Abstract
Aims
This work examines the available scientific evidence about the efficiency of essential oils (EO) as an alternative therapy to traditional treatment of fungal infections, including onychomycosis, assessing the effect of the three EO most frequently studied for their antifungal activity (thyme, cinnamon and tea tree EO) against three causative agents of fungal diseases in humans: Trichophyton rubrum, Trichophyton mentagrophytes complex and Candida albicans.
Methods and Results
The PRISMA statement protocol was followed to conduct a bibliographical search and 54 articles that met all the inclusion criteria were retrieved. Differences were observed in the MIC and MFC values depending on the micro‐organism strain and the EO used. The lowest MIC were observed with Cinnamomum zeylanicum EO (0.013–1120 μl ml−1) against the three micro‐organisms. For MFC, the lowest value was found for Thymus vulgaris EO (4.2 μl ml−1) against Trichophyton rubrum.
Conclusions
The antifungal effects of EO could be a very promising solution to overcome the therapeutic shortcomings of antimycotic medication. More experiments are needed to examine the properties of these oils to devise effective and nonaggressive therapies for treatment of dermatophytosis.
Significance and Impact of Study
The results indicate that EO remain good candidates for future treatments and could provide a solution for failed medications and/or adverse reactions to current pharmacological treatments.
Keywords: antifungal treatment, Candida albicans, Cinnamomum zeylanicum, dermatophytosis, Melaleuca alternifolia, Thymus vulgaris, Trichophyton mentagrophytes complex, Trichophyton rubrum
INTRODUCTION
Pathogenic fungi cause infections in humans, plants and animals. Dermatophytosis is a fungal infection caused by dermatophytes. It is the main cause of superficial mycosis and presents a significant public health problem (Mahmoudvand et al., 2014). Dermatophytes are divided into three genera (Trichophyton (T), Microsporum (M), Epidermophyton (E)) and have an affinity for keratinized tissues, in which they are able to grow, for example, hair, skin and nails (Abd Rashed et al., 2021; Ahmadi et al., 2015). Other fungal infections of human skin and its appendages can be caused by nondermatophyte fungi, such as yeasts (Candida (C), Pitorosporum (P), Cryptococcus (C)) and mould fungi (Aspergillus, Scopulariopsis) (Bhatia & Sharma, 2014). Onychomycosis is the dermatophytosis that affects the nails (Hoy et al., 2012; Vlahovic, 2016). Because the nail unit has no immunity mediated by effective cells, it is more susceptible than other parts of the skin to infection by these micro‐organisms (Lipner & Scher, 2019). Approximately 90% of toenail onychomycoses and 75% of all nail onychomycoses are caused by dermatophytes, in particular Trichophyton mentagrophytes complex and Trichophyton rubrum (Bodman & Krishnamurthy, 2021; Gupta et al., 2015; Joyce et al., 2019; Thomas et al., 2010; Youssef et al., 2018). In the case of yeast onychomycoses, Candida albicans accounts for approximately 70% of all onychomycoses caused by yeast (Hoy et al., 2012).
Fungal infections are increasing due to factors including their spread among at‐risk populations, population longevity, the use of immunosuppressive treatments, diabetes mellitus, obesity, hyperhidrosis and nail injury (Hoy et al., 2012; Leung et al., 2020; Papini et al., 2015; Tchernev et al., 2012; Thomas et al., 2010). However, oral treatment of these infections has disadvantages, including increased resistance due to overuse of antifungal agents, pharmacological interactions with other medicines and adverse side effects such as the risk of hepatic lesion, thus limiting the suitability of this type of treatment (Abd Rashed et al., 2021; Martín‐Aragón & Benedí, 2004; Valdes & M. P., 2000; Vlahovic, 2016). Moreover, some oral treatments, such as terbinafine, are ineffective against nondermatophyte fungi (Chang et al., 2007; Hoy et al., 2012). Another key consideration is that oral antimycotic therapies can affect the pharmacokinetics of previously prescribed medications and may change their effects, ranging from reduced efficiency to increased toxicity (Gupta et al., 2018). Because of this, a more appropriate strategy for antifungal treatment could be to develop alternative therapies to conventional treatments. Alternative therapies currently under investigation include the use of essential oils (EO) as possible antifungal agents (Abd Rashed et al., 2021; Hongpattarakere et al., 2008; Lopes et al., 2016; Mahmoudvand et al., 2014; Parrish et al., 2020).
The European Pharmacopoeia Commission recently adopted the following definition of EO (Agencia Española de Medicamentos y Productos Sanitarios, 2016; European Pharmacopoeia: Essential oils, 2021):
Odorous product, usually of complex composition, obtained from a botanically defined plant raw material by steam distillation, dry distillation, or a suitable mechanical process without heating.
Although numerous studies indicate the suitability of EO as an alternative treatment for fungal infections, the properties of each oil largely depend on its composition (da Cruz Cabral et al. 2013; D'agostino et al., 2019; Abd Rashed et al., 2021). As the definition of EO implies, composition can vary due to several factors, including the extraction method, the type and species of plant they are obtained from, the soil composition and the plant growth stage at harvest (European Pharmacopoeia: Essential oils, 2021). Due to the difficulty of attributing a specific effect to a particular compound or various compounds (Lopes et al., 2016), it is important to determine EO composition through chemical analyses such as gas chromatography and mass spectrometry (GC–MS) to verify and standardize the composition of EO and ensure consistency between batch lots over time (Parrish et al., 2020).
The specific mechanism of action of EO remains unclear (Abd Rashed et al., 2021; Parrish et al., 2020). Initially a process of permeabilization was proposed (Flores et al., 2015), in which the EO break down the fungal cell wall and cytoplasmic membranes. Changes in cytoplasmic membrane permeability affect electrolyte balance and may even cause a loss of cell content. Essential oils also impact the mitochondrial membranes by altering their polarity, affecting the flow of protons and the ability to produce ATP (Abd Rashed et al., 2021; Saad et al., 2013; Swamy et al., 2016).
According to some authors, certain EO components are irritating to the skin and mucous membranes and can cause significant damage at high doses (Carson et al., 2006; Lee et al., 2013). Even though the content of these components is minimal in EO, there are no guarantees that adverse reactions will be minimized.
Numerous studies have demonstrated the antifungal action of various commercial EO in studies in vitro (Córdoba et al., 2019; Gucwa et al., 2018; Michalczyk & Ostrowska, 2021; Villar Rodríguez et al., 2021; Wińska et al., 2019). The parameters most frequently used to assess their antimicrobial activity are minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC), that is, the minimum concentration of antifungal agents needed to inhibit growth of the fungus or reduce its growth by 99.9%, respectively (Abd Rashed et al., 2021; Natu & Tatke, 2019; Sharifi‐Rad et al., 2017). The EO most frequently studied for their antifungal activity are thyme (Thymus vulgaris), cinnamon (Cinnamomum zeylanicum) and tea tree (Melaleuca alternifolia) (D'agostino et al., 2019; Scalas et al., 2018). Thyme EO is used as an antifungal agent because of its high thymol and carvacrol content (Kowalczyk et al., 2020; Pinto et al., 2006), while cinnamon EO contains eugenol and cinnamaldehyde (Khan & Ahmad, 2011) and tea tree EO contains terpenic hydrocarbons, mainly monoterpenes and sesquiterpenes and their associated alcohols (Carson et al., 2006; Singh, 2001).
To examine the available scientific evidence on the efficiency of EO as an alternative therapy to traditional treatment of fungal infections, we analysed studies that used three EO (thyme, cinnamon and tea tree) against three of the causative agents of the most frequent fungal diseases in humans: T. mentagrophytes complex, T. rubrum and C. albicans.
MATERIAL AND METHOD
The study was conducted following the PRISMA statement protocol (Urrútia & Bonfill, 2010). A refined search of articles was carried out in September 2021 using two search engines (PubMed and WoS). The search terms were: “essential oils”, “onychomycosis”, “antifung*” and “fungal*”, combining them with the EO “thymus vulgari*”, “cinnamomum zeylanicu*”, “tea tree oil”, “melaleuca alternifol*” and the micro‐organisms “rubru*”, “mentagrop*”, “candida albican*” and the boolean operator AND. The search term “mentagrop*” includes all species of the Trichophyton mentagrophytes complex, composed of five species: T. mentagrophytes, T. interdigitale, T. erinacei, T. quinckeanum and T. benhamie.
