Abstract
Constant assessment of insecticide resistance levels is mandatory to implement adequate malaria control tools, but little information is available on the annual dynamics of resistance. We, therefore, monitored variations in resistance in Anopheles gambiae s.l. over four seasons during 2 years in two localities of Yaoundé: urban Etoa‐Meki and peri‐urban Nkolondom. Mosquitoes were collected seasonally at larval stage and reared to adults for insecticide susceptibility tests and molecular analysis of resistance mechanisms. Anopheles coluzzii was found in Etoa‐Meki and An. gambiae in Nkolondom. Low mortalities to pyrethroids were observed (permethrin <10%, deltamethrin <21%), and resistance extended to 5× and 10× diagnostic doses, revealing a marked increase compared to previous studies. A seasonal variation in resistance was observed with the highest levels within dry seasons in Etoa‐Meki and rainy seasons in Nkolondom. The 1014F kdr allele shows a high frequency (0.9), associated with overexpression of metabolic genes (Cyp6M2, Cyp6P4, Cyp9K1, Cyp6Z1, and Cyp6Z2) varying significantly seasonally. This study reveals an escalation in resistance to pyrethroids in Yaoundé's malaria vectors with seasonal variations. An adequate choice of the implementation period of punctual vector control actions according to the resistance profile will help to potentiate the desired effect and thus improve its efficiency.
Keywords: Anopheles gambiae, Anopheles coluzzii, insecticide resistance, resistance dynamic, resistance intensity, seasons, vector control
High resistance to permethrin wasobserved, extended up to 10x concentrations in Anophelescoluzzii (8%, 68% 87% mortalities for 1x, 5x,10x) and An.gambiae (9%, 75%, 96%) in urban settings.
Nearly fixed target site mutation (KDR 1014F, f:0.9) and cytochromes P450genes overexpression (cyp6M2, cyp6P4, cyp9K1, cyp6P3) found implicated in the observedresistance.
A significant seasonal variation in permethrin susceptibility wasdetected in urban Anopheles coluzzii with highest resistance recordedduring short dry season and lowest during long rainy season.
BACKGROUND
Despite significant progress made in the past decade in reducing the malaria burden, this disease remains a huge challenge for sub‐Saharan African countries including Cameroon in Central Africa. Prevention strategies for malaria rely mainly on insecticide‐based control interventions including long‐lasting insecticidal nets (LLINs) and Indoor Residual Spraying (IRS). However, the effectiveness of these tools is threatened by the increasing reports of resistance to main insecticides (WHO, 2019). In Cameroon, resistance is increasingly reported nationwide in several vectors notably in the main vectors Anopheles gambiae s.l Giles, 1902 (Antonio‐Nkondjio et al., 2019; Nwane et al., 2013) and Anopheles funestus Giles, 1900 (Tchouakui et al., 2019; Wondji et al., 2011). Furthermore, there are increasing reports of loss of efficacy of LLINs which remain the only mass intervention in the country (Etang et al., 2016, Menze et al., 2020), highlighting the need to continuously monitor the dynamics and evolution of resistance while continuing to assess its impact on vector control.
Resistance to two of the main insecticides used for LLINs (the pyrethroids permethrin and deltamethrin) has increased in the main vector An. gambiae s.l. (Menze et al., 2018) in Cameroon since first detected (Etang et al., 2006) although its annual dynamics and evolution in field populations remain less studied particularly in metropolitan areas such as Yaoundé, the capital city of Cameroon, where several factors can drive resistance (Tene et al., 2012). Previous studies highlighted a clear contrast in the vector's resistance profile between various environmental settings of Yaoundé classified as polluted, unpolluted and cultivated, pointing out a significant variation in the dynamics and evolution of resistance in the city with an implication of pollution in the selection of resistance (Tene et al., 2013). As some of those elements such as xenobiotics and agricultural pesticide concentration in breeding sites are dependent on rainfall levels, this could possibly result in a variation of the related insecticide resistance profile according to season.
Two mechanisms are mainly implicated in resistance to pyrethroids: target‐site and metabolic resistance. Target‐site mutations in the voltage‐gated sodium channel (VGSC), referred to as knock‐down resistance (kdr), impact response to pyrethroids and DDT. Two amino acid modifications at locus 1014; L1014F and L1014S were first identified in West and East Africa respectively (Martinez‐Torres et al., 1998; Ranson et al., 2000) but are now widely distributed. In Cameroon, they have both been detected in the main malaria vector An. gambiae s.l. (Nwane et al., 2013; Tene et al., 2013) and their frequencies have significantly increased, from 0% in 2000 to 21.0% for the L1014S and 98.0% for the L1014F variant in 2017 (Antonio‐Nkondjio et al., 2019). This is noted as a result of selection pressure from vector control insecticidal activities such as high distribution of LLINs in addition to the use of pesticides in agriculture, mainly in market gardening where accumulated water in furrows serves as larvae breeding sites (Antonio‐Nkondjio et al., 2011). A third substitution, the N1575Y, has been found in West and Central Africa and shown to increase resistance in association with L1014F (Jones, Liyanapathirana, et al., 2012) and recent studies detected and confirmed the link between new mutations in the VGSC gene and resistance phenotype in several African vectors populations (Clarkson et al., 2021, Williams et al., 2021).
The second important resistance mechanism is the increased detoxification enzyme activities in mosquitoes with over‐expression of some specific genes able to metabolize insecticides. Three gene families are involved in this process: esterases, glutathione S‐transferases, and P450 monooxygenases (Lui, 2015) and their over‐expression has been associated with resistance to pyrethroids, especially P450s in An. gambiae s.l. (Fagbohun et al., 2019). The use of some synergists that are able to inhibit the activity of specific metabolic enzyme families helps to evaluate their involvement in observed resistance (B‐Bernard & Philogène, 1993).
Mitigating the threat of insecticide resistance in African malaria vector populations requires comprehensive information on the distribution and strength of resistance, and how it is evolving. Estimating these trends is complicated by the sparse and heterogeneous distribution of resistance phenotypes in field populations. Previous studies in Cameroon have reported the presence of the two main modes of resistance to pyrethroids in malaria vectors, and a combination of both is producing high‐level resistance (Menze et al., 2016; Nwane et al., 2013). Characterization of the kdr mutations as well as the implication of metabolic processes has proven the association of these mechanisms in the observed and growing level of resistance (Antonio‐Nkondjio et al., 2015; Etang et al., 2007). In the urban area of Yaoundé, a longitudinal study on the distribution of vectors establish that there was a strong seasonal variation in anopheles density (Kamdem et al., 2012) as well as a strong correlation between the level of urban pollution and the overexpression of metabolic enzymes involved in insecticide resistance (Tene et al., 2013). As there are seasonal variations in breeding site pollution levels due to water refreshment by rainfall, the profile of resistance might also be impacted.
To optimize upcoming vector control activities by defining the best period according to the target and objectives, it is crucial to understand how resistance may fluctuate during the year. This study aimed to assess the level and the seasonal fluctuations of pyrethroids resistance in urban and peri‐urban areas of Yaoundé, and the involvement of various factors in the observed resistance intensity.
METHODS
In the urban area of Yaoundé, seasonal mosquito collections were carried out for 2 years to assess vector susceptibility to insecticides and the dynamics of this resistance across the year. Larval collections were performed using the dipping method, followed by lab rearing, bioassays, and molecular analysis of F0 adults.
The study was conducted after obtaining ethical approval from the Cameroon National Ethics Committee under the ethical clearance No 2019/10/1195/CE/CNERSH/SP and the institutional ethical committee of the Liverpool School of Tropical Medicine (Research Protocol 19‐011).
