Abstract
Cutaneous adnexal tumours (ATs) encompass a variegated group of hamartomas and benign or malignant tumours, originating from the hair follicle, sebaceous, eccrine or apocrine glands that may simulate other cutaneous neoplasms. This study aims to provide a comprehensive overview of the spectrum of clinical and dermoscopic features of ATs, to better define these lesions and assist in the differential diagnosis. We performed a two‐step systematic search of the literature in PubMed, Embase and Cochrane Library databases from inception until 4 September 2020. In the first step, we aimed to define histological variants of ATs with descriptions of dermoscopic criteria. The second step included a search for the name of each previously identified AT variants in the same databases adding ‘AND (epilum* or dermosc* or dermatosc*)’. All study types in English language reporting dermoscopic images of ATs were included. Collisions between ATs and other inflammatory or neoplastic skin lesions were excluded, with the exception of collisions with a sebaceous nevus. The protocol of this study was prospectively registered in PROSPERO (CRD42021244677). In total, 206 articles met our inclusion criteria, encompassing 372 ATs in 365 patients. Most ATs were apocrine‐eccrine (n = 217, 58.3%, n = 173 benign) with a prevalence of poromas (n = 82), followed by follicular ATs (n = 88, 23.7%, n = 83 benign) and sebaceous ATs (n = 67, 18.0%, n = 49 benign). Most patients had a single AT lesion (320, 86.0%), while 42 (11.3%) had multiple ATs. A syndrome causing multiple ATs was identified in 15 patients. Histopathological analysis revealed 82% benign (n = 305) and 18.0% malignant (n = 67). ATs were classified according to their ability to mimic four groups of more common skin tumours: basal cell carcinoma, squamous cell carcinoma, melanocytic lesions and benign cutaneous lesions. Moreover, we have highlighted the ability of malignant variants of ATs to simulate benign skin lesions. This systematic review offers a comprehensive overview of the common clinical and dermoscopic features of follicular, sebaceous and apocrine‐eccrine ATs and details possible differential dermoscopic features.
Keywords: adnexal skin tumours, dermoscopy, dermatoscopy, follicular, sebaceous, glandular, eccrine, apocrine
Abbreviations
- AT
adnexal tumor
- BCC
basal cell carcinoma
- nBCC
nodular basal cell carcinoma
- iBCC
infiltrative basal cell carcinoma
- sBCC
superficial basal cell carcinoma
- SCC
squamous cell carcinoma
- wdSCC
well‐differentiated squamous cell carcinoma
- m‐pdSCC
moderate ‐ poorly differentiated squamouscell carcinoma
- KA
keratoacanthoma
- RASD
reticular hamartoma with sebaceous differentiation.
- ePD
extramammary Paget’s disease
- TAA
tubular apocrine adenoma.
- TFI
tumor of the follicular infundibulum
- IFK
inverted follicular keratosis
- EMPSGC
endocrine mucin‐producing sweat gland carcinoma
Introduction
Cutaneous adnexal lesions encompass a variegated group of hamartomas and benign or malignant tumours, originating from the hair follicle, sebaceous, eccrine or apocrine glands. 1 , 2 This review considers adnexal tumours (ATs) as the entire group of cutaneous adnexal lesions. ATs are rare and often simulate basal cell or squamous cell carcinomas (BCC and SCC) and final histopathological diagnoses are usually unexpected. AT lesion diagnoses are associated with skin lesions mimicking either BCC or SCC, without typical dermoscopy features. 3
Our purpose is to provide a comprehensive overview of the spectrum of clinical and dermoscopic characteristics of ATs to better define these lesions and assist in the differential diagnosis.
Methods
We conducted a systematic review following the Meta‐analysis of Observational Studies in Epidemiology (MOOSE) reporting guidelines and the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. 4 , 5 The study protocol was prospectively registered in PROSPERO (CRD42021244677). 6
Search strategy
We performed a two‐step systematic search of the literature in PubMed, Embase and Cochrane Central Register of Controlled Trials electronic databases from inception until September 4, 2020.
In the first step, we aimed to define histological variants of ATs with descriptions of dermoscopic criteria.
The second step included a search for the name of each previously identified AT variants in the same databases adding ‘AND (epilum* or dermosc* or dermatosc*)’. See eMethods for the detailed search strategy. All study types in English language reporting dermoscopic images of ATs were included. Collisions between ATs and other inflammatory or neoplastic skin lesions were excluded, and collisions between different ATs, with the exception of collisions with a sebaceous nevus.
ATs were classified into three categories according to the reported histopathological features and the presumed origin: follicular (from hair follicle), sebaceous (from sebaceous glands) and apocrine‐eccrine (from sweat glands), following the classification of an eminent dermatopathology textbook. 1
Data extraction and quality assessment
Two investigators (M.L. and M.M.) independently performed title/abstract screening and data extraction, according to a standardized extraction form. Any discordance was resolved by a third expert in conducting a systematic review (R.P.).
Clinical, dermoscopic and histology images were also collected, when available. In the case of available histopathological images but incomplete histopathological information, an expert skin tumour pathologist (S.P.) was asked to review the histopathological images.
Two investigators (M.L. and R.P), not blinded to final diagnosis, evaluated clinical and dermoscopic images and jointly assigned each AT subtype to one or more categories of simulators, based on clinical and dermoscopic features. Final categories were reviewed by expert dermoscopists (C.L., G.P.). We assessed the quality of reporting according to previously published guidelines. 7 (Table S1).
Results
Study selection
The initial search strategy identified 1084 records, following the exclusion of duplicates (Figure S1). Of these records, 694 were excluded according to the title/abstract and the remaining studies were assessed for eligibility.
Following full‐text assessment, 166 studies were excluded. Of the 224 remaining articles, 18 were excluded due to reporting collisions between multiple variants of ATs or between ATs and other cutaneous inflammatory or neoplastic lesions.
Two hundred six records were finally selected including 405 cases of ATs with dermoscopic images available. See eReferences for the complete list of the included articles.
Finally, 33 cases were excluded, 19 because of collisions between different variants of ATs, 9 between ATs and other cutaneous lesions, 7 because of reporting follow‐up images of the same cases and 3 were duplicate cases.
Study population
A total of 372 ATs in 365 patients were included in the metanalysis. Of the included 45 histological variants of ATs, 33 were benign while 12 were malignant. A total of 168 (45.2%) were males and 133 (35.8%) females (missing data: 64, 19.0%), median age was 58 years (range: 1–102 years).
Most patients had a single AT lesion (320, 86.0%), while 42 (11.3%) had multiple Ats (missing data: 3, 2.7%). Ethnic group information was available for 226 patients (60.8%); most were Caucasians (n = 129) followed by Asians (n = 58). A syndrome causing multiple ATs was identified in 15 patients: Brooke‐Spiegler syndrome (n = 4), Muir‐Torre (n = 4), Birth‐Hogg‐Dubè (n = 3), Cowden (n = 2), multiple familiar trichoepithelioma (n = 1) and steatocystoma multiplex (n = 1). Histopathological analysis revealed 82% benign (n = 305) and 18.0% malignant (n = 67).