The following search formulas were used: “essential oils AND onychomycosis AND antifung* AND thymus vulgari*”, “essential oils AND antifung* AND thymus vulgari*”, “essential oils AND fungal* AND thymus vulgari*”, “essential oils AND antifung* AND thymus vulgari* AND rubru*”, “essential oils AND antifung* AND thymus vulgari* AND mentagrop*”, “essential oils AND antifung* AND thymus vulgari* AND candida albicans*”, “essential oils AND onychomycosis AND antifung* AND cinnamomum zeylanicum*”, “essential oils AND antifung* AND cinnamomum zeylanicum*”, “essential oils AND fungal* AND cinnamomum zeylanicum*”, “essential oils AND antifung* AND cinnamomum zeylanicum* AND rubru*”, “essential oils AND antifung* AND cinnamomum zeylanicum* AND mentagrop*”, “essential oils AND antifung* AND cinnamomum zeylanicum* AND candida albicans*”, “essential oils AND onychomycosis AND antifung* AND melaleuca alternifol*”, “essential oils AND antifung* AND melaleuca alternifol*”, “essential oils AND fungal* AND melaleuca alternifol*”, “essential oils AND antifung* AND melaleuca alternifol* AND rubru*”, “essential oils AND antifung* AND melaleuca alternifol* AND mentagrop*”, “essential oils AND antifung* AND melaleuca alternifol* AND candida albicans*”, *”, “essential oils AND onychomycosis AND antifung* AND tea tree oil*”, “essential oils AND antifung* AND tea tree oil*”, “essential oils AND fungal* AND tea tree oil*”, “essential oils AND antifung* AND tea tree oil* AND rubru*”, “essential oils AND antifung* AND tea tree oil* AND mentagrop*”, “essential oils AND antifung* AND tea tree oil* AND candida albicans*”.
The articles retrieved using this search strategy are shown in the flow diagram in Figure 1.
FIGURE 1.
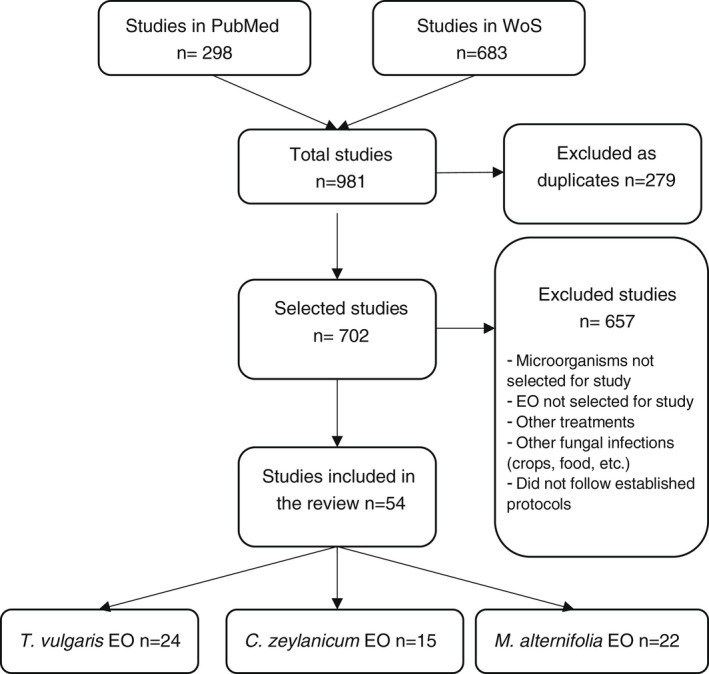
Flow diagram of the article selection process using the defined search strategy
The inclusion criteria for accepting articles for the study were: clinical studies performed in vitro and in vivo clinical trials with humans and animals with the commercial EO specified as antimycotic treatment against reference strains of the selected micro‐organisms and with clinical samples of nails infected with onychomycosis by T. rubrum and T. mentagrophytes complex, following the protocols CLSI M38‐A and/or EUCAST E. Def.11.0 for dermatophytes and CLSI M27‐A2 and/or E. Def.7.3.2 for the yeasts; studies that determined the MIC and MFC; concentration measurement expressed as a percentage (%) and/or v/v (μl ml−1); written in English and/or Spanish; and published from January 2001 to September 2021.
Articles were excluded when they had the search terms but addressed fungal infections elsewhere (food, crops, etc.). Studies in which the micro‐organisms came from clinical samples other than those specified and articles about other types of EO were also excluded. Similarly, others articles that did not follow the protocols indicated, were not written in English and/or Spanish, or were published outside the period 2001 to 2021 were not accepted for the study.
After the initial search in the databases, 981 articles were examined (298 from PubMed and 683 from WoS) and 279 were excluded as duplicates. After the articles had been read and the exclusion criteria had been applied, only 54 were selected for analysis and included in the final review. The 54 articles were classified into three categories: 24 for T. vulgaris EO, 15 for C. zeylanicum EO and 22 for M. alternifolia EO. Seven articles had several EO in common (see Figure 1).
RESULTS AND DISCUSSION
The data obtained from the articles reviewed show that the three EO have antifungal activity against C. albicans, T. mentagrophytes complex and T. rubrum, each with a specific concentration, as shown in the MIC and MFC data. Table 1 shows the range of MIC and MFC values of the three EO for the three micro‐organisms:
TABLE 1.
Range of MIC and MFC values of the EO for each micro‐organism
| C. albicans | T. Mentagrophytes sp. | T. rubrum | ||||
|---|---|---|---|---|---|---|
| EO | MIC | MFC | MIC | MFC | MIC | MFC |
| T. vulgaris |
0.016–3120 b (Alves et al., 2010; Baj et al., 2020; Bogavac et al., 2015; Chaftar et al., 2015; Das Neves et al., 2009; Gavanji & Larki, 2015; Giamperi et al., 2002; López et al., 2007; Nzeako & Bushra, 2008; Orchard et al., 2017; Pina‐Vaz et al., 2004; Pinto et al., 2006; Sacchetti et al., 2004; Sartoratto et al., 2004; Simo Kamdem et al., 2015; van Vuuren et al., 2009) |
0.32–12,500 b (Alves et al., 2010; Assiri et al., 2016; Baj et al., 2020; Bogavac et al., 2015; Gavanji & Larki, 2015; Giamperi et al., 2002; Pina‐Vaz et al., 2004; Pinto et al., 2006; Simo Kamdem et al., 2015) |
2.2–2000 b (Bozin et al., 2006; Giamperi et al., 2002; Inouye et al., 2001; Orchard et al., 2017; Simo Kamdem et al., 2015) |
3–460 b (Bozin et al., 2006; Giamperi et al., 2002; Simo Kamdem et al., 2015) |
0.32–100 b (Bozin et al., 2006; Inouye et al., 2001; Khan et al., 2014) |
4.2 b (Bozin et al., 2006) |
|
0.04–0.31 a (Donaldson et al., 2005; Gucwa et al., 2018; Rajkowska et al., 2014, 2016) | ||||||
| C. zeylanicum |
0.013–1120 b (Castro & Lima, 2013; Elgammal et al., 2020; Gavanji & Larki, 2015; Jantan et al., 2008; López et al., 2007; Miller et al., 2014; Orchard et al., 2017; Rangel et al., 2018; Unlu et al., 2010) |
52–2500 b (Castro & Lima, 2013; Gavanji & Larki, 2015; Rangel et al., 2018) |
0.08–250 b (Inouye et al., 2001; Jantan et al. 2008; Ayatollahi Mousavi & Kazemi, 2015; Orchard et al., 2017; Makimori et al., 2020) |
125–1000 b |
0.08–12.5 b (Inouye et al., 2001; Jantan et al. 2008) |
– |
|
<0.03–0.039 a |
<0.03–0.078 a |
|||||
| M. alternifolia |
9.7–8960 b (Francisconi et al., 2020; Juliano et al., 2008; Noumi et al., 2011; Orchard et al., 2017; Rosato et al., 2008; van Vuuren et al., 2009) |
10,000 – 17,929 b | 250–800 b | – |
400 b (Inouye et al., 2001) |
– |
|
0.008–0.5 a (Haba et al., 2014; Hammer et al., 2003; Mertas et al., 2015; Mondello et al., 2006; Rajkowska et al., 2014, 2016; Rosato et al., 2008, 2021) |
0.25–0.5 a |
0.3 a |
0.5 a |
0.06–0.3 a |
0.06–0.25 a |
|
Abbreviations: MIC, Minimum inhibitory concentration; MFC, Minimum fungicidal concentration. Ref., reference.