Study area
The study was carried out in the city of Yaoundé which is characterized by several hills and lowlands with small watercourses. Two collection sites were selected: Etoa‐Meki (urban) in the core of the city and Nkolondom (peri‐urban) in the outskirts. The urban collection site is located within the core of the town and was selected based on previous works which have shown a strong metabolic resistance linked to urban pollution with an exclusive presence of Anopheles coluzzii Coetzee & Wilkerson, 2013 (Kamdem et al., 2012). The collection points are located at Etoa‐Meki (3°52′ 56′′, 11°31′ 49′′), in the slums, and around the railway station (Figure 1). The area profile shows small temporal and semi‐temporal breeding sites. These water collections are replenished mainly by rains so are therefore very rare during dry seasons and those that persist show signs of pollution due to water evaporation and concentration of xenobiotics and organic matter. On the other hand, the peri‐urban site of Nkolondom (3°57′ 05′′, 11°29′ 24′′) is located around 10 km from the urban sites. It was selected to collect An. gambiae and have previously shown strong resistance to pyrethroids (Antonio‐Nkondjio et al., 2016). In this locality, agriculture especially market gardening in swampy areas is the main activity for decades (Figure 1) with aromatic herbs, lettuce, and other vegetables being the main products. The crops are constantly protected from plant pests through the intensive use of pesticides by the farmers. The standing water in the furrows serves as breeding sites for mosquitoes, Anopheles and Culex. They are mainly semi‐permanent and probably contain traces of the pesticides used on crops in soil and water as was found in a study in Burkina (Akogbeto et al., 2006). Here, cultivation is done throughout the year, but there are some short periods left for ground regeneration. These periods, just after the harvests, last from 3 weeks to a month during the long‐dry season.
FIGURE 1.
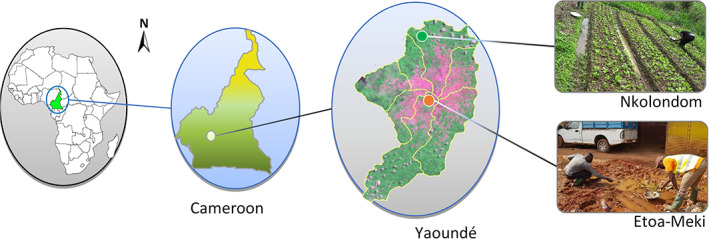
Study sites localization in Yaoundé (Cameroon): The urban collection areas Etoa‐Meki is in the core of the city and colonized by Anopheles coluzzii (□) while Nkolondom is in the peri‐urban area, about 10 km away, exclusively colonized by Anopheles gambiae (□). The map of Yaoundé was extracted from Landsat8 2010, the green representing vegetation and the reddish the structures.
Mosquito collection
The city of Yaoundé is situated within the Congo‐Guinean phytogeographical zone characterized by a typical equatorial climate named ‘type Yaoundéen’ (Suchel, 1972) with four seasons whose lengths vary slightly from 1 year to another, delimited as follows:
a long rainy season (LRS) from mid‐August to mid‐November;
a long dry season (LDS) from mid‐November to mid‐March;
a short rainy season (SRS) from mid‐March to mid‐June;
a short dry season (SDS) from mid‐June to mid‐August.
Collections were performed for 2 years, from March 2019 to December 2020 and were made over 2 weeks each season, around season's medium period to be sure to capture the real seasonal profile. Anopheles larvae were collected in breeding sites, following the dipping method (Service, 1993), and directly identified morphologically. The larvae were then brought to CRID's insectary for rearing to the adult stage, under controlled standard laboratory conditions (25 ± 2°C, 70%–80% relative humidity). They were kept in breeding site water in which distilled water was added progressively to avoid fermentation of organic matter. The F0 adults obtained were provided with a 10% sugar solution and used for susceptibility tests and molecular analysis.
Insecticide susceptibility tests
Insecticide susceptibility tests were done on F0 females obtained from reared field‐collected larvae and morphologically identified as members of An. gambiae complex (Gillies & de Meillon, 1968). This allowed us to be sure of mosquito ages (3 to 5 days) and physiological status (non‐blood‐fed). The exposure to insecticides follows the WHO tubes test protocol: 60 min exposure to insecticides and 24 h observation for 20 to 25 mosquitoes per test tube, with at least 4 tubes for the tested insecticide and 2 tubes as controls (WHO, 2016). The Ngousso lab susceptible strain was primarily used as a test control to confirm the quality of impregnated papers. Those insecticide‐impregnated papers were obtained from the WHO collaboration centre at the Vector Control Research Unit, University Sains, Malaysia. The insecticides included DDT 4%, type I pyrethroid, permethrin 0.75%, and type II pyrethroid deltamethrin 0.05%. An evaluation of the resistance intensity for pyrethroids was also done using 5× and 10× the discriminating concentration for permethrin (3.5% and 7.5%) and deltamethrin (0.25% and 0.5%) impregnated papers. WHO criteria were used to evaluate the resistance and susceptibility status of the tested mosquito population (WHO, 2016). Control mosquitoes were exposed to non‐impregnated papers and when their mortality was above 5%, Abbott's formula was used to correct the results (Abbott, 1987). The mortality rates were monitored 24 h post‐exposure to insecticide and resistance status was defined following WHO guidelines on insecticide susceptibility.
Synergist assays
To assess the contribution of metabolic enzymes in the resistance profile, especially cytochrome P450s, synergist assays were performed with Piperonyl Butoxide (PBO), an inhibitor of oxidases including P450s. For these tests, four replicates of 20–25 adult mosquitoes (3 to 5 days old) were pre‐exposed to PBO impregnated papers (4%) for 60 min and then immediately exposed to permethrin (0.75%) or deltamethrin (0.05%) for 60 min again. In addition, control assays using only the synergist impregnated papers for 60 min were also performed and mortality was recorded 24 h later. The mortality rate obtained was compared to those of insecticide tests without synergist's exposure to evaluate the level of susceptibility restoration and thus the implication of P450s in resistance to the tested insecticide.
Cone tests
To evaluate the efficacy of bed‐nets, cone bioassays were performed using two LLINs (Olyset® Net (containing 2% permethrin) and OlysetPlus® Net (containing 2% permethrin plus 1% PBO) from Sumitomo Chemical Co., obtained from the National Malaria Control Program in 2019 from a batch of nets for the mass distribution campaign. Following the WHO procedure, five unfed female mosquitoes aged 2–5 days were introduced into each cone placed on the LLIN for 3 min. After exposure, mosquitoes were immediately transferred to paper cups and supplied with 10% sucrose. Mosquitoes that were knocked down were recorded after 60 min and mortality was recorded 24 h after exposure. Two replicates were exposed to an untreated net as control.
Molecular analysis
Molecular identification was done as these subspecies belonging to the An. gambiae s.l. complex are not morphologically distinguishable. The aim is to check whether the geographical distribution of species is still following the same pattern as described by previous studies. Genomic DNA used for this identification of An. gambiae complex members was extracted from adult mosquitoes following the Livak technique (Livak, 1984). For each seasonal collection, a sub‐sample of 30 F0 adults mosquitoes randomly selected from larvae reared per collection site was used to perform the short interspersed elements PCR (SINE) that differentiate members of the An. gambiae s.l. complex (Santolamazza et al., 2008). This identification was made before the insecticide tests. In addition, a supplementary verification was done later, on a smaller set of samples (5–10) of female mosquitoes used for insecticide tests.
Genotyping of KDR mutations (L1014F, L1014S, and N1575Y) was done following TaqMan assays as previously described (Bass et al., 2007; Jones, Liyanapathirana, et al., 2012). Thus, 90 samples of An. coluzzii and 100 An. gambiae randomly selected in unexposed F0 were used to assess the presence of these KDR mutations. TaqMan reactions were undertaken using the Agilent MX3005P machine. Each reaction was conducted in a 10 μl final volume with 1xSensiMix (Bioline), 800 nM of each primer, and 200 nM of each probe. The primers used are listed in Table 1.
TABLE 1.