Most ATs were apocrine‐eccrine (n = 217, 58.3%, n = 173 benign) with a prevalence of poromas (n = 82), followed by follicular ATs (n = 88, 23.7%, n = 83 benign) and sebaceous ATs (n = 67, 18.0%, n = 49 benign). Clinical images were available for 324 cases and histological images for 243. Our expert pathologist confirmed all the uncertain diagnosis.
Ethnic information was provided in 206/372 (55.4%) cases, with the great majority involving fair‐skin individuals: Caucasians (129) and Asians (58) (Table S2). Nineteen cases instead involved darker ethnicities: 8 Indians, 5 Hispanics, 3 Africans, 2 African‐Americans and 1 Native American.
Notably, ATs occurring in darker phototypes were almost equally distributed between follicular (6), sebaceous (7) and sweat gland (6) tumours, with a prominence of benign ATs (14), while malignant tumours only derived from sweat glands, except for 1 case of sebaceous carcinoma in an Indian patient.
Concerning Caucasian patients, most ATs were from sweat glands (82, 63.6%), followed by follicular (40, 31%) and sebaceous (7, 5.4%) origin. Twelve out of 129 cases (9.3%) were malignant, all derived from sweat glands.
Cases belonging to Asian patients were also mainly originating from sweat glands (36, 62.1%), followed by sebaceous (15, 25.9%) and follicular tumours (7, 12.1%).
The ratio of malignant ATs was significantly higher in Asians than Caucasians with 29/58 (50%) cases, 10 of which were sebaceous carcinomas and 19 were malignant sweat gland tumours.
Concerning the ability of ATs to simulate other benign or malignant skin tumours, no differences were noted across different ethnicities.
Tables 1, 2, 3 report AT classifications the complete list of included follicular, sebaceous and apocrine‐eccrine ATs is reported, together with the number of cases and demographics, clinical and main dermoscopic features of each subtype.
Table 1.
Follicular adnexal tumours. 86 cases in 54 articles a
| Benign | Cases/Articles (81/51) | M/F (41/30) | Age range (4/93) | Max diameter range (1–30) | Clinical morphology aspect | Dermoscopy |
|---|---|---|---|---|---|---|
| Basaloid follicular hamartoma | 3/31,33,121 | 3/0 | 14–64 | 3–7 | 2 HN and truncal papules in 2 Indian patients with multiple lesions |
|
| Solitary HN macule in a 64 y/o patients from Singapore |
|
|||||
| Inverted follicular keratosis | 12/49,76,101,184 | 8/4 | 29–93 | 4–12 | 1 HN pigmented macule (black and brown) in a 93 y/o woman |
|
| 11 solitary nonpigmented pink‐reddish papules (6) and nodules (5), mainly on the HN (6) and limbs (3 upper, 3 lower). Patients were all Caucasians. |
|
|||||
| Tumour of the follicular infundibulum | 2/1140 | 1/1 | 53–54 | 5–11 | 2 pinkish macules on lower limbs and trunk of 2 Caucasian patients |
|
| Trichilemmoma (1 desmoplastic) | 7/5 40,77,86,105,128 | 4/3 | 6/84 | 3/10 |
5 solitary, 2 multiple (Cowden syndrome). All nonpigmented HN papules, except one keratotic horny plaque in a 6 y/o Asian girl, on a sebaceous nevus. |
All with 2 concentric areas:
|
| Trichoadenoma | 2/2115,139 | 1/1 | 45/65 | 5/6 | 2 solitary HN pinkish papules |
|
| Trichofolliculoma | 2/2141,66 | 2/0 | 48–62 | 8–10 | 2 HN brownish‐pinkish nodules |
|
| Trichoepitelioma desmoplastic | 5/58,46,91,97,136 | 2/1 | 16–83 | 2–14 | 5 solitary whitish‐pinkish plaques |
|
| Trichoepitelioma | 9/98,49,64,99,100,127,161,174,181 | 3/5 | 8–60 | 3–10 |
All HN papules: 7 white‐pink; 2 brown‐blue. 4 patients had multiple lesions (1 Brooke‐Spiegler syndrome; 1 multiple familial trichoepithelioma) |
|
| Trichoblastoma (1 adamantinoid) | 16/969,94,97,145,146,149,150,159,202 | 5/6 | 22–77 | 1–20 |
1 patient with multiple nodules on HN and trunk 15 solitary nodules or plaques, mainly on HN, of which 9 pigmented (blue‐grey). 6 on sebaceous nevus |
|
| Fibrofolliculoma and Trichodiscoma (1 mixed) | 5 (4 patients)/248,84 | 3/1 | 35–72 | 4 |
All pink‐red papules 1 on HN of a 72 y/o Caucasian woman, 3 on HN and 1 on trunk of 3 Asian patients with Birt‐Hogg Dubè syndrome |
|
| Panfolliculoma | 1/1103 | 1/0 | 60 | 7 | Pink‐reddish truncal nodule |
|
| Pilomatricoma (2 anetodermic; 1 bullous) | 16/123,12,28,68,86,93,97,111,115,182,194,200 | 7/8 | 4–75 | 4–30 |
All solitary nodules, mainly on HN (12) and upper limbs (3). 4 pigmented (blue). 1 on sebaceous nevus |
|
| Melanocytic matricoma | 1/1138 | 1/0 | 65 | 10 | Blue nodule on upper limbs of a Caucasian patient |
|
| Malignant | Cases/Articles (5/4) | M/F (4/0) | Age range (58/72) | Max diameter range (10–40) | Clinical morphology aspect | Dermoscopy |
|---|---|---|---|---|---|---|
| Pilomatrix carcinoma | 1/1155 | 1/0 | 69 | 14 | Solitary HN red‐purple nodule |
|
| Trichilemmal carcinoma | 3/210,97 | 2/0 | 65–72 | 10–40 | Solitary HN pink‐red nodules, 2 ulcerated |
|
| Trichoblastic carcinoma | 1/1205 | 1/0 | 58 | 20 | Ulcerated HN blue‐grey nodules |
|
HN, head and neck.
See eReferences.
Iris‐like structure: radial morphology aspect with erythematous radial lines surrounded by white shiny areas, the redlines appeared thickened and highlighted at their outer end, presenting as red triangles or red projections.
Table 2.