Concentration measurements of the oil in percentage (%).
Concentration measurements of the oil in v/v (μl ml−1).
The MIC values of T. vulgaris EO against C. albicans range from 0.16 to 3120 μl ml−1 and the MFC values range from 0.32 to 12,500 μl ml−1 (Alves et al., 2010; Assiri et al., 2016; Baj et al., 2020; Bogavac et al., 2015; Chaftar et al., 2015; Das Neves et al., 2009; Gavanji & Larki, 2015; Giamperi et al., 2002; López et al., 2007; Nzeako & Bushra, 2008; Orchard et al., 2017; Pina‐Vaz et al., 2004; Pinto et al., 2006; Sacchetti et al., 2004; Sartoratto et al., 2004; Simo Kamdem et al., 2015; van Vuuren et al., 2009). Other authors, for example, Donaldson et al. (2005), Rajkowska et al. (2014, 2016) and Gucwa et al. (2018), expressed the MIC of T. vulgaris EO against C. albicans as a percentage, from 0.04 to 0.31% (Donaldson et al., 2005; Gucwa et al., 2018; Rajkowska et al., 2014, 2016). For T. mentagrophytes sp., the MIC values are 2.2–2000 μl ml−1 and the MFC values are 3–460 μl ml−1 (Bozin et al., 2006; Giamperi et al., 2002; Inouye et al., 2001; Orchard et al., 2017; Simo Kamdem et al., 2015). For T. rubrum, the MIC values are 0.32–100 μl ml−1 and the MFC values are 4.2 μl ml−1 (Bozin et al., 2006; Inouye et al., 2001; Khan et al., 2014) (see Table 1).
The MIC values of C. zeylanicum EO against C. albicans range from 0.013 to 1120 μl ml−1 and the MFC values range from 52 to 2500 μl ml−1 (Castro & Lima, 2013; Elgammal et al., 2020; Gavanji & Larki, 2015; Jantan et al., 2008; López et al., 2007; Miller et al., 2014; Orchard et al., 2017; Rangel et al., 2018; Unlu et al., 2010). In the studies by Tran et al. (2020) and Veilleux and Grenier (2019), the antifungal action is expressed as a percentage: the MIC of C. zeylanicum EO against C. albicans is <0.03–0.039% and the MFC is <0.03–0.078% (Tran et al., 2020; Veilleux & Grenier, 2019). For T. mentagrophytes sp., the MIC values are 0.08–250 μl ml−1 and the MFC values are 125–1000 μl ml−1 (Ayatollahi Mousavi & Kazemi, 2015; Inouye et al., 2001; Jantan et al., 2008; Makimori et al., 2020; Orchard et al., 2017). For T. rubrum, only the MIC values were obtained, ranging from 0.08 to 12.5 μl ml−1 (Inouye et al., 2001; Jantan et al., 2008) (see Table 1).
The MIC values of M. alternifolia EO against C. albicans are 9.7–8960 μl ml−1 and the MFC are 10,000–17,929 μl ml−1 (Francisconi et al., 2020; Juliano et al., 2008; Noumi et al., 2011; Orchard et al., 2017; Rosato et al., 2008; van Vuuren et al., 2009). In the studies by Haba et al. (2014), Mertas et al. (2015), Mondello et al. (2006), Hammer et al. (2003), Rajkowska et al. (2014, 2016) and Rosato et al. (2021) on M. alternifolia EO against C. albicans, the MIC value was 0.008–0.5% and the MFC value was 0.25–0.5% (Haba et al., 2014; Hammer et al., 2003; Mertas et al., 2015; Mondello et al., 2006; Rajkowska et al., 2014, 2016; Rosato et al., 2021). For T. mentagrophytes sp. and T. rubrum, only the concentration in μl ml−1 of the MIC was obtained, with values of 250–800 μl ml−1 and 400 μl ml−1, respectively (Inouye et al., 2001; Orchard et al., 2017). For T. mentagrophytes sp. and T. rubrum, the MIC values are 0.3% and 0.06–0.3% and the MFC values are 0.5% and 0.06–0.25%, respectively (Hammer, 2002; Roana et al., 2021) (see Table 1).
Table 1 shows that C. zeylanicum EO has the lowest MIC values against the three micro‐organisms and T. vulgaris EO has the lowest MFC values against T. rubrum and T. mentagrophytes sp.
Table 2 shows the range of MIC and MFC values of the three EO against the micro‐organisms T. rubrum and T. mentagrophytes sp. isolated from onychomycosis clinical samples. It can be seen that T. rubrum requires a lower concentration of the two EO used to inhibit growth. No studies were found with onychomycosis clinical samples using C. zeylanicum EO.
TABLE 2.
Range of MIC and MFC values of the EO against T. rubrum and T. mentagrophytes isolated from onychomycosis clinical samples
| EO | T. Mentagrophytes sp. | T. rubrum | Ref. | ||
|---|---|---|---|---|---|
| MIC | MFC | MIC | MFC | ||
| T. vulgaris | 2.2 b | 4 b | 2 b | 4.2 b | (Bozin et al., 2006) |
| M. alternifolia | 0.3 a | 0.5 a | 0.06–0.3 a | 0.06–0.25 a | (Hammer, 2002; Roana et al., 2021) |
Abbreviations: MIC: Minimum inhibitory concentration; MFC: Minimum fungicidal concentration. Ref.: reference.
Concentration measurements of the oil in percentage (%).
Concentration measurements of the oil in v/v (μl ml−1).
The micro‐organism strain of C. albicans used in each study and the MIC and MFC values obtained in the two units of measure identified (μl ml−1 and %) are shown in Table 3. The final column indicates the study that reported the data for each strain used.
TABLE 3.