Primers used to evaluate metabolic and target site resistances in Yaoundé's malaria vectors
| Gene group | Gene name | Primer name | Primer sequences (5′ to 3′) | Efficiency (%) |
|---|---|---|---|---|
| House kipping genes | Elongation factor | EFq_F | GGCAAGAGGCATAACGATCAATGCG | 95.6 |
| EFq_R | GTCCATCTGCGACGCTCCGG | |||
| RPS7 | RPS7q_F | CCACCATCGAACACAAAGTTGA | 93.4 | |
| RPS7q_R | TGCTGCAAACTTCGGCTATTC | |||
| Metabolic genes | CYP6M2 | 6M2q_F | CTGGCGTTGAATCCAGAGGT | 80.7 |
| 6M2q_R | GATACTTGCGCAGTGATTCATTAAG | |||
| CYP6P3 | 6P3q_F | ACAATGTGATTGACGAAACCCT | 93 | |
| 6P3q_R | GGATCACATGCTTTGTGCCG | |||
| CYP9K1 | 9K1q_F | CCGACACGTGGTGATGGATAC | 101.9 | |
| 9K1q_R | CGTCGTCGGTCCAGTCAAC | |||
| CYP6P4 | 6P4q_F | CTGGACAACGTTATCAATGAAACC | 96.1 | |
| 6P4q_R | GCACGGTGTAATCACGCATC | |||
| CYP6Z1 | 6Z1q_F | CCCGCAACTGTATCGGTCTG | 98.6 | |
| 6Z1q_R | TTCGGTGCCAGTGTGATTGA | |||
| CYP6Z2 | 6Z2q_F | AGGCCACGAAGAACTACGAT | 106.1 | |
| 6Z2q_R | ACTTTTGCAGGAGTTGTGGC | |||
| Kdr mutations | Kdr primers | kdr_F | CATTTTTCTTGGCCACTGTAGTGAT | |
| kdr_R | CGATCTTGGTCCATGTTAATTTGCA | |||
| Kdr probes | FAM L1014F | ACGACAAAATTTC | ||
| FAM L1014S | ACGACTGAATTTC | |||
| HEX susceptible | CTTACGACTAAATTTC | |||
| 1575‐F | TGGATCGCTAGAAATGTTCATGACA | |||
| 1575‐R | CGAGGAATTGCCTTTAGAGGTTTCT | |||
| Y1575 | 3′NFQ‐TTTTTCATTGCATAATAGTAC | |||
| N1575 | 3′NFQ‐ATTTTTTTCATTGCATTATAGTAC |
Gene expression level was assessed for the two vector species collected during the main dry and rainy seasons. Six of the most reported detoxification genes listed by Mavridis (Mavridis et al., 2019) were selected for this study (Table 1). Using three biological replicates of 10 F0 females alive after exposure to permethrin and 3 batches of 10 susceptible females as control, total RNA was extracted followed by cDNA synthesis using Superscript III (Invitrogen®) based on manufacturer instructions. The qRT‐PCR reactions were performed as previously described by Riveron (Riveron et al., 2013). Fold change and expression of each gene in resistant and control samples were computed for each species according to the 2‐ΔΔCT method (Schmittgen & Livak, 2008) using housekeeping genes for standardization: ribosomal protein S7 (RPS7; VectoBase ID: AGAP010592) and Elongation factor (VectoBase ID: AGAP005128). The primers used, previously by Mavridis et al. (2019), are presented in Table 1.
Data analysis
Following the WHO guidelines on insecticide susceptibility, mosquito populations were considered susceptible if mortality was ≥98% and resistant if mortality was less than 90%. Mortality rates ranging between 90% and 97% indicate a potential resistance needing to be confirmed by additional tests (WHO, 2016). A chi‐square test was used to compare mortality data for insecticides and synergists, in addition to Fisher's exact test. The Pearson test helps to evaluate the correlation of susceptibility profiles between seasons. Data from the two‐year collections were computed by seasons as no significant difference was found between years. Microsoft Excel software was used to enter data and analyses were done with the R software version 4.0.2 (R_Core_Team, 2012).
RESULTS
Vector species composition
More than 6000 adult female Anopheles mosquitoes were obtained after larval rearing from the two sites and were morphologically identified as belonging to the An. gambiae complex. We used around 30 randomly selected adults per collection period for each of the two collection points for molecular identification. As result, we identified exclusively An. coluzzii in the urban area (Etoa‐Meki) on the 228 results for 240 samples tested, and An. gambiae in the rural market gardening area (Nkolondom) on 220 tested samples.
Resistance profile of malaria vectors
The phenotypic resistance to insecticides was done using WHO test tubes. Insecticide susceptibility tests were carried out on a total of 5209 samples (excluding the controls) for insecticides and in association with synergist (1952 for Nkolondom and 3257 for Etoa‐Meki). The bioassays results expressed as annual averages of the observed mortality rates for the whole collection period are presented in Figure 2.
FIGURE 2.
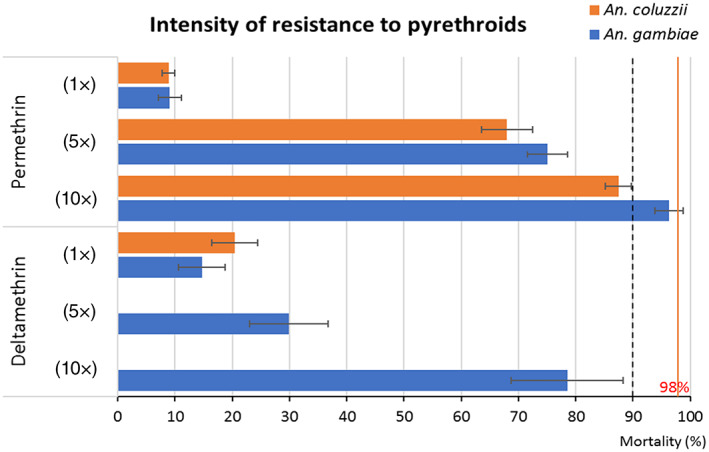
Resistance intensity to permethrin (1×, 5×, and 10×) and deltamethrin (1×, 5×, and 10×) for the two malaria vectors species collected from March 2019 to December 2020 in Yaoundé: Anopheles gambiae from Nkolondom (peri‐urban area) and Anopheles coluzzii from Ngusso (urban area), with 95% error bars
The profile of An. coluzzii showed resistance to all tested insecticides. Resistance to DDT was high, with 9.21% (±2.92) mortality recorded (Table 2). Deltamethrin and permethrin also showed low mortality, 20.4% (±4.01) and 8.87% (±2.82) respectively. An increase in mortality with PBO‐permethrin was observed, with 36.4% (±2.89) of mortality, that is a recovery of 23%. Resistance escalation tested for 5× and 10× permethrin‐impregnated papers showed a resistance of urban An. coluzzii to both, with 68.0% (±4.44) mortality to 5× and 87.5% (±2.27) to 10× indicating a high resistance level (Figure 2). Resistance escalation was not tested for deltamethrin in An. coluzzii due to an insufficient number of samples not allowing us to obtain valid results.
TABLE 2.
Annual means of mortalities of Anopheles gambiae and Anopheles coluzzii populations respectively from Nkolondom and Ngousso (Yaoundé, Cameroon) to DDT and pyrethroids in from March 2019 to December 2020
| Insecticides | An. coluzzii | An. gambiae | ||||
|---|---|---|---|---|---|---|
| Nb exp | Mortality | SD | Nb exp | Mortality | SD | |
| DDT | 164 | 9.21 | 2.92 | 112 | 0.00 | 0.00 |
| Permethrin | 976 | 8.87 | 1.10 | 571 | 9.07 | 2.04 |
| Permethrin (05×) | 600 | 68.00 | 4.44 | 197 | 75.02 | 3.49 |
| Permethrin (10×) | 493 | 87.47 | 2.27 | 84 | 96.33 | 2.49 |
| PBO + Permethrin | 738 | 36.37 | 2.90 | 270 | 46.99 | 9.13 |
| Deltamethrin | 246 | 20.41 | 4.01 | 252 | 14.67 | 4.08 |
| Deltamethrin (05×) | 117 | 29.88 | 6.83 | |||
| Deltamethrin (10×) | 83 | 78.50 | 9.79 | |||
| PBO + Deltamethrin | 102 | 79.38 | 1.94 | |||
| Total | 3217 | 1788 | ||||
Note: Nb exp, number of mosquitoes exposed to insecticides; SD, standard deviation.