Sebaceous adnexal tumours. 69 cases in 34 articles. a
| Benign | Cases/Articles (50/29) | M/F (18/14) | Age range (1/93) | Max diameter range (2–80) | Clinical morphology aspect | Dermoscopy |
|---|---|---|---|---|---|---|
| Folliculosebaceous cystic hamartoma | 2/22,192 | 1/1 | 29–44 | 5–15 | Pink‐white HN nodules in 1 Asian and 1 Indian patient |
|
| Reticulated acanthoma with sebaceous differentiation | 4/481,125,134,156 | 3/1 | 58–93 | 11–45 |
3 plaques, 1 macule. 3 pigmented (2 lower limbs, 1 trunk): brown‐red or ‐orange; 1 nonpigmented (red‐yellow) |
|
| Sebaceoma | 7/622,42,119,133,151,202 | 2/5 | 40–81 | 4–25 |
Solitary pink‐red nodules (4), papules (2), plaque (1), mainly on HN. 1 pigmented (dark brown‐blue). 1 on sebaceous nevus 1 patient with Muir‐Torre syndrome |
|
| Sebaceous adenoma | 6 (3 patients)/392,109,119 | 0/1 | 79 | 10 | Solitary pink‐yellow truncal nodule (Afro‐American woman) |
|
| 1/0 | 64 | 7 | 5 in 2 patients with Muir‐Torre syndrome with multiple pink‐yellow HN and truncal nodules |
|
||
| Sebaceous hyperplasia | 11/721,59,64,92,137,163,199 | 5/3 | 29–70 | 2–6 |
10 nonpigmented yellow‐orange papules and 1 bluish papule, mainly on HN. 3 patients had multiple lesions |
|
| Sebaceous nevus | 19/6 6,53,90,92,129,186 (+20/11 associated with another AT) | 5/3 | 1–25 | 7–80 | All HN solitary pink‐yellow plaques; 3 pigmented with brown colour |
|
| Steatocystoma multiplex | 1/1 174 | 1/0 | 28 | n.r. | Yellowish HN nodule (Indian) |
|
| Malignant | Cases/Articles (19/8) | M/F (11/8) | Age range (40/102) | Max diameter range (3–40) | Clinical morphology aspect | Dermoscopy |
|---|---|---|---|---|---|---|
| Sebaceous carcinoma | 19/8 31,37,79,97,118,124,152,165 | 11/8 | 40/102 | 3–40 |
All solitary nodules, mostly ulcerated (13) and located on HN (14). 16 pink‐red or yellow; 2 pigmented. 9 in Asian patients, 1 with Muir‐Torre syndrome. 1 on sebaceous nevus |
|
HN = head and neck; n.r. = not reported.
See eReferences.
well‐defined milky‐white cloud‐like structures.
Table 3.
Apocrine‐eccrine adnexal tumours. 217 cases in 122 articles a .
| Benign | Cases/Articles (173/93) | M/F (71/66) | Age range (6/88) | Max diameter range (2–70) | Clinical morphology aspect | Dermoscopy |
|---|---|---|---|---|---|---|
| Cylindroma | 8 (7 patients)/723,38,49,85,96,148,185 | 1/2 | 58–80 | 4–15 | 3: solitary pink‐red HN nodules |
|
| 3/1 | 43–80 | 8–30 | 5: pink nodules, 4 on HN, 1 on trunk, in patients with multiple lesions (3 had Brooke‐Spiegler syndrome) |
|
||
| Spiradenoma | 3/339,189,195 | 3/0 | 55–64 | 4–12 | All solitary HN pigmented nodules |
|
| Spiradenocylindroma | 3/385,164,168 | 1/2 | 37–76 | 8–33 | All pink‐blue nodules: 2 solitary, 1 multiple (Brooke‐Spiegler syndrome) |
|
| Hidradenoma (3 apocrine, 7 nodular, 1 clear cells, 1 eccrine) | 17/913,36,52,110,157,169, 170,198,202 | 7/10 | 19–88 | 5–15 |
11 pink‐yellow‐red solitary nodules (4 ulcerated) on HN (5), trunk (3), limbs (3). 6 pigmented bluish solitary nodules (1 ulcerated) on HN (3), trunk (1), limbs (2) 3 on sebaceous nevus |
|
| Hidradenoma papilliferum | 7/325,141,188 | 1/6 | 22–65 | 5–25 | All solitary nodules, 6 on genitalia (3 ulcerated and 2 pigmented), 1 on HN pigmented |
|
| Hidroacanthoma simplex | 11/832,65,106,113,123,162,170, 175,180,187 | 4/5 | 39–88 | 4–70 | 10 solitary plaques on lower limbs (5), HN (1), trunk (3), 1 unknown: 8 pigmented (brown), 3 ulcerated; 1 Asian 60 y/o patient with multiple plaques on lower limbs |
|
| Hidrocystoma (10 apocrine, 7 eccrine) | 17/1016,36,45,55,89,112,193,201,203,204 | 7/10 | 30–76 | 2–10 |
10 solitary (9 apocrine, 1 eccrine) nodules: 5 pinkish, 5 bluish, on HN (7), trunk (2), acral sites (1). 1 on sebaceous nevus 7 patients with multiple papules on HN (6 eccrine, 1 apocrine): 6 pink‐whitish, only 1 bluish (Robinson‐type) |
Solitary: 6 bluish background or homogenous areas + white lines (3) and linear irregular (2) or no visible (4) vessels; 2 pink background + orange hue and linear irregular vessels (1) or white peripheral and arborizing vessels (1); 2 grey‐white background and linear irregular vessels. Multiple: pink‐blue or brown‐grey background + white halo (4). Eccrine cases (no visible vessels), apocrine case (arborizing and linear irregular vessels |
| Poroma apocrine | 1/118 | 1/0 | 35 | 12 | Brown‐red HN plaque | 1: irregular pigmented globules, milia‐like cysts and focal arborizing vessels |
| Poroma eccrine | 81/354,5,11,15,19,32,41,47,50,54,58,62,63,71,78,80,83,88,95,97,98,108, 113,114,119,130,131,135,154,167,171,172,191,196,197 | 1/1 | 32–52 | 2 | 2: in 2 patients with multiple lesions (poromatosis). (1 Asian, 1 Caucasian) |
1: erosions and glomerular vessels over a pinkish background 1: pinkish areas with salmon halo, white speckles and linear, hairpin and comma vessels |
| 33/15 | 32–86 | 3–30 | 82: solitary pink‐white or reddish (59) or pigmented (23) nodules or plaques, ulcerated in one quarter of cases, on acral sites (20), trunk (14), lower limbs (12) |
Non pigmented: 57: white‐pink background + erosions or ulceration (26: 5 central), yellow structureless areas (12), milky‐red globules (11), white‐yellow scales (10), peripheral white collarette (9), milia‐like cysts (6). Vessels: mainly polymorphous (32), with cherry‐blossom or chalice‐like appearance (14 and 4) and surrounded by white halo (13); in 14 glomerular vesselswith white halo. Patterns: 2 globular (1 cobblestone), 2 white‐pink mesh bands reminiscent of a frog eggs aggregation (the mesh separates globules). Pigmented: 16: blue and/or brown background or structureless areas (in 5 brown‐blue peripheral and white‐pink central) + white‐yellow scales (9), brown‐black dots/globules (6), white lines (4), blue‐grey ovoid nests or leaf‐like areas (3). Vessels: mainly polymorphous or linear irregular (5 and 4). 7: pink‐white background + blue‐grey ovoid nests and arborizing vessels in 3 (surrounded by white halo in 2) brown‐blue globules in 3 + white peripheral halo in 2, central ulceration and polymorphous vessels in 2 brown‐black dots, brown peripheral globules and glomerular vessels surrounded by white halo in 1 |
||
| Dermal duct tumour | 2/132 | 1/n.r. | n.r. | 6 | Solitary brownish nodules on HN and lower limbs |