Reference strains of C. albicans with their respective MIC and MFC for the three EO: T. vulgaris, C. zeylanicum and M. alternifolia
| C. albicans | T. vulgaris | C. zeylanicum | M. alternifolia | Ref. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain | MIC* | MFC* | MIC† | MIC* | MFC* | MIC† | MFC† | MIC* | MFC* | MIC† | MFC† | |
| ATCC 10231 | 0.04 | (Gucwa et al., 2018) | ||||||||||
| ATCC 10231 | 0.64 | 0.64 | (Pinto et al., 2006) | |||||||||
| ATCC 10231 | 0.25 | 0.5 | (Rajkowska et al., 2014) | |||||||||
| ATCC 10231 | 0.25 | 0.5 | (Rajkowska et al., 2016) | |||||||||
| ATCC 10231 | 0.16–0.32 | 0.32 | (Pina‐Vaz et al., 2004) | |||||||||
| ATCC 10231 | 72 | (Sacchetti et al., 2004) | ||||||||||
| ATCC 10231 | 86 | 137 | 32 | 52 | (Gavanji & Larki, 2015) | |||||||
| ATCC 10231 | 200 | (Sartoratto et al., 2004) | ||||||||||
| ATCC 10231 | 320 | (Das Neves et al., 2009) | ||||||||||
| ATCC 10231 | 320 | (Assiri et al., 2016) | ||||||||||
| ATCC 10231 | 3300 | 6000 | (van Vuuren et al., 2009) | |||||||||
| ATCC 10231 | 460 | (Nzeako & Bushra, 2008) | ||||||||||
| ATCC 10231 | 1000 | 500 | 1.500 | (Orchard et al., 2017) | ||||||||
| ATCC 24433 | 550 | 550 | (Simo Kamdem et al., 2015) | |||||||||
| ATCC 10231 | 1250 | 2500 | (Baj et al., 2020) | |||||||||
| ATCC 10231 | 0.11 | 0.23 | (Bogavac et al., 2015) | |||||||||
| ATCC 10231 | 0.16–0.63 | (Jantan et al. 2008) | ||||||||||
| ATCC 10231 | 3.3 | (Elgammal et al., 2020) | ||||||||||
| ATCC 10231 | 70 | (Unlu et al., 2010) | ||||||||||
| ATCC 10231 | 0.03–0.13 | 0.03–0.25 | (Tran et al., 2020) | |||||||||
| ATCC 10231 | 500 | (Juliano et al., 2008) | ||||||||||
| ATCC 10231 | 3500 | (Rosato et al., 2008) | ||||||||||
| ATCC 10231 | 0.008 | (Haba et al., 2014) | ||||||||||
| ATCC 10231 | 0.125 | 0.25 | (Mertas et al., 2015) | |||||||||
| ATCC 10231 | 0.25 | (Mondello et al., 2006) | ||||||||||
| ATCC 10231 | 0.5 | 0.5 | (Hammer et al., 2003) | |||||||||
| ATCC 10231 | 0.5 | (Rosato et al., 2021) | ||||||||||
| ATCC 2091 | 525 | 0.60 | (Giamperi et al., 2002) | |||||||||
| ATCC 2091 | <0.03–0.13 | <0.03–0.25 | (Tran et al., 2020) | |||||||||
| ATCC 2091 | 9.7 | >10,000 | (Noumi et al., 2011) | |||||||||
| ATCC 90028 | 0.31 | (Donaldson et al., 2005) | ||||||||||
| ATCC 90028 | 0.31–1.25 | (Miller et al. 2015) | ||||||||||
| ATCC 90028 | 1120 | (Unlu et al., 2010) | ||||||||||
| ATCC 90028 | 312 | >10,000 | (Noumi et al., 2011) | |||||||||
| ATCC 90028 | 0.5 | (Rosato et al., 2008) | ||||||||||
| ATCC 90028 | 8960 | 17,920 | (Francisconi et al., 2020) | |||||||||
| ATCC 90028 | 0.25 | 0.5 | (Hammer et al., 2003) | |||||||||
| ATCC 90029 | 125 | 125 | (Rangel et al., 2018) | |||||||||
| ATCC 90029 | 0.25 | (Mondello et al., 2006) | ||||||||||
| ATCC 64550 | 0.026 | 0.013 | (López et al., 2007) | |||||||||
| ATCC 3153 | 300 | (Chaftar et al., 2015) | ||||||||||
| ATCC 40277 | 312.5 | 2500 | (Castro & Lima, 2013) | |||||||||
| ATCC 60193 | 250 | 250 | (Rangel et al., 2018) | |||||||||
| ATCC 28366 | 0.039 | 0.078 | (Veilleux & Grenier, 2019) | |||||||||
| ATCC 14053 | 3500 | (Rosato et al., 2008) | ||||||||||
| ATCC 76615 | 0.25 | (Mondello et al., 2006) | ||||||||||
| ATCC 24433 | 0.25 | (Mondello et al., 2006) | ||||||||||
| CBS‐562 | 3120 | 12,500 | (Alves et al., 2010) | |||||||||
Abbreviations: ATCC/CBS, strain of standard reference micro‐organism; MIC*, Minimum inhibitory concentration in μl ml−1; MFC†, Minimum fungicidal concentration in μl ml−1; MIC†, Minimum inhibitory concentration in %; MFC‡, Minimum fungicidal concentration in % and Ref., reference.
Tables 4 and 5 show the same data as Table 3 but refer to the micro‐organism strain of T. mentagrophytes sp. and T. rubrum used in each study and the MIC and MFC values obtained in μl ml−1.
TABLE 4.
Reference strains of T. mentagrophytes sp. with their respective MIC and MFC for the three EO: T. vulgaris, C. zeylanicum and M. alternifolia
| T. Mentagrophytes sp. | T. vulgaris | C. zeylanicum | M. alternifolia | Ref. | |||
|---|---|---|---|---|---|---|---|
| Strain | MIC | MFC | MIC | MFC | MIC | MFC | |
| ATCC 9533 | 500 | 190 | 250 | (Orchard et al., 2017) | |||
| ATCC 4808 | 2000 | 3 | (Giamperi et al., 2002) | ||||
| E 1425 | 260 | 460 | (Simo Kamdem et al., 2015) | ||||
| TIMM 1189 | 200 | 12.5 | 800 | (Inouye et al., 2001) | |||
| T14– Australian QC | 0.08 | (Jantan et al., 2008) | |||||
| ATCC 9533 | 71 | 125 | (Ayatollahi Mousavi & Kazemi, 2015) | ||||
| ATCC 200099 | 125 | 122 | |||||
| ATCC 11480 | 250 | 1000 | (Makimori et al., 2020) | ||||
Abbreviations: ATCC/TIMM/E, strain of standard reference micro‐organism; MIC, Minimum inhibitory concentration; MFC, Minimum fungicidal concentration. Concentration measurements of the oil in v/v (μl ml−1). Ref., reference.
TABLE 5.
Reference strains of T. rubrum with their respective MIC and MFC (μl ml−1) for the three EO: T. vulgaris, C. zeylanicum and M. alternifolia
| T. rubrum | Thymus vulgaris | C. zeylanicum | M. alternifolia | Ref. | |||
|---|---|---|---|---|---|---|---|
| Strain | MIC | MFC | MIC | MFC | MIC | MFC | |
| TIMM 2659 | 100 | 12.5 | 400 | (Inouye et al., 2001) | |||
| IOA9 | 72 | (Khan et al., 2014) | |||||
| T28 – Australian QC | 0.08 | (Jantan et al., 2008) | |||||
Note: TIMM/T28/IO‡: strain of standard reference micro‐organism. MIC: Minimum inhibitory concentration; MFC: Minimum fungicidal concentration. Concentration measurements of the oil in v/v (μl ml−1). Ref.: reference.
Tables 2, 3, 4, 5 show that T. vulgaris EO has been more frequently mentioned in studies than the other two EO chosen, as 24 articles address the activity of this EO against the micro‐organisms selected. The micro‐organism found in more in vitro tests is C. albicans, for the three EO. The most frequently used reference strain of this micro‐organism is ATCC 10231 (26), followed by strain ATCC 90028 (7) (see Table 3).
Only Orchard et al. (2017) and Inouye et al. (2001) studied M. alternifolia EO against T. mentagrophytes sp. and T. rubrum with standard strains, and no data were obtained about their MFC except against C. albicans (see Tables 3, 4 and 5) (Inouye et al., 2001; Orchard et al., 2017). In contrast, Hammer (2002) and Roana et al. (2021) determined the MIC and MFC values for T. mentagrophytes sp. and T. rubrum using onychomycosis clinical samples (Hammer, 2002; Roana et al., 2021). The same occurred with T. vulgaris and C. zeylanicum EO for T. rubrum, for which no MFC data were obtained (Das Neves et al., 2009; Miller et al., 2014; van Vuuren et al., 2009) (see Table 5). However, Bozin et al. (2006) reported data on the MFC of T. vulgaris EO against T. rubrum using onychomycosis clinical samples (Bozin et al., 2006). Only three studies using onychomycosis clinical samples were found, with the micro‐organisms T. rubrum and T. mentagrophytes sp. and T. vulgaris and M. alternifolia EO. The two EO show very similar values for the two micro‐organisms, but are not comparable because the authors measured MIC and MFC with different concentration measures (see Table 2). These two micro‐organisms are particularly significant for podiatrists, as 90% of toenail onychomycoses are caused by these dermatophytes (Bodman & Krishnamurthy, 2021; Gupta et al., 2015; Joyce et al., 2019; Thomas et al., 2010; Youssef et al., 2018).
With regard to in vivo studies, tests were found about the antifungal activity of EO on the selected micro‐organisms. We found three in vivo clinical tests on humans reporting antifungal efficacy of EO against T. rubrum (Romero‐Cerecero et al., 2008, 2009), but only one of the tests used M. alternifolia EO among its components, showing complete cure in 12 months in 78.5% (n = 14) of patients with onychomycosis caused by T. rubrum (Alessandrini et al., 2020). Another in vivo clinical test was performed on patients with palatal inflammation due to denture stomatitis (n = 27) caused by C. albicans, in which the inflammation in the mouth decreased (Catalán et al., 2008). Various clinical tests on animals (mice, rats and guinea pigs) were also found, as shown in Table 6.
TABLE 6.