For the peri‐urban strain An. gambiae, full resistance to DDT with no single mortality (0%) was obtained on the 112 tested samples. For pyrethroids, this population was also resistant with low mortality observed: 14.7% (±4.08) for deltamethrin and 9.69% (±2.11) for permethrin. The implication of metabolic enzymes in this resistance to pyrethroids was confirmed by mortality rising to 79.4% (±1.94) for PBO‐deltamethrin and 46.9% (±9.13) for PBO‐permethrin, showing partial recovery of susceptibility of 64% and 37% respectively. Given these results, we ran a test with 5× and 10× concentrated permethrin and deltamethrin and obtained high levels of resistance with 75% (±3.49) mortalities for 5× and 96.3% (±2.49) for 10× permethrin, 29.9% (±6.83) for 5× and 78.5% (±9.79) for 10× deltamethrin (Figure 2).
Bed nets efficacy evaluation
Low mortality rates were recorded against Olyset® Net compared to the PBO‐based OlysetPlus® Net (Figure 3). The mortality rates with Olyset® Net were 10.85% (±1.52) and 13.68% (±3.78) for An. gambiae and An. coluzzii, respectively. For the net containing PBO as a synergist (OlysetPlus®), the recorded mortalities were 93.33% (±4.08) and 96.28% (±1.52) respectively, above the threshold of suspected resistance.
FIGURE 3.
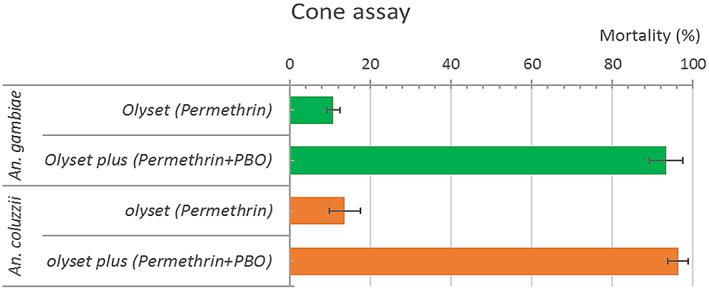
Mortalities of female Anopheles gambiae and Anopheles coluzzii populations respectively from Nkolondom and Ngousso (Yaoundé, Cameroon) to WHO cone bioassay with two LLINs (Olyset® and OlysetPlus®) in November 2020 with error bars at 95% confidence interval
KDR mutations frequencies
The L1014F mutation was found at a very high frequency in tested field populations with allelic frequencies of 0.90 for An. coluzzii and 0.93 for An. gambiae. Only four susceptible homozygotes (SS) were found in An. coluzzii and just a single one in An. gambiae on 90 and 73 samples tested respectively (Table 3). No L1014S or N1575Y mutations were detected in all tested samples despite an increase in the sample size.
TABLE 3.
KDR mutation genotypic frequencies in the two malaria vectors species of Yaoundé Anopheles gambiae and Anopheles coluzzii populations, respectively, from Nkolondom and Ngousso (Yaoundé, Cameroon) from March to December 2019
| Species | |||||
|---|---|---|---|---|---|
| An. coluzzii | An. gambiae | ||||
| Mutation | Genotype | Nb. samples | Frequency | Nb. samples | Frequency |
| L1014F | RR | 76 | 0.84 | 70 | 0.96 |
| RS | 10 | 0.11 | 2 | 0.03 | |
| SS | 4 | 0.04 | 1 | 0.01 | |
| L1014S | RR | 0 | 0 | 0 | 0 |
| RS | 0 | 0 | 0 | 0 | |
| SS | 180 | 1 | 180 | 1 | |
| N1575Y | RR | 0 | 0 | 0 | 0 |
| RS | 0 | 0 | 0 | 0 | |
| SS | 178 | 1 | 177 | 1 | |
Seasonal permethrin resistance monitoring
Larval collections were performed for 2 years, during the four different seasons. Initially, an analysis was done on samples from the same season but collected in different years in urban areas, to assess the level of variation between the two annual datasets. The average permethrin mortality 1×, 5× and 10× and with PBO was compared between the two collection years, season by season (Table S1). A strong correlation was obtained (r: 0.994, p = 0.006) showing that there is no significant variation from 2019 to 2020. In addition, a more in‐depth analysis was performed for the season presenting a complete set of data on the two collection years, the SDS. A multivariate ANOVA analysis shows that the general SDS profile is not significantly different between 2019 and 2020 (p = 0.47) nor by insecticide concentrations per year (p = 0.79), but rather shows a strong similarity (r: 0.994). Following these observations, we decided to mix the 2 years' results and analyse them as a single data set.
The general resistance profile observed in the urban area for An. coluzzii was analysed seasonally. For each season, permethrin was used at three concentrations: the recommended dose here named 1× (0.75%) and two higher concentrations at 5× and 10×. For An. gambiae unfortunately, some seasons did not provide sufficient data to perform a statically valid analysis on a full‐year period.
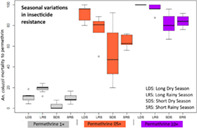
For An. coluzzii, the highest resistance to permethrin 1× was observed within the SDS, with the lowest mortality of only 2.1% (±0.73) (Figure 4). The lowest resistance was observed during the long rainy season (LRS) with 18.9% (±1.52) mortality. The observed mortalities to the 3 concentrations were significantly different between the four seasons (F‐stat = 33.92, p = 0), presuming a probable impact of parameters linked to seasons on pyrethroid resistance in this vector species. Concerning susceptibility to 5× and 10× permethrin, the highest resistances were observed during SDS with 54.4% (±7.84) and 81.2% (±3.41) mortalities respectively for 5× and 10× and the lowest resistance in LDS with the highest mortalities of 91.4% (±3.8) for 5× and 100% for 10× (Figure 4). During the LRS, 10× mortality reached 95.9% (±2.28) thus remaining in the resistance interval defined by WHO (under 98% mortality).
FIGURE 4.
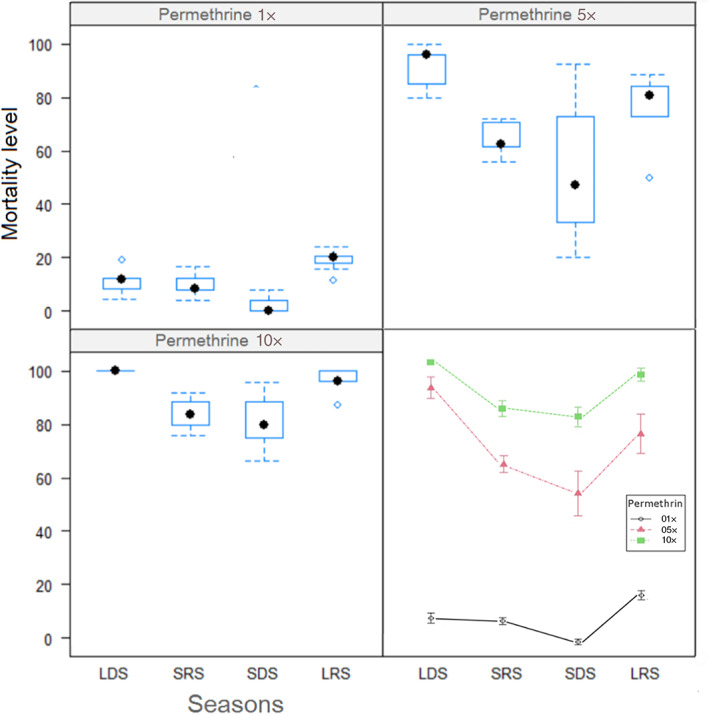
Plots of seasonal mortalities (%) of Anopheles coluzzii from Ngousso, an urban area of Yaoundé (Cameroon) to three permethrin concentrations (1×, 5×, and 10×) and the overall view of resistance profiles for a year with SE on means from March 2019 to December 2020. LDS, long dry season; LRS, the long rainy season; SDS, short dry season; SRS, the short rainy season.