1: grey‐brown background, with brown‐black dots and globules 1: yellowish papillomatous pattern with white circles Vessels: polymorphic in both cases (cherry‐blossom, glomerular and atypical) and surrounded by a white halo. |
| Syringocystadenoma papilliferum | 10/920,27,29,51,56,87,104,176,202 | 5/5 | 6–74 | 3–15 |
9 solitary pink‐yellow or reddish nodules and plaques, mainly on the HN (7). 4 ulcerated, 5 on sebaceaous nevus. 1 Asian 12 y/o girl with multiple truncal lesions |
most had erythematous background with white‐yellow areas and polymorphous vessels (linear irregular, glomerular, dotted, arborizing and horseshoe‐shaped) + white lines demarcating milky‐red or yellowish lobular structures (4), papillomatous projections surrounded by white circles (2), ulceration (4) |
| Syringoma | 11 (10 patients)/917,44,74,143,153,160,178,186,206 | 3/4 | 23–57 | 3–5 |
8 multiple small brownish‐reddish papules mainly on trunk (7 cases). 7 were reported as eruptive, 1 with linear distribution. |
All: light brownish‐pinkish + peripheral light brown pigmented network in the majority of cases. No visible vessels |
| 0/3 | 22–84 | 4–35 |
3 solitary nonpigmented papule, plaque and nodule, 2 on HN, 1 on genital area (2 Caucasians, 1 Asian). 1 on sebaceous nevus |
All: white‐yellowish structures (scales, streaks) on a pinkish background + clustered dotted, short linear or classical arborizing vessels. | ||
| Tubular apocrine adenoma | 2/282,107 | 2/0 | 49–63 | 3–7.5 | HN black papule and ulcerated nodule |
1: large blue‐grey ovoid nests and fine‐short vessels 1: white globules scattered uniformly over the entire surface, central red‐brown ulceration, fine arborizing, hairpin and glomerular vessels |
| Malignant | Cases/Articles (44/30) | M/F (23/15) | Age range (42/87) | Max diameter range (3–150) | Clinical morphologyaspect | Dermoscopy |
|---|---|---|---|---|---|---|
| Endocrine mucin‐producing sweat gland carcinoma | 1/173 | 1/0 | 78 | 8 | HN pink‐blue nodule | 1: papillomatous appearance with pink‐red globules separated with a white to pink mesh of band and some blue globules. Fine linear irregular vessels. |
| Extrammary Paget's disease | 17/107,26,34,35,43,75,122,126,144,166 | 6/1 | 48–80 | 25–130 |
13 pink‐reddish solitary plaques: 9 nonulcerated on genital area, excepted 1 patient with 1 additional lesion on trunk 4 ulcerated: 3 on genitalia and, 1 on the trunk |
All: pink or milky‐red background + white dots/globules (2), or grey‐black dots (2), or red globules focally surrounded by white colour (1), or white clods surrounded by dotted vessels (1) Other criteria: white lines (2), white scales (6), ulceration (2). Vessels: 8 dotted (1 surrounded by white halo), 2 glomerular (1 surrounded by white halo), 1 polymorphous, 1 not visible Pattern: 1 strawberry, 1 cloud‐like structureless area defined as a combination of a white structureless area resembling diffuse layering of stratus clouds and white small round clods resembling fluffy cumulus clouds |
| 2/2 | 55–86 | 40–150 | 4 pigmented (brown‐black) plaques on genital area (2) or trunk (2) |
1: brown‐white structureless areas with dark brown dots/globules surrounded by greyish halo, regression‐like areas and no visible vessels 1: brown‐red background with blue‐grey structureless areas and brown, blue‐grey and black globules sometimes linearly arranged and negative pigment network 1: white‐pink background with milky‐red areas, brown dots, grey rhomboidal structures, white scales and no visible vessels 1: pink background, white clods, glomerular vessels surrounded by white halo and lava lake structure: combination of branching white reticular lines and intermingling white clods resembling a lava lake inside a live volcanic crater |
||
| Microcystic adnexal carcinoma | 4/424,46,72,177 | 3/1 | 42–78 | 4–30 | 2 solitary pink‐reddish papules, 1 plaque and 1 nodule, on HN and trunk. |
1: central light brown pigmentation, structureless white areas, linear irregular vessels 1: brown‐grey dots on a pinkish‐white background with white‐yellow clods and scales and focused arborizing vessels 1: perilesional light brown pigmentation and a whitish structureless central area, with white‐yellowish clods of variable sizes, no visible vessels. 1: structureless white globules with monomorphic linear/serpentine vessel not crossing the midline. |
| Mucinous carcinoma | 6/330,60,183 | 4/2 | 48–66 | 3–12 |
5 solitary nodules (1 ulcerated) and 1 macule on HN (5 Asian, 1 Caucasian) 4 nonpigmented (pink‐red or yellowish), 2 pigmented (brown‐grey or blue‐red) |
3: white‐grey background with blue‐grey ovoid nests, grey dots and white structures + purple lacunae (2) or thin linear irregular vessels (2) 2: pink‐whitish background, yellow globules, white scales and polymorphous vessels (dotted, linear irregular, hairpin, atypical) 1: pinkish‐bluish background with central ulceration + white scales and thin linear irregular vessels (1) |
| Porocarcinoma (1 mixed between poroma and porocarcinoma) | 12/814,57,61,70,147,171,179,190 | 6/6 | 48–87 | 6–40 |
8: pink‐reddish ulcerated nodules on lower limbs or acral sites; 2 on HN. 2: pigmented (blue‐brown) ulcerated nodule and papule |
Most verrucous surface, pink‐whitish background with erosions or central ulceration and white‐yellowish scales and crusts. 3: pigmented criteria (brown or blue‐grey structureless areas, blue‐grey globules, black dots) Vessels: mostly polymorphic surrounded by white halo (dotted, glomerular, hairpin and linear irregular) |
| Squamoid eccrine ductal carcinoma | 2/2102,158 | 1/1 | 75–76 | 6–20 | 1 pinkish papule and 1 blue‐grey ulcerated nodule both on HN |
1: pinkish background with white globules and arborizing vessels 1: blue‐white colour, reddish‐purple lacunae, linear irregular peripheral vessels |
| Syringoid eccrine carcinoma | 1/1117 | 0/1 | 43 | 13 | 1 HN pink‐red ulcerated plaque | 1: pinkish background, central ulceration and yellow crusts, glomerular and dotted vessels with linear distribution |
| Syringomatous carcinoma | 1/1132 | 0/1 | 83 | 8 | 1 Truncal red nodule | 1: fine linear unfocused branched vessels, white‐pink homogenous backgrounds and surface scales |
HN, head and neck.
See eReferences.