Clinical trials with animals infected by the micro‐organisms for the three EO: T. vulgaris, C. zeylanicum and M. alternifolia
| Animals | n | Location | Micro‐organism | EO | Action | Ref. |
|---|---|---|---|---|---|---|
| Male guinea pigs | 55 | Dermal | T. mentagrophytes sp. | C. zeylanicum | Complete cure at 9–11 days | (Ayatollahi Mousavi & Kazemi, 2015) |
| Mice | 12 | Oral | C. albicans | M. alternifolia | Protective activity | (Ninomiya et al., 2012) |
| Mice | 6 | Oral | C. albicans | M. alternifolia | Anti‐inflammatory action | (Ninomiya et al., 2013) |
| Mice | 12 | Oral | C. albicans | M. alternifolia | Reduced microscopic lesions | (Campos Rasteiro et al., 2014) |
| Rats | 10 | Vaginal | C. albicans | M. alternifolia | Removed infections | (Mondello et al., 2006) |
| Wistar rats | 20 | Dermal |
T. rubrum T. mentagrophytes sp. |
T. vulgaris |
Complete cure at 8–20 days Anti‐inflammatory action |
(Soković et al., 2008) |
Abbreviations: EO, Essential Oil; n: sample size; Ref., References.
Of the three micro‐organisms studied, C. albicans shows the lowest MIC values with the three EO (see Table 1). Both T. rubrum and T. mentagrophytes sp. show the lowest MIC values with C. zeylanicum.
The studies reviewed revealed different MIC and MFC values depending on the micro‐organism strain and the commercial EO used. This may depend on where the EO is from, its chemical composition and the research methodology used to obtain the values (Parrish et al., 2020), although further study is necessary to demonstrate this. The MIC and MFC values of C. albicans with the three EO are very broad, due to the high number of studies addressing this pathogen (see Table 1). The lowest MIC range found was for C. zeylanicum EO against T. rubrum, and the lowest MFC was for T. vulgaris EO against T. rubrum, which may be due to the few studies with these EO for this micro‐organism, and therefore more studies are needed to determine the ideal concentration (see Table 1). Each specific oil needs to be studied against each specific pathogen species, and it would be worthwhile studying the effect of each particular compound in the EO.
Identifying the effects of the EO, at different concentrations, on certain pathogenic micro‐organisms could help to combat acquired resistance of micro‐organisms to medications and may also reduce medication side effects.
Although recent studies are promising, we agree with Bogavac et al. (2015) and Mutlu‐Ingok et al. (2020) that we must continue to consider the possible toxicity of these products, because EO are known to present a high risk of skin allergy and irritation. It is therefore important to continue studying EO and accurately determine the safety (harmlessness) of their use and the associated risks (Bogavac et al., 2015; Mutlu‐Ingok et al., 2020).
CONCLUSION
Essential oils are a possible alternative therapy with empirical evidence of good results, and the antifungal effects of EO may be a very promising solution to overcome the therapeutic shortcomings of antimycotic medication, which are increasing with immunosuppressive treatments and the appearance of resistant strains, among other factors. Some studies have highlighted the fungicidal action of EO against diseases caused by fungi in humans, including onychomycosis, a high prevalence condition.
Cinnamomum zeylanicum and T. vulgaris EO have been shown to be effective against C. albicans, T. rubrum and T. mentagrophytes sp. However, M. alternifolia EO has little scientific evidence as yet, and the MIC has been reported only for T. rubrum and T. mentagrophytes sp. in isolated strains. Candida albicans is the most frequently studied micro‐organism against EO, but more studies with EO against T. rubrum and T. mentagrophytes sp. are needed, as these are the most frequent causative agents of onychomycosis.
Despite all the progress found in studies on EO, more experiments are needed to examine the properties of the oils already studied, and other oils, to devise effective and nonaggressive therapies for treatment of dermatophytosis.
CONFLICT OF INTEREST
No conflict of interest declared.
ACKNOWLEDGEMENTS
This research was funded by the Extremadura Regional Government and the European Regional Development Fund (ERDF) through a grant to the research group [code CTS020, reference GR21077]. Acknowledgments: We thank Jane McGrath for assistance with the translation and final language review.
Villar Rodríguez, J. , Pérez‐Pico, A.M. , Mingorance‐Álvarez, E. & Mayordomo Acevedo, R. (2022) Meta‐analysis of the antifungal activities of three essential oils as alternative therapies in dermatophytosis infections. Journal of Applied Microbiology, 133, 241–253. Available from: 10.1111/jam.15539
Funding informationThis study was funded by the Extremadura Regional Government and the European Regional Development Fund (ERDF) through a grant to the research group (code CTS020, reference GR20077).
Contributor Information
Julia Villar Rodríguez, Email: juliavr@unex.es.
Ana María Pérez‐Pico, Email: aperpic@unex.es.
Esther Mingorance‐Álvarez, Email: emingorance@unex.es.
Raquel Mayordomo Acevedo, Email: rmayordo@unex.es.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study
REFERENCES
- Abd Rashed, A. , Rathi, D.N.G. , Ahmad Nasir, N.A.H. & Abd Rahman, A.Z. (2021) Antifungal properties of essential oils and their compounds for application in skin fungal infections: conventional and nonconventional approaches. Molecules, 26(4), 109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agencia Española de Medicamentos and Productos Sanitarios (AEMPS) , (2016) Guía Aceites Esenciales En Productos Cosméticos.
- Ahmadi, B. , Mirhendi, H. , Shidfar, M.R. , Nouripour‐Sisakht, S. , Jalalizand, N. , Geramishoar, M. et al. (2015) A comparative study on morphological versus molecular identification of dermatophyte isolates. Journal de Mycologie Médicale, 25(1), 29–35. [DOI] [PubMed] [Google Scholar]
- Alessandrini, A. , Starace, F.M. , Bruni, F. & Piaccini, B.M. (2020) An open study to evaluate effectiveness and tolerability of a nail oil composed of vitamin E and essential oils in mild to moderate distal subungual onychomycosis. Skin Appendage Disorders, 6(1), 14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves, I.P.T. , Garza‐Ramos, M.A.D.l. , Wong, L.J.G. , Niño, K.A. & García, A.R. (2010) In vitro tests of antifungal activity of plants used in Mexican traditional medicine. In: Microorganisms in industry and environment. London: World Scientific Publishing Co. Pte. Ltd., pp. 488–491. [Google Scholar]
- Assiri, A.M.A. , Elbanna, K. , Abulreesh, H.H. & Ramadan, M.F. (2016) Bioactive compounds of cold‐pressed thyme (thymus vulgaris) oil with antioxidant and antimicrobial properties. Journal of Oleo Science, 65(8), 629–640. [DOI] [PubMed] [Google Scholar]
- Ayatollahi Mousavi, S.A. & Kazemi, A. (2015) In vitro and in vivo antidermatophytic activities of some Iranian medicinal plants. Medical Mycology, 53(8), 852–859. [DOI] [PubMed] [Google Scholar]
- Baj, T. , Biernasiuk, A. , Wróbel, R. & Malm, A. (2020) Chemical composition and in vitro activity of Origanum vulgare L., Satureja hortensis L., Thymus serpyllum L. and Thymus vulgaris L. essential oils towards oral isolates of Candida albicans and Candida glabrata. Open Chemistry, 18(1), 108–118. [Google Scholar]
- Bhatia, V.K. & Sharma, P.C. (2014) Epidemiological studies on dermatophytosis in human patients in Himachal Pradesh, India. Springerplus, 3(1), 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodman, M.A. & Krishnamurthy, K. (2021) Onychomycosis. StatPearls: StatPearls Publishing. [PubMed] [Google Scholar]