Seasonal implication of P450s metabolic detoxification genes in resistance
The insecticide tests with pre‐exposure to synergist showed different levels of restoration of susceptibility for each season (Figure 5), significantly different from the permethrin alone tests (t‐test p = 0.03). The mortalities with PBO were 31.8% (±1.74), 24.0% (±1.68), 39.6% (±2.53) and 63.1% (±5.04) for SRS, SDS, LRS, and LDS, giving a restoration of susceptibility respectively of 22.1%, 21.9%, 20.7% and 52.3%. The results show a quite similar restoration for all seasons apart from the LDS showing more than the double of metabolic genes implication in resistance. To evaluate the level of implication of metabolic genes in permethrin resistance, we did a ratio between PBO + permethrin and permethrin1x mortalities. We found that the SDS had the highest ratio of P450‐based metabolic implication in resistance (11.35) due to the low permethrin mortality (2.11%), followed by LDS (5.83), SRS (3.27) and LRS (2.09). An analysis of the annual correlations between permethrin1x mortality and PBO + permethrin is showing that the correlation is stronger when analysing 3 seasons SDS, SRS, and LRS (r: 0.99, p = 0.03) than when the LDS is included (r: 0.41, p = 0.58). This is linked to the high resistance restoration by PBO observed during this season.
FIGURE 5.
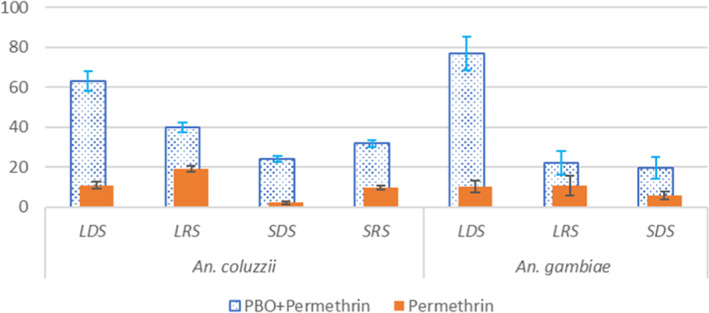
Synergist susceptibility profile of Anopheles gambiae and Anopheles coluzzii populations respectively from Nkolondom and Ngousso (Yaoundé, Cameroon) after 60 min exposure to permethrin and combined permethrin and PBO from March 2019 to December 2020. Data shown as mean mortality with ±95% confidence interval bars. LDS, long dry season; LRS, the long rainy season; SDS, short dry season; SRS, the short rainy season.
Seasonal variation in metabolic genes expression level
The profile of six of the main metabolic genes was assessed during the main dry and rainy seasons in the two localities.
In the urban site, a comparison of the differences in the gene expression fold changes for An. coluzzii between the rainy and dry seasons (Figure 6a) shows three genes overexpressed during the rainy season and not the dry season (Cyp6M2, Cyp6P4 and Cyp9K1) and one gene with the opposite profile (Cyp6Z2). A comparison by seasons shows three genes with significant differences in expression: Cyp6M2, Cyp6Z2 and Cyp9K1.
FIGURE 6.
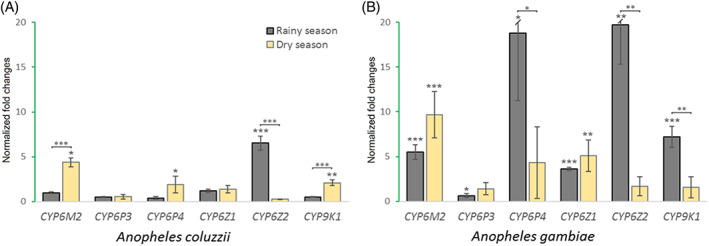
The normalized values of fold change variation in main metabolic genes expression level between rainy and dry seasons in malaria vectors populations Anopheles coluzzii (a) and Anopheles gambiae (b) populations respectively from Nkolondom and Ngousso (Yaoundé, Cameroon) in October 2019. The stars indicate the significance of the expression level of a single gene, or the difference in expression levels between wet and dry seasons. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; the first level analyses single gene expression significance while the upper level is comparing difference in expression between two seasons.
For the peri‐urban area, An. gambiae presented higher levels of over‐expression with all the six tested genes showing significant fold change at least during one of the two seasons (Figure 6b): During the rainy season, when farmers are doing high cropping, all the 6 genes were significantly over‐expressed. Within the dry season, Cyp6M2 and Cyp6Z1 were the only significantly over‐expressed genes. By comparing the fold changes between seasons, we observed three genes with significant variations: Cyp6P4, Cyp6Z2, and Cyp9K1.
Do we have any similarities between both collection areas in terms of gene expression? A r. comparing the 6 genes expression profiles for the two areas reveals a strong similarity within the dry season (r: 0.83, p = 0.02) but not for rainy seasons (r: 0.59, p = 0.16) thus the two areas show similar profile during the dry season but differ within the rainy season.
DISCUSSION
In this study, we investigated the level of resistance to insecticides of malaria vectors in the urban environment of Yaoundé, the implication of different resistance mechanisms in the process, the seasonal variations in resistance, as well as the mechanism involved. A high level of resistance was observed in the selected areas to all insecticides used, with an implication of metabolic genes in association with target‐site mutations. Pyrethroid resistance has been widespread in Cameroon for several years and especially in our study sites (Antonio‐Nkondjio et al., 2017).
Vector species composition, correlation with previous studies
The species collected in both localities follow our predictions on the spatial distribution of An. gambiae complex members in the urban area and periphery of Yaoundé. This situation has been observed for nearly 10 years (Aguilar et al., 2010; Antonio‐Nkondjio et al., 2016; Tene et al., 2013) and has not changed up to today. While this could be predictable in an urban area, it was not the case for the peri‐urban site where the ongoing increase in population and urbanization should have been accompanied by a progressive introduction of An. coluzzii in the area, following the patterns of both the species distribution in the town (Kamdem et al., 2012; Tene et al., 2010). The evolution may therefore be slower and there are probably other ecological factors involved that are not well clarified. Also, the continuous presence of vectors during all seasons stipulates a perennial transmission of malaria throughout the year.
Observed resistance and intensity at 5× and 10× for pyrethroids
The bioassays carried on the two targeted populations with diagnostic doses of DDT, deltamethrin, and permethrin resulted in mortality rates below 25% after 60 min of insecticide exposure, regardless of collection season. Furthermore, the results of the bioassays for DDT are of particular interest, as they show that the populations are extremely resistant, with no single mortality (0%) in the peri‐urban market‐gardening area.
Since 2016, WHO decides to incorporate for certain insecticides the 5× and 10× doses when discriminant concentrations are not providing information on the full resistance profile in the field (WHO, 2016). The current high level of resistance to permethrin and deltamethrin at 5× and 10× is likely to be the result of prolonged selection pressure resulting in mosquitoes with multiple resistance mechanisms: target‐site resistance and metabolic resistance. In our vectors, the KDRw mutation is nearly fixed in both populations and is associated throughout the year with the overexpression of genes linked to metabolic resistance. This particular profile of multiplicative resistance could be at the base of the observed resistance intensity (Yewhalaw et al., 2011). ‘When resistance is confirmed at the 5× and especially at the 10× concentrations, operational failure is likely’ (WHO, 2016). There is thus a need to move in this area to new insecticides for IRS/LLIN or implement new methods such as larval control and attractive toxic sugar baits.
Causes of high resistance to pyrethroid
In Nkolondom, the peri‐urban area, the use of agricultural pesticides may have contributed to the increase in pyrethroid resistance. Many studies, among which some in this particular area, have confirmed that the use of pesticides in agriculture can lead to the selection of resistance in breeding sites due to the bio‐accumulation of those pesticides in soils (Diabate et al., 2002; Yadouleton et al., 2009). In Etoa‐Meki, the urban area, the selection of resistance could be a result of the pollution observed in breeding sites in the collection area, associated with the domestic use of insecticides and LLINs. Previous studies comparing this area to others in the same town but with the lowest breeding sites pollution (Tene et al., 2013) support this hypothesis, and similar observations on the link between water pollutants and resistance have been made in other countries (Awolola et al., 2007; Diabate et al., 2002; Djouaka et al., 2007; Jones, Toé, et al., 2012; Poupardin et al., 2012). It is nevertheless necessary to remember that selection of resistance is not occurring only in larval stages. For adult mosquitoes, the intensive use of insecticides in LLINs and domestic indoor sprayings is also a factor of selection as observations have been made on a reduction of susceptibility of malaria vectors after LLINs implementations (Czeher et al., 2008). Knowing that in Cameroon the coverage of LLINs was around 73% of houses with at least one LLIN in 2018 (USAID 2020), we can assume that the observed high level of resistance should also be linked to selection pressure from the insecticides used in LLINs. A selection of metabolic resistant strains, in association with kdr mutations, could be one of the factors underlying this figure, with cross‐resistance leading to loss of susceptibility to other tested insecticides (Chandre et al., 1999).