Differential diagnosis
Cutaneous ATs are commonly misinterpreted as more common benign and malignant skin lesions. The most important differential diagnoses are reported in this section, according to the clinical‐dermoscopic patterns, highlighting the main similarities and differences.
Of note, we found that the poroma family, embracing eccrine and apocrine poroma, and hidroacanthoma simplex and dermal ductal tumour, potentially simulated the largest variety of benign and malignant skin lesions, well deserving the title of the ‘great imitator’. 8
When considered all together, most of the AT subtypes were observed at dermoscopy as BCC (21/45) or invasive SCC (12/45) simulators, while only six had a melanoma‐like appearance. The benign ATs were more likely to simulate nodular (n)BCCs (10/33), except for sebaceous benign tumours, which mostly revealed a well‐differentiated (wd)SCC‐ or keratoacanthoma (KA)‐like appearance. No benign AT subtypes were considered as moderate‐poor differentiated (m‐pd)SCC mimickers, while only four had melanoma‐like appearance: reticular hamartoma with sebaceous differentiation (RASD), hidradenoma papilliferum, hidroacanthoma simplex and poroma.
Benign skin lesions more frequently simulated by benign ATs included: dermal (n: 5) and blue (n: 3) nevi, solar lentigo‐SK (n: 5), dermatofibroma (n: 4), viral wart (n: 4) and molluscum contagiosum (n: 4). Malignant AT subtypes more commonly had an SCC‐like appearance (7/12) compare to BCC‐like (6/12) or melanoma‐like (2/12) appearances, with five simulating m‐pdSCC. Finally, only two malignant ATs simulated benign skin lesions: extramammary Paget's disease (ePD) simulating melanosis and porocarcinoma simulating viral wart.
BCC‐like
The most common BCC simulators were apocrine‐eccrine ATs, both benign and malignant, followed by benign follicular ATs (Fig. 1).
Figure 1.
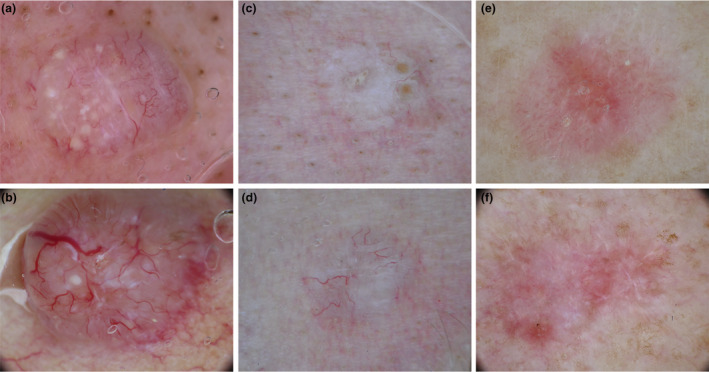
Dermoscopic images of adnexal tumours (top) mimicking basal cell carcinomas (bottom). Trichoepithelioma (a) mimicking a nodular BCC (b). Desmoplastic trichoepithelioma (c) simulating an infiltrative BCC (d). Tumour of the follicular infundibulum (e) mimicking a superficial BCC (f). [Colour figure can be viewed at wileyonlinelibrary.com]
Nodular BCC
Among benign ATs the following subtypes were listed as nodular BCC (nBCC) simulators: trichoblastoma, trichoepithelioma, panfolliculoma, cylindroma, spiradenocylindroma, hidradenoma, hidrocystoma, poroma and tubular apocrine adenoma (TAA). Most of these tumours appeared as translucid papules or nodules, mainly located on the face or scalp, with arborizing vessels (Fig. 1) and occasionally pigment BCC‐specific criteria, mainly blue‐grey ovoid nests, upon dermoscopy evaluation. However, compared with nBCC, arborizing vessels were thinner and less branched (Fig. 1a); vessels were also frequently unfocused and sometimes barely visible, particularly in hidradenoma, hidrocystoma and TAA. Furthermore, AT background colour was paler (pink‐whitish) (Fig. 1a) than the usual reddish colour of nBCC (Fig. 1b). Specific differential features compared with nBCC included: orange‐salmon background hue and interlacing white lines in cylindroma; white roundish structures, histologically correlated to small cysts, sign of follicular differentiation, in trichoepithelioma (Fig. 1a) 9 , 10 , 11 ; homogenous blue‐grey background pigmentation with a symmetrical distribution instead of asymmetric ovoid nests in pigmented tumours. 12 , 13 , 14
Trichoblastoma and its malignant counterpart, trichoblastic carcinoma, were the main nBCC simulators. Although thin, arborizing vessels in trichoblastoma and trichoblastic carcinoma were frequently in‐focus and no additional specific features could be seen in the majority of cases, rendering differential diagnosis with dermoscopy with nBCC impossible. This could be correlated to the lower differentiation and common origin from the hair follicle. 15 Besides trichoblastic carcinoma, several subtypes of apocrine‐eccrine malignant ATs simulated nBCC. As for benign ATs, the background colour was paler and vessels were thinner, less branching and less focused than would be expected in nBCC. Poroma was an exception to this rule, with thicker vessels and a reddish background when simulating nonpigmented nBCC or a bluish homogenous background when mimicking pigmented nBCC. In both cases, convoluted vessels could be found, occasionally with a blossom‐like appearance.
Infiltrative BCC
A minority of ATs were infiltrative BCC (iBCC) simulators (Fig. 1c, d). Specifically, trichoadenoma, desmoplastic trichoepithelioma, spiradenoma and microcystic adnexal carcinoma simulated iBCC because of the presence of ill‐defined borders, a pink‐whitish background and thin arborizing vessels, with few branches and a generally unfocused image (Fig. 1c). These tumours were usually palpable but flatter than those simulating nBCC and nonpigmented, with the exception of spiradenoma frequently displaying blue‐grey homogenous areas, located peripherally. One specific feature allowing to differentiate ATs from iBCC was the presence of small whitish structures in trichoadenoma and desmoplastic trichoepithelioma; in the latter subtype, an ivory background and central umbilication were also commonly described (Fig. 1c).
Superficial BCC
Tumour of the follicular infundibulum (TFI) and hydroacanthoma simplex were considered superficial (s)BCC simulators due to their frequent appearance as flat isolated macules with short and fine vessels, brown structures (maple‐leaf‐like or concentric) and erosions (Fig. 1e, f). However, TFI also displayed more convoluted vessels in the central area of the lesion, separated by a whitish network (Fig. 1e). Hidroacanthoma simplex, however, commonly had additional dermoscopic features simulating solar lentigo or seborrheic keratosis.
SCC‐like
Squamous cell carcinoma was the second most common skin tumour potentially simulated by ATs. The SCC‐like appearance encompasses a spectrum of distinct patterns, ranging from in‐situ to invasive forms. Among invasive SCC, the global clinical‐dermoscopic characteristics vary depending on the degree of differentiation, with wdSCC showing hyperkeratotic, verrucous or KA‐like appearance and m‐pdSCC appearing as proliferating nodules, with atypical vessels and low keratin production. We observed that benign ATs only simulated in‐situ or wdSCC (Fig. 2).