- Bogavac, M. , Karaman, M. , Janjušević, L. , Sudji, J. , Radovanović, B. , Novaković, Z. et al. (2015) Alternative treatment of vaginal infections ‐ in vitro antimicrobial and toxic effects of Coriandrum sativum L. and Thymus vulgaris L. essential oils. Journal of Applied Microbiology, 119(3), 697–710. [DOI] [PubMed] [Google Scholar]
- Bozin, B. , Mimica‐Dukic, N. , Simin, N. & Anackov, G. (2006) Characterization of the volatile composition of essential oils of some lamiaceae spices and the antimicrobial and antioxidant activities of the entire oils. Journal of Agricultural and Food Chemistry., 54(5), 1822–1828. [DOI] [PubMed] [Google Scholar]
- Campos Rasteiro, M.V. , Pereira, B. , da Costa, A.C. , Fernandes Araújo, C. , Pimentel De Barros, P. , Rossoni, R.D. et al. (2014) Essential oil of Melaleuca alternifolia for the treatment of Oral candidiasis induced in an immunosuppressed mouse model. BMC Complementary Medicine and Therapies, 14(489), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson, C.F. , Hammer, K.A. & Riley, T.V. (2006) Melaleuca alternifolia (tea tree) oil: a review of antimicrobial and other medicinal properties. Clinical Microbiology Reviews, 19(1), 50–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro, R.D. & Lima, E.O. (2013) Anti‐Candida activity and chemical composition of Cinnamomum zeylanicum blume essential oil. Brazilian Archives of Biology and Technology, 56(5), 749–755. [Google Scholar]
- Catalán, A. , Pacheco, J.G. , Martínez, A. & Mondaca, M.A. (2008) In vitro and in vivo activity of Melaleuca alternifolia mixed with tissue conditioner on Candida albicans. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics, 105(3), 327–332. [DOI] [PubMed] [Google Scholar]
- Chaftar, N. , Girardot, M. , Labanowski, J. , Ghrairi, T. , Hani, K. , Frère, J. et al. (2015) Comparative evaluation of the antimicrobial activity of 19 essential oils. Advances in Experimental Medicine and Biology, 901, 1–15. [DOI] [PubMed] [Google Scholar]
- Chang, C.‐H. , Young‐Xu, Y. , Kurth, T. , Orav, J.E. & Chan, A.K. (2007) The safety of oral antifungal treatments for superficial dermatophytosis and onychomycosis: a meta‐analysis. The American Journal of Medicine, 120(9), 791–798. [DOI] [PubMed] [Google Scholar]
- Córdoba, S. , Vivot, W. , Szusz, W. & Albo, G. (2019) Antifungal activity of essential oils against candida species isolated from clinical samples. Mycopathologia, 184(5), 615–623. [DOI] [PubMed] [Google Scholar]
- D'agostino, T. , Frippiat, M. & Debourgogne, L. (2019) Essential oils and their natural active compounds presenting antifungal properties. Molecules, 24(20), 3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Cruz Cabral, L. , Fernández Pinto, V. & Patriarca, A. (2013) Application of plant derived compounds to control fungal spoilage and mycotoxin production in foods. International Journal of Food Microbiology, 166(1), 1–14. [DOI] [PubMed] [Google Scholar]
- Das Neves, J. , Pinto, E. , Amaral, M.H. & Bahia, M.F. (2009) Antifungal activity of a gel containing Thymus vulgaris essential oil against Candida species commonly involved in vulvovaginal candidosis. Pharmaceutical Biology, 47(2), 151–153. [Google Scholar]
- Donaldson, J.R. , Warner, S.L. , Cates, R.G. & Gary Young, D. (2005) Assessment of antimicrobial activity of fourteen essential oils when using dilution and diffusion methods. Pharmaceutical Biology, 43(8), 687–695. [Google Scholar]
- Elgammal, E. , El Gendy, A.E.N. & Elgamal, A.E.‐B. (2020) Mechanism of action and bioactivities of Cinnamomum zeylanicum essential oil against some pathogenic microbes. Egyptian Pharmaceutical Journal, 19(2), 162. [Google Scholar]
- European Pharmacopoeia . Essential Oils. 2021.
- Flores, F.C. , Beck, R.C.R. & da Silva, C.D.B. (2015) Essential oils for treatment for onychomycosis: a mini‐review. Mycopathologia, 181(1–2), 9–15. [DOI] [PubMed] [Google Scholar]
- Francisconi, R.S. , Huacho, P.M.M. , Tonon, C.C. , Bordini, E.A.F. , Correia, M.F. , Sardi, J.d.C.O. et al. (2020) Antibiofilm efficacy of tea tree oil and of its main component terpinen‐4‐ol against Candida albicans. Brazilian Oral Research, 34, 1–9. [DOI] [PubMed] [Google Scholar]
- Gavanji, S. & Larki, B. (2015) Comparative effect of propolis of honeybee and some herbal extracts on Candida albicans. Chinese Journal of Integrative Medicine, 23(3), 201–207. [DOI] [PubMed] [Google Scholar]
- Giamperi, L. , Fraternale, D. & Ricci, D. (2002) The in vitro action of essential oils on different organisms. Journal of Essential Oil Research., 14(4), 312–318. [Google Scholar]
- Gucwa, K. , Milewski, S. , Dymerski, T. & Szweda, P. (2018) Investigation of the antifungal activity and mode of action of thymus vulgaris, citrus limonum, pelargonium graveolens, cinnamomum cassia, ocimum basilicum, and eugenia caryophyllus essential oils. Molecules, 23(5), 1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, A.K. , Mays, R.R. , Versteeg, S.G. , Piraccini, B.M. , Takwale, A. , Shemer, A. et al. (2018) Global perspectives for the management of onychomycosis. International Journal of Dermatology, 58(10), 1118–1129. [DOI] [PubMed] [Google Scholar]
- Gupta, A.K. , Sibbald, R.G. , Andriessen, A. , Belley, R. , Boroditsky, A. , Botros, M. et al. (2015) Toenail onychomycosis—a Canadian approach with a new transungual treatment. Journal of Cutaneous Medicine and Surgery, 19(5), 440–449. [DOI] [PubMed] [Google Scholar]
- Haba, E. , Bouhdid, S. , Torrego‐Solana, N. , Marqués, A.M. , Espuny, M.J. , García‐Celma, M.J. et al. (2014) Rhamnolipids as emulsifying agents for essential oil formulations: antimicrobial effect against Candida albicans and methicillin‐resistant Staphylococcus aureus. International Journal of Pharmaceutics, 476(1–2), 134–141. [DOI] [PubMed] [Google Scholar]
- Hammer, K.A. (2002) In vitro activity of Melaleuca alternifolia (tea tree) oil against dermatophytes and other filamentous fungi. Journal of Antimicrobial Chemotherapy, 50(2), 195–199. [DOI] [PubMed] [Google Scholar]
- Hammer, K.A. , Carson, C.F. & Riley, T.V. (2003) Antifungal activity of the components of Melaleuca alternifolia (tea tree) oil. Journal of Applied Microbiology, 95(4), 853–860. [DOI] [PubMed] [Google Scholar]
- Hongpattarakere, T. , Chanthachum, S. & Chanthaphon, S. (2008) Antimicrobial activities of essential oils and crude extracts from tropical citrus spp. against food‐related microorganisms. Songklanakarin Journal of Science and Technology, 30, 125–131. [Google Scholar]
- Hoy, N.Y. , Leung, A.K.C. , Metelitsa, A.I. & Adams, S. (2012) New concepts in median nail dystrophy, onychomycosis, and hand, foot, and mouth disease nail pathology. ISRN Dermatology, 2012, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye, S. , Uchida, K. & Yamaguchi, H. (2001) In‐vitro and in‐vivo anti‐trichophyton activity of essential oils by vapour contact. Mycoses, 44(3–4), 99–107. [DOI] [PubMed] [Google Scholar]
- Jantan, I.B. , Karim Moharam, B.A. , Santhanam, J. & Jamal, J.A. (2008) Correlation between chemical composition and antifungal activity of the essential oils of eightcinnamomum. Species. Pharmaceutical Biology, 46(6), 406–412. [Google Scholar]
- Joyce, A. , Gupta, A.K. , Koenig, L. , Wolcott, R. & Carviel, J. (2019) Fungal diversity and onychomycosis. Journal of the American Podiatric Medical Association, 109(1), 57–63. [DOI] [PubMed] [Google Scholar]