The investigations of kdr mutation frequencies show that the L1014F mutation is nearly (but not fully) fixed in our field populations, this being the result of long‐term selection pressure by agricultural pesticides, LLINs insecticides, and domestic insecticides (Yadouleton et al., 2009; Yahouédo et al., 2016). Also, it has recently been shown that the substitutions in the amino acid sequence of the voltage gate sodium channel gene that induce resistance are more complex than actually described (Clarkson et al., 2021). The involvement of some of these not yet well‐described mutations in target‐site resistance (Williams et al., 2021) could explain the non‐fixation of L1014F mutations in our populations since time.
Bed‐nets efficacy tests
The World Malaria Report (WHO, 2020) noted that global progress in the fight against malaria has stalled since 2015 showing an increase in malaria cases between 2015 and 2019, with a possible explanation being the widespread pyrethroid resistance in vectors. A test was done with two formulations of one of the most distributed nets in the country the Olyset® and his newer version OlysetPlus® containing a synergist (piperonyl butoxide) in addition to permethrin. Despite the high resistance profile detected in‐field populations, the cone tests gave us satisfying results in the end. If the first version has already lost its efficacy on‐field mosquitoes for many years (Pennetier et al., 2013), the combined version, thanks to the presence of synergist, remains well efficient with a mortality level above 90% today. This result is surprising given the presence of many resistance mechanisms in our field populations and the results of WHO tests but remains good as there is still a solution against these resistant field strains. Such information in addition to field condition bed nets efficacy tests in experimental hosts that shows loss of efficacy of some new nets formulations (PBO base nets) (Menze et al., 2020) will help the National Malaria Control Program in the choice of LLINs to be used in the areas of the country where resistance have been described while waiting for the new insecticides formulations to be available for mass usage.
Factors underlying seasonal dynamic of resistance
The seasonal survey was done on An. coluzzii in the urban area and reveals an interesting profile in the variation of susceptibility along the year. The mortality rates show various levels of resistance per season. Our sample collections were made in two consecutive years. A comparison of the SDS data from the 2 years showed a strong similarity in the resistance profile over the 2 years. This observation shows that the variation from 1 year to the next is quite low. However, caution should be taken when interpreting these results as they only include data from two consecutive years.
The kdr mutations, showed high frequencies with almost no seasonal variation, in contrast to the expression levels of the metabolic genes. Previous studies in similar areas have incriminated urban pollution as the factor stimulating overexpression of metabolic genes (Poupardin et al., 2012; Tene et al., 2012, 2013) and, knowing that there is multiplicative resistance in such situations (Taskin et al., 2016), we expected to have during low rains periods in an urban area the highest pollution in breeding sites leading to high levels of detoxication enzymes production and thus the lowest permethrin mortality. Our results follow this viewpoint for three seasons: LRS, SRS, and SDS with statistically similar profiles. Among them, the LRS is the one having the lowest resistance to permethrin for the three concentrations, and the lowest ratio of PBO susceptibility restoration. Knowing that in this season the rainfalls are renewing frequently water in breeding sites, the level of pollution is low and thus the linked metabolic enzymes expression also. The resistance profiles of SRS and SDS follow the same pattern, with the SDS having less mortality than the rainy seasons and a higher restoration ratio. The only season not following the pattern is LDS. Here, the level of restoration rises to more than the double of the other seasons. This high implication of metabolic enzymes could be a result of the high level of pollution caused by water stagnation and organic matter decomposition in breeding sites followed by a selection of individuals producing a high level of detoxification enzymes among which some can metabolize insecticides molecules (Muller et al., 2008; Poupardin et al., 2012). However, such high overexpression of detoxification enzymes to metabolize xenobiotics may have weakened those mosquitoes and reduced their ability to overcome insecticides. This fitness cost should be an explanation for the mortality observed during this season. The resistance to 5× and 10× also follow the seasonal pattern, increasing from SDS to LDS.
In addition to the detected target‐site mutation, the implication of metabolic enzymes has been proven firstly by the partial restoration of susceptibility with the synergist piperonyl butoxide (PBO) and over‐expression of some metabolic enzymes. The implication of P450s in DDT, permethrin, and deltamethrin have been proven many times, and some specific P450s enzymes have previously been found over‐expressed in these vectors species, mainly cyp6M2, cyp6P4, cyp9K1, and cyp6P3 (Adolfi et al., 2019; Fagbohun et al., 2019). This is the first study comparing gene expression profiles between dry and rainy seasons. The observed profiles of gene expressions showed a similarity between the dry season in both studied areas and a huge difference between the rainy seasons. In the urban area, over‐expression is higher during the dry season, this may probably be in link with the accumulation of organic matter decomposition into breeding sites and the concentration of other xenobiotics after water volume reduction due to evaporation (Jones, Toé, et al., 2012; Poupardin et al., 2012; Tene et al., 2013). During the rainy season, constant rains will increase breeding site size and frequently renew their water, resulting in a lower level of xenobiotics concentration. There will then be lower pressure on Anopheles larvae in breeding sites, resulting in a less stringent selection of individuals capable of producing high concentrations of detoxification enzymes.
In the peri‐urban area, the rainy season is the period of the rapid growth of crops. There is then an intensive usage of insecticides to protect them from pests. This element will then place the maximum selection pressure rather in the rainy season, contrasting what is observed in the other site and resulting in the highest enzyme expressions. Within the dry season, after harvesting, the plantations are abandoned for a period. We then observed a profile similar to that of the urban area, but with a higher overexpression of detoxification enzymes probably due to the presence of pesticide residues in the soil. The presence of these pesticides will lead to a selection at the larval level of individuals with the appropriate genetic background to resist the insecticides, resulting in the emergence of insecticide‐resistant adults.
CONCLUSION
Resistance to pyrethroids is spreading in Cameroon, due to selection pressure exerted in others by agricultural pesticides, urban pollution, and insecticides on urban malaria vectors. The mechanisms involved are very complex, with resistance mechanisms combining to define the high level of resistance observed. This resistance shows a temporal dynamic that seems to be linked to the seasonal variation in the specific selection pressure exerted on vectors in breeding sites. This information is crucial for the implementation of vector control programs, precisely in the choice of techniques to be implemented, the adequate LLINs to be deployed, the insecticide molecules to be used at given seasons for the IRS, or other vector control activity to have the best results possible. A period of lower resistance linked to lower vector densities will be the best to implement vector control activities.
AUTHOR CONTRIBUTIONS
Billy Tene‐Fossog, Charles S. Wondji and Hilary Ranson conceptualized and designed the study. Billy Tene‐Fossog, Yvan Gaetan Fotso‐Toguem and Nathalie Amvongo‐Adjia performed the field collection, bioassays and molecular analysis. Billy Tene‐Fossog analysed the data and drafted the manuscript, which was revised by all co‐authors. All authors read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Supporting information
Table S1. Mortalities recorded for various insecticides during the four seasons in Etoa‐Meki (Anopheles coluzzii) and Nkolondom (Anopheles gambiae) in 2019 and 2020.
ACKNOWLEDGEMENTS
We thank all the farmers in Nkolondom who gave us access to their fields, and all the members of the PIIVeC team who provided us with many good trainings. This work was supported by the Medical Research Council, UK, and Global Challenge Research Fund, through the PIIVeC project based at LSTM (grant number MR/P027873/1).