Figure 2.
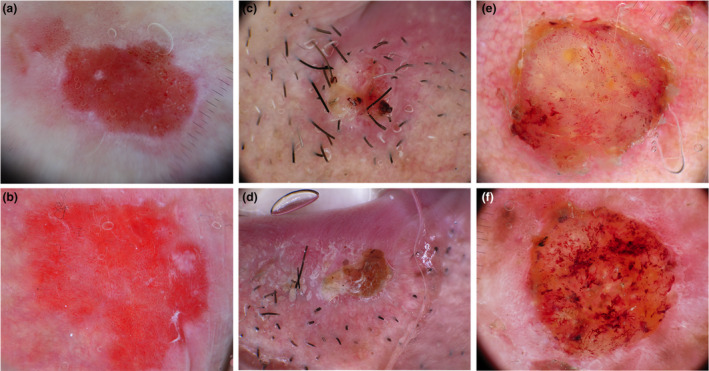
Dermoscopic images of adnexal tumours (top) mimicking squamous cell carcinomas (bottom). Extramammary Paget disease (a) mimicking Bowen's disease (b). Trichilemmoma (c) simulating a well‐differentiated SCC (d). Trichilemmal carcinoma (e) mimicking a poorly‐differentiated SCC (f). [Colour figure can be viewed at wileyonlinelibrary.com]
Bowen's disease
Two benign follicular tumours (inverted follicular keratosis [IFK] and poroma) and two malignant sweat gland tumours (syringoid eccrine carcinoma and ePD) were classified as Bowen's disease mimickers (Fig. 1a, b), mainly because of the presence of dotted and glomerular vessels and scales/crusts. However, differently from Bowen's disease, dotted/glomerular vessels were not clustered and were often combined with hairpin, convoluted or linear unfocused vessels. In ePD, white lines arranged as negative pigment networks were also observed. (Fig. 1a).
Well‐differentiated invasive SCC and keratoacanthoma
Almost all the sebaceous benign tumours were classified as KA or wdSCC mimickers because they commonly appeared as isolated papules or nodules with white circles or roundish structures and linear irregular vessels. Moreover, a central crater was also reported in sebaceoma and sebaceous adenoma. Specific features associated with sebaceous tumours include the presence of a yellowish background without crusts or keratin masses in folliculosebaceous cystic hamartoma, yellowish roundish structures in sebaceous adenoma and sebaceoma, commonly more numerous in the latter. Sebaceous hyperplasia less commonly displayed yellow structures and was generally more easily diagnosed because of its common occurrence in adults as multiple umbilicated papules located on the face and because of the presence of some specific dermoscopic signs, including the bonbon toffee sign and the cumulus sign. Further, the IFK and trichilemmoma follicular benign ATs subtypes were classified as wdSCC or KA simulators. IFK mainly showed a KA‐like appearance with a central keratin mass or crater and peripheral linear vessels. Trichilemmoma (Fig. 2c), however, varied from a KA‐like to hyperkeratotic SCC‐like appearance (cutaneous horn). A specific pattern was also described for trichilemmoma, called iris‐like structure, consisting of a peripheral area with erythematous radial lines surrounded by white shiny areas. 16 Porocarcinoma was the only malignant adnexal tumour classified as wdSCC simulator together with its benign counterpart, poroma. In particular, they mostly simulated verrucous SCC because of their exophytic and papillomatous surface and common acral location, or KA because of the presence of a central crater. However, vessels were more polymorphic and convoluted than those commonly observed in verrucous SCC and KA.
Moderate‐poor differentiated SCC
Almost all the follicular and sebaceous malignant ATs were m‐pdSCC simulators (pilomatrix carcinoma, trichilemmal carcinoma, sebaceous carcinoma), together with one malignant sweat gland tumours (Endocrine mucin‐producing sweat gland carcinoma [EMPSGC]). (Fig. 2e, f) These lesions appeared as solitary proliferating papules or nodules with atypical vessels, ulcerations and crusts. Specific signs prompting their adnexal origin were the presence of white irregular structures in pilomatrix carcinoma, 17 histologically correlated with calcification and the yellow background with yellow roundish structures in sebaceous carcinoma.
Melanocytic lesion
Melanocytic lesions more commonly simulated by ATs were melanoma and dermal or blue nevi. Both benign and malignant ATs can simulate melanoma whereas only benign AT subtypes seemed to simulate dermal and blue nevi (Fig. 3).
Figure 3.
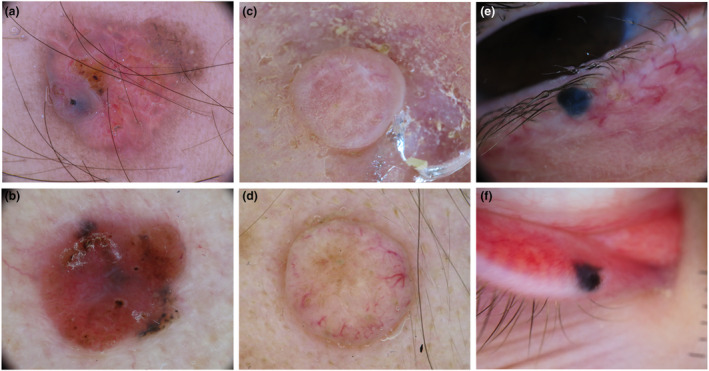
Dermoscopic images of adnexal tumours (top) mimicking melanocytic lesions (bottom). Sebaceoma (a) mimicking a nodular melanoma (b). Eccrine spiradenoma (c) simulating a dermal nevus (d). Apocrine hidrocystoma (e) mimicking a blue nevus (f). [Colour figure can be viewed at wileyonlinelibrary.com]
Melanoma
Malignant ATs potentially simulating melanoma included pilomatrix carcinoma and ePD. The former appeared as an isolated papule or nodule, with atypical vessels and red‐purple areas corresponding to vascular lacunae and haemorrhages. The presence of white irregular structures histologically correlated to calcification can be considered as a specific feature of pilomatrix carcinoma. 17 Pigmented ePD, however, was listed as a melanoma simulator because of the presence of a negative pigment network on a brown‐blue background. Benign ATs simulating melanoma included: RASD, hidroacanthoma simplex, hidradenoma papilliferum and poroma. RASD and hidroacanthoma simplex more commonly appeared as flat pigmented lesions, potentially simulating both melanoma and SK because of the presence of a brown network or pseudonetwork with defined borders, brown globules and blotches. RASD also showed a yellowish negative pigment network and yellow roundish structures, according to its sebaceous origin. Hidroacanthoma simplex also appeared as a homogeneously blue‐black pigmented lesion with atypical vessels. Hidradenoma papilliferum was considered a melanoma simulator mainly because of its nodular morphology and genital location. It generally appeared as a well‐circumscribed bluish or reddish nodule with atypical vessels on labia majora of middle‐aged women. Finally, poroma potentially simulated nodular melanoma when appearing as a pigmented nodule with blue and black homogenous areas or as a nonpigmented nodule with atypical vessels. In the latter case, however, the common finding of a papillomatous or verrucous surface is mainly oriented towards the verrucous SCC hypothesis.