- Juliano, C. , Demurtas, C. & Piu, L. (2008) In vitro study on the anticandidal activity of Melaleuca alternifolia (tea tree) essential oil combined with chitosan. Flavour and Fragrance Journal, 23(4), 227–231. [Google Scholar]
- Khan, M.S.A. & Ahmad, I. (2011) In vitro antifungal, anti‐elastase and anti‐keratinase activity of essential oils of Cinnamomum‐, Syzygium‐ and Cymbopogon‐species against aspergillus fumigatus and trichophyton rubrum. Phytomedicine, 19(1), 48–55. [DOI] [PubMed] [Google Scholar]
- Khan, M.S.A. , Ahmad, I. & Cameotra, S.S. (2014) Carum copticum and Thymus vulgaris oils inhibit virulence in trichophyton rubrum and aspergillus spp. Brazilian Journal of Microbiology., 45(2), 523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk, A. , Przychodna, M. , Sopata, S. , Bodalska, A. & Fecka, I. (2020) Thymol and thyme essential oil—new insights into selected therapeutic applications. Molecules, 25(18), 4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, C.J. , Chen, L.W. , Chen, L.G. , Chang, T.L. , Huang, C.W. , Huang, M.C. et al. (2013) Correlations of the components of tea tree oil with its antibacterial effects and skin irritation. Journal of Food and Drug Analysis, 21(2), 169–176. [Google Scholar]
- Leung, A.K.C. , Lam, J.M. , Leong, K.F. , Hon, K.L. , Barankin, B. , Leung, A.A.M. et al. (2020) Onychomycosis: an updated review. Recent Patents on Inflammation & Allergy Drug Discovery, 14(1), 32–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipner, S.R. & Scher, R.K. (2019) Onychomycosis. Journal of the American Academy of Dermatology, 80(4), 835–851. [DOI] [PubMed] [Google Scholar]
- Lopes, G. , Pinto, E. & Salgueiro, L. (2016) Natural products: an alternative to conventional therapy for dermatophytosis? Mycopathologia, 182(1–2), 143–167. [DOI] [PubMed] [Google Scholar]
- López, P. , Sánchez, C. , Batlle, R. & Nerín, C. (2007) Vapor‐phase activities of cinnamon, thyme, and oregano essential oils and key constituents against foodborne microorganisms. Journal of Agricultural and Food Chemistry., 55(11), 4348–4356. [DOI] [PubMed] [Google Scholar]
- Mahmoudvand, H. , Sepahvand, A. , Jahanbakhsh, S. , Ezatpour, B. & Ayatollahi Mousavi, S.A. (2014) Evaluation of antifungal activities of the essential oil and various extracts of Nigella sativa and its main component, thymoquinone against pathogenic dermatophyte strains. Journal de Mycologie Médicale, 24(4), e155–e161. [DOI] [PubMed] [Google Scholar]
- Makimori, R.Y. , Endo, E.H. , Makimori, J.W. , Zanqueta, E.B. , Ueda‐Nakamura, T. , Leimann, F.V. et al. (2020) Preparation, characterization and antidermatophytic activity of free‐ and microencapsulated cinnamon essential oil. Journal de Mycologie Médicale., 30(2), 1009–1033. [DOI] [PubMed] [Google Scholar]
- Martín‐Aragón, S. & Benedí, J. (2004) Antimicóticos dermatológicos. Revisión. Farmacia Profesional, 18(7), 38–49. [Google Scholar]
- Mertas, A. , Garbusińska, A. , Szliszka, E. , Jureczko, A. , Kowalska, M. & Król, W. (2015) The influence of tea tree oil (Melaleuca alternifolia) on fluconazole activity against fluconazole‐resistantcandida albicansstrains. BioMed Research International, 2015, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalczyk, A. & Ostrowska, P. (2021) Essential oils and their components in combating fungal pathogens of animal and human skin. Journal of Medical Mycology, 31(2), 101–118. [DOI] [PubMed] [Google Scholar]
- Miller, A.B. , Cates, R.G. , Lawrence, M. , Soria, J.A.F. , Espinoza, L.V. , Martinez, J.V. et al. (2014) The antibacterial and antifungal activity of essential oils extracted from Guatemalan medicinal plants. Pharmaceutical Biology, 53(4), 548–554. [DOI] [PubMed] [Google Scholar]
- Mondello, F. , De Bernardis, F. , Girolamo, A. , Cassone, A. & Salvatore, G. (2006) In vivo activity of terpinen‐4‐ol, the main bioactive component of Melaleuca alternifolia Cheel (tea tree) oil against azole‐susceptible and ‐resistant human pathogenic Candida species. BMC Infectious Diseases, 6(1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutlu‐Ingok, A. , Devecioglu, D. , Dikmetas, D.N. , Karbancioglu‐Guler, F. & Capanoglu, E. (2020) Antibacterial, antifungal, antimycotoxigenic, and antioxidant activities of essential oils: an updated review. Molecules, 25(20), 4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natu, K.N. & Tatke, P.A. (2019) Essential oils – prospective candidates for antifungal treatment? Journal of Essential Oil Research, 31(5), 347–360. 10.1080/10412905.2019.1604437 [DOI] [Google Scholar]
- Ninomiya, K. , Hayama, K. , Ishijima, S.A. , Maruyama, N. , Irie, H. , Kurihara, J. et al. (2013) Suppression of inflammatory reactions by Terpinen‐4‐Ol, a Main constituent of tea tree oil, in a murine model of Oral candidiasis and its suppressive activity to cytokine production of macrophages in vitro. Biological & Pharmaceutical Bulletin, 36(5), 838–844. [DOI] [PubMed] [Google Scholar]
- Ninomiya, K. , Maruyama, N. , Hiroko Ishibashi, S.I. , Haruyuki Oshima, T.T. & Abe, S. (2012) The essential oil of Melaleuca alternifolia (tea tree oil) and its Main component, Terpinen‐4‐Ol protect mice from experimental Oral candidiasis. Biological & Pharmaceutical Bulletin, 35(6), 861–865. [DOI] [PubMed] [Google Scholar]
- Noumi, E. , Snoussi, M. & Hafedh, H. (2011) Chemical composition, antioxidant and antifungal potential of Melaleuca alternifolia (tea tree) and eucalyptus globulus essential oils against Oral Candida species. Journal of Medicinal Plants Research, 5(17), 4147–4156. [Google Scholar]
- Nzeako, B.C. & Lawati, B.A. (2008) Comparative studies of antimycotic potential of thyme and clove oil extracts with antifungal antibiotics on Candida albicans. African Journal of Biotechnology, 7(11), 1612–1619. [Google Scholar]
- Orchard, A. , Sandasi, M. , Kamatou, G. , Viljoen, A. & van Vuuren, S. (2017) The in vitro antimicrobial activity and chemometric modelling of 59 commercial essential oils against pathogens of dermatological relevance. Chemistry & Biodiversity., 14(1), 160–218. [DOI] [PubMed] [Google Scholar]
- Papini, M. , Piraccini, B.M. , Difonzo, E. & Brunoro, A. (2015) Epidemiology of onychomycosis in Italy: prevalence data and risk factor identification. Mycoses, 58(11), 659–664. [DOI] [PubMed] [Google Scholar]
- Parrish, N. , Fisher, S.L. , Gartling, A. , Craig, D. , Boire, N. , Khuvis, J. et al. (2020) Activity of various essential oils against clinical dermatophytes of microsporum and trichophyton. Frontiers in Cellular and Infection Microbiology, 10, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pina‐Vaz, C. , Goncalves Rodrigues, A. , Pinto, E. , Costa‐de‐Oliveira, S. , Tavares, C. , Salgueiro, L. et al. (2004) Antifungal activity of thymus oils and their major compounds. Journal of the European Academy of Dermatology and Venereology, 18(1), 73–78. [DOI] [PubMed] [Google Scholar]
- Pinto, E. , Pina‐Vaz, C. , Salgueiro, L. , Gonçalves, M.J. , Costa‐de‐Oliveira, S. , Cavaleiro, C. et al. (2006) Antifungal activity of the essential oil of Thymus pulegioides on Candida, aspergillus and dermatophyte species. Journal of Medical Microbiology, 55(10), 1367–1373. [DOI] [PubMed] [Google Scholar]
- Rajkowska, K. , Kunicka‐Styczyńska, A. , Maroszyńska, M. & Dąbrowska, M. (2014) The effect of thyme and tea tree oils on morphology and metabolism of candida albicans. Acta Biochimica Polonica, 61(2), 305–310. [PubMed] [Google Scholar]
- Rajkowska, K. , Nowak, A. , Kunicka‐Styczyńska, A. & Siadura, A. (2016) Biological effects of various chemically characterized essential oils: investigation of the mode of action against Candida albicans and HeLa cells. RSC Advances, 6(99), 97199–97207. [Google Scholar]