Tene‐Fossog, B. , Fotso‐Toguem, Y.G. , Amvongo‐Adjia, N. , Ranson, H. & Wondji, C.S. (2022) Temporal variation of high‐level pyrethroid resistance in the major malaria vector Anopheles gambiae s.l. in Yaoundé, Cameroon, is mediated by target‐site and metabolic resistance. Medical and Veterinary Entomology, 36(3), 247–259. Available from: 10.1111/mve.12577
Funding information UK Research and Innovation Global Challenges Research Fund, Grant/Award Number: MR/P027873/1
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Abbott, W.S. (1987) A method of computing the effectiveness of an insecticide. Journal of the American Mosquito Control Association, 3, 302–303. [PubMed] [Google Scholar]
- Adolfi, A. , Poulton, B. , Anthousi, A. , Macilwee, S. , Ranson, H. & Lycett, G.J. (2019) Functional genetic validation of key genes conferring insecticide resistance in the major African malaria vector, Anopheles gambiae . Proceedings of the National Academy of Sciences of the United States of America, 116(51), 201914633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar, R. , Simard, F. , Kamdem, C. , Shields, T. , Glass, G.E. , Garver, L.S. et al. (2010) Genome‐wide analysis of transcriptomic divergence between laboratory colony and field Anopheles gambiae mosquitoes of the M and S molecular forms. Insect Molecular Biology, 19, 695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akogbeto, M.C. , Djouaka, R. & Kinde‐Gazard, D. (2006) Screening of pesticide residues in soil and water samples from agricultural settings. Malaria Journal, 5, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonio‐Nkondjio, C. , Fossog, B.T. , Ndo, C. , Djantio, B. , Togouet, S. , Awono‐Ambene, P. et al. (2011) Anopheles gambiae distribution and insecticide resistance in the cities of Douala and Yaounde (Cameroon): influence of urban agriculture and pollution. Malaria Journal, 10, 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonio‐Nkondjio, C. , Ndo, C. , Njiokou, F. , Bigoga, J.D. , Awono‐Ambene, P. , Etang, J. et al. (2019) Review of malaria situation in Cameroon: technical viewpoint on challenges and prospects for disease elimination. Parasites & Vectors, 12, 501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonio‐Nkondjio, C. , Poupardin, R. , Tene, B.F. , Kopya, E. , Costantini, C. , Awono‐Ambene, P. et al. (2016) Investigation of mechanisms of bendiocarb resistance in Anopheles gambiae populations from the city of Yaoundé, Cameroon. Malaria Journal, 15, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonio‐Nkondjio, C. , Sonhafouo‐Chiana, N. , Ngadjeu, C.S. , Doumbe‐Belisse, P. , Talipouo, A. , Djamouko‐Djonkam, L. et al. (2017) Review of the evolution of insecticide resistance in main malaria vectors in Cameroon from 1990 to 2017. Parasites & Vectors, 10, 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonio‐Nkondjio, C. , Tene Fossog, B. , Kopya, E. , Poumachu, Y. , Menze Djantio, B. , Ndo, C. et al. (2015) Rapid evolution of pyrethroid resistance prevalence in Anopheles gambiae populations from the cities of Douala and Yaounde (Cameroon). Malaria Journal, 14, 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awolola, T.S. , Oduola, A.O. , Obansa, J.B. , Chukwurar, N.J. & Unyimadu, J.P. (2007) Anopheles gambiae s.s. breeding in polluted water bodies in urban Lagos, southwestern Nigeria. Journal of Vector Borne Diseases, 44, 241–244. [PubMed] [Google Scholar]
- Bass, C. , Nikou, D. , Donnelly, M.J. , Williamson, M. , Ranson, H. , Ball, A. et al. (2007) Detection of knockdown resistance (kdr) mutations in Anopheles gambiae: a comparison of two new high‐throughput assays with existing methods. Malaria Journal, 6, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- B‐Bernard, C. & Philogène, B.J.R. (1993) Insecticide synergists: role, importance, and perspectives. Journal of Toxicology and Environmental Health, 38, 199–223. [DOI] [PubMed] [Google Scholar]
- Chandre, F. , Darriet, F. , Manga, L. , Akogbeto, M. , Faye, O. , Mouchet, J. et al. (1999) Pyrethroid cross resistance spectrum among populations of Anopheles gambiae s.s. from Côte d'Ivoire. Journal of the American Mosquito Control Association, 15, 53–59. [PubMed] [Google Scholar]
- Clarkson, C.S. , Miles, A. , Harding, N.J. , O'Reilly, A.O. , Weetman, D. , Kwiatkowski, D. et al. (2021) The genetic architecture of target‐site resistance to pyrethroid insecticides in the African malaria vectors Anopheles gambiae and Anopheles coluzzii . Molecular Ecology, 30, 5303–5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeher, C. , Labbo, R. , Arzika, I. & Duchemin, J.‐B. (2008) Evidence of increasing Leu‐Phe knockdown resistance mutation in Anopheles gambiae from Niger following a nationwide long‐lasting insecticide‐treated nets implementation. Malaria Journal, 7, 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabate, A. , Baldet, T. , Chandre, F. , Akogbeto, M. , Guiguemde, T.R. , Darriet, F. et al. (2002) The role of agricultural use of insecticides in resistance to pyrethroids in Anopheles gambiae s.l. in Burkina Faso. American Journal of Tropical Medicine and Hygiene, 67, 617–622. [DOI] [PubMed] [Google Scholar]
- Djouaka, R.F. , Bakare, A.A. , Bankole, H.S. , Doannio, J.M. , Coulibaly, O.N. , Kossou, H. et al. (2007) Does the spillage of petroleum products in Anopheles breeding sites have an impact on the pyrethroid resistance? Malaria Journal, 6, 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etang, J. , Fondjo, E. , Chandre, F. , Morlais, I. , Brengues, C. , Nwane, P. et al. (2006) First report of knockdown mutations in the malaria vector Anopheles gambiae from Cameroon. The American Journal of Tropical Medicine and Hygiene, 74, 795–797. [PubMed] [Google Scholar]
- Etang, J. , Manga, L. , Toto, J.C. , Guillet, P. , Fondjo, E. & Chandre, F. (2007) Spectrum of metabolic‐based resistance to DDT and pyrethroids in Anopheles gambiae s.l. populations from Cameroon. Journal of Vector Ecology, 32, 123–133. [DOI] [PubMed] [Google Scholar]
- Etang, J. , Pennetier, C. , Piameu, M. , Bouraima, A. , Chandre, F. , Awono‐Ambene, P. et al. (2016) When intensity of deltamethrin resistance in Anopheles gambiae s.l. leads to loss of Long Lasting Insecticidal Nets bio‐efficacy: a case study in north Cameroon. Parasites & Vectors, 9, 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagbohun, I.K. , Oyeniyi, T.A. , Idowu, T.E. , Otubanjo, O.A. & Awolola, S.T. (2019) Cytochrome P450 mono‐oxygenase and resistance phenotype in DDT and deltamethrin‐resistant Anopheles gambiae (Diptera: Culicidae) and Culex quinquefasciatus in Kosofe, Lagos, Nigeria. Journal of Medical Entomology, 56, 817–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies, M.T. & de Meillon, B. (1968) The Anophelinae of Africa south of the Sahara (Ethiopian Zoogeographical Region), 2nd edition. Johannesburg: The South African Institute for Medical Research. [Google Scholar]
- Jones, C.M. , Liyanapathirana, M. , Agossa, F.R. , Weetman, D. , Ranson, H. , Donnelly, M. et al. (2012) Footprints of positive selection associated with a mutation (N1575Y) in the voltage‐gated sodium channel of Anopheles gambiae . Proceedings of the National Academy of Sciences of the United States of America, 109, 6614–6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, C.M. , Toé, H.K. , Sanou, A. , Namountougou, M. , Hughes, A. , Diabaté, A. et al. (2012) Additional selection for insecticide resistance in urban malaria vectors: DDT resistance in Anopheles arabiensis from Bobo‐Dioulasso, Burkina Faso. PLoS One, 7, e45995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamdem, C. , Tene Fossog, B. , Simard, F. , Etouna, J. , Ndo, C. , Kengne, P. et al. (2012) Anthropogenic habitat disturbance and ecological divergence between incipient species of the malaria mosquito Anopheles gambiae . PLoS One, 7, e39453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak, K. (1984) Organization and mapping of a sequence on the Drosophila melanogaster X‐chromosome and Y‐chromosome that is transcribed during spermatogenesis. Genetics, 107, 611–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui, N. (2015) Insecticides resistance in mosquitoes: impact, mechanisms, and research directions. Annual Review of Entomology, 60, 537–559. [DOI] [PubMed] [Google Scholar]