Dermal‐blue nevus
Three apocrine‐eccrine benign ATs (hidradenoma, hidrocystoma and poroma), potentially appeared as bluish homogenous or pinkish with linear unfocused vessels papules and nodules, thus simulating blue and dermal nevi (Fig. 3e,f). Specific features enabling the recognition of hidradenoma and hidrocystoma were their shiny and cystic appearance, and the common location of hidrocystoma on the palpebral region. Poroma, however, had more convoluted and atypical vessels than dermal nevi. Other two benign lesions potentially simulating dermal nevi were trichodiscoma and basaloid follicular hamartoma. They both appeared as pinkish‐brownish nodules, with linear unfocused vessels and brown globules. Brown globules were prominent in basaloid follicular hamartoma, arranged in a cobblestone pattern and were also combined with blue‐grey globules.
Benign nonmelanocytic lesions
Several benign nonmelanocytic lesions might be simulated by ATs, including the solar lentigo‐SK spectrum, but also viral infectious, vascular lesions, dermatofibroma and sebaceous cyst (Fig. 4).
Figure 4.
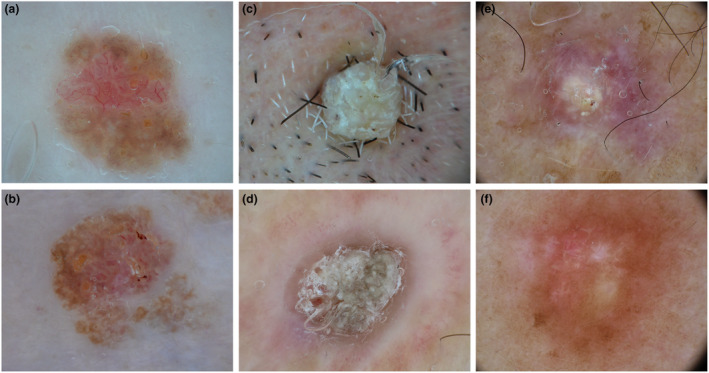
Dermoscopic images of adnexal tumours (top) mimicking benign nonmelanocytic lesions (bottom). Poroma (a) mimicking a seborrheic keratosis (b). Trichilemmoma (c) simulating a viral wart (d). Pilomatricoma (e) mimicking a dermatofibroma (f). [Colour figure can be viewed at wileyonlinelibrary.com]
Solar lentigo—Seborrheic keratosis
A few benign ATs potentially simulated solar lentigo or SK. Specifically, pigmented syringoma commonly appeared as multiple small macules or papules in the periocular region of young women, simulating small solar lentigo. RASD also simulated solar lentigo, appearing as a flat brownish lesion, with pale colours and defined borders; a yellowish negative pigment network or roundish yellow structures were described as specific features suggesting its sebaceous origin. Hidroacanthoma simplex and pilomatricoma may appear as SK simulators. They both appeared as exophytic plaques and nodules with a blue‐brownish background, well‐defined borders, a cerebriform or crusting surface and roundish white structures simulating milia‐like cysts. Of note, Hidroacanthoma simplex frequently simulated clonal SK because of the coexistence of mixed dermoscopic features between SK and BCC. Further, sebaceous nevus may also be considered an SK simulator, especially when small in size and first observed during adulthood, because of its cerebriform surface, well‐defined borders and brownish colour.
Furthermore, one benign follicular tumour (trichofolliculoma) simulated lichen‐planus‐like keratosis, showing a so‐called firework pattern, characterized by course brown‐greyish granules with radial distribution at the periphery. Finally, pigmented ePD potentially simulated melanosis when located in the genital area because of its brownish homogenous appearance. However, pigmented ePD was also characterized by whitish reticular lines arranged as a negative pigment network.
Infectious diseases
Viral warts and molluscum contagiosum were listed as infectious diseases potentially simulated by ATs. In particular, four benign (dermal duct tumour, syringocystoadenoma papilliferum, syringoma and poroma) and one malignant (porocarcinoma) sweat gland tumours were considered as viral wart simulators because of their exophytic morphology and papillomatous surface. However, vessels were mostly polymorphic (cherry, blossom, glomerular) and not dotted or linear vessels with white halo, particularly in poroma and porocarcinoma. 18 , 19 Syringocystoadenoma papilliferum also simulated molluscum contagiosum, together with sebaceous adenoma, sebaceous hyperplasia and trichilemmoma, mainly because of the presence of central umbilication. However, some specific signs could help differentiate these tumours from molluscum contagiosum, such as the common occurrence of syringocystoadenoma papilliferum on sebaceous nevus, the presence of yellowish roundish structures in sebaceous adenoma and of specific signs in trichilemmoma (Iris‐like structures) and sebaceous hyperplasia (bonbon toffee and cumulus sign). 16 , 20 , 21 , 22 , 23 , 24
Other benign lesions
Dermatofibroma was potentially simulated by four benign follicular ATs. Trichofolliculoma, pilomatricoma and melanocytic matricoma mainly simulated aneurysmatic dermatofibroma, because of the presence of whitish and purple‐reddish‐bluish areas in a nodular firm lesion; (Fig. 4e, f) TFI also potentially simulated dermatofibroma because of the presence of a whitish central reticular area and a peripheral brownish pigmentation. Two benign ATs had a cystic nature (hidradenoma and hidrocystoma) appearing as translucent soft papules or nodules with a bluish or pinkish background and sometimes linear unfocused vessels. Fibrofolliculoma, however, potentially simulated milia because of the common finding of roundish yellowish areas covering the majority of the lesion. Hidradenoma papilliferum was listed as a pyogenic granuloma simulator because of the common presence of a reddish background and occasionally a whitish peripheral collarette. Finally, steatocystoma multiplex and sebaceous nevus were listed as xantogranuloma simulators, because of their homogenous yellowish background with some roundish structure. Sebaceous nevus, in particular, potentially simulated xantogranuloma in early stages, when appearing as a flat yellowish patch or plaques on the scalp of young people.
Conclusions
Our systematic review offers a comprehensive overview of the current knowledge on clinical and dermoscopic features of cutaneous ATs and provides an extensive guide that can assist clinicians in differentiating these tumours from other benign and malignant skin lesions.
A limitation is the selective inclusion of lesions with dermoscopic pictures available. However, since the interpretation of confounding dermoscopic criteria may differ depending on the expertise of the evaluator, we would like to offer the readers a uniform evaluation done by experts. Moreover, we were not able to revise the histopathological slides of the included ATs, and we relied on the diagnosis provided by the authors of the included articles.
In conclusion, when dealing with adnexal tumours, dermoscopy reveals a variegated kaleidoscope of shapes and colours, piquing the curiosity of physicians interested in improving their specificity when looking at skin lesions.
Metaphoric terminology
Cherry‐blossom vessels = branched vessels with rounded endings.
Iris‐like structures = peripheral area with erythematous radial lines surrounded by white shiny areas.