- Rangel, M.d.L. , Aquino, S.G.D. , Lima, J.M.D. , Castellano, L.R. & Castro, R.D.D. (2018) In vitro effect of Cinnamomum zeylanicum blume essential oil on Candida spp. involved in oral infections. Evidence‐Based Complementary and Alternative Medicine, 2018, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roana, J. , Mandras, N. , Scalas, D. , Campagna, P. & Tullio, V. (2021) Antifungal activity of Melaleuca alternifolia essential oil (TTO) and its synergy with itraconazole or ketoconazole against Trichophyton rubrum . Molecules, 26(2), 461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero‐Cerecero, O. , Román‐Ramos, R. , Zamilpa, A. , Jiménez‐Ferrer, J.E. , Rojas‐Bribiesca, G. & Tortoriello, J. (2009) Clinical trial to compare the effectiveness of two concentrations of the Ageratina Pichinchensis extract in the topical treatment of onychomycosis. Journal of Ethnopharmacology, 126(1), 74–78. [DOI] [PubMed] [Google Scholar]
- Romero‐Cerecero, O. , Zamilpa, A. , Jiménez‐Ferrer, J.E. , Rojas‐Bribiesca, G. , Román‐Ramos, R. & Tortoriello, J. (2008) Double‐blind clinical trial for evaluating the effectiveness and tolerability of Ageratina Pichinchensis extract on patients with mild to moderate onychomycosis. A comparative study with ciclopirox. Planta Medica, 74(12), 1430–1435. [DOI] [PubMed] [Google Scholar]
- Rosato, A. , Altini, E. , Sblano, S. , Salvagno, L. , Maggi, F. , de Michele, G. et al. (2021) Synergistic activity of new diclofenac and essential oils combinations against different candida spp. Antibiotics, 10(6), 688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosato, A. , Vitali, C. , Gallo, D. , Balenzano, L. & Mallamaci, R. (2008) The inhibition of Candida species by selected essential oils and their synergism with amphotericin B. Phytomedicine, 15(8), 635–638. [DOI] [PubMed] [Google Scholar]
- Saad, N.Y. , Muller, C.D. & Lobstein, A. (2013) Major bioactivities and mechanism of action of essential oils and their components. Flavour and Fragrance Journal, 28(5), 269–279. [Google Scholar]
- Sacchetti, G. , Medici, A. , Maietti, S. , Radice, M. , Muzzoli, M. , Manfredini, S. et al. (2004) Composition and functional properties of the essential oil of amazonian basil, ocimum micranthum willd., labiatae in comparison with commercial essential oils. Journal of Agricultural and Food Chemistry, 52(11), 3486–3491. [DOI] [PubMed] [Google Scholar]
- Sartoratto, A. , Machado, A.L.M. , Delarmelina, C. , Figueira, G.M. , Duarte, M.C.T. & Rehder, V.L.G. (2004) Composition and antimicrobial activity of essential oils from aromatic plants used in Brazil. Brazilian Journal of Microbiology, 35(4), 275–280. [Google Scholar]
- Scalas, D. , Mandras, N. , Roana, J. , Tardugno, R. , Cuffini, A.M. , Ghisetti, V. et al. (2018) Use of Pinus sylvestris L. (Pinaceae), Origanum vulgare L. (Lamiaceae), and Thymus vulgaris L. (Lamiaceae) essential oils and their main components to enhance itraconazole activity against azole susceptible/not‐susceptible Cryptococcus neoformans strains. BMC Complementary and Alternative Medicine, 18(1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi‐Rad, J. , Sureda, A. , Tenore, G. , Daglia, M. , Sharifi‐Rad, M. , Valussi, M. et al. (2017) Biological activities of essential oils: from plant chemoecology to traditional healing systems. Molecules, 22(1), 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simo Kamdem, M. , Lambert Sameza, M. , Jazet Dongmo, P.M. , Fekam Boyom, F. , Bakargna‐Via, I. , Hzounda Fokou, J.B. et al. (2015) Antiradical, anti‐inflammatory and antifungal activities of essential oils of two aromatic plants: apium graveolens (apiaceae) and thymus vulgaris (lamiaceae). Journal of Life Sciences, 10(2), 51–64. [Google Scholar]
- Singh, N. (2001) Trends in the epidemiology of opportunistic fungal infections: predisposing factors and the impact of antimicrobial use practices. Clinical Infectious Diseases, 33(10), 1692–1696. [DOI] [PubMed] [Google Scholar]
- Soković, M. , Glamočlija, J. , Ćirić, A. , Kataranovski, D. , Marin, P.D. , Vukojević, J. et al. (2008) Antifungal activity of the essential oil of thymus vulgaris L. and thymol on experimentally induced dermatomycoses. Drug Development and Industrial Pharmacy, 34(12), 1388–1393. [DOI] [PubMed] [Google Scholar]
- Swamy, M.K. , Akhtar, M.S. & Sinniah, U.R. (2016) Antimicrobial properties of plant essential oils against human pathogens and their mode of action: an updated review. Evidence‐Based Complementary and Alternative Medicine, 2016, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchernev, G. , Penev, P.K. , Nenoff, P. , Zisova, L.G. , Cardoso, J.C. , Taneva, T. et al. (2012) Onychomycosis: modern diagnostic and treatment approaches. Wiener Medizinische Wochenschrift, 163(1–2), 1–12. [DOI] [PubMed] [Google Scholar]
- Thomas, J. , Jacobson, G.A. , Narkowicz, C.K. , Peterson, G.M. , Burnet, H. & Sharpe, C. (2010) Toenail onychomycosis: an important global disease burden. Journal of Clinical Pharmacy and Therapeutics, 35(5), 497–519. [DOI] [PubMed] [Google Scholar]
- Tran, H.N.H. , Graham, L. & Adukwu, E.C. (2020) In vitro antifungal activity of Cinnamomum zeylanicum bark and leaf essential oils against Candida albicans and Candida auris . Applied Microbiology and Biotechnology, 104(20), 8911–8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unlu, M. , Ergene, E. , Unlu, G.V. , Zeytinoglu, H.S. & Vural, N. (2010) Composition, antimicrobial activity and in vitro cytotoxicity of essential oil from Cinnamomum zeylanicum Blume (Lauraceae). Food and Chemical Toxicology, 48(11), 3274–3280. [DOI] [PubMed] [Google Scholar]
- Urrútia, G. & Bonfill, X. (2010) Declaración PRISMA: una propuesta para mejorar la publicación de revisiones sistemáticas y metaanálisis. Medicina Clínica, 135(11), 507–511. [DOI] [PubMed] [Google Scholar]
- Valdes, A.M.P. (2000) Nuevos antimicóticos orales: alternativas en el tratamiento de las micosis superficiales. Revista chilena de infectología, 17(2), 161–166. [Google Scholar]
- van Vuuren, S.F. , Suliman, S. & Viljoen, A.M. (2009) The antimicrobial activity of four commercial essential oils in combination with conventional antimicrobials. Letters in Applied Microbiology, 48(4), 440–446. [DOI] [PubMed] [Google Scholar]
- Veilleux, M.P. & Grenier, D. (2019) Determination of the effects of cinnamon bark fractions on Candida albicans and oral epithelial cells. BMC Complementary and Alternative Medicine, 19(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar Rodríguez, J. , Pérez Pico, A.M. , Mingorance Álvarez, E. & Mayordomo Acevedo, R. (2021) Revisión del uso de aceites esenciales como tratamiento antifúngico frente a onicomicosis. Conocimientos, Investigación y prácticas en el campo de la salud: actualización de competencias . España: ASUNIVEP, 28, 215–222. [Google Scholar]
- Vlahovic, T.C. (2016) Onychomycosis. Clinics in Podiatric Medicine and Surgery, 33(3), 305–318. [DOI] [PubMed] [Google Scholar]
- Wińska, K. , Mączka, W. , Łyczko, J. , Grabarczyk, M. , Czubaszek, A. & Szumny, A. (2019) Essential oils as antimicrobial agents—myth or real alternative? Molecules, 24(11), 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef, A.B. , Kallel, A. , Azaiz, Z. , Jemel, S. , Bada, N. , Chouchen, A. et al. (2018) Onychomycosis: which fungal species are involved? Experience of the laboratory of parasitology‐mycology of the rabta hospital of Tunis. Journal de Mycologie Médicale, 28(4), 651–654. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study


