recommendations on First‐Line and Second‐Line Antiretroviral Regimens and Post‐Exposure Prophylaxis and Recommendations on Early Infant Diagnosis of HIV‐Interim Guidance [Internet]. WHO Publication; 2018: 14‐36. Accessed May 11, 2021. https://apps.who.int/iris/bitstream/handle/10665/277395/WHO‐CDS‐HIV‐18.51‐eng.pdf [Google Scholar]
- 31. Ryom L, Cotter A, de Miguel R, et al. 2019 update of the European AIDS Clinical Society Guidelines for treatment of people living with HIV version 10.0. HIV Med. 2020;21(10):617‐624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. The RESPOND Study group . How to RESPOND to modern challenges for people living with HIV: a profile for a new cohort consortium. Microorganisms. 2020;8(8):1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Neesgaard B, Mocroft A, Zangerle R, et al. Virologic and immunologic outcomes of treatment with integrase inhibitors in a real‐world setting: the RESPOND cohort consortium. PLoS One. 2020;15(12):e0243625. doi: 10.1371/journal.pone.0243625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thiébaut R, El‐Sadr WM, Friis‐Møller N, et al. Predictors of hypertension and changes of blood pressure in HIV‐infected patients. Antivir Ther. 2005;10(7):811‐823. [DOI] [PubMed] [Google Scholar]
- 35. Crane HM, van Rompaey SE, Kitahata MM. Antiretroviral medications associated with elevated blood pressure among patients receiving highly active antiretroviral therapy. AIDS. 2006;20(7):1019‐1026. [DOI] [PubMed] [Google Scholar]
- 36. Friis‐Møller N, Ryom L, Smith C, et al. An updated prediction model of the global risk of cardiovascular disease in HIV‐positive persons: the data‐collection on adverse effects of anti‐HIV drugs (D:A:D) study. Eur J Prev Cardiol. 2016;23:214‐223. [DOI] [PubMed] [Google Scholar]
- 37. Gorwood J, Bourgeois C, Pourcher V, et al. The integrase inhibitors dolutegravir and raltegravir exert proadipogenic and profibrotic effects and induce insulin resistance in human/simian adipose tissue and human adipocytes. Clin Infect Dis. 2020;71(10):e549‐e560. [DOI] [PubMed] [Google Scholar]
- 38. Lamorde M, Atwiine M, Owarwo NC, et al. Dolutegravir‐associated hyperglycaemia in patients with HIV [Internet]. Lancet HIV. 2020;7(7):e461‐e462. [DOI] [PubMed] [Google Scholar]
- 39. Guzik TJ, Touyz RM. Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension. 2017;70(4):660‐667. [DOI] [PubMed] [Google Scholar]
- 40. Wang F, Han L, Hu D. Fasting insulin, insulin resistance and risk of hypertension in the general population: a meta‐analysis. Clin Chim Acta. 2017;464:57‐63. [DOI] [PubMed] [Google Scholar]
- 41. Rebeiro PF, Jenkins CA, Bian A, et al. Risk of incident diabetes mellitus, weight gain, and their relationships with integrase inhibitor–based initial antiretroviral therapy among persons with human immunodeficiency virus in the United States and Canada. Clin Infect Dis. 2021;73(7):e2234‐e2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tebas P, Sension M, Arribas J, et al. Lipid levels and changes in body fat distribution in treatment‐naive, HIV‐1–Infected adults treated with rilpivirine or efavirenz for 96 weeks in the ECHO and THRIVE trials. Clin Infect Dis. 2014;59(3):425‐434. [DOI] [PubMed] [Google Scholar]
- 43. Okeke NL, Davy T, Eron JJ, Napravnik S. Hypertension among HIV‐infected patients in clinical care, 1996–2013. Clin Infect Dis. 2016;63(2):242‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Greenberg L, Ryom L, Wandeler G, et al. Uptake and discontinuation of integrase inhibitors (INSTIs) in a large cohort setting. J Acquir Immune Defic Syndr. 2020;83(3):240‐250. [DOI] [PubMed] [Google Scholar]
- 45. Stecher M, Schommers P, Kollan C, et al. Treatment modification after starting cART in people living with HIV: retrospective analysis of the German ClinSurv HIV Cohort 2005–2017. Infection. 2020;48(5):723‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chkhartishvili N, Rukhadze N, Sharavdze L, et al. Uptake and outcomes of generic dolutegravir based antiretroviral therapy in Georgia. Transl Clin Med Georgian Med J. 2020;5(1):18‐22. [Google Scholar]
- 47. Okello S, Kanyesigye M, Muyindike WR, et al. Incidence and predictors of hypertension in adults with HIV‐initiating antiretroviral therapy in south‐western Uganda. J Hypertens. 2015;33(10):2039‐2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rodríguez‐Arbolí E, Mwamelo K, Kalinjuma AV, et al. Incidence and risk factors for hypertension among HIV patients in rural Tanzania‐A prospective cohort study. PLoS One. 2017;12(3):e0172089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Table S1
Table S2
Table S3
Table S4
Table S5
Table S6