- Martinez‐Torres, D. , Chandre, F. , Williamson, M.S. , Darriet, F. , Berge, J.B. , Devonshire, A.L. et al. (1998) Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Molecular Biology, 7, 179–184. [DOI] [PubMed] [Google Scholar]
- Mavridis, K. , Wipf, N. , Medves, S. , Erquiaga, I. , Müller, P. & Vontas, J. (2019) Rapid multiplex gene expression assays for monitoring metabolic resistance in the major malaria vector Anopheles gambiae . Parasites & Vectors, 12, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menze, B.D. , Kouamo, M.F. , Wondji, M.J. , Tchapga, W. , Tchoupo, M. , Kusimo, M.O. et al. (2020) An experimental hut evaluation of PBO‐based and pyrethroid‐only nets against the malaria vector Anopheles funestus reveals a loss of bed nets efficacy associated with GSTe2 metabolic resistance. Genes, 11, 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menze, B.D. , Riveron, J.M. , Ibrahim, S.S. , Irving, H. , Antonio‐Nkondjio, C. , Awono‐Ambene, P.H. et al. (2016) Multiple insecticide resistance in the malaria vector Anopheles funestus from Northern Cameroon is mediated by metabolic resistance alongside potential target site insensitivity mutations. PLoS One, 11, e0163261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menze, B.D. , Wondji, M.J. , Tchapga, W. , Tchoupo, M. , Riveron, J.M. & Wondji, C.S. (2018) Bionomics and insecticides resistance profiling of malaria vectors at a selected site for experimental hut trials in central Cameroon. Malaria Journal, 17, 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller, P. , Warr, E. , Stevenson, B.J. , Pignatelli, P.M. , Morgan, J.C. , Steven, A. et al. (2008) Field‐caught permethrin‐resistant Anopheles gambiae overexpress CYP6P3, a P450 that metabolises pyrethroids. PLoS Genetics, 4, e1000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwane, P. , Etang, J. , Chouaibou, M. , Toto, J. , Koffi, A. , Mimpfoundi, R. et al. (2013) Multiple insecticide resistance mechanisms in Anopheles gambiae s.l. populations from Cameroon, Central Africa. Parasites & Vectors, 6, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennetier, C. , Bouraima, A. , Chandre, F. , Piameu, M. , Etang, J. , Rossignol, M. et al. (2013) Efficacy of Olyset® Plus, a new long‐lasting insecticidal net incorporating permethrin and piperonil‐butoxide against multi‐resistant malaria vectors. PLoS One, 8, e75134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poupardin, R. , Riaz, M.A. , Jones, C.M. , Chandor‐Proust, A. , Reynaud, S. & David, J.P. (2012) Do pollutants affect insecticide‐driven gene selection in mosquitoes? Experimental evidence from transcriptomics. Aquatic Toxicology, 114‐115, 49–57. [DOI] [PubMed] [Google Scholar]
- R_Core_Team . (2012) R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Ranson, H. , Jensen, B. , Vulule, J.M. , Wang, X. , Hemingway, J. & Collins, F.H. (2000) Identification of a point mutation in the voltage‐gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect Molecular Biology, 9, 491–497. [DOI] [PubMed] [Google Scholar]
- Riveron, J.M. , Irving, H. , Ndula, M. , Barnes, K.G. , Ibrahim, S.S. , Paine, M.J.I. et al. (2013) Directionally selected cytochrome P450 alleles are driving the spread of pyrethroid resistance in the major malaria vector Anopheles funestus . Proceedings of the National Academy of Sciences of the United States of America, 110, 252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santolamazza, F. , Mancini, E. , Simard, F. , Qi, Y. , Tu, Z. & della Torre, A. (2008) Insertion polymorphisms of SINE200 retrotransposons within speciation islands of Anopheles gambiae molecular forms. Malaria Journal, 7, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen, T.D. & Livak, K.J. (2008) Analyzing real‐time PCR data by the comparative CT method. Nature Protocols, 3, 1101–1108. [DOI] [PubMed] [Google Scholar]
- Service, M.W. (1993) Mosquito ecology: field sampling methods, 2nd edition. London: Springer, Elsevier Applied Sciences. [Google Scholar]
- Suchel, B. (1972) Les climats du Cameroun. Thèse Doctorat d'état Université de Bordeaux III: p. 186.
- Taskin, B.G. , Dogaroglu, T. , Kilic, S. , Dogac, E. & Taskin, V. (2016) Seasonal dynamics of insecticide resistance, multiple resistance, and morphometric variation in field populations of Culex pipiens . Pesticide Biochemistry and Physiology, 129, 14–27. [DOI] [PubMed] [Google Scholar]
- Tchouakui, M. , Tene Fossog, B. , Ngannang, B. , Djonabaye, D. , Tchapga, W. , Njiokou, F. et al. (2019) Investigation of the influence of a glutathione S‐transferase metabolic resistance to pyrethroids/DDT on mating competitiveness in males of the African malaria vector, Anopheles funestus . Wellcome Open Research, 4, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tene, B.F. , Antonio, C.N. , Kamdem, C. , Bousses, P. , Fontenille, D. , Besansky, N.J. et al. (2010) Spatial distribution, habitat characterization and dynamics of Anopheles gambiae molecular forms larval biotopes along an urbanization gradient in the city of Yaounde, Cameroon. American Journal of Tropical Medicine and Hygiene, 83, 384–385. [Google Scholar]
- Tene, F.B. , Kopya, E. , Ndo, C. , Menze‐Djantio, B. , Costantini, C. , Njiokou, F. et al. (2012) Water quality and Anopheles gambiae larval tolerance to pyrethroids in the Cities of Douala and Yaoundé (Cameroon). Journal of Tropical Medicine, 10, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tene, F.B. , Poupardin, R. , Costantini, C. , Awono‐Ambene, P. , Wondji, C.S. , Ranson, H. et al. (2013) Resistance to DDT in an urban setting: common mechanisms implicated in both M and S forms of Anopheles gambiae in the City of Yaoundé Cameroon. PLoS One, 8, e61408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. President's Malaria Initiative . (2020) Malaria operational plan FY 2020. Cameroon: USAID/PMI. [Google Scholar]
- WHO . (2016) Test procedures for insecticide resistance monitoring in malaria vectos. Geneva: World Health Organization. [Google Scholar]
- WHO . (2019) World malaria report 2019. Geneva: World Health Organization. [Google Scholar]
- WHO . (2020) World malaria report 2020: 20 years of global progress & challenges. Geneva: WHO Press. [Google Scholar]
- Williams, J. , Cowlishaw, R. , Sanou, A. , Ranson, H. & Grigoraki, L. (2021) In vivo functional validation of the V402L voltage gated sodium channel mutation in the malaria vector An. gambiae. Pest Management Science, 78(3), 1155–1163. [DOI] [PubMed] [Google Scholar]
- Wondji, C.S. , Dabire, R.K. , Tukur, Z. , Irving, H. , Djouaka, R. & Morgan, J.C. (2011) Identification and distribution of a GABA receptor mutation conferring dieldrin resistance in the malaria vector Anopheles funestus in Africa. Insect Biochemistry and Molecular Biology, 41, 484–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadouleton, A.W. , Asidi, A. , Djouaka, R.F. , Braima, J. , Agossou, C.D. & Akogbeto, M.C. (2009) Development of vegetable farming: a cause of the emergence of insecticide resistance in populations of Anopheles gambiae in urban areas of Benin. Malaria Journal, 8, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahouédo, G.A. , Cornelie, S. , Djègbè, I. , Ahlonsou, J. , Aboubakar, S. , Soares, C. et al. (2016) Dynamics of pyrethroid resistance in malaria vectors in southern Benin following a large scale implementation of vector control interventions. Parasites & Vectors, 9, 385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yewhalaw, D. , Wassie, F. , Steurbaut, W. , Spanoghe, P. , Van Bortel, W. , Denis, L. et al. (2011) Multiple insecticide resistance: an impediment to insecticide‐based malaria vector control program. PLoS One, 6, e16066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Mortalities recorded for various insecticides during the four seasons in Etoa‐Meki (Anopheles coluzzii) and Nkolondom (Anopheles gambiae) in 2019 and 2020.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