Bonbon toffee sign = white‐yellow globules surrounding an umbilicated center resembling a bonbon toffee.
Cumulus sign = single or grouped ovoid milky white areas resembling a cumulus cloud.
Firework pattern = presence of course brown‐greyish granules with radial distribution at the periphery.
Supporting information
Appendix S1. Methos.
Figure S1. Flow chart of search results and study selection.
Table S1. Summary of quality of evidence of the included studies.
Table S2. Ethnic distribution of adnexal tumours.
Appendix S2. References.
Acknowledgements
Open Access Funding provided by Universita degli Studi di Modena e Reggio Emilia within the CRUI‐CARE Agreement.
Reprint requests: Caterina Longo
Funding sources
None.
Conflicts of Interest
The authors have no conflicts of interest relevant to this article to disclose. Reprint requests: Caterina Longo.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Patterson JW. Tumors of cutaneous appendages. In Patterson JW, Hosler GA, Prenshaw KL, eds. Weedon's Skin Pathology, 5th edn. Elsevier, Edinburgh, Scotland, 2020: 951–1015 e25. [Google Scholar]
- 2. Elder D, Massi D, Scolyer R, Willemze R. WHO Classification of Skin Tumours, Vol. 11, 4th edn. International Agency for Research on Cancer, Lyon, France, 2018. [Google Scholar]
- 3. Zaballos P, Gómez‐Martín I, Martin JM, Bañuls J. Dermoscopy of adnexal tumors. Dermatolic Clin 2018; 36: 397–412. [DOI] [PubMed] [Google Scholar]
- 4. Stroup DF, Berlin J, Morton S et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis of observational studies in epidemiology (MOOSE) group. JAMA 2000; 283: 2008–2012. [DOI] [PubMed] [Google Scholar]
- 5. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. J Clin Epidemiol 2009; 62: 1006–1012. [DOI] [PubMed] [Google Scholar]
- 6. National Institute for Health Research . Clinical and dermoscopic features of adnexal skin tumors: a systematic review of the literature. Accessed December 17, 2021. https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=244677
- 7. CEBM (Centre for Evidence‐Based Medicine) . 2009. Oxford Centre for Evidence‐based Medicine—Levels of Evidence (March 2009). Accessed December 16, 2021. https://www.cebm.ox.ac.uk/resources/levels‐of‐evidence/oxford‐centre‐for‐evidence‐based‐medicine‐levels‐of‐evidence‐march‐2009
- 8. Lallas A, Chellini PR, Guimarães MG et al. Eccrine poroma: the great dermoscopic imitator. J Eur Acad Dermatol Venereol 2016; 30: e61–e63. [DOI] [PubMed] [Google Scholar]
- 9. Navarrete‐Dechent C, Bajaj S, Marghoob A, Gonzalez S, Munoz D. Multiple familial trichoepithelioma: confirmation via dermoscopy. Dermatol Pract Concept 2016; 6: 51–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lei D, Zhang S, Li Z, Zhang S, Li J, Wang G. Trichoepithelioma: reflectance confocal microscopy features and dermoscopic features. Skin Res Technol 2021; 27: 283–284. [DOI] [PubMed] [Google Scholar]
- 11. Lazaridou E, Fotiadou C, Patsatsi A et al. Solitary trichoepithelioma in an 8‐year‐old child: clinical, dermoscopic and histopathologic findings. Dermatol Pract Concept 2014; 4: 55–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zaballos P, Bañuls J, Medina C, Salsench E, Serrano P, Guionnet N. Dermoscopy of apocrine hidrocystomas: a morphological study. J Eur Acad Dermatol Venereol 2014; 28: 378–381. [DOI] [PubMed] [Google Scholar]
- 13. Tosti G, Salvini C, Barisani A et al. Vulval hidradenoma papilliferum: a clinical and dermoscopic study. Clin Exp Dermatol 2020; 45: 1035–1039. [DOI] [PubMed] [Google Scholar]
- 14. Zaballos P, Serrano P, Flores G et al. Dermoscopy of tumours arising in naevus sebaceous: a morphological study of 58 cases. J Eur Acad Dermatol Venereol 2015; 29: 2231–2237. [DOI] [PubMed] [Google Scholar]
- 15. Schirren CG, Rütten A, Kaudewitz P, Diaz C, McClain S, Burgdorf WHC. Trichoblastoma and basal cell carcinoma are neoplasms with follicular differentiation sharing the same profile of cytokeratin intermediate filaments. Am J Dermatopathol 1997; 19: 341–350. [DOI] [PubMed] [Google Scholar]
- 16. Horcajada‐Reales C, Avilés‐Izquierdo JA, Ciudad‐Blanco C et al. Dermoscopic pattern in facial trichilemmomas: red iris‐like structure. J Am Acad Dermatol 2015; 72: S30–S32. [DOI] [PubMed] [Google Scholar]
- 17. Ravaioli GM, Lambertini M, Pazzaglia M, Corti B, Fanti PA, Dika E. Pilomatrix carcinoma of the nose: clinical and dermoscopic presentation. JAAD Case Rep 2018; 4: 376–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zalaudek I, Giacomel J, Cabo H et al. Entodermoscopy: a new tool for diagnosing skin infections and infestations. Dermatology 2008; 216: 14–23. [DOI] [PubMed] [Google Scholar]
- 19. Errichetti E. Dermoscopy in monitoring and predicting therapeutic response in general dermatology (non‐tumoral dermatoses): an up‐to‐date overview. Dermatol Ther 2020; 10: 1199–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moscarella E, Argenziano G, Longo C et al. Clinical, dermoscopic and reflectance confocal microscopy features of sebaceous neoplasms in Muir‐Torre syndrome. J Eur Acad Dermatol Venereol 2013; 27: 699–705. [DOI] [PubMed] [Google Scholar]
- 21. Marques‐Da‐Costa J, Campos‐Do‐Carmo G, Ormiga P, Cuzzi T, Ramos‐E‐Silva M. Sebaceous adenoma: clinics, dermatoscopy, and histopathology. Int J Dermatol 2015; 54: e200–e2002. [DOI] [PubMed] [Google Scholar]
- 22. Kim NH, Zell DS, Kolm I, Oliviero M, Rabinovitz HS. The Dermoscopic differential diagnosis of yellow Lobularlike structures. Arch Dermatol 2008; 144: 962. [DOI] [PubMed] [Google Scholar]
- 23. Oztas P, Polat M, Oztas M, Alli N, Ustun H. Bonbon toffee sign: a new dermatoscopic feature for sebaceous hyperplasia. J Eur Acad Dermatol Venereol 2008; 22: 1200–1202. [DOI] [PubMed] [Google Scholar]
- 24. Bryden AM, Dawe RS, Fleming C. Dermatoscopic features of benign sebaceous proliferation. Clin Exp Dermatol 2004; 29: 676–677. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Methos.
Figure S1. Flow chart of search results and study selection.
Table S1. Summary of quality of evidence of the included studies.
Table S2. Ethnic distribution of adnexal tumours.
Appendix S2. References.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


